Abstract
Background and Objective
Abnormally high glucose levels (dysglycemia) increase with age. Epidemiological studies suggest that dysglycemia is a risk factor for cognitive impairment but the underlying pathophysiological mechanisms remain unclear. The objective of this study was to examine the relation of dysglycemia clinical categories (Normal glucose tolerance (NGT), pre-diabetes, undiagnosed diabetes, known diabetes) with brain structure in older adults. We also assessed the relation between dysglycemia and cognitive performance.
Design, Setting, Participants
Cross-sectional and longitudinal analyses in 618 non-demented elderly from the multiethnic Washington Heights Inwood Columbia Aging Project (WHICAP).
Measurements
Dysglycemia categories were based on HBA1c or history of type 2 diabetes (diabetes). Brain structure (brain infarcts, white matter hyperintensities (WMH) volume, total gray matter volume, total white matter volume, total hippocampus volume) was assessed with brain MRI; cognitive function (memory, language and visuospatial function, speed) was assessed with a validated neuropsychological battery.
Results
Dysglycemia, defined with HbA1c as a continuous variable or categorically as pre-diabetes and diabetes, was associated with a higher number of brain infarcts, WMH volume and decreased total white matter, gray matter and hippocampus volumes cross-sectionally, and a significant decline in gray matter volume longitudinally. Dysglycemia was also associated with lower performance in language, speed and visuospatial function although these associations were attenuated when adjusted for education, APOE-ε4, ethnic group and vascular risk factors.
Conclusion
Our results suggest that dysglycemia affects brain structure and cognition even in elderly survivors, evidenced by higher cerebrovascular disease, lower white and gray matter volume, and worse language and visuospatial function and cognitive speed.
Keywords: dysglycemia, structural brain changes, cognition
INTRODUCTION
Dysglycemia, defined as the presence of type 2 diabetes (diabetes) or pre-diabetes, is one of the most common public health problems in the United States (US). According to 2011 prevalence data from the Centers for Disease Control and Prevention (CDC), diabetes affects 25.8 million people in the United States (US), corresponding to 8.3% of the total US population, while 79 million have pre-diabetes, more than a quarter of the US population.1 This problem is more common in the elderly, the group also at highest risk for cognitive impairment. In 2010, 26.9% of the population 65 years and older had diabetes, another 50% of elderly had pre-diabetes as measured by fasting glucose or hemoglobin A1c (HBA1c) levels and the prevalence of diabetes and pre-diabetes is trending upwards.2 An estimated 5.2 million Americans have late-onset Alzheimer’s disease (LOAD) with an annual incidence rate increasing from 1% at ages 60 years to 8% at ages 85 years and older3.
Epidemiological studies suggest that diabetes, and several diabetes-related factors are risk factors for cognitive decline4, mild cognitive impairment (MCI)5, and dementia6 but the underlying structural correlates and pathophysiological mechanisms remain unclear. Diabetes is related to a higher risk of cerebrovascular disease,7 including high white matter hyperintensities (WMH) volume,8 and infarcts. A limitation of most epidemiological studies examining this question is that persons without diabetes are treated as having normal glycemia, without taking into account whether they have undiagnosed diabetes or pre-diabetes. This can potentially attenuate the association between diabetes and cognition outcomes because pre-diabetes and undiagnosed diabetes may also be associated with brain structure and cognitive abnormalities.
The objective of the current study was to examine the relation of dysglycemia with various structural brain changes in a cohort of 618 non-demented elderly from the multiethnic Washington Heights Inwood Columbia Aging Project (WHICAP). In secondary analyses, we also assessed the relation between dysglycemia and cognitive performance in this brain-imaging sample.
METHODS
Subjects
The sample for this analysis was subjects from WHICAP who underwent brain imaging, were assessed for dysglycemia by testing for HbA1c or based on clinical records, and did not have dementia at the time of brain imaging.
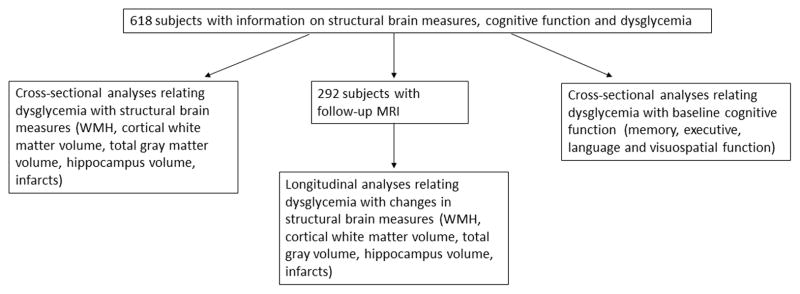
Participants were selected from a cohort participating in the prospective study of aging and dementia in Medicare recipients, 65 years and older and residing in northern Manhattan. The cohort was recruited in two waves in 1992 and 1999 and followed up at regular intervals of 18–24 months. The sampling strategies and recruitment outcomes have been described in detail.9 MRI was obtained in 769 participants. Participants were deemed eligible for MRI if they did not meet criteria for dementia at the visit (2002–2004) before the second follow-up (2005–2007), when brain imaging was performed. Of the 769 persons with MRI, 52 were excluded due to dementia at the time of MRI, and 99 due to no information on dysglycemia variables (diabetes history, HbA1c). The final sample comprised 618 non-demented participants of whom 292 had a follow-up MRI conducted approximately 4 years (2 follow-up intervals) later. Recruitment, informed consent and study procedures were approved by the Institutional Review Boards of Columbia University Medical Center and Columbia University Health Sciences and the New York State Psychiatric Institute.
Measures of Dysglycemia and other Covariates
HbA1C was measured by boronate affinity chromatography with the Primus CLC 385 (Primus, Kansas City, MO). “Dysglycemia” categories were defined based on HBA1C levels following American Diabetes Association guidelines10 as follows: 1. Normal glucose tolerance (NGT; HbA1c < 5.7%); 2. pre-Diabetes (HbA1c 5.7 to 6.49%); 3. undiagnosed diabetes (HbA1c of 6.5% or higher); 4. known diabetes. Known diabetes was defined by self-report at baseline and at each follow-up interval and by the use of disease-specific medications. At baseline, all participants were also asked whether or not they had a history of hypertension any time during their life. If affirmative, they were asked whether or not they were under treatment and the specific type of treatment. Blood pressure was also recorded at each visit. The blood pressure cuff was placed on the right arm while the individual was seated, and a recording was obtained every 3 minutes over 9 minutes. The third measurement was recorded in the database. Values above 140 mm Hg (systolic) and 90 mm Hg (diastolic) were used as criteria for hypertension. Body mass index (BMI) was calculated using the formula BMI=mass(kg)/(height(m))2. Fasting plasma total cholesterol and triglyceride levels were determined using standard techniques. High-density lipoprotein cholesterol (HDL-C) levels were determined after precipitation of apolipoprotein B containing lipoproteins with phosphotungstic acid. Low-density lipoprotein cholesterol was recalculated using the formula of Friedewald et al.11 Non-HDL cholesterol levels were calculated using the following formula: non-HDL-C = total cholesterol – HDL-C. At baseline, all participants were asked if they had ever been treated with statins. For assessment of smoking habit, a trigger question asked whether or not the individual ever smoked at least 1 cigarette per day for a period of 1 year or more. If the answer to the trigger question was no, the subject was classified as a nonsmoker and no further questions were asked. Participants who answered the question affirmatively were classified as current smokers if they were still smoking or past smokers if they had quit smoking. Current and past smokers were additionally asked at what age they began smoking and how many cigarettes on average they had smoked or still smoked per day. Past smokers were also asked at what age they stopped smoking. APOE genotypes were determined as described by Hixson and Vernier12 with slight modification. We classified persons as homozygous or heterozygous for the APOE ε4 allele or as not having any ε4 allele.
MRI protocol
Scan acquisition was performed on a 1.5T Philips Intera scanner at Columbia University Medical Center. T1-weighted (TR=20ms, TE=2.1ms, FOV 240cm, 256×160 matrix, 1.3mm slice thickness) and T2-weighted fluid attenuated inversion recovery (FLAIR; TR=11,000ms, TE=144.0ms, inversion time=2800, FOV 25cm, 2 nex, 256×192 matrix with 3mm slice thickness) images were acquired in the axial orientation. Scan acquisition sequence parameters were identical for the second MRI scan, which was performed on the same scanner.
Total grey matter, total white matter, hippocampus, and total intracranial volumes were derived with FreeSurfer version 5.1 (http://surfer.nmr.mgh.harvard.edu/) applied to the T1-weighetd MRI scans. Each segmented image was visually inspected by an expert operator and manually corrected if necessary. Volumes from left and right hippocampi were averaged to yield a single hippocampal volume measurement.
Regional WMH volumes were derived as described previously13. Briefly, FLAIR images were skull stripped, a Gaussian curve was fit to map the voxel intensity values, and values falling above 3.0SD the image mean were labeled as WMH. Labeled images were inspected and corrected manually in the event of false positive or false negative labels.
The presence or absence of brain infarction on MRI was determined using all available images, as previously described.14 Only lesions ≥3 mm qualified for consideration as brain infarcts.
Clinical Assessment
At each follow-up evaluation, each participant underwent an assessment of medical history, a physical/neurological examination and a neuropsychological battery that included measures of memory, orientation, language, abstract reasoning, and visuospatial ability. Memory was evaluated using the multiple choice version of the Benton Visual Retention Test15 and the seven subtests of the Selective Reminding Test:16 total recall, long-term recall, long-term storage, continuous long-term storage, words recalled on last trial, delayed recall, and delayed recognition. Orientation was evaluated using parts of the modified Mini-Mental State Examination.17 Language was assessed using the Boston Naming Test,18 the Controlled Word Association Test,19 category naming, and the Complex Ideational Material and Phrase Repetition subtests from the Boston Diagnostic Aphasia Evaluation.20 Abstract Reasoning was evaluated using WAIS-R Similarities subtest,21 and the non-verbal Identities and Oddities subtest of the Mattis Dementia Rating Scale.22 Visuospatial ability was examined using the Rosen Drawing Test,23 and a matching version of the Benton Visual Retention Test.15 This neuropsychological test battery has established norms for the same community and has been shown to effectively distinguish between normal aging and dementia.24
Statistical Methods
Included in the final analytic sample were the 618 non-demented subjects with brain imaging data. First, we evaluated the distributions of HbA1c levels, dysglycemia, other vascular risk factors, demographic variables, and clinical characteristics at baseline using ANOVA for continuous variables and χ2 test for categorical variables. Then, logistic regression and ANCOVA models were used to relate HbA1c levels and dysglycemia categories with structural brain measures adjusting first for intracranial volume only (Model 1), then in addition for age and sex (Model 2), education, ethnic group and APOE genotype (Model 3), and finally hypertension, smoking, BMI, and HDL levels (Model 4). Using linear mixed models we assessed the longitudinal effect of dysglycemia on available repeated measures of structural brain changes (WMH, cortical white matter volume, total gray volume, hippocampus volume).
For the analyses relating dysglycemia with cognitive function, we constructed a composite score for each cognitive domain using factor analysis. Specific tests included in each composite were for the memory domain the total recall, delayed recall, and delayed recognition subtests from the Selective Reminding Test, for the language domain the modified 15-item Boston Naming Test total score, Letter Fluency total, Category Fluency total, Similarities subtest of the Wechsler Adult Intelligence Scale – Revised, Boston Diagnostic Aphasia Evaluation Repetition and Comprehension subtests, for processing speed/executive functioning Color Trails 1 and 2, and for visuospatial abilities the recognition and matching tests from the Benton Visual Retention Test, the Rosen Drawing Test, and Identities/Oddities subtests of the Mattis Dementia Rating Scale. Invariance analyses showed that these measures are assessing similar constructs across English and Spanish speakers.
We then conducted ANCOVA analyses relating HbA1c levels and dysglycemia categories with the derived factor scores at baseline first performing crude models and then adjusting in a stepwise fashion for age, sex, education, ethnic group, APOE genotype, hypertension, smoking, BMI, and HDL levels. To assess a modification of APOE genotype on these associations conducted analyses including an interaction term for APOE genotype and dysglycemia in the models. All data analysis was performed using SPSS version 21.
RESULTS
Table 1 shows the characteristics of the dataset for the whole sample and across dysglycemia categories. Out of the 618 subjects included in this analysis, 115 had normal glucose tolerance (NGT), 224 had pre-diabetes, 81 subjects had undiagnosed diabetes, and 198 had known diabetes. Compared to persons with NGT, persons with dysglycemia were less educated, more often Black or Caribbean Hispanic, had higher levels of non-HDL-C and lower levels of HDL-C, had a higher BMI, had more often hypertension, and were less often smokers. On brain MRI, persons with dysglycemia showed lower cortical gray matter, cortical white matter and intracranial brain volumes than persons with NGT. On neuropsychological testing, dysglycemia was associated with lower performance on executive function, language function and speed.
Table 1.
Comparison of baseline characteristics for the whole sample and across dysglycemia categories. Comparisons for continuous variables was conducted using Analysis of Variance, and chi-squared was used for categorical outcomes.
| Characteristic† | All (n=618) | NGT (n=115) | Pre-Diabetes (n=224) | Undiagnosed Diabetes (n=81) | Known Diabetes (n=198) |
|---|---|---|---|---|---|
| Age | 80.0 (5.4) | 79.8 (5.4) | 80.6 (5.6) | 79.8 (5.4) | 79.6 (5.1) |
| Female, n (%) | 428 (69.3) | 76 (66.1) | 159 (71.0) | 55 (67.9) | 138 (69.7) |
| Education | 10.6 (4.8) | 12.1 (4.7) | 10.7 (4.9) | 10.0 (4.6)* | 9.7 (4.7)* |
| Ethnic group, n (%) | |||||
| White | 168 (27.2) | 51 (44.3) | 66 (29.5)* | 16 (19.8)* | 35 (17.7)* |
| Black | 202 (32.7) | 28 (24.3) | 62 (27.7)* | 35 (43.2)* | 77 (38.9)* |
| Hispanic | 238 (38.5) | 34 (29.6) | 93 (41.5)* | 30 (37.0)* | 81 (40.9)* |
| Weight (pounds) | 157.9 (34.1) | 146.6 (31.9) | 151.9 (31.5) | 161.9 (35.7)* | 169.6 (33.8)* |
| Height (cm) | 161.1 (9.9) | 161.4 (10.4) | 160.8 (9.5) | 161.3 (9.3) | 161.1 (10.7) |
| BMI | 27.7 (5.6) | 25.5 (4.7) | 26.6 (4.9) | 28.4 (5.8)* | 29.9 (6.0)* |
| APOE 4, n (%) | 146 (23.6) | 29 (25.2) | 57 (25.4) | 22 (27.2) | 38 (19.2) |
| Diabetes, n (%) | 198 (32.0) | - | - | - | 198 (32.0) |
| HbA1c | 6.5 (1.2) | 5.4 (0.2) | 6.1 (0.2)* | 7.0 (0.6)* | 7.5 (1.6)* |
| Hypertension, n (%) | 546 (88.4) | 91 (79.2) | 194 (86.6) | 70 (86.4) | 191 (96.4)* |
| Non-HDL | 128.9 (36.1) | 131.4 (33.8) | 131.7 (32.8) | 135.6 (35.8) | 121.1 (40.3)* |
| HDL | 57.6 (16.9) | 63.3 (17.8) | 58.6 (16.5) | 55.8 (15.4) | 53.5 (16.7)* |
| Systolic BP | 142.4 (21.7) | 139.6 (23.8) | 142.3 (21.9) | 141.9 (18.4) | 144.3 (21.4) |
| Smoking, n (%) | 312 (50.5) | 72 (62.6) | 107 (47.8)* | 36 (44.4)* | 99 (49.0)* |
| Memory score | 0.06 (0.78) | 0.15 (0.84) | 0.03 (0.77) | 0.08 (0.75) | 0.01 (0.77) |
| Executive score | 0.10 (1.13) | 0.39 (0.94) | 0.12 (1.19)* | 0.02 (1.13)* | −0.05 (1.12)* |
| Language score | 0.26 (0.67) | 0.42 (0.69) | 0.31 (0.67)* | 0.20 (0.60)* | 0.12 (0.68)* |
| Visuospatial score | 0.26 (0.61) | 0.44 (0.60) | 0.28 (0.62)* | 0.23 (0.50)* | 0.14 (0.61)* |
| Infarcts, n (%) | 200 (31.0) | 37 (31.4) | 66 (28.3) | 23 (27.7) | 74 (35.1) |
| White matter hyperintensities | 8.5 (10.5) | 7.6 (9.6) | 7.9 (9.7) | 11.2 (13.7) | 8.5 (10.0) |
| Total gray matter volume | 535159.6 (50782.6) | 553703.9 (61334.6) | 541412.2 (46802.3) | 534264.1 (41596.9)* | 514316.4 (44688.4)* |
| Total white matter volume | 396059.7 (52354.8) | 412060.9 (63956.0) | 400916.9 (53170.4) | 401267.9 (37885.3) | 375901.0 (41953.3)* |
| Hippocampal volume | 6670.6 (860.8) | 6784.6 (1043.0) | 6746.9 (854.4) | 6632.6 (724.5) | 6510.5 (771.8) |
| Intracranial volume | 1303967.1 (155245.0) | 1356590.6 (167875.7) | 1309562.3 (151701.5) | 1297898.0 (141741.2) | 1269556.0 (148689.8)* |
Significant vs. persons with normal glucose tolerance (NGT)
numbers represent mean (SD) unless otherwise indicated
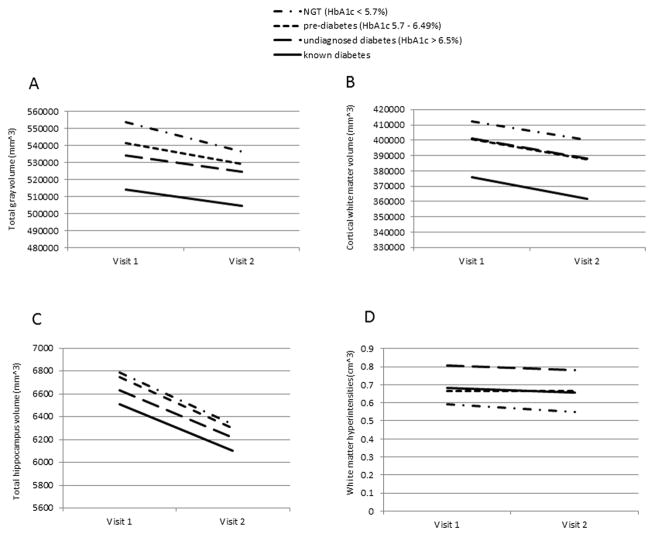
In analyses relating dysglycemia categories to measures of brain structure, presence of dysglycemia was associated with a higher number of brain infarcts, volume of WMH and decreased cortical white matter and gray matter volumes in all models performed (Table 2). In addition, known diabetes was associated with decreased hippocampus volume in the fully adjusted model. When using continuous levels of HbA1c as the predictor variable restricting the sample to persons without diabetes, results were consistent: higher levels of HbA1c were associated with an increased volume of WMH, and there were trends towards an association with lower gray matter and hippocampus volumes in the fully adjusted model (Table 2). In longitudinal analyses, dysglycemia was associated with a significant decline in total gray matter volume (p=0.04; Figure 2a) that was worse for persons with NGT. While there was no significant difference in the rate of change in total hippocampus or total white matter volume over time, the slopes of change were parallel and separate across dysglycemia categories with appreciable lower volumes in persons with diabetes and pre-diabetes as compared with those with NGT (Figures 2b,c). There was no change in WMH volume over follow-up (Figure 2d), but the dysglycemia categories showed persistently higher WMH as compared with persons with NGT. When assessing possible interaction of these associations with APOE genotype, for none of the outcomes interaction terms were significant (Supplemental table).
Table 2.
Results from multivariable models examining the cross sectional relation of HBA1c levels and dysglycemia categories to brain structure measures. Linear regression models were used for all outcomes except infarcts, for which logistic regression was used.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | p | Beta | SE | p | Beta | SE | p | Beta | SE | p | |
| Presence of Infarcts | ||||||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| HbA1c continuous | 1.03 | 0.77–1.39 | 0.81 | 1.02 | 0.75–1.37 | 0.89 | 1.01 | 0.74–1.37 | 0.91 | 0.96 | 0.68–1.34 | 0.82 |
| Known Diabetes | 1.34 | 0.81–2.19 | 0.24 | 1.28 | 0.78–2.10 | 0.32 | 1.34 | 0.79–2.27 | 0.26 | 1.17 | 0.64–2.14 | 0.60 |
| Undiagnosed diabetes | 0.96 | 0.51–1.80 | 0.90 | 0.91 | 0.48–1.73 | 0.79 | 0.93 | 0.48–1.79 | 0.83 | 0.92 | 0.45–1.87 | 0.83 |
| Pre-diabetes | 0.92 | 0.56–1.50 | 0.74 | 0.90 | 0.55–1.48 | 0.69 | 0.94 | 0.56–1.56 | 0.82 | 0.79 | 0.45–1.38 | 0.41 |
| NGT | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
|
| ||||||||||||
| Number of Infarcts | ||||||||||||
| HbA1c continuous | 0.01 | 0.06 | 0.77 | 0.01 | 0.06 | 0.77 | 0.02 | 0.07 | 0.70 | −0.01 | 0.06 | 0.94 |
| Known Diabetes | 0.30 | 0.13 | 0.02 | 0.29 | 0.13 | 0.03 | 0.32 | 0.14 | 0.02 | 0.39 | 0.16 | 0.01 |
| Undiagnosed diabetes | −0.03 | .17 | 0.82 | −0.05 | 0.17 | 0.75 | −0.01 | 0.17 | 0.92 | 0.05 | 0.18 | 0.75 |
| Pre-diabetes | −0.06 | .13 | 0.63 | −0.07 | 0.13 | 0.58 | −0.03 | 0.14 | 0.81 | −0.01 | 0.14 | 0.89 |
| NGT | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
|
| ||||||||||||
| WHI | ||||||||||||
| HbA1c continuous | 0.12 | 0.05 | 0.02 | 0.12 | 0.05 | 0.01 | 0.12 | 0.05 | 0.02 | 0.13 | 0.05 | 0.01 |
| Known Diabetes | 0.11 | 0.08 | 0.21 | 0.13 | 0.08 | 0.12 | 0.11 | 0.08 | 0.21 | 0.12 | 0.09 | 0.19 |
| Undiagnosed diabetes | 0.22 | 0.10 | .02 | 0.24 | 0.10 | 0.01 | 0.19 | 0.10 | 0.04 | 0.21 | 0.10 | 0.04 |
| Pre-diabetes | 0.08 | 0.08 | 0.30 | 0.07 | 0.08 | 0.34 | 0.08 | 0.08 | 0.26 | 0.09 | 0.08 | 0.25 |
| NGT | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
|
| ||||||||||||
| Cortical White Matter Volume | ||||||||||||
| HbA1c continuous | −2272.14 | 4211.70 | 0.59 | −2767.90 | 4112.41 | 0.50 | −3015.86 | 4196.86 | 0.47 | −3229.08 | 4498.28 | 0.47 |
| Known Diabetes | −14994.23 | 6180.31 | 0.01 | −16328.29 | 6115.73 | 0.008 | −14617.85 | 6400.05 | 0.02 | −12068.02 | 7097.68 | 0.09 |
| Undiagnosed diabetes | −131.44 | 7285.55 | 0.98 | −1356.19 | 7194.74 | 0.85 | 832.36 | 7368.44 | 0.91 | 1928.95 | 7895.34 | 0.80 |
| Pre-diabetes | −1291.98 | 5806.45 | 0.82 | −1106.48 | 5717.18 | 0.84 | −255.21 | 5790.24 | 0.96 | 137.05 | 6248.04 | 0.98 |
| NGT | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
|
| ||||||||||||
| Total Gray Volume | ||||||||||||
| HbA1c continuous | −4076.14 | 3250.25 | 0.21 | −4819.65 | 2983.14 | 0.10 | −5016.86 | 3020.89 | 0.09 | −6118.22 | 3172.79 | 0.05 |
| Known Diabetes | −16592.17 | 5119.89 | .001 | −19375.01 | 4758.23 | <0.0001 | −17535.02 | 4969.88 | <0.0001 | −19074.56 | 5340.42 | <0.0001 |
| Undiagnosed diabetes | −7957.34 | 6035.50 | 0.18 | −10502.05 | 5597.72 | 0.06 | −8460.54 | 5721.87 | 0.14 | −9802.54 | 5940.60 | 0.10 |
| Pre-diabetes | −1681.04 | 4810.18 | 0.72 | −1411.10 | 4448.14 | 0.75 | −1104.79 | 4496.34 | 0.80 | −1846.03 | 4701.14 | 0.69 |
| NGT | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
|
| ||||||||||||
| Total Hippocampus Volume | ||||||||||||
| HbA1c continuous | −18.96 | 36.91 | 0.60 | −32.78 | 34.60 | 0.34 | −38.64 | 35.45 | 0.27 | −61.28 | 36.56 | 0.09 |
| Known Diabetes | −97.56 | 138.8 | 0.48 | −136.45 | 129.90 | 0.29 | −170.44 | 136.52 | 0.21 | −328.53 | 148.56 | 0.02 |
| Undiagnosed diabetes | −63.04 | 163.66 | 0.70 | −99.01 | 152.82 | 0.51 | −119.27 | 157.18 | 0.44 | −227.44 | 165.26 | 0.17 |
| Pre-diabetes | 44.46 | 130.44 | 0.73 | 53.04 | 121.43 | 0.66 | 35.56 | 123.51 | 0.77 | −19.24 | 130.78 | 0.88 |
| NGT | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
Model 1=adjusted for intracranial volume; Model2=adjusted for intracranial volume, age, sex; Model3= adjusted for intracranial volume, age, sex, education, ethnic group, APOE; Model4= adjusted for intracranial volume, age, sex, education, ethnic group, APOE; hypertension, smoking, BMI, HDL
Figure 2a–d.
Mean value of structural brain changes at MRI visits 1 and 2 by dysglycemia category
a) Total gray volume
b) Cortical White Matter Volume
c) Total Hippocampus Volume
d) Volume of WHI
In analyses relating dysglycemia to cognitive function, both pre-diabetes and diabetes as well as higher HbA1c levels were associated with lower performance in language, speed and visuospatial function in crude models and models adjusted for age and sex. In models adjusted for education, ethnic group, APOEe4 genotype or vascular risk factors, these associations were attenuated (table 3).
Table 3.
Results from multivariable linear regression models examining the cross sectional relation of HBA1c levels and dysglycemia categories to cognitive performance.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | p | Beta | SE | p | Beta | SE | p | Beta | SE | p | |
| Memory | ||||||||||||
| HbA1c continuous | −0.07 | 0.06 | 0.27 | −0.06 | 0.05 | 0.28 | −0.01 | 0.05 | 0.82 | −0.02 | 0.05 | 0.69 |
| Known Diabetes | −0.15 | 0.09 | 0.11 | −0.16 | 0.08 | 0.06 | 0.03 | 0.08 | 0.73 | 0.01 | 0.09 | 0.84 |
| Undiagnosed diabetes | −0.07 | 0.11 | 0.53 | −0.07 | 0.11 | 0.50 | 0.10 | 0.10 | 0.31 | 0.11 | 0.11 | 0.29 |
| Pre-diabetes | −0.12 | 0.09 | 0.18 | −0.09 | 0.08 | 0.26 | −0.004 | 0.08 | 0.96 | 0.01 | 0.08 | 0.88 |
| NGT | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
|
| ||||||||||||
| Language | ||||||||||||
| HbA1c continuous | −0.11 | 0.05 | 0.02 | −0.10 | 0.04 | 0.02 | −0.02 | 0.03 | 0.46 | −0.01 | 0.03 | 0.79 |
| Known Diabetes | −0.30 | 0.08 | <0.0001 | −0.31 | 0.07 | <0.0001 | −0.06 | 0.06 | 0.29 | −0.04 | 0.06 | 0.54 |
| Undiagnosed diabetes | −0.22 | 0.09 | 0.02 | −0.22 | 0.09 | 0.01 | 0.008 | 0.07 | 0.91 | 0.04 | 0.07 | 0.57 |
| Pre-diabetes | −0.11 | 0.07 | 0.15 | −0.08 | 0.07 | 0.27 | 0.05 | 0.05 | 0.34 | 0.08 | 0.06 | 0.17 |
| NGT | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
|
| ||||||||||||
| Speed | ||||||||||||
| HbA1c continuous | −0.22 | 0.08 | 0.01 | −0.20 | 0.08 | 0.01 | −0.08 | 0.07 | 0.23 | −0.09 | 0.07 | 0.23 |
| Known Diabetes | −0.44 | 0.14 | 0.002 | −0.46 | 0.13 | 0.001 | −0.21 | 0.12 | 0.10 | −0.21 | 0.13 | 0.12 |
| Undiagnosed diabetes | −0.36 | 0.17 | 0.03 | −0.34 | 0.16 | 0.03 | −0.09 | 0.15 | 0.51 | −0.11 | 0.15 | 0.45 |
| Pre-diabetes | −0.26 | 0.13 | 0.05 | −0.22 | 0.13 | .09 | −0.02 | 0.12 | 0.83 | −0.01 | 0.12 | 0.89 |
| NGT | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
|
| ||||||||||||
| Visuospatial | ||||||||||||
| HbA1c continuous | −0.09 | 0.04 | 0.04 | −0.08 | 0.04 | 0.05 | −0.01 | 0.03 | 0.63 | −0.01 | 0.03 | 0.69 |
| Known Diabetes | −0.29 | 0.07 | <0.0001 | −0.30 | 0.07 | <0.0001 | −0.09 | 0.05 | 0.09 | −0.09 | 0.06 | 0.14 |
| Undiagnosed diabetes | −0.22 | 0.08 | 0.02 | −0.19 | 0.08 | 0.02 | −0.008 | 0.06 | 0.91 | −0.005 | 0.07 | 0.94 |
| Pre-diabetes | −0.15 | 0.07 | 0.02 | −0.12 | 0.06 | 0.05 | −0.008 | 0.05 | 0.87 | 0.004 | 0.05 | 0.94 |
| NGT | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
Model 1=crude; Model2=adjusted for age, sex; Model3= adjusted for age, sex, education, ethnic group, APOE; Model4= adjusted for age, sex, education, ethnic group, APOE-ε4; hypertension, smoking, Body Mass Index, High Density Lipoprotein.
DISCUSSION
In this study of elderly ethnically diverse community dwellers, presence of dysglycemia (diabetes, undiagnosed diabetes, and pre-diabetes) or higher HBA1c levels was associated with a higher number of brain infarcts, WMH volume and decreased total white matter, gray matter volumes and hippocampus volume in cross-sectional analyses and a significant decline in gray matter volume in longitudinal analyses. In addition, dysglycemia was associated with lower performance in language, speed and visuospatial function although these associations were attenuated when adjusting for education, APOE genotype, ethnic group or vascular risk factors.
Many studies have shown associations of diabetes with cognitive decline,25 mild cognitive impairment,26 LOAD27,28 and vascular dementia,29 which predominantly used a history of diabetes as the predictor variable. The continuum of dysglycemia has also been shown to be related to a higher risk of cognitive decline30 and dementia.31 However, there is a paucity of data assessing brain structure and cognition as a function of long-term changes in glucose control and prediabetic stages, particularly in very old adults, as represented in our sample. Our observations are important because the impact of pre-diabetes and diabetes in very old adults is a matter of debate.32
Our findings are consistent with previous longitudinal studies reporting an association between diabetes and structural brain changes. In the Framingham Offspring Study midlife diabetes was associated with an annual increase in temporal horn volume,33 hippocampal atrophy and brain infarcts.34 In the Atherosclerosis in Communities cohort35 and the Leukoaraiosis and DISability in the Elderly Study35 midlife diabetes was associated with reduced brain volume or brain atrophy. In The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial36 midlife diabetes was associated with brain volume loss, particularly in the gray matter. Persons with diabetes have been reported to have more brain atrophy compared with persons with diabetes and have higher WMH,37 representative of neurodegeneration and small vessel cerebrovascular disease respectively. We found that the associations between dysglycemia and white matter hyperintensities, cortical white matter volume, total gray volume and total hippocampus volume were independent of APOE genotype. Some studies have found that the association between diabetes and cognitive outcomes is strongest among persons with the APOE-ε4 allele38,39 but this finding is not consistent, 40 and was not supported by our findings (see supplemental tables a,b,c).
Dysglycemia, cognitive impairment and structural brain changes may have common underlying risk factors such as older age, which could confound the observed associations. However, our results are in line with the notion that dysglycemia may affect cognition through both vascular and neurodegenerative pathways. Dysglycemia is known to be a risk factor for cerebrovascular disease.41 Strokes, ascertained by clinical history or as brain infarcts on MRI are related to a higher risk of dementia including LOAD.42 While the mechanisms for this association are not clear, pathology studies have demonstrated that the presence of amyloid plaques is lower in brains of persons with dementia who also have infarcts43 suggesting that the presence of infarcts lowers the threshold of amyloid in the brain necessary to cause dementia. White matter hyperintensities are related to a higher risk of cognitive impairment,44 but the pathological underpinnings and etiological factors related to WMH are still not fully understood. There is a lot of evidence that WMH are ischemic in origin in the same way that infarcts are45 and have can be thought of as surrogate markers of cerebrovascular disease45. However, recent evidence also shows that WMH are common in LOAD and may be related to cerebral amyloid angiopathy.46
We found that dysglycemia is related to reduced grey matter volume, a surrogate of neurodegeneration. The medial temporal lobe (including the hippocampus and parahippocampus), the first region to be affected by neurofibrillary tangles and amyloid plaques as well as the greatest loss of neurons in AD,47 most consistently exhibits decreased grey-matter volume in AD and MCI48. A study based on the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset has demonstrated that grey-matter atrophy reflects clinically defined disease stages better than CSF biomarkers (such as Aβ and total tau).49
While a relation of dysglycemia with cerebrovascular markers is not surprising, neurodegeneration is also a plausible mechanism linking dysglycemia and brain atrophy. Hyperinsulinemia50 and advanced glycation end products (AGE)51 may link dysglycemia and neurodegeneration. One of our primary exposures, HbA1C, is the most common example of AGE. HbA1c is a form of hemoglobin bound by glucose through the non-enzymatic glycation pathway, and is the primary measure of prolonged (8–12 weeks) average ambient plasma glucose concentration in the circulation as well as an AGE.
Our results were stronger in cross-sectional analyses compared with longitudinal analyses. However, there was a clear separation in slopes for brain structure variables suggesting a detrimental association with dysglycemia. While we cannot exclude the possibility that people who developed relatively smaller brains—for example due to low lower socioeconomic status or poor nutrition- may be more likely to develop diabetes and poor cognition, these findings are in line with the notion that the effect of dysglycemia on brain structure occurred earlier in the lifespan and might have stabilized to a level of change similar to the general population. The same observation was made for cognitive decline. This observation has been previously reported for both changes in cognitive performance and brain structure.37,52
Our study has several strengths. First, our cohort is community-based. Second, the diagnosis of dysglycemia was based on HbA1c levels reflecting a more stable measure of long term glucose concentration in the circulation than blood glucose levels and allowing us to ascertain undiagnosed diabetes or glucose intolerance. Third, we had measures of structural brain changes from two time-points allowing us to explore the longitudinal effect of pre-diabetes on changes in volumetric measures. Fourth, the cohort includes a high proportion of Hispanic and African-American participants, who have been significantly underrepresented in previous studies on prediabetes/diabetes and structural MRI measures or cognition, and who are at a higher risk of both dysglycemia and cognitive impairment. Fifth, we had concurrent comprehensive brain imaging and cognitive performance data. Finally, measures for multiple potential confounders were carefully recorded and adjusted for in the analyses.
Limitations of our study include that we only had one measurement of HBA1c levels which can lead to measurement error and ignores past glycemia trajectories. Measurement error could have resulted in underestimation of the associations of dysglycemia with changes in volumetric measures in longitudinal analyses. Second, it would be interesting to examine lifetime cumulative or mid-life effects of dysglycemia, but we only had a measure from the time of brain imaging and no information on how long the subjects have had diabetes and what has been the status of their over-all glycemic control. Although we controlled for a wide variety of potential confounders, we cannot rule out the possibility of residual confounding (ie. distortion remaining after controlling for confounders). Dysglycemia may be a marker of lower education and lower socio-economic status, which in turn is related to a higher risk of cognitive impairment.53
It is important to point out that our findings are generalizable to relatively very old community dwelling persons without dementia. The main clinical implication of our findings is that dysglycemia may impact brain structure and cognition even in very old persons, suggesting that dysglycemia should not be considered “benign” in this age group and the need for interventions may be assessed.
Supplementary Material
Figure 1.
Project Flow
Acknowledgments
Funding Sources
Primary support for this project came from 1R01AG037212 (Mayeux), AG037212 (Mayeux) and 1R01AG034189 (Brickman). Dr. Reitz was further supported by a Paul B. Beeson Career Development Award (K23AG034550) and Dr. Luchsinger was also supported by P60 MD000206, RF1AG051556, and R01AG050440, and K24AG045334.
Footnotes
Conflict of Interest Information
| Elements of Financial/Personal Conflicts | Christiane Reitz | Vanessa Guzman | Atul Narkhede | Charles DeCarli | Adam Brickman | Jose Luchsinger | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | x | x | ||||||
| Grants/Funds | x | x | x | x | x | x | ||||||
| Honoraria | x | x | x | x | x | x | ||||||
| Speaker Forum | x | x | x | x | x | x | ||||||
| Consultant | x | x | x | x | x | x | ||||||
| Stocks | x | x | x | x | x | x | ||||||
| Royalties | x | x | x | x | x | x | ||||||
| Expert Testimony | x | x | x | x | x | x | ||||||
| Board Member | x | x | x | x | x | x | ||||||
| Patents | x | x | x | x | x | x | ||||||
| Personal Relationship | x | x | x | x | x | x | ||||||
References
- 1.http://www.cdc.gov//diabetes/pubs/factsheet11.htm CfDCaPNDFS.
- 2.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Annals of internal medicine. 2014 Apr 15;160(8):517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayeux R. Epidemiology of neurodegeneration. Annual review of neuroscience. 2003;26:81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- 4.Ravona-Springer R, Luo X, Schmeidler J, et al. Diabetes is associated with increased rate of cognitive decline in questionably demented elderly. Dement Geriatr Cogn Disord. 2010;29(1):68–74. doi: 10.1159/000265552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts RO, Geda YE, Knopman DS, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008 Aug;65(8):1066–1073. doi: 10.1001/archneur.65.8.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnaider Beeri M, Goldbourt U, Silverman JM, et al. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004 Nov 23;63(10):1902–1907. doi: 10.1212/01.wnl.0000144278.79488.dd. [DOI] [PubMed] [Google Scholar]
- 7.Sacco RL, Benson RT, Kargman DE, et al. High-density lipoprotein cholesterol and ischemic stroke in the elderly: the Northern Manhattan Stroke Study. JAMA. 2001 Jun 6;285(21):2729–2735. doi: 10.1001/jama.285.21.2729. [DOI] [PubMed] [Google Scholar]
- 8.Manschot SM, Brands AM, van der Grond J, et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006 Apr;55(4):1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 9.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001 Jan 9;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Standards of medical care in diabetes--2010. Diabetes Care. Jan;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 12.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548. [PubMed] [Google Scholar]
- 13.Brickman AM, Provenzano FA, Muraskin J, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Archives of neurology. 2012 Dec;69(12):1621–1627. doi: 10.1001/archneurol.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008 Aug;65(8):1053–1061. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benton A. The Benton Visual Retention Test. The Psychological Corporation; New York: 1955. [Google Scholar]
- 16.Buschke H, Fuld P. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974 Nov;24(11):1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- 19.Benton A. FAS Test. University of Victoria; Victoria, B.C: 1967. [Google Scholar]
- 20.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. 2. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- 21.Wechsler D. Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; New York, NY: 1981. [Google Scholar]
- 22.Mattis S. Mental status examination for organic mental syndrome in the elderly patient. Grune & Stratton; New York, NY: 1976. [Google Scholar]
- 23.Rosen W. The Rosen Drawing Test. Veterans Administration Medical Center; Bronx, NY: 1981. [Google Scholar]
- 24.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49(5):453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 25.Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K. Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo study cohort. Arch Intern Med. 2004 Jun 28;164(12):1327–1333. doi: 10.1001/archinte.164.12.1327. [DOI] [PubMed] [Google Scholar]
- 26.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007 Apr;64(4):570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 27.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53(9):1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 28.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004 Oct 12;63(7):1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 29.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001 Oct 1;154(7):635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 30.Diabetes impaired fasting glucose, and development of cognitive impairment in older women. [summary for patients in Neurology. 2004 Aug 24;63(4):E9–10; PMID: 15326277] Neurology. 2004 Aug 24;63(4):658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 31.Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. N Engl J Med. 2013 Aug 8;369(6):540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012 Dec;35(12):2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011 Aug 2;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korf ES, van Straaten EC, de Leeuw FE, et al. Diabetes mellitus, hypertension and medial temporal lobe atrophy: the LADIS study. Diabet Med. 2007 Feb;24(2):166–171. doi: 10.1111/j.1464-5491.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- 35.Knopman DS, Mosley TH, Catellier DJ, Sharrett AR. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology. 2005 Sep 27;65(6):876–881. doi: 10.1212/01.wnl.0000176074.09733.a8. [DOI] [PubMed] [Google Scholar]
- 36.Roberts RO, Knopman DS, Przybelski SA, et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology. 2014 Apr 1;82(13):1132–1141. doi: 10.1212/WNL.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brundel M, Kappelle LJ, Biessels GJ. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. Mar 15, 2014. Brain imaging in type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- 38.Irie F, Fitzpatrick AL, Lopez OL, et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the Cardiovascular Health Study Cognition Study. Arch Neurol. 2008 Jan;65(1):89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia Aging S. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002 Apr;51(4):1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 40.Cheng D, Noble J, Tang MX, Schupf N, Mayeux R, Luchsinger JA. Type 2 diabetes and late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;31(6):424–430. doi: 10.1159/000324134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee M, Saver JL, Hong KS, Song S, Chang KH, Ovbiagele B. Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ. 2012;344:e3564. doi: 10.1136/bmj.e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honig LS, Tang MX, Albert S, et al. Stroke and the risk of Alzheimer disease. Archives of Neurology. 2003 Dec;60(12):1707–1712. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- 43.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007 Dec 11;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 44.Luchsinger JA, Brickman AM, Reitz C, et al. Subclinical cerebrovascular disease in mild cognitive impairment. Neurology. 2009 Aug 11;73(6):450–456. doi: 10.1212/WNL.0b013e3181b1636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pantoni L. White matter ischemia: Time to begin integrating experimental and clinical data. Eur Neurol. 2006;56(2):71–73. doi: 10.1159/000095542. [DOI] [PubMed] [Google Scholar]
- 46.Haglund M, Englund E. Cerebral amyloid angiopathy, white matter lesions and Alzheimer encephalopathy - a histopathological assessment. Dement Geriatr Cogn Disord. 2002;14(3):161–166. doi: 10.1159/000063606. [DOI] [PubMed] [Google Scholar]
- 47.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995 May-Jun;16(3):271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
- 48.Whitwell JL, Przybelski SA, Weigand SD, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007 Jul;130(Pt 7):1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009 Jul 28;73(4):287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007 Apr;4(2):147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 51.Yan SD, Bierhaus A, Nawroth PP, Stern DM. RAGE and Alzheimer’s disease: a progression factor for amyloid-beta-induced cellular perturbation? J Alzheimers Dis. 2009 Apr;16(4):833–843. doi: 10.3233/JAD-2009-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. The lancet. Diabetes & endocrinology. 2014 Mar;2(3):246–255. doi: 10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- 53.Scarmeas N, Stern Y. Cognitive reserve and lifestyle. Journal of Clinical & Experimental Neuropsychology. 2003 Aug;25(5):625–633. doi: 10.1076/jcen.25.5.625.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.