Abstract
Fingolimod, a sphingosine-1-phosphate receptor (S1PR) agonist, is clinically available to treat multiple sclerosis and is showing promise in treating stroke. We investigated if fingolimod provides long-term protection from experimental neonatal germinal matrix hemorrhage (GMH), aiming to support a potential mechanism of acute fingolimod-induced protection. GMH was induced in P7 rats by infusion of collagenase (0.3 U) into the right ganglionic eminence. Animals euthanized at four weeks post-GMH received low or high dose fingolimod (0.25 or 1.0 mg/kg) or vehicle, and underwent neurocognitive testing before histopathological evaluation. Subsequently, a cohort of animals euthanized at 72 hours post-GMH received 1.0 mg/kg fingolimod; the specific S1PR1 agonist, SEW2871; or fingolimod co-administered with the S1PR1/3/4 inhibitor, VPC23019, or the Rac1 inhibitor, EHT1864. All drugs were injected intraperitoneally 1, 24, and 48 hours post-surgery. At 72 hours post-GMH, brain water content, extravasated Evans blue dye, and hemoglobin were measured as well as the expression levels of phospho-Akt, Akt, GTP-Rac1, Total-Rac1, ZO1, occludin, and claudin-3 determined. Fingolimod significantly improved long-term neurocognitive performance and ameliorated brain tissue loss. At 72 hours post-GMH, fingolimod reduced brain water content and Evans blue dye extravasation as well as reversed GMH-induced loss of tight junctional proteins. S1PR1 agonism showed similar protection, whereas S1PR or Rac1 inhibition abolished the protective effect of fingolimod. Fingolimod treatment improved functional and morphological outcomes after GMH, in part, by tempering acute post-hemorrhagic blood-brain barrier disruption via the activation of the S1PR1/Akt/Rac1 pathway.
Keywords: Germinal Matrix Hemorrhage, Fingolimod, Blood-Brain Barrier, Neuroprotection, Brain Edema, Behavior
Graphical abstract
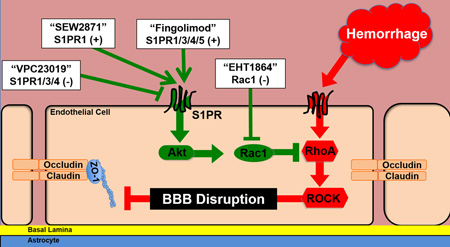
Neonatal germinal matrix hemorrhage (GMH) often results in lasting deficits. This study aimed to determine the protective effect of fingolimod-induced sphingosine-1-phosphate receptor (S1PR) modulation after experimental GMH. Fingolimod reduced long-term markers of brain injury. Acute protective effects were dependent on functionality of S1PRs and Rac1. This study supports manipulation of S1PRs and the Rac1 pathway as potentially protective clinical strategies.
INTRODUCTION
Neonatal germinal matrix hemorrhage (GMH), resulting from the rupture of immature blood vessels in the subependymal germinal matrix, is the most common brain injury of premature infants. Twenty percent of all infants delivered before 32 weeks gestation suffer from GMH and more than 12,000 newborns develop this condition in the United States annually (Kochanek et al., 2012). Long-term consequences of GMH include developmental delays, cerebral palsy, and post-hemorrhagic hydrocephalus (Ballabh, 2010). Clinical management is limited and has yet to be proven effective, thus intensive rehabilitation remains the best opportunity to improve quality of life (Manaenko et al., 2014).
Hemorrhage-induced brain injury develops due to direct mechanical tissue destruction followed by secondary events, such as activation of the coagulation cascade, cerebral infiltration of peripheral immune cells, oxidative damage, and delayed cell death (Xue and Del Bigio, 2000, Xi et al., 2004). Additionally, thrombin-mediated activation of downstream effectors, like Rho-associated protein kinase (ROCK), further open the blood-brain barrier (BBB) by forming stress fibers and disrupting junctional proteins (van Nieuw Amerongen et al., 2000, Stamatovic et al., 2003). BBB disruption permits extravasation of serum components into the brain parenchyma, leading to brain edema formation and exacerbation of cell death. However, activated Rac1 inhibits ROCK activity (Wojciak-Stothard et al., 2001) and has been associated with preserving BBB integrity after adult intracerebral hemorrhage (Huang et al., 2012).
Fingolimod, clinically used to treat relapsing-remitting multiple sclerosis, interacts with four of the five sphingosine-1-phosphate receptors (S1PR) to regulate cellular responses, like inflammation, apoptosis, and barrier integrity (Groves et al., 2013). Fingolimod has been reported to augment transendothelial electrical resistance in the lung and is associated with Rac1 activation (Sarai et al., 2009). Furthermore, studies have shown fingolimod provides neuroprotection following adult experimental ischemic and hemorrhagic stroke [Reviewed (Liu et al., 2013, Brunkhorst et al., 2014)]. However, its effects have not been evaluated in neonatal GMH.
In this study, we first aimed to determine if fingolimod provides significant long-term protection from GMH-induced neurological deficits and brain tissue injury. Moreover, we hypothesized fingolimod reduces acute BBB disruption and brain edema formation after GMH via S1PR1/Akt/Rac1 signaling pathway, thereby preserving endothelial tight junction proteins.
MATERIALS AND METHODS
Animals
The Institutional Animal Care and Use Committee of Loma Linda University approved all procedures. Timed pregnant Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN). One hundred and eighty three rat pups of both sexes were subjected to sham or GMH surgery. In order to determine the most efficacious dosage of fingolimod, 36 pups were used for the long-term branch of our study and randomly divided into: Sham; GMH + Vehicle; GMH + Low Dose Fingolimod; and GMH + High Dose Fingolimod. Subsequently, 147 animals were used for the short-term branch of our study and were randomly divided into: Sham; GMH + Vehicle; GMH + Fingolimod; GMH + S1PR1 agonist; GMH + Fingolimod + S1PR antagonist; and GMH + Fingolimod + Rac1 antagonist. Animals were maintained on a 12:12 light/dark cycle and fed ad libitum.
Drugs and Administration
Fingolimod (2-amino-2-[4-octylphenyl)ethyl]-1,3-propanediol, hydrochloride) and the specific S1PR1 agonist (SEW2871) were purchased from Cayman Chemical Co. (Ann Arbor, MI). The S1PR1, S1PR3, S1PR4 antagonist (VPC20391) and the Rac1 inhibitor (EHT1864) were purchased from Tocris Bioscience (Minneapolis, MN). Low dose fingolimod (0.25 mg/kg), high dose fingolimod (1.0 mg/kg), SEW2871 (5 mg/kg), VPC23019 (0.5 mg/kg), and EHT1864 (40 mg/kg) were given by daily intraperitoneal injection (1, 24, and 48 hours after GMH). The dissolving manner and dosages were followed as previously described (Desire et al., 2005, Awad et al., 2006, Landeen et al., 2008). Sham and vehicle animals received the same volume of 5% DMSO in saline.
Surgical Procedures
GMH was achieved by stereotactic infusions of bacterial collagenase into the right anterior ganglionic eminence of P7 rats, as described previously (Lekic et al., 2012). Briefly, pups were gently anesthetized with 3% isoflurane (in a 30/70 mix of oxygen and medical air), placed prone, and their head secured onto a small rodent stereotactic frame. After sterilizing the scalp, an incision was made in the longitudinal plane to expose the skull and reveal bregma. A 1 mm burr hole was drilled at 1.8 mm rostral and 1.5 mm lateral from bregma. A 26-gauge needle was stereotactically inserted 2.8 mm deep relative to the dura. Subsequently, 0.3 units of bacterial collagenase VII-S (Sigma, St. Louis, MO) was infused at a rate of 0.25 µl/minute. Leaving the needle in place for an additional 10 minutes after infusion prevented back-leakage. After needle removal, the burr hole was sealed with bone wax, the incision sutured closed, and the animals were allowed to recover on a 37 °C heating blanket. Upon recovery, the animals were returned to their dams. Sham surgery consisted of needle insertion alone.
Long-Term Behavioral Assessment
Sensorimotor deficits were evaluated via the foot fault test, rotarod test, and a composite neuroscore, while learning and memory performance was evaluated using the Morris water maze test. All neurobehavioral testing was conducted between the 21st and 27th day after GMH. These tests are routinely performed to test neurobehavioral function in juvenile rats (Zhou et al., 2009, Lekic et al., 2012, Leitzke et al., 2013, Manaenko et al., 2014). The foot fault test, the number of complete limb missteps through wire-grid openings, was recorded for each limb over 120 seconds while the animal explored an elevated wire suspension grid (20 cm × 40 cm). To perform the rotarod test, rats were placed onto a rotarod (Columbus Instruments, Columbus, OH) consisting of a horizontal rotating cylinder (7 cm-diameter × 9.5 cm-wide) and rotations were started at either 4 or 10 RPM with an acceleration of 2 RPM every 5 seconds. The latency of falling from the rotarod was recorded using a photobeam circuit. The percent neurodeficit was quantified using a series of six tests that measure functional deficits (100 = severe, 50 = moderate, 0 = none): 1) proprioceptive limb placing, 2) lateral limb placement, 3) forelimb placement, 4) postural reflex, 5) back pressure, and 6) lateral pressure. The Morris water maze test required the finding of a slightly submerged platform in a pool of water (diameter: 100 cm). An overhead camera recorded the swim path of each rat, which allowed for quantification of swim distance as well as spatial distance from the platform by a computerized tracking system (Noldus Ethovision, Tacoma, WA). Water maze testing was done over a 4-day period. Following the cued trials (platform visible) on day 1, the platform was submerged for all subsequent testing (days 2–4). The location of the platform was changed at the beginning of every testing day and the rats were released into the water on several sites around the platform. At the end of days 2–4, each animal was subjected to a “probe” trial, in which the platform was removed. The time spent in each quadrant (one of which formerly contained the platform) was recorded for 60 seconds.
Long-Term Histopathological Analysis
At four weeks after GMH, rats were sacrificed under deep isoflurane anesthesia and underwent transcardial perfusion with ice-cold PBS, followed by 10% paraformaldehyde. Brain samples were fixed and then dehydrated with 30% sucrose in PBS (at 4°C for three days). Frozen coronal brain slices (10 µm) were cut every 600 µm on a cryostat (Leica Microsystems, LM3050S) and mounted onto poly-L-lysine coated glass slides (Richard Allen Scientific, Kalamazoo, MI). Samples were stained with cresyl violet and morphometric analysis was conducted using computer-assisted (Image J 4.0, Media Cybernetics, Silver Springs, MD) hand delineation of the following areas: caudate putamen, thalamus, hippocampus, and corpus callosum. The borders of cerebral structures were based on criteria previously defined from stereologic studies using optical dissector principles (Oorschot, 1996, Avendano et al., 2005).
Brain Edema Measurement
Brain water content (edema) was measured in rats at 72 hours after GMH using the wet brain weight/dry brain weight method as previously described (Tang et al., 2004). Rats were decapitated under deep isoflurane anesthesia, and brains were removed. Immediately after brain removal, a 4 mm wide tissue section was made through the forebrain in the coronal plane (2 mm rostral and 2 mm caudal to the injection site) and the cerebellum was collected. All samples were weighed with an electronic analytical balance (AE100; Mettler Instrument Co, Columbus OH) to obtain a wet weight (WW). Tissues were then dried at 100 °C for 24 hours to determine the dry weights (DW). The brain water content (%) was calculated as (WW-DW)/WW ×100.
Tandem Evans Blue and Hemoglobin Assays
Approaching 72 hours after GMH, rats were given intraperitoneal injections of 2% Evans blue in normal saline (4 ml/kg of body weight) 3 hours before euthanasia, as described previously (Manaenko et al., 2011). Afterwards, under deep isoflurane anesthesia, rats were transcardially perfused with ice cold PBS. The brain tissue was removed, frozen in liquid nitrogen, and stored at −80 °C until analysis. Brains were homogenized in 1100 µl of PBS, sonicated, centrifuged (30 minutes, 15,000 rcf, 4 °C), and supernatants collected. Hemoglobin assay was performed by adding Drabkin’s reagent (0.8 ml, Sigma-Aldrich) to 0.2 ml supernatant aliquots, which were allowed to stand for 15 minutes at room temperature. Sample absorbance was measured via spectrophotometry (Thermo Spectronic Genesys 10 UV, Thermo Fisher Scientific Inc., Waltham, MA) at 540 nm, and quantified using a standard curve. Results are presented as µl of blood. Additionally, Evans blue assays were performed by adding an equal volume of 50% trichloroacetic acid to supernatant aliquots, after which they were incubated overnight at 4 °C and then centrifuged (30 minutes, 15,000 rcf, 4 °C). Evans blue content was then measured by spectrophotometry at 610 nm and quantified according to a standard curve. The results are presented as (µg of Evans blue dye)/(g of tissue).
Western Blotting
At 72 hours after GMH, following transcardial PBS perfusion, ipsilateral hemispheres were collected and processed as previously described (Huang et al., 2012). Homogenates were separated by SDS-PAGE and transferred onto nitrocellulose membranes, which were incubated with the following primary antibodies: anti-phospho-Akt (Ser473), anti-Akt (both from Cell Signaling Technology, Beverly, MA, 1:2000), anti-ZO1, anti-IL-17 (both from AbCam, Cambridge, MA, 1:1000), as well as anti-occludin and anti-claudin-3 (both from Santa Cruz Biotechnology, Santa Cruz, CA, 1:1000). GTP-Rac1 and total-Rac1 were detected using Rac1 Activation Assay Kits (Millipore, Temecula, CA). Secondary antibodies consisted of goat anti-mouse IgG-HRP and goat anti-rabbit IgG-HRP (both from Santa Cruz Biotechnology, 1:5000, sc-2005 and sc-2004 respectively). Immunoblots were visualized with the Amersham ECL Prime Western Blotting Detection Reagent (GE Life Sciences, Piscataway, NJ) and semiquantitatively analyzed using Image J software (4.0, Media Cybernetics, Silver Springs, MD). Results are expressed as relative density ratio, normalized to mean density of the sham group.
Peripheral Leukocyte Count by Flow Cytometry
At 72 hours after GMH, under deep isoflurane anesthesia, blood samples were collected via cardiac puncture and mixed with citrate-phosphate dextrose anticoagulant solution (Sigma-Aldrich) in a 1:5 ratio. Peripheral blood mononuclear cells (PBMCs) were then isolated by ammonium chloride lysis of red blood cells. PBMCs were then washed and pelleted by centrifugation (1500 rpm for 5 minutes at 4 °C). The resulting cell suspensions were counted and diluted to approximately 1×106 cells/ml. For multi-color flow cytometry staining, cells were incubated with monoclonal antibodies in PBS for 30 minutes in the dark at 4 °C. Cells were subsequently washed and fixed with 1% paraformaldehyde/PBS before analysis on a MACSQuant Analyzer (Miltenyi Biotec). The anti-rat antibodies used were CD45-APC-eFluor780, CD3-APC (clones: Ox1 and eBioG4.18 respectively, eBioscience, San Diego, CA) and CD11b-PE (clone: Ox42, Biolegend, San Diego, CA). Isotype controls consisted of mouse IgG1κ-APC-eFluor780, IgG1κ-APC and IgG2a,κ-PE. Living cells were identified by forward scatter and side scatter gating along with exclusion of the fixable viability dye FVD eFluor450 (eBioscience), added immediately prior to fixation. Flow cytometry data analysis was conducted using Flowjo data analysis software (Tree Star, Ashland, OR).
Statistical Analysis
Sample size estimates were made by power analysis using a type I error rate of 0.05 and a power of 0.8 on a 2-sided test. Behavior data were statistically analyzed using Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks, followed by the Student-Newman-Keuls method. All other data were statistically analyzed using one-way ANOVA, followed by Tukey post-hoc test. A P value <0.05 was considered statistically significant.
RESULTS
Fingolimod Improved Long-Term Behavioral and Histopathological Outcomes after GMH
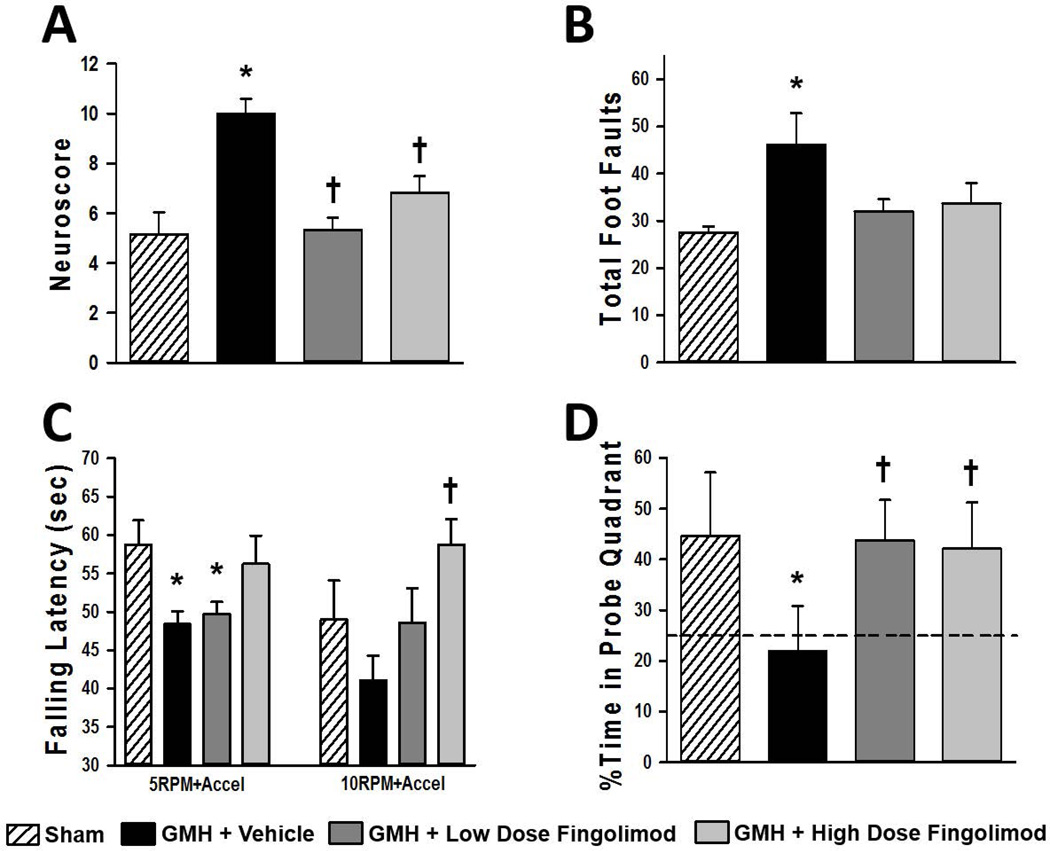
Following the third week after surgery, a cohort of sham and GMH animals treated with low or high dose fingolimod (0.25 mg/kg and 1.0 mg/kg, respectively) were subjected to a battery of sensorimotor and cognitive evaluations using a composite neuroscore, foot fault test, rotarod test, and Morris water maze test. When utilizing the composite neuroscore for evaluation, vehicle-treated GMH animals showed significant deficits compared to sham, while both the low and high dose fingolimod treated groups exhibited significant protection from GMH-induced neurodeficits (Fig. 1A, P<0.05). The foot fault test showed similar results, with vehicle-treated GMH animals exhibiting a significantly higher number of total foot faults compared to sham. Both fingolimod treated groups demonstrated fewer foot faults, however neither group reached significance compared to the vehicle group (Fig. 1B, P>0.05). Motor coordination was further evaluated using the rotarod test. At a starting rotation velocity of 5 RPM, both the vehicle-treated group and the low dose fingolimod group had a shorter falling latency. At this speed, the high dose fingolimod group was not significantly different from the sham group. Later, when the starting rotation velocity began at 10 RPM, the high dose fingolimod group spent significantly longer on the rotarod than the vehicle group (Fig. 1C, P<0.05). We found vehicle-treated GMH animals exhibited significant deficits in spatial memory on the Morris water maze test, compared to sham. However, both fingolimod treated groups showed a significant protection from the observed GMH-induced spatial memory deficits. (Fig. 1D, P<0.05)
Figure 1.
Composite neuroscore (A), total foot faults (B), falling latency during the rotarod test (C), and time spent in the target quadrant during probe trials of the Morris water maze test (D) between 21–27 days post-GMH. All data are expressed as mean ± SEM, except data from the Morris water maze test is expressed as mean ± 95% confidence. N=9 rats/group. * P<0.05 versus sham. † P<0.05 versus vehicle.
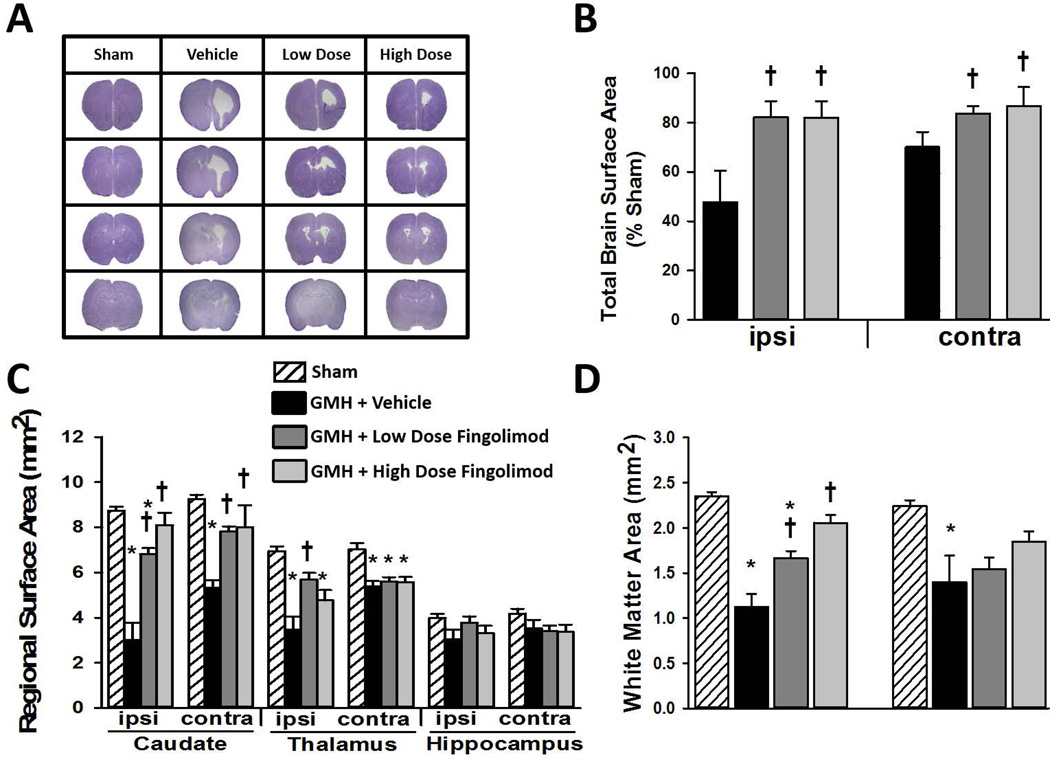
At four weeks after GMH, brain samples were prepared for histopathological analysis to quantify regional brain surface area (representative sections shown in Fig. 2A). Low and high dose fingolimod treatments significantly reduced GMH-induced gross tissue loss in both ipsilateral and contralateral hemispheres (Fig. 2B, P<0.05). Furthermore, fingolimod treated groups showed significant reductions in brain injury in bilateral caudate (Fig. 2C, P<0.05). High dose fingolimod treatment also provided significant protection from brain injury in the ipsilateral thalamus. Furthermore, both low and high dose fingolimod treated animals showed significant protection from white matter loss in the ipsilateral hemisphere (Fig. 2D, P<0.05).
Figure 2.
Representative microphotographs of Nissl stained brain sections four weeks after GMH (A) and quantifications of total brain surface area (B), individual areas of ipsilateral and contralateral caudate, thalamus, and hippocampus (C), and area of ipsilateral and contralateral corpus callosum (D). N=9 rats/group. Data expressed as mean ± SD. * P<0.05 versus sham. † P<0.05 versus vehicle.
Fingolimod and S1PR1 Agonism Reduced Brain Edema and Extravasated Evans Blue Dye, while S1PR1 and Rac1 Blockade Reversed Fingolimod-Induced Protection
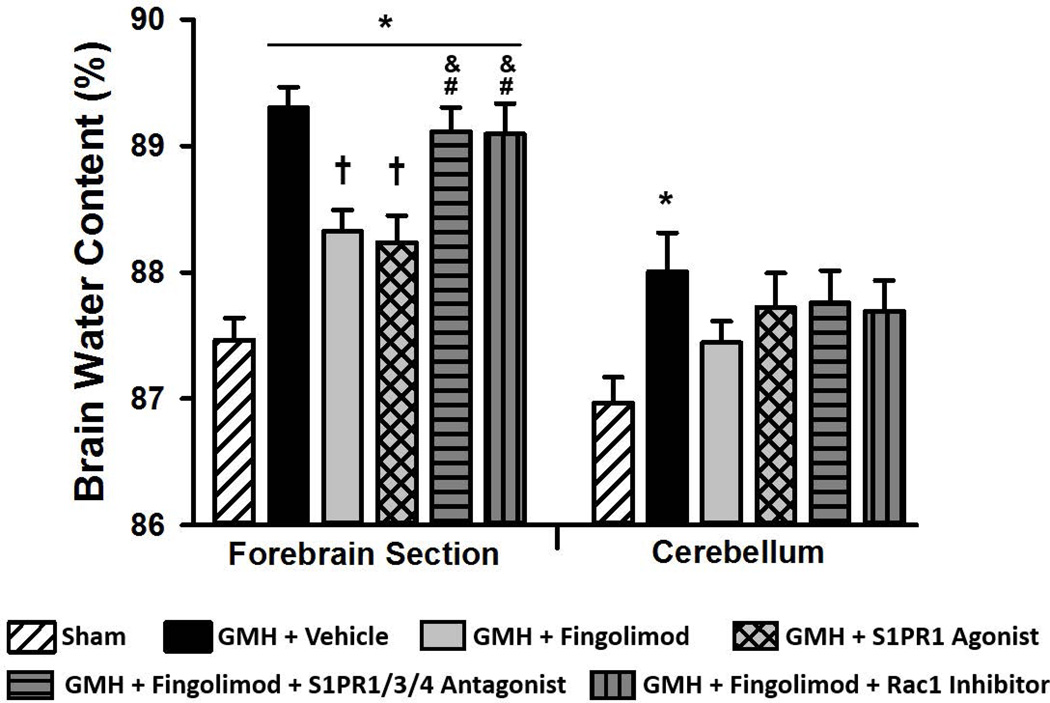
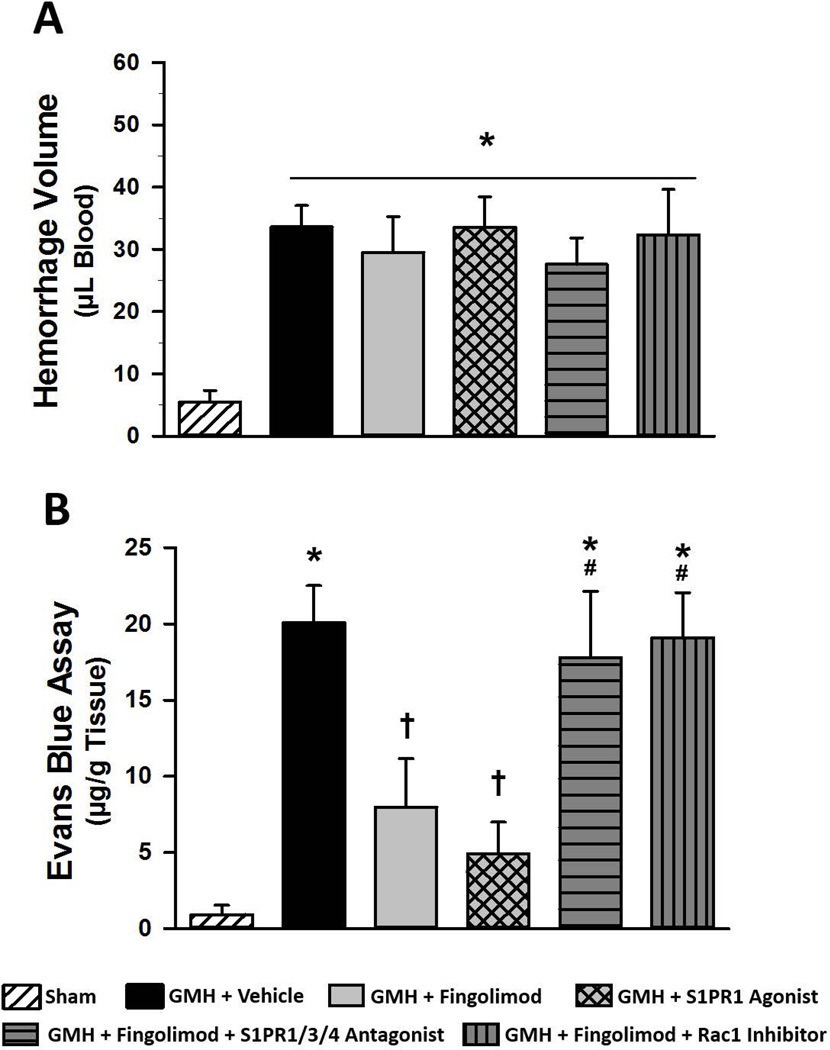
We found a significant elevation in brain edema in the forebrain of all GMH animals at 72 hours post-surgery (Fig 3, P<0.05). Both the fingolimod and the S1PR1 agonist groups showed a significant reduction in forebrain edema compared to vehicle (P<0.05). However, blockade of S1PR1/3/4 or Rac1 signaling abolished the fingolimod-induced reduction in forebrain edema (P<0.05). Cerebellar brain edema was significantly elevated in the vehicle-treated group, compared to the sham group. We found that Evans blue dye content was significantly elevated in vehicle-treated animals compared to sham at 72 hours after GMH (Fig. 4A, P<0.05). Fingolimod (1.0 mg/kg) and S1PR1 agonist treatment significantly decreased Evans blue dye extravasation (P<0.05). Furthermore, blockade of S1PR1/3/4 or Rac1 greatly reversed the effects of fingolimod (P<0.05, compared to sham and S1PR1 agonist groups). Brain hemorrhage volume was found to be significantly elevated in all groups, compared to sham, but was not significantly different between any GMH group (Fig. 4B). Representative images of brain sections after Evan’s blue injection shown in figure 4C.
Figure 3.
Brain water content at 72 hours after GMH in rats treated with fingolimod (1.0 mg/kg), the S1PR1 agonist, or in rats co-administered fingolimod with a S1PR1/3/4 antagonist or a Rac1 inhibitor. Data expressed as mean ± SD. N=7 rats/group. * P<0.05 versus sham. † P<0.05 versus vehicle. & P<0.05 versus fingolimod. # P<0.05 versus S1PR1 Agonist.
Figure 4.
Spectrophotometric quantification of brain extravasated Evans blue dye (A) and hemoglobin (B) at 72 hours after GMH in rats treated with fingolimod (1.0 mg/kg), the S1PR1 agonist, or in rats co-administered fingolimod with a S1PR1/3/4 antagonist or a Rac1 inhibitor. Representative images of brain sections are shown (C). Data expressed as mean ± SD. N=7 rats/group. * P<0.05 versus sham. † P<0.05 versus vehicle. # P<0.05 versus S1PR1 Agonist.
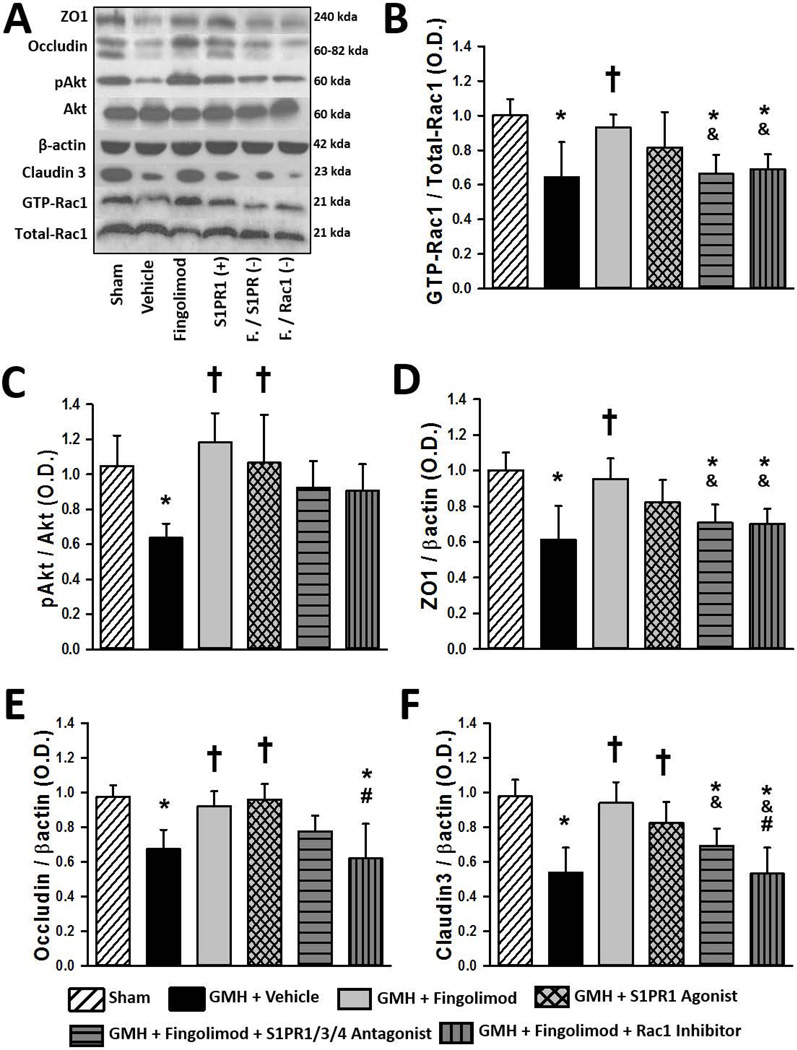
Fingolimod Improves ZO1, Occludin, and Claudin-3 Expression after GMH, via S1PR-Mediated Rescue of Akt and Rac1 Activation
Phosphorylated-Akt, Akt, GTP-Rac1, Total Rac1, ZO1, occludin and claudin-3 expression levels were measured by Western blot at 72 hours post-GMH. GMH resulted in significant decreases in the ratios of pAkt/Akt and GTP-Rac1/Total-Rac1 as well as the expression of ZO1, occludin, and claudin-3 (Fig. 5A–F, P<0.05). Fingolimod treatment significantly increased the ratios of pAkt/Akt and GTP-Rac1/Total-Rac1 as well as the expression of ZO1, occludin, and claudin-3 (P<0.05). S1PR1 agonist treatment protected against the GMH-induced changes, causing a significant increase in the pAkt/Akt ratio, occludin levels as well as claudin-3 levels (P<0.05). Though the GTP-Rac1/Total-Rac1 ratio and ZO1 protein levels only tended to be improved by S1PR1 agonist treatment, they were no longer significantly different compared to sham. Blockade of S1PR1/3/4 receptors significantly reversed fingolimod-induced elevations in the GTP-Rac1/Total-Rac1 ratio and ZO1 expression as well as claudin-3 expression (P<0.05, compared to fingolimod) and tended to reduce the elevations in the pAkt/Akt ratio and occludin expression. Inhibition of Rac1 reversed the fingolimod-induced increase in the GTP-Rac1/Total-Rac1 ratio as well as the expression of ZO1 (P<0.05, compared to fingolimod) and occludin (P<0.05, compared to S1PR1 agonist) as well as claudin-3 (P<0.05, compared to fingolimod and to S1PR1 agonist).
Figure 5.
Representative immunoblots (A) and quantification of GTP-Rac1/total Rac1 (B), pAkt/Akt (C), ZO1 (D), occludin (E), and claudin-3 (F) 72 hours after GMH in rats treated with fingolimod (1.0 mg/kg), the S1PR1 agonist, or in rats co-administered fingolimod with a S1PR1/3/4 antagonist or a Rac1 inhibitor. Data expressed as mean ± SD. N=7 rats/group. * P<0.05 versus sham. † P<0.05 versus vehicle. & P<0.05 versus fingolimod. # P<0.05 versus S1PR1 Agonist.
DISCUSSION
In a recent Chinese clinical pilot study, fingolimod decreased perihematomal edema and neurological impairment following primary intracerebral hemorrhage in adults (Fu et al., 2014). Fingolimod is a clinically available immunosuppressive agent that effectively penetrates the brain, has a long half-life, and bears structural similarity to the endogenous sphingosine-1-phosphate (S1P). Fingolimod activates four of the five G-protein coupled S1P receptors (S1PR1/3/4/5) to regulate numerous processes, including cell survival, immune cell migration, and endothelial barrier integrity (Cohen and Chun, 2011). While several studies have described the efficacy of fingolimod in murine models of adult ischemic (Czech et al., 2009, Shichita et al., 2009, Hasegawa et al., 2010, Wei et al., 2011, Brunkhorst et al., 2013, Campos et al., 2013, Kraft et al., 2013) and hemorrhagic stroke (Rolland et al., 2011, Rolland et al., 2013, Lu et al., 2014), this is the first study to evaluate fingolimod as a therapeutic modality in a rat model of neonatal GMH.
We first conducted long-term evaluations to demonstrate lasting protection. Treatment time points were chosen with the hypothesis that fingolimod targets early brain injury processes. We found both low and high dose fingolimod provided significant protection from long-term GMH-induced sensorimotor and cognitive deficits. Subsequently, in this cohort, histopathological analysis revealed both treatment groups had significantly more tissue area in bilateral caudate putamen and in the corpus callosum (white matter). Moreover, the high dose fingolimod group showed significantly greater surface area in the ipsilateral thalamus. Of particular interest, fingolimod treatment protected against GMH-induced white matter injury, since white matter dysfunction in infants with GMH have a higher risk of impairments in motor and executive function at 7 years (Thompson et al., 2014). Furthermore, recent experiments targeting oligodendrocyte lineage cells reported that remyelination by oligodendrocyte precursor cells may be augmented with fingolimod or specific S1PR mimetics, thereby ameliorating demyelination following a CNS insult (Miron et al., 2010). However, our experiments did not include analysis of oligodendrocyte lineage cells or other specific CNS cell types. Having provided evidence for the long-term efficacy of fingolimod in our GMH model and with support from previous adult murine stroke studies, we elected to include only the high dose of fingolimod for subsequent short-term experiments.
One of the hallmarks of secondary brain injury after stroke is the formation of brain edema, either from cell swelling (cytotoxic edema) or from disrupting the BBB structural foundation, leading to extravasation of osmotic agents into the brain parenchyma and higher paracellular permeation of fluid from the blood to the brain (vasogenic edema) (Xi et al., 2001). Research is underway to determine the primary mechanisms involved in the generation of GMH-induced brain injury. While the overall mechanism is still enigmatic, a myriad of pathogenic factors, such as excitotoxicity, oxidative stress, thrombin, and cerebral inflammation are related to BBB disruption and edema development, and are liable to contribute to secondary brain injury progression after GMH (Lekic et al., 2015, Tang et al., 2015). In recent reports, intranasal administered insulin-like growth factor 1 or osteopontin significantly reduced brain edema at 72 hours post-GMH, which was associated with improved long-term neurofunctional outcomes (Lekic et al., 2016, Malaguit et al., 2016). Therefore, we suggest brain edema formation is an important injury mechanism following GMH. Our model generated significant elevations in brain edema and BBB disruption at 72 hours, which is consistent with previous studies measuring acute post-GMH brain injury.
Assays for extravasated cerebral hemoglobin showed consistent cerebral hemorrhage volumes in all GMH groups. This indicates that our modeling technique is consistent and our therapeutic interventions do not potentially affect cerebral hemorrhage size after GMH. From our Evans blue results, we can conclude the forebrain edema had a significant vasogenic component. Both fingolimod and specific S1PR1 agonism significantly ameliorated GMH-induced forebrain edema. Furthermore, blockade of S1PR1/3/4 or Rac1 reversed the beneficial effects of fingolimod. These results indicate that fingolimod therapy protects against GMH-induced brain edema formation via activation of S1PR1 and Rac1.
In our experiment, cerebellar edema was significantly elevated in the vehicle group at 72 hours. Lekic, et al. showed that cerebellar edema tended to increase as a result of experimental GMH at 24 hours post-surgery (Lekic et al., 2012). The mechanism of cerebellar edema formation after GMH is unclear, though several lines of evidence may provide some explanation. Adults suffering from supratentorial ischemic stroke showed significantly increased apparent diffusion coefficients, an indicator of edema, on magnetic resonance imaging in cerebellar gray matter at 48 hours and both gray and white matter at 8 days post-ictus (Liu et al., 2010). It has been proposed that interrupting the excitatory afferent corticopontocerebellar and cerebellorubrothalamic tracts removes needed input to the cerebellum, resulting in a profound physiological imbalance that causes edema formation distal to the site of the supratentorial stroke. Not only is the cerebellum of neonates vulnerable to injurious insults, (Gallini et al., 2006) but also GMH with intraventricular extension can occur, (Lekic et al., 2012) releasing damaging cytokines and serum factors into the cerebrospinal fluid that increases stress on the cerebellum. Therefore, induction of experimental unilateral GMH will not only damage perihematomal tissue, but will potentially damage distal structures and the brain globally.
Endothelial cell-cell junctional contacts of the BBB require tight junctions (TJs) to maintain proper paracellular permeability. Composed of claudins, occludin, and junctional adhesion molecules, TJs are intracellularly bound to adaptor proteins, like zona occludins (ZO) proteins, which anchor the TJ complex with the actin cytoskeleton. The coagulation factor, thrombin, is elevated after experimental GMH (Lekic et al., 2012), inducing activation of the small GTPase RhoA and the downstream Rho-associated kinase (ROCK), which open the BBB by forming stress fibers and disrupting cell-cell junctional proteins (van Nieuw Amerongen et al., 2000, Stamatovic et al., 2003). The small GTPase Rac1 has been shown to stabilize barrier functions of endothelial cells (Wojciak-Stothard et al., 2001) by several pathways, including inhibition of RhoA and subsequent ROCK activity. Fingolimod has also been reported to augment transendothelial electrical resistance in the lung, which was associated with Rac1 activation (Sarai et al., 2009). Furthermore, ZO1 is redistributed to cell-cell junctions following S1P stimulation via the S1P1/Akt/Rac1 pathway (Lee et al., 2006). Fingolimod and the specific S1PR1 agonist significantly reduced the amount of extravasated Evans blue dye at 72 hours post-GMH. However, blockade of S1PR1/3/4 or Rac1 signaling reversed the effects of fingolimod treatment. Our results indicate fingolimod and S1PR1 stimulation is associated with reduced GMH-induced BBB permeability, and S1PR1 and Rac1 functionality are likely important for fingolimod’s BBB protective effects.
Akt and Rac1 activation and the expression levels of occludin and ZO1 were significantly reduced at 72 hours after GMH. Fingolimod treatment normalized Akt and Rac1 activity as well as restored occludin and ZO1 expression. Specific S1PR1 stimulation normalized Akt activation and occludin expression, but only showed a trend towards increasing Rac1 activation and ZO1 expression. S1PR1/3/4 blockade significantly reversed fingolimod-induced elevation in Rac1 activity and ZO1 expression with more subtle reversal of Akt activity and occludin expression. These results may be explained when considering the broad spectrum S1PR1/3/4/5 stimulatory activity of fingolimod as well as fingolimod’s inherent ability to inhibit S1P–Lyase (Brunkhorst et al., 2014), thus increasing S1P concentrations and further activating S1PRs. Finally, blockade of Rac1 was found to prevent fingolimod-induced elevations in ZO1, occludin, and claudins-3. Our results indicate fingolimod prevents BBB disruption after GMH by activating the S1PR axis and consequent Akt and Rac1 signaling. However, it is likely that other S1PRs, GTPases, and other intracellular effects are required for fingolimod-induced protection.
Inflammation is well known to contribute to BBB disruption and secondary brain injury after hemorrhage (Wang and Dore, 2007). We recently reported fingolimod decreases cerebral lymphocyte infiltration and IL-17 expression at 72 hours after experimental intracerebral hemorrhage (Rolland et al., 2013). Given that fingolimod is well known for its immunosuppressive properties, we measured the numbers of circulating blood leukocytes and the cerebral expression of IL-17 at 72 hours after GMH. We found fingolimod caused profound lymphopenia (Online Supplement, Fig. I) and significantly ameliorated GMH-induced expression of IL-17 in the brain (Online Supplement, Fig. II) at 72 hours post-surgery. Although IL-17 was significantly elevated in vehicle-treated animals, flow cytometric analysis showed no difference in leukocyte populations between vehicle and sham animals. Therefore, we hypothesize the peripheral immune cell flux occurred well before 72 hours post-GMH. Whether the reduction in cerebral IL-17 is due to decreased mobilized cell populations or due to the BBB protective effect of fingolimod limiting the transmigration of immune cells and/or their secretory products into the brain remains to be determined. Future studies utilizing lymphocyte deficient animals (nude rats or RAG1 knockout mice) should provide a better insight into the mechanisms contributing to fingolimod-induced protection.
There are several potential limitations of this study. First, we utilized stereotactic infusions of bacterial collagenase to generate our GMH model; which has the potential to cause a significant inflammatory reaction (Wang and Dore, 2007) above and beyond that of a spontaneous cerebral hemorrhage in pre-term neonates. This necessitates our findings to be repeated with alternative neonatal brain hemorrhage modeling techniques, such as intracerebroventricular blood infusion or intravenous dextrose infusion. Second, perturbations in physiological parameters (blood pressure, blood gasses, etc.) are not generally observed in experimental rodent studies with fingolimod, although a short-term induction of bradycardia has been observed in humans within the first 6 hours after treatment. Physiological parameters were not measured in our experimental animals. Third, sex differences have been documented in adult spontaneous intracerebral hemorrhage where female rats generally have a less severe injury through an estrogen receptor-dependent mechanism (Nakamura et al., 2005). However, data on sex differences in neonatal GMH is lacking. Both male and female pups were used, as previously reported (Leitzke et al., 2013, Klebe et al., 2014, Manaenko et al., 2014, Guo et al., 2015), but future studies should examine whether sex differences are present regarding fingolimod-induced neuroprotection from GMH. Fourth, we used ipsilateral hemisphere homogenates for our Western blot analysis of our proposed pathway and target proteins. This method does not distinguish between the various cell types in the brain. Further studies are needed to investigate the cell specific changes after GMH and the possible effects of fingolimod on neurons and non-neuronal brain cells. An ideal future experimentation plan would include flow cytometry of whole brain cell suspensions followed by cell sorting to isolate immunologic cells as well as those of the BBB in order to make final protein and mRNA assessments. Fifth, inhibition of the immune response following experimental hemorrhagic stroke has been widely linked to improved outcomes. However inflammation is also necessary for phagocytosis of cellular debris and for induction of an array of repair mechanisms (Zhao et al., 2007). The potentially protective effect of the adaptive immune system following GMH was not evaluated in the present study. Sixth, taking into account that fingolimod is an agonist of four of the five S1PRs (S1PR1/3/4/5) and its inherent ability to inhibit S1P–Lyase (Berdyshev et al., 2011), thus increasing S1P concentrations and further activating S1PRs, it is likely that other S1PRs and downstream second messengers are playing a role in fingolimod-induced protection. Seventh, we did not evaluate the changes in S1P concentrations, nor the S1PR1 expression time course in our experiments. However, based on previous studies, the time course of S1P production following adult experimental photochemical induced brain ischemia was reported to initially decrease up to day 3, after which it increased and peaked at day 14, remaining elevated thereafter (Kimura, Ohmori et al. 2008). Leitzke et al. reported GMH was associated with reduced cerebral expression of sphingosine kinases 1 and 2 (Leitzke et al., 2013), the enzymes responsible for phosphorylating sphingosine to S1P (Spiegel and Milstien, 2003). While, S1PR1 is expressed ubiquitously in the body, S1PR1 is enriched in the germinal matrix of fetuses and premature infants (Braun et al., 2007). Fingolimod, unlike S1P, can cause prolonged S1PR1 internalization and therefore drastically change the receptor expression profile (Chiba, 2005). Additionally, fingolimod has the ability to inhibit S1P lyase, the enzyme responsible for breaking down S1P (Bandhuvula et al., 2005). Therefore, we are interested in measuring blood and brain S1P levels, expression and activity of S1P metabolizing enzymes, as well as to localize and quantify S1PR expression in future studies. Lastly, several putative neuroprotective mechanisms have been linked to this pharmacological agent and its cognate receptors. S1PR1 is expressed ubiquitously throughout the body and its activation results in a plethora of downstream effects like cell mobilization, and regulation of survival, proliferation, and differentiation (Spiegel and Milstien, 2011). Stimulation of S1PR1 has generally been reported to reduce inflammation, cell stress, and cell death. Fingolimod treatment reduced neuronal apoptosis in a rat model of middle cerebral artery occlusion via an S1PR1 Akt and ERK-mediated pathway (Hasegawa et al., 2010). In the context of in-vitro studies, fingolimod reduced NMDA-mediated toxicity in cortical neuron cultures in a S1PR1 dependent manner (Di Menna et al., 2013). In HT22 cell cultures subjected to glucose deprivation followed by glucose reload, fingolimod and the specific S1PR1 agonist SEW2871 were found to enhance cell viability via activation of Bcl-2 and Bcl-XL (Czubowicz et al., 2015). S1PR1 in microglial cultures reduced fingolimod’s suppressive effects on TNF-α production by activated microglia (Noda et al., 2013). Interestingly, these authors found that fingolimod upregulated microglial production of brain-derived neurotrophic factor and glial cell-derived neurotrophic factor. The observed efficacy of fingolimod in our study likely occurred due to a combined effect involving BBB stabilization, a reduced immune response, and anti-apoptotic effects.
In conclusion, the present study showed fingolimod-induced activation of S1PR/Akt/Rac1 signaling ameliorated long-term behavioral deficits and brain injury after neonatal GMH in rats, likely in part by reducing acute BBB breakdown and brain edema formation. Furthermore, this study showed that the protective effect of fingolimod is associated with increased Akt and Rac1 activation and expression of the integral tight junction proteins ZO1 occludin and claudin-3. This conclusion is drawn from experiments using two S1PR1 agonists, fingolimod and SEW2871, the S1PR1/3/4 antagonist, VPC23019, and the Rac1 inhibitor, EHT1864. Additionally, we determined fingolimod induced peripheral lymphopenia and decreased GMH-induced IL-17 expression (supplemental material).
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by the National Institutes of Health (R01 NS078755 to Dr Zhang).
Abbreviations
- BBB
blood-brain barrier
- GMH
germinal matrix hemorrhage
- p-Akt
phosphorylated-Akt
- ROCK
Rho-associated protein kinase
- S1P
sphingosine-1-phosphate
- S1PR
sphingosine-1-phosphate receptor
- S1PR1
sphingosine-1-phosphate receptor1
- S1PR3
sphingosine-1-phosphate receptors3
- S1PR4
sphingosine-1-phosphate receptors4
- ZO
zona occludins
Footnotes
Author Contributions
William B. Rolland: Designed study, conducted animal surgery, obtained and analyzed data, wrote manuscript
Paul R. Krafft: Designed study, conducted behavioral testing, wrote manuscript
Tim Lekic: Conducted histopathological volumetric evaluation
Damon Klebe: Conducted behavioral testing, assisted with obtaining and analyzing data
Julia LeGrand: Assisted with obtaining and analyzing data
Abby Jones Weldon: Assisted with obtaining and analyzing data
Liang Xu: Conducted behavioral testing
John H. Zhang MD: Supervised study design, experimental execution, data analysis, and writing of the manuscript
Disclosures
The authors do not have any conflict of interest.
References
- Avendano C, Machin R, Bermejo PE, Lagares A. Neuron numbers in the sensory trigeminal nuclei of the rat: A GABA- and glycine-immunocytochemical and stereological analysis. The Journal of comparative neurology. 2005;493:538–553. doi: 10.1002/cne.20778. [DOI] [PubMed] [Google Scholar]
- Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. American journal of physiology Renal physiology. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatric research. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandhuvula P, Tam YY, Oskouian B, Saba JD. The immune modulator FTY720 inhibits sphingosine-1-phosphate lyase activity. The Journal of biological chemistry. 2005;280:33697–33700. doi: 10.1074/jbc.C500294200. [DOI] [PubMed] [Google Scholar]
- Berdyshev EV, Goya J, Gorshkova I, Prestwich GD, Byun HS, Bittman R, Natarajan V. Characterization of sphingosine-1-phosphate lyase activity by electrospray ionization-liquid chromatography/tandem mass spectrometry quantitation of (2E)-hexadecenal. Analytical biochemistry. 2011;408:12–18. doi: 10.1016/j.ab.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Xu H, Hu F, Kocherlakota P, Siegel D, Chander P, Ungvari Z, Csiszar A, Nedergaard M, Ballabh P. Paucity of pericytes in germinal matrix vasculature of premature infants. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12012–12024. doi: 10.1523/JNEUROSCI.3281-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkhorst R, Kanaan N, Koch A, Ferreiros N, Mirceska A, Zeiner P, Mittelbronn M, Derouiche A, Steinmetz H, Foerch C, Pfeilschifter J, Pfeilschifter W. FTY720 treatment in the convalescence period improves functional recovery and reduces reactive astrogliosis in photothrombotic stroke. PloS one. 2013;8:e70124. doi: 10.1371/journal.pone.0070124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkhorst R, Vutukuri R, Pfeilschifter W. Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Frontiers in cellular neuroscience. 2014;8:283. doi: 10.3389/fncel.2014.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F, Qin T, Castillo J, Seo JH, Arai K, Lo EH, Waeber C. Fingolimod reduces hemorrhagic transformation associated with delayed tissue plasminogen activator treatment in a mouse thromboembolic model. Stroke; a journal of cerebral circulation. 2013;44:505–511. doi: 10.1161/STROKEAHA.112.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacology & therapeutics. 2005;108:308–319. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Annals of neurology. 2011;69:759–777. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- Czech B, Pfeilschifter W, Mazaheri-Omrani N, Strobel MA, Kahles T, Neumann-Haefelin T, Rami A, Huwiler A, Pfeilschifter J. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochemical and biophysical research communications. 2009;389:251–256. doi: 10.1016/j.bbrc.2009.08.142. [DOI] [PubMed] [Google Scholar]
- Czubowicz K, Cieslik M, Pyszko J, Strosznajder JB, Strosznajder RP. Sphingosine-1-phosphate and its effect on glucose deprivation/glucose reload stress: from gene expression to neuronal survival. Molecular neurobiology. 2015;51:1300–1308. doi: 10.1007/s12035-014-8807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desire L, Bourdin J, Loiseau N, Peillon H, Picard V, De Oliveira C, Bachelot F, Leblond B, Taverne T, Beausoleil E, Lacombe S, Drouin D, Schweighoffer F. RAC1 inhibition targets amyloid precursor protein processing by gamma-secretase and decreases Abeta production in vitro and in vivo. The Journal of biological chemistry. 2005;280:37516–37525. doi: 10.1074/jbc.M507913200. [DOI] [PubMed] [Google Scholar]
- Di Menna L, Molinaro G, Di Nuzzo L, Riozzi B, Zappulla C, Pozzilli C, Turrini R, Caraci F, Copani A, Battaglia G, Nicoletti F, Bruno V. Fingolimod protects cultured cortical neurons against excitotoxic death. Pharmacological research. 2013;67:1–9. doi: 10.1016/j.phrs.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, Yan Y, Huang D, Yu C, Shi FD. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA neurology. 2014;71:1092–1101. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- Gallini F, Luciano R, Pane M, De Carolis MP, Romagnoli C, Mercuri E. Crossed cerebellar atrophy of prenatal onset. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2006;22:734–736. doi: 10.1007/s00381-006-0067-x. [DOI] [PubMed] [Google Scholar]
- Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. Journal of the neurological sciences. 2013;328:9–18. doi: 10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Chen Q, Tang J, Zhang J, Tao Y, Li L, Zhu G, Feng H, Chen Z. Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain research. 2015;1594:115–124. doi: 10.1016/j.brainres.2014.10.046. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke; a journal of cerebral circulation. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Krafft PR, Ma Q, Rolland WB, Caner B, Lekic T, Manaenko A, Le M, Tang J, Zhang JH. Fibroblast growth factors preserve blood-brain barrier integrity through RhoA inhibition after intracerebral hemorrhage in mice. Neurobiology of disease. 2012;46:204–214. doi: 10.1016/j.nbd.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe D, Krafft PR, Hoffmann C, Lekic T, Flores JJ, Rolland W, Zhang JH. Acute and delayed deferoxamine treatment attenuates long-term sequelae after germinal matrix hemorrhage in neonatal rats. Stroke; a journal of cerebral circulation. 2014;45:2475–2479. doi: 10.1161/STROKEAHA.114.005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek KD, Kirmeyer SE, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2009. Pediatrics. 2012;129:338–348. doi: 10.1542/peds.2011-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft P, Gob E, Schuhmann MK, Gobel K, Deppermann C, Thielmann I, Herrmann AM, Lorenz K, Brede M, Stoll G, Meuth SG, Nieswandt B, Pfeilschifter W, Kleinschnitz C. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke; a journal of cerebral circulation. 2013;44:3202–3210. doi: 10.1161/STROKEAHA.113.002880. [DOI] [PubMed] [Google Scholar]
- Landeen LK, Dederko DA, Kondo CS, Hu BS, Aroonsakool N, Haga JH, Giles WR. Mechanisms of the negative inotropic effects of sphingosine-1-phosphate on adult mouse ventricular myocytes. American journal of physiology Heart and circulatory physiology. 2008;294:H736–H749. doi: 10.1152/ajpheart.00316.2007. [DOI] [PubMed] [Google Scholar]
- Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, Hla T, Wang E, Lee MJ. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. The Journal of biological chemistry. 2006;281:29190–29200. doi: 10.1074/jbc.M604310200. [DOI] [PubMed] [Google Scholar]
- Leitzke AS, Rolland WB, Krafft PR, Lekic T, Klebe D, Flores JJ, Van Allen NR, Applegate RL, 2nd, Zhang JH. Isoflurane post-treatment ameliorates GMH-induced brain injury in neonatal rats. Stroke; a journal of cerebral circulation. 2013;44:3587–3590. doi: 10.1161/STROKEAHA.113.001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekic T, Flores J, Klebe D, Doycheva D, Rolland WB, Tang J, Zhang JH. Intranasal IGF-1 Reduced Rat Pup Germinal Matrix Hemorrhage. Acta neurochirurgica Supplement. 2016;121:209–212. doi: 10.1007/978-3-319-18497-5_37. [DOI] [PubMed] [Google Scholar]
- Lekic T, Klebe D, McBride DW, Manaenko A, Rolland WB, Flores JJ, Altay O, Tang J, Zhang JH. Protease-activated receptor 1 and 4 signal inhibition reduces preterm neonatal hemorrhagic brain injury. Stroke; a journal of cerebral circulation. 2015;46:1710–1713. doi: 10.1161/STROKEAHA.114.007889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekic T, Manaenko A, Rolland W, Krafft PR, Peters R, Hartman RE, Altay O, Tang J, Zhang JH. Rodent neonatal germinal matrix hemorrhage mimics the human brain injury, neurological consequences, and post-hemorrhagic hydrocephalus. Experimental neurology. 2012;236:69–78. doi: 10.1016/j.expneurol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang C, Tao W, Liu M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. The International journal of neuroscience. 2013;123:163–169. doi: 10.3109/00207454.2012.749255. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nuutinen J, Laakso MP, Karonen JO, Kononen M, Vanninen E, Kuikka JT, Vanninen RL. Cerebellar apparent diffusion coefficient changes in patients with supratentorial ischemic stroke. Acta neurologica Scandinavica. 2010;122:316–322. doi: 10.1111/j.1600-0404.2009.01289.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Barfejani AH, Qin T, Dong Q, Ayata C, Waeber C. Fingolimod exerts neuroprotective effects in a mouse model of intracerebral hemorrhage. Brain research. 2014;1555:89–96. doi: 10.1016/j.brainres.2014.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguit J, Casel D, Dixon B, Doycheva D, Tang J, Zhang JH, Lekic T. Intranasal Osteopontin for Rodent Germinal Matrix Hemorrhage. Acta neurochirurgica Supplement. 2016;121:217–220. doi: 10.1007/978-3-319-18497-5_39. [DOI] [PubMed] [Google Scholar]
- Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. Journal of neuroscience methods. 2011;195:206–210. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaenko A, Lekic T, Barnhart M, Hartman R, Zhang JH. Inhibition of transforming growth factor-beta attenuates brain injury and neurological deficits in a rat model of germinal matrix hemorrhage. Stroke; a journal of cerebral circulation. 2014;45:828–834. doi: 10.1161/STROKEAHA.113.003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, Antel JP. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. The American journal of pathology. 2010;176:2682–2694. doi: 10.2353/ajpath.2010.091234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Hua Y, Keep RF, Park JW, Xi G, Hoff JT. Estrogen therapy for experimental intracerebral hemorrhage in rats. Journal of neurosurgery. 2005;103:97–103. doi: 10.3171/jns.2005.103.1.0097. [DOI] [PubMed] [Google Scholar]
- Noda H, Takeuchi H, Mizuno T, Suzumura A. Fingolimod phosphate promotes the neuroprotective effects of microglia. Journal of neuroimmunology. 2013;256:13–18. doi: 10.1016/j.jneuroim.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Oorschot DE. Total number of neurons in the neostriatal, pallidal, subthalamic, and substantia nigral nuclei of the rat basal ganglia: a stereological study using the cavalieri and optical disector methods. The Journal of comparative neurology. 1996;366:580–599. doi: 10.1002/(SICI)1096-9861(19960318)366:4<580::AID-CNE3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rolland WB, 2nd, Manaenko A, Lekic T, Hasegawa Y, Ostrowski R, Tang J, Zhang JH. FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta neurochirurgica Supplement. 2011;111:213–217. doi: 10.1007/978-3-7091-0693-8_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland WB, Lekic T, Krafft PR, Hasegawa Y, Altay O, Hartman R, Ostrowski R, Manaenko A, Tang J, Zhang JH. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Experimental neurology. 2013;241:45–55. doi: 10.1016/j.expneurol.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarai K, Shikata K, Shikata Y, Omori K, Watanabe N, Sasaki M, Nishishita S, Wada J, Goda N, Kataoka N, Makino H. Endothelial barrier protection by FTY720 under hyperglycemic condition: involvement of focal adhesion kinase, small GTPases, and adherens junction proteins. American journal of physiology Cell physiology. 2009;297:C945–C954. doi: 10.1152/ajpcell.00606.2008. [DOI] [PubMed] [Google Scholar]
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nature medicine. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature reviews Molecular cell biology. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nature reviews Immunology. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. Journal of cell science. 2003;116:4615–4628. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- Tang J, Chen Q, Guo J, Yang L, Tao Y, Li L, Miao H, Feng H, Chen Z, Zhu G. Minocycline Attenuates Neonatal Germinal-Matrix-Hemorrhage-Induced Neuroinflammation and Brain Edema by Activating Cannabinoid Receptor 2. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9154-x. [DOI] [PubMed] [Google Scholar]
- Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN, Zhang JH. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Lee KJ, Egan GF, Warfield SK, Doyle LW, Anderson PJ, Inder TE. Regional white matter microstructure in very preterm infants: predictors and 7 year outcomes. Cortex; a journal devoted to the study of the nervous system and behavior. 2014;52:60–74. doi: 10.1016/j.cortex.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circulation research. 2000;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- Wang J, Dore S. Inflammation after intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, Guo S, Qin T, Alsharif N, Brinkmann V, Liao JK, Lo EH, Waeber C. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Annals of neurology. 2011;69:119–129. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. Journal of cell science. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Xi G, Fewel ME, Hua Y, Thompson BG, Jr, Hoff JT, Keep RF. Intracerebral hemorrhage: pathophysiology and therapy. Neurocritical care. 2004;1:5–18. doi: 10.1385/ncc:1:1:5. [DOI] [PubMed] [Google Scholar]
- Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT. Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity. Stroke; a journal of cerebral circulation. 2001;32:2932–2938. doi: 10.1161/hs1201.099820. [DOI] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neuroscience letters. 2000;283:230–232. doi: 10.1016/s0304-3940(00)00971-x. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Annals of neurology. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fathali N, Lekic T, Tang J, Zhang JH. Glibenclamide improves neurological function in neonatal hypoxia-ischemia in rats. Brain research. 2009;1270:131–139. doi: 10.1016/j.brainres.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.