Abstract
Cerenkov luminescence (CL) is an emerging imaging modality that utilizes the light generated during the radioactive decay of many clinical used isotopes. Although it is increasingly used for background-free imaging and deep tissue photodynamic therapy, in vivo applications of CL suffer from limited tissue penetration. Here, we propose to use quantum dots (QDs) as spectral converters that can transfer the CL UV-blue emissions to near-infrared light that is less scattered or absorbed in vivo. Experiments on tissue phantoms showed enhanced penetration depth and increased transmitted intensity for CL in the presence of NIR QDs. To realize this concept for in vivo imaging applications, we developed three types of NIR QDs and 89Zr dual-labeled nanoparticles based on lipid micelles, nanoemulsions, and polymeric nanoplatforms, which enable co-delivery of the radionuclide and the QDs for maximized spectral conversion efficiency. We finally demonstrated the application of these self-illuminating nanoparticles for imaging of lymph nodes and tumors in a prostate cancer mouse model.
Graphical Abstract
Introduction
Cerenkov luminescence (CL) is the light generated when charged subatomic particles travel in a dielectric medium faster than the speed of light in that medium.1–2 CL is observed with many clinically used radionuclides (e.g. 18F, 124I, 68Ga) that emit high-energy particles during their decay (e.g. β+ or β−).3–5 It is a relatively weak, but self-luminescent light, which allows for background-free imaging6–7 and possibly deep tissue photodynamic therapy,8–9 hence sparking increasing interest for biomedical applications.10
However, CL has intrinsic limitations for in vivo applications. Since its emission spectrum is mostly dominant in the UV-blue region (Figure 1a),11–12 CL intensity and penetration depth greatly suffer from scattering and tissue absorption. A possible solution could be to use an in vivo spectral converter to down-convert the high energy, blue-biased CL to near-infrared (NIR) light, where a biological optical window is located, thus improving the signal intensity and penetration depth.
Figure 1. Spectral conversion of Cerenkov luminescence by NIR QD.
a, Cerenkov emission spectrum and absorption spectra of typical tissue components. The theoretical Cerenkov emission spectrum is reproduced from ref. 10. b, Emission and absorption spectra of synthesized CdSeTe/CdSe/CdZnS NIR QD used in this work. c–d, Emission spectra of QD micelles and 89Zr oxalate mixtures in PBS. Each sample contains either (c) 80 µCi of 89Zr and indicated dilution of QD micelles, or (d) a fixed concentration of QD micelles and 89Zr with indicated activity. e, Under excitation of 80 µCi of 89Zr, the emission light intensity from the QD is linearly related to QD concentration.
NIR emitting quantum dots (QDs) are one of the best spectrum converters for CL due to their excellent optical properties.13 Compared to conventional organic NIR dyes (e.g. ICG), NIR QDs have continuous broad absorption spectra, which overlap well with the spectrum of CL. In addition, they also provide higher quantum yields, large spectral shifts and tunable narrow emissions down to the NIR range. Because of these advantages, in a similar role, QDs have widely served in many other applications as spectral converters, such as down-conversion for white-light LEDs,14 displays,15 solar cells,16 luminescent solar concentrators,17 etc.
To achieve the spectral conversion in applications requires spatial colocalization of light sources and spectral converters. A few recent reports have validated this concept and tested it for in vivo optical imaging.7, 18–19 However, in those works, QDs and radionuclides were administered as two separate chemical entities. Since QDs and radionuclides normally have different pharmacokinetics and biodistributions, their degree of spatial overlapping is low, thus limiting spectral conversion efficiency and hampering further in vivo applications.20–22 Therefore, developing a biocompatible nanoplatform, which enables efficient co-delivery of radionuclides and QDs to specifically targeted areas, is of great interest.21, 23
Here, we first studied in vitro the spectral converting properties of QD on CL, and investigated the effect of QD on enhancing CL in tissue depth penetration. Thereafter, to apply this concept for in vivo CL imaging applications, we developed dual labeling with NIR-QD and 89Zr of three types of widely-used self-assembled nanoparticles, including a lipidic nanoparticle, a nanoemulsion, and a polymeric nanoparticle. 89Zr were used as the emission source due to its relatively high β energy and long radiation half-life (78.4h). Finally, we applied them in in vivo imaging applications, i.e. sentinel lymph node (SLN) mapping and Cerenkov-guided resection and tumor visualization in a mouse model of prostate cancer.
Results and Discussion
QD spectral conversion of CL
We first synthesized highly efficient and water-stable CdTeSe/CdZnSe/ZnS core-shell-shell structured NIR QDs through a modified successive ionic layer adsorption and reaction (SILAR) method.24 Their emission wavelength was tuned to around 710 nm and their absorption profile has excellent spectral overlap with the Cerenkov emission (Figure 1b). To investigate the NIR QD’s spectral conversion function on the CL, we mixed different concentrations of 89Zr oxalate solution and PEG-lipid coated QDs in PBS, and recorded the emission spectra using an optical imaging system equipped with a range of emission filters. As shown in Fig 1c, with fixed activity of 89Zr and increasing QD concentration, the shape of the emission spectra changed gradually: the blue region of the CL spectra decreased and QD emission in the NIR increased correspondingly. On the other hand, keeping the QD concentration constant and gradually increasing the activity concentration, did not result in an obvious changes of the shapes of the spectra (Figure 1d). Instead, the overall intensity augmented due to an intensity increase of the light source. These results indicate that the spectral conversion efficiency mainly depends on QD concentration (Figure 1e) while the total intensity of CL is determined by the amount of activity used. In Figure 1e, the intensity of the light emitted by QDs is plotted against QD concentration. The intensity of converted light is linearly related with the concentration of the spectral converter, and the slope is related with the quantum yield of the fluorophore.
Light penetration through tissue phantoms
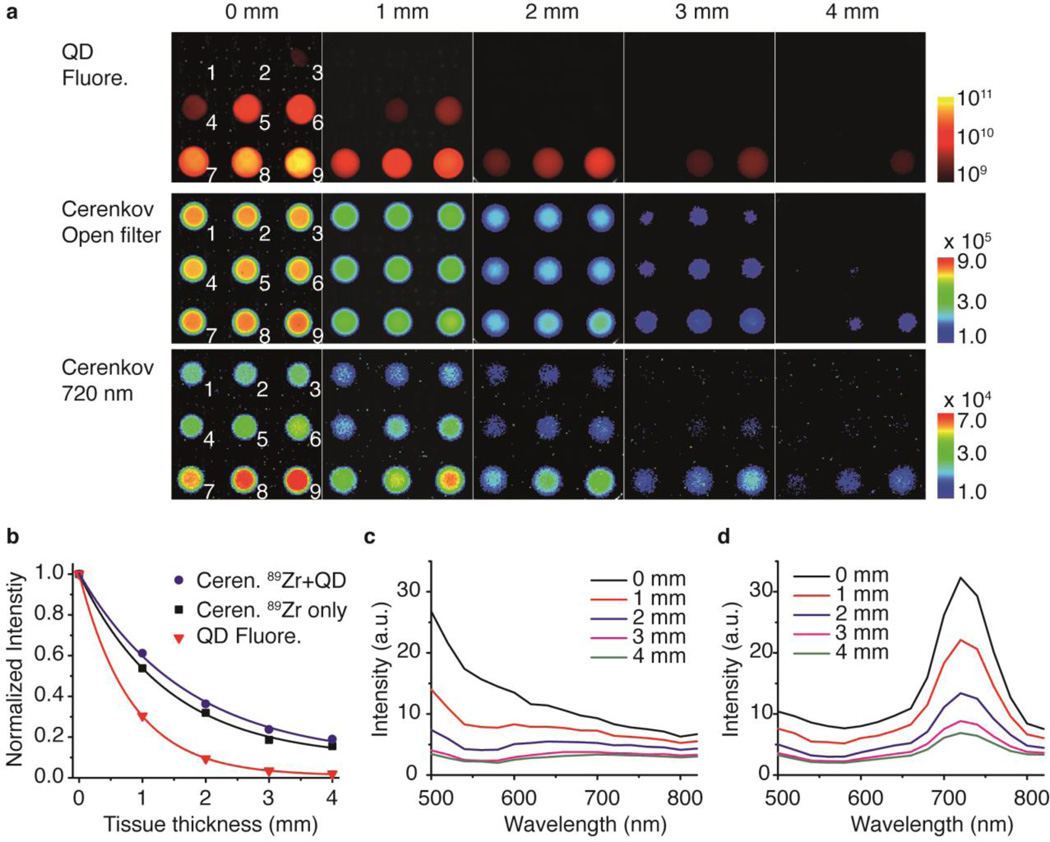
To test if the presence of QDs improves CL’s tissue penetration depth, we performed imaging experiments on tissue phantoms, in which experimental conditions are better controlled compared to in vivo testing. The intensity of the light that penetrated through the tissue phantoms was imaged by means of QD fluorescence with external excitation, CL with open filter, and CL with a 720 nm emission filter. Images in Figure 2a show that, in general, increasing tissue thickness attenuated the transmitted light’s intensity. However, in the presence of QDs – especially at higher QD concentrations – this QD-assisted CL penetrated the tissue phantom to a higher extent. A quantitative analysis of the tissue attenuation effect is summarized in figure 2b, and through an exponential fitting of the data, the effective attenuation coefficients, µeff, were obtained. Therefore, the penetration depth, δp, which is defined as the tissue depth at which the light’s intensity falls to 1/e (about 37%) of its original value, were 0.8 mm, 1.38 mm, and 1.70 mm for QD fluorescence, CL from 89Zr only, and CL with QD conversion, respectively. Fluorescence signal decayed most drastically with increasing tissue thickness, because both excitation and emission light are attenuated while traveling through the tissue. To the contrary, the self-illuminating Cerenkov emission has better preservation of the penetrated light because the attenuation only happens on the emission route. Most interestingly, for the QD-assisted CL, we observed an even better preservation of the intensity (Figure 2a). Spectral analysis on the Cerenkov emission with and without presence of QDs explains well the mechanism: with increasing tissue thickness, the short-wavelength regions of the emission spectra are more strongly suppressed than the long-wavelength regions (see all the emission spectra in Supporting Information, SI, Figure S1). As the original Cerenkov emission is most prominent in the blue region, at 4 mm tissue thickness the transmitted emission spectrum is flattened with almost complete signal extinction (Figure 2c). On the other hand, adding QDs greatly altered the transmitted emission spectrum profile and, in this case, the maximum shifted to 720 nm, which was less attenuated with increasing tissue thickness (Figure 2d). Even at a thickness of 4 mm, a substantial amount of light in the NIR region penetrated through. Of note, at given tissue conditions, if all the Cerenkov emission were converted to 720 nm light, the estimated theoretical δp value would be 2.27 mm. However, in practice, the δp will always be lower than this value due to incomplete spectral conversion in a specific system (SI, Figure S2).
Figure 2. Light penetration experiment on tissue phantoms.
a, Fluorescence, Cerenkov (without filter) and Cerenkov (filtered) images of QD and 89Zr mixtures with increasing tissue phantom thickness. Each well contains 80 µCi of 89Zr and increasing dilution of NIR QD, 1, 0%; 2, 2%; 3, 5%; 4, 10%; 5, 20%, 6, 30%, 7, 50%; 8, 70% and 9, 100%. b, Intensity of transmitted light is plotted against the tissue thickness. Solid curves are the fittings based on Beer-lambert law. I/I0 = exp(−µeff l), where l is the tissue thickness, I is the penetrated light, I0 is the original emission and µeff is the effective attenuation coefficient. c–d, Cerenkov emission spectra of transmitted light from wells No.1 (c, 89Zr only) and No. 9 (d) at different tissue thicknesses.
Although the in vitro optical studies above were carried out using mixtures of QDs and free 89Zr, in later applications 89Zr was directly associated with the nanoparticle. We found no significant difference in spectral conversion when 89Zr was bound to the QD surface compared to the equivalent mixtures of QDs and free 89Zr in solution (SI, Figure S3). It can be understood that, since the mean travel distance of β+ particles in solution or in tissue is normally in the millimeter range,25 this energy transfer from CL to QD actually does not require the radionuclides to be in close proximity to QDs.18 It is possible that the energy transfer process for this spectral conversion is mainly through radiative absorption. In this regard, it is very different from fluorescence resonance energy transfer (FRET), which is based on nonradiative dipole–dipole coupling that normally requires the donor-acceptor distance to be less than 10 nm.26
Synthesis and Characterization of QD and 89Zr dual-labeled nanoparticles
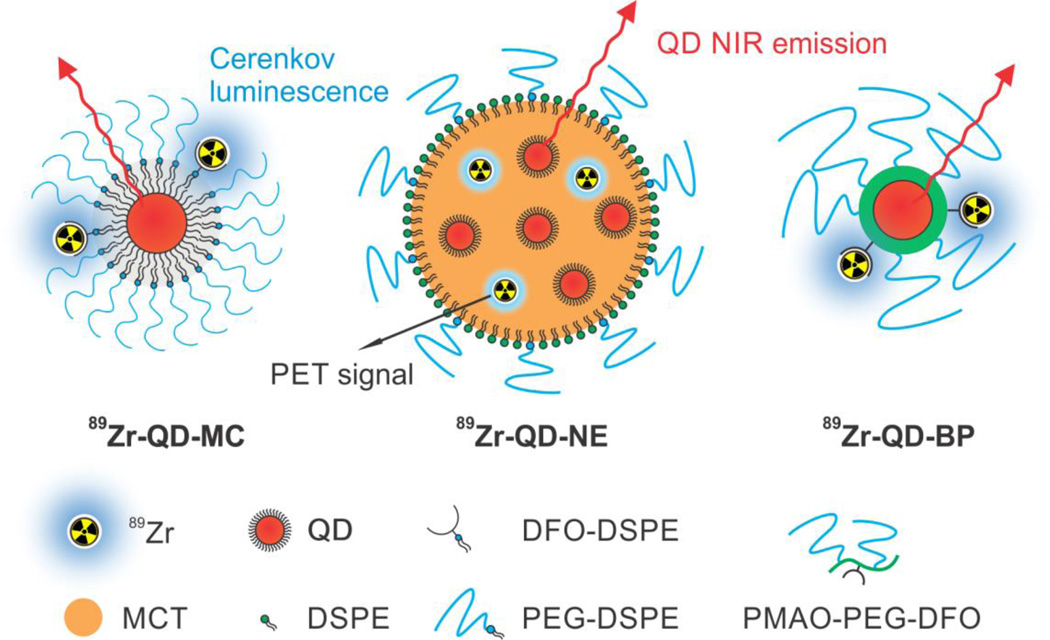
For in vivo applications, incorporating both QDs and radionuclides into one nanoparticle platform not only enables facile co-delivery of both components to the target area with maximized colocation, but also generates a radiolabeled self-illuminating nanoparticle that allows fluorescent, QD-assisted Cerenkov, and PET multi-modality imaging. Here, we realized this concept with three widely used and flexible nanoplatforms. The first nanoparticle, a micelle dually labeled with QD and 89Zr (89Zr-QD-MC), was prepared through the self-assembly of a hydrophobic ligand-capped QD core and a PEGylated-lipid shell (Figure 3a). In the lipid corona we incorporated a phospholipid functionalized with the chelator desferrioxamine (DFO; see synthetic route displayed in SI, Figure S4a), which allows complexation of 89Zr4+ ions (SI Figure S5a).27 The second nanoparticle, a QD nanoemulsion (89Zr-QD-NE), consists of phospholipid-stabilized medium chain triglycerides (MCT) with a high payload of QDs dispersed in the oil phase (Figure 3b). The size of the nanoemulsion was mainly controlled by the oil-to-phospholipid ratio.28 To achieve radiolabeling, 89Zr4+ ions can be transferred into the MCT core from aqueous phase using DFO-NCS as a phase transfer agent. Both 89Zr4+ and DFO-NCS were added to the PBS buffer containing the QD-NE. After complexation, the 89Zr-DFO-NCS complex turned to become hydrophobic, which facilitated its association with the MCT core (the detailed mechanism is unknown, the complex may be incorporated in the nanoemulsion core or stayed at the MCT/water interface, SI Figure S5b). The third platform was an amphiphilic block copolymer-coated QD (89Zr-QD-BP, Figure 3c). The coating polymer was synthesized by conjugating PEG2000-NH2 polymer chains and DFO moieties onto poly(maleic anhydride-alt-1-octadecene) (PMAO) backbone to achieve PEGylation and DFO functionalization (see synthetic route in SI Figure S4b). The hydrodynamic diameters of the three nanoparticles were determined through dynamic light scattering, ranging from 40 to 75 nm (DLS; SI, Figure S6). All three QD-containing nanoplatforms can be pre-synthesized, stably stored in solution for months, and then radiolabeled with 89Zr right before imaging with high radiochemical yields. The co-elution of Q trace (absorbance at 650 nm) and radioactive trace on size exclusion chromatography (SEC) at size over 50 nm indicate the successful association of 89Zr and QD with all three nanoparticle platforms (SI, Figure S5 d–f).
Figure 3. Schematic illustration of three designs of NIR QD, 89Zr dual-labeled nanoparticles.
89Zr-QD-MC (left) is a phospholipid-coated QD micelle with 89Zr labelled on the lipids; 89Zr-QD-NE (middle) is a phospholipid-stabilized nanoemulsion loaded with 89Zr and QD in the oil core; 89Zr-QD-BP (right) is a PMAO-PEG amphiphilic block copolymer-coated QD with 89Zr labelled on the polymer backbone.
To study the labeling stability of QDs and radionuclide in serum, the dual-labeled nanoparticles were incubated with fetal bovine serum (FBS) at 37 °C. Aliquots of the incubation solution at selected time points were analyzed by size exclusion chromatography (SEC; see results in SI Figure S7). For all three types of nanoparticles, no detectable dissociation of QDs from the nanoparticles was observed for over 24 hours. For 89Zr-QD-MC and 89Zr-QD-BP, the association of 89Zr with the nanoparticles was also strong, with very low activity leak-out (~6% and ~3% respectively) to other serum fractions for up to 24 hours. However, 89Zr-QD-NE was less stable and showed some dissociation of 89Zr (~15%) from the nanoparticle, either being associated to plasma proteins with an estimated molecular weight of 40 kDa or presenting as free ions.27 This release of small radiolabeled species was detectable at 1 h point and gradually increased with incubation time. Collectively, the FBS incubation results suggest that these dual-labeled nanoparticles are sufficiently stable for in vivo applications.
Cerenkov sentinel lymph node (SLN) mapping
The successful incorporation of QD and radionuclides into one bio-compatible platform enables important in vivo applications. Here we applied and evaluated them for lymph node mapping and tumor imaging through injection at three different sites in a prostate cancer mouse model. The lymphatic system is a vital part of the immune system and plays an important role in the pathology of many inflammatory diseases and cancers. Sentinel lymph node (SLN) mapping is widely performed for guiding lymph node dissection and biopsy in the diagnosis of metastasis. Conventionally, albumin-binding organic dyes (e.g. ICG, isosulfan blue) and radioactive sulfa-colloids (labeled with 99mTc) are used in the clinic.29 Recently new nanoparticle-based imaging agents for SLN mapping have been developed using various imaging modalities, such as NIR fluorescence,30 photoacoustic imaging,31 and MRI32 etc. As a new modality, Cerenkov-based SLN imaging has been achieved recently with 18F-FDG, and has shown its ability to guide LN resection.33 Here, we tested the LN imaging applications of QD and 89Zr dual-labeled nanoparticles at two injection sites, namely the footpad and the peri-tumoral area.
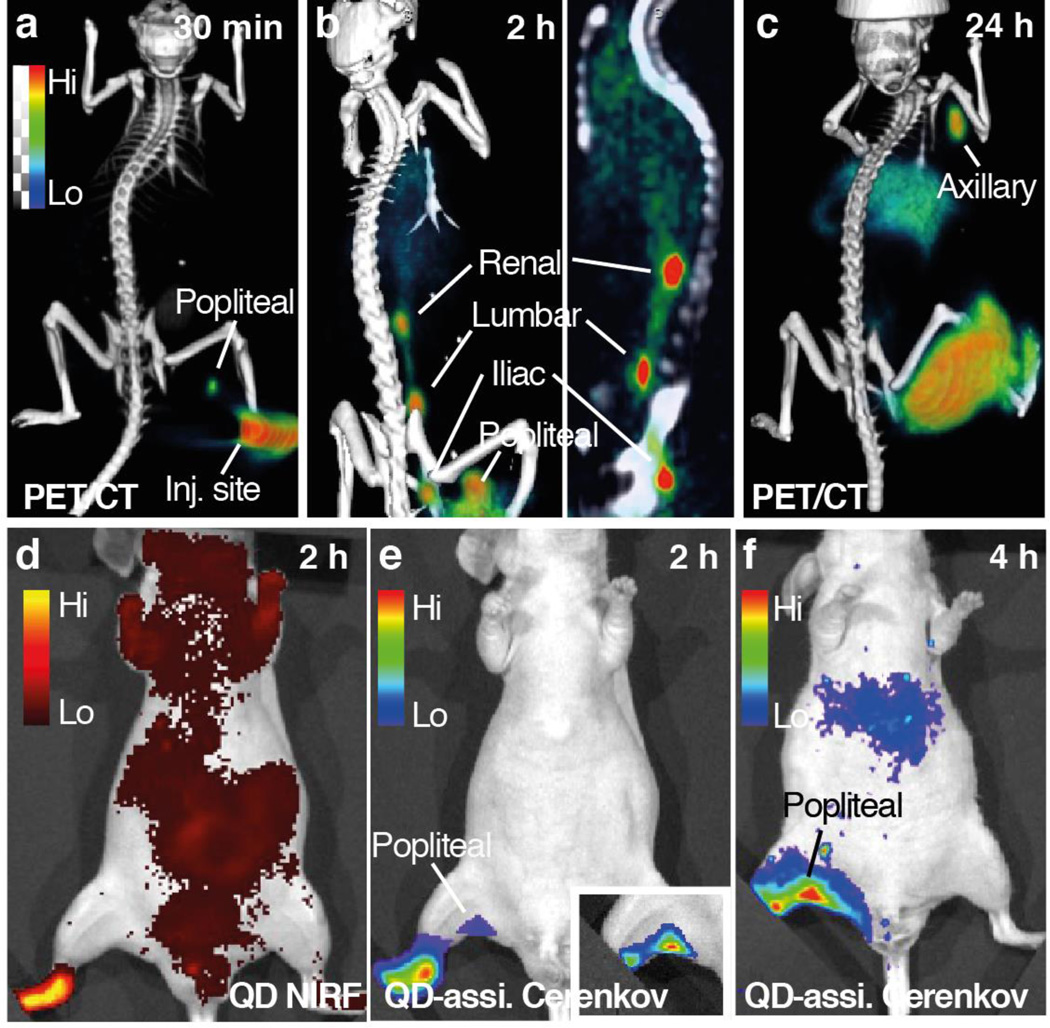
We first performed LN imaging using all three types of dual-labeled nanoparticles through footpad injection. After injecting the radiolabeled nanoparticle solution (50 µCi, 1.89 × 109 mBq, 30 µl) into the rear footpad of anesthetized nude mice (n=3), we conducted lymph node imaging scans at different time points using PET/CT, QD NIRF fluorescence imaging (with external excitation) and QD-assisted CL (self-luminescence) imaging. Representative results for 89Zr-QD-BP are displayed in Figure 4, while similar results were obtained for the other two nanoparticle types (SI Figure S8). PET/CT imaging clearly showed the progress of 89Zr-QD-BP lymphatic drainage: 30 min after injection we observed radioactivity accumulation in the closest popliteal lymph node. Within the next two hours, the nanoparticles had drained further along the lymphatic vessels and sequentially reached iliac LN, lumbar LN, and renal LN (Figure 4b).34 The structure of the nodes and the lymphatic vessels can be seen clearly, and some uptake in the liver was also observed. At 24 hours after injection, the image was dominated by axillary node and liver uptake (Figure 4c). In QD-assisted CL imaging with an open filter setting, the 89Zr-generated CL was partly converted by QD for better tissue penetration, and correspondingly, the popliteal node was observed distinctively with low background. The QD-assisted CL image shows a superior signal-to-background ratio compared to the QD fluorescence image, which suffered from high background signals due to auto-fluorescence (Figure 4d). After 2 hours, more nanoparticles drained to and retained in the popliteal nodes and the CL at the node became more intense. At later time points, the nanoparticles diffused from the footpad to the leg and interfered with the node signal, and thus no clear lymph node CL image can be appreciated.
Figure 4. Lymph node imaging with 89Zr-QD-BP.
a–c, 3-dimensional maximum intensity projection (MIP) rendering of PET/CT fusion images of a mouse injected on the right footpad with 89Zr-QD-BP at 30 min (a), 2 h (b) and 24 h (c) post-injection. d, The same animal imaged by NIRF imaging at 2h post-injection with excitation set at 600 nm and emission at 720 nm. e–f, QD-assisted Cerenkov luminescence (CL) imaging on the same animal at 2 h (e) and 4 h (f) post-injection. Inset of e shows popliteal node image with covering of injection site.
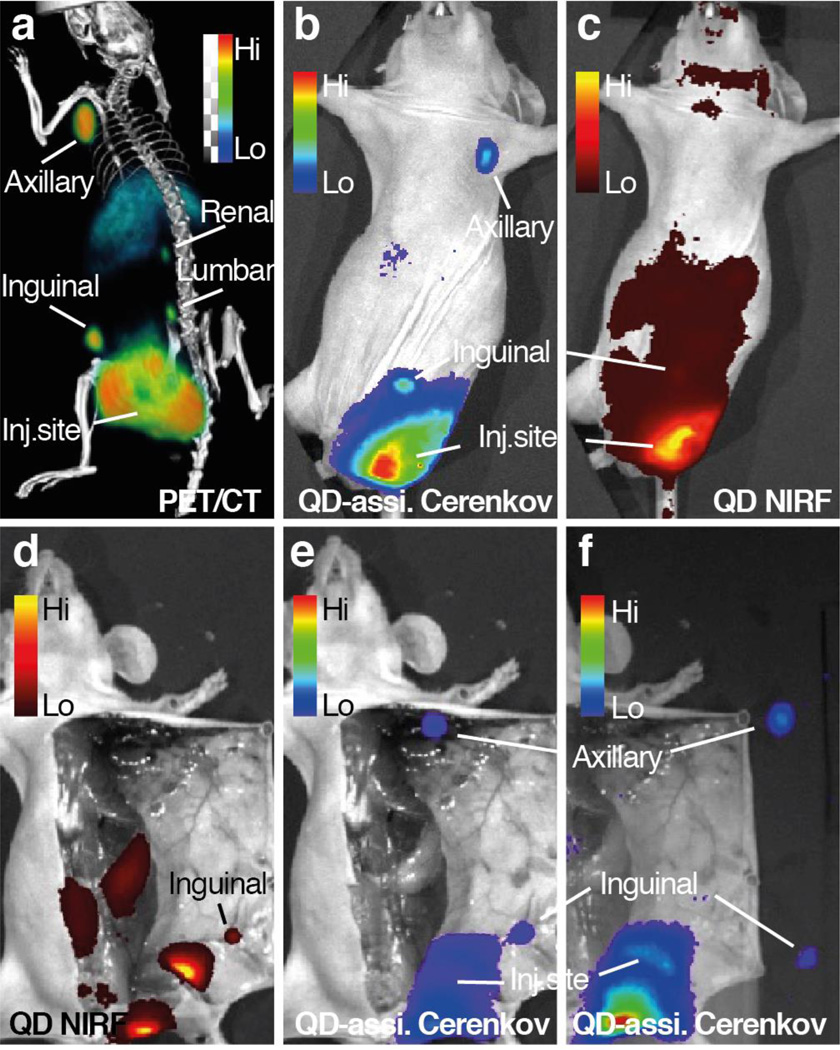
Next, we performed CL imaging-guided SLN resection using dual-labeled nanoparticles. Each nanoparticle formulations were injected intradermally in the periphery of the prostate tumor xenograft to allow the migration of nanoparticles into the lymphatic system. Twenty-four hours after injection, the mice were imaged using PET/CT, QD fluorescence, and QD-assisted CL imaging. The results from 89Zr-QD-BP imaging are displayed in Figure 5 and similar results from 89Zr-QD-NE are included in SI Figure S9. PET imaging clearly showed two paths of lymphatic drainage from the tumor: one through inguinal to axillary LNs, the other through the lumbar to renal LNs (Figure 5a). By QD-assisted CL imaging, the inguinal and axillary LNs were also clearly visualized with little background signal. To the contrary, in the QD NIRF imaging with external excitation, only the inguinal LN were visible and the axillary LN can be hardly perceived from the high background signal (Figure 5d). Finally, we performed resection of inguinal and axillary LNs under intraoperative guidance of QD-assisted CL imaging. This background-free imaging indicated the precise location of SLN, which finally led to successful and clean SLN removal (Figure 5e, f).
Figure 5. Sentinel lymph node imaging after peri-tumoral injection.
a–c, MIP of a mouse bearing a DU145 prostate tumor on the left flank was injected with 89Zr-QD-BP on the peri-tumoral area and imaged by PET/CT (a), QD-assisted CL imaging (b) and QD NIRF imaging with external excitation (c) at 24 h post-injection. d–f, Cerenkov imaging-guided lymph node removal performed on the same animal, showing QD-assisted CL images before node removal (e), compared with QD NIRF imaging (d), and after node removal (f).
Tumor Cerenkov imaging
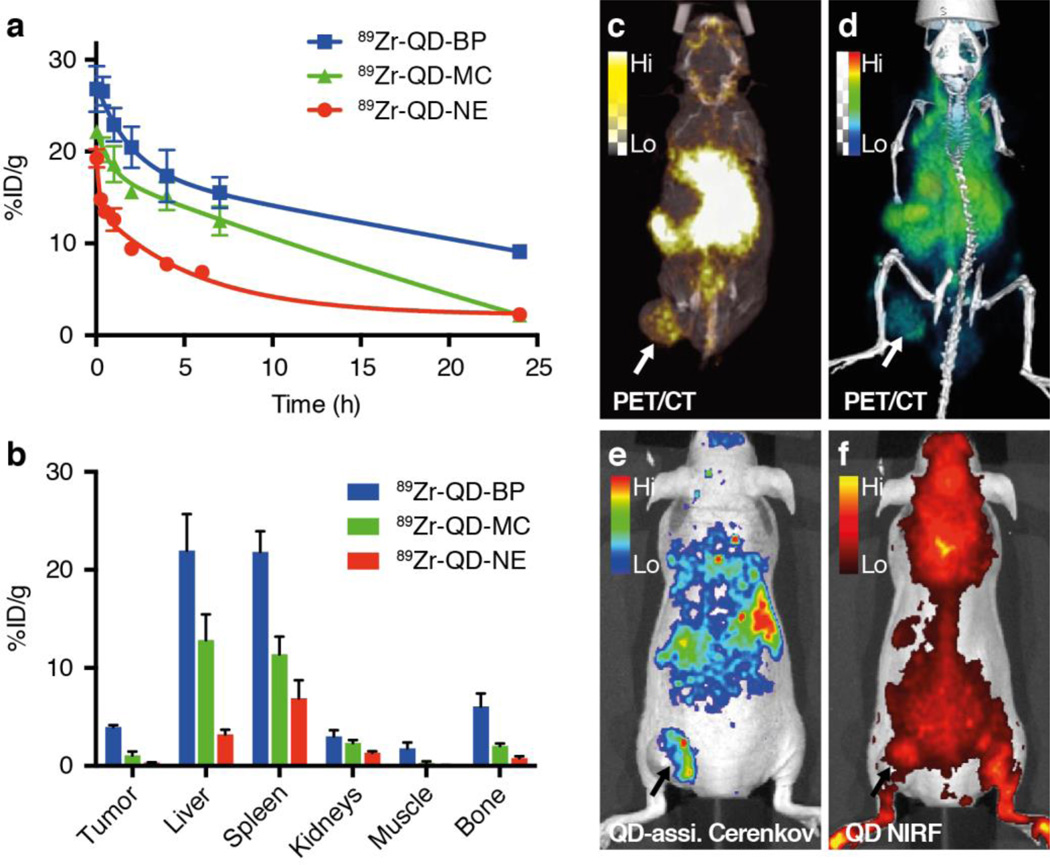
Having constructed the three different nanoparticles, we used the opportunity to study their passive targeting through the enhanced permeability and retention (EPR) effect. Before conducting tumor imaging with those dual-labeled nanoparticles via intravenous injection, their basic pharmacokinetics and biodistributions were evaluated. Blood clearance in healthy male nude mice was clearly different for the three particles: 89Zr-QD-BP and 89Zr-QD-MC had relatively longer blood radioactivity weighted half-lives of 4.1 h and 3.9 h, respectively (Figure 6a), compared to 89Zr-QD-NE’s 2.6 h. Biodistribution studies and tumor imaging based on EPR were completed on mice bearing flank DU145 prostate xenografts at 24 h post-injection. Biodistribution data from selected tissues are presented in Figure 6b. 89Zr-QD-BP generally showed higher accumulation in tumor as well as in other organs compared to 89Zr-QD-MC and 89Zr-QD-NE. PET/CT imaging was used to monitor nanoparticle in vivo distribution after administration at 4 and 24 h post injection (n=3). We found low tumor accumulation for both QD-MC-89Zr and QD-NE-89Zr at 24 hours after injection. For QD-MC-89Zr, activity was found mainly in the liver and spleen, and clearly in small and large intestine, suggesting hepatobiliary clearance of radiolabeled fragments (SI, Figure S10). For QD-NE-89Zr, activity was already seen intensely in the kidneys and bladder at 2 h post administration, indicating that the nanoemulsion likely disintegrates in the circulation and activity is cleared renally. These results are consistent with the shorter circulation half-lives and lower tissue accumulations of 89Zr-QD-MC and 89Zr-QD-NE, indicating lower particle or labeling stability in the circulation, which may be caused by interactions with blood components and subsequent renal or hepatic clearence.13 On the contrary, radioactivity accumulation was clearly observed for 89Zr-QD-BP in the tumor as early as one hour after injection. The images were dominated by a strong blood pool signal due to the long circulation time of 89Zr-QD-BP (Figure 6b), causing high background signal at early time points. At 24 h post-injection, a higher tumor-to-blood signal ratio was observed, resulting in higher tumor imaging quality (Figure 6c, d). Correspondingly, similar tumor accumulation trends and imaging patterns can be appreciated by QD-assisted CL, while in traditional QD NIRF imaging the signal from the tumor imaging can be hardly distinguished from body auto-fluorescence. These results show that although all three dual-labeled nanoparticles can be successfully used as LN imaging agent, only 89Zr-QD-BP is suitable for tumor imaging through intravenous injection, possibly due to its higher in vivo stabilities.35 89Zr-QD-BP has 89Zr-DFO moieties covalently conjugated on the QD coating thus is a more stable structure, compared to the 89Zr-DFO-lipids associated on the corona of QD micelles and 89Zr-DFO-NCS complex loaded in the MCT oil core.
Figure 6. Pharmacokinetics, biodistribution and whole-body imaging of QD and 89Zr dual-labeled nanoparticles.
a, Blood circulation half-life and b, radioactivity biodistribution in selected tissues after intravenous administration of 89Zr-QD-BP (blue), 89Zr-QD-MC (green) and 89Zr-QD-NE (red) in mice bearing DU145 tumor. c–f, Whole-body imaging of mice intravenously injected with 89Zr-QD-BP at 24 h post-injection, showing MIP PET/CT fusion image (c), 3-D rendered PET/CT fusion image (d), QD-assisted CL imaging (e), and QD NIRF imaging (excitation 600 nm, emission 720 nm) (f).
Conclusion
We have proved the concept that using NIR QD as spectral converters can greatly change the Cerenkov emission spectra by converting UV-blue luminescence into NIR light, resulting in significant increase in CL’s tissue penetration depth and output signal intensity. To realize this concept in in vivo imaging applications, we have developed three types of QD and 89Zr dual-labeled nanoparticles, including QD lipid micelles, QD containing nanoemulsions and block copolymer coated QDs that enable co-delivery of radionuclides and QDs to the desired location for maximized spectral conversion effect. The applications of these dual-labeled self-illuminating nanoparticle for background-free QD-assisted CL imaging of LN and tumor were demonstrated.
Experimental Procedures
Synthesis of CdSeTe/CdS/ZnS core-shell-shell (CSS) QD
Water-and-air-stable CdSeTe/CdS/ZnS NIR QD was synthesized through a modified core-shell two-step method.13, 24 First, CdSeTe alloyed core was synthesized through a hot-injection method. In a three-neck flask, 0.4 mmol CdO powder (≥99.99%, Sigma), 0.8 mmol tetradecylphosphonic acid (TDPA, 97%, Sigma), 3.5 ml 1-octadecene (ODE, 90%, Sigma), 2 ml oleylamine (OLA, 70%, Sigma) and 2 ml trioctylphosphine (TOP, 97%, Sigma) were added and sealed under Schlenk line. The reaction mixture was degased three times at 100 °C, kept under N2 atmosphere and then heated up to 280 °C until the mixture turned into a clear solution. At 280 °C, 0.2 mmol Se (powder, 99.99%, Sigma) and 0.1 mmol Te (powder, 99.8%, Sigma) dissolved in 1 ml TOP were swiftly injected into the reaction mixture, and the temperature was gradually cooled down to 230 °C. At this temperature, about 1 ml of 0.1 M Se in TOP was dripped into the reaction mixture to allow the continuous growth of the alloyed core, shifting the emission peak to 690 nm. The synthesized CdSeTe core was precipitated by adding ethanol and washed twice with hexane and methanol. For the growth of CdS/ZnS shell, the CdSeTe core in hexane was added to 5 ml of trioctylamine (TOA, 98%, Sigma) and degassed at 100 °C, removing hexane and moisture. Then at 230 °C, 0.2 ml of 0.1 M Cd(OA)2, 0.2 ml of 0.1 M S in TOP, 0.2 ml of 0.5M Zn(OA)2 and 1 ml of 0.1 M S were sequentially slowly dripped into the reaction mixture. The emission peak shifted to about 710 nm after shell coating. The final product was washed twice with a hexane/methanol (1:2) mixture and precipitated with acetone, and redispersed in chloroform for further use.
Radiochemistry
89Zr was produced through the 89Y(p,n)89Zr reaction on an EBCO TR19/9 variable-beam energy cyclotron (Ebco Industries Inc., British Columbia, Canada) at Memorial Sloan Kettering Cancer Center. The crude product was purified in accordance with previously reported methods to yield 89Zr with a specific activity of 5.3−13.4 mCi/µg.36 Dose measurements were made using a Capintec CRC-15R Dose Calibrator (Capintec, Ramsey, NJ).
Tissue phantom experiment
The tissue phantom was made from gelatin gel containing 2% of Intralipid (20%, emulsion, Sigma) mimicking the scattering from the tissue and 100 µM of hemoglobin (porcelain, Sigma) mimicking tissue absorption.37–38 Briefly, 5 g of gelatin, 1 ml of Intralipid (20% emulsion), 320 mg of hemoglobin and 0.5 g glutaraldehyde were dissolved in 50ml Tris buffer (pH=7.4, 50 mM Tris, 150 mM NaCl and 0.1% w/v sodium azide) at about 50 °C. The homogenous solution was then poured to a model with the thickness of 1, 2, 3, and 4 mm. The gel was formed after refrigeration overnight. For the tissue penetration experiment, 80 µCi (2.96 MBq) of 89Zr and an increasing concentration of QDs were added to wells in a 96-well plate, reaching a total volume of 250 µl. The tissue phantoms with different thickness were placed on top of the well plate and imaged in a PerkinElmer IVIS Spectrum optical imaging system. QD fluorescence was imaged with excitation at 605 nm, emission at 720 nm, and 2 s exposure time. CL was imaged without blocked excitation, either open emission filter or 720 nm filter, and 2 min exposure time.
Preparation of QD micelle and radiolabeling of 89Zr-QD-MC
DSPE-DFO was synthesized through the conjugation of DFO-NCS (Macrocyclics Inc.) and DSPE (Avanti) with details already described elsewhere.27 The QD micelle was synthesized through dripping a 0.5 ml chloroform solution containing 20 µmol of DSPE-PEG2000, 200 nmol of DSPE-DFO (1% of total lipids) and 1 nmol of NIR QDs into 5 ml of PBS at 80 °C under vigorous stirring.11 The organic phase was evaporated and the contents were transferred into aqueous phase forming micelles. The crude product solution was then centrifuged at 4,000 rpm for 10 min to remove aggregates and precipitates. To remove empty micelles and free lipids, the product solution was ultra-centrifuged twice, each for 2 hours, at RCF 480,000 × g, on a Beckman Coulter Optima XE100 ultracentrifuge equipped with a 70 Ti fixed angle rotor. The supernatant was removed after each run, and the precipitation pellet was finally redispersed in PBS. Radiolabeling was performed by reacting the QD micelles with 89Zr-oxalate in PBS (pH= 6.8–7.1) at 37 °C for 2 hours at an activity-to-QD ratio of ~ 1 mCi/nmol. The radiolabeled QD micelle, 89Zr-QD-MC, was purified through spin filtration using 100 kDa molecular weight cut-off (MWCO) centrifugal filters.
Preparation of QD nanoemulsion and radiolabeling of 89Zr-QD-NE
The QDs were first transferred to oil phase through dissolving the 2.5 nmol QD and 50 µl MCT in chloroform as a homogenous solution, followed by evaporation of the solvent under reduced pressure. The QD in MCT dispersion was placed under high vacuum for several hours to remove any trace of chloroform. A stock solution of mixed phospholipids was prepared in ethanol at 20 µmol/ml, containing 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) 90 mol % and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000] (DSPE-PEG2000) 10 mol%. To prepare the QD nanoemulsion, 10 mg of QD MCT dispersion and 500 µl of phospholipid stock solution were added into 5 ml PBS and vortexed. Subsequently, this crude emulsion was sonicated for 30 min using a 150 V/T ultrasonic homogenizer (BioLogics, Inc.) at 30% power output. The QD nanoemulsion was washed 3 times with fresh PBS by spin filtration using 100 kDa MWCO centrifugal filters. Radiolabeling was performed by adding 89Zr-oxalate and DFO-NCS (6 µg) in DMSO subsequently into the QD nanoemulsion solution in PBS (pH= 6.8–7.1) and reacted at 37 °C for 2 hours at an activity-to-QD ratio of ~ 1 mCi/ nmol, and purified by spin filtration using 100 kDa MWCO centrifugal filters.
Preparation of block-copolymer coated QD and radio labeling of 89Zr-QD-BP
The PMAO-PEG-DFO block co-polymer was synthesized by reacting PMAO (Sigma-Aldrich) 35 mg, PEG2k-NH2 (JenKem) 200 mg, and DFO-NH2 1.68 mg (3 mol% of PEG) in 10 ml anhydrous dichloromethane in a 25 ml round flask. The reaction solution was sealed under N2, and stirred overnight at room temperature. Afterwards, the solvent was removed in a rotary evaporator, and the product was redispersed in deionized water. After centrifugal removal of aggregates and undissolved materials, the unreacted PEG-NH2 and DFO-NH2 were removed by washing with DI water using 100 kDa MWCO centrifugal filters. Finally, the PMAO-PEG-DFO product was lyophilized. The method for the preparation of block copolymer coated QD and the radiolabeling were similar to that for QD-MC-89Zr.
HPLC and Radio-HPLC
HPLC and radio-HPLC were performed on a Shimadzu HPLC system equipped with two LC-10AT pumps and an SPD-M10AVP photodiode array detector. Radioactivity was detected using a Lablogic Scan-RAM Radio-TLC/HPLC detector. A Superdex 10/300 column (GE Healthcare Life Sciences, Pittsburgh, PA) was employed to run size exclusion chromatography using PBS as eluent at a flow rate of 1 ml/min.
Serum stability
The serum stabilities of QD-MC-89Zr, QD-NE-89Zr and QD-BP-89Zr were evaluated by incubating 200 µCi (7.4 MBq) of each type of particle with 0.5 ml of pure FBS at 37 °C. At 0 min, 10 min, 1 h, 4 h and 24 h after incubation, aliquots of 10 µCi (1.85 MBq) was sampled and immediately injected into the HPLC SEC column.
Cell culture and animal models
Four to five week old male NCR/NU athymic nude mice were obtained from Charles River Laboratories (Wilmington, MA). The prostate cancer cell line DU145 (ATCC) was cultured as recommended by ATCC. Briefly, Eagle's Minimum Essential Medium was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin streptomycin. DU145 cells were grown to confluence and 1.5 × 106 cells in 100 µl matrigel were injected into the left flank of the nude mice. The animals were used for imaging experiment when the tumor size reached approximately 400 mm3. All animal experiments were done in accordance with protocols approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center and followed National Institutes of Health guidelines for animal welfare.
Blood half-life and biodistribution
Healthy male nude mice (8–10 weeks old, n = 3 per type of particle) were injected with about 50 µCi (1.85 MBq) of nanoparticle solution in 200 µl PBS via the lateral tail vein. Blood samples were collected at 5, 15, and 30 min, 1, 2, 4, 6, and 24 h. Biodistribution experiments were conducted on male nude mice (8–10 weeks old, n = 3) bearing DU145 tumors. Around 300 µCi (11.1 MBq) of nanoparticles in 200 µl PBS were injected via tail vein. After 24 h, mice were sacrificed and perfused with 40 ml PBS through heart left ventricle before tissues were harvested. The blood and tissue samples were weighed and their radioactivity measured on a Wizard2 2470 automatic gamma counter (Perkin Elmer, Waltham, MA).
Lymph node imaging
The QD and 89Zr dual-labeled nanoparticle were concentrated in PBS using centrifugal filters. For lymph node imaging via footpad injection, about 30 µl of nanoparticle solution containing 100 µCi (3.7 MBq) were injected on the right footpad of healthy male nude mice. The injected mice underwent PET/CT, fluorescence and Cerenkov imaging at 30 min, 1 h, 2h, 4h, and 24h. For sentinel lymph node (SLN) imaging, about 10 µl of nanoparticle solution containing 30 µCi (1.11 MBq) were injected intradermally in the peritumoral area. The injected mice were then subjected to PET/CT and Cerenkov imaging at 1 h, 4h, and 24h.
For in vivo imaging, animals were anesthetized with isoflurane (Baxter Healthcare, Deerfield, IL) and oxygen gas mixture (2% for induction, 1% for maintenance. Whole body PET static scans were performed using an Inveon PET/CT scanner (Siemens) recording a minimum of 40 million coincident events, with scan durations of 5–30 min. The energy and coincidence timing windows were 350−750 keV and 6 ns, respectively. The image data were normalized to correct for non-uniformity of response of the PET, dead-time count losses, positron branching ratio, and physical decay to the time of injection, but no attenuation, scatter, or partial-volume averaging correction was applied. Images were analyzed using Inveon research software (Siemens). Whole body standard low magnification CT scans were performed with the X-ray tube setup at a voltage of 80 kV and current of 500 µA. The CT scan was acquired using 120 rotational steps for a total of 220 degrees yielding an estimated scan time of 120–140 s with an exposure time of 145 ms per frame.
Tumor imaging
Male nude athymic mice (8–10 weeks old, n = 3) bearing DU145 tumors were injected with about 300 µCi (11.1 MBq) of nanoparticle in 200 µl saline solution via the lateral tail vein. At predetermined time points (1, 2, 4 and 24h) animals were anesthetized and subjected to PET/CT (Inveon, Siemens), fluorescent and QD-assisted CL imaging (IVIS spectrum, Perkin Elmer).
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health grants: Cancer Center Core Grant to MSKCC (Craig Thompson; P30-CA008748), R01 EB014944 (J.G), R01 CA183953 (J.G), R01 HL118440 (W.J.M.M.), R01 HL125703 (W.J.M.M.), R01 CA155432 (W.J.M.M.), R01 EB009638 (Z.A.F.), NWO Vidi (W.J.M.M.).
Footnotes
Conflict of Interest
The authors declare no competing financial interest.
Supporting information
The Supporting Information is available free of charge on the ACS Publications website at DOI:
Additional information and data on tissue phantom experiments, synthesis of functional lipids and block-copolymers, radiolabeling characterization, nanoparticle characterization, and PET/CT imaging.
References
- 1.Cherenkov PA. Visible emission of clean liquids by action of γ radiation. Dokl. Akad. Nauk SSSR. 1934;2:451. [Google Scholar]
- 2.Robertson R, Germanos MS, Li C, Mitchell GS, Cherry SR, Silva MD. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys. Med. Biol. 2009;54:N355–N365. doi: 10.1088/0031-9155/54/16/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruggiero A, Holland JP, Lewis JS, Grimm J. Cerenkov luminescence imaging of medical isotopes. J. Nucl. Med. 2010;51:1123–1130. doi: 10.2967/jnumed.110.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell GS, Gill RK, Boucher DL, Li C, Cherry SR. In vivo Cerenkov luminescence imaging: a new tool for molecular imaging. Philos Trans A Math Phys Eng Sci. 2011;369:4605–4619. doi: 10.1098/rsta.2011.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaffer T, Drain CM, Grimm J. Optical imaging of ionizing radiation from clinical sources. J. Nucl. Med. 2016;57:1661–1666. doi: 10.2967/jnumed.116.178624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das S, Thorek DL, Grimm J. Cerenkov imaging. Adv. Cancer Res. 2014;124:213–234. doi: 10.1016/B978-0-12-411638-2.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorek DLJ, Ogirala A, Beattie BJ, Grimm J. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat. Med. 2013;19:1345–1350. doi: 10.1038/nm.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nano. 2015;10:370–379. doi: 10.1038/nnano.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouyang Z, Liu B, Yasmin-Karim S, Sajo E, Ngwa W. Nanoparticle-aided external beam radiotherapy leveraging the Čerenkov effect. Phys. Med. 2016;32:944–947. doi: 10.1016/j.ejmp.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Qu Y, Wang K, Zhang X, Zha J, Song T, Bao C, Liu H, Wang Z, Wang J, Liu Z, Liu H, Tian J. In vivo nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging. Nat. Commun. 2015;6:7560. doi: 10.1038/ncomms8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorek D, Robertson R, Bacchus WA, Hahn J, Rothberg J, Beattie BJ, Grimm J. Cerenkov imaging - a new modality for molecular imaging. Am J Nucl Med Mol Imaging. 2012;2:163–173. [PMC free article] [PubMed] [Google Scholar]
- 12.Allada K, Hurlbut C, Ou L, Schmookler B, Shahinyan A, Wojtsekhowski B. PMT signal increase using a wavelength shifting paint. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2015;782:87–91. [Google Scholar]
- 13.Zhao Y, van Rooy I, Hak S, Fay F, Tang J, Davies CdL, Skobe M, Fisher EA, Radu A, Fayad ZA, et al. Near-infrared fluorescence energy transfer imaging of nanoparticle accumulation and dissociation kinetics in tumor-bearing mice. ACS Nano. 2013;7:10362–10370. doi: 10.1021/nn404782p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coe-Sullivan S. Quantum dot developments. Nat. Photonics. 2009;3:315–316. [Google Scholar]
- 15.Jang E, Jun S, Jang H, Lim J, Kim B, Kim Y. White-light-emitting diodes with quantum dot color converters for display backlights. Adv. Mater. 2010;22:3076–3080. doi: 10.1002/adma.201000525. [DOI] [PubMed] [Google Scholar]
- 16.van Sark WGJHM, Meijerink A, Schropp REI, van Roosmalen JAM, Lysen EH. Enhancing solar cell efficiency by using spectral converters. Sol. Energy Mater. Sol. Cells. 2005;87(1–4):395–409. [Google Scholar]
- 17.Krumer Z, Pera SJ, van Dijk-Moes RJA, Zhao Y, de Brouwer AFP, Groeneveld E, van Sark WGJHM, Schropp REI, de Mello Donegá C. Tackling self-absorption in luminescent solar concentrators with type-II colloidal quantum dots. Sol. Energy Mater. Sol. Cells. 2013;111:57–65. [Google Scholar]
- 18.Dothager RS, Goiffon RJ, Jackson E, Harpstrite S, Piwnica-Worms D. Cerenkov radiation energy transfer (CRET) imaging: a novel method for optical imaging of PET isotopes in biological systems. PLoS ONE. 2010;5:e13300. doi: 10.1371/journal.pone.0013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Zhang X, Xing B, Han P, Gambhir SS, Cheng Z. Radiation-luminescence-excited quantum dots for in vivo multiplexed optical imaging. Small. 2010;6:1087–1091. doi: 10.1002/smll.200902408. [DOI] [PubMed] [Google Scholar]
- 20.Paik T, Chacko A-M, Mikitsh JL, Friedberg JS, Pryma DA, Murray CB. Shape-controlled synthesis of isotopic yttrium-90-labeled rare earth fluoride nanocrystals for multimodal imaging. ACS Nano. 2015;9:8718–8728. doi: 10.1021/acsnano.5b03355. [DOI] [PubMed] [Google Scholar]
- 21.Guo W, Sun X, Jacobson O, Yan X, Min K, Srivatsan A, Niu G, Kiesewetter DO, Chang J, Chen X. Intrinsically radioactive [64cu]cuins/zns quantum dots for pet and optical imaging: improved radiochemical stability and controllable cerenkov luminescence. ACS Nano. 2015;9:488–495. doi: 10.1021/nn505660r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Liu Y, Luehmann H, Xia X, Wan D, Cutler C, Xia Y. radioluminescent gold nanocages with controlled radioactivity for real-time in vivo imaging. Nano Lett. 2013;13:581–585. doi: 10.1021/nl304111v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X, Huang X, Guo J, Zhu W, Ding Y, Niu G, Wang A, Kiesewetter DO, Wang ZL, Sun S, Chen X. Self-illuminating 64Cu-doped CdSe/ZnS nanocrystals for in vivo tumor imaging. J. Am. Chem. Soc. 2014;136:1706–1709. doi: 10.1021/ja410438n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pons T, Lequeux N, Mahler B, Sasnouski S, Fragola A, Dubertret B. Synthesis of near-infrared-emitting, water-soluble cdtese/cdzns core/shell quantum dots. Chem. Mater. 2009;21(8):1418–1424. [Google Scholar]
- 25.Beattie BJ, Thorek DL, Schmidtlein CR, Pentlow KS, Humm JL, Hielscher AH. Quantitative modeling of cerenkov light production efficiency from medical radionuclides. PLoS ONE. 2012;7(2):e31402. doi: 10.1371/journal.pone.0031402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakowicz JR. Principles of Fluorescence Spectroscopy. Springer London, Limited; 2009. [Google Scholar]
- 27.Perez-Medina C, Abdel-Atti D, Zhang Y, Longo VA, Irwin CP, Binderup T, Ruiz-Cabello J, Fayad ZA, Lewis JS, Mulder WJ, Reiner T. A modular labeling strategy for in vivo PET and near-infrared fluorescence imaging of nanoparticle tumor targeting. J. Nucl. Med. 2014;55:1706–1711. doi: 10.2967/jnumed.114.141861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hak S, Helgesen E, Hektoen HH, Huuse EM, Jarzyna PA, Mulder WJM, Haraldseth O, Davies CdL. The effect of nanoparticle polyethylene glycol surface density on ligand-directed tumor targeting studied in vivo by dual modality imaging. ACS Nano. 2012;6:5648–5658. doi: 10.1021/nn301630n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valdes-Olmos RA, Jansen L, Hoefnagel CA, Nieweg OE, Muller SH, Rutgers EJ, Kroon BB. Evaluation of mammary lymphoscintigraphy by a single intratumoral injection for sentinel node identification. J. Nucl. Med. 2000;41:1500–1506. [PubMed] [Google Scholar]
- 30.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, Cohn LH, Bawendi MG, Frangioni JV. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song KH, Kim C, Cobley CM, Xia Y, Wang LV. Near-infrared gold nanocages as a new class of tracers for photoacoustic sentinel lymph node mapping on a rat model. Nano Lett. 2009;9:183–188. doi: 10.1021/nl802746w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torabi M, Aquino SL, Harisinghani MG. Current concepts in lymph node imaging. J. Nucl. Med. 2004;45:1509–1518. [PubMed] [Google Scholar]
- 33.Thorek DL, Riedl CC, Grimm J. Clinical Cerenkov luminescence imaging of (18)F-FDG. J. Nucl. Med. 2014;55(1):95–98. doi: 10.2967/jnumed.113.127266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J. Immunol. Methods. 2006;312(1–2):12–19. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Fay F, Hak S, Manuel Perez-Aguilar J, Sanchez-Gaytan BL, Goode B, Duivenvoorden R, de Lange Davies C, Bjorkoy A, Weinstein H, et al. Augmenting drug-carrier compatibility improves tumour nanotherapy efficacy. Nat. Commun. 2016;7 doi: 10.1038/ncomms11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009;36(7):729–739. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong L, Shuhendler AJ, Rao J. Self-luminescing BRET-FRET near-infrared dots for in vivo lymph-node mapping and tumour imaging. Nat. Commun. 2012;3:1193. doi: 10.1038/ncomms2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Grand AM, Lomnes SJ, Lee DS, Pietrzykowski M, Ohnishi S, Morgan TG, Gogbashian A, Laurence RG, Frangioni JV. Tissue-like phantoms for near-infrared fluorescence imaging system assessment and the training of surgeons. J. Biomed. Opt. 2006;11:014007. doi: 10.1117/1.2170579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.