Abstract
Objectives
The relative contribution of depression and anxiety to the risk of developing diabetes is unknown, particularly in samples with greater racial/ethnic and socioeconomic diversity. Therefore, we examined depression and anxiety screens, and their individual items, as simultaneous predictors of incident diabetes.
Design
10-year follow-up study of patients screened for the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) trial.
Setting
Two large urban primary care clinics in Indianapolis, IN
Participants
Diverse sample (53% African American, 80% of lower socioeconomic status) consisted of 2,156 older patients initially free of diabetes
Measurements
Depression and anxiety screens completed during routine primary care visits between 1999-2001. Incident diabetes data were obtained from an electronic medical record system and the Centers for Medicare and Medicaid Services analytic files though 2009.
Results
Over the 10-year period, 558 (25.9%) patients had diabetes onset. Cox proportional hazards models adjusted for demographic and diabetes risk factors revealed that a positive screen for anxiety, but not for depression, predicted incident diabetes when entered into separate models (Anxiety HR = 1.36, 95% CI: 1.15-1.61, p < .001; Depression HR = 1.18, 95% CI: 0.95-1.46, p = .129) and when entered simultaneously into one model (Anxiety HR = 1.35, 95% CI: 1.12-1.61, p < .001; Depression HR = 1.04, 95% CI: 0.83-1.31, p = .726). Both the feeling anxious item (p = 0.031) and the worry item (p = 0.016) predicted incident diabetes independent of the depression screen.
Conclusion
Our findings suggest that screening positive for anxiety is a risk factor for diabetes among older adults, independent of depression and traditional diabetes risk factors. Anxiety requires increased consideration and awareness in the context of diabetes risk assessment and primary prevention.
Keywords: Anxiety, depression, comorbidity, diabetes, primary care, prospective
INTRODUCTION
Emotional factors, namely depression and anxiety, have been increasingly linked to diabetes incidence. Depression has received the most attention, with meta-analyses indicating that depressed adults have a 37-60% greater risk of developing diabetes than nondepressed adults.1,2 Anxiety has received much less attention, and the bulk of studies assessing anxiety are cross-sectional.3 As a consequence, the directionality of the anxiety-diabetes association remains undetermined.
Another critical limitation of these literatures is that nearly all existing studies have examined these emotional factors in isolation, which is problematic given their considerable overlap. To illustrate, depressive and anxiety disorders are highly comorbid (55-60%),4 and self-report measures of depressive and anxiety symptoms are strongly correlated (r = 0.45-0.75).5 When only one emotional factor is examined at a time, it remains unclear whether an observed association reflects the measured factor, a closely related but unmeasured factor, or a broader construct encompassing both factors.6 Unfortunately, the few prospective studies that have examined more than one emotional factor predicting diabetes outcomes cannot distinguish between depression and anxiety due to their heterogeneous methodology and operationalization of variables.7-10 To illustrate, two recent Swedish studies used a composite index of psychological distress.8,9 A third study combined participants with depression and/or anxiety disorders,7 and a fourth study examined depression and anxiety separately.10 The lack of diversity of the study samples is also noteworthy, given that all but one of these studies were conducted with European samples. To date, depression and anxiety have not been simultaneously examined as predictors of incident diabetes in samples with greater racial/ethnic and socioeconomic diversity.
Determining whether depression, anxiety, or both are independent predictors of future diabetes could have important implications for the design of diabetes prevention programs. Yet, the relative contribution of these emotional factors to the risk of developing diabetes is currently unknown. Therefore, the primary objective of this study was to simultaneously examine depression and anxiety screens as predictors of 10-year incidence of diabetes. A secondary objective was to examine individual depression and anxiety screen items to explore whether specific symptoms are stronger predictors of incident diabetes than are others. Characteristics of the study, which increase the clinical relevance and ecological validity of the findings, include sample diversity (high percentage of African Americans and the medically uninsured/underinsured), patient risk factor profile (older adults with a higher diabetes risk factor burden), and the clinical setting (primary care).
METHODS
Participants
The Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) trial was a multisite randomized controlled trial examining the effectiveness of collaborative care for late-life depression [See Unutzer et al.11 for detailed methods; ClinicalTrials.gov Identifier: NCT01561105]. From July 1999 to August 2001, all 3,675 patients aged ≥ 60 years attending two large urban primary care clinics in a safety net healthcare system in Indianapolis, IN, were approached for screening for the IMPACT trial during routine visits. Roughly 80% of these patients were economically disadvantaged as indicated by receiving federal or county health insurance assistance.12
Of these 3,675 patients, 172 patients refused screening, and five patients had incomplete screens. For an additional 25 patients, we could not match their IMPACT trial data with data from other sources. Of the remaining 3,473 patients, we excluded 1,317 (37.9%) patients who had diabetes (type 1 or type 2) at baseline, given that our focus is predicting new-onset diabetes. To determine baseline diabetes status, we used data from the Regenstrief Medical Record System (RMRS),13 one of largest and longest operating electronic medical records (earliest data from 1978), merged with Medicare/Medicaid claims data from the Centers for Medicare and Medicaid Services (CMS) analytic files (earliest data from 1999). Baseline diabetes was defined as the presence of any of the following in the RMRS or CMS data before the participant's IMPACT screening date: (a) a diabetes diagnosis (ICD-9 code of 250); (b) a diabetes diagnosis or complication in an RMRS text field; (c) a fasting glucose value ≥ 126 mg/dL; (d) an HbA1c value ≥ 8.0%; or (e) a prescription for insulin or oral hypoglycemic medication. A cut point of ≥ 8.0% was chosen for HbA1c rather than the American Diabetes Association's cut point of ≥ 6.5%14 because more recently published guidelines15 recommend the use of a higher cut point (between 8.0-9.0%) for diabetes diagnosis among older adults with comorbid conditions, such as those who underwent screening for IMPACT trial. We chose the more conservative cut point in this range. We decided to include 123 individuals who were enrolled in the IMPACT trial in the current analyses because (a) a separate analysis including the 123 individuals revealed that the depression intervention had no effect on incident diabetes, and (b) sensitivity analyses excluding the 123 individuals revealed that the pattern of results and magnitude of effects did not change (data not shown). Table 1 presents the baseline characteristics of the 2,156 patients in our final sample.
Table 1.
Baseline Characteristics of Participants
| Demographic Factors | |
| Age (years), mean (SD) | 69.0 (7.3) |
| Female, n (%) | 1,486 (68.9) |
| African American, n (%) | 1,150 (53.3) |
| Diabetes Risk Factors | |
| Hypertension, n (%) | 1,496 (69.4) |
| Hypercholesterolemia (total cholesterol ≥240 mg/dL), n (%) | 346 (16.1) |
| Smoker, n (%) | 872 (40.5) |
| Body-Mass Index (kg/m2), mean (SD) | 29.0 (7.79) |
| Depression and Anxiety Screens | |
| Positive PRIME-MD Depression Screen, n (%) | 365 (16.9) |
| Positive PRIME-MD Anxiety Screen, n (%) | 958 (44.4) |
Note. N = 2,156. PRIME-MD = Primary Care Evaluation of Mental Disorders.
The IUPUI Institutional Review Board and the CMS Privacy Board approved the use of RMRS and CMS follow-up data for the Indiana participants of the IMPACT trial. The depression and anxiety screens were administered as part of routine care. A waiver of consent was obtained to link RMRS and CMS data.
Measures and Procedures
Baseline Depression and Anxiety Screens
During routine primary care visits occurring between July 1999 and August 2001, patients underwent screening for the IMPACT trial by completing a modified version of the Primary Care Evaluation of Mental Disorders (PRIME-MD) Patient Questionnaire.16 In the modified version, some items were omitted due to screening time constraints. The Patient Questionnaire is a self-report symptom measure that is used to screen medical patients for common psychiatric disorders. Patients responded yes or no to the question: “During the past month, have you been bothered a lot by [symptom]?” Our modified version included both depression items (“little interest or pleasure in doing things” and “feeling down, depressed, or hopeless”) and two of the three anxiety items (“nerves or feeling anxious or on edge” and “worrying about a lot of different things”) of the original questionnaire. Consistent with the PRIME-MD instructions,16 we coded patients who endorsed either of the two depression or anxiety items as screening positive for depression or anxiety, respectively. The complete PRIME-MD procedure includes a follow-up interview to confirm diagnoses of patients who screen positive for a disorder. This structured interview was not completed in our study. Nonetheless, the Patient Questionnaire depression screen has moderate sensitivity (69%) and high specificity (82%) for depressive disorder diagnoses made by mental health professionals, and the anxiety screen has higher sensitivity (94%) and lower specificity (53%) for anxiety disorder diagnoses.16
Incident Diabetes
Cases of incident diabetes were identified using RMRS data merged with CMS data (i.e., Medicare and Medicaid claims). Incident diabetes was defined as the first occurrence of any of the following between the participant's IMPACT screening date (1999-2001) and December 31, 2009: (a) diabetes diagnosis (ICD-9 code of 250); (b) a fasting glucose value ≥ 126 mg/dL; (c) an HbA1c value ≥ 8.0%; (d) a prescription for insulin or oral hypoglycemic medication; or (e) a death due to diabetes (ICD-10 codes E10-E14 the first-listed cause of death). Death dates were extracted from the Medicare data, and causes of death were obtained from death certificates provided by the Indiana State Department of Health included in the RMRS. Patients were followed for a maximum of 10.5 years (median = 8.5 years).
Other Baseline Factors
Baseline factors, assessed at or before each participant's IMPACT screening date, included demographic factors (age, sex, and race) and diabetes risk factors (hypertension, hypercholesterolemia, smoking, and body mass index). Demographic characteristics and the presence of physician-diagnosed hypertension were extracted from the RMRS, as has been described previously.12 For each patient, the most recent total cholesterol value during the 5-year window preceding each participant's IMPACT screening date was extracted from the RMRS, and patients with a value ≥ 240 mg/dL were coded as having hypercholesterolemia. We did not extract data regarding lipid lowering medication use and thus did not use this information in determining baseline hypercholesterolemia status. Smoking data was extracted from the RMRS during the same 5-year period preceding each participant's IMPACT screening date, and patients with a positive marker were coded as smokers. Height and weight data recorded in the RMRS before each participant's IMPACT screening date were used to compute body mass index (BMI; kg/m2). For 6 (0.3%) patients, missing values for height and weight were imputed with sex-specific median values. In addition to these factors, supplemental analyses utilized items from PRIME-MD Patient Questionnaire administered as part of the screening for the IMPACT trial assessing general perceived health (“Overall, would you say your health is: excellent, very good, good, fair, or poor”) and sleep disturbance (“feeling tired or having low energy” and “trouble sleeping”).
Data Analysis
To examine the relative contribution of a positive screen for depression and anxiety to the risk of developing diabetes, we performed a series of Cox proportional hazards models that included demographic factors and diabetes risk factors as covariates. Patients were censored at their date of death or December 31, 2009. In the individual models, the depression and anxiety screens were entered into separate models as predictor variables. In the simultaneous-entry model, both screens were entered into the same model. For screens that predicted incident diabetes, Kaplan-Meier survival curves were constructed to illustrate the time to diabetes onset for patients with positive versus negative screens. We also examined the individual items for screens that predicted incident diabetes. Specifically, the two items were entered as predictor variables in separate models, with and without inclusion of the other screen as a covariate. The proportional hazards assumption was not rejected for models examining either depression or anxiety screens or their constituent items (all p-values > .096).
In supplemental analyses, we first sought to reduce the possibility that impending diabetes onset results in a patient screening positive for depression and/or anxiety (i.e., reverse causality). To do so, we reran Cox models after excluding patients who had diabetes onset in the first year of follow-up. Second, analyses were performed to explore the effect excluding (a) all patients who died during the follow-up and (b) only patients who died without incident diabetes. Third, we sought to decrease the possibility that any observed relationships were due to confounding by poor overall health by further adjusting for the Patient Questionnaire general perceived health item. Finally, we examined the influence of sleep disturbance on any observed associations by further adjusting for the Patient Questionnaire feeling tired and trouble sleeping items. Analyses were conducted using SAS 9.3 statistical software.
RESULTS
Depression and Anxiety Screens
A total of 365 (16.9%) patients screened positive for depression (2.8% little interest item only, 6.3% feeling depressed item only, 7.8% both items), and 958 (44.4%) screened positive for anxiety (10.3% feeling anxious item only, 14.1 % worry item only, 20.0% both items). Our positive anxiety screen rate is similar to, but our depression screen rate is lower than, the rates reported for the Patient Questionnaire in the PRIME-MD validation study (anxiety: 48.6%; depression: 32.5%).16 We may have observed a lower rate of positive depression screens due to our older sample,17 and our exclusion of patients with baseline diabetes.18 In our sample, 302 (14,0%) patients screened positive for both depression and anxiety, a comorbidity rate similar to that reported in prior studies of primary care patients using brief screeners.19 Correlations between the depression and anxiety screen variables were in the moderate range (phi coefficients: 0.35-0.44).
Incident Diabetes
Over the 10 years of follow-up, 558 (25.9%) patients had new-onset diabetes. For these patients, the first diabetes event was an ICD-9 diagnosis for 228, a fasting glucose value ≥ 126 mg/dL for 226, an HbA1c value ≥ 8.0% for 45, a prescriptions for insulin or oral hypoglycemic medication for 58, and a diabetes death for one. The higher rate of incident diabetes in our sample than in the general population is likely accounted for by demographic factors (higher percentages of older adults, African Americans, and those of lower socioeconomic status) and diabetes risk factors (higher rates of hypertension, hypercholesterolemia, and smoking).20
Depression and Anxiety Screens as Predictors of Incident Diabetes
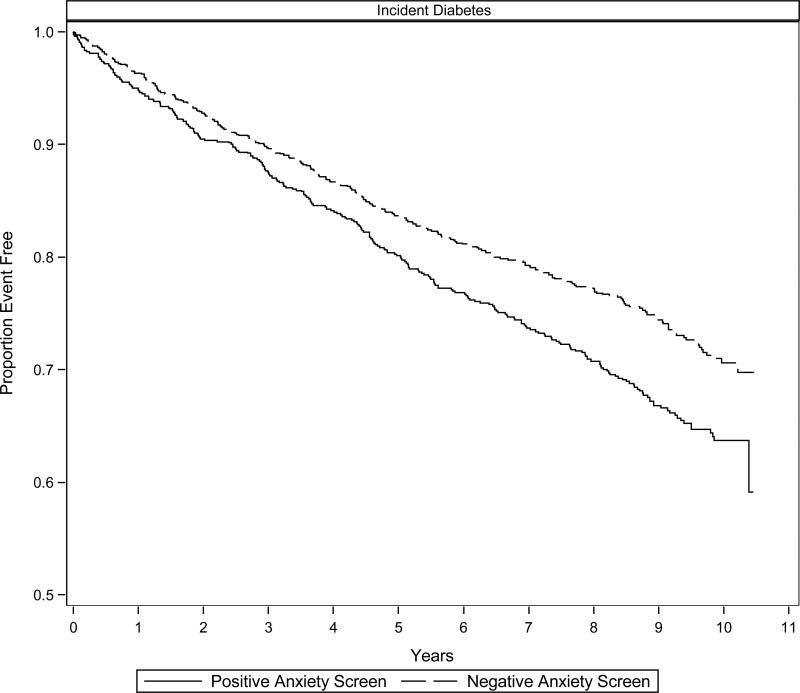
Individual Cox proportional hazards models adjusted for demographic and diabetes risk factors revealed that a positive anxiety screen (p < .001), but not a positive depression screen (p = .129), predicted incident diabetes (Table 2). A positive anxiety screen was associated with a 36% increased risk of incident diabetes over the 10-year follow-up period. The simultaneous-entry Cox model similarly revealed that a positive anxiety screen (p < .001), but not a positive depression screen (p = .726), predicted incident diabetes. In this model, a positive anxiety screen was associated with a 35% increased diabetes risk. In addition to the anxiety screen, BMI (HR = 1.04, 95% CI: 1.03-1.05, p < .001), male sex (HR = 1.24, 95% CI: 1.03-1.50, p = .021), smoking status (HR = 1.21, 95% CI: 1.01-1.45, p = .034) also predicted incident diabetes. The depression screen x anxiety screen interaction was not significant (p = .630). Figure 1 displays the Kaplan-Meier survival curves illustrating the time to diabetes onset for patients with a positive versus negative anxiety screen.
Table 2.
Cox Proportional Hazards Models Examining Depression and Anxiety Screens as Predictors of Incident Diabetes
| Individual Models a HR (95% CI) | Simultaneous-Entry Model b HR (95% CI) | |
|---|---|---|
| Incident Diabetes (n = 558, 25.9%) | ||
| Depression Screen | 1.18 (0.95-1.46) | 1.04 (0.83-1.31) |
| Little Interest Item | --- | --- |
| Depressed Mood Item | --- | --- |
| Anxiety Screen | 1.36* (1.15-1.61) | 1.35* (1.12-1.61) |
| Feeling Anxious Item | 1.26* (1.06-1.51) | 1.23* (1.02-1.49) |
| Worry Item | 1.28* (1.07-1.52) | 1.25* (1.04-1.51) |
Note. N = 2,156. HR = hazard ratio. CI = confidence interval.
Adjusted for baseline age, sex, race, hypertension, hypercholesterolemia, smoking, and body-mass index.
Adjusted for baseline age (HR = 1.00, 95% CI: 0.99-1.02, p = .685), sex (HR = 1.29, 95% CI: 1.08-1.58, p = .007), race (HR = 1.09, 95% CI: 0.91-1.29, p = .354), hypertension (HR = 1.11, 95% CI: 0.91-1.35, p = .298), hypercholesterolemia (HR = 0.98, 95% CI: 0.78-1.23, p = .856), smoking (HR = 1.20, 95% CI: 1.01-1.44, p = .044), body-mass index (HR = 1.04, 95% CI: 1.03-1.05, p < .001), and depression or anxiety screen (HRs reported here are from the simultaneous-entry model including both screens).
p < .05
Figure 1. Depression-incident Diabetes Survival Curves.
Kaplan-Meier survival curves illustrating the time to diabetes onset over 10-years of follow-up for older, primary care patients initially fee of diabetes with a positive versus negative anxiety screen.
Given that the anxiety screen independently predicted incident diabetes, the items of this measure were examined (Table 2). In individual Cox models, both the feeling anxious item (p = .011, 26% increased risk) and the worry item (p = .006, 28% increased risk) predicted incident diabetes. In models further adjusting for depression screen, a similar pattern of results was observed, as both the feeling anxious item (p = .031; 23% increased risk) and the worry item (p = .016, 25% increased risk) remained predictive of incident diabetes.
Supplemental Analyses
Examining the positive anxiety screen-incident diabetes association only, Cox models were repeated after excluding the 91 patients who had diabetes onset in the first year of follow-up. A positive anxiety screen remained predictive of incident diabetes (HR = 1.36, 95% CI: 1.13-1.64, p = .001), as did the feeling anxious item (HR = 1.28, 95% CI: 1.06-1.56, p = .012) and the worry item (HR = 1.24, 95% CI: 1.02-1.50, p = .029) in their individual models. A positive anxiety screen also predicted incident diabetes when Cox models were rerun after excluding all 809 patients who died during the follow-up (HR = 1.33, 95% CI: 1.08-1.64, p = .007), and excluding 622 patients who died without incident diabetes (HR = 1.38, 95% CI: 1.17, 1.64, p = .001). Moreover, when further adjusted for general perceived health (PRIME-MD perceived health item) or sleep disturbance (PRIME-MD feeling tired and trouble sleeping items), a positive anxiety screen continued to predict incident diabetes in its individual model (perceived health adjusted model: HR = 1.30, 95% CI: 1.08-1.55, p = .005; sleep disturbance adjusted model: HR = 1.26, 95% CI: 1.05-1.52, p = .013). For the constituent items, both the feeling anxious item and the worry item continued to predict incident diabetes when adjusted for perceived health (feeling anxious item: HR = 1.21, 95% CI: 1.01-1.47, p = .038; worry item: HR = 1.21, 95% CI: 1.01-1.46, p = .039) but fell short of significance when adjusted for sleep disturbance (feeling anxious item: HR = 1.15, 95% CI: 0.95-1.39, p = .145; worry item: HR = 1.18, 95% CI: 0.99-1.42, p = .070).
DISCUSSION
In a large cohort of older primary care patients, we found that patients initially free of diabetes who screened positive for anxiety had a 35% increased risk of developing diabetes over the 10-year follow-up period, even after adjusting for demographic factors, diabetes risk factors, and depression screen status. Item-level analyses indicated that both the feeling anxious item and the worry item contributed to the overall predictive utility of the PRIME-MD anxiety screen. Results of supplemental analyses suggest that the observed anxiety-incident diabetes association was not due impending diabetes onset leading to anxiety or to poor perceived health or sleep disturbance. In contrast to anxiety, patients screening positive of depression were not at a significantly increased risk of diabetes. Our findings suggest that anxiety is risk factor for incident diabetes in older adults, independent of traditional risk factors and depression.
Few studies have evaluated the anxiety-diabetes association longitudinally, and even fewer have simultaneously examined more than one emotional factor. Prior studies that have prospectively examined depression and anxiety in the same study have either used composite measures reflecting the presence of depression and/or anxiety or examined depression and anxiety in separate models only.7-10 Consequently, none can speak to the relative importance of one emotional factor over the other. Another cross-sectional study21 examined the association of mood, anxiety, impulse control, and substance use disorders assessed via diagnostic interview with diabetes diagnosis. When examined simultaneously, major depressive disorder was independently associated with a small elevation in diabetes prevalence. While these findings are seemingly inconsistent with those of our study, they are also not directly comparable, given their cross-sectional nature, and the inclusion of impulse control and substance use disorders in the same model. As is evident by the sparsity of this literature, additional prospective studies that simultaneously examined depression and anxiety as predictors of diabetes-related outcomes are needed. Nonetheless, our findings are in line with studies reporting a positive anxiety-incident diabetes association,22,23 and the magnitude of the association we observed is consistent with results of a recent meta-analysis of cross-sectional studies.3
What might account for the excess risk of diabetes conferred by anxiety? Candidate biological mechanisms include sympathetic overactivity,24,25 hypothalamic-pituitary-adrenal reactivity and other neuroendocrine changes,26,27 and systemic inflammation.26,28 For example, sympathetic overactivity has been strongly associated with anxiety disorders.29 In turn, a marker of sympathetic overactivity – namely, elevated resting heart rate – has been found to predict to diabetes development.30 Another example is systemic inflammation, wherein the exaggerated neurobiological sensitivity to threat common in anxiety is hypothesized to increase synthesis of various inflammatory markers.28 In turn, these markers can inhibit insulin's intracellular signaling cascade, ultimately decreasing insulin sensitivity and accelerating diabetes development.31,32 Worthy of equal attention are the candidate behavioral mechanisms, including smoking,33,34 sleep disturbance,35,36 and non-adherence to diabetes prevention strategies.37,38 Of note, the anxiety-incident diabetes association we observed remained significant after adjustment for smoking status, suggesting that this association is independent of smoking. Similarly, the anxiety screen also continued to predict incident diabetes after adjustment for sleep disturbance. However, sleep disturbance remains a viable mechanism, as our measure of sleep disturbance included only two items, which may have failed to capture important aspects of this construct. Lastly, anxiety is associated with non-adherence to diabetes prevention strategies, including physical activity and dietary behaviors,37,38 which could lead to a sedentary lifestyle, high energy intake, and ultimately obesity and diabetes onset. Future studies formally testing for mediation are needed to elucidate the mechanisms underlying the anxiety-incident diabetes relationship.
It is surprising that we did not observe an association between a positive depression screen and incident diabetes in light of findings from prior meta-analyses to the contrary.1,2 Indeed, in our own past work,39 we have shown that depressive symptoms predict 6-year increases in insulin resistance, a key stage in the etiology of type 2 diabetes. One possible explanation for the present results is the older age of our sample. Older adults are more likely to endorse the somatic-vegetative symptoms (e.g., fatigue, sleep disturbance, and appetite changes) versus cognitive-affective symptoms (e.g., depressed mood, anhedonia, and concentration difficulties) of depression.40 Because the two items comprising the PRIME-MD depression screen were cognitive-affective in nature, depressed patients with a somatic presentation may have been misclassified as negative for depression. Such misclassification could decrease the predictive utility of the depression screen. A related potential explanation arises from some evidence suggesting that the somatic-vegetative symptoms of depression may be stronger predictors of diabetes-related outcomes than the cognitive-affective symptoms.39,41 The failure of the depression screen to assess the potentially more predictive somatic-vegetative symptoms could have also lowered its predictive utility. A final possible explanation may be the moderate sensitivity of our depression measure, due to which patients with subthreshold symptoms may not have been identified as having depression. This may have attenuated our positive depression screen-incident diabetes association, since both depressive disorders and subthreshold depressive symptoms predict incident diabetes.1 In contrast, the anxiety screen likely identified patients with subthreshold symptoms due to its higher sensitivity, resulting in this screen's greater predictive utility.
Limitations of the present study warrant discussion. First, our use of brief screeners to detect depression and anxiety cases, rather than a structured clinical interview, certainly led to some misclassification. Specifically, the anxiety screen had higher sensitivity and lower specificity, while the depression screen demonstrated lower sensitivity and higher specificity. For the anxiety screen particularly, it is likely that patients with subthreshold symptom elevations were classified as anxiety cases. Additional studies that use structured clinical interviews to assess depressive and anxiety disorders are needed to augment our findings. Second, because only two of the three PRIME-MD anxiety items were used to screen participants for enrollment in the IMPACT trial, we could not include the third item [“Have you had an anxiety attack (suddenly feeling fear or panic)”] in our computation of the anxiety screen variable. However, the impact of this omission may be limited because the third item would have further increased the sensitivity of what appears to be an already highly sensitive anxiety screen (44% screen positive rate; Table 1). Third, our diabetes indicators at baseline and follow-up do not distinguish between type 1 and type 2 diabetes. However, by using a broad definition of diabetes at baseline, we probably excluded any patients with either type 1 or type 2 diabetes. Moreover, we almost certainly identified patients with type 2 diabetes during the follow-up period for the following reasons: (a) type 2 diabetes is overwhelmingly the most common type in adults, accounting for 90-95% of all diagnosed cases,42 and (b) new-onset type 1 diabetes is particularly rare in adults aged ≥ 60 years.43 Finally, our findings may not generalize to younger adults or those of higher socioeconomic status, given that our sample was primarily older and economically disadvantaged.
In summary, despite anxiety symptoms and disorders being highly prevalent, the association between a positive anxiety screen and diabetes risk is understudied compared to that between depression and diabetes risk. We report unique evidence that anxiety may be a risk factor for diabetes among older adults, independent of depression screening status and traditional risk factors for the disease. Methodological strengths of the study include the large, diverse sample, a comprehensive analytic approach, and the high rate of incident diabetes identified via multiple data sources. Our findings indicate that a positive anxiety screen requires increased consideration and awareness in the context of diabetes risk assessment and primary prevention. For example, primary care providers may need to be cognizant of the potentially elevated diabetes risk of older patients who screen positive for anxiety, and may need to implement earlier and more aggressive therapies for managing both anxiety symptoms and traditional diabetes risk factors in this at-risk group. Furthermore, research is needed to evaluate whether incorporating anxiety treatments in existing diabetes prevention efforts can further prevent or delay diabetes.
ACKNOWLEDGMENTS
Some data management tasks were performed by Joseph G. Kesterson, MA, Regenstrief Institute, Inc., Indianapolis, IN.
Source of Funding: This research was supported by the National Institutes of Health through the National Institute on Aging (C.M.C., AG031222, AG024078).
Sponsor's Role: The funding organizations had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, nor the preparation, review, or approval of the manuscript
Conflict of Interest Checklist:
| Elements of Financial/Personal Conflicts | *Tasneem Khambaty | Anthony J. Perkins | Christopher M. Callahan | Jesse C. Stewart | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
Footnotes
Prior Presentation: 2015 annual meeting of the American Psychosomatic Society
Author Contributions:
Study concept and design: TK, JCS
Acquisition of subjects and/or data: AJP, CMC
Analysis and interpretation of data: AJP, TK, JCS
Preparation of manuscript: TK, JCS, CMH, AJP
Disclosure of Potential Conflicts of Interest: None of the authors has any relationships with industry, or any actual or potential conflicts of interest related to this project.
REFERENCES
- 1.Knol M, Twisk J, Beekman A, Heine R, Snoek F, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49(5):837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 2.Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. The Journal of clinical psychiatry. 2013;74(1):31–37. doi: 10.4088/JCP.12r07922. [DOI] [PubMed] [Google Scholar]
- 3.Smith KJ, Béland M, Clyde M, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. Journal of psychosomatic research. 2013;74(2):89–99. doi: 10.1016/j.jpsychores.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Clark LA. The anxiety and depressive disorders: Descriptive psychopathology and differential diagnosis. In: Kendall PC, Watson D, editors. Anxiey and Depression: Distinctive and Overlapping Features. Academic Press; San Diego: 1989. [Google Scholar]
- 5.Clarke LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100(3):316. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 6.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychological bulletin. 2005;131(2):260. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 7.Atlantis E, Vogelzangs N, Cashman K, Penninx BJ. Common mental disorders associated with 2-year diabetes incidence: the Netherlands Study of Depression and Anxiety (NESDA). Journal of affective disorders. 2012;142:S30–S35. doi: 10.1016/S0165-0327(12)70006-X. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson AK, Ekbom A, Granath F, Hilding A, Efendic S, Östenson CG. Psychological distress and risk of pre-diabetes and Type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabetic Medicine. 2008;25(7):834–842. doi: 10.1111/j.1464-5491.2008.02463.x. [DOI] [PubMed] [Google Scholar]
- 9.Engum A. The role of depression and anxiety in onset of diabetes in a large population-based study. J Psychosom Res. 2007 Jan;62(1):31–38. doi: 10.1016/j.jpsychores.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Demmer RT, Gelb S, Suglia SF, et al. Sex Differences in the Association Between Depression, Anxiety, and Type 2 Diabetes Mellitus. Psychosomatic medicine. 2015;77(4):467–477. doi: 10.1097/PSY.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting. JAMA: the journal of the American Medical Association. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 12.Sha MC, Callahan CM, Counsell SR, Westmoreland GR, Stump TE, Kroenke K. Physical symptoms as a predictor of health care use and mortality among older adults. Am. J. Med. 2005 Mar;118(3):301–306. doi: 10.1016/j.amjmed.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 13.McDonald CJ, Tierney WM, Overhage JM, Martin DK, Wilson GA. The Regenstrief Medical Record System: 20 years of experience in hospitals, clinics, and neighborhood health centers. MD Computing. 1992 Jul-Aug;9(4):206–217. [PubMed] [Google Scholar]
- 14.American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2012 Jan 1;33(Supplement 1):S62–S69. doi: 10.2337/dc11-S062. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Geriatrics Society Expert Panel on the Care of Older Adults with Diabetes Mellitus Guidelines Abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 Update. Journal of the American Geriatrics Society. 2013;61(11):2020–2026. doi: 10.1111/jgs.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994 Dec 14;272(22):1749–1756. [PubMed] [Google Scholar]
- 17.Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5:363–389. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamura F, Tashiro A, Utumi A, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism. 2000;49(10):1255–1260. doi: 10.1053/meta.2000.9515. [DOI] [PubMed] [Google Scholar]
- 19.Holt R, Phillips D, Jameson K, Cooper C, Dennison E, Peveler R. The relationship between depression and diabetes mellitus: findings from the Hertfordshire Cohort Study. Diabetic medicine. 2009;26(6):641–648. doi: 10.1111/j.1464-5491.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 20.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes care. 2001;24(1):89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 21.de Jonge P, Alonso J, Stein DJ, et al. Associations between DSM-IV mental disorders and diabetes mellitus: a role for impulse control disorders and depression. Diabetologia. 2014;57(4):699–709. doi: 10.1007/s00125-013-3157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyko EJ, Seelig AD, Jacobson IG, et al. Sleep Characteristics, Mental Health, and Diabetes Risk A prospective study of US military service members in the Millennium Cohort Study. Diabetes care. 2013;36(10):3154–3161. doi: 10.2337/DC13-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyko EJ, Jacobson IG, Smith B, et al. Risk of diabetes in US military service members in relation to combat deployment and mental health. Diabetes care. 2010 doi: 10.2337/dc10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott KM. Depression, anxiety and incident cardiometabolic diseases. Current opinion in psychiatry. 2014;27(4):289–293. doi: 10.1097/YCO.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 25.Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. Journal of the American College of Cardiology. 2013;62(11):970–978. doi: 10.1016/j.jacc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pego J, Sousa J, Almeida O, Sousa N. Behavioral Neurobiology of Anxiety and its Treatment. Springer; 2010. Stress and the neuroendocrinology of anxiety disorders. pp. 97–118. [DOI] [PubMed] [Google Scholar]
- 27.Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Hormones and behavior. 2006;50(4):550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Donovan A, Slavich GM, Epel ES, Neylan TC. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neuroscience & Biobehavioral Reviews. 2013;37(1):96–108. doi: 10.1016/j.neubiorev.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurston RC, Rewak M, Kubzansky LD. An anxious heart: anxiety and the onset of cardiovascular diseases. Progress in cardiovascular diseases. 2013;55(6):524–537. doi: 10.1016/j.pcad.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Grantham N, Magliano D, Tanamas S, Söderberg S, Schlaich M, Shaw J. Higher heart rate increases risk of diabetes among men: The Australian Diabetes Obesity and Lifestyle (AusDiab) Study. Diabetic Medicine. 2013;30(4):421–427. doi: 10.1111/dme.12045. [DOI] [PubMed] [Google Scholar]
- 31.Ismail K. Unraveling the Pathogenesis of the Depression–Diabetes Link. Depression and Diabetes. 2010:29–61. [Google Scholar]
- 32.Stuart MJ, Baune BT. Depression and type 2 diabetes: inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neuroscience & Biobehavioral Reviews. 2012;36(1):658–676. doi: 10.1016/j.neubiorev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: a critical review of interrelationships. Psychological bulletin. 2007;133(2):245. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- 34.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2007;298(22):2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 35.Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. Journal of applied physiology. 2005;99(5):2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 37.Strine TW, Mokdad AH, Dube SR, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. General hospital psychiatry. 2008;30(2):127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Bonnet F, Irving K, Terra J-L, Nony P, Berthezène F, Moulin P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178(2):339–344. doi: 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 39.Khambaty T, Stewart JC, Muldoon MF, Kamarck TW. Depressive symptom clusters as predictors of 6-year increases in insulin resistance: data from the Pittsburgh Healthy Heart Project. Psychosomatic medicine. 2014;76(5):363–369. doi: 10.1097/PSY.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y, Pilkonis PA, Frank E, Thase ME, Reynolds CF. Differential functioning of the Beck depression inventory in late-life patients: use of item response theory. Psychology and aging. 2002;17(3):379. doi: 10.1037//0882-7974.17.3.379. [DOI] [PubMed] [Google Scholar]
- 41.Austin A, Gordon J, Lavoie K, Arsenault A, Dasgupta K, Bacon S. Differential association of insulin resistance with cognitive and somatic symptoms of depression. Diabetic Medicine. 2014;31(8):994–1000. doi: 10.1111/dme.12465. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention . National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, Georgia: 2011. [Google Scholar]
- 43.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes care. 2012;35(12):2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]