Abstract
Background
Erlotinib is a standard first-line therapy for metastatic non-small-cell lung cancers (NSCLC) with epidermal growth factor receptor (EGFR) mutations. The recommended dose of 150mg daily is the maximum tolerated dose (MTD). Little clinical data is available regarding its efficacy at doses less than MTD.
Methods
An institutional database was queried for patients with advanced NSCLC positive for EGFR L858R mutations or exon 19 deletions treated with erlotinib. Treatment course, including erlotinib dose at initiation of treatment, at four months into therapy, and at disease progression, was studied retrospectively. Progression-free survival (PFS) was compared between patients taking MTD (150mg) and reduced-dose (≤100mg) erlotinib.
Results
198 eligible patients were identified. Thirty-one (16%) were initiated on reduced-dose erlotinib; they were older (p=0.001) with a lower performance status (p=0.01) compared to those initiated at MTD. Response rate to reduced-dose erlotinib was 77%. Median PFS of patients initiated on reduced-dose erlotinib was 9.6 months versus 11.4 months for those initiated at MTD, a difference that was not statistically significant (HR 0.81, 95% CI: 0.54-1.21, p=0.30). There was a non-significant trend towards higher rates of progression within the CNS with reduced-dose erlotinib.
Conclusions
At doses below MTD, erlotinib treatment results in a high response rate and prolonged median PFS. Review of the literature indicates that 15 out of 30 small molecule inhibitors approved or in late-stage development for cancer therapy have recommended doses below MTD. When the toxicities of MTD dosing are a concern, investigation of small molecule inhibitors at doses below MTD is warranted.
Keywords: Non-small-cell lung carcinoma, EGFR gene, Erlotinib, Pharmacokinetics, Molecular Targeted Therapy
Introduction
The presence of an epidermal growth factor receptor (EGFR) mutation identifies a subset of non-small cell lung cancers (NSCLC) with exquisite sensitivity to EGFR tyrosine kinase inhibitors (TKIs).1, 2 The identification of this link between a specific genetic mutation and susceptibility to particular targeted small molecule inhibitors has been followed by a search for other driver mutations and corresponding inhibitors. These parallel discoveries now include lung adenocarcinomas with ALK or ROS1 rearrangements and corresponding sensitivity to crizotinib,3, 4 and melanomas with BRAF mutations and sensitivity to BRAF/MEK pathway inhibitors 5, 6.
Despite the success of using molecularly targeted agents to treat molecularly-selected cancers, there remains lack of clarity regarding the appropriate dose of these agents to use. New agents are conventionally developed at their maximum tolerated dose (MTD), an approach used with erlotinib (150mg daily) and vemurafenib (960mg bid).6, 7 This stems from the rationale that clinical benefit increases with an increase in dose, and then wanes with excessive toxicity, a historical approach derived from the development of cytotoxic chemotherapies. On the other hand, this rationale may not apply to molecularly targeted agents, which may achieve biological activity at a dose lower than the MTD, at which point clinical benefit may begin to plateau. Indeed, administering a targeted drug at its MTD has the potential to reduce one of the main perceived benefits of this type of therapy -- that by targeting tumor-specific aberrations, one can increase the therapeutic window and decrease toxicity.
Erlotinib might be efficacious at doses below MTD. The MTD of erlotinib (150mg daily) leads to plasma levels that are 10-100 fold higher than concentrations needed to inhibit proliferation of EGFR-mutant cell lines in vitro.7-9 Anecdotal responses to doses as low as 25mg/day have been reported.10, 11 However, concentrations of erlotinib achieved in the cerebrospinal fluid are 3-5% of plasma drug levels,12 suggesting that dose reduction might particularly impair drug delivery to the central nervous system (CNS).
We undertook this analysis of a large cohort of patients with EGFR-mutant NSCLC to determine if treatment with reduced-dose erlotinib (≤100mg daily) results in similar outcomes compared to treatment at MTD (150mg daily). Based on the in vitro data described above, we hypothesized that reduced-dose erlotinib has similar efficacy to the MTD of erlotinib and represents a viable treatment option for EGFR-mutant NSCLC.
Methods
An institutional database, which consisted of NSCLC patients seen at the Dana-Farber Cancer Institute from 2002 until 2014 who had provided written informed consent for the collection of clinical parameters and outcome, was queried. Subjects were eligible if they had (1) advanced NSCLC, (2) erlotinib exposure, with advanced disease (stage IV or recurrent) at time of initial exposure to erlotinib, (3) documentation of either EGFR exon 19 deletion or L858R mutation, (4) absence of EGFR T790M mutation at baseline, (5) known initial dose of erlotinib, and (6) adequate documentation present to determine date of clinical progression and date of death, if such events had occurred.
Date of erlotinib initiation and baseline demographic characteristics were determined from retrospective chart review. Performance status at erlotinib initiation was extracted from the treating oncologist's note if documented at the time (69%), was estimated from the treating oncologist's note if not documented (22%), or was deemed unknown if insufficient data were present (9%). Brain metastases were considered present at erlotinib initiation if the subject had any prior history of brain metastases or if they had positive brain imaging within one month of starting the medication.
Erlotinib dose at initiation, at four months, and at progression was determined by retrospective chart review. Erlotinib dose at initiation was defined as the dose the patient was taking at the end of the first week of therapy. Evaluation of erlotinib dose at four months was chosen to reflect an early timepoint when most patients would not have progressed but may have been dose reduced.
Date of disease progression was the date of the clinical encounter (or the date of the imaging study leading to this encounter, whichever occurred first) where a change in therapy due to disease progression was first discussed in the clinical record.13 Progression within the CNS was recorded if new brain or leptomeningeal metastases were noted on imaging or if the reading radiologist at the time noted that the size of known brain metastases had increased. Progression-free survival was defined as the time from start of erlotinib therapy to date of disease progression or death, whichever occurred first. Overall survival was calculated from the date of start of erlotinib therapy until death from any cause. Patients alive at the time of analysis were censored at the date of their last clinic visit. Time-to-event comparisons were made using log-rank tests. Multivariate analyses were performed using Cox's proportional hazards regression models with R version 2.10.0 (R Found Stat Comput, Vienna, Austria). All reported p values are two sided, and no adjustments have been made for multiple comparisons.
Post-progression therapy was defined as any systemic regimen that included chemotherapy, alternative TKIs besides erlotinib, a clinical trial, or any combination of the above (with or without erlotinib). Continuation of erlotinib monotherapy beyond the time of clinical progression was not considered an additional line of post-progression therapy.
For all patients initiated on reduced-dose erlotinib with serial CT imaging available during therapy, maximal tumor shrinkage was determined by a retrospective radiology review blinded to the doses of erlotinib, using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria, as described previously.14 Objective response rate (ORR) was calculated with a partial response defined as at least a 30% decrease in the sum of the diameters of the target lesions.
Results
A total of 345 patients with advanced EGFR-mutant NSCLC were identified. Of these, 147 patients were excluded for the following reasons: 82 had EGFR mutations other than exon 19 deletion or L858R, 32 had insufficient records for review, 22 received a TKI other than erlotinib, 7 were never exposed to a TKI, and 4 had the T790M co-mutation at baseline (Supplemental Figure 1). Excluding T790M, nine patients had second EGFR mutations in addition to either exon 19 deletion or L858R EGFR mutations; these patients were included in the analysis. The remaining group was composed of 198 patients who had been exposed to erlotinib to treat advanced NSCLC with an exon 19 deletion or an L858R mutation in EGFR.
Out of these 198 patients, 31 (16%) were initiated on reduced-dose erlotinib of 100mg/day or less, and 167 (84%) were initiated on the MTD of erlotinib at 150mg/day. Patients were balanced between dosing groups with the exceptions of age and performance status (Table 1). Patients in the reduced-dose group tended to be older (median age 71 years vs. 60 years, p = 0.001) and also tended to have a lower performance status, with 23% of patients having a PS of 2-3 in the reduced-dose group versus 7% of patients in the MTD group (p=0.01).
Table 1. Baseline Characteristics of Non-small-cell Lung Cancer Patients Initiated on Reduced-Dose Erlotinib as Compared to Patients Initiated on Standard-dose Erlotinib.
| Characteristics | Reduced Dose, n (%) | Full Dose, n (%) | Total, n (%) |
|---|---|---|---|
|
| |||
| N | 31 | 167 | 198 |
|
| |||
| Age at erlotinib start -- yr | |||
| Median | 71 | 60 | 62 |
| Range | 34-95 | 31-90 | 31-95 |
|
| |||
| Sex – no | |||
| Male | 10 (32) | 45 (27) | 55 (28) |
| Female | 21 (68) | 122 (73) | 143 (72) |
|
| |||
| Smoking Status | |||
| Never smoker | 13 (42) | 96 (57) | 109 (55) |
| Ever smoker | 18 (58) | 71 (43) | 89 (45) |
|
| |||
| PS at erlotinib start | |||
| PS 0-1 | 24 (77) | 141 (93) | 165 (91) |
| PS 2-3 | 7 (23) | 10 (7) | 17 (9) |
| Unknown | 0 | 16 | 16 |
|
| |||
| Erlotinib line of therapy | |||
| 1st line | 23 (74) | 119 (71) | 142 (72) |
| 2nd line | 6 (19) | 34 (20) | 40 (20) |
| 3rd line | 2 (6) | 10 (6) | 12 (6) |
| > 3rd line | 0 (0) | 4 (2) | 4 (2) |
|
| |||
| Chemo prior to erlotinib | |||
| No | 23 (74) | 119 (71) | 142 (72) |
| Yes | 8 (26) | 48 (29) | 56 (28) |
|
| |||
| Baseline Brain Metastases | |||
| No | 24 (77) | 114 (68) | 138 (70) |
| Yes | 7 (23) | 53 (32) | 60 (30) |
|
| |||
| EGFR Mutation | |||
| Exon 19 Deletion | 15 (48) | 101 (60) | 116 (59) |
| L858R | 16 (52) | 66 (40) | 82 (41) |
|
| |||
| Date of erlotinib initiation | |||
| Before 12/31/2009 | 10 (32) | 92 (55) | 102 (52) |
| After 12/31/2009 | 21 (68) | 75 (45) | 96 (48) |
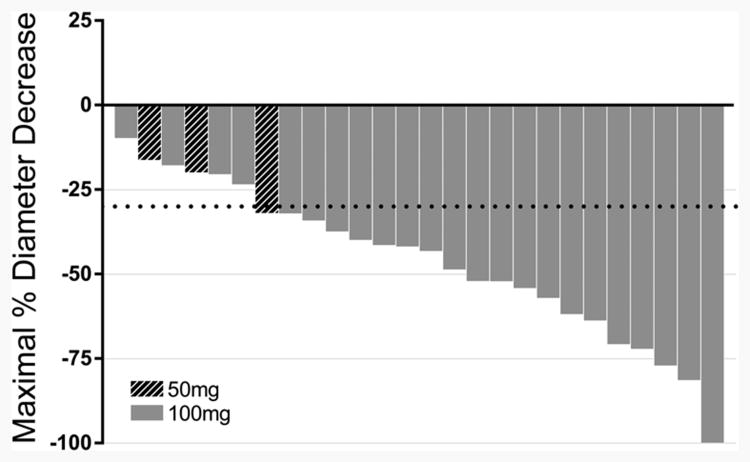
Of the 31 patients initiated on reduced-dose erlotinib, 26 had baseline and follow-up scans available for response assessment: 6 had stable disease and 20 had a partial response, corresponding to an ORR of 77% (95% exact binomial confidence interval [CI]: 56-91%; Figure 1). The median time from drug initiation to best overall response was 3.4 months (range 0.4-18.6 months). This ORR of 77% is similar to ORRs of 60-80% that have been reported with the use of erlotinib at 150mg daily in patients with TKI-sensitive EGFR mutations 1, 2.
Figure 1.
Waterfall plot depicting best percentage change from baseline in tumor size for individual patients in the reduced-dose erlotinib group. Twenty out of 26 patients had a maximum response of over 30%, leading to an ORR of 77% for reduced-dose erlotinib.
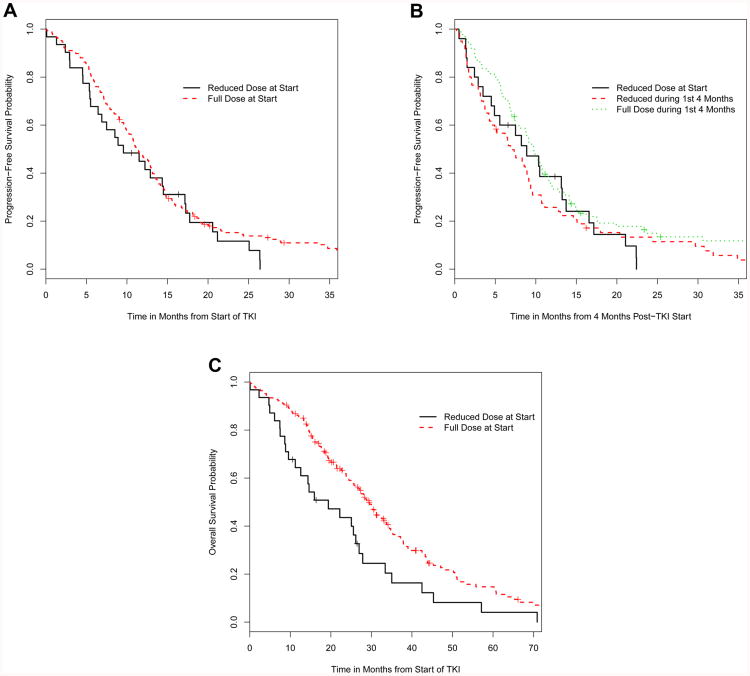
Progression-free survival (PFS) was compared between patients who initiated erlotinib at MTD and those who initiated reduced-dose erlotinib. Patients started at the MTD had a median PFS of 11.4 months (95% CI: 10.2-13.1 months), while those initiated on reduced-dose erlotinib had a median PFS of 9.6 months (95% CI: 6.4-17.1 months), a nonsignificant difference (HR 0.81, 95% CI: 0.54-1.21, p=0.30, Figure 2A).
Figure 2.
(A) Kaplan-Meier plot of progression-free survival in patients who were initiated on standard-dose erlotinib versus patients initiated on reduced-dose erlotinib. (B) PFS in patients who were initiated and maintained on standard-dose erlotinib, patients who were initiated and maintained on reduced-dose erlotinib, and patients who were initiated on standard-dose erlotinib but on a reduced dose by the four month landmark. (C) OS in patients who were initiated on the reduced dose versus the MTD of erlotinib.
Dose reductions were common. Out of the 167 patients initiated at the MTD, 81 (49%) were dose reduced by the time of progression, 7 (4%) stopped erlotinib prior to progression and 3 (2%) were taking an unknown dose at progression. Dose reductions occurred early. Of the population without progression and taking erlotinib at four months, 80% of all eventual dose reductions had already occurred by that time, justifying the selection of four months as the landmark timepoint. Of the 173 patients without progression and taking erlotinib at four months, 85 (49%) were taking the MTD of erlotinib, 85 (49%) were taking a reduced dose, and 3 (2%) were taking an unknown dose (Table 2).
Table 2.
Variation in erlotinib doses over time.
| Standard dose | Reduced dose | Unknown dose | ||||
|---|---|---|---|---|---|---|
| Erlotinib dose | 150mg/day | 100mg/day | 75mg/day | 50mg/day | 25mg/day | |
| Dose at Initiation | 167 (84%) | 28 (14%) | 0 (0%) | 3 (2%) | 0 (0%) | |
| Dose at 4 months1 | 85 (49%) | 622 (36%) | 6 (3%) | 15 (9%) | 2 (1%) | 3 (2%) |
| Dose at Progression3 | 76 (41%) | 694 (37%) | 12 (6%) | 24 (13%) | 3 (2%) | 3 (2%) |
Three patients who had stopped erlotinib prior to the four month time point, but had still not progressed, were excluded from this analysis
One patient received 112.5mg/day.
These numbers include patients who may have progressed prior to the four month time point. Eleven patients who stopped erlotinib prior to progression were excluded from this analysis. Patients who had not progressed were classified by their most recent dose.
One patient received 112.5mg/day and one patient received 125mg/day.
Outcomes were compared according to dose at the landmark time of four months on therapy. Patient who were initiated on the MTD of erlotinib but then dose-reduced during the first four months of therapy had a median PFS of 6.9 months (95% CI: 4.6-9.4 months). Those initiated at MTD and then remaining on this dose after four months had a median PFS of 9.8 months (95% CI: 7.9-11.6 months). Those initiated at a reduced dose and who continued on a reduced dose after four months of therapy had a median PFS of 8.9 months (95% CI: 4.9-16.6 months), with no significant difference in PFS between the three groups (log-rank p=0.10, Figure 2B). Out of the 108 patients on reduced-dose erlotinib at time of progression, 19 (17.5%) progressed in the CNS. Out of the 76 patients on the MTD at time of progression, 6 (7.8%) progressed in the CNS, a nonsignificant trend (p=0.08).
The use of post-progression therapy differed between dosing groups. Within the group initiated on reduced-dose erlotinib, 15 (48%) received additional therapy beyond the time of progression on erlotinib, consisting of an alternative TKI only (n=2, 13%), chemotherapy only (n=9, 60%), or chemotherapy and an alternative TKI (n=4, 27%). Within the group initiated at MTD, a significantly higher number received post-progression therapy (n=119, 72%, p=0.02). They also received more lines of therapy, with 12 patients (10%) receving solely an alternative TKI; 42 patients (35%) receiving chemotherapy only, and 65 patients (55%) receiving chemotherapy and an alternative TKI.
Studying overall survival (OS), 157 of 198 patients had died after a median follow-up time of 24.2 months (range: 0.03-102.9 months). Of the 31 subjects initiated on reduced-dose erlotinib, 28 (90%) had died; of the 167 subjects initiated at MTD, 129 (77%) had died. With univariate analysis, there was a significant difference in median OS between patients initiated at MTD (29.4 months; 95% CI: 25.7-33.7) and those initiated at reduced dose (19.4 months; 95% CI: 11.2-27.9; p=0.006; Figure 2C). However, dose of erlotinib initiation did not remain significant in a multivariable model (p=0.510) adjusting for age, gender, the presence of brain metastases, performance status, year of erlotinib initiation, and an interaction between dose and the year of erlotinib initiation Supplementary Table 1).
Discussion
In this, the largest study of patients with EGFR-mutant NSCLC on reduced-dose erlotinib, we report that use of a reduced dose of 100mg/day or less results in a high response rate, comparable to that previously reported for erlotinib at MTD. Treatment with reduced-dose erlotinib also results in a PFS that does not differ from the PFS seen in the population dosed at the MTD of 150mg/day, despite the fact that patients initiated on reduced-dose erlotinib were older and had a lower performance status. Dose reduction is a common event. At the time of progression only 41% of patients remain on the MTD of 150mg/day; however, a landmark analysis stratified by dose at 4 months found no decrement in outcomes in patients receiving a dose reduction. There was a difference in OS between the dosing levels, but this was not maintained in a multivariate analysis. While this study did not examine adverse effects at reduced doses, it has been shown that lower doses of erlotinib lead to a decreased incidence of side effects such as diarrhea.7, 10 Therefore, reduced-dose erlotinib is a reasonable alternative to the MTD that does not significantly compromise efficacy.
Our results have potential implications for the future development of targeted agents. We reviewed the reported MTD and recommended clinical dose for all non-hormonal small molecule inhibitors that have been approved by the Food and Drug Administration for the treatment of solid malignancies since the introduction of imatinib, as well as all new kinase inhibitors granted breakthrough status by the FDA for the treatment of lung cancer (Table 3). Of the 30 inhibitors examined, 15 have been developed at either the MTD or maximum dose tested, and 15 have been developed below the MTD. Newer drugs were more likely to have a recommended dose lower than the MTD. Ten out of the fifteen drugs with phase I studies reported in 2011 or later are now recommended to be used at doses less than the MTD, while only 5 out of 15 drugs with phase I doses reported prior to 2011 have a similar recommendation, perhaps reflecting a change in practice. This evolving approach to the dosing of small molecule inhibitors suggests that there is uncertainty regarding the efficacy of submaximal doses.
Table 3.
Comparison of MTD to package insert dose of small molecule inhibitors used to treat solid malignancies.
| Drug | MTD1 | Package Insert Dose / Dose under development | Dosed below MTD |
|---|---|---|---|
| afatinib | 50mg daily | 40mg daily | X |
| alectinib | 900mg bid | 600mg bid | X |
| axitinib | 5mg bid | 5mg bid | |
| brigatinib2 | 300mg daily | 180mg daily | X |
| cabozantinib | 175mg daily | 140mg daily | X |
| ceritinib | 750mg daily | 750mg daily | |
| cobimetinib | 60mg daily3 | 60mg daily3 | |
| crizotinib | 250mg bid | 250mg bid | |
| dabrafenib | 300mg bid | 150mg bid | X |
| erlotinib | 150mg daily | 150mg daily | |
| everolimus | 10mg daily4 | 10mg daily | |
| gefitinib | 600mg daily | 250mg daily | X |
| imatinib | 400mg bid | 400-600mg daily | X |
| lapatinib | 1500mg daily4,5 | 1500mg daily5 | |
| lenvatinib | 25mg daily | 24mg daily | X |
| olaparib | 400mg bid | 400mg bid | |
| olmutinib2 | 800mg daily | 800mg daily | |
| osimertinib | 240mg daily4 | 80mg daily | X |
| palbociclib | 125mg daily3 | 125mg daily3 | |
| pazopanib | 2000mg daily | 800mg daily | X |
| regorafenib | 160mg daily3 | 160mg daily3 | |
| rociletinib2 | 1000mg bid4 | 500mg bid | X |
| sonidegib | 800mg daily | 200mg daily | X |
| sorafenib | 400mg bid | 400mg bid | |
| sunitinib | 50mg daily6 | 50mg daily6 | |
| temsirolimus | 250mg weekly4 | 25mg weekly | X |
| trametinib | 3mg daily | 2mg daily | X |
| vandetanib | 300mg daily | 300mg daily | |
| vemurafenib | 960mg bid | 960mg bid | |
| vismodegib | 540mg daily4 | 150mg daily | X |
References for determination of MTD provided in Supplementary Table 2
Given breakthrough status by FDA for the treatment of lung cancer
Administered in a 3 weeks on, 1 week off cycle
Maximum tested dose rather than maximum tolerated dose
MTD tested and FDA-approved in combination with letrozole for HER2 positive metastatic breast cancer
Admininsterd in a 4 weeks on, 2 weeks off cycle
In this regard, the need for prospective studies of various dosing regimens is clear. For example, this approach demonstrated that imatinib dosed at 400mg twice daily had no significant OS advantage compared to imatinib at 400mg once daily;15 the recommended dose of imatinib for the treatment of most genetic subtypes of metastatic gastrointestinal stromal tumor is now less than its MTD.16 In a counterexample, a randomized trial in patients with renal cell carcinoma compared standard intermittent dosing of sunitinib at the MTD of 50mg (4 weeks on, 2 weeks off) with a more convenient continuous dosing of 37.5mg daily. The higher 50mg dose was superior in an analysis of a composite endpoint of death, disease progression, and disease related symptoms.17 In early phase clinical trials, identification of reliable biomarkers that reflect intratumoral target inhibition may allow for dose escalation strategies that determine the optimum biological dose rather than the MTD. For example, effective hedgehog pathway inhibition was demonstrated in skin biopsies taken from subjects exposed to vismodegib at doses less than its MTD of 540mg,18 and the drug entered phase 2 studies at a dose of 150mg daily. An alternative approach was taken with development of osimertinib. In its phase I trial, dose expansion cohorts were opened at any dose level where a response was seen, thus collecting early efficacy data at a wide range of doses 19.
In the absence of prospective trials, retrospective studies can provide guidance regarding acceptable dosing. Prior to this study there was limited retrospective data to support the use of reduced-dose erlotinib. A study of 7 patients with EGFR-mutant NSCLC treated with 25mg/day reported a 71% response rate.10 An additional study found no difference in time to progression between 18 patients receiving reduced doses of erlotinib (only 4 of whom had EGFR-mutant NSCLC) to 31 patients receiving the MTD.20 As our data demonstrate, retrospective studies examining submaximal dosing of targeted agents must take into account selection bias, as lower doses are often prescribed to patients with higher age and worse performance status.
The development of drugs at less than the MTD may facilitate combination therapy. For example, the combination of erlotinib and crizotinib was beneficial when a patient with EGFR-mutant cancer developed MET amplification, but resulted in significant toxicity requiring reduction of erlotinib to 75mg/day.21 A phase I study identified that the MTD of erlotinib when given with crizotinib is 100mg daily.22 Our study suggests that the use of erlotinib at lower doses in combination therapy might not impact its efficacy.
One argument against the use of a reduced dose could be an increased tendency to progress in the CNS. Previous studies demonstrated CNS concentrations of 14nM in a patient on 75mg/day of erlotinib and 27-201nM in patients on 150mg/day erlotinib,12, 23-25 which fall within the range of the in vitro IC50 for erlotinib. CNS progression can be due to pharmacokinetic failure, as many patients who progress in the CNS while on erlotinib do not display the T790M resistance mutation and respond to high-dose, pulsatile dosing of erlotinib, which achieves higher CNS concentrations.26, 27 In our study, the rate of CNS progression with reduced-dose erlotinib was slightly but not significantly higher than with erlotinib dosed at MTD. When molecularly targeted agents are developed at less than MTD, rates of CNS progression should be outcomes of interest.
Limitations of the study include its retrospective design. Subjects were not randomly assigned to dose levels, introducing the possibility of selection bias. These patients were all treated at a single academic medical center, although patterns of dose reductions in the community are similar to those in trials at academic centers.28 Our sample size of 198 patients may be too small to detect subtle effects of dose on PFS or on infrequent events such as brain metastases.
In conclusion, we have found that reduced-dose erlotinib for patients with advanced EGFR-mutant NSCLC results in a response rate of 77% and median PFS of 9.6 months, similar to the results seem with erlotinib administered at the MTD. In circumstances where toxicity is a concern, erlotinib can be used effectively below the MTD. Broadly, small molecule inhibitors represent a class of therapeutics where additional studies on the efficacy of lower doses are needed.
Supplementary Material
Acknowledgments
Funding Source: This work is supported by NIH 5R01CA114465-09.
P.A.J. has received consulting fees from AstraZeneca, Boehringer-Ingelheim, Clovis, Chugai, Merrimack Pharmaceuticals, Ariad Pharmaceuticals, and Genentech; sponsored research from AstraZeneca and Astellas Pharmaceuticals; is co-inventor on a patent held by D.F.C.I. for the use of EGFR genotyping; and receives a share of post-market licensing revenue distributed by D.F.C.I. G.R.O. has received consulting fees from AstraZeneca, Boehringer-Ingelheim, Clovis, Genentech, Novartis, and Sysmex. M.N. has served as a consultant for Bristol-Myers-Squibb and received research grants from Merck Investigator Studies Program and Canon, Inc.
Footnotes
Conflict of Interest: For the remaining authors, none were declared.
Contributions: Study conception and design: B.L.L. and G.R.O. Data collection and/or analysis: All authors. Manuscript drafting and critical revision: All authors. Final manuscript approval: All authors. G.R.O. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for european patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 5.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 8.Kancha RK, von Bubnoff N, Peschel C, Duyster J. Functional analysis of epidermal growth factor receptor (EGFR) mutations and potential implications for EGFR targeted therapy. Clin Cancer Res. 2009;15:460–467. doi: 10.1158/1078-0432.CCR-08-1757. [DOI] [PubMed] [Google Scholar]
- 9.Thomas RK, Greulich H, Yuza Y, et al. Detection of oncogenic mutations in the EGFR gene in lung adenocarcinoma with differential sensitivity to EGFR tyrosine kinase inhibitors. Cold Spring Harb Symp Quant Biol. 2005;70:73–81. doi: 10.1101/sqb.2005.70.056. [DOI] [PubMed] [Google Scholar]
- 10.Yeo WL, Riely GJ, Yeap BY, et al. Erlotinib at a dose of 25 mg daily for non-small cell lung cancers with EGFR mutations. J Thorac Oncol. 2010;5:1048–1053. doi: 10.1097/JTO.0b013e3181dd1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lind JS, Postmus PE, Heideman DA, Thunnissen EB, Bekers O, Smit EF. Dramatic response to low-dose erlotinib of epidermal growth factor receptor mutation-positive recurrent non-small cell lung cancer after severe cutaneous toxicity. J Thorac Oncol. 2009;4:1585–1586. doi: 10.1097/JTO.0b013e3181bbb2b9. [DOI] [PubMed] [Google Scholar]
- 12.Togashi Y, Masago K, Fukudo M, et al. Efficacy of increased-dose erlotinib for central nervous system metastases in non-small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Chemother Pharmacol. 2011;68:1089–1092. doi: 10.1007/s00280-011-1691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo PC, Dahlberg SE, Nishino M, et al. Delay of treatment change after objective progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer. 2015;121:2570–2577. doi: 10.1002/cncr.29397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishino M, Cardarella S, Jackman DM, et al. RECIST 1.1 in NSCLC patients with EGFR mutations treated with EGFR tyrosine kinase inhibitors: Comparison with RECIST 1.0. AJR Am J Roentgenol. 2013;201:W64–71. doi: 10.2214/AJR.12.9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST) Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: A meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247–1253. doi: 10.1200/JCO.2009.24.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(3):iii21–6. doi: 10.1093/annonc/mdu255. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, Olsen MR, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol. 2012;30:1371–1377. doi: 10.1200/JCO.2011.36.4133. [DOI] [PubMed] [Google Scholar]
- 18.LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 20.Binder D, Buckendahl AC, Hubner RH, et al. Erlotinib in patients with advanced non-small-cell lung cancer: Impact of dose reductions and a novel surrogate marker. Med Oncol. 2012;29:193–198. doi: 10.1007/s12032-010-9767-x. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich MF, Yan SX, Schiller JH. Response to crizotinib/erlotinib combination in a patient with a primary EGFR-mutant adenocarcinoma and a primary c-met-amplified adenocarcinoma of the lung. J Thorac Oncol. 2015;10:e23–5. doi: 10.1097/JTO.0000000000000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou SI, Govindan R, Eaton KD, et al. Phase I/II dose-finding study of crizotinib (CRIZ) in combination with erlotinib (E) in patients (pts) with advanced non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2012;30:2610. [Google Scholar]
- 23.Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol. 2014;2:116–120. doi: 10.3892/mco.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Togashi Y, Masago K, Fukudo M, et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol. 2010;5:950–955. doi: 10.1097/JTO.0b013e3181e2138b. [DOI] [PubMed] [Google Scholar]
- 25.Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399–405. doi: 10.1007/s00280-012-1929-4. [DOI] [PubMed] [Google Scholar]
- 26.Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010;99:283–286. doi: 10.1007/s11060-010-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stepanski EJ, Houts AC, Schwartzberg LS, Walker MS, Reyes CM, Blakely J. Second- and third-line treatment of patients with non-small-cell lung cancer with erlotinib in the community setting: Retrospective study of patient healthcare utilization and symptom burden. Clin Lung Cancer. 2009;10:426–432. doi: 10.3816/CLC.2009.n.080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.