Abstract
The heterogeneous breast cancers can be classified into different subtypes according to their histopathological characteristics and molecular signatures. Foxa1 expression is linked with luminal breast cancer (LBC) with good prognosis, while Twist1 expression is associated with basal-like breast cancer (BLBC) with poor prognosis due to its role in promoting epithelial-to-mesenchymal transition (EMT), invasiveness and metastasis. However, the regulatory and functional relationships between Twist1 and Foxa1 in breast cancer progression are unknown. In this study, we demonstrate that in the estrogen receptor (ERα)-positive LBC cells Twist1 silences Foxa1 expression, which plays an essential role in relieving Foxa1-arrested migration, invasion and metastasis of breast cancer cells. Mechanistically, Twist1 binds to Foxa1 proximal promoter and recruits the NuRD transcriptional repressor complex to de-acetylate H3K9 and repress RNA Polymerase II recruitment. Twist1 also silences Foxa1 promoter by inhibiting AP-1 recruitment. Twist1 expression in MCF7 cells silenced Foxa1 expression, which was concurrent with the induction of EMT, migration, invasion and metastasis of these cells. Importantly, restored Foxa1 expression in these cells largely inhibited Twist1-promoted migration, invasion and metastasis. Restored Foxa1 expression did not change the Twist1-induced mesenchymal cellular morphology and the expression of Twist1-regulated E-cadherin, β-catenin, vimentin and Slug, but it partially rescued Twist1-silenced ERα and cytokeratin 8 expression and reduced Twist1-induced integrin α5, integrin β1 and MMP9 expression. In a xenografted mouse model, restored Foxa1 also increased Twist1-repressed LBC markers and decreased Twist1-induced BLBC markers. Furthermore, Twist1 expression is negatively correlated with Foxa1 in the human breast tumors. The tumors with high Twist1 and low Foxa1 expressions are associated with poor distant metastasis-free survival. These results demonstrate that Twist1's silencing effect on Foxa1 expression is largely responsible for Twist1-induced migration, invasion and metastasis but less responsible for Twist1-induced mesenchymal morphogenesis and expression of certain EMT markers.
Keywords: breast cancer, gene regulation, cancer progression, metastasis
Introduction
Molecular profiling studies have classified breast cancers into 5 subtypes, including luminal A, luminal B, normal-like, HER2-positive and basal-like breast cancers 1-3. Luminal A tumors have the most favorable prognosis. Luminal B and normal-like tumors have intermediate prognosis. HER2-positive and basal-like tumors are associated with poor relapse-free and overall survivals 3-6. Due to the lack of estrogen receptor (ERα) or overexpressed HER2, no targeted therapy is currently available for the basal-like breast cancer (BLBC). Although BLBC initially responses to chemotherapy, it usually relapses with drug resistance and thus, represents a major clinical challenge 7. Therefore, it is important to identify key molecules responsible for BLBC progression and metastasis. Recent studies showed that BLBC possesses an activated epithelial-mesenchymal transition (EMT) program and many stem cell-like properties 8,9, suggesting transcriptional factors that induce EMT may play critical roles in BLBC progression.
Twist1, a member of the basic helix-loop-helix transcription factor family, is one of the master transcription factors that induce EMT, cell migration and invasion during embryonic development and in cancer cells 10-12. Twist1 is expressed in multiple types of invasive cancer cells including some breast cancer cells 11,12. Ectopic expression of Twist1 in Twist1-negative breast cancer cells is sufficient to induce EMT and cancer stem-like cell properties. Twist1 expression also enhances cell invasion and metastasis, while depletion of Twist1 inhibits cell invasion and metastasis 12-15. Twist1 can either silence or activate genes to regulate cancer cell behaviors 11. We have reported that Twist1 recruits the nucleosome remodeling deacetylase (NuRD) complex to repress E-cadherin and ERα expression to drive EMT, cell invasion and metastasis 14,16. Another study also showed that Twist1 recruits DNA methyltransferase 3B (DNMT3B) to silence ERα expression and contribute to hormone resistance 17. On the other hand, Twist1 directly upregulates AKT2, Bmi1, Wnt5a, PDGFRα, and Jagged1 to promote cancer cell survival, drug resistance, EMT, cancer stem-like cell number, invadopodia formation for extracellular matrix degradation, and cancer cell transition into endothelial cells, respectively 13,18-22. In addition, Twist1 overexpression can override oncogene-induced premature senescence by abrogating key regulators of the p53 and Rb-dependent pathways 23. These studies indicate that Twist1 controls cancer cell behaviors through regulating key genes involved in multiple cellular pathways.
Foxa1 is a forkhead box transcription factor expressed in the mammary luminal epithelial cells (LECs). It serves as a pioneer factor by facilitating ERα binding to chromatin and plays an important role in maintaining mammary ductal morphology and epithelial differentiation 24-27. Foxa1 is also a marker of luminal A breast cancer and is required for many ERα-regulated gene expression and cell proliferation 24,25,28,29. Foxa1 has been recognized as a determinant of breast tumor response to endocrine therapy and a marker for favorable patient prognosis 27,30,31. Another study showed that Foxa1 silencing allows breast cancer cells to partially shift their transcriptome signatures toward those of BLBC and progress to more aggressive cancer cells 32. Furthermore, Foxa1 and ERα expressions are decreased during the progression from luminal A to luminal B breast tumors in mice 33. These studies suggest that silenced Foxa1 expression may facilitate breast cancer progression toward BLBC. However, it remains unknown how Foxa1 expression is silenced during breast cancer progression and how the silenced Foxa1 expression is involved in the BLBC-associated EMT, migration, invasion and metastasis of breast cancer cells.
In this study, we found that Twist1 directly silences Foxa1 expression in breast cancer cells. This silenced Foxa1 expression is largely responsible for Twist1-induced migration, invasion and metastasis of breast cancer cells and for Twist1-repressed luminal breast cancer (LBC) marker gene expression and Twist1-induced BLBC marker gene expression. However, the Twist1-silenced Foxa1 expression is dispensable for Twist1-induced mesenchymal cellular morphogenesis and for the expression of some of the Twist1-induced EMT markers.
Results
Twist1 expression is associated with reduced Foxa1 expression and basal-like human breast tumors
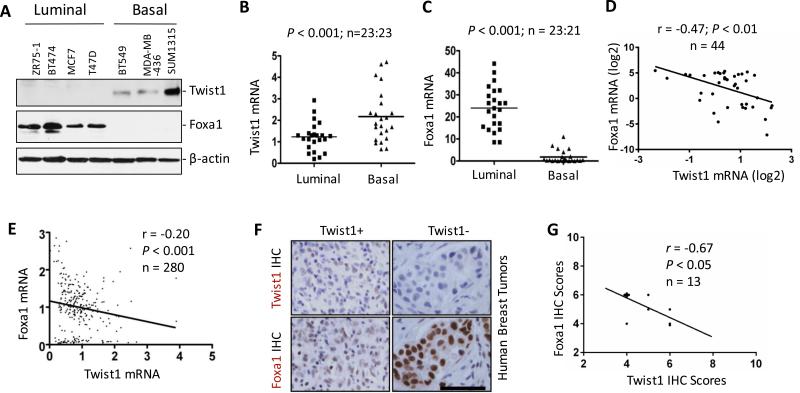
To examine the expressional relationship between Twist1 and Foxa1, we assayed Twist1 and Foxa1 proteins in the luminal and BLBC cell lines. We found Twist1 is only expressed in the three examined BLBC cell lines including BT549, MDA-MB-436 and SUM1315 and Foxa1 is only expressed in the four examined LBC cell lines including ZR75-1, BT474, MCF7 and T47D (Fig. 1A). Analysis of a dataset from NCBI GEO database (GSE15361) 34 also revealed that the average expression level of Twist1 mRNA is significantly higher in BLBC cell lines versus LBC cell lines. However, the average expression level of Foxa1 mRNA is markedly reduced in BLBC cell lines versus LBC cell lines. Accordingly, the level of Twist1 expression is negatively correlated with the level of Foxa1 expression in these cell lines (Fig. 1B-D). A similar negative correlation between Twist1 and Foxa1 mRNA expression was also identified from another dataset of 281 human breast tumors in NCBI GEO (GSE2034) 35 (Fig. 1E).
Figure 1. Twist1 expression is negatively associated with Foxa1 expression in human breast cancers.
A. Western blotting analysis of Twist1 and Foxa1 in human breast cancer cell lines. B and C. Expression levels of Twist1 (Panel B) and Foxa1 (Panel C) mRNAs in luminal and basal breast cancer cell lines. Data analysis was based on a published data set 34. D. Pearson's Correlation analysis between Twist1 and Foxa1 mRNA expression levels in log2 values in breast cancer cell lines shown in Panels B and C. E. Pearson's Correlation test of Twist1 and Foxa1 mRNA expression levels in another published data set of human breast tumor cohort 35. F and G. Representative images of IHC for Twist1 and Foxa1 in human breast tumors. Adjacent tumor sections were used for Twist1 and Foxa1 IHC (Panel F). Twist1 and Foxa1 protein levels are inversely correlated in Twist1-positive breast tumors. The r and P values were obtained by Pearson's correlation analysis (Panel G). The scale bar in Panel F, 50 μm.
To validate the negative correlation at protein levels, we performed immunohistochemistry (IHC) for Twist1 and Foxa1 in a cohort of 276 human breast tumors. Foxa1 and Twist1 proteins were respectively detected in 245 out of 261 (94%) and 13 out of 276 (5%) tumors, indicating that this cohort mainly consists of luminal breast tumors. Interestingly, 179 out of 261 (68.6%) tumors showed very high (>6) Foxa1 immunoreactive scores (IRSs). However, the 13 Twist1-positive tumors had IRSs of 6 or lower. Twist1 and Foxa1 IRSs and ERα, PR and HER2 expression profiles for the 13 Twist1-positive tumors are provided in Supplementary Table S1. Foxa1 immunoreactivity is significantly reduced in 5 out of the 13 tumors when compared with Twist1-negative tumors (Fig. 1F). Twist1 protein levels were also negatively correlated with Foxa1 protein levels among these 13 Twist1-positive tumors (Fig. 1G). Of note, all Twist1-positive tumors are also Foxa1 positive because Twist1 was only detected in a subpopulation of tumor cells. Together, these results demonstrate that the levels of Twist1 expression are negatively correlated with the levels of Foxa1 expression in human breast tumors.
Twist1 binds to Foxa1 promoter and represses its transcription
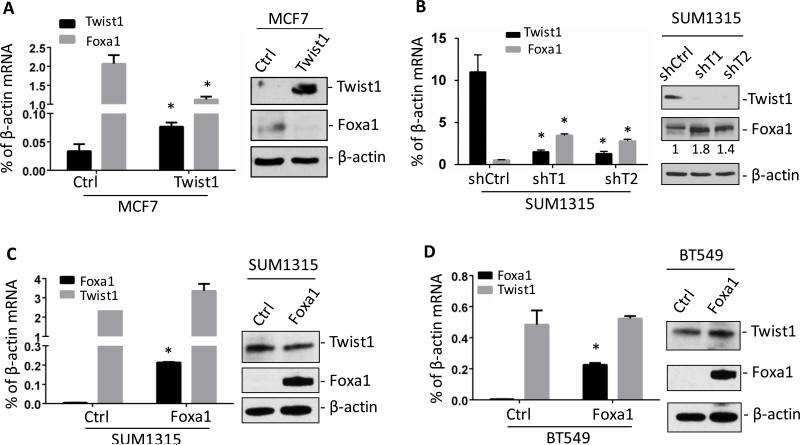
The negative correlation between Twist1 and Foxa1 expression in breast cancer cells and tumors hinted that Twist1 might repress Foxa1 expression to promote breast cancer progression. To test this hypothesis, we generated stable MCF7 cell lines with either the empty control vector (MCF7Ctrl) or Twist1-expressing vector (MCF7Twist1) from ERα-positive MCF7 LBC cells. We found that Twist1 drastically decreased Foxa1 mRNA and protein in MCF7Twist1 cells versus MCF7Ctrl cells (Fig. 2A). Knockdown of Twist1 is known to reduce migration, invasion and metastasis of invasive breast cancer cells 12,14,36,37. Here, we found that stable knockdown of Twist1 mRNA in SUM1315 and MDA-MB-436 BLBC cells increased Foxa1 mRNA and protein (Fig. 2B and supplementary Fig. S1). On the other hand, ectopic expression of Foxa1 in either SUM1315 or BT549 breast cancer cells with endogenous Twist1 expression did not alter Twist1 mRNA and protein expression (Fig. 2C and D), suggesting Foxa1 does not regulate Twist1 expression.
Figure 2. Twist1 silences Foxa1 expression in breast cancer cells.
A. Expression of Twist1 in MCF7 cells reduced Foxa1 mRNA and protein as measured by Q-PCR and Western blotting. B. Knockdown of Twist1 mRNA in SUM1315 cells by Twist1 shRNAs increased Foxa1 mRNA and protein. The indicated relative levels of Foxa1 protein were obtained by normalizing the Foxa1 band intensity to the β-actin band intensity. shCtrl, non-targeting shRNA as negative control; shT1 and shT2, two shRNAs for targeting 2 different regions of Twist1 mRNA. C and D. Overexpression of Foxa1 did not influence Twist1 mRNA and protein in both BT549 and SUM1315 cells. All experiments were repeated 3 times and the representative data are presented. *, P < 0.05 by Student's t test between the control and testing groups.
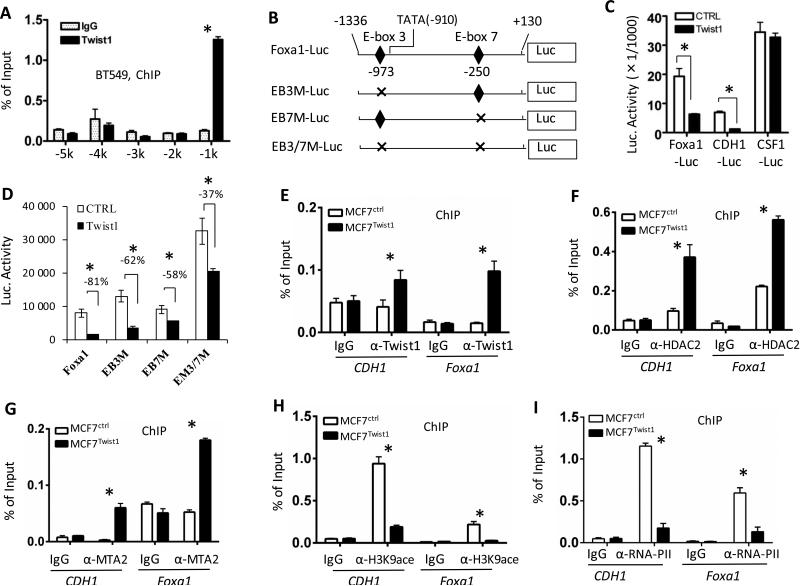
To determine whether Twist1 directly represses Foxa1 expression, we performed chromatin immunoprecipitation (ChIP) assays to examine whether Twist1 is associated with the enhancer/promoter regions of the Foxa1 gene. In BT549 cells, we found that Twist1 was associated with the 5’ regulatory region at -1 kb location from the transcriptional starting site (TSS), but it was not associated with the 5’ regulatory regions at -2, -3, -4 and -5 kb locations (Fig. 3A). We then constructed and tested a Foxa1 promoter-luciferase (Foxa1-Luc) reporter containing the 1.46-kb 5’ regulatory sequence with the Twist1-binding region (Fig. 3B). Expression of Twist1 significantly decreased the activity of the Foxa1-Luc reporter in MCF7 cells. As demonstrated previously, Twist1 decreased the activity of CDH1-Luc reporter (a positive control), but did not affect the activity of CSF1-Luc reporter (a negative control) (Fig. 3C) 14,38. By contrast, stable knockdown of Twist1 increased the activities of Foxa1 and CDH1 promoter-Luc reporters but did not alter the activity of CSF1-Luc reporter in SUM1315 cells (Supplementary Fig. S2A). These results indicate that Twist1 represses Foxa1 promoter by its direct association with the 5’ regulatory region proximal to Foxa1 promoter.
Figure 3. Foxa1 is a direct target gene of Twist1.
A. ChIP assays performed with BT549 breast cancer cells and Twist1 antibody. Non-immune IgG served as a negative control. Immunoprecipitated DNA was assayed by real time PCR with primers specific to the Foxa1 5’ regulatory sequences at the indicated locations. B. The wild type and mutated constructs of the Foxa1 promoter-luciferase reporter (Foxa1-Luc). EB3M-Luc, EB7M-Luc and ER3/7M-Luc had mutated E-box3, E-box7 or both of them, respectively. C & D. Cell transfection assays. Twist1 expression in MCF7 cells repressed the activities of Foxa1-Luc (the tester) and CDH1-Luc (a positive control), while it did not affect the activity of CSF1-Luc (a negative control) (Panel C). Twist1 showed much less repression on the activities of EB3M-Luc, EB7M-Luc and EM3/7MLuc versus Foxa1-Luc in MCF7 cells (Panel D). Control cells were transfected with equal amount of the empty vector DNA. E–I. ChIP assays. These assays were performed in MCF7Ctrl and MCF7Twist1 stable cell lines using antibodies against Twist1 (Panel E), HDAC2 (Panel F), MTA2 (Panel G), H3K9-ace (Panel H) and RNA-PII (Panel I). Non-immune IgG served as a negative control. Immunoprecipitated DNA was measured by real time PCR using primers for amplifying the Twist1-binding regions in the CDH1 gene (a positive control) and the Foxa1 gene (the E-box 3 region in Panel B) as indicated. All experiments were repeated 3 times, and the representative data with 3 technical replicates are presented as Mean ± SEM. *, P< 0.05 by Student's t test.
In silico analysis of the proximal Foxa1 promoter region revealed 11 E-boxes. Twist1 is more likely to bind two (EB3 and EB7) of these E-boxes as predicted by MatInspector Software (Genomatix) (Fig. 3B). Deletion of either EB3 in Foxa1-Luc (EB3M-Luc) reporter or EB7 in Foxa1-Luc (EB7M-Luc) reporter significantly relieved Twist1-repressed Foxa1 promoter activity in MCF7 cells. Deletions of both EB3 and EB7 in Foxa1-Luc (EB3/7M-Luc) reporter additively relieved Twist1-repressed Foxa1 promoter activity (Fig. 3B and D). In SUM1315 cells with endogenous Twist1 expression, mutation of EB3 and/or EB7 increased Foxa1-Luc reporter activity, while knockdown of Twist1 resulted in lesser fold increases in the mutant Foxa1 promoter activities versus the wild type promoter (Supplementary Fig. S2B). These results demonstrate that Twist1 can use both EB3 and EB7 sites to silence the transcriptional activity of Foxa1 promoter. Since the activity of EB3/7M-Luc reporter was still partially silenced by Twist1 in MCF7 cells, Twist1 might also bind to other E-boxes to repress Foxa1 promoter.
Twist1 recruits NuRD complex to Foxa1 promoter and deacetylates H3K9
We reported that Twist1 recruits NuRD complex to repress CDH1 and ERα promoters 14,16. Here, our ChIP assays revealed that Twist1 was recruited to the proximal promoter regions of both CDH1 and Foxa1 genes in MCF7Twist1 cells (Fig. 3E). The endogenous HDAC2 and MTA2 proteins, two of the NuRD complex components, were also recruited to the Twist1-binding regions of both CDH1 and Foxa1 genes in a Twist1 expression-dependent manner (Fig. 3F and G). In agreement with the Twist1-recruited NuRD complex containing histone deacetylase, the levels of acetylated histone H3K9 (H3K9-ace) were markedly reduced in the same Twist1-binding regions of both CDH1 and Foxa1 genes in MCF7Twist1 cells versus MCF7Ctrl cells (Fig. 3H). Accordingly, RNA polymerase II recruited to these gene promoters was significantly reduced in MCF7Twist1 cells versus MCF7Ctrl cells (Fig. 3I). Furthermore, knockdown of Twist1 in SUM1315 cells decreased HDAC2 and MTA2 recruitments and increased H3K9-ace and RNA polymerase II recruitment on Foxa1 promoter (Supplementary Fig. S3). These results demonstrate that Twist1 strongly silences the transcriptional activity of the Foxa1 promoter by recruiting the NuRD gene-repressing complex.
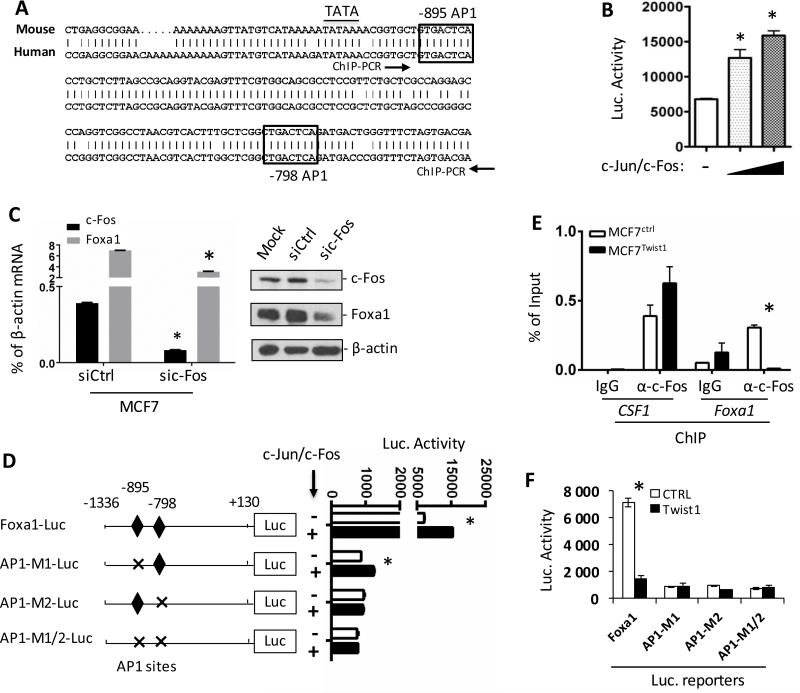
Twist1 silences Foxa1 expression through inhibiting AP-1-promoted activation of the Foxa1 promoter
Analysis of the Foxa1 promoter region identified two AP-1 sites conserved in both human and mouse Foxa1 genes (Fig. 4A). We found that in MCF7 cells overexpressed c-Jun and c-Fos significantly increased the activity of Foxa1-Luc reporter in a dose-dependent manner, while knockdown of c-Fos drastically reduced both Foxa1 mRNA and protein expression (Fig. 4B and C). This c-Fos-activated Foxa1 expression should be independent of Twist1 since Twist1 protein was undetectable in MCF7 cells with and without c-Fos knockdown (data not shown) and the low level Twist1 mRNA was not altered in MCF7 cells with c-Fos knockdown (Supplementary Fig. S4). Furthermore, deletion of either AP-1 site at bp -895 or bp -798 or both diminished AP-1-promoted activities of the Foxa1 AP1M1-Luc, AP1M2-Luc and AP1M1/2-Luc reporters (Fig. 4D). These results demonstrate that AP-1 is an esential transcriptional activator of Foxa1 promoter.
Figure 4. Twist1 inhibits AP1-mediated activation of the Foxa1 promoter by inhibiting AP1 recruitment.
A. The two conserved AP1 sites in both human and mouse Foxa1 promoter regions. B. Luciferase activity of MCF7 cells transfected with Foxa1-Luc plasmid with the empty vector (−) or c-Jun and c-Fos expression vectors. C. Q-PCR and Western blot assays showing Foxa1 mRNA and protein changes upon c-fos knockdown in MCF7 cells. D. Luciferase assay of MCF7 cells co-transfected with the indicated luciferase reporter and the empty vector (−) or c-Jun and c-Fos expression vectors. E. ChIP assays performed with MCF7Ctrl and MCF7Twist1 cells and non-immune IgG (negative control) or c-Fos antibody as indicated. The DNA fragment between the two arrowheads in Panel A was amplified by PCR from the immunoprecipitated DNA. Amplification of the known AP1-binding region by PCR in the CSF1 promoter served as a positive control. F. Luciferase assay of MCF7 cells co-transfected with the indicated luciferase reporter and the empty vector (Ctrl) or Twist1 expression plasmid. Experiments were repeated 3 times and the representative data with 3 technical replicates are presented as Mean ± SEM. * in Panels B–F, P<0.05 by Student's t test.
ChIP assays revealed that Twist1 expression in MCF7 cells abolished c-Fos recruitment to Foxa1 promoter (Fig. 4E), while knockdown of Twist1 in SUM1315 cells increased c-Fos recruitment to Foxa1 promoter (Supplementary Fig. S5A). As a control, Twist1 did not inhibit c-Fos recruitment to CSF1 promoter (Fig. 4E) 38. Mutation of the AP-1 sites in Foxa1 promoter, which simulates Twist1-prevented AP-1 binding, drastically reduced the basal activity of Foxa1 promoter. Thus, Twist1 expression was unable to further reduce the little activities of these mutant promoters as it did to the wild type promoter (Fig. 4F). Consistent results were also observed in SUM1315 cells with Twist1 knockdown (Supplementary Fig. S5B). These results suggest that silencing AP-1-mediated activation of Foxa1 promoter is an important mechanism for Twist1-repressed Foxa1 expression.
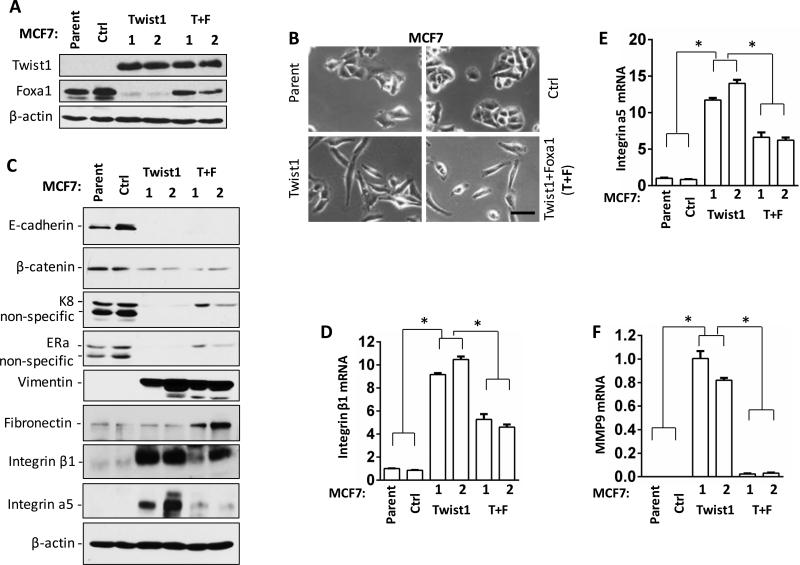
Twist1-silenced Foxa1 expression is not responsible for Twist1-induced cell morphological change but it mediates the expression of some Twist1-regulated genes
To examine whether Twist1-silenced Foxa1 expression is responsible for Twist1-induced morphology and gene expression changes in breast cancer cells, we stably restored Foxa1 expression in MCF7Twist1 cell lines (designated as MCF7Twist1+Foxa1 cell lines) with Twist1-silenced Foxa1 expression (Fig. 5A). As expected, MCF7Ctrl cells showed epithelial tumor cell morphology identical to their parent MCF7 cells, while MCF7Twist1 cells had an elongated spindle shape consistent with their EMT phenotype. MCF7Twist1+Foxa1 cells with restored Foxa1 expression showed a cellular morphology very similar to MCF7Twist1 cells (Fig. 5B), suggesting that the silenced Foxa1 expression is not responsible for Twist1-induced morphological change of MCF7 cells. Furthermore, Twist1 expression effectively repressed E-cadherin, β-catenin, cytokeratin 8 (K8) and ERα expression in MCF7Twist1 cells as compared to MCF7Ctrl cells, while restored Foxa1 expression only partially rescued K8 and ERα expression in MCF7Twist1+Foxa1 cells as compared to MCF7Twist1 cells (Fig. 5C). Moreover, Twist1 strongly induced vimentin, integrin β1, integrin α5, and MMP9 expression but did not change fibronectin expression in MCF7Twist1 cells as compared to MCF7Ctrl cells. Restored Foxa1 expression had no effect on vimentin expression but inhibited Twist1-induced integrin β1, integrin α5 and MMP9 expression and slightly increased fibronectin in MCF7Twist1+Foxa1 cells as compared to MCF7Twist1 cells (Fig. 5C-F and data not shown). In addition, Slug (Snail2) expression was increased in MCF7Twist1 cells versus MCF7Ctrl cells, but restored Foxa1 expression did not affect Twist1-induced Slug expression in MCF7Twist1+Foxa1 cells versus MCF7Twist1 cells. The expression levels of Snail1, Zeb1 and Zeb2 mRNAs were very low and remained unchanged in MCF7Ctrl, MCF7Twist1 and MCF7Twist1+Foxa1 cells (Data not shown). These results suggest that silenced Foxa1 is not responsible for Twist1-induced mesenchymal cell morphology and most EMT marker gene expression, but responsible for permitting the expression of ERα, K8 and other selective Twist1-regulated genes related to cell migration and invasion such as integrin β1, integrin α5 and MMP9.
Figure 5. The effects of silenced Foxa1 expression on Twist1-induced EMT cell morphogenesis and gene expression.
A. Western blot analysis of the indicated proteins in MCF7 parent cells, MCF7Ctrl cells, two lines (1 and 2) of MCF7Twist1 cells, and two lines (1 and 2) of MCF7Twist1+Foxa1 (T+F) cells. B. The images of MCF7 parent, MCF7Ctrl, MCF7Twist1 and MCF7Twist1+Foxa1 (T+F) cells. Scale bar, 50 μm. C. Western blot analysis of the indicated proteins in the indicated cell lines. D-F. qPCR analysis of integrin β1, integrin α5 and MMP9 mRNA levels in the indicated cell lines. *, P < 0.05 by Student's t test.
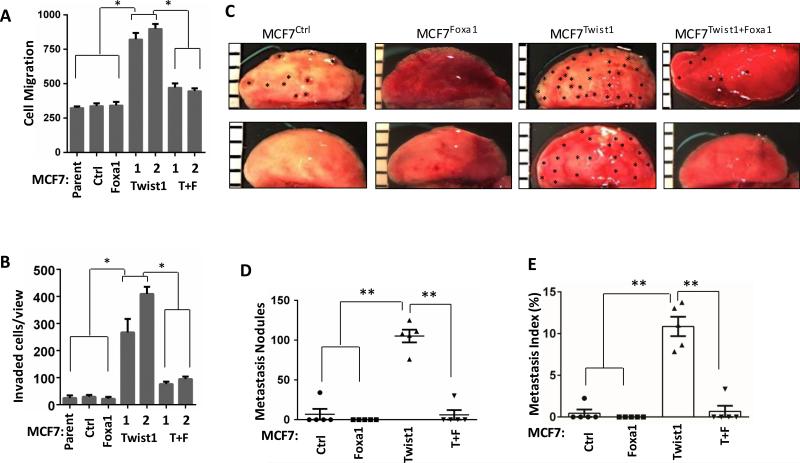
Silencing Foxa1 expression is required for Twist1 to promote migration, invasion and metastasis in breast cancer cells
Expression of Foxa1 alone showed no effects on MCF7 cell migration and invasion. However, restored Foxa1 expression in MCF7Twist1+Foxa1 cells significantly suppressed Twist1-induced cell migration on the culture plate and invasion through a Matrigel layer (Fig. 6A and B). To examine whether Foxa1 could inhibit Twist1-promoted metastasis in vivo, we injected MCF7Ctrl, MCF7Foxa1, MCF7Twist1 and MCF7Twist1+Foxa1 cells into the tail veins of SCID mice. After 4 weeks, we found metastatic tumor foci in 20% (1/5), 0% (0/5), 100% (5/5) and 20% (1/5) of lungs in mice received MCF7Ctrl, MCF7Foxa1, MCF7Twist1 and MCF7Twist1+Foxa1 cells, respectively (Fig. 6C). The average number of metastasis foci developed in each lung was significantly increased in MCF7 Twist1 group versus MCF7Ctrl, MCF7Foxa1 or MCF7Twist1+Foxa1 group (Fig. 6D). Accordingly, the lung metastasis index reflecting the percentage of tumor area to lung tissue area was more than 25 fold higher in the recipient mice of MCF7Twist1 cells versus the recipient mice of MCF7Ctrl, MCF7Foxa1 or MCF7Twist1+Foxa1 cells (Fig. 6E). To examine if restored Foxa1 could partially reverse the Twist1-induced basal tumor phenotype in vivo, we injected MCF7Ctrl, MCF7Foxa1, MCF7Twist1 and MCF7Twist1+Foxa1 cells into the mammary gland fat pads of SCID mice to form xenograft tumors and profiled the expression levels of well-established luminal and basal breast cancer marker genes. Luminal markers including FOXA1, PGR, GRP160, BAG1, BLVRA, PDEF, XBP1 and MUC1 were repressed and basal markers including JAG1, EGFR, FOXC1, CK5, CDC20 and ITGB1 were induced in MCF7Twist1 tumors versus MCF7Ctrl and most MCF7Foxa1 tumors. Restored Foxa1 expression increased the expression of many luminal markers including BLVRA, PDEF and XBP1 and decreased the expression of many basal markers including JAG1, CK5, CDC20 and ITGB1 in MCF7Twist1+Foxa1 tumors versus MCF7Twist1 tumors (Supplementary Fig. S6A). Again, Twist1 promoted MCF7Twist1 tumor metastasis, while restored Foxa1 inhibited MCF7Twist1+Foxa1 tumor metastasis in SCID mice (Supplementary Fig. S6B and C). Together, these results demonstrate that Twist1-repressed Foxa1 expression may be important for BLBC progression and is required for Twist1-promoted migration, invasion and metastasis of breast cancer cells.
Figure 6. Restored Foxa1 expression inhibited Twist1-promoted migration, invasion and metastasis of breast cancer cells.
A & B. Cell migration and invasion assays for MCF7 parent, MCF7Ctrl, MCF7Foxa1, MCF7Twist1 and MCF7Twist1+Foxa1 cells. *, P<0.05 by Student's t test. C. Lung photographs of SCID mice after receiving intraveinous injection of MCF7Ctrl, MCF7Foxa1, MCF7Twist1 or MCF7Twist1+Foxa1 cells for 4 weeks. Asterisks indicate visible metastasis nodules. D. Metastasis nodules identified on the H&E-stained serial lung sections of SCID mice (n=5) injected with MCF7Ctrl, MCF7Foxa1, MCF7Twist1 or MCF7Twist1+Foxa1 cells for 4 weeks. **, P<0.01 by Student's t test. F. Metastasis indexes were presented as the average ratios of tumor areas to lung areas measured on the images taken from H&E-stained serial lung sections. **, P<0.01 by Student's t test.
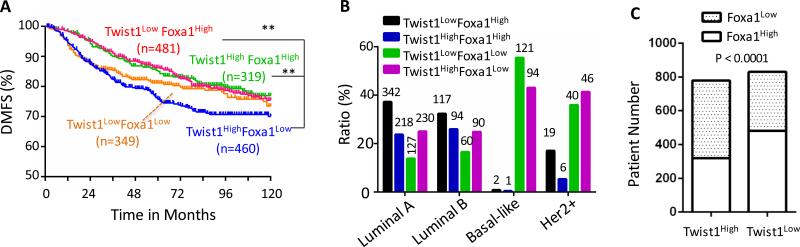
Breast tumor patients with high Twist1 and low Foxa1 expression exhibit poor distant metastasis-free survival
To assess the clinical relevance of the Twist1-Foxa1 regulatory axis in human breast cancer progression, we analyzed the association between the expression levels of Twist1 and Foxa1 and the clinical outcomes in a large breast cancer cohort with distant metastasis-free survival (DMFS) data 39. All patients were divided into four groups according to the median Twist1 and Foxa1 expression. The Twist1High/Foxa1Low subgroup exhibited significantly worse DMFS than the Twist1Low/Foxa1High and the Twist1High/Foxa1High subgroups (Fig. 7A). Interestingly, when the cohort was further subgrouped into luminal A, luminal B, HER2-positive and basal-like subtypes, only the Twist1High/Foxa1Low patients with luminal A tumors exhibited significantly worse DMFS as compared to Twist1Low/Foxa1High and Twist1High/Foxa1High patients with luminal A tumors (Supplementary Fig. S7). Further analyses revealed that the percentages of Twist1LowFoxa1High and Twist1HighFoxa1High subgroup tumors in luminal A and luminal B subtypes are much higher than those in Basal-like and HER2-positive subtypes, while the percentages of Twist1Low/Foxa1Low and Twist1high/Foxa1Low tumors in luminal A and luminal B subtypes are much lower than those in the more aggressive basal-like and HER2-positive subtypes (Fig. 7B). Moreover, Fisher's exact test revealed that Twist1High tumors are significantly associated with Foxa1Low tumors, while tumors with Twist1Low tumors are associated with Foxa1High tumors (Fig. 7C), indicating a negative correlation between Twist1 and Foxa1 expression profiles. These results suggest that Twist1-mediated repression of Foxa1 expression plays an important role in breast cancer metastasis and Foxa1 can effectively inhibit metastasis in tumors with high expression of both Twist1 and Foxa1.
Figure 7. The association of Twist1 and Foxa1 expression levels with the clinical outcomes of the breast cancer patients.
A. The distant metastasis-free survival (DMFS) curves of women with breast tumors expressing high or low levels of Twist1 and Foxa1 as indicated. The cohort (n=1609) was divided into high and low expression subgroups by the median. **, P<0.01 by Log-rank test. B. The distribution of patients with the indicated expression patterns of Twist1 and Foxa1 mRNAs in the 4 subtypes of breast cancer. The number of cases in each group is indicated above each bar. C. The distribution of Foxa1low and Foxa1high tumors in Twist1high and Twist1low subgroups of breast tumors in the cohort. The indicated P value was obtained from Fisher's Exact test.
Discussion
Breast cancer is heterogeneous and it is unclear how ERα-positive LBC could lose their ERα and progress toward BLBC. Foxa1 is associated with LBC with good prognosis, while Twist1 is associated with BLBC with poor prognosis due to its potential to drive cell invasiveness and metastasis. However, the regulatory and functional relationship between Twist1 and Foxa1 in breast cancer progression remains unknown. In this study, we found Twist1 expression negatively correlates with Foxa1 expression in human breast cancer cells and tumors. Mechanistically, Twist1 directly associates with Foxa1 promoter to recruit NuRD complex and prevent AP-1 binding to Foxa1 promoter, resulting in decreased H3K9 acetylation, reduced RNA polymerase II recruitment and silenced Foxa1 expression. It is conceivable that constitutive AP-1 binding in LBC cells with no Twist1 plays a major role to maintain a high level of Foxa1 expression and thus a luminal phenotype. Twist1 expression in partial or full EMT breast cancer cells may partially or fully inhibit AP-1 recruitment to Foxa1 promoter through NuRD complex-mediated changes in histone codes and thus reduce or silence Foxa1 expression, causing breast cancer progression toward BLBC. Therefore, our findings indicate that Foxa1 is a direct target of Twist1 during breast cancer progression and provides a possible new mechanism for the loss of Foxa1 during breast cancer progression from a luminal ERα-positive subtype to an ERα-negative BLBC subtype.
In agreement with the established function of Twist1 11, ectopic expression of Twist1 in MCF7 cells induced a mesenchymal morphology and gene expression signature but decreased epithelial gene expression. Using this cellular model, we addressed how much the Twist1-silenced Foxa1 expression contributes to Twist1-induced mesenchymal morphogenesis and gene expression in breast cancer cells by restoring Foxa1 in Twist1-expressing MCF7 cells. Interestingly, though previous studies have shown Foxa1 represses EMT-linked mesenchymal morphogenesis in pancreatic cancer cells 40, we found that restored Foxa1 did not change the mesenchymal cell morphology induced by Twist1 in MCF7Twist1+Foxa1 cells. In agreement with this, restored Foxa1 did not rescue the expression levels of some Twist1-repressed epithelial genes such as E-cadherin and β-catenin and also did not repress the expression of some Twist1-induced mesenchymal genes such as vimentin and Slug. We can also estimate the role of Foxa1 silencing in EMT by comparing the genes regulated by Foxa1 in the LBC cells to the previously published EMT signature genes 32,41. Among the 1118 Foxa1-regulated genes 32, we only found ARHGAP8, LMCD1, NMU, PPAP2B, PRKCH and SLPI in the 251 EMT signature gene list 41. None of these 6 genes has been shown to regulate the mesenchymal morphogenesis of breast cancer cells. Together, these findings suggest that Twist1-silenced Foxa1 expression is not a major regulatory pathway for the mesenchymal morphogenesis and for the expression of most typical EMT signature genes in breast cancer cells.
Interestingly, restored Foxa1 does partially rescue ERα and K8 expression and strongly downregulate Twist1-induced integrin β1, integrin α5 and MMP9 expression. Since ERα and K8 are markers of the LBC and integrins β1 and α5 are preferentially expressed in BLBC, it is conceivable that Twist1-silenced Foxa1 expression plays a crucial role in promoting BLBC progression. Foxa1 could either directly or indirectly regulate these genes. It has been reported that Foxa1 upregulates but Twist1 directly represses ERα expression 16,17,26. The results of the present study suggest that both the loss of Foxa1-mediated activation due to Twist1-silenced Foxa1 expression and the Twist1-mediated active repression are required for shutting down ERα expression and developing endocrine resistance during breast cancer progression toward BLBC. These interpretations are consistent with the previous studies showing that Foxa1 expression is associated with good responses to endocrine therapy and the loss of Foxa1 is associated with BLBC progression 24,25,27-32. More importantly, restored Foxa1 robustly inhibited Twist1-promoted migration, invasion and metastasis. These could be partially attributed to the downregulation of Twist1-induced expression of MMP9 and integrins β1 and 5α, since these proteins are known to promote cell invasiveness and metastasis 42-47. In addition, we demonstrate that Twist1 expressed in MCF7 cells increased most BLBC markers but decreased most LBC markers in these cell-derived xenograft tumors, while restored Foxa1 in Twist1-expressing MCF7 cells decreased many BLBC markers but increased many LBC markers. These results further support the notion that Twist1-repressed Foxa1 expression may play an important role to promote BLBC progression.
In previous studies, the role of Twist1 in promoting metastasis was largely attributed to its capability to induce EMT and enhance cancer stem cell (CSC) features 11-13,48. Now, the consensus from present and previous studies suggests that Twist1 may serve as a master regulator in breast cancer progression by regulating multiple genes involved in multiple pathways. On one hand, Twist1 may regulate one subgroup of genes, such as downregulating E-cadherin and ERα 14,16,17 and upregulating Bmi1, AKT2, Wnt5a and vimentin 18-20 to promote EMT, CSC features, cell migration, invasion and metastasis. On the other hand, Twist1 may regulate another subgroup of genes, such as upregulating PDGFRα to induce invadopodia formation for cell invasion 21 and downregulating Foxa1 to decrease ERα, CK8 and other LBC markers and increase integrin α5, integrin β1, MMP9 and other BLBC markers for promoting BLBC progression and breast cancer cell migration, invasion and metastasis. Although these multiple Twist1-regulated genes and pathways may not be equally important, they may work cooperatively to drive breast cancer progression and metastasis. A single targeting event such as silencing Foxa1 or upregulating AKT2 can be required but may not be sufficient for Twist1-promoted migration, invasion and metastasis of breast cancer cells.
Analysis of a large cohort dataset showed that tumors with Twist1HighFoxa1Low expression are associated with worse DMFS versus Twist1LowFoxa1High and Twist1HighFoxa1High tumors, which supports the notion that Twist1-silenced Foxa1 expression can promote breast cancer metastasis. Interestingly, this association is only observed in the mixed subtypes and luminal A subgroup, but not in the luminal B, HER2-positive or basal-like subgroups. We noticed that the tumor numbers with each expression profile are more evenly distributed in the luminal A and luminal B subgroups versus basal-like and HER2-positive subgroups. The later two subtypes contain too few tumors with Twist1LowFoxa1High and Twist1HighFoxa1High expressions and thus do not support a valid statistical analysis to compare Twist1LowFoxa1High or Twist1HighFoxa1High tumors with Twist1LowFoxa1Low or Twist1HighFoxa1Low tumors. Nevertheless, the facts that Twist1 is expressed at high levels in more than 40% of basal-like and HER2-positive tumors and Foxa1 is expressed at low levels in 96% of basal-like and 77% of HER2-positive tumors also support the notion that silenced Foxa1 expression is associated with more malignant breast cancer subtypes. For the luminal B subtype group, other factors in addition to Twist1 and Foxa1 may determine DMFS of these patients.
Analysis of tumor tissue microarrays by IHC also revealed an overall negative correlation between Twist1 and Foxa1 proteins. However, we did not found many Twist1-positive tumors from the examined breast tumor microarrays either because of not many BLBCs in the cohort or missed identification of some positive tumors due to the small area of tissue microarrays insufficient to cover heterogeneous regions for Twist1 expression in a tumor. IHC revealed that Twist1 is usually expressed only in a small subpopulation of tumor cells or a small region in a tumor. Most of these Twist1-positive tumors also contain many Foxa1-positive luminal tumor cells. These double positive tumors may contain Twist1-positive/Foxa1-positive, Twist1-positive/Foxa1-negative and/or Twist1-negative/Foxa1-positive cells. These observations support the notion that Twist1 expressed in a subset of breast tumor cells represses Foxa1 expression and drive EMT to promote BLBC progression from this subset of tumor cells.
In summary, we found that Twist1 directly represses the transcriptional activity of the Foxa1 promoter in breast cancer cells. Twist1-silenced Foxa1 expression is largely responsible for Twist1-mediated migration, invasion and metastasis but less responsible for Twist1-induced EMT morphology and some EMT marker expression of breast cancer cells. Therefore, maintenance or restoration of Foxa1 expression and function in Twist1-expressing breast cancer cells may be an effective approach to inhibit Twist1-promoted breast cancer cell migration, invasion and metastasis as well as BLBC progression.
Methods
Cell culture
HEK293T, MCF7, BT474 and ZR75-1 cells were obtained from ATCC and cultured in MDEM medium containing 10% of fetal bovine serum (FBS). T47D and BT549 cells were obtained from ATCC and cultured in RPMI-1640 medium with 10% of FBS. SUM1315 cells were cultured in Hyclone Nutrient Mixture with 5% of FBS, 5 μg/ml of insulin and 10 ng/ml of epidermal growth factor. All cells were cultured at 37°C in a tissue culture incubator supplied with 5% CO2.
Western blotting
Western blotting was carried out as described previously 49,50. Primary antibodies included antibodies against Twist1 (ab50887), Foxa1 (ab23738), c-Fos (ab53036, Abcam, Cambridge, MA), E-cadherin (610182, BD Bioscience, San Jose, CA), Vimentin (5741), c-Jun (9165), HA (3724, Cell Signaling, Danvers, MA) and β-actin (Sigma, St Louis, MO) and horseradish peroxidase (HRP) conjugated secondary antibody (Bio-rad, Hercules, CA) were used in the study.
Human breast cancer tissue microarray immunohistochemistry (IHC)
IHC was performed as described previously 49,51. Primary antibodies against Twist1 (ab50887), Foxa1 (ab23738, Abcam, Cambridge, MA), and secondary antibodies biotinylated anti-Rabbit IgG and biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA) were used.
Quantitative RT-PCR (qPCR)
Total RNA was isolated using the TRIZOL reagent (Life technologies, Grand Island, NY). Reverse transcription was performed with 1 μg of RNA by using the Reverse Transcriptase Core kit (Eurogentec; Fremont, CA). qPCR was performed by using the matched Universal Taqman probes (Roche, Nutley, NJ) and gene-specific primer pairs (Supplemental Table S2). The measurement of 18 S or β-actin mRNA was used as an endogenous normalizer.
Plasmids and cell transfection
The 1.5 kb human Foxa1 proximal promoter was amplified by PCR using specific primers (Supplemental Table S2) from the gemomic DNA of MCF7 cells and subcloned into pGL3-basic-luciferase plasmid to get Foxa1-Luc plasmid. The human E-cadherin-Luc and CSF1-Luc plasmids were described previously 14,38. The pCDNA-Twist1-2×flag expression plasmid was constructed by subcloning human Twist1 cDNA into the pCDNA-2×flag plasmid. The pCDH-Foxa1 plasmid was a gift from Dr. Bin He at Baylor College of Medicine.
For promoter-reporter assay, MCF7 cells were transfected with the expression vectors and promoter-luciferase reporter plasmid using the polyethylenimine reagent (23966-2, Polyscience, Niles, IL) 52. The transfected cells were harvested 24 hours and lysed with the Reporter Lysis buffer (Promega, Madison, WI) for luciferase assay as described 38. The relative luciferase activity was normalized to the total amount of protein assayed.
To generate MCF7Twist1 cell lines, MCF7 cells were transfected with pCDNA-2×flag and pCDNA-Twist1-2×flag plasmids using the Polyethylenimine reagent. Transfected cells were cultured in DMEM medium containing 2 μg/ml of Hygromycin for 2 weeks. To generate MCF7Twist1+Foxa1 cell lines, two MCF7Twist1 cell lines were transfected with pCDH-Foxa1 plasmid and growth-selected for 14 days with 4 μg/ml of Puromycin.
In siRNA-based knocking down experiments, non-targeting control siRNAs and siRNAs were purchased from Dharmacon (Lafayette, CO) and transfected using the HiPerFect Transfection Reagent (301705, QIAGEN, Valencia, CA).
ChIP assay
The DNA-bound proteins were cross-linked using 1% formaldehyde for 10 minutes. ChIP assays were performed as described previously 14,38. Antibodies used in these ChIP assays were for Twist1 (ab50887), c-Jun (ab31419, Abcam, Cambridge, MA), MTA2 (28791), HDAC2 (7899x, Santa Cruz, Dallas, TX), c-Fos (2250, Cell Signaling, Danvers, MA), Histone H3K9ac (39585, Active motif, Carlsbad, CA), RNA pol II (PLA0127, Sigma-Aldrich, St. Louis, MO) and M2 Flag antibody beads (A2220, Sigma-Aldrich, St. Louis, MO). ChIP-grade mouse IgG and rabbit IgG (Abcam, Cambridge, MA) were used as negative controls. The sequences of PCR primers used for amplifying the precipitated DNA samples are listed in Supplementary Table S2.
Cell migration and invasion assays
Cell migration and invasion capabilities were measured as described previously 53. Briefly, individual cell migration was directly traced in a 96-well plate for 18 hours using the Cell Motility HCS Reagent kit (K08-000-11, Thermo Scientific, Rockford, IL). The track areas on the electronic images were quantitatively measured using the Image J software. Cell invasion was assayed by using BioCoat Matrigel Invasion Chambers (BD Biosciences, San Jose, CA) according to the manufactures in instruction.
Metastasis assay
Animal protocols were approved by IACUC at Baylor College of Medicine. MCF7Ctrl, MCF7Foxa1, MCF7Twist1 or MCF7Twist1+Foxa1 cells (1×106) were injected into the tail vein of each 8-week-old female SCID mouse. Mice were randomly grouped with 5 mice in each group. This sample size was estimated to have adequate statistical power. These mice were euthanized in 4 weeks after the injection according to the NIH guidelines. The investigator was blinded to the group allocation during experiment. Mouse lung tissues were collected, photographed and processed for paraffin section. Metastasis was evaluated by counting the visible metastatic foci and measuring metastasis tumor area versus the lung area on H&E-stained lung sections as described previously 54,55.
Statistical analyses
All data were collected from several independent experiments to ensure adequate power (>80%). Each assay was performed in triplicate whenever applicable. All data were expressed as Mean ± SEM. Prism 4 Software (GraphPad, La Jolla, CA) was used to perform two-sided Student's t-test to analyze the data sets of mRNA level, tumor volume, luciferase activity, cell migration and invasion, metastatic tumor number and index, which are normal-distributed. Log-rank test was used to analyze the data sets of mice tumor-free survival and human breast cancer patient DMFS. Fisher's exact test was used to analyze the data sets of Foxa1 IHC scores and patient distribution in cohort. Person's Correlation test was used to analyze the Twist1 and Foxa1 expression data sets. In all statistical analyses, p<0.05 was considered significant.
Supplementary Material
Acknowledgements
We thank Yuqing Xiong for assisting manuscript preparation and the Genetically Engineered Mouse Core (GEMC) partially supported by the National Institutes of Health (NIH) grant P30CA125123 at Baylor College of Medicine for assisting mouse models. This study is supported by NIH grants CA112403 and CA193455 and Cancer Prevention and Research Institute of Texas grants RP120732-P5 and RP150197. This study is also partially supported by National Natural Science Foundation of China grants 81572619 and Sichuan Education Department research grant 15TD0020.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- 1.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 3.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 4.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan C, Oh DS, Wessels L, Weigelt B, Nuyten DS, Nobel AB, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S, Reis-Filho JS, Ashley S, Steele D, Ashworth A, Lakhani SR, et al. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J Clin Pathol. 2006;59:729–735. doi: 10.1136/jcp.2005.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer research. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- 9.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZF, Behringer RR. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 11.Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 2012;22:90–106. doi: 10.1038/cr.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelialmesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu J, Qin L, He T, Qin J, Hong J, Wong J, et al. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Res. 2010;21:275–289. doi: 10.1038/cr.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer research. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 16.Fu J, Zhang L, He T, Xiao X, Liu X, Wang L, et al. TWIST represses estrogen receptor-alpha expression by recruiting the NuRD protein complex in breast cancer cells. Int J Biol Sci. 2012;8:522–532. doi: 10.7150/ijbs.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vesuna F, Lisok A, Kimble B, Domek J, Kato Y, van der Groep P, et al. Twist contributes to hormone resistance in breast cancer by downregulating estrogen receptor-alpha. Oncogene. 2012;31:3223–3234. doi: 10.1038/onc.2011.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng GZ, Zhang W, Wang LH. Regulation of cancer cell survival, migration, and invasion by Twist: AKT2 comes to interplay. Cancer research. 2008;68:957–960. doi: 10.1158/0008-5472.CAN-07-5067. [DOI] [PubMed] [Google Scholar]
- 19.Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 20.Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, et al. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer cell. 2014;25:210–225. doi: 10.1016/j.ccr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen HF, Huang CH, Liu CJ, Hung JJ, Hsu CC, Teng SC, et al. Twist1 induces endothelial differentiation of tumour cells through the Jagged1-KLF4 axis. Nat Commun. 2014;5:4697. doi: 10.1038/ncomms5697. [DOI] [PubMed] [Google Scholar]
- 23.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Laganiere J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguere V. From the Cover: Location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci U S A. 2005;102:11651–11656. doi: 10.1073/pnas.0505575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, et al. FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development. 2010;137:2045–2054. doi: 10.1242/dev.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 2011;43:27–33. doi: 10.1038/ng.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naderi A, Meyer M, Dowhan DH. Cross-regulation between FOXA1 and ErbB2 signaling in estrogen receptor-negative breast cancer. Neoplasia. 2012;14:283–296. doi: 10.1593/neo.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20:1707–1714. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- 30.Badve S, Turbin D, Thorat MA, Morimiya A, Nielsen TO, Perou CM, et al. FOXA1 expression in breast cancer--correlation with luminal subtype A and survival. Clin Cancer Res. 2007;13:4415–4421. doi: 10.1158/1078-0432.CCR-07-0122. [DOI] [PubMed] [Google Scholar]
- 31.Thorat MA, Marchio C, Morimiya A, Savage K, Nakshatri H, Reis-Filho JS, et al. Forkhead box A1 expression in breast cancer is associated with luminal subtype and good prognosis. J Clin Pathol. 2008;61:327–332. doi: 10.1136/jcp.2007.052431. [DOI] [PubMed] [Google Scholar]
- 32.Bernardo GM, Bebek G, Ginther CL, Sizemore ST, Lozada KL, Miedler JD, et al. FOXA1 represses the molecular phenotype of basal breast cancer cells. Oncogene. 2013;32:554–563. doi: 10.1038/onc.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCune K, Mehta R, Thorat MA, Badve S, Nakshatri H. Loss of ERalpha and FOXA1 expression in a progression model of luminal type breast cancer: insights from PyMT transgenic mouse model. Oncol Rep. 2010;24:1233–1239. doi: 10.3892/or_00000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 36.Xue G, Restuccia DF, Lan Q, Hynx D, Dirnhofer S, Hess D, et al. Akt/PKB-mediated phosphorylation of Twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGF-beta signaling axes. Cancer Discov. 2012;2:248–259. doi: 10.1158/2159-8290.CD-11-0270. [DOI] [PubMed] [Google Scholar]
- 37.Yang F, Sun L, Li Q, Han X, Lei L, Zhang H, et al. SET8 promotes epithelialmesenchymal transition and confers TWIST dual transcriptional activities. EMBO J. 2012;31:110–123. doi: 10.1038/emboj.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin L, Wu YL, Toneff MJ, Li D, Liao L, Gao X, et al. NCOA1 Directly Targets M-CSF1 Expression to Promote Breast Cancer Metastasis. Cancer research. 2014;74:3477–3488. doi: 10.1158/0008-5472.CAN-13-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 40.Song Y, Washington MK, Crawford HC. Loss of FOXA1/2 is essential for the epithelial-to-mesenchymal transition in pancreatic cancer. Cancer research. 2010;70:2115–2125. doi: 10.1158/0008-5472.CAN-09-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin L, Liao L, Redmond A, Young L, Yuan Y, Chen H, et al. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol. 2008;28:5937–5950. doi: 10.1128/MCB.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer research. 2008;68:2329–2339. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sottnik JL, Daignault-Newton S, Zhang X, Morrissey C, Hussain MH, Keller ET, et al. Integrin alpha2beta 1 (alpha2beta1) promotes prostate cancer skeletal metastasis. Clin Exp Metastasis. 2013;30:569–578. doi: 10.1007/s10585-012-9561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta SK, Oommen S, Aubry MC, Williams BP, Vlahakis NE. Integrin alpha9beta1 promotes malignant tumor growth and metastasis by potentiating epithelial-mesenchymal transition. Oncogene. 2013;32:141–150. doi: 10.1038/onc.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E. Ligand-independent activation of c-Met by fibronectin and alpha(5)beta(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene. 2011;30:1566–1576. doi: 10.1038/onc.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, et al. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer research. 2011;71:1989–1998. doi: 10.1158/0008-5472.CAN-10-2833. [DOI] [PubMed] [Google Scholar]
- 48.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 49.Xu Y, Liao L, Zhou N, Theissen SM, Liao XH, Nguyen H, et al. Inducible knockout of Twist1 in young and adult mice prolongs hair growth cycle and has mild effects on general health, supporting Twist1 as a preferential cancer target. Am J Pathol. 2013;183:1281–1292. doi: 10.1016/j.ajpath.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tien JC, Liao L, Liu Y, Liu Z, Lee DK, Wang F, et al. The steroid receptor coactivator-3 is required for developing neuroendocrine tumor in the mouse prostate. Int J Biol Sci. 2014;10:1116–1127. doi: 10.7150/ijbs.10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee DK, Liu Y, Liao L, Wang F, Xu J. The prostate basal cell (BC) heterogeneity and the p63-positive BC differentiation spectrum in mice. Int J Biol Sci. 2014;10:1007–1017. doi: 10.7150/ijbs.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Longo PA, Kavran JM, Kim MS, Leahy DJ. Transient mammalian cell transfection with polyethylenimine (PEI). Methods Enzymol. 2013;529:227–240. doi: 10.1016/B978-0-12-418687-3.00018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin L, Liu Z, Chen H, Xu J. The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer research. 2009;69:3819–3827. doi: 10.1158/0008-5472.CAN-08-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuang SQ, Liao L, Zhang H, Lee AV, O'Malley BW, Xu J. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer research. 2004;64:1875–1885. doi: 10.1158/0008-5472.can-03-3745. [DOI] [PubMed] [Google Scholar]
- 55.Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O'Malley BW, et al. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci U S A. 2009;106:151–156. doi: 10.1073/pnas.0808703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.