Abstract
Our previous studies demonstrated that Jab1/Csn5 overexpression is correlated with low survival rates in cancer patients including nasopharyngeal carcinoma (NPC), breast cancer, hepatocellular carcinoma, and contributes to NPC’s resistance to radiotherapy and cisplatin by regulating DNA damage and repair pathways. However, the molecular mechanism by which Jab1/Csn5 expression is upregulated in NPCs has yet to be determined. In the present study, we identified the upstream regulator of Jab1/Csn5 expression and demonstrated its role in intrinsic resistance of NPC cells to treatment with cisplatin. Signal transducer and activator of transcription-3 (Stat3) expression correlates with and contributes to Jab1/Csn5 transcription. Consistently, silencing of Stat3 in tumors reduced Jab1/Csn5 expression, thereby sensitizing NPC cells to cisplatin-induced apoptosis both in vitro and in vivo. Mechanistically, Stat3 transcriptionally regulated Jab1/Csn5. Further, high mRNA expression levels of Stat3 or Jab1 in colon cancer, breast cancer, and glioblastoma are associated with significantly shorter survival times from the R2 online database. These findings identify a novel Stat3-Jab1/Csn5 signaling axis in cancer pathogenesis with therapeutic and prognostic relevance.
Keywords: nasopharyngeal carcinoma, Stat3, Jab1, cisplatin, therapeutic target
Introduction
Nasopharyngeal carcinoma (NPC) is a head and neck cancer that has a remarkable ethnic and geographic distribution 1, 2, with a high prevalence in southern China, Southeast Asia, northern Africa, and Alaska 2, 3. In endemic regions, the annual incidence of NPC peaks at 50 cases per 100,000 people, but in the Western hemisphere, the disease is rare, with an annual incidence of 1 case per 100,000 people 2, 3. Etiologic studies have indicated that Epstein-Barr virus (EBV) infection, environmental factors, and genetic susceptibility are associated with NPC 1.
Although radiotherapy and chemotherapy have improved NPC survival rates 4, the prognosis for patients with metastatic NPC remains poor; even with combined radiotherapy and chemotherapy, relapse rates are as high as 82% 5. Thus, additional targeted therapies for NPC are needed, and the molecular mechanisms leading to NPC tumorigenesis must be clarified.
One molecular mechanism leading to NPC tumorigenesis involves the fifth component of the COP9 signalosome complex (Csn5 or COPS5, more commonly known as Jab1; Jab1 hereafter), which we initially identified as a c-Jun coactivator 6. Jab1 acts as a modulator of intracellular signaling and affects cellular proliferation, apoptosis, and DNA damage response by interacting with several key regulatory proteins and affecting these proteins’ subcellular localization, degradation, phosphorylation, and deneddylation 7. Jab1 plays a crucial role in regulating several key tumor suppressors, including the p27Kip1 cyclin-dependent kinase inhibitor, p53, and Smad4/7 8, 9. Abnormal overexpression of Jab1, which has been detected in several human cancers, often is correlated with low-level expression of p27 and contributes to poor prognosis 7, 10–13.
Recently, we found that the aberrant activation of Jab1 overexpression is correlated with a lower survival rate in NPC patients 14 and that Jab1 positively regulates the DNA repair gene Rad51 and contributes to NPC cells’ response to radiotherapy and cisplatin 15. These findings suggest that Jab1 has an important role in NPC. However, the molecular mechanism by which Jab1 is upregulated in NPC remains unclear. Additional mechanisms governing Jab1’s transcriptional control through transcription factor regulation may link Jab1 regulation to upstream signaling pathways or other key events in this process.
In the present study, we investigated the relationship between Jab1 and signal transducer and activator of transcription-3 (Stat3), an oncogenic transcription factor that has been studied in a wide range of human cancers and whose expression is often correlated with poor prognosis 16, 17. Stat3 signaling plays critical roles in cell proliferation, metastasis, angiogenesis, host immune evasion, and therapy resistance 18, 19. Stat3 normally resides in the cytosol, but the activated Stat3 complex translocates into the nucleus to initiate the transcription of Stat3 target genes, including the genes encoding cyclin D1 20, Bcl-xL 21, c-Myc 20, Mcl1 22, survivin 23, and vascular endothelial growth factor 24. In NPC cells, Stat3 is constitutively activated and translocated into the nucleus 25. We hypothesized that Jab1 overexpression in NPC is due to Stat3 overexpression in the tumor cells relative to its expression in normal tissue.
To test our hypothesis, we assessed Stat3 and Jab1 expression in tissue samples from 45 patients with NPC and 30 patients with nasopharyngeal inflammation. We found that Stat3 was overexpressed in NPC specimens and was correlated with poor prognosis in NPC patients, while NPC patients whose specimens had the highest expression of both Stat3 and Jab1 had the poorest prognosis. Moreover, we found that in the NPC samples, overexpression of Stat3 was associated with high expression of Jab1. In addition, transfecting NPC cell lines with ectopic Stat3 significantly increased both the protein and RNA levels of Jab1, and inhibition of endogenous Stat3 expression with specific short interfering RNAs (siRNAs) substantially decreased Jab1 levels and inhibited cell proliferation, indicating that Stat3 positively regulates Jab1 in NPC. Luciferase reporter assays with the 5′ deletions of the Jab1 promoter and chromatin immunoprecipitation (ChIP) assay analyses revealed that Stat3 binds to Jab1 promoter sites. Finally, Stat3 depletion enhanced the antitumor effects of cisplatin in NPC cells and NPC xenografts. These findings reveal a novel mechanism of Jab1 regulation and provide functional and mechanistic links between Jab1-activating Stat3 signaling axes and Jab1 regulation.
Results
Patient Characteristics and Demographics
Samples from 45 patients with NPC (median age, 41 years; range, 20–73 years) and 30 patients with nasopharyngeal inflammation (median age, 36 years; range, 14–65 years) were used in the present study. The NPC patients’ clinical characteristics are shown in Supplementary Table 1.
Stat3 and Jab1 Expression Patterns
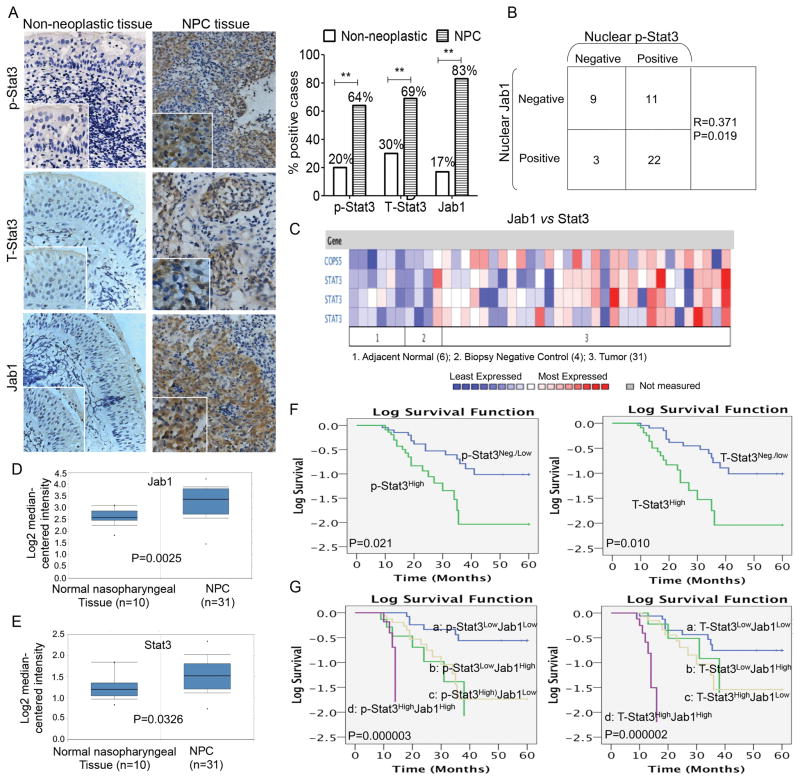
Immunohistochemical analysis revealed that 64% of the NPC samples had positive staining for phospho-Stat3 (p-Stat3). This proportion was higher than that in the noncancerous inflamed nasopharyngeal tissues, which was 20% (P < 0.01; Figure 1A). Total Stat3 (T-Stat3) levels in NPC tissues (69%) were also higher than those in the noncancerous inflamed nasopharyngeal tissues (30%; P < 0.01; Figure 1A), (examples of positive and negative tumor cases and inflamed nasopharyngeal tissues are provided in Supplemental Figure S1). Furthermore, nuclear p-Stat3 expression in the NPC tissues was associated with nuclear Jab1 expression patterns (R = 0.371, P = 0.019; Figure 1B). To determine whether Stat3 and Jab1 gene expression are correlated with clinical parameters in NPC patients, we analyzed in vivo cDNA microarrays using the Oncomine gene expression tool (https://www.oncomine.com) (Figure 1C). Compared with the normal nasopharyngeal tissue, NPC had higher expression of both Jab1 (P = 0.0025; Figure 1D) and Stat3 (P = 0.0025; Figure 1E). These findings suggest that Stat3 and Jab1 expression are potential prognostic biomarkers in NPC.
Figure 1. Expression patterns of Stat3 and Jab1 in nonneoplastic tissues and NPC tissues.
(A) Overall p-Stat3, T-Stat3, and Jab1 immunoreactivity in nonneoplastic tissues (left) was lower than that in NPC (right). The percentages of nonneoplastic nasopharyngeal tissues or NPC specimens with p-Stat3, T-Stat3, and Jab1 expression are shown at right. Original magnification, ×200; insets, ×400. (B) Nuclear p-Stat3 expression was associated with nuclear Jab1 in NPC tissues. R and P values were obtained using the Spearman test. (C–E) Jab1 and Stat3 gene expression in normal nasopharynx and NPC using the Oncomine gene expression tool (https://www.oncomine.com). The clinical data were downloaded from Oncomine Data Portal; C, Heat-map of Stat3 and Jab1 gene expression. Jab1 gene (D) and Stat3 gene (E) expression in normal nasopharynx and NPC. (F and G) Kaplan-Meier analyses of the association between p-Stat3 or T-Stat3 protein expression and survival (F) and the association between combined Stat3 and Jab1 protein expression (G) and survival.
Correlation of Stat3/Jab1 Expression with Clinical Outcome
Survival analysis using the Kaplan-Meier method showed that high expression of either T-Stat3 or p-Stat3 tended to correlate with poor prognosis (P < 0.05; Figure 1F). In our previous study, increased Jab1 expression was significantly associated with poorer overall survival (P = 0.001) 14. In the present study, we analyzed Stat3 and Jab1 expression phenotypes in combination and found that patients with high expression of p-Stat3 and Jab1 or high expression of T-Stat3 and Jab1 had the shortest mean survival durations (P = 0.000003 and P = 0.000002, respectively; Figure 1G).
The median survival time of patients with negative and weakly positive p-Stat3 tumors (35 months) was significantly longer than that of patients with highly positive p-Stat3 tumors (19 months; P = 0.021). The median survival time of patients with high T-Stat3 expression (18 months) was significantly shorter than that of patients with negative and weakly positive T-Stat3 expression (35 months; P = 0.01).
Stat3-Induced Jab1 Transcriptional Activation and Protein Expression
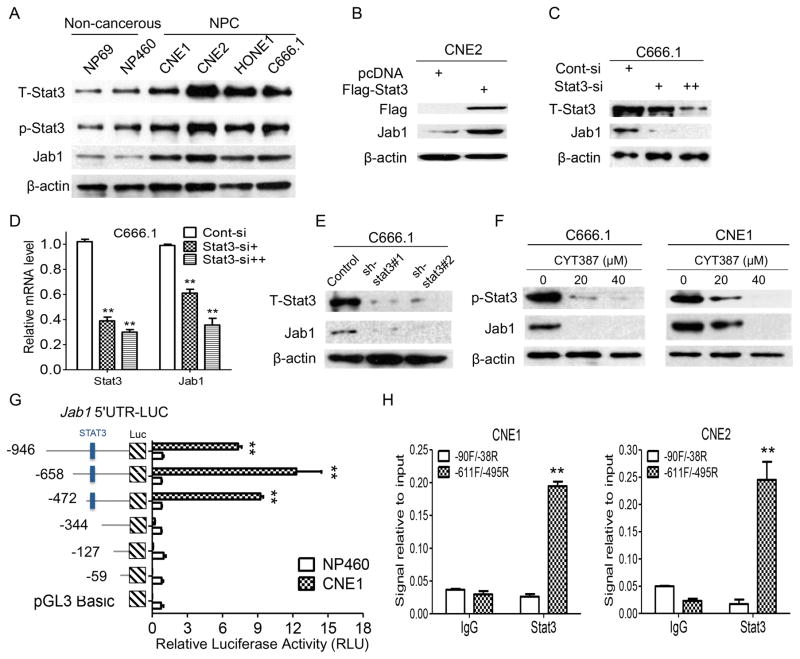
To determine the biological significance of Stat3-mediated Jab1 expression, we first used Western blotting to assess Jab1 and Stat3 expression levels in NPC cells. T-Stat3 and p-Stat3 expression was strong in NPC cells but not in normal nasopharyngeal epithelial cell lines (Figure 2A). Similarly, we found strong Jab1 expression in NPC cells.
Figure 2. Stat3 is overexpressed and regulates Jab1 levels in NPC cells.
(A) Whole-cell lysates were prepared from the cells as indicated. β-actin was used as a control for protein loading and integrity. The relative p-Stat3, T-Stat3, and Jab1 intensities for six samples are shown. (B) Ectopic Stat3 increased Jab1 expression in NPC cells. NPC cells were transfected by incubation with the Flag-Stat3 plasmid for 48 hours. The cells were then lysed and subjected to Western blotting for detection of Flag and Jab1 protein levels. (C) Knockdown of Stat3 downregulated endogenous Stat3 levels in NPC cell lines. Lysates were prepared from Stat3 siRNA-infected cells. (D) NPC cells were transfected with increasing [10 pM (+) and 40 pM (++)] doses of Stat3 siRNA for 48 hours, and Stat3 and Jab1 RNA levels were examined via quantitative PCR. (E) NPC cells with stable knockdown of Stat3 were established, and two clones for each cell line were selected for Stat3 and Jab1 detection. (F) NPC cells were exposed to CYT387 at the indicated concentration for 48 ho, and then were detected for p-Stat3 and Jab1 protein expression by western blotting. (G) Progressive deletions of the 5′ region of the Jab1 promoter in luciferase (Luc) constructs were transfected into NP460 and CNE1 cells and subjected to luciferase reporter assays. Promoter activity was higher in CNE1 cells than in NP460 cells. Deletion of the region −472 to −344 containing Stat3 binding site (−446/−423) 30 resulted in a loss of promoter activity in CNE1 cells. (H) ChIP assay was carried out using Stat3 and immunoglobulin G (IgG) antibodies, and the extracted DNA was amplified by real-time PCR. The data are the means with standard deviations for three independent experiments. **P < 0.01. Cont, Control.
Because Stat3 expression in NPC was associated with Jab1 expression, we sought to determine whether the overexpression of Stat3 could enhance Jab1 transcription in NPC cells. Ectopic expression of Stat3 in CNE1, CNE2, and HONE1 NPC cells increased Jab1 expression (Figure 2B and Supplementary Figure S2A). To assess the effect of silencing Jab1 in human NPC cells, we transfected NPC cells with Stat3 siRNA or control siRNA. Forty-eight hours after transfection, the Jab1 protein and RNA levels in the Stat3 siRNA-transfected cell lines were substantially decreased in a dose-dependent manner, compared with those in the cells transfected with the control siRNA oligonucleotides (Figure 2C, 2D, and Supplementary Figures S2B and S2C). We also established stable Stat3 short hairpin RNA (shRNA)-transfected NPC cell lines to assess the effects of Stat3 depletion on Jab1 and observed similar results: NPC cells transfected with Stat3 shRNA had a significant reduction in Jab1 levels (Figure 2E and Supplementary Figure S2D). Furthermore, treatment of NPC cells with different concentrations of the Jak/Stat3 inhibitor (CYT387) resulted in inhibition of Stat3 activation and decrease of Jab1 expression (Figure 2F), associated with decreased cell viability (Supplementary Figure S2E). These data suggest that Stat3 has biological importance in regulating Jab1 in NPC.
In order to delineate whether Stat3 regulates Jab1 transcription, we performed a luciferase reporter assay with 5′ deletion analysis of the human Jab1 promoter. The activity of the deletion constructs demonstrated differing ratios of luciferase activity between NP460 and CNE1, suggesting that different dominant regulatory factors may exist in NPC cells (Figure 2G). Interestingly, differential promoter activity was seen between the −472 and −344 constructs in CNE1 cells (Figure 2G). We next performed a ChIP assay to determine whether Stat3 could bind to the Stat3-binding site (position −446/−423) on the Jab1 promoter in NPC cells. When chromatin was incubated with the anti-Stat3 antibody, a product was observed (Figure 2H), which suggests that Stat3 binds specifically to the Jab1 promoter and thus is responsible for promoting Jab1 transcription in NPC cells.
Depletion of Stat3 Inhibits Cell Proliferation and Invasion in NPC Cell Lines
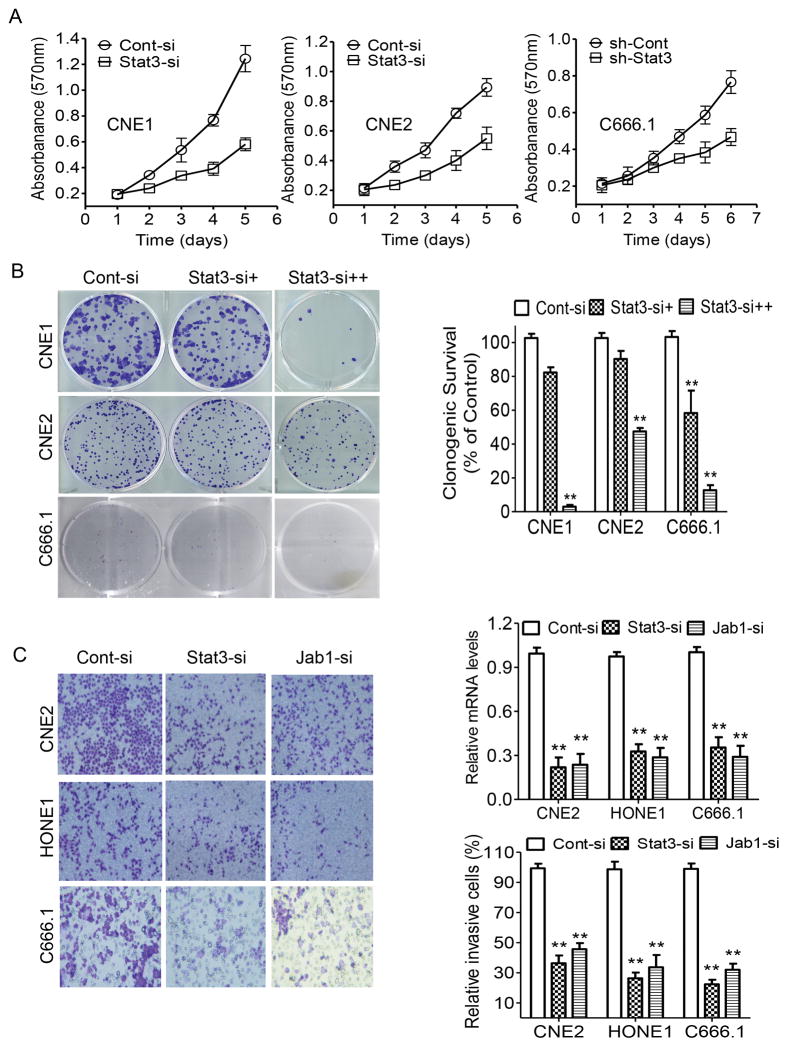
Our previous data showed that Jab1 depletion is involved in growth suppression 14. We therefore sought to determine whether siRNA-mediated Stat3 inhibition could recapitulate this tumor-suppressor effect in NPC cell lines. The expression levels of Stat3 protein in Stat3 siRNA–transfected or Stat3 shRNA (sh-Stat3) lentivirus–infected NPC cells were significantly lower than those in the control siRNA-transfected cells (Figure 2C and 2D). Knockdown of Stat3 also significantly inhibited in vitro growth (Figure 3A) and colony formation in NPC cells (Figure 3B). We also assessed the effect of Stat3 and Jab1 depletion on cell invasion. Using an in vitro invasion assay, we found that knockdown of either Stat3 or Jab1 significantly decreased NPC cell invasion (Figure 3C). Notably, the growth-inhibitory effects of Stat3 knockdown indicate that targeting Stat3 and Jab1 could suppress NPC growth and invasion.
Figure 3. Depletion of Stat3 inhibits cell proliferation and invasion in NPC.
(A) NPC cells were transfected with Stat3 siRNA for 48 hours and C666.1 cells stably infected with Stat3 shRNA (sh-Stat3)- or control shRNA (sh-Cont) carrying lentivirus, and cell growth was determined via an MTT assay. (B) Representative results of colony formation assays with NPC cells treated with control siRNA or 10 pM (+) or 40 pM (++) Stat3 siRNA. The relative numbers of colonies are shown at the right panel. (C) NPC cells transfected with Stat3 siRNA or Jab1 siRNA were exposed to invasion chamber assay. Stat3 and Jab1 mRNA levels were determined by RT-QPCR (Right, top). Matrigel membranes containing invading cells were observed via optical microscopy (Left), and the cells were counted (Right, bottom). The number of invading cells from each cell population was quantified. Cont-si, Control siRNA; Jab1-si, Jab1 siRNA. The data are means with standard deviations for three independent experiments. **P < 0.01.
Loss of Stat3 Enhances the Antitumor Effects of Cisplatin in NPC
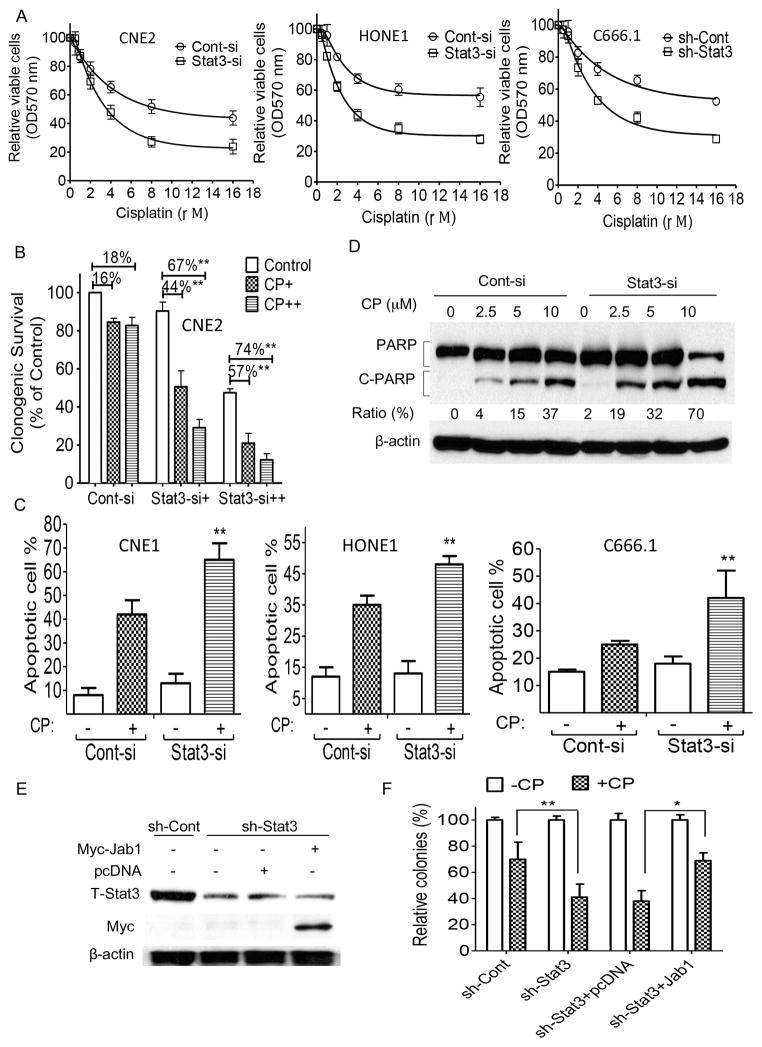
Cisplatin is the main treatment for NPC; therefore, we sought to determine whether Stat3 alters the antitumor effects of cisplatin. Using a MTT assay, we first sought to determine whether inhibition of Stat3 enhances the antitumor effects of cisplatin. We found that knockdown of Stat3 in NPC cells resulted in increased sensitivity to cisplatin treatment compared to control cells (Figure 4A). We also performed a colony formation assay in NPC cells treated with Stat3 siRNA and cisplatin. We found that the colony formation of Stat3 knockdown cells was significantly inhibited compared with that of the control siRNA-treated cells in response to cisplatin (Figure 4B).
Figure 4. Effect of Stat3 depletion on NPC cells’ sensitivity to cisplatin.
(A–D) Stat3 expression levels and cisplatin (CP) responses of CNE2 and HONE1 cells transiently transfected with Stat3 siRNA (Stat3-si) or scrambled control siRNA (Cont-si) and of C666.1 cells stably infected with Stat3 shRNA (sh-Stat3)- or control shRNA (sh-Cont)-carrying lentivirus as determined by MTT assay (A), colony formation assay (B), annexin-V/PI staining (C), and PARP cleavage in CNE2 cells (D). (E, F) CNE2 cells stably expressing sh-Stat3 were transfected with pcDNA or Myc-Jab1 plasmid DNA. (E) Western blot analyses demonstrated the effective knockdown and ectopic expression. (F) Colonies were stained with crystal violet 10 days after CP exposure. The data are means with standard deviations for three independent experiments. **P < 0.01. OD, optical density.
We further investigated whether Stat3 knockdown could enhance cisplatin-induced apoptosis in NPC cells. Using annexin V and propidium iodide (PI) staining, we found that cisplatin induced higher rates of apoptosis in Stat3 knockdown cells than in control siRNA-treated cells: the apoptosis rates of the Stat3 knockdown CNE1 cells and HONE1 cells were 23% and 13% higher, respectively, than those of the control cells (Figure 4C). We also assessed the effect of Stat3 siRNA on the proteolytic cleavage of poly ADP ribose polymerase (PARP) in response to cisplatin and found that cisplatin consistently induced the proteolytic cleavage of PARP in Stat3 knockdown CNE2 cells to a greater degree than it did in the control siRNA-treated cells (Figure 4D). In addition, transiently transfecting Jab1 plasmid DNA into Stat3-deficient cells (Figure 4E) altered and reversed their survival patterns: the pattern of colony formation in these cells was similar to that of the control shRNA-treated cells, indicating that adding back Jab1 can rescue the growth suppression of Stat3-deficient cells (Figure 4F).
Our in vitro findings indicated that Stat3 has a critical role in NPC. To confirm the function of Stat3 in NPC in vivo, we first established NPC cells with or without stable knockdown of Stat3 by transducing CNE2, HONE1 and C666.1 cells with a lentivirus carrying sh-Stat3 or sh-control. Two colonies were selected and subjected to Western blotting to confirm Stat3 expression levels (Figure 2D and Supplementary Figure S1C).
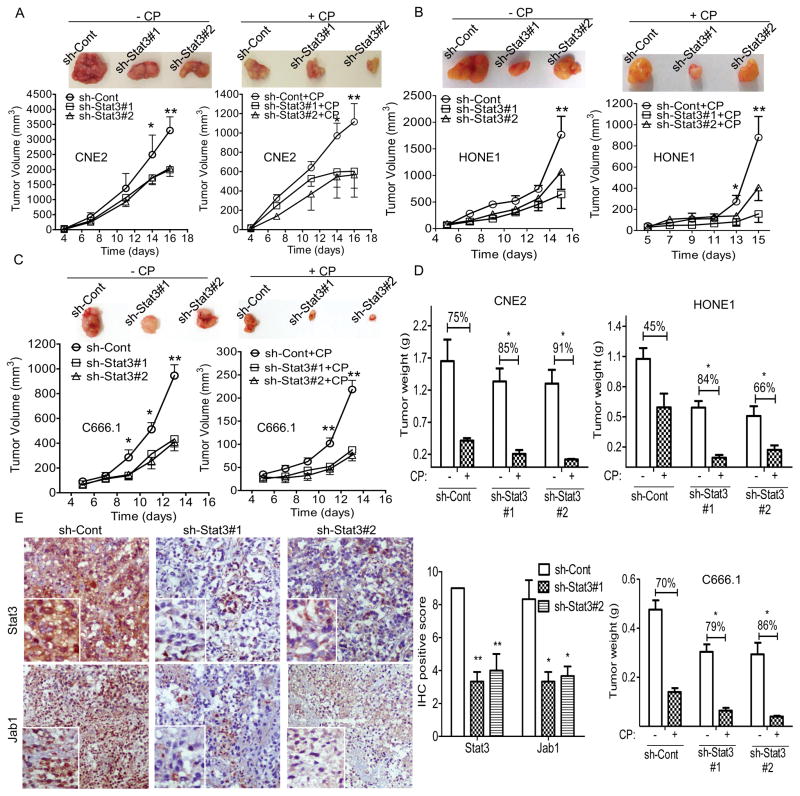
We then transplanted the NPC cells with stable knockdown of Stat3 into nude mice and treated the mice with cisplatin once tumors became palpable (Figure 5A–5C). The suppressive effects of Stat3 depletion on tumor growth were evident in the CNE2 mouse model (Figure 5A). The sh-Stat3 xenograft grew much more slowly than the sh-control xenograft did, and the injection of cisplatin substantially reduced tumor growth. Tumor weight was consistently significantly lower in the sh-Stat3 groups than in the sh-control group (Figure 5D). Similarly, HONE1 and C666.1 xenograft tumor growth was markedly reduced compared with that of the control, and the tumors with Stat3 knockdown were more responsive to cisplatin (Figure 5B–5D). These data suggest that suppression of Stat3 sensitizes NPC to cisplatin in vitro.
Figure 5. Inhibition of Stat3 reduces the growth of human NPC tumor xenografts in athymic nude mice.
(A–C) Representative photographs of harvested tumors (top) and the corresponding tumor growth curves (bottom) are shown. Tumor weights measured at the indicated times are shown at right. Female nude mice bearing xenograft tumors derived from CNE2 cells (A), HONE1 cells (B), or C666.1 cells (C) with stable Stat3 knockdown were intraperitoneally injected with phosphate-buffered saline or cisplatin (CP) at 5 mg/kg of body weight once every 2 days. (D) At the end of the experiments, the mice were humanely killed, and the tumors were excised and weighed. The data are means with standard errors. (E) Representative T-Stat3, and Jab1 expression in tissues from the same mice. Correlations of Stat3 and Jab1 staining scores are shown at right. IHC, immunohistochemistry. *P < 0.05, **P < 0.01.
Stat3 Associates with Jab1 Expression in NPC Tumor Xenografts
Because both Stat3 and Jab1 were overexpressed in NPC patient samples and nuclear p-Stat3 expression was associated with nuclear Jab1 expression patterns (R = 0.371, P = 0.019; Figure 1B), we further assessed Stat3 and Jab1 expression in tumor xenograft tissues. Immunostaining showed that Stat3 and Jab1 were abundantly coexpressed in the sh-control tumor, whereas both Stat3 and Jab1 had reduced expression in the sh-Stat3 tumor, as revealed by staining of serial sections of the same tumor (Figure 5E). These data confirmed that Stat3 associates with Jab1 in NPC.
Correlation of Stat3 and Jab1 Expression with Patient Survival
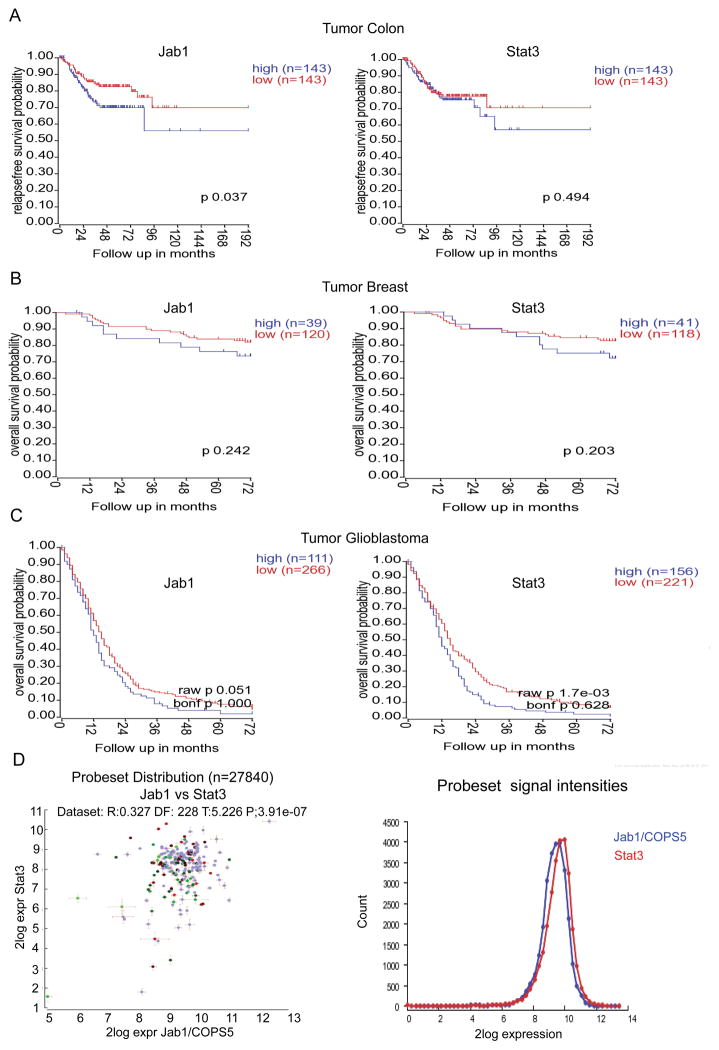
To determine whether the expression of both Stat3 and Jab1 are correlated with clinical parameters in cancer patients, we analyzed in vivo cDNA microarrays using data from patients with various cancers, including colon cancer, breast cancer, and glioblastoma, obtained from the R2 online data base. Compared with the patient cohorts characterized by low mRNA expression levels of both Stat3 and Jab1, those characterized by high mRNA expression levels of Stat3 or Jab1 had shorter survival times (Figure 6A, 6B and 6C). Furthermore, analyzing the two genes across datasets revealed that Stat3 was correlated with Jab1 (R=0.327, P=3.91e-07) (Figure 6D). The expression levels of Stat3 and Jab1 were directly correlated with patients’ survival durations. These findings suggest that Stat3 and Jab1 expression are potential prognostic biomarkers in various cancer types.
Figure 6. Correlation of the expression of Stat3 and Jab1 with the survival of patients with various cancers.
In vivo cDNA microarray data and Kaplan-Meier plots were used to assess correlations between Stat3 and Jab1 expression and patient survival. (A–C) Higher expression of Stat3 and Jab1 is associated with worse survival in patients with colon cancer (A), breast cancer (B), and glioblastoma (C). (D) Overview of the two genes across datasets.
Discussion
The present study yielded strong evidence that Stat3 overexpression mediates Jab1 overexpression in NPC oncogenesis. Our findings suggest that the expression of Stat3 and Jab1 could be used to predict the clinical outcomes of patients with NPC treated with cisplatin. To our knowledge, this is the first study of Stat3-regulated Jab1 expression in NPC to be reported.
Stat3 activation (p-Stat3 or nuclear Stat3 expression) or overexpression has been observed in NPC 26, 27 and has been clinically associated with advanced (stage III or IV) NPC 27. These findings, which are in agreement with the in vitro finding that Stat3 blockade inhibits NPC cell invasion 25, 28, suggest that Stat3 has a critical role in driving NPC progression and metastasis.
Our previous studies demonstrated that Jab1 is overexpressed and plays a critical role in the pathogenesis of NPC 9, 14, 15; however, the molecular basis of Jab1 overexpression in NPC remains undefined. In the current study, we found that Stat3 expression in NPC tissues was aberrant compared with that in noncancerous inflamed nasopharyngeal tissue and that that Stat3 protein is overexpressed in NPC cell lines but not in normal keratinocytes. These findings confirm those reported by Hsiao et al., who detected constitutive activation of Stat3 in 43 of 61 NPC specimens 29. Moreover, we found that nuclear Stat3 expression in NPC tissues was positively associated with nuclear Jab1 patterns (R = 0.371, P = 0.019). The clinical data suggest that Stat3 has a physiologic role in controlling Jab1 levels. Furthermore, we found that transfecting NPC cell lines with Stat3 plasmids significantly increased Jab1 levels. These results agree with those we previously obtained in breast cancer 30. In accordance with these data, our analysis of cDNA microarrays with known clinical parameters, including patients’ survival data, revealed that Stat3 was correlated with Jab1 and that their high expression levels were correlated with poor overall survival. Furthermore, our knockdown of endogenous Stat3 expression in NPC cell lines led to a significant decrease in Jab1 levels, and our luciferase reporter assays and ChIP assay analysis revealed a direct interaction between Stat3 and the Jab1 promoter. These results provide additional evidence that Stat3 controls Jab1 promoter activity in NPC.
Stat3 is a critical driver of cell growth in cancer 31, and constitutive Stat3 signaling has been implicated in aberrant cell growth and proliferation in many cancers, including head and neck squamous cell carcinoma and colorectal carcinoma 32–34. Constitutively active Stat3 also contributes to cell metastasis and invasion in human melanoma 35. Studies have demonstrated that enhanced Stat3 activation is associated with increased cell-cell contact or increased confluence, suggesting that Stat3 serves as a sensor of tumor cell contact and upregulates the genes necessary for cell invasion and migration 36, 37. In the present study, we also showed that loss of Stat3 inhibited NPC growth and invasion. Similarly, Lui et al. showed that JSI-124, a small-molecule inhibitor of JAK/Stat3, inhibited Stat3 activation in HONE-1-EBV cells and subsequently inhibited these cells’ growth and invasion 25. These findings suggest that Stat3 overexpression contributes to cancer cell proliferation and invasion.
Chemotherapy is the most common cancer treatment but does not always produce a response. Resistance to chemotherapy may result from the failure of the apoptosis pathways that are activated in response to drug treatment. Cancer cells with aberrant Stat3 activation have increased levels of antiapoptotic proteins (Mcl-1 and Bcl-xL) and cell cycle–regulating proteins (cyclin D1 and c-Myc) 38, 39. Therefore, cancer cells with aberrantly activated Stat3 are more resistant to chemotherapy-induced apoptosis. In the present study, we sought to determine whether Stat3 influences the antitumor effects of cisplatin and found that NPC cells with reduced Stat3 are more sensitive to cisplatin than control cells are both in vitro and in vivo. In addition, we found that the degree of apoptosis was higher in Stat3 knockdown NPC cells than in control cells. This finding is in agreement with the findings reported by Ji et al., who showed that knockdown of Stat3 by specific siRNA restored cisplatin sensitivity in ovarian cancer cells 40. Indeed, Studies have shown that Stat3 inhibitors have antitumor effects both in vitro and in vivo 41, 42. A growing number of preclinical studies conducted in numerous cancer types suggest that Stat3 is a valid therapeutic target 43. Our findings establish Stat3 as a potential marker for predicting the response of NPC to cisplatin, which could help to guide future treatment of NPC.
In conclusion, we found Stat3 to be overexpressed and correlated with Jab1 expression levels in NPC. Our findings that Stat3 positively regulates Jab1 and that Stat3 depletion sensitizes NPC cells to cisplatin suggest that assessing Stat3 and Jab1 levels in NPC patients will help predict the response of their disease to cisplatin treatment and enable the design of individualized treatment strategies for these patients.
Materials and Methods
Patients and Tissue Samples
Tissue samples were obtained in 2003 from patients at the Cancer Center of Sun Yat-Sen University, who have been described previously 14. The study group consisted of 45 NPC patients (36 men and 9 women), and the control group consisted of 30 patients (13 men and 17 women) with nasopharyngeal inflammation. Patients were selected for this study based on the availability of archived paraffin-embedded NPC and nasopharyngitis tissue blocks for immunohistochemical analysis. Surgical staging of tumors was done according to the American Joint Committee on Cancer tumor-nodes-metastasis system, and tumor grading was based on currently used histopathologic criteria. Ethics approval was given by the cancer center, and written informed consent was obtained from all patients.
Reagents
Cell culture media were purchased from Mediatech, Inc. (Manassas, VA), and fetal bovine serum (FBS) was obtained from Gibco (Grand Island, NY). Phosphate-buffered saline (PBS) and the chemiluminescent Western blotting substrate were from Thermo Scientific (Rockford, IL). Antibodies to the following proteins were used: Jab1 (Santa Cruz Biotechnology, Santa Cruz, CA, Cat#sc-13157); PARP (BD Pharmingen, San Diego, CA, Cat#556494); p-Stat3 and T-Stat3 (Cell Signaling Technology, Beverly, MA, Cat#9145 and Cat#12640); Myc-tag (Roche Applied Biosciences, Indianapolis, IN, Cat#11667149001); and Flag and β-actin (Sigma-Aldrich, St. Louis, MO, Cat#F3165 and Cat#A5441). The Lipofectamine Plus and Oligofectamine reagents were purchased from Invitrogen (Carlsbad, CA). The Annexin V/PI kit was purchased from BD Biosciences (Palo Alto, CA). The Cell Proliferation Kit was purchased from Roche.
Immunohistochemical Analysis
A total of 75 formalin-fixed, paraffin-embedded human specimens (45 primary NPC specimens and 30 noncancerous nasopharyngeal inflammation specimens) and a total of 9 paraffin-embedded tumor xenograft specimens from CNE-2 and HONE-1 athymic nude mice were analyzed. Briefly, the tumor xenograft specimens were deparaffinized and sectioned; the sections were then treated with 10 mM sodium citrate buffer (pH 6.0) for heat-induced antigen retrieval, immersed in 3% hydrogen peroxide solution to inhibit endogenous peroxidase activity, and then incubated in 5% bovine serum albumin to block nonspecific binding. The sections were then incubated with primary antibodies against Jab1, T-Stat3, and p-Stat3 at 4°C overnight, incubated with the biotinylated secondary antibody, and then subjected to the Liquid DAB+ Substrate Chromogen System (Dako, Carpinteria, CA) according to the manufacturer’s instructions. Stat3 and Jab1 protein expression levels were evaluated in at least 500 tumor cells (for NPC) or normal cells (for nasopharyngeal inflammation) in at least five representative high-power microscopy fields. For each specimen, protein staining in the nucleus and protein staining in the cytoplasm were scored separately and then combined as described previously 14. The proportion score represented the estimated fraction of positively stained cells (0 = less than 5%; 1 = 5% to 35%; 2 = greater than 35%). We defined the scores 0, 1, and 2 as negative (−), weakly positive (+), and highly positive (++), respectively.
Cell Cultures
The EBV-negative NPC cell lines CNE1, CNE2, and HONE1 and the EBV-positive NPC cell line C666.1 were cultured in RPMI medium containing 10% FBS and penicillin-streptomycin sulfate as described previously 14. Cells of the noncancerous human immortalized nasopharyngeal epithelial cell lines NP460 and NP69 were cultured in keratinocyte-SFM medium. Human embryonic kidney cells (293T cells) were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% FBS and penicillin-streptomycin. All cell lines were incubated at 37°C in a 5% CO2 atmosphere.
Cell Extracts and Immunoblotting
Cells in the log phase of growth were collected and lysed as described previously 14. Western blotting was then performed using antibodies against p-Stat3, T-Stat3, Jab1, Myc, and PARP. β-actin served as a control for protein load and integrity in all immunoblots. Protein bands were quantified using Image J software (National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij). PARP activity was recorded as the percentage of cleavage band intensity relative to total band intensity.
siRNA and DNA Transfection
NPC cells were transfected with Flag-Stat3-pcDNA3.1 and vector control plasmids using Lipofectamine Plus reagent as described previously 15. The negative siRNA control gene products and the siRNA targeting human Stat3 were obtained from Dharmacon (Lafayette, CO). shRNA targeting Stat3 was from Open Biosystems. Transient transfections of NPC cells were performed using the oligofectamine (Invitrogen) protocol and 5-nmol siRNAs in RPMI with 10% FBS and no penicillin or streptomycin as described previously 15.
Cell Viability Assay and Colony Formation Assay
The MTT assay was used to evaluate the proliferation of viable cells as described previously 15. The absorbance at 570 nm was read using an enzyme-linked immunosorbent assay microplate reader. For the colony-formation assay, NPC cells (400 cells/well) were plated in 6-well plates containing RPMI-1640 medium with 8% FBS. The following day, the cells were exposed to the indicated treatments. The NPC cells were grown at 37°C for 10 days. The cells were then stained with 0.1% crystal violet, and colonies (defined as 50 or more cells) were counted using an inverted microscope. Cell viability was calculated as the percentage of optical density (colony number) of the treatment group relative to the optical density (colony number) of the control group.
Cell Invasion Assay
A cell invasion assay was performed using a BD BioCoat Matrigel invasion chamber (Becton-Dickinson, Franklin Lakes, NJ). NPC cells were suspended in RPMI containing 1% FBS before incubation in the upper chamber at a density of 2000 cells/well. Cell invasion into Matrigel was determined after 12 hours of culture at 37°C. The cells invading the membrane were fixed with ice-cold methanol and stained with 0.01% crystal violet. After noninvading cells on the upper side of the membrane were removed with cotton swabs, cell invasion was quantified using an inverted-contrast microscope.
Flow Cytometry Analysis of Apoptosis
For dual annexin V–fluoroisothiocyanate (FITC) and PI staining, NPC cells were exposed to the indicated treatments and then labeled with annexin V–FITC and PI according to the manufacturer’s recommendations. Annexin V–FITC and PI binding was quantified using a FACScan flow cytometer (BD Biosciences).
Establishment of shRNA-Stable Cells
shRNA-stable cells were generated as described previously 15. Briefly, the packaging cell line 293T was cotransfected with Stat3 shRNA–vector DNA along with the helper vectors pLP and pVSVG using Lipofectamine Plus reagent. The supernatant was collected 48 hours after transfection, filtered through a 0.45-μm syringe filter, supplemented with 1.2 μg/ml Polybrene, and then used to infect the target cells. After 12 hours, the cell-containing medium was replaced with RPMI-1640 medium with 10% FBS. Following treatment with 0.8 μg/ml puromycin for 2 weeks, stable clones were selected. Positive clones were further confirmed with immunoblot analysis and maintained in 0.2 μg/ml puromycin.
Human Jab1 promoter and luciferase assay
Progressive 5′ deletion mutants of the pGL3-Jab1 promoter were constructed previously30. The following primers were used: −946F, −658F, −472F, −344F, −127F, and −59F.
NP460 and CNE1 cells were plated into 24-well plates overnight. Transfections were performed in triplicate according to the manufacturer’s protocol using Lipofectamine PLUS reagent. Briefly, 0.4 μg reporter plasmid Jab1-Luc (Firefly luciferase) together with 10 ng of pRL (Renilla luciferase) were cotransfected. Luciferase assays were performed 36 hours after transfection using a Dual-Luciferase Reporter Assay System (Promega, Madison, W, USA). Firefly and Renilla luciferase activities were read on a Monolight 3010 luminometer (BD Biosciences, Rockville, MD). Firefly luciferase activity was normalized to Renilla luciferase readings in each well. Each experiment was conducted in triplicate.
ChIP Assay
A ChIP assay (EMD Millipore, Billerica, MA) was performed according to the manufacturer’s instructions. Immunoprecipitations were performed with antibodies against Stat3 and immunoglobulin G (control), and the immunoprecipitated DNA was analyzed with polymerase chain reaction (PCR). The ChIP primers used to analyze protein binding were −611F (forward), 5′-CCATCTGCAAGTGAAGTGCC-3′, and −495R (reverse), 5′-CTGGAAGCTGGTTGAGAGGA-3′; and control primers were −90F (forward), 5′-ACCAACTTCACCTCCGGTTC-3′, and −38R (reverse), 5′-GCACCACGGGAACAAACTCT-3′.
Tumorigenicity Assay in Nude Mice
Four-week-old femal athymic nude (nu/nu) mice obtained from the National Cancer Institute received subcutaneous injections of 5 × 106 NPC cells with stable knockdown of Stat3 (sh-Stat3) or control vector (sh-Cont) in the right flank (five mice per group). Mice were checked every 2 days for xenograft development and were randomly divided into control group and cisplatin treated group. Treatment—intraperitoneal injections of 5 mg/kg cisplatin once every 2 days—was started after the tumors became palpable (about 0.1 mm3). Tumor volumes and mice’s body weights were measured twice weekly. Tumor volume (in cubic millimeters) was calculated as tumor length × tumor width2/2. The percentage of tumor growth inhibition, used to evaluate the tumor’s response to the drugs, was calculated as 100% × (1 – the average tumor weight of treated mice/the average tumor weight of control mice). At the end of the experiments, the mice were humanely killed under general anesthesia; the tumors were excised and weighed, and necropsies were performed. All animal experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center.
Analysis of Clinical cDNA Microarrays for the Detection of Correlations between Stat3, Jab1, and Patient Survival
The percentages of NPC samples with Jab1/COPS5 amplification were 4%. The processed copy number data were downloaded from cBioPortal (www.cbioportal.org) using the R package cgdsr.
Transcriptome data from patient samples of various cancers were analyzed using the free online database R2: microarray analysis and visualization platform (http://r2.amc.nl) to determine whether the expression of Stat3 and/or Jab1 is correlated with the cancer patients’ overall survival.
Statistical Analysis
Descriptive data are expressed as means ± standard deviations; a one-way analysis of variance or the chi-square test was used to determine whether the distributions of categorical variables differed. A stratified survival analysis was performed using the Kaplan-Meier method followed by the log-rank test. Differences between groups were considered statistically significant if P was < 0.05. Calculations were performed using SPSS 16.0 (SPSS, Chicago, IL). In addition, some of the data were analyzed using GraphPad Prism 6 (GraphPad Software).
Supplementary Material
Acknowledgments
The authors thank Dr Kwok-Wai Lo, The Chinese University of Hong Kong for providing EBV-positive NPC cell line C666.1. We thank Markeda Wade in the Department of Scientific Publications at MD Anderson Cancer Center for editing the manuscript. This study was supported by in part by grants from the National Natural Science Foundation of China (81372816), Fundamental Research Funds for the Central Universities (JUSRP115A31), NIH grant R01CA90853, University Cancer Foundation via the Sister Institution Network Fund of The University of Texas MD Anderson Cancer Center and by the NIH award P30CA016672.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- 1.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 2.Spano JP, Busson P, Atlan D, Bourhis J, Pignon JP, Esteban C, et al. Nasopharyngeal carcinomas: an update. European journal of cancer. 2003;39:2121–2135. doi: 10.1016/s0959-8049(03)00367-8. [DOI] [PubMed] [Google Scholar]
- 3.Ong YK, Heng DM, Chung B, Leong SS, Wee J, Fong KW, et al. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. European journal of cancer. 2003;39:1535–1541. doi: 10.1016/s0959-8049(03)00310-1. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 5.Cheng SH, Jian JJ, Tsai SY, Yen KL, Chu NM, Chan KY, et al. Long-term survival of nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy. International journal of radiation oncology, biology, physics. 2000;48:1323–1330. doi: 10.1016/s0360-3016(00)00779-3. [DOI] [PubMed] [Google Scholar]
- 6.Claret FX, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383:453–457. doi: 10.1038/383453a0. [DOI] [PubMed] [Google Scholar]
- 7.Shackleford TJ, Claret FX. JAB1/CSN5: a new player in cell cycle control and cancer. Cell division. 2010;5:26. doi: 10.1186/1747-1028-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan Y, Yang H, Claret FX. Emerging roles of Jab1/CSN5 in DNA damage response, DNA repair, and cancer. Cancer biology & therapy. 2014;15:256–262. doi: 10.4161/cbt.27823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan Y, Claret FX. Targeting Jab1/CSN5 in nasopharyngeal carcinoma. Cancer letters. 2012;326:155–160. doi: 10.1016/j.canlet.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouvaraki MA, Rassidakis GZ, Tian L, Kumar R, Kittas C, Claret FX. Jun activation domain-binding protein 1 expression in breast cancer inversely correlates with the cell cycle inhibitor p27(Kip1) Cancer research. 2003;63:2977–2981. [PubMed] [Google Scholar]
- 11.Rassidakis GZ, Claret FX, Lai R, Zhang Q, Sarris AH, McDonnell TJ, et al. Expression of p27(Kip1) and c-Jun activation binding protein 1 are inversely correlated in systemic anaplastic large cell lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:1121–1128. [PubMed] [Google Scholar]
- 12.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tai Y, et al. Jab1 expression is associated with inverse expression of p27(kip1) and poor prognosis in epithelial ovarian tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2001;7:4130–4135. [PubMed] [Google Scholar]
- 13.Kouvaraki MA, Korapati AL, Rassidakis GZ, Tian L, Zhang Q, Chiao P, et al. Potential role of Jun activation domain-binding protein 1 as a negative regulator of p27kip1 in pancreatic adenocarcinoma. Cancer research. 2006;66:8581–8589. doi: 10.1158/0008-5472.CAN-06-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Y, Zhang Q, Tian L, Wang X, Fan X, Zhang H, et al. Jab1/CSN5 negatively regulates p27 and plays a role in the pathogenesis of nasopharyngeal carcinoma. Cancer research. 2012;72:1890–1900. doi: 10.1158/0008-5472.CAN-11-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan Y, Zhang Q, Atsaves V, Yang H, Claret FX. Suppression of Jab1/CSN5 induces radio- and chemo-sensitivity in nasopharyngeal carcinoma through changes to the DNA damage and repair pathways. Oncogene. 2013;32:2756–2766. doi: 10.1038/onc.2012.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nature reviews Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 17.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8:945–954. [PubMed] [Google Scholar]
- 18.Real PJ, Sierra A, De Juan A, Segovia JC, Lopez-Vega JM, Fernandez-Luna JL. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene. 2002;21:7611–7618. doi: 10.1038/sj.onc.1206004. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nature medicine. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 20.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 21.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 22.Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 23.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 24.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 25.Lui VW, Wong EY, Ho Y, Hong B, Wong SC, Tao Q, et al. STAT3 activation contributes directly to Epstein-Barr virus-mediated invasiveness of nasopharyngeal cancer cells in vitro. International journal of cancer Journal international du cancer. 2009;125:1884–1893. doi: 10.1002/ijc.24567. [DOI] [PubMed] [Google Scholar]
- 26.Lo AK, Lo KW, Tsao SW, Wong HL, Hui JW, To KF, et al. Epstein-Barr virus infection alters cellular signal cascades in human nasopharyngeal epithelial cells. Neoplasia. 2006;8:173–180. doi: 10.1593/neo.05625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu YP, Tan YN, Wang ZL, Zeng L, Lu ZX, Li LL, et al. Phosphorylation and nuclear translocation of STAT3 regulated by the Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma. International journal of molecular medicine. 2008;21:153–162. [PubMed] [Google Scholar]
- 28.Lui VW, Yau DM, Wong EY, Ng YK, Lau CP, Ho Y, et al. Cucurbitacin I elicits anoikis sensitization, inhibits cellular invasion and in vivo tumor formation ability of nasopharyngeal carcinoma cells. Carcinogenesis. 2009;30:2085–2094. doi: 10.1093/carcin/bgp253. [DOI] [PubMed] [Google Scholar]
- 29.Hsiao JR, Jin YT, Tsai ST, Shiau AL, Wu CL, Su WC. Constitutive activation of STAT3 and STAT5 is present in the majority of nasopharyngeal carcinoma and correlates with better prognosis. British journal of cancer. 2003;89:344–349. doi: 10.1038/sj.bjc.6601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shackleford TJ, Zhang Q, Tian L, Vu TT, Korapati AL, Baumgartner AM, et al. Stat3 and CCAAT/enhancer binding protein beta (C/EBP-beta) regulate Jab1/CSN5 expression in mammary carcinoma cells. Breast cancer research: BCR. 2011;13:R65. doi: 10.1186/bcr2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlessinger K, Levy DE. Malignant transformation but not normal cell growth depends on signal transducer and activator of transcription 3. Cancer research. 2005;65:5828–5834. doi: 10.1158/0008-5472.CAN-05-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. The Journal of clinical investigation. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 34.Weerasinghe P, Garcia GE, Zhu Q, Yuan P, Feng L, Mao L, et al. Inhibition of Stat3 activation and tumor growth suppression of non-small cell lung cancer by G-quartet oligonucleotides. International journal of oncology. 2007;31:129–136. [PubMed] [Google Scholar]
- 35.Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer research. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 36.Steinman RA, Wentzel A, Lu Y, Stehle C, Grandis JR. Activation of Stat3 by cell confluence reveals negative regulation of Stat3 by cdk2. Oncogene. 2003;22:3608–3615. doi: 10.1038/sj.onc.1206523. [DOI] [PubMed] [Google Scholar]
- 37.Vultur A, Cao J, Arulanandam R, Turkson J, Jove R, Greer P, et al. Cell-to-cell adhesion modulates Stat3 activity in normal and breast carcinoma cells. Oncogene. 2004;23:2600–2616. doi: 10.1038/sj.onc.1207378. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher S, Drewry JA, Shahani VM, Page BD, Gunning PT. Molecular disruption of oncogenic signal transducer and activator of transcription 3 (STAT3) protein. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2009;87:825–833. doi: 10.1139/o09-044. [DOI] [PubMed] [Google Scholar]
- 39.Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anti-cancer drugs. 2005;16:601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Ji T, Gong D, Han Z, Wei X, Yan Y, Ye F, et al. Abrogation of constitutive Stat3 activity circumvents cisplatin resistant ovarian cancer. Cancer letters. 2013;341:231–239. doi: 10.1016/j.canlet.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Adachi M, Cui C, Dodge CT, Bhayani MK, Lai SY. Targeting STAT3 inhibits growth and enhances radiosensitivity in head and neck squamous cell carcinoma. Oral oncology. 2012;48:1220–1226. doi: 10.1016/j.oraloncology.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan Y, Zhou F, Zhang R, Claret FX. Stat3 inhibitor Stattic exhibits potent antitumor activity and induces chemo- and radio-sensitivity in nasopharyngeal carcinoma. PloS one. 2013;8:e54565. doi: 10.1371/journal.pone.0054565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page BD, Ball DP, Gunning PT. Signal transducer and activator of transcription 3 inhibitors: a patent review. Expert opinion on therapeutic patents. 2011;21:65–83. doi: 10.1517/13543776.2011.539205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.