Abstract
AIM
To evaluate the feasibility of chemotherapy including fluoropyrimidine, platinum and taxane with modified dosages for unresectable gastric cancer in Japanese patients.
METHODS
We performed a feasibility study of a modified docetaxel, cisplatin and capecitabine (DCX) regimen for stage IV gastric cancer. In particular, 30 or 40 mg/m2 of docetaxel on day 1, 60 mg/m2 of cisplatin on day 1, and 2000 mg/m2 of capecitabine for 2 wk were administered every three weeks.
RESULTS
Three patients were treated with modified DCX (mDCX) with 30 mg/m2 docetaxel, and five patients were treated with this regimen with 40 mg/m2 docetaxel. Grade 3 or 4 neutropenia was observed in six of the eight patients; no patients exhibited febrile neutropenia. Partial response was achieved in four of the eight patients. Three patients underwent gastrectomy, which achieved R0 resection without residual tumors in dissected lymph nodes. In one of these three patients, resected specimens revealed pathological complete response in the primary lesion and in lymph nodes.
CONCLUSION
mDCX was well tolerated by Japanese patients with stage IV gastric cancer. This regimen might be useful for allowing gastric cancer patients with distant lymph node metastasis to undergo conversion surgery.
Keywords: Docetaxel, Cisplatin, Capecitabine, Gastric cancer
Core tip: A combination of fluoropyrimidine and platinum is a standard treatment for unresectable gastric cancer. Although the addition of a taxane to this doublet is expected to improve effectiveness, research has demonstrated that such triplet regimens often cause adverse effects, including neutropenia. To reduce adverse events but maintain therapeutic effectiveness, we devised a triplet regimen with modified dosages. Modified docetaxel, cisplatin and capecitabine treatment was safe and effective for stage IV gastric cancer. Three of the eight treated patients underwent conversion surgery and achieved long-term survival without recurrence.
INTRODUCTION
The prognosis of stage IV gastric cancer is poor, and the median overall survival time is approximately one year. The standard treatment for Stage IV gastric cancer is chemotherapy with agents such as fluoropyrimidines and platinum compounds. In Japan, the oral fluoropyrimidine S-1 plus cisplatin (SP) is the standard regimen for HER2-negative advanced gastric cancer because SP was proven to be superior to S-1 alone in a phase III randomized trial[1]. Because capecitabine, similarly to S-1, is an effective oral fluoropyrimidine, capecitabine plus cisplatin (XP) is also a possible regimen[2].
The addition of docetaxel to fluoropyrimidine and cisplatin was expected to improve therapeutic efficacy. A combination of docetaxel, cisplatin and 5-fluorouracil (DCF) produced longer overall survival than cisplatin plus 5-fluorouracil; however, the use of DCF is limited due to severe side effects, including hematologic toxicity[3]. Various modified DCF regimens have been tested in attempts to improve tolerability without losing efficacy[4-8]. Research has also examined regimens that replace the infusion of 5-fluorouracil with an oral fluoropyrimidine, such as docetaxel, cisplatin and S-1 (DCS)[9] and docetaxel, cisplatin and capecitabine (DCX)[10]. Although DCX has been reported to be effective for unresectable gastric cancer, this regimen often causes adverse events, including hematologic toxicities[10-12]. We believed that a modification of the doses used for DCX might reduce toxicity but maintain effectiveness. In previous reports, doses of docetaxel used for DCX ranged from 60 mg/m2 to 75 mg/m2[10-12]. In certain studies of DCS, the dose of docetaxel was set to 30-40 mg/m2, and good effectiveness and adequate safety were achieved[13-15]. In the present study, we set the dose of docetaxel to 30 or 40 mg/m2, which was a lower dose than that used in previous reports on DCX, and evaluated the safety and efficacy of our modified DCX (mDCX) regimen in Japanese patients.
MATERIALS AND METHODS
Patient eligibility
The eligibility criteria included stage IV unresectable HER2-negative gastric cancer, an age of 20-75 years, Eastern Cooperative Oncology Group performance status 0-1, conserved organ functions, and no prior chemotherapy.
Treatment
The treatment regimen, which consisted of 1000 mg/m2 capecitabine twice per day on days 1-14, 60 mg/m2 cisplatin on day 1 and 30 or 40 mg/m2 docetaxel on day 1, was administered every three weeks. The dosage of docetaxel was 30 mg/m2 for the first three patients and was planned to increase to 40 mg/m2 for subsequent patients if no dose-limiting toxicities (DLTs) were observed after the first three patients' first treatment cycle. The treatment was continued until the disease progressed, patients experienced intolerable side effects, or curative resection was expected.
Treatment was interrupted if a patient developed grade ≥ 3 hematologic toxicity. If a patient experienced grade 4 neutropenia for more than 5 d or grade 3 febrile neutropenia, the dosage of all agents was decreased to 75% for the next course. If a patient exhibited grade 4 thrombocytopenia, dosages of all agents were decreased to 50%. If a patient had grade ≥ 2 diarrhea and/or grade ≥ 2 hand-foot syndrome, the treatment course was interrupted. If creatinine clearance (Ccr) was < 60 mL/min and ≥ 50 mL/min, cisplatin was decreased to 75%. If Ccr was < 50 mL/min and ≥ 40 mL/min, cisplatin was decreased to 50%. If Ccr was < 40 mL/min, the treatment protocol was terminated. Supportive treatment, including G-CSF and anti-emetics, was permitted.
Safety and anti-tumor activity assessments
Adverse events were assessed using the National Cancer Institute's CTCAE v4.0. DLTs were defined as adverse events that occurred after the beginning of the first cycle and before the beginning of the second cycle that satisfied any of the following criteria: (1) non-hematologic toxicities ≥ grade 3 that did not resolve to grade 0 or grade 1 within two consecutive days, except for nausea, vomiting, anorexia and asymptomatic electrolyte imbalance; (2) neutropenia ≥ grade 3 for > 5 consecutive days; (3) febrile neutropenia (absolute neutrophil count < 1.0 × 109/L and fever ≥ 38 °C); (4) grade 4 thrombocytopenia or platelet transfusion; or (5) delay of the treatment cycle for > 2 wk.
Radiological tumor assessments were conducted using computed tomography every eight weeks in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.
RESULTS
Three patients received mDCX with 30 mg/m2 of docetaxel. Because no DLTs were observed in the first three patients, treatment with 40 mg/m2 docetaxel was administered to five subsequent patients.
The patients' characteristics and clinical courses are summarized in Table 1, and adverse events are summarized in Table 2. No DLTs were observed after the first treatment cycles in any patient. The relative dose intensities of DCX were 90.6%, 90.0% and 76.2%, respectively. Four of the eight patients exhibited a partial response (PR). Three patients (cases 2, 5 and 8) underwent gastrectomy with lymph node dissection; for all of these patients, R0 resection was achieved, and no viable tumors were detected in resected lymph nodes (ypN0). Case 2 achieved pathological complete response in both the primary lesion and lymph node metastasis. Regarding case 5, the observed therapeutic effect was grade 2, and she received capecitabine for ten weeks as adjuvant chemotherapy. The clinical courses of three representative cases are presented below.
Table 1.
Patient characteristics and prognoses
| Age | Sex | Macroscopic type | Histopathology | Metastasis | Number of courses | Objective tumor response | Prognosis (mo) | Conversion surgery | ||
| 1 | 64 | M | 3 | tub2/por | Liver, LNs | 7 | PR | 21.9 | Dead | |
| 2 | 59 | M | 3 | tub2/por | LNs | 5 | PR | 50.9 | Alive | Yes |
| 3 | 62 | M | 2 | por | Liver, LNs | 6 | SD | 7.4 | Dead | |
| 4 | 65 | M | 3 | por | LNs | 5 | PR | 7.7 | Dead | |
| 5 | 67 | F | 3 | tub2/por | LNs | 3 | non-CR/non-PD | 31.3 | Alive | Yes |
| 6 | 66 | M | 3 | por | LNs | 4 | non-CR/non-PD | 12.0 | Dead | |
| 7 | 62 | M | 2 | por | Liver, LNs | 3 | SD | 5.4 | Dead | |
| 8 | 63 | F | 2 | tub2/por | LNs | 4 | PR | 24.4 | Alive | Yes |
tub2: Moderately differentiated tubular adenocarcinoma; por: Differentiated adenocarcinoma; LNs: Lymph nodes; PR: Partial response; SD: Stable disease; CR: Complete response.
Table 2.
Hematologic and non-hematologic adverse events n (%)
| Any grade | Grade 3 | Grade 4 | |
| Leukopenia | 7 (87.5) | 2 (25) | 0 |
| Neutropenia | 7 (87.5) | 4 (50) | 2 (25) |
| Anemia | 6 (75) | 1 (12.5) | 0 |
| Thrombocytopenia | 7 (87.5) | 0 | 0 |
| Hyperbilirubinemia | 3 (37.5) | 0 | 0 |
| Elevated serum aspartate aminotransferase | 6 (75) | 0 | 0 |
| Elevated serum alanine aminotransferase | 8 (100) | 0 | 0 |
| Elevated serum creatinine | 3 (37.5) | 0 | 0 |
| Fever | 4 (50) | 0 | 0 |
| Fatigue | 2 (25) | 0 | 0 |
| Alopecia | 1 (12.5) | 0 | 0 |
| Skin rash | 1 (12.5) | 0 | 0 |
| Anorexia | 7 (87.5) | 4 (50) | 0 |
| Diarrhea | 4 (50) | 2 (25) | 0 |
| Nausea | 3 (37.5) | 0 | 0 |
| Vomiting | 3 (37.5) | 0 | 0 |
| Constipation | 1 (12.5) | 0 | 0 |
| Peripheral neuropathy | 1 (12.5) | 0 | 0 |
| Infection | 1 (12.5) | 1 (12.5) | 0 |
Case 1
A 64-year-old man had type III advanced cancer in the fornix of the stomach and multiple liver metastases (Figure 1). Biopsy specimens were pathologically diagnosed as moderately and poorly differentiated adenocarcinoma. The patient received three courses of mDCX, and the liver metastases shrank, a phenomenon judged to be PR. After five courses, PR was confirmed. After seven courses, shrinkage of the liver metastases was sustained. The treatment protocol was discontinued due to grade 2 sensory peripheral neuropathy. Other adverse events were grade 2 anemia, grade 1 aspartate aminotransferase elevation, grade 1 alanine aminotransferase elevation, and grade 1 anorexia. The patient received post-protocol treatment that included irinotecan and weekly paclitaxel. He died 22 mo after enrollment in the study.
Figure 1.
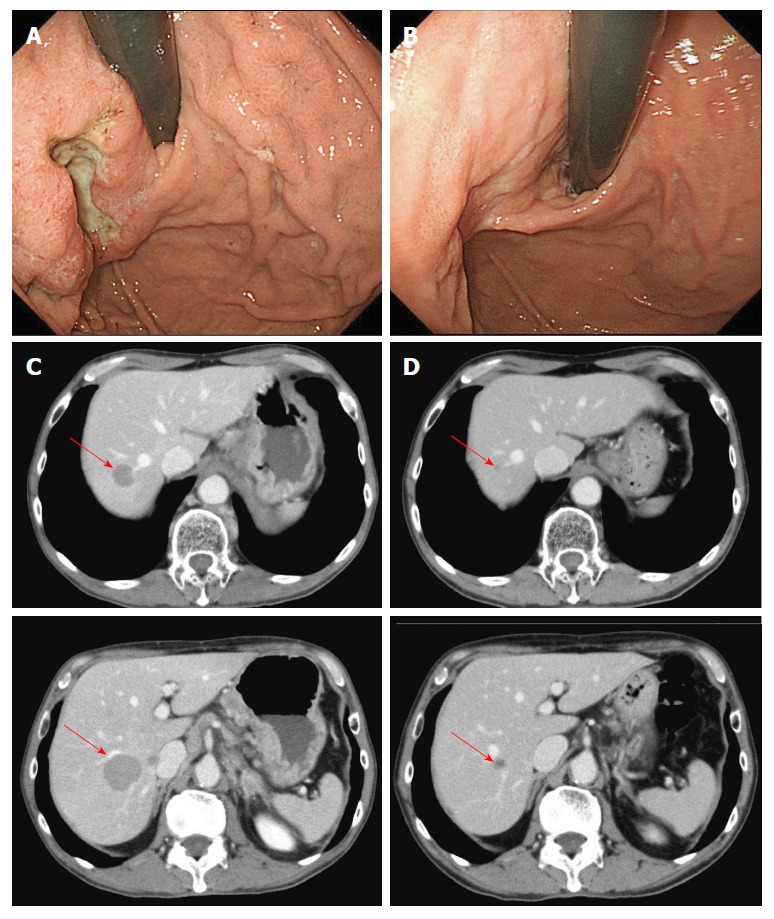
Case 1. Endoscopic findings (A, B) and computed tomography images (C, D) before (A, C) and after (B, D) seven courses of modified docetaxel, cisplatin and capecitabine (DCX) (mDCX).
Case 2
A 59-year-old man had type III advanced cancer at the small curvature of the angulus with lymph node metastasis along the superior mesenteric artery (#14a; Figure 2). Biopsy specimens were pathologically diagnosed as moderately and poorly differentiated adenocarcinoma. After three courses of mDCX, the lymph node metastasis shrank; after five courses, PR was confirmed. Adverse events included grade 2 leukopenia, grade 3 neutropenia, and grade 2 anemia. The patient underwent subtotal gastrectomy with lymph node dissection. Pathological findings revealed no residual carcinoma, and the observed therapeutic effect was grade 3. He received S-1 for one year as adjuvant chemotherapy. He remains alive without any findings indicative of recurrence four years after enrollment.
Figure 2.
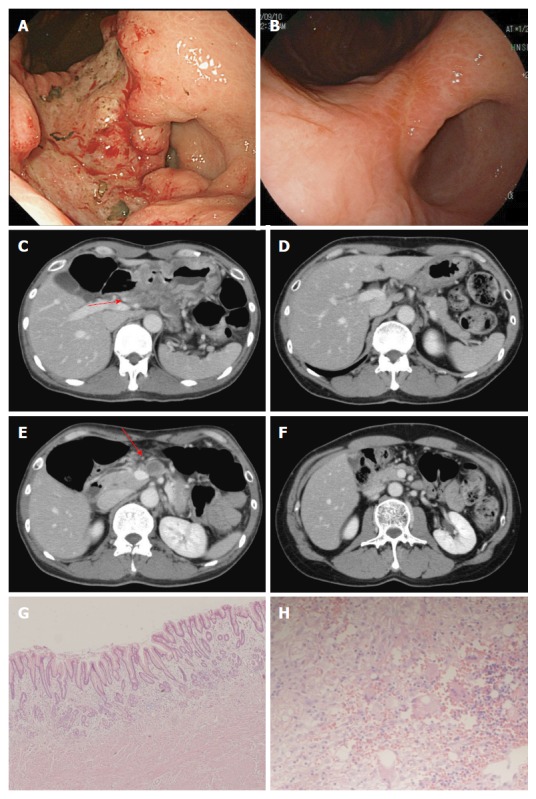
Case 2. Endoscopic findings (A, B) and computed tomography images (C, D, E, F) before treatment (A, C, E) and after (B, D, F) five courses of modified docetaxel, cisplatin and capecitabine (DCX) (mDCX). The primary lesion (A, B), swollen lymph nodes along the common hepatic artery (#8) (C, D) and lymph nodes along the superior mesenteric artery (#14a) (E, F) markedly shrank. Microscopic findings for the resected specimens of the primary lesion (E, magnification × 100) and lymph nodes (F, magnification × 400) revealed no residual tumor.
Case 8
A 63-year-old woman had type III advanced cancer at the gastric antrum with lymph node metastasis that included left supraclavicular lymph nodes and para-aortic lymph nodes (Figure 3). Biopsy specimens were pathologically diagnosed as moderately and poorly differentiated adenocarcinoma. After two courses of mDCX, the lymph nodes shrank; after four courses, PR was confirmed. Adverse events included grade 2 leukopenia, grade 4 neutropenia, grade 3 anorexia, and grade 3 diarrhea. Because the patient's lymph node metastasis became undetectable by computed tomography, she underwent subtotal gastrectomy with D2 dissection; the para-aortic lymph nodes were not dissected. Pathological findings revealed no residual tumors in the lymph nodes, and the observed therapeutic effect was grade 1b. As adjuvant chemotherapy, she underwent two courses of capecitabine plus oxaliplatin which were discontinued due to grade 2 nausea and fatigue. She subsequently received S-1 for one year. The patient remains alive without any findings indicative of recurrence two years after enrollment.
Figure 3.
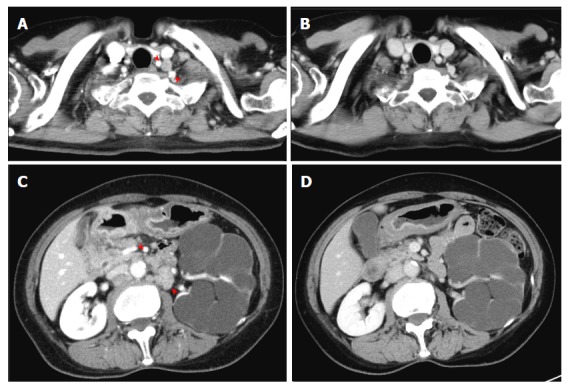
Case 8. Computed tomography images before (A, C) and after (B, D) four courses of modified docetaxel, cisplatin and capecitabine (DCX) (mDCX). Left supraclavicular nodes (A, B) and para-aortic nodes (C, D) became undetectable.
DISCUSSION
Reports have described the effectiveness of DCX for unresectable gastric cancer[10,16], as preoperative chemotherapy[17], as adjuvant chemotherapy[18] and as perioperative chemotherapy[12]. These reports indicate that DCX is highly effective but also rather toxic, particularly in hematologic respects. To our knowledge, no prior reports have described a DCX trial in Japan.
In the present study, six of the eight patients (75%) experienced grade 3 or grade 4 neutropenia, although no patients experienced febrile neutropenia. Although the modifications to the DCX regimen decreased the dose of docetaxel to 30 or 40 mg/m2, the frequency of hematologic toxicity in this study was similar to those reported in prior studies of DCX (neutropenia ≥ grade 3, 40%-76.5%; Table 3). However, we regarded the observed side effects as tolerable because no DLT was observed after the first cycle, and all patients were able to receive three or more courses of treatment with appropriate supportive care. In addition, the median admission period was five days (data not shown), indicating that for the most part, admission was primarily necessary for hydration due to the administration of cisplatin.
Table 3.
Previous reports regarding docetaxel, cisplatin and capecitabine
| Ref. | Setting | Capecitabine | Cisplatin | Docetaxel | Interval (d) | Number of patients | Grade 3-4 neutropenia | Febrile neutropenia | RR (95%CI) | PFS (95%CI) | OS (95%CI) | R0 resection | pCR | |
| (mo) | (mo) | |||||||||||||
| Kang et al[10], 2010 | Metastatic or recurrent | 1875 mg, days 1-14 | 60 mg, day 1 | 60 mg, day 1 | 21 | 40 | 62.5% | 10% | 68% (53%-83%) | 7.6 (6.9-8.4) | 14.4 (7.3-21.5) | 10.0% | ||
| Sym et al[17], 2010 | Neoadjuvant | 1875 mg, days 1-14 | 60 mg, day 1 | 60 mg, day 1 | 21 | 49 (36 resected cases) | 69% | 4% | R0: 54.3 (0-112.9) | R0: not reached | 63.0% | |||
| Non-R0: 5.1 (3.6-6.6) | Non-R0: 11.5 (7.3-15.7) | |||||||||||||
| Thuss-Patience et al[12], 2012 | Perioperative | 1875 mg, days 1-14 | 60 mg, day 1 | 75 mg, day1 | 21 | 51 | Preoperative | 76.5% | 21.5% | 90.2% | 13.7% | |||
| Postoperative | 62.9% | 11.1% | ||||||||||||
| Polyzos et al[16], 2012 | Metastatic | 2000 mg, days 2-15 | 60 mg, day 1 | 60 mg, day 1 | 21 | 36 | 50% | 16% | 44.4% (28%-60%) | 5 (3-6)1 | 12 (5-24) | |||
| Yoon et al[18], 2015 | Adjuvant for stage IIIB-IV | 1875 mg, days 1-14 | 60 mg, day 1 | 60 mg, day 1 | 21 | 46 | 40% | 15% | 26.9 (7.5-46.4)2 | 43.9 (29.2-58.7) |
Time to progression;
Relapse-free survival. RR: Response rate; PFS: Progression-free survival; OS: Overall survival; pCR: Pathological complete response.
Three of the five patients with only distant lymph node metastasis underwent conversion surgery, and all three patients have remained alive for more than two years without recurrence. Therefore, we believe that intensive treatment with a triplet regimen could be a useful preoperative treatment that enables conversion surgery for patients with distant lymph node metastasis. However, in certain cases, survival time was shorter than one year; in particular, one case died 7.7 mo after the start of treatment, although PR was achieved. It is necessary to identify certain biomarkers to select patients suitable for a triplet regimen. Furthermore, a randomized control study is needed to evaluate whether the proposed triplet regimen is superior to a standard platinum and oral fluoropyrimidine doublet.
In conclusion, mDCX is safe and effective for Stage IV gastric cancer in Japanese patients.
COMMENTS
Background
For stage IV gastric cancer, doublet regimens including fluoropyrimidine and platinum agents are standard chemotherapy. The addition of taxanes to the doublet regimen may improve effectiveness but may also increase toxicity.
Research frontiers
The addition of a smaller amount of docetaxel than previously reported to capecitabine and cisplatin was evaluated.
Innovations and breakthroughs
The authors set the dose of docetaxel to 30 or 40 mg/m2, which was a lower dose than used in previous reports on docetaxel, cisplatin and capecitabine (DCX).
Applications
Intensive treatment with a triplet regimen could be a useful preoperative treatment that allows for conversion surgery in patients with distant lymph node metastasis.
Terminology
DCX: A combination chemotherapy regimen including docetaxel, cisplatin and capecitabine. Conversion surgery: Surgical operation for patients with cancer that was unresectable before chemotherapy and became to be resectable after chemotherapy.
Peer-review
The authors designed the dose of docetaxel to 30 or 40 mg/m2, which was a lower dose than used in previous reports on DCX, and evaluated the safety and efficacy.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was approved by the review boards of Nagoya University Hospital.
Clinical trial registration statement: This study was registered at http://www.umin.ac.jp/ctr/index.htm, registration identification number is UMIN000006009.
Informed consent statement: All patients provided written informed consent.
Conflict-of-interest statement: Kodera Y has received research funding from Chugai Pharmaceutical Co., Ltd., Sanofi, Yakult Honsha Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer Inc., and Bristol-Myers Squib. Goto H has received research funding from Bristol-Myers Squibb, and Takeda Pharmaceutical Co., Ltd. Ando Y has received research funding from Sanofi, Chugai Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Yakult Honsya Co., Ltd., and Mochida Pharmaceutical Co., Ltd.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 24, 2016
First decision: October 28, 2016
Article in press: January 17, 2017
P- Reviewer: Arigami T, Chuang SM S- Editor: Yu J L- Editor: A E- Editor: Liu WX
References
- 1.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 2.Tsuburaya A, Morita S, Kodera Y, Kobayashi M, Shitara K, Yamaguchi K, Yoshikawa T, Yoshida K, Yoshino S, Sakamoto J. A randomized phase II trial to elucidate the efficacy of capecitabine plus cisplatin (XP) and S-1 plus cisplatin (SP) as a first-line treatment for advanced gastric cancer: XP ascertainment vs. SP randomized PII trial (XParTS II) BMC Cancer. 2012;12:307. doi: 10.1186/1471-2407-12-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 4.Ozdemir NY, Abali H, Oksüzoğlu B, Budakoglu B, Uncu D, Güler T, Odabaşi H, Zengin N. The efficacy and safety of reduced-dose docetaxel, cisplatin, and 5-fluorouracil in the first-line treatment of advanced stage gastric adenocarcinoma. Med Oncol. 2010;27:680–684. doi: 10.1007/s12032-009-9268-y. [DOI] [PubMed] [Google Scholar]
- 5.Keskin S, Yıldız I, Sen F, Aydogan F, Kilic L, Ekenel M, Saglam S, Sakar B, Disci R, Aykan F. Modified DCF (mDCF) regimen seems to be as effective as original DCF in advanced gastric cancer (AGC) Clin Transl Oncol. 2013;15:403–408. doi: 10.1007/s12094-012-0942-8. [DOI] [PubMed] [Google Scholar]
- 6.Koca D, Dogan E, Yardim H, Duzen O, Karaca S. A modified DCF regimen as primary treatment for patients with metastatic gastric cancer. J BUON. 2013;18:377–384. [PubMed] [Google Scholar]
- 7.Shah MA, Janjigian YY, Stoller R, Shibata S, Kemeny M, Krishnamurthi S, Su YB, Ocean A, Capanu M, Mehrotra B, et al. Randomized Multicenter Phase II Study of Modified Docetaxel, Cisplatin, and Fluorouracil (DCF) Versus DCF Plus Growth Factor Support in Patients With Metastatic Gastric Adenocarcinoma: A Study of the US Gastric Cancer Consortium. J Clin Oncol. 2015;33:3874–3879. doi: 10.1200/JCO.2015.60.7465. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Xu R, Li J, Bai Y, Liu T, Jiao S, Dai G, Xu J, Liu Y, Fan N, et al. Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. 2016;19:234–244. doi: 10.1007/s10120-015-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato Y, Takayama T, Sagawa T, Takahashi Y, Ohnuma H, Okubo S, Shintani N, Tanaka S, Kida M, Sato Y, et al. Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother Pharmacol. 2010;66:721–728. doi: 10.1007/s00280-009-1215-2. [DOI] [PubMed] [Google Scholar]
- 10.Kang YK, Ryu MH, Yoo C, Chang HM, Yook JH, Oh ST, Kim BS, Kim TW. Phase I/II study of a combination of docetaxel, capecitabine, and cisplatin (DXP) as first-line chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2011;67:1435–1443. doi: 10.1007/s00280-010-1444-4. [DOI] [PubMed] [Google Scholar]
- 11.Sym SJ, Ryu MH, Kang HJ, Lee SS, Chang HM, Lee JL, Kim TW, Yook JH, Oh ST, Kim BS, et al. Phase I study of 3-weekly docetaxel, capecitabine and oxaliplatin combination chemotherapy in patients with previously untreated advanced gastric cancer. Cancer Chemother Pharmacol. 2010;66:373–380. doi: 10.1007/s00280-009-1171-x. [DOI] [PubMed] [Google Scholar]
- 12.Thuss-Patience PC, Hofheinz RD, Arnold D, Florschütz A, Daum S, Kretzschmar A, Mantovani-Löffler L, Bichev D, Breithaupt K, Kneba M, et al. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO){dagger} Ann Oncol. 2012;23:2827–2834. doi: 10.1093/annonc/mds129. [DOI] [PubMed] [Google Scholar]
- 13.Fushida S, Fujimura T, Oyama K, Yagi Y, Kinoshita J, Ohta T. Feasibility and efficacy of preoperative chemotherapy with docetaxel, cisplatin and S-1 in gastric cancer patients with para-aortic lymph node metastases. Anticancer Drugs. 2009;20:752–756. doi: 10.1097/CAD.0b013e32832ec02b. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama N, Koizumi W, Sasaki T, Higuchi K, Tanabe S, Nishimura K, Katada C, Nakatani K, Takagi S, Saigenji K. A multicenter, phase I dose-escalating study of docetaxel, cisplatin and S-1 for advanced gastric cancer (KDOG0601) Oncology. 2008;75:1–7. doi: 10.1159/000151613. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi W, Nakayama N, Tanabe S, Sasaki T, Higuchi K, Nishimura K, Takagi S, Azuma M, Ae T, Ishido K, et al. A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with unresectable or recurrent gastric cancer (KDOG 0601) Cancer Chemother Pharmacol. 2012;69:407–413. doi: 10.1007/s00280-011-1701-1. [DOI] [PubMed] [Google Scholar]
- 16.Polyzos A, Felekouras E, Karatzas T, Griniatsos J, Dimitroulis D, Polyzos K, Kontzoglou K, Mantas D, Karavokyros J, Nikiteas N, et al. Modified docetaxel-cisplatin in combination with capecitabine as first-line treatment in metastatic gastric cancer. a phase II study. Anticancer Res. 2012;32:4151–4156. [PubMed] [Google Scholar]
- 17.Sym SJ, Chang HM, Ryu MH, Lee JL, Kim TW, Yook JH, Oh ST, Kim BS, Kang YK. Neoadjuvant docetaxel, capecitabine and cisplatin (DXP) in patients with unresectable locally advanced or metastatic gastric cancer. Ann Surg Oncol. 2010;17:1024–1032. doi: 10.1245/s10434-009-0838-1. [DOI] [PubMed] [Google Scholar]
- 18.Yoon S, Yoo C, Ryu MH, Kang MJ, Ryoo BY, Park SR, Yook JH, Oh ST, Yoo MW, Kim BS, et al. Phase 2 study of adjuvant chemotherapy with docetaxel, capecitabine, and cisplatin in patients with curatively resected stage IIIB-IV gastric cancer. Gastric Cancer. 2017;20:182–189. doi: 10.1007/s10120-015-0580-2. [DOI] [PubMed] [Google Scholar]


