Abstract
AIM
To determine the procedure-related factors that affect sedation satisfaction and to make a suggestion to improve it.
METHODS
We prospectively enrolled a total of 456 patients who underwent outpatient endoscopy procedures with midazolam sedation between March 2014 and August 2014. All patients completed both pre- and post-endoscopy questionnaires about sedation expectations and satisfaction.
RESULTS
The study cohort included 167 (36.6%) patients who underwent esophagogastroduodenoscopy (EGD), 167 (36.6%) who underwent colonoscopy, and 122 (26.8%) who underwent a combined procedure (EGD and colonoscopy). Over 80% of all patients were satisfied with sedation using midazolam. In univariate and multivariate analyses, total procedure time in the EGD group, younger age (≤ 50 years), and longer colonoscopy withdrawal time in the colonoscopy group were related to decreased satisfaction with sedation. However, in active monitoring and intervention group, there was no decrease in grade of satisfaction despite longer procedure time due to more procedures during colonoscopy. Younger age (≤ 50 years), longer inter-procedure time gap, and colonoscopy withdrawal time were related to decreased satisfaction in the combined EGD and colonoscopy group.
CONCLUSION
Midazolam is still a safe and effective sedative for gastrointestinal endoscopy. Satisfaction with sedation depends on several factors including age (≤ 50 years) and procedure time duration. To improve patient satisfaction with sedation, active monitoring of sedation status by the endoscopist should be considered for patients who require long procedure time.
Keywords: Conscious sedation, Patient satisfaction, Endoscopy, Midazolam, Surveys and questionnaires
Core tip: This was a prospective study of 456 patients that evaluated procedure-related factors with midazolam sedation satisfaction. Satisfaction with sedation depends on several factors including age (≤ 50 years) and procedure duration. To improve patient satisfaction with sedation, active monitoring of sedation status by an endoscopist should be considered for patients whose procedures take a long time.
INTRODUCTION
Esophagogastroduodenoscopy (EGD) and colonoscopy are important examinations for screening, diagnosing, and treating a variety of gastrointestinal diseases. Specifically, endoscopy is one of the best surveillance tools for early detection of several cancers, but some patients refuse endoscopic examinations because of fear and anxiety of discomfort during the procedure[1]. Previous studies have reported that conscious sedation endoscopy improves patient satisfaction, reduces fear and discomfort, and increases compliance with repeat endoscopic procedures[2,3]. Recently, conscious sedation endoscopy has become commonplace in clinical practice[4-6].
As more procedures emerge that are appropriate for sedation endoscopy, sedation quality becomes an important factor because it is directly related to patient satisfaction and could have an effect on performance of endoscopy. Thus, satisfaction with sedation has become an important outcome measure and surveys of satisfaction are critical for quality assurance in many endoscopy centers[7]. In previous studies, young age, high level of anxiety, female sex, and increased gag reflex have been proposed as factors related to decreased patient satisfaction with non-sedation endoscopy[8,9]. However, results varied about factors related to satisfaction with sedation in endoscopy[7,10], and no survey of satisfaction with sedation endoscopy has yet been validated.
Worldwide, midazolam is the most commonly used drug for sedation during endoscopy, followed by fentanyl, propofol, and meperidine[4-6]. Midazolam is a short-acting benzodiazepine with anxiolytic, amnestic, and hypnotic effects. Appropriate sedation level could be adjusted by intravenous titration of midazolam. Because it is possible to evaluate the subject's level of sedation by medical staff during procedure through the Richmond Agitation-Sedation Scale[11] or Observer's Assessment of Alertness/Sedation Scale[12]. Flumazenil, a specific benzodiazepine receptor antagonist, can be used to treat benzodiazepine overdoses in emergency situations and to help reverse anesthesia[13]. In the present study, all patients received midazolam for sedation, and meperidine was added for patients undergoing colonoscopy.
The purpose of this study was to evaluate patient satisfaction with conscious sedation endoscopy, to determine procedure-related factors that affect satisfaction with sedation during endoscopic examinations, and to make a suggestion to improve it.
MATERIALS AND METHODS
Patient selection
We prospectively enrolled 466 patients who underwent outpatient endoscopy procedures between March 2014 and August 2014 at Seoul National University Hospital (SNUH), which is a tertiary referral center in Korea. Ten (2.1%) patients were excluded because they did not complete the satisfaction questionnaire. A total of 456 patients were eligible for this study.
All participants provided written informed consent before completing study interviews and undergoing endoscopy. The procedure for our review of clinical records for this study was approved by the Institutional Review Board of SNUH (IRB No. 1402-083-558).
Pre-endoscopy interview
Each patient completed an interview before the endoscopic procedure. An investigator administered a questionnaire in the waiting room after the patient had received explanations of the endoscopic procedure and sedation. The following patient information was recorded: age, sex, body mass index, previous sedation endoscopy, anxiety about procedure, cause of anxiety, and patient expectations of sedation depth according to the Richmond Agitation-Sedation Scale (drowsy, light, or deep sedation)[11]. Before the procedure started, nurses checked and recorded vital signs including oxygen saturation and blood pressure.
Endoscopy procedure
After completing the pre-endoscopy questionnaire, all patients were moved from the waiting room to the endoscopy procedure room. Before EGD, patients received topical anesthesia by pharyngeal spray with lidocaine. All patients underwent examinations with sedation by intravenous midazolam; meperidine at a dose of 25 mg was added for all patients undergoing colonoscopy. The examinations were performed by 14 board-certified endoscopists using an esophagogastroduodenoscope (GIF-260; Olympus, Tokyo, Japan) and/or a colonoscope (CF H260AL; Olympus, Tokyo, Japan). A nurse and an assistant monitored the patient during the procedure by periodically assessing pulse, blood pressure, ventilator status, and neurologic status. Nurses also completed records that included adverse effects of midazolam, the doses and frequency of midazolam injections, and the durations of the procedure and sedation. Three stages of sedation have been described: minimal, moderate, and deep[14]. In our study, most patients underwent endoscopy with moderate sedation referred to as "conscious sedation".
Post-endoscopy questionnaire
After the endoscopy procedure, patients were allowed sufficient time to recover from sedation, and then they completed a post-procedure questionnaire before discharge. Patients subjectively evaluated the depth of sedation and memory loss during the procedure. The questionnaire was self-administered and collected information regarding patient satisfaction with sedation (very satisfied, satisfied, neutral, dissatisfied, or very dissatisfied) and the cause of dissatisfaction, if patients answered "dissatisfied" or "very dissatisfied".
Definitions
Paradoxical response was defined as unexpected movement after midazolam injection. Decreased respiration was defined as oxygen saturation below 88% despite stimulation. In the case of decreased respiration, oxygen was administered via nasal prong. Procedure time was subdivided into the following periods: midazolam injection to procedure start, procedure duration, and procedure finish to antidote injection. For colonoscopy procedures, we further divided the procedure time into two periods: insertion time (anal verge to cecum) and withdrawal time (cecum to anal verge). For patients in the combined EGD and colonoscopy group, the inter-procedure time gap was defined as the waiting time from the end of the first endoscopy procedure to the beginning of the second endoscopy procedure.
Statistical analysis
Results are expressed as frequencies and percentages for categorical variables and means for continuous variables. We compared the three procedure groups using the χ2-test for ordinal variables and analysis of variance for quantitative variables.
Patient satisfaction outcomes were grouped according to satisfaction: very satisfied, satisfied, neutral, dissatisfied, and very dissatisfied. We constructed univariate and multivariate proportional odds logistic models to determine which factors were related to satisfaction in each procedure group. Results with P values less than 0.05 were considered statistically significant. Data were analyzed with statistical software R, version 3.2.2.
RESULTS
A total of 456 patients were eligible for this study and completed the post-endoscopy questionnaire. The patient group comprised 224 men and 232 women and the mean age of the group was 57.2 years. The study cohort included 167 (36.6%) patients who underwent EGD, 167 (36.6%) who underwent colonoscopy, and 122 (26.8%) who underwent a combined procedure (EGD and colonoscopy together). The characteristics of the three groups are shown in Table 1. Compared with the other procedure groups, the combined group had slightly higher first and total midazolam doses; the combined group was also more likely to receive more frequent injections and have longer procedure time. The EGD group was the most satisfied with conscious sedation (Figure 1).
Table 1.
Baseline characteristics of patients according to procedure group n (%)
| EGD (n = 167) | Colonoscopy (n = 167) | Combined group1 (n = 122) | P value | |
| Sex | 0.099 | |||
| Male | 71 (42.5) | 89 (53.3) | 64 (52.5) | |
| Female | 96 (57.5) | 78 (46.7) | 58 (47.5) | |
| Age (yr) | 0.878 | |||
| ≤ 50 | 43 (25.7) | 44 (26.3) | 29 (76.2) | |
| > 50 | 124 (74.3) | 123 (73.7) | 93 (76.2) | |
| Body mass index (kg/m2) | 22.6 | 22.9 | 23.3 (76.2) | 0.245 |
| Previous sedation endoscopy | 0.310 | |||
| Yes | 137 (82.5) | 135 (80.8) | 92 (75.4) | |
| No | 29 (17.5) | 32 (19.2) | 30 (24.6) | |
| Midazolam (mg) | ||||
| First dose | 4.1 | 4.3 | 4.4 | 0.014 |
| Second dose | 1.5 | 1.6 | 1.7 | 0.055 |
| Third dose | 1.0 | 1.6 | 1.5 | 0.269 |
| Fourth dose | 1.5 | 1.5 | 1.9 | 0.881 |
| Total midazolam dose (mg) | 4.4 | 5.0 | 6.4 | < 0.005 |
| No. of midazolam injections | 1.2 | 1.4 | 2.2 | < 0.005 |
| Time (min) | ||||
| Midazolam injection to procedure start | 5.1 | 4.5 | 4.7 | 0.327 |
| Total procedure time | 3.5 | 23.7 | 42.4 | < 0.005 |
| Inter-procedure gap | 19.5 | |||
| Procedure finish to antidote injection | 12.8 | 13.1 | 12.1 | 0.482 |
| Satisfaction with sedation during endoscopy | 0.016 | |||
| Very satisfied | 81 (48.5) | 51 (30.5) | 46 (37.7) | |
| Satisfied | 74 (44.3) | 86 (51.5) | 58 (47.5) | |
| Neutral | 7 (4.2) | 19 (11.4) | 11 (9.0) | |
| Dissatisfied | 5 (3) | 11 (6.6) | 7 (5.7) | |
| Very dissatisfied | - | - | - |
Combined group: Esophagogastroduodenoscopy and colonoscopy together. EGD: Esophagogastroduodenoscopy.
Figure 1.
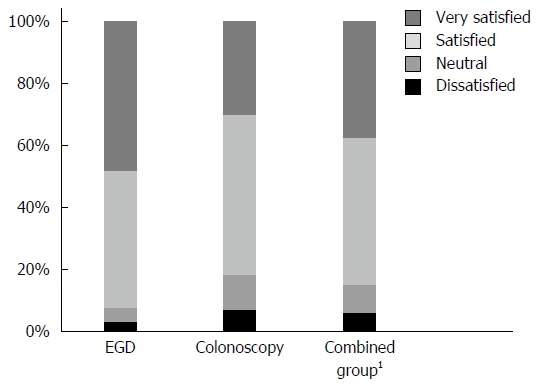
Patient sedation satisfaction according to endoscopy procedure. 1Combined group: Esophagogastroduodenoscopy and colonoscopy together. EGD: Esophagogastroduodenoscopy.
In all, 280 (61.4%) patients reported no anxiety before endoscopy; only 149 (32.7%) patients had mild anxiety and 19 (4.2%) patients had moderate anxiety. The most common cause of anxiety was fear of endoscopy procedure (n = 69, 41.1%), followed by fear of abdominal pain during endoscopy (n = 34, 20.2%), fear of insufficient sedation (n = 19, 11.3%), and fear of paradoxical response (n = 9, 5.4%). Most patients (50.2%) expected moderate sedation with movement or eye-opening to voice, followed by light sedation (41.9%) with brief awakenings to voice (Table 2).
Table 2.
Patient anxiety and expected sedation depth before endoscopy n (%)
| Anxiety before endoscopy | |
| No anxiety | 280 (61.4) |
| Mild anxiety | 149 (32.7) |
| Moderate anxiety | 19 (4.2) |
| No answer | 8 (1.8) |
| Cause of anxiety | |
| Fear of endoscopy procedure | 69 (41.1) |
| Fear of abdominal pain during endoscopy | 34 (20.2) |
| Fear of insufficient sedation | 19 (11.3) |
| Fear of paradoxical response | 9 (5.4) |
| None of the above | 36 (21.4) |
| Expected sedation depth1 | |
| Drowsy | 19 (4.2) |
| Light sedation | 191 (41.9) |
| Moderate sedation | 229 (50.2) |
| No answer | 9 (2) |
Drowsy: Not fully alert, but experiences sustained wakening to voice; Light sedation: Briefly awakens to voice; Moderate sedation: Movement or eye-opening to voice.
In the EGD group, 81 (48.5%) patients were very satisfied, 74 (44.3%) were satisfied, 7 (4.2%) were neutral, and 5 (3.0%) were dissatisfied with sedation according to the post-procedure questionnaire. Total procedure time was only the factor associated with decreased satisfaction (OR = 0.97, P = 0.041) in the EGD group (Table 3).
Table 3.
Factors associated with sedation satisfaction in the esophagogastroduodenoscopy group
|
Univariate analysis |
Multivariate analysis |
|||||||
| Beta | SE (β) | P value | OR (95%CI) | Beta | SE (β) | P value | OR (95%CI) | |
| Sex (Female) | 0.063 | 0.302 | 0.834 | 1.07 (0.59-1.92) | ||||
| Body mass index (kg/m2) | 0.001 | 0.005 | 0.798 | 1.00 (0.99-1.01) | ||||
| Previous sedation endoscopy | 0.642 | 0.395 | 0.104 | 1.90 (0.88-4.12) | ||||
| Midazolam, first dose (mg) | -0.094 | 0.143 | 0.509 | 0.91 (0.69-1.20) | ||||
| Midazolam, total dose (mg) | -0.022 | 0.116 | 0.852 | 0.98 (0.78-1.23) | ||||
| Time (min) | ||||||||
| Midazolam injection to procedure start | -0.033 | 0.052 | 0.525 | 0.98 (0.78-1.23) | ||||
| Total procedure time | 0.127 | 0.062 | 0.041 | 0.97 (0.87-1.07) | 0.127 | 0.062 | 0.041 | 0.97 (0.87-1.07) |
| Procedure finish to antidote injection | -0.036 | 0.024 | 0.127 | 1.14 (1.01-1.28) | ||||
In the colonoscopy group, 51 (30.5%) patients were very satisfied, 86 (51.5%) were satisfied, 19 (11.4%) were neutral, and 11 (6.6%) were dissatisfied with sedation. In our univariate analysis, younger age (≤ 50 years), total midazolam dose, and colonoscopy withdrawal time were associated with decreased patient satisfaction in this group. Age (> 50 years) (OR = 0.38, P = 0.005) and colonoscopy withdrawal time (OR = 1.03, P = 0.036) were significantly associated with sedation satisfaction in the multivariate analysis (Table 4). In colonoscopy cases, an endoscopist directly commanded nurse to inject additional doses of midazolam under active monitoring of sedation status. These patients were similarly satisfied with sedation despite longer procedure time due to more procedures (28.8 ± 12.2 min vs 22.3 ± 12.2 min, P = 0.005) (Table 5).
Table 4.
Factors associated with sedation satisfaction in the colonoscopy group
|
Univariate analysis |
Multivariate analysis |
|||||||
| Beta | SE (β) | P value | OR (95%CI) | Beta | SE (β) | P value | OR (95%CI) | |
| Sex (female) | 0.197 | 0.294 | 0.503 | 1.22 (0.68-2.17) | ||||
| Age (> 50 yr) | -0.937 | 0.339 | 0.006 | 0.39 (0.20-0.76) | -0.956 | 0.341 | 0.005 | 0.38 (0.20-0.75) |
| Midazolam, first dose (mg) | 0.253 | 0.165 | 0.127 | 1.29 (0.93-1.78) | ||||
| Midazolam, total dose (mg) | 0.223 | 0.108 | 0.039 | 1.25 (1.01-1.54) | ||||
| No. of midazolam injections | 0.441 | 0.244 | 0.070 | 1.55 (0.96-2.51) | ||||
| Time (min) | ||||||||
| Midazolam injection to procedure start | 0.009 | 0.051 | 0.863 | 1.01 (0.91-1.12) | ||||
| Procedure time | ||||||||
| Colonoscopy insertion time | 0.015 | 0.036 | 0.670 | 1.02 (0.95-1.09) | ||||
| Colonoscopy withdrawal time | 0.030 | 0.014 | 0.035 | 1.03 (1.00-1.06) | 0.030 | 0.014 | 0.036 | 1.03 (1.00-1.06) |
| Procedure finish to antidote injection | 0.016 | 0.020 | 0.447 | 1.02 (0.98-1.06) | ||||
Table 5.
Patients’ satisfaction through active monitoring and intervention by endoscopist during colonoscopy
| Active monitoring (n = 39) | Non-active monitoring (n = 128) | P value | |
| Sex (Male, %) | 19 (48.7) | 70 (54.7) | 0.584 |
| Age (mean ± SD) | 56.1 ± 13.0 | 57.7 ± 13.5 | 0.514 |
| Proportion of EMR, n (%) | 32 (82.1) | 51 (39.8) | < 0.001 |
| Satisfaction, n (%) | 0.968 | ||
| Very satisfied | 12 (30.8) | 39 (30.5) | |
| Satisfied | 20 (51.3) | 66 (51.6) | |
| Fair | 5 (12.8) | 14 (10.9) | |
| Unsatisfied | 2 (5.1) | 9 (7.0%) | |
| Midazolam, first dose (mg, mean ± SD) | 4.5 ± 1.0 | 4.2 ± 0.9 | 0.159 |
| Midazolam, total dose (mg, mean ± SD) | 5.8 ± 1.2 | 4.8 ± 1.5 | 0.002 |
| Midazolam, No. of injections (mean ± SD) | 1.6 ± 0.5 | 1.4 ± 0.7 | 0.025 |
| Procedure time (min, mean ± SD) | 28.8 ± 12.2 | 22.3 ± 12.2 | 0.005 |
In the combined EGD and colonoscopy group, 46 (37.7%) patients were very satisfied, 58 (47.5%) were satisfied, 11 (9.0%) were neutral, and 7 (5.7%) were dissatisfied with sedation. In our univariate analysis, female sex, younger age (≤ 50 years), total midazolam dose, number of midazolam injections, procedure time, and number of endoscopic mucosal resections were associated with decreased patient satisfaction in the combined group. In the multivariate analysis, age (> 50 years) (OR = 0.38, P = 0.022), inter-procedure time gap (OR = 1.02, P = 0.027), and colonoscopy withdrawal time (OR = 1.08, P = 0.002) were associated with dissatisfaction with sedation (Table 6).
Table 6.
Factors associated with sedation satisfaction in the combined esophagogastroduodenoscopy and colonoscopy group
|
Univariate analysis |
Multivariate analysis |
|||||||
| β | SE (β) | P value | OR (95% CI) | β | SE (β) | P value | OR (95%CI) | |
| Sex (Female) | 0.689 | 0.349 | 0.049 | 1.99 (1.00-3.95) | ||||
| Age (> 50 yr) | -0.868 | 0.407 | 0.033 | 0.42 (0.19-0.93) | -0.978 | 0.427 | 0.022 | 0.38 (0.16-0.87) |
| Body mass index (kg/m2) | -0.001 | 0.006 | 0.878 | 1.00 (0.99-1.01) | ||||
| Previous sedation endoscopy | -0.013 | 0.395 | 0.974 | 0.99 (0.46-2.14) | ||||
| Midazolam, first dose (mg) | 0.105 | 0.210 | 0.619 | 1.11 (0.74-1.68) | ||||
| Midazolam, total dose (mg) | 0.278 | 0.113 | 0.014 | 1.32 (1.06-1.65) | ||||
| No. of midazolam injections | 0.690 | 0.284 | 0.015 | 1.99 (1.14-3.48) | ||||
| Time (min) | ||||||||
| Midazolam injection to procedure start | 0.041 | 0.033 | 0.215 | 1.04 (0.98-1.11) | ||||
| Procedure time | ||||||||
| Inter-procedure time gap | 0.021 | 0.010 | 0.043 | 1.02 (1.00-1.04) | 0.024 | 0.011 | 0.027 | 1.02 (1.00-1.05) |
| Colonoscopy insertion time | 0.084 | 0.038 | 0.027 | 1.09 (1.01-1.17) | ||||
| Colonoscopy withdrawal time | 0.069 | 0.025 | 0.006 | 1.07 (1.02-1.13) | 0.081 | 0.027 | 0.002 | 1.08 (1.03-1.14) |
| Procedure finish to antidote injection | 0.014 | 0.029 | 0.637 | 1.01 (0.96-1.07) | ||||
Five (1.1%) patients experienced a paradoxical response, 10 (2.2%) patients complained of pain during the procedure, and 7 patients complained of decreased respiration during the endoscopy procedure. Among these patients, 3 patients with paradoxical response and 2 patients with decreased respiration were given an antidote to the sedative. Of 23 dissatisfied patients, 16 complained of insufficient sedation.
DISCUSSION
Using a multivariate analysis in this prospective study, we found that longer procedure time in EGD, younger age, and longer colonoscopy withdrawal time were procedure-related factors that influenced patient satisfaction with conscious midazolam sedation. Young age, long inter-procedure time, and long colonoscopy withdrawal time were associated with decreased satisfaction in the combined EGD and colonoscopy group, as determined by the multivariate analysis. If a procedure is prolonged, the concerned endoscopist and other health care personnel should pay attention to the sedation status, especially for younger patients.
Few studies have assessed procedure-related factors that affect satisfaction with sedation. In previous studies, endoscopy-associated sedation satisfaction was related to organizational factors such as waiting time, personal considerations, and comfort of the hospital environment[7,10]. Patient factors such as nervousness and chronic use of psychotropic drugs have also been associated with sedation satisfaction[15]. The satisfaction survey mGHAA-9 has been used to evaluate the general satisfaction with hospital systems and subjective aspects of endoscopy centers; however, mGHAA-9 is insufficient to evaluate satisfaction with the sedation itself[3,7].
Previous studies found that female and young patients experienced more discomfort during endoscopy and received more sedatives than male and older patients for achieving similar comfort levels[4,16]. Our findings support the fact that female and younger patients (≤ 50 years) were less satisfied with sedation in the combined EGD and colonoscopy group. However, female sex was not a significant factor for dissatisfaction with sedation during endoscopy in our multivariate analysis.
Longer procedure time was strongly associated with dissatisfaction in our analysis. When we divided procedure time for colonoscopy procedures, colonoscopy withdrawal time was associated with sedation satisfaction. When additional procedures such as biopsies and endoscopic mucosal resections were performed, withdrawal time was longer. In colonoscopy cases, an endoscopist directed a nurse to inject additional doses of midazolam while actively monitoring sedation status. Interestingly, over 80% of these patients were satisfied with sedation and there was no decrease in the degree of satisfaction despite longer procedure time due to the additional procedures being carried out (Table 5). However, the endoscopist, as a single variable, was not statistically significant in initial univariate analysis and was not included in multivariate analyses because only one endoscopist was involved in active monitoring of patient groups. Active monitoring and intervention by an endoscopist could be an important way to improve a patient's sedation satisfaction. For active monitoring, endoscopists have to pay close attention to sedation status by observing spontaneous eye opening, verbal arousal, and complaints of pain. As a result of active monitoring, timely dose titrations of midazolam might help maintain the desired conscious sedation during the procedure.
Same-day EGD and colonoscopy are commonly used in clinical practice[17], and carried out in clinical settings when digestive disease is suspected. Performing both EGD and colonoscopy as a combined procedure is convenient for patients, efficient for providers, and saves costs for the health care system[18]. Although the combined procedure group had a longer procedure time than the single-colonoscopy group in our data, patients in the combined group were more satisfied with conscious sedation than those in the colonoscopy group. Patients in the combined group tended to have higher midazolam doses and more midazolam injections than those in the colonoscopy group. This finding is likely because the endoscopist verified the sedation status of the patient and administered additional midazolam before performing the second procedure. In the combined EGD and colonoscopy group, the inter-procedure time gap (the waiting time from the end of the first endoscopy procedure to the start of the second procedure) was related to sedation satisfaction. Therefore, this waiting time should be reduced as much as possible in clinical practice.
In recent years, the sedative propofol use has increased in community medical practice compared to academic medical practice[19,20]. In a previous study, propofol increased sedation satisfaction by reducing fear and pain compared to other types of sedation[19]. Because propofol provided more rapid recovery than midazolam[21], it has the merit of post-procedure neuropsychologic function over midazolam[22]. Moreover, a previous study showed that propofol was cost-effective in critical illness and emergency situations[23]. However, its cost-effectiveness in outpatient endoscopy is yet unknown. It is important to select sedative medication not only for economic reasons but also for its safe use. The narrow therapeutic window of propofol necessitates close patient monitoring because of the risk of adverse cardiopulmonary events[14]. Therefore, midazolam was still the best option as a sedative during endoscopy in terms of both safety and cost-effectiveness. Administration of another sedative flumazenil results in a safe and cost-effective shortening of the recovery time[24].
This study has some limitations that must be considered. First, we collected the post-procedure survey from patients on site, usually in the recovery room. Patients may have been hesitant to provide responses indicating dissatisfaction in the presence of clinical staff. For this reason, our study showed higher satisfaction scores in on-site surveys than in mail-back surveys[25]. In addition, patients in the recovery room may still have been under the influence of midazolam and, as such, unable to answer all questions accurately. While the patients in this study answered our surveys on the day of the endoscopy examination, previous studies collected such data a few days after the examination via telephone surveys or using a mail-back system[7,16]. However, the response rate to telephone or mail back surveys could be lower than that to the on-site survey[25]. Even though the on-site survey has weaknesses, the magnitude of the differences is small, and the on-site method is simple and associated with a higher response rate than mail-back surveys.
Second, the surveys were not anonymous: each survey had the name of the patient and the date of the procedure printed at the top of the questionnaire. This unblinded format could also have led patients to overestimate satisfaction because most patients anticipated a return visit to the hospital to discuss the results of the endoscopy. However, anonymous questionnaires were impossible for this study because we analyzed clinical procedure data such as procedure time and midazolam doses. Third, we used a satisfaction survey that has not been formally validated. A few validated surveys exist for evaluating the general satisfaction of endoscopy, but currently no validated survey specifically evaluates sedation satisfaction.
In conclusion, midazolam is still a safe and effective sedative for gastrointestinal endoscopy. Satisfaction with sedation depends on total procedure time in EGD; younger age and colonoscopy withdrawal time in colonoscopy; and younger age, inter-procedure time gap, and colonoscopy withdrawal time in combined procedures. To improve patient satisfaction with midazolam sedation, active monitoring and intervention by the endoscopist should be considered for patients who require long procedure time.
COMMENTS
Background
The use of endoscopy is important for the early detection of gastrointestinal cancers, but some patients refuse endoscopic examinations owing to fear and anxiety over expected discomfort during the procedure. Conscious sedation endoscopy is the best option to relieve patient discomfort. Therefore, satisfaction with sedation endoscopy is critical for quality assurance in many endoscopy centers. This study was designed to evaluate patient satisfaction with conscious sedation endoscopy, to determine which procedure-related factors affect satisfaction with sedation, and to offer suggestions for improvement.
Research frontiers
In this study, the authors determined which procedure-related factors affect patient satisfaction with sedation during endoscopic examinations. Those factors varied in significance depending on the type of procedure (e.g., esophagogastroduodenoscopy, colonoscopy, and combined group). This outcome suggests that the endoscopist should closely monitor sedation status and pay attention to procedure-related factors, such as procedure time or patient factor (e.g., age), depending on procedure type.
Innovations and breakthroughs
An interesting finding of this study was that active monitoring and intervention by an endoscopist could be an important way to improve patient sedation satisfaction. In addition, midazolam was still found to be a safe and effective medication for conscious sedation.
Applications
The results of this study could help an endoscopist make decisions concerning midazolam titration and when to administer additional doses of midazolam.
Terminology
Midazolam is a short-acting benzodiazepine with anxiolytic, amnestic, and hypnotic effects. Propofol is an intravenous sedative-hypnotic agent used in the induction and maintenance of anesthesia.
Peer-review
A pleasure to read about this interesting topic regarding the patient/customer’s perception of adequate sedation that corresponds to the use of drug. A discussion regarding cost comparison of the drugs may add another dimension to drug selection by the Endoscopist/Medical center.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the institutional review board of Seoul National University Hospital (IRB No. 1402-083-558).
Informed consent statement: All study participants, or their legal guardian, provided written consent prior to study enrollment.
Conflict-of-interest statement: The authors of this manuscript have no conflicts of interest to disclose.
Data sharing statement: There is no additional data available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 11, 2016
First decision: November 9, 2016
Article in press: December 19, 2016
P- Reviewer: Hay JM, Kumaran SV, Triantafillidis JK S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu WX
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Abraham NS, Fallone CA, Mayrand S, Huang J, Wieczorek P, Barkun AN. Sedation versus no sedation in the performance of diagnostic upper gastrointestinal endoscopy: a Canadian randomized controlled cost-outcome study. Am J Gastroenterol. 2004;99:1692–1699. doi: 10.1111/j.1572-0241.2004.40157.x. [DOI] [PubMed] [Google Scholar]
- 3.Loftus R, Nugent Z, Graff LA, Schumacher F, Bernstein CN, Singh H. Patient satisfaction with the endoscopy experience and willingness to return in a central Canadian health region. Can J Gastroenterol. 2013;27:259–266. doi: 10.1155/2013/615206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Childers RE, Williams JL, Sonnenberg A. Practice patterns of sedation for colonoscopy. Gastrointest Endosc. 2015;82:503–511. doi: 10.1016/j.gie.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froehlich F, Harris JK, Wietlisbach V, Burnand B, Vader JP, Gonvers JJ. Current sedation and monitoring practice for colonoscopy: an International Observational Study (EPAGE) Endoscopy. 2006;38:461–469. doi: 10.1055/s-2006-925368. [DOI] [PubMed] [Google Scholar]
- 6.Porostocky P, Chiba N, Colacino P, Sadowski D, Singh H. A survey of sedation practices for colonoscopy in Canada. Can J Gastroenterol. 2011;25:255–260. doi: 10.1155/2011/783706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko HH, Zhang H, Telford JJ, Enns R. Factors influencing patient satisfaction when undergoing endoscopic procedures. Gastrointest Endosc. 2009;69:883–891, quiz 891.e1. doi: 10.1016/j.gie.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Mulcahy HE, Kelly P, Banks MR, Connor P, Patchet SE, Farthing MJ, Fairclough PD, Kumar PJ. Factors associated with tolerance to, and discomfort with, unsedated diagnostic gastroscopy. Scand J Gastroenterol. 2001;36:1352–1357. doi: 10.1080/003655201317097245. [DOI] [PubMed] [Google Scholar]
- 9.Campo R, Brullet E, Montserrat A, Calvet X, Moix J, Rué M, Roqué M, Donoso L, Bordas JM. Identification of factors that influence tolerance of upper gastrointestinal endoscopy. Eur J Gastroenterol Hepatol. 1999;11:201–204. doi: 10.1097/00042737-199902000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Yacavone RF, Locke GR, Gostout CJ, Rockwood TH, Thieling S, Zinsmeister AR. Factors influencing patient satisfaction with GI endoscopy. Gastrointest Endosc. 2001;53:703–710. doi: 10.1067/mge.2001.115337. [DOI] [PubMed] [Google Scholar]
- 11.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 12.Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251. [PubMed] [Google Scholar]
- 13.Whitwam JG, Amrein R. Pharmacology of flumazenil. Acta Anaesthesiol Scand Suppl. 1995;108:3–14. doi: 10.1111/j.1399-6576.1995.tb04374.x. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein DR, Jagannath S, Baron TH, Anderson MA, Banerjee S, Dominitz JA, Fanelli RD, Gan SI, Harrison ME, Ikenberry SO, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:815–826. doi: 10.1016/j.gie.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Peña LR, Mardini HE, Nickl NJ. Development of an instrument to assess and predict satisfaction and poor tolerance among patients undergoing endoscopic procedures. Dig Dis Sci. 2005;50:1860–1871. doi: 10.1007/s10620-005-2952-7. [DOI] [PubMed] [Google Scholar]
- 16.Seip B, Bretthauer M, Dahler S, Friestad J, Huppertz-Hauss G, Høie O, Kittang E, Nyhus S, Pallenschat J, Sandvei P, et al. Patient satisfaction with on-demand sedation for outpatient colonoscopy. Endoscopy. 2010;42:639–646. doi: 10.1055/s-0030-1255612. [DOI] [PubMed] [Google Scholar]
- 17.Urquhart J, Eisen G, Faigel DO, Mattek N, Holub J, Lieberman DA. A closer look at same-day bidirectional endoscopy. Gastrointest Endosc. 2009;69:271–277. doi: 10.1016/j.gie.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Serag HB, Xu F, Biyani P, Cooper GS. Bundling in medicare patients undergoing bidirectional endoscopy: how often does it happen? Clin Gastroenterol Hepatol. 2014;12:58–63. doi: 10.1016/j.cgh.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilgert B, Rybizki L, Grottke M, Neurath MF, Neumann H. Prospective long-term assessment of sedation-related adverse events and patient satisfaction for upper endoscopy and colonoscopy. Digestion. 2014;90:42–48. doi: 10.1159/000363567. [DOI] [PubMed] [Google Scholar]
- 20.Faulx AL, Vela S, Das A, Cooper G, Sivak MV, Isenberg G, Chak A. The changing landscape of practice patterns regarding unsedated endoscopy and propofol use: a national Web survey. Gastrointest Endosc. 2005;62:9–15. doi: 10.1016/s0016-5107(05)00518-3. [DOI] [PubMed] [Google Scholar]
- 21.Patterson KW, Casey PB, Murray JP, O’Boyle CA, Cunningham AJ. Propofol sedation for outpatient upper gastrointestinal endoscopy: comparison with midazolam. Br J Anaesth. 1991;67:108–111. doi: 10.1093/bja/67.1.108. [DOI] [PubMed] [Google Scholar]
- 22.Ulmer BJ, Hansen JJ, Overley CA, Symms MR, Chadalawada V, Liangpunsakul S, Strahl E, Mendel AM, Rex DK. Propofol versus midazolam/fentanyl for outpatient colonoscopy: administration by nurses supervised by endoscopists. Clin Gastroenterol Hepatol. 2003;1:425–432. doi: 10.1016/s1542-3565(03)00226-x. [DOI] [PubMed] [Google Scholar]
- 23.Hohl CM, Nosyk B, Sadatsafavi M, Anis AH. A cost-effectiveness analysis of propofol versus midazolam for procedural sedation in the emergency department. Acad Emerg Med. 2008;15:32–39. doi: 10.1111/j.1553-2712.2007.00023.x. [DOI] [PubMed] [Google Scholar]
- 24.Mathus-Vliegen EM, de Jong L, Kos-Foekema HA. Significant and safe shortening of the recovery time after flumazenil-reversed midazolam sedation. Dig Dis Sci. 2014;59:1717–1725. doi: 10.1007/s10620-014-3061-2. [DOI] [PubMed] [Google Scholar]
- 25.Lin OS, Schembre DB, Ayub K, Gluck M, McCormick SE, Patterson DJ, Cantone N, Soon MS, Kozarek RA. Patient satisfaction scores for endoscopic procedures: impact of a survey-collection method. Gastrointest Endosc. 2007;65:775–781. doi: 10.1016/j.gie.2006.11.032. [DOI] [PubMed] [Google Scholar]


