Abstract
Acute pancreatitis (AP) is a serious inflammatory disease with rising incidence both in the adult and pediatric populations. It has been shown that mitochondrial injury and energy depletion are the earliest intracellular events in the early phase of AP. Moreover, it has been revealed that restoration of intracellular ATP level restores cellular functions and defends the cells from death. We have recently shown in a systematic review and meta-analysis that early enteral feeding is beneficial in adults; however, no reviews are available concerning the effect of early enteral feeding in pediatric AP. In this minireview, our aim was to systematically analyse the literature on the treatment of acute pediatric pancreatitis. The preferred reporting items for systematic review (PRISMA-P) were followed, and the question was drafted based on participants, intervention, comparison and outcomes: P: patients under the age of twenty-one suffering from acute pancreatitis; I: early enteral nutrition (per os and nasogastric- or nasojejunal tube started within 48 h); C: nil per os therapy; O: length of hospitalization, need for treatment at an intensive care unit, development of severe AP, lung injury (including lung oedema and pleural effusion), white blood cell count and pain score on admission. Altogether, 632 articles (PubMed: 131; EMBASE: 501) were found. After detailed screening of eligible papers, five of them met inclusion criteria. Only retrospective clinical trials were available. Due to insufficient information from the authors, it was only possible to address length of hospitalization as an outcome of the study. Our mini-meta-analysis showed that early enteral nutrition significantly (SD = 0.806, P = 0.034) decreases length of hospitalization compared with nil per os diet in acute pediatric pancreatitis. In this minireview, we clearly show that early enteral nutrition, started within 24-48 h, is beneficial in acute pediatric pancreatitis. Prospective studies and better presentation of research are crucially needed to achieve a higher level of evidence.
Keywords: pediatric pancreatitis, enteral nutrition, nil per os diet, ATP restoration, length of hospitalization
Core tip: Acute pancreatitis is a serious inflammatory disease with rising incidence both in adult and pediatric medicine. Despite the existing research activities in this field, no specific therapy is available to treat this disease. Results in basic science strongly suggest that early energy restoration could be the first-line treatment for acute pancreatitis. Our minireview suggests that early enteral nutrition should have priority in the treatment of acute pediatric pancreatitis.
INTRODUCTION
Acute pancreatitis (AP) is a serious inflammatory disease with rising incidence both in adult and pediatric populations[1,2]. Common characteristics in both age groups are that no specific therapy is available to treat the disease and that the general supportive treatments at the early phase of the disease are usually volume resuscitation and a nil per os (NPO) diet[3-6]. However, while there is clear evidence in the literature that volume therapy is beneficial, the latter treatment is questionable.
One of the main reasons for the debate is that the pathogenesis of the disease clearly suggests the opposite. Irrespective of the etiological factors, mitochondrial damage and energy depletion are the leading intracellular responses in the early phase of the disease in the exocrine pancreas[7-10]. Bile acids[11-14], ethanol, fatty acids and their non-oxidative metabolites, fatty acid ethyl esthers[8,9,15-18] were shown to elevate the intracellular Ca2+ concentration, causing mitochondrial damage and a resultant decrease of intracellular ATP concentration. This will lead to inhibited fluid and bicarbonate secretion and CFTR Cl- channel dysfunction in the ductal cells and secretory block and intracellular trypsinogen activation in the acinar cells (Figure 1)[9,16,19,20]. Very importantly, restoration of ATP levels both in acinar and ductal cells prevents (at least in part) the toxic effects of the etiological factors[7,21,22] noted above. These data strongly suggest that an energy supply, for example, via enteral nutrition, should be beneficial for patients as compared to nil energy.
Figure 1.
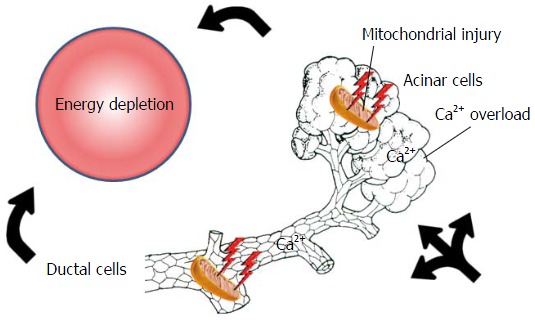
Early events in acute pancreatitis. Bile acids, ethanol, fatty acids or their non-oxidative metabolites, fatty acid ethyl esthers, induce calcium overload, causing mitochondrial damage and a resultant decrease in intracellular ATP concentration both in acinar and ductal cells. This will lead to general energy depletion in the pancreas.
Notably, early enteral nutrition (EEN) either via oral, nasogastric- or nasojejunal tube feeding is beneficial as regards systemic infections, complications, multi-organ failure, need for surgical interventions and mortality[6,23-30]. Enteral nutrition has already been proven to be beneficial in other inflammatory gastrointestinal diseases. The first-line recommendation to induce remission in pediatric Crohn's disease is exclusive enteral nutrition[31]. Enteral nutrition could also be effective in the maintenance of pediatric inflammatory bowel disease remission[32]. With regard to acute pancreatitis, three of the recent and most up-to-date guidelines for acute pancreatitis in adults clearly show the positive effect of enteral nutrition in moderate and severe AP[6,23,24]. Besides the energy supply, enteral nutrition in patients can also have other advantages as a first-line treatment for patients. It is well documented that the gut plays an important role as an immune barrier in the immune system and that EEN facilitates this barrier function. EEN significantly decreases pathogenic bacteria in the stool, alteration of intestinal flora and levels of serum endotoxins. EEN has a favourable effect on immune dysregulation caused by severe acute pancreatitis, which can reduce APACHE II scores, pancreatic sepsis, initial incidences of systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome[33,34].
Recent meta-analyses of adult data showed that EEN is beneficial in all severity groups in AP; however, no systematic review is available concerning the role of EEN in pediatrics[35]. Therefore, the aim was to review the literature to analyse the effect of EEN vs NPO therapy on the outcome of acute pediatric pancreatitis (APP) and aggregate the information in APP leading to a higher statistical power and more robust point estimate than is possible from the individual studies.
The preferred reporting items for systematic review and meta-analysis protocol (PRISMA-P) were followed[36]. Our structured literature search was based on the participants, intervention, comparison and outcomes format: P: patients under the age of twenty-one suffering from acute pancreatitis; I: early enteral nutrition (per os and nasogastric- or nasojejunal tube started within 48 h); C: NPO therapy [per os/nasogastric- or enteral tube after 48 h and total parenteral nutrition (TPN) within or after 48 h]; O: length of hospitalization, need for intensive care unit (ICU), complications, necessity of antibiotics, surgical/non-surgical interventions and mortality.
In February 2016, a literature search was performed on the PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and EMBASE (https://www.embase.com) databases using the following Medical Subject Headings and search terms: "pediatric" OR "paediatric" AND "pancreatitis". The search was limited to human studies, full-text publications with abstracts in English with no time period, resulting in 632 articles altogether (PubMed: 131; EMBASE: 501).
The articles were checked separately. Meta-analyses, reviews, case reports and articles on chronic pancreatitis were excluded and duplicates were removed (Figure 2). Potentially eligible papers were selected, and, finally, five of them with relevant data on EEN or/with NPO therapy in acute pediatric pancreatitis in patients under twenty-one years old were included (Table 1)[37-41]. To reduce the risk of bias, the literature search was independently performed by three researchers following the inclusion criteria noted above.
Figure 2.
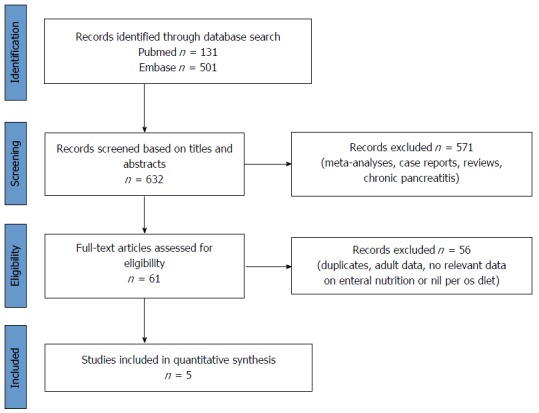
Flow chart on the methods used in the literature search.
Table 1.
Studies included in the quantitative synthesis
| Ref. | Data | Groups | NO. of patients |
| Abu-El-Haija et al[37], 2016 | Yes | EEN | 24 |
| NPO | 14 | ||
| Flores-Calderón et al[41], 2009 | only NPO | 18 | |
| Goh et al[40], 2003 | only NPO | 12 | |
| Raizner et al[39], 2013 | only NPO | 7 | |
| Szabo et al[38], 2015 | Yes | EEN + IVF lo | 55 |
| NPO + IVF lo | 20 | ||
| Yes | EEN + IVF hi | 96 | |
| NPO + IVF hi | 30 |
EEN: Early enteral nutrition; NPO: Nil per os.
The details in the collected articles were checked, and only articles where both EEN and NPO were presented separately were used. Two articles met this criterion. The two articles contained three separate data pairs, where EEN was compared to NPO (Figure 3). The following parameters were collected: length of hospitalization (LOH), need for treatment at an ICU, development of severe AP, lung injury (including lung oedema and pleural effusion), white blood cell count and pain score on admission. Only one of the five investigated parameters (LOH) contained a minimum of three items, which were analysed statistically.
Figure 3.
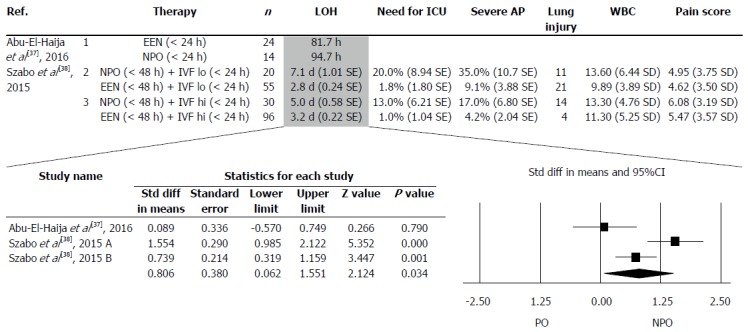
Two articles contained three separate data pairs where early enteral nutrition was compared to nil per os. LOH: Length of hospitalization; EEN: Early enteral nutrition; NPO: Nil per os. ICU: Intensive care unit; AP: Acute pancreatitis; WBC: White blood cell count.
The meta-analytic calculation was made with Comprehensive MetaAnalysis (V3) software using the random effects model (the DerSimonian-Laird method). We calculated a weighted standard difference in means and 95%CI. In the case of one study (Abu-El-Haija et al[37], 2016), we converted the median and range values to means and standard deviation using the modified Hozo's formula by Wan et al[42]. For a visual inspection, we used a forest plot.
Figure 3 shows the parameters collected from the articles. It was only possible to perform forest plot analyses on LOH. EEN significantly decreased LOH (SD = 0.806, P = 0.034) compared to the standard NPO diet (Figure 3).
DISCUSSION
Several therapeutic recommendations are available in the literature on nutrition in acute pancreatitis. The IAP/APA guideline suggests enteral tube feeding as the first-line therapy in patients requiring nutritional support with predicted severe and severe acute pancreatitis[6]. According to the Japanese guideline, enteral nutrition in the early phase of severe acute pancreatitis can decrease the incidence of complications and elevate the survival rate[24]. Recent meta-analyses of adult studies revealed that EEN decreases mortality, rate of interventions and the incidence of multi-organ failure in severe acute pancreatitis. Moreover, group analyses of 17 parameters including laboratory parameters (such as CRP and white blood cells) and symptoms (such as pain or presence of SIRS) suggested that EEN also has merits in mild acute pancreatitis. Since the incidence of APP has risen in the past twenty years (with 3.6 and 13.2/100000 children affected annually), we systematically reviewed the literature to understand whether there is any beneficial effect of EEN vs NPO in children[43,44].
We faced several difficulties during our review: (1) APP is still underdiagnosed, thus decreasing the possibility of performing clinical trials[45]; (2) the number of studies on the management of these patients is very low, and there is still only a small number of studies focused on understanding the characteristics of the disease[46]; (3) the studies have not focused on the early management of the patients; the groups were therefore not separated; and (4) finally, but very importantly, the methods sections and the quality of data presentation in these articles are very low. Consequently, in many cases, it was impossible to obtain quality analysable data from the manuscripts for a proper broad-spectrum meta-analysis[37-39].
By the end of the search, we identified five articles containing relevant data on nutritional management during the early phase of APP. Raizner et al[39] published a retrospective analysis involving seven children with necrotizing pancreatitis. All the children received a strict NPO diet, five patients received TPN and just one patient was treated with nasojejunal feeding for seven days. All the children required a prolonged hospital stay (with a mean of 20 d) for acute complications, with three of them suffering from late complications[39]. Goh et al[40] included twelve patients in their retrospective study. One patient needed a distal pancreatectomy, and eleven patients recovered with conservative management, with none of them receiving EEN. Two patients had acute complications, and two patients had recurrent AP[40]. Flores-Calderon et al[41] studied eighteen patients with acute pancreatitis caused by L-asparaginase due to acute lymphoblastic leukemia. All the patients were treated with bowel resting for a mean of 22 d, fourteen of the patients received TPN and four had an elementary diet. Two of the patients required intensive care unit admission, with local complications developing in twelve patients. None of the patients died from complications related to AP. Although these studies point out several disadvantages of that NPO diet, none of them could be enrolled in our meta-analysis.
Finally, it was possible to collect three sets of analysable data pairs where both NPO and EEN were present. Abu-El-Haija et al[37] conducted a prospective study of 38 children suffering from mild AP and retrospectively investigated the relationship of nutrition with pain and LOS. EEN feeding meant per os feeding and NPO was identified as oral feeding not being allowed for 24 h. Importantly, EEN, even with high fat intake, did not cause an elevation in pain in children, suggesting that EEN is a well tolerable nutritional possibility in children. The fact that LOS was much shorter in group EEN vs NPO points to EEN as a better way of treating APP[37]. The most advanced study was performed by Szabo et al[38], where several parameters were collected to understand the effect of EEN on the course of APP. Two hundred and one children suffering from mild AP were enrolled retrospectively. They compared EEN vs NPO both with and without aggressive fluid resuscitation. Fluid therapy was administered during the first 24 h, and the type of nutrition was determined during the first 48 h. Besides the beneficial effects of EEN on LOS, they also showed that EEN reduces the severity of the disease. Although our aim was to perform a meta-analysis on several parameters to understand the differences between EEN and NPO, we were only able to perform the statistical analyses on LOS, which clearly showed that EEN is not only a safe method of nutrition but also substantially decreases LOS, resulting in a better and less expensive treatment of mild APP[38].
CONCLUSION
The information collected by basic scientists, retrospective clinical studies and meta-analyses suggests that EEN should have priority in treating APP. However, it is perhaps self-evident that randomized multicenter clinical intervention trials would be crucial to achieving a higher level of evidence.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Hungary
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: All the authors disclaim any form of conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 7, 2016
First decision: October 20, 2016
Article in press: January 11, 2017
P- Reviewer: Cosen-Binker LI, Fujino Y, Luo HS, Peng SY, Sperti C S- Editor: Gong ZM L- Editor: A E- Editor: Liu WX
References
- 1.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187.e1-3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pant C, Deshpande A, Olyaee M, Anderson MP, Bitar A, Steele MI, Bass PF, Sferra TJ. Epidemiology of acute pancreatitis in hospitalized children in the United States from 2000-2009. PLoS One. 2014;9:e95552. doi: 10.1371/journal.pone.0095552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Párniczky A, Czakó L, Dubravcsik Z, Farkas G, Hegyi P, Hritz I, Kelemen D, Morvay Z, Oláh A, Pap Á, Sahin-Tóth M, Szabó F, Szentkereszti Z, Szmola R, Takács T, Tiszlavicz L, Veres G, Szücs Á, Lásztity N. [Pediatric pancreatitis. Evidence based management guidelines of the Hungarian Pancreatic Study Group] Orv Hetil. 2015;156:308–325. doi: 10.1556/OH.2015.30062. [DOI] [PubMed] [Google Scholar]
- 4.Hritz I, Czakó L, Dubravcsik Z, Farkas G, Kelemen D, Lásztity N, Morvay Z, Oláh A, Pap Á, Párniczky A, Sahin-Tóth M, Szentkereszti Z, Szmola R, Szücs Á, Takács T, Tiszlavicz L, Hegyi P. [Acute pancreatitis. Evidence-based practice guidelines, prepared by the Hungarian Pancreatic Study Group] Orv Hetil. 2015;156:244–261. doi: 10.1556/OH.2015.30059. [DOI] [PubMed] [Google Scholar]
- 5.Morinville VD, Husain SZ, Bai H, Barth B, Alhosh R, Durie PR, Freedman SD, Himes R, Lowe ME, Pohl J, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr. 2012;55:261–265. doi: 10.1097/MPG.0b013e31824f1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1–15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 7.Maléth J, Hegyi P. Ca2+ toxicity and mitochondrial damage in acute pancreatitis: translational overview. Philos Trans R Soc Lond B Biol Sci. 2016;371:pii: 20150425. doi: 10.1098/rstb.2015.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maléth J, Hegyi P, Rakonczay Z, Venglovecz V. Breakdown of bioenergetics evoked by mitochondrial damage in acute pancreatitis: Mechanisms and consequences. Pancreatology. 2015;15:S18–S22. doi: 10.1016/j.pan.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Hegyi P, Petersen OH. The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev Physiol Biochem Pharmacol. 2013;165:1–30. doi: 10.1007/112_2013_14. [DOI] [PubMed] [Google Scholar]
- 10.Hegyi P, Pandol S, Venglovecz V, Rakonczay Z. The acinar-ductal tango in the pathogenesis of acute pancreatitis. Gut. 2011;60:544–552. doi: 10.1136/gut.2010.218461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venglovecz V, Rakonczay Z, Ozsvári B, Takács T, Lonovics J, Varró A, Gray MA, Argent BE, Hegyi P. Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea pig. Gut. 2008;57:1102–1112. doi: 10.1136/gut.2007.134361. [DOI] [PubMed] [Google Scholar]
- 12.Hegyi P. Bile as a key aetiological factor of acute but not chronic pancreatitis: a possible theory revealed. J Physiol. 2016;594:6073–6074. doi: 10.1113/JP273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venglovecz V, Hegyi P, Rakonczay Z, Tiszlavicz L, Nardi A, Grunnet M, Gray MA. Pathophysiological relevance of apical large-conductance Ca²+-activated potassium channels in pancreatic duct epithelial cells. Gut. 2011;60:361–369. doi: 10.1136/gut.2010.214213. [DOI] [PubMed] [Google Scholar]
- 14.Voronina SG, Gryshchenko OV, Gerasimenko OV, Green AK, Petersen OH, Tepikin AV. Bile acids induce a cationic current, depolarizing pancreatic acinar cells and increasing the intracellular Na+ concentration. J Biol Chem. 2005;280:1764–1770. doi: 10.1074/jbc.M410230200. [DOI] [PubMed] [Google Scholar]
- 15.Hegyi P, Rakonczay Z. The role of pancreatic ducts in the pathogenesis of acute pancreatitis. Pancreatology. 2015;15:S13–S17. doi: 10.1016/j.pan.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Maléth J, Balázs A, Pallagi P, Balla Z, Kui B, Katona M, Judák L, Németh I, Kemény LV, Rakonczay Z, et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology. 2015;148:427–439.e16. doi: 10.1053/j.gastro.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maléth J, Hegyi P. Calcium signaling in pancreatic ductal epithelial cells: an old friend and a nasty enemy. Cell Calcium. 2014;55:337–345. doi: 10.1016/j.ceca.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Criddle DN. The role of fat and alcohol in acute pancreatitis: A dangerous liaison. Pancreatology. 2015;15:S6–S12. doi: 10.1016/j.pan.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Hegyi P, Wilschanski M, Muallem S, Lukacs GL, Sahin-Tóth M, Uc A, Gray MA, Rakonczay Z, Maléth J. CFTR: A New Horizon in the Pathomechanism and Treatment of Pancreatitis. Rev Physiol Biochem Pharmacol. 2016;170:37–66. doi: 10.1007/112_2015_5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallagi P, Venglovecz V, Rakonczay Z, Borka K, Korompay A, Ozsvári B, Judák L, Sahin-Tóth M, Geisz A, Schnúr A, et al. Trypsin reduces pancreatic ductal bicarbonate secretion by inhibiting CFTR Cl⁻ channels and luminal anion exchangers. Gastroenterology. 2011;141:2228–2239.e6. doi: 10.1053/j.gastro.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judák L, Hegyi P, Rakonczay Z, Maléth J, Gray MA, Venglovecz V. Ethanol and its non-oxidative metabolites profoundly inhibit CFTR function in pancreatic epithelial cells which is prevented by ATP supplementation. Pflugers Arch. 2014;466:549–562. doi: 10.1007/s00424-013-1333-x. [DOI] [PubMed] [Google Scholar]
- 22.Criddle DN, Murphy J, Fistetto G, Barrow S, Tepikin AV, Neoptolemos JP, Sutton R, Petersen OH. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology. 2006;130:781–793. doi: 10.1053/j.gastro.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415; 1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 24.Yokoe M, Takada T, Mayumi T, Yoshida M, Isaji S, Wada K, Itoi T, Sata N, Gabata T, Igarashi H, et al. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci. 2015;22:405–432. doi: 10.1002/jhbp.259. [DOI] [PubMed] [Google Scholar]
- 25.Petrov MS, Whelan K. Comparison of complications attributable to enteral and parenteral nutrition in predicted severe acute pancreatitis: a systematic review and meta-analysis. Br J Nutr. 2010;103:1287–1295. doi: 10.1017/S0007114510000887. [DOI] [PubMed] [Google Scholar]
- 26.Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg. 1997;84:1665–1669. [PubMed] [Google Scholar]
- 27.Abou-Assi S, Craig K, O’Keefe SJ. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: results of a randomized comparative study. Am J Gastroenterol. 2002;97:2255–2262. doi: 10.1111/j.1572-0241.2002.05979.x. [DOI] [PubMed] [Google Scholar]
- 28.Eckerwall GE, Tingstedt BB, Bergenzaun PE, Andersson RG. Immediate oral feeding in patients with mild acute pancreatitis is safe and may accelerate recovery--a randomized clinical study. Clin Nutr. 2007;26:758–763. doi: 10.1016/j.clnu.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Xue GJ, Liu YL, Javed MA, Zhao XL, Wan MH, Chen GY, Altaf K, Huang W, Tang WF. Early oral refeeding wisdom in patients with mild acute pancreatitis. Pancreas. 2013;42:88–91. doi: 10.1097/MPA.0b013e3182575fb5. [DOI] [PubMed] [Google Scholar]
- 30.Petrov MS, McIlroy K, Grayson L, Phillips AR, Windsor JA. Early nasogastric tube feeding versus nil per os in mild to moderate acute pancreatitis: a randomized controlled trial. Clin Nutr. 2013;32:697–703. doi: 10.1016/j.clnu.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8:1179–1207. doi: 10.1016/j.crohns.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Penagini F, Dilillo D, Borsani B, Cococcioni L, Galli E, Bedogni G, Zuin G, Zuccotti GV. Nutrition in Pediatric Inflammatory Bowel Disease: From Etiology to Treatment. A Systematic Review. Nutrients. 2016;8:pii: E334. doi: 10.3390/nu8060334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capurso G, Zerboni G, Signoretti M, Valente R, Stigliano S, Piciucchi M, Delle Fave G. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol. 2012;46 Suppl:S46–S51. doi: 10.1097/MCG.0b013e3182652096. [DOI] [PubMed] [Google Scholar]
- 34.Flint RS, Windsor JA. The role of the intestine in the pathophysiology and management of severe acute pancreatitis. HPB (Oxford) 2003;5:69–85. doi: 10.1080/13651820310001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Márta K, Farkas N, Szabó I, Illés A, Vincze Á, Pár G, Sarlós P, Bajor J, Szűcs Á, Czimmer J, Mosztbacher D, Párniczky A, Szemes K, Pécsi D, Hegyi P. Meta-Analysis of Early Nutrition: The Benefits of Enteral Feeding Compared to a Nil Per Os Diet Not Only in Severe, but Also in Mild and Moderate Acute Pancreatitis. Int J Mol Sci. 2016;17:pii: E1691. doi: 10.3390/ijms17101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 37.Abu-El-Haija M, Wilhelm R, Heinzman C, Siqueira BN, Zou Y, Fei L, Cole CR. Early Enteral Nutrition in Children With Acute Pancreatitis. J Pediatr Gastroenterol Nutr. 2016;62:453–456. doi: 10.1097/MPG.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 38.Szabo FK, Fei L, Cruz LA, Abu-El-Haija M. Early Enteral Nutrition and Aggressive Fluid Resuscitation are Associated with Improved Clinical Outcomes in Acute Pancreatitis. J Pediatr. 2015;167:397–402.e1. doi: 10.1016/j.jpeds.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 39.Raizner A, Phatak UP, Baker K, Patel MG, Husain SZ, Pashankar DS. Acute necrotizing pancreatitis in children. J Pediatr. 2013;162:788–792. doi: 10.1016/j.jpeds.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goh SK, Chui CH, Jacobsen AS. Childhood acute pancreatitis in a children’s hospital. Singapore Med J. 2003;44:453–456. [PubMed] [Google Scholar]
- 41.Flores-Calderón J, Exiga-Gonzaléz E, Morán-Villota S, Martín-Trejo J, Yamamoto-Nagano A. Acute pancreatitis in children with acute lymphoblastic leukemia treated with L-asparaginase. J Pediatr Hematol Oncol. 2009;31:790–793. doi: 10.1097/MPH.0b013e3181b794e8. [DOI] [PubMed] [Google Scholar]
- 42.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morinville VD, Barmada MM, Lowe ME. Increasing incidence of acute pancreatitis at an American pediatric tertiary care center: is greater awareness among physicians responsible? Pancreas. 2010;39:5–8. doi: 10.1097/MPA.0b013e3181baac47. [DOI] [PubMed] [Google Scholar]
- 44.Lopez MJ. The changing incidence of acute pancreatitis in children: a single-institution perspective. J Pediatr. 2002;140:622–624. doi: 10.1067/mpd.2002.123880. [DOI] [PubMed] [Google Scholar]
- 45.Zsoldos F, Párniczky A, Mosztbacher D, Tóth A, Lásztity N, Hegyi P. Pain in the Early Phase of Pediatric Pancreatitis (PINEAPPLE Trial): Pre-Study Protocol of a Multinational Prospective Clinical Trial. Digestion. 2016;93:121–126. doi: 10.1159/000441352. [DOI] [PubMed] [Google Scholar]
- 46.Párniczky A, Mosztbacher D, Zsoldos F, Tóth A, Lásztity N, Hegyi P. Analysis of Pediatric Pancreatitis (APPLE Trial): Pre-Study Protocol of a Multinational Prospective Clinical Trial. Digestion. 2016;93:105–110. doi: 10.1159/000441353. [DOI] [PubMed] [Google Scholar]


