Abstract
AIM
To investigate a suitable long-term culture system and optimal cryopreservation of intestinal organoid to improve organoid-based therapy by acquiring large numbers of cells.
METHODS
Crypts were isolated from jejunum of C57BL/6 mouse. Two hundred crypts were cultured in organoid medium with either epidermal growth factor/Noggin/R-spondin1 (ENR) or ENR/CHIR99021/VPA (ENR-CV). For subculture, organoids cultured on day 7 were passaged using enzyme-free cell dissociation buffer (STEMCELL Technologies). The passage was performed once per week until indicated passage. For cryopreservation, undissociated and dissociated organoids were resuspended in freezing medium with or without Rho kinase inhibitor subjected to different treatment times. The characteristics of intestinal organoids upon extended passage and freeze-thaw were analyzed using EdU staining, methyl thiazolyl tetrazolium assay, qPCR and time-lapse live cell imaging.
RESULTS
We established a three-dimensional culture system for murine small intestinal organoids using ENR and ENR-CV media. Both conditions yielded organoids with a crypt-villus architecture exhibiting Lgr5+ cells and differentiated intestinal epithelial cells as shown by morphological and biochemical analysis. However, during extended passage (more than 3 mo), a comparative analysis revealed that continuous passaging under ENR-CV conditions, but not ENR conditions induced phenotypic changes as observed by morphological transition, reduced numbers of Lgr5+ cells and inconsistent expression of markers for differentiated intestinal epithelial cell types. We also found that recovery of long-term cryopreserved organoids was significantly affected by the organoid state, i.e., whether dissociation was applied, and the timing of treatment with the Rho-kinase inhibitor Y-27632. Furthermore, the retention of typical morphological characteristics of intestinal organoids such as the crypt-villus structure from freeze-thawed cells was observed by live cell imaging.
CONCLUSION
The maintenance of the characteristics of intestinal organoids upon extended passage is mediated by ENR condition, but not ENR-CV condition. Identified long-term cryopreservation may contribute to the establishment of standardized cryopreservation protocols for intestinal organoids for use in clinical applications.
Keywords: intestinal organoid, Rho kinase inhibitor, three-dimensional culture, cryopreservation, long-term culture
Core tip: The phenotypes of intestinal organoids under epidermal growth factor/Noggin/R-spondin1 (ENR) medium were maintained over a long duration, whereas organoid under ENR/CHIR99021/VPA medium exhibited morphological change, reduced numbers of Lgr5+ cells and inconsistent expression of markers for differentiated intestinal epithelial cell types upon extended passages. We also demonstrated an efficacious long-term cryopreservation method for intestinal organoids through optimization of the organoid state and timing of treatment with the Rho kinase inhibitor Y-27632. Thus, the suitable long-term culture system and optimal cryopreservation of small intestinal organoid may contribute to the establishment of standardized cryopreservation protocols for intestinal organoids and subsequent clinical applications of these cell sources.
INTRODUCTION
The gastrointestinal (GI) tract is lined by a monolayer of epithelial cells that separates the intestinal lumen and underlying tissues. The epithelial cells of the small intestine are organized into villi and crypt structures. Intestinal stem cells (ISCs), which express leucine-rich repeat containing G protein-coupled receptor 5 (Lgr5), and their progenitors are located in crypts. ISCs generate daughter cells called transit-amplifying (TA) cells, which either return to stemness or differentiate into a secretory epithelial cells lineage, such as Paneth cells, goblet cells, enteroendocrine cells, or enterocytes[1,2]. The GI tract is highly vulnerable to the external environment, such as radiation. Exposure to high levels of ionizing radiation induces the clonogenic loss of crypt cells and villus depopulation and leads to malabsorption of nutrients and impaired physical barrier function. The resulting breach in the GI barrier, accompanied by immune suppression, results in a high risk of life-threatening infection[3-5]. Many studies have been focused on understanding the mechanisms of radiation-induced gastrointestinal syndrome (RIGS). However, in vitro analysis of RIGS has been hampered by the lack of a suitable culture system.
Long-term maintenance of crypts in traditional two-dimensional (2D) cultures of primary intestinal crypts is difficult due to the poor survival of crypts in vitro[6,7]. Based on three-dimensional (3D) culture systems, long-term cultures in which crypts are able to differentiate and recapitulate normal crypt-villus architecture have been established using crypts isolated from the mouse and human intestine using two different media[8,9]. Initial defined factors present in epidermal growth factor (EGF)/Noggin/R-spondin1 (ENR) medium, which are associated with growth requirements of intestinal epithelium, include EGF to enhance intestinal proliferation, bone morphogenic protein antagonists to induce expansion of crypt numbers, and Wnt agonists to increase crypt proliferation[8,10]. Additionally, small molecule screening showed that ENR/CHIR99021/valproic acid (VPA) (ENR-CV) medium, which was associated with enrichment of intestinal stem cells (Lgr5+) and growth of the intestinal epithelium, included a combination of ENR components and small molecules, such as CHIR99021 (a glycogen synthase kinase 3 inhibitor) and VPA [a histone deacetylase (HDAC) inhibitor][9]. Although both media can support the formation of organoid containing crypt-villus structures that recapitulate the native intestinal epithelium, there is little comparative study of the characteristics of the resulting cells, particularly after long-term continual passage.
In vitro expanded organoids have recently been applied to treat gastrointestinal diseases in preclinical models, supporting the establishment of potential organoid-based therapies for repairing damaged intestine[11,12]. Because clinical applications require large numbers of cells, it may be indispensable to in vitro expansion of organoids in long-term culture with retaining their initial characteristics. In addition, the cells should be capable of being preserved for prolonged periods, while maintaining cell functionality for off-the-shelf use. Cryopreservation may be an attractive technique for maintaining the functional properties and genetic characteristics of cells through long-term storage in order to facilitate the experimental and clinical applications of cell-based therapies[13-15]. However, although various methods have been developed for cryopreservation of different types of stem cells, such as mesenchymal, hematopoietic, and pluripotent stem cells[16-18], protocols for cryopreservation of intestinal organoids have not been described. Therefore, it is necessary to develop an efficient method for optimal cryopreservation of cultured organoids.
In the present study, we performed quantitative assessments to compare the characteristics (e.g., cell morphological phenotype, proliferation, and composition of differentiated intestinal epithelial cell types) of small intestinal organoids subjected to long-term culture under two different media. We also sought to optimize the cryopreservation method by elucidation of the survival of cryopreserved small intestinal organoids through a combination of dissociation and treatment with a Rho kinase (ROCK) inhibitor during freezing. Our findings provided important insights into our understanding of 3D culture systems with similarities to the intestine and contribute to the establishment of standardized cryopreservation protocols for intestinal organoid for use in clinical applications.
MATERIALS AND METHODS
Isolation of small intestinal crypts from mice
All animal experiments were approved by the Animal Investigation Committee of the Korea Institute of Radiological and Medical Sciences in South Korea and were performed according to institutional guidelines and national animal protection laws. Isolation of small intestinal crypts from mice was conducted as described previously with some modifications[8]. Briefly, the jejunum (10 cm from the stomach) of C57BL/6 male mice (8-10 wk age, n = 4) was opened longitudinally, cut into 5-mm pieces, washed three times with cold phosphate-buffered saline (PBS), and incubated with 2 mmol/L ethylenediaminetetraacetic acid (EDTA) in PBS for 15 min at 37 °C. After removal of EDTA solution, the supernatant containing villi was replaced with cold PBS. Crypts were isolated from the basal membrane by vigorous hand shaking for 1 min. This procedure was repeated until enriched crypts could be observed in the supernatant using microscopy. After collection of isolated crypts from tubes by centrifugation, the crypts were resuspended in 2% D-sorbitol (Sigma, St. Louis, MO, United States) in PBS, passed through a 70-μm cell strainer (BD Biosciences, Heidelberg, Germany), and centrifuged at 100 × g for 3 min at 4 °C. The pellet was resuspended in 10 mL basic medium [advanced Dulbecco's modified Eagle's medium/F12, 2 mmol/L L-glutamine, 10 mmol/L HEPES, 100 mg/mL streptomycin, 100 U/mL penicillin, 1 mmol/L N-acetylcysteine, 1% B27, and N2 supplement], and crypt numbers were counted using microscopy.
3D culture of crypts and organoid passage
The isolated crypts were cultured in organoid medium with either ENR or ENR-CV, as previously reported[8,9]. Two hundred crypts in 50 μL matrigel (BD Biosciences) were seeded in each well of a pre-warmed 24-well flat-bottomed plate. Crypts were then incubated for 30 min at 37 °C, and 500 μL of complete crypt culture medium was added. The ENR medium contained basic medium plus 50 ng/mL murine EGF (Invitrogen, Carlsbad, CA, United States), 100 ng/mL murine Noggin (Peprotech, Hamburg, Germany), and 500 ng/mL human R-spondin-1 (R&D Systems, Minneapolis, MN, United States), whereas the ENR-CV medium contained ENR medium plus 1 mmol/L valproic acid (Invitrogen) and 10 μmol/L CHIR99021 (Invitrogen). The crypts were cultured at 37 °C in an atmosphere containing 5% CO2 for the indicated number of days. The medium was changed every 2-3 d. For subculture, the organoids cultured on day 7 were passaged using enzyme-free cell dissociation buffer (STEMCELL Technologies Inc., Vancouver, BC, Canada). Briefly, cultured organoids were washed with cold PBS, and 500 μL cell dissociation buffer was added to the wells and incubated for 5 min at 37 °C. After washing with 0.1% BSA in PBS, dissociated organoids were passaged (a 1:5 ratio). Freshly prepared medium and Matrigel were then added for organoid culture. The passage of organoids cultured under ENR or ENR-CV medium was performed once per week until the indicated passage.
Cell proliferation and crypt viability
For analysis of cell proliferation in organoids by 5-ethynyl-2′-deoxyuridine (EdU) staining, the cultured organoids on the indicated day were incubated with fresh medium containing 10 μmol/L EdU (Molecular Probes, Eugene, OR, United States) for 30 min and then fixed in 4% paraformaldehyde in PBS overnight at 4 °C. The fixed organoids were permeabilized with 0.5% Triton X-100 for 1 h, and following steps were performed using a Click-iT EdU Imaging kit (Molecular Probes) according to the manufacturer's protocol. Hoechst (1:2000) was used for nuclear staining to facilitate cell counting. Images were acquired using an immunofluorescence microscope (Olympus, Shinjuku, Tokyo, Japan). For quantitative analysis of growing organoids, cultured crypts were examined at the indicated time point under bright-field of microscope. Organoids exhibiting at least two budding structures in each group were counted. Experiments were performed in triplicate. The data were expressed as the mean ± SD. For quantitative analysis of crypt viability after freezing and thawing, we performed methyl thiazolyl tetrazolium (MTT) assays as previously reported[19]. Briefly, on the indicated days, cultured organoids were incubated with 10% MTT (AMRESCO, Solon, OH, United States) for 2-3 h at 37 °C. After cell lysis by treatment with 2% sodium dodecyl sulfate (SDS) and dimethyl sulfoxide (DMSO), the optical density (OD) value of the solution was measured at 562 nm using a Synergy HT (BioTek, Winooski, VT, United States). Experiments were performed in triplicate. The data were expressed as the mean ± SD.
Immunofluorescence staining
For immunofluorescence staining, cultured organoids were fixed in 4% paraformaldehyde in PBS overnight at 4 °C. After washing with PBS, organoids were incubated with PBS containing 1% BSA and 0.5% Triton X-100 for 1 h at room temperature, followed by incubation with primary antibodies against lysozyme 1 (1:100; Abcam, Cambridge, MA, United States), mucin 2 (1:100; Dako, Carpenteria, CA, United States), or chromogranin A (1:200; Thermo Scientific). Fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG or anti-mouse IgG (1:100; Invitrogen) was used as a secondary antibody. Images were acquired using a Zeiss 710 confocal microscope (Carol Zeiss, Oberkochen, Germany) and analyzed by imaging software (Olympus America, Center Valley, PA, United States).
Quantitative real-time polymerase chain reaction
Total RNA was prepared from raw crypts (freshly isolated crypts from mice) and cultured crypts using an RNase mini kit (Qiagen, Valencia, CA, United States) according to the manufacturer's protocol. A total of 1 μg of RNA was reverse transcribed using an AccuPower RT PreMix kit (Bioneer, Seoul, South Korea). Real-time PCR was performed with FastStart Essential DNA Green Master Mix (Roche, Indianapolis, IN, United States). All reactions were performed in triplicate. mRNA expression was normalized to endogenous glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression and expressed relative to ENR-derived cells or raw crypts. The primers sequences are listed in Table 1.
Table 1.
Primer sequences used in quantitative polymerase chain reaction analysis
| Gene | Primer sequences (5’-3’) | Annealing temperatures (°C) | |
| mLgr5 | F | ACATTCCCAAGGGAGCGTTC | 60 |
| R | ATGTGGTTGGCATCTAGGCG | ||
| mLyz1 | F | GCCAAGGTCTACAATCGTTGTGAGTTG | 60 |
| R | CAGTCAGCCAGCTTGACACCACG | ||
| mMuc2 | F | ATGCCCACCTCCTCAAAGAC | 60 |
| R | GTAGTTTCCGTTGGAACAGTGAA | ||
| mChgA | F | CCCACTGCAGCATCCAGTT | 60 |
| R | AGTCCGACTGACCATCATCTTTC | ||
| mALP | F | AACTCACCTCATGGGCCTCTT | 60 |
| R | GGGTTTCGGTTGGCATCATA | ||
| mGAPDH | F | TCATCAACGGGAAGCCCATCAC | |
| R | AGACTCCACGACATACTCAGCACCG | ||
GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
Freezing-thawing of in vitro cultured organoids
For organoid cryopreservation, organoids cultured under ENR conditions were left intact or dissociated into single crypt-like colonies using enzyme-free cell dissociation buffer (STEMCELL Technologies Inc.). Undissociated and dissociated organoids were resuspended in freezing medium, e.g., 10% DMSO and 10% fetal bovine serum or recovery cell culture freezing medium (RCCFM; Invitrogen). To determine the effects of Y-27632, a specific inhibitor of ROCK (STEMCELL Technologies Inc.) on the recovery of organoids, organoids were treated with the ROCK inhibitor for different times, including pretreatment for 30 min prior to freezing (before freezing), direct addition into freezing medium (during freezing), and post-thaw treatment for 3 d (after thawing). After storage in liquid nitrogen for 1-3 mo, vials were quickly thawed, and thawed organoids were then cultured for 7 d.
Time-lapse live cell imaging
Live cell imaging was performed on a JuLi stage system (NanoEnTek, Seoul, South Korea). A culture dish placed on the microscope stage was covered with a chamber in 5% CO2 at 37 °C. Images for the growth of crypts were an acquired at 60-min intervals. The data were processed using JuLi stage software v1.0 (NanoEnTek).
Animal care and use statement
All procedures involving were reviewed and approved by the Institutional Animal Care and Use Committee of the South Korea Institute of Radiological and Medical Sciences in Korea, and performed according to the Guidelines for Animal Experimentation of Korea Institute of Radiological and Medical Sciences. The animals were acclimatized to laboratory conditions (23 °C ± 1 °C, 12 h/12 h light/dark, 50% ± 5% humidity and libitum access to food and water) for two, three or four weeks prior to experimentation. All appropriate protocols for study were taken to minimize pain and discomfort of animals.
Statistical analysis
Data are expressed as the mean ± SD or ± SEM of at least two independent samples. Statistical comparisons between groups were performed with two-tailed Student's t-tests or two-way analysis of variance (ANOVA) with Dunnett's T3 tests. Differences with P values of less than 0.05 were considered significant.
RESULTS
Establishment of a small intestinal organoid culture system using ENR and ENR-CV media
In an attempt to establish a conventional culture for intestinal organoids using two different conditions[8,9], freshly isolated crypts from the jejunum of C57/B6 mice were cultured in ENR or ENR-CV medium. Representative images of crypt growth into organoids are shown in Figure 1A. On day 1, crypts formed a round shape, called an enterosphere, and these structures became larger over time. Budding of enterospheres was observed beginning on day 3, and robust budding was observed on day 10, demonstrating a morphology typical of small intestinal organoids with a crypt-villus structure. We found that organoids cultured under ENR-CV conditions yielded increased budding length and size compared with those of organoids cultured under ENR conditions (Figure 1A). Consistent with the results of previous reports (Sato et al[8], 2009; Yin et al[9], 2014), ENR-based organoids exhibited proliferating cells within the crypt domains, whereas proliferating cells were present throughout the organoids under ENR-CV conditions, as shown by EdU staining (Figure 1B). We further confirmed the effects of the ENR-CV medium on enhancement of cell proliferation within organoids by counting the numbers of organoids exhibiting at least two budding structures (Figure 1C).
Figure 1.
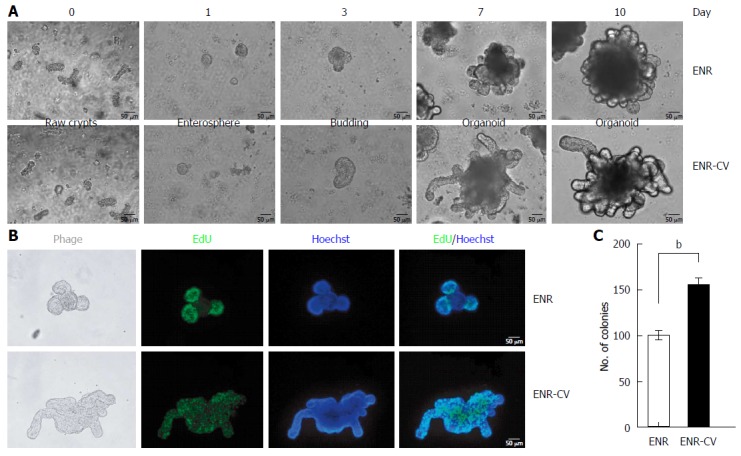
Establishment of small intestinal organoid culture under epidermal growth factor/noggin/r-spondin1 and epidermal growth factor/noggin/r-spondin1-/CHIR99021/VPA conditions. Crypts were isolated from the small intestines of C57/B6 mice at ages 9-12 wk and were resuspended in growth factor-reduced Matrigel. A: Time course of the growth of isolated crypts at passage 0 (P0) under two different culture media. Enterospheres formed on day 1, budding appeared on day 3, and robust budding was observed on days 5-10. Scale bars: 50 μm. B: Organoids were incubated with the thymidine analog EdU (green) for 1 h, and freshly isolated crypts were cultured for 6 d. Images were analyzed by fluorescence microscopy, and nuclei were double stained with Hoechst (blue). Scale bars: 50 μm. C: Numbers of organoids grown in two different media for 7 d. Organoids exhibiting at least two budding structures in each group were counted. The data are shown as means ± SDs of triplicate experiments (bP < 0.01, Student’s t-tests).
Long-term culture induced phenotypic differences in organoids under ENR-CV culture conditions, but not ENR culture conditions
The two different types of medium used in this study have been shown to support long-term culture of intestinal organoids[8-10]. Thus, we aimed to confirm the long-term culture of organoids under our experimental conditions. As shown in Figure 2A, during continuous passage, the morphology of ENR-based organoids was constant, whereas the enhanced size and budding length of organoids in ENR-CV culture conditions were gradually diminished after passage 8 (P8). For a more extensive comparative analysis, we classified organoid into early phase (P0-4) and late phase (P8-12) based on morphological criteria, as shown in Figure 2A, and data are presented the mean of the sum of organoids from P0 to P4 or from P8 to P12 after performing two independent experiments with each passage.
Figure 2.
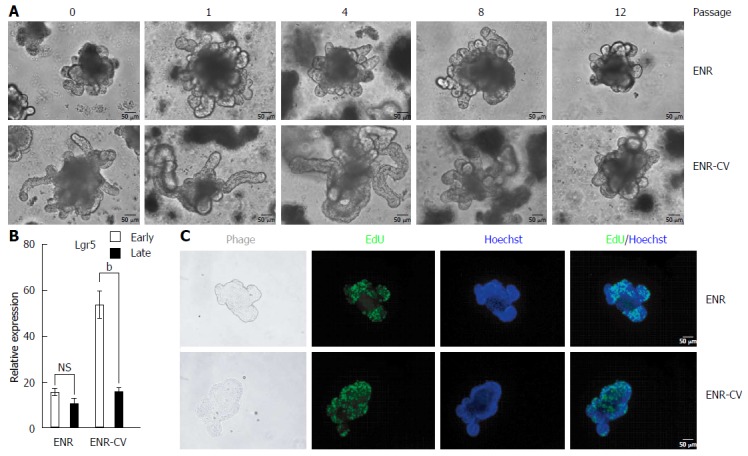
Phenotypic differences of intestinal organoids cultured under epidermal growth factor/noggin/r-spondin1-/CHIR99021/VPA or epidermal growth factor/noggin/r-spondin1 conditions upon continual passage. Organoids cultured for 7 d from freshly isolated crypt were split (1:4) and were cultured. Passage was performed once per week. A: Representative morphology of organoids cultured on day 7 under ENR or ENR-CV conditions upon continual passage. Scale bars; 50 μm. B: Quantitative real-time polymerase chain reaction analysis of relative mRNA expression levels of markers for intestinal stem cells (Lgr5) in organoids at early passage (P0-4) or late passage (P8-12) after culture for 6 d under ENR or ENR-CV conditions. GAPDH was used as an internal control. The data are shown as means ± SEMs of two independent experiments (bP < 0.01, two-way analysis of variance with Dunnett’s T3 tests) and normalized to the value for the ENR condition. Note that the mean of the sum from each passage with triplicate experiments in the indicated early and late passages was used. C: Organoids cultured on day 6 at late passage (P10) were incubated with the thymidine analog EdU (green) for 1 h. Images were analyzed by fluorescence microscopy. Nuclei were double stained with Hoechst (blue). Scale bars: 50 μm. GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; ENR: Epidermal growth factor/Noggin/R-spondin1; ENR-CV: ENR/CHIR99021/VPA.
To evaluate the characteristics of organoids at early and late passages, we analyzed the expression of Lgr5, known marker of ISCs[20], in organoids cultured in the two different media during continuous passage. At early passages, organoids cultured under ENR-CV conditions showed a dramatic increase in Lgr5 expression compared with that of organoids cultured under ENR conditions. These findings were consistent with a previous study showing that the expression level of Lgr5 was upregulated more than 3-fold in organoids cultured under ENR-CV conditions[9]. However, at later passages, Lgr5 expression under ENR-CV conditions was dramatically decreased to a level similar to that under ENR conditions. In contrast, Lgr5 expression levels in organoids cultured under ENR conditions were similar during both early and late passages (Figure 2B). Furthermore, reduced numbers of proliferating cells, which were generally positive for Lgr5, were observed in organoids cultured under ENR-CV conditions during the late phase, as observed by EdU staining (Figure 2C). Consistent with these results, we also detected decreased number of colonies in organoids cultured under ENR-CV conditions upon continual passage, but not in those cultured under ENR conditions, as shown by low-magnification observation of morphology and counting of organoid colonies (Supplementary Figure 1).
Next, we compared the compositions of intestinal epithelial cells in long-term cultured organoids under ENR and ENR-CV conditions. qPCR of intestinal epithelial marker expression showed that the expression levels of Lyz (a paneth cell marker), Muc2 (a goblet cell marker), ChgA (an enteroendocrine cell marker), and ALP (an enterocyte marker) were low and unstable under ENR-CV conditions compared with that under ENR conditions, similar to the expression of epithelial markers in primary raw crypts (Figure 3A). Consistent with this, the result of immunostaining showed the reduced expression of some epithelial markers in organoids under ENR-CV conditions upon continual passage (Figure 3B). In contrast, no changes in these markers were observed in organoids cultured under ENR conditions (data not shown). Therefore, these findings suggested that ENR-CV culture conditions could be susceptible to phenotypic alterations in organoids upon extended passage and may be less relevant to the in vivo composition of intestine cell types.
Figure 3.
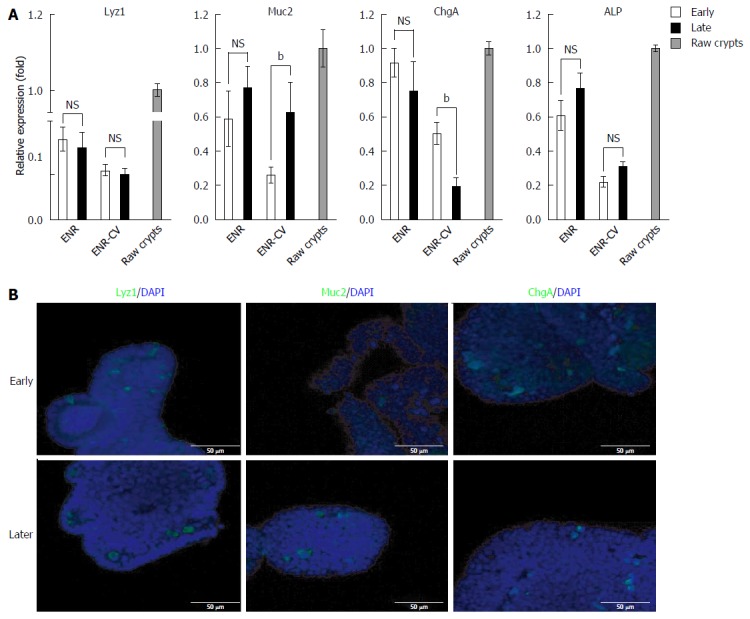
Comparison of the compositions of differentiated intestinal epithelial cells in long-term cultured intestinal organoids under two different media. A: Quantitative real-time polymerase chain reaction analysis of relative mRNA expression of markers for intestinal epithelial cells (Muc2 for goblet cells, ChgA for enteroendocrine cells, Alp for enterocytes, and Lyz1 for paneth cells) in organoids at early passage (P0-4) or late passage (P8-12) cultured for 6 d under ENR or ENR-CV conditions. GAPDH was used as an internal control. The data are shown as means ± SEMs of two independent experiments (bP < 0.01, two-way analysis of variance with Dunnett’s T3 test) and normalized to the ENR value. Note that the mean of the sum from each passage with triplicate experiments in the indicated early and late passages was used. Raw crypts: freshly isolated crypts from mice. B: Representative images show lysozyme (paneth cells), Muc2 (goblet cells), and ChgA (enteroendocrine cells) staining of organoids cultured for 6 d at passages 3 and 10 under ENR-CV conditions. Three-dimensional reconstructed confocal images are shown. Nuclei were double stained with DAPI (blue). Scale bars: 50 μm. GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; ENR: Epidermal growth factor/Noggin/R-spondin1; ENR-CV: ENR/CHIR99021/VPA.
Direct addition of the ROCK inhibitor Y-27632 into freezing media was superior for the recovery of cryopreserved organoids without dissociation
Recent studies have reported the use of continuously cultured intestinal organoids to treat GI disease in mice[11,12], suggesting that organoid-based therapy may have applications in repairing damaged intestines. In order to improve therapeutic technologies, we have examined the optimal conditions for cryopreservation of organoids. To explore the cryopreservation of cultured intestinal organoids under ENR conditions, we first performed freezing-thawing of undissociated and dissociated organoids using 10% DMSO, a traditional cryopreservative[21]. After 1 mo, cryopreserved organoids were thawed in medium and incubated for 7 d. Organoids with a crypt-villus structure were visible from frozen stock only for undissociated organoids (Figure 4A), indicating that undissociated organoids showed better recovery from cryopreservation with 10% DMSO compared with that of dissociated organoids. Similar results were obtained from RCCFM (data not shown). We also extended the storage period of cryopreserved organoids up to 3 mo, which has been used for long-term cryopreservation in previous studies[21,22]. The viability of organoids was dramatically decreased, even in commercial freezing medium, in a time-dependent manner, as shown by MTT assays (Figure 4B).
Figure 4.
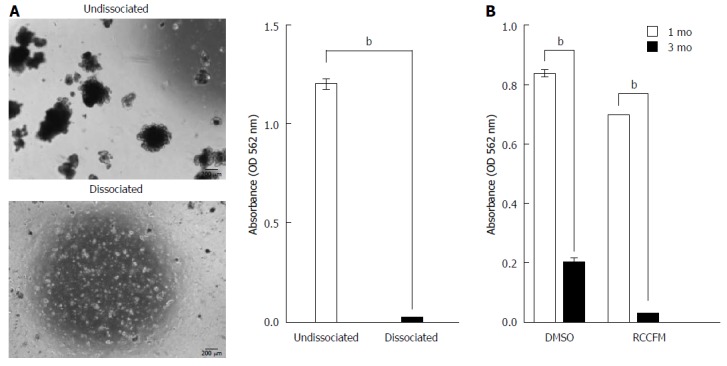
Low recovery of cryopreserved intestinal organoids upon long-term cryopreservation. Cultured organoids (P3) under ENR medium were subjected to dissociation or were left undissociated, followed by cryopreservation. A: Representative morphology (left panel) and quantification of recovery (right panel) for organoids on day 7 cultured under ENR conditions after thawing organoid cryopreserved for 1 mo in the presence of 10% DMSO as a cryoprotectant. Scale bars: 200 μm. The data are shown as the means ± SDs of triplicate MTT assays (bP < 0.01, Student’s t-tests). B: Quantification of recovery from cryopreserved organoid was performed using MTT assay. After 1 or 3 mo of cryopreservation with 10% DMSO or RCCFM (commercial freezing media), organoids were cultured for 7 d under ENR conditions. The data are shown as the mean ± SDs of triplicate experiments (bP < 0.01, two-way analysis of variance with Dunnett’s T3 tests). MTT: Methyl thiazolyl tetrazolium; ENR: Epidermal growth factor/Noggin/R-spondin1.
The survival of various types of stem cells, including ISCs, is enhanced by ROCK inhibition during subculture[8,16]. In addition, ROCK activity and cytoskeletal phenotypes are almost completely inhibited by 10 μmol/L Y-27632[23]. Thus, in this study, we aimed to further optimize the cryopreservation of cultured organoids by examining the effects of Y-27632, a specific inhibitor of ROCK activity, on the recovery of organoids from cryopreserved stocks when added before freezing, during freezing, and after thawing of organoids. By evaluating the densities of grown organoids after freezing-thawing, we found that direct addition of Y-27632 into freezing medium during freezing resulted in superior recovery compared with that of untreated organoids, organoids pretreated with Y-27632, or organoids treated with Y-27632 after thawing (Figure 5A). Consistent with this, MTT analysis revealed that there was a higher rate of recovery from direct addition of Y-27632 during freezing (> 2.5 fold) upon cryopreservation, compare with that observed under other conditions (Figure 5B). We also observed similar effects of Y-27632 in commercial freezing medium when the drug was directly added during freezing (data not shown), and the typical organoid morphology with a crypt-villus structure was further confirmed by tracing the growth of organoids for 7 d after freezing-thawing, as shown by live-imaging analysis (Video data). In contrast to undissociated organoids, we did not observe improvements in dissociated organoids following treatment with Y-27632 (Supplementary Figure 2). Taken together, these results suggested that the recovery of cryopreserved intestinal organoids was significantly improved when the ROCK inhibitor Y-27632 was used for treatment of undissociated organoids rather than dissociated organoids during freezing.
Figure 5.
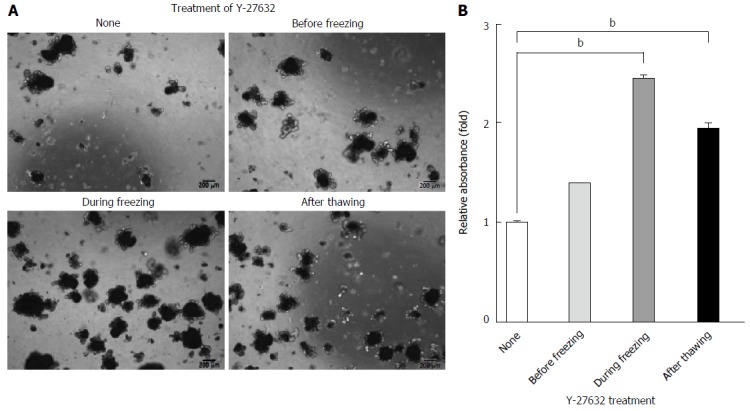
Enhanced recovery of long-term cryopreserved intestinal organoids was dependent on the timing of Y-27632 treatment. Undissociated organoids (P3) were treated with 10 μmol/L Y-27632 at the indicated time points. A: Representative morphology of organoids cultured on day 7 in the presence of ENR medium after thawing organoids cryopreserved for 3 mo in the presence of 10% DMSO. Scale bars, 200 μm. B: Quantification of recovery from cryopreserved organoids was performed using MTT assays. The data are shown as means ± SDs of triplicate experiments (bP < 0.01, two-way analysis of variance with Dunnett’s T3 tests) and normalized to untreated samples. MTT: Methyl thiazolyl tetrazolium; ENR: Epidermal growth factor/Noggin/R-spondin1.
DISCUSSION
Previous studies reported that long-term culture of intestinal organoids could be supported through either ENR or ENR-CV medium in a 3D culture system with a Matrigel matrix[8,10,12] , and in vitro cultured intestinal organoids may have applications in organoid-based therapy as shown in studies investigating the repair of damaged intestines in mice[11,12]. Here, we have extended these studies to determine a suitable long-term culture system and optimal cryopreservation of small intestinal organoid. We found that the phenotypes of intestinal organoids under ENR media were maintained over a long duration, whereas organoid under ENR-CV media exhibited morphological alterations, reduced numbers of Lgr5+ cells and inconsistent expression of markers for differentiated intestinal epithelial cell types upon extended passages. We also identified an efficacious cryopreservation method for expansion of undissociated intestinal organoids. For undissociated intestinal organoids, direct addition of the ROCK inhibitor Y-27632 during freezing permitted superior recovery of crypts after long-term cryopreservation.
Using established cultures of intestinal organoids under two different media, we confirmed that the characteristics of intestinal organoids under ENR-CV medium in the early passage were consistent with a previous report showing enrichment of Lgr5+ expression, enhanced organoid size and budding length, and rapid proliferating cells[9]. However, upon extended passaging under ENR-CV conditions, but not ENR conditions, we observed phenotypic changes, such as reduced size and budding length of organoid, accompanied by reduced expression of Lgr5, an ISC marker, and upregulation of Muc2, a goblet cell marker (Figures 2 and 3). Although these findings are contradictory to the report by Yin et al[9] , who showed maintenance of Lgr5+ stem cells during long-term passage, our findings were consistent with other reports demonstrating conversion of proliferating progenitors into secretory cells, along with loss of stem cells expressing Lgr5 in the context of inhibited Notch signaling[24]. The Notch signaling pathway contributes to enhancement of Lgr5+ stem cell proliferation and suppresses the differentiation of these ISCs into secretory cells, such as goblet and enteroendocrine cells. In contrast, Wnt signaling is associated with the formation of paneth cells, which we found to be unaltered as shown by Figure 3A[25-27]. Thus, we analyzed the expression of Notch signaling-associated molecules, including Notch family members and Hes1, in ENR and ENR-CV cultured organoids upon extended passage. However, the gene expression patterns were similar for organoids cultured under both conditions (data not shown). This suggested that the Notch signaling pathway was not involved in the observed changes under our culture conditions. Interestingly, although the expression of Lgr5 in intestinal organoids cultured in ENR-CV medium was reduced to a level similar to that of ENR-cultured intestinal organoids during late passages, indicating that these events may result from the reduced effects of small molecules, this relationship did not seem to be causal because the composition ratio of differentiated epithelial cells in long-term cultured intestinal organoids under ENR and ENR-CV conditions was not well correlated (Figure 3A). It is unclear why enhanced expression of Lgr5 was diminished upon continual passage in this study; however, a recent report demonstrated that loss of Lgr5+ stem cells is often observed as an unexpected side effect in patients treated with HDAC inhibitors[28]. Therefore, it is likely that changes in the phenotype and composition ratio of functionally differentiated cells in intestinal organoids under ENR-CVin long-term culture may be attributed to prolonged treatment with valproic acid, a known HDAC inhibitor[29].
In order to determine the mechanisms underlying RIGS at the cellular level, in-depth characterization of intestinal epithelial cells within in vitro cultured intestinal organoids is necessary. A previous study compared the characteristics of these cells under two different media[9]. Moreover, our current findings further showed that both media could support the long-term culture of intestinal organoids, recapitulating the crypt-villus architecture in vivo with ISCs (Lgr5) and differentiated intestinal epithelial cells, consistent with previous reports[8,9]. Based on the comparative analysis in our study, including analysis of raw crypts, we found that the expression levels of most markers of differentiated intestinal epithelial cells in ENR-cultured organoid were higher than those in ENR-CV-cultured organoids, regardless of whether the organoids were cultured long term. Furthermore, upon continuous passaging, the expression levels of epithelial cell markers in intestinal organoids under ENR conditions were constant and similar to the expression levels of corresponding markers in primary raw crypts, suggesting that ENR conditions may be appropriate for long-term culture of intestinal organoids and that the characteristics of ENR culture were relevant to determining the in vivo composition of small intestine cell types. Given that the specialized cellular niche plays an important role in the maintenance of intestinal homeostasis by creating a unique environment in vivo[30], our data emphasized that the ENR-based intestinal organoid system may be useful for analysis of the mechanisms of radiation induced-intestinal cell death and that results obtained from the ENR-CV culture system, particularly for long-term culture, should be interpreted cautiously.
One of the most important findings in this study was that recovery of cryopreserved intestinal organoids was dependent on the timing of Y-27632 treatment and the absence of dissociation. We found that intact organoids, not dissociated organoids, were efficiently cryopreserved in the presence of 10% DMSO as standard components in slow-freezing protocols[15,21]. Among current cryopreservation methods, including slow or fast freezing (vitrification), conventional slow-freezing protocols are generally effective in presence of DMSO as a cryoprotectant, are less labor intensive, and allow for handling of bulk quantities of cells[15,31]. However, DMSO is known to be toxic to tissues and cells and is considered an appropriate cryoprotectant for short-term storage owing to its time-dependent toxicity[31]. Indeed, we observed that low survival rates after freeze-thaw of cryopreserved organoids following extended storage (Figure 4). Importantly, however, addition of Y-27632 at the time of freezing improved the recovery of freeze-thawed intestinal organoids. Although Y-27632 is known to be a potent inhibitor of apoptosis and to facilitate the survival of dissociated stem cells during subculture including ISCs[8,16,32], we did not observe efficient recovery of cryopreserved intestinal organoids when dissociated organoids were treated with ROCK inhibitor directly into the freezing medium (Supplementary Figure 2). These differences may be explained by the toxicity of DMSO, which varies from cell type to cell type during cryopreservation[31].
Our live-imaging data indicated the characteristics of long-term cryopreserved intestinal organoids by tracing the growth of organoids having a typical intestinal organoid phenotype with a crypt-villus structure. Further studies are required to determine whether subtle genetic alterations can be induced by cryopreservation with the ROCK inhibitor Y-27632. In the present study, undissociated intestinal organoids, but not dissociated organoids, were effectively cryopreserved and propagated after long-term cryopreservation by incorporating the ROCK inhibitor Y-27632 directly into the freezing medium.
In conclusion, using a comparative analysis of the characteristics of long-term cultured small intestinal organoids under two different culture conditions, we demonstrated that ENR-CV condition, but not ENR conditions, induced phenotypic transition in in vitro cultured small intestinal organoids upon extended passaging. We also identified an efficacious long-term cryopreservation method for intestinal organoids through optimization of the organoid state and timing of treatment with the ROCK inhibitor Y-27632. This method may contribute to the establishment of standardized cryopreservation protocols for intestinal organoids and subsequent clinical applications of these cell sources.
ACKNOWLEDGMENTS
The authors would like to thank Songwon Seo, Chief of the laboratory of Low Dose Risk Assessment, National Radiation Emergency Medical Center, Korea Institute of Radiological and Medical Science for his statistical support.
COMMENTS
Background
Recent studies have suggested that in vitro cultured intestinal organoids can be introduced to manage gastrointestinal diseases, supporting the development of promising organoid-based therapies for repair of damaged intestines. To improve organoid-based therapeutic technologies by acquiring large numbers of cells for clinical application, it is essential for long-term maintenance of characteristics and optimal cryopreservation method of intestinal organoid.
Research frontiers
Two different media [epidermal growth factor/Noggin/R-spondin1 (ENR) and ENR/CHIR99021/VPA (ENR-CV)] can support the formation of organoid containing crypt-villus structures that recapitulate the native intestinal epithelium. However, there is little comparative study of the characteristics of the resulting cells, particularly after long-term continual passage. In addition, it has not been well described for optimal cryopreservation methods for maintaining the functional properties of intestinal organoids in order to facilitate the experimental and clinical applications of organoid-based therapies.
Innovations and breakthroughs
This is the first study to report a continuous passages-induced phenotypic difference of intestinal organoid under ENR-CV condition, but not ENR condition which is suitable to long-term culture. The authors also demonstrate that efficient long-term cryopreservation of organoids is associated with a combination of organoid state and timing of treatment with the Rho kinase (ROCK) inhibitor.
Applications
This study provide important insights into our understanding of 3D culture systems for intestine-related organs and contribute to the establishment of standardized cryopreservation protocols for intestinal organoids on application of organoid-based therapy.
Peer-review
The manuscript by Han et al described that phenotypes of mouse intestinal organoids under ENR media were maintained over a long duration, and organoids under ENR-CV media exhibited morphological alterations. They also found that adding the Rock inhibitor Y-27632 during freezing benefits recovery of undissociated intestinal organoids after long-term cryopreservation. The manuscript is succinct and the conclusions are well supported by the data.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was revised and approved by the Korea Institute of Radiological and Medical Sciences Institutional Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Korea Institute of Radiological and Medical Sciences in Korea (IACUC protocol number: kirams2016-0043).
Conflict-of-interest statement: The authors declare that they have no potential conflicts of interest.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 6, 2016
First decision: October 20, 2016
Article in press: November 23, 2016
P- Reviewer: Pan QW S- Editor: Gong ZM L- Editor: A E- Editor: Liu WX
References
- 1.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Baer AR, Cheeseman CI, Thomson AB. The assessment of recovery of the intestine after acute radiation injury. Radiat Res. 1987;109:319–329. [PubMed] [Google Scholar]
- 4.Shim S, Jang WS, Lee SJ, Jin S, Kim J, Lee SS, Bang HY, Jeon BS, Park S. Development of a new minipig model to study radiation-induced gastrointestinal syndrome and its application in clinical research. Radiat Res. 2014;181:387–395. doi: 10.1667/RR13207.1. [DOI] [PubMed] [Google Scholar]
- 5.Williams JP, McBride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS) Int J Radiat Biol. 2011;87:851–868. doi: 10.3109/09553002.2011.560996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossmann J, Maxson JM, Whitacre CM, Orosz DE, Berger NA, Fiocchi C, Levine AD. New isolation technique to study apoptosis in human intestinal epithelial cells. Am J Pathol. 1998;153:53–62. doi: 10.1016/S0002-9440(10)65545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaeffer B. Mammalian intestinal epithelial cells in primary culture: a mini-review. In Vitro Cell Dev Biol Anim. 2002;38:123–134. doi: 10.1290/1071-2690(2002)038<0123:MIECIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 9.Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11:106–112. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 11.Shaker A, Rubin DC. Stem cells: One step closer to gut repair. Nature. 2012;485:181–182. doi: 10.1038/485181a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5⁺ stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 13.Davies OG, Smith AJ, Cooper PR, Shelton RM, Scheven BA. The effects of cryopreservation on cells isolated from adipose, bone marrow and dental pulp tissues. Cryobiology. 2014;69:342–347. doi: 10.1016/j.cryobiol.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Hubel A. Parameters of cell freezing: implications for the cryopreservation of stem cells. Transfus Med Rev. 1997;11:224–233. doi: 10.1053/tmrv.1997.0110224. [DOI] [PubMed] [Google Scholar]
- 15.Hunt CJ. Cryopreservation of Human Stem Cells for Clinical Application: A Review. Transfus Med Hemother. 2011;38:107–123. doi: 10.1159/000326623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Ibañez R, Unger C, Strömberg A, Baker D, Canals JM, Hovatta O. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum Reprod. 2008;23:2744–2754. doi: 10.1093/humrep/den316. [DOI] [PubMed] [Google Scholar]
- 17.Son JH, Heo YJ, Park MY, Kim HH, Lee KS. Optimization of cryopreservation condition for hematopoietic stem cells from umbilical cord blood. Cryobiology. 2010;60:287–292. doi: 10.1016/j.cryobiol.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Yong KW, Pingguan-Murphy B, Xu F, Abas WA, Choi JR, Omar SZ, Azmi MA, Chua KH, Wan Safwani WK. Phenotypic and functional characterization of long-term cryopreserved human adipose-derived stem cells. Sci Rep. 2015;5:9596. doi: 10.1038/srep09596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabinger T, Luks L, Kostadinova F, Zimberlin C, Medema JP, Leist M, Brunner T. Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis. 2014;5:e1228. doi: 10.1038/cddis.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 21.Katkov II, Kim MS, Bajpai R, Altman YS, Mercola M, Loring JF, Terskikh AV, Snyder EY, Levine F. Cryopreservation by slow cooling with DMSO diminished production of Oct-4 pluripotency marker in human embryonic stem cells. Cryobiology. 2006;53:194–205. doi: 10.1016/j.cryobiol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Lee YA, Kim YH, Kim BJ, Kim BG, Kim KJ, Auh JH, Schmidt JA, Ryu BY. Cryopreservation in trehalose preserves functional capacity of murine spermatogonial stem cells. PLoS One. 2013;8:e54889. doi: 10.1371/journal.pone.0054889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20:242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240.e1-7. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreu P, Peignon G, Slomianny C, Taketo MM, Colnot S, Robine S, Lamarque D, Laurent-Puig P, Perret C, Romagnolo B. A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Dev Biol. 2008;324:288–296. doi: 10.1016/j.ydbio.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529.e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 27.VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimberlin CD, Lancini C, Sno R, Rosekrans SL, McLean CM, Vlaming H, van den Brink GR, Bots M, Medema JP, Dannenberg JH. HDAC1 and HDAC2 collectively regulate intestinal stem cell homeostasis. FASEB J. 2015;29:2070–2080. doi: 10.1096/fj.14-257931. [DOI] [PubMed] [Google Scholar]
- 29.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 30.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 31.Thirumala S, Goebel WS, Woods EJ. Clinical grade adult stem cell banking. Organogenesis. 2009;5:143–154. doi: 10.4161/org.5.3.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76:722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]


