ABSTRACT
Mouse embryos conditionally lacking Tgif1 and Tgif2 have holoprosencephaly and defects in left-right asymmetry. To identify pathways affected by loss of Tgif function during embryogenesis, we performed transcriptome profiling on whole mouse embryos. Among the genes with altered expression in embryos lacking Tgifs were a number with links to cilium function. One of these, Evi5l, encodes a RabGAP that is known to block the formation of cilia when overexpressed. Evi5l expression is increased in Tgif1; Tgif2-null embryos and in double-null mouse embryo fibroblasts (MEFs). Knockdown of Tgifs in a human retinal pigment epithelial cell line also increased EVI5L expression. We show that TGIF1 binds to a conserved consensus TGIF site 5′ of the human and mouse Evi5l genes and represses Evi5l expression. In primary MEFs lacking both Tgifs, the number of cells with primary cilia was significantly decreased, and we observed a reduction in the transcriptional response to Shh pathway activation. Reducing Evi5l expression in MEFs lacking Tgifs resulted in a partial restoration of cilium numbers and in the transcriptional response to activation of the Shh pathway. In summary, this work shows that Tgifs regulate ciliogenesis and suggests that Evi5l mediates at least part of this effect.
KEYWORDS: Evi5l, Tgif, Tgif1, Tgif2, cilium, regulation of gene expression, transcription factors
INTRODUCTION
Tgif1 and Tgif2 are related homeodomain-containing transcriptional repressors that are members of the TALE superfamily (1–4). The best-characterized role of Tgif1 and Tgif2 is in the regulation of transforming growth factor β (TGF-β)-responsive gene expression (3, 5, 6). In response to TGF-β family signaling, receptor-activated Smads are phosphorylated, bind the shared Smad4, and accumulate in the nucleus, where they regulate gene expression (7–9). Smad2 and Smad3 are the primary receptor-activated Smads that respond to TGF-β, Activin, and Nodal, whereas Smad1, -5, and -9 mediate the majority of BMP signaling. Tgifs interact with Smad2 and Smad3, resulting in the recruitment of Tgifs to DNA (3, 5). The binding of Tgifs limits expression of genes that are normally activated by TGF-β signaling, in part by competing with coactivators for Smad interaction and in part via active repression. Tgif1 interacts with general transcriptional corepressors, including CtBP1 and mSin3a, and the related Tgif2 binds mSin3a but lacks the CtBP interaction motif (10–12). Repression of TGF-β-dependent gene expression by Tgifs likely involves recruitment of general corepressors to the Smad complex, although other mechanisms for regulating TGF-β responses have been proposed (13, 14).
TGIF1 was first identified by its ability to bind to a specific retinoid response element (RXRE) from the rat CRBPII gene (1). TGIF1 was shown to limit transcription via this element. An interaction of Tgif1 with certain nuclear receptors has been shown, resulting in recruitment to nuclear receptor binding sites and repression of target genes (15, 16). Tgifs can also regulate gene expression when bound directly to DNA in the absence of Smads or nuclear receptors (4). Tgifs bind to a 6- to 7-base consensus site, and binding of TGIF1 to the site represses transcription, partly by recruiting general corepressors (1–4). In addition to active repression, Tgifs may also compete for binding to their cognate element with related TALE family homeodomain proteins, such as Meis2, which is a transcriptional activator (17, 18). However, relatively few direct Tgif target genes have been analyzed, although recent chromatin immunoprecipitation sequencing (ChIP-seq) analysis in mouse embryonic stem (ES) cells suggests there are a large number of potential Tgif1 binding sites throughout the genome (19).
Loss-of-function mutations in the human TGIF1 gene are associated with holoprosencephaly (HPE), a severe craniofacial disorder affecting forebrain development and midline patterning (20–22). Although these human TGIF1 mutations are heterozygous, deletion of Tgif1 in mice had relatively mild phenotypes even as a homozygous mutation (15, 23–25). However, deletion of both Tgif1 and Tgif2 resulted in gastrulation defects and altered Nodal responses, consistent with a role for Tgifs in TGF-β family signaling (26). Conditional mutation of Tgif1 in the background of a Tgif2-null mutation allowed bypass of gastrulation defects, and the resulting embryos had HPE and changes in the Shh signaling pathway, confirming Tgifs as regulators of forebrain development (26, 27). In addition to HPE, conditional double mutants have defects in left-right (L-R) asymmetry and do not survive past approximately 11 days of embryogenesis.
Primary cilia are specialized organelles that are assembled over the basal body during the G1 phase of the cell cycle (28, 29). The cilium, which protrudes from the surface in many cell types, consists of a central core of acetylated tubulin that is surrounded by a compartmentalized cell membrane domain. Transport into and out of the primary cilium is mediated by specific intraflagellar transport (IFT) complexes, which are also responsible for the regulated assembly and disassembly of cilia as cells progress through the cell cycle (30, 31). Primary cilia are important sensory organelles and mediate certain signaling pathways, such as Shh signaling (30, 32). Components of the Shh signaling pathway, including the Patched and Smoothened transmembrane receptors and the intracellular mediators, the Gli proteins, are present in primary cilia (33–35). This localization is required for the majority of Shh signaling, with the Gli proteins being activated in the primary cilium prior to translocation to the nucleus. Defects in ciliogenesis in certain mouse mutants cause phenotypes that are similar to those seen in mice with mutations affecting the Shh pathway, further reinforcing the importance of the organelle in Shh signaling (32).
In an effort to identify genes and pathways regulated by Tgifs, we performed transcriptome profiling by high-throughput RNA sequencing (RNA-seq) on wild-type (WT) embryos and embryos lacking both Tgif1 and Tgif2. This analysis identified a large number of genes that were differentially expressed, and here, we have focused on a small subset of these genes that may play a role in ciliogenesis. We show increased expression of a RabGAP, encoded by the Evi5l gene, which was previously shown to limit ciliogenesis (36). Deleting Tgifs results in increased Evi5l expression and reduced numbers of cells with cilia. Finally, we show that Tgif1 binds directly to a conserved element in the Evi5l gene and represses its expression. This work identifies Evi5l as a direct Tgif target gene and suggests that Tgifs play a role in regulating ciliogenesis.
RESULTS
Transcriptomic analysis of cdKO embryos.
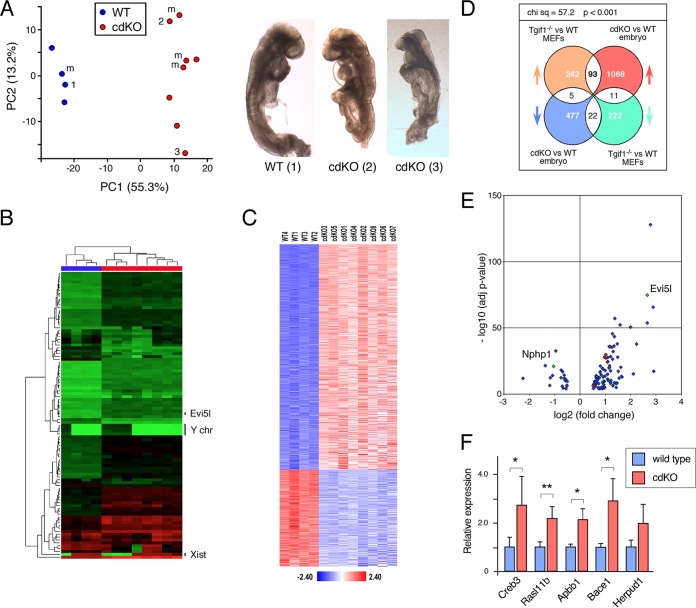
We have previously characterized embryos lacking both Tgif1 and Tgif2, generated with Sox2Cre and a conditional Tgif1 allele, which results in deletion of Tgif1 throughout the epiblast (Tgif1f/f; Tgif2−/−; Sox2Cre+, referred to as cdKO, for conditional double knockout). These cdKO embryos have a number of phenotypes, including HPE, defects in Shh signaling, and L-R asymmetry, and do not generally survive beyond ∼11.0 days postcoitum (dpc) (26, 27). In an effort to identify pathways or transcriptional programs that are altered in the absence of Tgifs, we performed RNA-seq analysis on whole embryos isolated at 8.5 to 9.0 dpc, a stage at which defects are clearly evident. Wild-type and cdKO embryos were isolated from separate litters and approximately stage matched so that the wild types had 9 to 10 somites, equivalent to 8.5 to 8.75 dpc in this strain background. Since somite structure was poorly defined in the cdKO embryos, we stage matched cdKOs by embryo size and overall appearance, focusing on litters isolated at approximately 9.0 dpc. Representative images of embryos used for this analysis are shown in Fig. 1A. Although analysis of whole embryos could mask changes in gene expression restricted to part of the embryo, this approach might identify more fundamental changes in gene expression in the absence of Tgifs. As shown in Fig. 1A, principal-component analysis (PCA) showed that the embryos clustered quite tightly by genotype, with some spread in the cdKOs. However, the majority of the variation in the data is accounted for by PC1, with only 13.2% being attributed to PC2. The two cdKO embryos shown span most of the range of the data as separated by PC2. We also performed unsupervised hierarchical clustering of the data based on the 100 most variable genes, and again, the wild-type and cdKO embryos clustered into two separate groups (Fig. 1B). Within each group in this analysis, embryos also clustered by expression of Xist and the reciprocal expression of a small group of genes on the Y chromosome. Using this as an indicator of gender, it appears that gender was not a major driver of the clustering in the PCA (Fig. 1A).
FIG 1.
Transcriptome analysis of cdKO embryos. RNA was isolated from four wild-type and eight cdKO embryos and analyzed by RNA-seq. (A) Principal-component analysis of the RNA-seq data. The three numbered points correspond to the three numbered embryos shown on the right. m, male embryos as determined by analysis of the RNA-seq data. (B) Unsupervised hierarchical clustering of the RNA-seq data, based on the 100 most variable genes. The positions of Xist and a small cluster of male-specific genes from the Y chromosome (chr) are shown, as is the position of Evi5l. (C) RNA-seq data were filtered using a log fold change of ±0.5 and an adjusted P value cutoff of <0.0001 and are displayed as the Z-scores for the 1,676 genes that passed this cutoff when comparing the wild type to the cdKO. (D) Analysis of the overlap between the data and Affyemtrix expression array data from wild-type and Tgif1-null primary MEFs. The distributions of the genes in the four overlapping segments were compared by chi-square analysis using a 2-by-2 contingency table. (E) Volcano plot for the P value versus the fold change for the 93 and 22 genes that increase or decrease, respectively, in both analyses shown in panel D. The red dots are genes tested for panel F, and Nphp1 and Evi5l are indicated. (F) Four wild-type and four cdKO embryos were analyzed by qRT-PCR for a selection of the genes in the overlap between the embryo RNA-seq and the MEF expression analysis. Relative expression compared to the wild type is shown. *, P < 0.05; **, P < 0.01. The error bars indicate standard deviations (SD).
Comparison of wild-type and cdKO embryos identified 1,676 genes that were differentially expressed (log2 fold change, greater than ±0.5; adjusted P value, <0.0001), of which 1,172 (70%) increased in the cdKO embryos compared to the wild type (see Tables S1 to S3 in the supplemental material). Displaying the Z-scores of each of these genes for all embryos revealed some variability, particularly in the cdKOs; however, it was not possible to identify any embryos as extreme outliers in their overall patterns from this analysis or from the PCA (Fig. 1A and C). We previously analyzed gene expression in primary mouse embryo fibroblasts (MEFs) lacking Tgif1 by Affymetrix expression array. This identified 2,095 probe sets that collapsed to 340 genes that increased in the absence of Tgif1 and 255 that decreased (6). We used this to filter the RNA-seq data generated here in an effort to focus on higher-confidence potential Tgif target genes. Comparing these two data sets identified 131 differentially expressed genes in common (see Table S4 in the supplemental material), with the majority (93 genes) increased in both analyses (Fig. 1D and E). We therefore focused on this list and tested a small panel of genes (Fig. 1E, red dots) by quantitative reverse transcription (qRT)-PCR in a set of four wild-type and four cdKO embryos that were distinct from those used for the initial RNA-seq analysis. Of the five genes tested, four increased significantly, and while the fifth increased, it was too variable to reach significance in this set of embryos (Fig. 1F). This analysis suggested that genes within the overlap between the two data sets were likely to be Tgif targets.
Increased Evi5l expression in cdKO embryos.
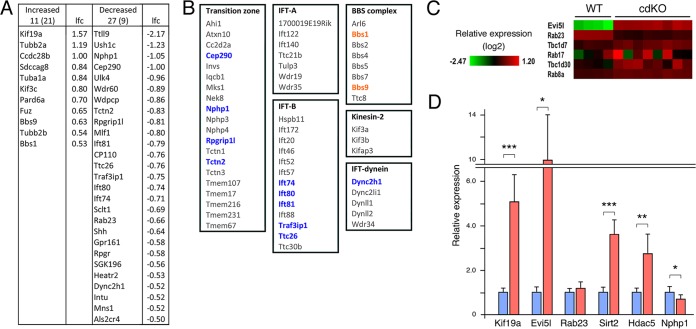
Gene ontology (GO) term analysis of the RNA-seq data revealed enrichment for genes involved in DNA metabolic processes and patterning in the downregulated gene list, consistent with previously identified phenotypes (Table 1). There was enrichment for extracellular matrix and cytoskeleton among the upregulated genes, suggesting alterations in cell architecture. Among the most significantly upregulated genes, we noticed the Evi5l gene, which encodes a RabGAP (Fig. 1E; see Tables S1 and S2 in the supplemental material). EVI5L was identified in a screen for RabGAPs, which when overexpressed reduced cilium numbers in retinal pigment epithelial cells. (36). Additionally, expression of a small group of Ift genes that encode components of the ciliary transport machinery was lower in the cdKOs (Fig. 2A; see Table S3 in the supplemental material). To examine if there were changes in multiple genes with functions in cilia, we compared our RNA-seq data to a curated list of cilium components (SYSCILIA [http://www.syscilia.org/goldstandard.shtml; 37]). As shown in Fig. 2A, there was some overlap with genes that were significantly altered in cdKO embryos. While this suggests that Tgifs do not regulate a large number of cilium-related genes, there were changes in expression of more genes than would be expected by chance. As shown in Fig. 2B, expression of the genes encoding four of the transition zone components (38) and five components of the IFT-B complex (39) decreased significantly in the absence of Tgifs. Although these changes could be consistent with cilium defects, putative direct Tgif target genes would be expected to show increased expression in the cdKO. Among the genes that significantly increased in the cdKO were Evi5l, Kif19a, and two potential tubulin deacetylase genes (Hdac5 and Sirt2 [40, 41]). Kif19a limits the formation of motile cilia in multiciliated cells, as mice with a targeted mutation in Kif19a had longer cilia (42). As mentioned above, Evi5l encodes a RabGAP that when overexpressed in human RPE cells decreased the number of cells with cilia (36). Two other RabGAPs (Tbc1d7 and Tbc1d30) also decreased cilium numbers in this screen but did not change significantly in our RNA-seq data (Fig. 2C). Similarly, no change in their cognate Rabs was observed here, whereas Rab23 (the Evi5l Rab) showed a small but significant decrease in the RNA-seq data. To validate the change in Evi5l expression, we analyzed embryos by qRT-PCR for Evi5l and a small number of other genes for comparison. As shown in Fig. 2D, Evi5l expression was significantly higher in cdKO than in wild-type embryos, whereas Rab23 expression did not change in this set of embryos. We also confirmed the changes observed by RNA-seq for Kif19a, Hdac5, and Sirt2 and the decrease in Nphp1 expression (Fig. 2D).
TABLE 1.
GO analysisa
| GO term | Fold enrichment | FDRb | Regulation |
|---|---|---|---|
| Extracellular matrix | 2.63 | 3.71E−06 | Up |
| Proteinaceous extracellular matrix | 2.62 | 9.64E−06 | Up |
| Cytoskeleton | 1.56 | 0.0085 | Up |
| Intracellular signaling cascade | 1.64 | 0.0094 | Up |
| Endosome | 2.26 | 0.0309 | Up |
| Muscle system process | 3.90 | 0.0370 | Up |
| Regionalization | 4.55 | 4.94E−06 | Down |
| DNA metabolic process | 2.99 | 2.94E−04 | Down |
| Anterior/posterior pattern formation | 4.77 | 3.69E−04 | Down |
| Pattern specification process | 3.43 | 9.47E−04 | Down |
| DNA repair | 3.65 | 0.0041 | Down |
| Response to DNA damage stimulus | 3.25 | 0.0042 | Down |
| Cellular response to stress | 2.61 | 0.0382 | Down |
Genes with a log2 fold change of more than ±0.5 and an adjusted P value of <0.0001 were analyzed for GO terms (DAVID [https://david.ncifcrf.gov/]).
FDR, false-discovery rate. Only terms with an FDR of <0.05 are shown.
FIG 2.
Analysis of ciliogenesis-related genes in cdKO embryos. (A) Genes that were significantly differently expressed by RNA-seq were compared to the SYSCILIA database, and the overlap is shown in separate lists for genes with increased and decreased expression in the cdKO, together with the log2 fold change (lfc) for each. The numbers above the columns indicate the numbers of genes that changed, with the expected numbers in parentheses. (B) Summary of components of specific cilium subcomplexes, with genes that decreased in the cdKO shown in blue and those that increased in orange. (C) Relative expression (RNA-seq) of three RabGAPs and their associated Rabs. (D) Wild-type and cdKO embryos were analyzed by qRT-PCR for five genes with significantly different expression by RNA-seq and for Rab23. Relative expression compared to the wild type is shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The error bars indicate SD.
Primary MEFs lacking Tgifs have reduced cilium numbers.
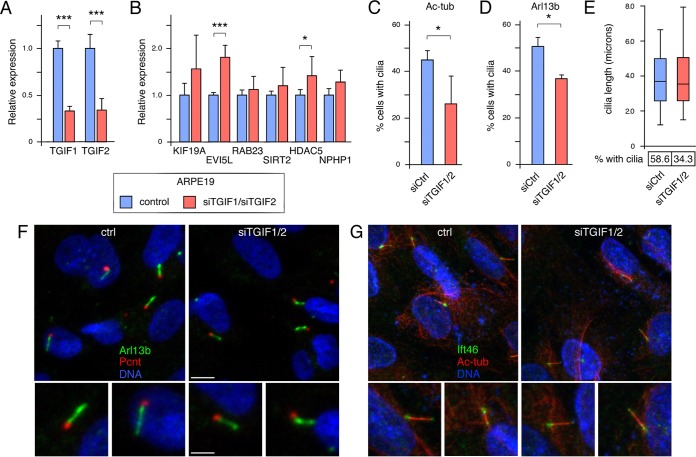
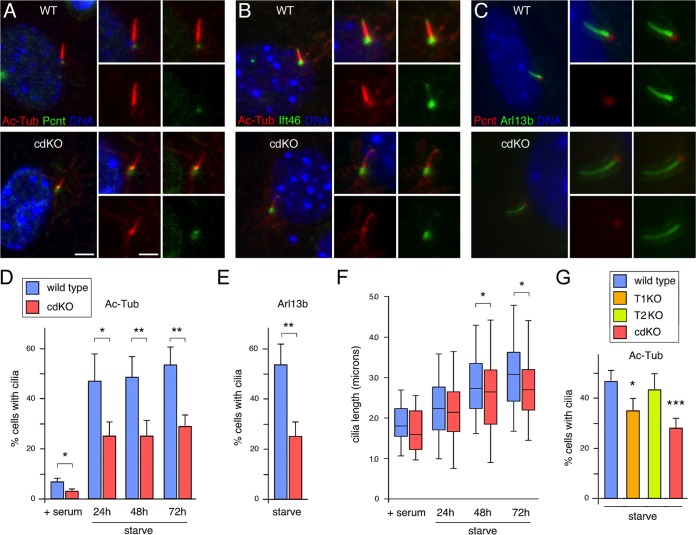
To begin to test if loss of Tgif function affects primary cilia, we transiently knocked down TGIF1 and TGIF2 in the human retinal pigment epithelial cell line ARPE19. As shown in Fig. 3A, we achieved approximately 70% knockdown of both genes in cells transfected with small interfering RNA (siRNA) pools against TGIF1 and TGIF2. Analysis of these samples for expression of several of the genes previously shown to change in cdKO embryos revealed a significant increase in expression of EVI5L and HDAC5 (Fig. 3B). To determine whether reduced TGIF levels affected the number of cells with primary cilia, we starved control and knockdown cells for 72 h, analyzed them by immunofluorescence (IF) microscopy for acetylated tubulin and pericentrin, and determined the proportion of cells with cilia. There were significantly fewer cells with primary cilia when both TGIFs were knocked down, suggesting that TGIFs may play a relatively direct role in regulating ciliogenesis (Fig. 3C). Similar results were obtained when cilium numbers were evaluated by staining with an antibody against Arl13b (Fig. 3D). Analysis of cilium length in knockdown cells that still had cilia did not reveal any significant differences compared to control cells, despite the decrease in the proportion of cells with cilia (Fig. 3E). Examination of cilia imaged with antibodies to Arl13b or acetylated tubulin did not reveal any differences between cilia that were present on control cell and those on knockdown cells (Fig. 3F and G). We also examined control and knockdown cells for cilium structure by confocal imaging of cells stained for acetylated tubulin and Ift46. This did not reveal any consistent changes in cilium length, shape, or overall structure at this level of resolution (Fig. 3G).
FIG 3.
Analysis of TGIFs in ARPE19 cells. (A) TGIF1 and TGIF2 were transiently knocked down in ARPE19 cells, and expression levels were tested by qRT-PCR 48 h after knockdown. (B) Expression of a panel of cilium-related genes was tested by qRT-PCR in ARPE19 cells with and without TGIF1/2 knockdown. Relative expression compared to the control is shown. *, P < 0.05; ***, P < 0.001. (C) Forty-eight hours after knockdown, ARPE cells were transferred to medium without serum for 72 h, and cilia were counted after staining for acetylated tubulin (Ac-tub). Means and SD for four replicates are shown. *, P < 0.05. (D) The proportion of ciliated cells was analyzed using an Arl13b antibody, as in panel C. (E) Cilium length was measured after staining for acetylated tubulin and is presented as the median, 25th and 75th percentiles (box), and 5th and 95th percentiles (whisker) plots The percentages of cells with cilia in the experiment are shown below. (F) Representative images of cilia in control and TGIF1/2 knockdown cells stained for pericentrin (Pcnt; red), for Arl13b (green), and for DNA with Hoechst (blue). (G) Representative images of cilia in control and TGIF1/2 knockdown cells stained for acetylated tubulin (red), for Ift46 (green), and for DNA with Hoechst (blue). Enlarged images of individual cilia are shown below. Scale bars, 4 μm (2 μm in the enlarged images).
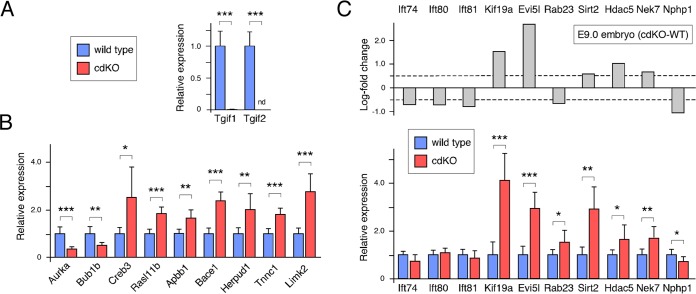
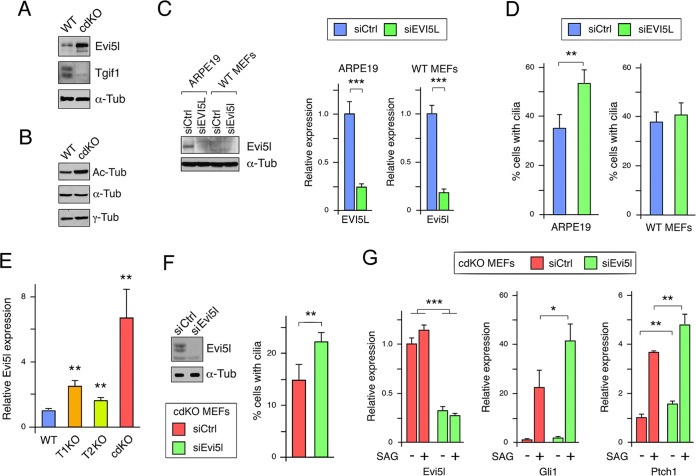
To generate a more amenable system to test potential effects of Tgifs on cilia, we created cdKO primary MEFs. MEFs were isolated at embryonic day 13.5 (E13.5) from mice with a Tgif2-null allele, loxP-flanked Tgif1 alleles, and a tamoxifen-inducible Cre transgene (Tgif1ff; Tgif2−/−; CreER+). At passage 2, the cells were treated with 4-hydroxy-tamoxifen for 48 h and then analyzed for gene expression after one more passage and one additional day in culture. For comparison, we used wild-type MEFs that were passaged similarly. As expected, Tgif2 was not detectable using primers to the deleted region, and Tgif1 expression was reduced to less than 1% of that seen in the wild-type cells after tamoxifen treatment, confirming efficient Cre-mediated deletion (Fig. 4A). We next tested the expression of a panel of genes that were present in the overlap between our RNA-seq data and our expression array analysis of Tgif1-null MEFs or that we had previously analyzed in Tgif1-null MEFs (6). As shown in Fig. 4B, we observed significant changes by qRT-PCR that were consistent with those seen by RNA-seq and/or Affymetrix array analysis. To examine changes in potential ciliogenesis genes, we tested expression of Evi5l and several other genes by qRT-PCR. In cdKO MEFs, Evi5l, Kif19a, Sirt2, and Hdac5 expression increased significantly, further verifying them as potential Tgif1 target genes (Fig. 4C). No significant changes were seen in three Ift genes tested; however, very few of these cells generated cilia when grown in high-serum medium.
FIG 4.
Analysis of gene expression in cdKO MEFs. (A) Expression of Tgif1 and Tgif2 was analyzed by qRT-PCR in wild-type and cdKO MEFs after tamoxifen treatment. (B) Expression of a selection of genes (primarily from the overlap between Tgif1 null MEFs and cdKO embryo RNA-seq) was analyzed by qRT-PCR in wild-type and cdKO MEFs. (C) Analysis of cilium-related gene expression by qRT-PCR in wild-type and cdKO MEFs. The relative expression based on embryo RNA-seq is shown at the top for comparison. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To test effects of Tgifs on cilia in MEFs, we maintained wild-type and cdKO MEFs in medium lacking serum to induce cilium formation and then examined the cilia by immunofluorescence microscopy with antibodies that detect acetylated tubulin and either pericentrin or Ift46. Cilia were present in both wild-type and cdKO MEFs and did not appear to be different between the two genotypes, as imaged with antibodies for Ift46 and acetylated tubulin or with antibodies to Arl13b and pericentrin (Fig. 5A to C). We next determined the proportions of cells with cilia when maintained in 10% serum or after 24 to 72 h in starvation medium. At all time points analyzed, we observed a significant decrease in the proportion of cdKO MEFs with cilia compared to the wild-type controls (Fig. 5D). Imaging cells with Arl13b revealed a similar decrease in cilium numbers in the cdKO MEFs (Fig. 5E). The fraction of cells with cilia was relatively constant after 24 h in starvation medium, although the length of the cilia increased somewhat over time (Fig. 5F). Loss of Tgifs had minimal effect on cilium length but significantly decreased the overall proportion of cells that had cilia. To test if both Tgif1 and Tgif2 were required, we compared cilium numbers in wild-type and cdKO MEFs to those in primary MEFs lacking either Tgif1 or Tgif2. As shown in Fig. 5G, cilium numbers were lower in Tgif1-null MEFs and were further reduced in the cdKO compared to the wild type. In contrast, deletion of Tgif2 alone did not have a significant effect on cilium numbers, suggesting that Tgif1 plays the major role. To test if loss of Tgifs affected cilium-dependent signaling, we starved wild-type and cdKO MEFs and then treated them with a cell-permeable Smoothened agonist (SAG) to activate the Shh pathway. In wild-type cells, we observed more than 100-fold activation of Gli1 expression by SAG, whereas SAG-induced Gli1 expression was significantly reduced in cdKO MEFs (Fig. 6). Similarly, expression of Ptch1 was induced by SAG to a significantly lower level in the cdKO MEFs than in wild-type cells. In contrast, we observed no changes in Gli3 gene expression, which is not known to be activated by Shh signaling (Fig. 6). Thus, it appears that loss of Tgif function in primary MEFs results in fewer cells with primary cilia and reduced Shh pathway activity.
FIG 5.
Analysis of cilia in MEFs. (A) Wild-type and cdKO MEFs were analyzed by immunofluorescence microscopy with antibodies against acetylated tubulin (red) and pericentrin (green). (B) MEFs were analyzed as in panel A for acetylated tubulin (red) and Ift46 (green). Confocal images of representative cilia are shown for each. (C) Wild-type and cdKO MEFs were analyzed by immunofluorescence microscopy for pericentrin (red) and Arl13b (green). (A to C) All the images show DNA stained with DAPI, and enlarged images of individual cilia are shown on the right in each panel. Scale bars, 2 μm (1 μm for enlarged images). (D) Wild-type and cdKO MEFs were incubated in regular medium (+ serum) or starved for 24 to 72 h and then analyzed for cilium numbers by staining for acetylated tubulin and pericentrin. The means and SD of quadruplicates are shown, together with P values for comparison of the wild type to the cdKO. (E) Cilium numbers were scored in wild-type and cdKO MEFs as for panel D after 72 h in starvation medium. (F) Cilium length was analyzed after staining for acetylated tubulin, as in Fig. 3E. (G) Cilia were counted in primary MEFs that were either wild type, Tgif1 null (T1KO), or Tgif2 null (T2KO) or lacked both Tgif1 and Tgif2 (cdKO). The means and SD of quadruplicates are shown, together with P values for comparison to the wild type. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by Student's t test.
FIG 6.
Decreased Shh pathway response in cdKO MEFs. Wild-type or cdKO MEFs were grown to confluence in 12-well plates and then incubated in 1% serum for 24 h with or without 400 nM SAG. Expression of the indicated genes was analyzed by qRT-PCR and is presented as fold induction, with the level in the absence of SAG in WT MEFs set to 1. The fold activation for each is shown below. *, P < 0.05; **, P < 0.01 by Student's t test.
Analysis of Evi5l function.
To confirm that the change in Evi5l gene expression in primary MEFs translated into changes in protein levels, we analyzed wild-type and cdKO MEFs by Western blotting. Expression of Evi5l was higher in the cdKO MEFs than in the wild-type control (Fig. 7A). When we analyzed similar samples for acetylated tubulin, we observed an increase in acetylated tubulin relative to bulk α-tubulin in the cdKO MEFs, suggesting that increased histone deacetylase (HDAC) expression is unlikely to account for the decrease in cilium numbers (Fig. 7B). To test the effects of Evi5l on cilia, we knocked it down in ARPE19 cells and in wild-type MEFs. As shown in Fig. 7C, the basal expression of Evi5l protein in wild-type MEFs was much lower than in ARPE19 cells. Nonetheless, we observed a clear reduction in EVI5L protein in the knockdown ARPE19 cells and significant reductions in the mRNA in both cell types. In ARPE19 cells, we observed a small but significant increase in the proportion of cells with primary cilia after EVI5L knockdown, whereas in primary MEFs there was little change (Fig. 7D). Given that cdKO MEFs had higher Evi5l expression than the wild type, we next tested whether reducing Evi5l expression would affect the reduction in cilium numbers seen in cdKO MEFs. We chose to test cdKO MEFs here, as the decrease in cilium numbers and increase in Evi5l expression in cdKO MEFs were greater than in either Tgif1 or Tgif2 single-null MEFs, suggesting that both Tgifs may contribute to ciliogenesis (Fig. 5G and 7E). As shown in Fig. 7F, Evi5l protein levels were reduced by transient siRNA knockdown in cdKO MEFs, but we did not see any change in expression of a panel of other cilium-related genes after Evi5l knockdown (data not shown). We next determined the proportion of cdKO MEFs with cilia after 48 h in serum-free medium following transfection with control or Evi5l siRNA. As shown in Fig. 7F, reducing Evi5l levels in cdKO MEFs resulted in a small but significant increase in cilium numbers, consistent with the idea that excess Evi5l in the cdKO MEFs may limit ciliogenesis. To test whether reducing the increased Evi5l expression in cdKO MEFs could also restore the transcriptional response to SAG, we transiently knocked down Evi5l in cdKO MEFs. Control and Evi5l knockdown cells were starved for 48 h to induce cilia, and we then compared expression of Gli target genes in cells treated or not with SAG for the final 24 h. Evi5l expression was reduced about 4-fold with or without SAG, and we observed a significant increase in expression of both Gli1 and Ptch1 in the presence of SAG when Evi5l was knocked down (Fig. 7G). Together, these data suggest that excess Evi5l expression contributes to the reduced cilium numbers and to the reduced response to activation of Shh signaling in the absence of Tgifs.
FIG 7.
Analysis of Evi5l function. (A) Evi5l expression was analyzed by Western blotting in wild-type and cdKO MEFs. Tgif1 and tubulin are shown as controls. (B) Relative levels of acetylated tubulin, α-tubulin, and γ-tubulin were analyzed by Western blotting from wild-type and cdKO MEFs. (C) Evi5l was transiently knocked down (siEvi5l, siRNA against Evi5l; siCtrl, control nontargeting siRNA) in ARPE19 and wild-type MEFs, and expression was analyzed by Western blotting and qRT-PCR. (D) Evi5l was transiently knocked down in ARPE19 and wild-type MEFs, and cilium numbers were analyzed by staining for acetylated tubulin. The means and SD of triplicates are shown. (E) Primary MEFs of the indicated genotypes were analyzed by qRT-PCR for Evi5l expression, which is shown relative to the wild type, with P values for comparison to the wild type. (F) Evi5l was transiently knocked down in cdKO MEFs, and expression of Evi5l was analyzed by Western blotting. Cilium numbers were analyzed in cdKO MEFs with control or Evi5l knockdown. The means and SD of quadruplicates are shown. (G) Evi5l was transiently knocked down in cdKO MEFs, which were then starved for 48 h, with SAG added for the final 24 h, as indicated. Expression of Evi5l, Gli1, and Ptch1 was analyzed by qRT-PCR. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by Student's t test.
Evi5l is a direct TGIF target gene.
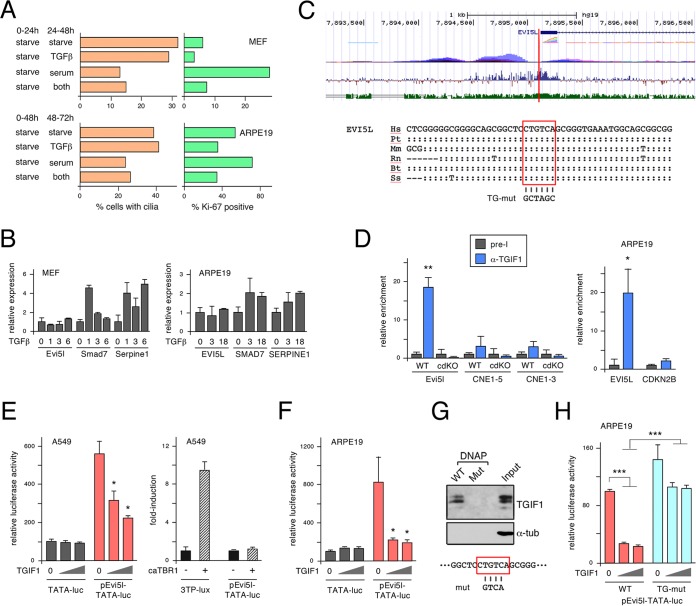
Tgif1 and Tgif2 are transcriptional corepressors for TGF-β-activated Smads. They have been shown to limit TGF-β-responsive gene expression in primary MEFs and to regulate Nodal-dependent phenotypes in early mouse embryos. However, Tgifs can also bind directly to DNA, allowing transcriptional regulation independent of TGF-β signaling. To test whether TGF-β signaling affected cilium formation in primary MEFs, we starved cells for 24 h and then added back serum or TGF-β and counted cilia by immunofluorescence microscopy 24 h later. TGF-β addition had no effect on the number of ciliated cells but clearly reduced proliferation, as measured by the proportion of Ki-67-positive cells (Fig. 8A). Treating MEFs with TGF-β resulted in induction of expression of Smad7 and Serpine1, two well-characterized TGF-β-responsive genes, whereas we observed no change in Evi5l expression (Fig. 8B). Experiments in ARPE19 cells yielded similar results, although the reduction in Ki-67 labeling with TGF-β addition and the induction of SMAD7 and SERPINE1 were less dramatic. Taken together, these data suggest that changes in cilium numbers and Evi5l expression on knockdown or deletion of Tgifs cannot be mimicked by increased TGF-β signaling.
FIG 8.
Evi5l is a direct Tgif target gene. (A) Wild-type MEFs or ARPE19 cells were starved and then treated with serum, TGF-β, or both, and effects on cilium numbers and proliferation were analyzed. (B) Expression of Evi5l and two known TGF-β target genes was analyzed in MEFs and ARPE19 cells. The data are plotted as fold induction relative to untreated cells. (C) Screen shot from the UCSC browser with the region of human chromosome 19 surrounding the transcriptional start site of the EVI5L gene. The gene prediction is shown in blue, composite RNA expression is in rainbow, and composite H3K27Ac ChIP-seq data are in purple. The bottom two tracks show vertebrate conservation (blue) and mouse-human identity (green). The vertical red bar indicates the position of the conserved element shown below, in which the conserved TGIF consensus site is boxed in red. Colons show identity to the human sequence, dashes indicate deletions, and differences from the human sequence are shown. Hs, Homo sapiens; Mm, Mus musculus; Pt, Pan troglodytes; Rn, Rattus norvegicus; Bt, Bos taurus; Ss, Sus scrofa. (D) Binding of TGIF1 to the EVI5L upstream region was analyzed by ChIP-qPCR in primary wild-type and cdKO MEFs and in ARPE19 cells. Relative binding compared to preimmune serum is shown, together with negative-control regions. *, P < 0.01; **, P < 0.001. (E and F) The activity of the mouse Evi5l upstream region (cloned upstream of a minimal TATA element) was analyzed by transient-transfection and luciferase assays. Activity is shown for the Evi5l reporter and the parental TATA-luc, with increasing TGIF1 coexpression in A549 (E) and ARPE19 (F) cells. The right-hand graph in panel E shows the effects of activating TGF-β signaling on the Evi5l reporter or a positive-control TGF-β-responsive reporter in A549 cells. The data are shown as mean (plus SD) relative activity of triplicates in arbitrary units. *, P < 0.01. (G) Binding of TGIF1 from ARPE19 cells was assayed by DNAP. Following incubation with biotinylated double-stranded oligonucleotides and precipitation on streptavidin-agarose, TGIF1 was detected by Western blotting. Binding to a wild-type sequence or a mutant version (mut) was tested. (H) The activity of the mouse Evi5l upstream region was analyzed by transient transfection and luciferase assay in ARPE19 cells. Activity is shown for the wild-type Evi5l reporter and a mutant form in which the TGIF consensus site was abolished. ***, P < 0.001.
Since Evi5l expression did not appear to respond to TGF-β, we considered the possibility that Tgifs bind directly to Evi5l regulatory elements to limit its expression. As a way to reduce the amount of sequence to search, we first scanned the human EVI5L gene for regions with peaks of H3K27Ac ChIP-seq signal from the ENCODE data using the UCSC genome browser (GRC37/hg19, 2009 assembly [http://genome.ucsc.edu/ENCODE/]). We chose the human genome over the mouse genome because the ENCODE project contains more information on the human genome, and we focused on H3K27Ac, as this histone mark is generally associated with transcriptionally active regulatory elements. The search identified four broad regions within or flanking the EVI5L gene with high H3K27Ac signal. We then scanned sequences in these regions for consensus TGIF binding sites (CTGTCA) (1) that were conserved between human and mouse. As shown in Fig. 8C, a single conserved TGIF consensus site was identified within a highly conserved region overlapping the 5′-most transcriptional start sites of EVI5L, adjacent to an H3K27Ac peak.
To test whether Tgif1 could bind to this region in MEFs, we performed ChIP with a TGIF1-specific antiserum or with preimmune serum as a control. The Evi5l 5′ region was clearly enriched in Tgif1 immunoprecipitates from wild-type MEFs but not in those from the cdKO MEFs (Fig. 8D). Additionally, we observed no Tgif1 binding to two intronic regions from the Gli3 gene, supporting the specificity of the binding. Similar results were obtained in ARPE19 cells, with clear enrichment of the EVI5L 5′ region and no binding to a control region from 5′ to the CDKN2B gene (Fig. 8D). To test whether this region could respond to TGIF1, we amplified a 471-bp region surrounding the putative TGIF site from mouse Evi5l and generated a luciferase reporter in which the sequence was placed upstream of a minimal TATA element. We first tested pEvi5l-TATA-luc in A549 lung carcinoma cells, as they respond well to transfected TGIF1 and to TGF-β. As shown in Fig. 8E, pEvi5l-TATA-luc was 5-fold more active than the parental TATA-luc, and its activity was significantly repressed by coexpression of TGIF1. As with the endogenous Evi5l gene, we did not observe any response of the luciferase reporter to TGF-β signaling, whereas the activity of a canonical TGF-β pathway reporter (3TP-lux) was clearly induced (Fig. 8E). When TGIF1 was coexpressed with the pEvi5l-TATA-luc reporter in ARPE19 cells, we again observed strong repression by TGIF1, with no effect of TGIF1 on the basic TATA-luc reporter (Fig. 8F). To determine whether TGIF1 could bind to the putative consensus site from the Evi5l promoter region, we tested binding of endogenously expressed TGIF1 from ARPE19 whole-cell lysates to biotinylated double-stranded oligonucleotides. TGIF1 bound to the wild-type sequence but was unable to bind to a mutant form in which the central four bases had been altered (Fig. 8G). To confirm that repression by TGIF1 was dependent on the TGIF site, we next created a mutant reporter in which the TGIF consensus sequence was replaced with an unrelated sequence. As shown in Fig. 8H, this mutant reporter was not significantly repressed by TGIF1 expression and was significantly more active than the wild type in the presence of coexpressed TGIF1. Taken together, these data suggest that EVI5L is a conserved TGIF target gene that is repressed by direct binding of TGIF1 to a consensus element in the proximal promoter.
DISCUSSION
Here, we show that Tgifs directly repress expression of the Evi5l gene by binding a conserved TGIF consensus site immediately upstream of the gene. In the absence of Tgif function, Evi5l expression is increased and cilium numbers are reduced. While the effects of reduced Tgif levels on ciliogenesis may depend on multiple factors, we show that excess Evi5l expression can contribute to the decreased numbers of ciliated cells and to a reduction in Shh pathway activation.
Transcriptome profiling of whole embryos identified a large number of genes that are differentially expressed between wild-type embryos and those lacking Tgif function. The majority (70%) of genes that were considered differentially expressed showed higher expression in the mutant than in the wild-type embryos, consistent with the function of Tgifs as transcriptional repressors. Any genes that showed differential expression in this analysis could be direct targets of Tgifs or could be secondary to altered expression of primary Tgif targets. It is also possible that some of the transcriptional changes are a more indirect result of the phenotypic alterations in the mutant embryos. Thus, definitively identifying direct targets will require analysis of Tgif binding, either genome-wide or on a gene-by-gene basis. Of the four overexpressed genes with potential links to ciliogenesis we analyzed in more detail here, we identified conserved Tgif consensus sites in two of them (Evi5l and Hdac5). By multiple assays, we confirmed that Evi5l is indeed a Tgif target gene, with Tgif1 binding to a conserved consensus site 5′ to the transcriptional start site. To identify putative Tgif sites, we focused on the body of the gene and the 5 kb upstream of the start site and searched close to peaks of H3K27Ac signal from human ENCODE data. It is possible, therefore, that some potential sites have been missed, and a more comprehensive analysis would be of interest to identify not just putative Tgif sites but also other enriched motifs associated with these genes. However, a more reliable way of distinguishing direct from indirect targets will be required for this sort of comprehensive analysis to be successful.
Gene ontology analysis of the genes with altered expression in the cdKO embryos compared to the wild type revealed enrichment for genes involved in patterning and DNA metabolism among the genes with lower expression in the cdKO. This is consistent with the overall patterning defects and reduced proliferation seen in cdKO embryos and with decreased proliferation observed in cdKO MEFs (6, 27). The most enriched categories among the genes with higher expression in the cdKO included extracellular matrix and cytoskeleton. However, GO term analysis revealed relatively few enriched categories in these gene lists. This may due to the fact that the cdKO embryos have multiple defects and that we isolated RNA from whole embryos, rather than an isolated tissue or a single cell type. Comparing the data with gene expression analysis from Tgif1-null MEFs also revealed a relatively small set of overlapping changes, likely in part due to the difference between whole embryos and cultured primary MEFs (which were null only for Tgif1) and also possibly due to the different platforms used for these analyses (RNA-seq versus expression arrays). The majority of the genes that changed in both analyses were either increased or decreased in both, as would be expected, and this allowed us to focus on what may be a higher-confidence set of potential Tgif targets. Recent work in mouse ES cells has identified a large number of potential Tgif1 binding sites across the genome (19). Comparison of these ChIP-seq peaks with our data revealed a large overlap, but without much selectivity. Based simply on ChIP peak enrichment, the authors predicted almost 10,000 Tgif target genes in their analysis. If we rank the peaks by enrichment score and use a more stringent cutoff, then we observe greater overlap among these possibly higher-confidence ChIP-seq peaks and genes that have increased expression in our cdKO embryos than with genes that have lower expression in the cdKO. Among the higher-confidence peaks are those that span the regions we identified in the Evi5l and Hdac5 genes by scanning the ENCODE data. However, the relatively low level of similarity between these multiple data sets suggests that Tgifs may perform cell-type-specific functions.
Our previous work identified phenotypes in cdKO embryos that could be secondary to defects in cilia, including Shh pathway defects, HPE, and L-R asymmetry defects. L-R asymmetry determination requires the appropriate regulation of the Nodal signaling pathway, both at the posterior notochord (PNC) and in the lateral plate mesoderm (LPM), and is dependent on the structure of the PNC and the presence of motile cilia (43, 44). Mutations that affect ciliogenesis can also result in phenotypes, including HPE and polydactyly, similar to those seen in Shh mutants (32). This raises the possibility that the HPE and lower-forebrain Shh expression seen in cdKO embryos are due to defects in ciliogenesis. Our analysis of the ventricular surface of the ventral forebrain suggests that there is disruption of the neuroepithelial surface but only a relatively modest decrease in the proportion of cells with cilia (unpublished observations). Even in cdKO MEFs where we observed a significant decrease in cilium numbers, there was not a complete loss of ciliated cells. This is similar to the incomplete loss of ciliated cells seen with EVI5L overexpression in RPE1 cells (36). However, our analysis of the Shh pathway in cdKO MEFs is clearly consistent with the possibility that even a relatively small reduction in the proportion of ciliated cells can have a significant effect on the Shh response. Alternatively, it is possible that altered signaling within the remaining cilia also contributes to the reduction in Shh-responsive gene expression. In the forebrains of cdKO embryos, it is possible that combined effects of neuroepithelial disorganization and a reduction in the number of ciliated cells result in lower Shh expression.
Although we have focused on Evi5l as a target for Tgifs, it is still possible that other Tgif target genes play a role in the effects of Tgif loss of function on cilium numbers. Possibilities include deacetylase genes, such as Sirt2 and Hdac5, although we did not observe such clearly reproducible effects on the expression of these genes in the different systems analyzed here as we did with Evi5l. Additionally, cdKO MEFs appeared to have increased rather than decreased tubulin acetylation, further arguing against a role for HDACs in the reduction in cilium numbers. It is also possible that disruption of other cellular processes in the absence of Tgifs contributes indirectly to the effects on ciliated cells. However, given that overexpression of EVI5L was shown to decrease cilium numbers (36), this remains our best candidate. The demonstration that reducing Evi5l expression in primary MEFs lacking Tgifs results in increased cilium numbers and an increase in the activation of Shh target genes clearly supports a role for Evi5l downstream of Tgifs. It will now be of interest to determine if other genes that are deregulated by loss of Tgif function can contribute to altered cilium function. The cognate GAP for EVI5L has been identified as Rab23 (36), although relatively little is known about the functions of Evi5L, and it remains possible that it performs functions other than regulating Rab23 and ciliogenesis. Interestingly, Rab23 has been shown to regulate the localization of Shh pathway components to the primary cilium (45, 46). We do not know whether this process is defective in our cdKO embryos, although it is tempting to speculate that this might provide an explanation of the defects in Shh expression in the forebrain. Rab23 has also been shown to play a role in L-R patterning in both mouse and zebra fish (47), although it is not known whether this is Evi5l dependent.
In summary, our data clearly suggest that Tgifs play a role in regulating ciliogenesis and that loss of Tgif function can affect the transcriptional response to Shh. In addition, we show that Evi5l expression is directly regulated by Tgifs, and it appears that Evi5l functions downstream of Tgifs to regulate primary cilia and the response to Shh pathway activation.
MATERIALS AND METHODS
Ethics statement.
All animal procedures were approved by the Animal Care and Use Committee of the University of Virginia, which is fully accredited by the AAALAC.
Mice and DNA analysis.
The loxP-flanked Tgif allele (25) and Tgif2-null alleles (26) were as described previously. The Sox2-Cre line (48) was obtained from Jax (Bar Harbor, ME) (number 4783). All mouse lines were maintained on a mixed C57BL/6J × 129Sv/J background. The cdKO MEFs were generated from Tgif1f/f; Tgif2−/− mice with a tamoxifen-inducible Cre transgene (Jax; number 4847). Genomic DNA for PCR genotype analysis was purified from an ear punch at postnatal day 21 (P21) or from a yolk sac (7.0 to 10.0 dpc) with HotShot (49).
Cell culture and luciferase assays.
Primary MEFs were isolated from 13.5-day mouse embryos and cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) with 10% fetal bovine serum (FBS) (HyClone) as described previously (6). The cells were transfected using TurboFect (Fisher) according to the manufacturer's instructions. The cells were transfected with firefly luciferase reporters, a Renilla transfection control (phCMVRLuc; Promega), and the indicated constructs. After 48 h, firefly luciferase activity was assayed using firefly substrate (Biotium) and Renilla luciferase was assayed with 0.09 μM coelenterazine (Biosynth), using a Berthold LB953 luminometer. Luciferase constructs were generated in a modified version of pGL3 basic (Promega) containing the TATA element from the adenovirus major late promoter.
RNA-seq.
RNA was isolated and purified using an Absolutely RNA kit (Agilent) and quality checked with a Bioanalyzer. Poly(A) RNA-seq libraries were generated at HudsonAlpha with Illumina barcodes and sequenced (Illumina HiSeq) to a target depth of ∼25 million paired-end 50-bp reads per sample. This resulted in 17 to 21 million mapped reads per sample. Raw FASTQ sequencing reads were chastity filtered to remove clusters having outlying intensities corresponding to bases other than the called base. The filtered reads were assessed for quality using FastQC. The reads were splice-aware aligned to the reference genome using STAR (50), and reads overlapping gene regions were counted using featureCounts (51). The DESeq2 Bioconductor package (52) in the R statistical computing environment (http://www.R-project.org/) was used for normalizing count data, performing exploratory data analysis, estimating dispersion, and fitting a negative binomial model for each gene comparing the expression from Tgif mutants to that from wild-type samples. After obtaining a list of differentially expressed genes, log fold changes, and P values, the Benjamini-Hochberg false-discovery rate procedure was used to correct P values for multiple testing. A cutoff of ±0.5 log2 unit and an adjusted P value of <0.0001 was considered significant. qRT-PCR verification of gene expression differences identified by RNA-seq was performed on RNA from an independent set of WT and cdKO embryos isolated at the same stage. GO term analysis was performed using DAVID (https://david.ncifcrf.gov/) (53, 54), and heat maps were generated using AutoSOME (55).
RNA analysis by qRT-PCR.
RNA was isolated and purified using an Absolutely RNA kit (Agilent). cDNA was generated using Superscript III (Invitrogen), and analyzed in triplicate by real-time PCR using a Bio-Rad MyIQ cycler and SensiMix plus SYBR green plus fluorescein isothiocyanate (FITC) mix (Bioline), with intron-spanning primer pairs selected using Primer3 (http://frodo.wi.mit.edu/). Expression was normalized to Rpl4 and actin using the ΔCT method and is shown as the mean plus standard deviation of triplicates. For knockdown, cells were plated in 6-well plates and transfected with Dharmacon Smartpool oligonucleotides against human TGIF1 and TGIF2 or mouse Tgif1 and Tgif2 using DharmaFect reagent 1. The control pool (siGenome Nontargeting siRNA pool number 3) was used for the nontargeting control. For Shh pathway responses, MEFs were grown to confluence and then incubated for 24 h in medium with 1% FBS with SAG (400 nM; Cayman Chemicals) or without prior to isolation of RNA and qRT-PCR.
IF.
For cilium IF microscopy, cells were plated in chamber slides, washed for 1 min with PHEM {PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], HEPES, EGTA, MgSO4} buffer, and then fixed in 4% paraformaldehyde in PHEM for 15 min. The cells were permeabilized in 0.25% Triton X-100 in PBS for 20 min and blocked in 10% normal goat serum (NGS)–0.25% Triton X-100 in phosphate-buffered saline (PBS) for 1 h. The cells were incubated in mouse monoclonal acetylated tubulin (1:400; Sigma T6793), rabbit polyclonal pericentrin (1:400; Abcam ab4448), mouse monoclonal Arl13B (1:400; Abcam ab136648), or rabbit polyclonal IFT46 (1:100; Abcam ab122422), Alexa Fluor 488-labeled goat anti-rabbit antibody (1:500; Invitrogen), Alexa Fluor 594-labeled goat anti-mouse antibody (1:500; Invitrogen), and Hoechst 33342 (1:5,000; Sigma). IF images were captured on a Nikon Eclipse NI-U and captured with a DS-QI1 camera with NIS Elements software. Confocal images were captured using a Zeiss LSM710 Multiphoton confocal microscope and adjusted in Adobe Photoshop. Cilia were counted, and their lengths were measured using NIS Elements BR software.
ChIP and DNAP.
Chromatin immunoprecipitation was performed as previously described (16, 56). Briefly, primary MEFs or ARPE19 cells were cross-linked with 1% formaldehyde for 20 min at 37°C. Following chromatin isolation, DNA was sheared by sonication to between 200 bp and 1,000 bp in length using a Branson digital sonifier with a microtip. Immunoprecipitations were carried out using 10 μl of a polyclonal Tgif1 antiserum (5) or preimmune serum. Bound and input fractions were analyzed by qPCR on a Bio-Rad MyIQ cycler using SensiMix plus SYBR green plus FITC mix (Bioline). DNA affinity purification (DNAP) was performed essentially as described previously (56). Briefly, biotinylated double-stranded oligonucleotides were incubated with whole-cell lysate and precipitated on streptavidin-agarose. Bound proteins were detected by Western blotting. Binding to wild-type oligonucleotide or a mutant version was tested. Biotin was added to the 5′ end of the lower strand (WT sequence, GGGCAGCGGCTCCTGTCAGCGGGTGAAATGGCAGTGGCGGAGC; mutant, GGGCAGCGGCTCCGTCAAGCGGGTGAAATGGCAGTGGCGGAGC) (TGIF sites are in boldface, and mutated bases are underlined). Upper strands only are shown.
Supplementary Material
ACKNOWLEDGMENTS
We thank the UVA Advanced Microscopy Facility for technical assistance and members of the Wotton laboratory for helpful discussions.
We declare that we have no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MCB.00527-16.
REFERENCES
- 1.Bertolino E, Reimund B, Wildt-Perinic D, Clerc R. 1995. A novel homeobox protein which recognizes a TGT core and functionally interferes with a retinoid-responsive motif. J Biol Chem 270:31178–31188. doi: 10.1074/jbc.270.52.31178. [DOI] [PubMed] [Google Scholar]
- 2.Hyman CA, Bartholin L, Newfeld SJ, Wotton D. 2003. Drosophila TGIF proteins are transcriptional activators. Mol Cell Biol 23:9262–9274. doi: 10.1128/MCB.23.24.9262-9274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melhuish TA, Gallo CM, Wotton D. 2001. TGIF2 interacts with histone deacetylase 1 and represses transcription. J Biol Chem 276:32109–32114. doi: 10.1074/jbc.M103377200. [DOI] [PubMed] [Google Scholar]
- 4.Wotton D, Lo RS, Swaby LA, Massague J. 1999. Multiple modes of repression by the smad transcriptional corepressor TGIF. J Biol Chem 274:37105–37110. doi: 10.1074/jbc.274.52.37105. [DOI] [PubMed] [Google Scholar]
- 5.Wotton D, Lo RS, Lee S, Massague J. 1999. A Smad transcriptional corepressor. Cell 97:29–39. doi: 10.1016/S0092-8674(00)80712-6. [DOI] [PubMed] [Google Scholar]
- 6.Zerlanko BJ, Bartholin L, Melhuish TA, Wotton D. 2012. Premature senescence and increased TGFbeta signaling in the absence of Tgif1. PLoS One 7:e35460. doi: 10.1371/journal.pone.0035460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heldin C-H, Miyazono K, ten Dijke P. 1997. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 8.Massague J, Seoane J, Wotton D. 2005. Smad transcription factors. Genes Dev 19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 9.Schmierer B, Hill CS. 2007. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 10.Melhuish TA, Wotton D. 2000. The interaction of C-terminal binding protein with the Smad corepressor TG-interacting factor is disrupted by a holoprosencephaly mutation in TGIF. J Biol Chem 275:39762–39766. doi: 10.1074/jbc.C000416200. [DOI] [PubMed] [Google Scholar]
- 11.Melhuish TA, Wotton D. 2006. The Tgif2 gene contains a retained intron within the coding sequence. BMC Mol Biol 7:2. doi: 10.1186/1471-2199-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wotton D, Knoepfler PS, Laherty CD, Eisenman RN, Massague J. 2001. The Smad transcriptional corepressor TGIF recruits mSin3. Cell Growth Differ 12:457–463. [PubMed] [Google Scholar]
- 13.Seo SR, Ferrand N, Faresse N, Prunier C, Abecassis L, Pessah M, Bourgeade MF, Atfi A. 2006. Nuclear retention of the tumor suppressor cPML by the homeodomain protein TGIF restricts TGF-beta signaling. Mol Cell 23:547–559. doi: 10.1016/j.molcel.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Seo SR, Lallemand F, Ferrand N, Pessah M, L'Hoste S, Camonis J, Atfi A. 2004. The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. EMBO J 23:3780–3792. doi: 10.1038/sj.emboj.7600398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartholin L, Powers SE, Melhuish TA, Lasse S, Weinstein M, Wotton D. 2006. TGIF inhibits retinoid signaling. Mol Cell Biol 26:990–1001. doi: 10.1128/MCB.26.3.990-1001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melhuish TA, Chung DD, Bjerke GA, Wotton D. 2010. Tgif1 represses apolipoprotein gene expression in liver. J Cell Biochem 111:380–390. doi: 10.1002/jcb.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pramfalk C, Melhuish TA, Wotton D, Jiang ZY, Eriksson M, Parini P. 2014. TG-interacting factor 1 acts as a transcriptional repressor of sterol O-acyltransferase 2. J Lipid Res 55:709–717. doi: 10.1194/jlr.M045922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Hwang CK, D'Souza UM, Lee SH, Junn E, Mouradian MM. 2000. Tale homeodomain proteins Meis2 and TGIF differentially regulate transcription. J Biol Chem 275:20734–20741. doi: 10.1074/jbc.M908382199. [DOI] [PubMed] [Google Scholar]
- 19.Lee BK, Shen W, Lee J, Rhee C, Chung H, Kim KY, Park IH, Kim J. 2015. Tgif1 counterbalances the activity of core pluripotency factors in mouse embryonic stem cells. Cell Rep 13:52–60. doi: 10.1016/j.celrep.2015.08.067. [DOI] [PubMed] [Google Scholar]
- 20.Geng X, Oliver G. 2009. Pathogenesis of holoprosencephaly. J Clin Invest 119:1403–1413. doi: 10.1172/JCI38937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gripp KW, Wotton D, Edwards MC, Roessler E, Ades L, Meinecke P, Richieri-Costa A, Zackai EH, Massague J, Muenke M, Elledge SJ. 2000. Mutations in TGIF cause holoprosencephaly and link NODAL signalling to human neural axis determination. Nat Genet 25:205–208. doi: 10.1038/76074. [DOI] [PubMed] [Google Scholar]
- 22.Muenke M, Beachy PA. 2001. Holoprosencephaly, p 6203–6230. In Scriver CR. (ed), The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, NY. [Google Scholar]
- 23.Jin JZ, Gu S, McKinney P, Ding J. 2006. Expression and functional analysis of Tgif during mouse midline development. Dev Dyn 235:547–553. doi: 10.1002/dvdy.20642. [DOI] [PubMed] [Google Scholar]
- 24.Mar L, Hoodless PA. 2006. Embryonic fibroblasts from mice lacking Tgif were defective in cell cycling. Mol Cell Biol 26:4302–4310. doi: 10.1128/MCB.02156-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J, Walsh CA. 2005. Targeted disruption of Tgif, the mouse ortholog of a human holoprosencephaly gene, does not result in holoprosencephaly in mice. Mol Cell Biol 25:3639–3647. doi: 10.1128/MCB.25.9.3639-3647.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powers SE, Taniguchi K, Yen W, Melhuish TA, Shen J, Walsh CA, Sutherland AE, Wotton D. 2010. Tgif1 and Tgif2 regulate Nodal signaling and are required for gastrulation. Development 137:249–259. doi: 10.1242/dev.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi K, Anderson AE, Sutherland AE, Wotton D. 2012. Loss of Tgif function causes holoprosencephaly by disrupting the Shh signaling pathway. PLoS Genet 8:e1002524. doi: 10.1371/journal.pgen.1002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S, Dynlacht BD. 2013. Assembling a primary cilium. Curr Opin Cell Biol 25:506–511. doi: 10.1016/j.ceb.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plotnikova OV, Pugacheva EN, Golemis EA. 2009. Primary cilia and the cell cycle. Methods Cell Biol 94:137–160. doi: 10.1016/S0091-679X(08)94007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goetz SC, Anderson KV. 2010. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen LB, Rosenbaum JL. 2008. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol 85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 32.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. 2003. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 33.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. 2005. Vertebrate Smoothened functions at the primary cilium. Nature 437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 34.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. 2005. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohatgi R, Milenkovic L, Scott MP. 2007. Patched1 regulates hedgehog signaling at the primary cilium. Science 317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. 2007. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Dam TJ, Wheway G, Slaats GG, SYSCILIA Study Group, Huynen MA, Giles RH. 2013. The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia 2:7. doi: 10.1186/2046-2530-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Gonzalo FR, Reiter JF. 2012. Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access. J Cell Biol 197:697–709. doi: 10.1083/jcb.201111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sung CH, Leroux MR. 2013. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat Cell Biol 15:1387–1397. doi: 10.1038/ncb2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho Y, Cavalli V. 2012. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J 31:3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. 2003. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11:437–444. doi: 10.1016/S1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 42.Niwa S, Nakajima K, Miki H, Minato Y, Wang D, Hirokawa N. 2012. KIF19A is a microtubule-depolymerizing kinesin for ciliary length control. Dev Cell 23:1167–1175. doi: 10.1016/j.devcel.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Shiratori H, Hamada H. 2006. The left-right axis in the mouse: from origin to morphology. Development 133:2095–2104. doi: 10.1242/dev.02384. [DOI] [PubMed] [Google Scholar]
- 44.Vandenberg LN, Levin M. 2013. A unified model for left-right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Dev Biol 379:1–15. doi: 10.1016/j.ydbio.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boehlke C, Bashkurov M, Buescher A, Krick T, John AK, Nitschke R, Walz G, Kuehn EW. 2010. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates smoothened levels. J Cell Sci 123:1460–1467. doi: 10.1242/jcs.058883. [DOI] [PubMed] [Google Scholar]
- 46.Eggenschwiler JT, Bulgakov OV, Qin J, Li T, Anderson KV. 2006. Mouse Rab23 regulates Hedgehog signaling from smoothened to Gli proteins. Dev Biol 290:1–12. doi: 10.1016/j.ydbio.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 47.Fuller K, O'Connell JT, Gordon J, Mauti O, Eggenschwiler J. 2014. Rab23 regulates Nodal signaling in vertebrate left-right patterning independently of the Hedgehog pathway. Dev Biol 391:182–195. doi: 10.1016/j.ydbio.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi S, Lewis P, Pevny L, McMahon AP. 2002. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev 119(Suppl 1):S97–S101. doi: 10.1016/S0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 49.Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. 2000. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29:52–54. [DOI] [PubMed] [Google Scholar]
- 50.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 52.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 54.Huang da W, Sherman BT, Lempicki RA. 2009. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newman AM, Cooper JB. 2010. AutoSOME: a clustering method for identifying gene expression modules without prior knowledge of cluster number. BMC Bioinformatics 11:117. doi: 10.1186/1471-2105-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bjerke GA, Hyman-Walsh C, Wotton D. 2011. Cooperative transcriptional activation by Klf4, Meis2, and Pbx1. Mol Cell Biol 31:3723–3733. doi: 10.1128/MCB.01456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.