ABSTRACT
The current study investigated the effect of environmental stressors (i.e., weather changes) on Salmonella shedding in free-range production systems and the correlations with behavioral and physiological measures (i.e., fecal glucocorticoid metabolites). This involved longitudinal and point-in-time surveys of Salmonella shedding and environmental contamination on four commercial free-range layer farms. The shedding of Salmonella was variable across free-range farms and in different seasons. There was no significant effect of season on the Salmonella prevalence during this investigation. In this study, the combined Salmonella most probable number (MPN) counts in environmental (including feces, egg belt, dust, nest box, and ramp) samples were highest in samples collected during the summer season (4th sampling, performed in February). The predominant serovars isolated during this study were Salmonella enterica serovar Mbandaka and Salmonella enterica serovar Typhimurium phage types 135 and 135a. These two phage types were involved in several egg product-related Salmonella outbreaks in humans. Multilocus variable-number tandem-repeat analysis (MLVA) results indicated that MLVA types detected from human food poisoning cases exhibited MLVA patterns similar to the strains isolated during this study. All Salmonella isolates (n = 209) were tested for 15 different genes involved in adhesion, invasion, and survival of Salmonella spp. We also observed variations for sopA, ironA, and misL. There were no positive correlations between fecal corticosterone metabolite (FCM) and Salmonella prevalence and/or shedding in feces. Also, there were no positive correlations between Salmonella prevalence and Salmonella count (log MPN) and any of the other welfare parameters.
IMPORTANCE In this study, the welfare of laying hens and Salmonella shedding were compared over a prolonged period of time in field conditions. This study investigated the long-term shedding of Salmonella serovars in a free-range egg production system. Given that there is increasing demand for free-range eggs, it is essential to understand the risks associated with such a production system.
KEYWORDS: eggs, free range, hens, Salmonella, welfare
INTRODUCTION
In Australia and the rest of the world, there has been an increase in egg production over the last decade. The Australian egg industry produced 421.3 million dozen eggs in 2015, and per capita consumption of the eggs increased to 226 eggs (1). Consumer preferences focus on perceived animal welfare and the environmentally friendly production of eggs. As a result, the free-range production system is becoming a major source of egg production in Australia and in other parts of the world. In Australia, free-range and conventional cage eggs have a market share of 49% and 39% by value (39% and 51% by volume), respectively (1). Challenging aspects of the free-range production system involve implementing biosecurity and controlling environmental stressors. Free-range hens are exposed to more environmental stressors, such as extreme weather conditions, predation, exposure to wild birds, and aggression, in comparison with hens from barn and cage systems (2–4). The findings from a recent Australian survey revealed that free-range egg producers imputed financial losses to heat stress, cannibalism, grass impaction, diseases, and malnutrition (5). According to this free-range survey, Australian free-range hens face extremely hot (>40°C, 16% of respondents) or cold (<0 to 10°C, 64% of respondents) temperatures on the range (6).
The exposure to extreme weather conditions might be stressful for birds and may increase the environmental shedding of bacteria. These factors may result in an increasing bacterial contamination of eggs. Higher bacterial loads on eggs may lead to increases in food poisoning outbreaks. Earlier studies reported increasing human Salmonella enterica serovar Typhimurium notifications with increasing temperatures during the warm season (7). In recent decades, foodborne illness has emerged as a serious problem throughout the world. In 2015, in Australia and the United States, the incidences of Salmonella notifications were 72.6 and 15.8 per 100,000 people, respectively (8, 9). Although there was a decreasing incidence of salmonellosis from 2008 to 2014 in the European Union, there was a 15.3% increase in the rate of Salmonella infections in 2014 compared with that in 2013 (10). Many salmonellosis outbreaks were traced back to raw egg products contaminated with Salmonella.
A previous epidemiology study in cage production systems found that the odds of an eggshell testing positive for Salmonella were higher when the environmental samples, such as fecal, egg belt, and dust samples, tested positive for Salmonella (11). However, little attention has been given to estimating the prevalence of Salmonella on eggs collected from free-range production systems or to determining the factors responsible for contamination. To maintain body temperature, birds maintain homeostasis through behavioral and physiological changes. High environmental stressors affect the neuroendocrine system of poultry, ultimately increasing plasma corticosterone levels (12). Hence, the current study investigated the effect of environmental stressors (i.e., weather changes) on Salmonella shedding in free-range production systems and the correlations with behavioral (i.e., panting and ranging activity) and physiological measures (i.e., fecal glucocorticoid metabolites).
RESULTS
Selection of sampling areas for cross-sectional study.
Culture isolation results indicated that, in all four flocks, 51 samples (32.28%; confidence interval [CI], 25.47 to 39.93) were positive for Salmonella spp. (Fig. 1). Flock B had the highest prevalence of Salmonella-positive samples (75.00%; CI, 61.67 to 84.89) followed by flock C (21.15%, CI, 12.08 to 34.20). On other hand, only one sample in flock A was Salmonella positive, and all samples collected from flock D were negative.
FIG 1.
Percentages of various locations within the sheds that were positive for Salmonella spp. during the cross-sectional sampling.
For each positive sample, three colonies were isolated and sent for serotyping. All Salmonella isolates from flock A were Salmonella enterica serovar Mbandaka. Serovars isolated from flock B were serovars Mbandaka and Agona, whereas flock C samples were positive for serovars Mbandaka, Anatum, and Worthington. Based on the prevalence result from the cross-sectional study (Fig. 1), different areas of the sheds were selected for longitudinal sampling.
Salmonella prevalence in flock A, B, C and D in longitudinal study.
In the longitudinal study during the period of samplings, the highest prevalence of Salmonella was observed in flock C (environmental samples: 29.89%, CI, 25.50 to 34.70; egg shells: 5%, CI, 2.93 to 8.29), followed by flock D (environmental samples: 3.97%, CI, 2.37 to 6.50; egg shells: 0%, CI, 0 to 1.63), flock A (environmental samples: 1.85%, CI, 0.82 to 3.85; egg shells: 0.36%, CI, 0 to 2.20), and flock B (environmental samples: 1.85%, CI, 0.82 to 3.85; egg shells: 0%, CI, 0 to 1.63). The overall prevalence of Salmonella in environmental samples was 9.39% with a CI of 8.02 to 10.97. The overall prevalence of Salmonella on egg shells (including clean and floor eggs) was 1.34% with a CI of 0.79 to 2.22. Overall (all flocks combined), the Salmonella prevalence was higher in dust samples (14.19%, CI, 10.64 to 18.90) than in shoe covers out on ranges (12.50%, CI, 5.88 to 23.93), feces (11.79%, CI, 8.48 to 16.12), ramps (8.21% CI, 5.49 to 12.07), nest boxes (7.86%, CI, 5.20 to 11.66), egg belts (5.36%, CI, 3.21 to 8.72), shoe covers inside sheds (3.57%, CI, 0.28 to 12.82), floor eggshells (3.57%, CI, 1.87 to 6.53), and eggshells (0.6%, CI, 0.21 to 1.43). All internal content samples from table and floor eggs were Salmonella negative. The prevalences of Salmonella in different types of samples from flocks A, B, C, and D are shown in Tables S1, S2, S3, and S4 in the supplemental material.
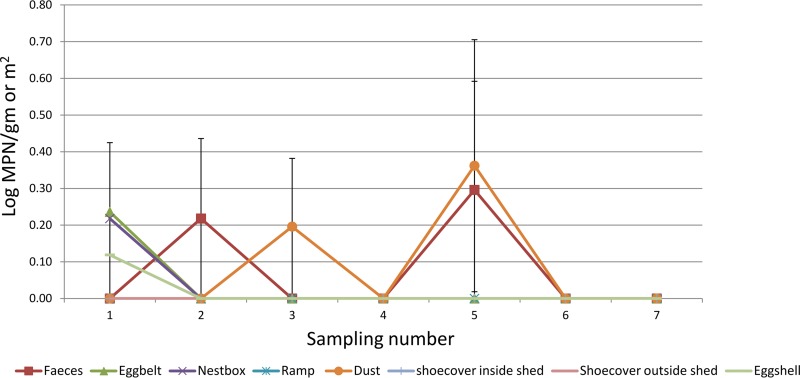
In flock A, the prevalences of Salmonella were similar in dust, feces, and nest box samples (2.85%), followed by egg belts (1.42%). In this flock, only one eggshell was Salmonella positive. All other samples, such as ramps, shoe covers inside sheds and out on ranges, and floor eggshells, were Salmonella negative. The log most probable number (MPN) values for dust (0.36 ± 0.1 per/m2 of swab) and feces (0.3 ± 0.1 per g) were highest during the 5th sample collection (performed in March 2015) (Fig. 2).
FIG 2.
Log MPN values in different samples collected at different sampling points in Flock A.
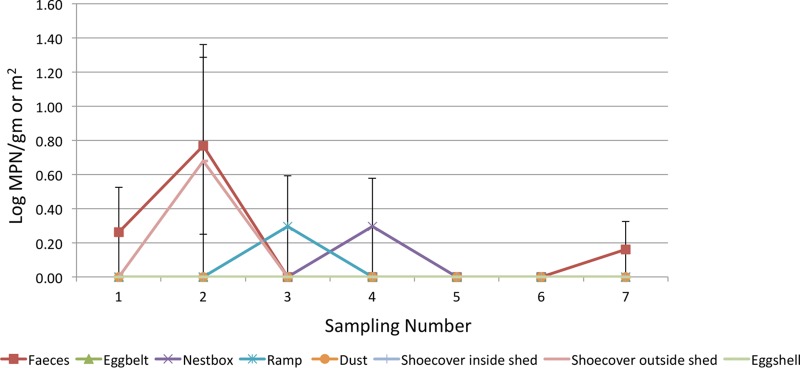
In flock B, the highest prevalence of Salmonella was in shoe covers out on the ranges (7.41%), followed by feces (5.71%), nest boxes, and ramps (1.43%). All eggshells and floor eggshells were Salmonella negative. The log MPN values for feces (0.77 ± 0.1 per g) and shoe cover samples collected from ranging areas (0.68 ± 0.1 per pair of shoe covers) were highest during the 2nd sample collection (performed in November 2014) (Fig. 3).
FIG 3.
Log MPN values in different samples collected at different sampling points in Flock B.
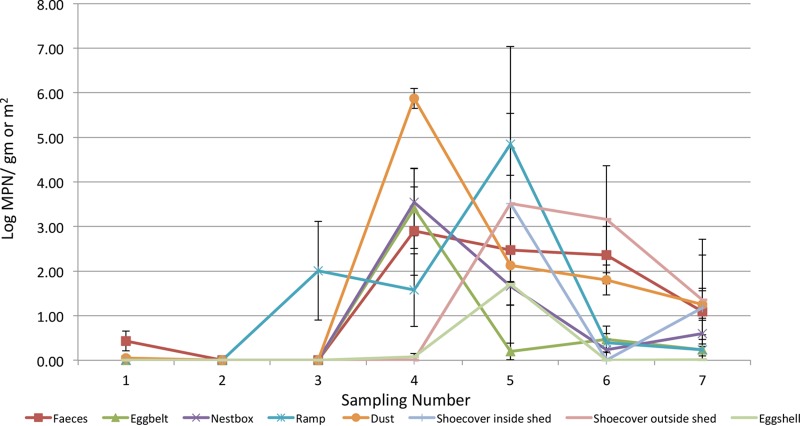
The highest numbers of samples that were Salmonella positive were from flock C. From the 4th sampling, there was an increasing prevalence of Salmonella in all types of samples, but it was highest in dust samples (48.57%). This was followed by feces (35.71%), shoe covers outside ranges (28.57%), ramps (25.71%), nest boxes (25.71%), egg belts (17.14%), and shoe covers inside sheds (14.28%). Flock C had the highest number of floor eggshells (14.28%) that were Salmonella positive compared with those from the other flocks. Of all the table eggs collected during sampling periods from flock C, 1.90% (4/840) were Salmonella positive. The log MPN values for dust (5.8 ± 0.1 per/m2 of swab) and nest boxes (3.7 ± 0.1 per g of feces) were highest during the 4th sample collection (performed in February 2015) (Fig. 4). During the 5th sample collection (performed in March 2015), the level of Salmonella was highest in swabs collected from ramps (4.8 ± 0.1 per/m2 of swab). The level of Salmonella on egg shells during this sample collection was 1.71 ± 0.1 per egg.
FIG 4.
Log MPN values in different samples collected at different sampling points in Flock C.
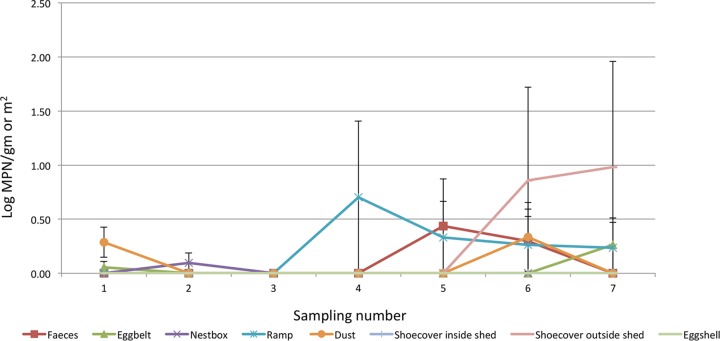
In contrast, in flock D, all eggshells, floor eggshells, and ramp samples were Salmonella negative. The highest prevalence was observed in shoe covers from ranges (14.28%), followed by dust (5.71%), ramp (5.71%), feces (2.85%), egg belt (2.85%), and nest box (1.43) samples. The level of Salmonella was highest (log MPN 0.70 ± 0.1) at the 5th sampling point (conducted in March 2015) in swabs collected from ramps (Fig. 5).
FIG 5.
Log MPN values in different samples collected at different sampling points in Flock D.
Overall, the Salmonella MPN values were highest for swabs collected from ramps at the 3rd and 5th sampling points, whereas the Salmonella MPN was highest for dust swabs collected at the 5th sampling point (see Fig. S1).
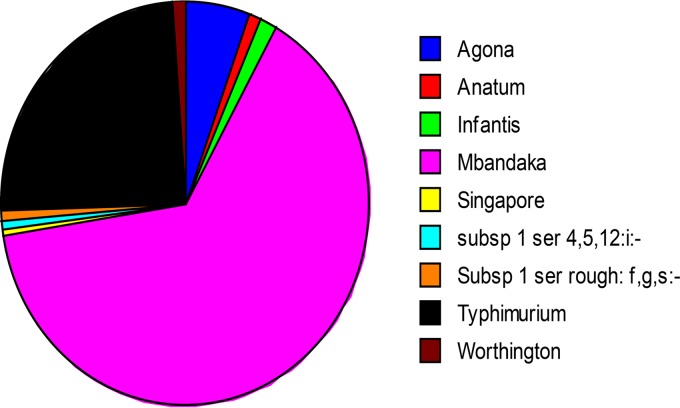
In total, 209 Salmonella isolates were obtained from all samples types during this longitudinal study. Salmonella serotyping suggested that the four farms selected in this study were positive for S. Mbandaka (64.5%), Typhimurium phage type 135 (24.4%), Agona (5.7%), Infantis (1.4%), Anatum (0.95%), Worthington (0.95%), Singapore (0.47%), subspecies 1 serovar 4,5,12:i:− (0.47%), and subspecies 1 serovar rough:f,g,s:− (0.47%). Figure 6 provides the percentage of various Salmonella serovars isolated from different type of samples.
FIG 6.
Distributions of Salmonella serovars isolated from the current epidemiological investigation.
Salmonella Typhimurium MLVA typing.
S. Typhimurium strains were isolated from flocks C and D. In flock C, S. Typhimurium was detected in all shoe covers collected from outdoor ranges. At the 6th sampling point, out of all Salmonella-positive dust and fecal samples, 70% and 60%, respectively, were S. Typhimurium (see Fig. S2). In flock D, S. Typhimurium was isolated from all shoe covers collected from outdoor ranges during the 6th and 7th sample collections (see Fig. S3l). Multilocus variable-number tandem-repeat analysis (MLVA) indicated that S. Typhimurium strains isolated from flocks C and D were genetically different. In flock C, the S. Typhimurium phage type 135 possessed three different MLVA patterns (03 13 10 12 523, 03 14 10 12 523, and 03 14 11 12 523), whereas S. Typhimurium phage type 135a isolated from flock D exhibited two different MLVA patterns (03 12 09 10 523 and 03 12 09 11 523).
Sampling time and Salmonella shedding.
Salmonella shedding was highest in dust samples collected during the 4th collection (conducted in February 15) (log MPN, 1.4 ± 0.83). The overall Salmonella count was lowest in fecal samples collected during the 3rd collection (performed in December 2014/January 2015) (log MPN, 0.0 ± 0.0). There was a significant difference between Salmonella counts in fecal samples from the 1st and 4th sample collections. There was also a significant difference in Salmonella MPN values for the 1st and 5th sample collections. S. Typhimurium was isolated from different samples collected in all seasons.
Fecal glucocorticoid metabolites and relationship with Salmonella shedding.
Results showed that fecal glucocorticoid concentrations as measured by the selected assay increased significantly by 1 h after adrenocorticotropin (ACTH) administration (analysis of variance [ANOVA], time × treatment interaction, P < 0.001, with all post hoc comparisons at P values of ≤0.01), and returned to concentrations that were not significantly different from control hens by 6.5 h after the administration. Hence, this assay provides valid measurements of glucocorticoid metabolites.
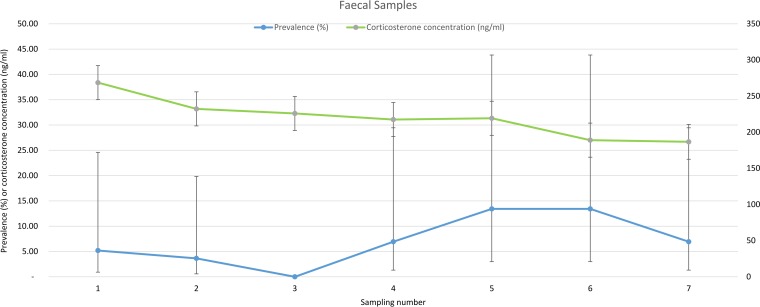
The highest concentration of fecal glucocorticoid metabolites was found in the 1st sampling (268.5 ± 22.3 ng/ml). Fecal glucocorticoid metabolite concentrations dropped significantly in the 2nd (232.2 ± 19.3 ng/ml) and 3rd (225.9 ± 18.7) sample collections, and were lowest (186.6 ± 15.1 ng/ml) in the last sampling. There was no association between fecal glucocorticoid metabolite concentrations and the loads of Salmonella in feces (Fig. 7).
FIG 7.
Levels of fecal corticosterone metabolites at different sampling points.
Other hen welfare parameters and Salmonella shedding.
There were no correlations between Salmonella shedding or prevalences and comb colors, panting, plumage damage, noise levels, novel object tests, distance avoidance tests, or social interactions between birds (Table 1).
TABLE 1.
Comparisons of welfare parameters with Salmonella shedding and prevalence
| Welfare measure |
P value for Salmonella measure |
|
|---|---|---|
| Prevalencea | Count | |
| Panting | 0.918 | 0.057 |
| Noise level | 0.153 | 0.567 |
| Comb color | 0.463 | 0.057 |
| Plumage damage | 0.459 | 0.588 |
| Interaction | 0.162 | 0.773 |
| Novel object test | 0.244 | 0.364 |
| Avoidance distance test | 0.968 | 0.744 |
| Pariah | N/A | 0.762 |
Measured by the MPN method. N/A, not available.
Presence of virulence genes in different Salmonella serovars.
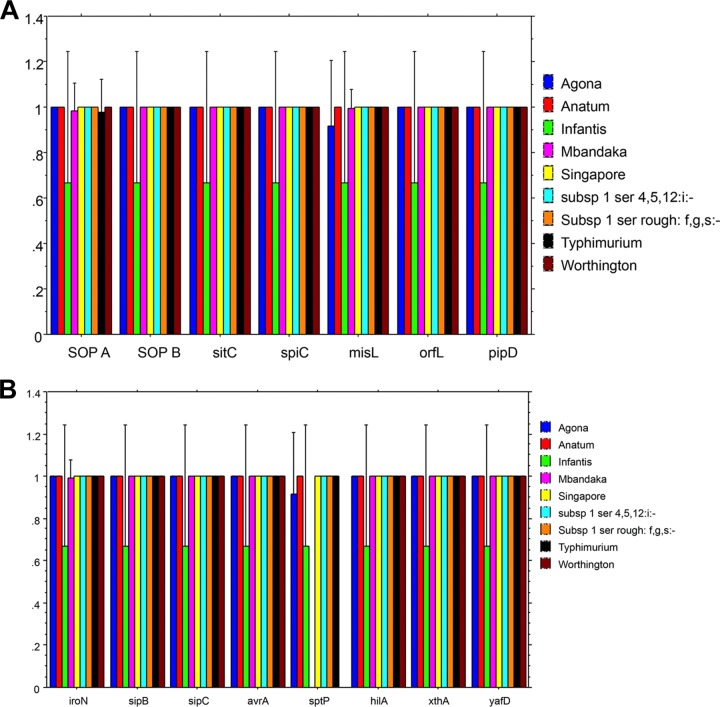
Salmonella serovars were screened for different virulence genes that are involved in adhesion, colonization, and survival in eggs. The results from PCRs for the presence of various genes are presented in Fig. 8. The majority of the selected virulence genes were detected in most of the serovars tested. All isolates of S. Mbandaka and S. Worthington were negative for sptP. sopA was not detected in one isolate of S. Typhimurium, one isolate of S. Infantis, or two isolates of S. Mbandaka (from flock C). One isolate of S. Mbandaka was also negative for iroN and misL.
FIG 8.
Distributions of genes tested in different Salmonella serovars isolated in this study. (A) sopA, sopB, sitC, spiC, misL, orfL, pipD; (B) iroN, sipB, sipC, avrA, sptP, hilA, xthA, and yafD.
DISCUSSION
The prevalences and shedding of Salmonella on four commercial free-range farms across various seasons were variable. The first cross-sectional study was conducted for selecting sampling sites in the free-range layer sheds. The results of the cross-sectional study performed on four different flocks indicated variable and mixed results for different sites in each flock across the sheds (Fig. 1). Hence, the longitudinal study involved all sites that were sampled during the cross-sectional study.
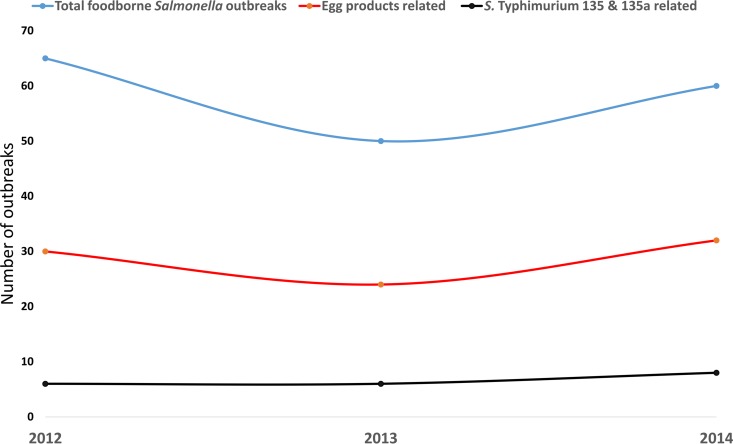
The shedding of Salmonella varied across free-range farms and during different seasons. This finding contrasts with an earlier study that found increasing Salmonella shedding over the sampling period (13). There was no significant effect of season on Salmonella prevalence during this study. There was no increase in Salmonella shedding in the summer season. Earlier, Traub-Dargatz et al. (14) reported that, under experimental conditions, heat stress did not influence the levels of Salmonella Typhimurium DT 104. Previous studies found increasing S. Typhimurium 135- and 135a-related human cases in the summer (7). These two phage types were isolated predominantly from free-range farms during this study. Our finding regarding high Salmonella shedding in summer is in agreement with that of a previous report that detected increasing levels of Salmonella on farms in the summer (15). During our study, variabilities between the detected prevalence and shedding were observed between flocks and farms. This observation is in agreement with previous reports (13, 16). There are numerous reports on the effects of the housing system on the shedding of Salmonella spp. (17). In Australia between 2012 and 2014, 213 Salmonella outbreaks were recorded. Of 213 outbreaks, 102 (47%) were attributed to the consumption of egg products. Twenty of 102 (19%) outbreaks were caused by S. Typhimurium phage types 135 and 135a (Fig. 9). MLVA results from S. Typhimurium strains isolated from flock C were distinct from and unrelated to those from flock D. The MLVA pattern of S. Typhimurium (both phage types 135 and 135a) evolved during the sample collection period. Earlier, we found that vectors, such as wild birds and foxes in the close vicinity of free-range farms, might play a major role in S. Typhimurium evolution (18). S. Typhimurium strains detected from human food poisoning cases exhibited MLVA patterns similar to the strains isolated from flocks C and D (19, 20) (Table 2). It is not clear why MLVA type 03 12 09 11 523 was isolated more frequently (from 59 human cases from 2013 to 2015) than MLVA type 03 12 09 10 523 isolated from the same flock. Our previous report suggested that MLVA profiles of S. Typhimurium isolated from cage farms and human Salmonellosis cases were similar (11). Although a majority of the previous studies were focused on S. enterica serovar Enteritidis, the results of these studies were highly variable. The contamination of eggs with Salmonella from production to plate is a complex issue and influenced by different variables, such as flock size, stocking density, flock age, stress, feed, the quality of eggs, disinfection procedures on the farm, the handling of eggs, storage temperature, the kitchen environment, and kitchen hygiene (21). Jones et al. (22) reported no significant differences in the prevalence of Salmonella between free-range and cage production systems. Findings from our study also reinforce the hypothesis that the level of egg contamination may be attributed largely to individual flock management and/or farm management. There are several possible sources of Salmonella contamination and/or infection from day-old hens to laying hens at the end of their commercial life span (23). In this study, sampling was conducted from week 24 onwards. Hence, further research is required to investigate the longitudinal epidemiology of S. Typhimurium from day-old hens to those at the end of their commercial life (75 to 80 weeks) in laying flocks on caged and free-range farms. Although such longitudinal investigations are valuable for understanding Salmonella-shedding dynamics, the successes of such studies also depend on the willingness of producers to participate.
FIG 9.
Salmonella outbreaks reported in Australia between 2012 and 2014. This information was obtained from OzFoodNet quarterly reports (2012 to June 2014) (19, 47–55) http://health.gov.au/internet/main/publishing.nsf/Content/cdna-ozfoodnet-reports.htm.
TABLE 2.
MLVA types isolated from human cases over three periods in Australiaa
| MLVA type | No. of cases |
||
|---|---|---|---|
| 2013 | 2014 | 2015 (Jan–June) | |
| 03 13 10 12 523 | 0 | 0 | 0 |
| 03 14 10 12 523 | 0 | 0 | 2 |
| 03 14 11 12 523 | 0 | 0 | 11 |
| 03 12 09 10 523 | 0 | 0 | 1 |
| 03 12 09 11 523 | 30 | 17 | 12 |
These MLVA types of S. Typhimurium were also isolated from free-range farms sampled in this study. Three MLVA types (03 14 10 12 523, 03 14 11 12 523, and 03 12 09 10 523) were not isolated from human cases in 2013 or 2014. However, these MLVA types started to appear in humans in 2015. This underlines the connection between the free-range layer industry and the potential threat of Salmonella to public health. Data are from quarterly reports of the Australian Salmonella Reference Centre (2013, 2014, and 2015), Institute of Medical and Veterinary Sciences, SA Pathology, Adelaide, Australia.
Currently, there is no nationwide prevalence database of S. Typhimurium on egg farms. A cross-sectional microbiological survey conducted by New South Wales Food Authority on 49 egg farms showed that 28% of the commercial free-range farms were positive for S. Typhimurium (24), whereas a survey conducted on 21 egg farms by Safe Food Queensland reported that 13.5% of farms were positive for S. Typhimurium (25). Current data suggest that shedding of S. Typhimurium from known-positive hens in both field conditions is highly variable and can be influenced by the stress experienced by hens. Cross-sectional sampling may not be sufficient for determining the true prevalence. Therefore, longitudinal sampling of flocks is essential.
During this study, the prevalence of Salmonella was highest in dust samples followed by those from shoe covers from outdoor ranging areas and in fecal samples. Our results regarding the persistence of Salmonella in dust samples from free-range production systems are in agreement with those of a previous study (26). The removal of dust by effective cleaning and disinfection of poultry sheds might reduce the levels of Salmonella contamination, although the recovery of Salmonella spp. from surfaces such as floors and dropping boards in cleaned and disinfected houses was variable (27). Salmonella spp., including S. Typhimurium, persisted in ranging areas. In practice, it is difficult to clean and disinfect ranging areas.
The predominant Salmonella serovars isolated during this study were S. Mbandaka and S. Typhimurium phage type 135. S. Mbandaka was the predominant pathogen in our previous longitudinal study conducted on cage farms (11, 28). S. Mbandaka has not been associated with egg-related Salmonella outbreaks in Australia. S. Typhimurium was the second most prevalent serovar in this study. This serovar has gained significant public attention over the last decade in Australia, as the majority of egg-related Salmonella outbreaks are associated with S. Typhimurium and its phage types (29). S. Typhimurium was isolated from all sample types, including egg shell wash, although its persistence was variable. It has been hypothesized that birds reared on the floor are less likely to harbor Salmonella spp. and that microorganisms present in the litter could play a role in competitive exclusion (30). Data from this field study do not support the hypothesis. However, further experimental studies are essential to confirm whether some Salmonella serovars act as seeding agents for the competitive exclusion of S. Typhimurium. Although the MLVA profiles of S. Typhimurium isolated in this study and from egg human outbreaks were similar, further studies are required for understanding the invasion potential of these isolates. It was demonstrated that the invasive potential of S. Typhimurium increased after enrichment (31). A hypothesis might be that the invasive potential of S. Typhimurium is influenced by the available favorable environment at the time of invasion.
The 15 genes analyzed in this study were tested because they are known to be involved in adhesion, invasion, and survival of Salmonella spp. The detection of these genes by PCR has been widely used as a predictive measure for Salmonella virulence (32, 33). sptP was not amplified among S. Mbandaka and Worthington isolates. We observed variability in the detections of sopA, ironA, and misL. Although the PCR results indicated that some of the Salmonella serovars were negative for these genes, it is possible that there was sufficient genetic variability preventing primer annealing and subsequent amplification. Full genome-sequence analysis studies are essential to investigate genetic variability. Although the health authorities in Australia are moving toward the whole-genome sequencing approach, PCR typing of virulence genes can still be used as a preliminary screening tool to select isolates for whole-genome sequencing. All but two S. Mbandaka isolates recovered from flock C tested positive for sopA. Also, three different MLVA types of S. Typhimurium were detected from flock C during the study period. It is possible that some Salmonella isolates either acquired virulence genes or evolved from a more virulent type (S. Typhimurium, in this case). Previous studies detected virulence genes in various Salmonella isolates collected from cage farms (31, 34). However, there is limited information on virulence typing of Salmonella serovars collected from free-range environments.
In this study, Salmonella MPN values for environmental (feces, egg belt, dust, nest box, and ramp) samples were highest in specimens collected during the summer season (4th sampling, performed in February). A recent study found an increase in cases of human S. Typhimurium phage types 9 and 108 during the warm season (7). It is important to note that none of these phage types were isolated in this study. Our previous study also indicated high Salmonella counts in feces in summer, with S. Typhimurium phage type 9 as a predominant serovar (18). Human gastrointestinal infections with Salmonella have been positively correlated with warmer temperatures (35, 36), as warmer temperatures can enhance the replication of bacteria. In this study, S. Typhimurium was detected in environmental samples and egg shell wash. However, their presence did not depend on the time of sampling and/or season. Earlier, Schulz et al. (37) reported that the probability of finding Salmonella spp. on farms did not depend on the time of sampling or season.
Other serovars, such as Singapore, Anatum, Agona, and Infantis, have also been associated with sporadic egg-related Salmonella cases in humans in Australia (38). The prevalence of these serovars was low in this study. Our previous study indicated that the presence of virulence genes may not necessarily dictate the virulence/invasive ability of Salmonella (31). The PCR results indicated that most of the above-listed serovars harbored virulence genes, but in vivo studies are required to study their virulence potential.
In this study, there were no correlations between fecal corticosterone metabolite (FCM) and Salmonella prevalence and/or shedding in feces. This observation is consistent with our previous finding (18). There are limited reports of FCM levels and Salmonella shedding in laying hens. The measurement of FCM is an established noninvasive method for measuring adrenocortical activity in chickens (39) as an indication of the hypothalamic-pituitary-adrenal axis component of the stress response. Borsoi et al. (40) reported high plasma corticosterone levels in cold-stressed and Salmonella-infected specific-pathogen-free broilers. A previous study found more pecks at waxworms (dummy worms) from Salmonella-infected birds than from uninfected birds (37). There were no correlations between the prevalence or shedding of Salmonella and any of the hen welfare measures, including social interaction, plumage damage (which may indicate occurrence of plumage damage), flock noise, fear tests (novel object and avoidance distance), comb colors, ranging activity, panting, or the prevalence of pariah birds. The breed of laying hens might influence their behavior (37). However, in this study, all birds were of the same breed. In Australia, free-range birds have a minimum of 8 h of range access, except during adverse weather conditions (41). Although flocks sampled during this study did not have access to ranging areas during adverse weather conditions, birds enclosed in the shed would have been exposed to heat. It is worth noting that, although Salmonella counts were high in fecal samples collected during the summer, the FCM levels were not high, and FCMs decreased from the first to the last visits, likely as a result of aging. During high temperatures, panting is one of the thermoregulatory mechanisms for laying hens (42). In this study, there was no correlation between panting and Salmonella shedding. The results from our study regarding high Salmonella shedding in warm seasons are in agreement with those reported by Wales et al. (13), but could not be linked with physiological stress based on FCM levels. It is important to note that corticosterone metabolites in fecal samples reflect the corticosterone production after a species-specific time period (43), and the detection of short peaks requires frequent sampling. In this study, hen welfare assessments were conducted at six weekly intervals, so it is possible that short-lasting deviations from these welfare states may have been missed. Further experiments are necessary to study the relationship between hen welfare and Salmonella shedding in a controlled environment.
The data provide important information for the egg industry regarding the dynamics of Salmonella shedding and the possible links between environment/bird/egg transmissions of Salmonella serovars of public health significance on free-range layer farms. This field study compared the shedding of Salmonella spp. with flock welfare in a free-range environment. The study highlights the challenges of implementing Salmonella control strategies in a free-range production system, because shedding and persistence varied across different farms and seasons. Some reports have indicated the possibility of increased human Salmonella cases in warmer months due to increases in Salmonella shedding on farms (7, 15). However, factors such as the handling of egg products or food handling practices in general, kitchen hygiene, changes in human life styles, and eating habits cannot be ignored. Salmonella organisms survived in various environmental samples (inside and outdoor environments), and their detection was variable. This finding highlights the challenge for establishing Salmonella prevalence based on single time point sampling. The incidences of Salmonella spp. need to be monitored regularly in the Australian egg industry. Regulatory authorities are involved in the testing of poultry farms in Australia. This study provides useful information for public health authorities, veterinarians, and regulators to design sampling strategies on free-range egg farms.
MATERIALS AND METHODS
Selection of farms.
Four commercial free-range flocks (A, B, C, and D) from four different farms were selected for this study on the basis of the willingness of egg producers to participate in the study. All farms had multiage flocks housed in separate sheds. The youngest flocks (ages ranged from 23 to 24 weeks) on each farm were selected for this study. All flocks had at least 8 h of outdoor range access. Flocks A and B had 10,000 birds each, whereas flocks C and D had 18,000 and 8,000 Hy-Line brown birds, respectively. To identify Salmonella serovars and determine the best sampling sites within the shed and range areas, a cross-sectional study was conducted on all four free-range flocks. This was followed by the longitudinal study to investigate the shedding of Salmonella in the free-range production systems. The stocking densities in the ranges were between 1,500 and 10,000 birds per hectare.
Cross-sectional study.
During this cross-sectional sampling, to identify areas of higher Salmonella prevalence and infection spread within a flock, 10 swabs (Whirl-Pak speci-sponge bags; Thermo Fisher Scientific, Australia) were collected from floor areas near pop holes (small openings from which birds went out to range), floor areas near drinker lines, nest boxes, and dust and ramps near pop holes (total 50 samples/flock) (Fig. 10). In addition, samples from shoe covers (n = 2) worn while collecting samples from the left and right sides of the sheds were also collected. At the end of the sampling, shoe covers were removed and placed in a 250-ml sterile plastic container (Pacific Laboratory Products, Australia).
FIG 10.
Graphical representation of sampling performed during the current study. The swabs from nest boxes (NB), ramps (L, R), and dust (D) were collected during each sampling. Fecal samples (FS) were collected during each sampling point.
Longitudinal sampling for investigating the dynamics of Salmonella shedding.
Based on the prevalence results from the cross-sectional study (Fig. 1), different areas of the sheds were selected for longitudinal sampling. Samplings were conducted from 2014 to 2015 during different seasons.
From each flock, samples were collected at 6-week intervals from October 2014 to July 2015. During the longitudinal sampling on each farm, 10 fecal samples were collected (five from each side) in a sterile Whirl-pak plastic bag (150 by 230 mm; Thermo Fisher Scientific, Australia). A clean and disinfected plastic tray was placed under the slats/flooring on the day before sampling for collecting fresh feces. Fresh feces were collected in a sterile plastic bag on the day of sampling, and stored at −20°C within 6 h. Swabs (Whirl-Pak Speci-sponge bags, 115 by 134 mm; Thermo Fisher Scientific, Australia) from egg belts, dust, nest boxes, and ramps near pop holes (n = 10 each) were collected at each sampling point. Samples from shoe covers (n = 4) worn while collecting samples from inside and outside (range area) of the sheds were collected from the left and right sides. Thirty visibly clean eggs, as well as 10 floor eggs, were collected at each sampling point.
Isolation and enumeration of Salmonella.
Salmonella organisms were isolated from egg belt, dust, nest box, ramp, swab, and shoe cover samples. The swabs were premoistened using 20 ml of buffered peptone water (BPW; Oxoid, Australia) and were dragged to cover 1 square meter of floor area. For Salmonella isolation, 10 g of feces was inoculated into 90 ml of BPW and further enriched. Shoe covers were placed in 100 ml of BPW and processed for further enrichment and isolation.
Each sample inoculated in BPW was incubated at 37°C overnight, and 100 μl of inoculated BPW was transferred to Rappaport Vassiliadis soya peptone (RVS) broth (Oxoid, Australia), which was then incubated at 42°C for 24 h. A loopful of the same sample was streaked on Brilliance Salmonella (Oxoid Australia) and xylose lysine deoxycholate (XLD; Oxoid, Australia) agar plates. The presumptive Salmonella colonies were also tested for ortho-nitrophenyl-β-d-galactopyranoside (Oxoid, Australia), lysine decarboxylase (LDC), and urease (Oxoid, Australia) activities. The suspected Salmonella cultures were sent for serotyping to the Salmonella Reference Laboratory, Adelaide, Australia. Salmonella organisms were isolated from shoe covers placed in 100 ml BPW as mentioned above.
Individual eggs were placed in 10 ml of sterile BPW in Whirl-Pak bags. To recover bacteria from eggshell surfaces, eggs were massaged out of Whirl-pak bags for 2 min. Before rinsing, BPW was prewarmed to 37°C to facilitate bacterial recovery. After a rinse sample was obtained, each egg was removed and transferred to a new sterile bag. The BPW samples were incubated at 37°C overnight, and 100 μl of this sample was inoculated into RVS broth (Oxoid, Australia), which was then incubated at 42°C for 24 h. The incubated RVS broths were further processed for Salmonella isolation as mentioned above. After eggshell surface processing, each egg was dipped into 70% alcohol for 60 s to eliminate any bacteria present on the outside of the shell and was allowed to air dry in a biosafety cabinet. After drying, the eggs were cracked open and the internal egg contents were collected in sterile containers, were thoroughly mixed, and 2 ml was inoculated into 8 ml of BPW. The inoculated BPW samples were further processed for Salmonella isolation as mentioned above. The miniaturized most probable number (MPN) method as described previously (44) was used for determining the loads of Salmonella in samples that tested positive by culture techniques. The results were interpreted as log MPN/g feces or m2 of area sampled.
Multilocus-variable tandem-repeat analysis of S. Typhimurium isolates.
After serotyping, all Salmonella strains that were identified as a Typhimurium were further analyzed by MLVA, as described previously by Ross et al. (45) at the Salmonella Reference Laboratory, Adelaide, Australia.
Assessment of bird welfare.
Hen welfare was measured on each visit to investigate the relationship between hen welfare and Salmonella shedding (Table 3). The sheds were sectioned into four quarters: 1, the front right corner; 2, the back right corner; 3, the back left corner, and 4, the front left corner. Each quarter was subdivided into two areas, with one area between the shed wall and the feeder and drinker line and the other area between the feeder and drinker line and the nest area in the center of the shed. Assessments were carried out in the middles of each of these areas. Observations were made between 11:00 AM and 02:30 PM, when hens are expected to be most active. Some measures were taken on 80 individual hens during each visit, with 10 birds in each of the 8 areas: panting, comb colors, plumage damage, and avoidance distance tests. Other measures were collected for each of the 8 areas: social interactions, novel object tests, pariah birds, and fecal sampling for glucocorticoid metabolite measurement. Finally, the last set of welfare measures was collected at flock level, including noise and ranging activity assessment.
TABLE 3.
Procedures and scoring system for hen welfare assessment
| Measure | Scoring method | Definition |
|---|---|---|
| Panting (adapted from 41) | Ten birds from four different areas were observed for panting. Numbers of panting birds in each section were recorded. | Breathing respiration in short gasps; visible signs are birds that sit upright, open their beaks, and often make visible respiratory movements; wings may be held out from the side of the body |
| Comb color (56) | Ten birds from each area were randomly selected and scored as follows: pale = 1–3, normal = 4–5, dark red = 6–7. | See comb color scale from the Bristol Welfare Assurance Programme hen assessment (56) |
| Noise (adapted from 57) | Each flock was observed inside the shed for 30 s and scored as follows for the noise level: quiet = 1, steady murmur = 2, loud chatter = 3, loud gakel calling = 4. | Self-explainable; see scoring method |
| Plumage damage (adapted from 58) | Ten birds were randomly selected in four different areas of the shed. Head/neck, back, and ventral area of each bird (without handling birds) was visibly assessed. No/minimal feather loss: no bare skin visible, no or slight wear, only single feathers missing = 0; moderate feather loss: moderate wear, i.e., damaged feathers or 1 or more featherless areas, bare skin visible <5 cm maximum dimension = 1; Severe feather loss: at least one area with bare skin visible ≥5 cm maximum dimension = 2. | Relates to plumage condition; see scoring method |
| Social interactions (adapted from 57) | Birds within an area of 1 m2 were observed for 3 min. No. of birds interacting with each other were measured (not the no. of interactions), accounting for the no. of hens in the area at the end of observation minus no. at the start. | Physical interactions between two or more hens, such as pecking or grooming |
| Novel object test (adapted from 57) | Red (comb color) sheet was placed on the floor in four different areas of sheds 2 m in front of the observer. Latency for 10 different birds to touch the paper was recorded or the no. of birds that have touched in 2 min, whichever came first. | Touching: physical contact (beak, foot) with novel object |
| Avoidance distance test (adapted from 58) | The assessor stops, turns around, and focuses on a random bird approx 2 m in front of him/her. The assessor holds his/her hand in a fixed position against the abdomen and walks slowly at one step per second toward the focal bird, looking at the bird's feet. When the bird turns away or retreats (both feet step aside or away), the distance (in mm) between the assessor's hand and where the bird's feet were originally is estimated. If the hen retreated for a reason other than the approach, another hen was selected. | Self-explainable; see scoring method |
| Pariah birds (adapted from 59) | No. of victimized birds within a 5 m radius in each of the eight areas of the sheds were counted. | Hyperactive individual that alternatively withdraws from or makes a frenzied dash through the body of the flock |
| Ranging activity assessment (adapted from 57) | No. of hens counted inside the veranda area, close to the veranda/pop holes, is within 2 m ranging, ranging away from the veranda/pop holes is 5–10 m, or more than halfway down the range is ≥20 m. This assessment was conducted outside the sheds on both the left and right sides of the ranges. | Self-explainable; see scoring method |
Measurement of fecal glucocorticoid metabolites.
Glucocorticoid metabolites were extracted from all fecal samples (n = 280) as described earlier (18). All fecal samples were homogenized and dried at 103°C overnight. After cooling at room temperature, the dried samples were milled to a fine powder. Fecal samples (0.2 g) were mixed with ethanol, vortexed for 30 min, and centrifuged at 10,000 × g for 15 min. The supernatants were dried using a nitrogen dryer, and the dried extracted samples were stored at −20°C. Immediately before the immunoassay, the extracts were dissolved in ethanol. Samples were analyzed for glucocorticoid metabolite concentrations by a radioimmunoassay (RIA) (ImmuChem double antibody corticosterone 125I RIA kit; MP Biomedicals LLC, Orangeburg, NY, USA) in accordance with the manufacturer's instructions using a 1:100 dilution. A preliminary validation was conducted by administrating 12.5 IU adrenocorticotropin (ACTH) subcutaneously in three laying hens, with two other hens administered saline as a control, followed by repeated fecal samplings every 30 min for 8 h. Samples were successfully obtained in 82% of the time points for all hens. Samples giving results with a coefficient of variation greater than 5% between duplicates were rerun. The results were interpreted as ng/ml.
DNA extraction and PCR.
Overnight cultures of the Salmonella serovars selected for this study were grown at 37°C in 4 ml Luria Bertani (LB) broth. DNA was purified from 109 bacteria/ml using the Promega genomic DNA kit (Promega, USA). Purified DNA was quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Australia). Working DNA solutions were prepared by diluting stock solutions to 5 ng/μl. Primers for each gene were designed using the primer design feature in GenBank or were obtained from Hughes et al. (46). Primers were obtained from GeneWorks (Adelaide, South Australia) (Table 4). The PCR mix comprised 1× Taq polymerase buffer (Fisher Biotech, Australia), 1.0 mM MgCl2, 0.5 μM forward and reverse primers, 0.2 μM deoxynucleoside triphosphates (dNTPs), 0.3 U Taq polymerase (Fisher Biotech, Australia), and 10 ng Salmonella DNA.
TABLE 4.
Details of the virulence genes used for Salmonella typing
| Virulence gene | Function | Primer sequence (5′ to 3′) |
Annealing temp (°C) | Reference | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| sitC | Iron transporter | CAGTATATGCTCAACGCGATGTGGGTCTCC | CGGGGCGAAAATAAAGGCTGTGATGAAC | 64 | 60 |
| spiC | Disrupts Golgi apparatus and lysosomes | CCTGGATAATGACTATTGAT | AGTTTATGGTGATTGCGTAT | 56 | 60 |
| misL | Adhesin | GTCGGCGAATGCCGCGAATA | GCGCTGTTAACGCTAATAGT | 58 | 60 |
| orfL | Survival within macrophages | GGAGTATCGATAAAGATGTT | GCGCGTAACGTCAGAATCAA | 56 | 60 |
| pipD | Colonization | CGGCGATTCATGACTTTGAT | CGTTATCATTCGGATCGTAA | 58 | 60 |
| iroN | Iron transport | ACTGGCACGGCTCGCTGTCGCTCTAT | CGCTTTACCGCCGTTCTGCCACTGC | 60 | 60 |
| sipB | Invasion protein | TGGCAGGCGATGATTGAGTC | CCCATAATGCGGTTCGTTTC | 58 | 31 |
| sipC | Invasion protein | TGCCCTGGCAAATAATGTCA | CATCGATTCGGGTCATATCC | 58 | 61 |
| sopA | Induces proinflammatory response | GCCCACGGTTTCTGAAGGTA | AAGAGTCCGCTGTGAGTGG | 60 | 31 |
| avrA | Modulates host immune response | ATACTGCTTCCCGCCGC | ACACCGAAGCATTGACCTGT | 58 | 31 |
| sptP | Disrupts actin cytoskeleton | TTCACCCTATCCGCCAGGTA | GTGTAGCCCGGTTCTCACAA | 58 | 46 |
| hilA | Activates expression of invasion genes | CACCAACCCGCTTCTCTCTT | ATTGTGGTCCAGCTCTGTCG | 58 | 62 |
| xth-a | Survival in egg | CGAAAAACACCAGCCCGATG | CCGGCAGGAAGGAGCATTTA | 55 | 62 |
| yafD | Survival in egg | CGGATCCGTATCCTCGTGTG | ATCGTCAGTGAAACGCACCT | 55 | 46 |
The product sizes for each of the genes were relatively similar. Therefore, the PCR conditions were similar, with differences only in the primer annealing temperatures (listed in Table 4). The general reaction protocol was 95°C for 5 min, 30 cycles of 95°C for 30 s (melt), annealing temperature (see Table 4) for 45 s, and 72°C for 1 min (extension), and then 72°C for 4 min and holding at 8°C. For data analysis, a positive PCR result was scored as 1 and a negative results was scored as 0.
Statistical analysis.
Descriptive and inference statistics were run using STATA v13.1 (StataCorp LP, Texas, USA). The apparent prevalences of Salmonella were compared at various time points using a mixed multifactorial logistic regression with a two-way cross-random effect for “sampling number” and “sample type” to account for nonindependent observations. For studying the relationship between fecal glucocorticoid metabolites and Salmonella shedding, for each fecal sample, the probability of isolating Salmonella spp. was compared to the measured concentration of fecal corticosterone using a mixed-effect logistic regression with “farm” and “sampling time” within farm as random effects. For fecal samples where Salmonella was detected (n = 33), the Salmonella cell count (log scaled) was compared to the fecal corticosterone concentration using a mixed-effect linear regression with “farm” and “sampling time” within farm as random effects. To study the relationships between hen welfare parameters and Salmonella prevalence and shedding for each flock at each sampling time, the means of each hen welfare parameter were collated with the mean Salmonella prevalences and counts (log scaled) from all samples but egg shells. Correlations between prevalence/shedding and hen welfare were estimated using mixed-effect regression models, where flock was included as a random effect to account for repeated measures across time within a flock. The relationship between prevalence/shedding and hen welfare was also explored using standard scatter plots.
Supplementary Material
ACKNOWLEDGMENTS
This research was conducted within the Poultry CRC, established and supported under the Australian Government's Cooperative Research Centres Program (number Poultry CRC 3.2.6).
We thank A. McWhorter and V. Pande for their valuable technical help during this study.
The authors have no conflicts of interest.
K.C., J.-L.R., and M.S. designed and developed the concept of the work, V.G., T.M., K.C., M.S., and R.W. performed the samplings on various farms, J.-L.R. and R.W. developed the welfare parameters, T.M. performed the PCRs, and C.C. and K.C. performed the statistical analyses.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.03313-16.
REFERENCES
- 1.Australian Egg Corporation Limited. 2015. Annual report, p 3. Australian Egg Corporation Limited, Sydney, New South Wales, Australia. [Google Scholar]
- 2.Daigle C, Siegford J. 2014. Welfare Quality parameters do not always reflect hen behaviour across the lay cycle in non-cage laying hens. Anim Welf 23:423–434. doi: 10.7120/09627286.23.4.423. [DOI] [Google Scholar]
- 3.Walker AW, Hughes BO. 1998. Egg shell colour is affected by laying cage design. Br Poult Sci 39:696–699. doi: 10.1080/00071669888593. [DOI] [PubMed] [Google Scholar]
- 4.Weeks CA, Lambton SL, Williams AG. 2016. Implications for welfare, productivity and sustainability of the variation in reported levels of mortality for laying hen flocks kept in different housing systems: a meta-analysis of ten studies. PLoS One 11:e0146394. doi: 10.1371/journal.pone.0146394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruhnke I. 2015. Free-range egg production in Australia—industry trends and challenges, Lohmann information. Lohmann Tierzucht, Cuxhaven, Germany. [Google Scholar]
- 6.Ruhnke I, de Koning C, Drake K, Glatz P, Walker T, Skerman A, Hunt P, Hinch G, Sommerlad M, Choct M, Singh M. 2015. Free range farm demographics and practices in Australia—preliminary data, p 260. In The 26th Australian Poultry Science Symposium, Sydney, Australia. [Google Scholar]
- 7.Milazzo A, Giles LC, Zhang Y, Koehler AP, Hiller JE, Bi P. 2016. The effect of temperature on different Salmonella serotypes during warm seasons in a Mediterranean climate city, Adelaide, Australia. Epidemiol Infect 144:1231–1240. doi: 10.1017/S0950268815002587. [DOI] [PubMed] [Google Scholar]
- 8.Huang JY, Henao OL, Griffin PM, Vugia DJ, Cronquist AB, Hurd S, Tobin-D'Angelo M, Ryan P, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Wolpert BJ, Patrick ME. 2016. Infection with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance–Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2012–2015. MMWR Morb Mortal Wkly Rep 65:368–371. doi: 10.15585/mmwr.mm6514a2. [DOI] [PubMed] [Google Scholar]
- 9.Australian Government Department of Health. 13 January 2016. National Notifiable Disease Surveillance System. Australian Government Department of Health, Canberra, ACT, Australia. [Google Scholar]
- 10.European Food Safety Authority, European Centre for Disease Prevention and Control. 2015. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J 13:4329. doi: 10.2903/j.efsa.2015.4329. [DOI] [Google Scholar]
- 11.Gole VC, Torok V, Sexton M, Caraguel CG, Chousalkar KK. 2014. Association between indoor environmental contamination by Salmonella enterica and contamination of eggs on layer farms. J Clin Microbiol 52:3250–3258. doi: 10.1128/JCM.00816-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lara LJ, Rostagno MH. 2013. Impact of heat stress on poultry production. Animals (Basel) 3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wales AD, Berslin M, Carter B, Sayers R, Davis R. 2007. A longitudinal study of environmental Salmonella contamination in caged and free range layer flocks. Avian Pathol 36:187–197. doi: 10.1080/03079450701338755. [DOI] [PubMed] [Google Scholar]
- 14.Traub-Dargatz JL, Ladely SR, Dargatz DA, Fedorka-Cray PJ. 2006. Impact of heat stress on the fecal shedding patterns of Salmonella enterica Typhimurium DT104 and Salmonella enterica infantis by 5-week-old male broilers. Foodborne Pathog Dis 3:178–183. doi: 10.1089/fpd.2006.3.178. [DOI] [PubMed] [Google Scholar]
- 15.Hellberg RS, Chu E. 2016. Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: a review. Crit Rev Microbiol 42:548–572. doi: 10.3109/1040841X.2014.972335. [DOI] [PubMed] [Google Scholar]
- 16.Snow LC, Davies RH, Christiansen KH, Carrique-Mas JJ, Wales AD, O'Connor JL, Cook AJ, Evans SJ. 2007. Survey of the prevalence of Salmonella species on commercial laying farms in the United Kingdom. Vet Rec 161:471–476. doi: 10.1136/vr.161.14.471. [DOI] [PubMed] [Google Scholar]
- 17.Van Hoorebeke S, Van Immerseel F, Haesebrouck F, Ducatelle R, Dewulf J. 2011. The influence of the housing system on Salmonella infections in laying hens: a review. Zoonoses Public Health 58:304–311. doi: 10.1111/j.1863-2378.2010.01372.x. [DOI] [PubMed] [Google Scholar]
- 18.Chousalkar K, Gole V, Caraguel C, Rault JL. 2016. Chasing Salmonella Typhimurium in free range egg production system. Vet Microbiol 192:67–72. doi: 10.1016/j.vetmic.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 19.OzFoodnet Working Group. 2015. OzFoodNet quarterly report, 1 July to 30 September 2013. Commun Dis Intell Q Rep 39:E280–E284. [PubMed] [Google Scholar]
- 20.Australian Salmonella Reference Centre. 2015. April to June 2015—quarterly report, p 1–14. In Microbiology and infectious diseases. SA Pathology, Flinders University, Adelaide, Australia. [Google Scholar]
- 21.Chousalkar KK, Sexton M, McWhorter A, Hewson K, Martin G, Shadbolt C, Goldsmith P. 2015. Salmonella Typhimurium in the Australian egg industry: multidisciplinary approach to addressing the public health challenge and future directions. Crit Rev Food Sci Nutr 11:0. doi: 10.1080/10408398.2015.1113928. [DOI] [PubMed] [Google Scholar]
- 22.Jones DR, Guard J, Gast RK, Buhr RJ, Fedorka-Cray PJ, Abdo Z, Plumblee JR, Bourassa DV, Cox NA, Rigsby LL, Robison CI, Regmi P, Karcher DM. 2016. Influence of commercial laying hen housing systems on the incidence and identification of Salmonella and Campylobacter. Poult Sci 95:1116–1124. doi: 10.3382/ps/pew036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chousalkar K, Gole VC. 2016. Salmonellosis acquired from poultry. Curr Opin Infect Dis 29:514–519. doi: 10.1097/QCO.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 24.New South Wales Farmers Association. 2013. Evaluation of the egg food safety scheme. New South Wales Farmers Association, Sydney, New South Wales, Australia. [Google Scholar]
- 25.Cuttell L, Groves M, Wilson A. 2014. 2014 microbiological baseline survey of the Queensland egg production environment. Safe Food Production Queensland, Brisbane, Queensland, Australia. [Google Scholar]
- 26.Carrique-Mas JJ, Breslin M, Sayers AR, McLaren I, Arnold M, Davies R. 2008. Comparison of environmental sampling methods for detecting Salmonella in commercial laying flocks in the UK. Lett Appl Microbiol 47:514–519. doi: 10.1111/j.1472-765X.2008.02450.x. [DOI] [PubMed] [Google Scholar]
- 27.Carrique-Mas JJ, Marin C, Breslin M, McLaren I, Davies R. 2009. A comparison of the efficacy of cleaning and disinfection methods in eliminating Salmonella spp. from commercial egg laying houses. Avian Pathol 38:419–424. doi: 10.1080/03079450903193768. [DOI] [PubMed] [Google Scholar]
- 28.Gole VC, Caraguel CG, Sexton M, Fowler C, Chousalkar KK. 2014. Shedding of Salmonella in single age caged commercial layer flock at an early stage of lay. Int J Food Microbiol 189:61–66. doi: 10.1016/j.ijfoodmicro.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Moffatt CR, Musto J, Pingault N, Miller M, Stafford R, Gregory J, Polkinghorne BG, Kirk MD. 2016. Salmonella Typhimurium and outbreaks of egg-associated disease in Australia, 2001 to 2011. Foodborne Pathog Dis 13:379–385. doi: 10.1089/fpd.2015.2110. [DOI] [PubMed] [Google Scholar]
- 30.Shields S, Greger M. 2013. Animal welfare and food safety aspects of confining broiler chickens to cages. Animals (Basel) 3:386–400. doi: 10.3390/ani3020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McWhorter AR, Davos D, Chousalkar KK. 2015. Pathogenicity of Salmonella strains isolated from egg shells and the layer farm environment in australia. Appl Environ Microbiol 81:405–414. doi: 10.1128/AEM.02931-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suez J, Porwollik S, Dagan A, Marzel A, Schorr YI, Desai PT, Agmon V, McClelland M, Rahav G, Gal-Mor O. 2013. Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PLoS One 8:e58449. doi: 10.1371/journal.pone.0058449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gharieb RM, Tartor YH, Khedr MH. 2015. Non-typhoidal Salmonella in poultry meat and diarrhoeic patients: prevalence, antibiogram, virulotyping, molecular detection and sequencing of class I integrons in multidrug resistant strains. Gut Pathog 7:34. doi: 10.1186/s13099-015-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manning J, Gole V, Chousalkar K. 2015. Screening for Salmonella in backyard chickens. Prev Vet Med 120:241–245. doi: 10.1016/j.prevetmed.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Akil L, Ahmad HA, Reddy RS. 2014. Effects of climate change on Salmonella infections. Foodborne Pathog Dis 11:974–980. doi: 10.1089/fpd.2014.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmutz C, Mausezahl D, Jost M, Baumgartner A, Mausezahl-Feuz M. 2016. Inverse trends of Campylobacter and Salmonella in Swiss surveillance data, 1988–2013. Euro Surveill 21:pii=30130 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21375 [DOI] [PubMed] [Google Scholar]
- 37.Schulz J, Van Hoorebeke S, Hald B, Hartung J, Van Immerseel F, Radtke I, Kabell S, Dewulf J. 2011. The dynamics of Salmonella occurrence in commercial laying hen flocks throughout a laying period. Avian Pathol 40:243–248. doi: 10.1080/03079457.2010.544290. [DOI] [PubMed] [Google Scholar]
- 38.Glass K, Fearnley E, Hocking H, Raupach J, Veitch M, Ford L, Kirk MD. 2016. Bayesian source attribution of salmonellosis in South Australia. Risk Anal 36:561–570. doi: 10.1111/risa.12444. [DOI] [PubMed] [Google Scholar]
- 39.Rettenbacher S, Mostl E, Hackl R, Ghareeb K, Palme R. 2004. Measurement of corticosterone metabolites in chicken droppings. Br Poult Sci 45:704–711. doi: 10.1080/00071660400006156. [DOI] [PubMed] [Google Scholar]
- 40.Borsoi A, Quinteiro-Filho WM, Calefi AS, Ferreira AJ, Astolfi-Ferreira CS, Florio JC, Palermo-Neto J. 2015. Effects of cold stress and Salmonella Heidelberg infection on bacterial load and immunity of chickens. Avian Pathol 44:490–497. doi: 10.1080/03079457.2015.1086976. [DOI] [PubMed] [Google Scholar]
- 41.Primary Industries Report Series. 2002. Model code of practice for the welfare of animals: domestic poultry. CSIRO, Collingwood, Australia. [Google Scholar]
- 42.Al-Ramamneh DS, Makagon MM, Hester PY. 2016. The ability of White Leghorn hens with trimmed comb and wattles to thermoregulate. Poult Sci 95:1726–1735. doi: 10.3382/ps/pew110. [DOI] [PubMed] [Google Scholar]
- 43.Mostl E, Palme R. 2002. Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74. doi: 10.1016/S0739-7240(02)00146-7. [DOI] [PubMed] [Google Scholar]
- 44.Pavic A, Groves PJ, Bailey G, Cox JM. 2010. A validated miniaturized MPN method, based on ISO 6579:2002, for the enumeration of Salmonella from poultry matrices. J Appl Microbiol 109:25–34. doi: 10.1111/j.1365-2672.2009.04649.x. [DOI] [PubMed] [Google Scholar]
- 45.Ross IL, Davos DE, Mwanri L, Raupach J, Heuzenroeder MW. 2011. MLVA and phage typing as complementary tools in the epidemiological investigation of Salmonella enterica serovar Typhimurium clusters. Curr Microbiol 62:1034–1038. doi: 10.1007/s00284-010-9820-1. [DOI] [PubMed] [Google Scholar]
- 46.Hughes LA, Shopland S, Wigley P, Bradon H, Leatherbarrow AH, Williams NJ, Bennett M, de Pinna E, Lawson B, Cunningham AA, Chantrey J. 2008. Characterisation of Salmonella enterica serotype Typhimurium isolates from wild birds in northern England from 2005–2006. BMC Vet Res 4:4. doi: 10.1186/1746-6148-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.OzFoodNet Working Group. 2012. OzFoodNet quarterly report, 1 January to 31 March 2012. Commun Dis Intell Q Rep 36:E353–E360. [PubMed] [Google Scholar]
- 48.OzFoodNet Working Group. 2013. OzFoodNet quarterly report, 1 April to 30 June 2012. Commun Dis Intell Q Rep 37:E73–E78. [PubMed] [Google Scholar]
- 49.OzFoodNet Working Group. 2013. OzFoodNet quarterly report, 1 July to 30 September 2012. Commun Dis Intell Q Rep 37:E260–E266. [PubMed] [Google Scholar]
- 50.OzFoodNet Working Group. 2014. OzFoodNet enhanced foodborne disease surveillance, 1 January to 31 March 2013. Commun Dis Intell Q Rep 38:E70-77. [PubMed] [Google Scholar]
- 51.OzFoodNet Working Group. 2014. OzFoodNet quarterly report, 1 April to 30 June 2013. Commun Dis Intell Q Rep 38:E376–E382. [PubMed] [Google Scholar]
- 52.OzFoodNet Working Group. 2015. OzFoodNet quarterly report, 1 October to 31 December 2013. Commun Dis Intell Q Rep 39:E479–E485. [PubMed] [Google Scholar]
- 53.OzFoodNet Working Group. 2015. OzFoodNet quarterly report, 1 January to 31 March 2014. Commun Dis Intell Q Rep 39:E612–E618. [PubMed] [Google Scholar]
- 54.OzFoodNet Working Group. 2013. OzFoodNet quarterly report, 1 October to 31 December 2012. Commun Dis Intell Q Rep 37:E418–E426. [PubMed] [Google Scholar]
- 55.OzFoodNet Working Group. 2016. OzFoodNet quarterly report, 1 April to 30 June 2014. Commun Dis Intell Q Rep 40:E290–E296. [PubMed] [Google Scholar]
- 56.Leeb C, Main DCJ, Whay HR, Webster AJF. 2005. Bristol Welfare Assurance Programme hen assessment, University of Bristol, version 2.0, vol 2 University of Bristol, Bristol, United Kingdom. [Google Scholar]
- 57.Whay HR, Main DC, Green LE, Heaven G, Howell H, Morgan M, Pearson A, Webster AJ. 2007. Assessment of the behaviour and welfare of laying hens on free-range units. Vet Rec 161:119–128. doi: 10.1136/vr.161.4.119. [DOI] [PubMed] [Google Scholar]
- 58.Welfare Quality Consortium. 2009. Welfare Quality assessment protocol for poultry. ASG Veehouderij BV, Lelystad, The Netherlands. [Google Scholar]
- 59.Freire R, Wilkins LJ, Short F, Nicol CJ. 2003. Behaviour and welfare of individual laying hens in a non-cage system. Br Poult Sci 44:22–29. doi: 10.1080/0007166031000085391. [DOI] [PubMed] [Google Scholar]
- 60.Hurley BP, McCormick BA. 2003. Translating tissue culture results into animal models: the case of Salmonella typhimurium. Trends Microbiol 11:562–569. doi: 10.1016/j.tim.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Wilkins W, Waldner C, Rajic A, McFall M, Muckle A, Mainar-Jaime RC. 2010. Comparison of bacterial culture and real-time PCR for the detection of Salmonella in grow-finish pigs in western Canada using a Bayesian approach. Zoonoses Public Health 57:115–120. doi: 10.1111/j.1863-2378.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 62.Chen S, Zhao SH, McDermott PF, Schroeder CM, White DG, Meng JH. 2005. A DNA microarray for identification of virulence and antimicrobial resistance genes in Salmonella serovars and Escherichia coli. Mol Cell Probes 19:195–201. doi: 10.1016/j.mcp.2004.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.