ABSTRACT
Legionella pneumophila in potable water installations poses a potential health risk, but quantitative information about its replication in biofilms in relation to water quality is scarce. Therefore, biofilm formation on the surfaces of glass and chlorinated polyvinyl chloride (CPVC) in contact with tap water at 34 to 39°C was investigated under controlled hydraulic conditions in a model system inoculated with biofilm-grown L. pneumophila. The biofilm on glass (average steady-state concentration, 23 ± 9 pg ATP cm−2) exposed to treated aerobic groundwater (0.3 mg C liter−1; 1 μg assimilable organic carbon [AOC] liter−1) did not support growth of the organism, which also disappeared from the biofilm on CPVC (49 ± 9 pg ATP cm−2) after initial growth. L. pneumophila attained a level of 4.3 log CFU cm−2 in the biofilms on glass (1,055 ± 225 pg ATP cm−2) and CPVC (2,755 ± 460 pg ATP cm−2) exposed to treated anaerobic groundwater (7.9 mg C liter−1; 10 μg AOC liter−1). An elevated biofilm concentration and growth of L. pneumophila were also observed with tap water from the laboratory. The Betaproteobacteria Piscinibacter and Methyloversatilis and amoeba-resisting Alphaproteobacteria predominated in the clones and isolates retrieved from the biofilms. In the biofilms, the Legionella colony count correlated significantly with the total cell count (TCC), heterotrophic plate count, ATP concentration, and presence of Vermamoeba vermiformis. This amoeba was rarely detected at biofilm concentrations of <100 pg ATP cm−2. A threshold concentration of approximately 50 pg ATP cm−2 (TCC = 1 × 106 to 2 × 106 cells cm−2) was derived for growth of L. pneumophila in biofilms.
IMPORTANCE Legionella pneumophila is the etiologic agent in more than 10,000 cases of Legionnaires' disease that are reported annually worldwide and in most of the drinking water-associated disease outbreaks reported in the United States. The organism proliferates in biofilms on surfaces exposed to warm water in engineered freshwater installations. An investigation with a test system supplied with different types of warm drinking water without disinfectant under controlled hydraulic conditions showed that treated aerobic groundwater (0.3 mg liter−1 of organic carbon) induced a low biofilm concentration that supported no or very limited growth of L. pneumophila. Elevated biofilm concentrations and L. pneumophila colony counts were observed on surfaces exposed to two types of extensively treated groundwater, containing 1.8 and 7.9 mg C liter−1 and complying with the microbial water quality criteria during distribution. Control measures in warm tap water installations are therefore essential for preventing growth of L. pneumophila.
KEYWORDS: Legionella pneumophila, predominating biofilm bacteria, threshold biofilm concentration, warm tap water
INTRODUCTION
Legionella pneumophila is the causative agent of Legionnaires' disease (LD), a life-threatening pneumonia, and proliferates in natural and engineered freshwater systems at warm temperatures. In 2014, the number of annually reported LD cases amounted to more than 6,000 in Europe (1) and more than 5,000 in the United States (2). The wide range of notifications per million inhabitants (<1 to 39.4) in European countries indicates that the number of reported cases is an underestimation of the true number of cases. Complicated diagnostics and reporting inefficiency are considered the main reasons for these different notification rates (3). The increased number of reported cases in the United States has been attributed to an increasing population of older persons and persons at high risk for infection and to improved diagnostics and reporting (4). More than two thirds of the cases reported in Europe are community acquired, implying that the source of the infection was not identified, with smaller percentages associated with travel (19%), both abroad and domestic, and with health care (8%) (1). In the United States, LD was the most frequently reported drinking water-associated disease from 2009 to 2012 (5, 6).
L. pneumophila is detected only incidentally in distributed drinking water in temperate regions, but the organism is commonly present in potable water installations in hotels, hospitals, and residential water systems (1, 6–8). L. pneumophila is a nutritionally fastidious organism requiring specific amino acids for growth (9, 10), but a variety of free-living amoebae grazing on biofilms and sediments can serve as hosts and enable its proliferation in the aquatic environment (11–16). The optimal growth temperature of L. pneumophila ranges from 37 to 42°C (17, 18), and a warm temperature is essential for its growth because host amoebae digest the bacterium at temperatures of <20°C (19, 20). Several studies showed that the colony counts of L. pneumophila in potable water installations correlated with the concentrations of organic carbon, iron, manganese, and corrosion products in the water (7, 21–23). However, in a recent study, no growth of culturable L. pneumophila was observed in water contained in glass bottles incubated at 32 to 37°C and supplemented with various amounts of ozonated fulvic acid (24). Colony counts of L. pneumophila in water from systems with iron pipes were higher than those in water from systems with pipes of steel and copper (25). Organic polymeric materials used in water installations, e.g., natural and synthetic rubber, polyethylene (cross-linked), polypropylene, and polybutylene, can also promote growth of L. pneumophila (26–31). The L. pneumophila colony counts in biofilms on a variety of materials in contact with tap water in experimental systems correlated significantly with the total cell count (TCC) (31) and the ATP level (30) but not with the heterotrophic plate count (HPC) (29).
Water temperature management and prevention of stagnation are essential for limiting the growth of L. pneumophila in potable water installations (32–35). Furthermore, the use of plumbing materials that do not promote biofilm formation has been advocated (26–29, 36). However, water quality also affects biofilm formation, but the relationship between the concentration of the water-induced biofilm and growth of Legionella is still unclear. Therefore, a test system, the boiler biofilm monitor (BBM), was developed to determine the effects of drinking water without disinfectant on the biofilm formation and growth of L. pneumophila under optimal temperature and hydraulic conditions resembling those in potable water installations (37). This system was tested in the laboratory by using the locally available drinking water and at two groundwater supplies distributing drinking water with either a very low concentration of natural organic matter (NOM) (<0.5 mg C liter−1) or a high NOM concentration (7.8 mg C liter−1). The abundances and identities of Legionella spp. and free-living protozoa in these unchlorinated supplies were reported earlier (38, 39). The objectives of the present study were to (i) assess the relationship between the biofilm concentration and the Legionella colony count in biofilms under controlled conditions in a model system, (ii) identify bacteria predominating in biofilms, (iii) determine the effect of water quality (NOM and assimilable organic carbon [AOC]) on biofilm formation, and (iv) compare the use of glass and chlorinated polyvinyl chloride (CPVC) in the test system.
RESULTS
BBM system.
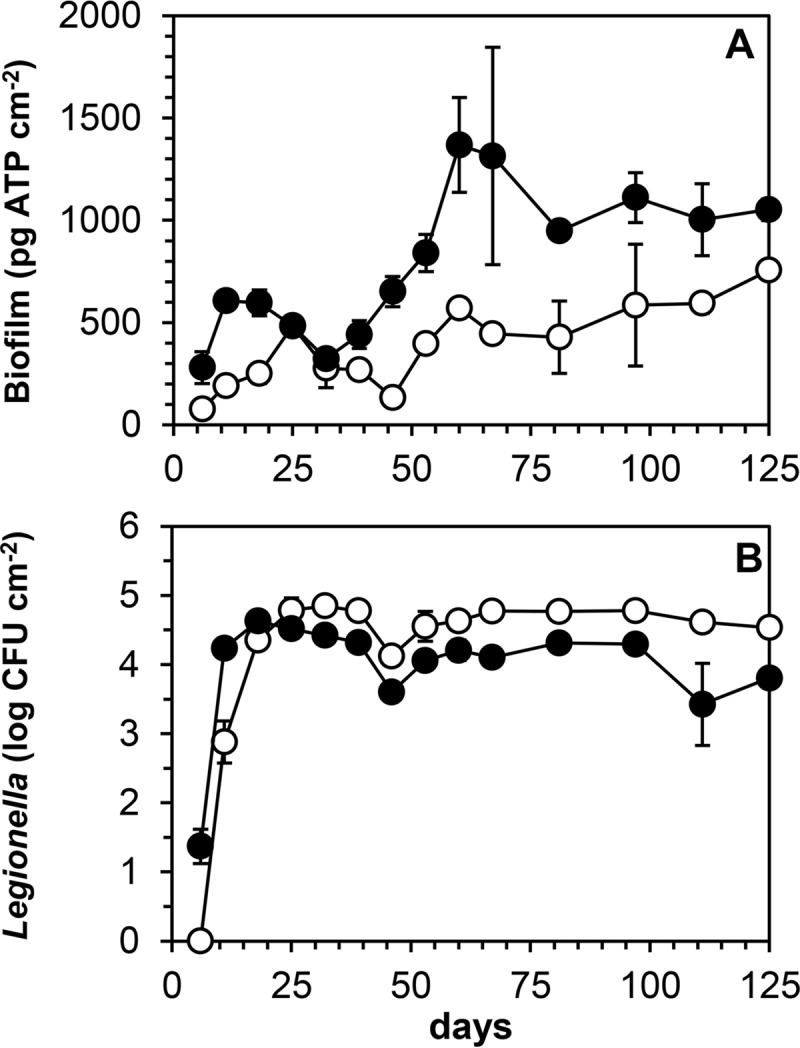
The locally available drinking water (supply C), with relatively low concentrations of total organic carbon (TOC), AOC, and ATP (Table 1), was used for testing of the model system. In a preliminary experiment, a glass column with glass cylinders placed on top of each other (biofilm monitor) (40) and glass cylinders (diameter, 1 cm; length, 2.5 cm) with glass beads (diameter, 0.4 cm) placed in parallel in an acrylate container were periodically flushed with warm drinking water to explore biofilm formation and growth of L. pneumophila in the biofilms on the glass surfaces. After 7 days of operation, a piece of silicone tubing with L. pneumophila and associated microbiota was inserted at the outlet of the thermostatic mixing valve. ATP analysis at day 14 revealed the presence of biofilms (200 to 300 pg ATP cm−2) on the beads and the cylinders. L. pneumophila multiplied in the biofilms and attained a level of about 103 CFU cm−2 at day 60 in both systems (results not shown). These observations showed the potential of a simple setup to assess the level of biofilm formation and multiplication of L. pneumophila in the biofilm. The column with cylinders was selected for further use because of easy sampling and the application of different materials. In two columns containing cylinders of either glass or CPVC, the biofilm concentration increased with fluctuations, with CPVC supporting a higher concentration than that with glass (Fig. 1 and 2A). Duplicate samples collected from each column gave similar concentrations in most cases, with median coefficients of variation (CV) of 8% for glass and 12% for CPVC. L. pneumophila multiplied in the biofilms at an exponential growth rate of approximately 1.3 day−1, and maximum colony counts were attained after about 3 weeks (Fig. 2B). Thereafter, the colony counts remained relatively stable, with significantly higher levels on glass than on CPVC. Table 2 shows the average biofilm concentrations and the means of the log-transformed Legionella colony counts. HPCs and TCCs, which were measured at a lower frequency than that for ATP and Legionella colony counts, attained levels of about 106 CFU cm−2 and 107 cells cm−2, respectively, on both materials, with significantly higher HPCs and TCCs on CPVC than on glass (Table 2).
TABLE 1.
Concentrations of total organic carbon (TOC), assimilable organic carbon (AOC), and ATP in the feed water at the test locationsc
| Water supply/locationa | TOC concn (mg liter−1) | AOC concn (μg acetate C equivalents liter−1) | % NOXb | AOC concn after 6 h at 70°C (μg acetate C equivalents liter−1) | % NOXb | ATP concn (ng liter−1) |
|---|---|---|---|---|---|---|
| AT | 0.32 ± 0.01 (4) | 1.1 ± 0.3 (3) | 75 | 1.9 ± 0.1 (2) | 70 | 0.7 ± 0.6 (13) |
| AD | 0.26 ± 0.03 (3) | 1.0 ± 0.1 (2) | 94 | NA | NA | 0.9 ± 0.7 (13) |
| BT | 7.9 ± 0.15 (3) | 10.9 ± 1.2 (5) | 89 | 22.9 ± 2.8 (5) | 94 | 10.3 ± 2.6 (29) |
| BD | 7.9 ± 0.16 (3) | 8.9 ± 0.7 (1) | 90 | 19.7 ± 3.3 (1) | 97 | 9.6 ± 3.2 (13) |
| CD | 1.8 ± 0.1 (2) | 3.4 ± 0.4 (3) | 77 | 7.9 ± 2.6 (2) | 87 | 3.0 ± 1.4 (14) |
AT, groundwater supply A, treated water (T); AD, supply A at a location in the distribution system; BT, supply B, treated water; BD, supply B at a location in the distribution system; CD, groundwater supply C at a location in the distribution system (D).
Average AOC fraction utilized by test strain NOX.
Numbers in parentheses show numbers of samples. NA, not analyzed.
FIG 1.
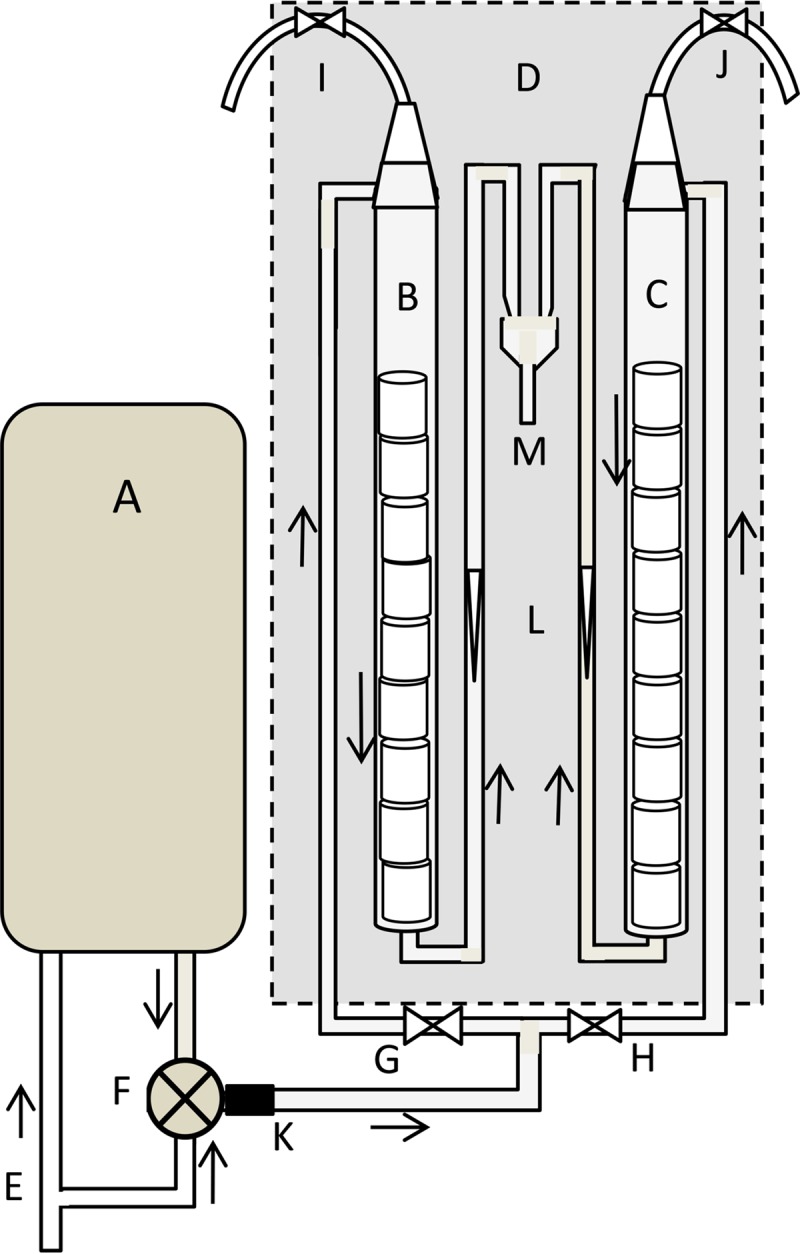
Scheme (not to scale) of the boiler biofilm monitor (BBM). (A) Electric boiler (30 liters); (B and C) glass columns with cylinders of either glass or CPVC; (D) wooden box; (E) cold water inlet; (F) thermostatic mixing valve; (G and H) magnetic valve; (I and J) deaeration valves; (K) location for insertion of inoculum; (L) flow meters; (M) water discharge. Arrows show the direction of water flow.
FIG 2.
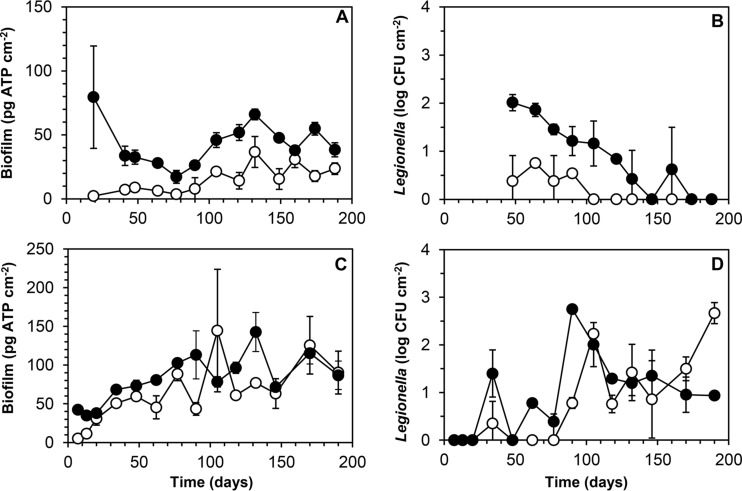
Biofilm concentrations (A) and colony counts of L. pneumophila (B) in biofilms developing on glass (○) and CPVC (●) in a boiler biofilm monitor supplied with tap water at a location in the distribution system of supply C. Error bars indicate standard deviations (SD). Colony counts below the detection limit are shown as 1 CFU cm−2.
TABLE 2.
Characteristics of biofilms on glass and CPVC in the boiler biofilm monitor with groundwater supplies A, B, and C
| Water supply and test materialb | Mean ± SD for biofilm characteristica |
|||||||
|---|---|---|---|---|---|---|---|---|
| Attached biomass concn (pg ATP cm−2) | Legionella count (log CFU cm−2) | TCC (log cells cm−2) | ATP/TCC ratio (fg cell−1) | HPC (log CFU cm−2) | HPC/TCC ratio (%) | Fe concn (mg m−2) | Mn concn (mg m−2) | |
| AT | ||||||||
| Glass | 23 ± 9 | 0 | 5.7 ± 0.4 | 0.03 ± 0.02 | 4.1 ± 0.3 | 3.6 ± 3.4 | 1.3 ± 0.7 | 0.11 ± 0.08 |
| CPVC | 49 ± 9* | 0.4 ± 0.5 | 6.0 ± 0.3 | 0.05 ± 0.03 | 4.7 ± 0.4* | 6.0 ± 5.4 | 1.6 ± 1.3 | 0.17 ± 0.18 |
| AD | ||||||||
| Glass | 82 ± 40 | 1.6 ± 0.8 | 6.2 ± 0.2 | 0.05 ± 0.02 | 5.2 ± 0.2 | 11 ± 6 | 1.9 ± 0.9 | 0.05 ± 0.03 |
| CPVC | 99 ± 25 | 1.7 ± 0.7 | 6.4 ± 0.2 | 0.04 ± 0.02 | 5.5 ± 0.2* | 12 ± 6 | 5 ± 3* | 0.13 ± 0.05* |
| BT1 | ||||||||
| Glass | 1,050 ± 225 | 4.2 ± 0.1 | 7.0 ± 0.2 | 0.13 ± 0.08 | 5.7 ± 0.3 | 5.5 ± 2.9 | 14 ± 8 | 3.5 ± 3.4 |
| CPVC | 2,755 ± 465* | 4.3 ± 0.1 | 7.5 ± 0.2* | 0.18 ± 0.16 | 6.4 ± 0.3* | 5.1 ± 1.4 | 23 ± 14* | 11 ± 11* |
| BT2 | ||||||||
| Glass | 630 ± 190 | 3.9 ± 0.4 | 7.2 ± 0.1 | 0.07 ± 0.04 | 6.3 ± 0.1 | 16 ± 11 | 6 ± 4 | 0.5 ± 0.5 |
| BD | ||||||||
| Glass | 580 ± 105 | 3.7 ± 0.6 | 7.2 ± 0.1 | 0.05 ± 0.01 | 6.1 ± 0.2 | 13 ± 7 | 10 ± 67 | 0.47 ± 0.3 |
| CPVC | 710 ± 175* | 4.0 ± 0.3 | 7.2 ± 0.1 | 0.07 ± 0.03 | 6.1 ± 0.2 | 15 ± 8 | 15 ± 8 | 0.45 ± 0.24 |
| CD | ||||||||
| Glass | 565 ± 165 | 4.6 ± 0.2 | 6.8 ± 0.3 | 0.11 ± 0.05 | 5.6 ± 0.2 | 20 ± 12 | ND | ND |
| CPVC | 1,130 ± 250* | 4.1 ± 0.4* | 7.0 ± 0.1* | 0.12 ± 0.05 | 6.2 ± 0.1* | 19 ± 8 | ND | ND |
TCC, total cell count (n = 4 to 7); HPC, heterotrophic plate count (n = 6 to 12); Fe, iron (n = 5 to 8); Mn, manganese (n = 5 to 8). The average concentrations for attached biomass (ATP), TCC, HPC, Fe, and Mn cover the steady-state phase attained after about 30 to 60 days. Elevated biofilm concentrations on CPVC at the end of the test period at locations BT1 and BD (Fig. 4) were not included in the calculations of the average steady-state biofilm concentrations; samples with no or initial growth of L. pneumophila in this phase were not included in the calculation of the mean. For location AT, the period of days 105 to 188 was used for the calculations (Fig. 3A and B), as well as for location BT1. ND, not determined; *, the concentration on CPVC was significantly (P < 0.05) different from the concentration on glass.
AT, groundwater supply A, treated water; AD, supply A at a location in the distribution system; BT1, supply B, treated water, first test; BT2, supply B, treated water, second test; BD, supply B at a location in the distribution system; CD, groundwater supply C at a location in the distribution system (D).
Groundwater supply A.
The BBM system with two columns was used to investigate treated water leaving the treatment facility of supply A (AT) and drinking water at a location in the distribution system (AD) 2 years later. This drinking water was characterized by low concentrations of TOC, AOC, and ATP at both locations (Table 1). Biofilm formation on glass was slow and remained below 50 pg ATP cm−2 at location AT (Fig. 3A). The biofilm concentration on CPVC, which obviously had reached an elevated concentration within 2 weeks, declined over a period of about 80 days and subsequently increased to a level of approximately 50 pg ATP cm−2. The elevated initial concentration is attributed to growth enhancement by CPVC (see Discussion). The Legionella colony count on glass remained <10 CFU cm−2 (Fig. 3B), and the bacteria disappeared from the biofilm (detection limit, 6 CFU cm−2). On CPVC, Legionella apparently had multiplied before the first analysis (day 50), but the colony count also declined to below the detection limit (13 CFU cm−2) after 150 days.
FIG 3.
Biofilm concentrations (A and C) and colony counts of L. pneumophila (B and D) in biofilms developing on glass (○) and CPVC (●) in a boiler biofilm monitor supplied with drinking water of supply A, at the treatment plant (A and B) or at a location in the distribution system (C and D). Error bars indicate SD. Colony counts below the detection limit are shown as 1 CFU cm−2.
The variable biofilm concentrations on glass and CPVC in the BBM at the distribution system location (AD) also remained low but were significantly higher than those observed at location AT (Fig. 3C). The Legionella colony counts in the biofilms showed large variations, with small differences between most duplicate samples (Fig. 3D). After 120 days, the Legionella counts on CPVC remained below 102 CFU cm−2, but a level of 460 CFU cm−2 was attained on glass after 190 days. The average TCC concentrations in the biofilms at locations AT and AD were below 3 × 106 cells cm−2 and were significantly higher on CPVC than on glass (Table 2). The concentrations of Fe and Mn on glass and CPVC were low at location AT and slightly higher at location AD, with significantly more Fe and Mn on CPVC than on glass (Table 2).
Groundwater supply B.
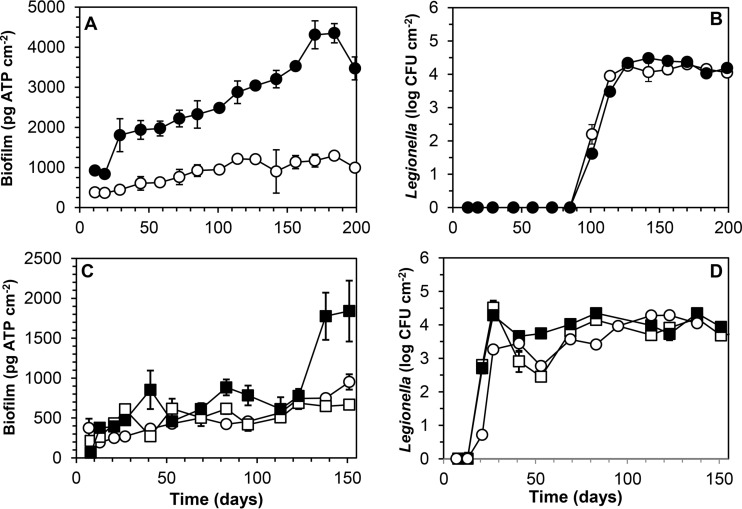
Drinking water from supply B was characterized by a relatively high TOC concentration and higher concentrations of AOC and ATP than those at supplies A and C (Table 1). In the first test with treated water (BT1), the biofilm on glass developed to concentrations exceeding 1,000 pg ATP cm−2 after 100 days, and on CPVC, a concentration of 3,500 pg ATP cm−2 was exceeded after 150 days (Fig. 4A). Legionella was not detected in the biofilm after 72 days, and therefore another silicone piece was inserted into the system at day 84. Thereafter, exponential growth (0.32 day−1) was observed, and Legionella levels of 2 × 104 to 3 × 104 CFU cm−2 were attained (Fig. 4B). HPCs exceeded 106 CFU cm−2, and TCCs exceeded 107 cells cm−2, with significantly higher levels on CPVC than on glass (Table 2). The concentrations of Fe and Mn on glass and CPVC were relatively high (Table 2) and increased after about 100 days of operation, to 25 mg Fe cm−2 and 20 mg Mn cm−2 at day 184. Treated water from supply B was tested a second time (BT2; glass only) in combination with water at a location (BD) in the distribution system (Fig. 4C and D). The biofilm concentration and the concentrations of Fe and Mn at location BT2 remained below those observed on glass in the first test (Table 2). L. pneumophila attained a maximum level of about 2 × 104 CFU cm−2. The biofilm concentration on CPVC at location BD was significantly higher than that on glass, but the Legionella colony count and the concentrations of other biofilm parameters did not differ significantly from those on glass (Table 2).
FIG 4.
Biofilm concentrations (A and C) and colony counts of L. pneumophila (B and D) in biofilms developing on glass (○ and □) and CPVC (● and ■) in a boiler biofilm monitor supplied with drinking water of supply B, leaving the treatment plant (○ and ●) or at a location in the distribution system (□ and ■). Error bars indicate SD. Colony counts below the detection limit are shown as 1 CFU cm−2.
Relationships between biofilm parameters and growth of L. pneumophila.
Linear regression analysis was used to identify relationships between the TCC, HPC, and ATP concentrations and between these concentrations and the colony counts of L. pneumophila in the biofilm. TCCs, HPCs, and ATP levels of the biofilms for the combined water types were significantly related at concentration ranges of several log units, but the relationships were weak (low R2 values) for most of the individual water types (Table 3; see Fig. S3 in the supplemental material). The colony count of L. pneumophila in the biofilm generally showed more variation than the biofilm concentration (ATP) and correlated significantly with TCC, HPC, and ATP for the water types combined (Fig. S4), but these relationships were weak (low R2 values) (Table 3). For the individual water types, the correlation was either low or not significant. The mean of the log-transformed colony counts of Legionella correlated significantly with the average of the log-transformed biofilm concentrations (Table 3; Fig. 5), with an exponential relationship at biofilm concentrations above 100 pg ATP cm−2 and no growth of Legionella at biofilm concentrations below 30 pg ATP cm−2.
TABLE 3.
Linear regression-based correlations and relationships between log-transformed data on biofilm characteristics, including Legionella colony counts, for biofilms in the boiler biofilm monitor for groundwater supplies A, B, and C
| Comparison (n)a | All data |
Data for water supplyb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
|||||||
| Mean slope ± SE | R2 value | P value | R2 value | P value | R2 value | P value | R2 value | P value | |
| TCC vs HPC (60) | 1.07 ± 0.07 | 0.78 | <10−20 | 0.55 | <10−3 | 0.20 | <0.05 | 0.64 | <10−3 |
| TCC vs ATP (68) | 1.05 ± 0.07 | 0.78 | <10−20 | 0.53 | <10−3 | 0.41 | <10−3 | 0.79 | <10−5 |
| HPC vs ATP (111) | 0.84 ± 0.04 | 0.78 | <10−20 | 0.65 | <10−8 | 0.22 | <10−3 | 0.59 | <10−6 |
| Legionella count vs TCC (50) | 2.09 ± 0.26 | 0.57 | <10−9 | 0.26 | NS | 0.31 | <0.01 | 0.27 | NS |
| Legionella count vs HPC (80) | 1.69 ± 0.19 | 0.49 | <10−12 | 0.29 | 0.01 | 0.04 | NS | 0.23 | 0.01 |
| Legionella count vs ATP (180) | 1.58 ± 0.09 | 0.62 | <10−12 | 0.19 | <0.01 | 0.18 | <10−4 | <0.01 | NS |
| Legionella countavg vs ATPavg (9)c | 2.00 ± 0.42 | 0.76 | 0.002 | NA | NA | NA | NA | NA | NA |
| Legionella countavg vs ATPavg (10)d | 1.99 ± 0.26 | 0.88 | 5 × 10−5 | NA | NA | NA | NA | NA | NA |
n = number of data pairs.
NS, not significant (P > 0.05); NA, not applicable.
Data pairs for location AT were excluded from the calculations.
FIG 5.
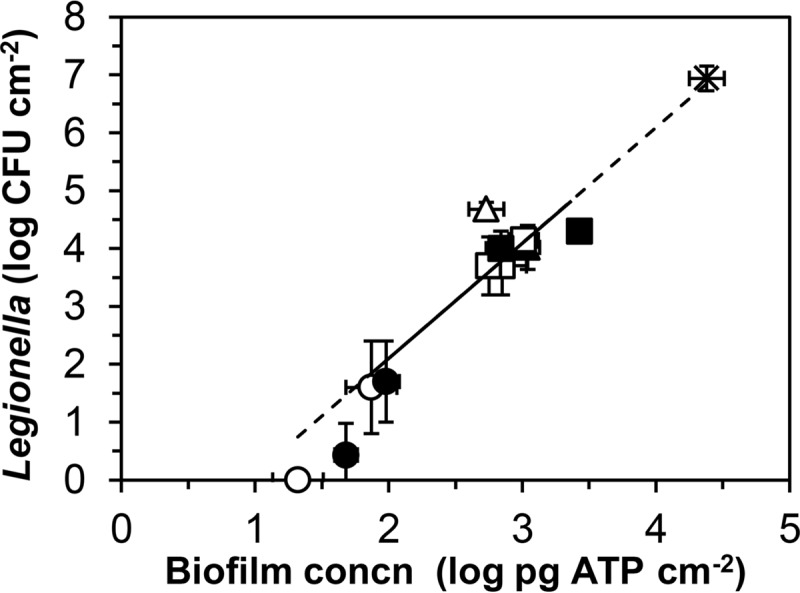
Relationship between the log mean of colony counts of L. pneumophila in the biofilm and the log mean of biofilm concentrations. Symbols: ○, glass, supply A; ●, CPVC, supply A; □, glass, supply B; ■, CPVC, supply B; △, glass, supply C; ▲, CPVC, supply C; ×, L. pneumophila SG1, ST1 strain LP25 on soft PVC (18). Error bars indicate SD. Data for location AT are shown (<1 log CFU cm−2) but were not included in the calculations. The solid line shows the relationship calculated for the data from this study; the dashed line includes the data for L. pneumophila on soft PVC (Table 3).
Free-living amoebae and predominating bacteria in biofilms.
The presence of the potential host amoebae Vermamoeba vermiformis and Acanthamoeba spp. in the biofilms was measured at two time points. The concentration of V. vermiformis was below the detection limit (0.5 cell equivalent cm−2) in most samples collected at locations AT and AD; the highest concentrations were observed with water type C (Table S2). V. vermiformis was present on day 41 at location BT1, before growth of L. pneumophila was observed (Fig. 4B). The colony count of L. pneumophila correlated significantly with the V. vermiformis concentration (P < 10−4; R2 = 0.81) (Fig. 6). The Acanthamoeba concentration was below the detection limit (0.5 cell equivalent cm−2) in all samples.
FIG 6.
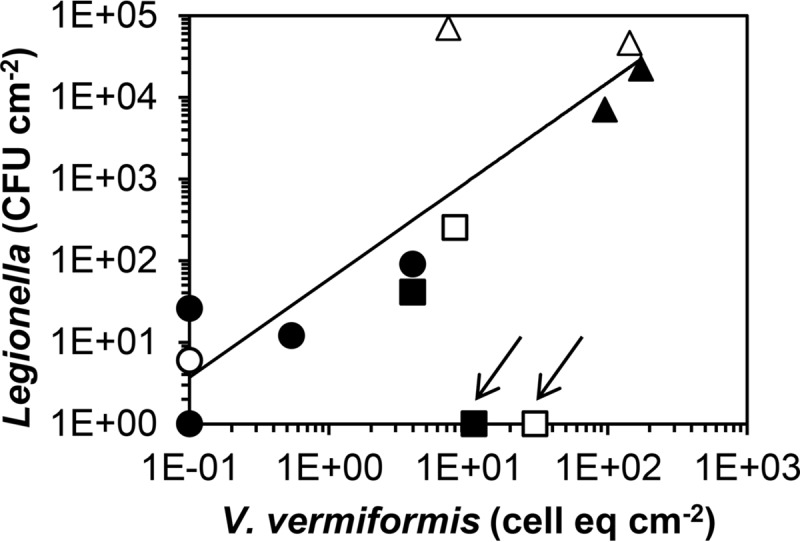
Relationship between the concentration of V. vermiformis and the L. pneumophila colony count in the biofilm (paired samples). V. vermiformis concentrations below the detection level are presented as 0.1 cell equivalent cm−2. Data for location BT at day 41 (arrows) were not included in the relationship (R2 = 0.81; P < 10−4). Symbols: ○, glass, supply A; ●, CPVC, supply A; □, glass, supply B; ■, CPVC, supply B; △, glass, supply C; ▲, CPVC, supply C.
Bacteria predominating in a biofilm are well adapted to the prevailing environmental conditions and may serve as prey for amoebae. To obtain information about the ecophysiological properties of these bacteria, a limited number of bacterial clones were retrieved and analyzed for identification. The predominating cultured bacteria were identified as well. The Betaproteobacteria accounted for 9 to 80% of the identified clones, with the highest percentages in the young biofilms (Table 4). Piscinibacter aquaticus was the most frequently observed species in the clone libraries and the bacteria cultured from the biofilms for water types A and B (Table 4; Table S4). Members of the Rhodocyclaceae, viz., Methyloversatilis discipulorum and an uncultured bacterium, predominated among the clones retrieved from the biofilms for water types B and C. Alphaproteobacteria accounted for 6 to 47% of the sequences, with the highest percentages in the older biofilms. Predominating representatives were an uncultured Xanthobacteraceae bacterium, Pseudorhodoplanes sinuspersici, and Sphingopyxis indica. Bradyrhizobium japonicum was the predominating cultured alphaproteobacterium (Table S4). For water types A and C, Gammaproteobacteria (3.7%) and Deltaproteobacteria (0.7%) represented small minorities, and Acidobacteria accounted for 13 to 26% of clones.
TABLE 4.
Identities and relative abundances of bacteria predominating in biofilms on glass and CPVC after different periods of exposure to warm drinking water types A (at a location in the distribution system; AD), B (treated water; BT2), and C (at a location in the distribution system; CD)
| Bacterial phylum and class as well as order, family, genus, and species (%)a | No. of OTUsb | No. (%) of clones |
Total no. (%) of clones | |||||
|---|---|---|---|---|---|---|---|---|
| AD |
BT2 |
CD |
||||||
| Day 118 |
Day 41 | Day 138 | Day 60 |
|||||
| Glass | CPVC | Glass | Glass | Glass | CPVC | |||
| Proteobacteria, Alphaproteobacteria | ||||||||
| Rhizobiales, Hyphomicrobiaceae, Hyphomicrobium aestuarii | 1 | 2 (4.7) | 2 (0.7) | |||||
| Rhizobiales, Hyphomicrobiaceae, Pedomicrobium (97) | 1 | 4 (9.5) | 4 (1.5) | |||||
| Rhizobiales, Pseudorhodoplanes, Pseudorhodoplanes sinuspersici | 2 | 5 (10.9) | 6 (13.9) | 11 (4.1) | ||||
| Rhizobiales, Xanthobacteraceae, uncultured bacterium (97) | 2 | 4 (9.3) | 3 (6.7) | 3 (7.0) | 4 (8.9) | 1 (2.2) | 15 (5.6) | |
| Rhodospirillales, Rhodospirillales IS,c Reyranella massiliensis | 1 | 4 (9.3) | 3 (6.7) | 1 (2.2) | 8 (3.0) | |||
| Rhodospirillales, KCM-B-60, uncultured bacterium (87) | 1 | 4 (9.5) | 4 (1.5) | |||||
| Sphingomonadales, Sphingomonadaceae, Sphingopyxis indica | 1 | 1 (2.3) | 14 (31.1) | 15 (5.6) | ||||
| Alphaproteobacteria (singletons)e | 2 (4.7) | 1 (2.2) | 1 (2.4) | 1 (2.2) | 1 (4.3) | 6 (2.2) | ||
| Proteobacteria, Betaproteobacteria | ||||||||
| Burkholderiales, Comamonadaceae, Curvibacter fontanusd | 1 | 4 (8.9) | 12 (26) | 16 (6.0) | ||||
| Burkholderiales, Comamonadaceae, Ideonella (98) | 1 | 1 (2.2) | 3 (6.5) | 4 (1.5) | ||||
| Burkholderiales, Comamonadaceae, Piscinibacter aquaticusd | 2 | 16 (37.2) | 4 (8.9) | 19 (41.3) | 4 (9.3) | 2 (4.4) | 45 (16.7) | |
| Burkholderiales, Ralstonia, Ralstonia (98) | 1 | 2 (4.7) | 2 (0.7) | |||||
| Nitrosomonadales, Nitrosomonadaceae, uncultured bacterium (≥97) | 3 | 5 (11.9) | 4 (8.9) | 4 (8.7) | 13 (4.9) | |||
| Rhodocyclales, Rhodocyclaceae, Methyloversatilus discipulorumd | 2 | 10 (21.7) | 4 (8.9) | 14 (30) | 28 (10.4) | |||
| Rhodocyclales, Rhodocyclaceae, uncultured bacterium (92) | 1 | 3 (6.5) | 4 (9.5) | 16 (35.5) | 2 (4.3) | 25 (9.3) | ||
| TRA3-20, uncultured bacterium (100) | 1 | 4 (8.7) | 1 (2.3) | 5 (1.9) | ||||
| Betaproteobacteria (singletons)e | 1 (2.3) | 1 (2.2) | 3 (7.0) | 2 (2.2) | 7 (2.6) | |||
| Proteobacteria, Gammaproteobacteria | ||||||||
| Pseudomonadales, Moraxellaceae, Perlucidibaca (97) | 1 | 8 (17.8) | 8 (3.0) | |||||
| Gammaproteobacteria (singletons)e | 1 (2.2) | 1 (4.4) | 2 (0.7) | |||||
| Proteobacteria, Deltaproteobacteria GR-WP33-30, uncultured (94) | 1 | 2 (4.4) | 2 (0.7) | |||||
| Fibrobacteres/Acidobacteria, subdivision 4, uncultured bacterium (100) | 1 | 11 (25.6) | 7 (15.6) | 5 (11.1) | 8 (17.4) | 31 (11.6) | ||
| Acidobacteria (singleton)e | 1 (2.2) | 1 (0.4) | ||||||
| Bacteroidetes (singleton)e | 1 (2.2) | 1 (0.4) | ||||||
| Gemmatimonadetes, Gemmatimonadeles, Gemmatimonadaceae, uncultured bacterium (≥90) | 3 | 2 (4.5) | 2 (4.3) | 5 (11.6) | 1 (2.2) | 10 (3.7) | ||
| Planctomycetes, Phycisphaerae, uncultured bacterium (89) | 1 | 3 (7.0) | 3 (1.1) | |||||
| Total no. of classified bacterial sequences | 43 (100) | 46 (100) | 45 (100) | 43 (100) | 45 (100) | 46 (100) | 268 (100) | |
Identity percentages at the family or genus level are based on SINA Alignment; identities at the species level are based on ≥97% sequence similarity with the type strain (Table S5).
OTU, operational taxonomic unit (sequences with ≥99% similarity).
IS, incertae sedis.
Also a predominating cultured bacterium (Table S4).
See Table S3 in the supplemental material.
Effect of water composition on biofilm formation.
The low biofilm concentrations at locations AT and AD corresponded to low concentrations of TOC, AOC, and ATP in the water (Table 1). The highest biofilm concentrations (water type B) were associated with clearly higher TOC, AOC, and ATP concentrations. The observations for water type C were between those for water types A and B. Storage of water in Erlenmeyer flasks at 70°C for 6 h, the average retention time of the water in the boiler, caused a 2.1 (± 0.3)-fold increase of the AOC concentration due to increased growth of the test strain NOX (Table 1). The increased AOC was strongly related (R2 = 0.97) to the TOC concentration (Fig. S5A). Based on the mixing ratio of hot (70°C) and cold (11 to 14°C) drinking water, the AOC concentration of the warm water was estimated to be about 1.5 times the AOC concentration of the cold water. The average biofilm concentration on glass (not CPVC) was significantly related to the AOC concentration of the warm water (Fig. 7) and to the TOC concentration (Fig. S5B). Investigations of more water types are needed to assess the effect of water quality on biofilm formation.
FIG 7.
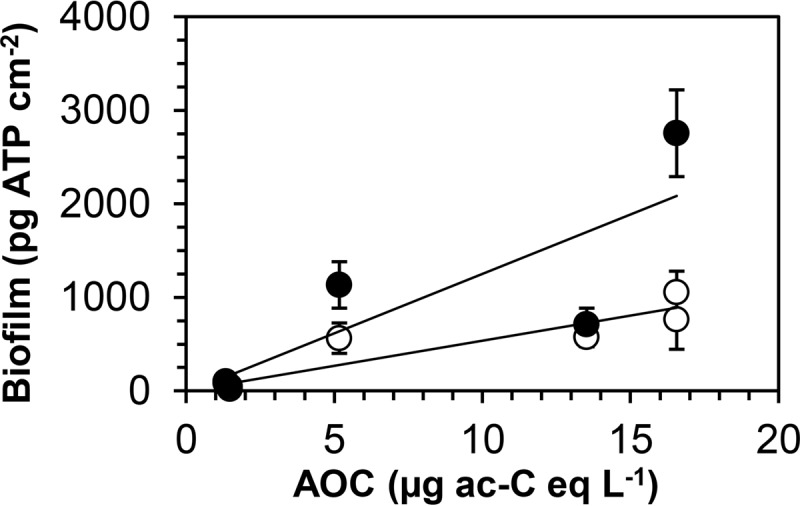
Relationship between the average steady-state biofilm concentrations on glass (○) and CPVC (●) in the boiler biofilm monitor and the assimilable organic carbon (AOC) concentration of the warm tap water. Error bars indicate SD. For glass, the relationship is given by the following equation: biofilm concentration (pg ATP cm−2) = 49 (±12) × AOC concentration (µg acetate-C equivalents liter−1) + 63 (±133) (R2 = 0.81; P < 0.05). For CPVC, the relationship is given by the following equation: biofilm concentration (pg ATP cm−2) = 128 (±53) × AOC (µg acetate-C equivalents liter−1) − 21 (±530) (R2 = 0.65; P > 0.05).
DISCUSSION
Boiler biofilm monitor.
The results of this study show that the BBM system enables assessment of the biofilm concentration and the associated growth of Legionella on surfaces exposed to warm water. Glass and CPVC are easy to handle and do not corrode, and glass does not release growth-promoting compounds. Furthermore, CPVC is commonly used in contact with warm water in potable water installations (41). Its properties compared to those of glass are discussed below. The operational conditions in the BBM simulate a worst-case situation in a tap water installation by using heated water to provide an optimal temperature for growth of L. pneumophila and host amoebae in the biofilm (18), with a regular, discontinuous water flow. Growth of L. pneumophila was measured in the biofilm only because the water volume contained in each of the columns (approximately 300 ml) was too small for accurately measuring Legionella counts at a high biofilm/water ratio (18). The advantages of biofilm analysis include a defined sample (material and location), a small sample size, and a low threshold level for Legionella detection.
Biofilm concentration assessment.
HPCs generally account for a small (<2%) and varying fraction of the TCC in drinking water biofilms at ambient temperatures (40, 42–44), but in a warm water system (35°C) an elevated culturability (38%) of the bacteria, defined as the HPC/TCC ratio, was observed, compared to 2% culturability in cold (11°C) water (45). The culturability of attached bacteria in the BBM ranged from <2% (aged biofilm for water type AT) to approximately 40% (young biofilm for water type C). The lowest culturability was associated with an average ATP content of 0.015 fg cell−1, and a culturability of >10% was associated with >0.1 fg ATP cell−1. Plating efficiencies of >20% have been reported for biofilm bacteria at >0.2 fg ATP cell−1 (49). These observations confirm that HPCs are not a suitable measure of biofilm concentration or activity. The TCCs of the biofilms in the BBM supplied with the three water types ranged from 2 × 105 to 4 × 107 cells cm−2 (Table 2; see Fig. S3 in the supplemental material), which covers the TCC range for biofilms on drinking water-exposed surfaces (42–44). However, TCCs do not represent the concentration of attached bacterial biomass, due to differences in cell size, or biomass activity, due to differences in viability and activity. Therefore, in this study, ATP was used as a measure of the concentration of attached active microbial biomass, reflecting the availability of energy sources in the biofilm. This parameter correlated well with TCCs over several orders of magnitude, but correlation was poor for TCC ranges of about 1 log unit for the individual water types (Table 3; Fig. S3B). The observed ATP concentrations covered a range of 3 orders of magnitude (5 to 4,500 pg ATP cm−2) (Fig. 1 to 3). ATP concentrations of biofilms on drinking water-exposed surfaces (40, 47), on surfaces in water treatment processes (48), in tests with materials (30, 36), or under other experimental conditions (18, 49) provide a framework for comparison, facilitating their interpretation.
ATP concentrations and HPCs on CPVC were significantly higher than those on glass at four of the five test locations where glass and CPVC were used. The TCC was significantly higher only at locations CD and BT1 (Table 2). At locations AD and BT1, the concentrations of Fe and Mn on CPVC were higher than those on glass. Glass is a hydrophilic, negatively charged material, whereas CPVC is hydrophobic and negatively charged (50). Hydrophobicity promotes bacterial attachment (51), and under the test conditions, the properties of CPVC seem to favor the attachment of active and culturable bacteria as well as the accumulation of Fe and Mn. At location AT, the biomass concentration on CPVC had increased shortly after the start of the experiment, followed by a decline lasting several months (Fig. 3A). An increase of the active biomass concentration within the first week, followed by a decline, was also observed in batch tests with CPVC (30) and unplasticized PVC (14). Washing with a detergent reduced the growth enhancement of unplasticized PVC by 90% (37). The low biofilm concentration after 50 days of exposure to water type AT (Fig. 3A) is consistent with the observation that the material did not enhance microbial growth after 8 weeks in the biomass production potential test (36). Therefore, CPVC may also have promoted the initial bacterial growth by the presence of biodegradable compounds on its surface.
Predominating biofilm bacteria.
The culturable Betaproteobacteria P. aquaticus and M. discipulorum predominated in the BBM biofilms (Table 4). P. aquaticus was first described as Methylibium aquaticum after its isolation from a eutrophic freshwater pond (52), but it was reclassified as P. aquaticus (53). M. discipulorum and the related species Methyloversatilis universalis have been isolated from lake sediment (54, 55). Piscinibacter and Methyloversatilis are facultative methylotrophic bacteria that can utilize single-carbon compounds but not methane (56). The biofilms developing in the BBM apparently provide a niche for these bacteria, which have not or have rarely been observed in studies of the identity of bacteria in drinking water supplies, potable water installations, or test systems at water temperatures below 25°C (57–59). Methylotrophic bacteria of other genera predominated in water meter biofilms at a groundwater supply distributing chlorinated drinking water (60). Their prevalence was attributed to the presence of methane in the finished water. Methane was also present in the anaerobic groundwater sources of drinking water types B and C, but intensive aeration and biofiltration (Fig. S1) effectively removed this compound. The predominance of methylotrophic bacteria in the BBM may be associated with the increased AOC due to heating part of the water to 70°C (Table 1). Compounds promoting the growth of test strain NOX accounted for the major proportion of the AOC. This organism is specialized for the utilization of low-molecular-weight (hydroxy)carboxylic acids, including formate (61). These compounds have been observed in water after exposition to ozone (62–64) or UV (65). We hypothesize that heating of the water to 70°C also causes the formation of low-molecular-weight carboxylic acids, including formate, from NOM. The presence of unidentified members of the Nitrosomonadaceae in the biofilms for water types B (16%) and C (9%) is consistent with another study of drinking water biofilms (59) and indicates the availability of ammonia, although this compound was not detected (<0.05 mg NH4 liter−1) in the water used (Table S1).
Approximately 40% of the Alphaproteobacteria clones classified as an uncultured Xanthobacteraceae bacterium or Pseudorhodoplanes sinuspersici (Table 4) showed 98 to 100% sequence similarity to bacteria isolated from a hospital warm tap water system (GenBank accession no. DQ123619 and DQ123621) (Table S5) by amoebal coculture using Acanthamoeba castellanii (66). These bacteria grew slowly on charcoal yeast extract agar, but most of their physiological properties are still unclear. Reyranella massiliensis has been isolated by amoebal coculture with Acanthamoeba polyphaga (67) and was also observed in a biofilm on Norprene tubing exposed to drinking water (60). The colony counts of the biofilm were predominated by B. japonicum (Table S4), a slowly growing nitrogen-fixing bacterium and a root nodule microsymbiont of soybean (68). This amoeba-resistant bacterium and related species are frequently observed in tap water installations (66) and ultrapure water systems (69). Endosymbiotic growth within V. vermiformis (70) may explain its presence in the biofilm, but the symbiotic relationship is not yet understood. Overall, these findings and the high percentage of Betaproteobacteria in the young biofilms suggest that biodegradable compounds present in the warm water promoted rapid growth of heterotrophic Betaproteobacteria, whereas most observed Alphaproteobacteria seem to grow in association with amoebae.
LGP.
Studies on the growth of L. pneumophila in biofilms on surfaces in contact with warm drinking water in a variety of test systems showed that Legionella amplification generally increased with an increasing biofilm concentration (29–31). A temperature of 34 to 39°C in the BBM promoted growth of the inoculated L. pneumophila. Legionella was not cultured from the biofilm at location BT1 within 2 months after inoculation, despite optimal growth conditions and the presence of V. vermiformis (Fig. 4 and 6). Testing of the stored biofilm culture used for inoculation showed that L. pneumophila was no longer detectable on the silicone pieces, and rapid growth was observed after inoculation with a fresh biofilm culture at day 84. Thus, inoculation with L. pneumophila is needed to ensure its timely growth in the test system. The typical and uniform colony type on buffered charcoal yeast extract (BCYE) agar plates represented the inoculated L. pneumophila strain, and no other colony type characteristic of Legionella spp. was observed at the test locations. The colony counts therefore show the ability of L. pneumophila to proliferate under the test conditions. L. pneumophila is the major causative agent of LD (1, 2). Currently, about 2,200 sequence types (STs) of this species have been identified (http://bioinformatics.phe.org.uk/legionella/legionella_sbt/php/sbt_homepage.php), most of which have not been implicated in LD (71). However, virulent STs could also grow well in an aquatic biofilm (18). The mean of the log-transformed colony counts of this organism in the biofilm is therefore a measure of the Legionella growth potential (LGP) of the water tested. Likewise, the average biofilm concentration is a measure of the biofilm formation potential (BFP) of the water.
A stable or declining Legionella colony count at a biofilm concentration increasing with age (Fig. 2 to 4) shows that the quantitative relationship with the biofilm concentration is complicated. Legionella proliferation is affected by the growth and nature of its protozoan host(s), whose replication depends on the concentration and nature of prey bacteria (72, 73). The significant relationship between the colony count of L. pneumophila and the concentration of V. vermiformis in the biofilm (Fig. 6) is consistent with other reports demonstrating that V. vermiformis is both a predominant free-living amoebae and an important host for L. pneumophila in potable water installations (11, 14, 15, 18, 74, 75). V. vermiformis produces about 10 times more cells on prey bacteria than those produced by Acanthamoeba spp. (73), which were not detected by quantitative PCR (qPCR) in the biofilms in the BBM. The exponential increase of the colony count of L. pneumophila in relation to the biofilm concentration is consistent with a colony count of approximately 107 CFU cm−2 of several L. pneumophila SG1 sequence types at a biofilm concentration of 2.5 × 104 pg ATP cm−2 on soft PVC exposed to flowing warm tap water (18) (Table 3; Fig. 5). This exponential increase may be explained by an increased growth of amoebae at an elevated concentration of prey bacteria in combination with a higher risk of infection by L. pneumophila. The predominating Betaproteobacteria, e.g., Piscinibacter, Methyloversatilis, and Thiobacillus, that utilize AOC-related compounds most likely served as prey bacteria in the biofilm developing in the BBM. Formation of the intracellular energy source poly-β-hydroxybutyrate (PHB) (52, 76), which is also present in exponentially grown L. pneumophila (77), may make these bacteria an attractive food source. A stable or declining Legionella colony count at a biofilm concentration increasing with age may be attributed to an increasing proportion of amoeba-resistant bacteria (Table 4). Establishment of a more accurate quantitative relationship between the biofilm concentration and growth of L. pneumophila and V. vermiformis therefore requires a more frequent analysis of the predominating bacteria in the biofilm.
Threshold biofilm concentration.
Biofilm concentrations of <100 pg ATP cm−2 in the BBM supplied with water types AT and AD were associated with Legionella colony counts of <100 CFU cm−2 in most samples (Fig. 3). The L. pneumophila colony count declined at a biofilm concentration of about 50 pg ATP cm−2 on CPVC after initial growth, and the organism also did not multiply on glass at biofilm concentrations of <30 pg ATP cm−2. Legionella colony counts at biofilm concentrations of 50 to 100 pg ATP cm−2 seem to be related to incidental increases of the active biomass concentration (Fig. 3). In 6 of 8 samples of these biofilms, the V. vermiformis concentration was ≤0.5 cell equivalent cm−2 (Fig. 6; Table S2), indicating amoebal growth limitation. The TCCs in the biofilms at locations AT and AD ranged from <1 × 106 cells cm−2 to 3 × 106 cells cm−2 for most samples from both glass and CPVC, with most HPCs being <3 × 105 CFU cm−2 (Table 2; Fig. S3). These TCCs are close to the half-saturation constants reported for growth of V. vermiformis on pure cultures of prey bacteria on agar at 20°C (72). The relationship between the Legionella colony count and the biofilm concentration (Fig. 5) confirms that the threshold biofilm concentration for growth of L. pneumophila under the test conditions was about 50 pg ATP cm−2, corresponding to a TCC of 1 × 106 to 2 × 106 cells cm−2 at 0.03 to 0.05 fg ATP cell−1. This threshold concentration most likely is based on the affinity of host amoebae for prey bacteria. In batch tests with glass and rigid PVC at 30°C, growth of L. pneumophila was also limited at a biofilm concentration of ca. 100 pg ATP cm−2 (37).
Practical implications.
The presented results show that drinking water prepared from groundwater by applying one (supplies A and C) or several (supply B) biofiltration processes (Fig. S1) can support growth of L. pneumophila in biofilms at elevated temperatures. Drinking water in these supplies is distributed without disinfectant residual and complies with the criteria for HPCs (annual geometric average, <100 CFU ml−1) and coliforms (<1 CFU 100 ml−1), but uncultured, mostly as yet undescribed Legionella spp. and free-living protozoa have been observed in the water and biofilms in the distribution systems (38, 39). The results of this study confirm that growth of Legionella in water systems depends on its ability to proliferate within protozoan hosts that graze on biofilms and sediments with bacteria adapted to oligotrophic conditions, but its growth efficiency decreases with decreasing biofilm concentrations. Drinking water with an AOC concentration of <10 μg acetate C equivalents liter−1 can support biofilm formation to concentrations of 106 to 107 cells cm−2 and >1,000 pg ATP cm2 (40, 43, 47), enabling proliferation of L. pneumophila at an elevated water temperature. Moreover, heating of the water increases the AOC concentration in relation to the TOC concentration (Table 1; Fig. S5A). The exponential relationship between the LGP and the BFP implies that a reduction of the biofilm concentration can result in a more-than-proportional LGP reduction. However, a reduction to below 100 pg ATP cm−2 requires an AOC concentration of <1 μg acetate C equivalents liter−1 (Fig. 7) at a low NOM concentration (<0.5 mg C liter−1). These levels are observed in drinking water supplies in the Netherlands distributing treated aerobic groundwater but are not achievable by biological filtration processes when the raw water contains a higher TOC concentration (78, 79). Limiting Legionella proliferation in warm tap water installations by distributing drinking water with very low concentrations of TOC and AOC would therefore require far-reaching treatment changes. Hence, application of control measures in water installations is essential. These measures include temperature management, selection of plumbing materials that do not enhance biofilm formation, and prevention of stagnancy and deposit accumulation, eventually in combination with additional physical or chemical barriers (34, 35, 80).
MATERIALS AND METHODS
BBM system.
An electric boiler (30 liters) with an enameled internal surface and a nickel-covered copper heating element was used to heat the water to 70°C. The heated water was mixed with cold water by using a thermostatic mixing valve (Watts) to achieve a temperature of 38 ± 1°C. The warm water was supplied via a stainless steel pipe and Teflon tubing to two vertical glass columns (internal diameter, 2.5 cm; length, 60 cm) situated in a box with air temperature regulation to maintain water temperature (37 ± 2°C) and to prevent light access. One column contained cylinders of glass (diameter, 1.8 cm; length, 1.6 cm; total surface area, 17.4 cm2), and the other contained chlorinated polyvinyl chloride (CPVC) pipe segments (diameter, 1.6 cm; length, 1.6 cm; total surface area, 15 ± 1 cm2). Every 20 min, 1.5 to 2 liters of warm water was supplied to each of the columns over 20 s to provide a regular, discontinuous water flow. The applied flow of approximately 120 liters per day in each column corresponds to the average drinking water usage per capita per day in the Netherlands (81). Figure 1 shows a scheme of the BBM system.
Inoculation.
The BBM system was inoculated with an L. pneumophila serogroup 1 (SG1), ST1 strain originating from a warm water installation and cultured on pieces of silicone tubing (diameter, 1 cm; length, 1.5 cm) incubated at 37°C in tap water as described previously (14). A silicone piece with 104 to 105 CFU of L. pneumophila and associated microbiota, including Vermamoeba vermiformis, previously named Hartmannella vermiformis (82), was inserted into the pipe directly after the thermostatic mixing valve 1 to 2 weeks after the operational start of the BBM and was removed when L. pneumophila was observed in the biofilm.
Biofilm sampling.
Periodically, usually every 14 days, two cylinders were collected from each column, placed in 10 ml of autoclaved tap water contained in a capped glass tube, and stored at 5 ± 3°C. Within 24 h, these samples were treated with low-energy ultrasound in a model 5510 ultrasonic cleaner (Branson Ultrasonic Corporation) at a 180-W power output and 40-kHz frequency. After each 2-min treatment, the water was taken from the tube and replaced with 10 ml of autoclaved tap water. Three ultrasonic treatments were applied to glass and six to CPVC to attain >95% attached biomass removal (83). The obtained suspensions (30 ml for glass and 60 ml for CPVC) were used for microbiological and chemical analyses.
Microbiological analyses.
Buffered charcoal yeast extract (BCYE) agar with antibiotics, prepared according to ISO standard 11731-2 (84), was used to measure the colony counts of Legionella. Aliquots of 0.1 ml of biofilm suspension or an appropriate decimal dilution in autoclaved tap water were spread over the surfaces of triplicate plates. The heterotrophic plate count (HPC) was measured by using R2A agar (Difco BD). Aliquots of 0.05 ml of the collected biofilm suspension or an appropriate decimal dilution were spread over the surfaces of triplicate plates, followed by incubation at 25°C for 10 days. The total cell count (TCC) in the biofilm suspension was measured by acridine orange staining and epifluorescence microscopy (85). The ATP concentrations (a measure of active biomass) of the suspension and the feed water were measured using a bioluminescence assay as described elsewhere (48). Membrane filtration (0.22-μm pore size) followed by the application of a FastDNA Spin kit for soil (MP Biomedicals) was used for the isolation of the DNAs of the microorganisms in the biofilm suspension as described previously (49). The concentrations of V. vermiformis and Acanthamoeba spp. in the biofilm suspensions were measured by qPCR targeting the 18S rRNA gene (15). Cloning and sequencing of the 16S rRNA gene insert (ca. 650 bp) for identification of the predominating uncultured bacteria were performed as described previously (49). Counts of colonies with identical appearances on R2A agar were recorded as percentages of the total colony count. Bacteria from predominating colonies were isolated, and the 16S rRNA genes of the isolates were amplified by PCR using primers 8f and 1392r (Biolegio, the Netherlands) (49). Fragments of approximately 1,030 bp were sequenced by using primer 8f. The SILVA Incremental Aligner (v1.2.11) (http://www.arb-silva.de/aligner/) was used for taxonomic classification of the partial 16S rRNA gene sequences of the clones and the isolates, which were also compared with the NCBI GenBank database by use of BLAST (http://www.ncbi.nlm.nih.gov/blast/).
Chemical analyses.
The concentrations of Fe and Mn in the biofilm suspensions were measured by inductively coupled plasma mass spectrometry (46) of samples acidified (pH < 2) with HNO3.
AOC concentration.
The concentration of easily assimilable organic carbon (AOC) in drinking water was measured by use of Pseudomonas fluorescens strain P17 and Spirillum sp. strain NOX (78). Introduction of a few micrograms of phosphate-P per liter with the inoculum of the strains into the test sample ensured the utilization of more than 100 μg of C liter−1. AOC concentrations were also measured in samples after 6 h of storage at 70°C in the glass-stoppered Erlenmeyer flasks used for sampling and testing.
Water types tested.
Three groundwater-derived drinking water types that are treated and distributed without disinfectant were included in the investigation. Water type A, with a low TOC concentration, is produced from aerobic groundwater by aeration for CO2 removal and limestone filtration. Water type B, with an elevated TOC concentration, is produced from anaerobic groundwater by intensive aeration, rapid sand filtration (RSF) followed by pellet softening, aeration, and RSF. Water type C, which is drinking water available at the laboratory, about 5 km from the treatment facility, is treated by aeration and RSF. Treatment schemes (Fig. S1) and water quality characteristics (Table S1) are included in the supplemental material. Water types A and B were investigated directly after treatment and at locations in the distribution system that are 2.7 km (supply A) and 19 km (supply B) from the treatment facility. At these locations, water entering the storage reservoir of the building was used. The investigation of the water types with the BBM covered a period of more than 2 years (Fig. S2).
Statistical analyses.
To determine the significance of differences between data, Student's t test was used on normally distributed data and verified with the Shapiro-Wilk test, after log transformation. The pairwise t test was applied as well. For non-normally distributed data, the nonparametric Mann-Whitney U test was used, and the Wilcoxon signed-rank test was used for paired data. Testing was two-tailed and had a 95% confidence. The analyses were conducted with Real Statistics, using Microsoft Excel 2010. Relationships between parameters were assessed by linear regression analysis.
Accession number(s).
Partial 16S rRNA gene sequences of the predominating uncultured and cultured bacteria have been deposited in NCBI GenBank under accession numbers KY247147 to KY247168 (clones) and KY284074 to KY284089 (isolates).
Supplementary Material
ACKNOWLEDGMENTS
This study was conducted within the framework of the Joint Research Program of the Water Supply Companies in the Netherlands.
We are much indebted to Anke Brouwer-Hanzens, Anita van der Veen-Lugtenberg, Marijan Uytewaal-Aarts, and Ton Braat for accurate analyses, to Meindert de Graaf for installation and sampling of the BBM system, and to Harry van Wegen and Sidney Meijering for constructing this system.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02737-16.
REFERENCES
- 1.European Centre for Disease Prevention and Control. 2014. Legionnaires' disease in Europe 2012. Surveillance report ECDC, Stockholm, Sweden. doi: 10.2900/203078. [DOI] [Google Scholar]
- 2.Garrison LE, Kunz JM, Cooley LA, Moore WR, Lucas C, Schrag S, Sarisky J, Whitney CG. 2016. Vital signs: deficiencies in environmental control identified in outbreaks of Legionnaires' disease—North America, 2000–2014. MMWR Morb Mortal Wkly Rep 65:576–584. doi: 10.15585/mmwr.mm6522e1. [DOI] [PubMed] [Google Scholar]
- 3.Beauté J, Robesyn E, de Jong B. 2013. Legionnaires' disease in Europe: all quiet on the eastern front? Eur Respir J 42:1454–1458. doi: 10.1183/09031936.00089113. [DOI] [PubMed] [Google Scholar]
- 4.Hicks LA, Garrison LE, Nelson GE, Hampton LM. 2011. Legionellosis—United States, 2000–2009. MMWR Morb Mortal Wkly Rep 60:1083–1086. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2013. Surveillance for waterborne disease outbreaks associated with drinking water and other nonrecreational water—United States 2009–2010. MMWR Morb Mortal Wkly Rep 62:714–720. [PMC free article] [PubMed] [Google Scholar]
- 6.Beer KD, Gargano JW, Robero VA, Hill VR, Garrison LE, Kurry PK, Hillborn ED, Wade TJ, Fullerton KE, Yoder JS. 2015. Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2011–2012. MMWR Morb Mortal Wkly Rep 64:842–848. doi: 10.15585/mmwr.mm6431a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borella P, Montagna MT, Stampi S, Stancanelli G, Romano-Spica V, Triassi M, Marchesi I, Bargellini A, Tatò D, Napoli C, Zanetti F, Leoni E, Moro M, Scaltritti S, D'Alcalà GR, Santarpia R, Boccia S. 2005. Legionella contamination of hot water in Italian hotels. Appl Environ Microbiol 71:5805–5813. doi: 10.1128/AEM.71.10.5805-5813.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Völker S, Schreiber C, Kistemann T. 2010. Drinking water quality in household supply infrastructure. A survey of the current situation in Germany. Int J Hyg Environ Health 213:204–209. doi: 10.1016/j.ijheh.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 9.George JR, Pine L, Reeves MW, Harrell WK. 1980. Amino acid requirements of Legionella pneumophila. J Clin Microbiol 11:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesh MJ, Miller RD. 1981. Amino acid requirements for Legionella pneumophila growth. J Clin Microbiol 13:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadowsky RM, Butler LJ, Cook MK, Verma SM, Paul MA, Fields BS, Keleth G, Sukora JL, Yee RB. 1988. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl Environ Microbiol 54:2677–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murga R, Forster TS, Brown E, Pruckler JM, Fields BS, Donlan RM. 2001. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147:3121–3126. doi: 10.1099/00221287-147-11-3121. [DOI] [PubMed] [Google Scholar]
- 13.Fields BS, Benson RF, Besser R. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev 15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuiper MW, Wullings BA, Akkermans ADL, Beumer RR, van der Kooij D. 2004. Intracellular proliferation of Legionella pneumophila in Hartmannella vermiformis in aquatic biofilms grown on plasticized polyvinyl chloride. Appl Environ Microbiol 70:6826–6833. doi: 10.1128/AEM.70.11.6826-6833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valster RM, Wullings BA, van der Kooij D. 2010. Detection of protozoan hosts for Legionella pneumophila in engineered water systems by using a biofilm batch test. Appl Environ Microbiol 76:7144–7153. doi: 10.1128/AEM.00926-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas JM, Thomas T, Stuetz RM, Ashbolt NJ. 2014. Your garden hose: a potential health risk due to Legionella spp. growth facilitated by free-living amoebae. Environ Sci Technol 48:10456–10464. doi: 10.1021/es502652n. [DOI] [PubMed] [Google Scholar]
- 17.Garrity GM, Bell JA, Tilburn T. 2005. Order VI. Legionellales. Family 1. Legionellaceae, p 210–237. In Brenner DJ, Krieg NR, Staley JT (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2 The Proteobacteria. Part B The Gammaproteobacteria. Springer, New York, NY. [Google Scholar]
- 18.van der Kooij D, Brouwer-Hanzens AJ, Veenendaal HR, Wullings BA. 2016. Multiplication of Legionella pneumophila sequence types 1, 47, and 62 in buffered yeast extract broth and biofilms exposed to flowing tap water at temperatures of 38 to 42°C. Appl Environ Microbiol 82:6691–6700. doi: 10.1128/AEM.01107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anand CM, Skinner RA, Malic A, Kurtz JB. 1983. Interaction of L. pneumophila and a free-living amoeba (Acanthamoeba palestinensis). J Hyg (Camb) 91:167–178. doi: 10.1017/S0022172400060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohno A, Kato N, Sakamoto R, Kimura S, Yamaguchi K. 2008. Temperature dependent parasitic relationship between Legionella pneumophila and a free-living amoeba (Acanthamoeba castellanii). Appl Environ Microbiol 74:4585–4588. doi: 10.1128/AEM.00083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habicht W, Müller HE. 1988. Occurrence and parameters of frequency of Legionella in warm water systems of hospitals and hotels in Lower Saxony. Zentralbl Bakteriol Mikrobiol Hyg B 186:79–88. [PubMed] [Google Scholar]
- 22.States SJ, Conley LF, Ceraso M, Stephenson TE, Wolford RS, Wadowsky RM, McNamara AM, Yee RB. 1985. Effects of metals on Legionella pneumophila growth in drinking water plumbing systems. Appl Environ Microbiol 50:1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bargellini A, Marchesi I, Righi E, Ferrari A, Cencitti S, Borella P, Rovesti S. 2011. Parameters predictive of Legionella contamination in hot water systems: association with trace elements and heterotrophic plate counts. Water Res 45:2315–2321. doi: 10.1016/j.watres.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Williams K, Pruden A, Falkinham JO III, Edwards M. 2015. Relationship between organic carbon and opportunistic pathogens in simulated glass water heaters. Pathogens 4:355–372. doi: 10.3390/pathogens4020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pringler N, Brydov P, Uldum SA. 2002. Occurrence of Legionella in Danish hot water systems, p 298–301. In Marre R, Kwaik YA, Bartlett C, Cianciotto NP, Fields BS, Frosch M, Hacker J, Lück PC (ed), Legionella. ASM Press, Washington, DC. [Google Scholar]
- 26.Colbourne JS, Pratt DJ, Smith MG, Fisher-Hoch SP, Harper D. 1984. Water fittings as sources of Legionella pneumophila in a hospital plumbing system. Lancet i:210–213. [DOI] [PubMed] [Google Scholar]
- 27.Niedeveld CJ, Pet FM, Meenhorst PL. 1986. Effect of rubbers and their constituents on proliferation of Legionella pneumophila in naturally contaminated hot water. Lancet ii:180–184. [DOI] [PubMed] [Google Scholar]
- 28.Schoenen D, Schulze-Röbbecke R, Schirdewan N. 1988. Microbial contamination of water by pipe and tubing material. 2. Growth of Legionella pneumophila. Zentralbl Bakteriol Mikrobiol Hyg B 186:326–332. [PubMed] [Google Scholar]
- 29.Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW. 1994. Influence of plumbing materials on biofilm formation and growth of Legionella pneumophila in potable water systems. Appl Environ Microbiol 60:1842–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Kooij D, Veenendaal HR, Slaats NPG, Vonk D. 2002. Biofilm formation and multiplication of Legionella on synthetic pipe materials in contact with treated water under static and dynamic conditions, p 176–180. In Marre R, Kwaik YA, Bartlett C, Cianciotto NP, Fields BS, Frosch M, Hacker J, Lück PC (ed), Legionella. ASM Press, Washington, DC. [Google Scholar]
- 31.Flemming HC, Bendinger B, Exner M, Gebel J, Kistemann T, Schaule G, Szewzyk U, Wingender J. 2014. The last meters before the tap: where drinking water quality is at risk, p 207–238. In van der Kooij D, van der Wielen PWJJ (ed), Microbial growth in drinking water supplies: problems, causes, control and research needs. IWA Publishing, London, United Kingdom. [Google Scholar]
- 32.Plouffe JF, Webster LR, Hackman B. 1983. Relationship between colonization of hospital buildings with Legionella pneumophila and hot water temperatures. Appl Environ Microbiol 46:769–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciesielski CA, Blaser MJ, Wang WLL. 1984. Role of stagnation and obstruction of water flow in isolation of Legionella pneumophila from hospital plumbing. Appl Environ Microbiol 48:984–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartram J, Chartier Y, Lee JV, Pond K, Surman-Lee S (ed). 2007. Legionella and the prevention of legionellosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 35.ASHRAE. 2011. BSR/ASHRAE standard 188P. Proposed new standard 188. Prevention of legionellosis associated with building water systems. Second public review (June 2011). American Society of Heating, Refrigerating and Air Conditioning Engineers, Atlanta, GA. [Google Scholar]
- 36.Hambsch B, Ashworth J, van der Kooij D. 2014. Enhancement of microbial growth by materials in contact with drinking water: problems and test methods, p 339–361. In van der Kooij D, van der Wielen PWJJ (ed), Microbial growth in drinking water supplies: problems, causes, control and research needs. IWA Publishing, London, United Kingdom. [Google Scholar]
- 37.van der Kooij D, Veenendaal HR. 2014. Regrowth problems and biostability assessment in the Netherlands, p 291–337. In van der Kooij D, van der Wielen PWJJ (ed), Microbial growth in drinking water supplies: problems, causes, control and research needs. IWA Publishing, London, United Kingdom. [Google Scholar]
- 38.Valster RM, Wullings BA, Bakker G, Smidt H, van der Kooij D. 2009. Free-living protozoa in two unchlorinated drinking water supplies identified by phylogenetic analysis of 18S rRNA gene sequences. Appl Environ Microbiol 75:4736–4746. doi: 10.1128/AEM.02629-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wullings BA, Bakker G, van der Kooij D. 2011. Concentration and diversity of uncultured Legionella spp. in two unchlorinated drinking water supplies with different concentrations of natural organic matter. Appl Environ Microbiol 77:634–641. doi: 10.1128/AEM.01215-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Kooij D, Vrouwenvelder JS, Veenendaal HR. 2003. Elucidation and control of biofilm formation processes in water treatment and distribution using the unified biofilm approach. Water Sci Technol 47:83–90. [PubMed] [Google Scholar]
- 41.WHO. 2006. Health aspects of plumbing. WHO Press, Geneva, Switzerland. [Google Scholar]
- 42.Block JC, Haudidier K, Miazga J, Levi Y. 1993. Biofilm accumulation in drinking water distribution systems. Biofouling 6:333–343. doi: 10.1080/08927019309386235. [DOI] [Google Scholar]
- 43.Boe-Hanzen R, Albrechtsen HJ, Arvin E, Jørgensen C. 2002. Dynamics of biofilm formation in a model drinking water distribution system. J Water Supply Aqua 51:399–406. [Google Scholar]
- 44.Lehtola MJ, Miettinen IT, Keinänen MM, Kekki TK, Laine O, Hirvonen A, Vartiainen V, Martikainen PJ. 2004. Microbiology, chemistry and biofilm development in a drinking water distribution system with pipes of copper and plastic. Water Res 38:3769–3779. doi: 10.1016/j.watres.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 45.Bagh LK, Albrechtsen HJ, Arvin E, Ovesen K. 2004. Distribution of bacteria in a domestic hot water system in a Danish apartment building. Water Res 38:225–235. doi: 10.1016/j.watres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 46.NEN. 2003. Water quality: application of inductively coupled plasma mass spectrometry (ICP-MS). Part 2. Determination of 62 elements. NEN-EN-ISO NEN 17294-1. NEN, Delft, the Netherlands. [Google Scholar]
- 47.van der Kooij D, Veenendaal HR, Scheffer WJH. 2005. Biofilm formation and multiplication of Legionella in a model warm-water system with pipes of copper, stainless steel and cross-linked polyethylene. Water Res 39:2789–2798. doi: 10.1016/j.watres.2005.04.075. [DOI] [PubMed] [Google Scholar]
- 48.Magic-Knezev A, van der Kooij D. 2004. Optimization and significance of ATP analysis for measuring active biomass in granular activated carbon filters used in water treatment. Water Res 38:3971–3979. doi: 10.1016/j.watres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Sack ELW, van der Wielen PWJJ, van der Kooij D. 2014. Polysaccharides and proteins added to flowing drinking water at microgram-per-liter levels promote the formation of biofilms predominated by Bacteroidetes and Proteobacteria. Appl Environ Microbiol 80:2360–2371. doi: 10.1128/AEM.04105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asadinezhad A, Lehocký M, Sáha P, Mozetič M. 2012. Recent progress in surface modification of polyvinyl chloride. Materials 5:2937–2959. doi: 10.3390/ma5122937. [DOI] [Google Scholar]
- 51.van Loosdrecht MCM, Norde W, Lyklema J, Zehnder AJB. 1990. Hydrophobic and electrostatic parameters in bacterial adhesion. Aquat Sci 52:103–114. doi: 10.1007/BF00878244. [DOI] [Google Scholar]
- 52.Song J, Cho JC. 2007. Methylibium aquaticum sp. nov., a betaproteobacterium isolated from a eutrophic freshwater pond. Int J Syst Evol Microbiol 57:2125–2128. doi: 10.1099/ijs.0.65179-0. [DOI] [PubMed] [Google Scholar]
- 53.Stackebrandt E, Verbarg S, Frühling A, Busse HJ, Tindall BJ. 2009. Dissection of the genus Methylibium: reclassification of Methylibium fulvum as Rhizobacter fulvus sp. nov., Methylibium aquaticum as Piscinibacter aquaticus gen. nov., comb. nov., Methylibium subsaxconicum as Rivibacter subsaxconicus gen. nov., comb. nov., and emended description of the genera Rhizobacter and Methylibium. Int J Syst Evol Microbiol 59:2252–2260. doi: 10.1099/ijs.0.008383-0. [DOI] [PubMed] [Google Scholar]
- 54.Smalley NE, Taipale S, De Marco P, Doronina NE, Kirpides N, Shapiro N, Woyke T, Kalyuzhnaya MG. 2015. Functional and genomic diversity of the methylotrophic Rhodocyclaceae: description of Methyloversatilis discipulorum sp. nov. Int J Syst Evol Microbiol 65:2227–2233. doi: 10.1099/ijs.0.000190. [DOI] [PubMed] [Google Scholar]
- 55.Kalyuzhnaya MG, De Marco P, Bowerman S, Pacheco CC, Lara JC, Lidstrom ME, Chistoserdova L. 2006. Methyloversatilus universalis gen. nov., sp. nov., a novel taxon within the Betaproteobacteria, represented by three methylotrophic isolates. Int J Syst Evol Microbiol 56:2517–2522. doi: 10.1099/ijs.0.64422-0. [DOI] [PubMed] [Google Scholar]
- 56.Kalyuzhnaya MG, Hristova KR, Lidstrom ME, Chistoserdova L. 2008. Characterization of a novel methanol dehydrogenase in representatives of the Burkholderiales: implication for environmental detection of methylotrophy and evidence for convergent evolution. J Bacterial 190:3817–3823. doi: 10.1128/JB.00180-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams MM, Domingo JWS, Meckes MC, Kelly CA, Rochon HS. 2004. Phylogenetic diversity of drinking water bacteria in a distribution system simulator. J Appl Microbiol 96:954–964. doi: 10.1111/j.1365-2672.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- 58.Liu G, Bakker GL, Li S, Vreeburg JHG, Verberk JQJC, Medema GJ, Liu WT, van Dijk JC. 2014. Pyrosequencing reveals bacterial communities in unchlorinated drinking water distribution system: an integral study of bulk water, suspended solids, loose deposits and pipe wall biofilm. Environ Sci Technol 48:5467–5476. doi: 10.1021/es5009467. [DOI] [PubMed] [Google Scholar]
- 59.Lu J, Buse HY, Gomez-Alvarez V, Struewing I, Santo Domingo J, Ashbolt NJ. 2014. Impact of drinking water conditions and copper materials on downstream microbial communities and Legionella pneumophila colonization. J Appl Microbiol 117:905–918. doi: 10.1111/jam.12578. [DOI] [PubMed] [Google Scholar]
- 60.Ling F, Hwang C, LeChevallier MW, Anderson G, Liu WT. 2016. Core-satellite populations and seasonality of water meter biofilms in a metropolitan drinking water distribution system. ISME J 10:582–595. doi: 10.1038/ismej.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Kooij D, Hijnen WAM. 1984. Substrate utilization by an oxalate-consuming Spirillum species in relation to its growth in ozonated water. Appl Environ Microbiol 47:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuo PPK, Chian ESK, Chang BJ. 1977. Identification of end products resulting from ozonation and chlorination of organic compounds commonly found in water. Environ Sci Technol 11:1177–1181. doi: 10.1021/es60136a012. [DOI] [Google Scholar]
- 63.Peldszus S, Huck PM, Andrews SA. 1996. Determination of short-chain aliphatic, oxo- and hydroxy-acids in drinking water at low microgram per liter concentrations. J Chromatogr A 723:27–34. doi: 10.1016/0021-9673(95)00838-1. [DOI] [PubMed] [Google Scholar]
- 64.Hammes F, Salhi E, Koster O, Egli T, von Gunten U. 2006. Mechanistic and kinetic evaluation of organic disinfection byproduct and assimilable organic carbon (AOC) formation during the ozonation of drinking water. Water Res 40:2275–2286. doi: 10.1016/j.watres.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 65.Brinkmann T, Hörsch P, Sartorius D, Frimmel FH. 2003. Photoformation of low-molecular-weight organic acids from brown water dissolved organic matter. Environ Sci Technol 37:4190–4198. doi: 10.1021/es0263339. [DOI] [PubMed] [Google Scholar]
- 66.Thomas V, Herrera-Rimann K, Blanc DS, Greub G. 2006. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl Environ Microbiol 72:2428–2438. doi: 10.1128/AEM.72.4.2428-2438.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pagnier I, Raoult D, La Scola B. 2011. Isolation and characterization of Reyranella massiliensis gen. nov., sp. nov. from fresh water samples by using an amoeba-coculture procedure. Int J Syst Evol Microbiol 61:2151–2154. doi: 10.1099/ijs.0.025775-0. [DOI] [PubMed] [Google Scholar]
- 68.Sadowsky MJ, Tully RE, Gregan PB, Kayser HH. 1987. Genetic diversity in Bradyrhizobium japonicum serogroup 123 and its relation to genotype-specific nodulation of soybean. Appl Environ Microbiol 53:2624–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kulakov LA, McAlister MB, Ogden KL, Larkin MJ, O'Hanlon JF. 2002. Analysis of bacteria contaminating ultrapure water in industrial systems. Appl Environ Microbiol 68:1548–1555. doi: 10.1128/AEM.68.4.1548-1555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delafont V, Brouke A, Bouchon D, Moulin L, Héchard Y. 2013. Microbiome of free-living amoebae isolated from drinking water. Water Res 47:6958–6965. doi: 10.1016/j.watres.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 71.Harrison TG, Afshar B, Doshi N, Fry NK. 2009. Distribution of Legionella pneumophila serogroups, monoclonal antibody subgroups and DNA sequence types in recent clinical and environmental isolates from England and Wales (2000–2008). Eur J Clin Microbiol Infect Dis 28:781–791. doi: 10.1007/s10096-009-0705-9. [DOI] [PubMed] [Google Scholar]
- 72.Pickup ZL, Pickup R, Parry JD. 2007. Effects of bacterial prey species and their concentration on growth of the amoebae Acanthamoeba castellanii and Hartmannella vermiformis. Appl Environ Microbiol 73:2631–2634. doi: 10.1128/AEM.02061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weekers PHH, Bodelier PLE, Wijen JPH, Volgels GD. 1993. Effects of grazing by the free-living soil amoebae Acanthamoeba castellanii, Acanthamoeba polyphaga, and Hartmannella vermiformis on various bacteria. Appl Environ Microbiol 59:2317–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang H, Edwards M, Falkinham JO III, Pruden A. 2012. Molecular survey of the occurrence of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa, and amoeba hosts in two chloraminated drinking water distribution systems. Appl Environ Microbiol 78:6285–6294. doi: 10.1128/AEM.01492-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buse HY, Lu J, Struewing IT, Ashbolt NJ. 2013. Eukaryotic diversity in premise drinking water using 18S rDNA sequencing: implications for health risk. Environ Sci Pollut Res 20:6351–6366. doi: 10.1007/s11356-013-1646-5. [DOI] [PubMed] [Google Scholar]
- 76.Spring S, Kämpfer P, Schleifer KH. 2001. Limnobacter thiooxidans gen. nov., sp. nov., a novel thiosulfate-oxidizing bacterium isolated from freshwater lake sediment. Int J Syst Evol Microbiol 51:1463–1470. doi: 10.1099/00207713-51-4-1463. [DOI] [PubMed] [Google Scholar]
- 77.James BW, Mauchline WS, Dennis PJ, Keevil CW, Wait R. 1999. Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low nutrient environments. Appl Environ Microbiol 65:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Kooij D. 1992. Assimilable organic carbon as an indicator of bacterial regrowth. J Am Water Works Assoc 84:57–65. [Google Scholar]
- 79.van der Kooij D, Martijn B, Schaap PG, Hoogenboezem W, Veenendaal HR, van der Wielen PWJJ. 2015. Improved biostability assessment of drinking water with a suite of test methods at a water supply treating eutrophic lake water. Water Res 87:347–355. doi: 10.1016/j.watres.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 80.Lin YE, Stout JE, Yu VL. 2011. Controlling Legionella in hospital drinking water: an evidence based review of disinfection methods. Infect Control Hosp Epidemiol 32:166–173. doi: 10.1086/657934. [DOI] [PubMed] [Google Scholar]
- 81.Geudens PJJG. 2012. Dutch drinking water statistics. Vewin, The Hague, the Netherlands. [Google Scholar]
- 82.Smirnov A. 2011. A revised classification of naked lobose amoebae (Amoebozoa: Lobosa). Protist 162:545–570. doi: 10.1016/j.protis.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 83.van der Kooij D, Albrechtsen HJ, Corfitzen CB, Ashworth J, Parry I, Enkiri F, Hambsch B, Hametner C, Kloiber R, Veenendaal HR, Verhamme D, Hoekstra EJ. 2003. CPDW project: assessment of the microbial growth support potential of products in contact with drinking water. Report EU 20832 EN JRC, Ispra, Italy. [Google Scholar]
- 84.ISO. 2004. Water quality—detection and enumeration of Legionella. Part 2. Direct membrane filtration method for waters with low bacterial counts. ISO 11731-2. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 85.Hobbie JE, Daley RJ, Jasper S. 1977. Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33:1225–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.