ABSTRACT
At least two-thirds of commercial antibiotics today are derived from Actinobacteria, more specifically from the genus Streptomyces. Antibiotic resistance and new emerging diseases pose great challenges in the field of microbiology. Cave systems, in which actinobacteria are ubiquitous and abundant, represent new opportunities for the discovery of novel bacterial species and the study of their interactions with emergent pathogens. White-nose syndrome is an invasive bat disease caused by the fungus Pseudogymnoascus destructans, which has killed more than six million bats in the last 7 years. In this study, we isolated naturally occurring actinobacteria from white-nose syndrome (WNS)-free bats from five cave systems and surface locations in the vicinity in New Mexico and Arizona, USA. We sequenced the 16S rRNA region and tested 632 isolates from 12 different bat species using a bilayer plate method to evaluate antifungal activity. Thirty-six actinobacteria inhibited or stopped the growth of P. destructans, with 32 (88.9%) actinobacteria belonging to the genus Streptomyces. Isolates in the genera Rhodococcus, Streptosporangium, Luteipulveratus, and Nocardiopsis also showed inhibition. Twenty-five of the isolates with antifungal activity against P. destructans represent 15 novel Streptomyces spp. based on multilocus sequence analysis. Our results suggest that bats in western North America caves possess novel bacterial microbiota with the potential to inhibit P. destructans.
IMPORTANCE This study reports the largest collection of actinobacteria from bats with activity against Pseudogymnoascus destructans, the fungal causative agent of white-nose syndrome. Using multigene analysis, we discovered 15 potential novel species. This research demonstrates that bats and caves may serve as a rich reservoir for novel Streptomyces species with antimicrobial bioactive compounds.
KEYWORDS: Actinobacteria, bats, caves, Pseudogymnoascus, Streptomyces, white-nose syndrome
INTRODUCTION
Presently, 90% of antibiotics are derived from microorganisms within the phylum Actinobacteria (1–3). The family Streptomycetaceae is particularly known for the production of chitinases capable of hydrolyzing the cell wall (4) and targeting ergosterol in the cell membrane of fungi (5). The rapid development of antibiotic resistance and the emergence of infectious diseases in humans and other animals, including bats and amphibians, have prompted the study of the bacterial microbiome and use for probiotic treatment in vertebrates (6, 7).
Actinobacteria are diverse and abundant in vertebrates, constituting the largest portion of skin microbiota in humans (i.e., >50%) (8). They are also one of the most dominant groups on the skin of different amphibian species (9), fish (10), and bats (11). Actinobacteria are well adapted to survive long periods and grow well in nutrient-depleted environments, such as caves (12). Current estimates reveal that less than 1% of actinobacteria have been cultivated (13).
In cave ecosystems, actinobacteria represent some of the most abundant microorganisms on cave walls and in guano (14–18), providing a rich reservoir for the discovery of novel bacterial species (19–21). Despite the high abundance of actinobacteria in caves, cultured-based studies are rare. Here, we study the diversity of naturally occurring actinobacteria associated with bats in New Mexico and Arizona and determine their antifungal activities against an emergent fungal pathogen in bats, Pseudogymnoascus destructans, the causative agent of white-nose syndrome (WNS).
White-nose syndrome is an invasive fungal disease that is currently threatening numerous bat species across the United States and Canada. WNS has spread from New York, throughout most of the eastern United States, and westward, killing millions of bats, and it has recently been confirmed in Washington state (22, 23). It has been estimated that the decline of bats in North America, which serve as substantial insect consumers, will lead to agricultural losses estimated at more than $3.7 billion/year (24). Severe wing damage affecting thermoregulation, blood electrolyte concentration, and gas exchange is commonly observed in infected bats (25). Bats also show an increase in arousals from torpor, depleting fat stores during hibernation and leading to starvation (26). Caves in the West are at high risk and are vulnerable to WNS due to their extraordinary bat diversity (27), appropriate temperatures, and relative humidity that supports the growth of P. destructans (28, 29).
In previous studies, bacteria have shown the capacity to inhibit P. destructans in vitro (30). For example, Hoyt et al. showed that bat bacterial isolates in the genus Pseudomonas obtained from Eptesicus fuscus and Myotis lucifugus inhibit P. destructans for at least 37 days (31). The present study constitutes, to our knowledge, the largest characterization of culturable actinobacteria from WNS-free bats and the largest collection of novel Actinobacteria strains showing antifungal activity against P. destructans. Here, we report an analysis of 632 isolates, 36 bacteria with antifungal activity, and 15 putative new species.
RESULTS
A total of 632 bacterial isolates from western bats were tested in this study, including 274 (43.4%) isolates that were isolated on humic acid-vitamin agar (HVA), 252 (39.9%) isolates on actinomycete isolation agar (AIA), 94 (14.9%) isolates on gellan gum (GG), and 12 (1.9%) isolates on glucose yeast extract agar (GYEA) (Table 1; see also Fig. S2 in the supplemental material). The collection is composed of 543 isolates (85.9%) from the phylum Actinobacteria and 62 isolates (9.8%) outside the phylum Actinobacteria, and the remaining 27 isolates (4.3%) could not be identified due to failure to amplify with 16S primers; none of these 27 showed antifungal activity (Table S1). Within the phylum Actinobacteria, 83 isolates (19.7%) had less than 97% similarity at the genus level, based on RDP Classifier. In addition, nine isolates had less than 97% similarity at the class level.
TABLE 1.
Number of bacterial isolates for each cave system
| Bat species | No. of isolates (no. of bats per species)a |
|||||
|---|---|---|---|---|---|---|
| CAVE | PARA | FS | ELMA | BLM | Total | |
| Corynorhinus townsendii | 10 (1) | 2 (1) | 53 (8) | 106 (18) | 0 (0) | 171 (28) |
| Myotis velifer | 62 (6) | 0 (0) | 0 (0) | 0 (0) | 26 (9) | 88 (15) |
| Antrozous pallidus | 39 (3) | 37 (3) | 0 (0) | 0 (0) | 0 (0) | 76 (6) |
| Myotis thysanodes | 40 (8) | 7 (2) | 15 (4) | 0 (0) | 0 (0) | 62 (14) |
| Eptesicus fuscus | 0 (0) | 0 (0) | 56 (6) | 0 (0) | 0 (0) | 56 (6) |
| Tadarida brasiliensis | 41 (5) | 10 (4) | 0 (0) | 0 (0) | 0 (0) | 51 (9) |
| Myotis californicus | 0 (0) | 41 (5) | 0 (0) | 0 (0) | 0 (0) | 41 (5) |
| Parastrellus hesperus | 0 (0) | 31 (5) | 0 (0) | 0 (0) | 0 (0) | 31 (5) |
| Myotis ciliolabrum | 0 (0) | 13 (4) | 5 (1) | 0 (0) | 0 (0) | 18 (5) |
| Myotis evotis | 0 (0) | 0 (0) | 0 (0) | 16 (4) | 0 (0) | 16 (4) |
| Lasionycteris noctivagans | 0 (0) | 8 (1) | 7 (1) | 0 (0) | 0 (0) | 15 (2) |
| Myotis volans | 0 (0) | 7 (2) | 0 (0) | 0 (0) | 0 (0) | 7 (2) |
| Total | 192 (23) | 156 (27) | 136 (20) | 122 (22) | 26 (9) | 632 (101) |
CAVE, Carlsbad Cavern National Park; PARA, Grand Canyon-Parashant National Monument; FS, Fort Stanton-Snowy River Cave National Conservation Area; ELMA, El Malpais National Monument; BLM, Bureau of Land Management caves 45 and 55.
Streptomyces was the dominant genus, with 422 isolates. Other Actinobacteria cultures were represented by 25 genera, including Microbacterium (22 isolates), Arthrobacter (16 isolates), Brevibacterium (18 isolates), and Rhodococcus (13 isolates) as the dominant taxa. Genera isolated outside the actinobacteria included, but were not limited to, Pseudomonas (13 isolates), Stenotrophomonas (7 isolates), and Advenella (5 isolates).
From our antifungal bioassays, 36 of the 632 bacterial isolates (5.7%) show inhibition zones against P. destructans (Table 2). Of the 36 positive actinobacteria, 32 (88.9%) were from the genus Streptomyces, whereas the remainder were members of the genera Rhodococcus, Streptosporangium, Luteipulveratus, and Nocardiopsis, with one isolate each. Of the inhibitory strains, the majority were isolated from Myotis velifer, Tadarida brasiliensis, Corynorhinus townsendii, and E. fuscus (Table 2). Zones of inhibition varied between isolates, but Streptomyces cyaneofuscatus (AC29), members of a novel clade (clade 9, Fig. 1), and Streptomyces anulatus (AC569) completely suppressed P. destructans growth even after 30 days of incubation (Table 2). A strain of Rhodococcus rhodochrous (AC 241) also showed complete inhibition of P. destructans.
TABLE 2.
Description of Actinobacteria inhibiting Pseudogymnoascus destructans
| Isolate | Speciesa | Caveb | Bat species | Accession no. | Inhibition activityc |
|---|---|---|---|---|---|
| AC29 | Streptomyces cyaneofuscatus | PARA | Parastrellus hesperus | KX458193 | High |
| AC52 | Streptomyces novel 1 | PARA | Parastrellus hesperus | KX458205 | Low |
| AC136 | Streptomyces novel 2 | PARA | Myotis californicus | KX458184 | Low |
| AC161 | Streptomyces novel 3 | ELMA | Corynorhinus townsendii | KX458185 | Low |
| AC162 | Streptomyces novel 3 | ELMA | Corynorhinus townsendii | KX458186 | Low |
| AC208 | Streptomyces novel 3 | ELMA | Corynorhinus townsendii | KX458187 | Low |
| AC230 | Streptomyces novel 3 | ELMA | Corynorhinus townsendii | KX458188 | Low |
| AC241 | Rhodococcus rhodochrous | BLM | Myotis velifer | KX458189 | High |
| AC245 | Streptomyces novel 1 | BLM | Myotis velifer | KX458190 | Low |
| AC250 | Streptomyces novel 1 | BLM | Myotis velifer | KX458191 | Low |
| AC260 | Streptomyces novel 1 | BLM | Myotis velifer | KX458192 | Medium |
| AC313 | Streptomyces novel 4 | FS | Corynorhinus townsendii | KX458194 | Low |
| AC331 | Streptomyces novel 5 | FS | Myotis ciliolabrum | KX458195 | Low |
| AC340 | Streptomyces diastaticus | FS | Eptesicus fuscus | KX458196 | Low |
| AC352 | Streptomyces halstedii | FS | Eptesicus fuscus | KX458197 | Medium |
| AC363 | Streptomyces novel 6 | FS | Eptesicus fuscus | KX458198 | Low |
| AC367 | Streptomyces rutgersensis | FS | Eptesicus fuscus | KX458199 | Low |
| AC373 | Unidentified Streptomyces | FS | Eptesicus fuscus | KX458200 | Low |
| AC469 | Streptosporangium | CAVE | Myotis velifer | KX458201 | Low |
| AC495 | Streptomyces novel 7 | CAVE | Myotis thysanodes | KX458202 | Medium |
| AC512 | Streptomyces novel 8 | CAVE | Myotis thysanodes | KX458203 | Medium |
| AC523 | Luteipulveratus | CAVE | Myotis velifer | KX458204 | Medium |
| AC536 | Streptomyces novel 9 | CAVE | Myotis velifer | KX458206 | High |
| AC538 | Streptomyces aureocirculatus | CAVE | Myotis velifer | KX458207 | Medium |
| AC541 | Streptomyces novel 9 | CAVE | Myotis velifer | KX458208 | High |
| AC542 | Streptomyces novel 10 | CAVE | Myotis velifer | KX458209 | Low |
| AC550 | Streptomyces novel 11 | CAVE | Tadarida brasiliensis | KX458210 | Low |
| AC555 | Streptomyces novel 12 | CAVE | Tadarida brasiliensis | KX458211 | Low |
| AC558 | Streptomyces novel 13 | CAVE | Tadarida brasiliensis | KX458212 | Medium |
| AC562 | Nocardiopsis | CAVE | Tadarida brasiliensis | KX458213 | High |
| AC563 | Streptomyces novel 9 | CAVE | Tadarida brasiliensis | KX458214 | Medium |
| AC564 | Streptomyces novel 9 | CAVE | Tadarida brasiliensis | KX458215 | High |
| AC569 | Streptomyces anulatus | CAVE | Corynorhinus townsendii | KX458216 | High |
| AC602 | Streptomyces novel 14 | CAVE | Antrozous pallidus | KX458217 | Medium |
| AC627 | Streptomyces novel 15 | CAVE | Tadarida brasiliensis | KX458218 | Medium |
| AC628 | Streptomyces novel 9 | CAVE | Tadarida brasiliensis | KX458219 | Medium |
Based on MLSA distance analysis (34).
PARA, Grand Canyon-Parashant National Monument; ELMA, El Malpais National Monument; BLM, Bureau of Land Management caves 45 and 55; FS, Fort Stanton-Snowy River Cave National Conservation Area; CAVE, Carlsbad Cavern National Park.
Inhibition activity was scored as low (1 to 15 mm), medium (16 to 30 mm), or high (31 to 45 mm).
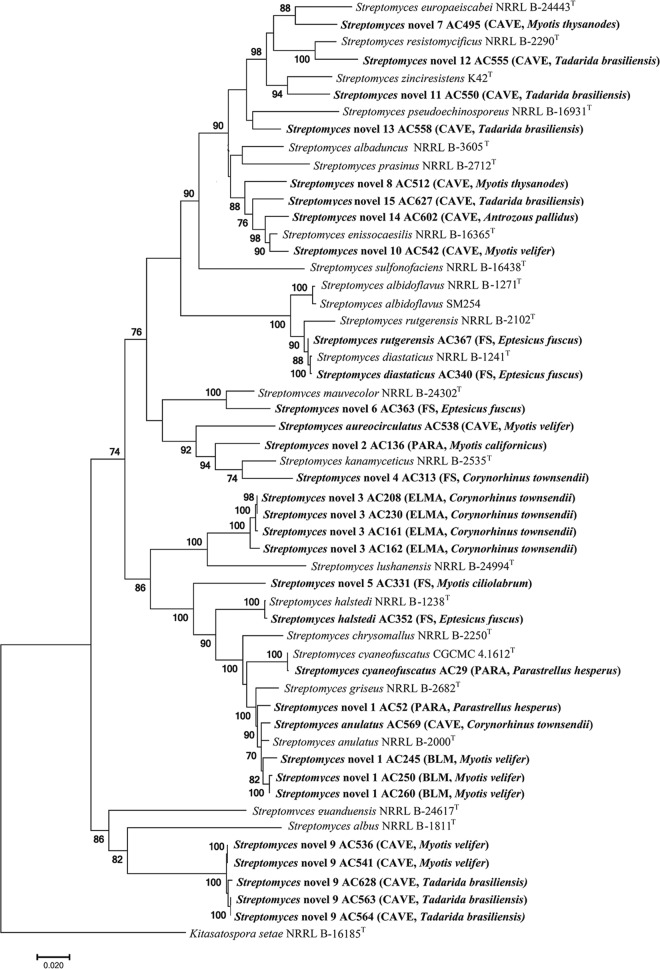
FIG 1.
Streptomyces spp. with antifungal activity were classified into 15 novel clades using multilocus sequencing analysis. A maximum likelihood tree of a 5-gene alignment based on the general time-reversible model is shown. Bootstrap values >70%, based on 500 pseudoreplicates, are indicated on branch points. The scale bar corresponds to 0.02 nucleotide substitution per site.
Identification of Streptomyces species.
The taxonomy of Streptomyces species is particularly challenging due to the high number of species and the limited resolution of the 16S rRNA gene for species circumscription (32). This has led to the adoption of multilocus sequence analysis approaches to resolve the taxonomy of this genus (33–35). Using a five-gene phylogeny, 31 of the 32 total Streptomyces isolates were placed in 21 different species, including 15 novel clades (Table 2 and Fig. 1). One isolate could not be assessed using the multigene sequence analysis due to failure to amplify all the target genes. One of the more phylogenetically distinct clades (novel clade 9) is composed of five strains isolated from two bat species (M. velifer and T. brasiliensis) in Carlsbad Cavern National Park (Fig. 1). This novel Streptomyces clade also contains some of the most antagonistic strains against P. destructans (Table 2). A second group of isolates (novel clade 3) clustered together to form another novel species (Fig. 1). These isolates came from the same location (El Malpais National Monument) and the same bat species (C. townsendii). We identified strain AC340 as belonging to Streptomyces diastaticus. This strain is also a near neighbor to Streptomyces strain SM254, which was reported as an antagonist of P. destructans (36). It is noteworthy that SM254 was previously described as Streptomyces albus, and in the present study, based on multilocus phylogenetic analysis utilizing gene locus sequence data extracted from the SM254, this strain actually is a representative Streptomyces albidoflavus (Fig. 1).
DISCUSSION
Fungal and bacterial surveys in caves have shown that microbial diversity in caves and bats is high and may have antagonistic properties against pathogens (31, 37–39). Microbial communities in caves show a high abundance of actinobacterial genera, with the most abundant being Streptomyces, especially on rock walls and soils (40, 41). Northup et al. (16) and Hathaway et al. (18) reported actinobacteria to be one of the most abundant groups in cave surveys.
This study represents one of the largest surveys of culturable actinobacteria in WNS-free caves. We explored the existing external microbial community of 12 species of healthy bats in New Mexico and Arizona inhabiting five cavernous and surface sites in the region. Southwestern caves in this study showed a high diversity of bacteria, with large numbers of novel species with antifungal activity. Caves represent a rich reservoir of novel species. For example, volcanic cave walls and ceiling surveys report more than 62% of sequences potentially representing novel species (15). Pasić et al., in a study of bacteria in cave walls, also reported 88 to 93% of bacterial sequences with the potential to represent novel species, with Actinobacteria as the most abundant group (42). In this study, we reported 15 new putative species using multilocus analysis.
The majority of the isolates with antifungal properties in this study belong to the genus Streptomyces. It is possible that the high abundance of actinobacteria recovered from bat skin is a result of inoculation from cave walls in which they are abundant (16, 18, 42). For example, Winter et al. reported that cave-caught bats possess a different external microbiome with respect to bats caught outside the caves (11), showing the potential influence of collection site on bat microbiota. Alternatively, actinobacteria are likely an active part of the bat microbiome, as they have been reported to be one of the most common skin habitants in multiple vertebrates, including humans (14), fish (10), amphibians (9), and bats (11). In humans, actinobacteria are prevalent and represent up to 50% of the human skin microbiome (6). In this particular study, it is unclear if these microorganisms are actively growing on the skin of their host or produced antifungals. It is also possible some of these taxa are only present as spores as a result of microbial dispersal in caves. Stable beneficial interactions between Streptomyces and many organisms are common, but they have not been reported yet in vertebrates (43). This study confirms that caves and bats are a rich reservoir of novel species, with the potential for the discovery of novel antifungal compounds, but additional work is necessary to determine the nature of these bacterial associations with bats.
Antagonists of P. destructans.
Previous reports have demonstrated positive results in the use of beneficial microbes as an alternative to toxic chemicals against infectious diseases caused by fungi in natural environments (44–46). For example, Becker et al. utilized a probiotic bath of the metabolite produce by a bacterium isolated from skin of amphibians to increase survival of amphibians infected with a lethal fungus, Batrachochytrium dendrobatidis (7, 44). In this study, we identify at least 36 naturally occurring isolates with potential to inhibit P. destructans. In similar studies, a Streptomyces isolate from the Soudan Iron Mine in Minnesota, USA, initially recognized as S. albus, has exhibited potent antagonistic activity against P. destructans (36). Experiments by Cornelison et al. also demonstrated that R. rhodochrous could completely inhibit P. destructans on bat tissue by slowing germination and reducing mycelial growth when cultured with shared air space at 15°C (30). We identified a similar isolate classified as R. rhodochrous (AC241) from a healthy M. velifer bat that completely inhibited P. destructans growth when using a bilayer plate assay. Sequence data were not reported for the strain in the Cornelison et al. study and thus could not be compared to our isolates.
In another study, six Pseudomonas isolates from M. lucifugus and E. fuscus bats were reported as antagonists of P. destructans (31). The 13 Pseudomonas cultures isolated from four species of western bats in this study did not exhibit inhibition of P. destructans using the bilayer assay. Hoyt et al. demonstrated that the suppression levels by bacteria on P. destructans depend on the concentration of the fungus and the type of bacteria (31). It is possible that the bioassay used in this study and the potential variation in spore loads and antifungal diffusion decreased the detection levels of activity of several of the isolates tested. However, little variation was observed on the replicates of the strains with activity, demonstrating that this bioassay allows for consistent selection of isolates that produce antifungals. This assay also eliminates the problem of other nontarget interactions of the fungus and bacteria observed in direct-contact bioassays. Nevertheless, as any other bioassay, it has limitations in the sensitivity of detection.
Novel cave isolates.
Isolates exhibiting antifungal activity against P. destructans were subjected to multilocus sequence analysis, and nearest-neighboring Streptomyces species were identified from phylogenetic analysis. Twenty-five (69%) of the isolates appeared in novel clades, representing 15 new Streptomyces species that will be described elsewhere (Table 2 and Fig. 1; see also Table S2 in the supplemental material). The high abundance of novel species previously described from caves (19, 20, 47–49) and the present report of 15 putative novel species with antifungal activity from bats caught in western U.S. caves illustrate the potential of caves as a valuable resource for the discovery of novel antimicrobials. It is possible that some of these bat-associated actinobacteria have the potential to be used as a probiotic in the control of WNS, with the additional environmental advantage that these microorganisms are already natural inhabitants of cave ecosystems (31). Western caves are currently free of WNS, and therefore, it is unknown if the bacteria present on the bats and cave walls are antagonistic to the fungus in vivo; however, the present study, together with that of Winter et al., provide a preinfection baseline and serve as a valuable resource to help assess differences in response if the bats in these caves become affected by the disease (11).
The use of probiotics is rapidly gaining importance as potential treatment for different conditions in humans and other animals (7, 50, 51), but additional studies are necessary to determine the potential use of probiotics in bats and their interactions with the overall bacterial community. This study represents an additional effort to characterize culturable novel actinobacteria with antifungal properties that may serve as an alternative preventative measures or treatment for bats infected with WNS and other mycotic diseases.
MATERIALS AND METHODS
Bat sampling.
We sampled bats on the surface and from caves posthibernation at the El Malpais Conservation Area, Grand Canyon-Parashant National Monument, Bureau of Land Management (BLM) caves 45 and 55, Fort Stanton-Snowy River Cave National Conservation Area, and Carlsbad Caverns National Park across New Mexico and Arizona (Fig. S1 and Table 1). Sampling of these cave and surface sites was performed during the spring and summer months (March to August) from 2013 to 2015. Bats were caught using mist nets or were hand plucked from cave walls (52), according to approved protocols under the following collection permits: 2014 Arizona and New Mexico Game and Fish Department Scientific Collecting Permit (SP670210, SCI#3423, and SCI#3350), National Park Service Scientific Collecting Permit (CAVE-2014-SCI-0012, ELMA-2013-SCI-0005, ELMA-2014-SCI-0001, and PARA-2012-SCI-0003), USGS Fort Collins Science Center Standard Operating Procedure (SOP) 2013-01, and an Institutional Animal Care and Use Committee (IACUC) permit from the University of New Mexico (protocol #12-100835-MCC) and from the National Park Service (protocol #IMR-ELMA.PARA-Northup-Bat-2013.A2).
Species, sex, reproductive conditions, and other metrics were recorded (53). The wings, muzzle, ears, and uropatagium were assessed for any tissue damage (necrosis), lesions, scarring, or skin mottling consistent with infection by P. destructans (25, 54). Most bats were easily identified using a key of standard morphological features (55).
Bat swabbing.
Skin (wing and tail membranes) and fur surfaces of the bats were thoroughly swabbed in situ using sterile polyester fiber-tipped application swabs (Falcon) moistened with sterile double-distilled water or Ringer's solution. Each bat was swabbed three times all over the fur and skin for humic acid-vitamin agar (HVA), on the right-side fur and skin for actinomycete isolation agar (AIA), and on the left-side fur and skin for gellan gum (GG) and for glucose yeast extract agar (GYEA) (see below). Plates were checked for contamination before being streaked with inoculated swabs and then stored in sleeves and kept cool for return to the laboratory. All bat work was in accordance with the guidelines of the American Society of Mammalogists for the use of wild mammals in research (56) and USGS Fort Collins Science Center Standard Operating Procedure (SOP) 2013-01 (57). A total of 101 bats, including 12 species, were sampled and identified: pallid bat (Antrozous pallidus), Townsend's big-eared bat (Corynorhinus townsendii), big brown bat (Eptesicus fuscus), silver-haired bat (Lasionycteris noctivagans), California myotis (Myotis californicus), Western small-footed myotis (Myotis ciliolabrum), long-eared myotis (Myotis evotis), fringed myotis (Myotis thysanodes), cave myotis (Myotis velifer), long-legged bat (Myotis volans), western canyon bat (Parastrellus hesperus), and Brazilian free-tailed bat (Tadarida brasiliensis).
Isolation of actinobacteria.
Three actinobacterium-selective media were used to isolate parent cultures of the bat microbiota: AIA (Difco, Sparks, MD), GG (7.0 g/liter gellan gum, 7 mM calcium chloride), or HVA (58) (Fig. S2). GYEA was used for a few plates due to low isolation rates (59). In an effort to target actinobacteria and actively discourage the growth of Gram-negative bacteria and fungi, media were supplemented with cycloheximide (50 mg/liter), nalidixic acid (50 mg/liter), trimethoprim (50 mg/liter) (Sigma-Aldrich, St. Louis, MO), and a vitamin solution (60). Isolates were subcultured for purification on Reasoner's 2A medium (R2A; Difco, Sparks, MD). Cultures were grown at 20°C to standardize incubation conditions, and pure cultures were stored at −80°C in 20% glycerol freezer stocks.
Initial identification of actinobacteria.
DNA from pure cultures was extracted using the MoBio UltraClean microbial DNA isolation kit (MoBio, Carlsbad, CA), according to the manufacturer's protocol, with the exception of using colonies from solid media and substituting 1.5 min of bead beating at medium speed for a vortexing step. The 16S rRNA was amplified with bacterial primers 8F (5′-AGAGTTTGATCCTGGCGCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) (61). Reactions were carried out in a 25-μl volume with 10× PCR buffer with 15 mM Mg2+, 0.3 μM each primer, 0.25 mM each dinucleoside triphosphate (dNTP), 5 μg of 50 mg/ml bovine serum albumin (BSA; Ambion, Austin, TX, USA), and 1 U of AmpliTaq LD (Applied Biosystems, Foster City, CA, USA). The PCR was performed with an MJ thermocycler with a program that consisted of preheating at 94°C for 5 min, 30 cycles at 94°C for 30 s, 55.5°C for 30 s, 72°C for 1.5 min, and a final extension at 72°C for 7 min. Amplicons were purified with ExoSAP-IT (Affymetrix, Santa Clara, CA) for in-house sequencing with BigDye 1.1, using 46F (5′-GCYTAAYACATGCAAGTCG-3′) for all sequences. In-house sequencing was performed with ABI 3130 and 377 automated DNA sequencers. Some of the extracted DNA was sequenced with the same primers by Beckman Coulter Genomics (Danvers, MA). In addition, reverse sequences using 1492R (5′-GTGACGGGCRGTGTGTRCAA-3′) were performed for bioactive isolates. Ambiguous portions were trimmed or edited, but the majority of the primer sequence was retained.
Phylogenetic analysis of Streptomyces isolates using multilocus analysis.
Genomic DNA was isolated from each strain using a modified cetyltrimethylammonium bromide (CTAB) method for Streptomyces isolates with antifungal activity after preliminary identification using 16S rRNA (62). Partial sequences of the housekeeping genes atpD (ATP synthase F1, beta-subunit), gyrB (DNA gyrase B subunit), rpoB (RNA polymerase beta-subunit), recA (recombinase A), and trpB (tryptophan synthetase, beta-subunit) were amplified and sequenced using the primers and protocols previously described (33). Amplified products were purified using ExoSAP-IT (Affymetrix, Santa Clara, CA) and sequenced using BigDye 3.1 on an ABI model 3730 sequencer at the National Center for Agricultural Utilization Research (NCAUR) core sequencing facility. Raw contigs for each locus were assembled and corrected from the traces using Sequencher version 5.2 (Gene Codes Corp., Ann Arbor, MI).
The gene sequences for the five housekeeping loci for each strain were organized using the Bacterial Isolate Genomic Sequence Database (BIGSdb) version 1.12.3 (63) on the ARS Microbial Genomic Sequence Database server (http://199.133.98.43). The sequences for the alleles of each locus for these strains and related strains obtained from GenBank or available locally were concatenated head to tail in-frame during export in FASTA format and subsequently aligned using MAFFT (64). The sequence alignment was analyzed in jModelTest 2 version 2.1.7 (65) to determine the optimal model for phylogenetic analysis. Evolutionary analyses were conducted in MEGA6 (66). The phylogenetic tree was inferred by using the maximum likelihood method based on the general time-reversible model (67). The phylogenetic relationships of the strains were also inferred using the Tamura-Nei evolutionary distance method (68) with the neighbor-joining model of Saitou and Nei (69) and maximum parsimony in MEGA6.0. All analyses were subjected to 500 bootstrap replicates. Multilocus sequence analysis (MLSA) evolutionary distances were determined using MEGA6 by calculating the Kimura 2-parameter distance (70), and strain pairs having distance less than 0.007 were considered conspecific based on the guideline empirically determined by Rong and Huang (34).
Inhibition assays.
A bilayer plate assay was modified from Montano and Henderson for testing antibiotic production (71). The results were a zone of inhibition measurement around a precise bacterial streak void of fungal growth. Plates were filled 0.5-cm deep with R2A medium (Difco, Sparks, MD) and left to solidify. The plate was then streaked in the center with a line 5 cm by 1 cm of sporulating bacterial inoculum. The bacteria were allowed to incubate at 25°C for a maximum of 14 days. Sabouraud dextrose agar (SDA; Oxoid, Basingstoke, Hampshire, England) was prepared without antibiotics and then allowed to cool to ∼60°C. The medium was poured indirectly and slowly on top of the grown bacteria so as to not disturb the isolate. After the second layer had solidified, 100 μl of a P. destructans spore suspension (105 to 107 conidia/ml) was spread evenly over the SDA medium with a triangular spreader. Espinel-Ingroff et al. proposed that 104 CFU/ml would be sufficient for antifungal susceptibility testing of filamentous fungi (72). The P. destructans culture was obtained from a WNS-positive Pipistrellus subflavus bat from Mesmore Cave, IN, isolated in 2010. Culture was maintained at the Western Illinois University Fungarium code TW250. Purity was confirmed by observing characteristic conidia of the fungus and psychrophilic growth preferences according to Gargas et al. (73). Plates were incubated at 6°C for a month and evaluated every 3 days. No inhibition was recorded if the plate was covered with a layer of fungus. The inhibition activity was characterized as low, medium, or high based on the width of the inhibition zone (area with no fungal growth) from the edge of the bacterial streak to the terminal growth of the fungus. Control plates were made without any bacterial streak. Plates with antifungal activity were done in triplicate. The zone of inhibition was ranked as low for average inhibition zones lower than 15 mm, medium for 15 to 30 mm, and high for more than 30 mm.
Accession number(s).
The 16S rRNA sequences for the cultures showing activity against P. destructans were deposited in GenBank with the accession numbers KX458184 to KX458219 (Table 2). Sequences of isolates without antifungal activity were deposited under accession numbers KX928078 to KX928646 (Table S1). The sequences used in the phylogenetic analysis are available at the ARS Microbial Genomic Sequence Database (http://199.133.98.43). GenBank numbers are listed in Table S2 in the supplemental material.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff at El Malpais and Grand Canyon-Parashant National Monuments, Carlsbad Caverns National Park, Bureau of Land Management, and the Fort Stanton Cave Study Project. Students from the Northup lab at University of New Mexico assisted with our bat netting efforts. We thank five anonymous reviewers for their valuable input.
Initial funding that began this study was provided by the Eppley Foundation. Building on this initial funding, further funding for this project was provided by the National Park Service through the Colorado Plateau Cooperative Ecosystem Studies unit (CPCESU) for work at the El Malpais National monument, Carlsbad Caverns National Monument, and Grand Canyon-Parashant National Monument. The Western National Park Association provided funding for the El Malpais National Monument work. The Bureau of Land Management and Fort Stanton Cave Study Project funded work in BLM caves 45 and 55 and Fort Stanton Cave, respectively. Additional funding was provided by the IDNR (principal investigator [PI] A.P.-A.), New Mexico Game and Fish Department Share with Wildlife Program, Cave Conservancy Foundation, National Speleological Society Rapid Response Fund, and T&E, Inc. P.S.H. was supported by WIU funding from the RISE (Research Inspiring Student Excellence) and WIS (Women in Science) programs and the Department of Biological Sciences. D.P.L. and the ARS Culture Collection CRIS project were supported by ARS National Program 301. C.A.D. was supported by ARS National Program 206.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. The mention of firm names or trade products does not imply that they are endorsed or recommended by the USDA or USGS over other firms or similar products not mentioned. USDA and USGS are equal opportunity providers and employers.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.03057-16.
REFERENCES
- 1.Wohlleben W, Mast Y, Stegmann E, Ziemert N. 2016. Antibiotic drug discovery. Microb Biotechnol 9:541–548. doi: 10.1111/1751-7915.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chater KF, Biro S, Lee KJ, Palmer T, Schrempf H. 2010. The complex extracellular biology of Streptomyces. FEMS Microbiol Rev 34:171–198. doi: 10.1111/j.1574-6976.2009.00206.x. [DOI] [PubMed] [Google Scholar]
- 3.Hopwood DA. 2007. Streptomyces in nature and medicine: the antibiotic makers. Oxford University Press, New York, NY. [Google Scholar]
- 4.Schrempf H. 2001. Recognition and degradation of chitin by streptomycetes. Antonie Von Leeuwenhoek 79:285–289. doi: 10.1023/A:1012058205158. [DOI] [PubMed] [Google Scholar]
- 5.Seco EM, Cuesta T, Fotso S, Laatsch H, Malpartida F. 2005. Two polyene amides produced by genetically modified Streptomyces diastaticus var. 108. Chem Biol 12:535–543. doi: 10.1016/j.chembiol.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Colston TJ, Jackson CR. 2016. Microbiome evolution along divergent branches of the vertebrate tree of life: what is known and unknown. Mol Ecol 25:3776–3800. doi: 10.1111/mec.13730. [DOI] [PubMed] [Google Scholar]
- 7.Bletz MC, Loudon AH, Becker MH, Bell SC, Woodhams DC, Minbiole KPC, Harris RN. 2013. Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecol Lett 16:807–820. doi: 10.1111/ele.12099. [DOI] [PubMed] [Google Scholar]
- 8.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML, Segre JA. 2009. Topographical and temporal diversity of the human skin microbiome. Science 324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kueneman JG, Parfrey LW, Woodhams DC, Archer HM, Knight R, McKenzie VJ. 2014. The amphibian skin-associated microbiome across species, space and life history stages. Mol Ecol 23:1238–1250. doi: 10.1111/mec.12510. [DOI] [PubMed] [Google Scholar]
- 10.Larsen A, Tao Z, Bullard SA, Arias CR. 2013. Diversity of the skin microbiota of fishes: evidence for host species specificity. FEMS Microbiol Ecol 85:483–494. doi: 10.1111/1574-6941.12136. [DOI] [PubMed] [Google Scholar]
- 11.Winter AS, Kimble JC, Young JM, Buecher DC, Valdez EW, Hathaway JJ, Porras-Alfaro A, Read KJ, Northup DE. 2016. External bacterial diversity on bats in the southwestern United States: changes in bacterial community structure above and below ground. PeerJ PrePrints 4:e2493v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton HA, Taylor MR, Pace NR. 2004. Molecular phylogenetic analysis of a bacterial community in an oligotrophic cave environment. Geomicrobiol J 21:11–20. doi: 10.1080/01490450490253428. [DOI] [Google Scholar]
- 13.Zang E, Brandes S, Tovar M, Martin K, Mech F, Horbert P, Henkel T, Figge MT, Roth M. 2013. Real-time image processing for label-free enrichment of actinobacteria cultivated in picolitre droplets. Lab Chip 13:3707–3713. doi: 10.1039/c3lc50572c. [DOI] [PubMed] [Google Scholar]
- 14.Cuezva S, Fernandez-Cortes A, Porca E, Pasic L, Jurado V, Hernandez-Marine M, Serrano-Ortiz P, Hermosin B, Canaveras JC, Sanchez-Moral S, Saiz-Jimenez C. 2012. The biogeochemical role of Actinobacteria in Altamira Cave, Spain. FEMS Microbiol Ecol 81:281–290. doi: 10.1111/j.1574-6941.2012.01391.x. [DOI] [PubMed] [Google Scholar]
- 15.Riquelme C, Hathaway JJM, Dapkevicius M, Miller AZ, Kooser A, Northup DE, Jurado V, Fernandez O, Saiz-Jimenez C, Cheeptham N. 2015. Actinobacterial diversity in volcanic caves and associated geomicrobiological interactions. Front Microbiol 6:1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Northup DE, Melim LA, Spilde MN, Hathaway JJM, Garcia MG, Moya M, Stone FD, Boston PJ, Dapkevicius M, Riquelme C. 2011. Lava cave microbial communities within mats and secondary mineral deposits: implications for life detection on other planets. Astrobiology 11:601–618. doi: 10.1089/ast.2010.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu YC, Tan LC, Liu WX, Wang BZ, Wang JJ, Cai YJ, Lin XG. 2015. Profiling bacterial diversity in a limestone cave of the western Loess Plateau of China. Front Microbiol 6:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hathaway JJM, Garcia MG, Balasch MM, Spilde MN, Stone FD, Dapkevicius M, Amorim IR, Gabriel R, Borges PAV, Northup DE. 2014. Comparison of bacterial diversity in Azorean and Hawai'ian lava cave microbial mats. Geomicrobiol J 31:205–220. doi: 10.1080/01490451.2013.777491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko KS, Kim Y, Seong CN, Lee SD. 2015. Rhodococcus antrifimi sp. nov., isolated from dried bat dung of a cave. Int J Syst Evol Microbiol 65:4043–4048. doi: 10.1099/ijsem.0.000534. [DOI] [PubMed] [Google Scholar]
- 20.Groth I, Schumann P, Schutze B, Augsten K, Stackebrandt E. 2002. Knoellia sinensis gen. nov., sp. nov. and Knoellia subterranea sp. nov., two novel actinobacteria isolated from a cave. Int J Syst Evol Microbiol 52:77–84. doi: 10.1099/00207713-52-1-77. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez-Patricio S, Jurado V, Laiz L, Saiz-Jimenez C. 2014. Two new species of bacteria isolated from white colonizations in Andalusian caves, p 281 In Rogerio-Candelera MA. (ed), Science, technology and cultural heritage. CRC Press, Boca Raton, FL. [Google Scholar]
- 22.Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, Buckles EL, Coleman JTH, Darling SR, Gargas A, Niver R, Okoniewski JC, Rudd RJ, Stone WB. 2009. Bat white-nose syndrome: an emerging fungal pathogen? Science 323:227–227. doi: 10.1126/science.1163874. [DOI] [PubMed] [Google Scholar]
- 23.Haman K, Hibbard C, Lubeck M. 2016. Bat with white-nose syndrome confirmed in Washington state. Washington Department of Fish and Wildlife and U.S. Geological Survey, Olympia, WA. [Google Scholar]
- 24.Boyles JG, Cryan PM, McCracken GF, Kunz TH. 2011. Economic importance of bats in agriculture. Science 332:41–42. doi: 10.1126/science.1201366. [DOI] [PubMed] [Google Scholar]
- 25.Cryan PM, Meteyer CU, Boyles JG, Blehert DS. 2010. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol 8:1–8. doi: 10.1186/1741-7007-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warnecke L, Turner JM, Bollinger TK, Misra V, Cryan PM, Blehert DS, Wibbelt G, Willis CKR. 2013. Pathophysiology of white-nose syndrome in bats: a mechanistic model linking wing damage to mortality. Biol Lett 9:20130177. doi: 10.1098/rsbl.2013.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphrey SR. 1975. Nursery roosts and community diversity of Nearctic bats. J Mammal 56:321–346. doi: 10.2307/1379364. [DOI] [Google Scholar]
- 28.Cunningham KI, LaRock EJ. 1991. Recognition of microclimate zones through radon mapping, Lechuguilla Cave, Carlsbad Caverns National Park, New Mexico. Health Phys 61:493–500. doi: 10.1097/00004032-199110000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Forbes J. 1998. Air temperature and relative humidity study: Torgac Cave, New Mexico. J Cave Karst Stud 60:27–32. [Google Scholar]
- 30.Cornelison CT, Keel MK, Gabriel KT, Barlament CK, Tucker TA, Pierce GE, Crow SA Jr. 2014. A preliminary report on the contact-independent antagonism of Pseudogymnoascus destructans by Rhodococcus rhodochrous strain DAP96253. BMC Microbiol 14:246. doi: 10.1186/s12866-014-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoyt JR, Cheng TL, Langwig KE, Hee MM, Frick WF, Kilpatrick AM. 2015. Bacteria isolated from bats inhibit the growth of Pseudogymnoascus destructans, the causative agent of white-nose syndrome. PLoS One 10:e0121329. doi: 10.1371/journal.pone.0121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kämpfer P. 2006. The family Streptomycetaceae, part I: taxonomy, p 538–604. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes: a handbook on the biology of bacteria, 3rd ed Volume 3: Archaea. Bacteria: Firmicutes, Actinomycetes; Springer, New York, NY. [Google Scholar]
- 33.Labeda DP, Doroghazi JR, Ju KS, Metcalf WW. 2014. Taxonomic evaluation of Streptomyces albus and related species using multilocus sequence analysis and proposals to emend the description of Streptomyces albus and describe Streptomyces pathocidini sp. nov. Int J Syst Evol Microbiol 64:894–900. doi: 10.1099/ijs.0.058107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rong X, Huang Y. 2012. Taxonomic evaluation of the Streptomyces hygroscopicus clade using multilocus sequence analysis and DNA-DNA hybridization, validating the MLSA scheme for systematics of the whole genus. Syst Appl Microbiol 35:7–18. doi: 10.1016/j.syapm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Labeda DP. 2016. Taxonomic evaluation of putative Streptomyces scabiei strains held in the ARS Culture Collection (NRRL) using multi-locus sequence analysis. Antonie Von Leeuwenhoek 109:349–356. doi: 10.1007/s10482-015-0637-6. [DOI] [PubMed] [Google Scholar]
- 36.Badalamenti JP, Erickson JD, Salomon CE. 2016. Complete genome sequence of Streptomyces albus SM254, a potent antagonist of bat white-nose syndrome pathogen Pseudogymnoascus destructans. Genome Announc 4(2):e00290-16. doi: 10.1128/genomeA.00290-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amelon S, Knudsen G, Eggert L. 2013. Biocontrol of fungi causing white nose syndrome. In Forest disturbance processes. Northern Research Station, U.S. Forest Service: https://www.nrs.fs.fed.us/disturbance/invasive_species/wns/control_management/biocontrol_fungus/. [Google Scholar]
- 38.Johnson LJAN, Miller AN, McCleery RA, McClanahan R, Kath JA, Lueschow S, Porras-Alfaro A. 2013. Psychrophilic and psychrotolerant fungi on bats and the presence of Geomyces spp. on bat wings prior to the arrival of white nose syndrome. Appl Environ Microbiol 79:5465–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanderwolf KJ, McAlpine DF, Malloch D, Forbes GJ. 2013. Ectomycota associated with hibernating bats in Eastern Canadian caves prior to the emergence of white-nose syndrome. Northeast Nat 20:115–130. doi: 10.1656/045.020.0109. [DOI] [Google Scholar]
- 40.Laiz L, Groth I, Gonzalez I, Saiz-Jimenez C. 1999. Microbiological study of the dripping waters in Altamira cave (Santillana del Mar, Spain). J Microbiol Methods 36:129–138. doi: 10.1016/S0167-7012(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 41.Laiz L, Gonzalez JM, Saiz-Jimenez C. 2003. Microbial communities in caves: ecology, physiology, and effects on paleolithic paintings, p 210–225. In Koestler RJ, Koestler VH, Charola AE, Nieto-Fernandez FE (ed), Art, biology, and conservation: biodeterioration of works of art. The Metropolitan Museum of Art, New York, NY. [Google Scholar]
- 42.Pasić L, Kovce B, Sket B, Herzog-Velikonja B. 2010. Diversity of microbial communities colonizing the walls of a Karstic cave in Slovenia. FEMS Microbiol Ecol 71:50–60. doi: 10.1111/j.1574-6941.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 43.Seipke RF, Kaltenpoth M, Hutchings MI. 2012. Streptomyces as symbionts: an emerging and widespread theme? FEMS Microbiol Rev 36:862–876. doi: 10.1111/j.1574-6976.2011.00313.x. [DOI] [PubMed] [Google Scholar]
- 44.Becker MH, Brucker RM, Schwantes CR, Harris RN, Minbiole KP. 2009. The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl Environ Microbiol 75:6635–6638. doi: 10.1128/AEM.01294-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gil-Turnes MS, Hay ME, Fenical W. 1989. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 246:116–118. doi: 10.1126/science.2781297. [DOI] [PubMed] [Google Scholar]
- 46.Sihag RC, Sharma P. 2012. Probiotics: the new ecofriendly alternative measures of disease control for sustainable aquaculture. J Fish Aquat Sci 7:72. doi: 10.3923/jfas.2012.72.103. [DOI] [Google Scholar]
- 47.Lee SD. 2008. Jiangella alkaliphila sp. nov., an actinobacterium isolated from a cave. Int J Syst Evol Microbiol 58:1176–1179. doi: 10.1099/ijs.0.65479-0. [DOI] [PubMed] [Google Scholar]
- 48.Jurado V, Groth I, Gonzalez JM, Laiz L, Saiz-Jimenez C. 2005. Agromyces subbeticus sp. nov., isolated from a cave in southern Spain. Int J Syst Evol Microbiol 55:1897–1901. doi: 10.1099/ijs.0.63637-0. [DOI] [PubMed] [Google Scholar]
- 49.Jurado V, Boiron P, Kroppenstedt RM, Laurent F, Couble A, Laiz L, Klenk HP, Gonzalez JM, Saiz-Jimenez C, Mouniee D, Bergeron E, Rodriguez-Nava V. 2008. Nocardia altamirensis sp. nov., isolated from Altamira Cave, Cantabria, Spain. Int J Syst Evol Microbiol 58:2210–2214. doi: 10.1099/ijs.0.65482-0. [DOI] [PubMed] [Google Scholar]
- 50.Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L. 2008. Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274:1–14. doi: 10.1016/j.aquaculture.2007.11.019. [DOI] [Google Scholar]
- 51.Fuller R. 1991. Probiotics in human medicine. Gut 32:439. doi: 10.1136/gut.32.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunz TH, Kurta A. 1988. Capture methods and holding devices, p 1–29. In Kunz TH. (ed), Ecological and behavioral methods for the study of bats. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- 53.Racey PA. 1982. Ecology of bat reproduction, p 57–104. In Kunz TH. (ed), Ecology of bats. Plenum Press, New York, NY. [Google Scholar]
- 54.Reichard JD, Kunz TH. 2009. White-nose syndrome inflicts lasting injuries to the wings of little brown myotis (Myotis lucifugus) Acta Chiropterol 11:457–464. [Google Scholar]
- 55.Hoffmeister DR. 1986. Mammals of Arizona. University of Arizona Press, Tuscon, AZ. [Google Scholar]
- 56.Sikes RS, Gannon WL, Animal Care and Use Committee of the American Society of Mammalogists . 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal 92:235–253. doi: 10.1644/10-MAMM-F-355.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellison LE, Valdez EW, Cryan PM, O'Shea TJ, Bogan MA. 2013. Standard operating procedure for the study of bats in the field. Fort Collins Science Center, Fort Collins, CO. [Google Scholar]
- 58.Hayakawa M, Momose Y, Yamazaki T, Nonomura H. 1996. A method for the selective isolation of Microtetraspora glauca and related four-spored actinomycetes from soil. J Appl Bacteriol 80:375–386. doi: 10.1111/j.1365-2672.1996.tb03232.x. [DOI] [Google Scholar]
- 59.Mueller GM, Bills GF, Foster MS. 2004. Biodiversity of fungi: inventory and monitoring methods. Elsevier Academic, Amsterdam, The Netherlands. [Google Scholar]
- 60.Hayakawa M, Nonomura H. 1987. Humic acid-vitamin agar, a new medium for the selective isolation of soil actinomycetes. J Ferment Technol 65:501–509. doi: 10.1016/0385-6380(87)90108-7. [DOI] [Google Scholar]
- 61.Lane DJ. 1991. 16S/23S rRNA sequencing, p 125–175. In Stackebrandt E, Goodfellow M (ed), Nucleic acid techniques in bacterial systematics, vol 5 John Wiley, New York, NY. [Google Scholar]
- 62.Gawel NJ, Jarret RL. 1991. A modified CTAB DNA extraction procedure for Musa and Ipomoea. Plant Mol Biol Rep 9:262–266. doi: 10.1007/BF02672076. [DOI] [Google Scholar]
- 63.Jolley KA, Maiden MCJ. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:1. doi: 10.1186/1471-2105-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. Oxford University Press, New York, NY. [Google Scholar]
- 68.Tamura K, Nei M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526. [DOI] [PubMed] [Google Scholar]
- 69.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- 70.Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 71.Montano ET, Henderson LO. 2012. Studies of antibiotic production by cave bacteria. Microbiology 1:109–130. [Google Scholar]
- 72.Espinel-Ingroff A, Bartlett M, Bowden R, Chin NX, Cooper C Jr, Fothergill A, McGinnis MR, Menezes P, Messer SA, Nelson PW, Odds FC, Pasarell L, Peter J, Pfaller MA, Rex JH, Rinaldi MG, Shankland GS, Walsh TJ, Weitzman I. 1997. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol 35:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gargas A, Trest MT, Christensen M, Volk TJ, Blehert DS. 2009. Geomyces destructans sp. nov. associated with bat white-nose syndrome. Mycotaxon 108:147–154. doi: 10.5248/108.147. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.