Abstract
Background
During pediatric cardiopulmonary resuscitation (CPR), vasoactive drug preparation for continuous infusion is both complex and time-consuming, placing children at higher risk than adults for medication errors. Following an evidence-based ergonomic-driven approach, we developed a mobile device app called Pediatric Accurate Medication in Emergency Situations (PedAMINES), intended to guide caregivers step-by-step from preparation to delivery of drugs requiring continuous infusion.
Objective
The aim of our study was to determine whether the use of PedAMINES reduces drug preparation time (TDP) and time to delivery (TDD; primary outcome), as well as medication errors (secondary outcomes) when compared with conventional preparation methods.
Methods
The study was a randomized controlled crossover trial with 2 parallel groups comparing PedAMINES with a conventional and internationally used drugs infusion rate table in the preparation of continuous drug infusion. We used a simulation-based pediatric CPR cardiac arrest scenario with a high-fidelity manikin in the shock room of a tertiary care pediatric emergency department. After epinephrine-induced return of spontaneous circulation, pediatric emergency nurses were first asked to prepare a continuous infusion of dopamine, using either PedAMINES (intervention group) or the infusion table (control group), and second, a continuous infusion of norepinephrine by crossing the procedure. The primary outcome was the elapsed time in seconds, in each allocation group, from the oral prescription by the physician to TDD by the nurse. TDD included TDP. The secondary outcome was the medication dosage error rate during the sequence from drug preparation to drug injection.
Results
A total of 20 nurses were randomized into 2 groups. During the first study period, mean TDP while using PedAMINES and conventional preparation methods was 128.1 s (95% CI 102-154) and 308.1 s (95% CI 216-400), respectively (180 s reduction, P=.002). Mean TDD was 214 s (95% CI 171-256) and 391 s (95% CI 298-483), respectively (177.3 s reduction, P=.002). Medication errors were reduced from 70% to 0% (P<.001) by using PedAMINES when compared with conventional methods.
Conclusions
In this simulation-based study, PedAMINES dramatically reduced TDP, to delivery and the rate of medication errors.
Keywords: resuscitation, medication errors, pharmaceutical preparations, pediatrics, biomedical technology
Introduction
Immediate (level 1) triage represents 175,000 patient visits every year in US pediatric emergency departments (PED) [1]. Among them, 6700 to 15,000 cases are due to out-of-hospital cardiac arrest (OHCA) [2-4], including 6000 related to nontraumatic origins [5], and 5800 to 10,000 to in-hospital cardiac arrest (INHCA) [6,7]. In our institution in 2014, cardiopulmonary resuscitation (CPR) accounted for 0.5% of almost 28,000 pediatric visits (0-15 years old). In CPR, time is a decisive success criterion. During the first 15 min, survival and favorable neurological outcome decrease linearly by 2.1% and 1.2% per minute, respectively [8], and are negatively affected by drug preparation (TDP) and delivery time (TDD) [9]. In a study with adults in cardiac arrest, the chance of return of spontaneous circulation (ROSC) was decreased by 4% for every 1-min delay in delivery of vasopressor [10].
Prolonged resuscitation time may result from TDP [11]. During some critical situations such as postcardiac arrest ROSC or septic shock, preparing intravenous (IV) vasoactive drugs for continuous infusion is particularly challenging. Quickly, accurately, and safely preparing and administering drugs in a stressful environment is complex and time-consuming [12-14]. The need for individual specific weight-based drug dose calculation and preparation and a lower dosing-error tolerance [15] place children at higher risk than adults for errors [16-18] and may result in life-threatening outcomes. Medication errors have been reported in up to 41% of pediatric resuscitations, the most common being incorrect medication dosage found in up to 65% of cases [19]. Proper preparation and delivery of these drugs could favorably affect the pediatric CPR outcomes.
To address these problems, we followed a cognitive and evidence-based ergonomic driven approach [20] to develop an innovative and customizable tablet app, called Pediatric Accurate Medication in Emergency Situations (PedAMINES). This app was designed to support nurses and physicians step-by-step from order to delivery of a wide range of drugs in real time, including those requiring continuous infusion [21]. The development of the app was followed by a study aiming to assess its impact on the error rate and time needed from drug prescription to administration. We hypothesized that PedAMINES would first reduce the TDP and TDD, and second, reduce medication errors during pediatric CPRs when compared with conventional preparation methods.
Methods
Study Design
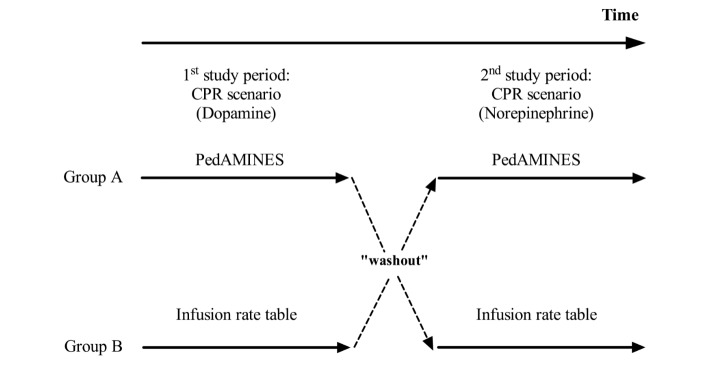
The study was a prospective, randomized controlled crossover trial with 2 parallel groups (Figure 1) comparing PedAMINES [21] with a conventional and internationally used drugs infusion rate table method (Frank Shann conventional drug infusion rate table [22]; Multimedia Appendix 1) in the preparation of continuous drug infusion, during a standardized simulation-based pediatric postcardiac arrest scenario. No changes were made on the app or on the intervention during the study.
Figure 1.
Study design: A two-period, randomized controlled, two-arm crossover study.
Selection of Participants
Certified pediatric emergency nurses were eligible if they were actively practicing in our PED. Shift-working nurses were randomly recruited on the day of the study by a blinded, noninvestigator, person on a random list. Written informed consent was obtained from all the participants before their voluntary involvement. Study participants were neither involved in the design of the app, nor in the study design, choice of outcome measures, or the study conduct. A senior specialized nurse in pediatric emergency medicine, simulation, and teaching, being an investigator of the study, has participated in the app and study design. No participants were asked to advice on interpretation or writing up of results. Results of the study were disseminated to the participants after the completion of the study.
Setting
The study was conducted in a PED of a tertiary hospital with approximately 28,000 visits per year. The PedAMINES app lists all the available resuscitation drugs, with doses automatically adapted to the weight or age of the patient. Evidence-based development of tools is an efficient way to develop apps that support clinicians [20]. The development of PedAMINES followed a user-centered approach with emergency department (ED) caregivers, as well as software developers and ergonomists. This team worked tightly together and the app development was mainly based on CPR observations and focus groups [21]. In this study, 6 drugs for continuous infusion and 19 drugs for direct IV injection were listed in the PedAMINES app and at the nurse’s disposal. The list can be expanded and customized according to users’ desires and to local drugs habits. By a simple touch, any of the listed drugs can be selected and its preparation detailed according to a standardized and simplified path. In the case of a continuous infusion, this path is composed of 3 steps: (1) drug selection, (2) dilution of the initial drug concentration, and (3) conversion of the prescribed dose rate in μg/kg/min into infusion pump rate in ml/h. For each drug, the exact amount to prepare is clearly displayed and thus avoids the necessity for calculations (Figure 2). This is based on the app’s ability to automatically calculate the optimal weight-based final infusion pump rate and to describe the preparation sequence required to achieve it, independently of the nurse competency in this domain. The nurse may, at any time, interact with the app. The user can start, pause, stop, increase, or diminish the perfusion rate. Multiple drugs can be prepared and run in parallel. All actions performed by the nurses were sequentially saved locally on the device in historic files to preserve information that can be retrieved at any time for debriefing or medicolegal purposes. Historic files can also be erased or safely exported and saved on the institutional electronic health record.
Figure 2.
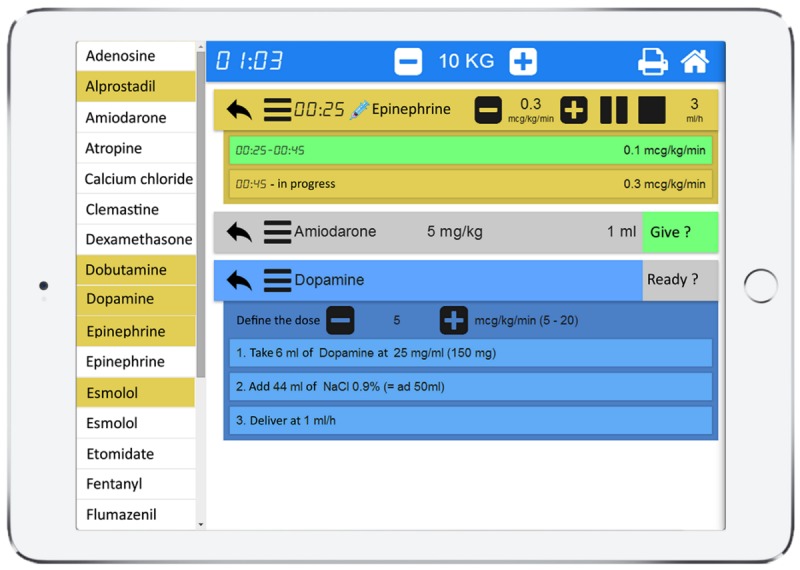
PedAMINES screenshot. List of bolus IV drugs (white boxes) and drugs for continuous infusion (yellow boxes) are selectable in the left margin of the application. The right window shows drugs selected by the nurse for a ten kilograms child. In this screenshot example, epinephrine is being delivered at an infusion rate of 0.3 mcg/kg/min. Amiodarone is selected and ready to be injected, waiting for nurse’s confirmation. Dopamine is being prepared by the nurse following a descriptive and detailed path automatically calculated by the application. The printer logo in the upper right corner indicates that all actions performed by the nurses are sequentially saved in historic files that can be retrieved and printed at any time.
Intervention and Resuscitation Scenario
We created a standardized simulation scenario on a high-fidelity manikin (Laerdal SimBaby) in the pediatric shock room of our PED. Consistent with standard emergency medicine practice, we created resuscitation teams comprising the study-participating nurse, an emergency pediatrician leading the resuscitation and being a Pediatric Advanced Life Support (PALS) instructor, and a second nurse (both part of the investigators team) to assist with resuscitation by performing chest compressions and bag-valve-mask ventilation according to the pediatrician instructions. A certified technician operated the simulator. Except for the participating nurse, the members of the resuscitation team were unchanged across all the scenarios and were the investigators of the study.
On the day of participation, nurses were given a survey collecting data regarding their demographics, nursing, and computer experience. After random allocation, each participating nurse received a standardized 5-min training session on how to use the app PedAMINES to familiarize them uniformly with it. Then, the nurses were asked to perform a 15-min highly realistic CPR scenario, including postreturn of spontaneous circulation (ROSC). It was standardized to follow the same chronological progression and provided on the same manikin. The scenario was conducted in situ in our shock room to increase realism. When entering the shock room, the nurses were asked to assist the physician to perform a 2-min full course massage and ventilation (15:2 ratio) maneuver for a child with asystole to increase their own stress level. On the basis of the American Heart Association (AHA), pediatric cardiac arrest algorithm for asystole, a bolus of 0.01 mg/kg epinephrine (0.1 mL/kg of 1:10,000 concentration) was administered. ROSC ensued with hypotension. At that time, a clinical statement to recognize the life-threatening condition of the patient, including his weight and age, was given to the nurses. The nurses were then asked to prepare a 5 μg/kg/min continuous infusion of dopamine for a 7-kg boy either with the help of PedAMINES first (allocation group A) or following the conventional method first (ie, Shann infusion rate table [22], group B; Multimedia Appendix 1). All participants had equal experience and competence with the Shann method. At the end of the instruction, the timed scenario began. To ensure that participants heard and understood the order correctly, they had to confirm it verbally, and written transcriptions were checked and video-recorded. When the drug was ready to be injected, the nurse was asked to deliver it to the patient using a syringe pump already in place. The nurse was then asked to perform a 1-min “washout” distraction maneuver by aspirating secretions in the throat of the manikin. At this moment, the crossover occurred. The nurse was asked to prepare a 0.1 μg/kg/min continuous infusion of norepinephrine by crossing the procedure (ie, group A allocation having used PedAMINES before was asked to use the conventional method, whereas group B allocation having used the conventional method before was asked to use PedAMINES; Figure 1). At the end of the order, the nurse was asked to prepare the drug. Given that norepinephrine preparation could present a challenge by requiring a more complex decimal-point dependent calculation with the Shann method, we rendered the tasks uniform by ordering a decimal-point final volume calculation for both Frank Shann and PedAMINES preparation methods. When the drug was ready to be injected, the nurse was asked to deliver it to the patient using a second syringe pump already in place. The delivery of both drugs required programming the same pump in a similar manner among all participants. Time elapsed after drug preparation until its delivery, that is, time needed to set up the pump, was assessed for all participants to ensure uniformity among participants. The measured deviation between the amount of drug delivered and the actual prescribed dose was given by the amount of drug in the syringe. This was verified by an examiner during the scenario and video-recorded. The beginning of the injection corresponded to the end of the scenario. At that time, the nurse had to recall and describe precisely how she prepared both the drugs.
Outcome Measures
The primary outcome was the elapsed time in seconds, in each allocation group, between the oral prescription by the physician and TDD by the nurse. TDD included TDP completion by the nurse. The secondary outcome was the medication dosage error rate in each allocation group, during the sequence from drug preparation to drug injection. Regarding both outcomes, we considered a 15 s difference in delivery of resuscitation drugs [23], and a 30% difference in the rate of medication errors to be clinically significant and sufficient to modify practice. At the end of the scenario, a 2 question questionnaire using a 10-point Likert scale (scored from 1 to 10 to avoid neutral answers) was submitted to participants. The questionnaire measured (1) the overall stress perceived (the question was “On a scale of 1 to 10, how much stress did you feel during the whole resuscitation scenario?”) and (2) the satisfaction about the preparation method used during the resuscitation scenario (the question was “On a scale of 1 to 10, how much satisfaction did you get during the resuscitation scenario with the help of PedAMINES, and with the help of the infusion rate table?”).
Methods of Measurement and Data Collection
Data collected during the scenario included (1) TDP, (2) TDD, and (3) final delivered drug concentration in μg/ml and infusion rate in μg/kg/min. All the actions (ie, primary and secondary outcomes) performed by the nurses during the scenario were automatically recorded and stored by the responsive simulator detectors and by several video cameras. To avoid assessment bias, 2 evaluators then independently reviewed these video recordings. In case of disagreement, a third independent evaluator helped reach a consensus. All actions performed with PedAMINES were automatically saved locally in log files for further analysis. Data were manually retrieved and entered into a Microsoft Excel spreadsheet (version 2011). The statistical software GraphPad Prism version 6.0h (GraphPad Software, Inc) was used for graph figures. Stata/IC version 14 (StataCorp) was used for descriptive analyses, and R version 2.15.2 (R Foundation for statistical computing) was used for statistical tests and 95% CI.
Sample Size
The primary objective of this study was to detect a difference in TDD of vasoactive drugs between the 2 groups. The sample size was calculated to detect a 15 s decrease in TDD between 2 independent groups with a power of 90% and a 2-sided risk alpha of .05. A previous study with pediatric emergency nurses has shown a median TDD of 69 s for the first dose of vasoactive drug to be given as direct IV infusion [23]. Assuming a standard deviation (SD) of 9 s for TDD in each group (based on a similar SD of 10 s estimated by Moreira et al [23]), 9 participants per group were required. To prevent a potential loss of power due to misspecification of assumptions, it was necessary to recruit 10 nurses per group (total sample size: 20 nurses). In case of a carryover effect, this sample size calculation was sufficient to evaluate PedAMINES’s effect within the first period of the trial.
Randomization and Blinding
We randomly assigned nurses in a 1:1 ratio with a Web-based software [24]. Blinding to the purpose of the study during recruitment was maintained to minimize preparation bias. Nurses were unblinded after randomization. Allocation concealment was ensured with sealed envelopes and was not released until the nurses started the scenario.
Statistical Analysis
Primary Outcome
For TDD (and TDP), the mean times were reported with 95% CI for each arm and each study period to investigate a potential carryover effect. As a carryover effect was suspected, intervention arms were compared within each study period using t tests for independent groups. No paired data were compared.
Secondary Outcomes
The rate of medication errors was the proportion of nurses making a preparation error. The rate of medication errors was reported with 95% CI (Clopper-Pearson method) with each method and by study period to investigate a potential carryover effect. The error rates for each method were globally assessed and compared using McNemar test as observations were paired. Differences in error rates were reported with 95% CIs.
Errors were also measured as the deviation in percent from the amount of delivered drug compared with the original dose prescribed by the physician. Absolute deviation was analyzed. The mean (SD) difference in deviation obtained with each method was reported with 95% CI. A t test for paired data was used to compare interventions. Mean differences were also reported by randomized group and by crossover period. Means and SDs were determined for stress and satisfaction scores of individuals for each questionnaire item and reported with descriptive statistics.
Ethics and Informed Consent
The study was approved by the institutional ethics committee and a trial registration number was not required. If we regard simulation as a translational science with outcomes categorized as T1 (results achieved in the simulated setting), T2 (improved health care delivery in the real clinical setting), and T3 (improved patient outcomes), our trial was a T-1 level study and, as for many other simulation studies, did not require registration according to ICMJE definition. Written informed consent was obtained from all participants before their voluntary involvement. It was conducted in accordance with the principles of the Declaration of Helsinki, the standards of Good Clinical Practice, and Swiss regulatory requirements.
Results
Study Participants
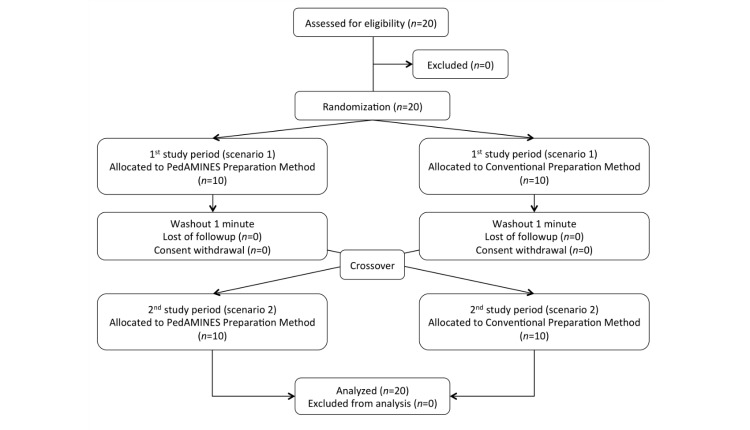
In June 2015, 20 certified pediatric emergency nurses participated and completed the study with no dropout (Figure 3). The demographic results are summarized in Table 1.
Figure 3.
Patient flowchart for randomized controlled trial on preparation of continuous drug infusion by nurses in simulation-based pediatric cardio-pulmonary resuscitation cardiac arrest scenario.
Table 1.
Participants’ demographic and clinical characteristics.
| Demographics and clinical characteristics | Randomization arm (first study period) | |
|
|
PedAMINESa (n=10) | Infusion rate table (n=10) |
| Age (years), mean (SD) | 42.9 (6.4) | 42.4 (10.5) |
| Sex (female), n (%) | 8 (80) | 7 (70) |
| Number of years since nurse certification, mean (SD) | 19.2 (7.7) | 18.5 (11.7) |
| Number of years since pediatric EDb certification, mean (SD) | 9.9 (6.4) | 9.8 (6.5) |
| Own and use a tab or mobile phone at home, n (%) | 9 (90) | 9 (90) |
| CPRsc having required vasoactive drugs Preparation for continuous infusion in the past 3 years, median (interquartile range) | 2.0 (0.0-2.30) | 1.0 (1.0-2.0) |
| Simulated CPR scenarios in the past 3 years, median (interquartile range) | 7.0 (3.8-12.0) | 5.5 (3.8-8.3) |
aPedAMINES: Pediatric Accurate Medication in Emergency Situations.
bED: emergency department.
cCPR: cardiopulmonary resuscitation.
TDP and TDD
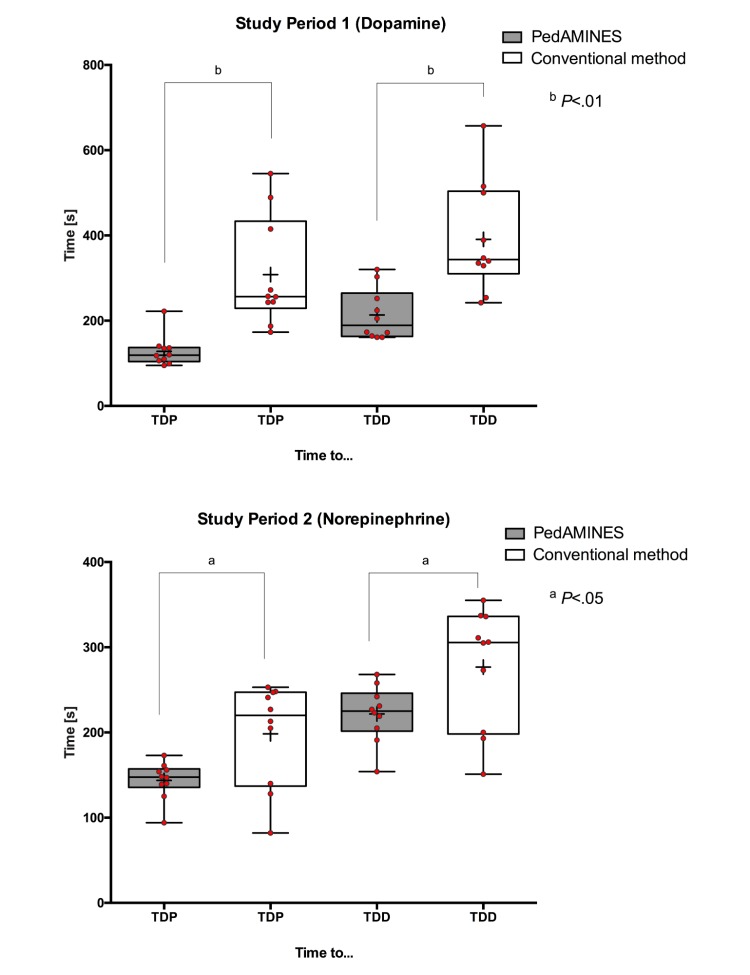
When using PedAMINES, the mean time either from drug prescription to preparation or to delivery was nearly equivalent in both study periods (128.1 s vs 143.7 s or 213.5 s vs 221.8 s, P=.71; Table 2). Using the conventional method, the mean time from prescription to preparation or to delivery was lower in the second period than in the first period (308.1 s vs 198.4 s, P=.03 and 390.8 s vs 276.7 s, P=.03, respectively; Table 2). These findings raised the suspicion of a carryover effect and comparisons between interventions were conducted for each period separately. In the first study period, the TDP was decreased by 180.0 s (95% CI 86.5-273.5, P=.002) with PedAMINES and TDD by 177.3 s (95% CI 79.7-274.9, P=.002; Figure 4). These gains in time represented 58% and 45% of the mean time, respectively, when using the conventional methods. The variability of individual recorded times was lower with PedAMINES (TDP upper range value=222 s, TDD=320 s) than with the conventional method (TDP upper range value=545 s [8 out of 10 measures were higher than 222], TDD=657 s; Figure 4). In the second study period, the TDP was decreased by 54.7 s (95% CI 10.3-99.1, P=.02) with PedAMINES and TDD by 54.9 s (95% CI 1.6-108.2, P=.04; Figure 4).
Table 2.
Mean time in seconds to drugs preparation and delivery.
| Mean time | First study period (Dopamine) | Second study period (Norepinephrine) | ||
|
|
PedAMINESa mean (95% CI) |
Conventional method mean (95% CI) |
PedAMINES mean (95% CI) |
Conventional method mean (95% CI) |
| TDPb | 128.1 (102.0-154.2) | 308.1 (216.3-399.9) | 143.7 (128.1-159.3) | 198.4 (155.3-241.5) |
| SD | 36.5 | 128.3 | 21.9 | 60.2 |
| Time differencec | 180.0 (86.5-273.5) | 54.7 (10.3-99.1) | ||
| TDDd | 213.5 (170.6-256.4) | 390.8 (298.3-483.3) | 221.8 (198.2-245.4) | 276.7 (226.2-327.2) |
| SD | 59.9 | 129.3 | 33.0 | 70.6 |
| Time differencec,e | 177.3 (79.7-274.9) | 54.9 (1.6-108.2) | ||
aPedAMINES: Pediatric Accurate Medication in Emergency Situations.
bTDP: time to drug preparation.
cTime difference represents time with the conventional method minus time with PedAMINES.
dTDD: time to drug delivery.
eSee Multimedia Appendix 2 for TDP and TDD details for each nurse and for each drug, by study period.
Figure 4.
Study period 1 (Dopamine) and 2 (Norepinephrine). Boxplots of elapsed time to drug preparation (TDP) and to drug delivery (TDD) in intervention group (PedAMINES) and control group (conventional method). Solid horizontal lines denote median and interquartile ranges; the whiskers go down to the smallest value and up to the largest; + denotes mean. Red open circles denote each individual value. Time is expressed in seconds.
Medication Error Rate
As data did not support a carryover effect in the medication error rate, both the study periods were pooled. Of the 20 drug doses delivered with PedAMINES, none (0%) was associated with medication errors. Of the 20 drug doses prepared with conventional methods, 14 were incorrect (70%; 95% CI 45.7-88.1, P<.001). Among the 8 errors committed during the first study period, 5 (63%, 5/8) were critical overdose errors ranging from 100% to 5233% (mean 1864%) of the normal prescribed dose (Table 3). Among the 6 errors committed during the second study period, 3 (50%, 3/6) ranged from 19% to 138% (mean 59%) of the normal prescribed dose and 2 (33%, 2/6) miscalculated preparations reached the right final dosage by chance (Table 3).
Table 3.
Drug doses errors and deviation from the prescribed doses.
| Outcomes | First study period (Dopamine) | Second study period (Norepinephrine)a | ||
|
|
PedAMINESb n (%), 95% CI |
Conventional method n (%), 95% CI |
Conventional method n (%), 95% CI |
PedAMINES n (%), 95% CI |
| All medication errorsc (n=10) | 0 (0), 0-31 | 8 (80), 44-98 | 6 (60), 26-88 | 0 (0), 0-31 |
| Unpaired medication errors difference (n=10) | 8 (80), 41.4-97.5 | 6 (60), 21.3-88.5 | ||
| Paired medication errors difference (N=20) | 14 (70), 42.2-88.1 | |||
aSee Multimedia Appendix 3 for drug doses errors details for each nurse and for each drug by study period.
bPedAMINES: Pediatric Accurate Medication in Emergency Situations.
cProportion of dosage with an error, where n denotes the number of drug dose errors that actually occurred and N denotes the total number of opportunities for errors to occur.
Questionnaire About Perceived Stress and Satisfaction
The questionnaire was completed and returned by 100% of the participants. Participants rated the overall perceived stress to be 7.1 (95% CI 6.1-8.1) on the Likert scale. They reported higher satisfaction when using PedAMINES for the preparation of drugs rather than conventional methods (9.3 [SD 1.2] vs 3.6 [SD 2.1], P<.001).
Discussion
Principal Findings
To our knowledge, this is the first study to investigate the benefit of a mobile app to improve delivery of continuous drug infusion during pediatric CPR. We found that TDP and TDD of vasoactive drugs for continuous infusion, as well as medication errors were dramatically reduced with the use of PedAMINES.
CPR is a continuum from the onset of resuscitation to immediate post-resuscitation care. Survival rate for CPR lasting less than 15 min is estimated at 41%, decreasing to 12% after 35 min [8]. A recent study reported that average time spent in the pediatric shock room was 46 min [25]. Care for postcardiac arrest patients is also time-sensitive. Postcardiac arrest syndrome (PCAS), characterized by brain injury, myocardial dysfunction, systemic ischemia or reperfusion response, and persistent precipitating disease, can develop precociously with poor outcome after ROSC [26,27]. Post-ROSC in-hospital mortality rates in children after nontraumatic OHCA or INHCA were estimated to be 70% [28] and 55% [29], respectively. If ROSC is quickly achieved and maintained after the onset of cardiac arrest, PCAS might be prevented [30]. Thus, early hemodynamic optimization improves the outcome of these patients [31,32]. The 2015 AHA guidelines recommended starting IV fluids and vasoactive drugs early in the postarrest phase, targeting a systolic blood pressure above the fifth percentile for age [33]. Commonly employed drugs for continuous infusion include dopamine (5-20 mcg/kg/min), norepinephrine, and epinephrine (both at 0.01-1 mcg/kg/min). These drugs should be available quickly. Moreira et al reported that the use of prefilled color-coded syringes during pediatric cardiac arrest can reduce the TDD of IV drugs by 27 s [23]. However, these results were limited to direct IV pushes requiring no prior preparation. Our work demonstrates that it is possible to drastically decrease TDD for IV drugs requiring complex upstream preparations and continuous infusion during the immediate postarrest phase. In our study, mean TDP, as well as mean TDD were significantly reduced by almost 180 s with PedAMINES. Even when considering the lower margins of errors of the CI, PedAMINES was still able to reduce TDP and TDD by approximately 1.5 min (4.5 min at the upper margins) and was largely higher than the minimal difference we set out to find when establishing the sample size. The interindividual upper time ranges were reduced by using the app, suggesting a worthwhile benefit if used in smaller hospitals where nurses and physicians are either rarely or not exposed to CPR, but have to use resuscitative drugs before patient transfer to a tertiary care center.
Although TDP and TDD were similar in the primary and secondary study periods when using PedAMINES, the gain provided by PedAMINES was less pronounced in the second study period, because TDP and TDD with the conventional method were lower among nurses with a PedAMINES experience than among nurses who had never used PedAMINES. The 1-min washout period may not have been long enough. Initially, we sought to estimate the effect of PedAMINES in the first study period. We wanted to determine whether changing habits with the use of our app by naïve nurses hitherto accustomed to a conventional preparation method resulted in a significant gain of time on the preparation of drugs compared with nurses using the conventional method. The second study period was intended to increase the overall power of our study. However, in this second period, the nurses could not be considered in exactly the same way as in the first period. Indeed, in the second period, we compared nurses who returned with a conventional preparation method after having used PedAMINES versus naïve nurses hitherto accustomed to a conventional preparation method that switched for PedAMINES for the first time. In any case, the intervention had a large impact in both periods. Given the carryover effect, the first study period was chosen as the most representative for the influence of PedAMINES on the primary outcome.
Medication errors are common in pediatric patients, accounting for 5-27% of all pediatric prescriptions and causing significant mortality and morbidity, including 7000 patient deaths each year in the United States [34]. One in every 32 prescriptions in a PED contains a 10-fold error on the recommended dose [17]. Kozer et al showed an error rate of 17% at the prescription level during pediatric resuscitations, with up to 10 times the recommended pediatric dose in more than 3% of these cases [35]. In the same study, 16% of the analyzed syringes showed a 20% dose deviation from the prescribed dose and up to 7% showed a deviation of more than 50%. Medication errors with infusions frequently result from mistake during preparation, due to wrong drug-volume calculations, imprecision with volume measurements or incorrect mixing during dilution [11,13,36]. Available conversion methods, such as infusion rate tables or nomograms [37], designed to simplify infusion rate calculation still remain difficult to use and subject to medication errors. Even small errors either in drug calculation or infusion pump flow rate may have a large detrimental impact on the amount of drug delivered [38-40]. This can be harmful for critical patients and even prove fatal [17]. In a study reporting 41% medication errors in pediatric CPRs, the most common error was drug dosage (65% of cases) [19]. Similarly, we found that using the conventional preparation method resulted in dosing errors in 70% of the preparations. Among them, 30% deviated from the original prescribed dose from 100% up to 5233% (ie, 2-fold to 53-fold overdose errors). Disruptive anxiety and exogenous conditions encountered during resuscitation increase the nurse’s cognitive workload and the risk of errors. The cognitive workload has also been shown to be higher when the task is less familiar, as typically seen during CPR [41], which remains uncommon in PED. For instance, at our PED, level one triage status patients represent 0.5% of the ED visits, a proportion similar to the 0.7% (+/- 0.2) seen in the US PED [1]. The lack of practice due to the rareness of certain pathologies or inexperience among the staff regarding vasoactive drugs preparation may add to the complexity of this phenomenon. Some authors have advocated replacing tasks requiring cognitive load during CPR by automated actions [23,41] as much as possible. By guaranteeing an automated, fast, and reliable conversion and preparation, medication errors were totally avoided using PedAMINES. It should be noted that we observed fewer medication errors in the control arm during the second study period, with only a single critical error of more than 20%. This was may be due to the higher number of vials required to prepare norepinephrine (3 vials) compared with that for dopamine (1 vial) or that nurses were a bit more trained (having just done the first scenario). While they had no impact on TDP and TDD, these additional steps of preparation have perhaps limited the rate of errors by strengthening the controls at each preparation step. As tasks were uniform, the complexity of dopamine and norepinephrine preparation by using either PedAMINES or Frank Shann method was similar and not expected to be responsible for the different rates of errors.
Limitations
This study has some limitations. First, it was conducted during a resuscitation simulation-based scenario. This choice was related to the ethical and organizational difficulties of conducting studies with patients in critical situations. However, several studies have demonstrated the benefit of simulation as an investigative research methodology to answer research questions that otherwise could not be answered during CPR [42]. Interindividual diversity among patients and their diseases make CPR studies hard to standardize. Simulation-based CPR scenarios may overcome these limitations by providing a standardized and controlled environment, detailed feedback analysis of the resuscitation stages using audiovisual recordings, and reproducibility. Therefore, high-fidelity simulation has become essential to study resuscitations skills or technologies. We acknowledge that a simulation cannot reveal whether the intervention improves clinical outcome. However, till date, none of the results obtained from simulation-based CPR studies disagreed with those obtained from studies in real life, confirming our study design choice. Although the survival rate has complex and numerous components, it would be interesting to determine in further studies whether saving time and decreasing medication errors owing to the use of PedAMINES would translate into increase in patient survival in real life.
Second, the lack of immersive realism provided during simulated scenarios might directly affect the assessed outcomes. In this study, we used a high-fidelity manikin simulator, which is currently and widely recognized as providing the most realistic and high-yield immersive environment achievable in emergency training [43]. Realism in our study was reflected by the stress levels experienced by the participants. They quoted the simulation as highly stressful when compared with real CPR situations.
Third, accuracy of intervention delivery times could also limit some CPR studies when assessing “times to” outcomes. Conventional paper-based documentation practices used during resuscitation—simulated or not—are often unreliable and inaccurate, either by misreporting intervention delivery times or by missing the delivery completely [44]. In this study, all drug preparation times were chronologically saved in historic log files and videorecorded, rendering our outcomes highly accurate.
Fourth, many different methods are used in PEDs to prepare and administer infusion drugs such as the Frank Shann method or Broselow pediatric resuscitation medication or infusion guide. They all commonly require the user to perform some kind of calculation that may lead to errors and a longer TDP. As described by many authors, the Broselow pediatric emergency tape and affiliate pediatric resuscitation medication or infusion guide have been used with mixed results in many countries and are somehow inaccurate to predict actual weight in almost 20-30% of children, especially for under- and overweight children [45-52]. This may lead to medical errors due to incorrect dosing selection. In 2007, the American Academy of Pediatrics committee on Pediatric Emergency Medicine acknowledged, “although helpful, the Broselow tape is not ideal” [53]. Frank Shann method offers the possibility to precisely adjust the drug doses on patient’s actual weights and is used in many hospitals worldwide. This was the rationale to use it as a comparator in our study.
Finally, our study was not intended to compare PedAMINES with “smart” IV pumps. Standardizing drug concentrations of premixed drug and varying infusion rates with smart pumps implicate to deal with poor dose and rate precision in already unstable and critically ill children. As recently reported, no conclusive evidence shows that smart pumps do prevent medication errors and adverse drug events [54,55]. In addition, little is known about the kind of errors that still occur with their use. Moreover, the number of premixed drugs required, their chemical stability over time [56-59] and the lack of specialized pharmacy facility in many smaller hospitals or in other countries around the world, limit their use. Further studies comparing PedAMINES and smart pumps would be valuable.
Conclusions
In summary, compared with a conventional and internationally used preparation method, we found that a mobile app developed following an evidence-based ergonomic-driven approach dramatically reduced TDP and TDD, as well as the medication error rate. The interindividual variance was also reduced by using the app, suggesting a worthwhile benefit to its use by nurses with different experience levels. A large multicenter randomized trial is further needed to assess this assumption in primary and secondary care hospitals.
Acknowledgments
We thank all the study participants for their contribution. All authors declare no source of funding, no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work, and no other relationships or activities that could appear to have influenced the submitted work.
Abbreviations
- CPR
cardiopulmonary resuscitation
- ED
emergency department
- PED
pediatric emergency department
- PedAMINES
Pediatric Accurate Medication in Emergency Situations
- TDD
time to drug delivery
- TDP
time to drug preparation
Frank Shann Drug Doses.
The table details the time expressed in seconds to drug preparation (TDP) and delivery (TDD) for each nurse and for each drug, by study period.
The table details the deviation expressed in percent in drug dosage (overdose) from the prescribed dose for each nurse and for each drug, by study period.
CONSORT-EHEALTH checklist V1.6.1 [60].
Footnotes
Conflicts of Interest: Some authors are the owners of the app PedAMINES that will be available in the near future on the Google Play Store and the Apple Store. The authors therefore declare a direct financial interest to market this app.
Authors' Contributions: JNS and SM did the literature search and reading. JNS wrote the manuscript and prepared the figures and tables. JNS, FE, CC, KH, and SM collected data and carried the statistical analysis. JNS, FE, CL, and SM carried out the development of the project software. FE, CC, LL, KH, OS, AG, CL, and SM were involved in the concept and design of the study and critical review of manuscript content. All authors have contributed to, seen and approved the final, submitted version of the manuscript and had full access to all the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author confirms that he had full access to the participants’ data and endorsed the final responsibility for the submission. He further affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained. Consent to publication was obtained from the participants.
References
- 1.CDC. 2011. [2017-01-22]. National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf .
- 2.Gerein RB, Osmond MH, Stiell IG, Nesbitt LP, Burns S, OPALS Study Group What are the etiology and epidemiology of out-of-hospital pediatric cardiopulmonary arrest in Ontario, Canada? Acad Emerg Med. 2006 Jun;13(6):653–8. doi: 10.1197/j.aem.2005.12.025. http://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=1069-6563&date=2006&volume=13&issue=6&spage=653 .j.aem.2005.12.025 [DOI] [PubMed] [Google Scholar]
- 3.Kuisma M, Suominen P, Korpela R. Paediatric out-of-hospital cardiac arrests--epidemiology and outcome. Resuscitation. 1995 Oct;30(2):141–50. doi: 10.1016/0300-9572(95)00888-z.030095729500888Z [DOI] [PubMed] [Google Scholar]
- 4.Sirbaugh PE, Pepe PE, Shook JE, Kimball KT, Goldman MJ, Ward MA, Mann DM. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest. Ann Emerg Med. 1999 Feb;33(2):174–84. doi: 10.1016/s0196-0644(99)70391-4.S0196064499000438 [DOI] [PubMed] [Google Scholar]
- 5.Atkins DL, Everson-Stewart S, Sears GK, Daya M, Osmond MH, Warden CR, Berg RA, Resuscitation Outcomes Consortium Investigators Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009 Mar 24;119(11):1484–91. doi: 10.1161/CIRCULATIONAHA.108.802678. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=19273724 .CIRCULATIONAHA.108.802678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knudson JD, Neish SR, Cabrera AG, Lowry AW, Shamszad P, Morales DL, Graves DE, Williams EA, Rossano JW. Prevalence and outcomes of pediatric in-hospital cardiopulmonary resuscitation in the United States: an analysis of the Kids' Inpatient Database*. Crit Care Med. 2012 Nov;40(11):2940–4. doi: 10.1097/CCM.0b013e31825feb3f. [DOI] [PubMed] [Google Scholar]
- 7.Topjian AA, Berg RA, Nadkarni VM. Pediatric cardiopulmonary resuscitation: advances in science, techniques, and outcomes. Pediatrics. 2008 Nov;122(5):1086–98. doi: 10.1542/peds.2007-3313. http://europepmc.org/abstract/MED/18977991 .122/5/1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matos RI, Watson RS, Nadkarni VM, Huang H, Berg RA, Meaney PA, Carroll CL, Berens RJ, Praestgaard A, Weissfeld L, Spinella PC, American Heart Association’s Get With The Guidelines–Resuscitation (Formerly the National Registry of Cardiopulmonary Resuscitation) Investigators Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013 Jan 29;127(4):442–51. doi: 10.1161/CIRCULATIONAHA.112.125625.CIRCULATIONAHA.112.125625 [DOI] [PubMed] [Google Scholar]
- 9.Andersen LW, Berg KM, Saindon BZ, Massaro JM, Raymond TT, Berg RA, Nadkarni VM, Donnino MW, American Heart Association Get With the Guidelines–Resuscitation Investigators Time to Epinephrine and Survival After Pediatric In-Hospital Cardiac Arrest. J Am Med Assoc. 2015 Aug 25;314(8):802–10. doi: 10.1001/jama.2015.9678.2429714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubble MW, Johnson C, Blackwelder J, Collopy K, Houston S, Martin M, Wilkes D, Wiser J. Probability of return of spontaneous circulation as a function of timing of vasopressor administration in out-of-hospital cardiac arrest. Prehosp Emerg Care. 2015;19(4):457–63. doi: 10.3109/10903127.2015.1005262. [DOI] [PubMed] [Google Scholar]
- 11.Adapa RM, Mani V, Murray LJ, Degnan BA, Ercole A, Cadman B, Williams CE, Gupta AK, Wheeler DW. Errors during the preparation of drug infusions: a randomized controlled trial. Br J Anaesth. 2012 Nov;109(5):729–34. doi: 10.1093/bja/aes257. http://bja.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=22850220 .aes257 [DOI] [PubMed] [Google Scholar]
- 12.Polischuk E, Vetterly CG, Crowley KL, Thompson A, Goff J, Nguyen-Ha P, Modery C. Implementation of a standardized process for ordering and dispensing of high-alert emergency medication infusions. J Pediatr Pharmacol Ther. 2012 Apr;17(2):166–72. doi: 10.5863/1551-6776-17.2.166. http://europepmc.org/abstract/MED/23118669 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen GY, Parker HB, Cash J, O'Connell M, Grant MC. Standard drug concentrations and smart-pump technology reduce continuous-medication-infusion errors in pediatric patients. Pediatrics. 2005 Jul;116(1):e21–5. doi: 10.1542/peds.2004-2452.116/1/e21 [DOI] [PubMed] [Google Scholar]
- 14.Moyen E, Camiré E, Stelfox HT. Clinical review: medication errors in critical care. Crit Care. 2008;12(2):208. doi: 10.1186/cc6813. https://ccforum.biomedcentral.com/articles/10.1186/cc6813 .cc6813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, Goldmann DA. Medication errors and adverse drug events in pediatric inpatients. J Am Med Assoc. 2001 Apr 25;285(16):2114–20. doi: 10.1001/jama.285.16.2114.joc01942 [DOI] [PubMed] [Google Scholar]
- 16.Hoyle JD, Davis AT, Putman KK, Trytko JA, Fales WD. Medication dosing errors in pediatric patients treated by emergency medical services. Prehosp Emerg Care. 2012;16(1):59–66. doi: 10.3109/10903127.2011.614043. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann J, Laschat M, Wappler F. Medication errors in pediatric emergencies: a systematic analysis. Dtsch Arztebl Int. 2012 Sep;109(38):609–16. doi: 10.3238/arztebl.2012.0609. doi: 10.3238/arztebl.2012.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzales K. Medication administration errors and the pediatric population: a systematic search of the literature. J Pediatr Nurs. 2010 Dec;25(6):555–65. doi: 10.1016/j.pedn.2010.04.002.S0882-5963(10)00106-5 [DOI] [PubMed] [Google Scholar]
- 19.Porter E, Barcega B, Kim TY. Analysis of medication errors in simulated pediatric resuscitation by residents. West J Emerg Med. 2014 Jul;15(4):486–90. doi: 10.5811/westjem.2014.2.17922. http://escholarship.org/uc/item/50g528cc .wjem-15-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrler F, Haller G, Sarrey E, Walesa M, Wipfli R, Lovis C. Assessing the usability of six data entry mobile interfaces for caregivers: a randomized trial. JMIR Hum Factors. 2015 Dec 15;2(2):e15. doi: 10.2196/humanfactors.4093. http://humanfactors.jmir.org/2015/2/e15/ v2i2e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagberg H, Siebert J, Gervaix A, Daehne P, Lovis C, Manzano S, Ehrler F. Improving drugs administration safety in pediatric resuscitation using mobile technology. Stud Health Technol Inform. 2016;225:656–7. [PubMed] [Google Scholar]
- 22.Shann F. Drug Doses RCH Intensive Care Unit, 16th Edition. Melbourne: Parkville, Victoria Collective Pty. Ltd; 2014. [Google Scholar]
- 23.Moreira ME, Hernandez C, Stevens AD, Jones S, Sande M, Blumen JR, Hopkins E, Bakes K, Haukoos JS. Color-coded prefilled medication syringes decrease time to delivery and dosing error in simulated emergency department pediatric resuscitations. Ann Emerg Med. 2015 Aug;66(2):97–106.e3. doi: 10.1016/j.annemergmed.2014.12.035. http://europepmc.org/abstract/MED/25701295 .S0196-0644(14)01661-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.sealedenvelope. [2016-11-17]. Randomisation and Online Databases for Clinical Trials https://www.sealedenvelope.com .
- 25.Lutz N, Vandermensbrugghe NG, Dolci M, Amiet V, Racine L, Carron P. Pediatric emergencies admitted in the resuscitation room of a Swiss university hospital. Pediatr Emerg Care. 2014 Oct;30(10):699–704. doi: 10.1097/PEC.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 26.Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bbttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T, International Liaison Committee on Resuscitation. Emergency Cardiovascular Care Committee‚ American Heart Association. Council on Cardiovascular Surgery and Anesthesia. Council on Cardiopulmonary‚ Perioperative‚ and Critical Care. Council on Clinical Cardiology. Council on Stroke Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke (Part II) Int Emerg Nurs. 2010 Jan;18(1):8–28. doi: 10.1016/j.ienj.2009.07.001.S1755-599X(09)00053-6 [DOI] [PubMed] [Google Scholar]
- 27.Checchia PA, Sehra R, Moynihan J, Daher N, Tang W, Weil MH. Myocardial injury in children following resuscitation after cardiac arrest. Resuscitation. 2003 May;57(2):131–7. doi: 10.1016/s0300-9572(03)00003-0.S0300957203000030 [DOI] [PubMed] [Google Scholar]
- 28.Donoghue AJ, Nadkarni V, Berg RA, Osmond MH, Wells G, Nesbitt L, Stiell IG, CanAm Pediatric Cardiac Arrest Investigators Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005 Dec;46(6):512–22. doi: 10.1016/j.annemergmed.2005.05.028.S0196-0644(05)00719-5 [DOI] [PubMed] [Google Scholar]
- 29.Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, Nichol G, Lane-Truitt T, Potts J, Ornato JP, Berg RA, National Registry of Cardiopulmonary Resuscitation Investigators First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. J Am Med Assoc. 2006 Jan 4;295(1):50–7. doi: 10.1001/jama.295.1.50.295/1/50 [DOI] [PubMed] [Google Scholar]
- 30.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth Jr WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008 Dec 2;118(23):2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=18948368 .CIRCULATIONAHA.108.190652 [DOI] [PubMed] [Google Scholar]
- 31.López-Herce J, del Castillo J, Matamoros M, Canadas S, Rodriguez-Calvo A, Cecchetti C, Rodríguez-Núnez A, Carrillo Á, Iberoamerican Pediatric Cardiac Arrest Study Network RIBEPCI Post return of spontaneous circulation factors associated with mortality in pediatric in-hospital cardiac arrest: a prospective multicenter multinational observational study. Crit Care. 2014 Nov 03;18(6):607. doi: 10.1186/s13054-014-0607-9. https://ccforum.biomedcentral.com/articles/10.1186/s13054-014-0607-9 .s13054-014-0607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topjian AA, French B, Sutton RM, Conlon T, Nadkarni VM, Moler FW, Dean JM, Berg RA. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med. 2014 Jun;42(6):1518–23. doi: 10.1097/CCM.0000000000000216. http://europepmc.org/abstract/MED/24561563 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Caen AR, Berg MD, Chameides L, Gooden CK, Hickey RW, Scott HF, Sutton RM, Tijssen JA, Topjian A, van der Jagt ÉW, Schexnayder SM, Samson RA. Part 12: pediatric advanced life support: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015 Nov 3;132(18 Suppl 2):S526–42. doi: 10.1161/CIR.0000000000000266. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=26473000 .CIR.0000000000000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinke ML, Bundy DG, Velasquez CA, Rao S, Zerhouni Y, Lobner K, Blanck JF, Miller MR. Interventions to reduce pediatric medication errors: a systematic review. Pediatrics. 2014 Aug;134(2):338–60. doi: 10.1542/peds.2013-3531. http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=25022737 .peds.2013-3531 [DOI] [PubMed] [Google Scholar]
- 35.Kozer E, Seto W, Verjee Z, Parshuram C, Khattak S, Koren G, Jarvis DA. Prospective observational study on the incidence of medication errors during simulated resuscitation in a paediatric emergency department. Br Med J. 2004 Dec 4;329(7478):1321. doi: 10.1136/bmj.38244.607083.55. http://europepmc.org/abstract/MED/15454495 .bmj.38244.607083.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ameer A, Dhillon S, Peters M, Ghaleb M. Systematic literature review of hospital medication administration errors in children. Integr Pharm Res Pract. 2015 Nov;:153. doi: 10.2147/IPRP.S54998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherry E, Burton GW, Wilkins DG. Infusion nomograms. Anaesthesia. 1993 May;48(5):396–401. doi: 10.1111/j.1365-2044.1993.tb07012.x. http://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0003-2409&date=1993&volume=48&issue=5&spage=396 . [DOI] [PubMed] [Google Scholar]
- 38.Allen EM, Van Boerum DH, Olsen AF, Dean JM. Difference between the measured and ordered dose of catecholamine infusions. Ann Pharmacother. 1995 Nov;29(11):1095–100. doi: 10.1177/106002809502901104. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann CU, Kim GR, Gujral R, Veltri MA, Clark JS, Miller MR. Decreasing errors in pediatric continuous intravenous infusions. Pediatr Crit Care Med. 2006 May;7(3):225–30. doi: 10.1097/01.PCC.0000216415.12120.FF. [DOI] [PubMed] [Google Scholar]
- 40.Parshuram CS, To T, Seto W, Trope A, Koren G, Laupacis A. Systematic evaluation of errors occurring during the preparation of intravenous medication. CMAJ. 2008 Jan 1;178(1):42–8. doi: 10.1503/cmaj.061743. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=18166730 .178/1/42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luten R, Wears RL, Broselow J, Croskerry P, Joseph MM, Frush K. Managing the unique size-related issues of pediatric resuscitation: reducing cognitive load with resuscitation aids. Acad Emerg Med. 2002 Aug;9(8):840–7. doi: 10.1111/j.1553-2712.2002.tb02175.x. http://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=1069-6563&date=2002&volume=9&issue=8&spage=840 . [DOI] [PubMed] [Google Scholar]
- 42.Cheng A, Auerbach M, Hunt EA, Chang TP, Pusic M, Nadkarni V, Kessler D. Designing and conducting simulation-based research. Pediatrics. 2014 Jun;133(6):1091–101. doi: 10.1542/peds.2013-3267. http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=24819576 .peds.2013-3267 [DOI] [PubMed] [Google Scholar]
- 43.Sahu S, Lata I. Simulation in resuscitation teaching and training, an evidence based practice review. J Emerg Trauma Shock. 2010 Oct;3(4):378–84. doi: 10.4103/0974-2700.70758. http://www.onlinejets.org/article.asp?issn=0974-2700;year=2010;volume=3;issue=4;spage=378;epage=384;aulast=Sahu . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peace JM, Yuen TC, Borak MH, Edelson DP. Tablet-based cardiac arrest documentation: a pilot study. Resuscitation. 2014 Feb;85(2):266–9. doi: 10.1016/j.resuscitation.2013.10.013. http://europepmc.org/abstract/MED/24157630 .S0300-9572(13)00807-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chavez H, Peterson RE, Lo K, Arel M. Weight estimation in an inner-city pediatric ED: the effect of obesity. Am J Emerg Med. 2015 Oct;33(10):1364–7. doi: 10.1016/j.ajem.2015.07.040.S0735-6757(15)00596-3 [DOI] [PubMed] [Google Scholar]
- 46.Clark M, Lewis R, Fleischman R, Ogunniyi AA, Patel DS, Donaldson RI. Accuracy of the Broselow Tape in South Sudan, “The hungriest place on earth”. Acad Emerg Med. 2016 Jan;23(1):21–8. doi: 10.1111/acem.12854. doi: 10.1111/acem.12854. [DOI] [PubMed] [Google Scholar]
- 47.Hofer C, Ganter M, Tucci M, Klaghofer R, Zollinger A. How reliable is length-based determination of body weight and tracheal tube size in the paediatric age group? The Broselow tape reconsidered. Br J Anaesth. 2002 Feb;88(2):283–5. doi: 10.1093/bja/88.2.283. http://bja.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=11878662 . [DOI] [PubMed] [Google Scholar]
- 48.Knight J, Nazim M, Riggs D, Channel J, Mullet C, Vaughan R, Wilson A. Is the Broselow tape a reliable indicator for use in all pediatric trauma patients? A look at a rural trauma center. Pediatr Emerg Care. 2011 Jun;27(6):479–82. doi: 10.1097/PEC.0b013e31821d8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milne WK, Yasin A, Knight J, Noel D, Lubell R, Filler G. Ontario children have outgrown the Broselow tape. CJEM. 2012 Jan;14(1):25–30. doi: 10.2310/8000.2011.110523. [DOI] [PubMed] [Google Scholar]
- 50.Nieman CT, Manacci CF, Super DM, Mancuso C, Fallon WF. Use of the Broselow tape may result in the underresuscitation of children. Acad Emerg Med. 2006 Oct;13(10):1011–9. doi: 10.1197/j.aem.2006.06.042. http://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=1069-6563&date=2006&volume=13&issue=10&spage=1011 .j.aem.2006.06.042 [DOI] [PubMed] [Google Scholar]
- 51.Sinha M, Lezine MW, Frechette A, Foster KN. Weighing the pediatric patient during trauma resuscitation and its concordance with estimated weight using Broselow Luten Emergency Tape. Pediatr Emerg Care. 2012 Jun;28(6):544–7. doi: 10.1097/PEC.0b013e318258ac2e. [DOI] [PubMed] [Google Scholar]
- 52.Wells M, Coovadia A, Kramer E, Goldstein L. The PAWPER tape: a new concept tape-based device that increases the accuracy of weight estimation in children through the inclusion of a modifier based on body habitus. Resuscitation. 2013 Feb;84(2):227–32. doi: 10.1016/j.resuscitation.2012.05.028.S0300-9572(12)00340-1 [DOI] [PubMed] [Google Scholar]
- 53.Committee on Pediatric Emergency Medicine, American Academy of Pediatrics. Krug S, Frush K. Patient safety in the pediatric emergency care setting. Pediatrics. 2007 Dec;120(6):1367–75. doi: 10.1542/peds.2007-2902.120/6/1367 [DOI] [PubMed] [Google Scholar]
- 54.Franklin BD. 'Smart' intravenous pumps: how smart are they? BMJ Qual Saf. 2017 Feb;26(2):93–94. doi: 10.1136/bmjqs-2016-005302.bmjqs-2016-005302 [DOI] [PubMed] [Google Scholar]
- 55.Schnock KO, Dykes PC, Albert J, Ariosto D, Call R, Cameron C, Carroll DL, Drucker AG, Fang L, Garcia-Palm CA, Husch MM, Maddox RR, McDonald N, McGuire J, Rafie S, Robertson E, Saine D, Sawyer MD, Smith LP, Stinger KD, Vanderveen TW, Wade E, Yoon CS, Lipsitz S, Bates DW. The frequency of intravenous medication administration errors related to smart infusion pumps: a multihospital observational study. BMJ Qual Saf. 2017 Feb;26(2):131–140. doi: 10.1136/bmjqs-2015-004465.bmjqs-2015-004465 [DOI] [PubMed] [Google Scholar]
- 56.Carr RR, Decarie D, Ensom MH. Stability of epinephrine at standard concentrations. Can J Hosp Pharm. 2014 May;67(3):197–202. doi: 10.4212/cjhp.v67i3.1356. http://europepmc.org/abstract/MED/24970939 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dix J, Weber RJ, Frye RF, Nolin TD, Mrvos R, Krenzelok E. Stability of atropine sulfate prepared for mass chemical terrorism. J Toxicol Clin Toxicol. 2003;41(6):771–5. doi: 10.1081/clt-120025341. [DOI] [PubMed] [Google Scholar]
- 58.Jäppinen A, Turpeinen M, Kokki H, Rasi A, Ojanen T, Pelkonen O, Naaranlahti T. Stability of sufentanil and levobupivacaine solutions and a mixture in a 0.9% sodium chloride infusion stored in polypropylene syringes. Eur J Pharm Sci. 2003 May;19(1):31–6. doi: 10.1016/s0928-0987(03)00041-1.S0928098703000411 [DOI] [PubMed] [Google Scholar]
- 59.Walker SE, Law S, Garland J, Fung E, Iazzetta J. Stability of norepinephrine solutions in normal saline and 5% dextrose in water. Can J Hosp Pharm. 2010 Mar;63(2):113–8. doi: 10.4212/cjhp.v63i2.896. http://europepmc.org/abstract/MED/22478966 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eysenbach Gunther, CONSORT-EHEALTH Group CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health interventions. J Med Internet Res. 2011 Dec 31;13(4):e126. doi: 10.2196/jmir.1923. http://www.jmir.org/2011/4/e126/ v13i4e126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Frank Shann Drug Doses.
The table details the time expressed in seconds to drug preparation (TDP) and delivery (TDD) for each nurse and for each drug, by study period.
The table details the deviation expressed in percent in drug dosage (overdose) from the prescribed dose for each nurse and for each drug, by study period.
CONSORT-EHEALTH checklist V1.6.1 [60].