Summary
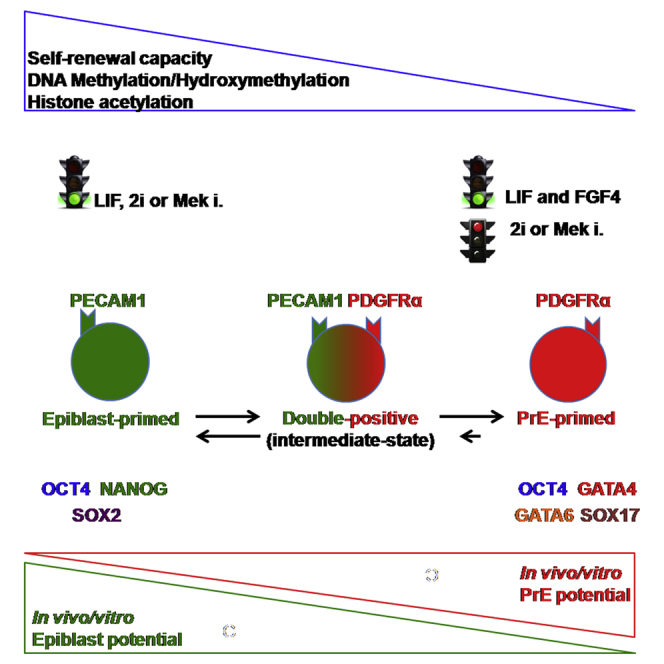
In early mouse pre-implantation development, primitive endoderm (PrE) precursors are platelet-derived growth factor receptor alpha (PDGFRα) positive. Here, we demonstrated that cultured mouse embryonic stem cells (mESCs) express PDGFRα heterogeneously, fluctuating between a PDGFRα+ (PrE-primed) and a platelet endothelial cell adhesion molecule 1 (PECAM1)-positive state (epiblast-primed). The two surface markers can be co-detected on a third subpopulation, expressing epiblast and PrE determinants (double-positive). In vitro, these subpopulations differ in their self-renewal and differentiation capability, transcriptional and epigenetic states. In vivo, double-positive cells contributed to epiblast and PrE, while PrE-primed cells exclusively contributed to PrE derivatives. The transcriptome of PDGFRα+ subpopulations differs from previously described subpopulations and shows similarities with early/mid blastocyst cells. The heterogeneity did not depend on PDGFRα but on leukemia inhibitory factor and fibroblast growth factor signaling and DNA methylation. Thus, PDGFRα+ cells represent the in vitro counterpart of in vivo PrE precursors, and their selection from cultured mESCs yields pure PrE precursors.
Keywords: embryonic stem cell heterogeneity, pre-implantation PrE precursors, in vitro model of early blastocyst development, PDGFRα+ subpopulations
Graphical Abstract
Highlights
-
•
Three subpopulations can be purified from mESC cultures by using PECAM1 and PDGFRα
-
•
Expression of PDGFRα is associated with a molecular and epigenetic PrE signature
-
•
PDGFRα+ cells are committed toward PrE derivatives in vitro and in vivo
In this article, Lo Nigro and colleagues further dissect the heterogeneity of mESC cultures by using the endogenous and heterogeneous expression of PECAM1 and PDGFRα. By combining the expression of these surface markers, the authors describe a strategy to sort out a subpopulation that exclusively gives rise to extraembryonic endodermal derivatives in vitro and in vivo.
Introduction
Totipotency is the capacity to form an entire organism, including embryonic and extraembryonic tissues. In mouse, totipotency lasts from fertilization at embryonic day (E)0 until the morula stage (∼E2.5). Loss of totipotency, early in pre-implantation development, is accompanied by segregation of the first lineage: the outer trophectoderm (TE) that separates from the inner cell mass (ICM). At implantation (∼E4.5), the ICM further generates two distinct layers: the epiblast and the primitive endoderm (PrE, also known as hypoblast) (Arnold and Robertson, 2009). At this stage, lineage identities are dictated by the expression of specific transcription factors (TFs). The pluripotent epiblast fate is induced by the expression of Oct4, Nanog, and Sox2 (Wicklow et al., 2014, Yamanaka et al., 2010); the segregated PrE layer is positive for Oct4, Gata4, Gata6, Sox7, and Sox17, whereas the cells of the TE express Cdx2 (Artus et al., 2011, Plusa et al., 2008). At earlier stages, these determinants are not specific: in the morula, embryonic and extraembryonic TFs are co-expressed in all blastomeres (Bessonnard et al., 2014, Dietrich and Hiiragi, 2007, Guo et al., 2010, Ohnishi et al., 2014, Schrode et al., 2014).
Proceeding with development, the epiblast forms all embryonic tissues but also the extraembryonic mesoderm of the visceral yolk sac, the chorion, the allantois, and the amnion. The PrE subsequently gives rise to the parietal endoderm (PE) of the transient parietal yolk sac and the visceral endoderm (VE). The VE consists of embryonic and extraembryonic VE. The extraembryonic VE, together with extraembryonic mesoderm, forms the visceral yolk sac, while the embryonic VE is necessary for correct anterior-posterior patterning of the embryo. In addition, recent findings suggest that embryonic VE also contributes to the gut (Kwon et al., 2008). The TE forms trophoblast giant cells, the extraembryonic ectoderm and its derivatives, the ectoplacental cone, and the chorionic ectoderm. TE is necessary for implantation of the conceptus and exchange of products between the maternal and fetal circulation.
Mouse embryonic stem cell (ESC) lines are derived from the ICM of developing blastocysts at ∼E3.5 (Evans and Kaufman, 1981, Martin, 1981). ESC lines capture many features of the epiblast and are defined as pluripotent because they can differentiate into the three definitive germ layers of the embryo when injected in recipient blastocysts or aggregated with morulas. In addition, pluripotent ESC lines can also generate trophoblast (Hayashi et al., 2010) and PrE cell types in vitro (i.e., extraembryonic endodermal cells [XENs]) (Kunath et al., 2005, Niakan et al., 2013), aside from cells of the three germ layers of the embryo. There is also evidence that ESCs rarely contribute to extraembryonic lineages in vivo (Beddington and Robertson, 1989). Taken together, these data indicate that ESC cultures contain precursors of extraembryonic lineages.
Traditionally, ESCs were derived and cultured in the presence of leukemia inhibitory factor (LIF) and either bone morphogenetic protein 4 (BMP4) or fetal bovine serum (BMP4/L or FBS/L) (Ying et al., 2003a). Under such conditions, ESC cultures are heterogeneous and contain metastable and fluctuating subpopulations, resembling later (post-implantation epiblast) or earlier (two-cell stage) developmental stages (Hayashi et al., 2008, Macfarlan et al., 2012). Recently, efficient and clonal derivation from ICM cells (Boroviak et al., 2014) was reported by using a defined medium containing two inhibitors of MEK and GSK3β kinases together with LIF (2i/L). ESC lines cultured in 2i/L maintain a less heterogeneous “naive” ground state (Marks et al., 2012, Ying et al., 2008).
Early in development, PDGFRα has a relatively weak but well visible expression in all blastomeres until it becomes stronger in PrE-committed cells around E3.75 (around 64 cells) (Artus et al., 2011, Grabarek et al., 2012, Plusa et al., 2008). Here, we demonstrate that PDGFRα+ cells can also be identified in undifferentiated ESC cultures. The PDGFRα+ subpopulations show a unique PrE-primed molecular and epigenetic signature, which is reflected by functional in vitro and in vivo differences when compared with the epiblast counterpart (PECAM1+). Despite these differences, the transcriptome of PDGFRα+ cells displays similarities with naive ESCs and with early/mid blastocyst cells. These findings suggest that PDGFRα+ cells are the equivalent of the in vivo PrE (hypoblast) precursors present at the pre-implantation stage.
Results
ESC Cultures Contain a PDGFRα+ Subpopulation When Cultured without 2i
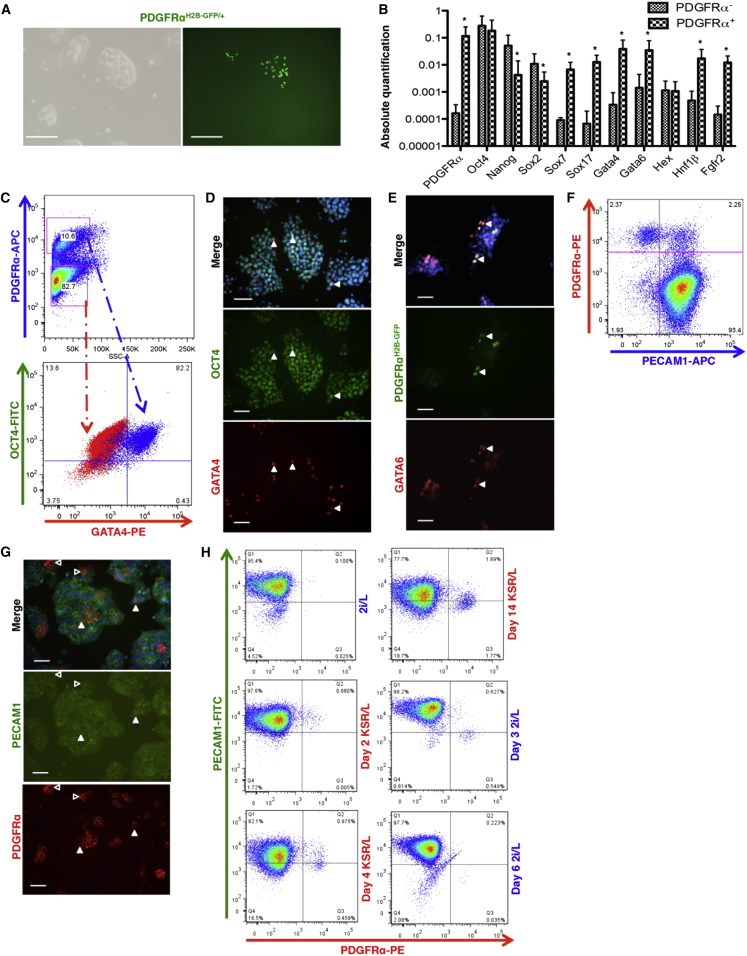
Expression of PDGFRα has been reported in differentiating ESCs and in XEN cells, but not in undifferentiated ESC lines. Here, we investigated its expression by using a PdgfrαH2B-GFP/+ reporter line (Hamilton et al., 2003) in which the H2B-GFP fusion protein tracks its presence. GFP+ cells were detected within colonies of ESC lines, cultured in LIF and knockout serum replacement (KSR/L) (Bryja et al., 2006) (Figure 1A). The comparison between GFP+ and negative cells by qRT-PCR, upon separation by fluorescence-activated cell sorting (FACS), showed that Oct4 transcript levels in PDGFRα+ cells were similar to those detected in PDGFRα− cells, while Nanog and Sox2 transcripts were expressed at lower levels (Figure 1B). Transcript levels of genes associated with early extraembryonic fate (Gata4, Gata6, Sox7, Sox17, Hnf1β, and Fgfr2) were higher in the PDGFRα+ fraction (Figure 1B). Although cells with a PrE profile (Canham et al., 2010) have been described as Hex+, the Hex transcript levels were identical in the two fractions (Figure 1B).
Figure 1.
Undifferentiated ESC Cultures Contain PDGFRα-Expressing Cells
(A) Bright field picture and GFP expression in PdgfrαH2B-GFP/+ ESC lines. Scale bar, 100 μm.
(B) qRT-PCR analysis for embryonic and extraembryonic markers in PdgfrαH2B-GFP +/− subpopulations. Data are presented as means ± SEM of each transcript from three independent experiments (normalized to β-Actin), ∗p < 0.05, t test.
(C) FACS analysis on E14 ESC lines for the expression of PDGFRα (top plot) and OCT4/GATA4 (bottom plot). The red cloud represents PDGFRα− cells, while the blue cloud represents PDGFRα+ cells, n = 3. The gating strategy was based on isotype controls.
(D) Immunostaining analysis for OCT4 and GATA4 on R1 ESC line. Arrowheads indicate cells co-expressing OCT4 and GATA4. Scale bar, 50 μm, n = 3.
(E) Immunostaining analysis for GATA6 on PdgfrαH2B-GFP/+ ESC line. Arrowheads indicate cells co-expressing PDGFRαH2B−GFP and GATA6. Scale bar, 50 μm, n = 3.
(F) Representative FACS analysis for PDGFRα and PECAM1 on the R1 line, n = 3. For isotype controls, gating strategies, and sorting purities, see Figure S1C.
(G) Immunostaining for PDGFRα and PECAM1 on the R1 line. Empty arrowheads indicate cells expressing only PDGFRα, full arrowheads indicate cells co-expressing PDGFRα and PECAM1. Scale bar, 50 μm, n = 3.
(H) Representative time course FACS analysis for PDGFRα and PECAM1 on the R1 line in 2i/L and KSR/L, n = 3. Gating strategy was based on isotype controls.
See also Figures S1 and S2.
We next stained E14 and R1 ESCs cultured in KSR/L with antibodies against PDGFRα, OCT4, and GATA4. This confirmed the presence of a PrE-primed subpopulation (Figure 1C, top plot), as ±80% of the PDGFRα+ cells co-expressed OCT4 and GATA4 (Figure 1C, bottom plot and 1D), differently from PDGFRα− cells, which expressed OCT4 only. We also found co-staining for PDGFRα and GATA6 (Figure 1E), using a Sox17:GFP/+ ESC line between SOX17 and PDGFRα (>50% of PDGFRα+ were GFP+, Figure S1A), and between SOX17 and OCT4 (Figure S1B). The molecular identity (OCT4, GATA4, GATA6, and SOX17) of PDGFRα+ cells strongly resembles the pre-implantation (∼E3.75) PrE precursor (Artus et al., 2011).
During the transition from morula to early blastocyst stage, cells co-express markers that later become specific for either epiblast or PrE. We tested whether PECAM1, a marker of epiblast in ICM and ESCs, was co-expressed with PDGFRα, to understand if expression of epiblast and PrE surface markers was mutually exclusive in vitro. We identified three different subpopulations: PECAM1+/PDGFRα− (epiblast-primed), PECAM1+/PDGFRα+ (double-positive), and PECAM1−/PDGFRα+ (PrE-primed) cells (Figures 1F, 1G, and S1C). Consistently, in the Sox17:GFP/+ ESC line, a subpopulation of PECAM1+ cells was also GFP+ (Figures S1D and S1E).
Previous reports suggested that culture in 2i/L maintains the expression of early endodermal genes (Canham et al., 2010, Marks et al., 2012). To test this, we cultured the PdgfrαH2B-GFP/+ ESCs for 3 days in 2i/L. As shown in Figure S2A, this resulted in a loss of GFP+ cells, a decrease of extraembryonic transcripts (Gata4, Gata6, Sox7, Sox17, FoxA2, and Hnf1β), and an increase of Nanog (Figure S2B); whereas an increase of extraembryonic transcripts levels was seen upon 2i withdrawal (Figure S2C). To confirm the effect of naive culture conditions, we adapted the R1 ESC line to 2i/L for 3 weeks (Figure 1H). In 2i/L, the PDGFRα+ subpopulations were strongly reduced. Subsequent withdrawal of 2i leads to the appearance of the double-positive subpopulation in 2 days and of the PrE-primed subpopulation in 4 days (Figure 1H, left plots). When culture conditions were again switched to 2i/L, the PDGFRα+ subpopulations almost completely disappeared in 6 days (Figure 1H, right plots). To determine whether the loss of PDGFRα+ cells was due to decreased proliferation or increased apoptosis of the PDGFRα+ cells, we performed triple intracellular staining for PECAM1, PDGFRα, and either KI67 (proliferation marker) or active CASPASE3 (apoptotic marker). This analysis showed that, under 2i/L, the proliferation of PDGFRα+ cells decreased (Figure S2D) without a significant increase in cell death (Figure S2E), demonstrating that the faster proliferating epiblast-primed subpopulation became predominant and took over the culture.
Molecular and Functional Differences of the PDGFRα+ Subpopulations
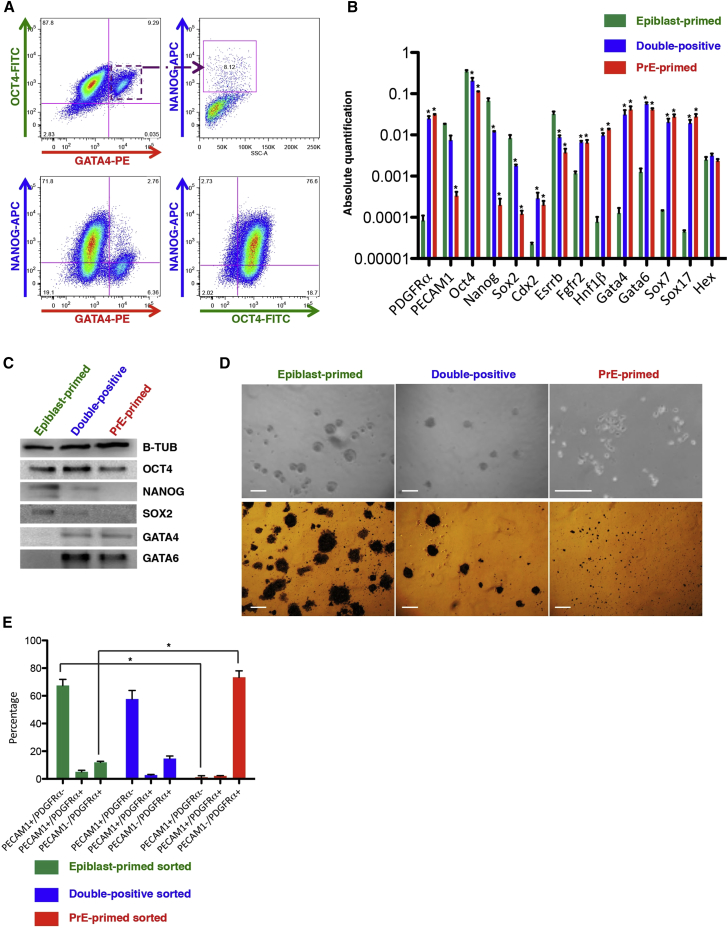
As we could co-detect epiblast and PrE surface markers (Figure 1F), we confirmed the expression of epiblast and PrE TFs at the single-cell level by performing triple intracellular staining for OCT4, GATA4, and NANOG (Figure 2A). This showed that ∼8% of the cells co-expressing OCT4 and GATA4 (top left plot) also expressed NANOG (top right plot).
Figure 2.
ESC Cultures Contain Different PDGFRα+ Subpopulations
(A) Representative intracellular FACS analysis for OCT4, GATA4, and NANOG on the R1 line, n = 3. The gating strategy was based on isotype controls.
(B) qRT-PCR analysis for embryonic and extraembryonic markers in the three subpopulations. Data are presented as means ± SEM of each transcript from three independent experiments (normalized to β-Actin), ∗p < 0.05, t test.
(C) Representative western blot of three independent experiments for OCT4, NANOG, SOX2, GATA4, and GATA6 on sorted cells. B-TUBULIN was used as normalizer.
(D) Bright field pictures and alkaline phosphatase staining on sorted subpopulations. Scale bar, 100 μm.
(E) Percentage of each subpopulation 1 week after their respective sorting, calculated from three independent experiments, ∗p < 0.05, t test.
See also Figure S3.
While comparing the different subpopulations, we detected in epiblast-primed cells high levels of the pluripotency transcripts (Oct4, Nanog, Sox2, and Esrrβ) as well as proteins (OCT4, NANOG, and SOX2), but low/no expression of PrE-related genes (Figures 2B and 2C). By contrast, in the PrE-primed cells, Oct4 transcripts and protein could be detected, whereas NANOG and SOX2 could not. Moreover, expression of markers specific for an extraembryonic (Figure 2B) but not of post-implantation epiblast fate (Fgf5, T, Nodal, Nr0b1, and Otx2; Figure S3A) (Brons et al., 2007) were significantly higher in the PrE-primed cells than in the other two-cell populations. Double-positive cells had an intermediate phenotype with respect to both single positive subpopulations.
To further characterize the three cell populations, they were isolated by FACS and subjected to in vitro functional tests. First, we cultured them at clonal density in KSR/L medium. In contrast to epiblast-primed and double-positive cells, PrE-primed cells poorly re-adhered to gelatin-coated plastic and rarely formed ESC colonies; they grew as single cells, resembling XEN cells (Kunath et al., 2005) and stained positive for alkaline phosphatase (Figure 2D). Time course FACS analysis showed that epiblast-primed and double-positive cells re-established the initial heterogeneity in a week when cultured in KSR/L, differently from PrE-primed cells, which strongly maintained a bias for the seeded subpopulation (Figures 2E and S3B), even when replated in 2i medium (Figure S3C).
We also tested whether PrE-primed sorted cells could be propagated in a stable and pure form by culturing them in medium that allows the derivation of OCT4+/GATA4+ PrE lines from rat blastocysts (Lo Nigro et al., 2012). However, prolonged culture (>3weeks) of PrE-primed sorted cells resulted in a mixture of cells with epiblast and PrE morphology and TFs (data not shown).
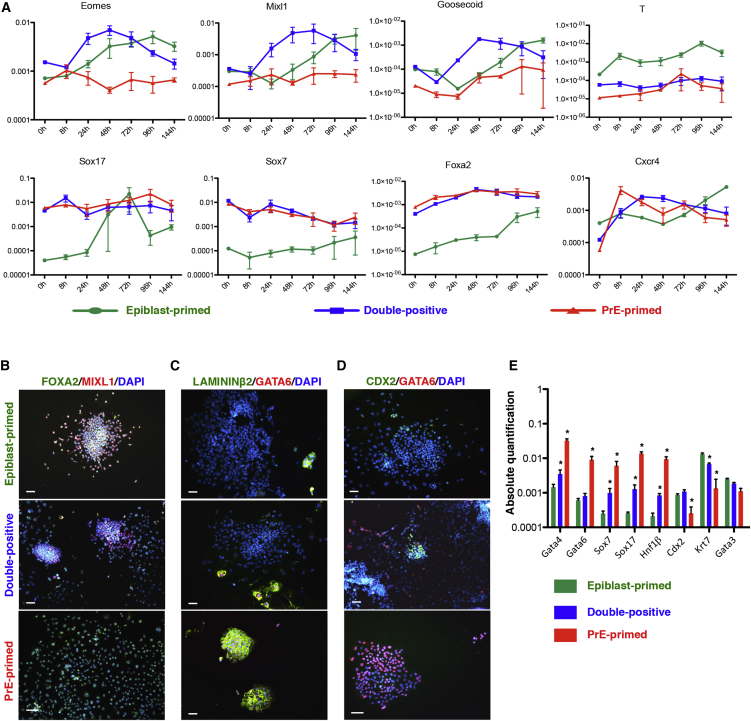
Second, we compared their differentiation potential into definitive endodermal by culturing them with Wnt3a and Activin A (Sancho-Bru et al., 2011). Time course analysis showed that Goosecoid, Eomes, and Mixl1 could be detected in epiblast-primed and double-positive cells, but not in PrE-primed cells, while T was upregulated specifically in epiblast-primed progeny (Figures 3A and 3B). Differently, Sox17, Sox7, Foxa2, and Cxcr4 (markers for definitive endoderm and for PrE-derivatives) were expressed from the beginning of the differentiation in PrE-primed and double-positive cells but not in epiblast-primed cells, wherein these markers were only upregulated at later stages. We also found that PDGFRα+ subpopulations fail to generate mesendoderm, suggesting a preferential differentiation toward PE/VE cell types. Similarly, upon induction of neuroectodermal lineage (Ying et al., 2003a), neural precursors were only detected in epiblast-primed progeny (arrows, Figure S3D). Double-positive and PrE-primed subpopulations formed vacuolated structures (empty arrows, Figure S3D), resembling differentiating XEN cells (Kunath et al., 2005).
Figure 3.
In Vitro Functional Differences of the Three Subpopulations
(A) qRT-PCR time course analysis for the indicated genes upon Wnt3a/Activin A treatment. Data are represented as means ± SEM of each transcript from three independent experiments (normalized to β-Actin).
(B) Immunostaining analysis for FOXA2 and MIXL1 on sorted subpopulations at day 4. Scale bar, 50 μm, n = 3.
(C) Immunostaining analysis for LAMININ-β2 and GATA6 on sorted subpopulations after 7 days in TSC medium. Scale bar, 50 μm, n = 3.
(D) Immunostaining analysis for CDX2 and GATA6 on the different sorted subpopulations after 7 days in TSC medium. Scale bar, 50 μm. n = 3.
(E) qRT-PCR analysis for XEN and trophoblast-related markers, upon sorting and differentiation. Data are presented as means ± SEM of each transcript from three independent experiments (normalized to β-Actin), ∗p < 0.05, t test.
See also Figure S3.
Third, we assessed the capacity of the three cell populations to generate extraembryonic cell types in trophoblast stem cell (TSC) medium, also shown to support the derivation of XEN cells (Niakan et al., 2013). PrE-primed cells but not the other two-cell populations formed XEN-like colonies, positive for GATA6 and LAMININ-β2 and expressing PrE transcripts (Figures 3C and 3E). Although ESCs are not thought to be capable of generating TSCs without genetic manipulation, we evaluated the presence of putative trophoblast cell progeny, i.e., CDX2+GATA6- cells (Figure 3D), as described (Morgani et al., 2013). Trophoblast-like progeny was only detected in epiblast-primed and double-positive cells cultures, as confirmed by qRT-PCR for Cdx2, Gata3, and Krt7 (Figure 3E).
Different Epigenetic State of PDGFRα+ Subpopulations
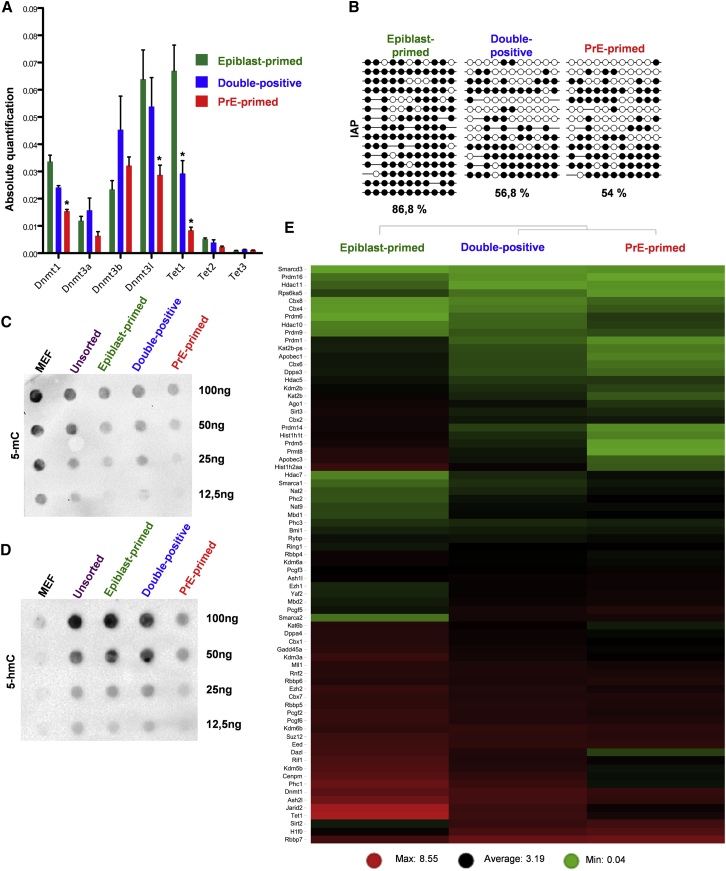
DNA methylation is dispensable for the growth of extraembryonic but not of embryonic tissues (Sakaue et al., 2010); moreover extraembryonic tissues have a lower level of DNA methylation than their embryonic equivalent (Rossant et al., 1986). We therefore compared the expression of DNA methylation and hydroxymethylation genes in the three subpopulations. Levels of Dnmt1, Dnmt3l, and Tet1 transcripts were significantly lower in PrE-primed cells compared with the other fractions (Figure 4A), suggesting a lower level of 5-mC and 5-hmC in PDGFRα+ cells. In addition, the promoter of intracisternal A-particle (IAP), which has repetitive elements with ±1,000 copies in the Mus musculus genome, was significantly less methylated in PDGFRα+ cells (±55%) compared with the epiblast-primed cells (±87%, Figure 4B). Accordingly, the genome-wide levels of 5-mC and 5-hmC in the genomic DNA were lower in PrE-primed cells compared with the other subpopulations (Figures 4C and 4D).
Figure 4.
Different Epigenetic State of PDGFRα+ Subpopulations
(A) qRT-PCR analysis for DNA methylation/hydroxymethylation genes upon sorting of the respective subpopulations. Data are presented as means ± SEM of each transcript from three independent experiments (normalized to β-Actin), ∗p < 0.05, t test.
(B) Bisulfite sequencing of IAP sequences. Open circles, unmethylated; closed circles, methylated.
(C) Representative dot blot for global 5-mC, from three independent experiments. Mouse embryonic fibroblasts (MEF) were used as control as they contain high levels of 5-mC and low levels of 5-hmC.
(D) Representative dot blot for global 5-hmC from three independent experiments. MEFs were used as control as they contain low levels of 5-hmC.
(E) Heatmap of epigenetic regulators on sorted subpopulations based on RNA-seq data. Transcript levels are based on FPKM (fragments per kilobase of exon per million fragments mapped). Red and green represent high and low gene expression, respectively.
The in vitro model described here also reflects these crucial differences in methylation between embryonic and extraembryonic tissues. A similar hypomethylated state has been reported for naive ESCs, where 2i reduces DNA methylation by increasing Prdm14 (Leitch et al., 2013). However, Prmd14 and other genes involved in primordial germ cells specification/imprinting (Dppa3, Dazl, and Prdm1) were lower in PDGFRα+ subpopulations (Figure 4E), suggesting that DNA hypomethylation depends on other mechanisms.
Finally, we compared the transcript levels of genes involved in chromatin regulation. Polycomb repressive complex (PRC)-1 and -2 and their histone modifications are crucial for the dynamic equilibrium and the plasticity of ESCs by acting as transcriptional repressors (Boyer et al., 2006). Of note, RNA sequencing (RNA-seq) analysis showed remarkable differences between the three subpopulations for the expression of these epigenetic regulators (Figure 4E). Compared with the epiblast-primed fraction, the PDGFRα+ subpopulations expressed significantly lower levels of Kdm2b, Jarid2 (which respectively recruit PRC1 and PRC2 complex to chromatin) and of Ezh2, Eed, and Suz12 (PRC2 components), suggesting a lower level of H3K27 methylation, known to be reduced in extraembryonic cell types (Alder et al., 2010, Rugg-Gunn et al., 2010).
The Developmental Potential of PDGFRα+ Cells Reflects Their Different Molecular Identity
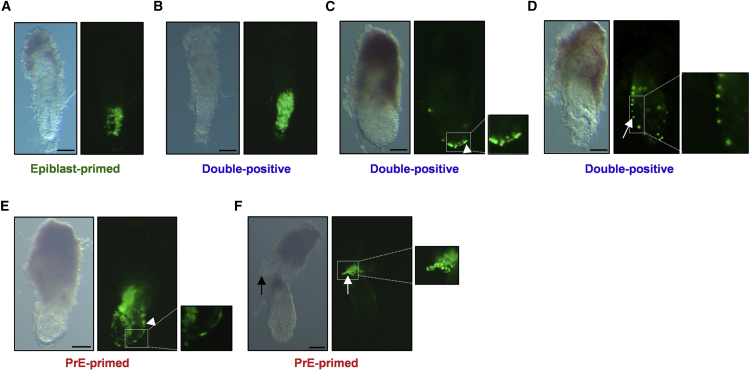
When ESCs are used to generate chimeras, chimerism is detected in the epiblast lineage that gives rise to all embryonic and to extraembryonic mesodermal tissues. However, ESC progeny has also been described to contribute very sporadically to TE or PrE-derived extraembryonic lineages (Beddington and Robertson, 1989). To compare their developmental potential, we injected GFP+ ESCs in recipient blastocysts after FACS sorting of the three subpopulations (Table S1). As expected, epiblast-primed cells efficiently colonized epiblast-derived tissues with high degrees of chimerism (Figure 5A) but not the TE/PrE-derived extraembryonic tissues. Injection of the double-positive subpopulation resulted in chimerism in the embryo proper (Figure 5B) as well as in the VE (Figure 5C) and PE (Figure 5D). PrE-primed cells contributed to both VE (Figure 5E) and PE (Figure 5F) but not to epiblast/TE derivatives. The behavior of the PrE-primed subpopulation differs from that of the Hex+ cells (Canham et al., 2010), which showed a low contribution (10%) to PrE-derived tissues while still colonizing the embryo.
Figure 5.
In Vivo Comparison of Developmental Potential
GFP+ ESCs were FACS sorted for PECAM and/or PDGFRα and injected into recipients blastocysts. Scale bar, 100 μm.
(A) E6.5 chimeric embryo generated from epiblast-primed cells showing the contribution to the embryo proper.
(B) E6.5 chimeric embryo generated from double-positive cells showing the contribution to the embryo proper.
(C) E6.5 chimeric embryo generated from double-positive cells showing the contribution to the VE (arrowhead).
(D) E6.5 chimeric embryo generated from double-positive cells showing the contribution to the PE (arrow).
(E) E6.5 chimeric embryo generated from PrE-primed cells showing the contribution to the VE (arrowhead).
(F) E6.5 chimeric embryo generated from PrE-primed cells showing the contribution to the PE (arrow).
See also Table S1.
PDGFRα+ cells have a distinct molecular identity, which is further reflected by different developmental potential in vivo.
PDGFRα+ Subpopulations Have a Unique Expression Profile that Resembles Early/Mid Blastocyst Cells
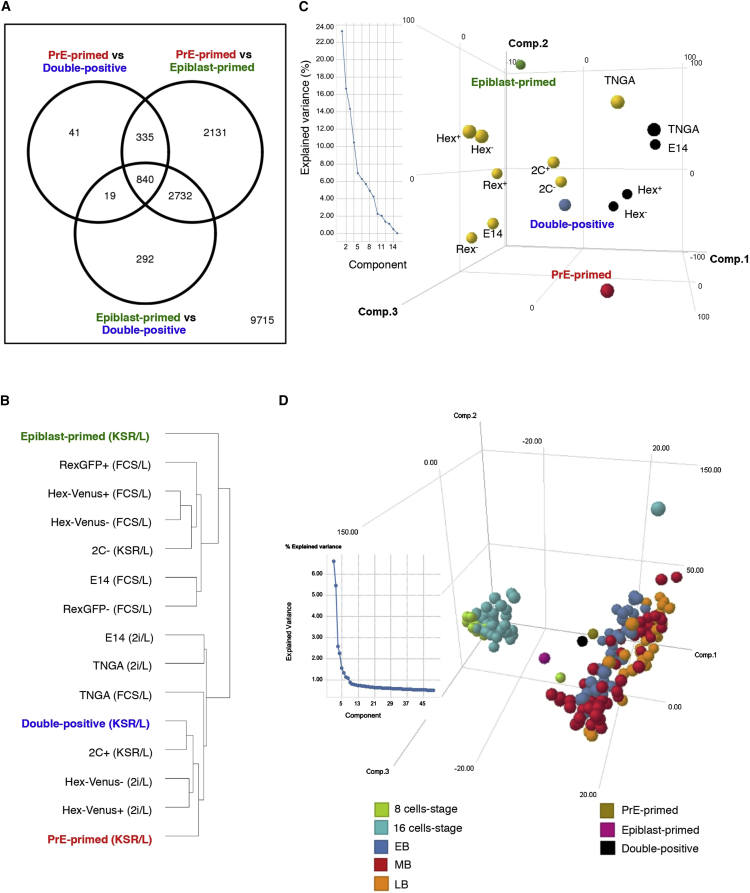
To investigate genome-wide differences/similarities between the three subpopulations, we performed RNA-seq. The comparison demonstrated that 292 genes were more than 2-fold differentially expressed in epiblast-primed cells, 41 in double-positive cells, and 2,131 in the PrE-primed subpopulation (Figure 6A and Table S2). The double-positive cells more closely resemble the PrE-primed state rather than the epiblast-primed state (Figures 6A and S4B). As PDGFRα+ subpopulations appear to have a PrE molecular phenotype, we compared the sorted subpopulations between each other but also with XEN isolated from embryo (eXEN) or converted from ESCs (cXEN), upon Activin A/retinoic acid treatment (Cho et al., 2012). Core and naive pluripotency genes (Nanog, Sox2, Esrrb, Klf2, Tdgf1, Gdf3, Nr0b1, and Fbxo15) were not expressed or expressed at a lower level in PrE-primed cells and in c/eXEN. Differently, Utf1, Tbx3, and Klf5 were expressed at higher levels in PDGFRα+ cells than in epiblast-primed cells (Figure S4A) or e/cXEN (not expressed). By contrast, genes involved in extraembryonic specification were exclusively detected in PDGFRα+ cells and e/cXEN. Remarkable differences were seen also for key pathway-associated genes. When compared with other analyzed lines, PDGFRα+ cells expressed higher levels of LIF regulators, such as Lifr and Il6st, and Wnt-associated genes, such as Lrp5/6 and Dkk1. Members of the fibroblast growth factor (FGF) signaling showed also a different pattern: Fgf4 and Fgfr1 levels were higher in epiblast-primed cells, while Fgf3, Fgfr2, and Fgfr4 were exclusively expressed in PDGFRα+ cells (Figure S4A).
Figure 6.
RNA-Seq Analysis and Comparison with In Vitro ESC Lines and In Vivo Single Cells
(A) Differentially expressed genes between sorted subpopulations. Adjusted p value < 0.05 with fold change (log2) > 1 or < −1.
(B) Unsupervised hierarchical clustering with previously published cell lines.
(C) PCA analysis and explained variance with previously published cell lines. Cell lines with black dots were culture in 2i/L; cell lines with yellow dots were cultured with FBS/L, with the exception of 2C+/−, which were cultured in KSR/L.
(D) PCA analysis and explained variance with in vivo single cells from early embryonic stages.
See also Figures S4–S6.
Next, we focused on these subpopulations and performed gene set enrichment analysis (GSEA) of the PANTHER (protein analysis through evolutionary relationships) biological process and KEGG (Kyoto encyclopedia of genes and genomes) pathways. Genes upregulated in the PDGFRα+ subpopulations were associated with metabolic processes and with lysosome, glutathione metabolism, and glycosphingolipid biosynthesis pathways (Tables S3 and S4). Epiblast-primed cells were enriched for terms associated with the cell cycle, focal adhesion, WNT and hedgehog signaling, cancer, developmental processes, and mesoderm/ectoderm development (Table S5). Remarkably, several terms enriched in the PDGFRα+ subpopulations (highlighted in yellow in Tables S3 and S4) have been reported for 2i/L ESCs, while many terms enriched in the epiblast-primed subpopulation (highlighted in yellow in Table S5) have been reported for FBS/L ESCs when comparing the naive with the primed state of pluripotency (Marks et al., 2012).
We also performed unsupervised hierarchical clustering and principal component analysis (PCA) to visualize the relationship of the three subpopulations with published transcriptomes: two-cell stage (Macfarlan et al., 2012) (2c+/−, KSR/L), Rex1+/− sorted cells, E14 and TNGA ESCs (Marks et al., 2012), and Hex+/- sorted cells (FBS/L and 2i/L) (Morgani et al., 2013). Unexpectedly, as ESCs cultured in 2i/L do not contain the PDGFRα+ subpopulations, these analyses showed that the PDGFRα+ subpopulations clustered more closely with naive than with other ESC lines (Figures 6B and 6C). Heatmap comparison of core/naive pluripotency and key pathway-associated genes with previously published transcriptomes (Figure S5) demonstrated that PDGFRα+ subpopulations have a unique transcriptome. PDGFRα+ cells, differently from the previously described Hex+ cells, have a pronounced PrE-primed signature, while still retaining some pluripotency-related genes (Oct4, Sall4, Utf1, Tbx3, Tfcp2l1, and Klf5). PDGFRα+ cells expressed Dkk1 in an exclusive manner and had higher levels of LIF regulators (Lifr and Il6st) and FGF signaling members (Fgf3/10, Fgfr2/3/4).
As major differences could be detected between epiblast- and PrE-primed cells (Figures 6A and S4B), we assessed their relationship with single cells obtained from 8-cell-stage morula to late blastocyst (LB)-stage embryos (Deng et al., 2014). PCA analysis grouped a subset of early (EB) and mid (MB) blastocyst single cells with PrE-primed and with epiblast-primed cells (Figures 6D and S6A). GSEA of PrE/epiblast-primed subpopulations with the five most similar in vivo cells revealed upregulation of Suz12 targets in the PrE cluster (Figure S6B) and DNA binding-related genes in the epiblast cluster (Figures S6C and S6D; Table S6). Thus, PDGFRα+ cells have a unique expression profile and surprisingly show similarities with naive ESCs and with EB/MB cells in vivo.
Epigenetic Modifications and Signaling Involved in the Regulation of the PDGFRα+ Subpopulations
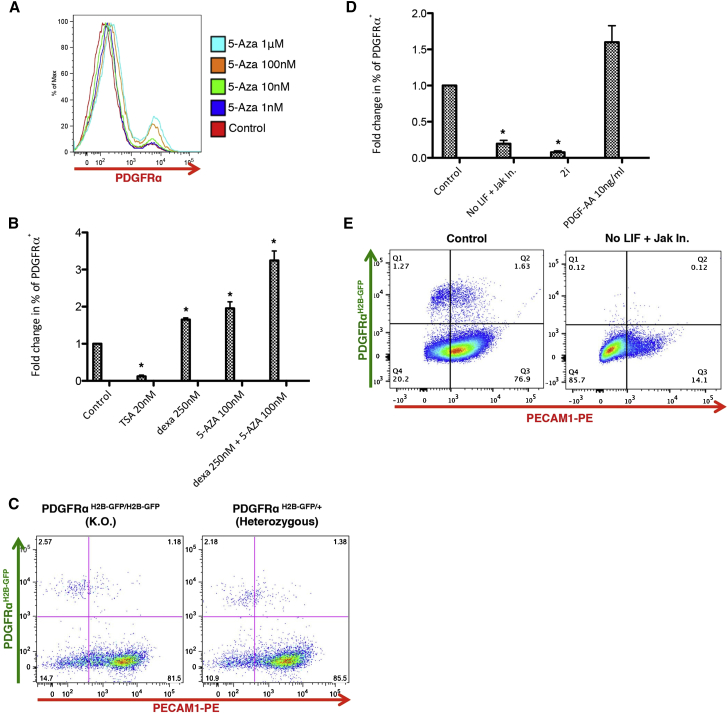
To better understand the mechanisms and signaling governing this heterogeneity, we tested the effect of known epigenetic modifiers and small molecules. Considering the lower level of 5-mC in PrE-primed cells (Figures 4B and 4C), we added the DNA methylation inhibitor 5-azacytidine (5-AZA) to KSR/L. 5-AZA enhanced the frequency of PDGFRα+ cells in a dose-dependent manner (Figure 7A). Likewise, the addition of dexamethasone, a glucocorticoid hormone involved in DNA demethylation and whose signaling interacts with the JAK/STAT pathway (Reddy et al., 2009), increased the PDGFRα+ cell frequency (Figure 7B). The effects of 5-AZA and dexamethasone were combinatorial, resulting in ∼3-fold increase in PDGFRα+ cells (Figures 7B and S7A). Addition of trichostatin A (TSA), a histone deacetylase inhibitor, resulted in a decrease of PDGFRα+ cells (Figure 7B), suggesting that histone acetylation negatively regulates the PrE-primed state.
Figure 7.
Epigenetic Modifications and Signaling Involved in the Regulation of PDGFRα+ Cells
(A) Dose response to 5-AZA treatment. Histograms show the percentage of PDGFRα+ cells in response to an increasing concentration of 5-AZA, n = 3.
(B) Fold change in percentage of PDGFRα+ cells after 72 hr of treatment under the indicated culture conditions for three independent experiments, ∗p < 0.05 by one-way ANOVA with subsequent Tukey honest significant difference(HSD) test.
(C) Representative FACS analysis for PDGFRα and PECAM1 in PDGFRα null and heterozygous ESC lines, n = 3. The gating strategy was based on isotype controls.
(D) Fold change in percentage of PDGFRα+ cells after 72 hr of treatment under the indicated culture conditions for three independent experiments, ∗p < 0.01 by one-way ANOVA with subsequent Tukey HSD test.
(E) Representative FACS analysis for PDGFRα and PECAM1 with LIF (left plot) or without LIF and with Jak Inhibitor (right plot), n = 3. The gating strategy was based on isotype controls.
See also Figure S7.
As PDGFRα was shown to be necessary for eXEN derivation (Artus et al., 2010) and for conversion of ESCs into cXEN (Cho et al., 2012), we compared PdgfrαH2B-GFP/+ (heterozygous) and PdgfrαH2B-GFP/H2B-GFP cells (a null knockin). The absence of the receptor did not alter the percentage of PDGFRα+ cells (Figure 7C) or the transcript levels of pluripotent/extraembryonic genes in the PDGFRα+ subpopulations (Figure S7B). Consistently, culture of ESCs with PDGF-AA did not significantly increase the percentage of PDGFRα+ cells (Figure 7D).
As LIF supports the expansion of PrE in pre-implantation development (Morgani and Brickman, 2015), we tested if LIF was also necessary for the propagation of PDGFRα+ cells. LIF withdrawal combined with the addition of a Janus kinase inhibitor (to block endogenous LIF), inhibited PDGFRα+ cell expansion (Figures 7D and 7E). FGF signaling regulates the segregation of the PrE layer (Yamanaka et al., 2010) by phosphorylation of extracellular-signal-regulated kinase. The simultaneous inhibition of Gsk3β and MEK kinases in 2i/L resulted in the disappearance of PDGFRα+ cells (Figure 7D); this effect was mediated by Mek (PD0325901), and not by the Gsk3β inhibitor, as shown by FACS for PDGFRα and OCT4/GATA4 (Figures S7C and S7D). This confirms the requirement of FGF also for the fluctuation of PDGFRα+ cells.
Discussion
During development, PDGFRα has an early, relatively weak but well visible expression from morula stage onward until it becomes stronger in PrE-fated cells at ∼E3.75 (64 cells) (Artus et al., 2011, Grabarek et al., 2012, Plusa et al., 2008). In this study, we investigated its presence in undifferentiated ESCs, further dissecting their known heterogeneity. By taking advantage of the endogenous expression of PECAM1 and PDGFRα, we defined three different subpopulations that were further characterized (Figures 1 and 2): PECAM1+/PDGFRα− (epiblast-primed), PECAM1+/PDGFRα+ (double-positive) and PECAM1-/PDGFRα+ (PrE-primed) cells. PrE-primed cells have a distinct molecular identity, as they co-express OCT4, GATA4, GATA6, and SOX17, which differs from epiblast-primed cells, which co-express OCT4, NANOG, and SOX2. Double-positive cells appear to be an intermediate between epiblast- and PrE-primed cells. In line with this, we also identified, at the single-cell level, cells co-expressing OCT4, GATA4, and NANOG. This is reminiscent of the simultaneous expression of epiblast and extraembryonic determinants in early pre-implantation development (Guo et al., 2010).
Although PrE-biased cells have already been described as Hex+ (Canham et al., 2010, Morgani et al., 2013), PDGFRα+ cells have a more pronounced PrE phenotype and a lower expression of epiblast determinants, which are still retained in Hex+ cells (Figure S5). Moreover, our model does not rely on signal amplification and on the use of reporter lines (Canham et al., 2010, Morgani et al., 2013), allowing the separation of these subpopulations in every ESC line of interest.
In vitro features of the three subpopulations appear to be drastically divergent in terms of self-renewal and differentiation capacity (Figure 3). Epiblast-primed cells and double-positive cells but not PrE-primed cells could re-establish the initial heterogeneity. When addressing their differentiation potential, PrE-primed cells efficiently generated XEN-like cells but not embryonic or presumptive trophoblast types diversely from epiblast-primed subpopulation.
PDGFRα+ cells have a distinct epigenetic state, characterized by a lower level of DNA methylation/hydroxymethylation and by a different pattern of epigenetic regulators (Figure 4), in line with the notion that extraembryonic tissues (Sakaue et al., 2010) and their stem cell models (Rugg-Gunn et al., 2010) are hypomethylated.
The distinct epigenetic and molecular profile of PDGFRα+ subpopulations was confirmed also by their developmental potential (Figure 5 and Table S1). The double-positive cells could still colonize the epiblast while PrE-primed cells exclusively contributed to PrE derivatives. Again, these in vivo experiments confirmed that PDGFRα+ cells closely represent the PrE precursors.
The comparative transcriptome analysis with epiblast-primed cells and with e/cXEN showed that PDGFRα+ subpopulations differentially express genes associated with core/naive pluripotency, and with JAK-STAT, WNT, and FGF signaling pathways (Figure S4). Unexpectedly, PCA, hierarchical clustering, and GSEA with previously available datasets, revealed that globally PDGFRα+ cells resemble more naive ESCs (Figures 6B and 6C; Tables S3, S4, and S5). When compared with single cells obtained from early embryos, PrE-primed cells, as their epiblast counterpart, clustered with cells from the EB-MB stage (∼E3.5–E4.0), further demonstrating that PDGFRα+ steady states mirror the pre-implantation developmental window (Figures 6D and S6A).
The mechanisms involved in the regulation of the heterogeneity in vitro (Figure 7) confirmed previous studies in early development. The percentage of PDGFRα+ cells was influenced by: JAK/STAT signaling, shown to support the expansion of PrE in pre-implantation development (Morgani and Brickman, 2015); FGF signaling, known to control the segregation of PrE and epiblast in the ICM (Yamanaka et al., 2010) and amount of DNA methylation, is a dispensable mechanism for the growth of extraembryonic lineages (Sakaue et al., 2010). By contrast, absence of PDGFRα, necessary for the derivation of eXEN (Artus et al., 2010) and cXEN (Cho et al., 2012), did not alter the abundance of PDGFRα+ cells in vitro. Together, these results confirm that PDGFRα+ cells are the in vitro equivalent of PrE precursors.
This model, which relies on the endogenous heterogeneous expression of PDGFRα, should facilitate and enable studies to gain insights in the factors regulating the early segregation of these different cell types within the ICM and to unravel the mechanisms involved in the different imprinting of embryonic and extraembryonic tissues (Hudson et al., 2010). Future studies are needed to determine whether PrE-primed cells recapitulate the imprinting associated with extraembryonic tissues (i.e., paternal imprinting of X chromosome) and whether a similar PrE-primed state is also present in human ESC cultures.
Experimental Procedures
Cell Culture
Undifferentiated ESCs were maintained feeder free on gelatin (EmbryoMax 0.1% gelatin solution, ES-006-B; Millipore)-coated plates, in knockout DMEM (10829-018; Gibco), 20% knockout serum replacement (KSR, 10828-028; Gibco), 2 mM L-glutamine (25030-024; Gibco), 1x minimal essential medium nonessential amino acids (11140-035; Gibco), 1× penicillin-streptomycin (15140-122; Gibco), 100 μM β-mercaptoethanol (31350; Gibco) and 1,000 U/mL recombinant LIF (ESG1107; Chemicon International).
qRT-PCR Analysis
For RNA isolation, the RNeasy Mini-kit/Micro-kit (74104 and 74004; QIAGEN) was used. DNase treatment was achieved using the Turbo DNase kit (1907, Ambion). cDNA synthesis was done with 1 μg of RNA with the Superscript III First-Strand synthesis system (18080-051; Invitrogen). Real-time PCR was analyzed with the SYBR Green Platinum qPCR Supermix-UDG (11733-046; Invitrogen) on a ViiA 7 Real-Time PCR System (Applied Biosystems). Expression was normalized to β-Actin. Primer sequences are listed in the Supplemental Information.
Flow Cytometry and Cell Sorting
Single-cell suspensions of ESCs were obtained by dissociating with cell-dissociation buffer (Invitrogen) at 37°C for 20 min. Cells were washed twice with PBS and incubated with conjugated primary antibodies for 30 min on ice in the dark. Cells were washed once with PBS and resuspended for FACS analysis in PBS + 5% FBS. Flow cytometry was performed at the KU Leuven Flow Cytometry Facility using an FACS AriaIII (Becton Dickinson) or an FACS Canto (Becton Dickinson) for analysis. Intracellular staining was performed with the Foxp3/Transcription Factor Staining Buffer Set kit (00-5523-00; Ebioscience), following the manufacturer's protocol. Antibodies are listed in the Supplemental Information.
Differentiation Assays
Upon sorting of the different subpopulations, specific differentiations were performed as described by Sancho-Bru et al. (2011) for mesendodermal differentiation; by Ying et al. (2003b) for neural differentiation and by Morgani et al. (2013) for TSC medium.
Culture Test
For the experiments described in Figures 7 and S7, 3 × 103 ESCs were sorted and plated in a 6-well plate in ESC medium under the following conditions: (1) no LIF and 1 μM InSolution JAK Inhibitor I (420097; Calbiochem); (2) 2i, 1 μM PD0325901 and 3 μM CHIR99021 (Axon Medchem); (3) 10 ng/mL PDGF-AA (315-18; Peprotech); (4) 20 nM Trichostatin A (TSA; T8552; Sigma); (5) 250 nM dexamethasone (D2915; Sigma); (6) 1 nM to 1 μM 5-AZA (A3656; Sigma); (7) 250 nM dexamethasone (Sigma) and 100 nM 5-AZA.
Immunoblotting
Sorted ESCs were lysed in RIPA buffer (R0278; Sigma) containing complete protease-inhibitor cocktail (04693116001; Roche) for 1 hr at 4°C. Protein concentrations of various samples were quantified using the Pierce BCA protein assay kit (23225; Thermo Scientific) following the manufacturer's instructions. To each protein sample, 1 volume of Bio-Rad loading buffer (161-0747, Bio-Rad) and β-mercaptoethanol (at 20:1, Sigma) was added. The samples were heated at 95°C for 10 min, followed by centrifugation at 13,000 × g for 10 min. Thirty micrograms of each protein sample was loaded in each lane of a 10% gradient Mini-PROTEAN TGXTM Precast gel (Bio-Rad) and electrophoresed. The resolved proteins were then transferred to Whatman Protan nitrocellulose membrane (Z613630; Sigma). Following blocking with 5% nonfat milk for 1 hr, membranes were incubated at 4°C overnight with primary antibodies. The following day, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies against rat, rabbit, and mouse IgG (Dako). Immunoreactive bands were visualized using Super Signal West Pico chemiluminescent substrate (34087; Thermo Scientific), and signals were detected using a ChemiDoc XRS+ System (Bio-Rad). Antibodies are listed in the Supplemental Information.
Bisulfite Sequencing
Extraction of the genomic DNA isolated from FACS-sorted ESCs was done with the EpiTect Bisulfite kit (59104; QIAGEN). The primer sequences and PCR conditions for amplification of IAP sequences were as described (Lane et al., 2003). The PCR products were cloned using the pGEM-T Easy Vector System I (A1360; Promega). At least 15 colonies for each sample were sequenced and analyzed using Quma software (http://quma.cdb.riken.jp/).
5HmC/5mC Dot Blot Assay
FACS-sorted ESC subpopulations of genomic DNA was extracted with the PureLink Genomic DNA Mini kit (K182001; Invitrogen). Two-fold serial dilutions were made by mixing DNA and Tris-EDTA in 96-well plates. Twenty microliters of 1 M NaOH/25 mM EDTA was added to each well, the plate sealed, and heated at 95°C for 10 min. Subsequently, plates were cooled on ice and 50 μL of ice-cold 2 M ammonium acetate (pH 7.0) was added to each well, the plates were incubated on ice for 10 min. Subsequently, the denatured DNA was loaded on the nitrocellulose membrane (Bio-Rad), which was washed with 500 μL of 0.4 M NaOH, and rinsed with water. The membrane was air dried for 5–10 min and placed under UV (at 120,000 μJ/cm2). The membrane was blocked with 5% nonfat milk in TBST (Tris-buffered saline and Tween 20) for 1 hr, and then incubated with antibodies against 5hmC/5mC O/N at 4°C. The membrane was washed with TBST for 10 min four times, and incubated with HRP-conjugated secondary antibodies (1: 5.000) at room temperature for 1 hr. The membrane was washed with TBST for 10 min four times, incubated with Enhanced ChemiLuminescence (ECL solution, Thermo Scientific) and developed using Chemidoc (Bio-Rad).
Statistical Analysis
p Values in the qRT-PCR analysis for pairwise differential expression against the epiblast-primed subpopulation were computed using Student's two-tailed t test. Experiments including three or more samples/treatment were subjected to one-way ANOVA with subsequent Tukey honest significant difference testing to establish significant changes between any two means.
Blastocyst Injections
Blastocyst injection studies were approved by the ethical committee for use of animals in research from KU Leuven (Belgium). C57BL/6 mouse ESCs were labeled with eGFP by lentiviral transduction. The eGFP transcription was under the control of elongation factor-1alpha promoter. Following culture in KSR/L, cells were dissociated and different subpopulations were sorted based on PDGFRα and PECAM1 labeling. Sorted cells (6–8 cells) were immediately injected in the blastocoel cavity of CD1 blastocysts. The embryos were transferred the same day to the uterus of pseudopregnant CD1 female mice. Post-implantation embryos were collected at E6.5 from pseudopregnant mice 3.5 days after embryo transfer. Intact post-implantation conceptuses were isolated from decidua, fixed with 4% paraformaldeyde, and immediately imaged using a SteREO Discovery V12 microscope (Zeiss) to determine chimerism.
Author Contributions
C.M.V. and A.L.N. designed the project and experiments. A.L.N, G.M.F. performed most of the experiments. I.P. helped with immunofluorescences and W.B., S.M.C.d.S.L. helped with the interpretation of the results. X.L.A., P.G.C., and K.-P.K provided materials. A.d.J. and F.L. performed differentiation experiments and helped with the revision, A.Z., R.K., and V.A.E. embedded/analyzed chimeric embryos. D.S.C. and W.S.H. performed RNA-seq analysis. A.L.N. and C.M.V. wrote the manuscript. F.L. and C.M.V. contributed equally. All the authors read the manuscript.
Acknowledgments
We thank the late Vik Van Duppen for FACS experiments, Rob Van Rossom and the KU LEUVEN FACS CORE for sorting, Zhiyong Zhang and Liesbeth Vermeire from InfraMouse for chimera production, and Kristel Eggermont for help with image acquisition/processing. We thank Dr. Hadjantonakis and Dr. Niakan for the FGF4/PDGFRα ESC lines and Dr. Morrison for the Sox17GFP/+ line. We thank Dr. Kian Koh and Joris Vande Velde for dot blots. The work was supported by grants obtained from FWO (G.0832) and KU Leuven (EIW-B4855-EF/05/11 and ETH-C1900-PF to C.M.V.; EME-C2161-GOA/11/012 to C.M.V./A.Z.; C14/16/078 to F.LL.), Hercules Foundation (ZW09/03 to A.Z.) and by the BELSPO-IUAP-DEVREPAIR grant (to C.M.V./S.M.C.d.S.L./A.Z.).
Published: January 12, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and six tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.12.010.
Accession Numbers
The gene expression data reported in this paper has been deposited at the GEO repository with accession number GEO: GSE65884.
Supplemental Information
References
- Alder O., Lavial F., Helness A., Brookes E., Pinho S., Chandrashekran A., Arnaud P., Pombo A., O'Neill L., Azuara V. Ring1B and Suv39h1 delineate distinct chromatin states at bivalent genes during early mouse lineage commitment. Development. 2010;137:2483–2492. doi: 10.1242/dev.048363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S.J., Robertson E.J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- Artus J., Panthier J.J., Hadjantonakis A.K. A role for PDGF signaling in expansion of the extra-embryonic endoderm lineage of the mouse blastocyst. Development. 2010;137:3361–3372. doi: 10.1242/dev.050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artus J., Piliszek A., Hadjantonakis A.K. The primitive endoderm lineage of the mouse blastocyst: sequential transcription factor activation and regulation of differentiation by Sox17. Dev. Biol. 2011;350:393–404. doi: 10.1016/j.ydbio.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddington R.S., Robertson E.J. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–737. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- Bessonnard S., De Mot L., Gonze D., Barriol M., Dennis C., Goldbeter A., Dupont G., Chazaud C. Gata6, Nanog and Erk signaling control cell fate in the inner cell mass through a tristable regulatory network. Development. 2014;141:3637–3648. doi: 10.1242/dev.109678. [DOI] [PubMed] [Google Scholar]
- Boroviak T., Loos R., Bertone P., Smith A., Nichols J. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat. Cell Biol. 2014;16:516–528. doi: 10.1038/ncb2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer L.A., Plath K., Zeitlinger J., Brambrink T., Medeiros L.A., Lee T.I., Levine S.S., Wernig M., Tajonar A., Ray M.K. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Brons I.G., Smithers L.E., Trotter M.W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Bryja V., Bonilla S., Cajanek L., Parish C.L., Schwartz C.M., Luo Y., Rao M.S., Arenas E. An efficient method for the derivation of mouse embryonic stem cells. Stem Cells. 2006;24:844–849. doi: 10.1634/stemcells.2005-0444. [DOI] [PubMed] [Google Scholar]
- Canham M.A., Sharov A.A., Ko M.S., Brickman J.M. Functional heterogeneity of embryonic stem cells revealed through translational amplification of an early endodermal transcript. PLoS Biol. 2010;8:e1000379. doi: 10.1371/journal.pbio.1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho L.T., Wamaitha S.E., Tsai I.J., Artus J., Sherwood R.I., Pedersen R.A., Hadjantonakis A.K., Niakan K.K. Conversion from mouse embryonic to extra-embryonic endoderm stem cells reveals distinct differentiation capacities of pluripotent stem cell states. Development. 2012;139:2866–2877. doi: 10.1242/dev.078519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q., Ramskold D., Reinius B., Sandberg R. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science. 2014;343:193–196. doi: 10.1126/science.1245316. [DOI] [PubMed] [Google Scholar]
- Dietrich J.E., Hiiragi T. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007;134:4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Grabarek J.B., Zyzynska K., Saiz N., Piliszek A., Frankenberg S., Nichols J., Hadjantonakis A.K., Plusa B. Differential plasticity of epiblast and primitive endoderm precursors within the ICM of the early mouse embryo. Development. 2012;139:129–139. doi: 10.1242/dev.067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G., Huss M., Tong G.Q., Wang C., Li Sun L., Clarke N.D., Robson P. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev. Cell. 2010;18:675–685. doi: 10.1016/j.devcel.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Hamilton T.G., Klinghoffer R.A., Corrin P.D., Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol. Cell. Biol. 2003;23:4013–4025. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Lopes S.M., Tang F., Surani M.A. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell. 2008;3:391–401. doi: 10.1016/j.stem.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Furue M.K., Tanaka S., Hirose M., Wakisaka N., Danno H., Ohnuma K., Oeda S., Aihara Y., Shiota K. BMP4 induction of trophoblast from mouse embryonic stem cells in defined culture conditions on laminin. In Vitro Cell. Dev. Biol. Anim. 2010;46:416–430. doi: 10.1007/s11626-009-9266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson Q.J., Kulinski T.M., Huetter S.P., Barlow D.P. Genomic imprinting mechanisms in embryonic and extraembryonic mouse tissues. Heredity. 2010;105:45–56. doi: 10.1038/hdy.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunath T., Arnaud D., Uy G.D., Okamoto I., Chureau C., Yamanaka Y., Heard E., Gardner R.L., Avner P., Rossant J. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. doi: 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- Kwon G.S., Viotti M., Hadjantonakis A.K. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev. Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N., Dean W., Erhardt S., Hajkova P., Surani A., Walter J., Reik W. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- Leitch H.G., McEwen K.R., Turp A., Encheva V., Carroll T., Grabole N., Mansfield W., Nashun B., Knezovich J.G., Smith A. Naive pluripotency is associated with global DNA hypomethylation. Nat. Struct. Mol. Biol. 2013;20:311–316. doi: 10.1038/nsmb.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Nigro A., Geraerts M., Notelaers T., Roobrouck V.D., Muijtjens M., Eggermont K., Subramanian K., Ulloa-Montoya F., Park Y., Owens J. MAPC culture conditions support the derivation of cells with nascent hypoblast features from bone marrow and blastocysts. J. Mol. Cell Biol. 2012;4:423–426. doi: 10.1093/jmcb/mjs046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlan T.S., Gifford W.D., Driscoll S., Lettieri K., Rowe H.M., Bonanomi D., Firth A., Singer O., Trono D., Pfaff S.L. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature. 2012;487:57–63. doi: 10.1038/nature11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks H., Kalkan T., Menafra R., Denissov S., Jones K., Hofemeister H., Nichols J., Kranz A., Stewart A.F., Smith A. The transcriptional and epigenomic foundations of ground state pluripotency. Cell. 2012;149:590–604. doi: 10.1016/j.cell.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgani S.M., Brickman J.M. LIF supports primitive endoderm expansion during pre-implantation development. Development. 2015;142:3488–3499. doi: 10.1242/dev.125021. [DOI] [PubMed] [Google Scholar]
- Morgani S.M., Canham M.A., Nichols J., Sharov A.A., Migueles R.P., Ko M.S., Brickman J.M. Totipotent embryonic stem cells arise in ground-state culture conditions. Cell Rep. 2013;3:1945–1957. doi: 10.1016/j.celrep.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niakan K.K., Schrode N., Cho L.T., Hadjantonakis A.K. Derivation of extraembryonic endoderm stem (XEN) cells from mouse embryos and embryonic stem cells. Nat. Protoc. 2013;8:1028–1041. doi: 10.1038/nprot.2013.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi Y., Huber W., Tsumura A., Kang M., Xenopoulos P., Kurimoto K., Oles A.K., Arauzo-Bravo M.J., Saitou M., Hadjantonakis A.K. Cell-to-cell expression variability followed by signal reinforcement progressively segregates early mouse lineages. Nat. Cell Biol. 2014;16:27–37. doi: 10.1038/ncb2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plusa B., Piliszek A., Frankenberg S., Artus J., Hadjantonakis A.K. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy T.E., Pauli F., Sprouse R.O., Neff N.F., Newberry K.M., Garabedian M.J., Myers R.M. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 2009;19:2163–2171. doi: 10.1101/gr.097022.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J., Sanford J.P., Chapman V.M., Andrews G.K. Undermethylation of structural gene sequences in extraembryonic lineages of the mouse. Dev. Biol. 1986;117:567–573. doi: 10.1016/0012-1606(86)90325-8. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn P.J., Cox B.J., Ralston A., Rossant J. Distinct histone modifications in stem cell lines and tissue lineages from the early mouse embryo. Proc. Natl. Acad. Sci. USA. 2010;107:10783–10790. doi: 10.1073/pnas.0914507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue M., Ohta H., Kumaki Y., Oda M., Sakaide Y., Matsuoka C., Yamagiwa A., Niwa H., Wakayama T., Okano M. DNA methylation is dispensable for the growth and survival of the extraembryonic lineages. Curr. Biol. 2010;20:1452–1457. doi: 10.1016/j.cub.2010.06.050. [DOI] [PubMed] [Google Scholar]
- Sancho-Bru P., Roelandt P., Narain N., Pauwelyn K., Notelaers T., Shimizu T., Ott M., Verfaillie C. Directed differentiation of murine-induced pluripotent stem cells to functional hepatocyte-like cells. J. Hepatol. 2011;54:98–107. doi: 10.1016/j.jhep.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Schrode N., Saiz N., Di Talia S., Hadjantonakis A.K. GATA6 levels modulate primitive endoderm cell fate choice and timing in the mouse blastocyst. Dev. Cell. 2014;29:454–467. doi: 10.1016/j.devcel.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklow E., Blij S., Frum T., Hirate Y., Lang R.A., Sasaki H., Ralston A. HIPPO pathway members restrict SOX2 to the inner cell mass where it promotes ICM fates in the mouse blastocyst. PLoS Genet. 2014;10:e1004618. doi: 10.1371/journal.pgen.1004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka Y., Lanner F., Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development. 2010;137:715–724. doi: 10.1242/dev.043471. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Nichols J., Chambers I., Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Stavridis M., Griffiths D., Li M., Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.