Abstract
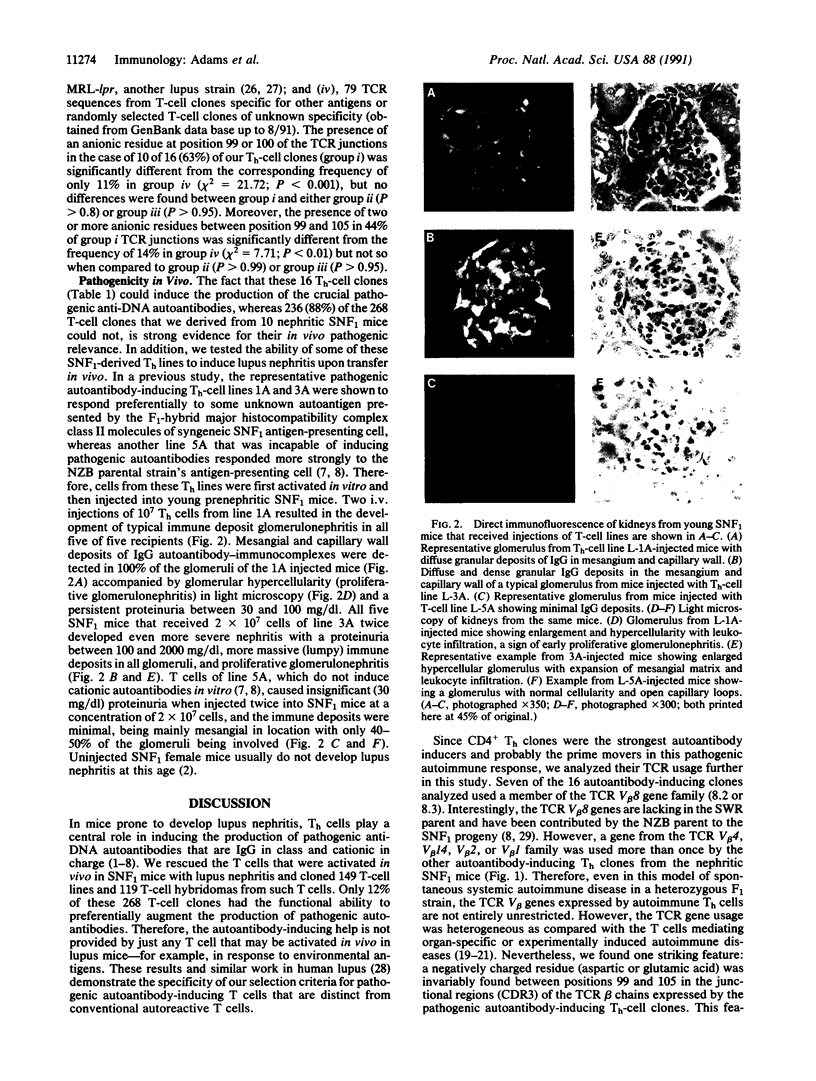
We rescued from the spleens of 10 (SWR x NZB)F1 (SNF1) mice with lupus nephritis the T cells that were activated in vivo and cloned 268 T-cell lines and hybridomas. Only 12% of these T-cell clones had the functional ability to preferentially augment the production of pathogenic anti-DNA autoantibodies. Among these, 16 helper T-cell (Th-cell) clones that were mostly CD4+ and had the strongest autoantibody-inducing ability were analyzed for T-cell receptor (TCR) beta-chain gene usage. Seven of the 16 Th-cell clones expressed beta-chain variable region (V beta) V beta 8 (8.2 or 8.3) genes and three expressed V beta 4, whereas two clones each used a V beta 1 or V beta 2 or V beta 14 gene, suggesting some restriction in TCR gene usage. Although heterogeneous, the V-D-J junctional region sequences of TCR beta-chain genes used by these Th-cell clones invariably encoded one or more negatively charged residues (aspartic or glutamic acid) that had been generated in most cases by unspecified nucleotide (N) additions. Representative pathogenic autoantibody-inducing Th-cell clones could rapidly induce the development of lupus nephritis when injected into young prenephritic SNF1 mice. The pathogenic autoantibody-inducing Th cells expressing the anionic residues in their TCR beta-chain junctions (complementarity-determining region CDR3) were probably selected by some cationic autoantigenic peptide presented by the anti-DNA B cells they preferentially helped. These results offer a clue regarding the nature of the primary autoantigen that may drive the pathogenic autoimmune response in lupus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Adams S., Zordan T., Sainis K., Datta S. T cell receptor V beta genes expressed by IgG anti-DNA autoantibody-inducing T cells in lupus nephritis: forbidden receptors and double-negative T cells. Eur J Immunol. 1990 Jul;20(7):1435–1443. doi: 10.1002/eji.1830200705. [DOI] [PubMed] [Google Scholar]
- Ando D. G., Sercarz E. E., Hahn B. H. Mechanisms of T and B cell collaboration in the in vitro production of anti-DNA antibodies in the NZB/NZW F1 murine SLE model. J Immunol. 1987 May 15;138(10):3185–3190. [PubMed] [Google Scholar]
- Barth R. K., Kim B. S., Lan N. C., Hunkapiller T., Sobieck N., Winoto A., Gershenfeld H., Okada C., Hansburg D., Weissman I. L. The murine T-cell receptor uses a limited repertoire of expressed V beta gene segments. Nature. 1985 Aug 8;316(6028):517–523. doi: 10.1038/316517a0. [DOI] [PubMed] [Google Scholar]
- Behlke M. A., Chou H. S., Huppi K., Loh D. Y. Murine T-cell receptor mutants with deletions of beta-chain variable region genes. Proc Natl Acad Sci U S A. 1986 Feb;83(3):767–771. doi: 10.1073/pnas.83.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel V., Dohlman J., Blaisdell B. E., Karlin S. Very long charge runs in systemic lupus erythematosus-associated autoantigens. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1536–1540. doi: 10.1073/pnas.88.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danska J. S., Livingstone A. M., Paragas V., Ishihara T., Fathman C. G. The presumptive CDR3 regions of both T cell receptor alpha and beta chains determine T cell specificity for myoglobin peptides. J Exp Med. 1990 Jul 1;172(1):27–33. doi: 10.1084/jem.172.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. K. A search for the underlying mechanisms of systemic autoimmune disease in the NZB x SWR model. Clin Immunol Immunopathol. 1989 May;51(2):141–156. doi: 10.1016/0090-1229(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Datta S. K., Patel H., Berry D. Induction of a cationic shift in IgG anti-DNA autoantibodies. Role of T helper cells with classical and novel phenotypes in three murine models of lupus nephritis. J Exp Med. 1987 May 1;165(5):1252–1268. doi: 10.1084/jem.165.5.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastcott J. W., Schwartz R. S., Datta S. K. Genetic analysis of the inheritance of B cell hyperactivity in relation to the development of autoantibodies and glomerulonephritis in NZB x SWR crosses. J Immunol. 1983 Nov;131(5):2232–2239. [PubMed] [Google Scholar]
- Engel I., Hedrick S. M. Site-directed mutations in the VDJ junctional region of a T cell receptor beta chain cause changes in antigenic peptide recognition. Cell. 1988 Aug 12;54(4):473–484. doi: 10.1016/0092-8674(88)90068-2. [DOI] [PubMed] [Google Scholar]
- Falcioni F., Dembic Z., Muller S., Lehmann P. V., Nagy Z. A. Flexibility of the T cell repertoire. Self tolerance causes a shift of T cell receptor gene usage in response to insulin. J Exp Med. 1990 May 1;171(5):1665–1681. doi: 10.1084/jem.171.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Gauthier V. J., Mannik M. A small proportion of cationic antibodies in immune complexes is sufficient to mediate their deposition in glomeruli. J Immunol. 1990 Nov 15;145(10):3348–3352. [PubMed] [Google Scholar]
- Ghatak S., O'Keefe T. L., Imanishi-Kari T., Datta S. K. Selective strain distribution pattern of a germline VH gene for a pathogenic anti-DNA autoantibody family. Int Immunol. 1990;2(10):1003–1012. doi: 10.1093/intimm/2.10.1003. [DOI] [PubMed] [Google Scholar]
- Hashim G., Vandenbark A. A., Gold D. P., Diamanduros T., Offner H. T cell lines specific for an immunodominant epitope of human basic protein define an encephalitogenic determinant for experimental autoimmune encephalomyelitis-resistant LOU/M rats. J Immunol. 1991 Jan 15;146(2):515–520. [PubMed] [Google Scholar]
- Hedrick S. M., Engel I., McElligott D. L., Fink P. J., Hsu M. L., Hansburg D., Matis L. A. Selection of amino acid sequences in the beta chain of the T cell antigen receptor. Science. 1988 Mar 25;239(4847):1541–1544. doi: 10.1126/science.2832942. [DOI] [PubMed] [Google Scholar]
- Lai M. Z., Jang Y. J., Chen L. K., Gefter M. L. Restricted V-(D)-J junctional regions in the T cell response to lambda-repressor. Identification of residues critical for antigen recognition. J Immunol. 1990 Jun 15;144(12):4851–4856. [PubMed] [Google Scholar]
- Liao N. S., Maltzman J., Raulet D. H. Positive selection determines T cell receptor V beta 14 gene usage by CD8+ T cells. J Exp Med. 1989 Jul 1;170(1):135–143. doi: 10.1084/jem.170.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- O'Keefe T. L., Bandyopadhyay S., Datta S. K., Imanishi-Kari T. V region sequences of an idiotypically connected family of pathogenic anti-DNA autoantibodies. J Immunol. 1990 Jun 1;144(11):4275–4283. [PubMed] [Google Scholar]
- Ohga S., Yoshikai Y., Kishihara K., Matsuzaki G., Ogimoto M., Nomoto K. Different expression of T-cell receptor beta-chain variable region genes in lymph nodes of lpr mice with different alleles of the major histocompatibility complex. Immunology. 1990 Jun;70(2):216–222. [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Potts W., Wakeland E. K., Kappler J., Marrack P. Surprisingly uneven distribution of the T cell receptor V beta repertoire in wild mice. J Exp Med. 1990 Jan 1;171(1):49–62. doi: 10.1084/jem.171.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S., Zordan T., Tsokos G. C., Datta S. K. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8- T helper cell lines that express the gamma delta T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7020–7024. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Marrack P., Kappler J. W. Antigen-specific, H-2-restricted helper T cell hybridomas. J Exp Med. 1982 Jul 1;156(1):191–204. doi: 10.1084/jem.156.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainis K., Datta S. K. CD4+ T cell lines with selective patterns of autoreactivity as well as CD4- CD8- T helper cell lines augment the production of idiotypes shared by pathogenic anti-DNA autoantibodies in the NZB x SWR model of lupus nephritis. J Immunol. 1988 Apr 1;140(7):2215–2224. [PubMed] [Google Scholar]
- Singer P. A., McEvilly R. J., Noonan D. J., Dixon F. J., Theofilopoulos A. N. Clonal diversity and T-cell receptor beta-chain variable gene expression in enlarged lymph nodes of MRL-lpr/lpr lupus mice. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7018–7022. doi: 10.1073/pnas.83.18.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991 Feb 22;64(4):767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- White J., Blackman M., Bill J., Kappler J., Marrack P., Gold D. P., Born W. Two better cell lines for making hybridomas expressing specific T cell receptors. J Immunol. 1989 Sep 15;143(6):1822–1825. [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- Zaller D. M., Osman G., Kanagawa O., Hood L. Prevention and treatment of murine experimental allergic encephalomyelitis with T cell receptor V beta-specific antibodies. J Exp Med. 1990 Jun 1;171(6):1943–1955. doi: 10.1084/jem.171.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]