Abstract
The history of science has many functions. Historians should consider how their work contributes to various functions, going beyond a simple desire to understand the past correctly. There are both internal and external functions of the history of science in relation to science itself; I focus here on the internal, as they tend to be neglected these days. The internal functions can be divided into orthodox and complementary. The orthodox function is to assist with the understanding of the content and methods of science as it is now practised. The complementary function is to generate and improve scientific knowledge where current science itself fails to do so. Complementary functions of the history of science include the raising of critical awareness, and the recovery and extension of past scientific knowledge that has become forgotten or neglected. These complementary functions are illustrated with some concrete examples.
Keywords: history of science, complementary science, critical awareness, replication
Beyond the common uses of the history of science
I have chosen a provocative title for this paper, but the question it contains is not simply intended to attract attention. It is a serious, literal question that I have been facing every day for over 20 years in my work as a practitioner and a teacher of the history of science, and in recent years in my work for the British Society for the History of Science and other similar organizations. From members of the public, from science educators, from practising scientists, we professional historians of science often get very sceptical questions about the usefulness of our work. The situation is similar for our sister discipline, the philosophy of science. The great physicist Richard Feynman is said to have quipped that the philosophy of science is only as useful to scientists as ornithology is to birds. I fear what he might have thought about the history of science, which would be more like palaeontology, obsessing about extinct birds.
In a progressive enterprise like science, why does one need history? Why worry about the past of science, which might be considered at best an inferior approximation of the present, and at worst a set of dead-ends that our ancestors thankfully managed to escape from? We can get some insight into such a perspective by reminding ourselves what aspects of the history of science do get mentioned in science textbooks, or in the popular media. They tend to be ‘human interest’ stories, appearing as mere garnishes to presentations of scientific content—stories of heroic scientists who overcame adversity, tragic scientists hampered by human limitations and circumstances, fortunate scientists who made great discoveries by exploiting chance happenings, strange scientists who engaged in bizarre experiments or devised fantastical theories, and so on. Such stories dominate the popular imagination concerning the history of science, whether they be about August Kekulé discovering the structure of the benzene ring by dreaming of a snake biting its own tail, Alexander Fleming being led to penicillin through his mouldy petri dish, Benjamin Franklin confirming the nature of lightning by flying a kite in a thunderstorm or Galileo Galilei dropping balls from the leaning tower of Pisa to refute Aristotelian physics.
Many of these stories are myths, even if they are not as crude as the iconic tale of how Isaac Newton discovered the universal law of gravitation thanks to an apple that fell on his head.1 We historians of science routinely groan or fulminate when presented with such stories; but in the rightful condemnation of such myths, there is something important that is often missed out, namely, the question of purpose: what exactly is it that we should be trying to achieve by writing and teaching history? The heroic tales or the bizarre anecdotes found in popular historical accounts do have positive functions in the right circumstances: they can give inspiration to students aspiring to enter science, or excite people's curiosity about the process of science. When we clear away the mythology, we need to ask what we are putting in place instead, and to what purpose. We must ask ourselves not only what good history is, but what good history is.
I am not disputing that historians of science should take pains to show that stories like the apple falling on Newton's head have no firm historical foundation. We should. Moreover, we should remind people that the idea of gravitation had a long and complex gestation in the thinking of Newton himself and many of his predecessors and contemporaries, that Newton's true achievement was not thinking up the idea of gravity but formulating it in precise mathematical form and applying it to important celestial and terrestrial phenomena, that his work in physics was intimately linked with his work in alchemy and theology, and so on. But we also need to ask: what benefits will follow when people understand all of that? Thirty years ago John Heilbron (the 2006 Wilkins Lecturer) urged his fellow historians of science to pay more serious attention to what he called ‘applied history of science’, focusing on its uses for general education, science education and science policy.2
We historians need to see beyond our intense desire to understand the past correctly. For the moment I am going to set aside the problem of whether there is really such a thing as the unvarnished truth (not to mention the unique best understanding). What I want to emphasize is that, in our condemnation of careless distortions of history, we often fall into another trap. This mis-step begins with the faultless observation that many of the errors we want to combat arise from trying to fit the past into frameworks defined by our present assumptions and concerns. In the words of Herbert Butterfield: ‘The study of the past with one eye, so to speak, upon the present is the source of all sins and sophistries in history, starting with the simplest of them, the anachronism.’3 Butterfield's anti-presentist advice was that ‘real historical understanding’ was achieved by ‘attempting to see life with the eyes of another century than our own’.4 But if we have followed Butterfield that far, we might just have fallen into the trap that I am referring to. We must not forget the fact that we historians are inevitably stuck in the present, no matter how much we might kick and scream about it. Even though history is about the past, history-writing is for the living, in the present. In our attempt to avoid the ills of presentism, we historians often decline to think about our own present and fall under the illusion of selfless objectivity. But we cannot be clear about our own purposes if we do not consider our own present situations. Understanding the past is good, but the point of that understanding must be in the present; we must articulate in which ways a misunderstanding of the past harms us and a better understanding of the past helps us. In the phrase of the radical American educationist, Neil Postman, we should be asking ‘how the past can improve our future’.5
Functions of history: a systematic view
Let us start by taking a quick systematic overview of what the possible functions of the history of science might be. I would start by drawing a distinction between those functions that are internal to science itself, and those that are external to it. Now, the traditional internal–external distinction is viewed with great suspicion by many historians, and for good reasons.6 However, it is possible to find a version of the distinction that is both sensible and useful. For example, Dudley Shapere understands the internal as what has been internalized in a particular epistemic community. Considerations internal to a science are based on a body of beliefs that have come to be accepted beyond specific and practical doubt within the community practising that science, owing to the success and coherence of inquiries made on their basis. Such beliefs ‘constitute a basis on which science can alter its domains and build further hypotheses, methods, rules of reasoning, and goals’. The internal–external distinction is ‘forged in the very process of investigation of nature, not laid down in some edict from heaven or philosophy which determines what counts as scientific and what does not’.7
These days the external side tends to be emphasized more, with the current trend within the history of science field being on understanding science as a social and cultural phenomenon, and the obsession outside academia being with science as a driver of technological and economic development rather than something worth doing for its own sake. So, we may hope that a better understanding of the history of science will help us use and control science more wisely and support it more effectively in economic and institutional terms.
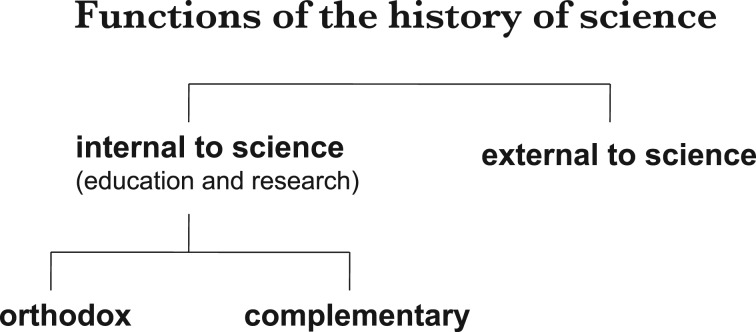
In the spirit of paying attention to what is neglected, I will focus my attention on the functions of the history of science that are internal to science. Roughly speaking, that means asking whether the study of the past of science can help us improve present scientific knowledge. This is generally not at the forefront of historians' thinking, though there has been a venerable tradition of attempts to use the history of science to improve science education.8 There are many ways in which the knowledge of the history of science may enhance scientific knowledge itself. To conceptualize this more precisely, I want to distinguish between the orthodox and the complementary functions of the history of science in relation to science itself (see figure 1). On the orthodox side of this divide, historical knowledge can help us understand better the scientific knowledge that we accept at present. To those who can handle some complexity, knowing how we came to know what we know can add a layer of depth to scientific knowledge, with a more nuanced understanding of concepts and a more assured justification of the results that we might otherwise be condemned to accept simply as gospel truth. Much of my own historical research has been in this direction.9
Figure 1.
Functions of the history of science.
Another orthodox function of the history of science is to teach us about scientific methods. Peter Medawar, one of the three greats in whose honour this lecture prize is named, thought that this was an area in which routine scientific training was woefully inadequate. His lament from half a century ago still rings true today:
Ask a scientist what he conceives the scientific method to be, and he will adopt an expression that is at once solemn and shifty-eyed: solemn because he feels he ought to declare an opinion; shifty-eyed because he is wondering how to conceal the fact that he has no opinion to declare.10
If we think science is working well, we must work to keep it working well, and it is necessary that scientists be trained in the methods of science. This does, of course, happen for the specialists by way of learning-by-doing, but that is not sufficient. Taking a historical viewpoint can be useful, rather than always being immersed in the current state of one's own narrow specialism. And for non-specialists, it is very useful to be able to learn about scientific methods by going back to the history, without having to master the formidable technical details of contemporary science. (The education of non-specialists links to the external functions of the history of science as well, since people with no understanding of the scientific method could not plausibly direct science policy.)
Three functions of history as ‘complementary science’
So far, I hope I have been saying obvious things that will be regarded merely as useful reminders. From this point on, I would like to focus on more unusual and controversial points, by addressing what I call the complementary function of the history of science, which is to generate and improve scientific knowledge where science itself fails to do so. This is in line with my notion of ‘complementary science’, which is how I see the task of the discipline of history and philosophy of science.11 The need for complementary science arises because scientific specialists need to agree on a particular set of research questions and tackle them by orthodox methods, rather than allow themselves to be distracted by unrestricted questioning and unlimited curiosity. Such narrowness of focus is both necessary and effective, as Thomas Kuhn has emphasized in characterizing what he called ‘normal science’,12 but it also inevitably leads to a neglect of other questions and other perspectives, which are in themselves perfectly valid and scientific. (The boundary between the orthodox and the complementary, being defined by where the orthodoxy happens to situate itself, is moveable and permeable, though it often feels like a brick wall when one runs up against it.) What better people to take up such neglected aspects of scientific knowledge, than historians and philosophers of science?
Critical awareness
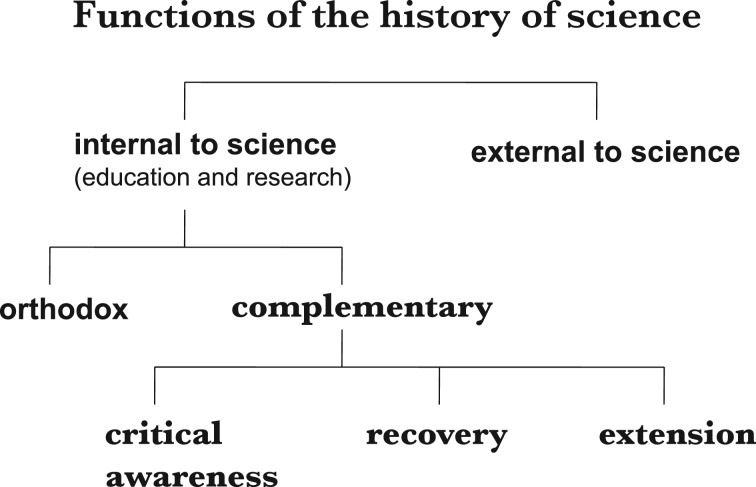
There are three main complementary functions of the history of science that I see (figure 2). The first is to enhance critical awareness. In my own work I find that history is one of the best critical tools for the philosopher. More generally speaking, learning history is a wonderful way of opening one's mind to new possibilities. This works in two different ways.
Figure 2.
Complementary functions of the history of science.
First, historical understanding reveals the contingency of our present situation. The Italian historian and philosopher, Benedetto Croce, once said: ‘Only historical judgment liberates the spirit from the pressure of the past.’13 Learning how scientists came to believe today's orthodoxy can aid our understanding of present science, as I mentioned earlier, but it may also reveal what we take as evident and necessary truths today as results of past decisions that were contingent and could have gone in a different way. It is significant that I learned Croce's dictum from its use as the epigraph in John Wheeler's preface to Thomas Kuhn and colleagues' description of the Archive for the History of Quantum Physics.14
An excellent expression of this liberatory function of history can be found in the work of James Cushing, which showed that the establishment of the Copenhagen interpretation of quantum mechanics as orthodoxy was due to various historical contingencies, including the order in which some key events happened to occur.15 John Heilbron, who was one of Kuhn's collaborators in the Archive project, went on to make a detailed analysis of one key aspect of that history, namely the fact that there happened to be a group of physicists around Niels Bohr who actively set out to persuade everyone else to adopt their new world-view, whom he called the ‘earliest missionaries of the Copenhagen spirit’.16 Against the consolidation of the Copenhagen orthodoxy, even Einstein's concerns had little force. One could argue that Albert Einstein only made philosophical complaints without putting forward any alternative quantum physics. But when David Bohm did provide a good alternative theory, as Cushing explains in detail, he was still side-lined and ignored. And I am not speaking as an adoring fan of Einstein. The absolute dominance of his own theories of relativity was itself a highly contingent event, and it would have been perfectly rational to maintain the ether-based theories of James Clerk Maxwell, Lord Kelvin (William Thomson), Hendrik Antoon Lorentz, Henri Poincaré and other great physicists.
In addition to teaching us about the general contingency of the present, the study of the past also serves to expand our conceptual horizons more specifically. It is said that truth is stranger than fiction, and actual past science can go beyond our imagination because our imagination is usually heavily constrained by our present situation. The motto here is provided by the novelist L. P. Hartley, who opened The Go-Between thus: ‘The past is a foreign country; they do things differently there.’17 Learning history is broadening like travelling is meant to be broadening. It is a common occurrence for those of us who delve into old scientific texts to slap our knees and exclaim: yes, one could think like that!
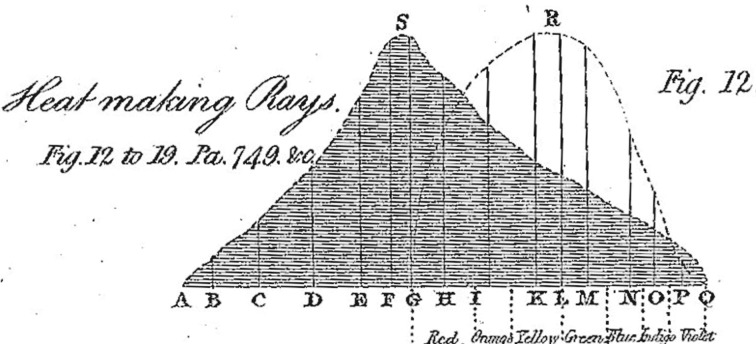
One quick example will illustrate this point well. The astronomer William Herschel, the discoverer of Uranus, is also known for his discovery of infrared light in 1800. However, that is not how Herschel himself and many of his contemporaries conceived the latter achievement; for them, what Herschel had done was to separate out, by means of the prism, rays of caloric (heat) from rays of light in the sunbeam.18 Herschel had probed the solar spectrum by placing a thermometer in various parts of it. Noting that the heating power increased as he moved towards the red end of the visible spectrum, Herschel tested whether it might continue into the dark space beyond the red, and indeed detected a great heating effect there. Joseph Banks, the long-time President of the Royal Society, wrote to Herschel in appreciation of his account of this discovery submitted to the Royal Society: ‘I have shown your second paper to Mr. Cavendish and to some other of my friends … and all are struck with the discovery, of Radiant Heat being separable from Radiant Light.’19 Figure 3 shows Herschel's own representation of this interpretation, showing the distributions of ‘heat making rays’ and the light rays as clearly distinct entities.20
Figure 3.
William Herschel's representation of heat and light rays from the sun.
Recovery
In addition to serving the role of opening our minds, past systems of science are valuable in themselves. This brings us to the function of history that consists in the recovery of lost scientific knowledge. Kuhn famously argued that when a scientific revolution happens, some knowledge that had been established in the old paradigm is liable to become lost. He accepted this as part of the normal course of scientific development, but there is no reason why historians should not contemplate with real appreciation what we dig up from the past. If the chemistry of phlogiston, and Antoine-Laurent Lavoisier's chemistry of oxygen and caloric, each once gave valid understanding of nature to the practitioners of those systems, then they can still provide that same understanding concerning the domains of phenomena in which they remain valid.21 This is how orthodox scientists in fact treat venerable old theories of physics such as geometric optics and Newtonian mechanics, which they still teach to every student of physics. That same appreciative attitude could be extended with benefit to some of the theories that orthodox science has now rejected.
This agenda of recovery may be quite difficult to accept when it comes to theories. It is difficult to go against the confidence of present experts that the old theories were rejected for good reasons, and deserve to be forgotten. This is why experimental work is so valuable in this context, because our own judgement can more easily be independent when we come face-to-face with phenomena themselves. Let me illustrate this point with a few examples.
In a study published in 1791, Marc-Auguste Pictet made a striking experiment in Geneva demonstrating the reality of radiant heat. He set up two concave metallic mirrors facing each other and placed a sensitive thermometer at the focus of one mirror; then he brought a hot but not glowing object into the focus of the other mirror, and observed that the thermometer reading began to rise immediately. The really surprising result came when Pictet made the same experiment with a cold object (a flask filled with snow): this time the temperature at the other focus began to sink immediately! Count Rumford, well-known to historians of science as a pioneer of the kinetic theory of heat (and the founder of the Royal Institution in London), unleashed a controversy by interpreting Pictet's result as a genuine action of ‘frigorific rays’, and performed striking new experiments to support his view.22 When faced with such reports from the past that seem alien to modern science, historians may try to confirm if the alleged phenomena can be reproduced; if so, then we will have recovered a piece of forgotten scientific knowledge. In this case, Pictet's experiment was duly replicated by two modern physicist–historians, James Evans and Brian Popp, though that replication itself seems to have been largely neglected.23
In another set of cases, many historians of science have noted that the Romantic literary giant Johann Wolfgang von Goethe had an intriguing theory of light and colours, which he set up against Newton's optics. Lately, some historians and philosophers of science have taken the additional step of reproducing many of Goethe's intriguing optical experiments in order to understand first-hand the phenomena that he was investigating.24
My own first experience with the replication of such forgotten experiments was at University College London in the summer of 2004, which I spent boiling water with utter fascination. I confirmed many implausible-sounding eighteenth- and nineteenth-century claims, that there were great variations in the boiling temperature and behaviour of pure distilled water under normal atmospheric pressure, depending on the material of the vessel in which the boiling happened, on the rate at which the heating was done and on the amount of dissolved gases in the water.25 Here I will describe just one experiment, for illustration.26 Heat water on a hotplate in a flask with a long, thin neck. This is an approximation of Jean-André De Luc's attempts to use slow heating in order to bring the whole body of water to the same temperature as that of the first layer in contact with the heat source. The hotplate is very hot but still much gentler than a naked flame. The behaviour of the water in this setup is very different from boiling driven by an open flame. As the temperature approaches 100°C, the water starts to boil in a normal way. As boiling continues, however, the temperature continues to rise, while the bubbles get bigger but less frequent; they also come more irregularly, often in bursts (this is what nineteenth-century observers termed ‘bumping’). The temperature goes over 100°C, easily reaching 101–102°C. With continued heating, the bubbles can become even less frequent, while temperature creeps up further; often there are long quiet periods punctuated by isolated large bubbles; sometimes the puffs are explosive, throwing some water out of the flask. My experiments routinely produced temperatures of 104°C or above during this very irregular boiling, which can continue indefinitely, maintaining clearly superheated temperatures even after explosive puffs. All of this is entirely consistent with what De Luc had reported in 1772. (The increasing ‘bumpiness’ of boiling is most likely the result of having more and more dissolved air taken out of the water.)
Historical boiling was a truly formative experience, which greatly heightened my sensitivity to lost phenomena from past science. The next striking case came up as I started looking into the intriguing early history of electrochemistry.27 This was an experiment reported by William Hyde Wollaston in 1801.28 He began with the familiar observation that certain metals were dissolved by acids, releasing bubbles of hydrogen. This phenomenon, first recorded with clarity by Henry Cavendish in 1766, can be observed very easily and safely by dipping a zinc wire in dilute hydrochloric acid or sulphuric acid; the zinc dissolves slowly, producing a fine stream of hydrogen bubbles.29 Now put a silver wire into the same pot of acid, and no reaction happens there, as hydrochloric and sulphuric acids do not attack silver. But just make the zinc and the silver wires touch each other (either inside or outside the solution), and hydrogen bubbles immediately start issuing from the silver as well as the zinc. Wollaston also observed the same phenomenon with various other metal–acid–metal combinations in which only one metal was soluble in the acid.
My attempt to replicate this experiment succeeded immediately, using a zinc wire and a copper wire in hydrochloric acid (see figure 4a and b).30 In case the phenomenon seems bewildering, it is useful to remember that Wollaston put forward this experiment in the context of the excited debate about the operation of Alessandro Volta's ‘pile’ (battery), an invention that had been publicized just a year earlier. It is easy to recognize that the topology of Wollaston's experiment is the same as that of the Voltaic cell: namely, two different metals with an electrolyte between them.31 In Wollaston's own view, his experiment demonstrated that the chemical reaction between the zinc and the acid was responsible for the release of the electrical fluid, and the silver (or copper) simply conducted an overflow of the electrical fluid, passing it into the liquid. The action of electricity, then, decomposed the water in the acid, releasing hydrogen bubbles from the surface of the silver or copper, which itself does not chemically react with the acid.32 Translated into modern terms (see figure 5), Wollaston's explanation is that electrons are let loose from the reaction between the zinc and the acid, and then some of them are conducted over to the copper wire; as they come out into the solution from the copper, they combine with hydrogen ions (H+) present in the acid and create hydrogen gas.
Figure 4.
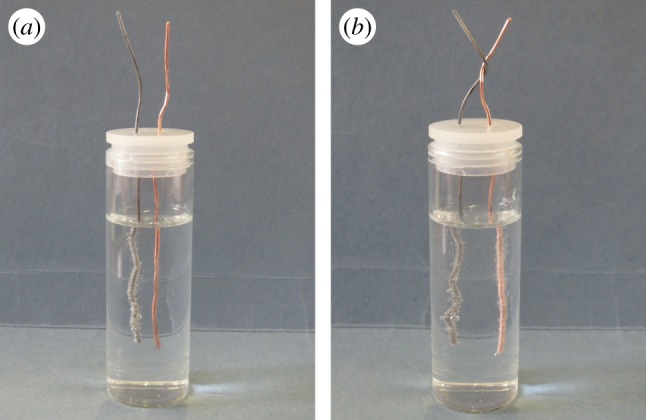
A modern reproduction of Wollaston's experiment, (a) and (b) using zinc and copper in hydrochloric acid (HCl).
Figure 5.
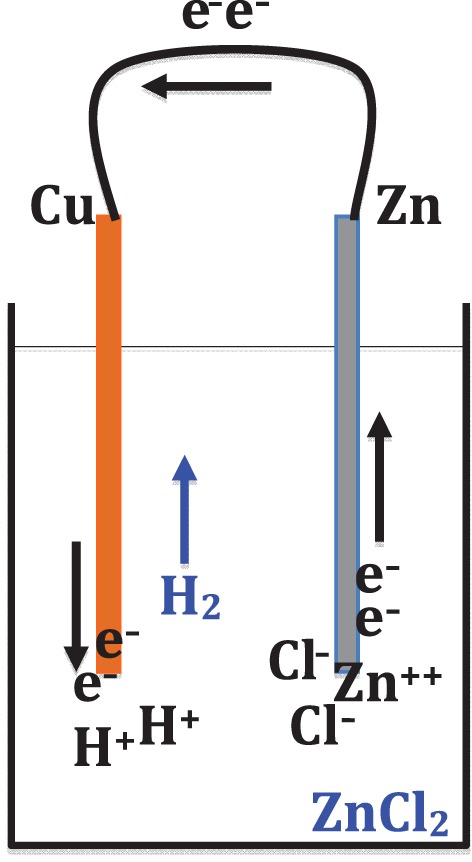
A semi-modern rendition of Wollaston's theoretical account of his experiment. (Online version in colour.)
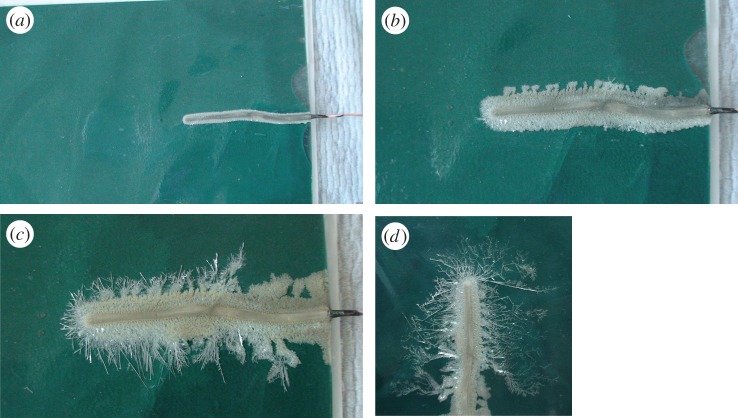
One other simple experiment from the early nineteenth century illustrates the process of recovery even more strikingly. In 1806 Charles Sylvester, chemist and inventor in Sheffield, published an account of various experiments including the following: ‘If a thin coat of a solution of nitrate of silver be laid upon a piece of plain glass, and in the centre of this be laid a bit of zinc wire, in a little time a beautiful tree of silver will appear as if growing from the wire.’33 I was incredulous at reading this: it seemed obvious that the zinc wire would be covered by a layer of silver, due to a zinc–silver replacement in the nitrate solution, but why would anything happen beyond that? Again, this was very easy to check by repeating Sylvester's procedure. Figure 6a–d shows the progress of Sylvester's experiment, which I made using a copper wire instead of zinc, and with the silver nitrate solution (roughly 1 molar concentration) contained in a flat transparent plastic envelope rather than spread on a glass pane.
Figure 6.
Sylvester's silver tree, after (a) 4 minutes, (b) 9 minutes, (c) 26 minutes and (d) 133 minutes. (Online version in colour.)
Such phenomena recovered from the dusty depositories of past science deserve to be remembered and appreciated. They may seem trivial, but such a judgement is most likely shaped by present orthodoxy. A seemingly trivial phenomenon can also stimulate much larger developments, as we know from Fleming's mouldy petri dish. About the recovery of valuable things from the past, the French historian and philosopher, Henri Marrou, had this to say: ‘I will assign to history, as one of its essential functions, the enrichment of my internal universe by recapturing cultural [valuables] salvaged from the past.’ These exist:
in the bosom of lost societies or civilizations. But to the extent that we are capable of grasping and understanding them, they again come to life in us. In a sense, they acquire a new reality and a second historical existence, in the womb of the historian's thought and in the contemporary culture to which he reintroduces them.34
More succinctly, Barthold Niebuhr in his History of Rome declared: ‘He who calls what has vanished back again into being, enjoys a bliss like that of creating.’35
Extension
Now I would like to address the function of the history of science that I call ‘extension’, meaning the extension of the neglected knowledge that we recover from the past. This may sound quite implausible, and again, it is easier to provide a proof of concept if we look to the experimental rather than theoretical end of things. I will give a few illustrations from my current project on the history of electrochemistry and batteries.
Almost every recovered experiment leads to open questions, which one can attempt to investigate by further experiments. This is, indeed, in the very nature of experimental science. So, having replicated Sylvester's beautiful experiment just mentioned, I was naturally led to a question as to how it happens. Why doesn't the reaction result in all of the copper surface getting covered in silver, and stop there? Why does more silver keep growing on silver? Sylvester's own view was that, when any silver gets deposited on the copper (or zinc), the two metals in contact with each other, and with the nitrate solution, form a Voltaic cell. The electricity flows out to the far reaches of the silver deposit and reduces further silver there. (In modern terms, electrons travel to the far end of the silver tree and meet silver ions in the solution in that vicinity, and produce and deposit more of the neutral silver there.) This is a plausible story, but it would be useful to check whether it is correct. It would also be interesting to investigate theoretically why the electrons disengaged from copper would travel across to the end of existing silver branches to meet silver ions there, instead of combining with the silver ions nearby, which would soon have the consequence of getting the entirety of the copper surface covered with silver, stopping the Voltaic action.
A very similar question also arises in relation to Wollaston's experiment. What exactly is the role of the inert metal (copper, in my version of the experiment)? For Wollaston, it was just receiving an overflow of electricity produced by the chemical reaction on the zinc side. For Volta, the zinc–copper contact was what actually caused the flow of electricity. This was a crucial point of contention between the ‘chemical theory’ and the ‘contact theory’ of Voltaic electricity.36 It is possible to engage with this 200-year-old debate in a new way. For example, figure 7 shows a very simple variation on Wollaston's experiment, in which the copper wire is replaced by a gold wire. Now most of the bubbles come out from the gold wire, though it is still only zinc that is dissolving in the acid. In a modern shorthand, I would say that the gold is clearly more effective than copper in pulling electrons over from the zinc. This would make perfect intuitive sense to modern physicists, in terms of bimetallic contact potentials arising from the differences in the work functions of different metallic surfaces, but such thinking is alien to electrochemists, whose conceptual framework is descended from the old chemical theory of Voltaic electricity.
Figure 7.

Wollaston's experiment, with zinc and gold wires.
If we look at Volta's original work, further questions come up. Instead of employing acids, as in Wollaston's experiment, Volta had used salt water in his original battery. This raises intriguing questions about the electrochemistry of sodium chloride (NaCl) solution, especially as to where electrons would go when they are released into it (what positive ions in the solution they would combine with); when a sodium chloride Voltaic cell is in operation, there is no visible hydrogen gas produced, not to mention any production of metallic sodium (see figure 8). Trying to make whatever is happening there more pronounced, I pumped electrons into a saturated salt solution at a very high rate by connecting a couple of batteries to copper electrodes inserted into the solution. A prodigious amount of hydrogen gas issued from the negative electrode, with an audible hiss. That raised an interesting puzzle, as there cannot be enough H+ ions in a solution of NaCl to receive electrons at such a rate. (Those who have made an electrolysis of water will know how slowly that reaction goes, even when it is aided by the addition of some acid into the water.) The only conclusion I could reach was that in the saturated salt solution the influx of electrons must result in a direct breakdown of H2O molecules at the electrode. Some scientists I have spoken to about this are quite sceptical about this idea, and others think it is obviously the case.37 If my thinking is correct, another interesting question is why the same does not happen in dilute solutions; there is probably no mystery there, but it would be instructive to work out a detailed answer. And while the negative electrode is producing hydrogen, the positive electrode dissolves, producing an orange precipitate whose identity is not immediately obvious—it does not seem to be copper (II) chloride (blue) or copper (II or I) oxide (red or black).
Figure 8.
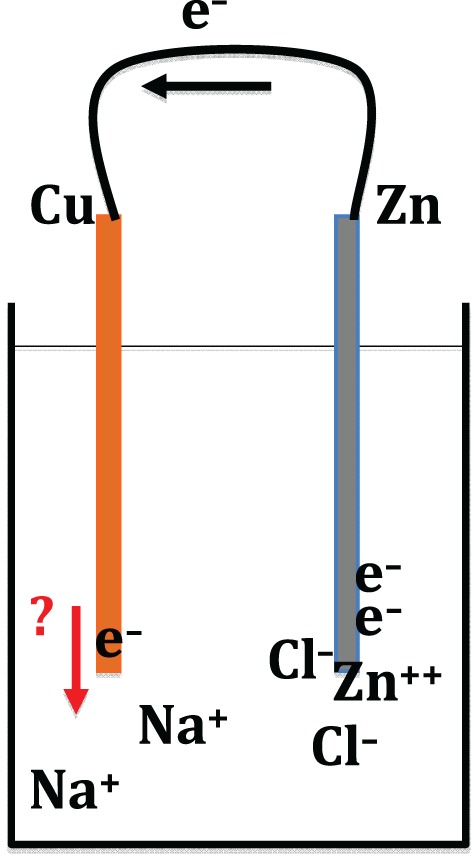
A semi-modern attempt to understand the action of a Voltaic cell employing salt water (NaCl) as the electrolyte. (Online version in colour.)
Rather than plunging further into the strangely intricate world of basic electrochemistry,38 I will finish with a brief account of a related experiment, whose interest can be much more readily appreciated. Interesting as the dissolution of the copper electrode was, I did not actually want it to happen because it complicated the question of what electrons did when they went into the NaCl solution, so I wanted to use chemically inert electrodes: there are various well-known options, such as gold and platinum, and graphite. Doing the experiment using a graphite electrode on the negative side and a gold electrode on the positive side, I was intrigued to learn that the gold electrode dissolved to form a bright yellow solution (whose chemical identity is not known to me as yet; see figure 9), in a narrow window of applied voltage ranging from 2.2 V to 3.5 V approximately, depending on the circumstances (beyond 3.5 V or so, the gold wire stops dissolving and instead serves as a location for the generation of chlorine gas). Dissolving gold in salt water with two household batteries was certainly not what I had set out to do when I innocently stepped into the history of early electrochemistry. There is much more to learn about this phenomenon.
Figure 9.

The dissolution of a gold electrode in salt water.
At this point many historians will be questioning whether I am still doing history: are these investigations not just science, rather than history of science? That question does not trouble me greatly, as I see the boundary between science and the history of science as moveable and permeable. And it is perfectly sensible that some functions of the history of science serve the aims of science itself; that is, after all, partly why I class them as ‘internal functions’. The scientific questions I address in the process of recovery and extension, however, are in the realm of the history of science (and the philosophy of science) because today's scientists tend to neglect them. That, again, reflects the sense of what I call ‘complementary science’.
Concluding remarks
I hope that I have done enough to illustrate the possibility that the history of science may serve the internal function of improving scientific knowledge itself, especially through the complementary recovery and extension of the knowledge that orthodox science has lost track of. I now want to take in the whole picture again, as a brief coda to this presentation.
The external functions of the history of science, which I have not spoken much about, have a close parallel to its internal functions. If we think that science is a force for the good in society, learning the history of what has enabled it to be such will help us maintain its institutions and keep its social and economic benefits flowing. If we think that science is not being supported properly or that it is developing in such a way as to have harmful social effects in its applications or cultural resonances, learning the history of science will facilitate the critical awareness, recovery and extension to help set things right. For those who do not really acknowledge the internal–external distinction, the parallel I am pointing out can be seen really as a continuum. I find inspiration in the work of Paul Forman, who has exhorted historians of science to embrace ‘the obligation to decide for ourselves what is the good of science, and by our historical research and writing to advance that good’.39 He laments the ‘extraordinary … intellectual subservience accepted by historians of science’ in comparison to the situation in the history of philosophy, literature, visual art and music, where the historian also functions ‘as a critic exercising independent judgment’.40 Such an active political conception of the history of science was also very important to J. D. Bernal, another one of the greats we are celebrating in this prize lecture series. It is also evident in the work of the 2009 Wilkins–Bernal–Medawar Lecturer, David Edgerton, whose lecture was titled ‘The social function of history’.41
Another parallel I must note is between the functions of the history of science and the functions of other branches of history. Here it is even clearer that the parallel must really be a continuum. Since science is undeniably a part of human society and culture, it makes sense that the history of science should be in large part like the history of any other aspects of human life. After all, some of my inspirations, such as Croce, Marrou and Niebuhr, were not historians of science at all. The particular importance of this continuity is an enhanced sense that in doing the history of science we should not shrink from addressing the intellectual and social relevance of the past to the present.
I would like to close by returning to one of my starting points. I noted that history is often used in order to excite curiosity and give inspiration for science, and that this motivation often encourages distortions and oversimplifications of history. I hope that my remarks go some way towards showing that curiosity and inspiration are not incompatible with doing full justice to history.
Acknowledgements
This is a slightly amended and expanded version of the Wilkins–Bernal–Medawar Lecture delivered at the Royal Society on 10 May 2016. I thank Jamie Upton for all his help with the organization of the lecture, and Uta Frith for kindly chairing the session. I also thank all those who gave helpful feedback to my plenary lecture at the International Congress of the History of Science, Technology and Medicine on 22 July 2013, in which some of the ideas discussed here were presented.
Notes
There is a better story (from my university classmate, Li Ho): gravity was actually discovered long before Newton by a Southeast Asian lying under a durian tree, but he didn't live to tell anyone about it.
John L. Heilbron, ‘HSS lecture: applied history of science’, Isis 78, 552–563 (1987).
Herbert Butterfield, The Whig interpretation of history (G. Bell, London, 1931), p. 32.
Ibid., p. 16.
Neil Postman, Building a bridge to the 18th century: how the past can improve our future (Random House, New York, 1999).
See Steven Shapin, ‘Discipline and bounding: the history and sociology of science as seen through the externalism–internalism debate’, Hist. Sci. 30, 333–369 (1992).
Dudley Shapere, ‘External and internal factors in the development of science’, Sci. Technol. Stud. 4, 1–9 (1986), at p. 6.
Many decades ago now, James Bryant Conant led the teaching of science through history in the General Education Program at Harvard University, and Gerald Holton and his colleagues achieved great success with the historically framed Project Physics course; see Holton, ‘The Project Physics course: then and now’, Sci. Educ. 12, 779–786 (2003). A great deal of current activity is coordinated and promoted by the International History, Philosophy and Science Teaching Group (IHPST) <http://ihpst.net/> (accessed 6 September 2016). Another effort worthy of note is Douglas Allchin's SHiPS (a resource centre for science teachers using Sociology, History and Philosophy of Science) <http://www.shipseducation.net/> (accessed 6 September 2016).
See, for instance, Hasok Chang, Inventing temperature: measurement and scientific progress (Oxford University Press, 2004), and Hasok Chang, Is water H2O? Evidence, realism and pluralism (Springer, Dordrecht, 2012).
Peter Medawar, Induction and intuition in scientific thought (Methuen, London, 1969), p. 11.
For a full exposition of this idea, see Chang, Inventing temperature, op. cit. (note 9), ch. 6.
Thomas S. Kuhn, The structure of scientific revolutions (University of Chicago Press, 1962).
Benedetto Croce, History as the story of liberty (trans. Sylvia Sprigge) (Norton, New York, 1941), p. 48.
Thomas S. Kuhn, John L. Heilbron, Paul Forman and Lini Allen, Sources for history of quantum physics: an inventory and report (American Philosophical Society, Philadelphia, 1967), p. v.
James T. Cushing, Quantum mechanics: historical contingency and the Copenhagen hegemony (University of Chicago Press, 1994).
John L. Heilbron, ‘The earliest missionaries of the Copenhagen spirit’, Rev. Hist. Sci. 38, 195–230 (1985).
David Lowenthal used Hartley's dictum as the title of his treatise on historiography, The past is a foreign country (Cambridge University Press, 1985).
For a detailed account of this episode, see Martin Hilbert, ‘Herschel's investigation of the nature of radiant heat: the limitations of experiment’, Ann. Sci. 56, 357–378 (1999); Hasok Chang and Sabina Leonelli, ‘Infrared metaphysics: the elusive ontology of radiation’, Stud. Hist. Philos. Sci. 36, 477–508 (2005).
Banks to Herschel, 26 March 1800, quoted in Constance A. Lubbock, The Herschel chronicle: the life-story of William Herschel and his sister Caroline Herschel (Cambridge University Press, 1933), p. 266.
William Herschel, ‘Experiments on the solar, and on the terrestrial rays that occasion heat; with a comparative view of the laws to which light and heat, or rather the rays which occasion them, are subject, in order to determine whether they are the same, or different. Part 2’, Phil. Trans. R. Soc. Lond. Abridged 18, 748–787 (1809); the figure reproduced here is Fig. 12 on Plate XIII. The original unabridged version of the paper is in Phil. Trans. R. Soc. Lond. 90, 437–538 (1800); the figure there appears without the ‘Heat making Rays’ label.
For a detailed account of this episode (the Chemical Revolution), see Chang, Is water H2O?, op. cit. (note 9), ch. 1.
For details of that episode see Hasok Chang, ‘Rumford and the reflection of radiant cold: historical reflections and metaphysical reflexes’, Phys. Perspect. 4, 127–169 (2002).
James Evans and Brian Popp, ‘Pictet's experiment: the apparent radiation and reflection of cold’, Am. J. Phys. 53, 737–753 (1985).
See, for example, Emir Korkut, Newton through the prism of Goethe (self-published, 2011); Olaf Müller, ‘Prismatic equivalence: a new case of underdetermination: Goethe vs Newton on the prism experiments’, Brit. J. Hist. Philos. (2016), 24, 323–347.
For making this work possible, I thank Andrea Sella and the UCL Department of Chemistry.
For a full account, with several video clips of experiments, see Hasok Chang, ‘The myth of the boiling point’ (2007), <http://www.hps.cam.ac.uk/boiling/> (accessed 6 September 2016). The experiment described here is Experiment 5 in the online paper.
For enabling this phase of work, I thank many other colleagues, especially Daren Caruana and Rosemary Coates at UCL, and Peter Wothers at Cambridge.
William Hyde Wollaston, ‘Experiments on the chemical production and agency of electricity’, Phil. Trans. R. Soc. Lond. 91, 427–434 (1801), at p. 427.
It will take a little while before the bubbles start coming, if there is an oxide layer on the wire.
For details of my replications of Wollaston's and similar experiments, see Hasok Chang, ‘How historical experiments can improve scientific knowledge and science education: the cases of boiling water and electrochemistry’, Sci. Educ. 20, 317–341 (2011).
This is, however, not how Volta himself conceived of the unit element in his pile: for him, the cell was a pair of metals in contact, with the wet layer on top to make a non-metallic conducting connection to the next cell.
William Hyde Wollaston, ‘Experiments on the chemical production and agency of electricity’, Phil. Trans. R. Soc. Lond. 91, 427–434 (1801), at pp. 428–429.
Charles Sylvester, ‘Observations and experiments on galvanism, the precipitation of metals by each other, and the production of muriatic acid’, [Nicholson's] A Journal of Natural Philosophy, Chemistry, and the Arts 14, 94–98 (1806), at p. 96.
Henri-Irénée Marrou, The meaning of history (Helicon, Baltimore and Dublin, 1959), pp. 260–261. I thank David d'Avray for introducing me to Marrou's work.
Niebuhr quoted by Charles Lyell, quoted by Roy Porter, quoted in James A. Secord, ‘Knowledge in transit’, Isis 95, 654–672 (2004), at p. 672.
For an instructive account of this debate, see Helge Kragh, ‘Confusion and controversy: nineteenth-century theories of the Voltaic pile’, in Nuova Voltiana: studies on Volta and his times, vol. 1 (ed. F. Bevilacqua and L. Fregonese), pp. 133–157 (Milan: Hoepli, 2000); online at <http://ppp.unipv.it/pagesIT/NuovaVoltFrame.htm> (accessed 6 September 2016).
For a more detailed report on this and other related lines of experimental work, see Chang, op. cit. (note 30), especially section 5.
Trying to isolate this anode-side reaction from what was going on at the other side, I put the two electrodes in different beakers connected by a long salt bridge filled with the same NaCl solution. In that setting, nothing seemed to happen on the anode side, but the solution acquired a slightly milky appearance after some time, indicating that something had indeed happened. I had reason to believe that the orange compound might form only in a highly alkaline environment; in the original experiment, if the influx of electrons at the cathode breaks down water molecules at a high rate and then removes the H+ ions by turning them into hydrogen gas, that would leave a great excess of OH− ions in the solution, making it highly alkaline. This suspicion was confirmed by pouring sodium hydroxide (NaOH) into the milky anode-side solution, which duly produced the orange precipitate. If my reasoning is correct, the orange precipitate is copper (I) hydroxide (CuOH); inorganic chemists will know that this is not a common compound. For some relevant technical background, see Chong‐Hong Pyun and Su‐Moon Park, ‘In situ spectroelectrochemical studies on anodic oxidation of copper in alkaline solution’, J. Electrochem. Soc. 133, 2024–2030 (1986).
Paul Forman, ‘Independence, not transcendence, for the historian of science’, Isis 82, 71–86 (1991), at p. 86.
Ibid., p. 77. I do not know if Forman would approve of the direction of my thought entirely, as he seems to imply that a critical stance in history of science requires a social focus of analysis. However, I do believe that he would be in favour of making independent judgements regarding the quality of scientific knowledge, as he has himself done in his early work, including the classic papers on Weimar physics and quantum electronics.
His activist vision of historiography is also exhibited clearly in David Edgerton, The shock of the old: technology and global history since 1900 (Oxford University Press, 2007).