Abstract
The balance of oxidants and antioxidants within the cell is crucial for maintaining health, and regulating physiological processes such as signalling. Consequently, imbalances between oxidants and antioxidants are now understood to lead to oxidative stress, a physiological feature that underlies many diseases. These processes have spurred the field of chemical biology to develop a plethora of sensors, both small-molecule and fluorescent protein-based, for the detection of specific oxidizing species and general redox balances within cells. The mitochondrion, in particular, is the site of many vital redox reactions. There is therefore a need to target redox sensors to this particular organelle. It has been well established that targeting mitochondria can be achieved by the use of a lipophilic cation-targeting group, or by utilizing natural peptidic mitochondrial localization sequences. Here, we review how these two approaches have been used by a number of researchers to develop mitochondrially localized fluorescent redox sensors that are already proving useful in providing insights into the roles of reactive oxygen species in the mitochondria.
Keywords: oxidative stress, fluorescent probes, mitochondrial reactive oxygen species, organelle targeting, reactive oxygen species
1. Introduction
Mitochondria are the sites of many critical physiological functions including oxidative phosphorylation, amino acid and fatty acid metabolism and the biosynthesis of hormones [1]. As these processes all involve oxidation–reduction reactions, mitochondrial redox regulation and dysregulation has far-reaching consequences that affect the health of cells and the whole body. Levels of reactive oxygen species (ROS) are key to the redox status of mitochondria, and indeed the rest of the cell.
The mitochondria are a major site of ROS production within the cell. This process begins with the premature leakage of electrons from the electron transport chain to molecular oxygen to form the superoxide radical, the predecessor of most other ROS [2]. These processes have been extensively reviewed elsewhere [2–5].
It is now well established that mitochondrial ROS are essential in both physiological and pathological processes. These small molecules play important roles in signalling, with various physiological outcomes, including controlling stem cell differentiation [6] and cardiac remodelling [7], and maintaining the morphology of mitochondria [8,9]. Furthermore, mitochondrially derived ROS are key to signalling processes elsewhere in the cell [10]. In the meantime, excessive mitochondrial ROS production can be damaging to the organelle and to the cell. For example, the hydroxyl radical, produced from the Fenton reaction between iron and hydrogen peroxide, can cause damage to mitochondrial DNA [11], the highly localized production of ROS can damage mitochondrial proteins [12], and severe mitochondrial oxidative stress leads to apoptosis [3].
There is an ongoing requirement to better understand mitochondrial redox processes, whether to screen for mitochondrial disease [13], to identify the most effective mitochondrially targeted drugs [14], or to uncover new roles for mitochondrial metabolites [15]. To achieve these aims, there is a need for the identification and development of the most suitable tools to elucidate and monitor mitochondrial redox state.
In studying mitochondrial ROS and redox processes as distinct from those elsewhere in the cell, it is important that techniques enable selective study of mitochondria, without capturing interfering events in other regions of the cell. Some studies, such as 31P magnetic resonance spectroscopy (MRS) [16] and electron paramagnetic resonance (EPR) [17] rely on a predominance of mitochondria-derived signal (from ADP/ATP and free radicals, respectively) over that from rest of the cell. A clearer picture of mitochondrial chemistry can be achieved by physical separation: studies of isolated mitochondria are routinely performed, and can give very useful information [5,18]. However, in cases where cells are to be monitored over long periods of time, and where there is an interest in investigating the effects of cytoplasmic events on mitochondrial ROS levels, it may be necessary to specifically study the mitochondria within a living cell. Electron microscopy enables the study of morphological changes in individual mitochondria of fixed cells, without providing any chemical information [19]. In order to gain information about the redox environment, or ROS levels, in mitochondria, it is necessary to use chemical tools, in the form of fluorescent sensors, or probes.
Fluorescent sensors may be genetically encoded or exogenously applied, and they change their fluorescence output (whether emission wavelength or intensity) in response to a chemical stimulus [20]. Fluorescent sensors of ROS have proved the mainstay of redox biology studies, and have been reviewed elsewhere [21–23]. Given the key importance of mitochondrial ROS, it is valuable to identify the most appropriate tools for studying this organelle, which will be the subject of this review.
Targeting cargo to cells and organelles can be achieved by employing passive or active transport mechanisms; both of these strategies have been successfully exploited to target redox probes to mitochondria (figure 1). Selective mitochondrial uptake via passive transport can be achieved by using lipophilic cations (figure 1a). The key feature of the mitochondrion enabling this strategy is an exceptionally negative potential across the inner membrane, established by the active proton efflux machinery, instrumental in ensuring the appropriate functioning of the cellular respiration and electron-transfer processes. The built-up potential across this most negatively charged membrane in the cell oscillates between −150 mV and −180 mV in healthy mitochondria, and enables increased accumulation of cationic species at its periphery [24]. The delocalization of charge over a lipophilic scaffold of the molecule, in turn, permits its membrane crossing. Commonly used lipophilic cations include triphenylphosphonium (TPP), cationic fluorophores and cationic short peptide sequences. Alternatively, larger molecules (such as proteins) can be reliably directed to the mitochondria by active cellular transport based on the recognition of naturally occurring mitochondrial localization sequences (MLS) (figure 1b).
Figure 1.
Mitochondrial targeting can be achieved by use of (a) lipophilic cations, which exploit the negative mitochondrial membrane potential; or (b) naturally occurring mitochondrial localization sequences (MLS), which use active mitochondrial transport pathways.
Total mitochondrial ROS levels are a function of mitochondrial number as well as health, so sensors must do more than just report on total mitochondrial ROS load. There are three main characteristics of such sensors, which can be included in the design to give valuable information about mitochondrial redox function.
(1) Temporal resolution (figure 2a). A reversible probe is one that is able to cycle between off and on states, and they can be used to monitor mitochondria during physiological events where analyte levels fluctuate over time. Such probes can be applied to differentiate between chronic oxidative stress and transient changes in redox equilibria, which occur during physiological signalling events; a single time-point reading of high ROS could result from either of these conditions.
(2) Specificity for individual ROS (figure 2b). It is known that individual ROS play unique roles in mitochondrial signalling processes—for example, peroxynitrite stimulates pentose phosphate pathway activity [25]—and therefore a better appreciation of these interactions relies upon the ability to distinguish individual ROS.
(3) Ratiometricity of response. The most common mode of fluorescence sensing is intensity-based (commonly termed ‘turn-on’), where the intensity of emission at a single wavelength increases with the sensing event. However, analysis of outputs from such sensors can be confounded by the fact that fluorescence intensity is also a function of probe concentration, and therefore also mitochondrial number. These challenges are particularly significant in the context of mitochondrial sensing, as mitochondrial membrane potential can also affect probe accumulation. This can be circumvented by the use of a ratiometric probe, for which the analyte induces a change in the fluorescence spectral form, and hence the emission or excitation colour rather than intensity (figure 2c). The ratio of emission outputs will not be a function of probe concentration, and will instead report only on the analyte concentration.
Figure 2.

Different modes of sensor design: (a) reversible sensors, which respond both to increases and decreases in ROS levels; (b) selective sensors, which specifically interact with an individual ROS; (c) ratiometric sensors, which signal environmental changes by changes in emission/excitation colour rather than emission intensity. (Online version in colour.)
In order to ensure the greatest efficiency of response, the probe should initially remain almost entirely reduced in the given environment, and then be oxidized only in the presence of the oxidation event of interest. This, in turn, strongly depends on matching the reduction potential of the probe and the investigated environment. Because mitochondria represent a highly reducing subcellular location, the optimal probes should exhibit low reduction potentials. Probes with a less negative reduction potential will therefore be less responsive to small changes in the environment potential.
Here, we review mitochondrially targeted redox sensors, grouped according to targeting strategy, whether (i) synthetic lipophilic cations such as TPP and other fluorophores or (ii) naturally occurring mitochondrial-targeting sequences (table 1).
Table 1.
Properties of fluorescent mitochondrial redox sensors discussed in this review. TPP, triphenylphosphonium; ROS, reactive oxygen species; ETC, electron transport chain; COX4, cytochrome oxidase subunit IV; GPD, glycerol phosphate dehydrogenase; Cyb2, cytochrome b2; COX8, cytochrome c oxidase 8.
| name | year of the first report | targeting group | Ex/Em | selectivity | fluoresence responsea | subsequent used |
|---|---|---|---|---|---|---|
| targeting strategy: lipophilic cations | ||||||
| MitoHE (MitoSOX) [26–28] | 2006 | TPP | 510 nm/580 nm | O2•−, OH•, ONOO− | turn on, irreversible | B,C |
| MitoPY1 [29] | 2008 | TPP | 515 nm/543 nm | H2O2, ONOO− (OCl−, hydroperoxides) | turn on, irreversible | B,C |
| SSH-Mito [30] | 2011 | TPP | 338 nm/462 nm and 545 nm | thiols | ratiometric (F545/F462 increases), irreversible | A,C |
| NpFR2 [31] | 2015 | TPP | 488 nm/545 nm | total ROS | turn on, reversible | A |
| MitoAR/MitoHR [32] | 2007 | rhodamine | 553 nm/574 nm | OH•, OCl− ONOO− | turn on, irreversible | A |
| RhoSS [33] | 2008 | NH3+-moiety attached to lipophilic rhodamine | 500 nm/530 nm | thiols | turn on, irreversible | A |
| FRR2 [34] | 2016 | rhodamine | 460 nm and 550 nm/590 nm | total ROS | excitation ratiometric (F550/F460 increases), reversible | A |
| MitoRP [35] | 2012 | coumarin 343b | 460 nm/495 nm | complex 1 of ETCc | turn on, reversible | A |
| Cy-O-EB [36] | 2013 | cyanine | 768 nm/794 nm | H2O2 | turn on, reversible | A |
| pep3-NP1 [37] | 2015 | styryl fluorophore | 455 nm (442 nm)/555 nm (646 nm) | H2O2 | ratiometric (F646/F555 increases), irreversible | A |
| HKSOX-1 m | 2015 | TPP | 509 nm/534 nm | O2•− | turn on, irreversible | A |
| targeting strategy: mitochondrial localization sequences (MLS) | ||||||
| roGFP1 and roGFP2 [38,39] | 2004 | MM: MLS of the pyruvate dehydrogenase E1, or COX4 (48 bp) IMS: sequence of GPD |
395 nm (after) and 475 nm (GFP1) or 490 nm (GFP2)/508 nm | general redox status | excitation ratiometric (F395/F475(490) increases), reversible | B,C |
| HyPer [40] | 2006 | MM: two tandem copies of the MLS of human COX8 | 420 nm and 500 nm/516 nm | H2O2 (pH sensitive) | excitation ratiometric (F500/F420 increases), reversible | B,C |
| rxYFP [41,42] | 2008 | MM: promoter and MLS (first 25 amino acids) of COX4. IMS: first 88 amino acids of S. cerevisiae Cyb2 |
512 nm/523 nm | general redox status | turn off upon oxidation, reversible (slowly) | B |
| Grx1-roGFP2 [43] | 2008 | MM: MLS from Neurospora crassa ATP synthase protein 9 | 395 nm and 488 nm/508 nm | GSH/GSSG redox couple | excitation ratiometric (F395/F488 increases), reversible | B,C |
| Orp1-roGFP2 [44] | 2011 | MM: MLS of human COX8 | 395 nm and 488 nm/508 nm | H2O2 | excitation ratiometric (F395/F488 increases), reversible | A,C |
| HyPerRed [45] | 2014 | MM: two tandem copies of the MLS of human COX8 | 560 nm/605 nm | H2O2 | turn on, reversible | B |
| sulfonyl-fluorescein [46] | 2015 | MM: MLS of human COX8 (first 25 aa) | 495 nm/519 nm | superoxide | turn on, irreversible | A |
aChange in fluorescence upon encountering a target.
bLipophilic electroneutral fluorophore at physiological pH.
cSpecificity not tested.
dThis column indicates the subsequent use of each probe to date beyond the initial publication: A, fewer than five papers; B, five or more papers; C, commercially available and/or open source access.
2. Triphenylphosphonium-targeted probes
TPP is extensively used as a mitochondrial-targeting group, [47] because its attachment to cargo is straight-forward and mitochondrial targeting is reliable. TPP is robust and versatile, and to date, it has been used to target a range of both nanoparticles and small molecules [48–50]. TPP has been used to successfully deliver a number of redox probes to the mitochondria, including MitoHE, MitoPY-1, SSH-Mito and NpFR2. These probes will be explored in the following paragraphs.
Hydroethidine (HE) is one of the most widely employed fluorescent sensors of cellular ROS [51]. It reacts with oxidants to form ethidium derivatives, which exhibit enhanced fluorescence properties. In this reaction, conjugation of the molecule is extended when the aniline moiety converts to an iminium cation upon oxidation (figure 3). This gives rise to a fluorescent ethidium derivative. Fluorescence intensity is further enhanced after intercalation with DNA. Hydroethidine has been applied to detect oxidants that are hydride acceptors including superoxide, and other ROS such as hydroxyl radical and peroxynitrite. Reaction with superoxide results in an additional hydroxylation (figure 3), which can be distinguished by high-performance liquid chromatography (HPLC) [52], and causes a fluorescence change that can be detected in vitro but not by microscopy [27]. While HE localizes in the cytoplasm, MitoHE (also called MitoSOX) is the mitochondrially targeted version, utilizing TPP as the targeting moiety [26–28]. MitoHE is commonly used, but can disrupt mitochondria function by inhibiting complex IV at micromolar concentrations [53]. It is also oxidized by iron or haem proteins such as cytochrome C, forming fluorescent and non-fluorescent dimers in a radical-mediated process [54].
Figure 3.

MitoHE (MitoSOX) detects superoxide and other ROS by modification of the ethidine core, which gives rise to a fluorescent product. (Online version in colour.)
MitoPY1 belongs to the broad family of hydrogen peroxide probes that utilize the selective unmasking of boronate by hydrogen peroxide [55], as well as peroxynitrite [56], hypochlorous acid [56] and amino acid hydroperoxides [57]. This probe contains the fluorophore fluorescein, for which keto–enol tautomerism and lactone ring-opening is essential for fluorescence. In MitoPY1, this tautomerization is prevented by the boronate-masking group, and deprotection by peroxide or peroxynitrite results in a sixfold increase in yellow fluorescence (figure 4). The authors reported use of this probe in HeLa cells to image hydrogen peroxide in mitochondria [29].
Figure 4.
MitoPY1 contains a boronate masking group removed upon selective reaction with hydrogen peroxide or peroxynitrite, enabling restoration of fluorescence. (Online version in colour.)
Levels of oxidized and reduced thiols are a good reflection of biological redox status, and SSH-Mito has been reported as a probe for mitochondrial thiols [30]. It contains 6-(benzo[d]thiazol-2′-yl)-2-(N,N-dimethylamino)naphthalene (BTDAN) as a fluorophore (figure 5). The wavelength of BTDAN fluorescence is greatly affected by the amine on the naphthalene ring, and in SSH-Mito, this amine is protected as a carbamate. Upon reduction of the redox sensitive disulfide bond, the free nucleophilic SH group can cleave the carbamate, unmasking the amine. This reaction irreversibly red-shifts the fluorescence of the probe from blue to yellow. The extent of reduction of the probe can be determined by monitoring the ratio of yellow to blue fluorescence (at 550 nm and 450 nm, respectively). Mitochondrial localization is again achieved by incorporation of TPP, and the efficiency of this strategy was confirmed in HeLa cells. This probe was also used to image thiol levels on rat hippocampal slices [30].
Figure 5.
SSH-Mito contains a redox-responsive disulfide bond. Upon reduction of this bond, the probe undergoes self-immolation to reveal a naphthylamine, with red-shifted fluorescence. (Online version in colour.)
Flavin is a naturally occurring co-enzyme involved in the catalysis of biological redox reactions. Flavin is fluorescent in its oxidized form, but its fluorescence is diminished upon two-electron reduction, as flavin undergoes a conformation change from planar to bent [58]. The flavin moiety has been employed in a number of reversible ROS sensors [59–61], including the mitochondrially targeted NpFR2, which exhibits a 115-fold increase in fluorescence upon oxidation (figure 6) [31]. NpFR2 responds non-selectively and reversibly to a range of ROS, and the probe was used to monitor the global redox state in primary mouse bone marrow, thymus and spleen cells.
Figure 6.

NpFR2 can be reversibly oxidized from a bent, non-fluorescent form to a planar, highly fluorescent form. (Online version in colour.)
HKSOX-1 m is a turn-on probe for imaging superoxide (figure 7) [62]. The 5-carboxy-2′,4′,5′,7′-tetrafluorofluorescein fluorophore core was selected for its low pKa of 3.7, which minimizes fluorescence quenching even in the most acidic cellular environments. In this probe, the fluorophore is initially switched off because the ketone electron acceptor and phenol electron donor are both masked with an aryl trifluoromethanesulfonate group, preventing their participation in the ICT fluorescence mechanism. In the presence of superoxide, the aryl trifluoromethanesulfonate groups are cleaved, unmasking the free phenol and ketone, thereby switching the fluorescence on. The probe shows good selectivity for O2•− over other strong oxidants, with a greater than 650-fold increase in fluorescence intensity upon superoxide treatment. Biological experiments were performed with differentiated human THP-1 cells and mitochondrial superoxide production was stimulated with antimycin A. HKSOX-1 m incorporates TPP as a targeting group, and cell staining patterns strongly suggests mitochondrial localization, but co-localization with a known mitochondrial stain was not reported.
Figure 7.

HKSOX-1 m contains aryl trifluoromethanesulfonate masking groups that are cleaved in the presence of superoxide. (Online version in colour.)
One disadvantage of using TPP is that there must be a free site on the probe that can be modified so that the targeting group can be attached. Not all probes have such reactive sites, and furthermore, required modifications to a fluorophore can often change its photophysical properties. Certain fluorophores can themselves act as targeting groups for the mitochondria, and can therefore have a dual role in the design of probes. These examples will be discussed in the following section.
3. Small-molecule cationic fluorophores as targeting groups
While TPP is a good mitochondrial-targeting group, many probes naturally localize to mitochondria without the aid of an additional targeting group. These probes often contain lipophilic cationic dyes including cyanine and rhodamine, which can localize into the mitochondria similarly to TPP. While eliminating the necessity of incorporating sterically bulky targeting groups that can interfere with the probe function, the localization of these fluorophores can change following synthetic modification, like the introduction of charge-altering sensing moieties, limiting their versatility. This method is therefore not as robust as the use of TPP.
Rhodamine 123 was among the first fluorophores reported to stain mitochondria [63], and many rhodamine-based mitochondrial stains are now available, including the CMXRos dyes (Thermo Fisher) and Mito Red (Sigma-Aldrich). Rhodamine has been employed as both a fluorophore and targeting group in a number of mitochondrial redox probes.
MitoAR and MitoHR are probes for detecting highly ROS (OH•, ONOO− and OCl−) in the mitochondria (figure 8) [32]. Both probes contain tetramethylrhodamine as the targeting group and fluorophore, and 4-aminophenyl aryl ether (for MitoAR) or 4-hydroxylphenyl aryl ether (for MitoHR) as the sensing group. The sensing group quenches the fluorescence of rhodamine via an intramolecular photoinduced electron-transfer (PET) mechanism. Highly ROS are able to irreversibly cleave the ether linkage and switch fluorescence on by removing the PET quencher. MitoHR was shown to be most sensitive towards the hydroxyl radical, displaying a greater than 500-fold fluoresence increase, while MitoAR showed greatest sensitivity to hypochlorite, with a 1500-fold fluorescence increase. Co-localization experiments were performed in HeLa cells, and highly ROS were imaged in HL-60 cells.
Figure 8.

The fluorescent rhodamine product of MitoAR and MitoHR is unmasked by oxidative cleavage of the ether linkage, removing the fluorescence quencher. (Online version in colour.)
RhoSS is a mitochondrial glutathione probe constructed on the rhodamine 101 scaffold (figure 9) [33]. Rhodamines contain two amino groups that are required for fluorescence, but in RhoSS, these amino groups are protected as carbamates and fluorescence is therefore switched off. Reduction of the disulfide bond attached to these carbamates leads to self-immolation and unmasking of the amines. One amine then undergoes tautomerization to an iminium cation, extending conjugation and opening the spirolactam ring. This generates the fluorescent rhodamine 110 as the product. Consequently, RhoSS must be fully deprotected to achieve fluorescence, and a 2000-fold increase in emission intensity can be observed in the presence of glutathione, but the response to other reductants was not investigated. Interestingly, mitochondrial localization in HeLa cells was observed, despite the fact that the positive charge on the probe before activation is highly localized on the alkyl NH3+ moiety. Therefore, the staining pattern could be due to the highly reducing nature of mitochondria, or the preferential re-distribution of the unmasked rhodamine 110 to this organelle. This probe was used to image intracellular glutathione in HeLa cells.
Figure 9.
RhoSS is a mitochondrial glutathione probe in which reduction of the disulfide bonds leads to unmasking of the fluorescent rhodamine core. (Online version in colour.)
FRR2 utilizes the same flavin redox switch as NpFR2, but incorporates a rhodamine group both as a mitochondrial-targeting group and acceptor fluorophore (figure 10) [34]. FRR2 operates by a Förster resonance energy transfer (FRET) mechanism, where oxidized flavin absorbs light and transfers excited state energy to rhodamine, which releases the energy as fluorescence. When flavin is reduced, its ability to transfer energy to rhodamine is diminished, leading to lower fluorescence output. FRR2 is termed an excitation-ratiometric probe because the extent of oxidation can be quantified by comparing the fluorescence output obtained after exciting the flavin, then directly exciting the rhodamine. FRR2 responds non-selectively and reversibly to a wide range of oxidants, and therefore can be considered to indicate the global redox state. The probe was used to study primary haematopoeitic cells from mice at different developmental stages.
Figure 10.
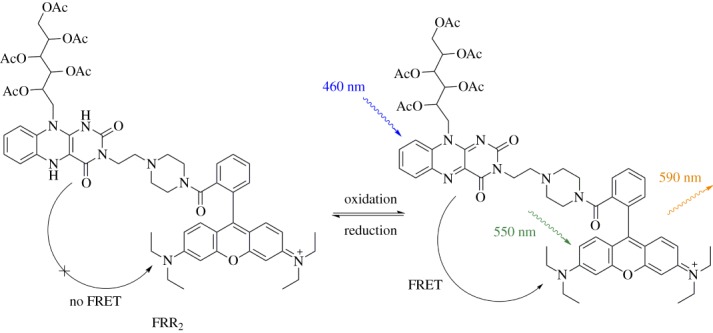
FRR2 is a FRET-based sensor of mitochondrial ROS/RNS, containing acetylated riboflavin as the FRET donor and rhodamine as the FRET acceptor. (Online version in colour.)
MitoRP consists of the coumarin 343 fluorophore linked to a redox-responsive stable nitroxyl radical, TEMPO (figure 11) [35] that can be reversibly transformed to its corresponding hydroxylamine upon reduction. While the nitroxide radical is able to quench the fluorescence of coumarin 343, reduction to the hydroxylamine form leads to a threefold increase in fluorescence intensity. MitoRP is particularly sensitive towards the activity of complex I of the electron transport chain, and therefore its staining pattern aligns with the mitochondria, despite the non-cationic, electroneutral character of the probe.
Figure 11.

MitoRP contains a coumarin 343 fluorophore linked to a redox-responsive stable nitroxyl radical, TEMPO, which when reduced, restores the fluorescence of the dye. (Online version in colour.)
Cyanines are another class of cationic fluorophores commonly used to stain mitochondria. Examples of commercially available cyanine-based mitochondrial stains include JC-1, DiOC6 and Mitotracker Deep Red FM (Thermo Fisher). Cy-O-EB is a mitochondrial redox probe that mimics the active site of glutathione peroxidase, in which a selenoenzyme uses glutathione to catalyse the rapid breakdown of hydrogen peroxide [36]. By mimicking this system, Cy-O-EB can reversibly sense the GSH/H2O2 redox couple. Cy-OEB integrates cyanine as both a fluorophore and lipophilic cationic targeting group, and ebselen as a reversible redox-sensing group (figure 12). Ebselen is a five-membered ring containing a redox-active Se–N bond which breaks upon reduction, generating NH and SeH functional groups. The reduced system contains electrons that effectively quench fluorescence of cyanine via an intramolecular PET mechanism. Ebselen can revert back to its closed-ring form upon oxidation, leading to a sixfold increase in near-IR fluorescence. The probe was reported to be selective to H2O2 and did not respond to ClO− or tBuOOH, but other oxidants were not tested. This probe was used to monitor fluctuations in oxidative stress in wounded zebra fish and mitochondrial localization was confirmed in Hep2G cells.
Figure 12.
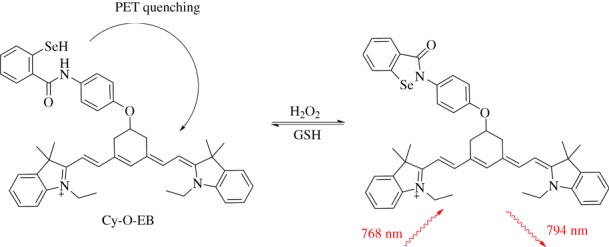
Cy-O-EB is a cyanine-containing fluorescent probe containing ebselen as the redox-responsive unit. (Online version in colour.)
As mentioned earlier, mitochondrial DNA is more prone to oxidative damage, because there are higher ROS levels in mitochondria compared with the nucleus. Pep1-NP is a probe that measures hydrogen peroxide near mitochondrial DNA, containing a cationic styryl dye that shows enhanced fluorescence upon intercalation with DNA (figure 13). Additional DNA targeting is achieved by integrating a DNA binding peptide, while the positively charged dye is thought to facilitate mitochondrial uptake. The hydrogen peroxide sensing unit comprises a 1,8-naphthalimide fluorophore with a boronate group at the eighth position which is reactive towards hydrogen peroxide. This boronate group prevents the intramolecular charge transfer (ICT) necessary for naphthalimide fluorescence, but the process can be restored upon unmasking of the electron donor following reaction of the boronate with hydrogen peroxide. Co-localization studies were performed in HeLa cells, and the probe response was studied in HeLa cells dosed with hydrogen peroxide [37].
Figure 13.

Pep1-NP localizes to mitochondrial DNA through its cationic styryl dye. Hydrogen peroxide selectively reacts with the boronate group to unmask naphthalimide fluorescence. (Online version in colour.)
The interpretation of the mitochondrial localization of the turn-on probes (e.g. MitoRP), unlike those with a constant-intensity fluorescent peak serving as an internal standard, must be done with care, as in these cases the mitochondrial staining pattern can arise due to an organelle-specific activation of the probe and not necessarily as a consequence of selective localization. In addition, TPP and lipophilic cations are limited in their use when studying metabolically disrupted mitochondria. As they operate by electrostatic accumulation at the negatively charged mitochondrial inner membrane, any loss or weakening of this negative membrane potential will affect the mitochondrial accumulation of these probes. Furthermore, by their proximity to the electron transport chain machinery, the probes can potentially disrupt this process and ultimately dissipate the membrane potential on their own.
4. Mitochondrial localization sequences as a targeting strategy
MLS are short peptides that are often found on the N-terminus of cytoplasmically expressed mitochondrial proteins. These sequences are recognized by the cell's own mitochondrial import machinery, and therefore can deliver cargo to mitochondria independently of their membrane potential. Therefore, MLS have been widely used to deliver protein probes to mitochondria, usually through attachment to their N-terminus [64]. The MLS is commonly attached to proteins using commercially available plasmids that contain the MLS gene in a region flanking the inserted gene for the protein of interest. Many of these ready-to-use plasmids are available via open source (for example Addgene). Depending on which MLS is selected, targeting regions within the mitochondria, such as the mitochondrial membrane (MM) or the intermembrane space (IMS) can be achieved. MLS typically contain several dozen amino acids, making them significantly larger than small-molecule redox probes. This not only poses a synthetic challenge, but can also potentially influence the reactivity and accessibility of the probes reactive centre. In addition, the high intrinsic charge and hydrophilicity of the MLS might hinder cellular uptake, a process that is indispensable for the delivery of the extracellularly applied small-molecule probes. Therefore, this section primarily comprises examples of genetically encoded protein-based redox sensors that have been successfully used to monitor redox state of mitochondria of live cells, with one recent example of the first small-molecule redox probe using this targeting strategy. For more details on redox-active fluorescent protein probes, the reader is referred to several excellent reviews on the topic [21,65–67].
The general strategy to develop redox indicators from fluorescent proteins is to artificially introduce a redox-active disulfide bond in the proximity of the fluorophore. Reduction or oxidation of this bond then leads to a subtle change in the protein conformation, altering the local environment around the fluorophore and thus leading to a change in the fluorescence [41,68]. In order to target these sensors to mitochondria, the redox protein sequence is inserted into a plasmid containing an MLS that targets either the mitochondrial matrix or IMS. In choosing the construct, matching it to the biological model of interest is of crucial importance: some plasmids will be suitable for human-derived cell lines, while others might be more appropriate for transfecting yeasts or other organisms. More information on the mechanisms of active protein uptake into mitochondria and selection of appropriate MLS can be found elsewhere [64,69,70]. It is important to keep in mind that the transfection efficiency will be different for different cells, which remains one of the major challenges in using genetically encoded sensors.
The first example of a redox-responsive genetically encoded indicator was rxYFP, [41] in which the oxidative formation of the disulfide bond facilitates the transition of the yellow fluorescent protein (YFP) fluorophore to a protonated, non-fluorescent form (λabs = 404 nm), with a subsequent decrease in the excitation peak at 512 nm (λem = 523 nm). The response of the probe to GSH/GSSG and other redox pairs is slow, and in cells is believed to depend on the activity of intracellular redox enzymes [71]. This, together with the sensitivity of YFP to pH and inorganic anions [41,72] limits its practical application. Mitochondrially targeted variants have been used in HeLa cells [73] and Saccharomyces cerevisiae [42].
Significant improvements have been achieved by the development of cytosolic [68] and mitochondrially targeted [38] roGFP1 and its brighter analogue roGFP2; excitation-ratiometric redox-responsive probes based on green fluorescent protein (GFP). Their response to the changes in the local redox state of GSH/GSSG buffer is mediated by the catalytic activity of glutaredoxins and other intracellular cofactors and is independent on the pH. roGFP1 and roGFP2 have been extensively employed to study the redox state in the mitochondrial matrix of a variety of biological models from cancerous and primary lines of human cells [38,74], to animal models including Caenorhabditis elegans [75], zebrafish [76], and mice [77–79], to plants [80,81]. They have also been successfully targeted to the mitochondrial IMS [39,82,83].
To decouple the oxidative response of the probe to the GSH/GSSG redox pair from other endogenous cofactors and improve the kinetics of the response, roGFPs have been covalently linked to the Grx1 (glutaredoxin) catalytic domain [43] enabling real-time selective monitoring of the changes in GSH/GSSG ratio in cells. Interestingly, no depletion of the endogenous levels of GSH has been observed with the use of Grx1-roGFP2 construct, suggesting its limited impact on intrinsic cellular redox homeostasis. Similarly to roGFPs, this probe has also been widely used in studying mitochondria in cultured cells [43], as well as in vivo in, for example, plants [84], Drosophila [44,84] and mice [85].
The fusion protein of roGFP2 and Orp1, a thiol peroxidase protein, gave an excitation-ratiometric probe, Orp1-roGFP2, highly selective for H2O2 [86]. In the presence of hydrogen peroxide, the initial highly sensitive oxidation of the Orp1 can then be communicated to the nearby roGFP2 by thiol–disulfide exchange leading to the fluorescence response. While the probe can detect H2O2 concentrations as low as 12.5 µM, the reduction of the probe back to its initial state is realized by GSH and thioredoxins, ensuring that the probe responds within the redox window of the cell. The Orp1-roGFP2 has been successfully targeted to the mitochondrial matrix of Drosophila to study the fluxes of mitochondrial H2O2 in this organism during development and ageing [44].
HyPer is another family of versatile genetically encoded probes, which are highly selective for H2O2, constructed on the basis of the S. cerevisiae OxyR domain that modulates the fluorescence of circularly permuted (cp) fluorescent proteins (cpYFP in excitation-ratiometric HyPer, HyPer-2 and HyPer-3 and mApple in HyPerRed) [40,66]. The cysteine in the OxyR domain, which upon oxidation leads to the conformational change sensed by the fluorescent protein, is initially buried in the hydrophobic pocket, enabling the access of H2O2 but not of other ionic oxidants, ensuring high selectivity of these probes for H2O2. In biological systems, similarly to Orp1-roGFP2, HyPer probes are then back-reduced by the thiol-reducing cellular machinery, enabling monitoring fluxes of H2O2 levels in time [40]. As little as 25 nM of H2O2 is sufficient to induce detectable response in HyPer protein, whereas treatment of cells with 5 µM H2O2 was required to induce the observable response in the cytoplasm [40]. Further modifications led to the development of HyPer-2 with a twofold greater dynamic range of response [87] and its variant, HyPer-3, with improved response kinetics, and demonstrated suitability for fluorescence lifetime imaging (FLIM) [88]. In order to account for the intrinsic pH sensitivity of cpYFP fluorescence in HyPer probes, parallel control experiments with pH sensors and subsequent signal intensity calibration should be performed [66]. The most reliable results with this approach can be achieved by using SypHer [89], a H2O2-irresponsive analogue of HyPer with identical pH-dependence of fluorescence. This case exemplifies the more general importance of considering pH-sensitivity of redox probes: fluorescence changes with pH should be routinely screened, and in cases where there are pH-induced perturbations in response, a pH sensor should be simultaneously employed to account for this effect.
The replacement of cpYFP with mApple led to the development of a HyPerRed [45], which responds to the presence of hydrogen peroxide by an increase in the intensity of red fluorescence (λexc = 560 nm, λem = 605 nm). Despite the non-ratiometric response, HyPerRed offers significantly larger Stokes shifts than GFP and YFP-based sensors, which together with the red emission colour makes it well suited for in vivo applications.
The HyPer probes have also been reliably targeted to the mitochondrial matrix [40,90] and mitochondrial IMS [45,90] of HEK and other cells, providing a robust tool to study the changes in the concentration of H2O2 in time.
Only recently, the first small-molecule fluorescent probe, sulfonyl-fluorescein, was reported, which was successfully targeted to mitochondria via the 25 amino acid long MLS sequence of the human COX8 enzyme, commonly used in targeting genetically encoded proteins to mitochondria (figure 14) [46]. The probe reacts selectively with superoxide, and subsequent non-redox cleavage of dinitrophenylsulfonyl ester liberates fluorescein, which leads to a fluorescence increase at 520 nm, with H2O2 being the only other potential activator with only 10% of the superoxide-induced response. The attachment of the MLS sequence to the probe increased its cellular uptake in RAW 264.7 macrophages and led to an approximately fivefold increase in the fluorescence in these cells upon treatment with KO2. Studying the efficiency of the targeting strategy and cellular uptake in other, less active cell lines would be of high interest for assessing the universality of MLS as targeting groups for small-molecule probes.
Figure 14.

Sulfonyl-fluorescein, a small-molecule probe that uses a 25 amino acid long MLS sequence for mitochondrial targeting. Reaction with superoxide liberates the fluorophore fluorescein. (Online version in colour.)
5. Employing diverse targeting strategies
This survey of the literature has revealed that mitochondrial ROS sensors can be principally divided into two groups: small-molecule probes that employ lipophilic cations (whether appended TPP or a lipophilic cationic fluorophore); and fluorescent protein-based sensors that use a natural mitochondrial-localizing peptide sequence. This division is by no means coincidental: the incorporation of an organic tag into an organic molecule and a peptide tag into a genetically encoded sensor is the most straightforward combination. However, it is possible that mitochondrial-localizing sequences could find broader use for small-molecule targeting: the superoxide-responsive MLS-tagged organic fluorophore described above demonstrates the validity of this method [46]. Furthermore, there is precedent for using mitochondrial-localizing peptides to transport non-protein cargo including quantum dots [91] and manganese-porphyrin photosensitisers [92]. Such a strategy is going to be most likely to be useful only for very large molecules for which a lipophilic cation as a tag does not have sufficient effect, as MLS can hamper cell penetration of a molecule.
One clear distinction between the two general methods of targeting is that lipophilic cations can only localize to the mitochondria in cells that have a negative (and therefore relatively healthy) MM potential, and can subsequently leak out of mitochondria with perturbed membrane potentials. By contrast, the subcellular distribution of MLS does not depend on the membrane potential, and cargo tagged in this way can be retained.
Interestingly, short cationic peptide sequences have found less application to date as fluorescent sensors of mitochondrial ROS. Such sequences, which typically contain positive residues such as arginine and lysine [93], have been successfully utilized to target other cargo to the mitochondria: organic and inorganic drugs including the anti-cancer agents chlorambucil [94] and a cisplatin analogue [95], respectively; DNA damaging agents to probe mitochondrial DNA repair factors [96]; and antioxidants [97]. The use of such sequences for targeting is therefore likely to be a valuable future research direction.
6. Future challenges in probe design
The task of imaging mitochondrial ROS is, without a doubt, a crucial role for chemical biologists. This is an area in which a number of untapped questions remain. It is important, however, that probe design should focus on providing new tools for unprobed questions, rather than exerting effort in duplicating current probe capabilities.
The selective sensing of individual ROS is particularly valuable in uncovering their specific roles in health and disease. However, apart from selective genetically encoded H2O2 sensors and despite the prevalence of selective cytoplasmic small-molecule sensors, few truly selective probes for other ROS and RNS are available. As many ROS/RNS already have acknowledged roles in the mitochondria, including hydrogen peroxide, peroxynitrite, nitric oxide and hydroxyl radicals [98], mitochondrially targeted sensors for these species are likely to be very valuable.
A key challenge that remains is the development of a mitochondrially localized small-molecule probe that can reversibly and selectively respond to an individual ROS. This has been achieved for the fluorescent proteins HyPer and Orp1-roGFP2, but not for any mitochondrially targeted small-molecule sensors. Indeed, purely organic systems may be unable to impart the required selectivity and reversibility; rather, it is likely that the solution will be found in metal complexes.
The use of a fluorescent sensor requires that the sensor itself does not perturb the system under study. In the case of a mitochondrial redox sensor, the probe should not alter mitochondrial redox homeostasis by the generation or consumption of ROS. While there have been reports of such perturbations, for example for the generation of ROS upon excitation of non-mitochondrially targeted GFP [99] and 2′,7′-dichlorodihydrofluorescein (DCFH) [100], this aspect is not routinely investigated for new probes, and should be the subject of further study. Another common concern, especially regarding the use of thiol-sensing probes is their potential unwanted interactions with endogenous thiol-containing proteins. However, the reversibility of disulfide-bond formation, and the very low (micromolar) concentrations of probe relative to cellular glutathione (millimolar) should limit this effect. This can be circumvented by the high selectivity of Grx1-roGFP2 towards glutathione.
While the advantages of ratiometric sensors are now widely accepted [101], there remains a dearth of mitochondrially targeted ratiometric small-molecule probes, and this is certainly a valuable area for future work. Ratiometric sensors can be readily prepared by tethering of two fluorophores, whether as FRET-based sensors, as for FRR2, or incorporating a separate control peak and responsive peak, as for pep3-NP1 [37]. A third method of ratiometric sensing is the use of an inherently ratiometric fluorophore, in which electronic changes elicited by oxidation–reduction result in the altered emission wavelength of a single fluorophore. This has been used for the probe SSH-Mito, where the HOMO-LUMO gap of the benzothiazolylnaphthylamine fluorophore is modulated upon reduction-induced autoimmolation [30].
7. The right probe for the right job
From this survey of the literature, it is clear that there are a number of tools available for measuring mitochondrial ROS. A key task for biological researchers interested in mitochondrial ROS is to collaborate closely with chemical biologists designing new probes: to guide synthetic effort to the most pertinent questions; and to provide an array of relevant systems in which to test and validate any new probes.
Alongside informing new probe design, biological studies must involve judicious selection the most appropriate probe for the question being investigated. Despite the broad array of mitochondrial ROS tools presented here, only a handful are routinely used in biological studies. Indeed, a survey of the literature from 2010 to 2015 reporting the use of a fluorescent mitochondrial ROS sensor reveals that over 75% of studies use MitoHE (MitoSOX), despite its limited selectivity, as highlighted above, with a further 20% employing a fluorescent protein-based sensor. This poor uptake of improved probes is due in part to the fact that many of the probes reviewed here are not commercially available, highlighting the need for more effort on commercialization or collaboration on the part of those who make probes. It is also important, however, that a better understanding of mitochondrial redox processes requires the routine use of a broader array of available probes.
This review has summarized key probe properties that should be considered when selecting the most appropriate sensor to use. The imaging modality, and the instrument specifications, will determine the excitation and emission wavelengths that can be employed. For any temporal studies, ratiometric probes should be sought to circumvent analytical problems associated with changes in probe concentration over time. Ratiometric probes are also essential in ensuring that changes in mitochondrial ROS levels can be distinguished from altered numbers of mitochondria: in cases where it is not possible to access or use a ratiometric probe, a second, non-responsive mitochondrial marker should be concurrently employed.
The selection of a small-molecule or a fluorescent protein-based sensor will depend on the biological system at hand. A small-molecule probe may be required for studying ex vivo samples, or for cell types that are difficult to transfect.
While there are distinct advantages to harnessing the breadth of probes now available, the use of relatively new probes must be approached with some caution. Initial validation is always required to ensure that the probe can be applied to the system under study: first validation steps might include confirming the response to exogenously applied oxidants or antioxidants.
8. Conclusion
The preparation and application of mitochondrially targeted ROS sensors is crucial to an understanding of the key redox processes involved in mitochondrial health and disease. In order to ensure productivity in this domain, it is necessary that there are thriving collaborations between those who prepare probes, and those who use them. Such cooperation will ensure that the most useful tools can be prepared to enable a far greater understanding of this essential organelle.
Authors' contributions
K.Y., J.L.K. and E.J.N. all equally contributed to the preparation of the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
K.Y. is funded by an Australian Postgraduate Award (Department of Education and Training) and the Vice-Chancellor's Research Scholarship (the University of Sydney). J.L.K. is funded by a Discovery Project from the Australian Research Council. E.J.N. is funded by a Westpac Research Fellowship from the Westpac Bicentennial Foundation and the Australian Research Council (grant nos DP150100649 and DP150103369).
References
- 1.Shadel GS, Horvath TL. 2015. Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569. ( 10.1016/j.cell.2015.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turrens JF. 2003. Mitochondrial formation of reactive oxygen species. J. Physiol. 552, 335–344. ( 10.1113/jphysiol.2003.049478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadenas E, Davies KJA. 2000. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 29, 222–230. ( 10.1016/S0891-5849(00)00317-8) [DOI] [PubMed] [Google Scholar]
- 4.Balaban RS, Nemoto S, Finkel T. 2005. Mitochondria, oxidants, and aging. Cell 120, 483–495. ( 10.1016/j.cell.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 5.Murphy MP. 2009. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13. ( 10.1042/BJ20081386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigarella CL, Liang R, Ghaffari S. 2014. Stem cells and the impact of ROS signaling. Development (Cambridge, England). 141, 4206–4218. ( 10.1242/dev.107086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolke C, Bukowska A, Goette A, Lendeckel U. 2015. Redox control of cardiac remodeling in atrial fibrillation. Biochim. Biophys. Acta (BBA) General Subjects 1850, 1555–1565. ( 10.1016/j.bbagen.2014.12.012) [DOI] [PubMed] [Google Scholar]
- 8.Murphy MP, et al. 2011. Unraveling the biological roles of reactive oxygen species. Cell Metab. 13, 361–366. ( 10.1016/j.cmet.2011.03.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willems Peter HGM, Rossignol R, Dieteren Cindy EJ, Murphy Michael P, Koopman Werner JH. 2015. Redox homeostasis and mitochondrial dynamics. Cell Metab. 22, 207–218. ( 10.1016/j.cmet.2015.06.006) [DOI] [PubMed] [Google Scholar]
- 10.Dröge W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95. ( 10.1152/physrev.00018.2001) [DOI] [PubMed] [Google Scholar]
- 11.Giulivi C, Boveris A, Cadenas E. 1995. Hydroxyl radical generation during mitochondrial electron-transfer and the formation of 8-hydroxydesoxyguanosine in mitochondrial-DNA. Arch. Biochem. Biophys. 316, 909–916. ( 10.1006/abbi.1995.1122) [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJA. 1990. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J. Biol. Chem. 265, 16 330–16 336. [PubMed] [Google Scholar]
- 13.Lightowlers RN, Taylor RW, Turnbull DM. 2015. Mutations causing mitochondrial disease: what is new and what challenges remain? Science 349, 1494–1499. ( 10.1126/science.aac7516) [DOI] [PubMed] [Google Scholar]
- 14.Heller A, Brockhoff G, Goepferich A. 2012. Targeting drugs to mitochondria. Eur. J. Pharm. Biopharm. 82, 1–18. ( 10.1016/j.ejpb.2012.05.014) [DOI] [PubMed] [Google Scholar]
- 15.Gaude E, Frezza C. 2014. Defects in mitochondrial metabolism and cancer. Cancer Metab. 2, 10 ( 10.1186/2049-3002-2-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Befroy DE, Falk PK, Rothman DL, Shulman GI. 2009. Chapter 21 Assessment of in vivo mitochondrial metabolism by magnetic resonance spectroscopy. In Methods in enzymology, pp. 373–393. Cambridge, MA: Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikalov S, Griendling KK, Harrison DG. 2007. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49, 717–727. ( 10.1161/01.HYP.0000258594.87211.6b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frezza C, Cipolat S, Scorrano L. 2007. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat. Protoc. 2, 287–295. ( 10.1038/nprot.2006.478) [DOI] [PubMed] [Google Scholar]
- 19.Mannella CA. 2006. The relevance of mitochondrial membrane topology to mitochondrial function. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1762, 140–147. ( 10.1016/j.bbadis.2005.07.001) [DOI] [PubMed] [Google Scholar]
- 20.New EJ. 2016. Harnessing the potential of small molecule intracellular fluorescent sensors. ACS Sensors 1, 328–333. ( 10.1021/acssensors.6b00148) [DOI] [Google Scholar]
- 21.Schwarzländer M, Dick TP, Meyer AJ, Morgan B. 2015. Dissecting redox biology using fluorescent protein sensors. Antioxid Redox Signal. 24, 680–712. ( 10.1089/ars.2015.6266) [DOI] [PubMed] [Google Scholar]
- 22.Kolanowski JL, Kaur A, New EJ. 2015. Selective and reversible approaches toward imaging redox signaling using small-molecule probes. Antioxid Redox Signal. 24, 713–730. ( 10.1089/ars.2015.6588) [DOI] [PubMed] [Google Scholar]
- 23.Kaur A, Kolanowski JL, New EJ. 2016. Reversible fluorescent probes for biological redox states. Angew. Chem. Int. Ed. 55, 1602–1613. ( 10.1002/anie.201506353) [DOI] [PubMed] [Google Scholar]
- 24.Smith RAJ, Porteous CM, Coulter CV, Murphy MP. 1999. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 263, 709–716. ( 10.1046/j.1432-1327.1999.00543.x) [DOI] [PubMed] [Google Scholar]
- 25.García-Nogales P, Almeida A, Bolaños JP. 2003. Peroxynitrite protects neurons against nitric oxide-mediated apoptosis: a key role for glucose-6-phosphate dehydrogenase activity in neuroprotection. J. Biol. Chem. 278, 864–874. ( 10.1074/jbc.M206835200) [DOI] [PubMed] [Google Scholar]
- 26.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. 2006. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl Acad. Sci. USA 103, 15 038–15 043. ( 10.1073/pnas.0601945103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zielonka J, Kalyanaraman B. 2010. Hydroethidine-and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic. Biol. Med. 48, 983–1001. ( 10.1016/j.freeradbiomed.2010.01.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson KM, Janes MS, Beckman JS. 2008. The selective detection of mitochondrial superoxide by live cell imaging. Nat. Protoc. 3, 941–947. ( 10.1038/nprot.2008.56) [DOI] [PubMed] [Google Scholar]
- 29.Dickinson BC, Chang CJ. 2008. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 130, 9638–9639. ( 10.1021/ja802355u) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim CS, Masanta G, Kim HJ, Han JH, Kim HM, Cho BR. 2011. Ratiometric detection of mitochondrial thiols with a two-photon fluorescent probe. J. Am. Chem. Soc. 133, 11 132–11 135. ( 10.1021/ja205081s) [DOI] [PubMed] [Google Scholar]
- 31.Kaur A, Brigden KWL, Cashman TF, Fraser ST, New EJ. 2015. Mitochondrially targeted redox probe reveals the variations in oxidative capacity of the haematopoietic cells. Org. Biomol. Chem. 13, 6686–6689. ( 10.1039/C5OB00928F) [DOI] [PubMed] [Google Scholar]
- 32.Koide Y, Urano Y, Kenmoku S, Kojima H, Nagano T. 2007. Design and synthesis of fluorescent probes for selective detection of highly reactive oxygen species in mitochondria of living cells. J. Am. Chem. Soc. 129, 10 324–10 325. ( 10.1021/ja073220m) [DOI] [PubMed] [Google Scholar]
- 33.Pires MM, Chmielewski J. 2008. Fluorescence imaging of cellular glutathione using a latent rhodamine. Org. Lett. 10, 837–840. ( 10.1021/ol702769n) [DOI] [PubMed] [Google Scholar]
- 34.Kaur A, Jankowska K, Pilgrim C, Fraser ST, New EJ. 2016. Studies of hematopoietic cell differentiation with a ratiometric and reversible sensor of mitochondrial reactive oxygen species. Antioxid Redox Signal. 24, 667–679. ( 10.1089/ars.2015.6495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirosawa S, Arai S, Takeoka S. 2012. A TEMPO-conjugated fluorescent probe for monitoring mitochondrial redox reactions. Chem. Commun. 48, 4845–4847. ( 10.1039/c2cc30603d) [DOI] [PubMed] [Google Scholar]
- 36.Xu K, Qiang M, Gao W, Su R, Li N, Gao Y, Xie Y, Kong F, Tang B. 2013. A near-infrared reversible fluorescent probe for real-time imaging of redox status changes in vivo. Chem. Sci. 4, 1079–1086. ( 10.1039/c2sc22076h) [DOI] [Google Scholar]
- 37.Wen Y, Liu K, Yang H, Liu Y, Chen L, Liu Z, Huang C, Yi T. 2015. Mitochondria-directed fluorescent probe for the detection of hydrogen peroxide near mitochondrial DNA. Anal. Chem. 87, 10 579–10 584. ( 10.1021/acs.analchem.5b03326) [DOI] [PubMed] [Google Scholar]
- 38.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. 2004. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 279, 13 044–13 053. ( 10.1074/jbc.M312846200) [DOI] [PubMed] [Google Scholar]
- 39.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. 2010. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ. Res. 106, 526–535. ( 10.1161/CIRCRESAHA.109.206334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. 2006. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Meth. 3, 281–286. ( 10.1038/nmeth866) [DOI] [PubMed] [Google Scholar]
- 41.Østergaard H, Henriksen A, Hansen FG, Winther JR. 2001. Shedding light on disulfide bond formation: engineering a redox switch in green fluorescent protein. EMBO J. 20, 5853–5862. ( 10.1093/emboj/20.21.5853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J, Dong L, Outten CE. 2008. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J. Biol. Chem. 283, 29 126–29 134. ( 10.1074/jbc.M803028200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutscher M, Pauleau A-L, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP. 2008. Real-time imaging of the intracellular glutathione redox potential. Nat. Meth. 5, 553–559. ( 10.1038/nmeth.1212) [DOI] [PubMed] [Google Scholar]
- 44.Albrecht Simone C, Barata Ana G, Großhans J, Teleman Aurelio A, Dick Tobias P. 2011. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 14, 819–829. ( 10.1016/j.cmet.2011.10.010) [DOI] [PubMed] [Google Scholar]
- 45.Ermakova YG, et al. 2014. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 5, 5222 ( 10.1038/ncomms6222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Si F, Liu Y, Yan K, Zhong W. 2015. A mitochondrion targeting fluorescent probe for imaging of intracellular superoxide radicals. Chem. Commun. 51, 7931–7934. ( 10.1039/C5CC01075F) [DOI] [PubMed] [Google Scholar]
- 47.Murphy MP. 2008. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta (BBA) Bioenergetics 1777, 1028–1031. ( 10.1016/j.bbabio.2008.03.029) [DOI] [PubMed] [Google Scholar]
- 48.Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. 2008. Targeting mitochondria. Acc. Chem. Res. 41, 87–97. ( 10.1021/ar700135m) [DOI] [PubMed] [Google Scholar]
- 49.Smith RAJ, Hartley RC, Murphy MP. 2011. Mitochondria-targeted small molecule therapeutics and probes. Antioxid Redox Signal. 15, 3021–3038. ( 10.1089/ars.2011.3969) [DOI] [PubMed] [Google Scholar]
- 50.Madak JT, Neamati N. 2015. Membrane permeable lipophilic cations as mitochondrial directing groups. Curr. Top. Med. Chem. 15, 745–766. ( 10.2174/1568026615666150302105622) [DOI] [PubMed] [Google Scholar]
- 51.Wojtala A, Bonora M, Malinska D, Pinton P, Duszynski J, Wieckowski MR. 2014. Methods to monitor ROS production by fluorescence microscopy and fluorometry. In Conceptual background and bioenergetic/mitochondrial aspects of oncometabolism (eds Galluzzi L, Kroemer G), pp. 243–262. San Diego, CA: Elsevier Academic Press Inc. [DOI] [PubMed] [Google Scholar]
- 52.Maghzal GJ, Stocker R. 2007. Improved analysis of hydroethidine and 2-hydroxyethidium by HPLC and electrochemical detection. Free Radic. Biol. Med. 43, 1095–1096. ( 10.1016/j.freeradbiomed.2007.06.023) [DOI] [PubMed] [Google Scholar]
- 53.Roelofs BA, Ge SX, Studlack PE, Polster BM. 2015. Low micromolar concentrations of the superoxide probe MitoSOX uncouple neural mitochondria and inhibit complex IV. Free Radic. Biol. Med. 86, 250–258. ( 10.1016/j.freeradbiomed.2015.05.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B. 2008. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic. Biol. Med. 44, 835–846. ( 10.1016/j.freeradbiomed.2007.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lippert AR, Van de Bittner GC, Chang CJ. 2011. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 44, 793–804. ( 10.1021/ar200126t) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zielonka J, Sikora A, Hardy M, Joseph J, Dranka BP, Kalyanaraman B. 2012. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem. Res. Toxicol. 25, 1793–1799. ( 10.1021/tx300164j) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michalski R, Zielonka J, Gapys E, Marcinek A, Joseph J, Kalyanaraman B. 2014. Real-time measurements of amino acid and protein hydroperoxides using coumarin boronic acid. J. Biol. Chem. 289, 22 536–22 553. ( 10.1074/jbc.M114.553727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Visser AJWG, Ghisla S, Massey V, Müller F, Veeger C. 1979. Fluorescence properties of reduced flavins and flavoproteins. Eur. J. Biochem. 101, 13–21. ( 10.1111/j.1432-1033.1979.tb04210.x) [DOI] [PubMed] [Google Scholar]
- 59.Yamada Y, Tomiyama Y, Morita A, Ikekita M, Aoki S. 2008. BODIPY-based fluorescent redox potential sensors that utilize reversible redox properties of flavin. ChembBioChem 9, 853–856. ( 10.1002/cbic.200700718) [DOI] [PubMed] [Google Scholar]
- 60.Yeow J, Kaur A, Anscomb MD, New EJ. 2014. A novel flavin derivative reveals the impact of glucose on oxidative stress in adipocytes. Chem. Commun. 50, 8181–8184. ( 10.1039/c4cc03464c) [DOI] [PubMed] [Google Scholar]
- 61.Kaur A, Haghighatbin MA, Hogan CF, New EJ. 2015. A FRET-based ratiometric redox probe for detecting oxidative stress by confocal microscopy, FLIM and flow cytometry. Chem. Commun. 51, 10 510–10 513. ( 10.1039/C5CC03394B) [DOI] [PubMed] [Google Scholar]
- 62.Hu JJ, et al. 2015. Fluorescent probe HKSOX-1 for imaging and detection of endogenous superoxide in live cells and in vivo. J. Am. Chem. Soc. 137, 6837–6843. ( 10.1021/jacs.5b01881) [DOI] [PubMed] [Google Scholar]
- 63.Baracca A, Sgarbi G, Solaini G, Lenaz G. 2003. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochim. Biophys. Acta (BBA) Bioenergetics 1606, 137–146. ( 10.1016/S0005-2728(03)00110-5) [DOI] [PubMed] [Google Scholar]
- 64.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. 2009. Importing mitochondrial proteins: machineries and mechanisms. Cell 138, 628–644. ( 10.1016/j.cell.2009.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meyer AJ, Dick TP. 2010. Fluorescent protein-based redox probes. Antioxid Redox Signal. 13, 621–650. ( 10.1089/ars.2009.2948) [DOI] [PubMed] [Google Scholar]
- 66.Bilan DS, Belousov VV. 2015. HyPer family probes: state of the art. Antioxid Redox Signal. 24, 731–751. ( 10.1089/ars.2015.6586) [DOI] [PubMed] [Google Scholar]
- 67.Booth DM, Joseph SK, Hajnoczky G. 2016. Subcellular ROS imaging methods: relevance for the study of calcium signaling. Cell Calcium 60, 65–73. ( 10.1016/j.ceca.2016.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. 2004. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 279, 22 284–22 293. ( 10.1074/jbc.M312847200) [DOI] [PubMed] [Google Scholar]
- 69.Gebert N, Ryan MT, Pfanner N, Wiedemann N, Stojanovski D. 2011. Mitochondrial protein import machineries and lipids: a functional connection. Biochim. Biophys. Acta (BBA) Biomembranes 1808, 1002–1011. ( 10.1016/j.bbamem.2010.08.003) [DOI] [PubMed] [Google Scholar]
- 70.Lithgow T. 2000. Targeting of proteins to mitochondria. FEBS Lett. 476, 22–26. ( 10.1016/S0014-5793(00)01663-X) [DOI] [PubMed] [Google Scholar]
- 71.Østergaard H, Tachibana C, Winther JR. 2004. Monitoring disulfide bond formation in the eukaryotic cytosol. J. Cell Biol. 166, 337–345. ( 10.1083/jcb.200402120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wachter RM, James Remington S. 1999. Sensitivity of the yellow variant of green fluorescent protein to halides and nitrate. Curr. Biol. 9, R628–R6R9. ( 10.1016/S0960-9822(99)80408-4) [DOI] [PubMed] [Google Scholar]
- 73.Banach-Latapy A, He T, Dardalhon M, Vernis L, Chanet R, Huang M-E. 2013. Redox-sensitive YFP sensors for monitoring dynamic compartment-specific glutathione redox state. Free Radic. Biol. Med. 65, 436–445. ( 10.1016/j.freeradbiomed.2013.07.033) [DOI] [PubMed] [Google Scholar]
- 74.Cheng W-Y, Tong H, Miller EW, Chang CJ, Remington J, Zucker RM, Bromberg PA, Samet JM, Hofer TPJ. 2010. An integrated imaging approach to the study of oxidative stress generation by mitochondrial dysfunction in living cells. Environ. Health Perspect. 118, 902–908. ( 10.1289/ehp.0901811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghose P, Park EC, Tabakin A, Salazar-Vasquez N, Rongo C. 2013. Anoxia-reoxygenation regulates mitochondrial dynamics through the hypoxia response pathway, SKN-1/Nrf, and stomatin-like protein STL-1/SLP-2. PLoS Genet. 9, e1004063 ( 10.1371/journal.pgen.1004063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O'Donnell KC, Vargas ME, Sagasti A. 2013. WldS and PGC-1α regulate mitochondrial transport and oxidation state after axonal injury. J. Neurosci. 33, 14 778–14 790. ( 10.1523/JNEUROSCI.1331-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michaelson LP, Shi G, Ward CW, Rodney GG. 2010. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve. 42, 522–529. ( 10.1002/mus.21724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dryanovski DI, Guzman JN, Xie Z, Galteri DJ, Volpicelli-Daley LA, Lee VMY, Miller RJ, Schumacker PT, Surmeier DJ. 2013. Calcium entry and α-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J. Neurosci. 33, 10 154–10 164. ( 10.1523/JNEUROSCI.5311-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolf AM, Nishimaki K, Kamimura N, Ohta S. 2014. Real-time monitoring of oxidative stress in live mouse skin. J. Invest. Dermatol. 134, 1701–1709. ( 10.1038/jid.2013.428) [DOI] [PubMed] [Google Scholar]
- 80.van Creveld SG, Rosenwasser S, Schatz D, Koren I, Vardi A. 2015. Early perturbation in mitochondria redox homeostasis in response to environmental stress predicts cell fate in diatoms. ISME J. 9, 385–395. ( 10.1038/ismej.2014.136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang K, et al. 2006. Expression and characterization of a redox-sensing green fluorescent protein (reduction–oxidation-sensitive green fluorescent protein) in Arabidopsis. Plant Physiol. 141, 397–403. ( 10.1104/pp.106.078246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez-Rocha H, et al. 2013. Compartmentalized oxidative stress in dopaminergic cell death induced by pesticides and complex I inhibitors: distinct roles of superoxide anion and superoxide dismutases. Free Radic. Biol. Med. 61, 370–383. ( 10.1016/j.freeradbiomed.2013.04.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi Hilton K, Santos Laila RB, Roma Letícia P, Duprez J, Broca C, Wojtusciszyn A, Jonas J-C. 2014. Acute nutrient regulation of the mitochondrial glutathione redox state in pancreatic β-cells. Biochem. J. 460, 411–423. ( 10.1042/BJ20131361) [DOI] [PubMed] [Google Scholar]
- 84.Albrecht SC, Sobotta MC, Bausewein D, Aller I, Hell R, Dick TP, Meyer AJ. 2014. Redesign of genetically encoded biosensors for monitoring mitochondrial redox status in a broad range of model eukaryotes. J. Biomol. Screen. 19, 379–386. ( 10.1177/1087057113499634) [DOI] [PubMed] [Google Scholar]
- 85.Breckwoldt MO, et al. 2014. Multiparametric optical analysis of mitochondrial redox signals during neuronal physiology and pathology in vivo. Nat. Med. 20, 555–560. ( 10.1038/nm.3520) [DOI] [PubMed] [Google Scholar]
- 86.Gutscher M, Sobotta MC, Wabnitz GH, Ballikaya S, Meyer AJ, Samstag Y, Dick TP. 2009. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 284, 31 532–31 540. ( 10.1074/jbc.M109.059246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Markvicheva KN, Bilan DS, Mishina NM, Gorokhovatsky AY, Vinokurov LM, Lukyanov S, Belousov VV. 2011. A genetically encoded sensor for H2O2 with expanded dynamic range. Bioorg. Med. Chem. 19, 1079–1084. ( 10.1016/j.bmc.2010.07.014) [DOI] [PubMed] [Google Scholar]
- 88.Bilan DS, et al. 2013. HyPer-3: a genetically encoded H2O2 probe with improved performance for ratiometric and fluorescence lifetime imaging. ACS Chem. Biol. 8, 535–542. ( 10.1021/cb300625g) [DOI] [PubMed] [Google Scholar]
- 89.Poburko D, Santo-Domingo J, Demaurex N. 2011. Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J. Biol. Chem. 286, 11 672–11 684. ( 10.1074/jbc.M110.159962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malinouski M, Zhou Y, Belousov VV, Hatfield DL, Gladyshev VN. 2011. Hydrogen peroxide probes directed to different cellular compartments. PLoS ONE 6, e14564 ( 10.1371/journal.pone.0014564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Derfus AM, Chan WCW, Bhatia SN. 2004. Intracellular delivery of quantum dots for live cell labeling and organelle tracking. Adv. Mat. 16, 961–966. ( 10.1002/adma.200306111) [DOI] [Google Scholar]
- 92.Sibrian-Vazquez M, Nesterova IV, Jensen TJ, Vicente MGH. 2008. Mitochondria targeting by guanidine− and biguanidine−porphyrin photosensitizers. Bioconjug. Chem. 19, 705–713. ( 10.1021/bc700393u) [DOI] [PubMed] [Google Scholar]
- 93.Jean SR, Ahmed M, Lei EK, Wisnovsky SP, Kelley SO. 2016. Peptide-mediated delivery of chemical probes and therapeutics to mitochondria. Acc. Chem. Res. 49, 1893–1902. ( 10.1021/acs.accounts.6b00277) [DOI] [PubMed] [Google Scholar]
- 94.Fonseca Sonali B, Pereira Mark P, Mourtada R, Gronda M, Horton Kristin L, Hurren R, Minden MD, Schimmer AD, Kelley SO. 2011. Rerouting chlorambucil to mitochondria combats drug deactivation and resistance in cancer cells. Chem. Biol. 18, 445–453. ( 10.1016/j.chembiol.2011.02.010) [DOI] [PubMed] [Google Scholar]
- 95.Wisnovsky Simon P, Wilson Justin J, Radford Robert J, Pereira Mark P, Chan Maria R, Laposa Rebecca R, Lippard SJ, Kelley SO. 2013. Targeting mitochondrial DNA with a platinum-based anticancer agent. Chem. Biol. 20, 1323–1328. ( 10.1016/j.chembiol.2013.08.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wisnovsky S, Jean SR, Kelley SO. 2016. Mitochondrial DNA repair and replication proteins revealed by targeted chemical probes. Nat. Chem. Biol. 12, 567–573. ( 10.1038/nchembio.2102) [DOI] [PubMed] [Google Scholar]
- 97.Szeto HH. 2006. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 8, E277–EE83. ( 10.1007/BF02854898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sena Laura A, Chandel Navdeep S. 2012. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 48, 158–167. ( 10.1016/j.molcel.2012.09.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoebe RA, Van Oven CH, Gadella TWJ, Dhonukshe PB, Van Noorden CJF, Manders EMM. 2007. Controlled light-exposure microscopy reduces photobleaching and phototoxicity in fluorescence live-cell imaging. Nat Biotech. 25, 249–253. ( 10.1038/nbt1278) [DOI] [PubMed] [Google Scholar]
- 100.Rota C, Chignell CF, Mason RP. 1999. Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Radic. Biol. Med. 27, 873–881. ( 10.1016/S0891-5849(99)00137-9) [DOI] [PubMed] [Google Scholar]
- 101.Lee MH, Kim JS, Sessler JL. 2015. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 44, 4185–4191. ( 10.1039/C4CS00280F) [DOI] [PMC free article] [PubMed] [Google Scholar]






