Abstract
Reports suggest that metformin exerts anti-cancer effects in diabetic individuals with pancreatic cancer. Thus, metformin is currently being repurposed as a potential drug in cancer treatment. Studies indicate that potent metformin analogues are required in cancer treatment because of the low bioavailability of metformin in humans at conventional antidiabetic doses. We proposed that improved mitochondrial targeting of metformin by attaching a positively charged lipophilic triphenylphosphonium group will result in a new class of mitochondria-targeted metformin analogues with significantly enhanced anti-tumour potential. Using this approach, we synthesized various mitochondria-targeted metformin analogues with different alkyl chain lengths. Results indicate that the antiproliferative effects increased with increasing alkyl chain lengths (100-fold to 1000-fold). The lead compound, mito-metformin10, potently inhibited mitochondrial respiration through inhibition of complex I, stimulation of superoxide and hydrogen peroxide formation and activation of AMPK. When used in combination with ionizing radiation, mito-metformin10 acted as a radiosensitizer of pancreatic cancer cells. Because of the 1000-fold-higher potency of mitochondria-targeted metformin10, therapeutically effective plasma concentrations likely can be achieved in cancer patients.
Keywords: metformin, anti-cancer agent, mitochondria, redox signalling, AMPK, reactive oxygen species
1. Repurposing metformin in cancer treatment: an old drug with a new potential
Approved for antidiabetic treatment in 1995, metformin has become the most prescribed antidiabetic drug in the world [1]. Metformin is widely used by patients with type 2 diabetes mellitus, who take several grams of it daily to decrease blood glucose levels. Metformin is relatively safe, with minimal side effects. Metformin causes minimal lactic acidosis, a side effect of phenformin—a related pharmaceutical, and accumulates only in normal tissues expressing organic cation transporters. Metformin is not metabolized—it enters the body and is excreted out unchanged in the urine [2]. However, its efficacy is attributed to the many metabolic pathways it induces or alters in the body, as reviewed elsewhere [3–6]. It is assumed that mitochondria are the major target of metformin, leading to inhibition of mitochondrial respiration, AMPK activation and bioenergetic reprogramming [6–8].
Epidemiological studies have recently shown an association between metformin use and decreased incidence of cancer [9–11]. In particular, diabetic patients taking metformin show a decreased incidence of pancreatic cancer, stimulating a flurry of research activity on the potential anti-tumour effects of metformin. Meta-analyses of the results of epidemiological studies indicated that patients taking metformin display approximately 30% reduced overall cancer incidence as well as cancer mortality [10]. However, after adjusting for body/mass index and time-related biases, the chemoprotective effects of metformin lost their statistical significance, suggesting that the actual effect is smaller than previously reported [10,11]. Other lipophilic analogues of metformin, such as phenformin, have increased bioavailability and exhibit more potent anti-tumour effects [12]. However, phenformin was discontinued more than 40 years ago in the USA because of enhanced incidence of acidosis (10- to 20-fold more in patients with renal dysfunction) [13,14].
A recent report indicates that cancers with mutations in mitochondrial genes encoding proteins of complex I of the mitochondrial electron transport chain are more susceptible to biguanides such as metformin [15]. Thus, pharmacological inhibition of mitochondrial energy metabolism compromised by mutations in complex I may further exacerbate mitochondrial energy deficits in cancer cells treated with metformin and analogues [15]. It was proposed that patients whose cancer harbours complex I mutations will be more sensitive to metformin. Additionally, it has been demonstrated that the availability of bioenergetic substrates may determine the sensitivity of cancer cells to metformin, implicating the tumour microenvironment as an additional factor to consider [15,16].
2. Mitochondria-targeted cations inhibit tumour proliferation
We and others have shown that mitochondria-targeted cationic compounds induce antiproliferative and/or cytotoxic effects in tumour cells without markedly affecting non-cancerous cells [17–19]. The selectivity has been attributed to an enhanced uptake of delocalized lipophilic cations by cancer cells owing to increased mitochondrial membrane potential [20]. A triphenylphosphonium cationic moiety (TPP+) tethered to a nitroxide, quinone or a chromanol group via an aliphatic linker side chain formed a new class of mitochondria-targeted cations that potently decreased the proliferation of various cancer cells [17–19]. Selective cytostatic and cytotoxic effects of TPP+-containing compounds in tumour cells when compared with normal cells could be attributed to enhanced uptake and retention in tumour mitochondria [18,21,22]. We hypothesized that attaching a positively charged lipophilic substituent will improve the mitochondrial targeting of metformin (i.e. mito-metformin), thus leading to a new class of compounds with improved anti-tumour potential.
3. Mitochondria-targeted metformins, inhibition of pancreatic ductal adenocarcinoma cell proliferation and cellular uptake
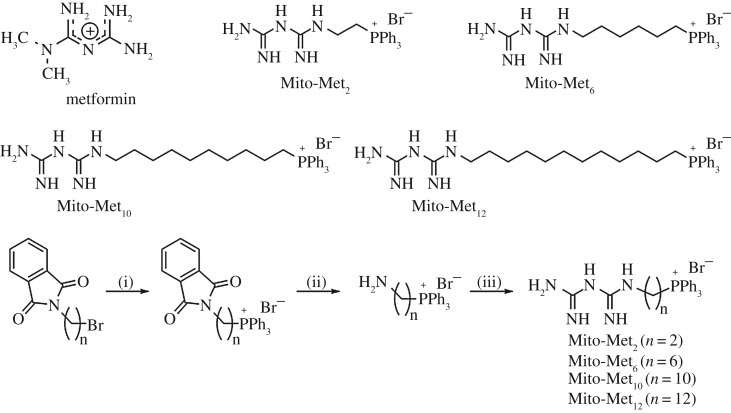
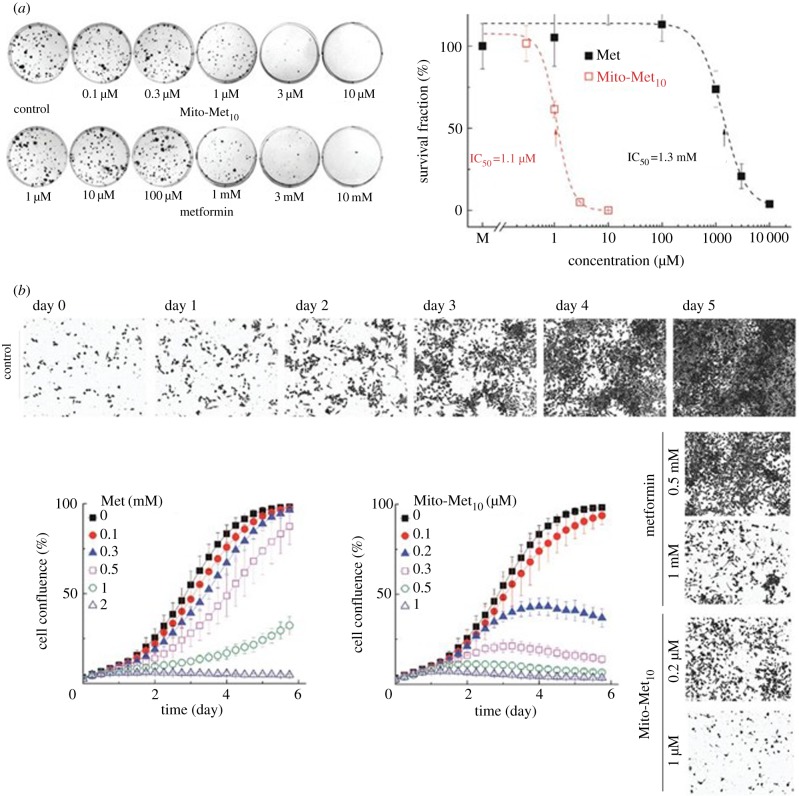
The base metformin is hydrophilic and weakly cationic (figure 1). We synthesized and characterized several metformin analogues (e.g. Mito-Met2, Mito-Met6, Mito-Met10 and Mito-Met12) conjugated to a TPP+ moiety via an alkyl linker chain (figure 1). We compared the relative antiproliferative potencies of mito-metformin homologues, phenformin and metformin in normal and pancreatic cancer cells. As shown in figure 2, Mito-Met10 more potently inhibited the colony formation of pancreatic ductal adenocarcinoma cells (PDACs) when compared with metformin. Furthermore, Mito-Met analogues are much more potent than metformin in inhibiting human pancreatic carcinoma (MiaPaCa-2) cell proliferation. These findings are consistent with their relative potencies to inhibit complex I-mediated cell respiration [23]. The cellular uptake of Mito-Met analogues increased as a function of increasing the carbon–carbon side chain length, and the most potent analogue, Mito-Met10, was 100-fold more potent than phenformin [23].
Figure 1.
Chemical structures of metformin and its mitochondria-targeted analogues and the synthetic pathway for the mito-metformins. Adapted from [23].
Figure 2.
Effects of Mito-Met10 and metformin on MiaPaCa-2 (a) cell colony formation and (b) cell proliferation. Adapted from [23]. (Online version in colour.)
4. Mitochondrial complex I inhibition
Recent studies have shown that inhibiting mitochondrial complex I in cancer cells causes a decrease in cell proliferation [5,16,23]. Metformin's inhibitory effects on tumourigenesis and cancer progression were attributed, in part, to its ability to inhibit mitochondrial complex I [5,6,16,24]. Reports also suggest that metformin-mediated inhibition of mitochondrial complex I is reversible, whereas rotenone, a classical complex I inhibitor, is an irreversible inhibitor [7]. Rotenone is relatively non-tissue selective and is highly toxic to both cancer cells and non-cancerous cells. Thus, not all agents inhibiting mitochondrial complex I are equally cytotoxic. Furthermore, rotenone uptake into mitochondria is not dependent on mitochondrial membrane potential, limiting its effectiveness as an anti-cancer therapy.
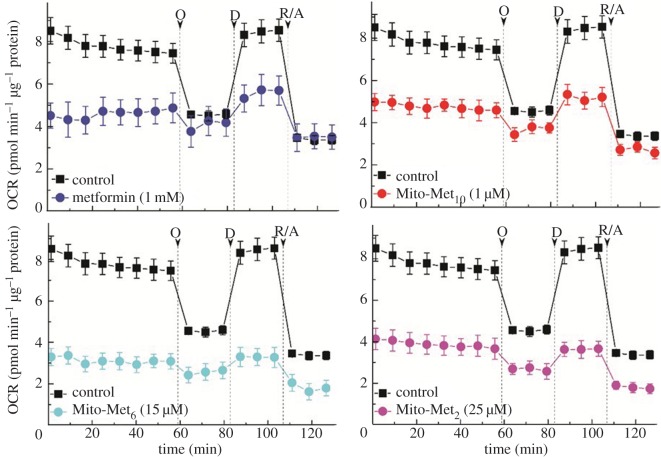
The oxygen consumption rate (OCR) in intact cells was measured as a readout of mitochondrial function (figure 3), and the complex I activity was determined by monitoring oxygen consumption in permeabilized cells in the presence of complex I substrates, using the Seahorse XF extracellular flux analyser (Agilent Technologies, Santa Clara, CA) [25]. Mitochondrial respiration was monitored in MiaPaCa-2 cells treated with varying concentrations of metformin and Mito-Met with different side chain lengths. Results indicate that the extent of OCR inhibition was dependent on the alkyl chain (linker) length with the mitochondrial inhibitory efficacy increasing in the order: metformin < Mito-Met2 < Mito-Met6 < Mito-Met10 (figure 3). The IC50 value for inhibition of mitochondrial complex I determined for metformin was 1.1 mM when compared with 0.4 µM observed for Mito-Met10 [23]. Interestingly, the complex I inhibitory activity of Mito-Met10 is much stronger in pancreatic cancer cells (MiaPaCa-2 and PANC-1, IC50 < 1 µM) than in non-tumourigenic cells (HPNE, IEC-6, IC50 > 10 µM), possibly owing to differential uptake of the compound [23].
Figure 3.
Effect of metformin and its mitochondria-targeted analogues on mitochondrial respiration in intact MiaPaCa-2 cells, as measured by real-time monitoring of the oxygen consumption rates in cell pretreated for 24 h with the compounds. O, oligomycin; D, 2,4-dinitrophenol; R/A, rotenone + antimycin A. Adapted from [23]. (Online version in colour.)
5. Enhanced formation of superoxide and hydrogen peroxide induced by mito-metformin
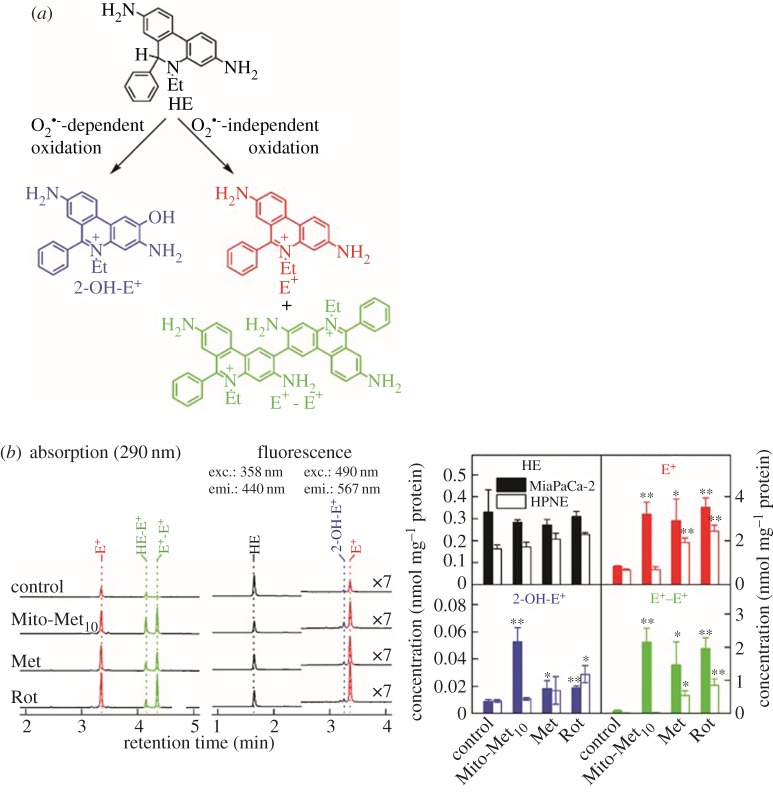
One of the consequences of complex I inhibition is enhanced cellular generation of redox species (superoxide radical anion, hydrogen peroxide (H2O2) and haem-derived oxidants). To the best of our knowledge, mitochondria-derived superoxide has never been unambiguously determined in cancer cells. This is due, in part, to artefacts associated with probes typically used for cellular superoxide and the lack of determination of specific products formed from reactions of probes with superoxide. This problem has been overcome because of the intense research efforts devoted to understanding the reaction mechanisms and kinetics of fluorescent probes and reactive oxygen species (ROS) [26–28]. We used the cell-permeable probe, hydroethidine (HE), to detect superoxide. As shown in figure 4, Mito-Met10 treatment of MiaPaCa-2 cells increased the formation of 2-hydroxyethidium (2-OH-E+), a specific marker product of the HE/superoxide reaction. In addition, a marked increase in ethidium and diethidium was observed (figure 4b), suggesting that the Mito-Met10 interaction with mitochondrial proteins induces generation of one-electron oxidant(s). Under similar conditions, Mito-Met10 did not stimulate O2•– (superoxide) formation in control human pancreatic epithelial nestin-expressing (HPNE) cells, used as a normal cell control for MiaPaCa-2 cells. This result is consistent with the lack of inhibition of mitochondrial complex I in HPNE cells under these conditions [23]. However, at higher concentrations, Mito-Met10 treatment led to inhibition of mitochondrial function and increased formation of 2-OH-E+ in HPNE cells [23].
Figure 4.
(a) Chemical structures of the specific oxidation products formed from hydroethidine (HE) probe; (b) intracellular oxidant formation induced by Mito-Met10 (1 µM, 24 h), metformin (1 mM, 24 h) and rotenone (1 µM, 1 h), as measured by profiling the HE oxidation products upon 1 h incubation with the HE probe (10 µM). Adapted from [23]. (Online version in colour.)
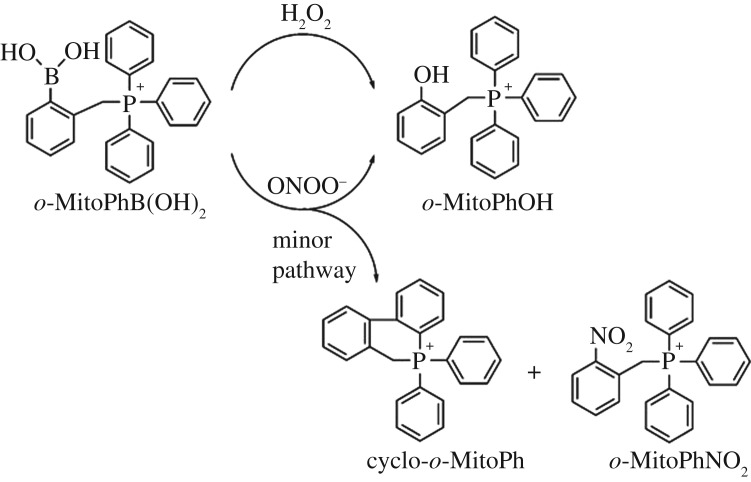
To detect H2O2 generated in Mito-Met10 treated cells, we used the probe o-MitoPhB(OH)2 [26,29–31]. The reason for using o-MitoPhB(OH)2 instead of m-MitoPhB(OH)2 (known also as the MitoB probe [29]) is that, whereas both probes react stoichiometrically with H2O2 to form the respective phenolic product (Mito-PhOH), only the ortho-substituted probe will react with peroxynitrite (ONOO–) to form ONOO–-specific cyclo-o-MitoPh [26] and o-MitoPhNO2 [30,31] products (figure 5). Failure to detect those peroxynitrite-specific products means that ONOO− was not the active oxidant responsible for oxidation of o-MitoPhB(OH)2 to o-MitoPhOH. Results showed that Mito-Met10 treatment of MiaPaCa-2 cells in the presence of o-MitoPhB(OH)2 leads to enhanced formation of o-MitoPhOH, indicative of H2O2, ONOO− or hypochlorous acid (HOCl) formation. However, the lack of formation of peroxynitrite- or hypochlorous-specific products accompanying o-MitoPhB(OH)2 oxidation [26] suggests that neither ONOO− nor HOCl is responsible for oxidation of o-MitoPhB(OH)2 to o-MitoPhOH (not shown).
Figure 5.
Chemical structures of products formed from hydrogen peroxide (H2O2) and peroxynitrite (ONOO–)-induced oxidation of o-MitoPhB(OH)2 probe.
6. Proposed signalling pathway
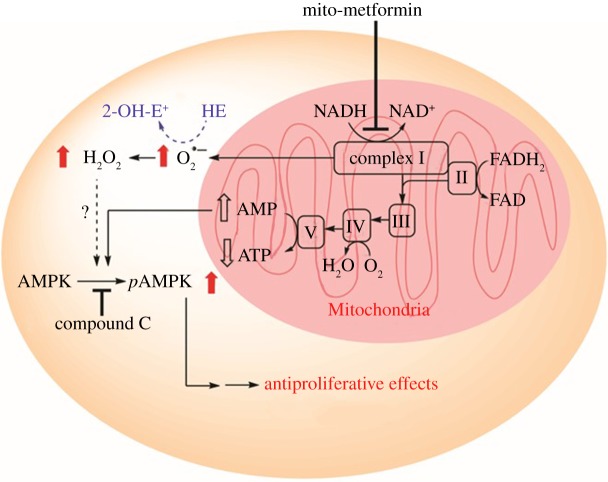
We showed that Mito-Met10 activated adenosine monophosphate (AMP)-activated protein kinase (AMPK) phosphorylation at a 1000-fold lower concentration than metformin (not shown) [23]. AMPK, a master regulator of cellular energy homeostasis, is typically activated by intracellular AMP. Under conditions wherein intracellular adenosine triphosphate (ATP) levels decrease along with an increase in AMP (enhanced AMP/ATP ratio), AMPK is activated via phosphorylation of its threonine-172 residue [32]. We proposed that Mito-Met10 likely exerts antiproliferative effects in PDACs via targeting the energy-sensing bioenergetics pathway (figure 6). H2O2, generated in mitochondria through dismutation of superoxide formed from complex I inhibition by Mito-Met10, likely leads to AMPK phosphorylation [33,34], resulting in antiproliferative effects in cancer cells. It is conceivable that Mito-Met10-induced increases in both intracellular AMP and H2O2 contribute to AMPK activation, leading to inhibition of cell proliferation (figure 6).
Figure 6.
Proposed signalling pathway activated by metformin analogues. Adapted from [23]. (Online version in colour.)
7. Metformin, mito-metformin and radiosensitization
Previously, mitochondria-targeted nitroxides have been shown to enhance radiosensitivity of neuroblastoma cells [35]. Metformin also increased the radiosensitivity of pancreatic cancer cells and inhibited tumour growth [36,37]. The enhanced radiosensitivity of metformin was attributed to activation of the AMPK pathway and/or improved tumour oxygenation owing to inhibition of mitochondrial complex I and tumour cell respiration in irradiated tumours [36,37]. Studies suggest that decreasing oxygen consumption with pharmacological drugs is an effective route for increasing tumour oxygenation and radiosensitivity [38–40]. As presented previously (figure 3), Mito-Met10 inhibited mitochondrial complex I activity and pancreatic cancer cell respiration at micromolar levels, a 1000-fold lower than metformin. Mito-Met10 induced AMPK activation at a 1000-fold lower concentration when compared with metformin. As shown previously [23], Mito-Met10 enhanced radiation sensitivity in PDAC at a 1000-fold lower concentration than metformin. This is a very significant finding that could have high clinical relevance as relatively non-toxic mitochondria-targeted metformin analogues could be used in combination with radiotherapy in treating pancreatic cancer. Mito-Met10 also decreased the growth of the pancreatic tumour in the mouse xenograft model in vivo, without any sign of toxicity under those conditions [23]. It is conceivable that administration of Mito-Met10 exhibiting a 1000-fold-higher potency, when compared with metformin, would result in a therapeutically effective plasma concentration in cancer patients.
8. Conclusion
This study shows how mitochondrial targeting of metformin enhances its antiproliferative effects in pancreatic cancer cells. The lead compound, Mito-Met10, potently inhibited mitochondrial respiration through inhibition of complex I activity, resulting in ROS-mediated stimulation of AMPK. Mito-Met10 acts as a radiosensitizer in pancreatic cancer cells.
Acknowledgements
The authors thank Donna McAllister and Kathleen Boyle from M.D.'s laboratory for their involvement in the in vitro and in vivo studies of the anti-cancer effects of Mito-Met10.
Authors' contributions
All authors prepared the manuscript and gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
This work was supported by National Institutes of Health National Cancer Institute grant no. U01 CA178960 to M.D. and B.K. A.S. was supported by a grant from the Polish National Science Centre No. 2015/18/E/ST4/00235.
References
- 1.Bailey C, Day C. 2004. Metformin: its botanical background. Pract. Diabetes Int. 21, 115–117. ( 10.1002/pdi.606) [DOI] [Google Scholar]
- 2.Graham GG, et al. 2011. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 50, 81–98. ( 10.2165/11534750-000000000-00000) [DOI] [PubMed] [Google Scholar]
- 3.Emami Riedmaier A, Fisel P, Nies AT, Schaeffeler E, Schwab M. 2013. Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol. Sci. 34, 126–135. ( 10.1016/j.tips.2012.11.005) [DOI] [PubMed] [Google Scholar]
- 4.Pryor R, Cabreiro F. 2015. Repurposing metformin: an old drug with new tricks in its binding pockets. Biochem. J. 471, 307–322. ( 10.1042/bj20150497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jara JA, López-Muñoz R. 2015. Metformin and cancer: between the bioenergetic disturbances and the antifolate activity. Pharmacol. Res. 101, 102–108. ( 10.1016/j.phrs.2015.06.014) [DOI] [PubMed] [Google Scholar]
- 6.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. 2014. Metformin: from mechanisms of action to therapies. Cell Metab. 20, 953–966. ( 10.1016/j.cmet.2014.09.018) [DOI] [PubMed] [Google Scholar]
- 7.Bridges H, Jones A, Pollak M, Hirst J. 2014. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 462, 475–487. ( 10.1042/BJ20140620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Romero IL, Litchfield LM, Lengyel E, Locasale JW. 2016. Metformin targets central carbon metabolism and reveals mitochondrial requirements in human cancers. Cell Metab. 24, 728–739. ( 10.1016/j.cmet.2016.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. 2005. Metformin and reduced risk of cancer in diabetic patients. BMJ (Clin. Res. ed.) 330, 1304–1305. ( 10.1136/bmj.38415.708634.F7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. 2014. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res. 7, 867–885. ( 10.1158/1940-6207.CAPR-13-0424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heckman-Stoddard BM, Gandini S, Puntoni M, Dunn BK, Decensi A, Szabo E. 2016. Repurposing old drugs to chemoprevention: the case of metformin. Semin. Oncol. 43, 123–133. ( 10.1053/j.seminoncol.2015.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang W, Finniss S, Cazacu S, Xiang C, Brodie Z, Mikkelsen T, Poisson L, Shackelford DB, Brodie C. 2016. Repurposing phenformin for the targeting of glioma stem cells and the treatment of glioblastoma. Oncotarget. 7, 56 456–56 470. ( 10.18632/oncotarget.10919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwong SC, Brubacher J. 1998. Phenformin and lactic acidosis: a case report and review. J. Emerg. Med. 16, 881–886. ( 10.1016/S0736-4679(98)00103-6) [DOI] [PubMed] [Google Scholar]
- 14.Dykens JA, Jamieson J, Marroquin L, Nadanaciva S, Billis PA, Will Y. 2008. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol. Appl. Pharmacol. 233, 203–210. ( 10.1016/j.taap.2008.08.013) [DOI] [PubMed] [Google Scholar]
- 15.Birsoy K, et al. 2014. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 508, 108–112. ( 10.1038/nature13110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gui DY, et al. 2016. Environment dictates dependence on mitochondrial complex I for NAD+ and aspartate production and determines cancer cell sensitivity to metformin. Cell Metab. 24, 716–727. ( 10.1016/j.cmet.2016.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng G, Zielonka J, Dranka BP, McAllister D, Mackinnon AC Jr, Joseph J, Kalyanaraman B. 2012. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 72, 2634–2644. ( 10.1158/0008-5472.can-11-3928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng G, Zielonka J, McAllister D, Hardy M, Ouari O, Joseph J, Dwinell MB, Kalyanaraman B. 2015. Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer Lett. 365, 96–106. ( 10.1016/j.canlet.2015.05.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng G, Zielonka J, McAllister DM, Mackinnon AC Jr, Joseph J, Dwinell MB, Kalyanaraman B. 2013. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer 13, 285 ( 10.1186/1471-2407-13-285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modica-Napolitano JS, Aprille JR. 2001. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug Deliv. Rev. 49, 63–70. ( 10.1016/S0169-409X(01)00125-9) [DOI] [PubMed] [Google Scholar]
- 21.Cheng G, Zielonka J, McAllister D, Tsai S, Dwinell MB, Kalyanaraman B. 2014. Profiling and targeting of cellular bioenergetics: inhibition of pancreatic cancer cell proliferation. Brit. J. Cancer 111, 85–93. ( 10.1038/bjc.2014.272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberg F, et al. 2010. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl Acad. Sci. USA 107, 8788–8793. ( 10.1073/pnas.1003428107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng G, et al. 2016. Mitochondria-targeted analogues of metformin exhibit enhanced antiproliferative and radiosensitizing effects in pancreatic cancer cells. Cancer Res. 76, 3904–3915. ( 10.1158/0008-5472.can-15-2534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheaton WW, et al. 2014. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3, e02242 ( 10.7554/eLife.02242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dranka BP, Zielonka J, Kanthasamy AG, Kalyanaraman B. 2012. Alterations in bioenergetic function induced by Parkinson's disease mimetic compounds: lack of correlation with superoxide generation. J. Neurochem. 122, 941–951. ( 10.1111/j.1471-4159.2012.07836.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zielonka J, et al. 2016. Mitigation of NADPH oxidase 2 activity as a strategy to inhibit peroxynitrite formation. J. Biol. Chem. 291, 7029–7044. ( 10.1074/jbc.M115.702787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalyanaraman B, Dranka BP, Hardy M, Michalski R, Zielonka J. 2014. HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes—the ultimate approach for intra- and extracellular superoxide detection. Biochim. Biophys. Acta 1840, 739–744. ( 10.1016/j.bbagen.2013.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalyanaraman B, Hardy M, Podsiadly R, Cheng G, Zielonka J. 2016. Recent developments in detection of superoxide radical anion and hydrogen peroxide: opportunities, challenges, and implications in redox signaling. Arch. Biochem. Biophys. ( 10.1016/j.abb.2016.08.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cochemé HM, et al. 2011. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 13, 340–350. ( 10.1016/j.cmet.2011.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sikora A, et al. 2013. Reaction between peroxynitrite and triphenylphosphonium-substituted arylboronic acid isomers: identification of diagnostic marker products and biological implications. Chem. Res. Toxicol. 26, 856–867. ( 10.1021/tx300499c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielonka J, Sikora A, Adamus J, Kalyanaraman B. 2015. Detection and differentiation between peroxynitrite and hydroperoxides using mitochondria-targeted arylboronic acid. Methods Mol. Biol. (Clifton NJ.) 1264, 171–181. ( 10.1007/978-1-4939-2257-4_16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardie DG, Ashford ML. 2014. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda, MD.) 29, 99–107. ( 10.1152/physiol.00050.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackenzie RM, et al. 2013. Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clin. Sci. (London, England: 1979) 124, 403–411. ( 10.1042/cs20120239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quintero M, Colombo SL, Godfrey A, Moncada S. 2006. Mitochondria as signaling organelles in the vascular endothelium. Proc. Natl Acad. Sci. USA 103, 5379–5384. ( 10.1073/pnas.0601026103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Z, Jiang J, Belikova NA, Stoyanovsky DA, Kagan VE, Mintz AH. 2010. Protection of normal brain cells from gamma-irradiation-induced apoptosis by a mitochondria-targeted triphenyl-phosphonium-nitroxide: a possible utility in glioblastoma therapy. J. Neuro-oncol. 100, 1–8. ( 10.1007/s11060-010-0387-2) [DOI] [PubMed] [Google Scholar]
- 36.Song CW, Lee H, Dings RP, Williams B, Powers J, Santos TD, Choi BH, Park HJ. 2012. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci. Rep. 2, 362 ( 10.1038/srep00362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fasih A, Elbaz HA, Huttemann M, Konski AA, Zielske SP. 2014. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat. Res. 182, 50–59. ( 10.1667/rr13568.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bol V, et al. 2015. Reprogramming of tumor metabolism by targeting mitochondria improves tumor response to irradiation. Acta Oncol. (Stockholm, Sweden) 54, 266–274. ( 10.3109/0284186x.2014.932006) [DOI] [PubMed] [Google Scholar]
- 39.Crokart N, et al. 2005. Tumor radiosensitization by antiinflammatory drugs: evidence for a new mechanism involving the oxygen effect. Cancer Res. 65, 7911–7916. ( 10.1158/0008-5472.can-05-1288) [DOI] [PubMed] [Google Scholar]
- 40.Durand RE, Biaglow JE. 1977. Radiosensitization of hypoxic cells of an in vitro tumor model by respiratory inhibitors. Radiat. Res. 69, 359–366. [PubMed] [Google Scholar]