Abstract
During the work cycle of elongation, the ribosome, a molecular machine of vast complexity, exists in a large number of states distinguished by constellation of its subunits, its subunit domains and binding partners. Single-particle cryogenic electron microscopy (cryo-EM), developed over the past 40 years, is uniquely suited to determine the structure of molecular machines in their native states. With the emergence, 10 years ago, of unsupervised clustering techniques in the analysis of single-particle data, it has been possible to determine multiple structures from a sample containing ribosomes equilibrating in different thermally accessible states. In addition, recent advances in detector technology have made it possible to reach near-atomic resolution for some of these states. With these capabilities, single-particle cryo-EM has been at the forefront of exploring ribosome dynamics during its functional cycle, along with single-molecule fluorescence resonance energy transfer and molecular dynamics computations, offering insights into molecular architecture uniquely honed by evolution to capitalize on thermal energy in the ambient environment.
This article is part of the themed issue ‘Perspectives on the ribosome’.
Keywords: free-energy landscape, molecular machines, mRNA–tRNA translocation, single-particle reconstruction
1. Background
The ribosome, once assembled around a new messenger RNA (mRNA), carries out many rounds of the peptide elongation cycle, one for each amino acid to be added to the nascent chain [1–5]. As a processive molecular machine, the ribosome remains engaged with the mRNA throughout this process until a stop codon is encountered. The ribosome's engagement with transfer RNA (tRNA), attached with its anticodon end to the mRNA, is also processive: the aminoacyl tRNA (aa-tRNA) cognate to the codon presented in the decoding centre, once accommodated in the A site, is carried forward in discrete steps in the immediately following cycles of elongation, until it exits the ribosome. These steps alternate between the apposed ‘handover sites’ on both ribosomal subunits, following the sequence of A/A, A/P, P/P, P/E, E/E. The analogy between the tRNA and a trapeze artist alternating between hands and feet for safe support during her acrobatics in the air is tempting. In the symbolic notation used above, ‘A’ stands for aminoacyl, ‘P’ for peptidyl and ‘E’ for exit site, and the designation ‘X/Y’ traditionally refers to a configuration of the tRNA with its anticodon in the ‘X’ site of the small subunit and the position of its acceptor arm in the ‘Y’ site of the large subunit. For completeness, the site occupied by the tRNA during the aa-tRNA selection step prior to accommodation is often designated A/T. (Note that a different nomenclature introduced by Alexander Spirin with the opposite designation, Aa, Ap, etc. has not attained purchase in the literature).
It can be easily appreciated that the task of transporting the large moiety formed by mRNA with its attached tRNAs in precise steps through the complex environment of the ribosome, while avoiding the risk of losing the reading frame initially defined by the start codon, is formidable. Alexander Spirin, who early on recognized the mechanistic challenge posed by this feat, spoke of the ribosome as a conveying machine [6], invoking the idea of conveyor belts used in industry and now at the cash registers of every grocery store, but the preferred term is mRNA–tRNA translocation or translocation for short. Indeed, the loss of reading frame, in most cases, would be a debacle leading to the formation of a product that is non-functioning at best and toxic for the cell at worst. Considering this critical requirement and the topology of the intersubunit cavity through which the tRNA has to find its way, one recognizes that the alternating tRNA trapeze steps described above are too coarse to ensure safe handover; that additional ‘baby steps’ are essential to keep the reading frame and ensure secure transit of the tRNA. Thus, both these a priori considerations and the experimental evidence of hybrid states have led us to expect multiple states of the ribosome–mRNA–tRNA complex, all with distinct structures closely related to one another in conformation.
This article expands on an earlier review [7] in highlighting the progress that has been made by cryogenic electron microscopy (cryo-EM) in identifying, resolving and capturing these multiple states. (Because of the close similarity in the structural basis of translation between eubacteria and eukaryotes, findings from both kingdoms will be reviewed concurrently and generically.) Historically, these attempts started with the use of chemical interventions (antibiotics, GTP analogues) or mutations to stabilize key states, one at a time, in separate experiments. However, the more recent development of single-particle cryo-EM, summarized in §2, enabled a much more efficient way to visualize multiple states in a single sweep, even at resolutions approaching those achieved by X-ray crystallography. As elongation factor G (EF-G; eEF2 in eukaryotes) plays a key part in translocation, the interaction between the ribosome and EF-G will be seen as an important determinant in the definition and distribution of intermediate states during translocation.
2. Single-particle cryo-electron microscopy
The general idea of reconstructing an asymmetric molecule from low-exposure projections cast by randomly oriented ‘copies' or realizations of that molecule was first formulated in 1975 [8] and later put into practice through the development of an image processing system, SPIDER [9]. Importantly, the method is based on the ability to align such low-exposure images by cross-correlation with sufficient accuracy to determine the molecule's structure [10]. A brief account of the early development of mathematical concepts and computational approaches has been given elsewhere [11]. What is important, in the context of this review, are two distinct advantages of the single-particle over crystallographic methods [12,13]. One is the fact that the imaging of the molecule in single, non-attached form is likely to yield a more authentic structure—unaffected by interaction of the molecule with its neighbours, and arguably more representative of the free molecule in solution. In contrast, both X-ray crystallography and electron crystallography [14] result in structures that are influenced by the energy minimization inherent in the formation of a closely packed crystal, and thus may significantly differ from the structures assumed by the functional molecule in the course of its work cycle. The second advantage of single-particle techniques is related to the fact that multiple equilibrating conformations and binding states of the native molecule coexist that, as will become apparent, can be recovered all at once in a single experiment. In contradistinction, crystallographic methods, with a few exceptions, yield a single structure, selected by the minimization of energy as the crystal is formed.
3. Evidence of conformational dynamics—the early days
First visualizations of tRNA and EF-G bound to the ribosome emerged in the 1990s [15–20]. Just at the time when the first X-ray structures of ribosomal subunits came out [21–23], evidence of large conformational changes accompanying translocation was found by single-particle cryo-EM [24]: the two subunits were seen to be rotated with respect to each other upon binding of EF-G stalled by GDPNP (in the GTP state) or fusidic acid (in the GDP state). In a good approximation, the subunits behaved as rigid bodies, at least at the resolutions reachable for asymmetric molecules by cryo-EM at the time. The inference obtained from a comparison of EF-G bound with unbound structures was that binding of EF-G in the GTP state induced a different conformation in the ribosome, conducive to completion of mRNA–tRNA translocation. As such, the observations were generally consistent with the ‘induced fit’ paradigm of enzyme catalysis [25]. Even though the resolution of this study, and of a follow-up study with ribosomes in a defined pre-translocational (‘PRE’) state [26], was insufficient to draw conclusions on the precise molecular mechanism, a dramatic reorganization of the ribosome and a concurrent change in EF-G conformation, resulting from a rearrangement of its domains, could be recognized and quantitatively described (figure 1). Fitting of the atomic structure of EF-G into the observed density revealed a joint rigid-body rotation of a ‘superdomain’ formed by domains II, IV and V, relative to domains I and II [16,17]. Soon similar discoveries regarding the structural basis of eukaryotic translocation followed suit [27].
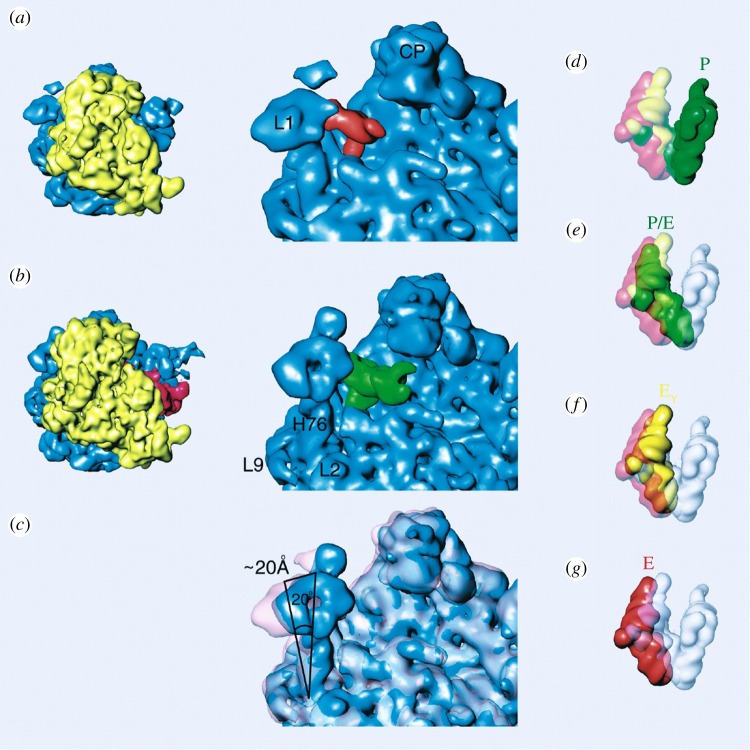
Figure 1.
Ratchet-like motion and associated L1 stalk motion accompanying the movement of tRNA. Cryo-EM maps of Escherichia coli 70S·MFTI-tRNAIle–puromycin complexes before (a) and after (b) binding of EF-G•GDPNP (a non-hydrolysable GTP analogue) [26]. The L1 stalk of the 50S subunit is ‘open’ before (a) and closed after (b) binding of EF-G. (c) Conformational change of L1 stalk, which moves the tRNA from P/E (b) into E/E (a) position. Landmarks: L9, ribosomal protein L9; L2, protein L2; H76, helix 76 from the 23S rRNA; L1, L1 protuberance or stalk in the 50S subunit. (Reproduced with permission from [26].)
Intersubunit rotation of the magnitude observed, in the range of 9° (many more sightings of rotations on this scale were made subsequently), is evidently pre-configured in the ribosome's architecture: in the core region, the two subunits are connected by tight rRNA–rRNA bridges. In the periphery, bridges are flexible/pliable, each involving at least one ribosomal protein (rprotein) [28]. One of the peripheral bridges, S13–L5, which appears to be rapidly remodelled during intersubunit rotation, has an intriguing ‘ruler’ configuration that will be highlighted further below (§8) in the context of intermediate states reachable by the PRE ribosome in thermal equilibrium.
In discussing the process of mRNA–tRNA translocation following peptidyl transfer, it is useful to distinguish two stages [28,29]: in the first stage, which is reversible, A- and P-site tRNAs are advanced on the large subunit as the small subunit rotates, while their anticodon stem–loops remain firmly anchored on the small subunit along with the mRNA. The sites assumed by the tRNAs intermittently, A/P and P/E, were discovered by Moazed & Noller [30], and are called hybrid sites. The second stage of translocation starts with the binding of EF-G/eEF2 to the ribosome, an event that results in a rotation of the small-subunit head domain [31] and the movement of the anticodon stem–loops of the tRNAs on the small subunit from A to P and P to E, respectively, together with the advance of the mRNA bound to the tRNAs by one codon. The precise function of GTP hydrolysis on EF-G/eEF2 in this process is still controversial, and it may not be strictly coupled with the second stage of translocation [32].
4. Overcoming two serious limitations of single-particle cryo-electron microscopy
These early studies showed both the power of single-molecule cryo-EM and its limitations, which have curtailed its ability to recover high-resolution structure of a molecular machine as complex and multifaceted as the ribosome. The potential of the technique lies in its ability to capture a molecule in action, in a state most populated at the exact moment of plunge-freezing. The initial limitations, at the time, were twofold: (i) heterogeneity of the population of molecules imaged, and (ii) the suboptimal performance of the recording medium (i.e. film or CCD camera) in terms of signal-to-noise ratio and modulation transfer function. Importantly, both hurdles have been overcome in the past decade, as will be highlighted below in §4a,b.
(a). Overcoming heterogeneity
Heterogeneity stems from the fact that often multiple states of the molecule are present, all differing in structure, so that a reconstruction from all projections will be a blurred superposition of several structures, representative of none. As a remedy, it is possible to use antibiotics or non-hydrolysable GTP analogues to trap the molecule in particular states. Antibiotics are typically small molecules that bind to a functional centre and disable its dynamical features [33]. Non-hydrolyzable GTP analogues may be used to trap the ribosome at a point where GTPase activity of an elongation factor is required for a forward move of the elongation cycle. Indeed, the first mapping of the elongation cycle [18] involved the trapping of three transition states: the 70S ribosome bound with EF-G after GTP hydrolysis but before release from the ribosome (fusidic acid; [16]) or at a point immediately prior to GTP hydrolysis (GDPNP; [17]), and of the 70S ribosome bound with the ternary complex formed by aa-tRNA, EF-Tu (eEF1α in eukaryotes) and GDP after GTP hydrolysis (kirromycin in bacteria; [19,34–38]). However, the problem is that these constitute only a few specific states out of a large spectrum of existing states within the cycle. Furthermore, when it comes to the precise molecular mechanism, it is unclear to what extent the states trapped by chemical means are truly representative of the native states [5]. To summarize, multiple states do coexist in the sample, and singling out a particular state for imaging by biochemical enrichment of the corresponding subpopulation ran into difficulties for two reasons: one is that only a few selected states can be captured in this way; the other is that there are legitimate questions about the authenticity of the stalled states.
Because of these limitations, the capture of multiple states present in the same sample remained a big challenge for quite some time. This challenge initially led to the introduction of multireference methods, using a comparison of the experimental data with a library of projections of several reference structures [34,39]. Here, depending on the value of the cross-correlation coefficient between experimental and predicted projection, the experimental projection is assigned to the class and the Eulerian angles that produce the best match. The disadvantage of this approach is obvious as it is built upon a priori assumptions as to which states are represented in the mix. Moreover, the use of reference structures is questionable as it is known to introduce reference bias. Still, careful applications of reference-based methods involving multiple hierarchical steps have been successful in sorting ribosome images [31,40,41].
However, another concern is that the very use of ‘hard’ deterministic class assignments is suboptimal for data with a low signal-to-noise ratio. This problem, along with the problem of reference bias, was subsequently overcome with the introduction of maximum-likelihood methods into cryo-EM. Fred Sigworth [42] had already used a maximum-likelihood approach to image alignment in electron microscopy. Sjors Scheres, in Carazo's group, was the first to use this approach in three-dimensional image classification (program ‘ML3D’) [43,44]. A later incarnation of this program is now in widespread use in the cryo-EM community under the name RELION [45]. Similar approaches to reference-free clustering were developed by other authors [46,47].
With these novel computational approaches, a new phase in ribosome research has started, taking maximum advantage of the capabilities of single-particle cryo-EM. It is the basis for the novel studies, featured in this article, that are able to capture multiple authentic states of the polypeptide elongation cycle from the same sample.
(b). Overcoming limitations of the recording medium
Another limitation, for the longest time, has been the suboptimal quality of the recording media—film or CCD cameras—which limited the resolution of ribosomes and other asymmetric molecules to the range 7–5 Å (e.g. 5.5 Å for the T. brucei ribosome; [48]). A few years ago, in 2012, the introduction of commercial direct electron detection cameras of the CMOS type revolutionized the field, now permitting close-to-atomic resolution to be achieved not only for ribosomes but smaller molecules also in the 100 kDa range [49]. Although numerous studies have demonstrated that a characterization of ribosomal states can already be accomplished on the basis of observed domain constellations and ligand occupancies, even without requiring resolution beyond 10 Å, the ability to reach atomic detail has dramatically transformed the scope and value of these studies. In terms of the goal of inventorying the states of the elongation cycle, this resolution improvement means that we are now able to move from the description of a few coarse-grained states to one of multiple, fine-grained states, which includes a precise accounting for the interactions between the ribosome and its ligands on the atomic level.
5. Quantitative characterization of domain movements
Observations accumulated in studies of ribosome dynamics, such as intersubunit movements and intrasubunit domain movements within the actively translating ribosome, and of binding states of ligands (tRNAs, factors) associated with a ribosome complex, are difficult to compare, for a lack of a common standard. Thus, even when we focus on the same organism, say Escherichia coli, descriptions of domain motions in the literature vary widely, pointing to the need for a common framework.
The problem has been concisely stated by Whitford et al. [50] as these authors set out to identify collective coordinates of ribosomal subunits in motion. When comparing two structures portraying the molecule in two different states, we may isolate a domain by segmentation and look at the movements of each individual residue. We then can distinguish between a (usually large) set of residues undergoing a collective motion—with small deviations in the usual order of thermal fluctuations—and some groups of residues that differ strongly from this common behaviour, representing internal conformational changes of the domain that go along with the domain's motion. Therefore, describing the motion of the domain as a rigid-body motion is usually an approximation as we express the collective motion of the majority of its residues by a single coordinate transformation.
There is no common framework for describing domain motions other than by reference to the entire set of coordinates in the respective atomic models. Thus, measurements of the angle of rotation of the small-subunit head domain are not strictly comparable between two laboratories since they depend on the definition of the domain and the choice of the axis of rotation (see [28] in this context). Agirrezabala et al. [51] proposed a method to describe the positions of domains uniquely in terms of their principal axes of inertia, as a basis for describing their motions between different states. This method was used extensively to characterize motions of ribosome domains and tRNA in intermediate states of the first stage of translocation [52] and will soon be available, together with a general tool for domain segmentation, in a new toolbox on the VMD platform [53].
6. Evidence of intersubunit rotation in the absence of elongation factors
While comparison of cryo-EM maps of the PRE ribosome (i.e. a translating ribosome after peptidyl transfer) with and without EF-G bound indicated that mRNA–tRNA translocation goes hand in hand with the ratchet-like intersubunit motion [24,26], the first proof that this motion occurs in real time was obtained by Harry Noller's group with the aid of fluorescence resonance energy transfer (FRET) experiments [54]. Here the placement of an acceptor–donor pair on the large subunit (L9) and small subunit (either S6 or S11), respectively, ensured that intersubunit rotation would either increase or decrease the distance between acceptor and donor upon binding of the factor. However, besides proving the existence of rotation catalysed by the binding of EF-G, these experiments also indicated that the intersubunit rotation deduced from the distance changes was sensitive to the state of acylation of the P-site tRNA in the absence of EF-G. Subsequently, single-molecule (sm) FRET experiments [55] using the same labelling strategy as in the bulk experiment [54] proved that the PRE ribosome fluctuates spontaneously between two states, ‘unrotated’ and ‘rotated’ (these simplified terms are now commonly used in preference to macrostate I and II or global state I and II earlier introduced in cryo-EM and smFRET). Ruben Gonzalez's group [56], using placements of donor–acceptor pair on the elbow of P-tRNA and protein L1, respectively, obtained results entirely consistent with these findings, as L1 is known to change its position in synchrony with the intersubunit rotation [26,52].
Based on the results of the smFRET studies of the PRE ribosome, it could be expected that cryo-EM of this kind of sample would yield strongly heterogeneous data. Indeed, reference-based classification, where density maps of rotated and unrotated ribosomes were used as references, showed a clear division of the data into two subpopulations [57,58] which, after separate reconstruction, yielded unambiguous evidence of the two states—namely unrotated along with classical A/A-, P/P-tRNA positions, and rotated with hybrid A/P, P/E positions.
At this point the evidence for the ribosome acting as a Brownian machine, an idea long advanced by Alexander Spirin (see early literature cited in refs [6,59]), was firmly established [60–62]. As a remarkable conclusion, energy barriers created by multiple intersubunit contacts that are strained or must be broken and reconnected are in the order of a few kT0, where T0 is room temperature. Thus, driven as it is by thermal energy, the freely equilibrating PRE ribosome is expected to oscillate between its two main states—canonical and fully rotated—with increasing frequency as the temperature is raised. This prediction was indeed proved to be correct in an smFRET experiment covering the range 22–37°C using a specially designed micro-heated chip [63].
The role of EF-G in translocation consequently appeared in a new light: rather than triggering intersubunit rotation by some kind of induced-fit mechanism, it now seemed likely that EF-G's role is more passive in the first stage of translocation (as defined above), as the factor chooses to bind the thermally fluctuating ribosome when its GTP conformation comes into a perfect match with the ribosome's binding surface (more about this interaction in §9). As later smFRET and cryo-EM experiments showed, the ribosome is partially rotated at this point [64,65].
7. Observations of multiple equilibrating states of the pre-translocational ribosome in the absence of elongation factor G
The observation of intersubunit rotation in the absence of EF-G motivated a closer examination of such factorless ribosome data for clues on the nature of the transition between the observed states of relative stability. Indeed, the paradigm shift concerning the role of EF-G, from induced fit to ‘opportunistic’ binding, left open the question in what configuration of the ribosome the interaction might take place. Application of the maximum-likelihood clustering method incorporated in the program ML3D [43] to the same data as previously analysed using reference-based classification by Agirrezabala et al. [57] showed the existence of at least two additional, intermediate states, characterized by intermediate intersubunit rotation angles and intermediate hybrid tRNA positions [52]. Such intermediate states had already been postulated by Ermolenko et al. [54] on theoretical grounds but thought to be undetectable by FRET, given the technical limitations of these experiments. At that time, Munro et al. [66], working with methods of smFRET, reported the observation of intermediate states in signals derived from samples in which mutants were deployed. Similar results were later found by Fu et al. [67] in cryo-EM visualization of ribosomes with a P-loop mutation. Remarkably, however, no intermediates were found in smFRET experiments with wild-type, factor-free ribosomes in the PRE configuration. This apparent paradox prompted an examination of experimental limitations in smFRET and the conditions under which intermediates would not register in smFRET while solidly supported by data from cryo-EM [68]. This analysis strongly suggested, as an explanation for the discrepancy between the results from the two experimental methods, that the lifetimes of the intermediates seen in cryo-EM are quite short compared with those in the canonical and fully rotated states, and that consequently their transitions were too fast to be captured by the camera used in these studies. Even employment of hidden Markov models in the computational analysis was not successful in finding these transitions.
However, as missing smFRET observations are plausibly explained, there is no doubt that intermediates are indeed captured by cryo-EM, and the only question remaining is how many of these exist. Because the data are notoriously noisy, and the conformational states might differ only slightly, it is quite conceivable that numerous ‘micro-states’ exist [60] but that they are elusive to capture by the maximum-likelihood approach. At this point the study by Fischer et al. [40] should be mentioned, in which a large dataset was collected by cryo-EM for a specimen undergoing back-translocation, a slow process that, in the end, leads to a pool of PRE ribosomes in thermal equilibrium. Apart from differences in buffer conditions, this PRE subset of their data is therefore comparable with the datasets analysed by Agirrezabala et al. [52,57] and Julian et al. [58]. Fischer et al. [40] found at least two intermediate states in this pool. The cryo-EM study by Spahn's group [69] provided a similar inventory of the PRE states for the eukaryotic ribosome.
As mentioned earlier, Agirrezabala et al. [52] compiled a tabulation of all intermediate PRE states of the E. coli ribosome published at the time, coming from both X-ray and cryo-EM studies, together with intersubunit rotation angles and other parameters describing ribosomal domain and tRNA positions. This tabulation of states in fact indicated up to eight different observed intersubunit rotation angles and concomitant domain and tRNA constellations, although it is not clear to what extent inclusion of data from X-ray crystallography in which two ribosomes contained in the asymmetric unit happen to be in different states of rotation and domain constellations [70–73] is relevant to a discussion of authentic functional states.
A new approach to resolving heterogeneity, by employing manifold embedding of cryo-EM projection data, promises to tackle this issue more conclusively as the technique is capable of rendering the full conformational spectrum of a molecular machine [74,75]. In this first application of the manifold embedding method to yeast ribosomes purified from cell extract, a sequence of structures characteristic for pre- and post-translocational states could be identified in an apparent continuum of states. However, application of this method to a complete, well-characterized in vitro translation system is still pending.
8. Structural basis of intersubunit motion in the absence of elongation factor G
Intersubunit rotation is a ‘natural’ motion of the PRE ribosome in thermal equilibrium, based on the unique bipartite architecture of this molecule [6,28,61]. Examination of the binding interactions between the two subunits, as revealed by X-ray and cryo-EM structures (see latest tabulation by Liu & Fredrick [76]) yields some clues on the distribution of rotation states as well as the molecular mechanism for the fine-grained control of motion. Some of these interactions are detailed in the following.
The peripheral intersubunit bridge B1b (bridge nomenclature introduced in [77,78]) mentioned earlier on is formed by a loop of large-subunit protein L5 and a long helix of protein S13 on the small subunit's head. As the subunits rotate relative to each other, the L5 loop glides along the S13 helix, which may act as a ruler, allowing contacts to be formed via salt bridges at multiple repeats of the helix turn [28,79]. Juxtaposition of GRASP-painted proteins L5 and S13 in the canonical and fully rotated states of the ribosome [28] and subsequent electrostatic calculations [79] confirmed that the subunits are stabilized by electrostatic interactions at the two ends of their relative rotation movement. Thus, the picture emerges of a malleable bridge that does not offer strong resistance and undergoes rapid changes during intersubunit rotation, but exhibits energetic preferences, via charge interactions, for intersubunit positions at both ends of the rotation range. Bridge B1b has in fact been implicated in the control of translocation in yeast [80]. Its immediate neighbour, B1a, is formed by a contact between the A-site finger, helix 38 of 23S rRNA, and proteins S13/S19. The long, bent helix of the A-site finger may be thought of as a mechanical lever that, through its contact with the small-subunit proteins, guides the small subunit in its movement [81]. A mutation leading to truncation of helix 38, and thereby abolition of its contacts with S13 and S19, accelerates mRNA–tRNA translocation but leads to enhanced frameshift activity [82], reinforcing the view of bridge B1a as another fine-tuning control element. Because both bridges B1a and B1b engage the small-subunit head, the constraints they impose on ribosome dynamics affect both relative motion of small-subunit body versus large subunit and small-subunit head swivel motion.
Using mutations that have the effect of deleting individual intersubunit bridges, Liu & Fredrick [83] undertook a systematic study of the contributions of individual bridges to the energy barriers that intersubunit motion has to overcome. Deletion of four of the bridges (B1a, B4, B7a and B8) that are known to be involved in constraining 30S subunit head movement (B1a) and intersubunit rotation (B4, B7a, B8), was found to accelerate the maximal rate of translocation.
9. Second stage of mRNA–tRNA translocation and the role of elongation factor G
The interaction of EF-G with the ribosome and its precise role in the second stage of translocation has been the subject of numerous studies, some of which have been mentioned above. The most recent research, benefiting from X-ray crystallography, advances in cryo-EM, and increased sensitivity of smFRET, has resulted in a complex picture of this process. According to an X-ray study, EF-G is in a compact conformation [84] when it binds to the PRE ribosome in the half-rotated state, in which the deacylated P-tRNA is found in a newly characterized, ‘chimeric’ position [64,85]. Additional light on the initial interaction between EF-G and the ribosome and GTP activation has been shed by four studies: molecular dynamics simulations [86], X-ray crystallography [87], smFRET [65] and cryo-EM [88].
This engagement of the elongation factor starts the second stage of translocation, involving a swivel movement of the small-subunit head domain [31] and removal of a physical barrier between P and E sites on the small subunit [27,89,90]. During this process, the connection between the mRNA•A–tRNA complex and the decoding centre is severed; both tRNAs move to the canonical P/P, E/E sites; the mRNA bound to their anticoon stem–loops is translocated by the length of one codon; the intersubunit rotation is fully reversed and the ribosome is reset to its post-translocational state, a process which involves re-establishment of the physical barrier. The triggering event for this stage of translocation has been thought to be GTP hydrolysis on EF-G, but interpretation of newer results by the Ermolenko group suggests that GTP hydrolysis and the second stage of translocation might not be strictly coupled [32]. The most detailed picture of this stage of translocation was recently obtained by the Blanchard laboratory [91], in which three transient intermediates are identified that can be trapped by antibiotics. The last two of these states are believed to interconvert through Brownian motion, until relocking takes place. Remarkably, these authors found that EF-G•GDP remains engaged with the ribosome throughout this entire process, until the final POST state of the ribosome, with a single tRNA in the P site, is reached.
Interesting in this context are smFRET data of Adio et al. [92]. The authors explain the catalytic power of EF-G in translocation (50 000-fold [32]) by the effect of its binding to the ribosome on the free-energy landscape of the rotating ribosome: upon binding of EF-G, a strongly increased dynamic behaviour of the ribosome is observed, prompting the authors to conclude that ‘the engagement of EF-G alters the energetics of translocation towards a flat energy landscape …’.
10. Multiple states of ribosomes purified from cell extracts
With structural knowledge having accumulated from cryo-EM and X-ray studies of elongation complexes prepared in vitro, and classification methods having been perfected, it has become possible now to identify the states of ribosome complexes purified from cell extracts directly by analysis of their structures. Entire inventories of complexes found in a sample have been recently characterized in this way [93,94]. In the study of the ribosome from Plasmodium falciparum, several complexes were identified, two of which could be placed in the known framework of the elongation cycle [93] (figure 2). The study of human ribosomes by [94] amounted to a tour de force in that virtually all key states of the elongation cycle were recovered and visualized from a polysome sample. As such, it is exemplary for the ability of cryo-EM to obtain an inventory of states of a molecular machine encountered in the living cell.
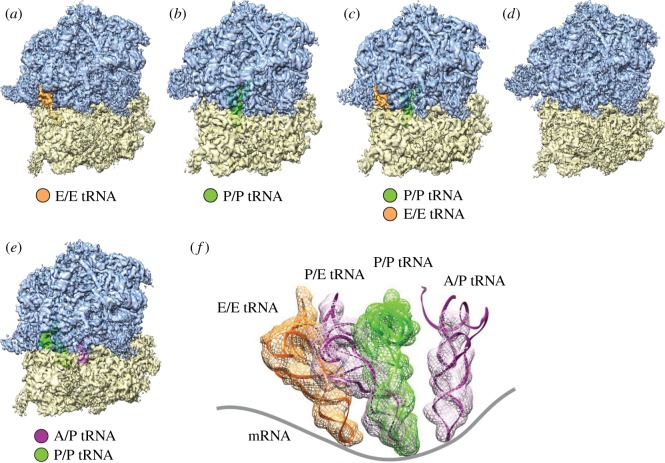
Figure 2.
Cryo-EM reconstructions of the P. falciparum 80S ribosomes purified from cell extract [93]. (a–d) Density maps of the P. falciparum 80S ribosome in non-rotated states. (a) Bound with E-tRNA (approx. 97 000 particles) at an average resolution of 4.7 Å; (b) bound with P-tRNA (approx. 14 500 particles) at 6.7 Å; (c) bound with P/P- and E/E-tRNAs (approx. 14 500 particles) at 6.7 Å and (d) without tRNAs (approx. 32 000 particles) at 5.1 Å. (e) Rotated state (approx. 23 000 particles) at 5.8 Å resolution; 60S subunits are coloured blue and 40S subunits are yellow. (f) Positions of tRNAs for all 80S complexes in (a–d). Structures and positions of E-, P- and P/E-tRNA were obtained by MDFF fitting, and the structure and position of A/P-tRNA was taken from the existing model, PDB 3J0Z, rigid-body fitted into the segmented map in UCSF Chimera. Contours of cryo-EM densities are displayed in mesh; structures of tRNA are displayed as ribbons and the mRNA path has been added as a cartoon. (Reproduced with permission from [93].)
11. Computational simulations of ribosomal dynamics
To a remarkable degree, computational modelling of ribosomal dynamics is now starting to help us appreciate the way ribosomal architecture has been shaped by evolution to use thermal energy in a functionally productive way. The point was made early on by normal mode analysis of the E. coli ribosome structure obtained by X-ray crystallography [95]. This study predicted the motion of the L1 stalk, observed in an independent cryo-EM study [26], which is correlated with the intersubunit rotation. Such normal-mode computations show that the shape of a molecule is intrinsically related to its dynamics in solution [96]. Molecular dynamics simulations of the full ribosome started with the groundbreaking work of Sanbonmatsu et al., who studied the process of aminoacyl-tRNA accommodation in the ribosomal A site [97]. Larger-scaled computations covering increasingly larger time frames have followed since [50,98–100]. For instance, Whitford et al. [50] conducted explicit solvent simulations at the atomic level (2.1 million atoms) for 1.3 µs in exploring mRNA–tRNA translocation. Recent molecular dynamics simulations of the first stage of translocation, at different levels of granularity, demonstrate the validity of a model of the translocating ribosome as a complex network of connections between subunits and tRNAs, constructed such that it ensures smoothness of the free-energy landscape and thereby allows rapid interconversion between the two relatively stable flanking states [101,102].
12. Conclusions
At the turn of the century, after X-ray crystallography solved the atomic structure of the ribosome [21–23], with pioneering contributions from Venki Ramakrishnan's laboratory [23,103], the field opened up for the exploration of its dynamics, and it is here where single-particle cryo-EM has stepped in as a technique that captures molecules in the process of performing their work. As structural probing of the ribosome is expanding, aided by much-increased resolution and the capability to resolve heterogeneity, the number of distinguishable states proliferates. The picture that emerges is of a machine of high complexity, whose structure is honed by evolution to take advantage of Brownian motion. The necessity to transport tRNA in precise steps over large distances, on the molecular scale, has given rise to an architecture in which three large domains—large subunit, small subunit body and small subunit head—are able to rotate with respect to one another, assuming multiple constellations separated by low energy barriers.
Looking at the achievements made to date and the tools of structure research and biophysical and genetic characterization available now, the author is convinced that it is just the start of a fascinating journey [104] to fully explore the way Nature solved the problem to make proteins in infinite varieties, in all life forms on Earth.
Acknowledgements
The author would like to thank Wen Li for a critical reading of the manuscript.
Competing interests
I declare I have no competing interests.
Funding
This work was supported by HHMI and NIH R01 GM29169 and GM55440.
References
- 1.Korostelev A, Ermolenko DN, Noller HF. 2008. Structural dynamics of the ribosome. Curr. Opin. Chem. Biol. 12, 674–683. ( 10.1016/j.cbpa.2008.08.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank J, Spahn CMT. 2006. The ribosome and the mechanism of protein synthesis. Rep. Prog. Phys. 69, 1383–1417. ( 10.1088/0034-4885/69/5/R03) [DOI] [Google Scholar]
- 3.Ramakrishnan V. 2008. What we have learned from rribosome structures. Biochem. Soc. Trans. 36, 567–574. ( 10.1042/BST0360567) [DOI] [PubMed] [Google Scholar]
- 4.Agirrezabala X, Frank J. 2009. Elongation in translation as a dynamic interaction among the ribosome, tRNA, and elongation factors EF-G and EF-Tu. Quart. Rev. Biophys. 42, 159–200. ( 10.1017/S0033583509990060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voorhees RM, Ramakrishnan V. 2013. Structural basis of the translational elongation cycle. Annu. Rev. Biochem. 82, 203–236. ( 10.1146/annurev-biochem-113009-092313) [DOI] [PubMed] [Google Scholar]
- 6.Spirin AS. 2009. The Ribosome as a conveying thermal ratchet machine. J. Biol. Chem. 284, 21 103–21 119. ( 10.1074/jbc.X109.001552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank J. 2012. Intermediate states during mRNA–tRNA translocation. Curr. Opin. Struct. Biol. 22, 778–785. ( 10.1016/j.sbi.2012.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank J. 1975. Averaging of low exposure electron micrographs of non-periodic objects. Ultramicroscopy 1, 159–162. ( 10.1016/S0304-3991(75)80020-9) [DOI] [PubMed] [Google Scholar]
- 9.Frank J, Shimkin B, Dowse H. 1981. SPIDER: a modular software system for image processing. Ultramicroscopy 6, 343–358. ( 10.1016/S0304-3991(81)80221-5) [DOI] [Google Scholar]
- 10.Saxton WO, Frank J. 1977. Motif detection in quantum noise-limited electron micrographs by cross-correlation. Ultramicroscopy 2, 219–227. ( 10.1016/S0304-3991(76)91385-1) [DOI] [PubMed] [Google Scholar]
- 11.Frank J. 2015. Generalized single-particle cryo-EM – a historical perspective. Microscopy 65, 3–8. ( 10.1093/jmicro/dfv358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank J. 2011. Visualization of molecular machines by cryo-electron microscopy. I: Molecular machines in biology: workshop of the cell, pp. 20–37. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Nogales E, Scheres SHW. 2015. Cryo-EM: a unique tool for the visualization of macromolecular complexity. Mol. Cell 58, 677–689. ( 10.1016/j.molcel.2015.02.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glaeser RM, Downing K, Chiu W, Frank J, DeRosier D. 2007. Electron crystallography of biological macromolecules. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Agrawal RK, Penczek P, Grassucci RA, Li Y, Leith A, Nierhaus KH, Frank J. 1996. Direct visualization of A-, P-, and E-site transfer RNAs in the Escherichia coli ribosome. Science 271, 1000–1002. ( 10.1126/science.271.5251.1000) [DOI] [PubMed] [Google Scholar]
- 16.Agrawal RK, Penczek P, Grassucci RA, Frank J. 1998. Visualization of elongation factor G on the Escherichia coli 70S ribosome: the mechanism of translocation. Proc. Natl Acad. Sci. USA 95, 6134–6138. ( 10.1073/pnas.95.11.6134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agrawal RK, Heagle AB, Penczek P, Grassucci RA, Frank J. 1999. EF-G-dependent GTP hydrolysis induces translocation accompanied by large conformational changes in the 70S ribosome. Nat. Struct. Biol. 6, 643–647. ( 10.1038/10695) [DOI] [PubMed] [Google Scholar]
- 18.Agrawal RK, Spahn CMT, Penczek P, Grassucci RG, Knud H, Nierhaus KH, Frank J. 2000. Visualization of tRNA movements on the Escherichia coli 70S ribosome during the elongation cycle. J. Cell Biol. 150, 447–460. ( 10.1083/jcb.150.3.447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stark H, Rodnina MV, Rinke-Appel J, Brimacombe R, Wintermeyer W, van Heel M. 1997. Visualization of elongation factor Tu on the Escherichia coli ribosome. Nature 389, 403–406. ( 10.1038/38770) [DOI] [PubMed] [Google Scholar]
- 20.Stark H, Rodnina MV, Wieden HJ, Zemlin F, Wintermeyer W, van Heel M. 2002. Ribosome interactions of aminoacyl-tRNA and elongation factor Tu in the codon-recognition complex. Nat. Struct. Biol. 9, 849–854. ( 10.1038/nsb859) [DOI] [PubMed] [Google Scholar]
- 21.Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289, 905–920. ( 10.1126/science.289.5481.905) [DOI] [PubMed] [Google Scholar]
- 22.Schluenzen F, et al. 2000. Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution. Cell 102, 615–623. ( 10.1016/S0092-8674(00)00084-2) [DOI] [PubMed] [Google Scholar]
- 23.Wimberly BT, Brodersen DE, Clemons WM Jr, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V. 2000. Structure of the 30S ribosomal subunit. Nature 21, 327–339. ( 10.1038/35030006) [DOI] [PubMed] [Google Scholar]
- 24.Frank J, Agrawal RK. 2000. A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature 406, 318–322. ( 10.1038/35018597) [DOI] [PubMed] [Google Scholar]
- 25.Koshland DE. 1958. Application of a theory of enzyme specificity to protein synthesis. Proc. Natl Acad. Sci. USA 44, 98–104. ( 10.1073/pnas.44.2.98) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valle M, Zavialov A, Sengupta J, Rawat U, Ehrenberg M, Frank J. 2003. Locking and unlocking of ribosomal motions. Cell 114, 123–134. ( 10.1016/S0092-8674(03)00476-8) [DOI] [PubMed] [Google Scholar]
- 27.Spahn CM, et al. 2004. Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J. 23, 1008–1019. ( 10.1038/sj.emboj.7600102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank J, Gao H, Sengupta J, Gao N, Taylor DJ. 2007. The process of mRNA–tRNA translocation. Proc. Natl Acad. Sci. USA 104, 19 671–19 678. ( 10.1073/pnas.0708517104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohan S, Donahue JP, Noller H. 2014. Molecular mechanics of 30S subunit head rotation. Proc. Natl Acad. Sci. USA 111, 13 325–13 330. ( 10.1073/pnas.1413731111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moazed D, Noller HF. 1989. Intermediate states in the movement of transfer RNA in the ribosome. Nature 342, 142–148. ( 10.1038/342142a0) [DOI] [PubMed] [Google Scholar]
- 31.Ratje AH, et al. 2010. Head swivel on the ribosome facilitates translocation via intra-subunit tRNA hybrid site. Nature 468, 713–716. ( 10.1038/nature09547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling C, Ermolenko DN. 2016. Structural insights into ribosome translocation. Wire's RNA 7, 620–636. ( 10.1002/wrna.1354). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spahn CM, Prescott CD. 2006. Throwing a spanner in the works: antibiotics and the translation apparatus. J. Mol. Med. Berl. 74, 423–439. ( 10.1007/BF00217518) [DOI] [PubMed] [Google Scholar]
- 34.Valle M, Sengupta J, Swami NK, Grassucci RA, Burkhardt N, Nierhaus KH, Agrawal RK, Frank J. 2002. Cryo-EM reveals an active role for aminoacyl-tRNA in the accommodation process. EMBO J. 21, 3557–3567. ( 10.1093/emboj/cdf326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valle M, et al. 2003. Incorporation of aminoacyl-tRNA into the ribosome as seen by cryo-electron microscopy. Nat. Struct. Biol. 10, 899–906. ( 10.1038/nsb1003) [DOI] [PubMed] [Google Scholar]
- 36.Villa E, et al. 2009. Ribosome-induced changes in elongation factor Tu conformation control GTP hydrolysis. Proc. Natl Acad. Sci. USA 106, 1063–1068. ( 10.1073/pnas.0811370106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuette JC, et al. 2009. GTPase activation of elongation factor EF-Tu by the ribosome during decoding. EMBO J. 28, 755–765. ( 10.1038/emboj.2009.26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer N, Neumann P, Konevega AL, Bock LV, Ficner R, Rodnina MV, Stark H. 2015. Structure of the E. coli ribosome–EF-Tu complex at <3 Å resolution by Cs-corrected cryo-EM. Nature 520, 567–570. ( 10.1038/nature14275) [DOI] [PubMed] [Google Scholar]
- 39.Gao H, Valle M, Ehrenberg M, Frank J. 2004. Dynamics of EF-G interaction with the ribosome explored by classification of a heterogeneous cryo-EM dataset. J. Struct. Biol. 148, 283–289. ( 10.1016/j.jsb.2004.02.008) [DOI] [PubMed] [Google Scholar]
- 40.Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H. 2010. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature 466, 329–333. ( 10.1038/nature09206) [DOI] [PubMed] [Google Scholar]
- 41.Loerke J, Giesebrecht G, Spahn CMT. 2010. Multiparticle cryo-EM of ribosomes. Methods Enzymol. 483, 161–177. ( 10.1016/S0076-6879(10)83008-3) [DOI] [PubMed] [Google Scholar]
- 42.Sigworth F. 1998. A maximum-likelihood approach to single-particle image refinement. J. Struct. Biol. 122, 328–339. ( 10.1006/jsbi.1998.4014) [DOI] [PubMed] [Google Scholar]
- 43.Scheres SHW, Gao H, Valle M, Herman GT, Eggermont PPB, Frank J, Carazo JM. 2007. Disentangling conformational states of macromolecules in 3D-EM through likelihood optimization. Nat. Methods 4, 27–29. ( 10.1038/nmeth992) [DOI] [PubMed] [Google Scholar]
- 44.Sigworth FJ. 2007. From cryo-EM, multiple protein structures in one shot. Nat. Methods 4, 20–21. ( 10.1038/nmeth0107-20) [DOI] [PubMed] [Google Scholar]
- 45.Scheres SH. 2012. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 415, 406–418. ( 10.1016/j.jmb.2011.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elmlund D, Elmlund H. 2012. SIMPLE: software for ab initio reconstruction of heterogeneous single particles. J. Struct. Biol. 180, 420–427. ( 10.1016/j.jsb.2012.07.010) [DOI] [PubMed] [Google Scholar]
- 47.Lyumkis D, Brilot AF, Theobald DL, Grigorieff N. 2013. Likelihood-based classification of cryo-EM images using FREALIGN. J. Struct. Biol. 183, 377–388. ( 10.1016/j.jsb.2013.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashem Y, et al. 2013. High-resolution cryo-electron microscopy structure of the Trypanosoma brucei ribosome. Nature 494, 385–389. ( 10.1038/nature11872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao M, Cao E, Julius D, Cheng Y. 2013. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112. ( 10.1038/nature12822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitford PC, Blanchard SC, Cate JH, Sanbonmatsu KY. 2013. Connecting the kinetics and energy landscape of tRNA translocation on the ribosome. PLoS Comput. Biol. 9, e1003003 ( 10.1371/journal.pcbi.1003003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agirrezabala X, Schreiner E, Trabuco LG, Lei J, Ortiz-Meoz RF, Schulten K, Green R, Frank J. 2011. Structural insights into cognate vs. near-cognate discrimination during decoding. EMBO J. 30, 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agirrezabala X, Liao H, Schreiner E, Fu J, Ortiz-Meoz RF, Schulten K, Green R, Frank J. 2012. Structural characterization of mRNA–tRNA translocation intermediates. Proc. Natl Acad. Sci. USA 109, 6094–6099. ( 10.1073/pnas.1201288109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maji S, Shahoei R, Schulten K, Frank J. In press. Quantitative characterization of domain motions in molecular machines J. Phys. Chem. B. [DOI] [PMC free article] [PubMed]
- 54.Ermolenko DN, Majumdar ZK, Hickerson RP, Spiegel PC, Clegg RM, Noller HF. 2007. Observation of intersubunit movement of the ribosome in solution using FRET. J. Mol. Biol. 370, 530–540. ( 10.1016/j.jmb.2007.04.042) [DOI] [PubMed] [Google Scholar]
- 55.Cornish PV, Ermolenko DN, Noller HF, Ha T. 2008. Spontaneous intersubunit rotation in single ribosomes. Mol. Cell 30, 578–588. ( 10.1016/j.molcel.2008.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fei J, Kosuri P, MacDougall DD, Gonzalez RL Jr. 2008. Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation. Mol. Cell 30, 348–359. ( 10.1016/j.molcel.2008.03.012) [DOI] [PubMed] [Google Scholar]
- 57.Agirrezabala X, Lei J, Brunelle JL, Ortiz-Meoz RF, Green R, Frank J. 2008. Visualization of the hybrid state of tRNA binding promoted by spontaneous ratcheting of the ribosome. Mol. Cell 32, 190–197. ( 10.1016/j.molcel.2008.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Julián P, Koneveg AL, Scheres SHW, Lázaro M, Gila D, Wintermeyer W, Rodnina MV, Valle M. 2008. Structure of ratcheted ribosomes with tRNAs in hybrid states. Proc. Natl Acad. Sci. USA 105, 16 924–16 927. ( 10.1073/pnas.0809587105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spirin AS, Finkelstein AV. 2011. The ribosome as a Brownian ratchet machine. In Molecular machines in biology – workshop of the cell, chapter 9 (ed. Frank J.), pp. 158–190. New York, NY: Cambridge University Press. [Google Scholar]
- 60.Munro JB, Sanbonmatsu KY, Spahn CM, Blanchard SC. 2009. Navigating the ribosome's metastable energy landscape. Trends Biochem. Sci. 34, 390–400. ( 10.1016/j.tibs.2009.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frank J, Gonzalez RL Jr. 2010. Structure and dynamics of a processive Brownian motor: the translating ribosome. Annu. Rev. Biochem. 79, 381–412. ( 10.1146/annurev-biochem-060408-173330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodnina MV, Wintermeyer W. 2011. The ribosome as a molecular machine: the mechanism of tRNA-mRNA movement in translocation. Biochem. Soc. Trans. 39, 658–662. ( 10.1042/BST0390658) [DOI] [PubMed] [Google Scholar]
- 63.Wang B, Ho J, Fei J, Gonzalez RL Jr, Lin Q. 2011. A microfluidic approach for investigating the temperature dependence of biomolecular activity with single-molecule resolution. Lab Chip 11, 274–281. ( 10.1039/C0LC00157K) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brilot AF, Korostelev AA, Ermolenko DN, Grigorieff N. 2013. Structure of the ribosome with elongation factor G trapped in the pretranslocation state. Proc. Natl Acad. Sci. USA 110, 20 994–20 999. ( 10.1073/pnas.1311423110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Petrov A, Tsai A, O'Leary SE, Puglisi JD. 2013. Coordinated conformational and compositional dynamics drive ribosome translocation. Nat. Struct. Mol. Biol. 20, 718–727. ( 10.1038/nsmb.2567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munro JB, Altman RB, O'Connor N, Blanchard SC. 2007. Identification of two distinct hybrid state intermediates on the ribosome. Mol. Cell. 25, 505–517. ( 10.1016/j.molcel.2007.01.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu J, Munro JB, Blanchard SC, Frank J. 2011. Cryo-EM structures of the ribosome complex in intermediate states during tRNA translocation. Proc. Natl Acad. Sci. USA 108, 4817–4821. ( 10.1073/pnas.1101503108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kinz-Thompson CD, Sharma AK, Frank J, Gonzalez RL Jr, Chowdhury D. 2015. Quantitative connection between ensemble thermodynamics and single-molecule kinetics: a case study using cryogenic electron microscopy and single-molecule fluorescence resonance energy transfer investigations of the ribosome. J. Phys. Chem. B 119, 10 888–10 901. ( 10.1021/jp5128805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Budkevich T, Giesebrecht J, Altman RB, Munro JB, Mielke T, Nierhaus KH, Blanchard SC, Spahn CMT. 2011. Structure and dynamics of the mammalian ribosomal pre-translocation complex. Mol. Cell 44, 214–224. ( 10.1016/j.molcel.2011.07.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Doudna Cate JH. 2005. Structures of the bacterial ribosome at 3.5 Å resolution. Science 310, 827–833. ( 10.1126/science.1117230) [DOI] [PubMed] [Google Scholar]
- 71.Zhang W, Dunkle JA, Cate J. 2009. Structures of the ribosome in intermediate states of ratcheting. Science 325, 1014–1017. ( 10.1126/science.1175275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dunkle JA, et al. 2011. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science 332, 981–984. ( 10.1126/science.1202692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pulk A, Cate HD. 2013. Control of ribosomal subunit rotation by elongation factor G. Science 340, 1235970 ( 10.1126/science.1235970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dashti A, et al. 2014. Trajectories of the ribosome as a Brownian nanomachine. Proc. Natl Acad. Sci. USA 111, 17 492–17 497. ( 10.1073/pnas.1419276111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frank J, Ourmazd A. 2016. Continuous changes in structure mapped by manifold embedding of single-particle data in cryo-EM. Methods 100, 61–67. ( 10.1016/j.ymeth.2016.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Q, Fredrick K. 2016. Intersubunit bridges of the bacterial ribosome. J. Mol. Biol. 428, 2146–2164. ( 10.1016/j.jmb.2016.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frank J, et al. 1995. A model of the translational apparatus based on a three-dimensional reconstruction of the Escherichia coli ribosome. Biochem. Cell Biol. 73, 757–765. ( 10.1139/o95-084) [DOI] [PubMed] [Google Scholar]
- 78.Cate JH, Yusupov MM, Yusupova GZ, Earnest TN, Noller HF. 1999. X-ray crystal structures of 70S ribosome functional complexes. Science 285, 2095–2104. ( 10.1126/science.285.5436.2095) [DOI] [PubMed] [Google Scholar]
- 79.Shasmal M, Chakraborty B, Sengupta J. 2010. Intrinsic molecular properties of the protein-protein bridge facilitate ratchet-like motion of the ribosome. Biochem. Biophys. Res. Commun. 399, 192–197. ( 10.1016/j.bbrc.2010.07.053) [DOI] [PubMed] [Google Scholar]
- 80.Rhodin MHJ, Dinman JD. 2011. An extensive network of information flow through the B1b/c intersubunit bridge of the yeast ribosome. PLoS ONE 6, e20048 ( 10.1371/journal.pone.0020048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reblova KRF, Li W, Gao H, Frank J, Sponer J. 2010. Dynamics of the base of ribosomal A-site finger revealed by molecular dynamics simulations and cryo-EM. Nucleic Acids Res. 38, 1325–1340. ( 10.1093/nar/gkp1057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komoda T, Sato NS, Phelps SS, Namba N, Joseph S, Suzuki T. 2006. The A-site finger in 23S rRNA acts as a functional attenuator for translocation. J. Biol. Chem. 281, 32 303–32 309. ( 10.1074/jbc.M607058200) [DOI] [PubMed] [Google Scholar]
- 83.Liu Q, Fredrick K. 2012. Contribution of intersubunit bridges to the energy barrier of ribosomal translocation. Nucleic Acids Res. 41, 565–574. ( 10.1093/nar/gks1074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin J, Gagnon MG, Bulkley D, Steitz TA. 2015. Conformational changes of elongation factor G on the ribosome during tRNA translocation. Cell 160, 219–227. ( 10.1016/j.cell.2014.11.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramrath DJF, Lancaster L, Sprink T, Mielke T, Loerke J, Noller HF, Spahn CMT. 2013. Visualization of two transfer RNAs trapped in transit during elongation factor G-mediated translocation. Proc. Natl Acad. Sci. USA 110, 20 964–20 969. ( 10.1073/pnas.1320387110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li W, Trabuco LG, Schulten K, Frank J. 2011. Molecular dynamics of EF-G during translocation. Proteins 79, 1478–1486. ( 10.1002/prot.22976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tourigny DS, Fernandez IS, Kelley AC, Ramakrishnan V. 2013. Elongation factor G bound to the ribosome in an intermediate state of translocation. Science 340, 1235490. ( 10.1126/science.1235490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li W, Liu Z, Koripella RK, Langlois R, Sanyal S, Frank J. 2015. Activation of GTP hydrolysis in mRNA–tRNA translocation by elongation factor G. Sci. Adv. 1, e1500169 ( 10.1126/sciadv.1500169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berk V, Cate JHD. 2007. Insights into protein biosynthesis from structures of bacterial ribosomes. Curr. Opin. Struct. Biol. 17, 302–309. ( 10.1016/j.sbi.2007.05.009) [DOI] [PubMed] [Google Scholar]
- 90.Borovinskaya MA, Shoji S, Holton JM, Fredrick K, Cate JH. 2007. A steric block in translation caused by the antibiotic spectinomycin. ACS Chem. Biol. 2, 545–552. ( 10.1021/cb700100n) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wasserman MR, Alejo JL, Altman RB, Blanchard SC. 2016. Multiperspective smFRET reveals rate-determining late intermediates of ribosomal translocation. Nat. Struct. Mol. Biol. 23, 333–341. ( 10.1038/nsmb.3177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adio S, Senyushkina T, Peske F, Fischer N, Wintermeyer W, Rodnina MV. 2015. Fluctuations between multiple EF-G-induced chimeric tRNA states during translocation on the ribosome. Nat. Commun. 6, 7442 ( 10.1038/ncomms8442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun M, Li W, Blomqvist K, Das S, Hashem Y, Dvorin JD, Frank J. 2015. Dynamical features of the Plasmodium falciparum ribosome during translation. Nucleic Acids Res. 43, 10 515–10 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Behrmann E, et al. 2015. Structural snapshots of actively translating human ribosomes. Cell 161, 845–857. ( 10.1016/j.cell.2015.03.052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tama F, Valle M, Frank J, Brooks CL 3rd. 2003. Dynamic reorganization of the functionally active ribosome explored by normal mode analysis and cryo-electron microscopy. Proc. Natl Acad. Sci. USA 100, 9319–9323. ( 10.1073/pnas.1632476100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu M, Ma J. 2005. The role of shape in determining molecular motions. Biophys. J. 89, 2395–2401. ( 10.1529/biophysj.105.065904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanbonmatsu KY, Joseph S, Tung CS. 2005. Simulating movement of tRNA into the ribosome during decoding. Proc. Natl Acad. Sci. USA 102, 15 854–15 859. ( 10.1073/pnas.0503456102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Whitford PC, Sanbonmatsu KY. 2013. Simulating movement of tRNA through the ribosome during hybrid-state formation. J. Chem. Phys. 139, 121919 ( 10.1063/1.4817212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Noel JK, Chahine J, Leite VBP, Whitford PC. 2014. Capturing transition paths and transition states for conformational rearrangements in the ribosome. Biophys. J. 107, 2881–2890. ( 10.1016/j.bpj.2014.10.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirmizialtin S, Loerke J, Behrmann E, Spahn CMT, Sanbonmatsu KY. 2015. Using molecular simulation to model high-resolution cryo-EM reconstructions. Methods Enzymol. 558, 497–514. ( 10.1016/bs.mie.2015.02.011) [DOI] [PubMed] [Google Scholar]
- 101.Bock LV, Blau C, Schröder GF, Davydov II, Fischer N, Stark H, Rodnina MV, Vaiana AC, Grubmüller H. 2013. Energy barriers and driving forces in tRNA translocation through the ribosome. Nat. Struct. Mol. Biol. 20, 1390–1396. ( 10.1038/nsmb.2690) [DOI] [PubMed] [Google Scholar]
- 102.Bock LV, Blau C, Vaiana AC, Grubmüller H. 2015. Dynamic contact network between ribosomal subunits enables rapid large-scale rotation during spontaneous translocation. Nucleic Acids Res. 43, 6747–6760. ( 10.1093/nar/gkv649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramakrishnan V. 2010. Unraveling the structure of the ribosome (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 49, 4355–4380. ( 10.1002/anie.201001436) [DOI] [PubMed] [Google Scholar]
- 104.Frank J. 2016. Whither ribosome structure and dynamics research? (A perspective). J. Mol. Biol. 428, 3565–3569. ( 10.1016/j.jmb.2016.04.034) [DOI] [PMC free article] [PubMed] [Google Scholar]