Abstract
Epigenetics, as a DNA signature that affects gene expression and enables rapid reaction of an organism to environmental changes, is likely involved in the process of biological invasions. DNA methylation is an epigenetic mechanism common to plants and animals for regulating gene expression. In this study we show, for the first time in any marine species, significant reduction of global methylation levels during the expansive phase of a pygmy mussel (Xenostrobus securis) recent invasion in Europe (two-year old), while in older introductions such epigenetic signature of invasion was progressively reduced. Decreased methylation was interpreted as a rapid way of increasing phenotypic plasticity that would help invasive populations to thrive. This epigenetic signature of early invasion was stronger than the expected environmental signature of environmental stress in younger populations sampled from ports, otherwise detected in a much older population (>90 year old) of the also invasive tubeworm Ficopomatus enigmaticus established in similar locations. Higher epigenetic than genetic diversity found in X. securis was confirmed from F. enigmaticus samples. As reported for introduced plants and vertebrates, epigenetic variation could compensate for relatively lower genetic variation caused by founder effects. These phenomena were compared with epigenetic mechanisms involved in metastasis, as parallel processes of community (biological invasion) and organism (cancer) invasions.
Epigenetics, particularly a noticeable shift in methylation status, is often associated with the process of colonization of new environments. This is a natural response to changes in abiotic factors1 or biotic environment2. Changes in methylation are thought to be involved in phenotypic plasticity3, that is an important prerequisite for adaptation to varying environmental conditions reported both in plants and animals1,4. Different types of stressing environmental conditions may alter global methylation levels5,6,7,8. The direction of the change in global methylation may depend on particular stressors; for example, some chemicals present in environment may reduce methylation while others tend to increase it5,9. A few in situ studies reported hypermethylation response in aquatic animals exposed to environmental stress6,10.
Due to its importance in adaptation mechanisms, epigenetics must be involved in the process of biological invasions facilitating the establishment of exotic organisms in recipient ecosystems11,12. The epigenetic variation in introduced populations may to a certain level compensate for reduced genetic diversity and serve as an alternative source of phenotypic variation. Evidences of epigenetic, not genetic, response to environmental variance in invaded habitats and therefore differentiation of populations are reported for introduced plants and birds13,14,15. These findings can add a new perspective to the understanding of biological invasions and underlying mechanisms16. Plasticity or new phenotypes acquired through epigenetic changes can explain the ability of invasive species to expand and colonize new ecosystems with very reduced genetic diversity due to founder effects17. Some invasive populations exhibit considerable genetic diversity resulting from repeated introduction events11,18, and have therefore a sufficient substrate of genetic variants for selection and response to different environmental conditions. However, epigenetic mechanisms provide a faster way of response because they may occur in one generation, while selection implies differential reproduction of genetic variants – so at least two generations are needed. Hence, successful invaders are expected to be prone to epigenetic variations and this might be reflected in their epigenetic signatures regardless the genetic diversity of the invasive population.
To date, the available data about invasion epigenetics refer predominantly to terrestrial species. The epigenetics of marine invasions is largely understudied yet. Biological invasions follow a sequential process of arrival, establishment, expansion, and eventually accommodation within the recipient ecosystem19,20,21. The maximum alteration at epigenomic level is expected to occur at the arrival through early expansion phase, when the species needs to boost its adaptive capacity to overcome the existing environmental constraints and establish a successful population.
Here we present a proof-of-concept study aimed at challenging our hypothesis of epigenetic signature in invasive populations, based on the New Zealand pygmy mussel Xenostrobus securis. This species is invading the coastal waters of Japan22 and south Europe23,24. A new arrival was detected in 2014 in a port from northwest Iberian Peninsula (southwest Bay of Biscay), where it is expanding dramatically fast18,25,26. Epigenetic and genetic variation of this new population were compared with older invasive European populations from another port and a lagoon protected under NATURA 2000. Considering that: environmental stress induces methylation changes (sometimes hypermethylation but not always); some introduced populations exhibit higher epigenetic than genetic diversity; and lower methylation would encompass higher phenotypic plasticity, expectations were: I) mussels would be differentially methylated in polluted ports compared to cleaner lagoons; II) higher epigenetic than genetic diversity would be expected in invasive mussels if the process described for vertebrates and plants is common to all invasions, that is, these introduced samples will differ more for epigenetic than for genetic variation; III) the newer population, undergoing the initial expansion phase, would exhibit lower global methylation than already established ones –i.e. for similar port environment the newer invader would be less methylated than the older one.
To check if the results may be generalizable we have also analyzed a few individuals from comparatively older introduced populations of Australian tubeworm Ficopomatus enigmaticus from markedly different environments. This is a worldwide well-established marine invader27,28,29, of very old introduction in the Bay of Biscay (back to 192130). Following the same rationale, these Bay of Biscay samples would be more methylated than newer ones. Contrasting environmental conditions were also considered for the newer populations: one more recent from a big port in New Zealand and another of intermediate age from a protected Mediterranean lagoon.
Material and Methods
Sampling sites and environmental conditions
The X. securis and F. enigmaticus specimens analyzed in this study were all adults, collected in winter time (December 2014–January 2015 in Europe, July in New Zealand). The sampling sites represented different environments (Table 1), from lower to higher environmental stress: protected lagoons in the Mediterranean; one local fishing port in a small village (Llanes in the Bay of Biscay); commercial ports with international maritime traffic nearby industrial cities (Aviles in the Bay of Biscay, Pontevedra in Northwest Spain, Napier in New Zealand). General environmental information for the sampling areas was collected from the online resources (e.g. National Agency of Meteorology, Global Sea Temperature website, ClimaTemps website, National Ports –Spanish ports at http://www.puertos.es/es-es, Napier port at http://www.napierport.co.nz/), published national and regional reports and research papers. The date of the first record of the species was verified with the published literature resources and on-line databases (Invasive Species Specialist Group, IUCN; WRIMS; AquaNIS31).
Table 1. Generalized environmental conditions at the sampling (specimen collection) sites.
| Sample code | Country | Region | First report | Location | Coordinates | Temperature | Sunlight | Rainfall | Habitat | Salinity | Pollution |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Xenostrobus securis | |||||||||||
| XCant | Spain | South Bay of Biscay | 201426 | Aviles estuary | 43° 33′ 22″N, 5° 55′ 20″W | 9.6 (6.8–19.8) | 1,670 | 1,048 | Marina, international port | 29.3 (17.9–35.8) | Urban, industrial |
| XAtl | Spain | Northwest Spain | 200253 | Pontevedra estuary | 42° 26′ 7.6″N, −8° 38′ 56″W | 14.8 (10.4–19.2) | 2,247 | 1,613 | Marina, international port | 33 (31.5–35)57 | Urban, industrial |
| XMed | France | Mediterranean Sea | 199254 | Vidourle Lagoon | 43°34′42.14″N 4°02′34.98″E | 15.1 (10.4–19.9) | 2,668.2 | 629.1 | Lagoon/Natura 2000 Network | 23.9 (20.4–27.3)58 | Urban, eutrophication |
| Ficopomatus enigmaticus | |||||||||||
| FCant | Spain | South Bay of Biscay | 1920s30 | Llanes | 43°25′16″N, 4°45′11″W | 13.5 (9.9–17.1) | 1,756 | 1,062 | Marina, small fishing port | 25.2 (21.4–29.5) | Urban, small village |
| FMed | France | Mediterranean Sea | 194759 | Saint-Nazaire Lagoon | 42°38′49.25″ N 3°01′27.75″ E | 15.7 (11.4–20.1) | 2,464.9 | 557.6 | Lagoon/Natura 2000 Network | 20.4 (5.55–35.2)60 | Urban, eutrophication61 |
| FNZ | New Zealand | South Pacific Ocean | 196762 | Napier, Hawke’s Bay | 39°29′S 176°55′E | 16.4 (12.2–22.1) | 2,281 | 809.7 | Marina, international port | 34–3563 | Urban, industrial |
In the sample code ‘X’ refers to X. securis samples and ‘F’ to F. enigmaticus samples. International ports receive >one million annual cargo tons. Temperature, average annual temperature in °C (min-max); sunlight, average sunlight hours per year; rainfall, annual rainfall in mm; salinity in ppm (min-max).
Ethical statement
This study has been carried out on invertebrate invasive species, thus measures of careful cleaning and disinfection of materials and clothes after sampling were taken, to avoid further dispersion of these organisms. This study adheres to the European Code of Conduct for Responsible Research.
Sample collection, DNA extraction and barcoding
The two species here studied have a short planktonic larval stage, are tolerant to wide salinity, temperature and pollution ranges and can disperse through shipping pathway27,32. Adult individuals of X. securis (25, 26 and 31 from Mediterranean, Atlantic and Cantabric respectively) were identified de visu and preserved in ethanol for further genetic and epigenetic analysis. Total DNA was extracted from a small piece of foot muscle with the E.Z.N.A Mollusc DNA kit (IOMEGA, bio-tek), following manufacturer´s instructions. Five individuals of F. enigmaticus were sampled from each site and preserved in ethanol as well. DNA was extracted from the whole body employing a method based on silica gel columns (QIAmp DNA Mini Kit, Qiagen), following manufacturer’s instructions.
The tubes with DNA samples were stored at 4 °C for immediate analysis and aliquots were frozen at −20 °C for long-time preservation. Isolated DNA was quantified using a fluorometric method with Qubit® 2.0, and normalized to 100 ng/μ for subsequent analysis.
DNA barcoding was performed for each individual to verify the identity of the species and for the further reference. The mitochondrial cytochrome c oxidase subunit I (COI) gene was amplified from X. securis samples using the universal primers designed by Geller et al.33 and the conditions described therein. The nuclear subunit 18 S rRNA gene was PCR amplified from F. enigmaticus due to the absence of COI gene references in publically available databases (BOLD Systems, NCBI) at the moment of this study (June, 2016), using the primers and protocol described in Distel et al.34. Bovine serum albumin (BSA, 200 ng/μl) was included in the PCR protocol described by Distel et al.34 to avoid interferences of possible inhibitors.
PCR products were examined on 2% agarose gel stained with SimplySafeTM (EURx, Poland). Positive amplicons (evidenced by clear single band of the expected size) were sequenced by Macrogen Inc. (The Netherlands) with ABI3730xl DNA sequencer (Applied Biosystems).
Obtained DNA sequences were edited with BioEdit v7.2.535 and compared with those published in online databases using nBLAST tool (www.ncbi.nlm.nih.gov/).
MSAP analysis
The methylation-sensitive amplified polymorphism approach or MSAP was used to detect polymorphism in DNA methylation patterns. The protocol for global methylation analysis was conducted following Díaz-Freije et al.36. An aliquot (100 ng) of DNA of each sample was split in two parts to be treated with either EcoRI/HpaII or EcoRI/MspI. Both enzymes (MspI and HpaII) recognize and cleave CCGG target sequences, but cleaving by HpaII is blocked when the inner or outer C is methylated at both strands; while cleaving in MspI is blocked when the outer cytosines are fully or hemi-methylated; cleaving in both enzymes is blocked when both cytosines are methylated –and/or when nucleotide polymorphism occurs in the restriction target thus the sequence is not recognized by the enzymes37.
The resulting DNA fragments were ligated with linkers and PCR amplified using two primer combination: EcoRI-AAG, HpaII-TCC and EcoRI-AAG, HpaII-TAC. HpaII primers were end-labeled using 6-FAM reporter molecule38. PCR products were loaded with a GeneScan GS-500 LIZ3130 size standard into an ABI Prism 3100 Genetic Analyzer (Applied Biosystem). Fragment analysis and AFLP scoring was performed using GeneMapper v.4.0 software (Applied Biosystem). To avoid confounding methylation sites and poorly reproducible fragments the following settings were applied: analysis range, 50–500 base pairs (bp); minimum peak height, 50 relative fluorescence units; pass range for sizing quality: 0.75–1.0; maximum peak width: 1.5 bp; maximum peak height ratio: 1.8 (higher peaks were removed); normalization method: sum of signals. To confirm AFLP reproducibility the five F. enigmaticus and five X. securis samples (XAv1–5) from the Bay of Biscay were analyzed again with the same protocols.
Data analysis
MSAP individual and population profiles were analyzed using the R package msap v.3.2.2.39. The software combines the information based on the four possible patterns from presence-absence matrices obtained with the EcoRI-HpaII and EcoRI-MspI primer combinations, yielding a new score matrix according to the methylation state. The type of epigenetic variation detected with MSAP loci was categorized following Salmon et al.37:
-Type I = restriction site no methylation: both enzymes cut at the restriction site,
-Type II = methylation of internal C: HpaII does not cut and MspI does cut,
-Type III = methylation of external C or hemimethylation: HpaII does cut and MspI does not, and
-Type IV = hypermethylation or mutation in restriction site: neither enzymes cut.
The study developed by Fulnecek and Kovarik40 indicated that type II and III variation cannot be interpreted as CG versus CGH methylation, because what looks like CHG methylation is in fact often caused by differently methylated internal restriction sites nested with fragments. Type IV variation is not employed for calculating methylation state because it could be also due to mutations in restriction sites, so methylation state cannot be specified37. Therefore, we pooled data in two categories, methylated (Type II and III) or not methylated (Type I) restriction sites. The global methylation level was thus measured following Nicotra et al.41 as the proportion of methylated loci (Types II and III) over the scorable loci (Types I, II and III), per dataset.
Every locus was classified as either Methylation-susceptible loci (MSL) or Non-methylated loci (NML), depending on whether the observed proportion of methylated states across all samples exceeded a user-defined error rate-based threshold (ERT; 5% by default). Only those fragments showing polymorphism, with at least two occurrences of each state, were used for subsequent analysis42. MSL were used to assess epigenetic variation and NML were analyzed in order to asses genetic variation among populations as their banding pattern depends exclusively on changes in the sequence at the restriction target.
The following analyses were performed in MSAP using the R package msap v.3.2.2.39, for both, MSL and NML. The amount of overall variation was estimated using the Shannon diversity index (I). Differences between Shannon’s indices between MSL and NML were tested using the Wilcoxon rank sum test with continuity correction (W).
The epigenetic (MSL) and genetic (NML) differentiation among populations and between pairs of populations was assessed by means of ɸST values (equivalent to FST values in codominant loci), and principal coordinates analyses (PCoA) followed by analysis of molecular variance (AMOVA)43, using the R package msap v.3.2.2.39 and GenAlEx software44,45.
The mean proportion of methylated loci was compared among populations using classic ANOVA. Analysis of residuals was done and normality was checked with Shapiro-Wilk test; if it was significant or not interpretable Welch F test was performed instead of ANOVA. In that case clear outlier data were removed for pairwise analyses. Medians were compared among populations with Kruskal-Wallis test. Post-hoc pairwise comparisons were made with Tukey’s honest significance tests for means, and Mann-Whitney for medians. Software PAST46 was employed to perform these statistical tests.
Finally, genetic differences between pairs of populations were also assessed by comparing EcoRI-HpaII and EcoRI-MspI profiles as standard AFLPs using the option meth(false) implemented in the R package msap. This is a second measure of genetic variation that scores all the loci, not only NML. Consistent results for the two measures would reinforce the conclusions about genetic differences between populations. For confirmation ɸST values were also obtained and AMOVA performed for population pairs using GenAlEx software44,45.
Results
In total 82 X. securis and 15 F. enigmaticus adults, from three different introduced populations for each species, were barcoded for species confirmation and analysed for AFLP and MS-AFLP variation. The most frequent haplotypes of COI and 18 S rRNA gene sequences obtained from the analyzed X. securis and F. enigmaticus individuals (648 and 656 nucleotides respectively), are available in NCBI GenBank database with the accession numbers KX129960-KX129962 and KX129957-KX129959. Comparison of the acquired sequences with existing references in nucleotide databases confirmed unambiguously the species identity of the individuals analyzed.
Detected AFLP variation was considerable in the two species. In total 380 AFLP loci were found in the X. securis samples (Supplementary Table 1 with Dataset 1) and 188 in F. enigmaticus (Supplementary Table 2 with Dataset 2). Of those, 200 (52.63%) and 105 (55.6%) were methylation-susceptible loci (MSL) in X. securis and F. enigmaticus respectively. The results were reproducible because the 5 individuals reanalyzed of each species gave the same AFLP and methylation patterns (data not shown). The genetic differentiation based on all the AFLP loci provided higher statistical significance for pairwise ɸST, as expected from the higher number of loci examined and distant populations of likely multiple origin18. Indeed significant differences were detected between all pairs of X. securis populations (data not shown). Significant differences occurred between F. enigmaticus FMed and FNZ samples (ɸST = 0.082, P = 0.018), but not between FAtl and FMed (ɸST = 0.015, P = 0.249), neither between FCant and FNZ (ɸST = 0.0501, P = 0.061), probably due to small sample sizes.
Xenostrobus securis
There was considerable variation in MSL between individuals within each population. The proportional occurrence of each type of methylation for X. securis from different locations is summarized in Fig. 1. All of the samples exhibited more fully methylated –or mutations in restriction sites- (Type IV) than hemimethylated (Type III), internally methylated (Type II) and unmethylated loci (Type I).
Figure 1. DNA methylation in methylation-sensitive loci detected from all analyzed specimens of Xenostrobus securis, per population.
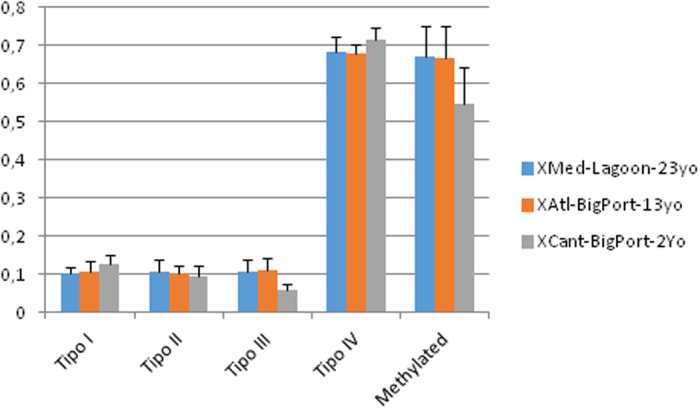
Type I to IV are respectively: no methylated, methylation of internal C, methylation of external C or hemimethylation, and hypermethylation or mutation in restriction site. Methylated: Global methylation level estimated following Nicotra et al. (Nicotra et al.41), as proportion of (Type II+Type III loci)/(scorable loci). The letters yo mean “year old”.
For the individual number of methylated loci in the analyzed mussels, Shapiro-Wilk test (value of 1) was not statistically significant, meaning the distribution did not deviate significantly from normality. The mean proportion of methylated MSL loci exhibited strong statistical significance among groups (F = 18.34, P ≪ 0.0001) and the same occurred for medians (Kruskal-Wallis test with P ≪ 0.0001; Table 2). The pygmy mussel population recently introduced into a big port, XCant, was hypomethylated in comparison with the other two populations of this species analyzed. The difference between this sample and the other two was statistically highly significant for both means and medians, as indicated from pairwise Tukey’s and Mann-Whitney tests (Table 2). Methylated loci (Types II and III) represented 55% of the total scorable loci (Fig. 1, column at right). In contrast the other two populations were quite similar to each other, with 67% methylated loci in both population: the relatively young population from the other big port (XAtl) and the older population introduced in a Mediterranean lagoon (XMed), without statistical differences between them (Table 2).
Table 2. Statistical analysis of individual methylated loci for X. securis (above) and F. enigmaticus (below). ANOVA or Welch F test, and Kruskal-Wallis H analysis, for comparing means and medians respectively; Tukey’s and Mann-Whitney post-hoc tests for respective pairwise comparisons of means and medians (in italics, statistically significant p-values).
| Xenostrobus securis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Test for equal means: ANOVA | Test for equal medians | |||||||
| Sum of squares | df | Mean square | F | p (same) | H (chi2): | 28.71 | ||
| Between groups | 0.27815 | 2 | 0.1391 | 18.34 | 0.0000003 | Hc (tie corrected): | 28.72 | |
| Within groups | 0.599038 | 79 | 0.0076 | p (same): | 0.0000006 | |||
| Total: | 0.877188 | 81 | ||||||
| Tukey’s post-hoc | Mann-Whitney post-hoc (P-values) | |||||||
| Atlantic | Bay of Biscay | Mediterranean | ||||||
| Atlantic | 0.0001154 | 0.9968 | Atlantic | Bay of Biscay | ||||
| Bay of Biscay | 7.126 | 0.0001135 | Bay of Biscay | 0.000003 | ||||
| Mediterranean | 0.1094 | 7.235 | Mediterranean | 0.6993 | 0.000009 | |||
| Ficopomatus enigmaticus | ||||||||
| Test for equal means: Welch F | Test for equal medians | |||||||
| F | df | P-value | H (chi2): | 5.41 | ||||
| 4.969 | 6, 186 | 0.051 | Hc (tie corrected): | 5.41 | ||||
| p (same): | 0.066 | |||||||
| Tukey’s post-hoc | ||||||||
| Bay of Biscay | Mediterranean | New Zealand | Mann-Whitney post-hoc (P-values) | |||||
| Bay of Biscay | 0.08647 | 0.1012 | Bay of Biscay | Mediterranean | ||||
| Mediterranean | 3.4 | 0.9947 | Mediterranean | 0.06619 | ||||
| Nwe Zealand | 3.26 | 0.1401 | New Zealand | 0.0606 | 0.9025 | |||
On the other hand, global epigenetic differences (i.e. in MSL) were detected among X. securis populations (highly significant AMOVA, ɸST = 0.1447, P < 0.0001). From a total variance of 42.323, 14.47% was due to variation among populations. For genetic variation (NML) the populations were also globally significantly different (ɸST = 0.1029, P < 0.0001), but the variance due to among-population variation was lower, 10.29% of the total variance (1.345 for a total variance of 13.065). Epigenetic diversity (at MSL) was indeed significantly higher than genetic diversity (NML), Shannon’s index being I = 0.5754 (SD = 0.1189) and I = 0.2510 (SD = 0.1483) respectively, W = 31834 (P < 0.0001).
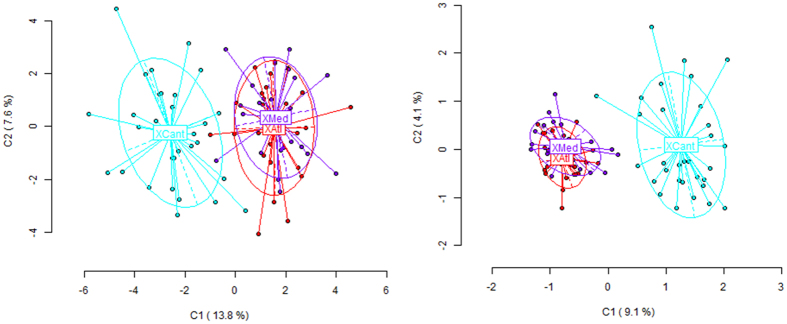
The difference between epigenetic and genetic variation in X. securis dataset could be visualized from the PCoA (Fig. 2). The methylation patterns (MSL, Fig. 2, left) reported for X. securis from the Mediterranean lagoon (XMed) and Atlantic international port (XAtl) samples overlapped partially, with the new expansive population from the Bay of Biscay port (XCant) clearly differentiated. For NML (Fig. 2, right) the variation was lower. Pairwise differences for MSL involving XCant were highly significant (Table 3, below diagonal). XMed (23 year-old population) was expected to be less methylated than XAtl (13 year old) because of less disturbed environment, but it was slightly more methylated –although not significantly (ɸST = 0.012, P = 0.1567 for MSL). Despite not significantly difference for epigenetic variation, these two populations did differ significantly for their genetic variation (ɸST = 0.016, P = 0.0035 for NML; Table 3 above diagonal).
Figure 2. Two-dimensional visualization of the Principal Component Analysis (PCoA) of the detected methylation patterns in Xenostrobus securis, with the epigenetic variation (methylation-sensitive loci) on the left and the genetic variation (no methylated loci, NML) on the right.
The individuals of each population are represented by the acronyms XAtl, XCant and XMed for the Atlantic international port, Cantabric international port and Mediterranean lagoon populations respectively.
Table 3. Pairwise differentiation between sampled populations measured as ɸST (P-value in parenthesis), for methylation sensitive or epigenetic loci (below diagonal) and non-methylated or genetic loci (above diagonal).
| XAtl | XMed | XCant | FNZ | FCant | FMed | |
|---|---|---|---|---|---|---|
| XAtl | 0.016 (P = 0.004) | 0.139 (p < 0.0001) | — | — | — | |
| XMed | 0.012 (P = 0.157) | 0.132 (p < 0.0001) | — | — | — | |
| XCant | 0.165 (p < 0.0001) | 0.182 (p < 0.0001) | — | — | — | |
| FNZ | — | — | — | 0.0203 (P = 0.387) | 0.067 (P = 0.168) | |
| FCant | — | — | — | 0.187 (P = 0.009) | −0.015 (P = 0.535) | |
| FMed | — | — | — | 0.205 (P = 0.018) | 0.021 (P = 0.343) |
F and X letters represent F. enigmaticus and X. securis respectively, and Atl, Cant, Med and NZ correspond accordingly to Atlantic, Bay of Biscay, Mediterranean and New Zealand populations.
Ficopomatus enigmaticus
The tubeworm Ficopomatus enigmaticus individuals examined were also variable for AFLP, with 105 MSL (50% polymorphic) and 83 NML (99% polymorphic). As in the case of X. securis, more fully methylated –or mutation in restriction site- (Type IV) than hemimethylated (Type III), internally methylated (Type II) and unmethylated loci (Type I) were detected (Fig. 3). The global methylation level was higher in the long-time established samples of the Bay of Biscay fishing port (FCant, 73%) than in the Mediterranean lagoon and Napier port (63.5% and 63.9% respectively) (Fig. 3, columns “Methylated”, on the right-side). The Shapiro-Wilk test provided a NaN value of 1.989 for the individual proportion of methylated loci in the 15 samples analyzed. The Welch F test for samples with unequal variances was marginally significant (F = 4.969, P = 0.052). Differences among medians measured from Kruskal-Wallis test were not significant but not far from significance (P = 0.067) (Table 2). After removing the two clearly divergent individuals FCant5 and FNZ1, and despite small sample sizes, the difference between FCant and the other two samples was nearly significant for the Mann-Whitney test, although not for the post-hoc Tukey’s test (Table 2).
Figure 3. DNA methylation in methylation-sensitive loci detected from the analyzed specimens of Ficopomatus enigmaticus.
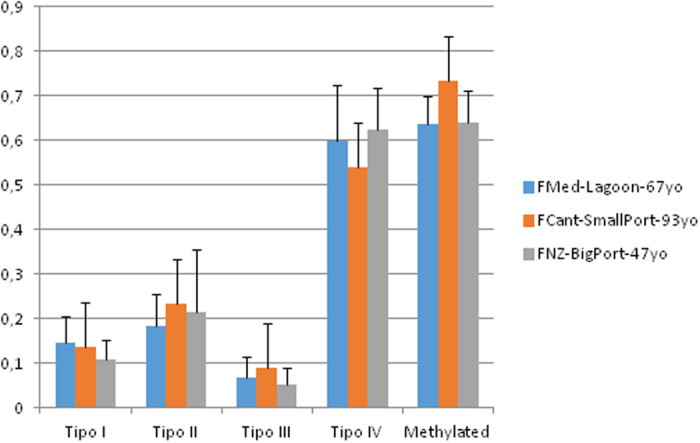
Type I to IV are respectively: no methylated, methylation of internal C, methylation of external C or hemimethylation, and hypermethylation or mutation in restriction site. Methylated: Global methylation level estimated following Nicotra et al.41, as proportion of (Type II+Type III loci)/(scorable loci). The letters yo mean “year old”.
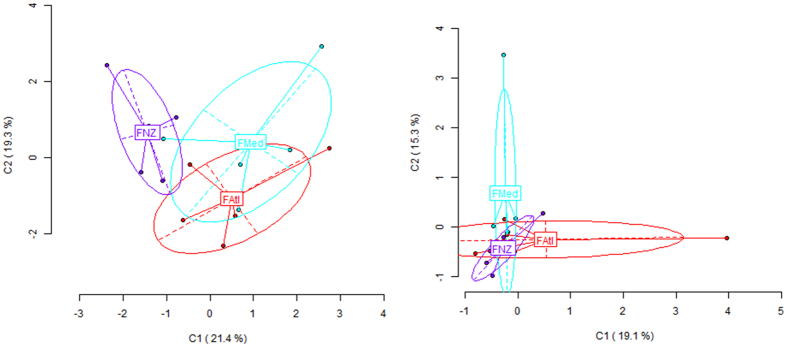
As it happened in X. securis, higher variation was found for MSL (epigenetic) than for NML (genetic). The average Shannon’s diversity indices were I = 0.5869 (SD: 0.1055) and I = 0.2843 (SD: 0.0873) for MSL and NML respectively, significantly different according to Wilcoxon signed rank test (W = 4206, P < 0.0001). According to lower genetic than epigenetic diversity, the PCoA for this species (Fig. 4) showed partial population overlapping for MSL (Fig. 4, left) and clearly lower variation with higher population overlaps for NML (Fig. 4, right).
Figure 4. Two-dimensional visualization of the Principal Component Analysis (PCoA) of the detected methylation patterns in Ficopomatus enigmaticus, with the epigenetic variation (methylation-sensitive loci) on the left and the genetic variation (no methylated loci, NML) on the right.
Each population is represented by the acronyms FNZ, FCant and FMed for samples, respectively, from the international Napier port in New Zealand, Cantabric fishing port and Mediterranean lagoon locations.
The three population samples analyzed did not exhibit significant genetic differences among them based on the 83 NML loci examined (ɸST = 0.0079, P = 0.375; variance among and within populations being 0.053 and 6.733 respectively). Accordingly, significant difference did not occur between any pair of samples (Table 3, above diagonal). In contrast with NML, the epigenetic differences (for MSL) among the analyzed samples were highly significant (ɸST = 0.1654, P = 0.0018, for variances among and within populations of 1.88, 9.488). The pairwise differences at MSL between the more recently introduced Napier international port population (FNZ) and the other two samples were significantly different (Table 3, below diagonal). The difference between the Mediterranean lagoon (FMed) and the Bay of Biscay port (FCant) samples was not significant (ɸST = 0.021, P = 0.3428).
Discussion
Our study is the first reporting changes in methylation patterns of invertebrates likely associated with the process of biological invasions. In invasive X. securis introduced to ports of similar size and pollution levels, significant hypomethylation was found in the most recent population XCant versus the 13-year older XAtl. This latter, also under high anthropogenic pressure, and an older population under lesser stress in a Natura 2000 lagoon (XMed) were similarly methylated. These populations (XAtl and XMed), with a significant differentiation in genetic variation and their very different stress level, were expected to be significantly different for global methylation4,9. The results however reported the opposite. X. securis is likely under expansion in the northwest Iberia sampling region24, and, although XMed was older than XAtl, its methylation level probably represented still the “invasive” signature of the species rather than its response to environmental stress. All this suggests that the hypomethylated signature of early invasions may override the expected environmental signature in the newer populations.
Despite limited sample sizes for F. enigmaticus analysis, the results pointed to the same direction. For this species the older population, sampled from a small fishing port, seemed to be more methylated than populations from differentially disturbed settings (an international port in New Zealand and a lagoon under Natura 2000 network protection). Although for quantitative tests (proportion of methylated loci) statistical significance of p < 0.05 was not reached, highly significant differences were found for ɸST analysis between the more recent population established in the New Zealand port and the two older ones (Table 3). Assuming ports are disturbed areas, absence of differential epigenetic patterns between the older Bay of Biscay port and the intermediate Mediterranean lagoon samples would contradict the expectations of higher methylation under environmental stress in polychaetes6. Perhaps the epigenetic signature of (relatively) recent invasion was overriding the environmental signature also in F. enigmaticus, as it seemed to happen in X. securis. The expected influence of environmental stressors on methylation4,9, principally due to disturbed environment in big ports in this case study, would be perhaps detected in native populations without the “invasive” signature.
Taken together, the results of this study would support our departure hypothesis of relaxed methylation control in early invasions. All the individuals here analyzed were adults, thus ontogenetic methylation changes can be reasonably excluded as a reason for global methylation differences. The phenomenon of reduced methylation could be a function of the invasion phase (expanding populations versus the accommodated ones), and would have a role in invasion processes. At organism level, in vertebrates DNA methylation switches-off gene expression during the development but can also arise when ageing, perturbing the animal organism and causing disease47. Uncontrolled growth is generally associated with decreased global methylation in cell populations48,49. Our results could suggest a parallelism between cancers and biological invasions. In a way, the uncontrolled spread of nuisance species in an ecosystem (bioinvasion outbreak) could be compared to the uncontrolled growth of cells in an organisms (cancer). The two invertebrates studied here are extremely invasive, populations grow rapidly and exhibit incredible dispersal potential27,32. They could be compared with potentially malignant tumors that can reproduce without control. Epigenetic changes involved in malignant cells that cause tumor growth affect some groups of specific loci, that are generally hypermethylated50. If the process advances and the malignant cells move to other tissues (metastasis) new epigenetic phenomena appear. Global hypomethylation coupled with hypermethylation of some specific DNA regions are typical signatures of some metastatic cancers51,52. From our modest but significant results, in recent invasive populations undergoing expansive growth (like our X. securis case) even adult individuals would exhibit a global signature of decreased methylation - compared to those from earlier established populations.
The clear understanding of epigenetic contribution to the species invasiveness is difficult from observational studies. There is poor knowledge on the current status of the local invasive populations (their abundance and distribution range, magnitude of environmental impacts) and the details of the invasion history (date of introduction, establishment, number and sources of repeated incursions, etc.). Yet, in the present case there is no doubt XCant population is very young and in early expansive phase18,25,26, while the other two populations of X. securis analyzed here were reported from the respective regions at least one decade ago53,54. In the expansive phase of the invasion, many genes might be activated for maximizing the ability of invaders to adapt and effectively utilize the resources available in the new habitat. Therefore, the invasive success of a species would benefit from relaxed gene expression control -low levels of DNA methylation would increase transcriptional opportunities and enhance activity of transposable elements55. However as long as the invasion process advances, global methylation would acquire normal levels, allowing intrinsic regulation mechanisms, control of population growth and exhibiting common response to environmental stress. In our study F. enigmaticus, especially the older introduced population in Bay of Biscay –although not the more recent introduction in New Zealand, could be an example. Epigenetic variation may thus be linked to the ‘age’ of the introduced population13,14,15 or the phase of an invasion. Although this should be confirmed from further studies, perhaps increased epigenetic polymorphism or decreased global methylation in a newly arrived population could serve as an indicator of invasiveness - and thus a proxy of its threat to local ecosystems.
Another interesting finding in our study was significantly higher epigenetic than genetic diversity in both highly invasive species, X. securis and F. enigmaticus. Not a minor discovery, we provided here the first evidence of methylation in the two studied species. To date, regulation of gene expression through DNA methylation has been described for other molluscs55,56 and polychaetes6, but not for these particular species. The methylation patterns described in those earlier studies were generally associated with ecological contexts56. In our results, despite the large geographical scope of this study involving populations from distant areas, the genetic variation (NML and total AFLP) in F. enigmaticus from the three analyzed populations did not differ much. It differed more among X. securis samples. Still, genetic diversity was clearly and significantly lower than epigenetic diversity in both species. Epigenetic differences with similar genetic variation were reported in a few studies of introduced populations of plants and vertebrates13,14,15. The present findings from six marine invertebrate populations would also support the idea of changes in methylation being involved in a general mechanism of biological invasions independently from genetic variation.
This proof-of-concept study could open new perspectives for better understanding marine biological invasions, their ecology and intrinsic mechanisms. The epigenome analysis of donor and introduced populations along with the population status assessment (i.e. established, expanding, outbreak, accommodated) is highly desirable. Discovering the key genes involved in adaptation mechanisms of marine invaders, as well as their epigenetics would provide useful information for prioritizing risks associated with particular invasive species and undertaking adequate response measures.
Additional Information
How to cite this article: Ardura, A. et al. Epigenetic signatures of invasive status in populations of marine invertebrates. Sci. Rep. 7, 42193; doi: 10.1038/srep42193 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study has been funded by Asturias Principality (Spain) with the Grant for Excellence Research Groups GRUPIN-2014-093, and the Spanish Ministry of Economy and Competitiveness MINECO Grant CGL2016-79209-R. A.A. holds a regional postdoctoral Marie Curie grant COFUND-CLARIN. Laura Clusa, Laura Miralles and Marta Muñoz-Colmenero helped with laboratory work. We are grateful to Fiona Gower (Cawthron Institute, New Zealand), and Martin Desmalades (University of Perpignan, France) for collaboration in sample collection. This is a contribution from the Marine Observatory of Asturias.
Footnotes
The authors declare no competing financial interests.
Author Contributions A.A. and P.M. performed laboratory work and analyzed output; E.G.V. performed statistical work, analyzed output and wrote the first drafts of the manuscript; A.Z. and S.P. and the rest of authors contributed substantially to revisions.
References
- Bossdorf O., Richards C. L. & Pigliucci M. Epigenetics for ecologists. Ecol. Lett. 11, 106–115 (2008). [DOI] [PubMed] [Google Scholar]
- Kardong K. V. Epigenomics: the new science of functional and evolutionary morphology. Anim. Biol. 53, 225–243 (2003). [Google Scholar]
- Zhang Y. Y., Fischer M., Colot V. & Bossdorf O. Epigenetic variation creates potential for evolution of plant phenotypic plasticity. New Phytol. 197, 314–22 (2013). [DOI] [PubMed] [Google Scholar]
- Suarez-Ulloa V., Gonzalez-Romero R. & Eirin-Lopez J. M. Environmental epigenetics: A promising venue for developing next-generation pollution biomonitoring tools in marine invertebrates. Mar. Poll. Bull. 98, 5–13 (2015). [DOI] [PubMed] [Google Scholar]
- Ruiz-Hernandez A. et al. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clinic. Epigenet. 7, 55. doi: 10.1186/s13148-015-0055-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A. G. & Pasqualone A. A. DNA methylation and temperature stress in an Antarctic polychaete, Spiophanes tcherniai. Front. Physiol. 5, 173. doi: 10.3389/fphys.2014.00173 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Zhang X., Wang D. & Baccarelli A. Environmental chemical exposures and human epigenetics. Int. J. Epidemiol. 41(1), 79–105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniagu S. O., Williams T. D., Allen Y., Katsiadaki I. & Chipman J. K. Global genomic methylation levels in the liver and gonads of the three-spine stickleback (Gasterosteus aculeatus) after exposure to hexabromocyclododecane and 17-β oestradiol. Environ. Int. 34, 310–317 (2008). [DOI] [PubMed] [Google Scholar]
- Head J. A., Mittal K. & Basu N. Application of the LUminometric Methylation Assay (LUMA) to ecological species; tissue quality requirements and a survey of DNA methylation levels in animals. Mol. Ecol. Res. doi: 10.1111/1755-0998.12244 (2014). [DOI] [PubMed] [Google Scholar]
- Fuzzinato C. F., Flohr L., Melegari S. P. & Matias W. G. Oxidative stress and hypermethylation induced by exposure of Oreochromis niloticus to complex environmental mixtures of river water from Cubatão do Sul, Brazil. Ecotox. Environ. Safe. 114, 190–197 (2015). [DOI] [PubMed] [Google Scholar]
- Chown S. L. et al. Biological invasions, climate change and genomics. Evol. App. 8, 23–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C. M., Pozo M. I. & Bazaga P. Jack of all nectars, master of most: DNA methylation and the epigenetic basis of niche width in a flower-living yeast. Mol. Ecol. 21, 2602–2616 (2012). [DOI] [PubMed] [Google Scholar]
- Liebl A. L., Schrey A. W., Richards C. L. & Martin L. B. Patterns of DNA Methylation Throughout a Range Expansion of an Introduced Songbird. Integr. Comp. Biol. 53(2), 351–358 (2013). [DOI] [PubMed] [Google Scholar]
- Richards C. L., Schrey A. W. & Pigliucci M. Invasion of diverse habitats by few Japanese knotweed genotypes is correlated with epigenetic differentiation. Ecol. Lett. 15, 1016–1025 (2012). [DOI] [PubMed] [Google Scholar]
- Schrey A. W. et al. Epigenetic Variation May Compensate for Decreased Genetic Variation with Introductions: A Case Study Using House Sparrows (Passer domesticus) on Two Continents. Genet. Res. Int. doi: 10.1155/2012/979751 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendorf F. W. & Lundquist L. L. Introduction: population biology, evolution, and control of invasive species. Conserv. Biol. 17, 24–30 (2013). [Google Scholar]
- Perez J. E., Nirchio M., Alfonsi C. & Muñoz C. The biology of invasions: the genetic adaptation paradox. Biol. Inv. 8, 1115–1121 (2006). [Google Scholar]
- Devloo-Delva F. et al. Detection and characterisation of the biopollutant Xenostrobus securis (Lamarck 1819) Asturian population from DNA Barcoding and eBarcoding. Mar. Poll. Bull. 105, 23–29 (2016). [DOI] [PubMed] [Google Scholar]
- Zaiko A., Minchin D. & Olenin S. “The day after tomorrow”: anatomy of an ‘r’ strategist aquatic invasion. Aquat. Inv. 9, 145–155 (2014). [Google Scholar]
- Reise K., Olenin S. & Thieltges D. W. Are aliens threatening European aquatic coastal ecosystems? Helgol. Mar. Res. 60, 77–83 (2006). [Google Scholar]
- Williamson M. & Fitter A. The varying success of invaders. Ecology. 77, 1661–1666 (1996). [Google Scholar]
- Iwasaki K. & Yamamoto H. Recruitment and population structure of the non-indigenous brackish-water mytilid Xenostrobus securis (Lamark, 1819) in the Kino River, Japan. Aquat. Inv. 9, 479–487 (2014). [Google Scholar]
- Barbieri M., et al. New records of the pygmy mussel Xenostrobus securis (Bivalvia: Mytilidae) in brackish-water biotopes of the western Mediterranean provide evidence of its invasive potential. Mar. Biodiv. Rec. 4, e48 (2011). [Google Scholar]
- Pascual S. et al. The mussel Xenostrobus securis: a well-established alien invader in the Ria de Vigo (Spain, NE Atlantic). Biol. Inv. 12, 2091–2103 (2010). [Google Scholar]
- Miralles L., Dopico E., Devloo-Delva F. & Garcia-Vazquez E. Controlling populations of invasive pygmy mussel (Xenostrobus securis) through citizen science and environmental DNA. Mar. Poll. Bull. doi: 10.1016/j.marpolbul.2016.06.072 (2016). [DOI] [PubMed] [Google Scholar]
- Pejovic I. et al. DNA barcoding for assessment of exotic molluscs associated with maritime ports in northern Iberia. Mar. Biol. Res. 12, 168–176 (2016). [Google Scholar]
- Galil B. S. et al. International arrivals: widespread bioinvasions in European Seas. Ethol. Ecol. Evol. 26(2–3), 152–171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid K. A. & Griffiths C. L. Alien reef-building polychaete drives long-term changes in invertebrate biomass and diversity in a small, urban estuary. Estuar. Coast. Shelf Sci. 138, 101–106 (2014). [Google Scholar]
- Bruschetti M., Bazterrica C., Luppi T. & Iribarne O. An invasive intertidal reef-forming polychaete affect habitat use and feeding behavior of migratory and locals birds in a SW Atlantic coastal lagoon. Ecology. 375, 76–83 (2009). [Google Scholar]
- Gollasch S. Ficopomatus enigmaticus. In: AquaNIS. Editorial Board, Information system on Aquatic Non-Indigenous and Cryptogenic Species. World Wide Web electronic publication. www.corpi.ku.lt/databases/aquanis. www.corpi.ku.lt/databases/aquanis. Version 2.36+. Accessed 2016-11-07 (2015).
- AquaNIS. Editorial Board. Information system on Aquatic Non-Indigenous and Cryptogenic Species. World Wide Web electronic publication www.corpi.ku.lt/databases/aquanis. Version 2.36+. Accessed 2016-11-07 (2015).
- Leppäkoski E., Gollasch S. & Olenin S. (Eds.) Invasive Aquatic Species of Europe. Distribution, Impacts and Management. Kluwer Academic Publishers. doi: 10.1007/978-94-015-9956-6 (2002). [DOI] [Google Scholar]
- Geller J., Meyer C., Parker M. & Hawk H. Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all‐taxa biotic surveys. Mol. Ecol. Res. 13(5), 851–861 (2013). [DOI] [PubMed] [Google Scholar]
- Distel D. L. et al. Molecular phylogeny of Pholadoidea Lamarck, 1809 supports a single origin for xylotrophy (wood feeding) and xylotrophic bacterial endosymbiosis in Bivalvia. Mol. Phyl. Evol. 61(2), 245–254 (2011). [DOI] [PubMed] [Google Scholar]
- Hall T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nuc. Acid. S. 41, 95–98 (1999). [Google Scholar]
- Díaz-Freije E., Gestal C., Castellanos-Martínez S. & Morán P. The role of DNA methylation on Octopus vulgaris development and their perspectives. Front. Physiol. 5, 62. doi: 10.3389/fphys.2014.00062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon A., Clotault J., Jenczewski E., Chable V. & Manzanares-Dauleux M. J. Brassica oleracea displays a high level of DNA methylation polymorphism. Plant Sci. 174, 61–70 (2008). [Google Scholar]
- Moran P. & Perez-Figueroa A. Methylation changes associated with early maturation stages in the Atlantic Salmon. BMC Genet. 12, 86 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Figueroa A. msap: a tool for the statistical analysis of methylation‐sensitive amplified polymorphism data. Mol. Ecol. Res. 13(3), 522–527 (2013). [DOI] [PubMed] [Google Scholar]
- Fulneček J. & Kovařík A. How to interpret methylation sensitive amplified polymorphism (MSAP) profiles? BMC Genet. 15(1), 1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra A. B. et al. Adaptive plasticity and epigenetic variation in response to warming in an Alpine plant. Ecol. Evol. 5(3), 634–647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C. M. & Bazaga P. Epigenetic differentiation and relationship to adaptive genetic divergence in discrete populations of the violet Viola cazorlensis. New Phytol. 187(3), 867–876 (2010). [DOI] [PubMed] [Google Scholar]
- Excoffier L., Smouse P. E. & Quattro J. M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 131(2), 479–491 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R. & Smouse P. E. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Not. 6, 288–295 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R. & Smouse P. E. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics, 28, 2537–2539 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer Ø., Harper D. A. T. & Ryan P. D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Paleon. Elec. 4(1), 4–9 (2001). [Google Scholar]
- Jaenisch R. & Adrian B. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33, 245–254 (2013). [DOI] [PubMed] [Google Scholar]
- Szyf M., Pakneshan P. & Rabbani S. A. DNA demethylation and cancer: therapeutic implications. Cancer Lett. 211, 133–143 (2014). [DOI] [PubMed] [Google Scholar]
- Jones P. A. & Baylin S. B. The Epigenomics of Cancer. Cell. 128(4), 683–692 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum. Mol. Genet. 16(R1), R50–R59 (2007). [DOI] [PubMed] [Google Scholar]
- Legendre C. et al. Whole-genome bisulfite sequencing of cell-free DNA identifies signature associated with metastatic breast cancer. Clinic. Epigenet. 7, 100. doi: 10.1186/s13148-015-0135-8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzese D. M. et al. Epigenome-wide DNA methylation landscape of melanoma progression to brain metastasis reveals aberrations on homeobox D cluster associated with prognosis. Hum. Mol. Genet. 23(1), 226–38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garci M. E. et al. Xenostrobus securis (Lamarck, 1819) (Mollusca:Bivalvia): first report of an introduced species in Galician waters. Aquac. Int. 15, 19–24 (2007). [Google Scholar]
- Gofas S. & Zenetos A. Exotic molluscs in the Mediterranean Basin: current status and perspectives. Ocean. Mar. Biol. Ann. Rev. 41, 237–277 (2003). [Google Scholar]
- Gavery M. R. & Roberts S. B. A context dependent role for DNA methylation in bivalves. Brief. Funct. Genom. 13(3), 217–222 (2014). [DOI] [PubMed] [Google Scholar]
- Gavery M. R. & Roberts S. B. DNA methylation patterns provide insight into epigenetic regulation in the Pacific oyster (Crassostrea gigas). BMC Genomics. 11, 483 http://dx.doi.org/10.1186/1471-2164-11-483 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez I., deCastro M., Gomez-Gesteira M. & Prego R. Inter- and intra-annual analysis of the salinity and temperature evolution in the Galician Rias Baixas–ocean boundary (northwest Spain). J. Geoph. Res. 110, C04008, doi: 10.1029/2004JC002504 (2005). [DOI] [Google Scholar]
- Esteves K. et al. Highly diverse recombining populations of Vibrio cholerae and Vibrio parahaemolyticus in French Mediterranean coastal lagoons. Front. Microbiol. http://dx.doi.org/10.3389/fmicb.2015.00708 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérès J. M. Contribution a l’etude des annelides polychetes de la Mediterranee occidentale. Recueil des travaux de la Station marine d’Endoume. 13, 83–162 (1954). [Google Scholar]
- Bec B. et al. Distribution of picophytoplankton and nanophytoplankton along an anthropogenic eutrophication gradient in French Mediterranean coastal lagoons. Aquat. Microb. Ecol. 63(1), 29–45 (2011). [Google Scholar]
- Vouvé F. et al. Bages-Sigean and Canet-St Nazaire lagoons (France): physico-chemical characteristics and contaminant concentrations (Cu, Cd, PCBs and PBDEs) as environmental quality of water and sediment. Environ. Sci. Poll. Res. 21(4), 3005–3020 (2014). [DOI] [PubMed] [Google Scholar]
- Read G. B. & Gordon D. P. Adventive occurrence of the fouling serpulid Ficopomatus enigmaticus (Polychaeta) in New Zealand. NZ J. Mar. Fresh. Res. 25, 269–273 (1991). [Google Scholar]
- Ridgway N. M. & Stanton B. R. Some hydrological features of Hawke Bay and nearby shelf waters. NZ J. Mar Fresh. Res. 3(4), 545–559 (1969). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.