Abstract
The data for the Ebola outbreak that occurred in 2014–2016 in three countries of West Africa are analysed within a common framework. The analysis is made using the results of an agent based Susceptible-Infected-Removed (SIR) model on a Euclidean network, where nodes at a distance l are connected with probability P(l) ∝ l−δ, δ determining the range of the interaction, in addition to nearest neighbors. The cumulative (total) density of infected population here has the form 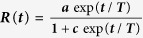 , where the parameters depend on δ and the infection probability q. This form is seen to fit well with the data. Using the best fitting parameters, the time at which the peak is reached is estimated and is shown to be consistent with the data. We also show that in the Euclidean model, one can choose δ and q values which reproduce the data for the three countries qualitatively. These choices are correlated with population density, control schemes and other factors. Comparing the real data and the results from the model one can also estimate the size of the actual population susceptible to the disease. Rescaling the real data a reasonably good quantitative agreement with the simulation results is obtained.
, where the parameters depend on δ and the infection probability q. This form is seen to fit well with the data. Using the best fitting parameters, the time at which the peak is reached is estimated and is shown to be consistent with the data. We also show that in the Euclidean model, one can choose δ and q values which reproduce the data for the three countries qualitatively. These choices are correlated with population density, control schemes and other factors. Comparing the real data and the results from the model one can also estimate the size of the actual population susceptible to the disease. Rescaling the real data a reasonably good quantitative agreement with the simulation results is obtained.
Mathematical modelling of the phenomena of disease spreading has a long history, the first such attempts being made in the early twentieth century1,2,3,4,5,6,7. Typically, an individual is assumed to be in either one of the three possible states: susceptible, infected and removed (or recovered) denoted by S, I, and R respectively in the simplest models. Diseases which can be contracted only once are believed to be described by the SIR model in which a susceptible individual gets infected by an infected agent who is subsequently removed (dead or recovered). A removed person no longer takes part in the dynamics. In SIS model, an infected person may become susceptible again. In the SIR model, S, I and R represent the densities of population in the three different states and are related through the normalization condition
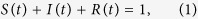 |
The following set of deterministic differential equations are obeyed by the densities:
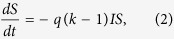 |
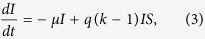 |
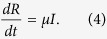 |
These equations can be interpreted as follows: infected nodes become recovered at a rate μ, while susceptible nodes become infected at a rate proportional to both the densities of infected and susceptible nodes. Here, q is the infection rate and k is the number of contacts or degree. Without loss of generality, one can take μ = 1. Due to the conservation of the total population (eq. 1), only two of the three variables are independent. For the SIS model, one has only two similar equations connecting S and I, one of which is independent only. In most theoretical models, the epidemic has a threshold behaviour as the infection probability q is varied. However, an estimate of q from real data is difficult as it is related to biological features like nature of the pathogen etc.
Plenty of variations and modifications of the SIR and SIS models have been considered over the last few decades. Resurgence of interest in these models has taken place following the discovery that social networks do not behave like random or regular networks8,9. The current emphasis has been to study these models on complex networks like small world and scale free networks. A few surprising results have been derived theoretically in the recent past9.
The test of a model lies in its ability to match real data. No appreciable success has been made so far for the familiar models although some qualitative consistency has been achieved9,10. The available data is usually in the form of number of newly infected patients and total (cumulative) number of cases. In the SIR model, the newly infected fraction shows an initial growth followed by a peak and a subsequent decay. This matches with the overall structure of the real data (e.g. for Severe Acute Respiratory Syndrome (SARS)11), which however, show local oscillatory behaviour in addition. Such a behaviour may be due to demographic non uniformity12.
The set of equations (2, 3, 4) represent only a mean field picture. The mean field equations do not depend on the topology of the network and are also essentially deterministic. It is therefore more meaningful to study the epidemic spreading by considering an agent based model on spatial networks where the dynamics of each agent can be tagged and the averages can be extracted easily. Agent based models for epidemic spreading on regular lattices have been studied quite extensively in the last few decades and in the more recent studies, the complex nature of the network connecting the individuals has been taken into consideration. It has been shown that the geographical factor plays an important role in the spreading process13,14,15,16,17,18,19,20,21. In particular, the SIR model on an Euclidean network, where the agents may be connected not only to their nearest neighbours but also to a few randomly chosen long range neighbours has been considered in detail20,21.
In 2014, the Ebola virus caused large scale outbreaks mainly in three West African countries and only recently it has been declared as over (June 2016). Ebola virus is transmitted through body fluids and it is also believed that a person can contract the disease only once. A few attempts have been made to analyse the data so far22,23,24,25,26,27. Different factors like demographic effect, hospitalization, vaccination and treatment plans have been incorporated in the traditional and well-known SIR model to understand the dynamics of Ebola disease25,26,27. However, in these models, a mean field approximation has been used which is rather unphysical. Using the results of an agent based SIR model on Euclidean network20, mentioned in the last paragraph, we have analysed the Ebola data for the three countries Guinea, Liberia and Sierra Leone in West Africa where the outbreak extended over approximately two years. We have also reproduced the comparative treads using appropriate parameter values in the model, albeit qualitatively.
Results
Data analysis
We have studied the available data for total (cumulative) number of cases R(t) as a function time t and extracted the data for number of new cases I(t) from these.
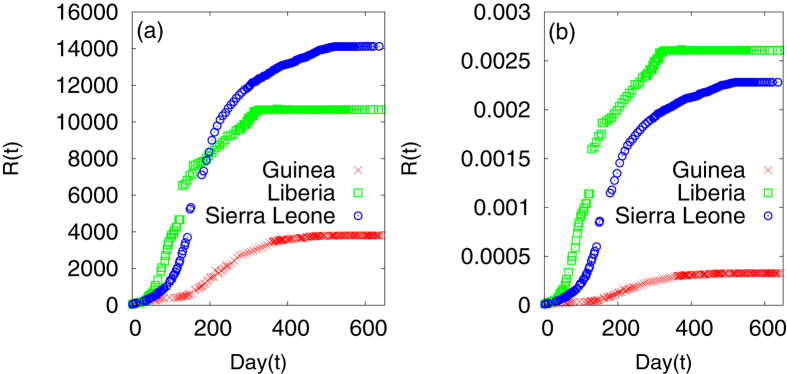
Most of the earlier studies have dealt with the actual numbers of cases. However, as we attempt to provide a comparative picture, we have taken the fraction i.e., divided the numbers by the total population Np for each country. One can easily see that the comparative trends become different in the two different approaches (Fig. 1). The disease is seen to affect the least fraction of the population in Guinea and the maximum in Liberia. However, the number of cases is maximum for Sierra Leone and not for Liberia. Considering the time at which the data reach a saturation value, one can also conclude that the disease has existed over a longer period in the case of Sierra Leone and Guinea.
Figure 1.
(a) Cumulative number of infected individuals as a function of time (day) for the three countries Guinea, Liberia and Sierra Leone. (b) Same data normalised by the population of each country.
Had the infection probability been the sole factor responsible for the spread, the patterns would have been the same for the three countries. We argue that the network structure is responsible for the different trends. Hence a theoretical model that yields results comparable to the observed data must have more than one parameter. A minimal model would consist of two parameters like the one considered in ref. 20. Here the agents have two nearest neighbour connections and a random long range connection to a agent located at a distance l with probability l−δ (details given in the Methods Section). The parameter δ essentially controls the network structure and the other parameter is of course the infection probability q. This study revealed that for a given δ, above a threshold value of q (which depends on δ), an epidemic can occur.
The removed population in the model in ref. 20 was fitted to the form:
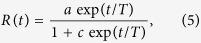 |
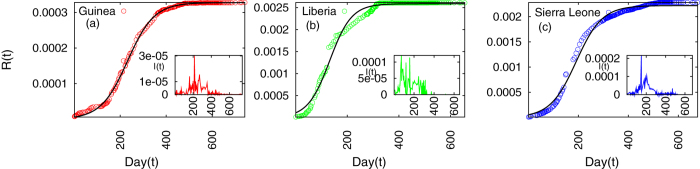
where a, c and T depend on the values of δ and q. Note that the removed population in the model essentially corresponds to the cumulative infected cases since in the model the infected agents were assumed to be removed immediately after being infected. This fitting form is used for the cumulative data of infected cases and shows very good agreement for Guinea (Fig. 2(a)), while there is fairly good agreement with the data of the other two countries (Fig. 2(b,c)). Rescaling R(t) such that it varies from 0 to 1, one can find out the goodness of fit. We performed the Kolmogorov-Smirnov test to evaluate the goodness of the fit for all the three sets of data. The values are: 1.1294 for Guinea (sample size Ns = 217), 1.1070 for Sierra Leone (Ns = 235) and 1.4959 for Liberia (Ns = 233). Thus the fittings are acceptable at the level of significance α = 0.10 for Guinea and Sierra Leone and at α = 0.01 for Liberia.
Figure 2.
Cumulative fraction of population infected and the fitted curve as a function of time (day) for countries (a) Guinea, (b) Liberia and (c) Sierra Leone. Insets are for the fraction of newly infected population as a function of time of each country.
From eq. (5), one can show that a peak value for I(t) will occur at tp = T log(1/c). The associated values of the exponents a, c and T are found out for the three countries and the values of tp also extracted. We have plotted the data for I(t) against t in the insets of the Fig. 2(a–c). We observe a lot of fluctuations and not a very clear peak in the data just as in the case of SARS11. Even then, the theoretically estimated values of tp tally with a large value of new cases occurring close to this time. The exponent values and tp are tabulated in Table 1. The errors in the estimation of exponents are  for a,
for a, 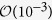 for c and
for c and 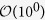 for T (beween 0.9 to 7.6 percent). One can see that tp is also directly proportional to the total duration, being least for Liberia and maximum for Guinea.
for T (beween 0.9 to 7.6 percent). One can see that tp is also directly proportional to the total duration, being least for Liberia and maximum for Guinea.
Table 1. Exponents a, c and T for three different countries using the total population as normalization factor.
| Country | a | c | T | tp | a/c |
|---|---|---|---|---|---|
| Guinea | 0.0000059 ± 0.0000002 | 0.0182146 ± 0.0006 | 57.9150 ± 0.503 | 231.98 | 0.0003239 |
| Liberia | 0.0001125 ± 0.0000085 | 0.0434763 ± 0.0032 | 42.1957 ± 1.043 | 132.30 | 0.0025876 |
| Sierra Leone | 0.0000549 ± 0.0000041 | 0.0247653 ± 0.0018 | 51.758 ± 1.040 | 191.41 | 0.0022168 |
Results from the model
The cumulative data for infected people has a sigmoid form in general and has been shown to have a form given by eq. (5) in a recent study as well27. To establish that indeed the Euclidean network is an appropriate model responsible for the epidemic spreading, one should be able to reproduce from the model the consistent results and trends using suitable values of the parameters, at least qualitatively.
Epidemic spreading on the Euclidean model with the two parameters δ and q, already mentioned in the context of data analysis, was first considered in ref. 20. The model and simulation methods are given in detail in the Methods section. The behaviour of the network depends on the value of δ. The network behaves as a small world network for δ < 1 and as a regular one dimensional lattice for δ > 2. For 1 < δ < 2, it shows short range behaviour. These properties of the network had been earlier detected by considering its network properties as well as critical phenomena on the network (see refs 20 and 28 and the references in these papers).
The Ebola virus spreads through actual body contact and in most cases the infection occurred within family members. Hence the underlying network must be short ranged. Therefore to get results comparable to the real data, one should use a value of δ larger than 1. Also, δ < 2 is chosen as a real network is more connected than a regular one. The values of q should be same in principle as it depends on biological factors. However, the value of q may be effectively altered using control schemes like contact tracing, quarantining the patient and efficiently treating the disease. Such possibilities have not been directly included in the model. We will address this issue in the next section again.
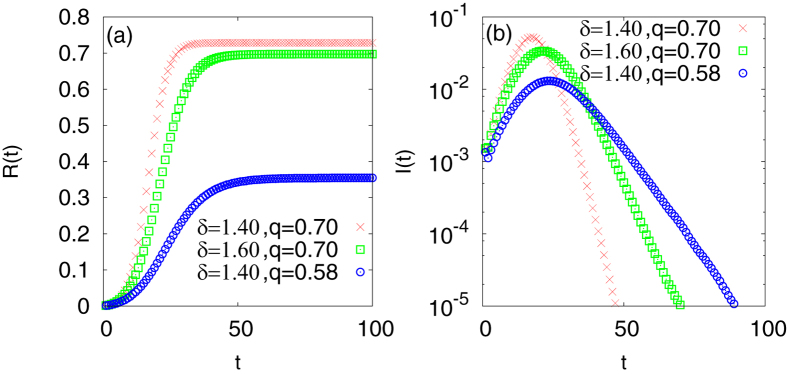
We first discuss the case of Liberia and Sierra Leone. We note that the saturation values are quite close while the saturation in Liberia has been reached earlier (Fig. 1). We find that the same value of q but a different value of δ can indeed reproduce these features; the red and green curves in Fig. 3(a) show the results for δ = 1.4 (for Liberia) and 1.6 (for Sierra Leone) while the q values are same (q = 0.70).
Figure 3.
(a) Fraction of population infected as a function of time (Monte Carlo time step) for different pairs of infection rate q and δ. (b) Fraction of newly infected population as a function of time (Monte Carlo time step) for same pairs of infection rate q and δ.
We next discuss the case for Guinea. It has the lowest saturation value of the cumulative data for infected population while the disease is of duration slightly longer than that of Sierra Leone. This makes it quite apparent that one has to use a smaller value of q to get data consistent with that of Guinea. We find that indeed one can get such values of q keeping δ = 1.4 such that the saturation value is smaller while the duration is larger comparatively. We show the data by the blue curve in Fig. 3(a) using q = 0.58.
Of course these are some typical values which yield results comparable to the real data. A range of values exist which more or less show the same behaviour. However, that range is not too large which would mean that the values are irrelevant. For δ, this range is ±0.05 while for q it is ±0.02.
Figure 3(b) shows the data for I(t) against t from the Euclidean model. Again we find consistency, the red curve has a peak occurring earliest while the disease lasts for the shortest duration which corresponds to Liberia. The green curve shows a peak occurring at a later time and the duration is also longer. This we claim to correspond to Sierra Leone. The peak value is slightly less in height for the green curve compared to the red which is also consistent with the real data (up to a multiplicative factor) if one takes the single spike occurring in Fig. 2(c) inset to be spurious. The data for Fig. 2(a) inset is easily comparable to the red curve in Fig. 3(b). The blue curve in Fig. 3(b) corresponds to Guinea as the peak value occurs at a slightly larger time compared to the green curve while the duration is longest. The quantitative values of tp shown in Table 2 are also consistent with the real data. The errors in the estimates lie between 0.04 to 0.49 percent. The argument behind the choices of δ and q are discussed in the next section.
Table 2. Exponents a, c and T for different values of parameters.
| Parameters | a | c | T | tp | a/c |
|---|---|---|---|---|---|
| δ = 1.4, q = 0.58 (Guinea) | 0.011842 | 0.032342 | 7.09658 | 24.35107 | 0.3661492 |
| δ = 1.4, q = 0.70 (Liberia) | 0.005116 | 0.006974 | 3.39171 | 16.8415 | 0.7335818 |
| δ = 1.6, q = 0.70 (Sierra Leone) | 0.011130 | 0.015718 | 5.10908 | 21.2175 | 0.7081053 |
The countries to which the data correspond are shown within the parenthesis.
As in the real data, one can quote here the goodness of fit for R(t) from the simulations. The Kolmogorov-Smirnov test for the three cases yield the largest errors as 0.1653 for δ = 1.40, q = 0.70; 0.3558 for δ = 1.60, q = 0.70; and 1.2261 for δ = 1.40, q = 0.58. The sample size is 2000 for each and therefore the first two results are acceptable at level of significance α = 0.2 and the last one at α = 0.05.
Comaparison of data by rescaling
While qualitative features of the data obtained from the model are quite similar to the real data, the actual values of the fitting parameters a, c and T (and consequently tp) are quite different (see Tables 1 and 2). It may be noted that a/c corresponds to the saturation value of R(t) and c and T determine the value of tp. The mismatch of the tp values is not surprising, unit of time in the model is just one Monte Carlo (MC) time step that has got nothing to do with real time. On the other hand, the saturation values depend heavily on the normalization factor. The actual population who are susceptible may form only a subset of the total population so saturation values can be different changing the values of a and c. Nevertheless, we find that the ratio of of a/c from the data and from the model for Sierra Leone and Liberia are very close which indicates that the fractions of susceptible population in these two countries were comparable while for Guinea it was smaller. Indeed, Table 3 shows that the density of infected population were same for Sierra Leone and Liberia and order of magnitude smaller in Guinea.
Table 3. Statistics of Ebola data for three different countries.
| Country | Total Cases | Density of Infected Population | Lab-Confirmed Cases | Total Deaths |
|---|---|---|---|---|
| Guinea | 3814 | 3.0 × 10−4 | 3358 | 2544 |
| Sierra Leone | 14124 | 2.2 × 10−3 | 8706 | 3956 |
| Liberia | 10678 | 2.2 × 10−3 | 3163 | 4810 |
| Total | 28616 | 15227 | 11310 |
However, one can still explore the possibility of rescaling the real data to obtain better quantitative agreement between the parameters. This may be possible by suitably choosing a normalization factor for each of the three data sets. Assuming the total population which has been removed at time t to be Rtot(t) and ρNp the actual population susceptible to the disease, we calculate the density
 |
where R(t) is the density calculated earlier by dividing Rtot(t) by the total population Np and ρ a proportionality constant. Taking Rs(t) to be the saturation value of R(t) obtained from the model and equating it to the saturation value of R(t) obtained from the real data, one can estimate the value of ρ. For example, for Guinea, the saturation value of R(t) is 0.000328 from the data and from the model it is 0.354720. Hence 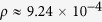 . Similarly for Sierra Leone and Liberia it is 3.27 × 10−3 and 3.58 × 10−3 respectively. On the other hand, one can compare the tp values from real data and the model and we find that the timescales in the real data are approximately 8–9.2 times the timescales in the Monte Carlo simulations. Hence we also rescale the time for the results obtained for the model. The rescaled data Rs(t) and R(t) are plotted against “real time” in Fig. 4 and show an excellent agreement for Guinea and a reasonably good agreement for Sierra Leone. The agreement for Liberia is not that good, however, the data for Liberia are somewhat irregular and it is difficult to fit them with a smooth function very accurately as already noted. Particularly for Liberia and Sierra Leone we find that before saturation, there is a slower increase in R(t); this might be due to an enhancement in the treatment and preventive measures against the disease.
. Similarly for Sierra Leone and Liberia it is 3.27 × 10−3 and 3.58 × 10−3 respectively. On the other hand, one can compare the tp values from real data and the model and we find that the timescales in the real data are approximately 8–9.2 times the timescales in the Monte Carlo simulations. Hence we also rescale the time for the results obtained for the model. The rescaled data Rs(t) and R(t) are plotted against “real time” in Fig. 4 and show an excellent agreement for Guinea and a reasonably good agreement for Sierra Leone. The agreement for Liberia is not that good, however, the data for Liberia are somewhat irregular and it is difficult to fit them with a smooth function very accurately as already noted. Particularly for Liberia and Sierra Leone we find that before saturation, there is a slower increase in R(t); this might be due to an enhancement in the treatment and preventive measures against the disease.
Figure 4. Solid curves are for the fraction of population infected (simulated data) R(t) as a function of real time (rescaled Monte Carlo time step) for different pairs of infection rate q and δ.
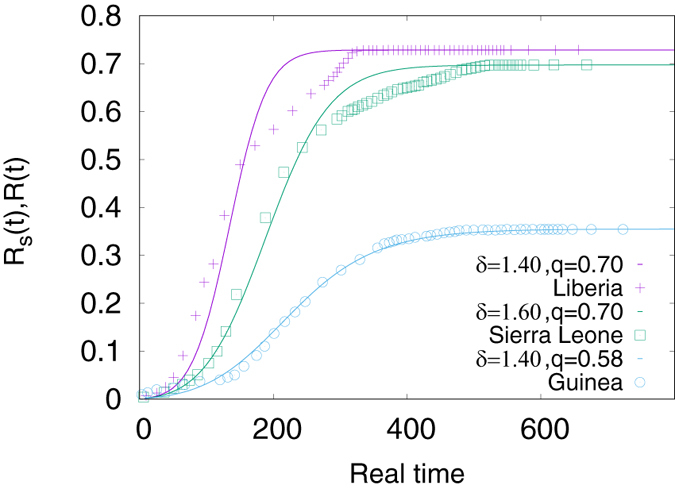
The rescaling factors for the MC time steps are 8.0 for Liberia, 9.0 for Sierra Leone and 9.2 for Guinea. Data points are for the rescaled fraction of population infected (real data) Rs(t) as a function of real time for the three countries Guinea, Liberia and Sierra Leone.
One can similarly rescale the newly infected density I(t), however, the data being too noisy, we do not attempt that. Nevertheless, we find that the peak values of the newly infected density I(t), when scaled by ρ shows order of magnitude agreement with the model data.
Further, we have fitted Rs using equation 5 and present the value of the parameters in Table 4. The values from the model and the real data are easily comparable now showing order of magnitude agreement for most of them.
Table 4. Exponents a, c and T for rescaled data R s (t) for three different countries.
| Country | a | c | T | tp | a/c |
|---|---|---|---|---|---|
| Guinea | 0.0077722 ± 0.00070 | 0.0214467 ± 0.00189 | 59.5716 ± 1.33 | 228.88 | 0.362399 |
| Liberia | 0.0320618 ± 0.00464 | 0.0443399 ± 0.00636 | 42.5638 ± 2.02 | 132.62 | 0.723091 |
| Sierra Leone | 0.0274868 ± 0.00450 | 0.0387566 ± 0.00603 | 57.3669 ± 2.41 | 186.47 | 0.709215 |
Discussion
In this section we justify the choice of the parameters used in the model to obtain the results consistent with the real data. One can of course attempt to get a full calibration of a and c for given values of δ and q so that the choice of δ and q are automatically obtained from this calibration, however, we have refrained from doing so as it involves a huge computational calculation.
We have already justified the choice of δ between 1 and 2 in the last section. We have used a larger value of δ for Sierra Leone and a smaller value of q for Guinea to get the consistency. To justify why δ should be larger for Sierra Leone we note the following. Sierra Leone and Liberia are comparable in size but the density of population is much higher in the former. The density of population is 79.4/km2 and 40.43/km2 respectively for these two countries29. Hence the number of neighbours within the same distance is larger for Sierra Leone which implies a larger value of δ effectively (more short ranged).
On the other hand, the population densities of Guinea (40.90/km2)30 and Liberia are quite close so that one should use the same δ value. However, we need to justify why a smaller value of q is able to reproduce the data for Guinea. A smaller value of q indicates less infection probability which is possible if proper medical care and control measurements are taken. This is indeed true as we find from several documents that the disease was tackled most effectively in Guinea. Table 3 clearly shows that the maximum percentage of cases for Guinea were laboratory-tested which indicates that the process of contact tracing and treatment were more efficient. This is supported by the fact that in Guinea, about 56 contacts per infected person were traced compared to 23 in case of Sierra Leone31. We find from ref. 32 that MSF treated the largest number of reported cases in Guinea, in Sierra Leone the minimum out of reported cases. Thus most cases in Sierra Leone, even when reported, had received less attention while in Liberia, a large number is not confirmed or reported at all. Apparently, medical centers by international organisations have also been set up much earlier in Guinea as it was the epicenter of the disease and the disease started as early as in 2013 December. However, later activities could control the disease in Liberia and Sierra Leone as well, and the final number of deaths had been far less than initially anticipated. We also note a curious fact - though Guinea may have recorded the minimum number of cases, yet the disease spanned a longer duration compared to Liberia. Further analysis, beyond the scope of the present paper, may be able to explain this.
Although we have shown that by rescaling the real data by ρNp and the MC time by a suitable factor, one can get fairly good agreement between the real data and the simulated data, it has to be emphasized that the rescaling is somewhat manipulated by the results of the model. The ratio of the saturation values for the real data and the simulated data corresponds to the factor ρ. In principle one should incorporate more factors in the model to fit the real data independently. However, at the present stage qualitative consistency is what we emphasize on. To achieve quantitative consistency one needs to introduce more parameters making the model complex. These parameters may be related to features like inhomogeneity, mobility, more general initial conditions to name a few. We have made simple assumptions like homogeneity, i.e., uniform number of contacts for all agents. The initial condition has been taken to be identical: the disease commences with only one infected person. Our assumption that agents are immobile is supported by ref. 27 in which it is argued that migration did not play a role in the spreading. Even so, this simple model is able to yield data which is consistent with real data and we conclude that it captures the basic mechanism of the epidemic spread. The effect of the Ebola outbreak has been devastating in the West African countries. Apart from the human losses, economic loss has also been considerable33. The present study shows that the Euclidean model can be treated as a basic starting point and can be further developed by adding other features. This will make it very useful and important for making accurate predictions.
Methods
How the database was handled
We consulted the Ebola data for the number of cases detected in the three countries Guinea, Liberia and Sierra Leone in West Africa (The Centers for Disease Control and Prevention (CDC)34). The data is available from 25th March 2014 to 13th April 2016 at the time interval of a few days. The data is noisy and contains obvious errors as sometimes the cumulative data is shown to decrease which is unphysical. The first available data is from March 2014 when Guinea was already struck with the disease for some time (first case in Guinea reported in December 2013) such that the data for the initial period is missing. For Liberia and Sierra Leone, the data for initial stage are available, however these are sparse and unreliable; often the data for number of death exceeds the number of cases. For this reason, the data has been analysed from the date when the number of cases detected is at least 50 for each country. Even then the errors cannot be fully avoided as for very late stages, the data being rare, also become somewhat unreliable. Hence, the entire data set has to be handled carefully.
In Table 3, a summary of the statistics of the Ebola data is presented and one can immediately note that all cases could not have been confirmed in the laboratory in the case of Liberia where number of deaths exceeds the laboratory confirmed cases. Obviously many cases were unreported. For Guinea, these two figures are closest and the data for Guinea is in fact the cleanest one.
Another point needs to be mentioned. The disease has been officially declared over on 1st June 2016 for Guinea, 9th June 2016 for Liberia and 17th March 2016 for Sierra Leone35. But one can see from Fig. 1 that the cumulative data shows a saturation over fairly long period of time. Apparently a few stray cases delayed the declaration of the disease being over. For Liberia, for example, the disease was originally declared to be over as early as in May 2015 but two small flare-ups were reported later. However the cumulative data is hardly affected by the later cases. The data can be downloaded by clicking on the link “Ebola Data” in the page http://www.physics-caluniv.in/parongama-sen/index.html
Model and Simulation method
In the Euclidean model, the nodes of the network are assumed to occupy the sites of a chain of length N. We generated random long range bonds by connecting nodes located at a distance l along the chain with a probability 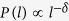 ; the probability is normalised by making the total probability equal to unity. Once N/2 such bonds are constructed, the network is completed. The average degree of each node is three and it is expected that the inhomogeneity of the degree distribution is negligible. The disease spreading process is then simulated by assuming a single infected agent at any randomly chosen site in the beginning. All the neighbours are likely to be infected with a probability q in the next step. One generates a random number between 0 and 1, if it is less than q, the agent is taken to be infected. From the agents who are infected in the second step, the disease spreads to their neighbours and the process continues. Infected people are removed within one unit of time, with the assumption that they are either dead or cured, and they can infect the susceptible agents during this one time step only. The dynamical evolution stops when there is either no infected agent at a particular step or when all of them have died. Several configurations are considered and the dynamical variables averaged.
; the probability is normalised by making the total probability equal to unity. Once N/2 such bonds are constructed, the network is completed. The average degree of each node is three and it is expected that the inhomogeneity of the degree distribution is negligible. The disease spreading process is then simulated by assuming a single infected agent at any randomly chosen site in the beginning. All the neighbours are likely to be infected with a probability q in the next step. One generates a random number between 0 and 1, if it is less than q, the agent is taken to be infected. From the agents who are infected in the second step, the disease spreads to their neighbours and the process continues. Infected people are removed within one unit of time, with the assumption that they are either dead or cured, and they can infect the susceptible agents during this one time step only. The dynamical evolution stops when there is either no infected agent at a particular step or when all of them have died. Several configurations are considered and the dynamical variables averaged.
In the present simulation, for the same network, the initial choice of infected site was repeated 400 times and the quantities averaged. A secondary averaging is made by considering 100 different network configurations. The number of nodes N and the total number of edges were kept fixed for any value of δ and q in the different realisations. Periodic boundary condition has been used in the simulation. Systems with size N = 211 has been considered.
Additional Information
How to cite this article: Khaleque, A. and Sen, P. An empirical analysis of the Ebola outbreak in West Africa. Sci. Rep. 7, 42594; doi: 10.1038/srep42594 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
P.S. acknowledges support from Council of Industrial and Scientific Research, Government of India grant.
Footnotes
The authors declare no competing financial interests.
Author Contributions The authors have jointly made the data analysis and contracted the model. A.K. prepared the figures. Both the authors participated in writing the manuscript.
References
- Ross R. An application of the theory of probabilities to the study of a priori pathometry -part i. Proceedings of the Royal Society A 92, 204 (1916). [Google Scholar]
- Ross R. An application of the theory of probabilities to the study of a priori pathometry -part ii and iii. Proceedings of the Royal Society A 93, 212, 225 (1917). [Google Scholar]
- Brownlee J. Certain aspects of the theory of epidemiology in special relation to plague. Proceedings of the Royal Society of medicine 11, 85 (1918). [PMC free article] [PubMed] [Google Scholar]
- Kermack W. O. & McKendrick A. G. A contribution to the mathematical theory of epidemics. Proceedings of the Royal Society of London A 115, 700 (1927). [Google Scholar]
- Soper E. Interpretation of periodicity in disease-prevalence. Journal of the Royal Statistical Society 92, 34 (1929). [Google Scholar]
- Kermack W. O. & McKendrick A. G. Contributions to the mathematical theory of epidemics. ii. The problem of epidemicity. Proceedings of the Royal Society of London A 138, 55 (1932). [Google Scholar]
- Kermack W. O. & McKendrick A. G. Contributions to the mathematical theory of epidemics. iii. Further studies of the problem of epidemicity. Proceedings of the Royal Society of London A 141, 94 (1933). [Google Scholar]
- Barrat A., Barthelemy M. & Vespignani A. Dynamical processes on complex networks. (Cambridge University Press, Cambridge, 2008). [Google Scholar]
- Sen P. & Chakrabarti B. K. Sociophysics: An Introduction (Oxford University Press, Oxford, 2013). [Google Scholar]
- Hethcote H. W. The mathematics of infectious diseases. SIAM Review 42, 599 (2000). [Google Scholar]
- Watts D. J., Muhamad R., Medina D. C. & Dodds P. S. Multiscale, resurgent epidemics in a hierarchical metapopulation model. Proceedings of the National Academy of Sciences of the United States of America 102, 11157 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hethcote H. W. Asymptotic behavior and stability in epidemic models. Springer 2, 83 (1974). [Google Scholar]
- Janssen H. K., Oerding K., Van Wijland F. & Hilhorst H. J. Lévy-flight spreading of epidemic processes leading to percolating clusters. The European Physical Journal B 7, 137 (1999). [Google Scholar]
- Linder F., Tran-Gia J., Dahmen S. R. & Hinrichsen H. Long-range epidemic spreading with immunization. Journal of Physics A 41, 185005 (2008). [Google Scholar]
- Bennett S. N. et al. Epidemic dynamics revealed in dengue evolution. Molecular biology and evolution 27, 811 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Rou K. & Cui H. The hiv/aids epidemic in china: History, current strategies and future challenges. AIDS Education and Prevention 16, 7 (2004). [DOI] [PubMed] [Google Scholar]
- Xu X., Peng H., Wang X. & Wang Y. Epidemic spreading with time delay in complex networks. Physica A 367, 525 (2006). [Google Scholar]
- Wang J., Liu Z. & Xu J. Epidemic spreading on uncorrelated heterogenous networks with non-uniform transmission. Physica A 382, 715 (2007). [Google Scholar]
- Zhao Z., Liu Y. & Tang M. Epidemic variability in hierarchical geographical networks with human activity patterns. Chaos 22, 023150 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaleque A. & Sen P. Susceptible-infected-recovered model on euclidean network. J. Phys. A: Math. Theor. 46, 095007 (2013). [Google Scholar]
- Grassberger P. Sir epidemics with long-range infection in one dimension. Journal of Statistical Mechanics: Theory and Experiment 2013, P04004 (2013). [Google Scholar]
- Lewnard J. A. et al. Dynamics and control of ebola virus transmission in montserrado, liberia: a mathematical modelling analysis. The Lancet Infectious Diseases 14, 1189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell D., Castillo-Chavez C., Krishna S., Qiu X. & Anderson K. S. Modelling the effect of early detection of ebola. The Lancet Infectious Diseases 15, 148 (2015). [DOI] [PubMed] [Google Scholar]
- Camacho A., Kucharski A. J., Funk S., Piot P. & Edmunds W. J. Potential for large outbreaks of ebola virus disease. Epidemics 9, 70 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachah A. & Torres D. F. M. Mathematical modelling, simulation, and optimal control of the 2014 ebola outbreak in west africa. Discrete Dynamics in Nature and Society 2015, 842792 (2015). [Google Scholar]
- Radulescu A. & Herron J. Ebola impact and quarantine in a network model. arXiv:1512.06305 (2015).
- Burghardt K. et al. Testing modeling assumptions in the west africa ebola outbreak. arXiv:1606.07497 (2016). [DOI] [PMC free article] [PubMed]
- Chatterjee A. & Sen P. Phase transitions in ising model on a euclidean network. Phys. Rev. E 74, 036109 (2006). [DOI] [PubMed] [Google Scholar]
- Liberia-Wikipedia, Sierra Leone-Wikipedia. https://en.wikipedia.org/wiki/Liberia, https://en.wikipedia.org/wiki/Sierra_Leone.
- Guinea-Wikipedia. https://en.wikipedia.org/wiki/Guinea.
- Global Ebola Response. http://ebolaresponse.un.org/guinea, http://ebolaresponse.un.org/sierra-leone.
- Key financial data on MSF’s response to the Ebola epidemic in West Africa. https://www.doctorswithoutborders.org/sites/usa/files/msf_ebola_accountability_report_final_05_11_2016_2_002.pdf.
- Songwe V. Fighting ebola: A strategy for action. https://www.brookings.edu/wp-content/uploads/2016/07/fighting-ebola-songwe-2.pdf (2015). [Google Scholar]
- Centers for disease control and prevention. https://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/previous-case-counts.html (Retreived December, 2014).
- West african ebola virus epidemic. https://en.wikipedia.org/wiki/West_African_Ebola_virus_epidemic.