1. Introduction
On a moonless night or in the depths of the sea, where light levels are many orders of magnitude dimmer than sunlight, animals rely on their visual systems to orient and navigate, to find food and mates and to avoid predators. To see well at such low light levels is far from trivial. The paucity of light means that visual signals generated in the light-sensitive photoreceptors of the retina can easily be drowned in neural noise. Despite this, research over the past 15 years has revealed that nocturnal and deep-sea animals—even very small animals like insects with tiny eyes and brains—can have formidable visual abilities in dim light. The latest research in the field is now beginning to reveal how this visual performance is possible, and in particular which optical and neural strategies have evolved that permit reliable vision in dim light. This flurry of research is rapidly changing our understanding of both the limitations and the capabilities of animals active in very dim light. For instance, while the long-held view was that night vision allows only an impoverished, noisy and monochrome view of the world, we now know that many nocturnal animals see the world more or less in the same manner as their day-active relatives. Many are able to see colour, to use optic flow cues to control flight, and to navigate using learned visual landmarks and celestial cues such as polarized light.
Much of our appreciation of the richness of the visual world seen by nocturnal animals has derived primarily from behavioural, anatomical and optical studies. More recently, enormous advances have also been made in understanding the neural basis of this performance in both single cells and circuits of cells from both nocturnal vertebrates (notably mice) and nocturnal invertebrates (notably insects). These studies indicate that the remarkable behavioural performance of these animals in dim light can only partially be explained by what we currently know of the performance of the underlying visual cells. We are thus now at an important point in the field where this gap is closing. It is thus particularly timely that this special issue brings together a unique combination of recent research on deep-sea and nocturnal animals and moreover from a wide spectrum of scientific disciplines, from ecology, evolution and quantitative visual behaviour to cellular electrophysiology, mathematical modelling and molecular biology. This landmark collection of papers is the first to exclusively address the topic of comparative vision in dim light.
2. The dimmest habitats on the Earth
For us, as day-active organisms, nocturnal and deep-sea habitats are perishingly dark places. At great depths in the ocean it can be totally dark, a darkness broken only by rare and unpredictable sparks of bioluminescence produced by animals themselves. Nonetheless, for the immense variety of animals that live in nocturnal and deep-sea habitats (figure 1), vision plays a surprisingly important role in the tasks of daily life [16–21].
Figure 1.
Nocturnal and deep-sea animals with excellent vision. (a) The nocturnal Indian carpenter bee Xylocopa tranquebarica has trichromatic colour vision at night [1]. Photo: Nicolas Vereeken. (b) The dung beetles Scarabaeus zambesianus (i) and Scarabaeus satyrus (ii) use nocturnal celestial polarized light and the Milky Way for navigation [2–4]. (c) The nocturnal bee Megalopta genalis uses learned visual landmarks to find its way back to its nest in Central American rainforests [5]. Photo: Ajay Narendra. (d) The deep-sea cockeyed squid Stigmatoteuthis dofleini has one eye greatly larger than the other—this it orients upwards to capture as much of dim downwelling daylight as possible [6]. Photo: Sönke Johnsen. (e) The nocturnal Australian bull ant Myrmecia pyriformis navigates to and from its nest using learned visual landmarks [7]. Photo: Ajay Narendra. (f,g) The Philippine tarsier Tarsius syrichta (f) hunts prey visually (and is here seen eating a katydid) [8]. Its huge eyes, whose very wide pupils maximize light catch, dominate the skull (g). Photos: David Haring (f) and Bone Clones, www.boneclones.com (g). (h) The nocturnal hawkmoth Deilephila elpenor uses trichromatic colour vision to search for flowers at night [9], and uses spatial and temporal summation to maximize visual performance in dim light [10–12]. Photo: Michael Pfaff. (i) Even though possessing an acute sense of hearing, the barn owl Tyto alba has excellent vision in dim light [13]. Photo: 123rf.com photo agency. (j) The net-casting spider Deinopis subrufa uses its exquisite visual sensitivity to trap passing prey by ensnaring them with a net of web spun between its front legs [14,15]. (k) A deep-sea hatchet fish with huge tubular eyes oriented upwards to maximize sensitivity to the dim downwelling daylight. Photo: David Wrobel, Monterey Bay Aquarium.
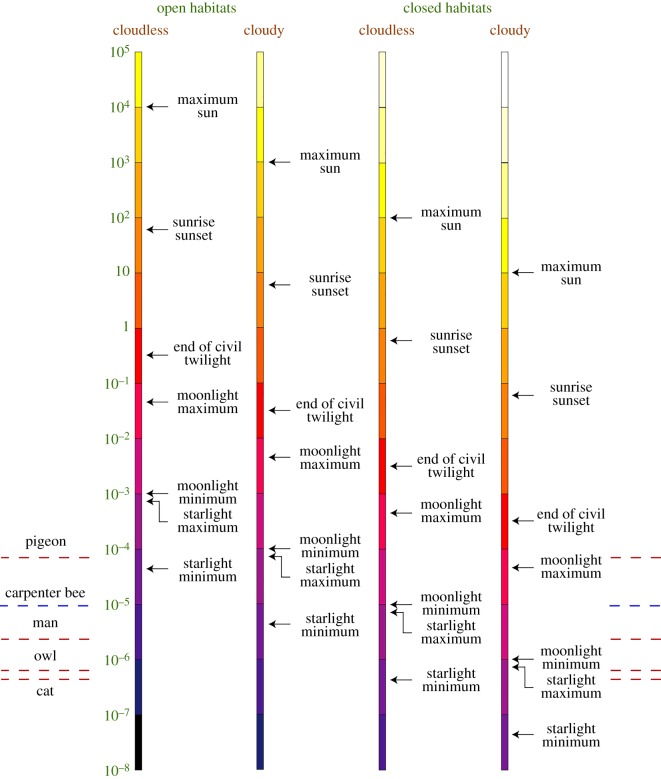
As in bright diurnal habitats, the primary source of illumination in nocturnal and deep-sea habitats is the sun, which bathes the Earth with light either directly, as during the day in terrestrial habitats and the deep ocean, or indirectly by reflection from the moon at night [22]. The spectra of sunlight and moonlight are thus similar, although on nights with a full moon light levels are approximately one million times dimmer than full daylight. On a clear moonless night, stars become the exclusive source of light, an illumination that is considerably redder than that provided by sunshine [23] and which is typically around 100 million times dimmer (figure 2). The passage of clouds, or the presence of a forest canopy, can dim the illumination by up to a further 100 times.
Figure 2.
The luminance (in units of cd m−2) of a natural substrate of leaf litter when illuminated by sunlight, moonlight or starlight during cloudy and cloudless conditions. The luminance is shown for two habitat conditions: an ‘open’ treeless field, and beneath the ‘closed’ canopy of a forest (which is considered here to reduce illumination by 100 times). Luminance is calculated assuming that the leaf litter substrate has an average reflectance of 25% and a fully overcast sky reduces illumination by a factor of 10. Dashed red lines show absolute visual thresholds (i.e. the minimum intensity of light which is just perceivable) measured for pigeons, humans, Tawny owls and cats. Dashed blue line indicates the very low intensity at which the nocturnal Indian carpenter bee Xylocopa tranquebarica (figure 1a)—despite small apposition compound eyes and a tiny brain—is able to use trichromatic colour vision while flying and foraging at night [1]. This intensity is likely to be 1–2 orders of magnitude higher than that at which absolute visual threshold occurs. Civil twilight is defined as the period during which the sun's disc is between the horizon and 6° below the horizon. Reproduced with permission from [17], having been adapted with kind permission from [24].
The open ocean can be even darker, with both the intensity and the spectrum of light changing rapidly with depth [19,25]. In terms of spectrum, downwelling daylight in a clear sea becomes near-monochromatic blue in colour from a depth of around 200 m [26]. Clear water is most transparent to blue light of 475 nm wavelength, and within the first 100 m the orange-red part of the spectrum (beyond 550 nm) is almost entirely absorbed. Ultraviolet (UV) light is also absorbed, but not quite as effectively: in the clearest oceans, biologically relevant intensities remain down to at least 200 m [27,28]. Owing to the absorption of light by water and its narrowing spectrum, the intensity of light available for vision thus falls rapidly with depth. Within the first 100 m, it declines by about 2.6 orders of magnitude [19,25]. Below this depth, presumably because the concentration of plankton and suspended organic matter is less and the spectrum of light is approaching its narrowest, light intensity declines less rapidly: about 1.5 orders of magnitude for every 100 m of depth. It reaches starlight levels (during the day) by approximately 600–700 m [29]. Below 1000 m almost no daylight penetrates, certainly not enough to be seen by deep-sea animals [30].
Thus, on a starlit night or in a deep ocean, vision is relentlessly pressed at the limits of the physically possible. While some species have given up the fight altogether, with their eyes having regressed to mere vestiges of their counterparts in bright habitats, in others the eyes have evolved extreme adaptations for extracting the most fleeting of visual cues. Indeed, in the world's dimmest habitats—terrestrial or aquatic—many animals depend on good vision to survive.
3. The problem of seeing in dim light
A number of landmark papers have addressed the topic of vision in dim light, particularly the absolute threshold of vision in humans and other animals. The classic psychophysical work of Hecht, Schlaer and Pirenne in the 1940s was the first to reveal that humans (and certainly most other animals) can consistently perceive a flash of light so dim that very few photons are simultaneously absorbed in the retina (for humans around five photons: [31,32]). The realization that these few photons are then spread across many (approx. 500) rod photoreceptors suggested that individual photoreceptors were very unlikely to absorb more than single photons during such flashes (see also [33]). This seminal finding suggested that photoreceptors must be able to respond to single photons, a notion later demonstrated more directly with electrophysiological recordings, first in invertebrates (in the horseshoe crab Limulus in 1958 [34], and the locusts Locusta and Schistocerca in 1965 [35]) and then two decades later in vertebrates (in the toad Bufo marinus in 1980 [36] and the monkey Macaca fascicularis in 1984 [37]).
Although absolute sensitivity to single photons is remarkable, the early workers in this field also recognized that this ability is insufficient on its own to perform many useful real-world visual tasks. Detecting objects, negotiating obstacles during locomotion or distinguishing colours are all tasks that require the discrimination of visual contrast, for which many more photons are needed. Part of the reason for this lies in the need for a larger and more reliable visual signal. Part also lies in the need to overcome visual noise, the scourge of vision in dim light that can drown the intended fluctuations of photon counts in the retina's matrix of photoreceptors induced by the presence of brighter and darker features in the visual scene.
There are several sources of this noise. The first of these, known as ‘photon shot noise’, derives from the sporadic and random nature of the stimulus itself—even when a photoreceptor experiences a light stimulus of apparently constant intensity, the rate of photon arrivals is not constant. During any given sampled period, a photoreceptor sometimes receives a greater-than-average number of photons, sometimes a lower-than-average number. This uncertainty in the photoreceptor's measure of the stimulus intensity is due to the random (Poisson) nature of photon arrivals, with the relative magnitude of shot noise (according to Poisson statistics) being equivalent to the square root of the average photon catch N. Thus, if the signal is N, the visual signal-to-noise ratio is N/√N, or simply √N. Consequently, the effect of shot noise is worse in dimmer light—for a decreasing photon catch, the signal-to-noise ratio (and the ability to discriminate contrast) declines.
Two further sources of noise derive from the imperfect nature of the phototransduction mechanism. Firstly, despite being responses to an invariant stimulus—the quantal energy of single photons—the graded voltage responses to these photons are highly irregular, with variable amplitudes, latencies and time courses. This ‘transducer noise’ originates in the biochemical enzyme cascades responsible for the massive signal amplification required to change ion fluxes enough for the photoreceptor voltage response to give a reliable response to single photons [38–40]. Although this transducer noise has the potential to degrade the reliability of vision, some recent work suggests it could, remarkably, have the opposite effect [41,42]. Secondly, as first predicted by Horace Barlow almost 60 years ago [43], and confirmed two decades later in toad rods [36], the biochemical pathways responsible for transduction are occasionally activated even in perfect darkness. These dark events can trigger responses that are in every respect identical to the responses to real photons, and perceptually the brain cannot tell them apart. In vertebrates at least, it turns out that such ‘dark noise’ sets the ultimate threshold for vision in dim light [44,45]. Interestingly, despite also using a transduction mechanism with amplification so high that single photons elicit responses of several millivolts, dark-noise events have been shown to be much less important—almost negligible—in insect photoreceptors [40,46]. This lack of dark noise provides many invertebrate photoreceptors with an outright performance advantage for dim-light vision, but one that comes at the cost of greater expenditure of energy in the form of ATP than in their vertebrate counterparts ([47], see also [48]).
To combat both problems—the paucity of light and the degrading effects of visual noise—nocturnal and deep-sea animals have evolved a suite of remarkable adaptations, both optical and neural, to improve vision at night or in the depths of the ocean. Optical adaptations improve the visual image by gathering more photons into the image formed on the retina, improving the ability to distinguish subtle contrasts, whether these be in luminance, colour or in the e-vector of polarized light. Equally important, neural adaptations allow the brain to adjust the way in which information gathered by the retina is processed—pooling signals over time or from neighbouring detectors to improve reliability, albeit at the expense of resolution in time or space. Many of these adaptations at both levels are explored in the collection of papers comprising this special issue. The overriding conclusion from this collection, and from other studies over the past 15 years, is that nocturnal and deep-sea animals do not live in an impoverished visual world, but many experience the world more or less as we do, being able to distinguish colour, negotiate obstacles during locomotion and navigate using learned visual landmarks.
4. A special issue devoted to vision in dim light
With the exception of a single monograph entitled Night Vision [49], as far as we are aware there are no previous books or collections of articles on the topic of vision in dim light. Moreover, with the exception of a single chapter, this previous monograph was entirely concerned with mammalian vision (and mostly humans). The initial idea for this special issue in the Philosophical Transactions of the Royal Society arose from a highly successful international symposium that we organized and hosted in Sweden in 2013 on the topic of Vision in Dim Light. To the best of our knowledge, this symposium was the first of its kind, but attracted 56 participants from all over the world (approximately half of them early career researchers) working on diverse animal systems, both vertebrate and invertebrate. Since that time, two of our present authors—Almut Kelber and Carola Yovanovich—also organized a further well-attended symposium on the topic of dim light vision in vertebrates that was held at the biannual congress of the International Society of Neuroethology (in Uruguay, April 2016), an indication of the growing interest in the field.
The papers in this issue are organized around two distinct themes, both strongly represented at these recent scientific meetings. Firstly, behavioural approaches provide a proof of concept that different aspects of vision at night or in the deep sea are possible with highly optimized optical image–forming mechanisms, appropriate sampling by the retina and additional processing by the brain. Animal behaviour shows that something is not only possible, but that the solution works in a practical sense. Secondly, physiological analysis using an array of modern techniques is providing great insights into the actual mechanisms employed by the brain to both transduce light efficiently and then process the resulting neural image to reject noise. Papers addressing both themes in this issue are introduced by two substantive reviews. In the first [12], Warrant introduces vision in dim light by insects, a group displaying enormous diversity in adapting to an enormous variety of habitats and visual tasks. Insects are arguably the animal group with the most remarkable visual abilities at night, particularly if considered in terms of the size of the eyes and the amount of neural tissue involved in nocturnal visual processing. Moreover, their accessibility for physiological experiments has permitted perhaps our best analysis of nocturnal vision, from the optics right through to the central neural adaptations within the brain that pool noisy signals. In the second review, Field & Sampath [33] explore the remarkable advances in understanding visual processing by the vertebrate retina, taking us from a description of the classic pioneering work that established the thresholds for visual performance in humans and other vertebrates (and led to a realization that photoreceptors can detect single photons of light), right through to our latest research into the mechanisms of visual transduction and the neural circuits of the retina that pool signals from rod photoreceptors.
Both of these reviews [12,33] highlight an important point: while recent research is finally unravelling the mysteries of dim light vision, this is still an immature field with many open questions. The remaining papers in the issue nevertheless exploit different animal groups (terrestrial insects, animals from the deep sea and terrestrial vertebrates) to address at least a subset of these. In the first of four papers using insects as a model system, Stöckl et al. [11] use a comparative approach to quantify the trade-offs between luminance of the scene and visual performance during flower-tracking by different species of hawkmoths (Sphingidae) that are normally active at different times of the day and night. The following two papers behaviourally dissect the specific visual cues that nocturnal dung beetles [4] and bull ants [7] use when navigating. Nocturnal dung beetles (figure 1b) have previously been shown to use the Milky Way for reliable orientation, and are well known to use this cue to maintain a straight-line course when rolling dung balls [3]. Foster et al. [4] here used artificial celestial cues to quantify the constraints on the visual contrast that underlies this orientation behaviour—with just 13% contrast being sufficient at starlight levels. Without relying on a scent trail like many other ants, nocturnal Australian bull ants (figure 1e) forage visually, using trees in the vicinity of the nest entrance as visual landmarks to find their way home again after a long and tortuous foraging trip [50]. Narendra & Ramirez-Esquivel [7] here used a field-based approach to quantify the behavioural adaptation of nocturnal ants to a change in their natural environment, in this case by removing dead trees from their normal foraging path. Ants initially became confused, but over the following nights could re-learn the new arrangements of landmarks to again navigate successfully. In the final paper in this section, Honkanen et al. [51] then review what we currently know about the physiological properties of photoreceptors in nocturnal insects, how they have evolved to extract as much information as possible from dim and noisy images and how recent advances in methodologies may ultimately be used to address remaining questions about their functional properties.
Studying animals active in the marine environment offers a different set of challenges to that of studying insects (whether in the laboratory or field). The animals that live here are often difficult to obtain, and even if caught, irreversible damage to their visual systems often ensues soon after (e.g. due to exposure to bright light). Nevertheless, this fascinating environment is the basis for the next four papers in the issue. Much of what we understand about their visual systems is based on morphological studies of specimens brought to the surface (as is the case with the second paper in this group) or from theoretical modelling (as with the first and third). It is extremely rare that one has the possibility to perform physiological studies on deep-sea eyes, although the final paper of this group does just that. Almost as rare is the possibility of observing deep-sea creatures—and their visual behaviour—first hand. But this is precisely what Thomas et al. [6] have done in their study of the cockeyed squid (figure 1d). They observed and filmed the animals in their natural habitat off the coast of California using remotely operated submersibles. Using these observations together with optical modelling, the authors quantify the remarkable differences between the left and right eyes as a unique adaptation to the completely different challenges of detecting objects above, silhouetted against the dim background of daylight downwelling from the surface (detected by one large eye), versus detection of small point sources of light produced by bioluminescent organisms below (detected by the other smaller eye). De Busserolles & Marshall [52] then review a remarkable body of work on lanternfishes (Myctophidae) a diverse group of 250 mesopelagic species that produce their own light as a partial solution to the problem of seeing in an environment where little light from the sun ever reaches. Cronin et al. [53] explore a fascinating question of whether right whales foraging at depths of hundreds of metres can potentially detect rich patches of their main food—clouds of tiny copepod crustacea—a very subtle visual cue. They use theoretical modelling to provide a tentative answer that yes, this may be possible, especially if the swarm is located above the whale and thus creates a silhouette against the background of dim downwelling light throughout the daylight hours. The final paper in this group, by Frank [54], uses electrophysiological analysis to explore significant effects of the gradient in temperature as a means to optimize temporal processing in crustacea that migrate from the surface to the deeper ocean as they mature—a fascinating example of a parsimonious link between physiology and habitat.
The remaining papers in the issue address different approaches to understanding the limits and mechanisms of night vision in a variety of terrestrial vertebrate species. Penteriani & del Mar Delgado [55] review current knowledge on visual signalling in crepuscular and nocturnal birds and mammals and the visual signals that they may use to communicate with others—another example of a topic within this field where many questions remain unanswered. Takeshita et al. [56] then review the mechanistic basis for the segregation of visual information from the same rod photoreceptors into two visual pathways that process increments of light intensity (ON) and the other decrements (OFF). Morshedian & Fain [48] then review the reasons why vertebrates evolved ciliary-type photoreceptors rather than the less-noisy rhabdomeric type used by insects and many other invertebrates, reaching the fascinating conclusion that this was primarily for reasons of energetic economy—they use less ATP. While for most humans, the day is enriched by our ability to perceive our world in full colour, at night these colours fade away as we have only a single type of rod photoreceptor that provides useful night vision. As Kelber et al. [57] review, however, the night world is far more colourful for a number of other animal groups which have evolved a variety of mechanisms to discriminate colours at night. A further new research paper on this topic, by Yovanovich et al. [58] uses the power of a comparative behavioural approach to show for the first time that amphibians can use two different types of rod photoreceptor to discriminate colour at night, with some frogs displaying the ability to distinguish blue from green in an orientation task right down to the absolute limits of vision, where vision relies only on rod signals. In the final paper, Moritz et al. [8] use a different approach—sequencing the genes coding for short wavelength sensitivity in the retina of nocturnal tarsiers (figure 1f,g)—to ask whether colour vision might be important for some nocturnal primates. They conclude that in some species the evolution of dichromatic night vision aids the discrimination of their insect prey by improving their contrast against natural foliage.
5. Concluding remarks
Given that this is still an immature field, many of the papers in this issue ask as many new questions as they answer. It will be exciting to revisit this topic a decade from now to see how many of these questions are answered. Hence we expect this issue to have broad interest to the vision research community because it summarizes and provides future directions in an important but relatively little-studied area of visual research. Beyond this specialist interest, we are all surrounded by ever smarter man-made imaging systems that play an increasingly important role in our everyday lives. With the recent interest in bioinspired and even biomimetic solutions to complex engineering problems, this issue is also likely to be of great interest to a broad range of computer vision engineers. As there are no current textbooks covering this subject matter, this special issue also promises to serve a useful role outside the vision science community. Vision is one of our richest senses and given our fascination with creatures active in the dark of night and of monsters from the deep sea, this is a subject that will also interest a broad range of scientists, high-school students and members of the general public—anyone wishing to learn about how animals can see in the dark.
Biographies
Guest editor biographies
 David C. O'Carroll is Professor of Animal Physiology at the University of Lund in Sweden. His research uses the insect brain as a ‘simple’ model system to study mechanisms for tracking and focusing attention on moving features and patterns. His approach combines electrophysiological recordings from multiple levels in the visual system from peripheral processing by the retina to neurons in higher order brain areas, with computational modelling of the underlying algorithms for feature extraction. O'Carroll's research has led to understanding of the adaptive neural mechanisms that allow motion detectors to be tuned to respond robustly to natural stimuli in both diurnal and nocturnal species.
David C. O'Carroll is Professor of Animal Physiology at the University of Lund in Sweden. His research uses the insect brain as a ‘simple’ model system to study mechanisms for tracking and focusing attention on moving features and patterns. His approach combines electrophysiological recordings from multiple levels in the visual system from peripheral processing by the retina to neurons in higher order brain areas, with computational modelling of the underlying algorithms for feature extraction. O'Carroll's research has led to understanding of the adaptive neural mechanisms that allow motion detectors to be tuned to respond robustly to natural stimuli in both diurnal and nocturnal species.
 Eric J. Warrant is Professor of Zoology at the University of Lund in Sweden. Warrant leads an active research group studying vision and visual navigation in animals from extremely dim habitats (nocturnal and deep sea). Using electrophysiological, optical, histological, behavioural and theoretical approaches, Warrant studies how animals as diverse as nocturnal insects, deep-sea cephalopods and fast-swimming predatory fishes are able to see well at very low light levels, and his research has led to the discovery of neural principles that permit vision in dim light.
Eric J. Warrant is Professor of Zoology at the University of Lund in Sweden. Warrant leads an active research group studying vision and visual navigation in animals from extremely dim habitats (nocturnal and deep sea). Using electrophysiological, optical, histological, behavioural and theoretical approaches, Warrant studies how animals as diverse as nocturnal insects, deep-sea cephalopods and fast-swimming predatory fishes are able to see well at very low light levels, and his research has led to the discovery of neural principles that permit vision in dim light.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by an Institutional grant from STINT, the Swedish Foundation for International Cooperation in Research and Higher Education (STINT 2012-2033), and project grants from the Australian Research Council’s Discovery Projects funding scheme (project number DP130104561), AFOSR (FA8655-07-C-4011 and FA9550-12-1-0237) and the Swedish Research Council (VR: 2016-04014).
References
- 1.Somanathan H, Borges RM, Warrant EJ, Kelber A. 2008. Nocturnal bees learn landmark colours in starlight. Curr. Biol. 18, R996–R997. ( 10.1016/j.cub.2008.08.023) [DOI] [PubMed] [Google Scholar]
- 2.Dacke M, Nilsson D-E, Scholtz CH, Byrne M, Warrant EJ. 2003. Insect orientation to polarized moonlight. Nature 424, 33 ( 10.1038/424033a) [DOI] [PubMed] [Google Scholar]
- 3.Dacke M, Baird E, Byrne M, Scholtz CH, Warrant EJ. 2013. Dung beetles use the Milky Way for orientation. Curr. Biol. 23, 298–300. ( 10.1016/j.cub.2012.12.034) [DOI] [PubMed] [Google Scholar]
- 4.Foster JJ, el Jundi B, Smolka J, Khaldy L, Nilsson D-E, Byrne MJ, Dacke M. 2017. Stellar performance: mechanisms underlying Milky Way orientation in dung beetles. Phil. Trans. R. Soc. B 372, 20160079 ( 10.1098/rstb.2016.0079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warrant EJ, Kelber A, Gislén A, Greiner B, Ribi W, Wcislo WT. 2004. Nocturnal vision and landmark orientation in a tropical halictid bee. Curr. Biol. 14, 1309–1318. ( 10.1016/j.cub.2004.07.057) [DOI] [PubMed] [Google Scholar]
- 6.Thomas KN, Robison BH, Johnsen S. 2017. Two eyes for two purposes: in situ evidence for asymmetric vision in the cockeyed squids Histioteuthis heteropsis and Stigmatoteuthis dofleini. Phil. Trans. R. Soc. B 372, 20160069 ( 10.1098/rstb.2016.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narendra A, Ramirez-Esquivel F. 2017. Subtle changes in the landmark panorama disrupt visual navigation in a nocturnal bull ant. Phil. Trans. R. Soc. B 372, 20160068 ( 10.1098/rstb.2016.0068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moritz GL, Ong PS, Perry GH, Dominy NJ. 2017. Functional preservation and variation in the cone opsin genes of nocturnal tarsiers. Phil. Trans. R. Soc. B 372, 20160075 ( 10.1098/rstb.2016.0075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelber A, Balkenius A, Warrant EJ. 2002. Scotopic colour vision in nocturnal hawkmoths. Nature 419, 922–925. ( 10.1038/nature01065) [DOI] [PubMed] [Google Scholar]
- 10.Stöckl AL, O'Carroll DC, Warrant EJ. 2016. Neural summation in the hawkmoth visual system extends the limits of vision in dim light. Curr. Biol. 26, 821–826. ( 10.1016/j.cub.2016.01.030) [DOI] [PubMed] [Google Scholar]
- 11.Stöckl AL, Kihlström K, Chandler G, Sponberg S. 2017. Comparative system identification of flower tracking performance in three hawkmoth species reveals adaptations for dim light vision. Phil. Trans. R. Soc. B 372, 20160078 ( 10.1098/rstb.2016.0078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warrant EJ. 2017. The remarkable visual capacities of nocturnal insects: vision at the limits with small eyes and tiny brains. Phil. Trans. R. Soc. B 372, 20160063 ( 10.1098/rstb.2016.0063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlowski J, Harmening W, Wagner H. 2012. Night vision in barn owls: visual acuity and contrast sensitivity under dark adaptation. J. Vis. 12, 1–8. ( 10.1167/12.13.4) [DOI] [PubMed] [Google Scholar]
- 14.Blest AD, Land MF. 1977. The physiological optics of Dinopis subrufus L. Koch: a fish-lens in a spider. Proc. R. Soc. Lond. B 196, 197–222. ( 10.1098/rspb.1977.0037) [DOI] [PubMed] [Google Scholar]
- 15.Laughlin SB, Blest AD, Stowe S. 1980. The sensitivity of receptors in the posterior median eye of the nocturnal spider Dinopis. J. Comp. Physiol. 141, 53–65. ( 10.1007/BF00611878) [DOI] [Google Scholar]
- 16.Warrant EJ. 2004. Vision in the dimmest habitats on earth. J. Comp. Physiol. A 190, 765–789. ( 10.1007/s00359-004-0546-z) [DOI] [PubMed] [Google Scholar]
- 17.Warrant EJ. 2008. Nocturnal vision. In The senses: a comprehensive reference (vol. 2: vision II) (eds Albright T, Masland RH; Series eds AI Basbaum, A Kaneko, GM Shepherd and G Westheimer), pp. 53–86. Oxford, UK: Academic Press. [Google Scholar]
- 18.Warrant EJ. 2008. Seeing in the dark: vision and visual behaviour in nocturnal bees and wasps. J. Exp. Biol. 211, 1737–1746. ( 10.1242/jeb.015396) [DOI] [PubMed] [Google Scholar]
- 19.Warrant EJ, Locket NA. 2004. Vision in the deep sea. Biol. Rev. 79, 671–712. ( 10.1017/S1464793103006420) [DOI] [PubMed] [Google Scholar]
- 20.Warrant EJ, Dacke M. 2011. Vision and visual navigation in nocturnal insects. Annu. Rev. Entomol. 56, 239–254. ( 10.1146/annurev-ento-120709-144852) [DOI] [PubMed] [Google Scholar]
- 21.Warrant EJ, Dacke M. 2016. Visual navigation in nocturnal insects. Physiology 31, 182–192. ( 10.1152/physiol.00046.2015) [DOI] [PubMed] [Google Scholar]
- 22.Warrant EJ, Johnsen S. 2013. Vision and the light environment. Curr. Biol. 23, R990–R994. ( 10.1016/j.cub.2013.10.019) [DOI] [PubMed] [Google Scholar]
- 23.Johnsen S, Kelber A, Warrant EJ, Sweeney AM, Widder EA, Lee RL Jr, Hernández-Andrés J. 2006. Twilight and nocturnal illumination and its effects on color perception by the nocturnal hawkmoth Deilephila elpenor. J. Exp. Biol. 209, 789–800. ( 10.1242/jeb.02053) [DOI] [PubMed] [Google Scholar]
- 24.Martin GR. 1990. Birds by night. London, UK: T & AD Poyser. [Google Scholar]
- 25.Jerlov NG. 1976. Marine optics. Amsterdam, The Netherlands: Elsevier Scientific Publishing Company. [Google Scholar]
- 26.Tyler JE, Smith RC. 1970. Measurement of spectral irradiance underwater. New York, NY: Gordon and Breach. [Google Scholar]
- 27.Frank TM, Widder EA. 1996. UV light in the deep sea: In situ measurements of downwelling irradiance in relation to the visual threshold sensitivity of UV-sensitive crustaceans. Mar. Freshw. Behav. Physiol. 27, 189–197. ( 10.1080/10236249609378964) [DOI] [Google Scholar]
- 28.Losey GS, Cronin TW, Goldsmith TH, Hyde D, Marshall NJ, McFarland WN. 1999. The UV visual world of fishes: a review. J. Fish Biol. 54, 921–943. ( 10.1111/j.1095-8649.1999.tb00848.x) [DOI] [Google Scholar]
- 29.Clarke GL, Denton EJ. 1962. Light and animal life. In The sea (ed. Hill MN.), pp. 456–468. New York, NY: Wiley-Interscience. [Google Scholar]
- 30.Denton EJ. 1990. Light and vision at depths greater than 200 metres. In Light and life in the sea (eds Herring PJ, Campbell AK, Whitfield M, Maddock L), pp. 127–148. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 31.Hecht S, Schlaer S, Pirenne MH. 1942. Energy, quanta, and vision. J. Gen. Physiol. 25, 819–840. ( 10.1085/jgp.25.6.819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pirenne MH. 1948. Vision and the eye. London, UK: The Pilot Press. [Google Scholar]
- 33.Field GD, Sampath AP. 2017. Behavioural and physiological limits to vision in mammals. Phil. Trans. R. Soc. B 372, 20160072 ( 10.1098/rstb.2016.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeandle S. 1958. Evidence of quantized slow potentials in the eye of Limulus. Am. J. Ophthalmol. 46, 82–87. [Google Scholar]
- 35.Scholes J. 1965. Discontinuity of the excitation process in locust visual cells. Cold Spring Harb. Symp. Quant. Biol. 30, 517–527. ( 10.1101/SQB.1965.030.01.050) [DOI] [PubMed] [Google Scholar]
- 36.Baylor DA, Matthews G, Yau KW. 1980. Two components of electrical dark noise in toad retinal rod outer segments. J. Physiol. 309, 591–621. ( 10.1113/jphysiol.1980.sp013529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baylor DA, Nunn BJ, Schnapf JL. 1984. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J. Physiol. 357, 575–607. ( 10.1113/jphysiol.1984.sp015518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lillywhite PG, Laughlin SB. 1979. Transducer noise in a photoreceptor. Nature 277, 569–572. ( 10.1038/277569a0) [DOI] [PubMed] [Google Scholar]
- 39.Lillywhite PG. 1981. Multiplicative intrinsic noise and the limits to visual performance. Vision Res. 21, 291–296. ( 10.1016/0042-6989(81)90123-1) [DOI] [PubMed] [Google Scholar]
- 40.Laughlin SB, Lillywhite PG. 1982. Intrinsic noise in locust photoreceptors. J. Physiol. 332, 25–45. ( 10.1113/jphysiol.1982.sp014398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Z, Postma M, Billings SA, Coca D, Hardie RC, Juusola M. 2012. Stochastic, adaptive sampling of information by microvilli in fly photoreceptors. Curr. Biol. 22, 1371–1380. ( 10.1016/j.cub.2012.05.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song Z, Juusola M. 2014. Refractive sampling links efficiency and costs of sensory encoding to stimulus statistics. J. Neurosci. 34, 7216–7237. ( 10.1523/JNEUROSCI.4463-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barlow HB. 1956. Retinal noise and absolute threshold. J. Opt. Soc. Am. 46, 634–639. ( 10.1364/JOSA.46.000634) [DOI] [PubMed] [Google Scholar]
- 44.Aho A-C, Donner K, Hydén C, Larsen LO, Reuter T. 1988. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature 334, 348–350. ( 10.1038/334348a0) [DOI] [PubMed] [Google Scholar]
- 45.Aho A-C, Donner K, Helenius S, Larsen LO, Reuter T. 1993. Visual performance of the toad (Bufo bufo) at low light levels, retinal ganglion cell responses and prey-catching accuracy. J. Comp. Physiol. A 172, 671–682. [DOI] [PubMed] [Google Scholar]
- 46.Lillywhite PG. 1977. Single photon signals and transduction in an insect eye. J. Comp. Physiol. 122, 189–200. ( 10.1007/BF00611889) [DOI] [Google Scholar]
- 47.Fain GL, Hardie R, Laughlin SB. 2010. Phototransduction and the evolution of photoreceptors. Curr. Biol. 20, R114–R124. ( 10.1016/j.cub.2009.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morshedian A, Fain GL. 2017. The evolution of rod photoreceptors. Phil. Trans. R. Soc. B 372, 20160074 ( 10.1098/rstb.2016.0074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hess RF, Sharpe LT, Nordby K. 1990. Night vision. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 50.Reid SF, Narendra A, Hemmi JM, Zeil J. 2011. Polarised skylight and the landmark panorama provide night-active bull ants with compass information during route following. J. Exp. Biol. 214, 363–370. ( 10.1242/jeb.049338) [DOI] [PubMed] [Google Scholar]
- 51.Honkanen A, Immonen E-V, Salmela I, Heimonen K, Weckström M. 2017. Insect photoreceptor adaptations to night vision. Phil. Trans. R. Soc. B 372, 20160077 ( 10.1098/rstb.2016.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Busserolles F, Marshall NJ. 2017. Seeing in the deep-sea: visual adaptations in lanternfishes. Phil. Trans. R. Soc. B 372, 20160070 ( 10.1098/rstb.2016.0070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cronin TW, Fasick JI, Schweikert LE, Johnsen S, Kezmoh LJ, Baumgartner MF. 2017. Coping with copepods: do right whales (Eubalaena glacialis) forage visually in dark waters? Phil. Trans. R. Soc. B 372, 20160067 ( 10.1098/rstb.2016.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank TM. 2017. Ontogenetic adaptations in the visual systems of deep-sea crustaceans. Phil. Trans. R. Soc. B 372, 20160071 ( 10.1098/rstb.2016.0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penteriani V, Delgado MM. 2017. Living in the dark does not mean a blind life: bird and mammal visual communication in dim light. Phil. Trans. R. Soc. B 372, 20160064 ( 10.1098/rstb.2016.0064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeshita D, Smeds L, Ala-Laurila P. 2017. Processing of single-photon responses in the mammalian On and Off retinal pathways at the sensitivity limit of vision. Phil. Trans. R. Soc. B 372, 20160073 ( 10.1098/rstb.2016.0073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelber A, Yovanovich C, Olsson P. 2017. Thresholds and noise limitations of colour vision in dim light. Phil. Trans. R. Soc. B 372, 20160065 ( 10.1098/rstb.2016.0065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yovanovich CAM, Koskela SM, Nevala N, Kondrashev SL, Kelber A, Donner K. 2017. The dual rod system of amphibians supports colour discrimination at the absolute visual threshold. Phil. Trans. R. Soc. B 372, 20160066 ( 10.1098/rstb.2016.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]