Abstract
Colour discrimination is based on opponent photoreceptor interactions, and limited by receptor noise. In dim light, photon shot noise impairs colour vision, and in vertebrates, the absolute threshold of colour vision is set by dark noise in cones. Nocturnal insects (e.g. moths and nocturnal bees) and vertebrates lacking rods (geckos) have adaptations to reduce receptor noise and use chromatic vision even in very dim light. In contrast, vertebrates with duplex retinae use colour-blind rod vision when noisy cone signals become unreliable, and their transition from cone- to rod-based vision is marked by the Purkinje shift. Rod–cone interactions have not been shown to improve colour vision in dim light, but may contribute to colour vision in mesopic light intensities. Frogs and toads that have two types of rods use opponent signals from these rods to control phototaxis even at their visual threshold. However, for tasks such as prey or mate choice, their colour discrimination abilities fail at brighter light intensities, similar to other vertebrates, probably limited by the dark noise in cones.
This article is part of the themed issue 'Vision in dim light’.
Keywords: colour vision, visual threshold, visual ecology, Purkinje shift
1. Humans are colour blind on starry nights
To the human eye, the sunlit world appears in sparkling colour, but when night falls, colours fade away until, with less than a half moon, we see the world in 50 shades of grey. The reason for this is our duplex retina. In bright light (above approx. 10 cd m−2), photopic vision based on three spectral types of cone photoreceptors allows colour vision, whereas in dim light (at intensities less than ≈10−2 to 10−3 cd m−2, see [1]), a single type of rod only allows colour-blind scotopic vision. At intermediate light intensities, both rods and cones contribute to varying degrees to our mesopic vision. When we move from brighter to darker light intensities, colours appear less and less saturated, and the hue as well as the brightness of colours shift considerably making colour perception less reliable in these light conditions [2 (p. 320), 3–5].
In 1825, Johan Purkinje [6] was the first to describe another basic difference in the appearance of colours between photopic, mesopic and scotopic conditions. On his early morning walks, he noted that the reds and yellows that appeared as the brightest colours in daylight still looked black to him, in the dim light of early dawn, when he already could see blue. This observation—that the brightest region of the spectrum shifted from the red towards the blue—has since been called the Purkinje shift. It results from the difference in the scotopic, rod-based spectral sensitivity, with a peak at 500 nm and the photopic, cone-based spectral sensitivity, which peaks at 555 nm. While other changes in colour appearance are more difficult to study in animals other than humans, the Purkinje shift has been observed in various vertebrate species, including frogs [7], cats [8], ground squirrels [9] and sharks [10], just to name a few examples. The general view was that, as in humans, such a change in spectral sensitivity—depending on light intensity and the adaptation state of the eye—was an indication of a duplex retina, with cone-based colour vision in bright light and rod-based colour-blind vision in dim light.
A Purkinje shift has even been described in flies [11], and it has long been assumed that the fly visual system uses a highly sensitive subset of photoreceptors (receptors 1–6) in a rod-like function, and the remaining two photoreceptors, 7 and 8, in a cone-like function, strictly separating colour vision and other visual modalities such as motion vision into parallel pathways [12]. A newer study has shown that flies use all their photoreceptors for colour vision [13], but another older study [14] described the honeybee as colour blind in dim light, seemingly confirming the belief that colour-blind vision at night was a general rule.
2. Why vision should not be colour blind at night
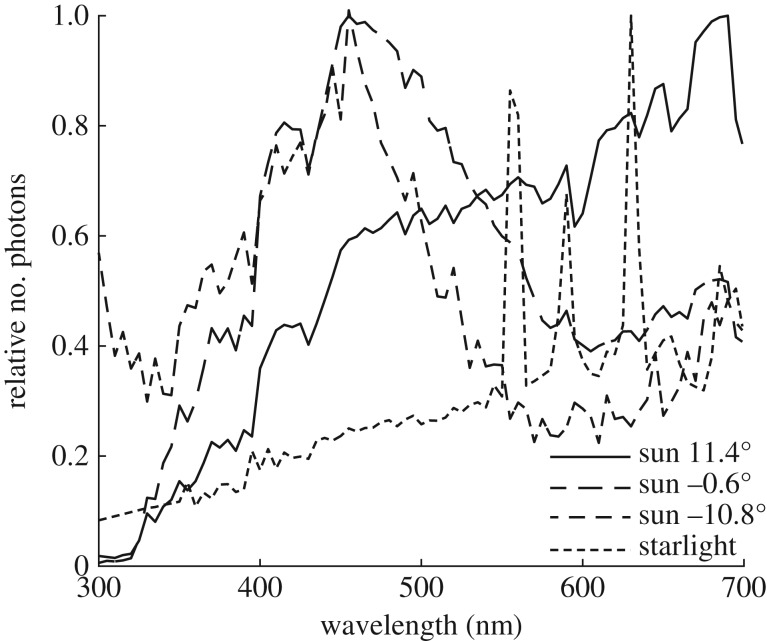
Evolution results in sensory systems that gather useful information and reject information that is not reliable. Thus, the obvious question to ask is: is spectral information less useful in dim light than in bright light? Most animals with advanced colour vision abilities [15] use the spectral information of light to detect and recognize objects such as their food, mates, habitats or homes. Although chromatic or colour signals have a generally lower information content [16], they are more reliable than achromatic (or luminance) signals because they depend less on the illumination spectrum. Colour vision goes along with colour constancy; it has been argued that colour vision may even have evolved for achieving constancy in the first place (see [17]), and this is particularly relevant during the dark half of the day. When the sun sets, the spectral composition of light (figure 1) shifts towards shorter wavelengths until, at astronomical twilight (when the sun no longer contributes to the illumination), it returns to the sunlight spectrum—now much dimmer reflected from the moon—or changes to the long-wavelength-shifted starlight spectrum. In the course of these dramatic illumination changes, coloured objects change dramatically in luminance, but, with the help of colour constancy mechanisms, they will always retain their hue [18]. Even in dim light, colour information is useful for reliable recognition of relevant objects.
Figure 1.
The spectrum of sunlight during daytime, sunset and late twilight, and starlight. Moonlight has a similar spectrum to sunlight. Data from Johnsen et al. [18].
3. Why vision should be colour blind at night
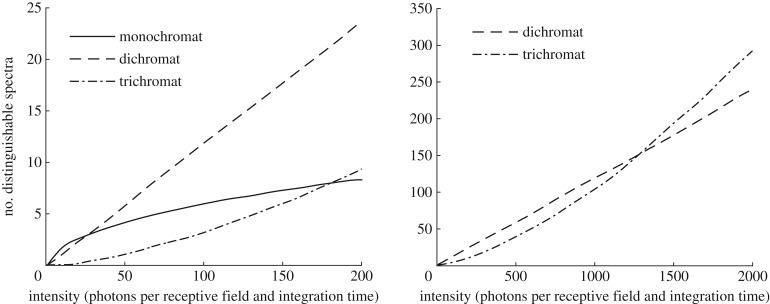
Things change when photons become too sparse, because noise increases. Colour discrimination is based on opponent interactions between receptor signals and is limited by receptor noise. Therefore, the number of discriminable colours is inversely correlated to the product of noise in the receptor types [19]. Noise levels, however, increase with decreasing light intensity. As photons (or quanta) of light are distributed randomly, photon shot noise, the uncertainty in an intensity measurement, is equivalent to √n, when, on average, n quanta reach the eye. The resulting signal-to-noise ratio √n, thus decreases with decreasing n, reducing the contrast sensitivity of the receptors. In very dim light, spontaneous activation of the phototransduction cascade by thermal energy adds further noise. This dark noise, often expressed as noise-equivalent dark light, differs strongly between photoreceptor classes and spectral sensitivity of the photopigment, and sets the absolute sensitivity limit of vision [20]. Estimated dark light is very low in insect photoreceptors [21], and equivalent to ≈0.01 photoisomerizations s−1 in human rods, but is in the range of ≈100 s−1 in human cones [22,23]. Comparing receptor signals in opponent channels instead of pooling signals, results in a lower signal-to-noise ratio given the same number of photons available. This will then lead to fewer distinguishable spectra (figure 2; [19] and see [24], for model equations).
Figure 2.
Number of discriminable colours, as function of the number of photons (the left graph shows a smaller range than the right graph) and the number of photoreceptors used for colour vision. Adapted from Vorobyev [19], with permission from the author.
Nocturnal animals have optical adaptations to increase photon capture [25]. Larger pupil diameters and shorter focal lengths result in a brighter retinal image, and photoreceptors with large volumes of visual pigment (large outer segments in vertebrate rods and cones, large rhabdoms in invertebrate rhabdomeric photoreceptors) allow for higher photon capture, but lead to higher levels of dark noise. The eyes of many nocturnal animals therefore have tapeta, mirror-like structures doubling the path length of light and increasing the probability of a photon being absorbed without adding noise. Finally, nonlinear synaptic transfer [26] and temporal and spatial summation of receptor signals [27] can improve signal reliability and signal-to-noise ratio on the neural level. Do animal eyes employ these adaptations to be able to use colour vision and avoid spectral pooling in dim light?
4. Evidence for colour vision in dim light
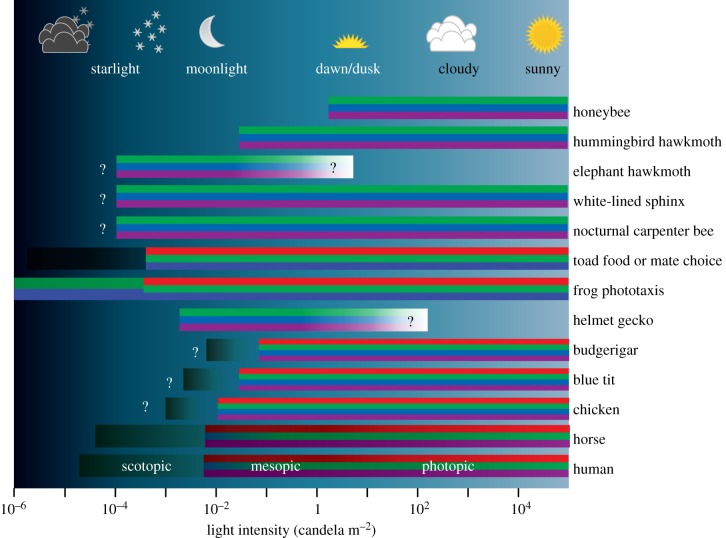
Large nocturnal insects such as hawkmoths have compound eyes with superposition optics [28] allowing light to reach the receptors in one ommatidium through several hundred facet lenses. Together with large facet lenses, wide rhabdoms and a tracheal tapetum, this adaptation has the effect that, at the same light intensity, a single photoreceptor in their eye absorbs over 4000 times as many photons as a photoreceptor in a honeybee's eye or the human retina [1]. Although they could improve absolute sensitivity further by expressing only a single long-wavelength-sensitive visual pigment, nocturnal and diurnal hawkmoths have three spectral types of photoreceptors with peak sensitivities around 350, 440 and 525 nm [29], and behavioural tests in three nocturnal species demonstrated their ability to use colour information even at dim starlight intensity levels (figure 3; [30]).
Figure 3.
Dim light colour vision and thresholds of species tested so far. Colours in the bars code for receptor types contributing to vision (red: peak sensitivity >550 nm, green: peak sensitivity 490–550 nm, blue: peak sensitivity 430–490 nm, purple: peak sensitivity <430 nm, grey indicates achromatic rod vision). Question marks indicate unknown thresholds. Honeybee Apis mellifera [14], hummingbird hawkmoth Macroglossum stellatarum [29], elephant hawkmoth Deilephila elpenor [30], white-lined sphinx Hyles lineata [30], nocturnal carpenter bee Xylocopa tranquebarica [31], common toads Bufo bufo and B. garganizans [32], common frog Rana temporaria [32], helmet gecko Tarentola chazaliae [33], budgerigar Melopsittacus undulatus [34], blue tit Cyanistes caeruleus [35], chicken Gallus gallus [36] and horse Equus caballus [37].
A large number of nocturnal insects, such as cockroaches, grasshoppers, beetles and ants have apposition compound eyes, and their sensitivity is restricted by the aperture of single ommatidial lenses [28]. So far, only one nocturnal species of carpenter bees has been shown to use colour cues for finding the nest entrance on very dark nights (figure 3; [31]). The sensitivity of a single photoreceptor of this bee is only ≈30 times higher than that of a human cone or honeybee receptor. As this bee has a very high spatial resolution (interommatidial angles less than or equal to 1°), we suggest that summation of receptor signals from many ommatidia, similar to summation of vertebrate rod signals [27], is one of the strategies allowing for this amazing ability.
Nocturnal colour vision is not restricted to insects. Nocturnal geckos, with pure cone retinae, were the first vertebrates shown to reliably discriminate colours in dim light (figure 3; [33]). The large pupil size and short focal length of their eyes, and their large outer segments, allow each of their rod-like cones to be ≈400 times as sensitive as a human cone [1].
5. Cone dark noise limits colour vision in vertebrates with duplex retinae
The animals mentioned so far use the same set of photoreceptors for colour vision at all light intensities. Very early in the evolution of vertebrates, however, a specialized type of photoreceptor for vision in dim light evolved: the rod [38]. Specific adaptations (see above) allow for reliable rod vision in about ≈100 times lower light intensities than cone vision [22,23]. The majority of mammal species are nocturnal, crepuscular or arrhythmic and have rod-dominated retinae. What are the consequences for colour discrimination in dim light?
Although horses, with dichromatic vision, have among the largest terrestrial eyes, they failed in behavioural colour discrimination tests at a very similar light intensity as that for which human subjects failed [37]. This is partly explained by the outer segments of horse cones being much shorter than those of human cones. In addition, while humans have a rod-free fovea in their otherwise rod-dominated retina, such an adaptation for colour vision does not exist in horses. In cats, the lower limit of the mesopic range has been estimated at ≈0.05 cd m−2 in physiological experiments ([8], corrected for pupil size, see [39]), thus higher than for humans and horses. Rather than using cone-based vision in dim light, horses, cats and likely the majority of mammals, use highly sensitive but colour-blind rod vision in dim light.
Unlike mammals, most birds have cone-dominated retinae with four spectral types of single cones used for colour vision, and many species (including raptors, passerines and even nocturnal owls) have rod-free foveae [40]. The absolute threshold of colour vision has been studied in four species of birds (figure 3): budgerigars and the more crepuscular Bourke's parrots, loose colour vision ≈0.1–0.4 cd m−2 [34]; blue tits at 0.05–0.2 cd m−2 [35]; and chickens between 0.025 and 0.08 cd m−2 [36]. These colour vision thresholds, which are up to 40 times higher than those of humans, may be due to the coloured oil droplets in the single cones of birds that absorb a substantial fraction of the light before it reaches the cone outer segments [41]. Oil droplet pigmentation in diurnal bird cones is beneficial for colour discrimination only at relatively bright light intensities [24].
In chickens, behaviourally measured thresholds indicate that photon shot noise impairs colour discrimination at intensities below ≈10 cd m−2, and likely at higher intensities for colours of low reflectance, and for smaller colour differences. Dark noise dominates at intensities below ≈0.1 cd m−2 and sets the absolute limit, not only for absolute sensitivity of the visual system [20], but also for colour discrimination [36]. The results also indicate that spatial summation of cone signals may rescue the signal-to-noise ratio in dim light.
6. Rod intrusion and rod-based colour vision
The ability of rods to contribute to colour vision has been discussed repeatedly, and experiments on humans indicate that in mesopic light intensities, the perceived saturation and hue of colours change as the result of rod intrusion [5]. It has been suggested that cone monochromats—species possessing rods and just one type of cone—may possess rod–cone-based colour vision but outside rare human subjects [42], no convincing behavioural evidence for this has been provided so far [43]. Retinal pathways receiving signals from both rod and cone signals are known, but only recently, in mice, a ganglion cell type mediating colour-opponent signals originating from rod and cone inputs has been identified [44]. However, as this study nicely demonstrates, the different sensitivity ranges of rods and cones, and temporal properties of rod and cone transduction, will make such a colour signal unreliable and thus difficult to interpret for tasks such as object detection by a moving animal. In summary, the function of rod–cone interactions in mammals may be related to improved visual capacity of animals with rod-dominated retinae in mesopic and photopic light intensities but does not extend colour vision to dimmer light levels.
The situation is different in amphibians. Common toads (Bufo bufo) and frogs (Rana temporaria) have large eyes with small F-numbers, rod-dominated retinae and a very low absolute visual threshold [45,46]. They differ from other vertebrates by having two spectral types of rods. Ninety per cent of the rods—the green-sensitive rods, often called ‘red rods’, according to their appearance in the dissected retina, see [47]—are homologous to the rods of other vertebrates and express an Rh1 pigment with peak sensitivity at 500 nm, but 10% of the rods (blue-sensitive rods, called ‘green rods’) express an SWS2 pigment [48] with the same spectral sensitivity (peaking at 430 nm) as the SWS1 pigment in their blue-sensitive cones [49].
Yovanovich et al. [32] have recently tested the intensity threshold of colour discrimination of Bufo sp. and R. temporaria by performing a set of three experiments, using three different behavioural contexts—mate choice, foraging and phototaxis (figure 3). Male toads (Bufo gargarizans) used colour for mate choice, and hungry toads (B. bufo) used colour at light levels of ≈10−4 cd m−2, about 10 times lower than the human threshold. In even dimmer light, the same toads continued making choices—they tried to mate, or snapped at the stimuli—but their choices were random [32] (this issue). Hungry frogs (R. temporaria) used achromatic cues in their choice of stimuli (that mimicked prey). However, in the phototaxis experiment, given the choice between a blue window, a green window and two dark windows, frogs (R. temporaria) behaved differently. At intensities higher than ≈10−4 cd m−2, they preferred to jump towards the blue window when it emitted the same number of photons visible for the green-sensitive rods as the green window, but, in addition, also emitted photons visible to the blue-sensitive rods. However, at lower intensities, down to their absolute visual threshold at ≈10−6 cd m−2, they preferred the green to the blue window, providing unequivocal evidence for an opponent mechanism that compares signals from both rod types, thus indicating rod-based colour vision [32].
We have no evidence that toads use such rod-based colour vision for mate or food choice, and frogs seem not to use colour for food choice even in bright light. Thus, this set of experiments confirms, once again, that different behavioural tasks recruit different visual pathways.
7. Outlook
Colour is a reliable cue to the identity of objects such as mates, food items or predators, but as vision is less reliable in dim light owing to high noise levels, nocturnal animals and animals living in generally dark habitats such as the deep sea rely, to a larger degree than diurnal animals, on senses other than vision. Insects, which possess photoreceptors with extremely low dark noise levels, and vertebrates without rods can make use of colour information, given they have highly sensitive eyes and neural mechanisms for spatial summation. Nocturnal vertebrates with rod-dominated retinae loose colour discrimination at brighter light levels, in favour of rod vision, whereas frogs can use rod-based colour vision to control phototaxis. Otherwise, the range of animals that use colour information in very dim light is probably small and restricted to those that do not have a duplex retina.
Acknowledgements
Fruitful discussions with Olle Lind, Miriam Henze and Misha Vorobyev over many years have shaped the ideas for this review, and without Hema Somanathan's, Lina Roth's and Anna Balkenius' patience, many of the reviewed results would not exist.
Authors' contributions
A.K. has taken the initiative to this review, all authors have contributed to it to substantial degrees and given final approval of the version to be published. None of the authors have any relationship to the guest editors that impedes the independence of either side.
Competing interests
We have no competing interests.
Funding
Funding from the Swedish Research Council and the K&A Wallenberg Foundation is gratefully acknowledged.
References
- 1.Kelber A, Lind O. 2010. Limits of colour vision in dim light. Ophthal. Physiol. Opt. 30, 454–459. ( 10.1111/j.1475-1313.2010.00721.x.) [DOI] [PubMed] [Google Scholar]
- 2.Helmholtz H. 1867. Handbook of physiological optics. Leipzig, Germany: Voss. [Google Scholar]
- 3.Schneider N, Campenhausen CV. 1998. Color and lightness constancy in different perceptual tasks. Biol. Cybern. 79, 445–455. ( 10.1007/s004220050494) [DOI] [PubMed] [Google Scholar]
- 4.Shin JC, Yaguchi H, Shiori S. 2004. Change of color appearance in photopic, mesopic and scotopic vision. Opt. Rev. 11, 265–271. ( 10.1007/s10043-004-0265-2) [DOI] [Google Scholar]
- 5.Cao D, Pokorny J, Smith VC, Zele AJ. 2008. Rod contributions to color perception: linear with rod contrast. Vision Res. 48, 2586–2592. ( 10.1016/j.visres.2008.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purkinje J. 1825. Beobachtungen und Versuche zur Physiologie der Sinne, zweites Bändchen, neue Beiträge zur Kenntnis des Sehens in subjektiver Hinsicht. Berlin, Germany: Reimer. [Google Scholar]
- 7.Himstedt F, Nagel WA. 1901. Die Verteilung der Reizwerte für die Froschnetzhaut im Dispersionsspektrum des Gaslichtes mittels der Aktionsströme untersucht. Ber. Physiol. Ges. Freiburg 11, 153–162. [Google Scholar]
- 8.Hammond P, James CR. 1971. The Purkinje shift in cat: extent of the mesopic range. J. Physiol. 216, 99–109. ( 10.1113/jphysiol.1971.sp009511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver PH. 1966. A Purkinje shift in the spectral sensitivity of grey squirrels. J. Physiol. 186, 439–450. ( 10.1113/jphysiol.1966.sp008045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruber S. 1977. The visual system of sharks: adaptations and capability. Am. Zool. 17, 453–469. ( 10.1093/icb/17.2.453) [DOI] [Google Scholar]
- 11.Fingerman M, Brown FA. 1952. A ‘Purkinje shift’ in insect vision. Science 116, 171–172. ( 10.1126/science.116.3007.171) [DOI] [PubMed] [Google Scholar]
- 12.Strausfeld NJ, Lee J-K. 1991. Neuronal basis for parallel visual processing in the fly. Vis. Neurosci. 7, 13–33. ( 10.1017/S0952523800010919) [DOI] [PubMed] [Google Scholar]
- 13.Schnaitmann C, Gerbers C, Wachtler T, Tanimoto H. 2013. Color discrimination with broadband photoreceptors. Curr. Biol. 23, 2375–2382. ( 10.1016/j.cub.2013.10.037) [DOI] [PubMed] [Google Scholar]
- 14.Menzel R. 1981. Achromatic vision in the honeybee at low light intensities. J. Comp. Physiol. A 141, 389–393. ( 10.1007/BF00609941) [DOI] [Google Scholar]
- 15.Kelber A, Osorio D. 2010. From spectral information to animal colour vision: concepts and terminology. Proc. R. Soc. B 277, 1617–1625. ( 10.1098/rspb.2009.2118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohta Y-I, Kanade T, Sakai T. 1980. Color information for region segmentation. Comput. Graphics Image Process. 13, 222–241. ( 10.1016/0146-664X(80)90047-7) [DOI] [Google Scholar]
- 17.Campenhausen Cv. 1986. Photoreceptors, lightness constancy and color vision. Naturwissenschaften 73, 674–675. ( 10.1007/BF00366692) [DOI] [PubMed] [Google Scholar]
- 18.Johnsen S, Kelber A, Warrant EJ, Sweeney AM, Widder EA, Lee RL, Hernandez-Andres J. 2006. Crepuscular and nocturnal illumination and its effects on color perception by the nocturnal hawkmoth Deilephila elpenor. J. Exp. Biol. 209, 789–800. ( 10.1242/jeb.02053) [DOI] [PubMed] [Google Scholar]
- 19.Vorobyev M. 1997. Cost and benefits of increasing the dimensionality of colour vision systems. In Biophysics of photoreception: molecular and phototransductive events (ed. Taddei-Ferreti C.), pp. 280–289. Singapore: World Scientific. [Google Scholar]
- 20.Barlow HB. 1956. Retinal noise and absolute threshold. J. Opt. Soc. Am. 46, 634–639. ( 10.1364/JOSA.46.000634) [DOI] [PubMed] [Google Scholar]
- 21.Laughlin SB, Lillywhite PG. 1982. Intrinsic noise in locust photoreceptors. J. Physiol. 332, 25–45. ( 10.1113/jphysiol.1982.sp014398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donner K. 1992. Noise and the absolute thresholds of cone and rod vision. Vision Res. 32, 853–866. ( 10.1016/0042-6989(92)90028-H) [DOI] [PubMed] [Google Scholar]
- 23.Fain GL, Hardie R, Laughlin SB. 2010. Phototransduction and the evolution of photoreceptors. Curr. Biol. 20, R114–R124. ( 10.1016/j.cub.2009.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toomey MB, et al. 2016. Complementary shifts in photoreceptor spectral tuning unlock the full adaptive potential of ultraviolet vision in birds. Elife 5, e15675 ( 10.7554/eLife.15675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warrant EJ. 2004. Vision in the dimmest habitats on Earth. J. Comp. Physiol. A 190, 765–789. ( 10.1007/s00359-004-0546-z) [DOI] [PubMed] [Google Scholar]
- 26.Field GD, Rieke F. 2002. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron 34, 773–785. ( 10.1016/S0896-6273(02)00700-6) [DOI] [PubMed] [Google Scholar]
- 27.Barlow HB. 1958. Temporal and spatial summation in human vision at different background intensities. J. Physiol. 141, 337–350. ( 10.1113/jphysiol.1958.sp005978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Land MF, Nilsson D-E. 2012. Animal eyes. 2nd edn New York, NY: Oxford University Press. [Google Scholar]
- 29.Kelber A, Balkenius A, Warrant EJ. 2003. Colour vision in diurnal and nocturnal hawkmoths. Integr. Comp. Biol. 43, 571–579. ( 10.1093/icb/43.4.571) [DOI] [PubMed] [Google Scholar]
- 30.Kelber A, Balkenius A, Warrant EJ. 2002. Scotopic colour vision in nocturnal hawkmoths. Nature 419, 922–925. ( 10.1038/nature01065) [DOI] [PubMed] [Google Scholar]
- 31.Somanathan H, Borges RM, Warrant EJ, Kelber A. 2008. Nocturnal bees learn landmark colours in starlight. Curr. Biol. 18, R996–R997. ( 10.1016/j.cub.2008.08.023) [DOI] [PubMed] [Google Scholar]
- 32.Yovanovich CAM, Koskela SM, Nevala N, Kondrashev SL, Kelber A, Donner K. 2017. The dual rod system of amphibians supports colour discrimination at the absolute visual threshold. Phil. Trans. R. Soc. B 372, 20160066 ( 10.1098/rstb.2016.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth LSV, Kelber A. 2004. Nocturnal colour vision in geckos. Proc. R. Soc. Lond. B 271(Suppl), S485–S487. ( 10.1098/rsbl.2004.0227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lind O, Kelber A. 2009. The intensity threshold of colour vision in two species of parrot. J. Exp. Biol. 212, 3693–3699. ( 10.1242/jeb.035477) [DOI] [PubMed] [Google Scholar]
- 35.Gomez D, Gregoire A, Del Rey Granado M, Bassoul M, Degueldre D, Perret P, Doutrelant C. 2014. The intensity threshold of colour vision in a bird, the blue tit. J. Exp. Biol. 217, 3775–3778. ( 10.1242/jeb.107573) [DOI] [PubMed] [Google Scholar]
- 36.Olsson P, Lind O, Kelber A. 2015. Bird colour vision: behavioural thresholds reveal underlying receptor noise. J. Exp. Biol. 218, 184–193. ( 10.1242/jeb.111187) [DOI] [PubMed] [Google Scholar]
- 37.Roth LSV, Balkenius A, Kelber A. 2008. The absolute threshold of colour vision in the horse. PLoS ONE 3 e3711, 1–6. ( 10.1371/journal.pone.0003711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morshedian A, Fain GL. 2015. Single-photon sensitivity of lamprey rods with cone-like outer segments. Curr. Biol. 25, 484–487. ( 10.1016/j.cub.2014.12.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilcox JG, Barlow HB. 1975. Size and shape of pupil in lightly anesthetized cats as a function of luminance. Vision Res. 15, 1363–1365. ( 10.1016/0042-6989(75)90191-1) [DOI] [PubMed] [Google Scholar]
- 40.Coimbra JP, Collin SP, Hart NS. 2014. Topographic specializations in the retinal ganglion cell layer of Australian passerines. J. Comp. Neurol 522, 3609–3628. ( 10.1002/cne.23624) [DOI] [PubMed] [Google Scholar]
- 41.Wilby D, Toomey MB, Olsson P, Frederiksen R, Cornwall MC, Oulton R, Kelber A, Corbo JC, Roberts NW. 2015. Optics of cone photoreceptors in the chicken (Gallus gallus domesticus). J. R. Soc. Interface 12, 20150591 ( 10.1098/rsif.2015.0591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reitner A, Sharpe L, Zrenner E. 1991. Is colour vision possible with only rods and blue-sensitive cones? Nature 352, 798–800. ( 10.1038/352798a0) [DOI] [PubMed] [Google Scholar]
- 43.Scholtyssek C, Kelber A, Dehnhardt G. 2015. Why do seals have cones? Behavioural evidence for colour-blindness in harbour seals. Anim. Cogn. 18, 551–560. ( 10.1007/s10071-014-0823-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joesch M, Meister M. 2016. A neuronal circuit for colour vision based on rod–cone opponency. Nature 532, 236–239. ( 10.1038/nature17158) [DOI] [PubMed] [Google Scholar]
- 45.Aho A-C, Donner K, Hydén C, Larsen LO, Reuter T. 1988. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature 334, 348–350. ( 10.1038/334348a0) [DOI] [PubMed] [Google Scholar]
- 46.Aho A-C, Donner K, Helenius S, Larsen LO, Reuter T. 1993. Visual performance of the toad (Bufo bufo) at low light levels: retinal ganglion cell responses and prey-catching accuracy. J. Comp. Physiol. A 172, 671–682. ( 10.1007/BF00195393) [DOI] [PubMed] [Google Scholar]
- 47.Govardovskii V, Reuter T. 2014. Why do green rods of frog and toad retinas look green? J. Comp. Physiol. A 200, 823–835. ( 10.1007/s00359-014-0925-z) [DOI] [PubMed] [Google Scholar]
- 48.Hisatomi O, Kayada S, Taniguchi Y, Kobayashi Y, Satoh T, Tokunaga F. 1998. Primary structure and characterization of a bullfrog visual pigment contained in small single cones. Comp. Biochem. Phys. B 119, 585–591. ( 10.1016/S0305-0491(98)00032-7) [DOI] [PubMed] [Google Scholar]
- 49.Koskelainen A, Hemilä S, Donner K. 1994. Spectral sensitivities of short- and long-wavelength sensitive cone mechanisms in the frog retina. Acta Physiol. Scand. 152, 115–124. ( 10.1111/j.1748-1716.1994.tb09790.x) [DOI] [PubMed] [Google Scholar]