Abstract
North Atlantic right whales (Eubalaena glacialis) feed during the spring and early summer in marine waters off the northeast coast of North America. Their food primarily consists of planktonic copepods, Calanus finmarchicus, which they consume in large numbers by ram filter feeding. The coastal waters where these whales forage are turbid, but they successfully locate copepod swarms during the day at depths exceeding 100 m, where light is very dim and copepod patches may be difficult to see. Using models of E. glacialis visual sensitivity together with measurements of light in waters near Cape Cod where they feed and of light attenuation by living copepods in seawater, we evaluated the potential for visual foraging by these whales. Our results suggest that vision may be useful for finding copepod patches, particularly if E. glacialis searches overhead for silhouetted masses or layers of copepods. This should permit the whales to locate C. finmarchicus visually throughout most daylight hours at depths throughout their foraging range. Looking laterally, the whales might also be able to see copepod patches at short range near the surface.
This article is part of the themed issue ‘Vision in dim light’.
Keywords: right whale, visual sensitivity, contrast vision, environmental radiometry
1. Introduction
North Atlantic right whales (Eubalaena glacialis) rank among the most impressive animals on Earth, reaching lengths exceeding 18 m and masses near 100 000 kg. The three species of right whales, along with the bowhead whale, are assigned to the family Balaenidae, possibly the earliest-branching taxon of the Cetacea [1]. Unlike other baleen whales (mysticetes), whose predatory behaviours include lunges (e.g. [2]) and sometimes the use of bubble nets to corral prey [3], right whales use their forward motion to ram filter feed [4]. They open their mouths to form a large and sophisticated whale-powered plankton net, swimming forward and filtering out zooplankton from seawater exiting the mouth through their baleen plates. When at the surface, this is called ‘skim feeding’.
Such feeding demands significant energy, which limits speed and thus the choice of prey to creatures that cannot easily evade the approaching whale [4]. Eubalaena glacialis specializes on small crustaceans, primarily the copepod Calanus finmarchicus. The whales' energetic demands require that they filter copepod patches of unusually high density in the highly productive waters in the northwestern Atlantic Ocean during their springtime residence [5]. Eubalaena glacialis unfailingly locates these [6] and can track the vertical migrations of its prey, often feeding at depths exceeding 100 m [5,7]. Diving is energetically costly and time-limited, so the whales must consistently locate prey just to recover the cost of the dive itself. If copepod distributions permit it, they conserve energy by feeding at or near the surface. Finding food probably involves both knowing how local oceanographic and hydrographic features concentrate copepod stocks and using specialized sensory systems to locate smaller-scale patches that contain the highest copepod densities (reviewed in [4]).
Right whales apparently earned their common name by being the ‘right whales’ for early whalers to hunt. They forage at slow speeds near coasts, often on the surface, and their bodies are rich in baleen and blubber. The same characteristics that placed them at the mercy of whalers endanger them from modern threats, especially in northwestern Atlantic waters. They live in some of the most active sea lanes in North America, they feed in shallow waters where boat traffic is heavy, and they feed where fixed fishing gear is abundant. Together with their tendency to linger on the surface and their limited swimming speeds, this puts them at constant risk of injury or death from ship strikes, collision with smaller boats, and entanglement in fishing gear. Such frequent interactions with humans and their numerous environmental impacts encouraged its researchers to dub E. glacialis ‘the urban whale’ [8].
Unlike toothed whales, mysticetes do not echolocate (see [9]), but some species use vision to locate prey [2]. We thus set out to learn whether E. glacialis could sight concentrations of C. finmarchicus at the depths at which they feed. To do this, we needed to know the visual sensitivity of the whales, the light available at feeding depths and the visual contrast of copepod patches against the background light.
Like most mysticetes, balaenids are rod monochromats [10–14]. Otherwise, their eyes are of typical mammalian design, resembling somewhat enlarged bovine eyes. Expression analysis of E. glacialis rod visual pigment places its peak wavelength of absorption at 493 nm [15,16], rather short for a coastal species [15,17]. Combining the visual pigment data with eye measurements available from other balaenids, we estimated the absolute spectral sensitivity of E. glacialis. Together with measurements of spectral attenuation by live C. finmarchicus and of the photic properties of water in locations where E. glacialis feeds, we evaluated the potential for the whales to sight copepod concentrations at feeding depths. Our results suggest that vision may indeed be used by these animals to find prey, particularly if they locate copepod aggregations by looking upwards.
2. Material and methods
(a). Estimation of visual spectral sensitivity
We used Land's [18,19] equation for sensitivity, which requires pupil diameter, lens focal length, photoreceptor (rod outer segment) diameter and length, and the rod visual pigment absolute absorbance spectrum. Of these, only the visual pigment's relative absorbance spectrum is known for E. glacialis [16], and no eyes sufficiently well preserved for dimensional measurements were available. However, published data from the related and similarly sized balaenids E. australis (southern right whale) and Balaena mysticetus (bowhead whale) [14,20,21] provide reasonable estimates for the ocular properties of E. glacialis. The methods used to estimate these measurements and associated data are described in the electronic supplementary material.
(b). Copepod spectral absorption and contrast
Live copepods, predominantly the fifth copepodite stage of C. finmarchicus, were gently collected with plankton nets in the Great South Channel, a springtime right whale habitat, kept chilled and shipped to Baltimore for measurements of spectral attenuation. The attenuations over a range of copepod concentrations were analysed in a cylindrical spectrophotometer cell 10 cm in length. Further details are available in the electronic supplementary material.
(c). Radiometric measurements in the field
Underwater spectral radiometry was done from the research vessels R/V Tioga and the NOAA Ship Gordon Gunter. At each station, three replicate casts were made with a free-falling Profiler II instrument (Satlantic, Halifax, Nova Scotia) equipped to measure spectral profiles of down-welling irradiance and horizontal radiance (348–802 nm at approx. 3 nm intervals). Measurements were averaged into 4 m deep bins beginning at the surface (giving averages at depths of 2 m, 6 m, 10 m, etc.). Four series at separate locations within right whale foraging habitats obtained measurements either to the bottom or to depths where readings were indistinguishable from the electronic noise of the instruments (typically approx. 40–70 m).
Two series were completed on 4 April 2012, at locations in Wilkinson Basin (42°23′ N, 69°86′ W; solar elevation approx. 47°) and near the entrance to Cape Cod Bay (42°07′ N, 70°27′ W; solar elevation approx. 23°). Two additional series were completed in May 2013, both in the Great South Channel southeast of Cape Cod (8 May: 41°15′ N, 69°18′ W; solar elevation approx. 58°; 9 May: 41°19′ N, 69°19′ W; solar elevation approx. 30.5°). Measurements occurred on afternoons (approx. 12.00 to approx. 17.00 local time) of days that were clear (2012) to overcast or with thin cloud cover (2013). All readings were interpolated into 1 nm intervals, 350–800 nm, and converted to photon fluxes for further use. As the Satlantic instruments were not configured to measure downward radiance, we also used an earlier dataset from a site near Cape Cod Bay off the coast of New Hampshire [22]. In that study, conducted at noon on 30 June 2000, downward radiance and horizontal radiance towards and away from the solar direction were computed at 5 m intervals.
3. Results and discussion
(a). Estimation of visual spectral sensitivity
The optical sensitivity (S) of receptors in an eye in photon units (n photons absorbed per receptor at a given radiance R, or n/R) can be found using the equation [18,19]
| 3.1 |
Here, λ is wavelength, D is pupil diameter, Δρ is the photoreceptor acceptance angle (radians) and Pabs is the proportion of photons absorbed by a photoreceptor (a function of λ). Pabs (absorptance) is calculated from total absorbance, the product of the absorbance per unit length (α) at wavelength λ and photoreceptor length (l). For rod-sized photoreceptors with diameter d in an eye of focal length f, Δρ2 is (d/f)2 in steradians (sr), so S is described in units of photons absorbed multiplied by square centimetres and steradians. Multiplying this by radiance (photons s−1 cm−2 nm−1 sr−1) gives photons absorbed per rod per second per nanometre. After substitution, equation (3.1) becomes
| 3.2 |
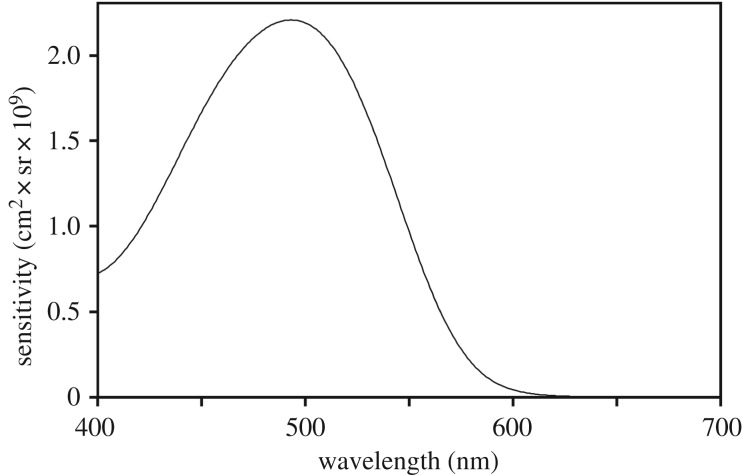
Thus, calculating the optical sensitivity of the E. glacialis eye requires pupil diameter, focal length and photoreceptor length and diameter. No material from E. glacialis was available for any of these measurements, so we used light and electron micrographs of B. mysticetus (bowhead) rods from [14], from which we estimated rod length to be 30 µm and diameter as 1.4 µm. We used published data from E. australis [20] to approximate the optical properties of eyes of E. glacialis, comparing the results with data from B. mysticetus [21] to check their validity (electronic supplementary material, tables S1 and S2). Using the E. australis results and the absorptance spectrum for a 30 µm rod based on an absorbance of 0.015 per µm at the 493 nm peak [23] and a standard visual pigment template for a 493 nm rhodopsin [24], we computed sensitivity at 1 nm intervals from 400 to 700 nm (figure 1). These measures of optical sensitivity do not include light lost during its passage through the ocular media or reflected by the tapetum, which we consider later on.
Figure 1.
Optical sensitivity spectrum of one Eubalaena glacialis rod photoreceptor. This curve is calculated using the sensitivity of the eye of E. australis (electronic supplementary material, table S1) multiplied by the absorptance spectrum of E. glacialis rod photopigment (λmax = 493 nm) from [16], assuming a rod length of 30 µm and a peak absorbance of 0.015 µm−1. See the text for details.
(b). Copepod light attenuation
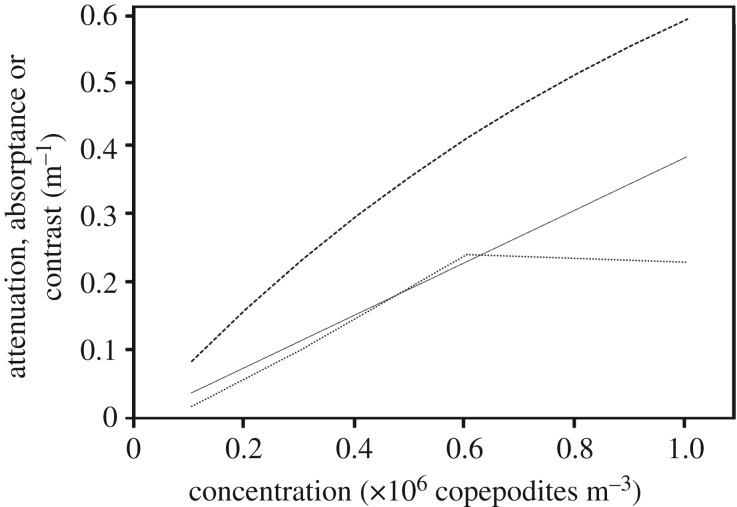
If right whales use vision to forage, copepods obviously must be visible to them. Individual C. finmarchicus are probably too small to see, but compact, dense copepod patches could form visible regions of contrast against the background radiance. Our attenuation measures (electronic supplementary material, figure S1, raw data, dotted line) were from a 10 cm spectrophotometer cell. For a 1 m path length (in other words, the same numbers of copepods but in a 1 m long cell), they correspond to attenuations for natural concentrations of 0.1, 0.3, 0.6 and 1.0 ml−1 (105–106 copepods m−3). Natural densities of copepods in locations where E. glacialis whales forage range from approximately 104–106 per cubic metre [5,6]. A curve was fitted to the mean spectral attenuation at the three lowest concentrations, forced to pass through the origin (figure 2, thin solid line). This fitted curve was converted to absorptance, the fraction of light absorbed (figure 2, dashed curve).
Figure 2.
Light absorption and visual contrast of living fifth copepodites of Calanus finmarchicus in seawater. Dotted curve: attenuation versus concentration (averages of the attenuation spectra electronic supplementary material, figure S1). Thin straight line: linear fit to average concentration data (through a concentration of 0.6 copepodites m−3) forced to pass through the origin y = 0.0386x. Dashed curve: absorptance (proportion of light removed) corresponding to the fitted curve, which is identical to the Weber contrast of copepod patches viewed at very short distances over this range of concentrations.
The Weber contrast of a copepod patch against the unobstructed background radiance is
| 3.3 |
where Nb is number of photons detected by retinal photoreceptors from the background radiance, and Np is the number of photons detected from light at the location of the copepod patch. (Weber contrast usually takes both positive and negative values, but we used absolute Weber contrast here because minimum contrast sensitivity concerns only the level, not the sign, of the contrast.) Both N values vary with the numbers of receptors involved per unit ‘pixel’ of the scene and their integration times, and of course with the actual spectra and radiances viewed. At zero range, for basically flat attenuation spectra like those through patches of copepods (electronic supplementary material, figure S1) and considering only attenuation, C is equal to patch absorptance (i.e. C ∼ 1 – Np/Nb; figure 2). Minimum contrast sensitivities (contrast thresholds) for visual systems across animals range from 0.01 to 0.05 [25], where the lower limit is probably set by photoreceptor and neural noise. Critically, there must also be enough light available to make detection of such a contrast statistically possible [18,19]. Light measurements in E. glacialis foraging habitats provided the data needed to compute photon detection rates and contrasts.
(c). Radiometric measurements in the field and Eubalaena glacialis photon detection rates
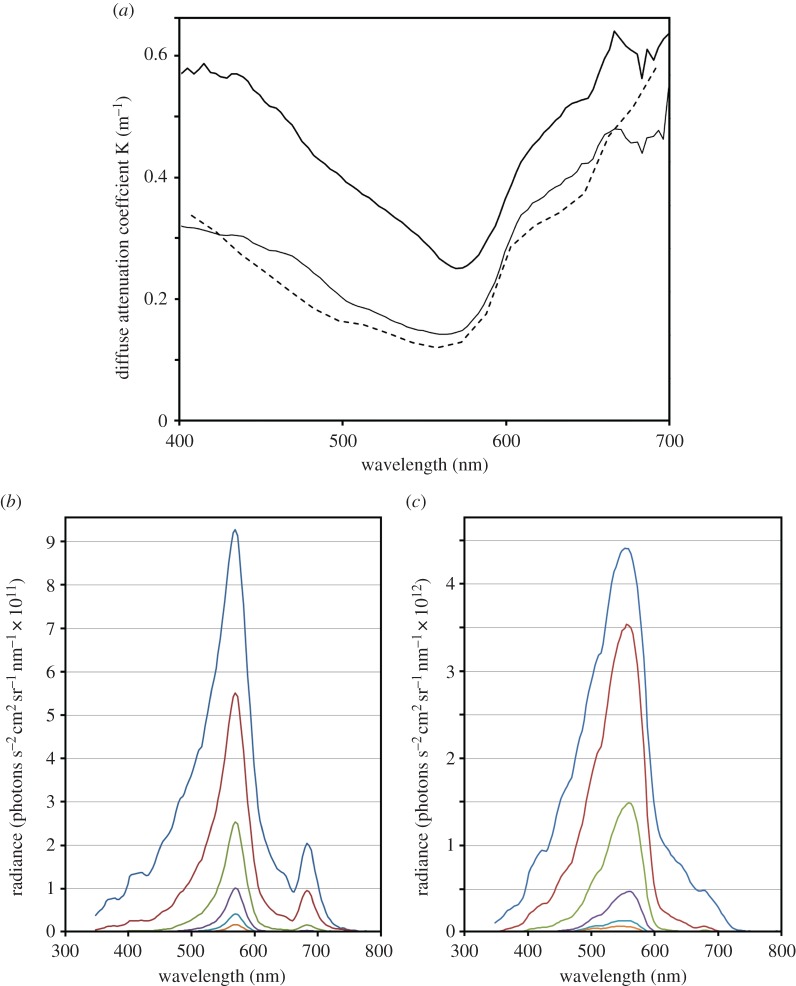
Peak primary production in the ocean near Cape Cod occurs during the late winter and early spring. Whales arrive a month or two later, when the copepod prey are well fed and often diapausing at depths of hundreds of metres [4]. Depth profiles of down-welling irradiance collected in E. glacialis feeding habitats were used to calculate diffuse attenuation coefficients, to characterize the waters there. Figure 3a shows these for the most turbid water (Wilkinson Basin, 4 April 2012) and the clearest water (Great South Channel, 8 May 2013) we encountered (figure 3a also shows attenuation coefficients from [22] discussed later). Waters where right whales forage are typical of coastal regions [26], with elevated light attenuation and peak transmission of green/yellow light (figure 3). Such conditions restrict visual foraging, not only because light levels are low, but also because these waters contain plankton and other suspended solids. Seeing through such water can be challenging. Horizontal radiance profiles for the Wilkinson Basin (figure 3b) and the Great South Channel series (figure 3c) are dominated by spectrally narrow, green/yellow light. Waters like these seem ill suited for visual foraging far below the surface, particularly because the spectral sensitivity of E. glacialis is poorly matched to the light it views at depths below just a few metres (compare figures 1 and 3).
Figure 3.
Photic properties of waters where E. glacialis feeds. (a) Thin solid curve: diffuse attenuation coefficients for the clearest water measured (Great South Channel, 8 May 2013). Thick solid curve: diffuse attenuation spectrum of the least clear water measured (Wilkinson Basin, 4 April 2012). Dashed curve: diffuse attenuation spectrum of waters off Portsmouth, New Hampshire (30 June 2000) measured in [22]. (b) Horizontal radiance spectra from Wilkinson Basin series averaged over 4 m depth ranges plotted for centre depths of 2, 6, 10, 14, 18 and 22 m. (c) Horizontal radiance spectra from the Great South Channel series averaged over 4 m depth ranges plotted for centre depths of 2, 6, 10, 14, 18 and 22 m.
For a North Atlantic right whale viewing a scene horizontally over the depth ranges where we measured horizontal radiance, the rate of photon capture is simply the product of the rod's sensitivity (figure 1) and the radiance at that depth, summed over the full sensitivity spectrum of the visual pigment in the retina (here we used 400–700 nm, because it encompassed essentially the full range of available light). Photon capture rates must be corrected for the integration time of a rod. When dark-adapted, rods have long integration times, approaching 2 s in some ectothermic vertebrates [25,27,28], but mammalian rod integration times are certainly shorter. We used 1 s integration times in our calculations (note that photon radiances were also measured per second). We then corrected for transmission loss through the eye (80% transmission, [29]) and the quantum efficiency for rhodopsin (0.69, [30]) to get actual photon detections per rod per 1 s integration time. However, we ignored tapetal reflection. Mysticete whales have well-developed and extensive tapeta [31,32], which reflect significant quantities of photons back through the retina. Potentially, this could increase photon absorption by 50% or more, but without actual measurements, we decided to ignore this effect.
In the coastal waters off Cape Cod, photon detection rates decrease rapidly with depth (electronic supplementary material, figure S2). Average rates reach one photon per second per rod at depths as shallow as approximately 35 m in Wilkinson Basin. While the photon detection rates we calculated depend on a number of assumptions, their rapid decrease with depth means that moderate errors in our assumptions (e.g. rod integration times, tapetal effects) have relatively minor effects on the outcomes of our calculations.
(d). Contrast vision and visual foraging by Eubalaena glacialis
We can now evaluate contrast vision. Owing to the statistics of the arrival rates of photons at single receptors, the minimum possible detectable Weber contrast (Cmin) of a visual object against background is equal to 2.77 over the square root of the average number of photons detected in one integration time (N) of the photoreceptors involved [29]
| 3.4 |
Using photon detection rates (electronic supplementary material, figure S2), we computed minimum discriminable contrast versus depth. Figure 4 shows results for sites with the lowest rates (Wilkinson Basin) and the greatest ones (Great South Channel). In horizontal radiance, rod photon capture rates sufficient to support a conservative mammalian contrast threshold (0.05) exist only down to approximately 12 m in Wilkinson Basin (figure 4a) and approximately 30 m in the Great South Channel (figure 4b). But rods do not act alone; in mammalian retinas commonly 1000 or more rods summate onto a single luminance-detecting retinal ganglion cell (RGC) [28,29,33]. Cetaceans in particular have exceptionally large RGCs called ‘giant retinal ganglion cells’ which probably acquire input from many rods [31,32]. Rod convergence ratios have never been directly measured in cetaceans, but RGC densities and distributions are known for many cetacean retinas [31,32] (in particular for two mysticetes, the minke whale [34] and the grey whale [35]), and rod densities are available for a few toothed whale species [12]. Like other cetaceans [31], minke and grey whales have two areas of elevated RGC density, in the temporal and nasal retinas (sampling forward-looking and lateral vision, respectively) [34,35]. Calculated rod : RGC ratios imply that many, many rods converge onto single RGCs in these whales, even in their regions of best vision. The current mammalian champion for convergence ratios is the highly nocturnal owl monkey, where between 800 and 10 000 rods converge per RGC, varying with retinal region [36]. Data from [12,34,35] suggest that mysticete whales reach similar ratios, approaching 2000 : 1 in area centralis regions in the temporal and nasal retina and perhaps 8000 : 1 in the periphery. These levels of summation limit spatial resolution, but might permit whales to see dim, fuzzy, low-contrast objects like plankton clouds well below the surface during the day.
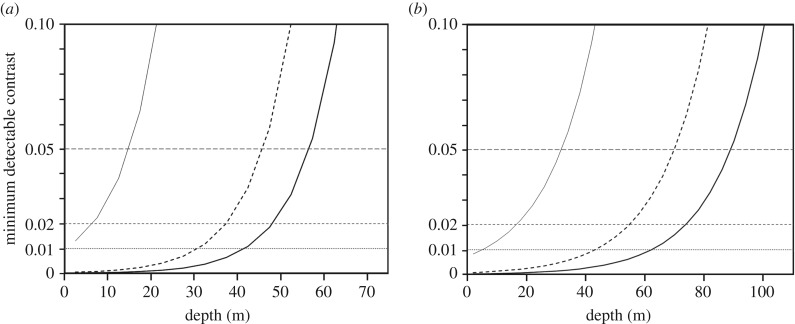
Figure 4.
Calculated minimum detectable contrast for extended objects viewed horizontally at approximately 0 m range versus depth for E. glacialis in (a) the most turbid waters we measured (Wilkinson Bay) and (b) the clearest waters we measured (Great South Channel). In both panels, thin solid lines plot trended data (based on photon absorption rates from trends in electronic supplementary material, figure S2) for contrast detectable by single rods; dashed curves are calculated from trended data for pools of 100 rods; and thick solid curves are calculated from trended data for pools of 1000 rods. Horizontal lines show minimum detectable contrasts for thresholds of 0.05, 0.02 and 0.01. Portions of the curve that are below each horizontal line correspond to depths at which there is sufficient light to view contrasts at or above the corresponding threshold.
Based on summations of 100 or 1000 rods, we recalculated contrast:depth relationships for right whale vision (figure 4). When 100 rod signals are summed, whales potentially could sight objects with contrast of 0.05 down to approximately 45 m depth even in the gloomy photic conditions of Wilkinson Basin (figure 4a) and to approximately 70 m in the Great South Channel (figure 4b). Summing 1000 rods per RGC extends these depths to approximately 55 and approximately 90 m, respectively. These are still well above the deepest foraging depths, which can exceed 150 m [5]. Given these results, is visual foraging likely over much of the whales' springtime feeding depth range in the western Gulf of Maine?
Unfortunately, we do not know precisely how copepods are distributed on fine spatial scales, nor the geometry of high-density micropatches found within the larger copepod swarms. If copepods are fairly evenly distributed within their preferred depth range during the day, there is little for a right whale to see, because it surely does not image individual plankton, and the overall haze produced by the copepod clouds fills the visual field. Perhaps, right whales look for contrasting patches of unusually high local density. If these exist, they are most useful to the whale if they are sufficiently small to spot against the background light passing through the lower-density regions of the swarm. Figure 2 suggests that for a patch thickness of 1 m, Weber contrast is above 0.05 for all copepod concentrations above 105 m−3. Such a contrast is only visible at ranges within fractions of a metre—probably not relevant to a whale. At greater distances, the intervening turbidity will quickly introduce veiling light, which will obscure the dim patch behind it.
Another potential problem is that copepods do not just absorb and scatter light coming from behind them. They also scatter down-welling light arriving from the surface, and this laterally scattered light will reduce—and possibly even eliminate—the contrast they produce against the background. The down-welling radiance is so much brighter than the horizontal radiance (figure 5a) that even weak scattering in the direction of the whale's eye may obliterate the background contrast. Indeed, brightly scattering patches of plankton could themselves become prominent visual targets [37]. At this point, we do not have the measurements or images to determine just what a patch of copepods looks like underwater at any depth. And as already noted, we lack definitive descriptions of patches of copepods. Based on the results reported here, however, we suggest that visual foraging by whales looking laterally into the water column is likely to be most useful during skim feeding or at very shallow depths.
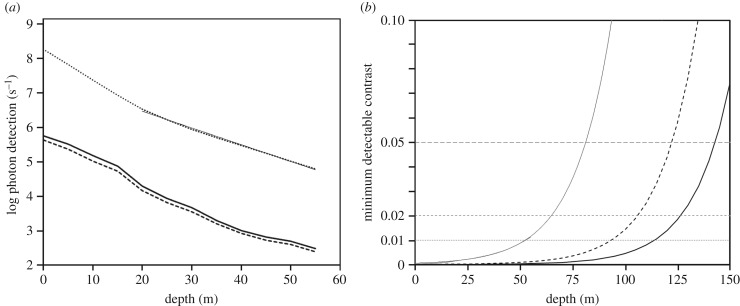
Figure 5.
(a) Calculated log photon detection for E. glacialis rods in waters off Portsmouth, New Hampshire [22]. Dark solid curve: horizontal viewing away from the Sun's azimuth. Dark dashed curve: horizontal viewing towards the sun's azimuth. Dotted curve: vertical viewing, looking upward (the thin straight line shows the log-linear fit to the data at depths below 20 m, used to compute contrasts plotted in (b); y = 27 400 000 e−0.0112x). (b) Calculated minimum detectable contrast for extended objects viewed vertically versus depth for E. glacialis looking vertically upward in the conditions illustrated in (a). Thin solid curve is calculated using trended data (photon catch rates given by the fit in (a)) for contrast detectable by single rods; dashed curve is calculated from trended data for pools of 100 rods; and thick solid curve is calculated from trended data for pools of 1000 rods. Horizontal lines show minimum detectable contrasts at thresholds of 0.05, 0.02 and 0.01; portions of the curve that are below each horizontal line correspond to depths at which there is sufficient light to view contrasts at or above the corresponding threshold.
This does not rule out visual foraging deeper down. Instead of looking horizontally, right whales could search overhead for copepods using their dorsal visual field. As forward scattering of light is included in our copepod attenuation measurements, we can reasonably accurately predict the downward light attenuation by any given concentration of copepods if we know patch dimensions. Even if the copepods are randomly dispersed, their lower average concentrations would be offset by the thickness of the horizontal pancake within which they exist, so the whale could visually experience something like a cloud passing in front of the down-welling light—which might be all it needs to localize the copepod mass. Work on zooplankton light absorption and scattering suggests that their visual predators should, in fact, look more laterally near the surface and more vertically at depth [37]. But for this to work for E. glacialis, the whale must monitor the overhead visual field, and we do not know if right whales do this.
Looking upwards is advantageous mostly because the downward-directed radiance is far brighter than the horizontal radiance and also because very little veiling light is added in this direction. We thus included data on such radiance collected in June 2000 at a location not far from Cape Cod, 80 km seaward from Portsmouth, New Hampshire [22]. The water quality at this location was similar to that of the Great South Channel in May 2013 (figure 2a). Viewing horizontal radiance (either towards or away from the sun) in these photic conditions, whale rods could detect photons with similar depth profiles for the 2000 and 2013 data (compare electronic supplementary material, figure S2 with figure 5a, dark solid and dashed lines). In comparison, the much brighter downward radiances produced photon detection rates some two orders of magnitude higher (figure 5a, dotted line). Using downward radiance, right whales may be able to spot cubic-metre-size, copepod patches with 100 000 or more copepods at depths of approximately 140 m by spatially summing 1000 rods (figure 5b). (If 8000 rods summate, a contrast of 0.05 is perceptible down to approx. 160 m, not shown.) In thick layers of copepods, even if they are randomly distributed, the contrast would nearly always be visible throughout the entire foraging depth range near Cape Cod. Thus, whales could easily detect patches with copepod densities known to occur at their foraging depths, at least in locations of fair water clarity. Densities and distributions of RGCs in ventral retinas of E. glacialis should be measured to learn whether this could be done even without postural changes by the whales.
Blue whales sight prey concentrations when swimming upwards, looking ahead and visually targeting patches of krill against surface light for a feeding lunge [2], so overhead monitoring by right whales should not be ruled out. It would be interesting to make depth casts of an upward-looking radiance metre coupled with an optical plankton recorder so that the effects of copepod layers on down-welling light could be monitored directly. It would also be revealing to attach releasable cameras (like the ‘Crittercams’ used with blue whales [38]) to foraging right whales. While the dim illumination could be problematic, it should be possible to monitor whether the whales occasionally roll or lift their heads to search the overhead light field. This could be coupled with recorders of depth, speed and orientation to reconstruct the dynamics of the whales as they approach foraging depths. Note that right whales need not penetrate the full thickness of a copepod layer to know that they are at the right depth; the dimming of light as they pass into the layer is likely to be a usable signal in itself. Of course, they also must be able to discriminate the quick dimming of light by a passing cloud from that of a copepod layer, which must present a challenge.
Right whales successfully feed even when copepods in a given region vertically migrate, primarily orienting to regions of high densities regardless of whether these occur at the surface or at depth [7]. These migrations should favour a visual role in predation, because migrating copepods are generally at their deep location during the day, when the brighter light could help to spot them, and move to the upper waters at night, when the whales could skim-feed or perhaps even sight them in surface waters at nocturnal light levels. On the other hand, our measurements were made over a somewhat limited range of weather conditions and solar elevations, and we did not explore the temporal limits of potential visually directed foraging. Whales are likely to be capable of doing this for only part of the day, at least away from surface waters.
Another challenge for right whales is their descent from sunlit surface waters to quite dark depths when they dive to feed. Tracks of diving whales indicate that they reach foraging depths in only a few minutes [5]. To see at all in this new photic environment, they would have to dark-adapt unusually rapidly. Humans require over half an hour for full dark adaptation, longer than the entire dive duration of E. glacialis. In comparison, deep-diving elephant seals fully dark-adapt within 6 min, to thresholds well below those of human controls [39], and bottle-nose dolphin pupils fully constrict within 8 s (although the relaxation times are not known) [40]. Retinas of B. mysticetus, and probably retinas of other (all?) mysticete whales retain the neural pathways used for cone signalling, even in the absence of cone outer segments, implying that rod-mediated vision can flow through the typical bright-light pathway [14]. Perhaps whales maintain separate light-adapted and dark-adaptive networks running in parallel, although such a dual system has not previously been described.
North Atlantic right whales make V-shaped dives when searching for plankton, which implies rapid or continuous dark adaption. These are thought to be used to track prey depths and locations. During subsequent foraging dives, the whales may fully adapt to dim light and become capable of continuously monitoring prey density and its changes in depth as they feed.
(e). Other methods of prey detection
Prey search is surely a multimodal process, perhaps involving a combination of olfactory senses (which are poorly developed in cetaceans, although perhaps not in balaenids [41]), sensory taste buds in the oral cavity (also limited in cetaceans, see [42]) and mechanoreception, where foraging whales may monitor impacts of plankton with bristles on their rostrum or on the tongue itself [4]. Our study supports the notion that whales can use their eyes in prey search, even in turbid and relatively dark environments. Nevertheless, we still have no clear understanding of whether they would search for prey as visible patches (which might be visible looking horizontally not far below the surface), as dim clouds stretching overhead, or in some other way. Equally distressing is that we do not understand how—or if—their vision might be involved in underwater object detection and control of avoidance behaviour. Knowing this could be critical for finding ways of reducing entanglements with fishing gear. On the bright side, this work identifies some promising research questions and approaches that could bring us closer to understanding the visual world of North Atlantic right whales.
Supplementary Material
Acknowledgements
We thank B. Arnold and K. Feller for help with the copepod spectroscopy, S. Laney for the use of the submersible radiometer, A. Greco for assistance with electron microscopy and L. Peichl for advice and comments on the manuscript.
Data accessibility
All new data reported in this paper are available upon request to the corresponding author.
Authors' contributions
T.W.C., J.I.F. and M.F.B. conceived and designed the study. T.W.C., S.J. and L.J.K. carried out the modelling and analysis. M.F.B collected field data. L.E.S. collected data on retinal measurements. T.W.C. wrote the final paper, with assistance from the other authors. All authors have approved the final manuscript.
Competing interests
We have no competing interests.
Funding
T.W.C. is supported by the Air Force Office of Scientific Research, under grant no. FA9550-12-1-0321. Support for field observations by M.F.B. was provided by the Woods Hole Oceanographic Institution and by the National Oceanic and Atmospheric Administration (NOAA) Northeast Fisheries Science Center.
References
- 1.Gatesy J, Geisler JH, Chang J, Buell C, Berta A, Meredith RW, Springer MS, McGowen MR. 2013. A phylogenetic blueprint for a modern whale. Mol. Phylogenet. Evol. 66, 479–506. ( 10.1016/j.ympev.2012.10.012) [DOI] [PubMed] [Google Scholar]
- 2.Goldbogen JA, Calambokidis J, Friedlaender AS, Francis J, DeRuiter SL, Stimpert AK, Falcone E, Southall BL. 2012. Underwater acrobatics by the world's largest predator: 360° rolling manoeuvres by lunge-feeding blue whales. Biol. Lett. 9, 20120986 ( 10.1098/rsbl.2012.0986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiley D, Ware C, Bocconcelli A, Cholewiak D, Friedlander A, Thompson M, Weinrich M. 2011. Underwater components of humpback whale bubble-net feeding behaviour. Behaviour 148, 575–602. ( 10.1163/000579511X570893) [DOI] [Google Scholar]
- 4.Baumgartner MF, Mayo CA, Kenney RD. 2007. Enormous carnivores, microscopic food, and a restaurant that's hard to find. In The urban whale: North Atlantic right whales at the crossroads (eds Kraus SD, Rolland RM), pp. 138–171. Cambridge, MA: Harvard University Press. [Google Scholar]
- 5.Baumgartner MF, Mate BR. 2003. Summertime foraging ecology of North Atlantic right whales. Mar. Ecol. Prog. Ser. 264, 123–135. ( 10.3354/meps264123) [DOI] [Google Scholar]
- 6.Wishner KF, Schoenherr JR, Beardsley R, Chen C. 1995. Abundance, distribution and population structure of the copepod Calanus finmarchicus in a springtime right whale feeding area in the southwestern Gulf of Maine. Cont. Shelf Res. 15, 475–507. ( 10.1016/0278-4343(94)00057-T) [DOI] [Google Scholar]
- 7.Baumgartner MF, Lysiak NSJ, Schuman C, Urban-Rich J, Wenzel FW. 2011. Diel vertical migration behavior of Calanus finmarchicus and its influence on right and sei whale occurrence. Mar. Ecol. Prog. Ser. 423, 167–184. ( 10.3354/meps08931) [DOI] [Google Scholar]
- 8.Kraus SD, Rolland RM. 2007. The urban whale: North Atlantic right whales at the crossroads. Cambridge, MA: Harvard University Press. [Google Scholar]
- 9.Beamish P. 1978. Evidence that a captive humpback whale (Megaptera novaeangliae) does not use sonar. Deep Sea Res. 25, 469–472. ( 10.1016/0146-6291(78)90554-4) [DOI] [Google Scholar]
- 10.McFarland WN. 1971. Cetacean visual pigments. Vision Res. 11, 1065–1076. ( 10.1016/0042-6989(71)90113-1) [DOI] [PubMed] [Google Scholar]
- 11.Meredith RW, Gatesy J, Emerling CA, York VM, Springer MS. 2013. Rod monochromacy and the coevolution of cetacean retinal opsins. PLoS Genet. 9, e1003432 ( 10.1371/journal.pgen.1003432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peichl L, Bermann G, Kröger R. 2001. For whales and seals the ocean is not blue: a visual pigment loss in marine mammals. Eur. J. Neurosci. 12, 1520–1528. ( 10.1046/j.0953-816x.2001.01533.x) [DOI] [PubMed] [Google Scholar]
- 13.Levenson DH, Dizon A. 2003. Genetic evidence for the ancestral loss of short-wavelength-sensitive cone pigments in mysticete and odontocete cetaceans. Proc. R. Soc. Lond. B 270, 673–679. ( 10.1098/rspb.2002.2278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweikert LE, Fasick JI, Grace MS. 2016. Evolutionary loss of cone photoreception in balaenid whales reveals circuit stability in the mammalian retina. J. Comp. Neurol. 524, 2873–2885. ( 10.1002/cne.23996) [DOI] [PubMed] [Google Scholar]
- 15.Fasick JI, Bischoff N, Brennan S, Velasquez S, Andrade G. 2011. Estimated absorbance spectra of the visual pigments of the North Atlantic right whale (Eubalaena glacialis). Mar. Mamm. Sci. 27, E321–E331. ( 10.1111/j.1748-7692.2011.00467.x) [DOI] [Google Scholar]
- 16.Bischoff N, Nickle B, Cronin TW, Velasquez S, Fasick JI. 2012. Deep-sea and pelagic rod visual pigments identified in the mysticete whales. Vis. Neurosci. 29, 95–103. ( 10.1017/S0952523812000107) [DOI] [PubMed] [Google Scholar]
- 17.Fasick JI, Robinson PR. 2000. Spectral-tuning mechanisms of marine mammal rhodopsins and correlations with foraging depth. Vis. Neurosci. 17, 781–788. ( 10.1017/S095252380017511X) [DOI] [PubMed] [Google Scholar]
- 18.Land MF. 1981. Optics and vision in invertebrates. In Handbook of sensory physiology, vol. 7/6B. Vision in invertebrates. Invertebrate visual centers and behavior I (ed. Autrum H.), pp. 471–592. Berlin, Germany: Springer. [Google Scholar]
- 19.Land MF, Nilsson D-E. 2012. Animal eyes, 2nd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 20.Buono MR, Fernández MS, Herrera Y. 2012. Morphology of the eye of the southern right whales (Eubalaena australis). Anat. Rec. 295, 355–368. ( 10.1002/ar.21541) [DOI] [PubMed] [Google Scholar]
- 21.Zhu Q, Hillmann DJ, Henk WG. 2001. Morphology of the eye and surrounding structures of the bowhead whale, Balaena mysticetus. Mar. Mamm. Sci. 17, 729–750. ( 10.1111/j.1748-7692.2001.tb01296.x) [DOI] [Google Scholar]
- 22.Johnsen S, Sosik HM. 2003. Cryptic coloration and mirrored sides as camouflage strategies in near-surface pelagic habitats: implications for foraging and predator avoidance. Limnol. Oceanogr. 48, 1277–1288. ( 10.4319/lo.2003.48.3.1277) [DOI] [Google Scholar]
- 23.Rodieck RW. 1998. The first steps in seeing. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 24.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. 2000. In search of the visual pigment template. Vis. Neurosci. 17, 509–528. ( 10.1017/S0952523800174036) [DOI] [PubMed] [Google Scholar]
- 25.Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 26.Jerlov NG. 1976. Marine optics. New York, NY: Elsevier. [Google Scholar]
- 27.Aho A-C, Donner K, Helenius S, Larsen LO, Reuter T. 1993. Visual performance of the toad (Bufo bufo) at low light levels: retinal ganglion cell responses and prey-catching accuracy. J. Comp. Physiol. A 172, 671–682. [DOI] [PubMed] [Google Scholar]
- 28.Warrant E. 2004. Vision in the dimmest habitats on earth. J. Comp. Physiol. A 190, 765–789. ( 10.1007/s00359-004-0546-z) [DOI] [PubMed] [Google Scholar]
- 29.Nilsson D-E, Warrant E, Johnsen S. 2014. Computational visual ecology in the pelagic realm. Phil. Trans. R. Soc. B 369, 20130038 ( 10.1098/rstb.2013.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronin TW, Goldsmith TH. 1982. Quantum efficiency and photosensitivity of the rhodopsin–metarhodopsin conversion in crayfish photoreceptors. Photochem. Photobiol. 36, 447–454. ( 10.1111/j.1751-1097.1982.tb04401.x) [DOI] [PubMed] [Google Scholar]
- 31.Supin AY, Popov VV, Mass AM. 2001. The sensory physiology of aquatic mammals. Boston, MA: Kluwer Academic Publishers. [Google Scholar]
- 32.Reuter T, Peichl L. 2008. Structure and function of the retina in aquatic tetrapods. In Sensory evolution on the threshold—adaptations in secondarily aquatic vertebrates (eds Thewissen JGM, Numella S), pp. 149–172. Berkeley, CA: University of California Press. [Google Scholar]
- 33.Warrant EJ. 1999. Seeing better at night: life style, eye design and the optimum strategy of spatial and temporal summation. Vision Res. 39, 1611–1630. ( 10.1016/S0042-6989(98)00262-4) [DOI] [PubMed] [Google Scholar]
- 34.Murayama T, Somiya H, Aoki I, Ishii T. 1992. The distribution of ganglion cells in the retina and visual acuity of minke whale. Nippon Suisan Gakkaishi 58, 1057–1061. ( 10.2331/suisan.58.1057) [DOI] [Google Scholar]
- 35.Mass AM, Supin AY. 1997. Ocular anatomy, retinal ganglion cell distribution, and visual resolution in the gray whale, Eschrichtius gibbosus. Aquat. Mamm. 23, 17–28. [Google Scholar]
- 36.Silveira LCL, Saito CA, Lee BB, Kremers J, Filho MS, Kilavik BE, Yamada ES, Perry VH. 2004. Morphology and physiology of primate M- and P-cells. Prog. Brain Res. 144, 21–46. ( 10.1016/S0079-6123(03)14402-0) [DOI] [PubMed] [Google Scholar]
- 37.Gagnon YL, Shashar N, Warrant EJ, Johnsen SJ. 2007. Light scattering by selected zooplankton from the Gulf of Aqaba. J. Exp. Biol. 210, 3728–3735. ( 10.1242/jeb.003251) [DOI] [PubMed] [Google Scholar]
- 38.Calambokidis H, Schorr GS, Steiger GH, Francis J, Bakhtiari M, Marshal G, Oleson EM, Gendron D, Robertson K. 2007. Insights into the underwater diving, feeding, and calling behavior of blue whales from a suction-cup-attached video-imaging tag (Crittercam). Mar. Technol. Soc. J. 41, 19–29. ( 10.4031/002533207787441980) [DOI] [Google Scholar]
- 39.Levenson DH, Schusterman RJ. 1999. Dark adaptation and visual sensitivity in shallow and deep-diving pinnipeds. Mar. Mamm. Sci. 15, 1303–1313. ( 10.1111/j.1748-7692.1999.tb00892.x) [DOI] [Google Scholar]
- 40.Dawson WW, Adams CK, Barris MC, Litzkow CA. 1979. Static and kinetic properties of the dolphin pupil. Am. J. Physiol. 6, R301–R305. [DOI] [PubMed] [Google Scholar]
- 41.Thewissen JGM, George J, Rosa C, Kishida T. 2010. Olfaction and brain size in the bowhead whale (Balaena mysticetus). Mar. Mamm. Sci. 27, 282–294. ( 10.1111/j.1748-7692.2010.00406.x) [DOI] [Google Scholar]
- 42.Kishida T, Thewissen JGM, Hayakawa T, Imai H, Agata K. 2015. Aquatic adaptation and the evolution of smell and taste in whales. Zool. Lett. 1, 9 ( 10.1186/s40851-014-0002-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All new data reported in this paper are available upon request to the corresponding author.