Abstract
The ability of ants to navigate when the visual landmark information is altered has often been tested by creating large and artificial discrepancies in their visual environment. Here, we had an opportunity to slightly modify the natural visual environment around the nest of the nocturnal bull ant Myrmecia pyriformis. We achieved this by felling three dead trees, two located along the typical route followed by the foragers of that particular nest and one in a direction perpendicular to their foraging direction. An image difference analysis showed that the change in the overall panorama following the removal of these trees was relatively little. We filmed the behaviour of ants close to the nest and tracked their entire paths, both before and after the trees were removed. We found that immediately after the trees were removed, ants walked slower and were less directed. Their foraging success decreased and they looked around more, including turning back to look towards the nest. We document how their behaviour changed over subsequent nights and discuss how the ants may detect and respond to a modified visual environment in the evening twilight period.
This article is part of the themed issue ‘Vision in dim light’.
Keywords: Myrmecia, learning walks, visual navigation, night-active, panorama
1. Introduction
Ants derive compass information from a range of cues including the pattern of polarized skylight [1], the landmark panorama [2,3], odours [4] and magnetic fields [5]. Among these, visual orientation has been extensively studied because the visual navigational information can be quantified [6], relevant sensory systems can be characterized [7–9] and the visually driven behaviour can be monitored under natural conditions [6,10,11]. Most of our current knowledge about ant navigation has come from day-active ants. However, a large number of ants are active at night and face navigational challenges similar to their diurnal counterparts. At night, light intensity is about a 100 million times dimmer than during the day [12], decreasing the detectability of visual information, which makes navigating at night a challenge. So, how then do nocturnal ants navigate?
Both diurnal and nocturnal ants navigate using celestial and terrestrial visual information [13–16]. Nocturnal ants derive compass information from the pattern of polarized skylight. However, when the pattern of polarized skylight is experimentally rotated, the ants only partially compensate for this change [14]. This is perhaps because they rely most on visual landmark information. Ants are thought to learn landmark information through a carefully constructed series of learning walks carried out in multiple orientations around a goal [17–20]. During homing, individuals move and compare their current view to a previously memorized view and travel in the direction that provides the least image difference. For this, they rely on the entire landmark panorama and indeed when an artificial panorama is rotated, ants travel in the direction predicted by that panorama [2]. Demonstrating the use of visual landmarks has often involved training animals to new landmarks, or making familiar landmarks unavailable or blocking parts of the panorama [14,21–23], which causes significant changes in the insect's visual field. So, is visual navigation affected by subtle changes in the landmark panorama?
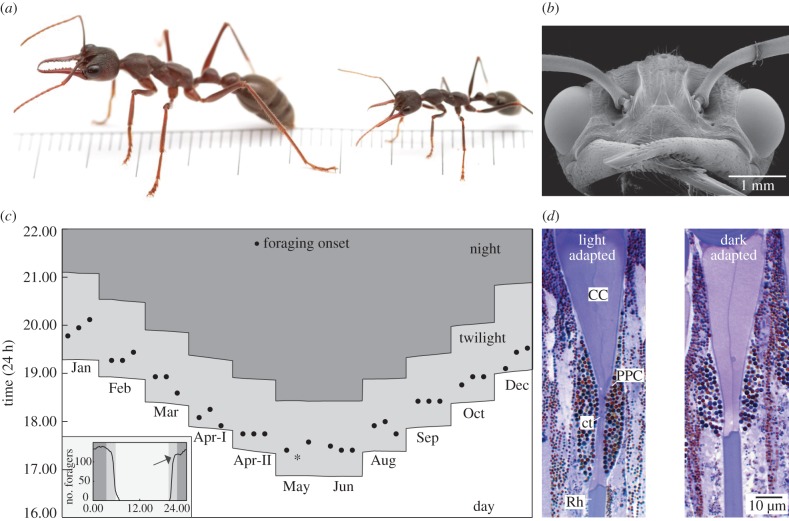
The nocturnal ant Myrmecia pyriformis (figure 1) presents an unusual opportunity to address this question, since they have predictable foraging patterns [25] and key aspects of their ecology [27] and visual anatomy [26] have been well documented. They are long lived, with individuals capable of surviving for more than a year in natural conditions (A. Narendra 2013, personal observation). Individual ants forage strictly on one tree over at least two months and each nest remains faithful to 1–3 trees for the entire lifetime of a colony. A majority of these solitary foraging ants carry out only one foraging trip each day [27], restricting the visually demanding task of navigating between the nest and the foraging tree to the dim-lit conditions of the evening and morning twilight [25]. Ambient light intensity during sunset triggers the onset of foraging and it most likely controls the cessation of foraging before sunrise (figure 1c) [25]. M. pyriformis workers have evolved nocturnal visual adaptations, which include large lenses and wide photoreceptors to increase their optical sensitivity by 27-fold compared with their diurnal relatives [9,28]. The workers have over 3500 facets in each eye [28], with the largest lenses present in the medio-frontal region of their eye [9], which is most likely the eye region with the best resolving power. Additionally, they control for light flux through a variable primary pigment pupil (figure 1d) that constricts the crystalline cone tract in bright light [26], which allows them to adjust the sensitivity of their eyes. Despite having developed nocturnal visual adaptations, these ants avoid navigating at night. However, when they are forced to travel at night, their navigational efficiency degrades dramatically, suggesting that their ability to detect visual navigational information reduces in low light [13]. In the twilight period, workers of M. pyriformis navigate by relying most on the landmark panorama [14]. This enables them to establish idiosyncratic routes to which they adhere for long periods [24]. Here, we investigated whether a subtle change in the landmark panorama, caused by the felling of three dead trees, two en route to their main foraging tree and one perpendicular to their foraging direction, affected the visual navigation ability of this nocturnal ant.
Figure 1.
The nocturnal Australian bull ant, Myrmecia pyriformis. (a) Photograph of major and minor worker of M. pyriformis. (b) Scanning electron micrograph of a frontal view of the head of a worker M. pyriformis showing the large compound eyes and three dorsally placed ocelli. (c) Activity schedule of ants on a single day (inset) and throughout the year. The onset of foraging (black circles) was recorded at every 30-min; change in sunset time shows that onset occurs during the evening twilight throughout the year; * indicates no activity. Inset: number of active workers averaged over three nests. (d) Longitudinal section of an ommatidium in the frontal region of the eye in light- and dark-adapted state. CC, crystalline cone; ct, crystalline cone tract; PPC, primary pigment cell; Rh, rhabdom. (a) Photo credit Ajay Narendra; (b) adapted from Reid [24]; (c) adapted from Narendra et al. [25]; inset in (c) adapted from Narendra et al. [9]; (d) adapted from Narendra et al. [26].
2. Material and methods
(a). Study location, tree removal and quantifying the change in the landmark panorama
We studied the behaviour of M. pyriformis from a single nest located at the campus field station at The Australian National University, in Canberra (35°16′50″ S, 149°06′43″ E). All individuals from this nest foraged on a Eucalyptus tree that was 15 m away (electronic supplementary material, figure S1). Two dead trees (T2 and T3) were located on either side of their typical foraging route. These trees were used by the ants until mid-2013, when the trees died. Perpendicular to the foraging direction and 10 m from the nest was another dead tree, a tree on which we never found foragers from this nest. These trees had a few tall branches, but no leafy cover. We coordinated with the Building and Service Facilities to organize the removal of the three dead trees on 18 February 2014, between 1200 and 1500 h. At this time of the day, workers of M. pyriformis typically remain within the nest and do not forage [25]. The dead trees were felled, stumps were pulverized and the ground was levelled. The ground was then cleared of sawdust, pieces of logs and fallen branches. During the tree removal process, an area of nearly 3 m in radius centred around the nest entrance was cordoned off to ensure there was no change to the natural substrate within the vicinity of the nest. We analysed the behaviour of the ants within this 3 m range of undisturbed terrain separately from the remainder of the ants' route.
We captured panoramic scenes using a Sony Bloggie camera (MSH-PM5) from the nest both before and after the trees were removed. The camera was levelled using a spirit level. The panoramic images were unwarped to rectangular panoramas, converted to grey scale and low-pass filtered with a σ = 3° Gaussian filter to match the visual acuity of these ants [9]. For the subsequent analysis, we used only the part of the images above the horizon, which had a vertical angular extent of 53°, which did not include the tree canopy. We computed image similarities using the rotational image difference function (rotIDF), by comparing root mean square (r.m.s.) pixel differences for every shift in pixel, between the views before the trees were removed with itself and between the views before and after the trees were removed (for detailed methods see [29,30]). The minima derived in this manner match to the highest similarity between the views.
(b). Filming ant departures from the nest
We filmed ants departing the nest for seven nights, including two consecutive nights before the trees were removed (N−2 and N−1), four consecutive nights immediately after removal (N0, N1, N2 and N3) and on the 28th night (N28). Ants were filmed at 50 fps using an infrared video camera (Manta IR, Allied Vision Technologies) and illuminated with infrared LEDs (peak λ 850 nm), which were beyond the spectral sensitivity of the nocturnal Myrmecia ants [31]. The camera covered an area of 80 × 60 cm around the nest, was connected to a laptop through a gigabit cable and footage was captured in an avi format using StreamPix software (Norpix Inc., Canada). Recording was carried out in the evening twilight which lasted for about 90–120 min. We marked the ants individually but it became difficult to reliably identify the colours under red lights at night and hence we had to ignore individual identification.
(c). Tracking ant paths from nest to foraging tree
We tracked the path of individual ants for the same seven nights either until they reached the tree or for 15 min. For tracking, we followed ants individually as they left the nest and placed miniature coloured flags about 10–15 cm behind them, ensuring not to disturb them. Subsequently, we recorded the position of the flags using a differential GPS (DGPS) ensuring errors of less than 20 cm. This tracking technique has been described in detail elsewhere [13,30].
(d). Data analysis
The videos were converted to image sequences in QuickTime (Apple Inc.) and processed in a custom written program (courtesy Jan Hemmi and Robert Parker) in Matlab (Mathworks, Nattick). We digitized the head, pronotum and propodeum position at 20 ms intervals on N−2, N−1 and N0 and at 100 ms intervals for the other nights. We measured for each individual the bearing when their paths crossed a circle of 10 cm diameter around the nest and calculated the mean heading direction for each night before and after the trees were removed. Ants that did not reach a distance of 10 cm from the nest were excluded from this analyses. We used the ratio between the path length and the straight-line distance from the nest as a measure of path straightness, where values ranged from zero to unity, with unity being a straight path. We used the pronotum position to calculate the walking speed. We identified the proportion of ants that turned and looked back towards the nest to within ±10° of their midline and the duration for which animals viewed the nest direction. We determined the retinal position of the animals and also the gaze direction relative to an external coordinate system for each night. The positional data acquired from the DGPS were used to record ant paths over 15 m. Analyses were carried out in custom written scripts in Matlab 2013b.
3. Results
(a). Quantifying change in the panorama
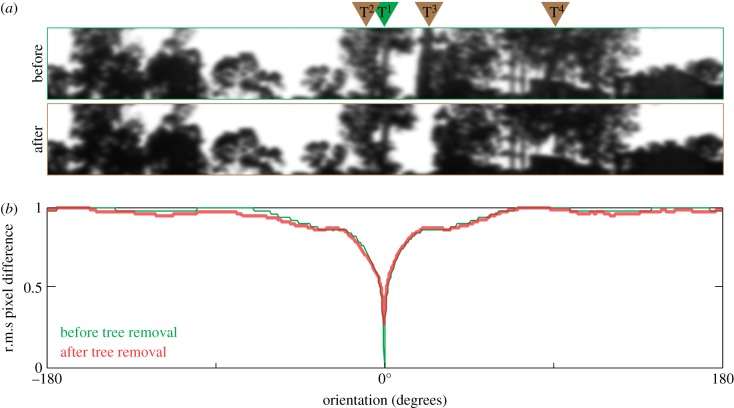
We quantified the change in the information content of the environment when the trees were removed using rotIDF. For this, we first compared the panoramic view from the nest with itself, before the trees were removed (figure 2). The analysis showed a clear minimum at 0° that corresponded to the direction of the main foraging tree (green curve in figure 2b). Next, we compared the panoramic views before and after the trees were removed. The analysis again showed a distinct, although shallower, minimum that corresponded to the direction of the main foraging tree (red curve in figure 2b).
Figure 2.
Quantifying the change in the landmark panorama after three trees were removed. (a) Panoramic view before the trees were removed (upper) and after the trees were removed (lower). Green arrow indicates the direction of the foraging tree T1; brown arrows indicate the trees T2, T3 and T4 that were removed. Panoramic images were low-pass filtered with a σ = 3° Gaussian filter to match the visual acuity of the ants. (b) The rotIDF compares the view before the trees were removed with itself (green) and compares views before and after the trees were removed (red). Both curves have a distinct minimum that points in the direction of the foraging tree (0°).
(b). Behaviour in the vicinity of the nest
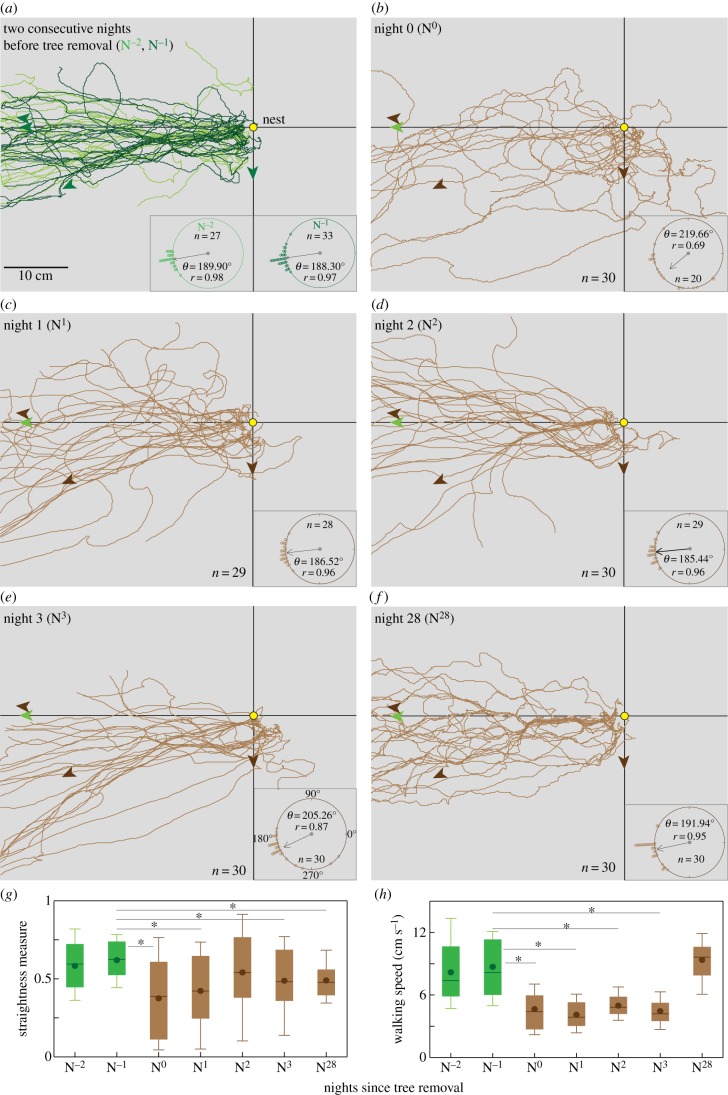
On the two consecutive nights before tree-felling, ants departed the nest and headed directly towards their foraging trees (figure 3a). The circular plot shows that the ants were well oriented towards their foraging tree at a distance of 10 cm from the nest (figure 3a; V-test: N−2: u = 7.136, p < 0.001; N−1: u = 7.842, p < 0.001). The first night following tree felling (N0), the ants were less directed, although most moved into the general direction of the foraging tree (figure 3b). After having reached a distance of 10 cm from the nest, these ants were less directed (mean ± s.d. = 219.66° ± 49.4, r = 0.69, n = 20), but were still significantly oriented towards the foraging tree (V-test: u = 3.358, p < 0.001). Thereafter on subsequent nights (figure 3c–f), the ants were again well directed towards the tree.
Figure 3.
Initial foraging paths of ants before and after the trees were removed. Paths of ants on (a) two consecutive nights before the trees were removed and after the trees were removed on (b) N0, (c) N1, (d) N2 (e) N3 and (f) N28. Nest (yellow circle), direction from the nest towards the trees before removal (green) and after removal (brown) are shown by arrows. Inset in each panel: circular plot shows the orientation of the ants at a path length of 10 cm (foraging tree direction = 180°). Ants that did not travel more than 10 cm were excluded from this analysis. Mean vector (θ), length of the home vector (r) and sample size (n) are shown. Box plots show (g) path straightness and (h) walking speed. Straightness is defined as the ratio between the path length and the straight line distance to the nest. Box plot shows mean (filled circle), median (thick line) and 25th and 75th percentile and whiskers extend to 10th and 90th percentile. Green: before the trees were removed; brown: after the trees were removed. *p < 0.001.
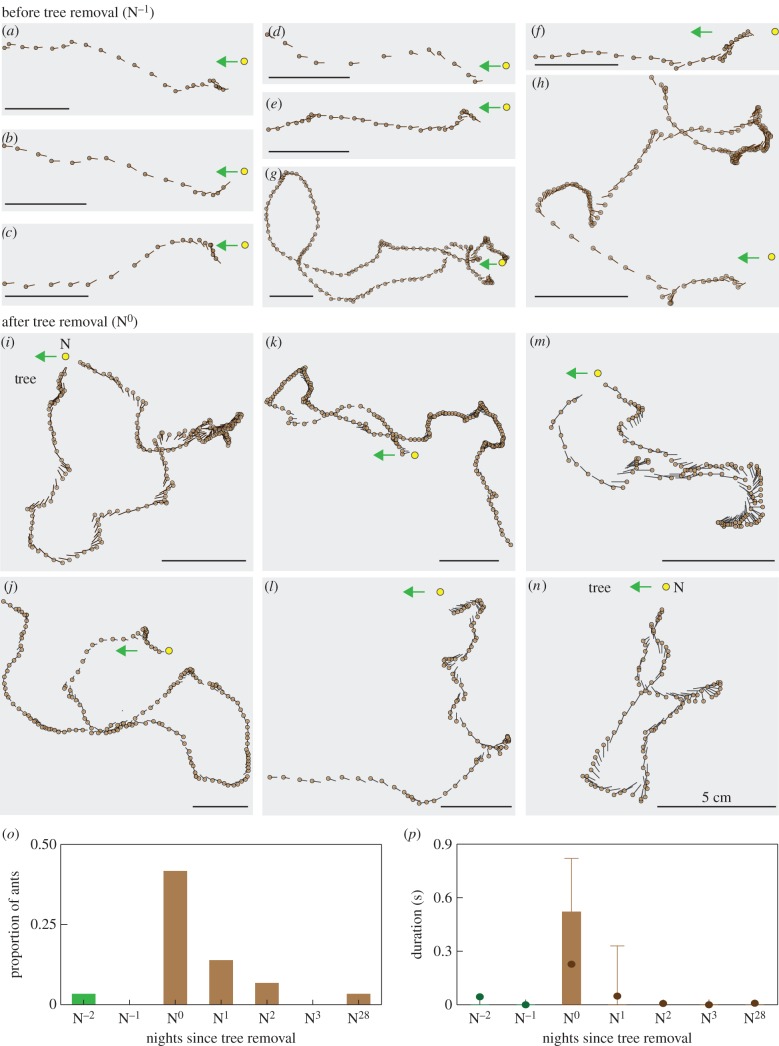
Overall, the path straightness of ants was significantly different (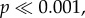 KW = 29.85, Kruskal–Wallis test; figure 3g), and a post hoc test showed that it did not differ between N−1 and N−2 (green box plots in figure 3g; p > 0.01, Dunn's test). Ant paths on N0, N1, N3 and N28 were less straight compared with paths on N−1 (ps < 0.001, Dunn's test). Overall, the walking speed of ants was significantly different (
KW = 29.85, Kruskal–Wallis test; figure 3g), and a post hoc test showed that it did not differ between N−1 and N−2 (green box plots in figure 3g; p > 0.01, Dunn's test). Ant paths on N0, N1, N3 and N28 were less straight compared with paths on N−1 (ps < 0.001, Dunn's test). Overall, the walking speed of ants was significantly different (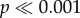 , KW = 101.3, Kruskal–Wallis test; figure 3h), and a post hoc test showed that it did not differ between N−1 and N−2 (p > 0.01, Dunn's multiple comparison test). Compared with N−1, the walking speed of ants reduced by nearly half on N0 and remained so for the next three nights (ps < 0.001, Dunn's test). Walking speed of ants on N28 increased to 9.3 ± 0.37 cm s−1 and this was not significantly different from the walking speed of ants prior to tree removal (p > 0.01, Dunn's test). This decrease in the path straightness and the walking speed for the first three nights after tree felling could also be due to the mismatch between the food vector and the landmark panorama.
, KW = 101.3, Kruskal–Wallis test; figure 3h), and a post hoc test showed that it did not differ between N−1 and N−2 (p > 0.01, Dunn's multiple comparison test). Compared with N−1, the walking speed of ants reduced by nearly half on N0 and remained so for the next three nights (ps < 0.001, Dunn's test). Walking speed of ants on N28 increased to 9.3 ± 0.37 cm s−1 and this was not significantly different from the walking speed of ants prior to tree removal (p > 0.01, Dunn's test). This decrease in the path straightness and the walking speed for the first three nights after tree felling could also be due to the mismatch between the food vector and the landmark panorama.
(c). Beyond the vicinity of the nest
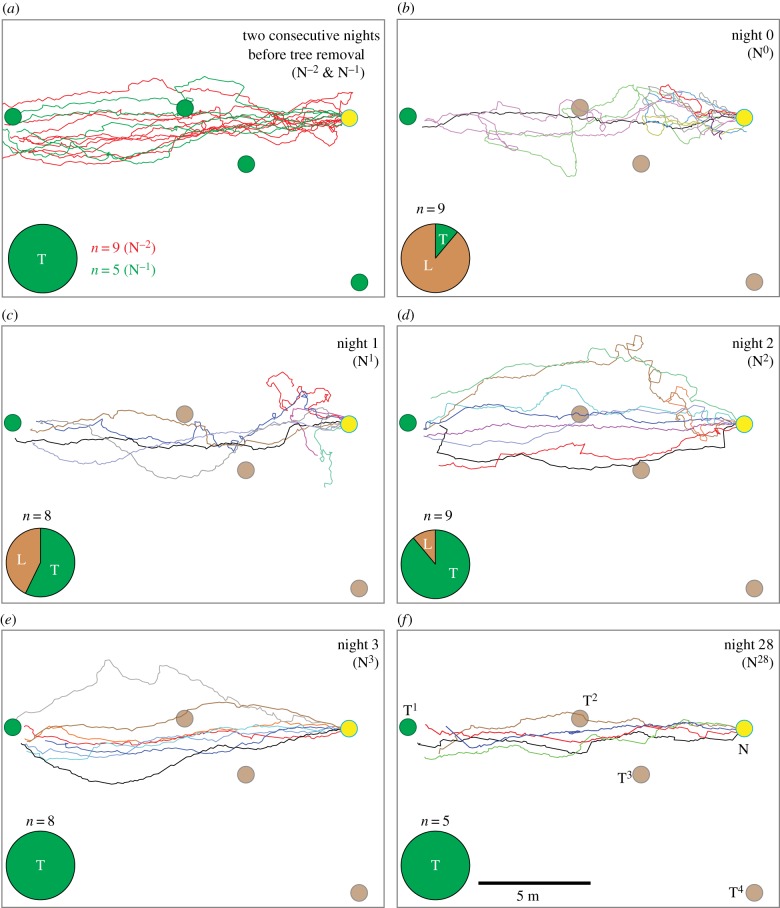
Recording the ant paths with a DGPS showed that before tree felling, ants travelled in a narrow corridor from the nest to their foraging tree (figure 4a). Every single ant we tracked on these two nights climbed the main foraging tree, T1. On N0 we tracked nine individual ants, and only one reached the tree and climbed it (figure 4b, in black; pie-graph). Two other ants walked all or most of the way to the tree (figure 4b, in purple and green) and subsequently returned back to the nest without prey, a behaviour we have never seen before in these ants. The remaining ants that we followed (and those we observed by eye), headed in the direction of the foraging tree and then either turned back to return to the nest or made sharp U-turns and remained within 4 m of the nest. On N1, the proportion of ants that reached the tree increased to greater than 50% (figure 4c), with some individuals often pausing and scanning when they were a metre away from the location of the trees that were removed, T2 and T3. On subsequent days (figure 4d–f), the proportion of ants that successfully headed to and climbed the main foraging tree, T1 increased gradually. It must be noted that the ant paths beyond 3 m from the nest may not be exclusively visually mediated and could be influenced by a change in the substrate or olfactory cues.
Figure 4.
Paths of ants recorded with a DGPS before and after the trees were removed. Paths of ants on (a) two consecutive nights before the trees were removed and after the trees were removed on (b) N0, (c) N1, (d) N2 (e) N3 and (f) N28. Pie-graph insets show the proportion of animals that successfully reached and climbed the tree (T, in green) and those that were lost (L, in brown). On N0 (b), only one ant reached the tree (in black) and two others that travelled close to the tree returned back to the nest (in green and purple). Paths are shown in different colours to distinguish the paths. Other conventions as in figure 3.
(d). Choreography of re-learning walks
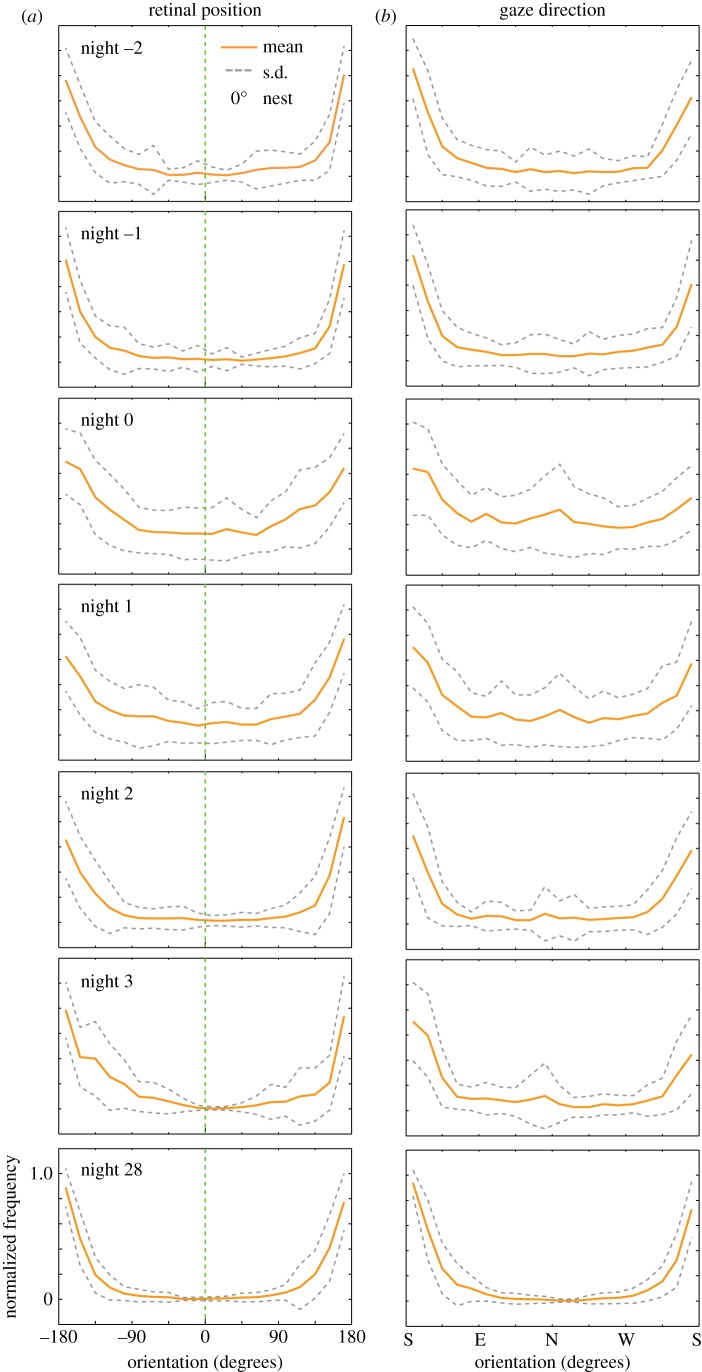
Animals clearly recover from the subtle change in their landmark panorama. Towards understanding what assisted this recovery, we analysed the changes in the viewing direction of ants before and after tree felling (figures 5 and 6). On N−2 and N−1, most ants left the nest without turning back and looking in the nest direction (figures 5a–h and 6a). On these two nights, the average nest viewing duration was 0.044 ± 0.17 s (figure 5p), which was contributed only by two ants (out of 60; figure 5o). One of these ants repeatedly turned back and looked towards the nest while heading out foraging (figure 5h), while the other ant walked in a loop and returned back to the nest (figure 5g). Their behaviour of looking back towards the nest from different distances and bearings (figure 5g,h) is reminiscent of learning walks [18,32], and hence we suspect that these two ants may have been inexperienced individuals. Most of the ants on N−2 and N−1, however, looked in the direction of the foraging trees and not towards the nest (figures 5o,p and 6a). On N0, there was an increase in the number of ants that turned and looked back towards the nest (figure 5o) and also in the duration for which animals viewed the nest (figure 5p). On N0, animals walked slower (figure 3h), and either returned home after walking in a loop around the nest (figure 5i,m,n) or walked in an arc looking away from the nest (e.g. figure 5l). They looked back towards the nest more frequently than earlier (figure 6a, note the increase in variance in N0; figure 6) and also looked around more than earlier (figure 6b). The behaviour of these ants suggests that even a slight modification of the visual environment drives animals to re-learn the visual information around the nest. On subsequent nights, both the proportion of ants that turned and looked towards the nest (figures 5o and 6a) and the extent to which animals looked around gradually decreased (figure 6b).
Figure 5.
Gaze direction of ants before and after tree removal. Quiver plots show gaze direction (line) with head position (brown circle) of ants on nights (a–h) before the trees were removed and (i–n) after the trees were removed. Ants were filmed at 20 ms interval, but for clarity data are displayed at a 100 ms interval. Nest (yellow circle, N) and the direction of the foraging tree (green arrow) are shown. Scale bar is 5 cm. (g,h) The only two ants, before tree felling, that turned back and looked in the direction of the nest. (o) Histogram showing the proportion of ants that turned back to look towards ±10° of the nest. (p) Box plot shows the duration that ants viewed ±10° of the nest. Sample size for panels o and p as indicated in figure 3. The ground was sloping into the nest entrance for 1–3 cm around the entrance. We tracked ants away from this region and hence the paths do not always start from the nest.
Figure 6.
Analysis of where ants look before and after the trees were removed. (a) Normalized frequency of the retinal position of the ants. Nest position is at 0° and is highlighted by a vertical line (green). Foraging tree direction is at±180°. (b) Normalized frequency of absolute gaze direction of ants. Gaze direction is relative to the external coordinate system of north (N), south (S), east (E) and west (W). For both (a,b) data were normalized to the maximum for each night.
4. Discussion
We have shown here that foragers of the nocturnal bull ant M. pyriformis detect subtle changes in their landmark panorama. Typically, ants leaving the nest are well oriented towards their foraging trees and do not turn back and look elsewhere. However, when ants first encounter a change in their visual panorama, a high proportion of animals turn back and look towards the nest direction and also look around extensively. This scanning behaviour decreases over 3 days, but their walking speed continues to remain slower than usual.
Ants carry out carefully orchestrated learning walks when leaving from a food resource or from the nest [18–20,32]. It is during these learning walks (or learning flights in bees [33–35] and wasps [36,37]) that insects are thought to develop a visual representation of the goal environment which they recall during the return trip to pinpoint home [36]. We do not know the extent of experience of ants in our study. However, the majority of the ants on N−2 and N−1 headed out foraging without looking back towards the nest and hence they are likely to be experienced animals. In addition, most workers of M. pyriformis travel each night for self-sustenance trips [27], and hence it is likely that a large proportion of ants on N0 and on subsequent days were also experienced. Nevertheless, on N0, ants for the first time experienced the slightly modified visual environment. Ants responded to the change by increasing the frequency of looking around and turning back to look towards the nest (figure 5), rather than heading straight to their foraging tree. Ants are most likely re-learning visual information around the nest. However, this was carried out by less than 50% of the ants, suggesting that other factors (e.g. experience and age) influence when animals perform re-learning walks. Experienced individuals of the related day-active ant Myrmecia croslandi are known to carry out such relearning walks when they encounter a mismatch of the visual information between their outbound and return journeys [18].
Some ants derive compass information from canopy patterns [38] but our panoramic images currently only capture 53° on the vertical angular extent. Hence, there is a need to incorporate the canopy information in the panoramic images in future studies. We are unsure whether animals extract and use salient features in their visual environment or whether they monitor the depth of the minimum of the rotIDF and rely on the entire panorama for guidance. Wystrach et al. [39] also attempted to identify this, where they trained desert ants (Melophorus bagoti) with a sheet behind the nest and then tested by rotating the sheet. Unlike in our experiments where ants responded to the change immediately, the desert ants ignored the change in their visual field for the first half of the journey. The behaviour on the second half of their journey varied with the degree of the rotation of the sheet: at a smaller rotation, ants searched at the fictive nest location based on the rotated sheet, but at a higher rotation, ants ignored the sheet and used other cues. In addition, the paths of the ants differed between the left and right rotation of the sheet, indicating that the ants were not guided by a single landmark. More recently, Buehlmann et al. [40] have shown that ants trained to different shapes in the panorama, use the centre of mass of each salient feature for guidance. It is unclear at this stage how this can be applied to highlight subtle changes in a landmark rich panorama.
Supplementary Material
Acknowledgements
We are grateful to Jochen Zeil for encouraging us to liaise with the University for the tree felling and also for his advice and fruitful discussions. We thank Chloé Raderschall and Sara Wood for their bravado in helping us track the bull ants at night. We thank in particular Tom Collett for his critical and constructive comments.
Data accessibility
The complete GPS dataset is available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1q269.
Authors' contributions
Conceptualized the study: A.N.; performed the experiments: A.N. and F.R.E.; digitization: A.N. and F.R.E., programming, analysis and first draft: A.N.; manuscript revision: A.N. and F.R.E.
Competing interests
No competing interests declared.
Funding
This work was supported by a PhD scholarship to F.R.E. from the Australian National University, and grants from the Australian Research Council (DE120100019, FT140100221, DP150101172).
References
- 1.Zeil J, Ribi WA, Narendra A. 2014. Polarisation vision in ants, bees and wasps. In Polarized light and polarization vision in animal sciences (ed. Horváth G.), pp. 41–60. Berlin, Germany: Springer. [Google Scholar]
- 2.Graham P, Cheng K. 2009. Ants use the panoramic skyline as a visual cue during navigation. Curr. Biol. 19, R935–R937. ( 10.1016/j.cub.2009.08.015) [DOI] [PubMed] [Google Scholar]
- 3.Zeil J, Narendra A, Stürzl W. 2014. Looking and homing: how displaced ants decide where to go. Phil. Trans. R. Soc. B. 369, 20130034 ( 10.1098/rstb.2013.0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steck K, Hansson B. 2011. Desert ants benefit from combining visual and olfactory landmarks. J. Exp. Biol. 214, 1307–1312. ( 10.1242/jeb.053579) [DOI] [PubMed] [Google Scholar]
- 5.Wajnberg E, Acosta-Avalos D, Alves OC, de Oliveira JF, Srygley RB, Esquivel DMS. 2010. Magnetoreception in eusocial insects: an update. J. R. Soc. Interface 7, S207–S225. ( 10.1098/rsif.2009.0526.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stürzl W, Grixa I, Mair E, Narendra A, Zeil J. 2015. Three-dimensional models of natural environments and the mapping of navigational information. J. Comp. Physiol. A 201, 563–584. ( 10.1007/s00359-015-1002-y) [DOI] [PubMed] [Google Scholar]
- 7.Schwarz S, Narendra A, Zeil J. 2011. The properties of the visual system in the Australian desert ant Melophorus bagoti. Arthr. Struct. Dev. 40, 128–134. ( 10.1016/j.asd.2010.10.003) [DOI] [PubMed] [Google Scholar]
- 8.Muscedere ML, Gronenberg W, Moreau CS, Traniello JFA. 2014. Investment in higher order central processing regions is not constrained by brain size in social insects. Proc. R. Soc. B 281, 20140217 ( 10.1098/rspb.2014.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narendra A, Reid SF, Greiner B, Peters RA, Hemmi JM, Ribi WA, Zeil J. 2011. Caste-specific visual adaptations to distinct daily activity schedules in Australian Myrmecia ants. Proc. R. Soc. B. 278, 1141–1149. ( 10.1098/rspb.2010.1378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ardin PB, Mangan M, Webb B. 2016. Ant homing ability is not diminished when traveling backwards. Front. Behav. Neurosci. 10, 69 ( 10.3389/fnbeh.2016.00069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raderschall CA, Narendra A, Zeil J. 2016. Head roll stabilisation in the nocturnal bull ant Myrmecia pyriformis: implications for visual navigation. J. Exp. Biol. 219, 1449–1457. ( 10.1242/jeb.134049) [DOI] [PubMed] [Google Scholar]
- 12.Warrant EJ, Dacke M. 2011. Vision and visual navigation in nocturnal insects. Annu. Rev. Entomol. 56, 239–254. ( 10.1146/annurev-ento-120709-144852) [DOI] [PubMed] [Google Scholar]
- 13.Narendra A, Reid SF, Raderschall CA. 2013. Navigational efficiency of nocturnal Myrmecia ants suffers at low light levels. PLoS ONE 8, e58801 ( 10.1371/journal.pone.0058801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid SF, Narendra A, Hemmi JM, Zeil J. 2011. Polarised skylight and the landmark panorama provide night-active bull ants with compass information during route following. J. Exp. Biol. 214, 363–370. ( 10.1242/jeb.049338) [DOI] [PubMed] [Google Scholar]
- 15.Legge ELG, Wystrach A, Spetch ML, Cheng K. 2014. Combining sky and earth: desert ants (Melophorus bagoti) show weighted integration of celestial and terrestrial cues. J. Exp. Biol. 217, 4159–4166. ( 10.1242/jeb.107862) [DOI] [PubMed] [Google Scholar]
- 16.Warrant EJ, Dacke M. 2016. Visual navigation in nocturnal insects. Physiology 31, 182–192. ( 10.1152/physiol.00046.2015) [DOI] [PubMed] [Google Scholar]
- 17.Fleischmann PN, Christian M, Müller VL, Rössler W, Wehner R. 2016. Ontogeny of learning walks and the acquisition of landmark information in desert ants, Cataglyphis fortis. J. Exp. Biol. 219, 3137–3145. ( 10.1242/jeb.140459) [DOI] [PubMed] [Google Scholar]
- 18.Jayatilaka P.2014. Individual foraging careers of the jack jumper ant, Myrmecia croslandi, pp. 1–166. See https://digitalcollections.anu.edu.au/handle/1885/13471 .
- 19.Jayatilaka P, Raderschall CA, Zeil J, Narendra A. 2013. Learning to forage: the learning walks of Australian jack jumper ants. Front. Physiol. Conference Abstract: International Conference on Invertebrate Vision. ( 10.3389/conf.fphys.2013.25.00081) [DOI] [Google Scholar]
- 20.Müller M, Wehner R. 2010. Path integration provides a scaffold for landmark learning in desert ants. Curr. Biol. 20, 1368–1371. ( 10.1016/j.cub.2010.06.035) [DOI] [PubMed] [Google Scholar]
- 21.Wystrach A, Philippides A, Aurejac A, Cheng K, Graham P. 2014. Visual scanning behaviours and their role in the navigation of the Australian desert ant Melophorus bagoti. J. Comp. Physiol. A 200, 1–12. ( 10.1007/s00359-014-0900-8) [DOI] [PubMed] [Google Scholar]
- 22.Julle Daniere E, Schultheiss P, Wystrach A, Schwarz S, Nooten SS, Bibost AL, Cheng K. 2014. Visual matching in the orientation of desert ants (Melophorus bagoti): the effect of changing skyline height. Ethology 120, 1–10. ( 10.1111/eth.12247) [DOI] [Google Scholar]
- 23.Collett M, Collett TS. 2009. The learning and maintenance of local vectors in desert ant navigation. J. Exp. Biol. 212, 895–900. ( 10.1242/jeb.024521) [DOI] [PubMed] [Google Scholar]
- 24.Reid SF. 2010. Life in the dark: vision and navigation in a nocturnal bull ant. PhD thesis, The Australian National University, Canberra, Australia.
- 25.Narendra A, Reid SF, Hemmi JM. 2010. The twilight zone: ambient light levels trigger activity in primitive ants. Proc. R. Soc. B 277, 1531–1538. ( 10.1098/rspb.2009.2324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narendra A, Greiner B, Ribi WA, Zeil J. 2016. Light and dark adaptation mechanisms in the compound eyes of Myrmecia ants that occupy discrete temporal niches. J. Exp. Biol. 219, 2435–2442. ( 10.1242/jeb.142018) [DOI] [PubMed] [Google Scholar]
- 27.Reid SF, Narendra A, Taylor RW, Zeil J. 2013. Foraging ecology of the night-active bull ant Myrmecia pyriformis. Aust. J. Zool. 61, 170–177. ( 10.1071/ZO13027) [DOI] [Google Scholar]
- 28.Greiner B, Narendra A, Reid SF, Dacke M, Ribi WA, Zeil J. 2007. Eye structure correlates with distinct foraging-bout timing in primitive ants. Curr. Biol. 17, R879–R880. ( 10.1016/j.cub.2007.08.015) [DOI] [PubMed] [Google Scholar]
- 29.Stürzl W, Zeil J. 2007. Depth, contrast and view-based homing in outdoor scenes. Biol. Cyber. 96, 519–531. ( 10.1007/s00422-007-0147-3) [DOI] [PubMed] [Google Scholar]
- 30.Narendra A, Gourmaud S, Zeil J. 2013. Mapping the navigational knowledge of individually foraging ants, Myrmecia croslandi. Proc. R. Soc. B. 280, 20130683 ( 10.1098/rspb.2013.0683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa Y, Falkowski M, Narendra A, Zeil J, Hemmi JM. 2015. Three spectrally distinct photoreceptors in diurnal and nocturnal Australian ants. Proc. R. Soc. B 282, 20150673 ( 10.1098/rspb.2015.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson DJ, Judd S, Cartwright B, Collett TS. 1999. Learning walks and landmark guidance in wood ants (Formica rufa). J. Exp. Biol. 202, 1831–1838. [DOI] [PubMed] [Google Scholar]
- 33.Zeil J, Kelber A. 1996. Structure and function of learning flights in bees and wasps. J. Exp. Biol. 199, 245–252. [DOI] [PubMed] [Google Scholar]
- 34.Dittmar L, Egelhaaf M, Stürzl W, Boeddeker N. 2011. The behavioral relevance of landmark texture for honeybee homing. Front. Behav. Neurosci, 5, 20 ( 10.3389/fnbeh.2011.00020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collett TS, de Ibarra NH, Riabinina O, Philippides A. 2013. Coordinating compass-based and nest-based flight directions during bumblebee learning and return flights. J. Exp. Biol. 216, 1105–1113. ( 10.1242/jeb.081463) [DOI] [PubMed] [Google Scholar]
- 36.Stürzl W, Zeil J, Boeddeker N, Hemmi JM. 2016. How wasps acquire and use views for homing. Curr. Biol. 26, 470–482. ( 10.1016/j.cub.2015.12.052) [DOI] [PubMed] [Google Scholar]
- 37.Collett TS, Lehrer M. 1993. Looking and learning: a spatial pattern in the orientation flight of the wasp Vespula vulgaris. Proc. R. Soc. B. 252, 129–134. ( 10.2307/49642) [DOI] [Google Scholar]
- 38.Rodrigues PAP, Oliveira PS. 2014. Visual navigation in the Neotropical ant Odontomachus hastatus (Formicidae, Ponerinae), a predominantly nocturnal, canopy-dwelling predator of the Atlantic rainforest. Behav. Proc. 109, 48–57. ( 10.1016/j.beproc.2014.06.007) [DOI] [PubMed] [Google Scholar]
- 39.Wystrach A, Beugnon G, Cheng K. 2011. Landmarks or panoramas: what do navigating ants attend to for guidance? Front. Zool. 8, 21 ( 10.1186/1742-9994-8-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buehlmann C, Woodgate JL, Collett TS. 2016. On the encoding of panoramic visual scenes in navigating wood ants. Curr. Biol. 26, 2022–2027. ( 10.1016/j.cub.2016.06.005) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete GPS dataset is available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.1q269.