Abstract
Ecological and behavioural constraints play a major role in shaping the visual system of different organisms. In the mesopelagic zone of the deep- sea, between 200 and 1000 m, very low intensities of downwelling light remain, creating one of the dimmest habitats in the world. This ambient light is, however, enhanced by a multitude of bioluminescent signals emitted by its inhabitants, but these are generally dim and intermittent. As a result, the visual system of mesopelagic organisms has been pushed to its sensitivity limits in order to function in this extreme environment. This review covers the current body of knowledge on the visual system of one of the most abundant and intensely studied groups of mesopelagic fishes: the lanternfish (Myctophidae). We discuss how the plasticity, performance and novelty of its visual adaptations, compared with other deep-sea fishes, might have contributed to the diversity and abundance of this family.
This article is part of the themed issue ‘Vision in dim light’.
Keywords: deep-sea, Myctophid, visual adaptations, dim-light vision, bioluminescence, sensitivity
1. Introduction
The visual system of different organisms generally reflects each species' ecology and behaviour by becoming adapted to their lifestyle and ambient environmental constraints. In the marine environment, visual conditions are dependent on the light intensity, turbidity (amount of particulate or dissolved organic matter), type of habitat (benthic, pelagic, deep-sea), prey and predator relationships (size, colour), season, the organism's size and developmental stage and sexual communication [1].
Light in the ocean, and particularly in the deep-sea, has been extensively reviewed elsewhere [2–4]. Briefly, the oceanic visual environment is driven by two main sources of light: the downwelling light, produced by the sun and stars, and reflected and scattered by the moon and sky; and bioluminescence, produced by the organisms themselves. While bioluminescence is found at all depths, downwelling light is predominant in the surface layers (upper 200 m in the epipelagic zone) and its intensity diminishes rapidly with depth though absorption and scattering. Below 200 m, residual daylight is present in sufficient levels to allow visual tasks but not photosynthesis. Here the euphotic zone transitions into the mesopelagic or twilight zone. From around 800 to 1000 m in the clearest ocean, light from the surface is insufficient to be detected by deep-sea organisms [2], creating a dark and featureless background where bioluminescent emissions are the only sources of light. In addition to changes in intensities, the spectral range and direction of light also vary greatly with depth, at least to start with. While daylight can be seen in all directions and covers the entire visibly useful light spectrum (300–800 nm) in the shallows, residual light becomes vertically symmetrical, and short and long wavelengths are rapidly absorbed with depth, only leaving blue–green light (around 480 nm in clear ocean) in deeper waters. Deep-sea organisms have adapted to these particular conditions by producing bioluminescent emissions mainly in the blue–green range of the spectrum, although some species emit light of shorter or longer wavelengths [5]. Moreover, bioluminescent emissions may vary in intensity, duration, frequency, angular distribution and have an effective visual range limited to about 100–150 m [2]. It is worth mentioning that a third type of light signal, biofluorescence (which is not directly produced by the animals but results from the absorption of light and its re-emission at longer wavelengths), has recently been observed in the two deep-sea cnidarians [6,7] and might be used by deep-sea organisms for interaction with prey, predators and congeners as also suggested for their shallow water counterparts [8]. However, because its occurrence in other deep-sea organisms and function is still unknown, we will not mention it further in this review.
Deep-sea organisms rely on different sensory systems (vision, olfaction, hearing, taste, electroreception, lateral line) to survive in such an inhospitable environment. Depending on the depth and type of habitat in which they live, organisms mainly rely on one or more of these sensory systems [9,10]. In this review, we are particularly interested in the mesopelagic zone or twilight zone (200–1000 m), a transitional zone of the deep-sea characterized by an increasingly dimmer and unidirectional blue–green downwelling light and where bioluminescence is prevalent [11]. In this zone, the greatest variation in visual scenes within the deep-sea is found, ranging from extended (downwelling light) to point-like (bioluminescence) sources [3,12], and vision appears to be one of the dominant senses used by its inhabitants [9]. One of the most abundant and studied groups of mesopelagic fishes, the lanternfish (Myctophidae), provides us with a useful model to investigate how the visual systems of deep-sea organisms have developed to such low light-intensity conditions.
2. Lanternfish model
The lanternfish or Myctophidae is one of the most abundant and diverse families of mesopelagic fishes in the world's ocean with at least 250 species (33 genera, two subfamilies) [13]. They are distributed worldwide in all major oceans and seas from the surface (night-time) to depths exceeding 1000 m (daytime), and all produce and emit bioluminescence using a luciferin–luciferase reaction [14]. What makes this family particularly interesting for visual adaptation studies is their exceptionally high diversity in ecology and behaviour, with different species inhabiting a wide range of depths during day- and night-time, and presenting varied diel vertical migration and bioluminescent patterns [13,15–17]. This diversity not only allows the study of a wide range of visual adaptations to the deep-sea environment, but also allows comparison between closely related species with high ecological variability in order to better understand how these visual adaptations occurred and the selective pressures that may have driven such changes.
To elucidate how lanternfishes perceive objects in their surroundings, we first need to define their visual environment, and determine which visual tasks they need to perform. Myctophids are carnivorous fishes living in the mesopelagic zone during the day in order to avoid the numerous predators present in the well-lit shallow waters [17]. However, at these depths, food is quite sparse and as a result, most species and individuals migrate at night to the upper 200 m to feed in the biomass-rich surface layers while still being able to hide from predators in a dark environment [18]. It is also at night that lanternfishes find potential mates to reproduce [19]. Although nightlight (moon and stars) is 1–100 millions times dimmer than daylight (sun) [20], its intensity, in the best conditions (clear sky, full moon), is sufficient to create an extended visual scene in the first 400 m [2,12]. The visual scene in the upper layers at night is then comparable to the one found at deeper levels during the day (400 m at night comparable to 800 m in the middle of the day [2]) but with a higher amount of bioluminescent signals owing to increased biomass in the shallows. Shadows may be seen when looking upward, and bioluminescent emissions may come from any direction, at several intensities and frequencies. Moreover, depending to some extent on the intensity of the downwelling light (in turn dependent on depth), bioluminescent emissions will be more or less visible against the background space light [12].
Lanternfishes may use bioluminescence in several ways: for seeing prey and predators, for camouflaging themselves and for inter- and intraspecific communication [20–25]. They possess two kinds of photophores or bioluminescent organs that light up independently. There are the ventral and ventrolateral photophores also called primary photophores arranged in a species-specific pattern, and the luminous organs and tissue patches of various sizes and shape, located on the caudal peduncle, head and body. Because the pattern of the primary photophores is species-specific, it has been hypothesized that lanternfishes might use these for species recognition [21,22]; however, this has not been tested taking into consideration their visual capabilities, and it is currently unknown if they can resolve single photoreceptors or respond to their patterns differentially. Luminous organs (head and caudal) appear to only produce brief light flashes [23] and are often sexually dimorphic [24]. They are thought to play a role in communication within and between species [24], to avoid predators (caudal organs [25]) and/or to illuminate potential prey (head luminous organs [26]).
Downwelling light from the surface might also be used by myctophids for several purposes: to set their circadian rhythms, to hold a particular depth station, to vertically migrate, to camouflage themselves and to detect potential prey and predators. In common with several other mesopelagic fishes, lanternfishes camouflage themselves by emitting bioluminescence through their ventral photophores. This light matches the colour and intensity of the downwelling light to counter-shade their silhouette and appear inconspicuous to predators situated below and looking up [27,28]. In order to achieve such camouflage a visual system might compare the difference between the downwelling light and the bioluminescent emissions produced [27,29]. The efficiency of camouflage mediated by counter-illumination is, of course, dependent not only on the visual sensitivity of the producer, but also on the predator's visual acuity. In fact, predators with an acute eye (0.11 degrees resolution) would be able to break myctophid camouflage at distances up to 4 m [28]. Similarly, prey and/or predators that do not counter-shade or do not counter-shade effectively enough for the lanternfish visual system, will then cast a shadow against the downwelling background when viewed from below.
3. Lanternfishes' visual adaptations
(a). Sensitivity
Because mesopelagic organisms live in a dim environment, their eyes need to be more sensitive than acute to be able to detect levels of downwelling light and bioluminescent flashes. To enhance sensitivity, lanternfishes possess several visual adaptations that optimize light collection and extend their visual field. At the ocular level, these include: large eyes, aphakic gaps and reflective tapetum lucida. While enlarged eyes are not always the norm in myctophids and a great diversity in sizes is observed within the family [30], aphakic gaps and/or tapetum lucida are very common. At least one of these two adaptations was observed in 52 of 53 species investigated by de Busserolles et al. [31]. At the retinal level, increased sensitivity is achieved through several mechanisms including a pure rod retina, a very high rod density (highest recorded for vertebrates [32]) and correspondingly very thin photoreceptors (smallest diameter recorded for both vertebrates and invertebrates [32]), a high summation ratio of rod photoreceptors to ganglion cells (200–600 : 1 for Myctophum brachygnathum [32,33]), a lack of pigment granules in the retinal pigment epithelium of adults fish [31] and retinal pigments specifically tuned to the blue–green light environment of the mesopelagic zone [34].
Sensitivity to extended light sources or point-like sources can be increased by a variety of adaptations, and different lanternfish species appear to be better specialized for the detection of one signal or the other [32]. While sensitivity to downwelling light is mainly set by the level of summation of rods to ganglion cells (the diameter of the ganglion cell's dendritic field), sensitivity to bioluminescent signals is more dependent on the size of the pupil and the amount of background space light (downwelling light) [32].
The optical sensitivity S of an eye to an extended light source is often estimated using the formulae of Land [35] for monochromatic light [4,32,36]:
| 3.1 |
where A is the diameter of the pupil, f the focal length, d, l and k the diameter, outer segment length and absorption coefficient of the photoreceptors, respectively. However, formula (3.1) only calculates sensitivity at the level of the photoreceptor matrix (d, diameter of a single photoreceptor) and does not take into account the ganglion cell matrix. Alternatively, individuals living in a dim environment usually have a large summation of photoreceptors onto ganglion cells and calculations using formula (3.1) will underestimate the true sensitivity of their eyes, a parameter ultimately set by the ganglion cell's receptive field [37]. As an example, lanternfish optical sensitivity at the level of the photoreceptor matrix ranges from 0.26 to 2.39 µm2 sr [32] which is similar to the sensitivity found for the human eye (0.93 µm2 sr [4]). However, the human eye, notably in the fovea, has a ratio of photoreceptor to ganglion cells of 1 : 2 [38], whereas myctophid ratio in the peak density of ganglion cells is 200 : 1 [32,33]. If we now replace d by the diameter of one ganglion cell dendritic field assuming it is circular
where N is the density of ganglion cells in cell mm−2, then equation (3.1) becomes
| 3.2 |
Using equation (3.2), lanternfishes then possess a much higher optical sensitivity to extended light sources than the human eye (10–100 times higher, table 1). Compared to some deep-sea fishes, such as the bathypelagic Platytroctes apus, myctophids are more sensitive to extended light sources, but less sensitive than other mesopelagic representatives (table 1). The reason for a lower sensitivity to downwelling light in lanternfishes, compared to others living in the same environment, might be explained by their greater reliance on bioluminescence signals, especially for communication. In fact, while myctophids possess several luminous organs used for communication and interactions with prey, predator and congeners, the other species investigated in table 1 do not possess any bioluminescent organs (the escolar Lepidocybium flavobrunneum) or only ventral and lateral photophores used exclusively for counter-illumination and possibly species recognition (lantersharks: Etmopterus spp. and Squaliodus sp.). Consequently, if one wants to compare the optical sensitivity (S) of several individuals from different environments, especially vertebrates from dim-light habitats, one might want to do so at the level of the ganglion cell matrix (diameter of the ganglion cell's dendritic field) instead of the photoreceptor matrix (photoreceptor diameter).
Table 1.
Optical sensitivity S, calculated at the level of the ganglion cell matrix, for a range of species. When possible a range of estimation per species is given based on the lowest and highest ganglion cell density. Note that the highest ganglion cell density gives the lowest sensitivity S and vice versa. For all species, we fixed the photoreceptor coefficient of absorption k at 0.035 µm−1, the average for vertebrates [36]. For fish species, we used the diameter of the lens for A and Matthiessen's ratio to calculate f (f = 1.275A). l, Photoreceptor outer segment length; A, pupil or lens diameter; f, focal length; N, ganglion cell density; d, diameter of a ganglion cell's dendritic field; n.a., not available.
| species | l (μm) | A (mm) | f (mm) | N (×103 cells mm−2) | d (μm) | S (μm2 sr) | refs |
|---|---|---|---|---|---|---|---|
| lanternfishes | |||||||
| Bolinichthys longipesd | 117a | 1.8 | 2.3 | 3.4–14.9 | 9–19 | 32–140 | [32,33] |
| Electrona rissod | 64a | 3.4 | 4.3 | 1.4–8.0 | 13–30 | 54–308 | [32,33] |
| Lampanyctus alatusd | 52 | 1.3 | 1.7 | 3.2–19.6 | 8–20 | 21–126 | [32,33] |
| Myctophum brachygnathumd | 91a | 3.3 | 4.2 | 1.1–10.6 | 11–34 | 44–421 | [32,33] |
| Notoscopelus kroeyerid | 37 | 2.7 | 3.4 | 1.1–6.0 | 15–34 | 58–317 | [32,33] |
| Symbolophorus rufinusd | 78a | 2.8 | 3.6 | 2.0–11.2 | 11–25 | 40–226 | [32,33] |
| other teleosts | |||||||
| Platytroctes apuse | 150 | 5.1 | 6.5 | n.a.–26.2 | 7–n.a. | 18–n.a. | [39,40] |
| Lepidocybium flavobrunneumd | 148a | 16.0 | 20.4 | 0.1–0.6 | 46–113 | 801–4804 | [37] |
| Makaira nigricansc,d | 57 | 19.0 | 24.2 | 0.1–1.6 | 28–113 | 261–4174 | [41] |
| sharks | |||||||
| Etmopterus luciferd | 52 | 5.4 | 6.9 | n.a.–25.3 | 22–n.a. | 159–n.a. | [42] |
| Etmopterus spinaxd | 51 | 7.1 | 9.1 | n.a.–10.7 | 35–n.a. | 377–n.a. | [42] |
| Etmopterus splendidusd | 69 | 3.8 | 4.8 | n.a.–20.5 | 25–n.a. | 215–n.a. | [42] |
| Squaliolus aliaed | 48 | 3.1 | 4.0 | n.a.–38.9 | 18–n.a. | 101–n.a. | [42] |
| Triaenodon obesusc,d | 31 | 9.1 | 10.3 | n.a.–15.2 | 29–n.a. | 265–n.a. | [43] |
| Orectolobus ornatusc | 23 | 5.7 | 7.3 | n.a.–16.2 | 28–n.a. | 165–n.a. | [43] |
| Hemiscyllium ocellatumc | 35 | 4.5 | 5.7 | n.a.–23.7 | 23–n.a. | 114–n.a. | [43] |
| human | |||||||
| Homo sapiens | 30 | 8b | 16.7 | n.a.–38 | 6–n.a. | 3.1–n.a. | [35,38] |
aOuter segment length doubled by the presence of a tapetum lucidum.
bPupil diameter at night-time.
cEpipelagic.
dMesopelagic.
eBathypelagic.
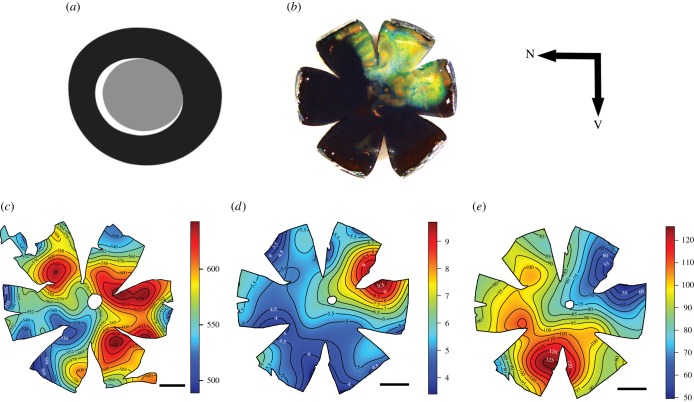
Finally, several of the lanternfish adaptations mentioned above are actually combined to optimize their visual system. In the popeye lanternfish Bolinichthys longipes, for example, the location of the aphakic gap aligns with the area of highest photoreceptor densities (the region of the visual field subtended by the area of high density of photoreceptors is increased by the aphakic gap; figure 1), a characteristic observed in most myctophid species [32]. The position of the aphakic gap also aligns with the position of the tapetum lucidum, enhancing the number of photons available in the dorsotemporal part of the retina where the highest density of ganglion cells is found. It may also provide better acuity in the frontal visual field, as well as ventrally (figure 1). Because the optical sensitivity S is dependent on the ganglion cell receptive field, S varies according to the ganglion cell densities and across the retina accordingly (figure 1). In B. longipes, the area of highest sensitivity is found in the ventral part of the retina, the part that directly receives downwelling light, assuming that the fish is positioned horizontally in the water column (figure 1e).
Figure 1.
Visual adaptations in the popeye lanternfish Bolinichthys longipes: (a) aphakic gap, represented in white, (b) tapetum lucidum, (c) photoreceptor topography, (d) ganglion cell topography, (e) sensitivity S topography. N, Nasal, V, ventral. Scale bars, 1 mm. Densities in (c,d) × 103 cells mm−2. Sensitivity S in (e) in μm2 sr. (a–d) Modified from [31–33] with the permission of S. Karger AG, Basel.
(b). Acuity and specialization
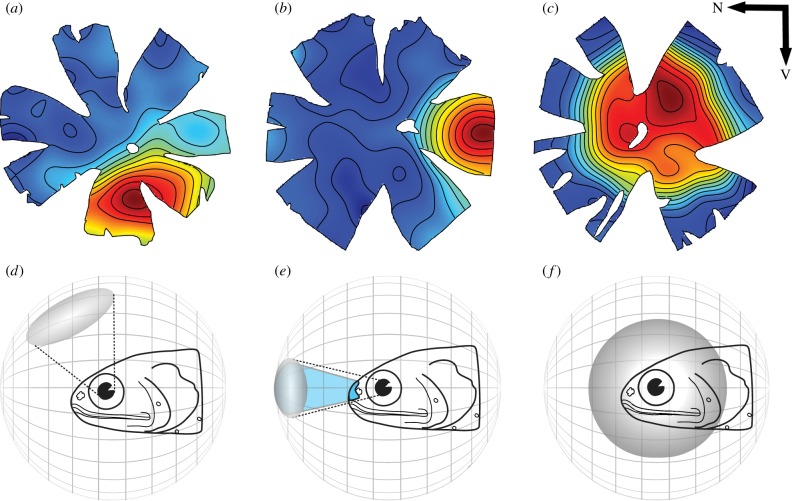
As a necessary trade-off for their high sensitivity, myctophids have very poor visual acuity with a spatial resolving power ranging from 1.6 to 4.8 cycles per degree, making them one of the less visually acute groups of deep-sea fishes [33,44]. Despite their poor acuity and contrary to previous findings [44], ganglion cell densities do vary across the retina in lanternfishes to form specific retinal specializations (areas of high cell densities) that provide higher acuity in a specific part of their visual field. Three main types of retinal specializations have been identified by analysing the ganglion cell (and excluding displaced amacrine cell) distribution across the retina in 21 species of lanternfishes [33,44]. These three specializations are: elongated areae ventro-temporales, areae temporales and large areae centrales. Each of these most likely corresponds to different behavioural and predatory strategies (figure 2 [33]).
Figure 2.
The three types of retinal specializations (a–c) found in lanternfishes and their corresponding ecological significance (d–f). (a–c) Topographic maps of ganglion cell densities: (a) elongated area ventrotemporalis, (b) area temporalis, (c) large area centralis. The colour gradient on the maps is similar to the one in figure 1, with red representing high cell densities and blue low cell densities. N, Nasal; V, ventral. Adapted from [33] with the permission of S. Karger AG, Basel. Panels (d–f) illustrate the visual significance of each topography (a–c), respectively. The shaded grey areas represent the regions of the visual field subtended by the retinal specializations (high density of ganglion cells represented in red to light blue on the maps). In (e), the lanternfish possesses a large head luminous organ that may emit light in front of the fish (in blue).
An elongated area ventrotemporalis, assuming that the fish is positioned horizontally in the water column, samples the frontal and dorsal part of the visual field with higher acuity, allowing the detection of silhouettes against the lighter background situated above (figure 2a,d). An area temporalis provides a higher acuity in the visual field situated right in front of the fish and may allow binocular vision, two common visual characteristics of predatory species (figure 2b,e [45]). Some of the myctophids with this specialization also possess large luminous organs on the front of their heads (Diaphus spp.) that may illuminate the area of high acuity to increase the chances of prey capture [33]. Finally, a large area centralis provides higher acuity in the centrolateral or monocular field of view, potentially allowing the animal to detect signals in a wide range of directions (figure 2c,f). This type of specialization is found in species with less specialized visual systems, indicating that they may be visual generalists using other sensory systems at least as much.
Another peculiarity of the lanternfish visual system is the very large population of ‘displaced’ amacrine cells (70–80%) found in their ganglion cell layer [33,44]. These amacrine cells are most likely of the type AII and may facilitate the detection of small bioluminescent flashes against the mesopelagic background, in the periphery of their visual field, where they are most abundant [33,46].
(c). A novel sexually dimorphic intraocular filter
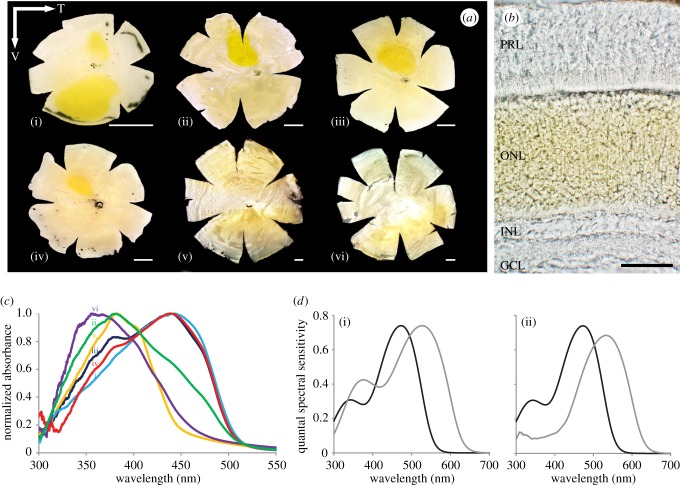
A novel retinal specialization was found in 10 species of lanternfishes out of 61 analysed, all from the Myctophinae subfamily (figure 3 [47]). This specialization is a yellow pigmentation present at the level of the outer nuclear layer and segregated into ‘patches’ of various numbers, size, shape and intensity, in specific parts of the retina (figure 3a,b). This yellow pigmentation was also found to be sexually dimorphic in two of the species analysed, making it the first example of a sexually dimorphic visual adaptation in any non-primate vertebrate. Further analyses including spectrophotometry, microspectrophometry, molecular biology and modelling, reveal that this yellow pigmentation acts as a filter, absorbing shorter wavelengths between 356 and 443 nm (figure 3c). It is found in species having two spectrally distinct rod photoreceptors resulting from the duplication of the Rh1 opsin gene; the pigmentation being specifically associated with the long-wavelength rods.
Figure 3.
Novel intraocular filter in lanternfishes. (a) Diversity in the yellow pigmentation distribution across the retina of six species of lanternfishes: Gonichthys tenuiculus (i), Hygophum proximum (ii), S. rufinus (iii), Symbolophorus evermanni (iv), Myctophum lychnobium (v) and Myctophum obtusirostre (vi). T, Temporal; V, ventral. (b) Location and distribution of the yellow pigmentation in the retina of G. tenuiculus. PRL, Photoreceptor layer; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar, 50 µm, (c) Normalized corrected absorbance spectra of the yellow pigment in each of the six species presented in (a). (d) Modelling of the quantal spectral sensitivity of the two visual pigments measured in M. obtusirostre, without the presence of the yellow pigmentation (i) and with the yellow pigmentation associated with the long-wave-shifted visual pigment (527 nm) (ii). Black line: visual pigment 473 nm, grey line: visual pigment 527 nm. Adapted from [47] with the permission of S. Karger AG, Basel.
The effect of the yellow pigmentation on the spectral sensitivity of the long-wavelength rods is to shift the overall sensitivity peak slightly towards longer wavelengths. It also narrows the spectral sensitivity function by absorbing short wavelengths, tuning the photoreceptor to longer wavelength signals only (figure 3d). The authors concluded that this new intraocular filter might, similar to yellow lenses in other deep-sea fish [48], increase the contrast of bioluminescent signals by differentially filtering the background irradiance resulting from the downwelling illumination. The result would be to improve the detection of bioluminescent signals in a specific part of the visual field of the animal. The ecological function of this pigment is still uncertain and might be species specific: to potentially break the counter-illumination camouflage of some predators, detect a specific signal from a potential mate in the case of the sexually dimorphic species, detect a specific bioluminescent prey or even help to detect predators emitting signals of longer wavelengths (red-light-emitting species such as stomiid fish [49,50]).
4. What drove the variability in lanternfish visual systems?
Interspecific variability in the visual system of lanternfishes is high at all levels (ocular, retinal and molecular [30–33,47]). Representative examples of this variation are the genera Myctophum and Lampanyctus. Species from the genus Myctophum possess eyes with a large size, a localized aphakic gap, a tapetum lucidum covering the entire retina, the highest rod density, the highest spatial resolving power and additional specialization such as the sexually dimorphic retinal yellow pigment and two rod opsins. Lampanyctus species, on the other hand, possess a less specialized visual system with a small eye size, low rod densities, a poorly specialized retina, lower acuity, and a variable tapetum lucidum and aphakic gap coverage.
In an attempt to explain this interspecific variability in visual adaptations, possible relationships between morphological variables (eye and photoreceptor size) and ecological variables (depth range, presence/absence of luminous organs) were assessed using phylogenetic comparative analyses [30,32]. The results revealed that both ecological and phylogenetic constraints drove the evolution of the visual system in lanternfishes [30,32]. Some visual specializations were strongly influenced by phylogeny (eye size [30], yellow pigment [47], type of retinal specializations [33] and visual pigments [34]), whereas others appeared to be driven by ecological constraints (aphakic gap and tapetum lucidum [31], rod diameter and length [32] and some types of retinal specializations [33]). The main ecological factor influencing photoreceptor design, and consequently sensitivity in lanternfishes, was the depth range at night, indicating that vision at night is of great importance in myctophids [32]. This is not surprising when most lanternfish are known to perform their main survival tasks (feeding and reproducing) in the surface layers at night.
We previously mentioned that the type of visual adaptations depends on which signal is more important: an extended source or a point-like source. Downwelling light, characterized by the night depth range, drives, to some extent, the evolution of the visual system in lanternfishes; however, because bioluminescence is used extensively by lanternfishes for a variety of tasks [23], it presumably also determined the evolution of their visual system. Several lines of evidence support this hypothesis:
(1) the evolution of species-specific bioluminescent structures allowed a higher and faster species diversification [22],
(2) visual pigments are spectrally tuned to maximize their sensitivity to the bioluminescent emissions present in their environment, rather than to the downwelling light [34,47,51],
(3) eyes are less sensitive to downwelling light compared with other mesopelagic species that do not bioluminescent at all or not in a (presumed) communicative way (this review),
(4) a high proportion of supposedly ‘AII amacrine cells’ is present in the ganglion cell layer, and may have evolved to potentially facilitate the detection of small bioluminescent flashes against the mesopelagic background [33],
(5) relationships between visual characteristics and sexual dimorphism in luminous tissues also exist [32]: species with a sexual dimorphism in luminous tissues possess higher rod densities (an adaptation for higher sensitivity) [32] and a species-specific sexually dimorphic intraocular filter (yellow pigment [47]) enhancing contrast and improving the detection of bioluminescent signals. Indeed, the fact that species with sexually dimorphic luminous tissues also possessed more sensitive eyes and a sexually dimorphic visual system strengthens the long-standing hypothesis that bioluminescence is used for intraspecific communication in lanternfishes, which has been proposed by a range of authors [23,24,52].
5. Visual arms race in the deep-sea
Lanternfishes possess a wide range of visual adaptations to their environment, but how do their specializations compare against different adaptations found in other groups of deep-sea fishes? The family is not only one of the most abundant in the world's oceans, in terms of densities [53], but also in term of species numbers having diversified at much quicker rates than other fish families [22]. But why are they so successful? In this section, we discuss how the diversity, performance and novelty of the lanternfish visual adaptations, compared to other deep-sea fishes, might have contributed to their success.
Several other visual adaptations that not are found in myctophids exist in other deep-sea fishes. These include tubular eyes, yellow lenses, multibank retinas, foveas and sensitivity to red light. Each of these adaptations has been reviewed in detail elsewhere [2,3,39,54], and so we will only give a brief account of each of them and focus on the comparison with the lanternfish system.
Tubular eyes are usually cylindrical and positioned dorsally to maximize pupil diameter and focal length in order to increase sensitivity and resolution in the dorsal field of view of the animal. In some cases, they have become specialized to look into a narrow field, taking in the narrow angle of light from the surface. Although visual capabilities to detect shadows against the downwelling background are dramatically improved, it is often at the expense of the rest of the visual field. As a result, some tubular eyed species have evolved additional specializations to re-extend signal detection in other parts of the visual field (frontal eye rotation [55], accessory retina [56], lens pad [39], retinal diverticulum [57]), but in most cases, their visual system still remains restricted to largely upward viewing. Lanternfishes, with their large eyes and slender body shape possess a very large monocular field of view in addition to possible binocular vision frontally, dorsally and ventrally [31]. Similar to tubular eyes, their elongated area ventro-temporalis at the level of the ganglion cell layer allows the detection of silhouettes situated above but without limiting visual detection in other directions.
Yellow lenses are present in few deep-sea fishes such as the scopelarchids or some hatchetfish [58], and similar to lanternfishes, yellow pigmentation might increase hue discrimination of bioluminescent signals against the downwelling background [48]. While yellow lenses will reduce the photon capture of the entire visual system of the animal, the lanternfish retinal yellow pigmentation, being segregated in specific parts of the retina, only affects a restricted part of the visual field of the animal, potentially allowing a wider range of visual capabilities.
Several mesopelagic fishes have increased the sensitivity of their eye by staking photoreceptors in several layers, also called multibank retinae [59,60]. In addition to increased sensitivity by enhancing photon capture, multibank retinae may also allow colour vision in single pigment species or at least increase hue discrimination in the blue to greenish-yellow part of the spectrum [2,59]. Although lanternfishes only have a single bank of photoreceptors, the following specializations: increase in rod length, tapetum lucida of different reflectivities and colours, rod opsin duplication, and retinal yellow pigmentation, associated together, as found in several myctophid species, may be directed at similar endpoints as the multibank strategy.
Three dragonfish genera (Malacosteus, Aristostomias, Pachystomias) are able to emit and see far-red bioluminescence [49,51,61], providing them with a private visual channel to illuminate and see potential prey or communicate with conspecifics without being seen. There is currently no evidence that lanternfishes are able to emit any other types of bioluminescence than the frequently used blue–green range. However, species possessing a second long-wavelength sensitive rod, associated with retinal yellow pigmentation, might be able to detect longer-wavelength light emitted by some of their predators (e.g. Aristostomis [47,50]) [47]. Moreover, myctophids with these long-wavelength sensitivities might construct their own discrete communication channel between potential mates with the sexually dimorphic luminous organs and retinal yellow pigmentation [47].
Over 30 species of deep-sea fishes have developed a fovea or retinal pit, an indentation of a particular region of the retina in which a high density of retinal receptors is present [62]. In the deep-sea environment, foveas might increase resolution and especially enhance the ability to detect movement. They might also play a role in depth perception and bioluminescent camouflage breaking by providing a skewed image as an object passes across the visual field [62,63]. Although lanternfishes do not possess any foveas, the visual system of some species has also adapted to enhance resolution (area temporalis coupled with head luminous organs) and the potential to break counter-illumination camouflage (yellow pigmentation).
The surprising variety of visual adaptations present in deep-sea fishes, given their relatively simple visual world, highlights the competitive evolutionary pressures or ‘visual arms race’, to select the most efficient visual systems in this extreme environment. Even though some deep-sea fishes have evolved more complex visual systems for the detection of a specific type of visual cue (e.g. the tubular eye of Rhynchohyalus natalensis [57], multibank and fovea of Bajacalifornia drakei [63], far-red bioluminescent light system in dragonfishes [61]), lanternfishes have developed a flexible visual system with a wide range of adaptations to allow the detection of diverse signals in any direction. Because myctophids are often targets for a variety of predators (fishes, squids, marine birds and mammals) as well as themselves predating on a wide range of trophic levels [64], the plasticity of their visual system may well be a response to this as well as the pressing need to find mates in the relatively sparse ocean world. Whatever the case, this plasticity most likely contributed to the great success of the family in the deep-sea.
Myctophids represent a valued fish family at several levels. Ecologically, they play an essential role (central link) in the marine ecosystem by providing energy to the deeper levels of the ocean [17,53]. Economically, their status as one of the most abundant fish on Earth [53] unfortunately makes them an attractive and still underexploited target for diverse human uses by fisheries [17,65]. Finally, their extremely high abundance and diversity at all levels (species, ecology and behaviour) make the family a prime model for comparative studies, especially to shed light on evolution in the deep-sea [65].
Acknowledgements
We thank our collaborators, Shaun P. Collin, John R. Paxton, John L. Fitzpatrick, Nathan S. Hart, David M. Hunt, Wayne I. Davies, Michael W. Clarke and Dorothee Hahne, for their contribution to previous research forming part of this review. We gratefully acknowledge Eric J. Warrant for his helpful discussions regarding sensitivity estimations, and Karen Cheney for providing helpful comments on this manuscript.
Authors' contributions
F.d.B. and N.J.M. contributed to writing this review.
Competing interests
We have no competing interests.
Funding
Australian Research Council.
References
- 1.Sandström A. 1999. Visual ecology of fish–a review with special reference to percids. Fiskeriverket Rapport 2, 45–80. [Google Scholar]
- 2.Denton EJ. 1990. Light and vision at depths greater than 200 metres. In Light and life in the sea (eds Herring PJ, Campbell AK, Whitfield M, Maddock L), pp. 127–148. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Warrant EJ, Locket NA. 2004. Vision in the deep sea. Biol. Rev. 79, 671–712. ( 10.1017/s1464793103006420) [DOI] [PubMed] [Google Scholar]
- 4.Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Widder EA. 2010. Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity. Science 328, 704–708. ( 10.1126/science.1174269) [DOI] [PubMed] [Google Scholar]
- 6.Haddock SHD, Dunn CW, Pugh PR, Schnitzler CE. 2005. Bioluminescent and red-fluorescent lures in a deep-sea siphonophore. Science 309, 263 ( 10.1126/science.1110441) [DOI] [PubMed] [Google Scholar]
- 7.Vogt A, D'Angelo C, Oswald F, Denzel A, Mazel CH, Matz MV, Ivanchenko S, Nienhaus GU, Wiedenmann J. 2008. A green fluorescent protein with photoswitchable emission from the deep sea. PLoS ONE 3, e3766 ( 10.1371/journal.pone.0003766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparks JS, Schelly RC, Smith WL, Davis MP, Tchernov D, Pieribone VA, Gruber DF. 2014. The covert world of fish biofluorescence: a phylogenetically widespread and phenotypically variable phenomenon. PLoS ONE 9, e83259 ( 10.1371/journal.pone.0083259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner H-J. 2001. Sensory brain areas in mesopelagic fishes. Brain Behav. Evol. 57, 117–133. ( 10.1159/000047231) [DOI] [PubMed] [Google Scholar]
- 10.Wagner H-J. 2001. Brain areas in abyssal demersal fishes. Brain Behav. Evol. 57, 301–316. ( 10.1159/000047249) [DOI] [PubMed] [Google Scholar]
- 11.Herring PJ. 2002. The biology of the deep-sea. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Nilsson D-E, Warrant E, Johnsen S. 2014. Computational visual ecology in the pelagic realm. Phil. Trans. R. Soc. B 369, 20130038 ( 10.1098/rstb.2013.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulley PA, Paxton JR. 2016. Myctophidae. In The living marine resources of the eastern Central Atlantic, Vol. 3, Bony fishes, part 1 (Elopiformes to Scorpaeniformes) (eds Carpenter KE, De Angelis N), pp. 1860–1928. Rome, Italy: FAO. [Google Scholar]
- 14.Haygood MG, Edwards DB, Mowlds G, Rosenblatt RH. 1994. Bioluminescence of myctophid and stomiiform fishes is not due to bacterial luciferase. J. Exp. Zool. 270, 225–231. ( 10.1002/jez.1402700212) [DOI] [Google Scholar]
- 15.Karnella C. 1987. Family Myctophidae, lanternfishes. In Biology of midwater fishes of the Bermuda Ocean Acre (eds Gibbs RH, Krueger WH), pp. 51–168. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 16.Watanabe H, Moku M, Kawaguchi K, Ishimaru K, Ohno A. 1999. Diel vertical migration of myctophid fishes (Family Myctophidae) in the transitional waters of the western North Pacific. Fish. Oceanogr. 8, 115–127. ( 10.1046/j.1365-2419.1999.00103.x) [DOI] [Google Scholar]
- 17.Catul V, Gauns M, Karuppasamy PK. 2011. A review on mesopelagic fishes belonging to family Myctophidae. Rev. Fish. Biol. Fish. 21, 339–354. ( 10.1007/s11160-010-9176-4) [DOI] [Google Scholar]
- 18.Kinzer J, Schulz K. 1985. Vertical distribution and feeding patterns of midwater fish in the central equatorial Atlantic. I. Myctophidae. Mar. Biol. 85, 313–322. ( 10.1007/BF00393252) [DOI] [Google Scholar]
- 19.Gartner JV. 1993. Patterns of reproduction in the dominant lanternfish species (Pisces: Myctophidae) of the eastern Gulf of Mexico, with a review of reproduction among tropical-subtropical Myctophidae. Bull. Mar. Sci. 52, 721–750. [Google Scholar]
- 20.Land MF, Nilsson D-E. 2002. Animals eyes. Oxford, UK: Oxford University Press. [Google Scholar]
- 21.Beebe W, Vander Pyl M. 1944. Eastern Pacific expeditions of the New York Zoological Society. XXXIII. Pacific Myctophidae (fishes). Zoologica 29, 59–95. [Google Scholar]
- 22.Davis MP, Holcroft NI, Wiley EO, Sparks JS, Smith WL. 2014. Species-specific bioluminescence facilitates speciation in the deep sea. Mar. Biol. 161, 1139–1148. ( 10.1007/s00227-014-2406-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards AS, Herring PJ. 1977. Observations on the comparative morphology and operation of the photogenic tissues of myctophid fishes. Mar. Biol. 41, 59–70. ( 10.1007/BF00390582) [DOI] [Google Scholar]
- 24.Herring PJ. 2007. Sex with the lights on? A review of bioluminescent sexual dimorphism in the sea. J. Mar. Biol. Assoc. UK 87, 829–842. ( 10.1017/S0025315407056433) [DOI] [Google Scholar]
- 25.Paxton JR. 1972. Oeteology and relationships of the lanternfishes (family Myctophidae). Sci. Bull. Los Angeles County Nat. Hist. Museum 13, 1–81. [Google Scholar]
- 26.Herring PJ. 1985. How to survive in the dark: bioluminescence in the deep sea. In Physiological adaptations in marine animals (ed. Laverack MS.), pp. 323–350. London, UK: Society for Experimental Biology. [PubMed] [Google Scholar]
- 27.Case JF, Warner J, Barnes AT, Lowenstine M. 1977. Bioluminescence of lantern fish (Myctophidae) in response to changes in light intensity. Nature 265, 179–181. ( 10.1038/265179a0) [DOI] [PubMed] [Google Scholar]
- 28.Johnsen S, Widder EA, Mobley CD. 2004. Propagation and perception of bioluminescence: factors affecting counterillumination as a cryptic strategy. Biol. Bull. 207, 1–16. ( 10.2307/1543624) [DOI] [PubMed] [Google Scholar]
- 29.Young RE, Roper CFE, Walters JF. 1979. Eyes and extra-ocular photoreceptors in midwater cephalopods and fishes - their roles in detecting downwelling light for counter-illumination. Mar. Biol. 51, 371–380. ( 10.1007/BF00389215) [DOI] [Google Scholar]
- 30.de Busserolles F, Fitzpatrick JL, Paxton JR, Marshall NJ, Collin SP. 2013. Eye-size variability in deep-sea lanternfishes (Myctophidae): an ecological and phylogenetic study. PLos ONE 8, e58519 ( 10.1371/journal.pone.0058519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Busserolles F, Marshall NJ, Collin SP. 2014. The eyes of lanternfishes (Myctophidae, Teleostei): novel ocular specializations for vision in dim light. J. Comp. Neurol. 522, 1618–1640. ( 10.1002/cne.23495) [DOI] [PubMed] [Google Scholar]
- 32.de Busserolles F, Fitzpatrick JL, Marshall NJ, Collin SP. 2014. The influence of photoreceptor size and distribution on optical sensitivity in the eyes of lanternfishes (Myctophidae). PLoS ONE 9, e99957 ( 10.1371/journal.pone.0099957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Busserolles F, Marshall NJ, Collin SP. 2014. Retinal ganglion cell distribution and spatial resolving power in deep-sea lanternfishes (Myctophidae). Brain Behav. Evol. 84, 262–276. ( 10.1159/000365960) [DOI] [PubMed] [Google Scholar]
- 34.Turner JR, White EM, Collins MA, Partridge JC, Douglas RH. 2009. Vision in lanternfish (Myctophidae): adaptations for viewing bioluminescence in the deep-sea. Deep Sea Res. Part I 56, 1003–1017. ( 10.1016/j.dsr.2009.01.007) [DOI] [Google Scholar]
- 35.Land MF. 1981. Optics and vision in invertebrates. In Handbook of sensory physiology, vol. VII/6B (ed. Autrum H.), pp. 471–492. Berlin, Germany: Springer. [Google Scholar]
- 36.Warrant EJ, Nilsson D-E. 1998. Absorption of white light in photoreceptors. Vis. Res. 38, 195–207. ( 10.1016/S0042-6989(97)00151-X) [DOI] [PubMed] [Google Scholar]
- 37.Landgren E, Fritsches K, Brill R, Warrant E. 2014. The visual ecology of a deep-sea fish, the escolar Lepidocybium flavobrunneum (Smith, 1843). Phil. Trans. R. Soc. Lond. B 369, 20130039 ( 10.1098/rstb.2013.0039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curcio CA, Allen KA. 1990. Topography of ganglion cells in human retina. J. Comp. Neurol. 300, 5–25. ( 10.1002/cne.903000103) [DOI] [PubMed] [Google Scholar]
- 39.Locket NA. 1977. Adaptations to the deep-sea environment. In Handbook of sensory physiology (ed. Crescitelli F.), pp. 67–192. Berlin, Germany: Springer. [Google Scholar]
- 40.Collin SP, Partridge JC. 1996. Retinal specializations in the eyes of deep-sea teleosts. J. Fish Biol. 49, 157–174. ( 10.1111/j.1095-8649.1996.tb06073.x) [DOI] [Google Scholar]
- 41.Fritsches KA, Marshall NJ, Warrant EJ. 2003. Retinal specializations in the blue marlin: eyes designed for sensitivity to low light levels. Mar. Freshw. Res. 54, 333–341. ( 10.1071/MF02126) [DOI] [Google Scholar]
- 42.Claes JM, Partridge JC, Hart NS, Garza-Gisholt E, Ho H-C, Mallefet J, Collin SP. 2014. Photon hunting in the twilight zone: visual features of mesopelagic bioluminescent sharks. PLoS ONE 9, e104213 ( 10.1371/journal.pone.0104213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litherland L, Collin SP. 2008. Comparative visual function in elasmobranchs: spatial arrangement and ecological correlates of photoreceptor and ganglion cell distributions. Vis. Neurosci. 25, 549–561. ( 10.1017/S0952523808080693) [DOI] [PubMed] [Google Scholar]
- 44.Wagner H-J, Fröhlich E, Negishi K, Collin SP. 1998. The eyes of deep-sea fish II. Functional morphology of the retina. Prog. Retin. Eye Res. 17, 637–685. ( 10.1016/S1350-9462(98)00003-2) [DOI] [PubMed] [Google Scholar]
- 45.Hughes A. 1977. The topography of vision in mammals of contrasting life style: comparative optics and retinal organisation. In Handbook of sensory physiology, vol. VII/5 (ed. Crescitelli F.), pp. 613–756. Berlin, Germany: Springer. [Google Scholar]
- 46.Nelson R. 1982. AII amacrine cells quicken time course of rod signals in the cat retina. J. Neurophysiol. 47, 928–947. [DOI] [PubMed] [Google Scholar]
- 47.de Busserolles F, Hart NS, Hunt DM, Davies WI, Marshall NJ, Clarke MW, Hahne D, Collin SP. 2015. Spectral tuning in the eyes of deep-sea lanternfishes (Myctophidae): a novel sexually dimorphic intra-ocular filter. Brain Behav. Evol. 85, 77–93. ( 10.1159/000371652) [DOI] [PubMed] [Google Scholar]
- 48.Muntz W. 1976. On yellow lenses in mesopelagic animals. J. Mar. Biol. Assoc. UK 56, 963–976. ( 10.1017/S0025315400021019) [DOI] [Google Scholar]
- 49.Widder EA, Latz MI, Herring PJ, Case JF. 1984. Far red bioluminescence from two deep-sea fishes. Science 225, 512–514. ( 10.1126/science.225.4661.512) [DOI] [PubMed] [Google Scholar]
- 50.Sutton TT, Hopkins TL. 1996. Trophic ecology of the stomiid (Pisces: Stomiidae) fish assemblage of the eastern Gulf of Mexico: strategies, selectivity and impact of a top mesopelagic predator group. Mar. Biol. 127, 179–192. ( 10.1007/bf00942102) [DOI] [Google Scholar]
- 51.Douglas RH, Partridge JC, Marshall NJ. 1998. The eyes of deep-sea fish I: lens pigmentation, tapeta and visual pigments. Prog. Retin. Eye Res. 17, 597–636. ( 10.1016/S1350-9462(98)00002-0) [DOI] [PubMed] [Google Scholar]
- 52.Barnes AT, Case JF. 1974. Luminescence of lanternfish (Myctophidae) - spontaneous activity and responses to mechanical, electrical, and chemical stimulation. J. Exp. Mar. Biol. Ecol. 15, 203–221. ( 10.1016/0022-0981(74)90046-X) [DOI] [Google Scholar]
- 53.Irigoien X, et al. 2014. Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 5, 3271 ( 10.1038/ncomms4271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warrant EJ, Collin SP, Locket NA. 2003. Eye design and vision in deep-sea fishes. In Sensory processing in aquatic environments (eds Collin SP, Marshall NJ), pp. 303–322. New York, NY: Springer. [Google Scholar]
- 55.Robison BH, Reisenbichler KR. 2008. Macropinna microstoma and the paradox of its tubular eyes. Copeia 2008, 780–784. ( 10.1643/CG-07-082) [DOI] [Google Scholar]
- 56.Collin SP, Hoskins RV, Partridge JC. 1997. Tubular eyes of deep-sea fishes: a comparative study of retinal topography. Brain Behav. Evol. 50, 335–357. ( 10.1159/000113345) [DOI] [PubMed] [Google Scholar]
- 57.Partridge JC, Douglas RH, Marshall NJ, Chung W-S, Jordan TM, Wagner H-J. 2014. Reflecting optics in the diverticular eye of a deep-sea barreleye fish (Rhynchohyalus natalensis). Phil. Trans. R. Soc. B 281, 20133223 ( 10.1098/rspb.2013.3223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Douglas RH, Thorpe A. 1992. Short-wave absorbing pigments in the ocular lenses of deep-sea teleosts. J. Mar. Biol. Assoc. UK 72, 93–112. ( 10.1017/S0025315400048815) [DOI] [Google Scholar]
- 59.Denton EJ, Locket NA. 1989. Possible wavelength discrimination by multibank retinae in deep-sea fishes. J. Mar. Biol. Assoc. UK 69, 409–435. ( 10.1017/S0025315400029507) [DOI] [Google Scholar]
- 60.Fröhlich E, Wagner HJ. 1998. Development of multibank rod retinae in deep-sea fishes. Vis. Neurosci. 15, 477–483. ( 10.1017/S095252389815304X) [DOI] [PubMed] [Google Scholar]
- 61.Herring P, Cope C. 2005. Red bioluminescence in fishes: on the suborbital photophores of Malacosteus, Pachystomias and Aristostomias. Mar. Biol. 148, 383–394. ( 10.1007/s00227-005-0085-3) [DOI] [Google Scholar]
- 62.Collin SP, Lloyd DJ, Wagner H-J. 2000. Foveate vision in deep-sea teleosts: a comparison of primary visual and olfactory inputs. Phil. Trans. R. Soc. Lond. B 355, 1315–1320. ( 10.1098/rstb.2000.0691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Locket NA. 1985. The multiple bank rod fovea of Bajacalifornia drakei, an alepocephalid deep-sea teleost. Proc. R. Soc. Lond. B. 224, 7–22. ( 10.1098/rspb.1985.0018) [DOI] [Google Scholar]
- 64.Cherel Y, Fontaine C, Richard P, Labat JP. 2010. Isotopic niches and trophic levels of myctophid fishes and their predators in the Southern Ocean. Limnol. Oceanogr. 55, 324–332. ( 10.4319/lo.2010.55.1.0324) [DOI] [Google Scholar]
- 65.Denton JSS. 2014. Seven-locus molecular phylogeny of Myctophiformes (Teleostei; Scopelomorpha) highlights the utility of the order for studies of deep-sea evolution. Mol. Phylogenet. Evol. 76, 270–292. ( 10.1016/j.ympev.2014.02.009) [DOI] [PubMed] [Google Scholar]