Abstract
Photoreceptors in animals are generally of two kinds: the ciliary or c-type and the rhabdomeric or r-type. Although ciliary photoreceptors are found in many phyla, vertebrates seem to be unique in having two distinct kinds which together span the entire range of vision, from single photons to bright light. We ask why the principal photoreceptors of vertebrates are ciliary and not rhabdomeric, and how rods evolved from less sensitive cone-like photoreceptors to produce our duplex retina. We suggest that the principal advantage of vertebrate ciliary receptors is that they use less ATP than rhabdomeric photoreceptors. This difference may have provided sufficient selection pressure for the development of a completely ciliary eye. Although many of the details of rod evolution are still uncertain, present evidence indicates that (i) rods evolved very early before the split between the jawed and jawless vertebrates, (ii) outer-segment discs make no contribution to rod sensitivity but may have evolved to increase the efficiency of protein renewal, and (iii) evolution of the rod was incremental and multifaceted, produced by the formation of several novel protein isoforms and by changes in protein expression, with no one alteration having more than a few-fold effect on transduction activation or inactivation.
This article is part of the themed issue ‘Vision in dim light’.
Keywords: evolution, photoreceptor, vision, rod, cone, lamprey
1. Introduction
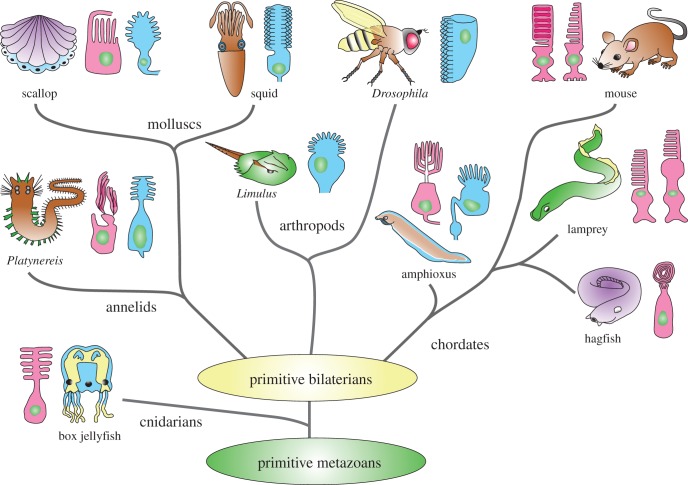
Phototransduction with rhodopsin pigments and a G-protein cascade emerged very early in the evolution of metazoans. Eyes containing photoreceptors are widespread among animals (figure 1) and are generally of two types: the ciliary or c-type with membrane developing from a basal body and cilium, and the rhabdomeric or r-type whose pigment-containing membranes are formed from microvilli. We do not know when these two types of photoreceptors first emerged. Ciliary photoreceptors have been found in some species of cnidarians (for example box jellyfish, see [2]), which as a group have been shown to express a large family of opsins [3,4]. These pigments, sometimes called cnidops, are closely related to the c-opsin family of photopigments found in the ciliary photoreceptors of some other invertebrate phyla and all vertebrates [4]. The more common photoreceptors among invertebrates are the rhabdomeric, which may also be very old, perhaps also emerging among cnidarians or their ancestors [5]. Their photopigments constitute the separate class of r-opsins.
Figure 1.
Phylogenetic tree of metazoans showing only representative animal groups or species with photoreceptor types in principal eyes illustrated as ciliary (red) or microvillar (blue). (Modified and reproduced with permission from Fain et al. [1]).
It is easy to explain how rhabdomeric photoreceptors and r-opsins became so common in the principal eyes of invertebrates, from the small ocelli of spiders to the massive lateral eyes of giant squid. Rhabdomeric photoreceptors are excellent single-photon detectors but can also adapt and function even in bright bleaching light [6]. They can regenerate pigment quickly with light and without an enzymatic pathway. Moreover, houseflies can detect flickering light up to 300 Hz [7], double the highest recorded value for a ciliary photoreceptor of 140 Hz in the pigeon [8] and much greater than that of the human eye, which can barely detect the flickering of a 50 Hz tungsten lamp.
Despite these obvious advantages, many invertebrate species nevertheless have ciliary photoreceptors instead of, or in addition to rhabdomeric photoreceptors (figure 1). The ciliary photoreceptors in invertebrates seem to be used as simple light detectors and for bright-light vision like our cones. There is no evidence for a rod-like photoreceptor in any invertebrate species. Moreover, comparison of the DNA sequences of a large number of vertebrate pigments indicates that all of the cone pigments emerged before the rod pigment evolved [9,10]. This and other evidence indicates that cones are older and that rods evolved from cells with at least some of the properties of cones. Vertebrates seem to be unique in having two distinct kinds of ciliary photoreceptors which together span the entire range of vision, from single-photons to bright-light intensities. The key step was the evolution of the rod.
These observations pose two questions. First, why are the principal photoreceptors of vertebrates ciliary and not rhabdomeric? How did rods evolve from less sensitive cone-like ciliary photoreceptors to produce our duplex retina?
2. Why are vertebrate photoreceptors ciliary?
Vertebrates evolved from primitive chordates, which may have had both rhabdomeric and ciliary eyes each containing only its own sort of photoreceptor. Both rhabdomeric and ciliary eyes are present in amphioxus [11], the organism generally thought to be the closest living relative to stem chordates [12,13]. Although nothing is known about the physiology of amphioxus ciliary photoreceptors, recordings from the rhabdomeric eyes show that their cells respond to both dim and bright light and behave in many respects like the rhabdomeric photoreceptors of arthropods [14]. If eyes of this kind were present in stem vertebrates, they were lost during the further evolution of the vertebrate line. The principal eyes of early vertebrates seem instead to have adopted c-opsins and ciliary photoreceptors, perhaps at first only cells like cones, but eventually both rods and cones and a duplex retina.
What advantage did the ciliary photoreceptors have that might have facilitated this development? It certainly was not single-photon detection or sensitivity to change or motion, because rhabdomeric photoreceptors are at least as good if not better in their sensitivity and speed of responsiveness. One possible explanation is that the density of photopigment that can be packed on the lamellae and discs of ciliary photoreceptors can be much larger than for the microvilli of rhabdomeric photoreceptors, increasing the probability of photon absorption [1]. Another is that the ciliary photoreceptors of vertebrates use less ATP and are therefore less of a drain on the energy budget of the organism. The minimizing of ATP and energy use has long been thought to play a role in the design and evolution of both sensory receptors [15] and the central nervous system [16].
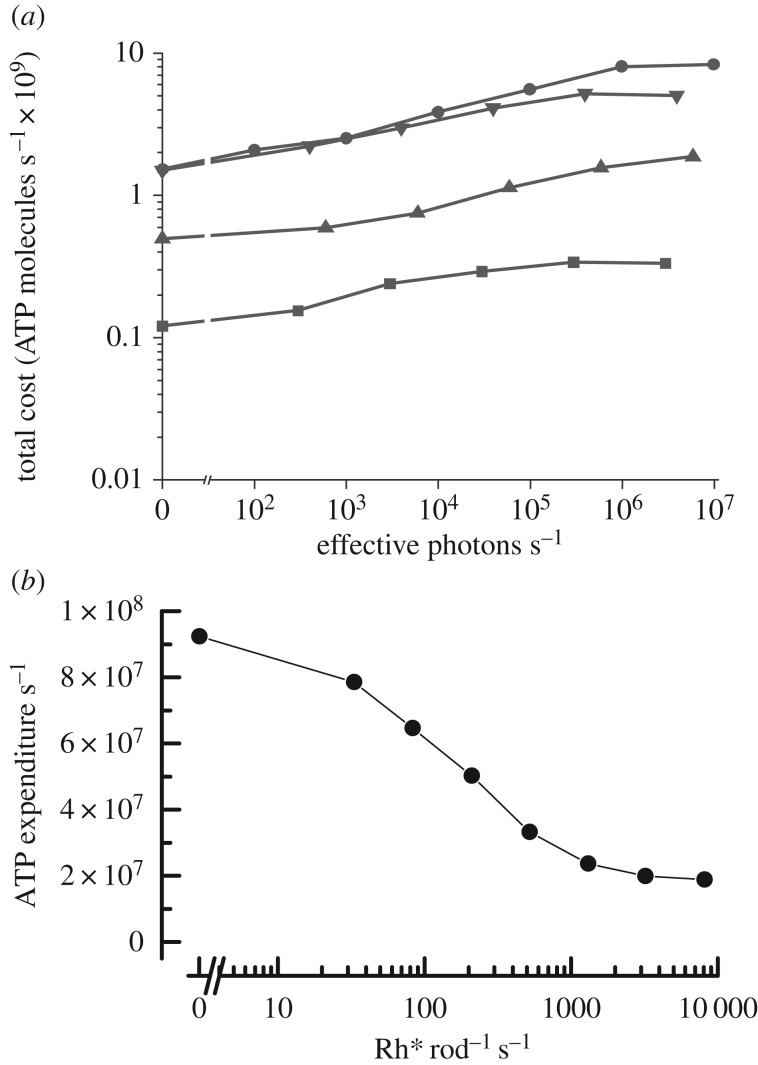
In figure 2, we compare the calculations of ATP utilization for fly rhabdomeric photoreceptors (figure 2a; [17]) and mammalian rods (figure 2b; [18]). The fly calculations take into account only the utilization of energy by the Na+/K+ ATPase, based on the resting and light-dependent conductances of the photoreceptor. They do not include the utilization of ATP by transduction or the pumping of Ca2+ at the synaptic terminal and are therefore likely to be underestimates. The rod calculations include the pumping of the Na+/K+ ATPase required by the cGMP-gated conductance of the outer segment as well as other conductances in the inner segment, with (in addition) the ATP used for transduction, for the pumping of Ca2+ at the synapse, and for regeneration of the visual pigment after light exposure.
Figure 2.
Comparison of ATP utilization by rhabdomeric and ciliary photoreceptors. (a) Mean rate of hydrolysis of ATP molecules per second calculated from membrane conductance and rate of ion pumping, as a function of rate of photon absorption for R1–6 rhabdomeric photoreceptors from four species of flies: Calliphora vicina (filled circles), Sarcophaga carnaria (filled downside triangles), Drosophila virilis (filled upside triangles), and Drosophila melanogaster (filled squares). (Reprinted with permission from Niven et al. [17]). (b) Mean rate of hydrolysis of ATP molecules per second from all sources. Calculated as a function of photon absorption for mouse ciliary rod photoreceptors. (Adapted and reprinted with permission from Okawa et al. [18]).
As the intensity of light is increased, ATP utilization increases in the fly but decreases in a mouse rod, for the simple reason that the biggest contributor to energy utilization in both photoreceptors comes from the pumping of ions; in fly, light opens channels, whereas in mouse, light closes them. ATP utilization for transduction is relatively minor by comparison. The energy requirement for pigment regeneration in a rod amounts to only two to three ATPs per retinal and is insignificant by comparison with the ATP required for ion pumping [18].
A surprising feature of figure 2 is that even for the small photoreceptors of Drosophila, the expenditure of ATP in darkness is as large as in a mouse rod [18]. This is because a significant number of channels are open in darkness in a fly eye. These include voltage-gated potassium channels, required to achieve sufficient signal bandwidth; and other unidentified channels with a more positive reversal potential [17], which are required to bias the membrane potential, so that single-photon responses can be reliably transmitted across the synapse. The energy required for pumping ions that have passed through these channels is about the same as the energy required in a mouse rod for voiding the ion influx in darkness through the cGMP-gated channels in the outer segment and the Ca2+ channels at the synaptic terminal.
The results in figure 2 show that rods are less costly to operate than typical rhabdomeric photoreceptors. Energy utilization is about the same in darkness but, in the light, increases in a fly and decreases in a rod. In bright light, nearly all of the channels in a rod are closed, and ATP utilization decreases to only about 20% of that in darkness [18]. ATP utilization is greater in cones, because even the brightest light does not close all of the cone cGMP-gated channels [19]. The same principles will however apply, and we expect energy utilization to decrease in a cone just as in a rod, though to a smaller extent. In order to explore this question in detail, we are voltage-clamping mouse cones to measure ion currents from both inner and outer segments, so that we can calculate cone energy utilization as a function of light intensity and compare it with energy utilization in rods and rhabdomeric photoreceptors.
These considerations suggest that early chordates and primitive vertebrates may have retained both rhabdomeric and ciliary eyes like those of amphioxus, perhaps using the rhabdomeric for dim-light detection and the ciliary for brighter light much as in some other invertebrates (for example scallop, see [20]). The gradual evolution of rods in addition to cones within the ciliary eye of stem vertebrates would have permitted these organisms to use ciliary photoreceptors over the whole range of light intensities. Because ciliary photoreceptors have higher photopigment densities and use less energy, the rhabdomeric eyes may have gradually disappeared with r-opsin retained only in the intrinsically light-sensitive ganglion cells [21].
3. How did rods evolve?
Although we cannot give a complete answer to this question, there are certain features of the evolution of rods that can be described with some assurance.
(a). Rods appeared very early
We know that rods are very old because they are present in cyclostomes, which are jawless vertebrates including present-day lamprey and hagfish. The line leading to cyclostomes split from the gnathostomes (the jawed vertebrates) about 500 million years ago (Ma) in the late Cambrian (figure 1). Although the physiology of cyclostome photoreceptors was initially uncertain [1,22], recent suction-electrode recordings have shown that the lamprey retina has rods and cones which behave much like those of fish, amphibians and even mammals [23,24].
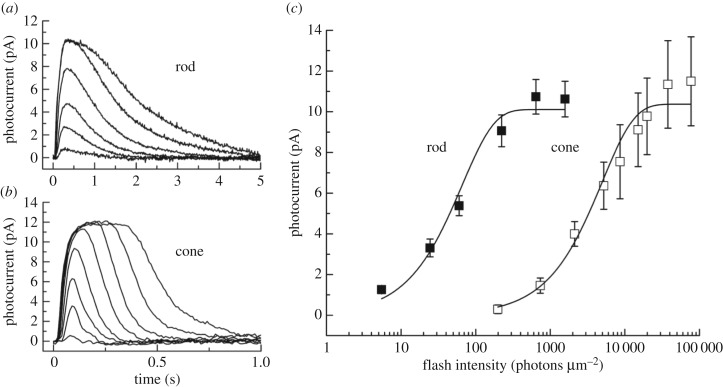
In figure 3a,b, we show averaged responses from lamprey rods and cones (from [23]). As in other vertebrate species, cone responses decay much more rapidly: exponential fits to the time course of decay give time constants about 10 times faster in cones than in rods. Moreover, rods are more sensitive. In figure 3c, we plot peak amplitudes of responses to brief flashes in both kinds of photoreceptors. Rods are about a factor of 70 more sensitive than cones (see also [24]), which is well within the range of the difference in other vertebrate species [19,25,26].
Figure 3.
Current responses and sensitivity of lamprey rod and cone photoreceptors to brief light stimuli. (a) Mean responses of 11 rods to 20 ms, 500 nm flashes given at t = 0 for the following intensities (in photons μm−2): 5, 24, 60, 222, 642 and 1576. (b) Mean responses of eight cones to 20 ms, 600 nm flashes given at t = 0 for the following intensities (in photons μm−2): 735, 2120, 5210, 1.98 × 104, 7.70 × 104, 2.28 × 105, 6.96 × 105 and 2.03 × 106. (c) Mean peak current response amplitudes plotted against flash intensity for 11 rods (filled squares) and eight cones (open squares). Error bars are standard errors of the mean. Cells are the same as in (a) and (b). The data for both cell types were fitted with the equation r = rmax [1 − exp(−kI)]. The best-fitting values of rmax and k were 10.1 pA and 1.52 × 10−2 photons−1 µm2 for rods and 10.4 pA and 2 × 10−4 photons−1 µm2 for cones. (Reproduced with permission from Morshedian & Fain [23]).
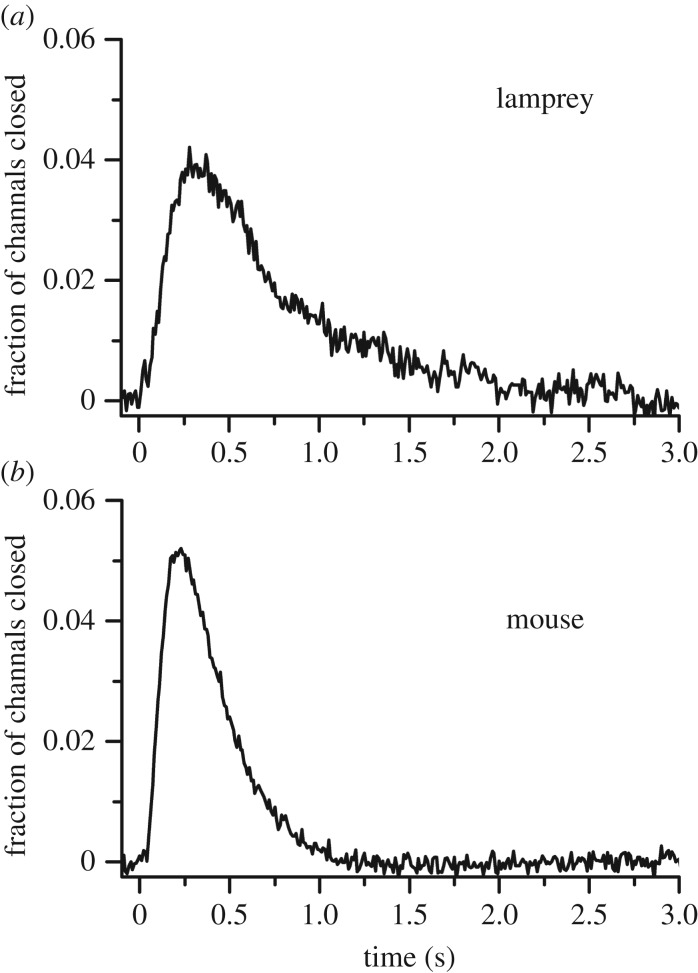
The ultimate test of rod function is the detection of light at the physiological limit of single photons. In figure 4 (from [23]), we compare single-photon responses from lamprey rods and mouse rods, with amplitudes plotted as a fraction of the total circulating current effectively providing the per cent channel closure as a function of time. Mouse single-photon responses decay somewhat more rapidly, perhaps in part because of the higher temperature of the recording, but single photons close about the same fraction of channels in both species. The most likely explanation of this result is that lamprey rods or their progenitors had all of the modifications in transduction required to provide high-sensitivity vision, and that a duplex retina evolved before cyclostomes split off from other vertebrates, probably in the late Cambrian and very early in the evolution of vertebrates [23,24].
Figure 4.
Single-photon responses of lamprey rods and mouse rods. Responses were calculated from the squared mean and variance (as in [27]) for 10 lamprey rods (a) and 41 mouse rods (b), normalized rod by rod to circulating current and averaged to give the mean fractional closure of channels as a function of time. (Reprinted with permission from Morshedian & Fain [23]).
(b). Outer-segment morphology makes no contribution to sensitivity
Outer-segment membranes in gnathostome rods and cones have a different configuration. In rods of amphibians or mammals, most of the rhodopsin lies within the membrane of intracellular discs, which are detached from and continuously surrounded by plasma membrane except at the very base of the outer segment [28,29]. In cones, on the other hand, the rhodopsin-containing membrane of the outer segment consists of invaginating lamellae continuous with the plasma membrane [30].
It has long been thought that this difference in morphology may be the key to the greater sensitivity of the rod. The recordings from lamprey show, however, that outer-segment morphology has no effect on absolute sensitivity. This is because lamprey rods and cones have the same outer-segment morphology resembling that of cones. Careful morphological investigations have been made of the photoreceptors of the lampreys Petromyson marinus [31], Lampetra fluviatilis [32–34] and Lampetra japonica [35]. All these studies have come to the same conclusion: the outer segments of the different kinds of photoreceptors in the lamprey retina are all identical in ultrastructure with clear regions where the plasma membrane makes infoldings to form lamellae much as in vertebrate cones.
Moreover, lamprey rods and cones both renew photopigment in the same way, which is quite different from the situation among the gnathostomes. Richard Young first showed that in an amphibian or mammalian rod, isotopic labelling of newly synthesized rhodopsin accumulates at the base of the rod outer segment to form a band, which then moves up the outer segment over a period of one to two weeks until the outer segment discs are shed and engulfed by the retinal pigment epithelium [36]. In an amphibian or mammalian cone, on the other hand, labelled rhodopsin is inserted at the base as in a rod but is then free to diffuse over the whole of the outer segment [37]. This is because rhodopsin is a membrane protein, and in a cone there are no barriers to its free diffusion throughout the outer segment.
In lamprey, both kinds of cells appear to renew pigment in the same way [31]: isotopic label has been reported to spread uniformly over the whole of the outer segments of both cell types without banding much as in an amphibian or mammalian cone. This is further evidence that lamprey rods do not have discs like other vertebrate rods. Because lamprey rods and cones have an identical morphology but behave in other respects like typical rods and cones, with the rods responding to single-photons like mouse rods (figure 4), we can conclude that a rod-like morphology, with discs detached from and surrounded by the plasma membrane, is not necessary for a rod-like physiology (see also [38]).
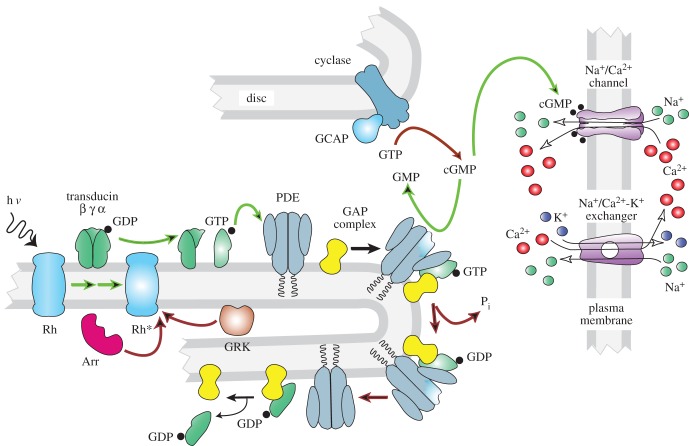
So why do rods have discs? We have speculated [23] that the discs provide a more efficient mechanism of membrane renewal. Because the protein in discs is kept together as it marches up the outer segment and then is finally engulfed by the pigment epithelium, all of the oldest transduction proteins are renewed in concert. Cones do not renew pigment in this way, perhaps because it is more important that 11-cis retinal have easy access to plasma-membrane opsin for rapid pigment regeneration [39]. There are, of course, other possible explanations for rod discs, which separate rod channels on the plasma membrane from the majority of rhodopsin and other transduction proteins on the discs (figure 5). It is possible that this separation leads to some increase in efficiency of signalling or protein function.
Figure 5.
Transduction cascade of vertebrate photoreceptor. Steps contributing to rate of activation are indicated by green arrows; steps contributing to rate of inactivation by red arrows. Arr, arrestin; cGMP, 3′, 5′-cyclic guanosine monophosphate; GAP, GTPase-activating protein; GDP, guanosine diphosphate; GCAP, guanylyl cyclase-activating proteins; GMP, guanosine monophosphate; GRK, G-protein receptor kinase (rhodopsin kinase); GTP, guanosine triphosphate; hν, light; PDE, phosphodiesterase 6; Pi, inorganic phosphate; Rh, rhodopsin; Rh*, light-activated rhodopsin.
(c). Evolution proceeded incrementally
In addition to outer segment ultrastructure, vertebrate rods and cones also differ in many of the protein isoforms used in the transduction cascade, including not only the pigment molecules but also the G-proteins, phosphodiesterases (PDEs) and cGMP-gated channel subunits [40,41]. Some proteins such as the GTPase-activating proteins (GAPs) are the same in the two kinds of cells but expressed at different levels in rods and cones [42,43]. These differences must in some way explain the differences in sensitivity and waveform between rods and cones present in nearly all vertebrate species (figure 3). Rods and cones also release synaptic transmitter at different numbers of release sites and process retinal signals in a different way [16,44], but these distinctions are beyond the scope of this review.
In mouse, the sensitivity per Rh* is about 20–25 times greater in a rod than in a cone (for rods, [45,46]; for cones, [19,47,48]). Which differences in the transduction cascade might account for this difference? One way of conceptualizing this question is to separate the transduction proteins into those that act on the rate of activation of the cascade and those that act on the rate of inactivation or decay [41]. The initial rate of rise of the photoreceptor response depends on the rate of formation of light-activated PDE, the rate of decline of cGMP concentration per activated PDE molecule and the Hill coefficient of binding of cGMP to the channels (figure 5, green arrows); and this initial rate is at least two to three times faster per light-activated rhodopsin (per Rh*) in rods than in cones [19,48,49]. Everything else being equal, a faster rate of activation per Rh* would produce a larger response in a rod and an increase in sensitivity. But everything else is not equal: rod responses also decay much more slowly than cone responses, and the mechanisms responsible for inactivation (red arrows in figure 5) seem also likely to contribute to the greater sensitivity of the rod.
In order to test the roles of activation and inactivation, much recent effort has been given to expressing cone isoforms of transduction proteins in rods to measure the resulting changes in sensitivity and waveform (see [41]). These studies have given mixed results. In some cases experiments expressing the cone photopigment, cone transducin or cone PDE catalytic subunits in rods have produced a clear decrease in activation rate and sensitivity, as well as a speeding of response decay [50–52], with response parameters in each case typically altered by about a factor of 2. In other experiments, little or no difference was detected [53–57]. Although no attempt has been made to express cone cGMP-gated channel subunits in rods, the Hill coefficient of rod and cone channels is similar ([58], see [59]), suggesting that the channels may not make a significant contribution to the difference in the rate of activation.
The rate of inactivation is governed by the rate of decay of Rh*, the rate of hydrolysis of transducin-alpha-GTP (TαGTP) to TαGDP followed by rebinding of PDE inhibitory to PDE catalytic subunits, and the rate of resynthesis of cGMP by guanylyl cyclase. Differences in the decay rate of Rh* between rods and cones seem unlikely to contribute at least in mouse, because both rods and cones use the same GRK1 kinase to phosphorylate Rh*, with no marked difference in expression level [60,61]. Moreover, mouse rods and cones use the same species of arrestin-1 to bind to phosphorylated Rh*; there is a small amount of arrestin-4 in cones, but it is unlikely to affect the rate of Rh* decay [62]. Expression of cone rhodopsin in rods produces little or no change in the waveform of the light response [53,54].
Rods and cones use the same GCAP proteins to modulate guanylyl cyclase activity, and deletion of the GCAPs produces about the same change in rate of inactivation and sensitivity for both kinds of photoreceptors [47,63]. The GCAPs seem therefore to make little or no contribution to the sensitivity difference. The rate of hydrolysis of TαGTP, on the other hand, is likely to be significantly slower in rods than in cones. This rate is accelerated when cone transducin or cone PDE is expressed in rods [51,52]; moreover, cones have a higher expression level of the GAP proteins, which are essential for rapid extinction of PDE activity [64]. The basal rate of cGMP resynthesis seems also to be faster in cones, and this difference could also contribute to the faster recovery of the cone response. The expression of guanylyl cyclase may be two to three times higher in cones (A. Dizhoor 2016, personal communication), and the rate of cGMP turnover in darkness is about threefold greater, at least in salamander, the only species where these measurements have been made [65,66].
We can summarize these observations in the following way. Many attempts have been made to compare the effects of individual elements of the transduction cascade between rods and cones, both by expressing cone genes in rods and by measuring differences in the biochemical or physiological properties of the two kinds of photoreceptors. Although not every protein in the transduction cascade has been investigated, we probably know enough to be able to say that substituting a rod protein isoform with a cone isoform or changing the expression level of guanylyl cyclase or the GAP proteins can produce a significant change in photoreceptor sensitivity or time course of response decay. These effects, however, are never very large, at least in mouse (for other species, see [67]), probably amounting to a change in sensitivity or rate of decay of no more than a factor of 2 in each case.
The clear implication is that no one change by itself is responsible for the increase in sensitivity and slower recovery of the rod response. Instead, evolution seems to have proceeded by a series of small steps of gene duplication and evolution of distinct isoforms, accompanied by changes in the expression of some of the transduction proteins. That is, evolution proceeded incrementally, gradually increasing the sensitivity of the photoreceptor by a series of small changes in many of the elements of the transduction cascade.
4. Evolution of rod vision
Early chordates may have had ciliary eyes with photoreceptors like cones and rhabdomeric eyes with photoreceptors like those in arthropods. Ciliary photoreceptors are widespread among invertebrates, but these cells seem to resemble cones and function in bright light. During the evolution of vertebrates, the duplication of genes and formation of new protein isoforms together with changes in protein expression seem to have permitted the formation of a new kind of ciliary photoreceptor sensitive enough to give detectable responses to single photons. Cones would have been retained, so that their greater sensitivity to change and motion could be exploited at brighter intensities, when photon flux is not as limiting. The evolution of the rod required many changes in the genetic make-up of the photoreceptor, each one of which seems by itself to have had only a small effect on sensitivity and rate of decay. The gradual accumulation of all of these changes over many generations would, however, have given early vertebrates a duplex retina capable of spanning the entire range of stimulus intensities.
Once the ciliary eye of early vertebrates was able to function at both scotopic and photopic light levels, rhabdomeric eyes seem to have disappeared in the line to the vertebrates, perhaps because of the greater energy burden they place upon the organism. Although the evolution of the rod and the duplex retina would have taken many generations, it must nevertheless have occurred very early: rods are present in every class of vertebrates including jawless cyclostomes, which split off from the rest of the vertebrates approximately 500 Ma. Moreover, the properties of lamprey rods and cones are very similar to those of rods and cones of gnathostomes. Recent experiments have shown, for example, that light adaptation in lamprey rods and cones is virtually indistinguishable from that in other vertebrates [68]. The early emergence of this new kind of ciliary photoreceptor together with the other retinal cells and synaptic pathways required for dim-light vision permitted the formation of the duplex retina, first postulated 150 years ago by Schultze [69], and now recognized to be a fundamental feature of vertebrate photodetection.
Acknowledgements
We are grateful to Jeremy Niven and Margery Fain for help with the figures, and to Simon Laughlin for reading the manuscript.
Authors' contributions
A.M. provided and analysed the data for figures 3 and 4, contributed to the drafting of the manuscript and approved the final version. G.L.F. was principally responsible for the conception and design of the experiments, contributed to the drafting of the manuscript and approved the final version.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by a contract to G.L.F. from the Great Lakes Fishery Commission (USA) and from R01 EY001844 to G.L.F. from the National Eye Institute of the NIH (USA).
References
- 1.Fain GL, Hardie R, Laughlin SB. 2010. Phototransduction and the evolution of photoreceptors. Curr. Biol. 20, R114–R124. ( 10.1016/j.cub.2009.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garm A, Coates MM, Gad R, Seymour J, Nilsson DE. 2007. The lens eyes of the box jellyfish Tripedalia cystophora and Chiropsalmus sp. are slow and colorblind. J. Comp. Physiol. A Neuroethol. Sens Neural Behav. Physiol. 193, 547–557. ( 10.1007/s00359-007-0211-4) [DOI] [PubMed] [Google Scholar]
- 3.Suga H, Schmid V, Gehring WJ. 2008. Evolution and functional diversity of jellyfish opsins. Curr. Biol. 18, 51–55. ( 10.1016/j.cub.2007.11.059) [DOI] [PubMed] [Google Scholar]
- 4.Porter ML, Blasic JR, Bok MJ, Cameron EG, Pringle T, Cronin TW, Robinson PR. 2012. Shedding new light on opsin evolution. Proc. R. Soc. B 279, 3–14. ( 10.1098/rspb.2011.1819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordstrom K, Wallen R, Seymour J, Nilsson D. 2003. A simple visual system without neurons in jellyfish larvae. Proc. R. Soc. Lond. B 270, 2349–2354. ( 10.1098/rspb.2003.2504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardie RC, Postma M. 2008. Phototransduction in microvillar photoreceptors of Drosophila and other invertebrates. In The senses - a comprehensive reference: Vision vol. 1 (eds Basbaum AI, Kaneko A, Shepherd GM, Westheimer G), pp. 77–130. San Diego, CA: Academic Press. [Google Scholar]
- 7.Tatler B, O'Carroll DC, Laughlin SB. 2000. Temperature and the temporal resolving power of fly photoreceptors. J. Comp. Physiol. A 186, 399–407. ( 10.1007/s003590050439) [DOI] [PubMed] [Google Scholar]
- 8.Emmerton J. 1983. Vision. In Physiology and behaviour of the pigeon (ed. Abs M.), pp. 245–266. New York, NY: Academic Press. [Google Scholar]
- 9.Nickle B, Robinson PR. 2007. The opsins of the vertebrate retina: insights from structural, biochemical, and evolutionary studies. Cell Mol. Life Sci. 64, 2917–2932. ( 10.1007/s00018-007-7253-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shichida Y, Matsuyama T. 2009. Evolution of opsins and phototransduction. Phil. Trans. R. Soc. B 364, 2881–2895. ( 10.1098/rstb.2009.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacalli TC. 2004. Sensory systems in amphioxus: a window on the ancestral chordate condition. Brain Behav. Evol. 64, 148–162. ( 10.1159/000079744) [DOI] [PubMed] [Google Scholar]
- 12.Holland LZ, et al. 2008. The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res. 18, 1100–1111. ( 10.1101/gr.073676.107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putnam NH, et al. 2008. The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1064–1071. ( 10.1038/nature06967) [DOI] [PubMed] [Google Scholar]
- 14.del Pilar Gomez M, Angueyra JM, Nasi E. 2009. Light-transduction in melanopsin-expressing photoreceptors of amphioxus. Proc. Natl Acad. Sci. USA 106, 9081–9086. ( 10.1073/pnas.0900708106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niven JE, Laughlin SB. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804. ( 10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 16.Sterling P, Laughlin S. 2015. Principles of neural design. Cambridge, MA: MIT Press. [Google Scholar]
- 17.Niven JE, Anderson JC, Laughlin SB. 2007. Fly photoreceptors demonstrate energy-information trade-offs in neural coding. PLoS Biol. 5, e116 ( 10.1371/journal.pbio.0050116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okawa H, Sampath AP, Laughlin SB, Fain GL. 2008. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr. Biol. 18, 1917–1921. ( 10.1016/j.cub.2008.10.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikonov SS, Kholodenko R, Lem J, Pugh EN Jr. 2006. Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J. Gen. Physiol. 127, 359–374. ( 10.1085/jgp.200609490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fain GL. 2003. Sensory transduction. Sunderland, MA: Sinauer, Inc. [Google Scholar]
- 21.Do MT, Yau KW. 2010. Intrinsically photosensitive retinal ganglion cells. Physiol. Rev. 90, 1547–1581. ( 10.1152/physrev.00013.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamb TD, Collin SP, Pugh EN Jr. 2007. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 8, 960–976. ( 10.1038/nrn2283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morshedian A, Fain GL. 2015. Single-photon sensitivity of lamprey rods with cone-like outer segments. Curr. Biol. 25, 484–487. ( 10.1016/j.cub.2014.12.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asteriti S, Grillner S, Cangiano L. 2015. A Cambrian origin for vertebrate rods. eLife 4, 57 ( 10.7554/eLife.07166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fain GL, Dowling JE. 1973. Intracellular recordings from single rods and cones in the mudpuppy retina. Science 180, 1178–1181. ( 10.1126/science.180.4091.1178) [DOI] [PubMed] [Google Scholar]
- 26.Tachibanaki S, Tsushima S, Kawamura S. 2001. Low amplification and fast visual pigment phosphorylation as mechanisms characterizing cone photoresponses. Proc. Natl Acad. Sci. USA 98, 14 044–14 049. ( 10.1073/pnas.241396898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CK, Burns ME, He W, Wensel TG, Baylor DA, Simon MI. 2000. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature 403, 557–560. ( 10.1038/35000601) [DOI] [PubMed] [Google Scholar]
- 28.Ding JD, Salinas RY, Arshavsky VY. 2015. Discs of mammalian rod photoreceptors form through the membrane evagination mechanism. J. Cell Biol. 211, 495–502. ( 10.1083/jcb.201508093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volland S, Hughes LC, Kong C, Burgess BL, Linberg KA, Luna G, Zhou ZH, Fisher SK, Williams DS. 2015. Three-dimensional organization of nascent rod outer segment disk membranes. Proc. Natl Acad. Sci. USA 112, 14 870–14 875. ( 10.1073/pnas.1516309112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mustafi D, Engel AH, Palczewski K. 2009. Structure of cone photoreceptors. Prog. Retin. Eye Res. 28, 289–302. ( 10.1016/j.preteyeres.2009.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickson DH, Graves DA. 1979. Fine structure of the lamprey photoreceptors and retinal pigment epithelium (Petromyzon marinus L.). Exp. Eye Res. 29, 45–60. ( 10.1016/0014-4835(79)90165-9) [DOI] [PubMed] [Google Scholar]
- 32.Öhman P. 1971. The photoreceptor outer segments of the river lamprey (Lampreta fluviatilis). An electron-, fluorescence- and light microscopic study. Acta Zool. 52, 287–297. ( 10.1111/j.1463-6395.1971.tb00564.x) [DOI] [Google Scholar]
- 33.Öhman P. 1976. Fine structure of photoreceptors and associated neurons in the retina of Lampetra fluviatilis (Cyclostomi). Vision Res. 16, 659–662. ( 10.1016/0042-6989(76)90014-6) [DOI] [PubMed] [Google Scholar]
- 34.Govardovskii VI, Lychakov DV. 1984. Visual cells and visual pigments of the lamprey, Lampetra fluviatilis. J. Comp. Physiol. A 154, 279–286. ( 10.1007/BF00604994) [DOI] [Google Scholar]
- 35.Ishikawa M, Takao M, Washioka H, Tokunaga F, Watanabe H, Tonosaki A. 1987. Demonstration of rod and cone photoreceptors in the lamprey retina by freeze-replication and immunofluorescence. Cell Tissue Res. 249, 241–246. ( 10.1007/BF00215506) [DOI] [PubMed] [Google Scholar]
- 36.Young RW. 1976. Visual cells and the concept of renewal. Invest. Ophthalmol. Vis. Sci. 15, 700–725. [PubMed] [Google Scholar]
- 37.Young RW. 1971. The renewal of rod and cone outer segments in the rhesus monkey. J. Cell Biol. 49, 303–318. ( 10.1083/jcb.49.2.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma J, et al. 2001. A visual pigment expressed in both rod and cone photoreceptors. Neuron 32, 451–461. ( 10.1016/S0896-6273(01)00482-2) [DOI] [PubMed] [Google Scholar]
- 39.Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, Cornwall MC, Yau KW. 2005. Breaking the covalent bond: a pigment property that contributes to desensitization in cones. Neuron 46, 879–890. ( 10.1016/j.neuron.2005.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamb TD. 2013. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog. Retin. Eye Res. 36, 52–119. ( 10.1016/j.preteyeres.2013.06.001) [DOI] [PubMed] [Google Scholar]
- 41.Ingram NT, Sampath AP, Fain GL. 2016. Why are rods more sensitive than cones? J. Physiol. 594, 5415–5426. ( 10.1113/JP272556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowan CW, Fariss RN, Sokal I, Palczewski K, Wensel TG. 1998. High expression levels in cones of RGS9, the predominant GTPase accelerating protein of rods. Proc. Natl Acad. Sci. USA 95, 5351–5356. ( 10.1073/pnas.95.9.5351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Wensel TG, Kraft TW. 2003. GTPase regulators and photoresponses in cones of the eastern chipmunk. J. Neurosci. 23, 1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masland RH. 2012. The neuronal organization of the retina. Neuron 76, 266–280. ( 10.1016/j.neuron.2012.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sampath AP, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Chen J, Kawamura S, Rieke F, Hurley JB. 2005. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron 46, 413–420. ( 10.1016/j.neuron.2005.04.006) [DOI] [PubMed] [Google Scholar]
- 46.Reingruber J, Holcman D, Fain GL. 2015. How rods respond to single photons: key adaptations of a G-protein cascade that enable vision at the physical limit of perception. Bioessays 37, 1243–1252. ( 10.1002/bies.201500081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakurai K, Chen J, Kefalov VJ. 2011. Role of guanylyl cyclase modulation in mouse cone phototransduction. J. Neurosci. 31, 7991–8000. ( 10.1523/JNEUROSCI.6650-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao LH, Luo DG, Yau KW. 2014. Light responses of primate and other mammalian cones. Proc. Natl Acad. Sci. USA 111, 2752–2757. ( 10.1073/pnas.1400268111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pugh EN Jr, Lamb TD. 1993. Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta 1141, 111–149. ( 10.1016/0005-2728(93)90038-H) [DOI] [PubMed] [Google Scholar]
- 50.Sakurai K, Onishi A, Imai H, Chisaka O, Ueda Y, Usukura J, Nakatani K, Shichida Y. 2007. Physiological properties of rod photoreceptor cells in green-sensitive cone pigment knock-in mice. J. Gen. Physiol. 130, 21–40. ( 10.1085/jgp.200609729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CK, Woodruff ML, Chen FS, Shim H, Cilluffo MC, Fain G. 2010. Replacing the rod with the cone transducin alpha subunit decreases sensitivity and accelerates response decay. J. Physiol. 588, 3231–3241. ( 10.1113/jphysiol.2010.191221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majumder A, Pahlberg J, Muradov H, Boyd KK, Sampath AP, Artemyev NO. 2015. Exchange of cone for rod phosphodiesterase 6 catalytic subunits in rod photoreceptors mimics in part features of light adaptation. J. Neurosci. 35, 9225–9235. ( 10.1523/JNEUROSCI.3563-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu Y, Kefalov V, Luo DG, Xue T, Yau KW. 2008. Quantal noise from human red cone pigment. Nat. Neurosci. 11, 565–571. ( 10.1038/nn.2110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi G, Yau KW, Chen J, Kefalov VJ. 2007. Signaling properties of a short-wave cone visual pigment and its role in phototransduction. J. Neurosci. 27, 10 084–10 093. ( 10.1523/JNEUROSCI.2211-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng WT, et al. 2009. Functional interchangeability of rod and cone transducin alpha-subunits. Proc. Natl Acad. Sci. USA 106, 17 681–17 686. ( 10.1073/pnas.0901382106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mao W, Miyagishima KJ, Yao Y, Soreghan B, Sampath AP, Chen J. 2013. Functional comparison of rod and cone Gαt on the regulation of light sensitivity. J. Biol. Chem. 288, 5257–5267. ( 10.1074/jbc.M112.430058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng WT, et al. 2013. Cone phosphodiesterase-6α′ restores rod function and confers distinct physiological properties in the rod phosphodiesterase-6β-deficient rd10 mouse. J. Neurosci. 33, 11 745–11 753. ( 10.1523/JNEUROSCI.1536-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Picones A, Korenbrot JI. 1992. Permeation and interaction of monovalent cations with the cGMP-gated channel of cone photoreceptors. J. Gen. Physiol. 100, 647–673. ( 10.1085/jgp.100.4.647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaupp UB, Seifert R. 2002. Cyclic nucleotide-gated ion channels. Physiol. Rev. 82, 769–824. ( 10.1152/physrev.00008.2002) [DOI] [PubMed] [Google Scholar]
- 60.Lyubarsky AL, Chen C, Simon MI, Pugh EN Jr. 2000. Mice lacking G-protein receptor kinase 1 have profoundly slowed recovery of cone-driven retinal responses. J. Neurosci. 20, 2209–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss ER, Ducceschi MH, Horner TJ, Li A, Craft CM, Osawa S. 2001. Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J. Neurosci. 21, 9175–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nikonov SS, Brown BM, Davis JA, Zuniga FI., Bragin A, Pugh EN Jr, Craft CM. 2008. Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron 59, 462–474. ( 10.1016/j.neuron.2008.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gross OP, Pugh EN Jr, Burns ME. 2012. Calcium feedback to cGMP synthesis strongly attenuates single-photon responses driven by long rhodopsin lifetimes. Neuron 76, 370–382. ( 10.1016/j.neuron.2012.07.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arshavsky VY, Wensel TG. 2013. Timing is everything: GTPase regulation in phototransduction. Invest. Ophthalmol. Vis. Sci. 54, 7725–7733. ( 10.1167/iovs.13-13281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cornwall MC, Fain GL. 1994. Bleached pigment activates transduction in isolated rods of the salamander retina. J. Physiol. (Lond.) 480, 261–279. ( 10.1113/jphysiol.1994.sp020358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cornwall MC, Matthews HR, Crouch RK, Fain GL. 1995. Bleached pigment activates transduction in salamander cones. J. Gen. Physiol. 106, 543–557. ( 10.1085/jgp.106.3.543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawamura S, Tachibanaki S. 2008. Rod and cone photoreceptors: molecular basis of the difference in their physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 150, 369–377. ( 10.1016/j.cbpa.2008.04.600) [DOI] [PubMed] [Google Scholar]
- 68.Morshedian A, Fain GL. 2017 Evolution of adaptation in vertebrate photoreceptors. Abstract 2687119, annual meeting of the Association for Research in Vision and Ophthalmology.
- 69.Schultze M. 1866. Zur Anatomie und Physiologie der Retina. Arch. Mikroskopische Anatomie 2, 175–286. ( 10.1007/BF02962033) [DOI] [Google Scholar]