Abstract
Night vision is ultimately about extracting information from a noisy visual input. Several species of nocturnal insects exhibit complex visually guided behaviour in conditions where most animals are practically blind. The compound eyes of nocturnal insects produce strong responses to single photons and process them into meaningful neural signals, which are amplified by specialized neuroanatomical structures. While a lot is known about the light responses and the anatomical structures that promote pooling of responses to increase sensitivity, there is still a dearth of knowledge on the physiology of night vision. Retinal photoreceptors form the first bottleneck for the transfer of visual information. In this review, we cover the basics of what is known about physiological adaptations of insect photoreceptors for low-light vision. We will also discuss major enigmas of some of the functional properties of nocturnal photoreceptors, and describe recent advances in methodologies that may help to solve them and broaden the field of insect vision research to new model animals.
This article is part of the themed issue ‘Vision in dim light’.
Keywords: night vision, compound eye, photoreceptor, phototransduction, quantum bump
1. Introduction
Visual guidance of behaviour is challenging when photons, the elementary particles of light, are scarce. To produce a reliable representation of the surroundings, the visual system must (i) ensure absorption of a sufficient number of photons into a photoreceptor, (ii) house photoreceptors that efficiently convert each photon absorption into a neural signal, and (iii) process these signals appropriately. Insects are a numerous and diverse class of arthropods that have evolved to occupy ecological niches from the brightest to the darkest. The accessibility and comparative simplicity of the insect visual system make it an attractive model for studying visual adaptations, including adaptations to vision in dim light.
The main photoreceptive organ of insects is the compound eye. As the name implies, it consists of more or less identical repeating optical units (ommatidia) that contain the photoreceptor cells. The two compound eye types, apposition and superposition, collect light in different ways (figure 1a). In an apposition eye, light is guided through optically isolated ommatidial lenses onto the light-sensitive structures, rhabdoms, lying immediately underneath each lens. This arrangement is thought to favour acuity over sensitivity, making it well suited for vision in bright light. By contrast, a dark-adapted superposition eye collects light from a wider angle through multiple lenses onto each rhabdom. As a result the number of photons reaching each photoreceptor in a superposition eye can be hundreds of times greater than in an apposition eye [3], albeit at the expense of spatial resolution. Therefore, not surprisingly, superposition eyes are typically found among nocturnal insects. Outstanding examples of nocturnal behaviour with superposition vision include colour discrimination and colour constancy in the hawkmoth Deilephila elpenor [4] and orientation to the night sky polarization pattern and the Milky Way [5] by nocturnal dung beetles. Remarkably, against the seemingly apparent logic, also various insects with apposition eyes have adapted to life in darkness. Some hymenopterans and bugs use canopy cues, landmarks, and skylight polarization for navigation [3,6,7]. The shade response of the American cockroach, Periplaneta americana, persists down to at least 0.01 lx [8] and its optomotor turning response to 0.005 lx [9].
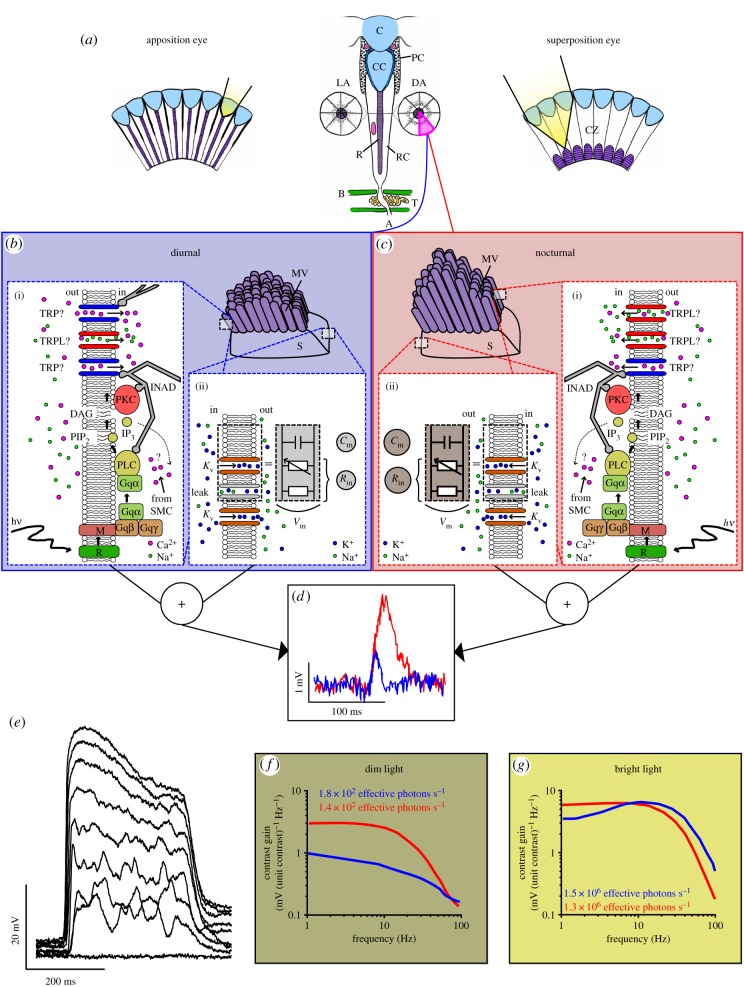
Figure 1.
(a) Schematic structure of an insect compound eye. An ommatidium (middle; from Periplaneta americana), the functional unit of the compound eye, consists of the corneal lens C, crystalline cone CC, pigment cells PC, the photoreceptive rhabdom R made up by the photoreceptor cells RC and the RC axons A traversing the basement membranes B and the tracheal layer T. A cross section of light- (LA) and dark-adapted (DA) cockroach ommatidium demonstrating pigment migration. In an apposition eye (left) ommatidia are optically isolated and each rhabdom (purple) receives light through a single lens. In a superposition eye (right), several lenses focus light onto a rhabdom across the clear zone, CZ. (b,c) Simplifications of a transverse slice of a diurnal (b) and a nocturnal (c) photoreceptor. The schematics consist of rhabdomere microvilli MV, and cell soma S. Note the size difference of the microvilli between (b) and (c). Simplified representations of (i) the phototransduction cascade and (ii) the electrical properties of the photoreceptor membrane with their molecular constituents (see §§3–5 for full explanation). Note the hypothetical difference in TRP/TRPL channel expression between (b(i)) and (c(i)), and the IP3-induced Ca2+ release from submicrovillar cisternae (SMC). Also note that Cm (due to larger rhabdomeres) and Rin (voltage-gated and leak channels combined) are larger in (c(i)) than in (b(i)). (d) Single photon responses, quantum bumps, intracellularly recorded from dark-adapted photoreceptors of the diurnal bee Lasioglossum leucozonium (blue) and the nocturnal bee Megalopta genalis (red). A quantum bump and its shape are the combined results of (i) and (ii). (e) Intracellularly recorded graded voltage responses of P. americana photoreceptor to 300 ms light pulses with incremental intensity. (f,g) The average contrast gain functions of Lasioglossum (blue) and Megalopta (red) photoreceptors to (e) a dim and (f) a bright white-noise modulated light stimulus. The nocturnal photoreceptors are more low-passing and provide more response amplification in dim light. (d,f,g) Modified from Frederiksen et al. [1] with permission. (e) Adapted from Heimonen et al. [2].
The lowest light intensity where a given visually guided behaviour can successfully take place is known as the behavioural threshold. Visual behaviour in dim light can be linked to photoreceptor physiology by determining both the behavioural threshold and the photon absorption rate in a photoreceptor, either by recording intracellularly [9,10] or by estimating it from the photoreceptor optics [11,12]. While the photon absorption rate in Periplaneta photoreceptors is 0.1 photons s−1 at the behavioural threshold, it is 17 times higher in the fly, 50 times higher in the nocturnal bee Megalopta genalis and 4400 times higher in the hawkmoth Deilephila [3,4,9,10]. This does not mean that these insects need brighter ambient light than the cockroach for seeing or behaviour. Megalopta, for example, has its behavioural threshold at one-tenth of the absolute light intensity of Periplaneta's threshold [3,9], yet each of its photoreceptors is able to capture 5 photons s−1. For flying insects the threshold probably is at higher absorption rates than for walking ones, because flight steering requires more visual information and thus more photons s−1 than terrestrial locomotion. The differences also emphasize how the moth, with its superposition eyes, is able to capture hundreds of photons s−1 at an intensity where diurnal flies can no longer be active.
Megalopta and Periplaneta have apposition eyes, yet they both are nocturnal, and the bee is even an active flyer. In fact, Megalopta retains its flight and landing performance under starlight intensities [3]. Although the apposition eye is traditionally considered as the day active eye type, structural modifications, such as increased lens and rhabdom diameter, may facilitate its operation at low light conditions. Moreover, various neuroanatomical and functional adaptations, resulting in spatial and temporal summation within the visual pathway from photoreceptors to the brain, can improve information acquisition from unreliable visual inputs. Spatial summation is preferred when temporal resolution cannot be sacrificed, such as during flight and landing in bees [13,14]. Slow tracking of small moving targets in darkness generally relies on temporal summation, as in shore crabs [15]. The hawkmoth visual system likely uses both spatial and temporal summation for the demanding task of hovering at night [16].
A major site of spatial (neural) summation is probably the first optic ganglion, lamina, where many insects have a retinotopic array of cartridges corresponding to individual ommatidia in the retina. The dendrites of second order large monopolar cells connect lamina cartridges laterally in nocturnal fireflies, bees and moths [17–19], and photoreceptor axon bundles in the cockroach [20]. However, the first steps in processing visual inputs occur in the photoreceptors that are responsible for the conversion of light information into relevant neural representations. Therefore, reliable vision in dim light does not come down to having appropriate optical and anatomical adaptations only, but also crucially relies on the ability of photoreceptors to transduce photon absorptions efficiently. In this review, we will highlight the known functional properties that can facilitate insect photoreceptors to succeed in this task and provide examples from nocturnal insects with apposition eyes.
2. Light responses in microvillar photoreceptors
The compound eye surface of an insect consists of small corneal lenses belonging to ommatidia, the optical units of a compound eye (figure 1a). Additionally, an ommatidium consists of a crystalline cone, pigment cells surrounding the ommatidium, and eight or nine photoreceptors. Each photoreceptor has a soma and a brush-like rhabdomere made of thousands of bristle-like microvilli, hence insect photoreceptors are often referred to as microvillar or rhabdomeric. Photoreceptors within an ommatidium are usually organized in a circle, with rhabdomeres facing each other and forming a rod-like structure known as the rhabdom.
When a photon of visible light enters the rhabdom, it may get absorbed by the visual pigment rhodopsin embedded in the microvillar membrane. This triggers the phototransduction cascade, a chain of biochemical reactions leading to the generation of a membrane voltage response known as a quantum bump. Quantum bumps were first described in the lateral compound eye of the horseshoe crab Limulus by Yeandle [21]. Later on, together with Fuortes, he confirmed that stimulation of dark-adapted Limulus photoreceptors with steady dim light or short flashes produces discrete voltage bumps with Poisson-distributed timing and occurrence [22]. Similar findings in locust eyes confirmed that these single photon absorption events occur also in insects [23].
The stochastic nature of photon arrival and absorption in the photoreceptor causes variance or noise of √N photons for every N photons absorbed. This shot noise increases relative to the signal with decreasing photon availability, until photoreceptors can no longer detect contrast [24]. Another source of noise is the inability of the photoreceptors to produce identical responses to sequentially absorbed photons. This transducer noise, i.e. variation in phototransduction, manifests itself as differences in bump latency, duration and amplitude. Fuortes and Yeandle also reported bumps appearing in total darkness. This dark noise, arising from the spontaneous (thermal) activation of phototransduction components, can severely limit vision. However, insect eyes generally have very little dark noise. For example, locust photoreceptors show a dark noise rate of only 10 h−1 [23]. In Drosophila, the dark noise rate is clearly higher (approx. 2 s−1), but unlikely to limit visual capabilities due to the fly's diurnal lifestyle [25].
Quantum bumps in nocturnal photoreceptors tend to be much larger than in diurnal photoreceptors. An example of this is provided by the tropical sweat bees Megalopta and Lasioglossum (figure 1d). They have apposition eyes and share a similar lifestyle, but the former is nocturnal producing large quantum bumps, while the latter is diurnal with small quantum bumps [1]. Importantly, similar findings are reported from nocturnal and diurnal Onitis dung beetles, both equipped with superposition eyes [26]. Other nocturnal insects with large bumps include the cockroach [2,27], locust [23], carpenter ant [28], crane fly [29] and stick insect [30]. Microvillar photoreceptors of spiders Dinopis [31] and Cupiennius [32] also produce very conspicuous bumps. The reason for having larger quantum bumps may be the need to ensure sufficiently undistorted delivery of single photon signals to the optic lobes. With increasing light intensity more photons are absorbed and thus more quantum bumps start to appear. Eventually, quantum bumps become so numerous that they start to overlap, fuse and build on each other to form a graded light response. Consequently, the size and shape of a graded light response will depend on the size and shape of the quantum bumps. Following this logic, having large quantum bumps may not be beneficial for a diurnal insect as its photoreceptors could become more easily blinded by bright light.
Stimulating a photoreceptor with incremental series of light flashes enables recording of an intensity–response relation. For dim light flashes the relation is linear, but with brighter flashes compressive nonlinearities set in. In the linear range, it is possible to record so-called impulse responses resulting from the superposition of a small number of quantum bumps. Since impulse response shape reflects photoreceptor dynamics, they have been commonly used for comparative studies. Like quantum bumps, impulse responses are typically slower in nocturnal than in diurnal species [28,33]. With increasing light intensity nonlinearities caused by photo- and electrochemical adaptation mechanisms gradually start to have more and more effect on the intensity–response relation. These nonlinearities allow a photoreceptor to compress a range of light intensities, spanning from starlight to daylight, into its narrow operating range (approx. 60 mV).
Photoreceptor dynamics, including light adaptation, can be studied using longer light pulses with varying intensities (figure 1e). In a dark-adapted photoreceptor a dim light pulse produces a rectangular graded light response with superimposed bump noise on top of it. With higher intensities the noise subsides and the graded response is characterized by a fast initial transient followed by a plateau with much reduced amplitude. This peak-to-plateau transition stems from the photoreceptor light adaptation mechanisms. Interestingly, a seminal comparative study on dipteran species by Laughlin & Weckström [29] showed that nocturnal flies not only had slower responses than their diurnal counterparts, but also poorer ability to adapt to increasing light levels.
These differences in quantum bumps and macroscopic responses raise two essential questions: (i) what mechanisms underlie the differences, and (ii) why are they different? The answer to the first question has two parts, phototransduction and electrical properties of the photoreceptor membrane.
3. Phototransduction in the diurnal Drosophila
The starting point of phototransduction is the visual pigment rhodopsin embedded in the microvillar membrane. Photon absorption and thus light sensitivity depend on the concentration of these molecules in the rhabdomere. Unsurprisingly, nocturnal insects have developed means to adjust the expression of rhodopsins with suitable spectral sensitivities [34] and translocate them in and out of the rhabdomere according to the prevailing light levels [35]. Despite investigation in many insects, everything downstream of rhodopsin activation by light has relied heavily on research on the photoreceptors of Drosophila. Ever since patch-clamping could be combined with molecular genetic tools [36], Drosophila phototransduction has been the single most important model representing all insects [37]. It was also the visually severely impaired Drosophila transient receptor potential (trp) mutant [38,39] that led to finding a whole superfamily of TRP ion channels.
When a rhodopsin (R) absorbs a photon, it isomerizes into metarhodopsin (M; figure 1b(i),c(i)). M causes the heterotrimeric Gq protein complex to release its α subunit (Gqα), which in turn activates the phospholipase C (PLC). The active PLC hydrolyses a phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2) into a proton [40], a hydrophilic inositol-1,4,5-trisphosphate (IP3) and a hydrophobic diacylglycerol (DAG). The released proton [40] and the mechanical contractions caused by PIP2 hydrolysis [41] result in the opening of approximately 15 TRP and TRP-like (TRPL) channels and a flow of cations into the microvillus, generating an ionic current responsible for the generation of a quantum bump, i.e. the bump current [42,43]. The majority of the approximately 10 pA [43] bump current is mediated by relatively Ca2+-selective TRP channels [44], which are 10 times more abundant in the photoreceptors than the non-selective TRPL [45].
Bump current generation is a fast process, characterized by a 20–100 ms latency and approximately 20 ms bump half-width [43]. In bright light, these kinetics become even faster and bump amplitudes attenuate minimizing transducer noise [46]. The rapid kinetics are enabled by the scaffolding protein INAD, binding together protein kinase C (PKC), PLC and TRP channels into a signalling complex [47], and the tight compartmentalization of the whole phototransduction machinery into single microvilli [37]. This has two major advantages. Firstly, the tight compartmentalization minimizes diffusional delays. Secondly, Ca2+ influx via a single open TRP channel can quickly increase microvillar Ca2+ levels [48] to facilitate the rapid opening of the remaining TRP/TRPL channels [49]. Also, IP3-induced Ca2+ release (IP3-Ca2+) from submicrovillar cisternae (SMC) of the endoplasmic reticulum, which are closely associated to microvilli, may be essential for sensitizing PLC for efficient channel activation [50]. Ca2+ build-up in the microvillar lumen provides negative feedback by stimulating Ca2+-dependent mechanisms that inactivate the bump current [51–54]. After the bump current has been inactivated, the microvillus remains refractory for 50–150 ms before another bump can be initiated [53], possibly due to the high Ca2+ concentration and the reversible dissociation of INAD from PLC and TRP [55]. Responsiveness is recovered after excess Ca2+ has been extruded and INAD reassociates with TRP and PLC. Since the differences in the size of bump currents are relatively small [43] and derive from differences between microvilli, the major determinants of the transducer noise are the latency and refractory period. Presumably, the variable latency is caused by diffusion-limited reactions of M and PLC with Gq [37], whose spontaneous activity creates most of the dark noise [56]. Although the refractory period varies, possibly mostly due to INAD complex reassociation time, it might also prevent more severe transducer noise by providing time for recovery processes, such as the replenishment of the PIP2 reserve [37].
4. Phototransduction and light-induced currents in nocturnal insects
Recently, through the successful use of the patch-clamp technique on isolated photoreceptors, new insect species with various lifestyles, including nocturnal, have entered into phototransduction research [57]. Bump currents (i.e. the ionic currents underlying the generation of quantum bumps) have been statistically analysed in the field cricket Gryllus bimaculatus [58] and the cockroach P. americana [59], both species having apposition eyes and being active in dim light. Compared with Drosophila, both cricket and cockroach photoreceptors produce bump currents with approximately fourfold higher amplitudes and slower waveforms [43,58,59]. The bump currents of the cricket have also clearly longer latencies, but in the cockroach the latencies and their dispersion are surprisingly close to those in Drosophila, suggesting that the phototransduction processes prior to channel opening are similar. The large bump currents are, in part, responsible for large quantum bumps (i.e. large voltage bumps), facilitating the delivery of messages from absorbed photons to the brain. Although definite evidence is lacking, there are indications that the higher current amplification may be due to higher expression of TRPL channels. The TRPL channels of Drosophila are 10-fold less Ca2+-selective, produce larger currents and have slower kinetics than TRP [43,44]. Proportionally higher expression of TRPL might also explain some of the Ca2+-dependent properties of the currents in the cockroach [59]. This theory is supported by a study where RNA interference (RNAi) was successfully used to prevent the expression of TRP and TRPL homologues in the photoreceptors of the cockroach [60]. According to this study TRPL is more abundant than TRP in the cockroach retina, and responsible for 75% of the electroretinographic response amplitude. In Drosophila, mutants lacking TRPL do not manifest dramatic changes in responses to bright light, but have trouble adapting to low illumination levels [61]. Also, TRPL channels translocate from the rhabdomere into the cell body in response to prolonged light stimulation [62,63]. All these observations point to a more significant role for the TRPL in dim light photoreception.
In response to a long, bright light pulse the photoreceptors of Drosophila and the cockroach show a conspicuous peak-to-plateau transition, with similar waveform to a corresponding graded voltage response [27,37,59]. When a long light pulse is presented to a Drosophila trp mutant the light-induced current (LIC) decays to baseline after the initial transient [39]. Any shortly following light stimulation fails to elicit a new response. This sensitivity loss is due to PIP2 depletion resulting from the lack of TRP-mediated Ca2+ influx necessary for PLC inhibition [51]. Interestingly, a minority of cockroach photoreceptors also show such transient hyper-adapting behaviour [27,59]. The trp phenotype can also be induced by omitting extracellular Ca2+ in Drosophila [39]. However, this phenotype does not appear in normally responding photoreceptors of the cockroach. When Ca2+ is omitted, cockroach LIC shows slow kinetics, large amplitude and no apparent light adaptation, but also no rundown. Only after intracellular Ca2+ is chelated, does cockroach LIC decay to the baseline while the light is still on [59]. This implies that cockroach photoreceptors might use an intracellular Ca2+ source during light responses. If indeed cockroach LIC is largely mediated by TRPL, additional Ca2+ sources might be necessary for response amplification and inactivation. The most plausible candidates for the Ca2+ release are the SMCs, which undergo light-dependent translocation together with mitochondria and screening pigments [64,65]. In dark-adapted photoreceptors SMCs are situated at the base of rhabdomeres, forming a palisade that helps to gather light more efficiently. Among arthropods, at least the ventral nerve photoreceptors of Limulus and the photoreceptors of the honeybee are known to rely on IP3-Ca2+ from SMCs [66,67], possibly due to the lack of sufficient Ca2+ influx via light-gated channels in the former. Since a quantum bump in Limulus is formed by the activation of several microvilli, and perhaps partially by the widespread Ca2+ after IP3-Ca2+ [66], it is possible that the larger bumps in photoreceptors of nocturnal insects might derive from more than one activated microvillus. It should be noted, however, that since the visual function of the extraocular ventral photoreceptors of Limulus is not known, and since the honeybee is a diurnal species, the IP3-Ca2+ mechanism might not be an exclusive specialization of nocturnal photoreceptors.
5. Electrical properties of the photoreceptor membrane
The conductance created by open TRP/TRPL channels creates an LIC, which changes the photoreceptor membrane potential, resulting in a graded response. LIC amplitude depends on the driving force (i.e. the difference between membrane potential and the LIC reversal potential) and TRP/TRPL conductance. The driving force is reduced during depolarization as membrane voltage approaches the LIC reversal potential, the upper limit of the photoreceptor's operating range, resulting in an effect called self-shunting. Together with other means of light adaptation self-shunting plays an important role in compressing a wide range of light intensities into the narrow functional range of a photoreceptor.
The conversion from conductance to voltage response is governed by the membrane impedance, which defines how much amplification a signal receives per temporal frequency [68]. Membrane impedance is formed by capacitive and resistive properties of the photoreceptor membrane resulting from the lipid bilayer and the ion channel proteins embedded in it, respectively (figure 1b(ii),c(ii)). Input resistance (Rin) results from passive resistance caused by persistently open leak channels, and active resistance due to ion channels whose open probability is controlled by a biophysical signal, e.g. chemical ligand or the membrane potential. Together Rin and the membrane capacitance (Cm) form a lowpass or bandpass impedance function that filters the phototransduction current signal [69]. The impedance filter is characterized by the upper cut-off frequency fc = 1/(2πτ), where τ is the membrane time constant τ = Rin · Cm, which describes how fast the transmembrane current is converted into a membrane voltage change. By modifying either Rin or Cm, τ can be regulated to allow faster voltage changes or to limit the responses to slow signals. Cm is relatively constant, although some variation may occur due to shedding of microvillar membrane [70]. Conversely, Rin can be dynamically adjusted by opening and closing ion channels, regulating their expression [71], or by modulating their open probability profile [72].
Cm and Rin in nocturnal photoreceptors are large compared to diurnal species (figure 1b(ii),c(ii)) [29]. The former is partly explained by the larger rhabdom size (see longer microvilli in figure 1c than in figure 1b) [12,57], and the latter by reduced non-selective leak conductance and smaller voltage-dependent conductance near the dark-resting potential [73]. The large τ and small fc promote temporal summation, a common property of nocturnal photoreceptors, as the membrane filters out faster signals and amplifies slow ones. Consequently, the photoreceptors will have improved sensitivity for low light signals, but poor temporal resolution.
6. Kv conductances
Voltage-gated potassium channels, Kv channels, are considered the most important modulators of light-independent membrane impedance in insect photoreceptors [74]. Kv channels opened during the light-induced depolarization can have various functions, such as prevention of voltage response saturation by decreasing the impedance, decreasing the membrane time constant to allow propagation of faster signals, or fine-tuning the time- and voltage-dependent amplification to optimize information processing of the photoresponse [75]. Based on the voltage-dependence of opening and closing kinetics, Kv channels in insect photoreceptors can be categorized roughly into two types: sustained delayed-rectifier and transient A-type channels.
Kv channels in Drosophila photoreceptors were the first to be described in detail [76]. They express at least four Kv channel types contributing to voltage light response modulation. The largest effects come from Shaker, a fast activating and inactivating transient conductance, and Shab, a slowly activating delayed-rectifier type conductance. Shaker improves information processing by selectively amplifying graded voltage responses and distributing the responses more effectively into the physiological voltage range [75]. Shab, typically for sustained conductances, prevents saturation during light-induced depolarization and accelerates the photoreceptor temporal properties [77–79].
Since different Kv channels modulate the electrical light response, the expression of specific channels in species with different visual ecologies has received special attention. In Diptera, diurnal species express a dominant sustained conductance and crepuscular species a transient Kv conductance [29]. In the locust, the Kv conductance profile follows a circadian rhythm induced by serotonin modulation, with a sustained conductance dominating during the day and a transient conductance during the night [71]. In flies, the Shab delayed-rectifier is upregulated by light through PIP2 hydrolysis [80], and serotonin modulates the voltage-dependence of Shab and Shaker conductances [72]. However, fly and locust studies give a simplified view on the relationship between the circadian activity and expressed Kv conductances, as shown by Frolov et al. [73]. Their comparative study of 15 species showed that while the fast flying diurnal insects as a rule express a dominant sustained Kv conductance, the nocturnal or crepuscular species may in fact express either a sustained, or both sustained and transient, conductance. Moreover, the transient Kv conductances in the nocturnal species do not seem to be active at the physiological voltage levels of light responses, leaving open the question about the significance of such conductances to photoreceptor function. Typecasting insects to specific circadian lifestyles based on Kv conductances is thus difficult, and more comparative studies are needed in order to make sense of the Kv channel palette in insect photoreceptors.
7. Signalling and information transfer in nocturnal photoreceptors
Together the phototransduction and the electrochemical properties of the membrane define the signalling properties of photoreceptors. A useful tool for comparing signalling differences between diurnal and nocturnal photoreceptors is the photoreceptor transfer function. The simplest way is to derive the transfer function from impulse responses by Fourier transformation. Another method is recording responses to a light contrast stimulus modulated by Gaussian-distributed white noise. This provides two temporal frequency-dependent measures, which together constitute the transfer function: contrast gain describing the size of a photoreceptor voltage response per unit contrast at a given frequency, and phase function describing the phase difference between the stimulus and response. As the white-noise method allows the analysis of the frequency dependence of the signal-to-noise ratio (SNR), it can be used to estimate photoreceptor information transfer rates, i.e. the number of bits processed per second.
Once again the closely related tropical bees, Megalopta and Lasioglossum, may serve as an example of differences in diurnal and nocturnal photoreceptor signalling [26]. Megalopta photoreceptors discriminate contrasts in dim light better due to higher contrast gain and a relatively narrow signalling bandwidth, which effectively filters out high frequency noise, but also reduces temporal resolution (figure 1f). In a bright background, Megalopta photoreceptors retain their lowpass filtering properties, while Lasioglossum photoreceptors assume bandpass properties, improving temporal discrimination (figure 1g). However, when information rates are compared, Lasioglossum triumphs at all background light levels due to the consistently poorer SNR and bandwidth in Megalopta photoreceptors. Only after accounting for the more sensitive optics, has Megalopta higher information rates than Lasioglossum in the dimmest backgrounds. Similar lowpass filtering (temporal summations) and relatively poor information transfer properties have been reported also in other dark-active insects [27,29,58,81]. In conclusion, nocturnal insects trade temporal (and spatial) resolving power to allow photoreceptors to distinguish meaningful intensity differences from an environment where photons are scarce.
8. Peculiar properties in cockroach photoreceptors underlie adaptation to dim light
Compared with diurnal and also other studied nocturnal species, the photoreceptors of the cockroach Periplaneta have some peculiar features. Light responses adapt with greatly varying kinetics and magnitude [2]. At the extreme, cockroach photoreceptors may enter a dark-adapted-like state within seconds after a prolonged bright light exposure, with the membrane potential returning close to the dark-resting potential value and discrete bumps forming the response despite the bright illumination (the trp phenotype).
Contrary to most of the studied insects where light adaptation mechanisms improve photoreceptor performance with increasing light intensity, cockroach photoreceptors do not generate significantly faster or more accurate responses at intensities above approximately 1000 absorbed photons s−1 [27]. Failure of adaptation to brighter backgrounds has also been observed in nocturnal flies and bees, although to somewhat lesser degrees than in cockroach [1,29]. A possible explanation for the saturation of the performance at modest light levels can be given using the stochastic sampling model by Song et al. [82], and by assuming that in comparison to Drosophila photoreceptors, cockroach photoreceptors have either fewer microvilli, longer refractory periods, or both. Activation of several microvilli during the generation of a quantum bump, or the first stage of TRPL channel translocation out of the microvilli [63], could also explain the early saturation.
Presumably to boost signal propagation in the long photoreceptor axons of Periplaneta, graded photoreceptor responses recorded from the axon are overlaid with spikes [83]. Although inter-axon variability is large, all of the photoreceptor axons in Periplaneta are very long and grouped seemingly randomly into bundles terminating in the optic lobe [2]. The random variation in photoreceptor properties, the random pooling of photoreceptor signals, and the peculiar spiking axons are actually adaptations improving visual reliability in low light [2].
9. Future perspectives
Drosophila is a useful model organism in vision research because of the vast research toolbox available to be used with it. However, the mainly day active [25] Drosophila represents a very narrow group of insects. Mounting evidence [57] warns against making generalizations of visual system functions between insects from different ecological niches.
Although research with dark-active insects lags behind Drosophila studies in available molecular methods, some advances have been made. RNA interference (RNAi) is a method of targeted gene silencing via a cascade initiated by introduction of double stranded RNA into the cell. RNAi has been successfully used in concert with electroretinogram recordings to determine the functional consequences of silencing visual opsins or channel proteins in the compound eye of Periplaneta [60]. Next, TRP and TRPL channel silencing by RNAi should be combined with behavioural and electrophysiological experiments in a controlled environment [9] to resolve whether TRPL channel prevalence in the photoreceptor membrane really is a prerequisite for vision in low light. Periplaneta has two green opsins [60], but the function, or lack thereof, of the less abundant duplicate is unknown. Its possible role in light adaptation could be examined with protein expression studies and immunohistochemistry (e.g. [35]). Also, there is the possibility of the recruitment of multiple microvilli during a quantum bump, possibly in conjunction with IP3-Ca2+.
Superposition compound eyes, typical of many nocturnal insects such as moths, pose a problem of their own for electrophysiology. Poor success of patch-clamp experiments on superposition photoreceptors means their electrical properties and phototransduction mechanisms are practically unknown. Also, conclusions about spatio-temporal pooling in the optic lobes have until recently [16] relied mainly on lamina cell anatomy (e.g. [18,20]).
The ideal animal model for studying night vision, or any vision for that matter, would manifest visual behaviour suitable for behavioural experiments, have a visual system fit for both various electrophysiological recording methods and histological and immunohistochemical studies, and be yielding to various molecular methods. The cockroach P. americana is joining Drosophila in the ranks of vision research workhorses meeting all these demands, but other model organisms are badly needed as well. Comparative studies between species possessing different eye types and behavioural needs will lead the way into a more thorough understanding of photoreception in dim light.
10. Conclusion
The compound eyes of night active insects produce bigger quantum bumps relative to the eyes of their diurnal counterparts. Big bumps are characterized by slow kinetics, and high phototransduction gain due to high input resistance and membrane capacitance. The high capacitance is partially explained by larger rhabdomeres and thus larger photoreceptive membrane area in the night active eyes. Together with the high-gain phototransduction these electrical properties adjust nocturnal photoreceptors to have high sensitivity to light with the cost of reduced temporal resolution and information transfer. Efficient adaptation mechanisms—such as trafficking organelles, screening pigments, rhodopsin and ion channels—are needed to optimize the vision to changing photic conditions during dusk and dawn. As more insects are being studied, it is becoming evident that the division between night- and day-active photoreceptor physiologies is not as straightforward as was thought. A varied set of experimental tools and new animal models are needed for the thorough study of dim light vision in insects.
Acknowledgements
We thank David O'Carroll and Eric Warrant for allowing us to complete this manuscript after the sudden passing of Matti Weckström; and Rikard Frederiksen for letting us reproduce his figures. Matti was the scientific father of all of us, and we thank him for his brilliant fatherly supervision and friendly guidance.
Authors' contributions
All authors contributed equally.
Competing interests
The authors declare no competing interests.
Funding
Funding was provided by the Academy of Finland to all authors.
References
- 1.Frederiksen R, Wcislo WT, Warrant EJ. 2008. Visual reliability and information rate in the retina of a nocturnal bee. Curr. Biol. 18, 349–353. ( 10.1016/j.cub.2008.01.057) [DOI] [PubMed] [Google Scholar]
- 2.Heimonen K, Salmela I, Kontiokari P, Weckström M. 2006. Large functional variability in cockroach photoreceptors: optimization to low light levels. J. Neurosci. 26, 13 454–13 462. ( 10.1523/JNEUROSCI.3767-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warrant EJ, Kelber A, Gislén A, Greiner B, Ribi W, Wcislo WT. 2004. Nocturnal vision and landmark orientation in a tropical halictid bee. Curr. Biol. 14, 1309–1318. ( 10.1016/j.cub.2004.07.057) [DOI] [PubMed] [Google Scholar]
- 4.Kelber A, Balkenius A, Warrant EJ. 2003. Colour vision in diurnal and nocturnal hawkmoths. Integr. Comp. Biol. 43, 571–579. ( 10.1093/icb/43.4.571) [DOI] [PubMed] [Google Scholar]
- 5.Dacke M, Baird E, Byrne M, Scholtz CH, Warrant EJ. 2013. Dung beetles use the Milky Way for orientation. Curr. Biol. 23, 298–300. ( 10.1016/j.cub.2012.12.034) [DOI] [PubMed] [Google Scholar]
- 6.Hölldobler B, Taylor R. 1983. A behavioral study of the primitive ant Nothomyrmecia macrops Clark. Insect. Soc. 30, 384–401. ( 10.1007/BF02223970) [DOI] [Google Scholar]
- 7.Hironaka M, Inadomi K, Nomakuchi S, Filippi L, Hariyama T. 2008. Canopy compass in nocturnal homing of the subsocial shield bug, Parastrachia japonensis (Heteroptera: Parastrachiidae). Naturwissenschaften 95, 343–346. ( 10.1007/s00114-007-0324-1) [DOI] [PubMed] [Google Scholar]
- 8.Okada J, Toh Y. 1998. Shade response in the escape behavior of the cockroach, Periplaneta americana. Zool. Sci. 15, 831–835. ( 10.2108/zsj.15.831) [DOI] [Google Scholar]
- 9.Honkanen A, Takalo J, Heimonen K, Vähäsöyrinki M, Weckström M. 2014. Cockroach optomotor responses below single photon level. J. Exp. Biol. 217, 4262–4268. ( 10.1242/jeb.112425) [DOI] [PubMed] [Google Scholar]
- 10.Dubs A, Laughlin SB, Srinivasan MV. 1981. Single photon signals in fly photoreceptors and first order interneurones at behavioral threshold. J. Physiol. 317, 317–334. ( 10.1113/jphysiol.1981.sp013827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warrant EJ, Nilsson DE. 1998. Absorption of white light in photoreceptors. Vision Res. 38, 195–207. ( 10.1016/S0042-6989(97)00151-X) [DOI] [PubMed] [Google Scholar]
- 12.Warrant EJ. 1999. Seeing better at night: life style, eye design and the optimum strategy of spatial and temporal summation. Vision Res. 39, 1611–1630. ( 10.1016/S0042-6989(98)00262-4) [DOI] [PubMed] [Google Scholar]
- 13.Theobald JC, Coates MM, Wcislo WT, Warrant EJ. 2007. Flight performance in night-flying sweat bees suffers at low light levels. J. Exp. Biol. 210, 4034–4042. ( 10.1242/jeb.003756) [DOI] [PubMed] [Google Scholar]
- 14.Reber T, Vähäkainu A, Baird E, Weckström M, Warrant E, Dacke M. 2015. Effect of light intensity on flight control and temporal properties of photoreceptors in bumblebees. J. Exp. Biol. 218, 1339–1346. ( 10.1242/jeb.113886) [DOI] [PubMed] [Google Scholar]
- 15.Doujak FE. 1985. Can a shore crab see a star? J. Exp. Biol. 116, 385–393. [Google Scholar]
- 16.Stöckl AL, O'Carroll DC, Warrant EJ. 2016. Neural summation in the hawkmoth visual system extends the limits of vision in dim light. Curr. Biol. 26, 821–826. ( 10.1016/j.cub.2016.01.030) [DOI] [PubMed] [Google Scholar]
- 17.Ohly KP. 1975. The neurons of the first synaptic region of the optic neuropil of the firefly, Phausis splendidula L. (Coleoptera). Cell Tissue Res. 158, 89–109. ( 10.1007/BF00219953) [DOI] [PubMed] [Google Scholar]
- 18.Greiner B, Ribi WA, Wcislo WT, Warrant EJ. 2004. Neural organisation in the first optic ganglion of the nocturnal bee Megalopta genalis. Cell Tissue Res. 318, 429–437. ( 10.1007/s00441-004-0945-z) [DOI] [PubMed] [Google Scholar]
- 19.Stöckl AL, Ribi WA, Warrant EJ. 2016. Adaptations for nocturnal and diurnal vision in the hawkmoth lamina. J. Comp. Neurol. 524, 160–175. ( 10.1002/cne.23832) [DOI] [PubMed] [Google Scholar]
- 20.Ribi WA. 1977. Fine structure of the first optic ganglion (lamina) of the cockroach, Periplaneta americana. Tissue Cell 9, 57–72. ( 10.1016/0040-8166(77)90049-0) [DOI] [PubMed] [Google Scholar]
- 21.Yeandle S. 1958. Electrophysiology of the visual system. Am. J. Ophthalmol. 46, 82–87. [Google Scholar]
- 22.Fuortes MG, Yeandle S. 1964. Probability of occurrence of discrete potential waves in the eye of Limulus. J. Gen. Physiol. 47, 443–463. ( 10.1085/jgp.47.3.443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lillywhite PG. 1977. Single photon signals and transduction in an insect eye. J. Comp. Physiol. A 122, 189–200. ( 10.1007/BF00611889) [DOI] [Google Scholar]
- 24.de Vries HL. 1943. The quantum character of light and its bearing upon threshold of vision, the differential sensitivity and visual acuity of the eye. Physica 10, 553–564. ( 10.1016/S0031-8914(43)90575-0) [DOI] [Google Scholar]
- 25.Vanin S, Bhutani S, Montelli S, Menegazzi P, Green EW, Pegoraro M, Sandrelli F, Costa R, Kyriacou CP. 2012. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature 484, 371–375. ( 10.1038/nature10991) [DOI] [PubMed] [Google Scholar]
- 26.Frederiksen R, Warrant EJ. 2008. The optical sensitivity of compound eyes: theory and experiment compared. Biol. Lett. 4, 745–747. ( 10.1098/rsbl.2008.0467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heimonen K, Immonen E-V, Frolov RV, Salmela I, Juusola M, Vähäsöyrinki M, Weckström M. 2012. Signal coding in cockroach photoreceptors is tuned to dim environments. J. Neurophysiol. 108, 2641–2652. ( 10.1152/jn.00588.2012) [DOI] [PubMed] [Google Scholar]
- 28.de Souza JM, Ventura DF. 1989. Comparative study of temporal summation and response form in hymenopteran photoreceptors. J. Comp. Physiol. A 165, 237–245. ( 10.1007/BF00619198) [DOI] [PubMed] [Google Scholar]
- 29.Laughlin SB, Weckström M. 1993. Fast and slow photoreceptors—a comparative study of the functional diversity of coding and conductances in the Diptera. J. Comp. Physiol. A 172, 593–609. ( 10.1007/BF00213682) [DOI] [Google Scholar]
- 30.Frolov R, Immonen E-V, Vahasoyrinki M, Weckström M. 2012. Postembryonic developmental changes in photoreceptors of the stick insect Carausius morosus enhance the shift to an adult nocturnal life-style. J. Neurosci. 32, 16 821–16 831. ( 10.1523/JNEUROSCI.2612-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laughlin S, Blest AD, Stowe S. 1980. The sensitivity of receptors in the posterior median eye of the nocturnal spider, Dinopis. J. Comp. Physiol. A 141, 53–65. ( 10.1007/BF00611878) [DOI] [Google Scholar]
- 32.Pirhofer-Walzl K, Warrant E, Barth FG. 2007. Adaptations for vision in dim light: impulse responses and bumps in nocturnal spider photoreceptor cells (Cupiennius salei Keys). J. Comp. Physiol. A 193, 1081–1087. ( 10.1007/s00359-007-0263-5) [DOI] [PubMed] [Google Scholar]
- 33.Howard J, Dubs A, Payne R. 1984. The dynamics of phototransduction in insects. A comparative study. J. Comp. Physiol. A 154, 707–718. ( 10.1007/BF01350224) [DOI] [Google Scholar]
- 34.Schmeling F, Wakakuwa M, Tegtmeier J, Kinoshita M, Bockhorst T, Arikawa K, Homberg U. 2014. Opsin expression, physiological characterization and identification of photoreceptor cells in the dorsal rim area and main retina of the desert locust, Schistocerca gregaria. J. Exp. Biol. 217, 3557–3568. ( 10.1242/jeb.108514) [DOI] [PubMed] [Google Scholar]
- 35.Moon YM, Metoxen AJ, Leming MT, Whaley MA, O'Tousa JE. 2014. Rhodopsin management during the light–dark cycle of Anopheles gambiae mosquitoes. J. Insect Physiol. 70, 88–93. ( 10.1016/j.jinsphys.2014.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardie RC. 1991. Whole-cell recordings of the light induced current in dissociated Drosophila photoreceptors: evidence for feedback by calcium permeating the light-sensitive channels. Proc. R. Soc. Lond. B 245, 203–210. ( 10.1098/rspb.1991.0110) [DOI] [Google Scholar]
- 37.Hardie RC. 2012. Phototransduction mechanisms in Drosophila microvillar photoreceptors. Wiley Interdiscip. Rev. Membr. Transp. Signal. 1, 162–187. ( 10.1002/wmts.20) [DOI] [Google Scholar]
- 38.Cosens DJ, Manning A. 1969. Abnormal electroretinogram from a Drosophila mutant. Nature 224, 285–287. ( 10.1038/224285a0) [DOI] [PubMed] [Google Scholar]
- 39.Hardie RC, Minke B. 1992. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8, 643–651. ( 10.1016/0896-6273(92)90086-S) [DOI] [PubMed] [Google Scholar]
- 40.Huang J, Liu C-H, Hughes SA, Postma M, Schwiening CJ, Hardie RC. 2010. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr. Biol. 20, 189–197. ( 10.1016/j.cub.2009.12.019) [DOI] [PubMed] [Google Scholar]
- 41.Hardie RC, Franze K. 2012. Photomechanical responses in Drosophila photoreceptors. Science 338, 260–263. ( 10.1126/science.1222376) [DOI] [PubMed] [Google Scholar]
- 42.Wu CF, Pak WL. 1975. Quantal basis of photoreceptor spectral sensitivity of Drosophila melanogaster. J. Gen. Physiol. 66, 149–168. ( 10.1085/jgp.66.2.149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henderson SR, Reuss H, Hardie RC. 2000. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+. J. Physiol. 524, 179–194. ( 10.1111/j.1469-7793.2000.00179.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reuss H, Mojet MH, Chyb S, Hardie RC. 1997. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron 19, 1249–1259. ( 10.1016/S0896-6273(00)80416-X) [DOI] [PubMed] [Google Scholar]
- 45.Xu XZ, Chien F, Butler A, Salkoff L, Montell C. 2000. TRPγ, a Drosophila TRP-related subunit, forms a regulated cation channel with TRPL. Neuron 26, 647–657. ( 10.1016/S0896-6273(00)81201-5) [DOI] [PubMed] [Google Scholar]
- 46.Juusola M, Hardie RC. 2001. Light adaptation in Drosophila photoreceptors: I. Response dynamics and signaling efficiency at 25°C. J. Gen. Physiol. 117, 3–25. ( 10.1085/jgp.117.1.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker CS. 1997. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature 388, 243–249. ( 10.1038/40805) [DOI] [PubMed] [Google Scholar]
- 48.Postma M, Oberwinkler J, Stavenga DG. 1999. Does Ca2+ reach millimolar concentrations after single photon absorption in Drosophila photoreceptor microvilli? Biophys. J. 77, 1811–1823. ( 10.1016/S0006-3495(99)77026-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardie RC. 1995. Photolysis of caged Ca2+ facilitates and inactivates but does not directly excite light-sensitive channels in Drosophila photoreceptors. J. Neurosci. 15, 889–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohn E, Katz B, Yasin B, Peters M, Rhodes E, Zaguri R, Weiss S, Minke B. 2015. Functional cooperation between the IP3 receptor and phospholipase C secures the high sensitivity to light of Drosophila photoreceptors in vivo. J. Neurosci. 35, 2530–2546. ( 10.1523/JNEUROSCI.3933-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardie RC, Raghu P, Moore S, Juusola M, Baines RA, Sweeney ST. 2001. Calcium influx via TRP channels is required to maintain PIP2 levels in Drosophila photoreceptors. Neuron 30, 149–159. ( 10.1016/S0896-6273(01)00269-0) [DOI] [PubMed] [Google Scholar]
- 52.Gu Y, Oberwinkler J, Postma M, Hardie RC. 2005. Mechanisms of light adaptation in Drosophila photoreceptors. Curr. Biol. 15, 1228–1234. ( 10.1016/j.cub.2005.05.058) [DOI] [PubMed] [Google Scholar]
- 53.Liu CH, Satoh AK, Postma M, Huang J, Ready DF, Hardie RC. 2008. Ca2+-dependent metarhodopsin inactivation mediated by calmodulin and NINAC myosin III. Neuron 59, 778–789. ( 10.1016/j.neuron.2008.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardie RC, Minke B. 1994. Calcium-dependent inactivation of light-sensitive channels in Drosophila photoreceptors. J. Gen. Physiol. 103, 409–427. ( 10.1085/jgp.103.3.409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu W, Wen W, Wei Z, Yu J, Ye F, Liu CH, Hardie RC, Zhang M. 2011. The INAD scaffold is a dynamic, redox-regulated modulator of signaling in the Drosophila eye. Cell 145, 1088–1101. ( 10.1016/j.cell.2011.05.015) [DOI] [PubMed] [Google Scholar]
- 56.Chu B, Liu C-H, Sengupta S, Gupta A, Raghu P, Hardie RC. 2013. Common mechanisms regulating dark noise and quantum bump amplification in Drosophila photoreceptors. J. Neurophysiol. 109, 2044–2055. ( 10.1152/jn.00001.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frolov RV. 2016. Current advances in invertebrate vision: insights from patch-clamp studies of photoreceptors in apposition eyes. J. Neurophysiol. 116, 709–723. ( 10.1152/jn.00288.2016) [DOI] [PubMed] [Google Scholar]
- 58.Frolov RV, Immonen E-V, Weckström M. 2014. Performance of blue- and green-sensitive photoreceptors of the cricket Gryllus bimaculatus. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 200, 209–219. ( 10.1007/s00359-013-0879-6) [DOI] [PubMed] [Google Scholar]
- 59.Immonen EV, Krause S, Krause Y, Frolov R, Vähäsöyrinki MT, Weckström M. 2014. Elementary and macroscopic light-induced currents and their Ca2+-dependence in the photoreceptors of Periplaneta americana. Front. Physiol. 5, 153 ( 10.3389/fphys.2014.00153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.French AS, Meisner S, Liu H, Weckström M, Torkkeli PH. 2015. Transcriptome analysis and RNA interference of cockroach phototransduction indicate three opsins and suggest a major role for TRPL channels. Front. Physiol. 6, 207 ( 10.3389/fphys.2015.00207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leung HT, Geng C, Pak WL. 2000. Phenotypes of trpl mutants and interactions between the transient receptor potential (TRP) and TRP-like channels in Drosophila. J. Neurosci. 20, 6797–6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bähner M, Frechter S, Da Silva N, Minke B, Paulsen R, Huber A. 2002. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron 34, 83–93. ( 10.1016/S0896-6273(02)00630-X) [DOI] [PubMed] [Google Scholar]
- 63.Cronin MA, Lieu M-H, Tsunoda S. 2006. Two stages of light-dependent TRPL-channel translocation in Drosophila photoreceptors. J. Cell Sci. 119, 2935–2944. ( 10.1242/jcs.03049) [DOI] [PubMed] [Google Scholar]
- 64.Horridge GA, Barnard PBT. 1965. Movement of palisade in locust retinula cells when illuminated. J. Cell Sci. 106, 131–136. [PubMed] [Google Scholar]
- 65.Butler R. 1973. The anatomy of the compound eye of Periplaneta americana L. 2. Fine structure. J. Comp. Physiol. 83, 239–262. ( 10.1007/BF00693677) [DOI] [Google Scholar]
- 66.Dorlöchter M, Stieve H. 1997. The Limulus ventral photoreceptor: light response and the role of calcium in a classic preparation. Prog. Neurobiol. 53, 451–515. ( 10.1016/S0301-0082(97)00046-4) [DOI] [PubMed] [Google Scholar]
- 67.Walz B, Baumann O, Zimmermann B, Ciriacy-Wantrup EV. 1995. Caffeine- and ryanodine-sensitive Ca2+-induced Ca2+ release from the endoplasmic reticulum in honeybee photoreceptors. J. Gen. Physiol. 105, 537–567. ( 10.1085/jgp.105.4.537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weckström M, Kouvalainen E, Juusola M. 1992. Measurement of cell impedance in frequency domain using discontinuous current clamp and white-noise-modulated current injection. Pflügers Arch. 421, 469–472. ( 10.1007/BF00370258) [DOI] [PubMed] [Google Scholar]
- 69.Juusola M, Weckström M. 1993. Band-pass filtering by voltage-dependent membrane in an insect photoreceptor. Neurosci. Lett. 154, 84–88. ( 10.1016/0304-3940(93)90177-M) [DOI] [PubMed] [Google Scholar]
- 70.Williams DS. 1983. Changes of photoreceptor performance associated with the daily turnover of photoreceptor membrane in locusts. J. Comp. Physiol. A 150, 509–519. ( 10.1007/BF00609577) [DOI] [Google Scholar]
- 71.Cuttle MF, Hevers W, Laughlin SB, Hardie RC. 1995. Diurnal modulation of photoreceptor potassium conductance in the locust. J. Comp. Physiol. A 176, 307–316. ( 10.1007/BF00219056) [DOI] [Google Scholar]
- 72.Hevers W, Hardie RC. 1995. Serotonin modulates the voltage dependence of delayed rectifier and Shaker potassium channels in Drosophila photoreceptors. Neuron 14, 845–856. ( 10.1016/0896-6273(95)90228-7) [DOI] [PubMed] [Google Scholar]
- 73.Frolov R, Immonen E-V, Weckström M. 2016. Visual ecology and potassium conductances of insect photoreceptors. J. Neurophysiol. 115, 2147–2157. ( 10.1152/jn.00795.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weckström M, Laughlin SB. 1995. Visual ecology and voltage-gated ion channels in insect photoreceptors. Trends Neurosci. 18, 17–21. ( 10.1016/0166-2236(95)93945-T) [DOI] [PubMed] [Google Scholar]
- 75.Niven JE, Vähäsöyrinki M, Kauranen M, Hardie RC, Juusola M, Weckström M. 2003. The contribution of Shaker K+ channels to the information capacity of Drosophila photoreceptors. Nature 421, 630–634. ( 10.1038/nature01384) [DOI] [PubMed] [Google Scholar]
- 76.Hardie RC. 1991. Voltage-sensitive potassium channels in Drosophila photoreceptors. J. Neurosci. 11, 3079–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weckström M, Hardie RC, Laughlin SB. 1991. Voltage-activated potassium channels in blowfly photoreceptors and their role in light adaptation. J. Physiol. 440, 635–657. ( 10.1113/jphysiol.1991.sp018729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salmela I, Immonen E-V, Frolov R, Krause S, Krause Y, Vähäsöyrinki M, Weckström M. 2012. Cellular elements for seeing in the dark: voltage-dependent conductances in cockroach photoreceptors. BMC Neurosci. 13, 93 ( 10.1186/1471-2202-13-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vähäsöyrinki M, Niven JE, Hardie RC, Weckström M, Juusola M. 2006. Robustness of neural coding in Drosophila photoreceptors in the absence of slow delayed rectifier K+ channels. J. Neurosci. 26, 2652–2660. ( 10.1523/JNEUROSCI.3316-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krause Y, Krause S, Huang J, Liu C-H, Hardie RC, Weckström M. 2008. Light-dependent modulation of Shab channels via phosphoinositide depletion in Drosophila photoreceptors. Neuron 59, 596–607. ( 10.1016/j.neuron.2008.07.009) [DOI] [PubMed] [Google Scholar]
- 81.Frolov R, Weckström M. 2014. Developmental changes in biophysical properties of photoreceptors in the common water strider (Gerris lacustris): better performance at higher cost. J. Neurophysiol. 112, 913–922. ( 10.1152/jn.00239.2014) [DOI] [PubMed] [Google Scholar]
- 82.Song Z, Postma M, Billings SA, Coca D, Hardie RC, Juusola M. 2012. Stochastic, adaptive sampling of information by microvilli in fly photoreceptors. Curr. Biol. 22, 1371–1380. ( 10.1016/j.cub.2012.05.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weckström M, Järvilehto M, Heimonen K. 1993. Spike-like potentials in the axons of nonspiking photoreceptors. J. Neurophysiol. 69, 293–296. [DOI] [PubMed] [Google Scholar]