Abstract
Flight control in insects is heavily dependent on vision. Thus, in dim light, the decreased reliability of visual signal detection also prompts consequences for insect flight. We have an emerging understanding of the neural mechanisms that different species employ to adapt the visual system to low light. However, much less explored are comparative analyses of how low light affects the flight behaviour of insect species, and the corresponding links between physiological adaptations and behaviour. We investigated whether the flower tracking behaviour of three hawkmoth species with different diel activity patterns revealed luminance-dependent adaptations, using a system identification approach. We found clear luminance-dependent differences in flower tracking in all three species, which were explained by a simple luminance-dependent delay model, which generalized across species. We discuss physiological and anatomical explanations for the variance in tracking responses, which could not be explained by such simple models. Differences between species could not be explained by the simple delay model. However, in several cases, they could be explained through the addition on a second model parameter, a simple scaling term, that captures the responsiveness of each species to flower movements. Thus, we demonstrate here that much of the variance in the luminance-dependent flower tracking responses of hawkmoths with different diel activity patterns can be captured by simple models of neural processing.
This article is part of the themed issue ‘Vision in dim light’.
Keywords: system identification, vision, flower tracking, motor control, flight, hawkmoth
1. Introduction
A hawkmoth zooming from flower to flower at a honeysuckle bush at night might seem effortless, yet there are considerable challenges to this performance. Flight control in insects, both during day and night foraging, has a strong visual component, which includes safely approaching the honeysuckle bush while avoiding obstacles [1–4], inserting the proboscis in the nectary [5,6], and stable tracking of the flowers swaying in the wind [7–10]. Mechanosensory systems also contribute to flight control, by providing information about ego-motion through sensors in the antennae [11], and about flower motion through sensors in the proboscis [12]. Yet, only the visual contribution is challenged by the lower reliability of its input signals at night, with more than six orders of magnitude lower light intensity than during the day ([13], figure 1a). To cope, the visual systems of nocturnal insects trade off sensitivity for spatial and/or temporal resolution [13]. Increased sensitivity in insects can arise from eye anatomy featuring coarser acceptance angles [14–16], photoreceptors reducing response speed [16–19] and the central nervous system integrating signals in space and time [19–21]. This trade off between sensitivity and resolution poses a dilemma for nocturnal flight: adaptations to increase sensitivity are crucial to obtain the necessary visual input for flight control, yet decreased spatial and temporal resolution reduce the visual information content, especially at high frequencies. How do the changes in visual sensitivity and resolution affect the flight performance of insects active at variable light intensities?
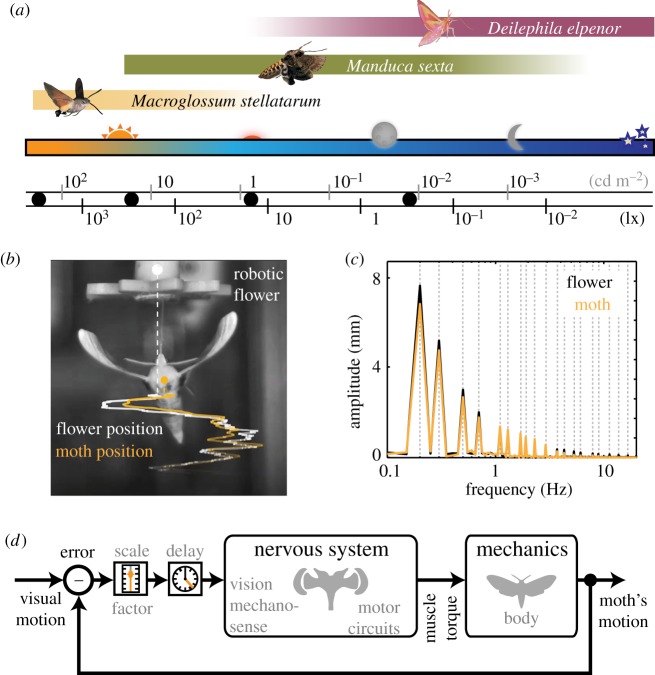
Figure 1.
Flower tracking in hawkmoths. We investigated the flower tracking performance of three hawkmoths species with different diel activity patterns (a). Black dots denote experimental light intensities. Illuminance in (a) was measured at the position of the flower, facing the light source, while luminance was measured from the flower face at a distance of 2 cm. Moths tracked and fed from robotic artificial flowers (b, example from D. elpenor; see the electronic supplementary material), moving in a combination of sines of different frequencies (c). The Fourier transformation of the tracked flower shows the stimulus frequencies and amplitudes (c), which were chosen to give equal velocities across frequencies. We used a system identification approach to describe the closed-loop behaviour of a moth's flower tracking (d). The inner part of the closed loop contains the nervous system (sensory and motor circuits) and the mechanics (body and wings). A simple time delay, as well as scaling factor, can be added to the inner part of the loop to model adaptations of the nervous system at different light intensities or across species.
The consequences of low-light intensity on flight performance have been observed in a number of species [9,22,23]. In hymenopterans, both spatial and temporal summation in the visual system have been proposed as the neural mechanisms for the behavioural changes in dim light. Bumblebees as well as hornets reduce their flight speed with decreased light intensity, which has been suggested as a mechanism to cope with the reduced temporal acuity of the visual system caused by temporal summation [22,24]. In contrast, nocturnal sweat bees do not change their flight speed during landing [23] or tunnel flight [25], and spatial summation has been suggested to underlay their high sensitivity at night. While qualitative similarities and species-specific differences are emerging, we do not yet understand from a quantitative, much less a mechanistic, standpoint how the physiological adaptations for low-light vision translate to behaviour differences across species.
Sponberg et al. [9] used system identification approaches to assess how flower tracking changed with light intensity in a crepuscular hawkmoth species. This approach enables explicit testing of simple dynamics models for how temporal processing could affect behaviour, while taking advantage of the inherent feedback nature of sensorimotor processing. They showed that the differences in flower tracking behaviour at different light intensities were consistent with a simple temporal delay in the nervous system, such as could result from increased temporal summation in the nervous system in dim light [13]. However, we do not yet know if this model generalizes across species, especially those with different diel activity patterns. Moreover, species active at different preferred light levels might show shifts in their neural processing. These adaptations could translate into behavioural differences, which might also be captured by simple models if the underlying sensorimotor processing is similar.
We thus chose to investigate different species of hawkmoth active in vastly different light intensities (figure 1a). The diurnal Macroglossum stellatarum, crepuscular Manduca sexta and nocturnal Deilephila elpenor all share very similar ecologies and flight strategies [26], enabling a natural comparison of neural and behavioural strategies for flight in dim light. Moreover, recent studies have provided detailed insight into the visual systems of all three species and their neural adaptations to different light intensities [16,19], allowing us to directly compare our behavioural predictions of temporal summation strategies to the corresponding physiological measurements.
We quantified the tracking behaviour of moths using a system identification approach, where animals freely fly and feed from a robotic flower. We investigated whether a luminance-dependent adjustment of flight control was a general feature in all three hawkmoth species, and whether the same simple temporal delay model could be generalized across species. Furthermore, we extended the investigation to differences between species, and tested whether simple delay dynamics explain the differences in tracking shown by the three hawkmoth species.
2. Material and methods
See the electronic supplementary material for further details.
(a). Behavioural experiments
Experiments on M. stellatarum and D. elpenor were performed as similarly as possible to the previous experiments on M. sexta [9], at Lund University, Sweden (see the electronic supplementary material, m). In brief, artificial flowers (diameter of flower face: 46 mm) were designed and 3D printed from ABS plastic (UPrint SE, Dimension), and mounted atop a fibreglass or stainless steel rod, which was connected to a bipolar stepper motor (0.9°/step resolution, 1/16 microstepping, Phidgets, Inc.). This allowed for high-frequency, precise movements of the flower. The flower was actuated with a sum-of-sinusoids stimulus composed of 20 frequencies (0.2–20 Hz), all of which were prime multiples in order to avoid harmonic overlap [27]. We analysed frequencies up to 13.7 Hz, at which all animals still consistently tracked the flower. The phase of each sinusoid was randomly determined. The amplitudes were scaled to have equal power in velocity (figure 1c). This scaling prevents the high frequencies from being much faster and potentially saturating the moth's ability to keep up [9,28].
A small, adjustable white LED panel and a diffuser (CN-126 LED video light, Neewer) was mounted above the chamber, to provide background illumination. The colour temperature of the panel was 5400 K (a blueish-white peak), which ranges between the colour temperature of horizon to overhead white daylight and is the closest match to daylight spectra of commercially available light sources [9]. Light intensity was adjusted on the panel and could be further lowered by neutral density filters placed in front of the light source (see figure 1a for illuminance and luminance values in the different experimental conditions). All sides of the arena were blacked out, except for the top. We illuminated the arena with 850 nm IR LED light sources (LEDLB-16-IR, Larson Electronics), which is outside the moth's visual spectrum. Moths were tracked using high-speed video cameras from above at 100 fps (MotionBLITZ EoSens mini, Mikrotron for experiments with the other two species).
Moths were placed in the arena, left to warm up and start flying, to then insert their proboscis into the nectary of the artificial flower and feed from it. Upon proboscis insertion, the stimulus was started, and the moths tracked the movement of the flower by adjusting their body position to stay centred with the nectary (figure 1b,c; electronic supplementary material, videos S1–S3). The lowest driving frequencies (0.2 and 0.3 Hz) were 0.1 Hz apart, so a continuous 20 s of tracking data were collected, giving a frequency resolution of 0.5 Hz and thereby separating these peaks. If moths touched the flower with their legs, or lost contact with their proboscis, we excluded the trial. In each video, we tracked a point on the moth's head and thorax, and a point on the nectary of the flower using the DLTdv5 software package [29]. From each moth, only one trial was obtained, resulting in the following sample sizes: M. stellatarum (n = 13/10/10 at 3000/300/15 lx), D. elpenor (n = 12/14/11 at 300/15/0.3 lx), M. sexta (n = 8/8/15 at 300/15/0.3 lx).
(i). System identification and data analysis
Flower tracking responses were confirmed to be linear and time invariant (electronic supplementary material, figures S1 and S2, methods), and can thus be described by two components: the gain and the phase [9,10]). Gain and phase are the magnitude and angle, respectively, of the complex-valued frequency response of the moth tracking the flower. Averages and 95% confidence intervals were calculated in the complex plane following prior methods [30]. Because performance is a combination of gain and phase, we also calculated the moth's tracking error [9,30] as a single metric to assess how well the moth tracked the flower. Tracking error, ε, is defined as the distance in the complex plane between the moth's actual frequency response H(s) to the ideal tracking conditions (gain = 1; phase = 0):
| 2.1 |
To characterize the dynamics, we measured three characteristic frequencies. The corner frequency is where the power in the tracking response falls below 0.5, corresponding to a gain of 0.71. The frequency at which the phase lag reaches π/2 radians indicates that the animal is more than a quarter period out of sync. The frequency where tracking error first exceeds unity indicates where the moth would perform better by remaining stationary.
(ii). Fitting simple delays and scaling factors to the differences within and between species
Flower tracking is an inherently closed-loop behaviour where the moth's sensory systems (vision and proboscis mechanoreception) do not detect and minimize the absolute flower position but rather the flower's position relative to its body (the sensory error, figure 1d). To determine whether simple models of luminance-dependent neural processing could account for the within- and across-species differences, we modelled the differences between behavioural responses using two simple elements: (i) a delay term, which is consistent with the slowing of nervous processing and (ii) an open loop gain, termed scale factor, a, which changes the strength of the responses to the perceived error between flower and moth motion. The scale factor represents an increase in sensitivity somewhere in the sensorimotor loop, including visual or motor circuits. As these adjustments are hypothesized to arise from luminance-dependent adaptations, they must be modelled within the closed-loop feedback response of the behaviour (see the electronic supplementary material, methods).
3. Results and discussion
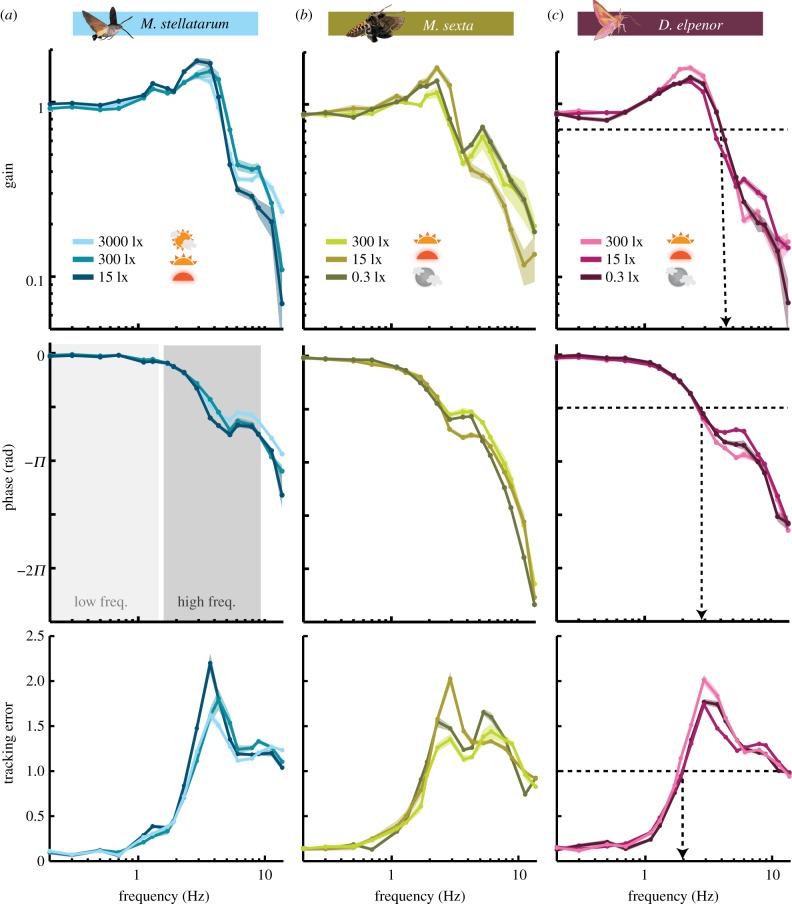
All three hawkmoth species successfully tracked the robotic flower and showed general similarities in their tracking responses across the frequency spectrum (figure 1c). At frequencies between 1 and 4 Hz, all three species showed a gain overshoot, thus producing larger tracking amplitudes than the actual flower amplitude at these frequencies (figure 2, first row). At the same time, the moths also lagged more and more behind the flower movement (figure 2, second row), and in combination, the overshoot and phase lag led to steeply increasing tracking errors (figure 2, last row). All phase responses demonstrated a flattening or local maximum at some point above 4 Hz, indicating that the response is not captured by simple first-order dynamics. For higher frequencies of the flower trajectory, the tracking gain decreased with increasing frequency, while the phase lag increased further. As a result, the tracking error decreased again to level out around unity (as the gain approached zero, figure 2, last row).
Figure 2.
Flower tracking performance at different light intensities. Gain, phase and tracking error of the diurnal (a), crepuscular (b) and nocturnal (c) species, each at three different luminance levels. Grey shades in (a) show frequency ranges of figure 3 summary statistics, based on the frequencies of natural flower movement [9]; dashed lines in (c) show gain, phase and error values (0.71, −π/2 radians, 1) of figure 4 summary statistics. Curves show the mean and 95% confidence intervals of the mean, calculated in the complex plane [28].
(a). All hawkmoth species showed behavioural adaptations to changes in light intensity
Despite the general similarity of tracking responses, there were distinct differences between species, as well as within species, across light intensities. We first investigated the latter, to quantify luminance-dependent adaptations in the three hawkmoth species. There was no significant difference in tracking performance at the characteristic frequency values (gain = 0.7, phase = −π/2, tracking error = 1) across light intensities (electronic supplementary material, figure S3b). This indicates that all the moths had similar tracking dynamics (figure 2a–c). However, the responses did diverge significantly in specific frequency bands. To broadly summarize these differences, we compared tracking behaviour in the range of natural flower movements (0.2–1.7 Hz [9]) and in a range of frequencies higher than those (1.7–8.9 Hz). Therefore, we averaged the gain, phase and tracking error in the two frequency bands (see also figure 2a, second row). While there were no significant differences in any of the species at low frequencies (electronic supplementary material, figure S3a), there were consistent differences at high frequencies (figure 3), suggesting that luminance-dependent processing manifests in behavioural changes at frequencies higher than natural flower movements. This is in line with other findings showing that it is at the higher frequencies where performance starts to fail, which indicates the differences in dynamics [2]. In M. stellatarum and M. sexta, the gain increased in this frequency band with decreasing light intensity (significantly so in M. sexta, figure 2a,b). Moreover, the phase lag increased with decreasing light intensity (significantly so in M. stellatarum, figure 3a; also visible in figure 2a,b). Tracking error at high frequencies increased with decreasing light intensity in both species (figure 3a,b). Interestingly, we did not observe a similar pattern in the nocturnal D. elpenor. Average gain, phase lag and tracking error were largest at the highest light intensity, and significantly different from the next lower intensity at 15 lx (figure 3c).
Figure 3.
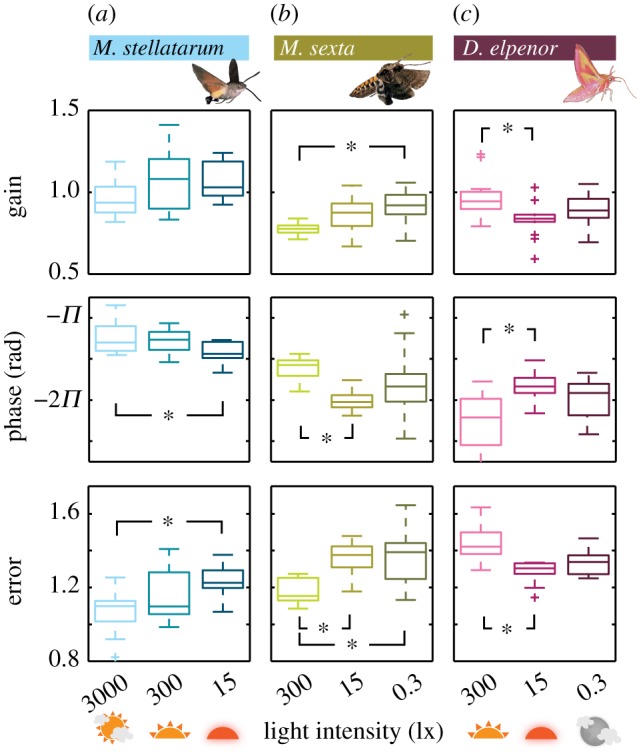
Summary statistics of flower tracking at high frequencies. Average gain, phase lag and tracking error for each individual of the diurnal (a), crepuscular (b) and nocturnal (c) species were calculated for the high-frequency band (1.7–7.9 Hz, for flower frequencies; see the electronic supplementary material, figure S3). Box shows interquartile range and median; whiskers denote quartile ± 1.5 × interquartile range. Asterisks denote p < 0.05 (Kruskall–Wallis test).
(b). A luminance-dependent delay can account for each species' response to changing light intensity
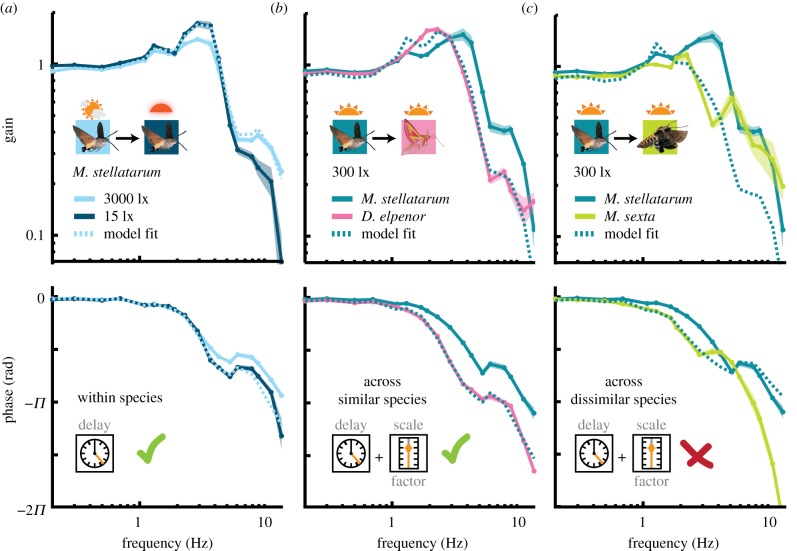
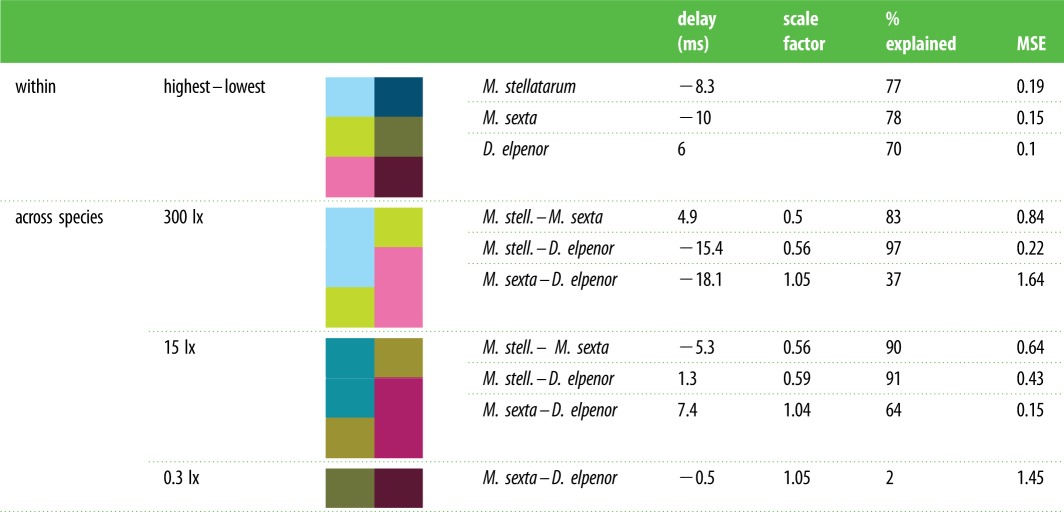
To test for evidence of consistent temporal processing strategies across species, we examined whether the same luminance-dependent delay model that has previously been shown to fit the tracking behaviour of M. sexta [9] could be generalized to the other two hawkmoth species (figure 1d; see the electronic supplementary material, methods). Indeed, the simple time-delay model provided good fits for the change in all three species (figure 5a and electronic supplementary material, figure S4). In M. stellatarum, a time delay of 8.3 ms applied to the tracking responses at 3000 lx could explain 77% of the difference to the responses at 15 lx (for all model results, see table 1). In M. sexta a delay of 10 ms explained 78% of the difference between the 3000 and 0.3 lx condition. While the model holds for nocturnal D. elpenor, the time shift was reversed, corresponding to a speeding up of the system by 6 ms in dim light, which explained 70% of the difference between conditions.
Figure 5.
Model fitting of intra- and interspecific differences in flower tracking. (a) A simple time delay was combined with the response of M. stellatarum at 3000 lx (light blue), to predict (light blue dots) the response at 15 lx (dark blue). While a simple delay gave good fits within species, it was not sufficient for fitting across species. Thus, a combination of delay and a scaling factor were used to model how well the response of M. stellatarum at 300 lx could predict the response of D. elpenor (b) and M. sexta (c) at the same light intensity, which provided good fits between M. stellatarum and D. elpenor, yet not between M. stellatarum and M. sexta (table 1).
Table 1.
Model parameters of inter- and intraspecific differences in flower tracking. We fitted average data of pairs of different tracking conditions, using the model outlined in figure 5. Within species, the high luminance condition was used as a template for the model, which was fitted the low luminance condition. Across species, the species named first, which typically preferred the brighter conditions, was used as the template to which the second species was fitted. Shown are the resulting time delay and scale factor, as well as the sum of squared errors (SSEs) between the model and the fitted-to condition, the percentage of difference between conditions explained by the model (see the electronic supplementary material).
 |
(c). Known neurophysiological responses can account for behavioural changes in the diurnal and crepuscular moths, but not the nocturnal species
It has been shown before that the visual system of insects adapts its temporal properties with light intensity [16–21]. Recent work on the spatial and temporal properties of the motion vision system in hawkmoths [16] allows us to compare our behavioural results with the visual physiology of the three species. In the optic flow neurons of the diurnal M. stellatarum, the temporal resolution decreased by 20% from the high- to low-light conditions tested in behaviour (assessed at peak and 50% cut-off response frequencies). Similarly, the temporal resolution decreased in the crepuscular and nocturnal species between the equivalent of 300 and 0.3 lx (M. sexta, peak decreased by 26%, cut-off by 2%; D. elpenor, peak by 18%, cut-off by 9%). This decrease in temporal resolution in the visual system is consistent with the temporal delay predicted by our model of luminance-dependent differences within M. stellatarum and M. sexta.
However, it also becomes evident that the behavioural performance of D. elpenor, which tracked higher temporal frequencies better at lower light intensities, does not match their visual physiology, since their photoreceptors and motion neurons respond to higher temporal frequencies at brighter light intensities rather than at dimmer ones. What could have caused this difference in the behaviour of the nocturnal moth compared to the other two species?
One difference between D. elpenor and the other two species is that the higher light intensities are well outside the range of D. elpenor's natural activity period and, under natural circumstances, they would not forage but would instead stay in their hiding places until nightfall [26]. At 300 lx, their superposition pupil is closed, thus their eye is adapted to diurnal vision (consistent with [19]). In our trials, 50–80% of the tested moths would feed from the flower at 15 and 0.3 lx, but only 5–10% of animals would eventually feed from the flower at 300 lx, while most fly until they find a resting place to settle, suggesting a strong decrease in motivation to approach and feed from flowers at bright light intensities. To a lesser degree, this has also been reported in M. sexta [9].
It has been shown in many experiments that the activity state of the animal, controlled by neuromodulators such as octopamine, strongly influences the output of the visual system and flight performance: high levels of octopamine increase visual response gain in the motion vision system [31]. This gain increase is enhanced at higher temporal frequencies, thus speeding up the motion vision system when octopamine was present (and the insect was in its active state) [32–35]. Octopamine also regulates olfactory sensitivity in synchrony with the circadian rhythm in hawkmoths, making moths more sensitive in their natural activity phase [36]. It is conceivable that octopamine has a similar circadian role on the motion vision system, which would be an intriguing explanation for the observed differences in behaviour of the nocturnal moth.
(d). Species with different diel preferences demonstrate behavioural differences in flower tracking
Luminance-dependent adaptations of flight performance might manifest not only as differences in tracking behaviour as light intensities change, but also in general features of tracking performance in species generally active in different light environments. We therefore compared tracking performance across species at the same luminance levels and found significant changes in the gain and phase characteristics of the behavioural frequency response (figure 4). At 300 lx, the diurnal species reached both a gain of 0.7, phase lag of –π/2 and tracking error of unity at higher frequencies than the other two species (a difference significant in all cases except for the difference in gain between M. stellatarum and D. elpenor, figure 4a). There was no significant difference between the characteristic frequencies of M. sexta and D. elpenor at 300 and 15 lx (figure 4a,b), and none in the tracking error at 0.3 lx (figure 4c), suggesting more similar temporal characteristics between those two species than the diurnal one. These differences between species were well matched with the expected consequences of their natural light environments on flight performance: the diurnal species presumably has the least selective pressure for adaptations to increase sensitivity, such as temporal summation, and thus would be expected to have the best performance at higher temporal frequencies.
Figure 4.
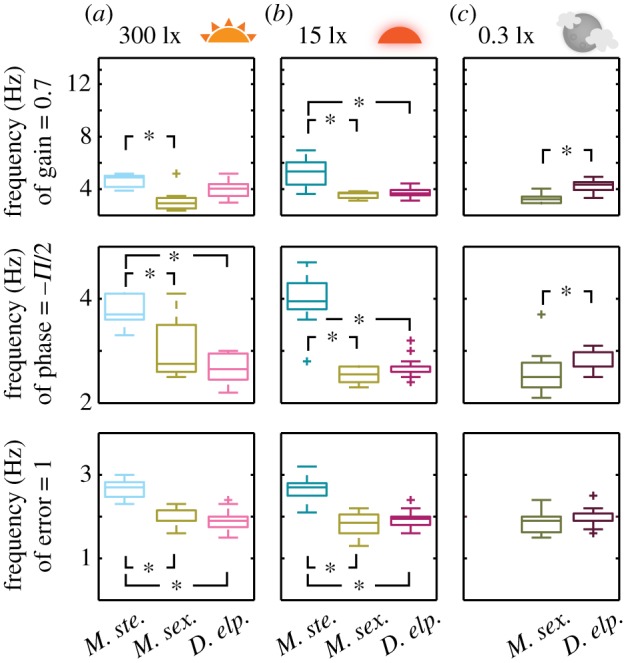
Summary statistics of the flower tracking performance across species. Shown are the frequencies where each species' flower tracking had a gain of 0.71 (50% power), a phase of −π/2 and a tracking error of 1. Box shows interquartile range and median; whiskers denote quartile ± 1.5 × interquartile range. Asterisks denote p < 0.05 (Kruskall–Wallis test).
(e). A luminance-dependent delay and a change in sensitivity can account for interspecific differences between diurnal and nocturnal moths
Since a variable time delay could account for the tracking differences between light conditions within species, we investigated whether a simple delay term could also account for the interspecific differences between species, which followed a similar order as the luminance-dependent differences: the diurnal species tracked the highest temporal frequencies, and the nocturnal species the lowest. This reasoning is furthermore supported by the physiology of the moth's motion-sensitive neurons, following a similar pattern: the diurnal species has the highest peak and cut-off temporal frequencies at all light intensities, the crepuscular species has an intermediate range and the nocturnal species has the lowest [16].
However, unlike the luminance-dependent differences, the differences in tracking performance across species could not be accounted for by a simple time-delay model (electronic supplementary material, table S1), likely due to the more pronounced differences in gain and phase across than within species (figure 4). We thus considered which other simple parameters in the nervous system might differ between species. The most prominent one is a difference in the sensitivity of the nervous system to a given error between flower and moth motion (figure 1d), which could be implemented as a multiplicative scale factor on the inside of the closed-loop response in addition to the time delay. With this factor included in the model, fits between species, especially diurnal and nocturnal, improved dramatically (figure 5b and table 1; see also the electronic supplementary material, figure S4). Fitting M. stellatarum to M. sexta at 300 lx captured 83% of the difference between species, while fitting to D. elpenor captured 97%. The fits between M. stellatarum and M. sexta need to be treated with caution: while they did explain 83% of the difference between species, the magnitude of the unexplained differences in frequency response were still large in absolute terms (see fitting error in table 1 and figure 5c). Finally, fits between M. sexta and D. elpenor with a scale factor and delay did not lead to good results, capturing only 37% of differences. Importantly, the efficacy of scaled, delayed model was consistent for fits between species at the other luminance levels (15 and 0.3 lx; electronic supplementary material, figure S4; table 1). At all light levels the best fits were obtained for the M. stellatarum and D. elpenor comparison, followed by M. stellatarum and M. sexta, and very poor fits between M. sexta and D. elpenor. Adding a scale factor did not substantially improve the intraspecific fits between different light intensities (electronic supplementary material, table S1).
An interesting consistency emerged in the predicted scale factor across species: for fits between the diurnal and either crepuscular or nocturnal species a scale factor of around 0.5 was predicted (thus the perceived error between flower and moth motion was translated into a twice as strong behavioural response in the diurnal species, compared to the other two). This pattern remained the same even when fits across light conditions were conducted. Despite large changes in the predicted temporal delay, these results suggest that the scale factor captures a light intensity independent aspect of the behavioural responses.
(f). Possible physiological correlates of a scale factor in the sensorimotor processing
Physiologically, a scale factor could be implemented as an interspecific difference in the visual system's response to the relative flower motion, at the integration stage between the sensory systems contributing to flower tracking, or between the sensory and motor systems.
Comparing the firing rate of motion-sensitive neurons in the three hawkmoth species suggests that the scaling is not implemented at this stage of visual processing, since the firing rate of motion neurons in the diurnal species is similar or lower than that of the other two species, rather than higher, as our scale factor would suggest (electronic supplementary material, figure S5; data replotted from [16]). There might, however, be other visual pathways contributing to flower tracking, such as a target fixation pathway [37], which could implement such a scaling.
The scaling shift could also arise from a rebalancing of vision with other sensory modalities that enable tracking. All species have their proboscis in the flower during tracking and could obtain mechanosensory information from the flower. As recently demonstrated, M. sexta integrates both visual and mechanosensory information in a linear sum when flower tracking [12], while there is some information suggesting the diurnal M. stellatarum does not rely on information from their proboscis for flower tracking [10]. Thus, different species might weight visual and proboscis inputs differently, with vision weighted stronger (scaling factor increased) in diurnal species and weaker in crepuscular and nocturnal species.
Finally, nothing is known about the transfer function between the visual and motor system in these hawkmoth species, yet an intriguing possibility is that the scale factor is implemented between the visual and the motor system. In the diurnal moth, visual fidelity is high and thus the possibility of erroneous visual perceptions of sensory error is low. Thus, the species active in the reduced reliability of dim light could benefit from translating visual perception into movement with less gain, while the diurnal species could afford high gains.
(g). Mechanical differences between hawkmoth species could be responsible for variation not accounted for by the simple models
The model fits using a time delay and scale factor assume that the rest of the closed-loop system comprising the nervous system and flight mechanics (figure 1d) is the same across conditions. This is a reasonable assumption within species, when comparing different light intensities, but a much stronger simplification when comparing different species. Thus, the variance between species, which is not explained by our simple model, might have its origin in differences in their anatomy and physiology, arising from distinct evolutionary histories (all three species are part of the family Sphingidae (D. elpenor and M. stellatarum are part of the same subfamily (Macroglossinae), while M. sexta belongs to the subfamily Sphinginae [38]).
Differences between species could, for example, arise from differences in their flight mechanics: M. sexta has twice the mass of D. elpenor, and about five to six times that of M. stellatarum, and exceeds the wingspan of the former by about 60% and the latter by almost 250% [39]. It is likely that this vast difference in size requires differences in flight kinetics which separate the tracking responses of M. sexta from the other two species.
Moreover, there are striking differences in proboscis length between M. stellatarum and D. elpenor on the one side and M. sexta on the other side: an average of 7.5 cm in M. sexta [40], and around 2.5 cm in M. stellatarum [41] and D. elpenor. Species with a long proboscis typically prefer flowers with a long nectary (e.g. [40]), where a bigger proportion of the proboscis can be inserted into the nectary, and a more stable mechanical contact between the flower and the proboscis exists. Mechanical feedback from the proboscis might thus be more reliable in long-tongued species and have a bigger impact on flower tracking. These anatomical differences could go hand in hand with physiological ones: physiological differences between species discussed as possible mechanisms for a scale factor could also, if not acting to the same degree on all frequencies, be responsible for the differences in flower tracking performance.
4. Conclusion
To summarize, we find many indications for luminance-dependent adaptations in hawkmoth behaviour. Species-specific changes in flower tracking performance with light levels are in agreement with a simple luminance-dependent time delay in the nervous system. However, the differences between species cannot generally be reduced to a simple temporal shift alone. Changing the responsiveness to the relative flower movement by a simple scale factor in addition to the timing change can describe the differences between the diurnal and nocturnal species very well. Our comparative system identification analysis on hawkmoth flight behaviour has been able to explain much of the variation in responses we found with simple models of neural processing. This mechanistic connection of behaviour and physiology requires further investigation, especially in D. elpenor, where behaviour seems to be at odds with the observed physiological responses. Nevertheless, we can propose a number of testable hypotheses on further adjustments in flight control, which might explain some of the differences not captured by our simple model. Thus, iterating between neurophysiological experiments and quantitative behaviour, especially in the context of a system identification framework, can bring us closer to understanding how variations in behaviour are brought about by differences in neuronal processing in insects.
Supplementary Material
Acknowledgements
We would like to thank Eatai Roth and Almut Kelber for valuable comments on the manuscript, and Eric Warrant for initiating and supporting this collaboration.
Data accessibility
Supporting information is part of the electronic supplementary material, and the raw data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.94kt2.
Authors' contributions
S.S. and A.S. conceived the study. A.S., K.K. and S.C. acquired the data. A.S. and S.S. analysed and interpreted the data. A.S. wrote the paper and drafted the figures with input from S.S. All authors commented on the manuscript.
Competing interests
We have no competing interests.
Funding
A.S. was supported by the Swedish Research Council (VR 621-2012-2205), The Knut and Alice Wallenberg Foundation and Air Force Office of Scientific Research (AFOSR, FA9550-12-1-0237) awarded to Eric Warrant.
References
- 1.Baird E, Kreiss E, Wcislo W, Warrant E, Dacke M. 2011. Nocturnal insects use optic flow for flight control. Biol. Lett. 7, 499–501. ( 10.1098/rsbl.2010.1205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird E, Srinivasan MV, Zhang S, Cowling A. 2005. Visual control of flight speed in honeybees. J. Exp. Biol. 208, 3895–3905. ( 10.1242/jeb.01818) [DOI] [PubMed] [Google Scholar]
- 3.Fry SN, Rohrseitz N, Straw AD, Dickinson MH. 2009. Visual control of flight speed in Drosophila melanogaster. J. Exp. Biol. 212, 1120–1130. ( 10.1242/jeb.020768) [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan MV, Zhang SW. 2000. Visual navigation in flying insects. Int. Rev. Neurobiol. 44, 67–92. ( 10.1016/S0074-7742(08)60738-2) [DOI] [PubMed] [Google Scholar]
- 5.Goyret J. 2010. Look and touch: multimodal sensory control of flower inspection movements in the nocturnal hawkmoth Manduca sexta. J. Exp. Biol. 213, 3676–3682. ( 10.1242/jeb.045831) [DOI] [PubMed] [Google Scholar]
- 6.Goyret J, Kelber A. 2012. Chromatic signals control proboscis movements during hovering flight in the hummingbird hawkmoth Macroglossum stellatarum. PLoS ONE 7, e34629 ( 10.1371/journal.pone.0034629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farina WM, Kramer D, Varjú D. 1995. The response of the hovering hawk moth Macroglossum stellatarum to translatory pattern motion. J. Comp. Physiol. A 176, 551–562. ( 10.1007/BF00196420) [DOI] [Google Scholar]
- 8.Sprayberry JDH, Daniel TL. 2007. Flower tracking in hawkmoths: behavior and energetics. J. Exp. Biol. 210, 37–45. ( 10.1242/jeb.02616) [DOI] [PubMed] [Google Scholar]
- 9.Sponberg S, Dyhr JP, Hall RW, Daniel TL. 2015. Luminance-dependent visual processing enables moth flight in low light. Science 348, 1245–1248. ( 10.1126/science.aaa3042) [DOI] [PubMed] [Google Scholar]
- 10.Farina WM, Varjú D, Zhou Y. 1994. The regulation of distance to dummy flowers during hovering flight in the hawk moth Macroglossum stellatarum. J. Comp. Physiol. A 174, 239–247. ( 10.1007/BF00193790) [DOI] [Google Scholar]
- 11.Sane SP, Dieudonné A, Willis MA, Daniel TL. 2007. Antennal mechanosensors mediate flight control in moths. Science 315, 863–866. ( 10.1126/science.1133598) [DOI] [PubMed] [Google Scholar]
- 12.Roth E, Hall RW, Daniel TL, Sponberg S. 2016. The integration of parallel mechanosensory and visual pathways resolved through sensory conflict. Proc. Natl Acad. Sci. USA 113, 12 832–12 837. ( 10.1073/pnas.1522419113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warrant EJ. 2017. The remarkable visual capacities of nocturnal insects: vision at the limits with small eyes and tiny brains. Phil. Trans. R. Soc. B 372, 20160063 ( 10.1098/rstb.2016.0063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greiner B, Ribi WA, Warrant EJ. 2004. Retinal and optical adaptations for nocturnal vision in the halictid bee Megalopta genalis. Cell Tissue Res. 316, 377–390. ( 10.1007/s00441-004-0883-9) [DOI] [PubMed] [Google Scholar]
- 15.Land MF, Gibson G, Horwood J, Zeil J. 1999. Fundamental differences in the optical structure of the eyes of nocturnal and diurnal mosquitoes. J. Comp. Physiol. A 185, 91–103. ( 10.1007/s003590050369) [DOI] [Google Scholar]
- 16.Stöckl AL, O'Carroll DC, Warrant E. Submitted. Higher-order neural processing tunes motion neurons to visual ecology in three species of hawkmoths. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frederiksen R, Wcislo WT, Warrant EJ. 2008. Visual reliability and information rate in the retina of a nocturnal bee. Curr. Biol. 18, 349–353. ( 10.1016/j.cub.2008.01.057) [DOI] [PubMed] [Google Scholar]
- 18.Laughlin S, Weckström M. 1993. Fast and slow photoreceptors: a comparative study of the functional diversity of coding and conductances in the Diptera. J. Comp. Physiol. A 172, 593–609. ( 10.1007/BF00213682) [DOI] [Google Scholar]
- 19.Stöckl AL, O'Carroll DC, Warrant EJ. 2016. Neural summation in the hawkmoth visual system extends the limits of vision in dim light. Curr. Biol. 26, 821–826. ( 10.1016/j.cub.2016.01.030) [DOI] [PubMed] [Google Scholar]
- 20.O'Carroll D, Warrant E. 2011. Computational models for spatiotemporal filtering strategies in insect motion vision at low light levels. In Proc. 7th Int. Conf. on Intelligent Sensors, Sensor Networks and Information Processing ( ISSNIP ), pp. 119–124. New York, NY: IEEE. ( 10.1109/ISSNIP.2011.6146593). [DOI]
- 21.Warrant EJ. 1999. Seeing better at night: life style, eye design and the optimum strategy of spatial and temporal summation. Vision Res. 39, 1611–1630. ( 10.1016/S0042-6989(98)00262-4) [DOI] [PubMed] [Google Scholar]
- 22.Reber T, Vähäkainu A, Baird E, Weckström M, Warrant E, Dacke M. 2015. Effect of light intensity on flight control and temporal properties of photoreceptors in bumblebees. J. Exp. Biol. 218, 1339–1346. ( 10.1242/jeb.113886) [DOI] [PubMed] [Google Scholar]
- 23.Theobald JC, Coates MM, Wcislo WT, Warrant EJ. 2007. Flight performance in night-flying sweat bees suffers at low light levels. J. Exp. Biol. 210, 4034–4042. ( 10.1242/jeb.003756) [DOI] [PubMed] [Google Scholar]
- 24.Spiewok S, Schmolz E. 2006. Changes in temperature and light alter the flight speed of hornets (Vespa crabro L.). Physiol. Biochem. Zool. 79, 188–193. ( 10.1086/498181) [DOI] [PubMed] [Google Scholar]
- 25.Baird E, Fernandez DC, Wcislo WT, Warrant EJ. 2015. Flight control and landing precision in the nocturnal bee Megalopta is robust to large changes in light intensity. Front. Physiol. 6, 305 ( 10.3389/fphys.2015.00305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittaway A. 1993. The Hawkmoths of the Western Palearctic. London, UK: Harley Books. [Google Scholar]
- 27.Roth E, Sponberg S, Cowan NJ. 2014. A comparative approach to closed-loop computation. Curr. Opin Neurobiol. 25, 54–62. ( 10.1016/j.conb.2013.11.005) [DOI] [PubMed] [Google Scholar]
- 28.Dyhr JP, Morgansen KA, Daniel TL, Cowan NJ. 2013. Flexible strategies for flight control: an active role for the abdomen. J. Exp. Biol. 216, 1523–1536. ( 10.1242/jeb.077644) [DOI] [PubMed] [Google Scholar]
- 29.Hedrick TL. 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001 ( 10.1088/1748-3182/3/3/034001) [DOI] [PubMed] [Google Scholar]
- 30.Roth E, Zhuang K, Stamper SA, Fortune ES, Cowan NJ. 2011. Stimulus predictability mediates a switch in locomotor smooth pursuit performance for Eigenmannia virescens. J. Exp. Biol. 214, 1170–1180. ( 10.1242/jeb.048124) [DOI] [PubMed] [Google Scholar]
- 31.Suver MP, Mamiya A, Dickinson MH. 2012. Octopamine neurons mediate flight-induced modulation of visual processing in Drosophila. Curr. Biol. 22, 2294–2302. ( 10.1016/j.cub.2012.10.034) [DOI] [PubMed] [Google Scholar]
- 32.Chiappe ME, Seelig JD, Reiser MB, Jayaraman V. 2010. Walking modulates speed sensitivity in Drosophila motion vision. Curr. Biol. 20, 1470–1475. ( 10.1016/j.cub.2010.06.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung SN, Borst A, Haag J. 2011. Flight activity alters velocity tuning of fly motion-sensitive neurons. J. Neurosci. 31, 9231–9237. ( 10.1523/JNEUROSCI.1138-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lüders J, Kurtz R. 2015. Octopaminergic modulation of temporal frequency tuning of a fly visual motion-sensitive neuron depends on adaptation level. Front. Integr. Neurosci. 9, 36 ( 10.3389/fnint.2015.00036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longden KD, Krapp HG. 2010. Octopaminergic modulation of temporal frequency coding in an identified optic flow-processing interneuron. Front. Syst. Neurosci. 4, 153 ( 10.3389/fnsys.2010.00153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schendzielorz T, Schirmer K, Stolte P, Stengl M. 2015. Octopamine regulates antennal sensory neurons via daytime-dependent changes in cAMP and IP3 levels in the hawkmoth Manduca sexta. PLoS ONE 10, e0121230 ( 10.1371/journal.pone.0121230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox JL, Frye MA. 2014. Figure-ground discrimination behavior in Drosophila. II. Visual influences on head movement behavior. J. Exp. Biol. 217, 570–579. ( 10.1242/jeb.080192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawahara AY, Mignault AA, Regier JC, Kitching IJ, Mitter C. 2009. Phylogeny and biogeography of hawkmoths (Lepidoptera: Sphingidae): evidence from five nuclear genes. PLoS ONE 4, e5719 ( 10.1371/journal.pone.0005719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henningsson P, Bomphrey RJ. 2013. Span efficiency in hawkmoths. J. R Soc. Interface 10, 20130099 ( 10.1098/rsif.2013.0099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haverkamp A, Bing J, Badeke E, Hansson BS, Knaden M. 2016. Innate olfactory preferences for flowers matching proboscis length ensure optimal energy gain in a hawkmoth. Nat. Commun. 7, 11644 ( 10.1038/ncomms11644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goyret J, Kelber A. 2011. How does a diurnal hawkmoth find nectar? Differences in sensory control with a nocturnal relative. Behav. Ecol. 22, 976–984. ( 10.1093/beheco/arr078) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting information is part of the electronic supplementary material, and the raw data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.94kt2.