Abstract
Hematopoietic stem cells (HSCs) are the therapeutic component of bone marrow transplants, but finding immune-compatible donors limits treatment availability and efficacy. Recapitulation of endogenous specification during development is a promising approach to directing HSC specification in vitro, but current protocols are not capable of generating authentic HSCs with high efficiency. Across phyla, HSCs arise from hemogenic endothelium in the ventral floor of the dorsal aorta concurrent with arteriovenous specification and intersegmental vessel (ISV) sprouting, processes regulated by Notch and Wnt. We hypothesized that coordination of HSC specification with vessel patterning might involve modulatory regulatory factors such as R-spondin 1 (Rspo1), an extracellular protein that enhances β-catenin-dependent Wnt signaling and has previously been shown to regulate ISV patterning. We find that Rspo1 is required for HSC specification through control of parallel signaling pathways controlling HSC specification: Wnt16/DeltaC/DeltaD and Vegfa/Tgfβ1. Our results define Rspo1 as a key upstream regulator of two crucial pathways necessary for HSC specification.
KEY WORDS: HSCs, Wnt signaling, Rspo1, Vegfaa, Zebrafish
Summary: R-spondin 1, a Wnt co-factor, regulates the expression of components of the parallel pathways Wnt16/DeltaC/DeltaD and Vegfa/Tgfβ1, required for specification of hematopoietic stem cells.
INTRODUCTION
Hematopoietic stem cells (HSCs) are tissue-specific stem cells that reside in the bone marrow of mammals and maintain hematopoiesis by production of all mature blood cell types (Clements and Traver, 2013). HSCs are used in treatment of various blood diseases including leukemias and anemias. Autologous or allotransplanation of bone marrow, mobilized or cord blood-derived HSCs is used in treatment, but is currently limited by low numbers of HSCs and a lack of suitable donors. A potential solution is by in vitro-directed differentiation of HSCs from induced pluripotent stem cells (iPSCs); however, current protocols are not capable of generating normal transplantable HSCs at high efficiency. A better understanding of embryonic development of HSCs and a fuller elucidation of the necessary signaling pathways might inform attempts at in vitro specification and expansion of HSCs.
HSCs arise from hemogenic endothelium in the ventral wall of the dorsal aorta (Chen et al., 2009; Taoudi and Medvinsky, 2007; Zovein et al., 2008). The fact that HSCs arise specifically from arterial endothelium, which is derived from splanchnopleural (in mammals) or lateral plate (in anamniotic vertebrates) mesoderm, suggests that this endothelium receives unique hematopoietic competence that derives from earlier patterning (Clements and Traver, 2013). Of particular interest, canonical Wnt signaling and downstream master regulators of anterior/posterior regionalization, Caudal type homeobox genes (Cdx) and their Hox gene targets, are crucial for definitive hematopoietic development (Clements and Traver, 2013). Furthermore, only arterial endothelium produces HSCs, indicating that regulation of arteriovenous specification, which relies on Sonic hedgehog (Shh), Vascular endothelial growth factor A (Vegfa), and Notch signaling must somehow be integrated with HSC programming (Clements and Traver, 2013). Overlapping the time when HSCs bud from the dorsal aorta, the vessel is also maturing through intersegmental vessel (ISV) sprouting (Gering and Patient, 2010). Sprouting, restriction of angiogenic tip cells, and arborization are also regulated by Vegfa, Notch, and Wnt signaling (Corada et al., 2010; Gering and Patient, 2010; Gore et al., 2011; Leslie et al., 2007; Siekmann and Lawson, 2007).
A number of signaling pathways converge on the dorsal aorta, resulting in initiation of the definitive hematopoietic program, which can be observed through expression of the conserved master hematopoietic regulator runt related transcription factor 1 (Runx1) (Kalev-Zylinska et al., 2002; North et al., 2002; Okuda et al., 1996). Notch (Burns et al., 2005; Butko et al., 2016; Clements and Traver, 2013; Gering and Patient, 2010; Lawson et al., 2001), Bone morphogenetic protein (Bmp) (Wilkinson et al., 2009), Wnt (Goessling et al., 2009; Luis and Staal, 2009; North et al., 2007; Ruiz-Herguido et al., 2012), Shh (Chen et al., 1996; Gering and Patient, 2005; Lawson et al., 2002; Wilkinson et al., 2009), Vegfa (Burns et al., 2005; Gering and Patient, 2005, 2010; Lawson et al., 2003), and Tgfβ (Monteiro et al., 2016) all contribute to HSC program initiation.
One of the earliest signaling axes determined to be involved in HSC specification was the Shh/Vegfa/Notch signaling axis (Clements and Traver, 2013; Gering and Patient, 2005; Wilkinson et al., 2012; Williams et al., 2010). Extensive work in mouse and zebrafish supports the idea that endothelial Notch1-mediated signaling is required for HSC specification and expression of the master hematopoietic regulator, Runx1 (Burns et al., 2005; Hadland et al., 2004; King et al., 2006; Kumano et al., 2003). It is not clear to what extent this role of Notch is distinct from its role in arteriovenous specification (Clements and Traver, 2013). Also, the activation of Runx1 is not direct, but likely involves intermediate activation of GATA binding protein 2 (Gata2) (Butko et al., 2015; King et al., 2006) and perhaps other factors. From work in fish, Notch receptor expression is regulated by both Shh and Vegfa and both are also required for HSC specification (Gering and Patient, 2010; Lawson et al., 2002; Williams et al., 2010). Shh can activate somite expression of vegfaa (Lawson et al., 2002). Together, these results lead to a pathway where Shh turns on vegfaa in the somites, and this ligand in turn activates Notch receptor display in the endothelium, allowing reception of Notch ligands required for HSC specification. More recently, published work demonstrated that Vegfa induces HSC specification by activating endothelial expression of tgfb1a and tgfb1b, as well as notochord expression of tgfb3, and that these ligands are required to stimulate autocrine endothelial expression of jag1a, leading to HSC specification (Monteiro et al., 2016). It thus seems that Vegfa has distinct roles in directing HSC specification.
Interestingly, in vertebrates multiple isoforms of Vegfa are expressed, although the in vivo significance of these isoforms has been difficult to access. In Xenopus, a short isoform of Vegfa, Vegfa-122 (equivalent toVegfa-121 in other vertebrates), seems to be responsible for arterial gene expression (Ciau-Uitz et al., 2010; Leung et al., 2013). Conversely, treatments causing a loss of the intermediate isoform Vegfa-170 (equivalent to Vegfa-165 in other vertebrates) cause HSC specification to be specifically disrupted without extensive alterations in arterial patterning (Ciau-Uitz et al., 2010). There are also a growing number of pathways that are able to bypass one or more steps of the ‘canonical’ Shh/Vegfa/Notch arteriovenous/HSC specification process under the right conditions (Ciau-Uitz et al., 2010; Wilkinson et al., 2012).
Further, Wnt signaling is required for HSC specification. Signaling downstream of the Wnt family has been loosely categorized into ‘canonical’ β-catenin-dependent and ‘non-canonical’ β-catenin-independent branches (Niehrs, 2012). We previously determined that non-canonical signaling downstream of Wnt16 is necessary for HSC specification (Clements et al., 2011). Rather than acting as a direct proximal specification signal, Wnt16 seems to work through a series of relay signals that are required both for proper somite patterning and eventual HSC specification (Clements et al., 2011). Wnt16 is required for somitic expression of the Notch ligands deltaC (dlc) and deltaD (dld) (Clements et al., 2011), which in turn signal through Notch3 (Kim et al., 2014) to activate expression of unknown specification signals that act on the hemogenic endothelium.
Canonical Wnt signaling is also involved in specification and early maintenance of HSCs, both at the level of establishing hematopoietic tissue competence (Clements and Traver, 2013), and evidently within the hemogenic endothelium and nascent HSCs themselves. Wnt3a is required for both normal numbers and behavior of developing HSCs (Luis and Staal, 2009), and an allelic series of β-catenin mutants indicates that canonical Wnt signaling is required for definitive hematopoietic development (Ruiz-Herguido et al., 2012). Prostaglandin E2 is required for early HSC persistence through a β-catenin-dependent mechanism (Goessling et al., 2009; North et al., 2007). These results demonstrate that HSC development relies on β-catenin-dependent Wnt signaling at multiple points during development.
One factor that modulates Wnt signaling and is crucial in vascular patterning is R-spondin (Kim et al., 2008, 2006; Nam et al., 2006; Wei et al., 2007), which has been shown to enhance low levels of Wnt signaling. R-spondin 1-4 are involved in numerous developmental and physiological processes through augmentation of both canonical and non-canonical Wnt signaling (Glinka et al., 2011; Jin and Yoon, 2012). R-spondins are also implicated in human disease and hold therapeutic potential as stem cell growth factors (Blaydon et al., 2006; Kim et al., 2005, 2006; Zhao et al., 2009). Mutations that alter the function of R-spondin 1 (Rspo1) have been shown to lead to an XX sex reversal, palmoplantar hyperkeratosis, and a predisposition to squamous cell carcinoma of the skin, showing that Rspo1 is essential for sex determination, skin differentiation and skin malignancy (Parma et al., 2006). R-spondins bind several receptors including frizzled (Fzd) (Nam et al., 2006), Lgr4 and Lgr5 (de Lau et al., 2011, 2014; Glinka et al., 2011), and LRP6 (Wei et al., 2007). In one mechanism, R-spondin causes augmentation of Wnt signaling through the suppression of the Wnt antagonist ZNRF3, causing a decreased turnover of Wnt receptors FZD and LRP6 (Hao et al., 2012). Embryonically, Rspo1 is involved in a variety of events including formation of the vasculature during development (Gore et al., 2011). Gore et al. described Rspo1 as necessary for ISV growth in the developing embryo through collaboration with canonical Wnt signaling and Vegfc/Vegfr3 (Gore et al., 2011).
Many of the signaling pathways involved in arteriovenous specification and angiogenesis in the developing embryo have also been shown to be important in HSC specification. Given the role of Rspo1 in vascular patterning, we speculated it could be involved in HSC specification. We took advantage of strong conservation of hematopoietic patterning and the experimental tractability of the zebrafish to examine HSC specification using established mutants, knockdown, gain-of-function, and visualization approaches in this model system. In this study, we find that Rspo1 is required to activate two key HSC specification pathways: Wnt16/Dlc/Dld and Vegfa/Tgfβ.
RESULTS
rspo1 is widely expressed in the developing embryo
HSCs are specified from the trunk dorsal aorta and the signaling pathways that regulate them are active there. Previously, rspo1 expression was described in the head and the dorsal aorta of the embryo (http://zfin.org/ZDB-PUB-010810-1; Gore et al., 2011). We wanted to examine rspo1 expression more widely over the time frame relevant to HSC specification, with particular emphasis on the trunk of the developing zebrafish embryo. Using a probe specific for the 3′UTR of Rspo1 and whole-mount in situ hybridization (WISH), rspo1 was expressed in both the head and the trunk of the developing embryo from 16 h post-fertilization (hpf) through to at least 51 hpf (Fig. S1). Trunk expression is ubiquitous at a low level, with stronger expression in the neural tube and nearby dorsal somite, as well as the ventromedial somite near the developing dorsal aorta. By reverse transcription-PCR, we showed that rspo1 is maternally expressed and is clearly visible through 36 hpf (Fig. S1).
rspo1 is required for HSC specification
Rspo1 was previously shown to control vascular patterning (Gore et al., 2011). As many vascular patterning pathways are also involved in HSC specification, we wanted to examine the possibility that Rspo1 might also be necessary for HSC specification. In a previous study, a role for Rspo1 in regulating primitive (not HSC-derived) erythrocyte development (as marked by gata1 expression at 18 hpf) was examined, but whether definitive HSC specification was affected was not determined (Gore et al., 2011).
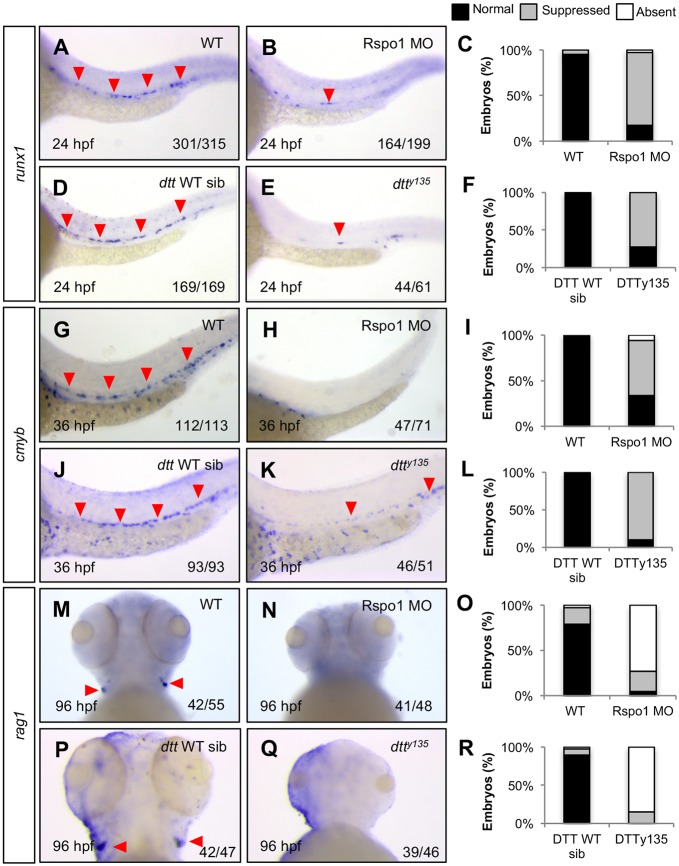
To determine if rspo1 is required for HSC specification, we performed loss-of-function studies using a previously published splice-blocking morpholino (Rspo1 MO) (Gore et al., 2011) as well as the rspo1 mutant, down the tubes (dtty135, a gift from B. Weinstein; Gore et al., 2011), which carries an S193L mutation and causes the same vascular patterning defects as morpholino knockdown. We confirmed that expression of rspo1 is greatly reduced in Rspo1 MO-injected embryos (Fig. S1M, Fig. S2). The primary vessels (dorsal aorta and posterior cardinal vein) in the trunk are maintained, but display the previously described (Gore et al., 2011) stunted intersegmental vessel sprouting morphology (Fig. S3G,H). rspo1 homozygous mutants can be positively identified by their vascular phenotype and yolk sac extension defect. Both Rspo1 MO-injected and dtty135 homozygous mutant embryos, compared with uninjected, wild-type (WT) or heterozygous siblings, had a significant loss of HSCs as indicated by comparative reduction in cells positive for the HSC markers runx1 at 24 hpf (Fig. 1A-F) and cmyb at 36 hpf (Fig. 1G-L). dtty135 and Rspo1 morphants also failed to develop thymic rag1+ T lymphocytes, which develop from an HSC precursor, from 96 hpf(Fig. 1M-R). These results indicate that rspo1 is required for HSC specification.
Fig. 1.
Rspo1 is required for HSC specification. The HSC markers runx1 (A,B,D,E) and cmyb (G,H,J,K), which identify HSCs emerging from the dorsal aorta, as well as the T lymphocyte marker rag1 (M,N,P,Q), found in thymic T cells, were examined at 24 hpf, 36 hpf and 96 hpf as indicated, by whole-mount in situ hybridization (WISH) in wild-type (WT; A,G,M), Rspo1 MO-injected (B,H,N), Rspo1 wild-type and heterozygous siblings (D,J,P), or rspo1 dtty135 homozygous mutants (E,K,Q). Red arrowheads indicate HSC precursors in the dorsal aorta (A,B,D,E,G,J,K) or T lymphocytes in the thymus (M,P), when present. Numbers of embryos displaying the depicted phenotype are indicated. (C,F,I,L,O,R) Histograms illustrate percentages of phenotypes. Lateral mid-trunk or ventral head views at 160× magnification.
To examine the specificity of the Rspo1 loss-of-function phenotype, we examined the surrounding tissues by looking at the expression of several genes marking important anatomical structures in Rspo1 morphants. Consistent with previous reports (Gore et al., 2011), Rspo1 morphants have normal primary vasculature [cadherin-5 (cdh5); Fig. S3A,B], dorsal aorta (notch1b, Fig. 3J,K; dlc, Fig. S3C,D; efnb2a; Fig. S3E,F), primitive blood (gata1; Fig. S4A,B), somite myotome (myoda; Fig. S4C,D), neural crest (crestin; Fig. S4E,F), and thymic epithelium (foxn1; Fig. S4G,H), indicating that the requirement for rspo1 in hematopoietic development is specific, and not the result of failure to generate arterial hemogenic endothelium or more global patterning defects.
Fig. 3.
Notch pathway gene expression in Rspo1 morphants. Wild-type (A,D,G,J,M,P) or Rspo1 MO-injected animals (B,E,H,K,N,Q) were examined by WISH for expression of the Notch ligands dlc (A,B), dld (D,E), or Notch receptors notch1a (G,H), notch1b (J,K), notch2 (M,N) and notch3 (P,Q) at the time points indicated. Compared with wild-type (A,D), dlc and dld are specifically decreased (B,E) in the formed somites (below red bars), but not the pre-somitic mesoderm. Notch receptor expression is unaffected. Flat mount dorsal views of the trunk or lateral views at 160× magnification. Numbers of embryos displaying the depicted phenotype are indicated. (C,F,I,L,O,R) Histograms illustrate percentages of phenotypes. (S,T) Decreased dlc and dld mRNA in Rspo1 knockdown (Rspo1 MO) was confirmed by qRT-PCR, normalized to expression of ef1a, of transcript isolated from dissected embryo trunks (mean±s.e.m.). *P≤0.0006, determined by one-way ANOVA.
Rspo1 regulates wnt16, dlc and dld expression
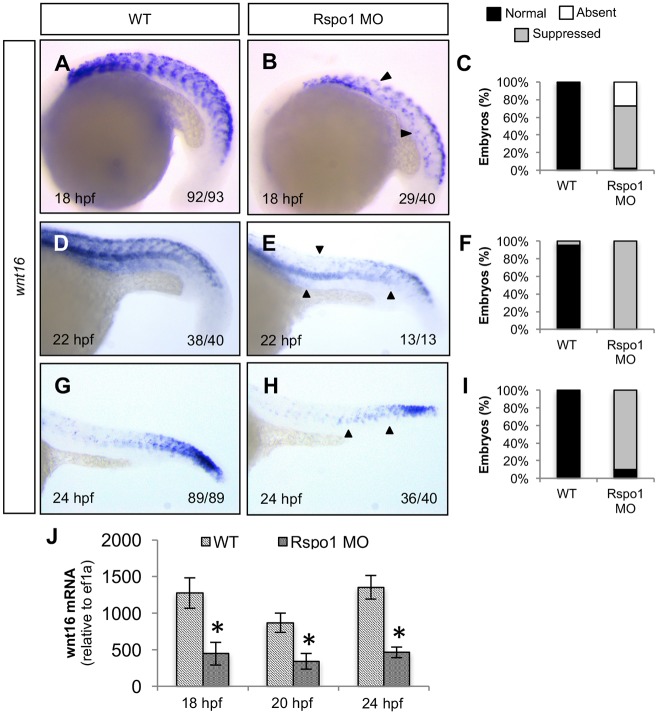
To determine the cause of HSC specification defects in Rspo1 loss-of-function animals, we systematically examined the state of signaling inputs known to be required for HSC specification. One pathway known to be involved in HSC specification is the Wnt16/Dlc/Dld pathway (Clements et al., 2011). The expression domains of wnt16 and rspo1 overlap in the somite (Figs S1 and S2), raising the possibility that Rspo1 might regulate wnt16 expression, which could explain HSC loss. To determine if there is any alteration in wnt16 expression in Rspo1 knockdown animals, we examined the expression of wnt16 in Rspo1 MO-injected embryos. Rspo1 morphants had decreased wnt16 expression in the dorsoanterior portion of each somite at all time points examined (18, 22 and 24 hpf) as compared with WT embryos (Fig. 2A-I). The region where wnt16 expression is diminished in Rspo1 morphants coincides with the endogenous rspo1 expression domain in the dorsal somite and nearby neural tube. We confirmed the apparent loss of expression observed by in situ hybridization using quantitative real time-PCR (qRT-PCR) (Fig. 2J), which demonstrated that wnt16 transcription was significantly decreased in embryos at 18, 20 and 24 hpf. Knockdown of wnt16 using a previously described Wnt16 splice-blocking morpholino (Clements et al., 2011) did not have a reciprocal effect on rspo1 expression but did cause the expected decrease in wnt16 (Fig. S2), runx1 (Fig. S2), dlc (Fig. S5), and dld expression (Fig. S5). Our results indicate that Rspo1 regulates expression of wnt16, which might explain the observed defects in HSC specification.
Fig. 2.
Rspo1 is required for normal wnt16 expression. Wild-type (WT; A,D,G) or Rspo1 MO-injected animals (B,E,H) were examined by WISH for expression of wnt16 at the time points indicated. Wnt16 is natively found in the dorsal anterior domain of each formed somite, overlapping the rspo1 expression domain, and is diminished in Rspo1 MO-injected animals. Black arrows indicate areas of lost wnt16 expression. Numbers of embryos displaying the depicted phenotype are indicated. (C,F,I) Histograms illustrate percentages of phenotypes. Lateral views of the mid trunk at 160× magnification. (J) qRT-PCR of wnt16 transcript at 18 hpf, 20 hpf, and 24 hpf, normalized to expression of ef1a, reveals decreased wnt16 in Rspo1 knockdown (Rspo1 MO) compared with wild-type (WT) (mean±s.e.m.). *P≤0.0189, determined by one-way ANOVA.
Wnt16 activates somitic expression of two Notch family ligands, dlc and dld, to specify HSCs (Clements et al., 2011). To determine if decreased wnt16 in Rspo1 leads to the expected decrease in dlc and dld, we examined their expression in Rspo1 morphants. Rspo1 morphants had a consistent decrease in dlc and dld expression by both in situ hybridization and qRT-PCR (Fig. 3A-F,S,T). As with the loss of dlc and dld observed in Wnt16 loss-of-function animals, this decrease was confined to the formed somites and not observed in the pre-somitic mesoderm (Clements et al., 2011), which we also confirmed by qRT-PCR analysis of the trunk (Fig. 3S,T). As predicted, Rspo1 morphants did not have altered Notch receptor expression (Fig. 3G-R). These results are consistent with the possibility that failure of HSC specification in Rspo1 loss-of-function animals is the result of abrogation of the Wnt16/Dlc/Dld pathway; however, we cannot exclude the possibility that Rspo1 regulates dlc and dld directly.
Rspo1 regulates vegfaa, tgfb1a, and tgfb1b
We wanted to examine the state of additional signaling pathways with known importance in HSC specification. Signaling through the Vegfa receptor, Kinase insert domain receptor like (Kdrl; also known as Flk1 and Vegfr2), has previously been shown to be required for HSC specification (Gering and Patient, 2005; Monteiro et al., 2016), and multiple perturbations where vegfa expression is altered lead to HSC defects in Xenopus (Ciau-Uitz et al., 2010; Leung et al., 2013). These results indicate that Vegfa signaling is required for HSC specification, although it has been difficult to discriminate this requirement from its role in arteriovenous specification (Lawson et al., 2002), which is likely a precondition for HSC specification. We found that Rspo1 morphants had decreased vegfaa expression in somites compared with WT controls by both in situ hybridization and qRT-PCR (Fig. 4A-C,S). As Shh signaling regulates vegfaa expression (Lawson et al., 2002), we examined the state of Shh target gene expression as well as shha itself to determine if decreased vegfaa expression is due to defects in Shh signaling. Both shha and its target ptch2 (previously known as ptc1) were unaffected in Rspo1 morphants (Fig. 4D-I). Our results indicate that Rspo1 regulation of vegfaa is distinct from its regulation by Shh.
Fig. 4.
Rspo1 is required for normal vegfaa expression. Wild-type (A,D,G,J,M,P) or Rspo1 MO injected animals (B,E,H,K,N,Q) were examined by WISH for expression of vegfaa (A,B), shha (D,E), the Shh transcriptional target ptch2 (G,H), tgfb1a (J,K), tgfb1b (M,N), or tgfbr2 (P,Q) at the time points indicated. vegfaa, which is expressed in the medial somite of uninjected embryos, is diminished in Rspo1 MO-injected animals (A,B). shha in the hypochord and its target, ptch2, in the somite are unaffected (D,E,G,H). Tgfb1a and tgfb1b are also suppressed in Rspo1 knockdown embryos (J,K,M,N). Red arrowhead in J indicates dorsal aorta. Tgfbr2 in the somite is unaffected (P,Q). Numbers of embryos displaying the depicted phenotype are indicated. (C,F,I,L,O,R) Histograms illustrate percentages of phenotypes. Lateral views of the mid trunk at 160× magnification. (S) Diminished vegfaa expression was confirmed by qRT-PCR normalized to expression of ef1a at 20 hpf, 22 hpf, and 24 hpf (mean±s.e.m.). *P≤0.0008, determined by one-way ANOVA.
Recent work has uncovered a role for vegfaa in HSC specification by controlling Tgfβ family autocrine signaling that in turn regulates jag1a expression (Monteiro et al., 2016). To determine whether this pathway might be affected in Rspo1 morphants, we examined the expression of tgfb1a, tgfb1b, and their putative receptor, tgfbr2. Expression of both ligands, tgfb1a and tgfb1b, was decreased in Rspo1 morphants whereas the receptor, tgfbr2, was unaffected (Fig. 4J-R). Our results are consistent with the idea that Rspo1 regulates the Vegfa/Tgfβ HSC specification axis.
We previously showed that vegfaa expression is not regulated by Wnt16 (Clements et al., 2011), suggesting that Rspo1 regulates two independent signaling inputs required for HSC specification, Wnt16/Dlc/Dld and Vegfa/Tgfβ. To better understand the separability of the Wnt16 and Vegfa pathways, we tested whether Vegfa could rescue dlc and dld expression in Rspo1 morpholino-injected animals. We found that neither injection of vegfaa-121, nor vegfaa-165 mRNA could rescue dlc or dld expression (Fig. S6). Similarly dlc, dld or wnt16 mRNA could not recover vegfaa expression in Rspo1 morphants (Fig. S7). Thus, altered vegfaa expression in Rspo1 morphants is independent of changes in the Wnt16/Dlc/Dld pathway, highlighting the separability of these inputs.
Dlc, dld and vegfaa can rescue HSCs in Rspo1 morphants
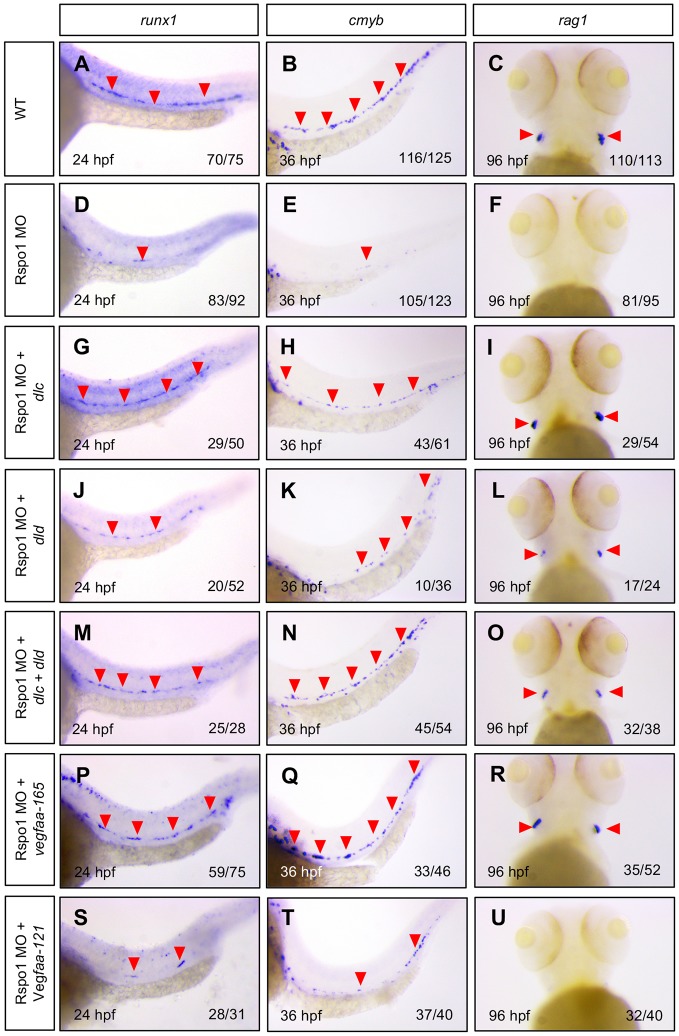
Our results show that in Rspo1 knockdown animals, expression of wnt16, as well as its downstream effectors dlc and dld, is diminished. Likewise, we observe loss of vegfaa, tgfb1a and tgfb1b expression in Rspo1 morphants. To determine whether either or both of these alterations might explain the loss of HSCs observed in Rspo1 knockdown animals, we performed a series of rescue experiments. mRNA encoding dlc and dld, alone or in combination, was injected at the 1-cell stage, and we then investigated HSC specification by examination of the markers, runx1 and cmyb, and the T lymphocyte marker, rag1, which provides an additional readout of the state of definitive hematopoietic development. We found that, compared with WT (Fig. 5A-F), dlc and dld alone could partially restore HSCs (Fig. 5G-L), and that dlc and dld together could fully restore HSC specification in Rspo1 morphants (Fig. 5M-O). These results indicate that decreased dlc and dld are responsible for hematopoietic defects observed in Rspo1 morphants.
Fig. 5.
Dlc, dld and vegfaa-165 can rescue HSC specification in Rspo1 morphants. Expression of the HSC markers runx1 (A,D,G,J,M,P,S) and cmyb (B,E,H,K,N,Q,T), and the T lymphocyte marker rag1 (C,F,I,L,O,R,U) in the thymus were examined at 24 hpf, 36 hpf, and 96 hpf as indicated, by WISH in wild-type embryos (A-C), or embryos injected with 5 ng Rspo1 MO (D-F), or 5 ng Rspo1 MO and mRNAs encoding candidate rescue genes dlc (100 ng; G-I), dld (50 ng; J-L), dlc+dld (50 ng and 25 ng, respectively; M-O), vegfaa-165 (100 ng; P-R) or vegfaa-121 (100 ng; S-U). dlc and dld mRNA were independently able to partially rescue HSCs. Co-injection of dlc and dld caused a more complete rescue of HSCs. vegfaa-165, but not vegfaa-121, was able to rescue HSCs. Red arrowheads indicate runx1+ (A,D,G,J,M,P,S), and cmyb+ (B,E,H,K,N,Q,T) HSCs, or rag1+ T lymphocytes (C,I,L,O,R), when visible. Numbers of embryos displaying the depicted phenotype are indicated. Lateral views of the mid trunk at 160× magnification.
In complementary experiments, we tested the ability of Vegfa to rescue HSC specification in Rspo1 morphants. Previous reports have suggested isoform specificity of Vegfa in regulating arterial gene expression and hematopoietic induction specifically, where the short form, Vegfa-121, regulates arterial gene expression and intermediate form, Vegfa-165/170, controls HSC specification (Ciau-Uitz et al., 2010; Leung et al., 2013). As arterial gene expression is unaffected in Rspo1 morphants (this study; Gore et al., 2011), we predicted that Vegfa-165 (the intermediate isoform in zebrafish) might be the form specifically affected. Consistent with this hypothesis, we found that injection of vegfaa-165, but not vegfaa-121, was able to rescue HSC specification (Fig. 5P-U).
DISCUSSION
Our results demonstrate that Rspo1 controls expression of genes in, and thus the activity of, two key HSC specification pathways: Wnt16/Dlc/Dld and Vegfa/Tgfβ. Importantly, HSC specification defects can be rescued by supplying mediating factors via mRNA injection in Rspo1 morphants, indicating that alterations in expression of these genes do indeed explain the loss of HSCs in Rspo1 loss-of-function. This is the first description of a requirement for Rspo1 in HSC specification. Our results provide an upstream integration of two pathways crucial for hematopoietic stem cell specification.
Rspo1 amplifies Wnt signaling by allowing Wnt receptors to stay on the cell membrane surface by blocking ubiquitination (Binnerts et al., 2007; Hao et al., 2012) and recycling of Wnt receptors, or through interruption of the activity of Wnt signaling antagonists (Kim et al., 2008). Rspo1 has been implicated in both canonical and non-canonical Wnt signaling (Glinka et al., 2011; Jin and Yoon, 2012; Ohkawara et al., 2011). Our data do not define the time at which Rspo1 is required for its regulation of either pathway. As canonical Wnt signaling directs somitic and lateral plate mesoderm cdx and hox gene expression, which are required for hematopoietic competence, it is possible that Rspo1 has a role in this regulation, consistent with its early expression.
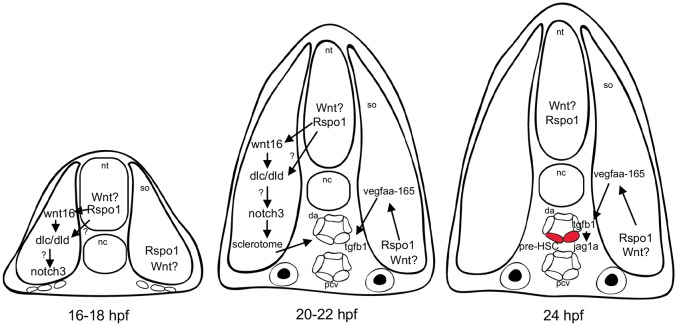
During later somitogenesis, rspo1 is expressed most strongly in the neural tube and dorsal and ventromedial somite (Fig. S1), and in the dorsal aorta (Gore et al., 2011). The fact that Rspo1 modifies the expression of somite genes [wnt16 (Fig. 2), dlc, dld (Fig. 3), and vegfaa (Fig. 4)] makes it probable that Rspo1 is acting within the somite, likely by modulating canonical Wnt signals known to regulate somite patterning (Geetha-Loganathan et al., 2008). Fig. 6 illustrates a model for Rspo1 regulation of HSC specification through key stages of development. In our model, Rspo1 acts to locally amplify signaling by canonical Wnt signal(s), for example Wnt1, which is expressed from the neural tube. Rspo1 is required for optimal expression of wnt16, which is expressed in the dorsal somite and regulates downstream Notch ligand expression, dlc and dld. rspo1 is also expressed in the ventromedial somite, where it likely potentiates Wnt activation of vegfaa expression there. Vegfaa-165 in turn is required for expression of tgfb1a, tgfb1b, and downstream jag1a expression in the dorsal aorta, ligands recently shown to be necessary for HSC specification (Monteiro et al., 2016). Our results strongly suggest that one or more unknown canonical Wnt ligands regulate wnt16 and vegfaa expression in conjunction with Rspo1.
Fig. 6.
Model of Rspo1 regulation of the Wnt16/Dlc/Dld and Vegfa/Tgfβ HSC specification pathways. Schematic depicting a transverse section through the trunk embryo of a zebrafish embryo at (left) 16-18 hpf prior to formation of the dorsal aorta, (middle) 20-22 hpf just prior to initiation of definitive hematopoiesis, and (right) 24 hpf when HSC precursors (red cells) are first visible in the formed dorsal aorta by expression of the marker runx1. Rspo1 regulates expression of key signaling factors in the somite (so), likely by amplifying the signaling activity of undetermined Wnt ligands. rspo1 is expressed throughout the somite but at higher levels in the neural tube (nt), dorsal and ventromedial somite, and axial vasculature (dorsal aorta, da; posterior cardinal vein, pcv). At 16 hpf rspo1 is expressed in the dorsal somite and is required for maximal expression of wnt16, which acts through relay Notch signals, including dlc and dld, and additional as-yet-unidentified downstream relay signals. rspo1 is also expressed in the medial somite where it is required for expression of vegfa-165, by directing expression of tgfb1a and tgfb1b expression in the dorsal aorta. Autocrine Tgfβ signaling activates jag1a expression required for HSC specification at 24 hpf. nc, notochord.
We previously showed that Wnt16 is necessary for HSC specification (Clements et al., 2011). Wnt16 activates expression of the Notch ligands dlc and dld, the activity of both of which is required within the somite through Notch3 to optimally specify HSCs, likely through the action of one or more additional downstream relay signals (Kim et al., 2014). Little is known about the signaling regulating wnt16 expression, although it has previously been shown to be negatively regulated by Shh (Wilkinson et al., 2012) and require meox (Nguyen et al., 2014). However, we found no difference in Shh signaling (Fig. 4) or in meox expression (data not shown) in Rspo1 morphants. This study confirms and extends our knowledge of the regulation of the Wnt16/Dlc/Dld pathway and begins to define its relationship to signaling through Vegfa/Tgfβ.
Vegfa signaling is crucial for vascular development (Carmeliet et al., 1996; Ferrara et al., 1996), and its role in HSC specification is becoming clearer. In Xenopus, Vegfa-122 regulates convergence of dorsal aorta angioblast precursors from the lateral plate mesoderm to the midline (Cleaver and Krieg, 1998). Work in Xenopus additionally suggests that Vegfa might have isoform-specific roles on vascular patterning with the smaller Vegfa isoform (122 in frogs, 121 in zebrafish) responsible for angioblast migration (Cleaver and Krieg, 1998) and arterial specification, whereas the intermediate Vegfa isoform (170 in frogs, 165 in zebrafish) seems to act more directly to potentiate the HSC program (Ciau-Uitz et al., 2010; Leung et al., 2013). It seems probable that arterial specification is a prerequisite to hematopoietic program development (Clements and Traver, 2013), which does not exclude a subsequent direct role of Vegfa in directing HSC specification. In Rspo1 knockdown animals, we and previous studies find intact arterial patterning (Gore et al., 2011), but loss of HSCs (this work). Hematopoietic defects can be rescued by injection of vegfaa-165, but not vegfaa-121, further supporting the idea that Vegfa-165 might have a discrete role in HSC specification, independent of arteriovenous specification. The major role of Vegfa in HSC specification was until recently posited to be activation of arterial notch1b expression, which would potentiate reception of the key cell-autonomous Notch signal(s) necessary for HSC specification. Surprisingly, we see no loss of notch1b expression, despite a clear decrease in vegfaa. Instead we observe decreased expression of key Tgfβ family ligands, tgfb1a and tgfb1b, which have recently been shown to be necessary for HSC specification (Monteiro et al., 2016). Our data fit the idea that Vegfa-165 has a role in HSC specification, independent of induction of notch1b, by directing Tgfβ ligand expression and downstream display of the Notch ligand, jag1a.
A full description of the endogenous processes leading to HSC specification during embryonic development will enhance our understanding of the geography and signaling environment that directs definitive hematopoietic development. A clear understanding of these elements will likely inform attempts to recapitulate normal signaling and derive culture environments that can efficiently replicate in vivo specification of HSCs for in vitro-directed differentiation of pluripotent cells. Multiple pathways required for HSC specification have now been described, including the Shh/Vegfa/Tgfβ/Notch1 and the Wnt16/Dlc/Dld pathways, and it is important to more clearly define how they interact with one another, as well as whether there are facets of the pathways that deviate from expectations, which might suggest the existence of as-yet-unknown regulatory inputs. Here, we describe a previously unknown role for Rspo1, a Wnt amplification factor, in upstream regulation of these pathways, and derive unexpected insight into the proximal signaling events.
MATERIALS AND METHODS
Zebrafish strains and husbandry
Wild-type (WT) zebrafish (Danio rerio, WIK and AB lines), the rspo1 mutant dtty135 and Tg(fli1a:EGFP)y1 embryos of both sexes in approximately equal numbers were obtained and maintained in accordance with standard husbandry procedures (Westerfield, 2007) and in compliance with IACUC guidelines and all relevant institutional and national animal welfare laws, guidelines and policies. Mating pairs, ages 3 to 18 months of age, were set up overnight and embryos were collected and maintained in E3 media (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) or 0.03% Instant Ocean (Spectrum Brands).
Cloning, constructs, probes and whole-mount in situ hybridization
rspo1 and rspo1 3′UTR were amplified from WIK whole-embryo cDNA at 24 h post fertilization (hpf), using primers Rspo1: forward, 5′-GGCGCTGGCACTGGTCTTCTTCAGCT-3′; reverse, 5′-CCATTCGAACATGATGGAGGAACTTCAG-3′; Rspo1 3′UTR: forward, 5′- GGATCCTTTCATCACAGCATCAGGTGGGC-3′; reverse, 5′-CAAGCACAGAATCTTTTATACACATACGTC-3′, designed based on the NCBI reference sequence NM_001002352, cloned into pCR4-TOPO (Invitrogen) and subcloned into pCS2+ (Turner and Weintraub, 1994). The sequenced clones conform to NM_001002352.1 (rspo1) and did not contain any non-silent alterations. tgfb1a, tgfb1b and tgfbr2 probe fragments were amplified from cDNA using primers tgfb1a: forward, 5′-GCATTATGAGGTTGGTTTGCTTGG-3′; reverse, 5′-CGCAGTATAACCTCAGCTCCAAGG-3′; tgfb1b: forward, 5′-GCAATGTCTGTTGGGATTTGTGC-3′; reverse, 5′-CACCTCTATTGCGGGACAAACCTGC-3′; tgfbr2, forward: 5′- CAAAGCCAAGCTGAGACAAAGCC-3′; reverse, 5′- CCTCCATTTGGCTCTAAAGTCCGAG-3′, cloned into pCRII (Invitrogen), and verified by sequencing.
Probe synthesis and whole mount in situ hybridization was performed as described previously (Clements et al., 2011, 2009) with a minimum of three independent replications. The following probe constructs were used: pBK-CMV cmyb (L. Zon, Boston Children's Hospital and Dana Farber Cancer Institute, Boston, MA, USA), pCRII crestin (D. Raible, University of Washington, Seattle, WA, USA), pBS dlc (J. Lewis, Cancer Research UK, London, UK), pCS2+ dld (S. Holley, Yale University, New Haven, CT, USA), pBS efnb2a (Clements et al., 2011), pBS gata1 (D. Ransom, National Cancer Institute, Rockville, MD, USA), pCRII myoda (Clements et al., 2011), pCR-Script notch1a (J. Campos Ortega, Institut für Entwicklungsbiologie, Köln, Germany), pCR-Script notch1b (M. Lardelli, University of Adelaide, Adelaide, Australia), pCR-Script notch2 (B. Appel, University of Colorado, Denver, CO, USA), pCR-Script notch3 (M. Lardelli), pBS ptch1 (J. Waxman, Cincinnati Children's Hospital, Cincinnati, OH, USA), pCRII rag1 (N. Trede, Acetylon Pharmaceuticals, Boston, MA, USA), pCR4 rpso1 3′UTR, pCS21 runx1 (C. Burns, Massachusetts General Hospital, Charlestown, MA, USA), pCS2 shha (Clements et al., 2011), pCRII tgfb1a, pCRII tgfb1b, pCRII tgfbr2, pCRII vegfaa-165 (Clements et al., 2011), and pCS2 wnt16 (Clements et al., 2011). The following constructs were subcloned into pCS2+ from pCRII via EcoRI: pCS2+ vegfaa-165 and pCS2+ vegfaa-121.
Transverse in situ hybridization sections
Embryos processed by in situ hybridization for rspo1 were embedded in 4.5% low melt agarose. Transverse sections were cut using Leica VT1200 vibratome. Sections were imaged using a Leica M205 FA stereo microscope.
Morpholinos, mRNA and microinjections
Antisense-morpholinos (MOs, Gene Tools, LLC) were diluted to 3 mM stock (25 mg/ml) in DEPC-treated water. The Rspo1 splice-blocking morpholino (Rspo1 MO, 5′-AGAAACATCAGCACTCACTCCGTCT-3′) was previously described (Gore et al., 2011). Embryos at the 1-cell stage of development as previously described (Clements et al., 2009) were injected with 5 ng of Rspo1 MO. The Wnt16 splice-blocking morpholino (Wnt16 MO2, 5′-GCGTGGAATACTTACATCCAACTTC-3′) was previously described (Clements et al., 2011) and 5 ng was injected at the 1-cell stage of development. Linearized expression constructs pCS2+ dlc (Clements et al., 2011), pCS2+ dld (Clements et al., 2011), pCS2+ vegfaa-165 and pCS2+ vegfaa-121 were synthesized in house from 5′-G-capped mRNA using mMessage mMachine kit (Ambion) transcription (Clements et al., 2009). For rescue experiments, mRNA was co-injected with 5 ng of Rspo1 MO into 1-cell-stage embryos at concentrations between 25 and 100 pg, as indicated in the text.
Reverse transcription PCR (RT-PCR)
RT-PCR was as previously described (Clements et al., 2011). Briefly, total cellular RNA was isolated from embryos using Trizol reagent and treated with DNase. cDNA from polyadenylated mRNA was synthesized using SuperScript III first-strand synthesis supermix kit (Invitrogen). PCR for rspo1 was carried out with custom primers: forward, 5′-GGCGCTGGCACTGGTCTTCTTCAGCT-3′; reverse, 5′-CCATTCGAACATGATGGAGGAACTTCAG-3′.
Quantitative real-time RT-PCR (qRT-PCR)
qRT-PCR was performed following a previously described protocol (Lan et al., 2009). Briefly, uninjected and Rspo1 morpholino-injected embryos were collected. Mid-trunk tissue from 17 hpf, whole 17 hpf, whole 20 hpf, whole 22 hpf, and whole 24 hpf embryos was collected. RNA was isolated from frozen embryos with Trizol. Samples were treated with DNase. cDNA was synthesized using Superscript III immediately following RNA extraction and purification. qRT-PCR was performed with an Applied Biosystems Step One Plus Real-Time PCR System. cDNA was diluted 1:10 and added to 1× Fast SYBR Green Master Mix (Thermo Fisher) plus 300 nM primer in a 20 µl reaction. Samples were run in triplicate and a total of three biological replicates per treatment were analyzed. qRT-PCR data was analyzed using the Step One Plus software. Data shown is relative to ef1a expression (mean± s.e.m.). Statistical significance was determined by one-way analysis of variance with a Fisher LSD post-hoc test using StatPlus:Mac Pro software (AnalystSoft). Statistical significance was reached with a P-value ≤0.05.
Primers for qRT-PCR were either previously published or designed as previously described (Lan et al., 2009). The following primers were used: dlc (Hamada et al., 2014) forward, 5′-GACCGGTGCAGCAGTGACCC-3′; reverse, 5′-TGTGCCCATGAAGCCTGCCG-3′; dld (Hamada et al., 2014) forward, 5′-AGCGACGGCGACAAAAACGGA-3′; reverse, 5′-TGTGGCGTTACACCTCGGTTGC-3′; ef1a (McCurley and Callard, 2008) forward, 5′-CTTCTCAGGCTGACTGTGC-3′; reverse, 5′-CCGCTAGCATTACCCTCC-3′; rspo1 (Zhang et al., 2011) forward, 5′-GAAAGGGGCGTCCTCAGT-3′; reverse, 5′-TGATGGAGGAACTTCAGTGCT-3′; vegfaa-165 forward, 5′-GCCAAAGGCAGAAGTCAAAG-3′; reverse, 5′-CTTGCATTGCATTTGTGTGA-3′; wnt16 forward, 5′-AAGGATTGCCACTGGATCAC-3′; reverse, 5′-AGCAGCGTCTTCGTTGACTT-3′.
Acknowledgements
We are grateful to Dr Brant Weinstein for gift of the dtty135 fish.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
J.R.G. and W.K.C.: Conception and design of research; interpretation of results of experiments; editing and revision of manuscript. J.R.G.: Performed experiments and analyzed data; prepared figures and drafted manuscript. W.K.C.: approved final version of manuscript.
Funding
This work was supported by the National Heart, Lung, and Blood Institute (grant R00HL097150 to W.K.C.) and March of Dimes Foundation Basil O'Connor Starter Scholar Research Award (#5-FY14-42 to W.K.C.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.139956.supplemental
References
- Binnerts M. E., Kim K.-A., Bright J. M., Patel S. M., Tran K., Zhou M., Leung J. M., Liu Y., Lomas W. E. III, Dixon M. et al. (2007). R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc. Natl. Acad. Sci. USA 104, 14700-14705. 10.1073/pnas.0702305104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon D. C., Ishii Y., O'Toole E. A., Unsworth H. C., Teh M.-T., Rüschendorf F., Sinclair C., Hopsu-Havu V. K., Tidman N., Moss C.. et al. (2006). The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat. Genet. 38, 1245-1247. 10.1038/ng1883 [DOI] [PubMed] [Google Scholar]
- Burns C. E., Traver D., Mayhall E., Shepard J. L. and Zon L. I. (2005). Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 19, 2331-2342. 10.1101/gad.1337005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butko E., Distel M., Pouget C., Weijts B., Kobayashi I., Ng K., Mosimann C., Poulain F. E., McPherson A., Ni C.-W.. et al. (2015). Gata2b is a restricted early regulator of hemogenic endothelium in the zebrafish embryo. Development 142, 1050-1061. 10.1242/dev.119180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butko E., Pouget C. and Traver D. (2016). Complex regulation of HSC emergence by the Notch signaling pathway. Dev. Biol. 409, 129-138. 10.1016/j.ydbio.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Mackman N., Moons L., Luther T., Gressens P., Van Vlaenderen I., Demunck H., Kasper M., Breier G., Evrard P. et al. (1996). Role of tissue factor in embryonic blood vessel development. Nature 383, 73-75. 10.1038/383073a0 [DOI] [PubMed] [Google Scholar]
- Chen J. N., Haffter P., Odenthal J., Vogelsang E., Brand M., van Eeden F. J., Furutani-Seiki M., Granato M., Hammerschmidt M., Heisenberg C. P.. et al. (1996). Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development 123, 293-302. [DOI] [PubMed] [Google Scholar]
- Chen M. J., Yokomizo T., Zeigler B. M., Dzierzak E. and Speck N. A. (2009). Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457, 887-891. 10.1038/nature07619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciau-Uitz A., Pinheiro P., Gupta R., Enver T. and Patient R. (2010). Tel1/ETV6 specifies blood stem cells through the agency of VEGF signaling. Dev. Cell 18, 569-578. 10.1016/j.devcel.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Cleaver O. and Krieg P. A. (1998). VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development 125, 3905-3914. [DOI] [PubMed] [Google Scholar]
- Clements W. K. and Traver D. (2013). Signalling pathways that control vertebrate haematopoietic stem cell specification. Nat. Rev. Immunol. 13, 336-348. 10.1038/nri3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements W. K., Ong K. G. and Traver D. (2009). Zebrafish wnt3 is expressed in developing neural tissue. Dev. Dyn. 238, 1788-1795. 10.1002/dvdy.21977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements W. K., Kim A. D., Ong K. G., Moore J. C., Lawson N. D. and Traver D. (2011). A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature 474, 220-224. 10.1038/nature10107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M., Nyqvist D., Orsenigo F., Caprini A., Giampietro C., Taketo M. M., Iruela-Arispe M. L., Adams R. H. and Dejana E. (2010). The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev. Cell 18, 938-949. 10.1016/j.devcel.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W., Barker N., Low T. Y., Koo B.-K., Li V. S. W., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M.. et al. (2011). Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293-297. 10.1038/nature10337 [DOI] [PubMed] [Google Scholar]
- de Lau W., Peng W. C., Gros P. and Clevers H. (2014). The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 28, 305-316. 10.1101/gad.235473.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O'Shea K. S., Powell-Braxton L., Hillan K. J. and Moore M. W. (1996). Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439-442. 10.1038/380439a0 [DOI] [PubMed] [Google Scholar]
- Geetha-Loganathan P., Nimmagadda S., Scaal M., Huang R. and Christ B. (2008). Wnt signaling in somite development. Ann. Anat. 190, 208-222. 10.1016/j.aanat.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Gering M. and Patient R. (2005). Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev. Cell 8, 389-400. 10.1016/j.devcel.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Gering M. and Patient R. (2010). Notch signalling and haematopoietic stem cell formation during embryogenesis. J. Cell. Physiol. 222, 11-16. 10.1002/jcp.21905 [DOI] [PubMed] [Google Scholar]
- Glinka A., Dolde C., Kirsch N., Huang Y.-L., Kazanskaya O., Ingelfinger D., Boutros M., Cruciat C.-M. and Niehrs C. (2011). LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055-1061. 10.1038/embor.2011.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W., North T. E., Loewer S., Lord A. M., Lee S., Stoick-Cooper C. L., Weidinger G., Puder M., Daley G. Q., Moon R. T.. et al. (2009). Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136, 1136-1147. 10.1016/j.cell.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A. V., Swift M. R., Cha Y. R., Lo B., McKinney M. C., Li W., Castranova D., Davis A., Mukouyama Y.-S. and Weinstein B. M. (2011). Rspo1/Wnt signaling promotes angiogenesis via Vegfc/Vegfr3. Development 138, 4875-4886. 10.1242/dev.068460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland B. K., Huppert S. S., Kanungo J., Xue Y., Jiang R., Gridley T., Conlon R. A., Cheng A. M., Kopan R. and Longmore G. D. (2004). A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood 104, 3097-3105. 10.1182/blood-2004-03-1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Watanabe M., Lau H. E., Nishida T., Hasegawa T., Parichy D. M. and Kondo S. (2014). Involvement of Delta/Notch signaling in zebrafish adult pigment stripe patterning. Development 141, 318-324. 10.1242/dev.099804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H.-X., Xie Y., Zhang Y., Charlat O., Oster E., Avello M., Lei H., Mickanin C., Liu D., Ruffner H.. et al. (2012). ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195-200. 10.1038/nature11019 [DOI] [PubMed] [Google Scholar]
- Jin Y.-R. and Yoon J. K. (2012). The R-spondin family of proteins: emerging regulators of WNT signaling. Int. J. Biochem. Cell Biol. 44, 2278-2287. 10.1016/j.biocel.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalev-Zylinska M. L., Horsfield J. A., Flores M. V., Postlethwait J. H., Vitas M. R., Baas A. M., Crosier P. S. and Crosier K. E. (2002). Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development 129, 2015-2030. [DOI] [PubMed] [Google Scholar]
- Kim K.-A., Kakitani M., Zhao J., Oshima T., Tang T., Binnerts M., Liu Y., Boyle B., Park E., Emtage P.. et al. (2005). Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309, 1256-1259. 10.1126/science.1112521 [DOI] [PubMed] [Google Scholar]
- Kim K.-A., Zhao J., Andarmani S., Kakitani M., Oshima T., Binnerts M. E., Abo A., Tomizuka K. and Funk W. D. (2006). R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle 5, 23-26. 10.4161/cc.5.1.2305 [DOI] [PubMed] [Google Scholar]
- Kim K.-A., Wagle M., Tran K., Zhan X., Dixon M. A., Liu S., Gros D., Korver W., Yonkovich S., Tomasevic N.. et al. (2008). R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 19, 2588-2596. 10.1091/mbc.E08-02-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. D., Melick C. H., Clements W. K., Stachura D. L., Distel M., Panakova D., MacRae C., Mork L. A., Crump J. G. and Traver D. (2014). Discrete Notch signaling requirements in the specification of hematopoietic stem cells. EMBO J. 33, 2363-2373. 10.15252/embj.201488784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I. N., Kathiriya I. S., Murakami M., Nakagawa M., Gardner K. A., Srivastava D. and Nakagawa O. (2006). Hrt and Hes negatively regulate Notch signaling through interactions with RBP-Jkappa. Biochem. Biophys. Res. Commun. 345, 446-452. 10.1016/j.bbrc.2006.04.097 [DOI] [PubMed] [Google Scholar]
- Kumano K., Chiba S., Kunisato A., Sata M., Saito T., Nakagami-Yamaguchi E., Yamaguchi T., Masuda S., Shimizu K., Takahashi T.. et al. (2003). Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18, 699-711. 10.1016/S1074-7613(03)00117-1 [DOI] [PubMed] [Google Scholar]
- Lan C. C., Tang R., Un San Leong I. and Love D. R. (2009). Quantitative real-time RT-PCR (qRT-PCR) of zebrafish transcripts: optimization of RNA extraction, quality control considerations, and data analysis. Cold Spring Harb. Protoc. 2009, pdb prot5314 10.1101/pdb.prot5314 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Scheer N., Pham V. N., Kim C. H., Chitnis A. B., Campos-Ortega J. A. and Weinstein B. M. (2001). Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128, 3675-3683. [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Vogel A. M. and Weinstein B. M. (2002). sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev. Cell 3, 127-136. 10.1016/S1534-5807(02)00198-3 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Mugford J. W., Diamond B. A. and Weinstein B. M. (2003). phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 17, 1346-1351. 10.1101/gad.1072203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie J. D., Ariza-McNaughton L., Bermange A. L., McAdow R., Johnson S. L. and Lewis J. (2007). Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development 134, 839-844. 10.1242/dev.003244 [DOI] [PubMed] [Google Scholar]
- Leung A., Ciau-Uitz A., Pinheiro P., Monteiro R., Zuo J., Vyas P., Patient R. and Porcher C. (2013). Uncoupling VEGFA functions in arteriogenesis and hematopoietic stem cell specification. Dev. Cell 24, 144-158. 10.1016/j.devcel.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis T. C. and Staal F. J. T. (2009). WNT proteins: environmental factors regulating HSC fate in the niche. Ann. N. Y. Acad. Sci. 1176, 70-76. 10.1111/j.1749-6632.2009.04566.x [DOI] [PubMed] [Google Scholar]
- McCurley A. T. and Callard G. V. (2008). Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 9, 102 10.1186/1471-2199-9-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro R., Pinheiro P., Joseph N., Peterkin T., Koth J., Repapi E., Bonkhofer F., Kirmizitas A. and Patient R. (2016). Transforming growth factor beta drives hemogenic endothelium programming and the transition to hematopoietic stem cells. Dev. Cell 38, 358-370. 10.1016/j.devcel.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J.-S., Turcotte T. J., Smith P. F., Choi S. and Yoon J. K. (2006). Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J. Biol. Chem. 281, 13247-13257. 10.1074/jbc.M508324200 [DOI] [PubMed] [Google Scholar]
- Nguyen P. D., Hollway G. E., Sonntag C., Miles L. B., Hall T. E., Berger S., Fernandez K. J., Gurevich D. B., Cole N. J., Alaei S.. et al. (2014). Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature 512, 314-318. 10.1038/nature13678 [DOI] [PubMed] [Google Scholar]
- Niehrs C. (2012). The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13, 767-779. 10.1038/nrm3470 [DOI] [PubMed] [Google Scholar]
- North T. E., de Bruijn M. F. T. R., Stacy T., Talebian L., Lind E., Robin C., Binder M., Dzierzak E. and Speck N. A. (2002). Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16, 661-672. 10.1016/S1074-7613(02)00296-0 [DOI] [PubMed] [Google Scholar]
- North T. E., Goessling W., Walkley C. R., Lengerke C., Kopani K. R., Lord A. M., Weber G. J., Bowman T. V., Jang I.-H., Grosser T.. et al. (2007). Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007-1011. 10.1038/nature05883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara B., Glinka A. and Niehrs C. (2011). Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev. Cell 20, 303-314. 10.1016/j.devcel.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Okuda T., van Deursen J., Hiebert S. W., Grosveld G. and Downing J. R. (1996). AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84, 321-330. 10.1016/S0092-8674(00)80986-1 [DOI] [PubMed] [Google Scholar]
- Parma P., Radi O., Vidal V., Chaboissier M. C., Dellambra E., Valentini S., Guerra L., Schedl A. and Camerino G. (2006). R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat. Genet. 38, 1304-1309. 10.1038/ng1907 [DOI] [PubMed] [Google Scholar]
- Ruiz-Herguido C., Guiu J., D'Altri T., Inglés-Esteve J., Dzierzak E., Espinosa L. and Bigas A. (2012). Hematopoietic stem cell development requires transient Wnt/beta-catenin activity. J. Exp. Med. 209, 1457-1468. 10.1084/jem.20120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann A. F. and Lawson N. D. (2007). Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781-784. 10.1038/nature05577 [DOI] [PubMed] [Google Scholar]
- Taoudi S. and Medvinsky A. (2007). Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc. Natl. Acad. Sci. USA 104, 9399-9403. 10.1073/pnas.0700984104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. L. and Weintraub H. (1994). Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 8, 1434-1447. 10.1101/gad.8.12.1434 [DOI] [PubMed] [Google Scholar]
- Wei Q., Yokota C., Semenov M. V., Doble B., Woodgett J. and He X. (2007). R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J. Biol. Chem. 282, 15903-15911. 10.1074/jbc.M701927200 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2007). The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press. [Google Scholar]
- Wilkinson R. N., Pouget C., Gering M., Russell A. J., Davies S. G., Kimelman D. and Patient R. (2009). Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev. Cell 16, 909-916. 10.1016/j.devcel.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. N., Koudijs M. J., Patient R. K., Ingham P. W., Schulte-Merker S. and van Eeden F. J. M. (2012). Hedgehog signaling via a calcitonin receptor-like receptor can induce arterial differentiation independently of VEGF signaling in zebrafish. Blood 120, 477-488. 10.1182/blood-2011-10-383729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C., Kim S.-H., Ni T. T., Mitchell L., Ro H., Penn J. S., Baldwin S. H., Solnica-Krezel L. and Zhong T. P. (2010). Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate. Dev. Biol. 341, 196-204. 10.1016/j.ydbio.2010.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li F., Sun D., Liu J., Liu N. and Yu Q. (2011). Molecular analysis shows differential expression of R-spondin1 in zebrafish (Danio rerio) gonads. Mol. Biol. Rep. 38, 275-282. 10.1007/s11033-010-0105-3 [DOI] [PubMed] [Google Scholar]
- Zhao J., Kim K.-A., De Vera J., Palencia S., Wagle M. and Abo A. (2009). R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/beta-catenin pathway. Proc. Natl. Acad. Sci. USA 106, 2331-2336. 10.1073/pnas.0805159106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein A. C., Hofmann J. J., Lynch M., French W. J., Turlo K. A., Yang Y., Becker M. S., Zanetta L., Dejana E., Gasson J. C.. et al. (2008). Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3, 625-636. 10.1016/j.stem.2008.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]