Abstract
Integrin αvβ6 is rapidly up-regulated on cells of epithelial lineage during tissue injury, where one of its primary functions is activation of latent transforming growth factor beta 1 (TGFβ1). In human liver cirrhosis, αvβ6 is overexpressed by cells comprising the ductular reaction, and its inhibition suppresses experimental biliary fibrosis in rodents. Here, we show that αvβ6 is expressed on the actively proliferating subset of hepatic progenitor cells and is required for their progenitor function in vivo and in vitro through integrin αvβ6-dependent TGFβ1 activation. Freshly isolated αvβ6+ liver cells demonstrate clonogenic potential and differentiate into cholangiocytes and functional hepatocytes in vitro, whereas colony formation by epithelial cell adhesion molecule-positive progenitor cells is blocked by αvβ6-neutralizing antibody and in integrin beta 6-deficient cells. Inhibition of progenitors by anti-αvβ6 antibody is recapitulated by TGFβ1 neutralization and rescued by addition of bioactive TGFβ1. Genetic disruption or selective targeting of αvβ6 with 3G9 antibody potently inhibits progenitor cell responses in mouse models of chronic biliary injury and protects from liver fibrosis and tumorigenesis, two conditions clinically associated with exacerbated ductular reaction.
Conclusion
These results suggest that αvβ6 is a promising target for chronic fibrotic liver diseases and associated cancers.
Primary sclerosing cholangitis (PSC) is a chronic, cholestatic liver disease of unknown etiology, characterized by inflammation and fibrosis of both intrahepatic and extrahepatic bile ducts, which ultimately progresses to biliary cirrhosis.1 Patients with PSC are at high risk of developing cholangiocarcinoma (10-year cumulative risk of 7%–9%). Cholangiocarcinoma is a devastating complication of PSC, difficult to diagnose, and associated with high mortality.2 Because no effective treatment exists outside liver transplantation in a low number of eligible patients, therapies that halt or slow down the progression of PSC are urgently needed.
Progression of biliary fibrosis to cirrhosis in PSC and other cholangiopathies is associated with a pronounced “ductular reaction,”3 characterized by proliferation of adult bipotent hepatic progenitors and reactive cholangiocytes. Prior studies have demonstrated that reactive cholangiocytes drive fibrogenesis through secretion of multiple proinflammatory and profibrogenic factors,4 acting in a paracrine fashion on hepatic stellate cells, the major fibrogenic effector cells in the liver.5 Importantly, clinicopathological evidence suggests that hepatic progenitors may give rise to cholangiocarcinoma6 and a subclass of hepatocellular carcinoma with poor clinical prognosis.7 Several proinflammatory cytokines, most notably TWEAK8 and lymphotoxin α/β,9 have been directly implicated in triggering progenitor (oval) cell responses. Elucidating the critical molecular mechanisms underlying hepatic progenitor activation and proliferation is essential to developing conceptually novel therapies targeting both fibrosis and the associated risk of cancer.
Integrins constitute a family of transmembrane heterodimeric cellular receptors that mediate cell-cell and cell-matrix interactions. The β6 integrin chain forms a heterodimer only with the αv subunit and is restricted exclusively to cells of epithelial lineage.10 While the αvβ6 integrin is present only at low levels in adult differentiated tissues, it is overexpressed during embryogenesis, tumorigenesis, and tissue injury.11 A unique function of integrin αvβ6 is the binding and activation of latent transforming growth factor beta 1 (TGFβ1) on epithelial surfaces.12,13 Due to its selective up-regulation in fibrosis and cancer, integrin αvβ6 emerges as a promising pharmacological target, particularly in fibrotic biliary diseases. We have previously demonstrated that αvβ6 is de novo overexpressed on biliary epithelia in patients with cirrhosis and mice with experimental biliary fibrosis,14 and others have reported up-regulation in human cholangiocarcinomas.15 Furthermore, studies by our group and others have established that αvβ6 is functionally required for biliary fibrosis progression and can be targeted therapeutically using selective inhibitors14,16 and blocking antibody.17 Expression of αvβ6 on progenitor-like cells was noted in human end-stage cirrhosis14,18 and attenuated ductular reaction upon αvβ6 inhibition16,19 in biliary fibrosis models. However, it remained unknown how far integrin αvβ6 is functionally involved in hepatic progenitor activation. Here, we performed mechanistic in vitro and in vivo studies to directly address the potential role of integrin αvβ6 in regulating progenitor (oval) cell biology in the context of chronic liver injury. We report that αvβ6 is expressed on activated hepatic progenitor cells and regulates their function. Isolated αvβ6+ liver cells are able to form colonies and differentiate into cholangiocytes and hepatocytes in vitro. Genetic and pharmacological inactivation of αvβ6 potently suppresses progenitor cell function in vitro and in vivo and subsequently inhibits hepatic fibrosis and tumorigenesis in murine cholangiopathy models.
Materials and Methods
Mouse Models of Sclerosing Cholangitis
All mouse experiments were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center (158–2008, 004–2012, 010–2015).
FVB.multidrug resistance protein 2 (Mdr2)(abcb4)−/− mice (referred to as Mdr2−/− here), deficient in the canalicular phospholipid flippase, that develop spontaneous biliary fibrosis with features of PSC have been characterized.20 Mdr2−/− and Mdr2−/− (wild-type) mice were obtained from Jackson Laboratory (Bar Harbor, ME) and bred at Beth Israel Deaconess Medical Center.
3,5-Diethoxycarbonyl-1,4-dihydrocollidine (DDC) feeding induces progressive cholangitis, pronounced ductular reaction, and bridging fibrosis.21 Eight-week-old male C57Bl/6 mice (Jackson Laboratory) or C57Bl/6.integrin beta 6 knockout (Itgb6−/−) mice were fed a DDC-supplemented diet (0.1%) for 3 weeks to induce advanced biliary fibrosis, as established.22
Itgb6−/− mice were generated as described on FVB23 and C57Bl/624 genetic backgrounds. Itgb6−/− mice functionally lack αvβ6 integrin because integrin β6 forms heterodimers only with the αv chain.
Mdr2(abcb4)−/−;Itgb6−/− double-mutant mice (FVB background) were generated by crossing FVB.Mdr2−/− with FVB. Itgb6−/− mice to obtain F1 double-heterozygous mutants, intercrossed, F2 progeny-genotyped at weaning, and phenotyped at age 8 weeks (see also Supporting Information). To assess progenitor response and fibrogenesis, 5-week-old male Mdr2−/− and Mdr2−/−;Itgb6−/− double-knockout mice were challenged by low-dose 0.1% cholic acid feeding, which aggravates liver injury and fibrosis in Mdr2−/− mice,25 for 4 weeks. For long-term outcome studies of fibrosis and primary liver cancers, three Mdr2−/−;Itgb6−/− mice (one male and two females) in F2 generation were bred to establish a double-knockout colony.
Human liver explants were obtained from the subset of patients with biliary cirrhosis (primary biliary cirrhosis [PBC] n = 4, PSC n = 3) undergoing orthotopic liver transplantation due to end-stage liver disease. Wedge biopsies of normal human livers not suitable for transplantation (n = 3) served as normal controls. The study protocol was approved by the Ethics Committee of the University of Heidelberg.
Therapeutic Anti-αvβ6 Monoclonal Antibody Treatment
The αvβ6-specific monoclonal antibody (mAb) 6.3G9 (3G9; Biogen Idec) was generated as described. Two doses of 3G9 mAb (1 and 10 mg/kg) or irrelevant isotype control antibody (1E6, 10 mg/kg; Biogen Idec) were administered intraperitoneally once a week in sterile phosphate-buffered saline into Mdr2−/− mice from week 4 through week 8 of age (early treatment protocol) or from week 6 through week 12 (delayed treatment protocol). Systemic TGFβ inhibition was achieved using recombinant soluble mouse TGFβ receptor II fusion protein (rsTGFβRII-Fc, 5 mg/kg intraperitoneally once a week; Biogen Idec).
Isolation of primary epithelial cell adhesion molecule-positive (EpCAM ) and integrin αvβ6+ cells was achieved through a two-step procedure. First, nonparen-chymal liver cells were freshly isolated from livers of 5-week-old Mdr2−/− mice through in situ collagenase perfusion, followed by three low-speed (50g) centrifugations/washes to remove hepatocyte and cell debris, as described.27 Second, a nonparenchymal cell pellet was immediately resuspended and EpCAM+ cells were purified with commercial EpCAM mAb-conjugated magnetic beads on an AutoMACS Pro Separator (Miltenyi). Similarly, integrin αvβ6+ cells were isolated from the nonparenchymal cell fraction through positive selection magnetic cell sorting using the 3G9 mAb directed against the extracellular domain of αvβ6 (conjugated to pan-mouse immunoglobulin G-coated magnetic beads [Miltenyi]), as described.28 Purity of isolated cell fractions was assessed by fluorescence-activated cell sorting and routinely exceeded 85%.
A colony formation and differentiation assay was performed according to Dorrell et al.,29 with modifications. Freshly isolated EpCAM+ and αvβ6+ cells from Mdr2−/− mice were seeded at a density of 10 cells/cm2 on collagen-coated 24-well or 96-well plastic plates (BD Falcon) or Nunc Lab-Tek II Chamber Slide System (Cole Parmer). Cell medium (10% fetal bovine serum, Dulbec-co’s modified Eagle’s medium) was supplemented with murine epidermal growth factor, murine hepatocyte growth factor (both at 10 ng/mL; Peprotech, Inc.), I× insulin-transferrin-selenium-ethanolamine (Life Technologies Corp.), and dexamethasone (10−7 mol/L; Sigma) and changed 24 hours after plating and every 3 days thereafter. Colonies were harvested on days 7 and 14, and cell supernatant was collected for albumin determination (mouse albumin enzyme-linked immunosorbent assay kit; Assaypro, St. Charles, MO). Colonies, derived from 105 isolated cells/condition and defined as organized cell clusters of at least 20 cells, were counted on day 14. Phase contrast and in selected cases 4′,6-diamidino-2-phenylindole nuclear staining were used except when colonies were to be analyzed by immunofluorescence or real-time reverse-transcriptase polymerase chain reaction (RT-PCR).
Hepatic hydroxyproline assay,30 quantitative RT-PCR,14,30 serum levels of alanine aminotransferase, immunohistochemistry, immunofluorescence, and western blotting for hepatic progenitor (oval) and hepatic stellate cell markers31,32 were performed as reported (see Supporting Information).
Statistical Analysis
Data are expressed as mean ± standard error of mean, and statistical analyses were performed using Graph-Pad Prism version 5.0 (GraphPad Software, San Diego, CA) Multiple comparisons were performed by one-way analysis of variance followed by Dunnett’s posttest and comparisons between two groups, by the Student t test. Differences among selected experimental groups with P < 0.05 were considered significant.
Results
Expansion of Integrin αvβ6-Expressing Ductal Cells Characterizes Human Biliary Cirrhosis and Parallels Fibrosis Progression in Mdr2−/− Mice
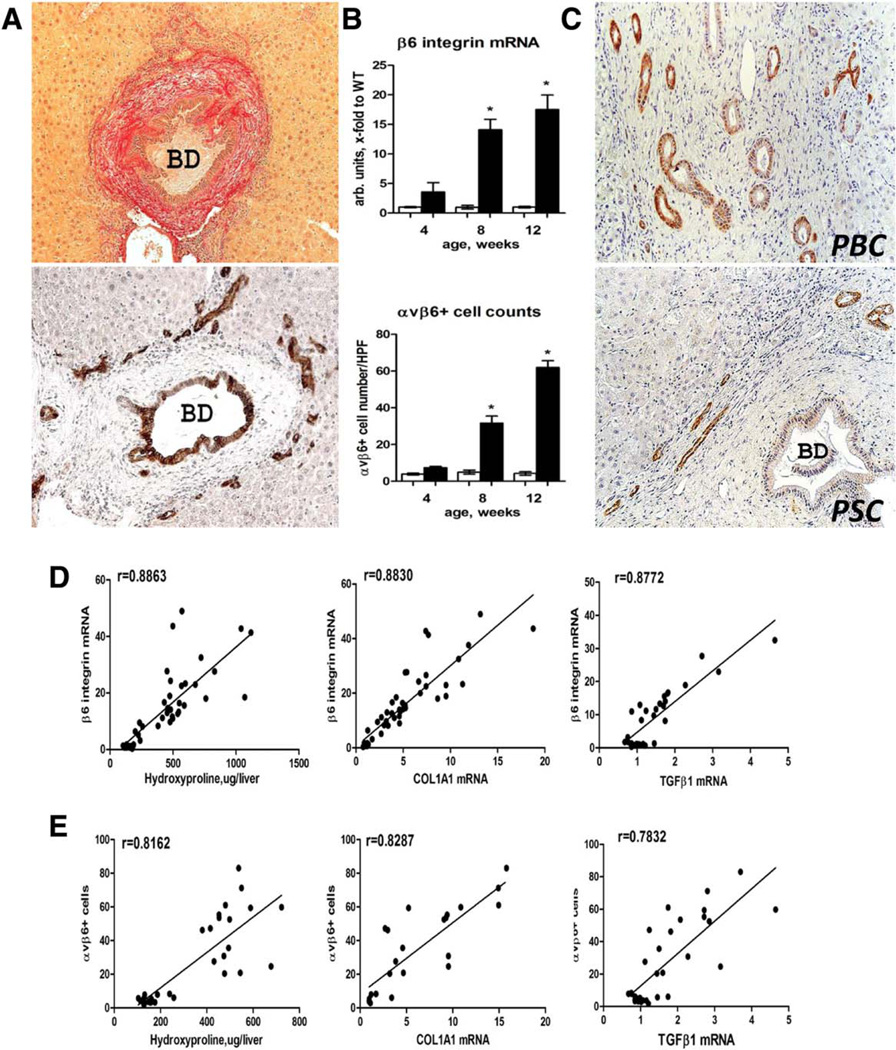
We have previously reported that messenger RNA (mRNA) expression of integrin αvβ6 is dramatically up-regulated and demonstrates a strong correlation with fibrosis progression (but not inflammation) in human subjects with chronic hepatitis C and experimental fibrosis models.14 Here, we further characterized αvβ6 expression at the cellular level by immunohistochemistry in relation to fibrosis progression in the Mdr2−/− mouse model of sclerosing cholangitis. Integrin αvβ6-positive cells were readily detected in portal areas of Mdr2−/− mice starting from 4 weeks of age. Injured bile ducts as well as ductular proliferation resembling reactive cholangiocytes/hepatic progenitors (“pseudoducts”) at the interface of expanding fibrotic septa demonstrated strong immunoreactivity for αvβ6 integrin (Fig. 1A). Numbers of αvβ6+ cells and Itgb6 mRNA dramatically increased from week 4 through week 12 of age, paralleling fibrosis progression in this model (Fig. 1B).14 A similar expression pattern was observed in human samples from end-stage biliary cirrhosis due to PSC and PBC (Fig. 1C). In contrast, integrin αvβ6 expression was absent from healthy human and murine livers (Supporting Figs. S1 and S2). Both αvβ6 integrin-positive cell numbers and Itgb6 mRNA expression strongly correlated with degree of fibrosis (hepatic collagen levels) and activity of fibrogenesis (hepatic TGFβ1 and collagen type 1 α1 [COL1A1] transcript levels) in Mdr2−/− mice analyzed at 4, 8, and 12 weeks of age (Fig. 1D).
Fig. 1.
Expansion of integrin αvβ6+ ductal cells in Mdr2−/− mice parallels fibrosis progression and is present in human biliary cirrhosis. (A) Typical “onion skin” lesion around large αvβ6+ bile duct, with clusters of αvβ6+ cells resembling reactive cholangiocytes/hepatic progenitors (“ductular reaction”) at the interface of fibrotic septa and hepatic lobule. Representative images from 12-week-old Mdr2−/− mice, sirius red (upper image) and αvβ6 immunohistochemistry (lower image). (B) Increases in integrin β6 mRNA expression and αvβ6+ cell numbers parallel hepatic fibrosis progression from week 4 (incipient fibrosis) to week 12 (advanced fibrosis) in Mdr2−/− mice (closed bars) compared to Mdr2+/+ healthy littermate controls (open bars). αvβ6-positive cells were counted in 10 random portal fields of four individual mice per time point at ×200 magnification. (C) Survey of αvβ6 integrin in human explant livers with end-stage biliary cirrhosis due to PBC and PSC demonstrates a ductular cell pattern of expression similar to Mdr2−/− mice. Representative immunohistochemistry images (PSC n = 3, PBC n = 4) at ×200 original magnification. (D,E) Hepatic αvβ6+ cell counts and integrin β6 mRNA levels strongly correlate with degree of fibrosis (hydroxyproline content) and fibrosis-related gene expression (COL1A1 and TGFβ1, Spearman test r as indicated) in Mdr2−/− mice analyzed at 4, 8, and 12 weeks of age. Abbreviations: BD, bile duct; HPF, high-power field; WT, wild type.
Integrin αvβ6 s Expressed by an Actively Proliferating Subset of Hepatic Progenitor (Oval) Cells in Mdr2−/− Mice
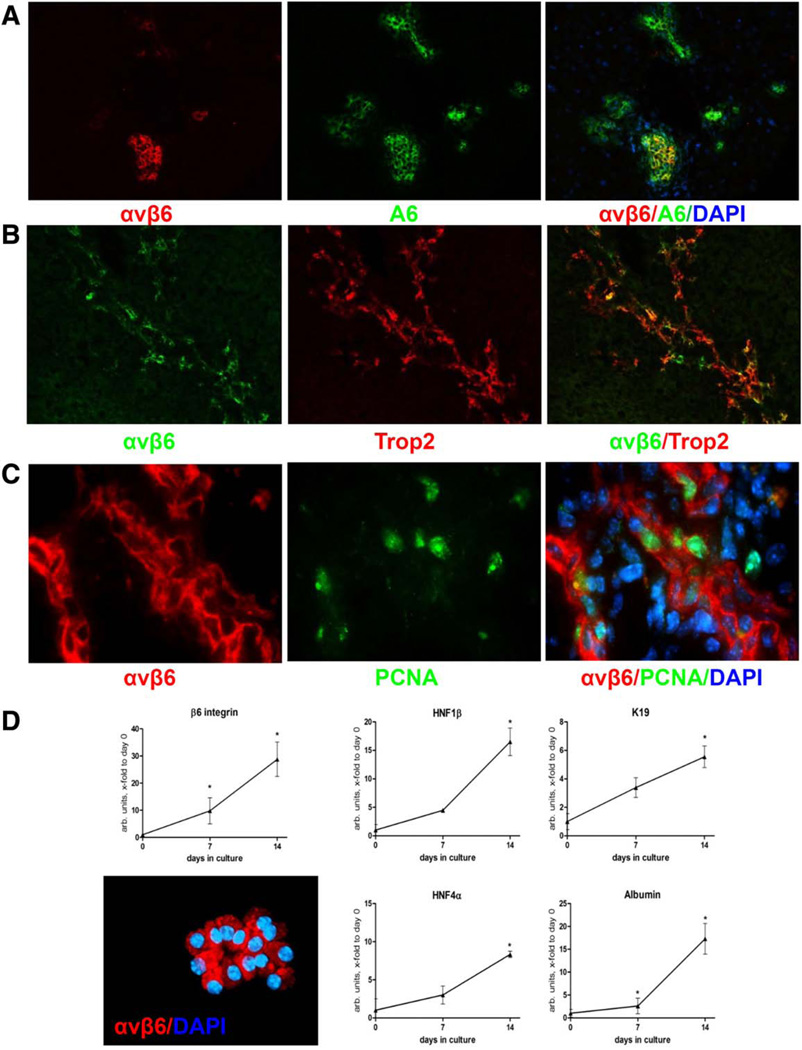
Based on the morphological appearance of αvβ6-expressing cells (Fig. 1A), we hypothesized that they may represent the cells arising from activated bipotent progenitor (oval) cells and performed double-immunofluorescence for integrin αvβ6+ cells and progenitor cell markers A6 and Trop2(Tacstd2) in livers from Mdr2−/− mice. Most, but not all, A6-positive oval cells coexpressed αvβ6 (Fig. 2A). Overlap appeared to be greater with Trop2, another hepatic progenitor cell-specific marker (Fig. 2B).33 Interestingly, αvβ6-expressing cells were also frequently positive for proliferating cell nuclear antigen, indicating that these cells actively proliferate in vivo (Fig. 2C). Primary oval cells isolated from Mdr2−/− livers using EpCAM antibody-coated magnetic beads expressed αvβ6 integrin (>90% by fluorescence-activated cell sorting, not shown) and further up-regulated Itgb6 mRNA in vitro, along with cholangiocyte (HNF1β, cytokeratin 19 [CK19]) and hepatocyte (HNF4α, albumin) transcriptional differentiation markers (Fig. 2D). Cell colonies generated by EpCAM+ cells were αvβ6-positive as assessed by immunofluorescence (Fig. 2D). Because a hepatocyte origin was recently proposed for hepatic progenitors,34,35 we analyzed αvβ6 expression by immunofluorescence in primary murine hepatocytes, which were αvβ6-negative for up to 7 days in culture (not shown).
Fig. 2.
Integrin αvβ6 is expressed by an actively proliferating subset of hepatic progenitor (oval) cells in Mdr2−/− mice. Double staining for αvβ6 integrin and hepatic progenitor (oval) cell markers A6 (A) and Trop2 (B). Original magnification ×100. (C) Nuclei within αvβ6-positive pseudoducts are frequently positive for the cell proliferation marker proliferating cell nuclear antigen (arrows), indicating that the αvβ6+ subset of progenitor cells actively proliferates (double-immunofluorescence for αvβ6 [red] and proliferating cell nuclear antigen [green]). Original magnification ×630. (D) Cell colonies derived from EpCAM+ progenitor (oval) cells express αvβ6 integrin and up-regulate both cholangiocyte (HNF1β, CK19) and hepatocyte (HNF4α, albumin) lineage differentiation markers in vitro. Primary EpCAM+ cells were isolated from Mdr2−/− mice and cultured for up to 14 days, as described in Materials and Methods. Original magnification ×200. Data are expressed as mean ± standard error of the mean relative to day 0 (from four individual mice). *P < 0.05. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; K19, cytokeratin 19; PCNA, proliferating cell nuclear antigen.
Isolated αvβ6+ Cells Express Progenitor (Oval) Cell Markers and Readily Differentiate Into Cholangiocytes and Hepatocytes in vitro
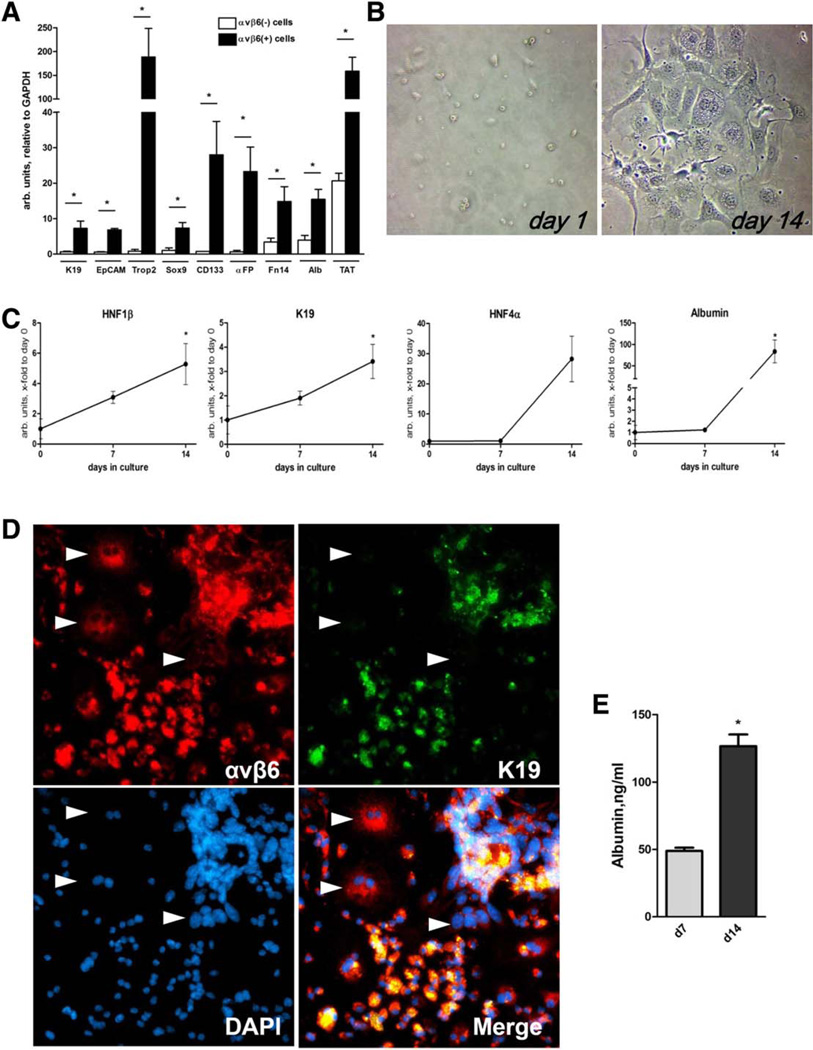
To further test whether αvβ6 integrin is expressed on bona fide progenitor (oval) cells, we isolated and characterized αvβ6+ cells from crude nonparenchymal liver cells of Mdr2−/− mice using positive selection with magnetic beads coupled to anti-αvβ6 antibody (magnetic cell sorting). Consistent with coexpression of αvβ6 and Trop2 in situ (Fig. 2B), RNA from freshly isolated αvβ6+ cells was highly enriched in Trop2 mRNA (>200-fold) and other hepatic progenitor (oval) cell markers (CD133, EpCAM, α-fetoprotein, Sox9, Fn14)36,37 and, to a lesser degree, cholangiocyte-specific (CK19, EpCAM) and hepatocyte-specific (albumin, TAT) mRNA (three-fold to 10-fold over the remaining αvβ6− nonparenchymal cell fraction) (Fig. 3A). When cultured in appropriate conditions in an oval cell colony formation assay,29 αvβ6+ cells readily formed multiple cell colonies, which became apparent from day 7. On day 14, large colonies consisted of cells having typical morphological features of either ductal cells (spindle-like shape) or hepatocytes (large, often diploid nuclei) (Fig. 3B). RT-PCR analysis of colonies revealed an up-regulation of differentiation markers of both cholangiocyte (HNF1β, CK19) and hepatocyte (HNF4α, albumin) lineages between day 7 and day 14, in a similar fashion to that observed in the EpCAM+ oval cell differentiation assay (Fig. 2D). At day 14, about 60%-70% of cells in colonies derived from αvβ6+ cells expressed biliary marker CK19. All cells in the colonies maintained expression of αvβ6, including CK19-negative cells with large and often diploid nuclei, morphologically resembling hepatocytes (Fig. 3D). Albumin secretion was readily detected in αvβ6+ cell culture supernatants from day 7 and increased 2.5-fold by day 14, suggesting differentiation of αvβ6+ cells into functional hepatocytes in vitro (Fig. 3E). Cells from αvβ6+-derived colonies maintained high proliferative capacity upon multiple passages up to 5 weeks in culture (not shown).
Fig. 3.
Isolated αvβ6+ cells express progenitor (oval) cell markers and differentiate into cholangiocytes and functional hepatocytes in vitro. (A) Freshly isolated αvβ6+ liver cells coexpress hepatocyte, cholangiocyte, and progenitor (oval) cell markers. αvβ6+ cells were purified from a nonparenchymal liver cell fraction of Mdr2−/− mice using magnetic cell sorting and analyzed in comparison with the remaining (αvβ6) cell fraction. αvβ6+ cells (closed bars) overexpress hepatic progenitor markers (Trop2, CD133, EpCAM, α-fetoprotein (Afp), Sox9, Fn14), as well as cholangiocyte (CK19, EpCAM) and hepatocyte (albumin and TAT) markers. Data are expressed as mean ± standard error of the mean. *P < 0.05. (B) Primary αvβ6+ cells form cell colonies and differentiate into both cholangiocyte-like and hepatocyte-like cells in vitro. Representative phase-contrast images from day 1 and 14 in vitro. Original magnification ×400. (C) Up-regulation of cholangiocyte-specific and hepatocyte-specific markers in cultured αvβ6+ cells. Total RNA was extracted at days 0, 7, and 14 and analyzed for cholangiocyte (CK19, HNF1β) and hepatocyte (albumin, HNF4α) markers using RT-PCR. Data are expressed as mean ± standard error of the mean, fold to day 0 (from four individual mice). *P < 0.05. (D) Double-immunofluorescence for αvβ6 integrin and CK19 demonstrate emergence of two distinct cell lineages in αvβ6+ cell colony formation assay. All cells were αvβ6-positive (red), and the majority coexpressed the cholangiocyte marker CK19 (green). CK19-negative cells represented a significant proportion of cells within colonies and demonstrate hepatocyte-like morphology with large and often diploid nuclei (arrows). Representative images of a single large colony derived from αvβ6+ cells after 14 days in culture (original magnification ×200). (E) Albumin levels in cell colony supernatants collected at days 7 and 14 (mouse albumin enzyme-linked immunosorbent assay). Abbreviations: αFP, α-fetoprotein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; K19, cytokeratin 19.
Integrin αvβ6 Expression Is Required for Colony Formation by Isolated EpCAM+ Progenitor (Oval) Cells in a TGFβ-Dependent Mechanism
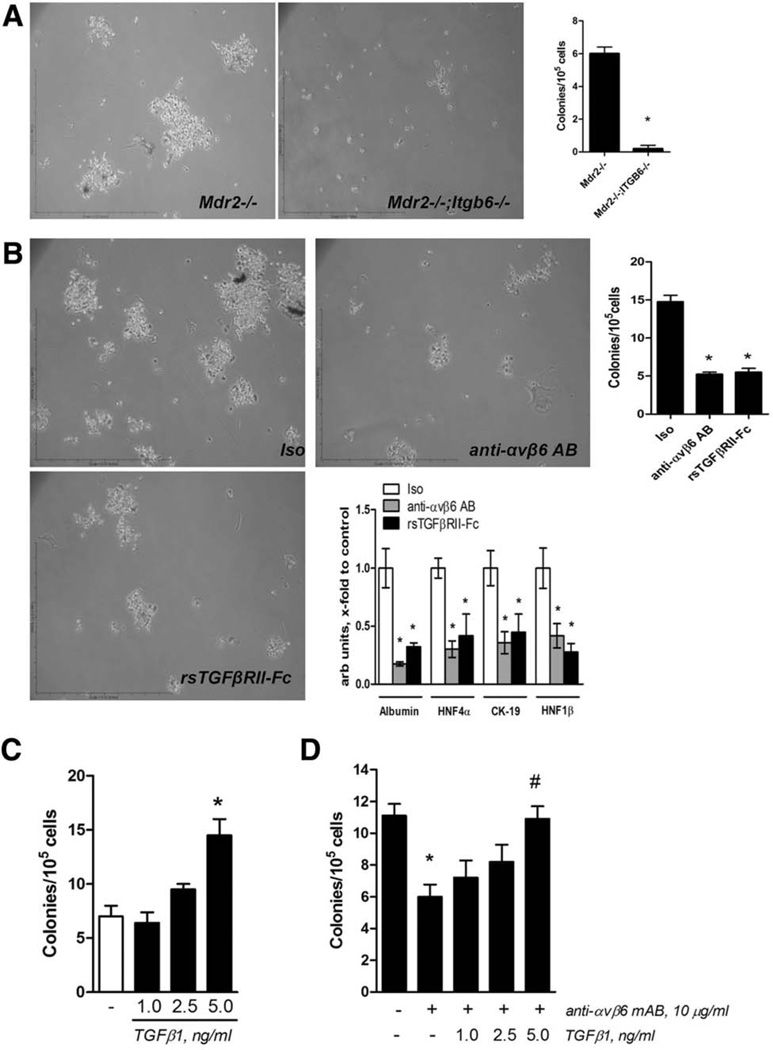
Having established αvβ6 expression by hepatic (bipotent) progenitor cells, we sought to clarify whether αvβ6 has a functional role in progenitor activation and differentiation using genetic ablation and pharmacologic inhibition approaches. As expected, EpCAM+ progenitors isolated from integrin-sufficient Mdr2−/− livers formed cell colonies from day 7 and differentiated into biliary and hepatocytic lineages in vitro (Fig. 2D). However, EpCAM+ cells from Mdr2−/− mice with αvβ6 deficiency (Mdr2−/−;Itgb6−/− double knockouts) failed to form cell colonies when cultured in identical conditions up to 14 days (Fig. 4A). Culturing integrin-sufficient EpCAM+ progenitors from Mdr2−/− mice in the presence of the function-blocking αvβ6-specific antibody 3G9 markedly reduced their ability to generate cell colonies (by 66.7% compared to isotype-treated EpCAM+ cells, P < 0.001; Fig. 4B). Interestingly, inhibition of bioactive TGFβ using rsTGFβRII-Fc blocked colony formation and differentiation markers of both biliary and hepatocytic lineages to the same extent as αvβ6 neutralization, suggesting a critical role of paracrine, αvβ6 integrin-dependent TGFβ activation in the regulation of progenitor function (Fig. 4B). Indeed, stimulation of EpCAM+ cells with exogenous TGFβ1 promoted colony formation in a dose-dependent fashion (Fig. 4C). Furthermore, the colony-forming capacity of EpCAM+ cells, suppressed in the presence of integrin αvβ6 neutralizing antibody, could be rescued completely by addition of bioactive TGFβ1 (Fig. 4D).
Fig. 4.
Integrin αvβ6 is functionally required for in vitro colony formation in isolated EpCAM+ progenitor (oval) cells through a TGFβ-dependent mechanism. (A) EpCAM+ progenitor (oval) cells isolated from integrin αvβ6-deficient mice (Mdr2−/−;Itgb6−/−) fail to form cell colonies in vitro. Representative low-magnification (×100) phase-contrast images shown. Colonies (>50 cells) derived from 1 × 105 EpCAM+ cells were counted at day 14 (n = 5 mice of each genotype). *P < 0.05. (B) Pharmacological inhibition of αvβ6 and TGFβ dramatically reduces the colony-forming ability of EpCAM+ progenitor (oval) cells (original magnification ×100) and inhibits both cholangiocyte-specific (CK19, HNF1β) and hepatocyte-specific (HNF4α, albumin) transcripts (C). (D) Exogenous bioactive TGFβ1 dose-dependently stimulates colony formation by EpCAM cells. (E) Inhibitory effect of αvβ6 integrin-neutralizing antibody on colony formation is rescued by addition of bioactive TGFβ1. EpCAM+ cells from integrin-sufficient Mdr2−/− mice were cultured for 14 days in the presence of TGFβ1 (1–5 ng/mL), isotype control antibody E15, anti-αvβ6 antibody 3G9, or recombinant soluble TGFβ receptor II (rsTGFβR-Fc, all at 10 µg/mL). Data are mean ± standard error of the mean (n = 4 mice). *P < 0.05. Abbreviations: AB, antibody; Iso, isotype.
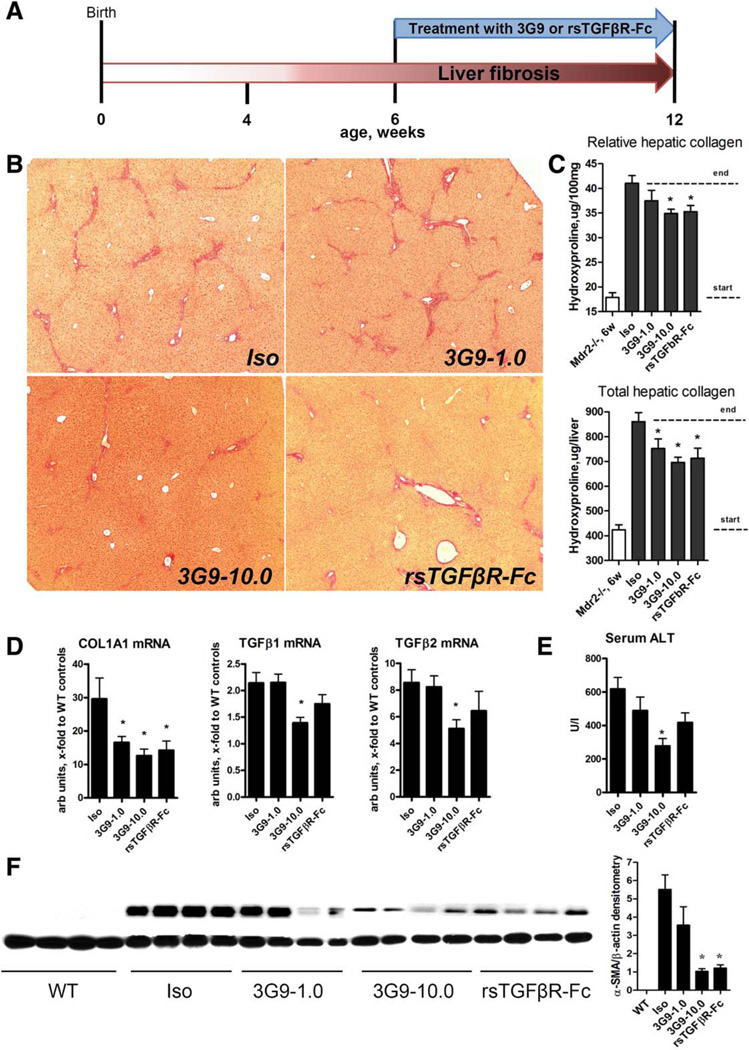
Selective Integrin αvβ6 Targeting Using the Function-Blocking 3G9 Antibody Inhibits Biliary Fibrosis in Mdr2−/− Mice
Next we evaluated the antifibrotic efficacy of 3G9, an anti-αvβ6 mAb, in comparison with systemic TGFβ inhibition (rsTGFβRII-Fc). The 3G9 mAb (1 and 10 mg/kg), rsTGFβRII-Fc (5 mg/kg), or isotype control immunoglobulin G (1E6, 10 mg/kg) was administered weekly in Mdr2−/− mice in a delayed treatment protocol (starting from 6 weeks of age, when significant fibrosis is already established) (Fig. 5A).20 While isotype control antibody-treated Mdr2−/− mice developed advanced bridging fibrosis, administration of 3G9 mAb resulted in a dose-dependent improvement in liver histology with notably diminished fibrosis and bridging, similar to the TGFβ inhibition group (Fig. 5B). This was accompanied by an up to 38% reduction in overall hepatic collagen deposition during treatment as determined by hydroxyproline in the high 3G9 dose-treated group (Fig. 5C), and >50% inhibition of profibrogenic COL1A1 and TGFβ1/2 mRNA expression (Fig. 5D). There was a clear trend toward lower serum alanine aminotransferase levels in 3G9-treated and rsTGFβRII-Fc-treated mice, which reached statistical significance in the high-dose 3G9 group (Fig. 5E). Notably, α-smooth muscle actin protein, a hepatic stellate cell activation marker, was dose-dependently suppressed in 3G9-treated and rsTGFβRII-Fc-treated livers at the protein level by up to 80% (Fig. 5F). No adverse side effects were observed as a result of treatment. The antifibrotic efficacy of high-dose 3G9 mAb was comparable to or even exceeded that of anti-TGFβ therapy with rsTGFβRII-Fc (37.7% and 33.8% reduction of collagen deposition, respectively) (Fig. 4C). Importantly, anti-αvβ6 therapy was most effective when applied in advanced compared to early disease stages (37.7% versus 19.7% collagen reduction, respectively; Supporting Fig. S3), in agreement with the dynamics of increasing numbers of αvβ6+ cells in advanced fibrosis (Fig. 1A).
Fig. 5.
Selective inhibition of integrin αvβ6 using function-blocking 3G9 antibody significantly reduces fibrosis in Mdr2−/− mice. (A) Scheme of experimental design. Integrin αvβ6-blocking antibody 3G9 mAb (1 and 10 mg/kg), 1E5 isotype control antibody (10 mg/kg), and rsTGFβRII-Fc protein (5 mg/kg) were administered six times (intraperitoneally once a week) starting from 6 weeks of age in Mdr2−/− mice with preestab-lished biliary fibrosis. (B) Representative low-magnification images of connective tissue (sirius red) staining (original magnification ×50). (C) Relative (per 100 mg of liver) and total (per whole liver) hepatic collagen levels (biochemically through hydroxyproline). Dotted lines indicate collagen levels at the start (Mdr2−/−, 6 weeks) and the end of treatment. (D) Hepatic transcript levels of COL1A1 and TGFβ1/2 as determined by quantitative RT-PCR in total liver RNA (relative to β-microglobulin). (E) Serum alanine aminotransferase levels and (F) α-smooth muscle actin immunoblotting with densitometric analysis in Mdr2−/− mice with αvβ6 and TGFβ inhibition. β-actin was used as a loading control. Data are expressed as means ± standard error of the mean (n = 6–8), *P ≤ 0.05 compared to isotype control group (analysis of variance with Dunnett’s posttest). Abbreviations: ALT, alanine aminotransferase; Iso, isotype; α-SMA, α-smooth muscle actin; WT, wild type.
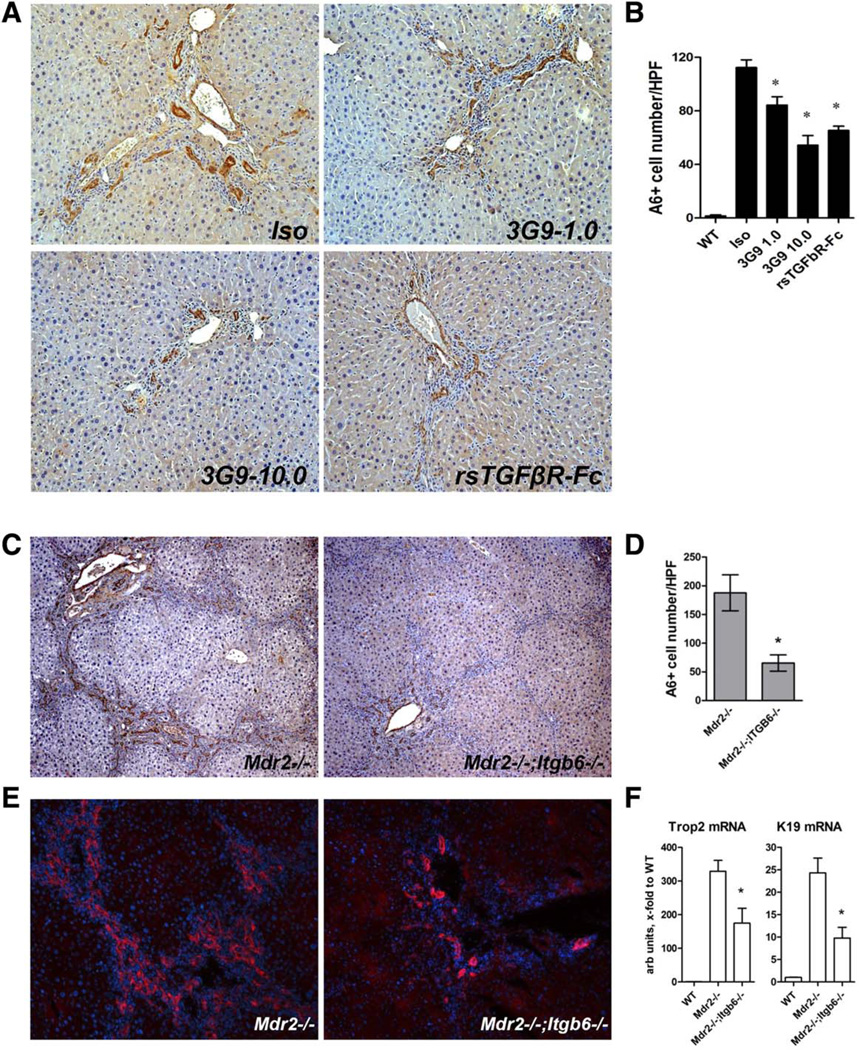
In Vivo Progenitor Responses in Mouse Models of Biliary Fibrosis Are Markedly Attenuated by Genetic or Pharmacological Inactivation of Integrin αvβ6
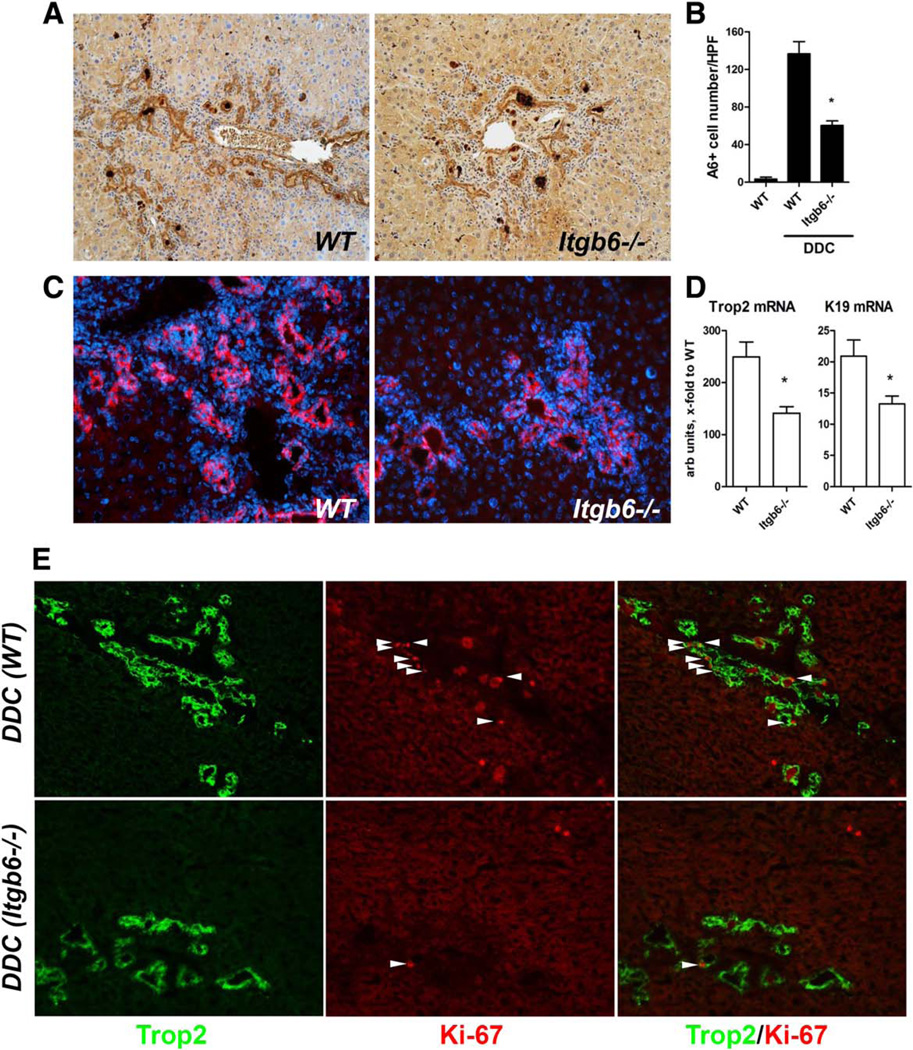
In order to validate the functional requirement of αvβ6 integrin for progenitor (oval) cell proliferation in vivo, we studied whether progenitor responses are altered by αvβ6 inhibition or deficiency in two models of biliary fibrosis. In the Mdr2−/− model, delayed pharmacological αvβ6 inhibition with 3G9 antibody (from 6 weeks of age for 6 weeks; Fig. 5A) resulted in significant, dose-dependent inhibitory effects on progenitor expansion, with up to 52% reduction in A6-positive cell counts compared to the isotype control antibody-treated group (Fig. 6A,B). Similar results were obtained using the alternative progenitor marker pan-cytokeratin (not shown). Systemic TGFβ inhibition with rsTGFβRII-Fc had an effect on the oval cell compartment comparable to 3G9 treatment at a high dose (Fig. 6A,B). A similar phenotype was observed due to genetic inactivation of integrin αvβ6 in the Mdr2−/− model, where Mdr2−/−; Itgb6−/− double mutants had A6-positive cell counts reduced by 65% compared to integrin-sufficient Mdr2−/− controls (Fig. 6C,D). These results were confirmed with the alternative progenitor marker Trop2 (Fig. 6E). Furthermore, progenitor (Trop2) and ductular/biliary (CK19) markers were reduced two-fold at the transcriptional level in livers of Mdr2−/−;Itgb6−/− mice (Fig. 6F). In order to validate these findings in a second, mechanistically different model, we fed Itgb6-null and wild-type control mice a DDC-supplemented diet to induce oval cell expansion38 and sclerosing cholangitis.21 DDC feeding resulted in a progressive increase in αvβ6 expression in wild-type mice, which paralleled fibrosis progression, similar to the Mdr2−/− model (Supporting Fig. S4). Progenitor cell expansion was markedly attenuated in DDC-fed mice lacking functional αvβ6 as assessed by A6 immunohistochemistry, with 67% reduction in A6-positive cells compared to wild-type controls (Fig. 7A,B). Similar results were obtained with the progenitor cell marker Trop2 (Fig. 7C), p-CK (not shown), and hepatic transcript levels of Trop2 and CK19, which were suppressed in Itgb6−/− mice to an extent comparable to the Mdr2−/− model (Fig. 6). Attenuated ductular reaction was apparently due to reduced progenitor replication in the absence of αvβ6 integrin because Trop2+ progenitors in DDC-fed Itgb6−/− mice (Fig. 7E) and Mdr2−/−;Itgb6−/− mice (Supporting Fig. S5) coexpressed proliferating nuclear antigen Ki-67 less frequently. Mdr2−/−;Itgb6−/− mice and DDC-fed Itgb6−/− mice were also significantly protected from biliary fibrosis (Supporting Figs. S4 and S6).
Fig. 6.
Progenitor cell response is markedly attenuated in mice with pharmacological or genetic inactivation of integrin αvβ6 in the Mdr2−/− model of biliary fibrosis. Representative images of A6 immunostaining (A, ×200 original magnification) and morphometric quantification of A6+ progenitor cell numbers (B) following 6 weeks of treatment of Mdr2−/− mice with isotype control antibody, anti-αvβ6 mAb (3G9), and TGFβ inhibition with rsTGFβRII-Fc (see Fig. 5A for experimental design). Data are means ± standard error of the mean (n = 4). *P ≤ 0.05 compared to isotype control group (analysis of variance with Dunnett’s posttest). Immunostaining for A6 (C) and Trop2 (E) demonstrates robust progenitor expansion in Mdr2−/− mice, which is markedly attenuated in Mdr2−/−;Itgb6−/− mice (original magnification ×200). (D) A6+ cell counts as quantified in 10 random high-power fields (n = 4). (F) Hepatic transcript levels of Trop2 and CK19 as determined by quantitative RT-PCR in total liver RNA (relative to β2-microglobulin, n = 6–8). Data are means ± standard error of the mean. *P ≤ 0.05 compared to Mdr2−/− controls (t test). Abbreviations: HPF, high-power field; Iso, isotype; K19, cytokeratin 19; WT, wild type.
Fig. 7.
Ductular reaction and progenitor cell proliferation are suppressed in integrin αvβ6-deficient mice in the DDC-induced biliary fibrosis model. Expansion of hepatic progenitors is notably reduced in integrin αvβ6-deficient mice (C57Bl/6.Itgb6−/−) compared to C57Bl/6 (wild-type) controls in DDC-induced biliary fibrosis. Representative images of A6 (A) and Trop2 (C) immunostaining, and (B) quantification of A6+ cell numbers following 3 weeks of DDC feeding (n = 4). (D) Hepatic transcript levels of Trop2 and CK19 as determined by quantitative RT-PCR in total liver RNA (relative to β2-microglobulin, n = 6–8). (E) Double-immunofluorescence for cell proliferation marker Ki-67 (red, arrows) and progenitor marker Trop2 (green) suggests diminished progenitor cell replication (arrows indicate double-positive cells) in DDC-fed Itgb6−/− mice (lower panel) compared to wild-type mice (upper panel). Representative images shown (original magnification ×200). Data are means ± standard error of the mean. *P ≤ 0.05 compared to DDC-fed wild-type controls (t test). Abbreviations: HPF, high-power field; WT, wild type.
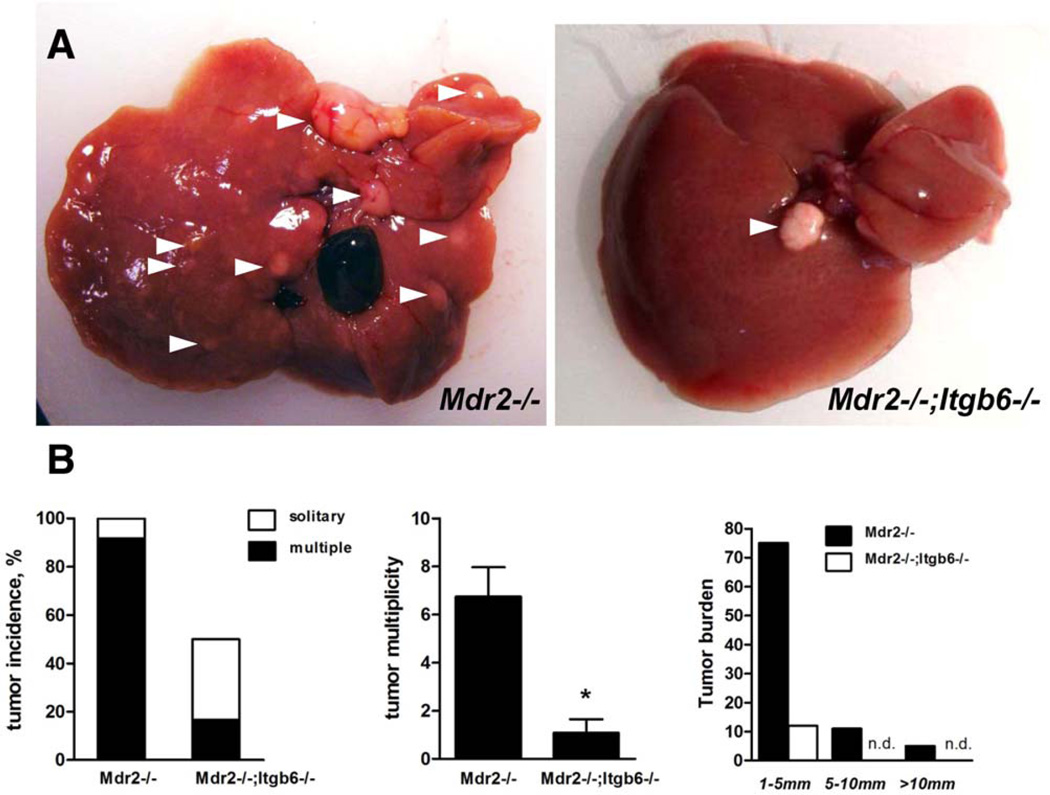
Integrin αvβ6 Deficiency Protects From Cirrhosis-Associated Primary Liver Cancer in the Mdr2−/− Model
Progenitor cell origins of liver cancer, at least for a subset of tumors, have been suggested for both hepatocellular carcinoma and cholangiocarcinoma based on histopathological observations6,39 and global gene expression analysis.7 Mdr2−/− mice develop cirrhosis and multiple primary liver cancers (hepatocellular carcinoma) with 100% penetrance, beginning at 10 months of age.32 To assess the effect of impaired progenitor responses in the absence of αvβ6 on the development of liver cancer, we evaluated tumor incidence and burden in aged Mdr2−/−;Itgb6−/− and Mdr2−/− mice (12 mice of each genotype). At the age of 12 months, macroscopic examination revealed that all Mdr2−/− mice developed liver cancers, with a median number of seven tumors per liver, with only one animal out of 12 having a solitary neoplasm (Fig. 8A,B). In contrast, six out of 12 age-matched Mdr2−/−;Itgb6−/− mice were tumor-free, four had a solitary liver tumor, and only 2 developed multiple neoplasms. Overall, the tumor burden was dramatically reduced in Mdr2−/−;Itgb6−/− mice, in terms of both tumor numbers and size (Fig. 8B,C). Aged Mdr2−/−;Itgb6−/− mice were also partially protected from liver scarring (Supporting Fig. S6). Thus, αvβ6 integrin deficiency confers significant protection both from fibrosis and from cirrhosis-associated liver cancer in Mdr2−/− mice.
Fig. 8.
Loss of αvβ6 integrin in aged Mdr2−/−;Itgb6−/− mice confers protection from cirrhosis-associated primary liver cancer. (A) Representative macroscopic findings in livers of aged Mdr2−/− and Mdr2−/−;Itgb6−/− mice. Arrowheads indicate visible tumors >1 mm in size. (B) Tumor incidence (left panel), tumor muliplicity (middle panel), and overall tumor burden (right panel) analyzed in a total of 12 mice of each genotype at age 12 months. Data are expressed as means ± standard error of the mean. *P < 0.05 compared to Mdr2−/− mice (two-way analysis of variance followed by Bonferroni’s posttest). Abbreviation: n.d., not detected.
Discussion
Our group and others have reported that up-regulation of integrin αvβ6 on reactive cholangiocytes is linked to liver fibrosis progression in humans and rodents.14,16,17,19 However, the mechanism by which αvβ6 promotes biliary fibrosis has not been firmly established. Here, we report that αvβ6 integrin expression marks proliferating adult progenitor (oval) cells and critically regulates their function in the injured liver through a TGFβ-dependent mechanism. Purified primary αvβ6+ liver cells demonstrate clonogenic potential and bipotency in vitro, and αvβ6 expression is required for colony formation of freshly isolated hepatic progenitors in vitro. Furthermore, genetic or pharmacologic inactivation of integrin αvβ6 potently suppresses ductular reaction in vivo and subsequently inhibits biliary fibrosis progression and tumorigenesis.
The “ductular reaction” is defined as the proliferation of small duct-like structures in response to liver injury3 and, besides being a classical feature in biliary diseases such as PSC, is found almost universally in chronic liver disease associated with fibrosis. It represents a heterogenic cell population, which includes proliferation of cells in preexisting ductules, activated bipotent progenitor cells and their progeny, reactive cholangiocytes, and intermediate hepatocytes.40 Clinically, progression of liver fibrosis to cirrhosis strongly correlates with ductular reaction, including nonbiliary chronic liver diseases such as infection with hepatitis B or C virus,41 alcoholic steatohepatitis, and nonalcoholic steatohehatitis.42 In earlier studies, our team14 and others18 noted that in a subset of human cirrhosis intermediate hepatocyte-like cells at the septa-parenchyma interface were strongly immunoreactive for αvβ6 integrin. This intriguing observation suggested a progenitor origin of these cells because it was extensively demonstrated in several species that in normal liver, including early developmental stages, hepatocytes do not express αvβ6.10,11 Our current findings reconcile these early data by providing direct evidence that purified αvβ6-expressing liver cells function as adult progenitors, capable of clonal growth and differentiation into (αvβ6+) cholangiocytes and hepatocytes in vitro (Fig. 3). Furthermore, αvβ6 expression appears to be essential for progenitor function because primary EpCAM+ cells isolated from integrin-deficient mice failed to form colonies in vitro and the colony-forming ability of integrin-sufficient EpCAM+ cells was markedly diminished in the presence of αvβ6-blocking antibody (Fig. 4). Proliferation of certain epithelial cells directly depends on αvβ6 integrin, with a specific sequence identified as critical within the β6 subunit cytoplasmic domain.43 In hepatic progenitor cells, several lines of evidence in our study suggest that the mechanism by which αvβ6 regulates progenitor proliferation is TGFβ-dependent. TGFβ blocking inhibited colony formation/differentiation by EpCAM+ cells to the same extent as αvβ6 blocking (Fig. 4B,C). Moreover, we found that TGFβ itself stimulates clonogenic growth in vitro in a dose-dependent manner (Fig. 4D) and that the inhibitory effects of αvβ6 neutralization on progenitors can be rescued by addition of exogenous bioactive TGFβ (Fig. 4E). The critical role of integrin αvβ6-activated TGFβ on progenitors has not been described to date and appears particularly relevant in the context of previous data showing that TGFβ exerts an opposing, inhibitory effect on hepatocyte replication,44 while it has been postulated that TGFβ promotes the stem cell transition to a progenitor cell phenotype.45 Our data in two mouse models of sclerosing cholangitis confirm that genetic or pharmacological inactivation of αvβ6 or TGFβ inhibition markedly inhibits progenitor expansion in vivo (Figs. 6 and 7; Supporting Fig. S3).
The role and even the existence of bipotent adult hepatic progenitors (called oval cells in rodents), capable of differentiating into both cholangiocytes and hepatocytes, in replenishing dying liver cells is hotly debated. An alternative theory suggests that mature hepatocytes in chronically injured liver can undergo transformation into progenitor-like cells that, in turn, can differentiate into hepatocytes and cholangiocytes.46 Recent fatetracing studies yielded conflicting data, possibly owing to inherent pitfalls of current cell-tracing techniques,47 supporting both progenitor (oval) cell48,49 and hepato-cyte34,35 origins of hepatic epithelial renewal in chronically injured liver. The design of our study, while clearly demonstrating αvβ6 expression on bipotent hepatic progenitors and its requirement for their progenitor function, does not allow us to unequivocally assign the exact origin of αvβ6+ progenitor cells. However, our overall data and previous reports on αvβ6 are most consistent with the concept of oval cell origin, in line with the lack of αvβ6 expression in hepatocytes in fibrotic mice and during prolonged primary hepatocyte culture in vitro. Regardless of the cellular origin of αvβ6+ cells, our data strongly suggest that progenitor cell activation and ductular reaction can be effectively suppressed by αvβ6 inhibition (Figs. 6 and 7). While we demonstrate the requirement of αvβ6 integrin for colony formation/ growth by EpCAM1 hepatic progenitors in vitro, future studies will need to address its possible role in regulating their lineage commitment and differentiation.
The strong association of progenitor activation and ductular reaction with liver fibrosis progression led to the hypothesis that progenitor activation drives fibrogenic response in advanced disease. In this vein, in the fibrotic liver, progenitor cell-mediated regeneration after partial hepatectomy elicits a severe fibrogenic response, which can be blocked by specifically targeting progenitor cell proliferation with anti-TWEAK antibody.31 Thus, we examined the relationship between deficient progenitor cell activation due to αvβ6 inactivation and liver fibrosis. In Mdr2−/− mice, αvβ6+ cells gradually accumulated in periportal areas during disease progression between 4 and 12 weeks of age and correlated with degree and activity of fibrosis (Fig. 1). A similar staining pattern was observed in human PSC and PBC liver explants, where proliferating “reactive” ducts were uniformly positive for αvβ6 integrin (Fig. 1C). The functional role of integrin αvβ6 in regulating experimental biliary fibrosis is presumed to be mediated by TGFβ activation.14,16,17 However, TGFβ can be activated in vivo through multiple pathways, and the relative contribution of local, biliary epithelial surface-restricted αvβ6-dependent TGFβ activation in biliary fibrosis is unknown. Thus, we evaluated the antifibrotic efficacy of a function-blocking anti-αvβ6 mAb, in direct comparison to systemic TGFβ1 inhibition using recombinant soluble TGFβ receptor II fusion protein (rsTGFβRII-Fc) on preestablished fibrosis progression in the Mdr2−/− model. Treatment with 3G9 mAb resulted in a dose-dependent improvement in liver histology with diminished fibrosis, an up to 40% reduction in collagen deposition, and a >50% inhibition of COL1A1, TGFβ1&2, and α-smooth muscle actin mRNA expression, the major profibrogenic determinants in this model (Fig. 5).20 Importantly, anti-αvβ6 therapy was as effective in suppressing fibrosis as anti-TGFβ therapy (37.7% and 33.8% reduction in collagen deposition, respectively), suggesting that in advanced stages αvβ6-mediated TGFβ activation accounts for most, if not all, of the TGFβ-driven fibrogenesis in the Mdr2−/− model. Interestingly, while systemic TGFβ blocking was equally effective in early and advanced stages of disease in Mdr2−/− mice, targeting αvβ6 was far more effective at suppressing fibrosis in advanced stages (delayed therapy protocol; Fig. 5) than in early stages (Supporting Fig. S3). This can plausibly be explained by (1) an increase in abundance of αvβ6+ cells (Fig. 1AB) and (2) a predominance of integrin-mediated TGFβ activation in advanced (but not early) fibrosis. This unique feature is particularly important because while liver fibrosis is diagnosed at advanced stages in most patients, many experimental antifibrotic therapies (e.g., imatinib50) achieve greater efficacy in prevention studies than in delayed treatment.5 The critical role of αvβ6 in driving biliary liver fibrosis was further validated in genetic deletion experiments, where Itgb6 loss protected from biliary fibrosis in two mechanistically different models of biliary fibrosis, Mdr2−/− and DDC feeding (Supporting Figs. S4 and S6). Overall, our study suggests that diminished biliary fibrosis upon αvβ6 integrin inhibition is likely a consequence of a suppressed (αvβ6+) hepatic progenitor cell response (ductular reaction).
Liver cirrhosis is a major risk factor for development of primary liver cancer, and the two conditions share common cellular and signaling pathways. We explored whether the impaired progenitor activation phenotype in Itgb6−/− mice may affect tumorigenesis in the Mdr2−/− model in long-term studies. Although Mdr2−/− mice develop hepatocellular carcinoma32 and not cholangiocarcinoma (as in human PSC), both tumors have been linked to hepatic progenitor cell activation based on clinicopathological evidence6 and global gene expression pro-file.7 Compared to aged Mdr2−/− mice, which all developed liver tumors, Mdr2−/−;Itgb6−/− mice were significantly protected from development of liver cancer, with half of the Mdr2−/−;Itgb6−/− mice remaining tumor-free at 12 months of age (Fig. 8). Tumors in Mdr2−/−;Itgb6−/− mice were often solitary and smaller in size, with the overall tumor burden reduced by about 90% (Fig. 8). To our knowledge, this is the first report directly demonstrating the important role of αvβ6 in promoting hepatic tumorigenesis. It is tempting to speculate, given the putative stem/progenitor cell origin of hepatocellular carcinoma, that protection from liver cancer in the absence of integrin αvβ6 in Mdr2−/− model is due to suppressed progenitor cell activation in Itgb6−/− mice. However, indirect mechanisms, such as inhibition of integrin αvβ6-mediated TGFβ1 activation on precancerous epithelia or a secondary effect due to attenuated fibrosis in Itgb6−/− mice, cannot be ruled out. Further mechanistic studies, including lineage tracing of αvβ6-expressing cells, will be necessary to elucidate the precise cellular and molecular mechanisms behind the role of αvβ6 in promoting liver cancer.
In conclusion, we demonstrate that integrin αvβ6 is expressed on hepatic progenitor cells and critically regulates their function in vivo and in vitro. Genetic disruption or selective pharmacologic antibody targeting of αvβ6 potently inhibits progenitor cell responses in mouse models of chronic biliary injury and provides protection from liver fibrosis and tumorigenesis, two conditions clinically associated with exacerbated ductular reaction.
Supplementary Material
Acknowledgments
We are grateful to Nelly Polyak (Harvard Medical School) for the protocols and expert advice on αvβ6(+) cell isolation, and Helmut Friess (Technical University of Munich) for access to explant tissue bank samples.
Supported by research grants from Biogen, Inc., and Stomedix, Inc.; an institutional grant from Department of Medicine, Beth Israel Deaconess Medical Center (to Y.P.); a career development award from The First Affiliated Hospital of Sun Yat-sen University (to Z.-W.P.); and grants from the National Natural Science Foundation of China (81301842) and the Pearl River S&T Nova Program (2014J2200087).
He holds intellectual property rights and received grants from Biogen. He advises Genentech. Dr. Schuppan consults and received grants from Boehringer-Ingelheim. He consults for MSD, Mitsubishi-Tanabe, and Takeda. Dr. Popov received grants from Biogen and Stromedix. Dr. Violette is employed by and owns stock in Biogen. Dr. Weinreb is employed by and owns stock in Biogen.
Abbreviations
- CK19
cytokeratin 19
- COL1A1
collagen type 1 α1
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- EpCAM
epithelial cell adhesion molecule
- Itgb6
integrin beta 6
- mAb
monoclonal antibody
- Mdr2
multidrug resistance protein 2
- mRNA
messenger RNA
- PBC
primary biliary cirrhosis
- PSC
primary sclerosing cholangitis
- RT-PCR
reverse-transcription polymerase chain reaction
- TGFβ
transforming growth factor β
Footnotes
Potential conflict of interest: Dr. Sheppard consults and owns stock in Pliant Therapeutics.
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.28274/suppinfo.
References
- 1.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2009;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholan-giocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 3.Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol. 1998;15:259–269. [PubMed] [Google Scholar]
- 4.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- 6.Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 8.Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, et al. TWEAK induces liver progenitor cell proliferation. J Clin Invest. 2005;115:2330–2340. doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108(Pt. 6):2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 11.Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem. 1993;41:1521–1527. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- 12.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, et al. Latent TGF-beta structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alphavbeta6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 14.Popov Y, Patsenker E, Stickel F, Zaks J, Bhaskar KR, Niedobitek G, et al. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J Hepatol. 2008;48:453–464. doi: 10.1016/j.jhep.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Patsenker E, Wilkens L, Banz V, Osterreicher CH, Weimann R, Eisele S, et al. The alphavbeta6 integrin is a highly specific immunohisto-chemical marker for cholangiocarcinoma. J Hepatol. 2010;52:362–369. doi: 10.1016/j.jhep.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology. 2008;135:660–670. doi: 10.1053/j.gastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, et al. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404–1412. doi: 10.1002/hep.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sipos B, Hahn D, Carceller A, Piulats J, Hedderich J, Kalthoff H, et al. Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro . Histopathology. 2004;45:226–236. doi: 10.1111/j.1365-2559.2004.01919.x. [DOI] [PubMed] [Google Scholar]
- 19.Pi L, Robinson PM, Jorgensen M, Oh SH, Brown AR, Weinreb PH, et al. Connective tissue growth factor and integrin alphavbeta6: a new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology. 2015;61:678–691. doi: 10.1002/hep.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)2/2 mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Fickert P, Stoger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwong GA, von Maltzahn G, Murugappan G, Abudayyeh O, Mo S, Papayannopoulos IA, et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol. 2013;31:63–70. doi: 10.1038/nbt.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, et al. Inactivation of the integrin beta6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, et al. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 25.Van Nieuwkerk CM, Elferink RP, Groen AK, Ottenhoff R, Tytgat GN, Dingemans KP, et al. Effects of ursodeoxycholate and cholate feeding on liver disease in FVB mice with a disrupted mdr2 P-glycoprotein gene. Gastroenterology. 1996;111:165–171. doi: 10.1053/gast.1996.v111.pm8698195. [DOI] [PubMed] [Google Scholar]
- 26.Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, Dolinski BM, et al. Function-blocking integrin alphavbeta6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem. 2004;279:17875–17887. doi: 10.1074/jbc.M312103200. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida S, Ikenaga N, Liu SB, Peng ZW, Chung J, Sverdlov DY, et al. Extrahepatic platelet-derived growth factor-beta, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology. 2014;147:1378–1392. doi: 10.1053/j.gastro.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, et al. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev. 2011;25:1193–1203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popov Y, Sverdlov DY, Sharma AK, Bhaskar KR, Li S, Freitag TL, et al. Tissue transglutaminase does not affect fibrotic matrix stability or regression of liver fibrosis in mice. Gastroenterology. 2011;140:1642–1652. doi: 10.1053/j.gastro.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuramitsu K, Sverdlov DY, Liu SB, Csizmadia E, Burkly L, Schuppan D, et al. Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol. 2013;183:182–194. doi: 10.1016/j.ajpath.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikenaga N, Liu SB, Sverdlov DY, Yoshida S, Nasser I, Ke Q, et al. A new mdr22/2 mouse model of sclerosing cholangitis with rapid fibrosis progression, early-onset portal hypertension, and liver cancer. Am J Pathol. 2015;185:325–334. doi: 10.1016/j.ajpath.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, et al. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–1960. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 34.Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, et al. The biliary tree—a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol. 2012;9:231–240. doi: 10.1038/nrgastro.2012.23. [DOI] [PubMed] [Google Scholar]
- 37.Itoh T, Miyajima A. Liver regeneration by stem/progenitor cells. Hepatology. 2014;59:1617–1626. doi: 10.1002/hep.26753. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100(Suppl. 1):11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma A possible progenitor cell origin. Histopathology. 2006;49:138–151. doi: 10.1111/j.1365-2559.2006.02468.x. [DOI] [PubMed] [Google Scholar]
- 40.Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 41.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–818. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 42.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, et al. Progressive fibrosis in nonalcoholic steato-hepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Dixit RB, Chen A, Chen J, Sheppard D. Identification of a sequence within the integrin beta6 subunit cytoplasmic domain that is required to support the specific effect of alphavbeta6 on proliferation in three-dimensional culture. J Biol Chem. 1996;271:25976–25980. doi: 10.1074/jbc.271.42.25976. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen LN, Furuya MH, Wolfraim LA, Nguyen AP, Holdren MS, Campbell JS, et al. Transforming growth factor-beta differentially regulates oval cell and hepatocyte proliferation. Hepatology. 2007;45:31–41. doi: 10.1002/hep.21466. [DOI] [PubMed] [Google Scholar]
- 45.Mishra L, Derynck R, Mishra B. Transforming growth factor-beta signaling in stem cells and cancer. Science. 2005;310:68–71. doi: 10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- 46.Michalopoulos GK. The liver is a peculiar organ when it comes to stem cells. Am J Pathol. 2014;184:1263–1267. doi: 10.1016/j.ajpath.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemaigre FP. Determining the fate of hepatic cells by lineage tracing: facts and pitfalls. Hepatology. 2015;61:2100–2103. doi: 10.1002/hep.27659. [DOI] [PubMed] [Google Scholar]
- 48.Shin S, Walton G, Aoki R, Brondell K, Schug J, Fox A, et al. Foxl1-Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev. 2011;25:1185–1192. doi: 10.1101/gad.2027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, et al. in vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neef M, Ledermann M, Saegesser H, Schneider V, Widmer N, Decosterd LA, et al. Oral imatinib treatment reduces early fibrogenesis but does not prevent progression in the long term. J Hepatol. 2006;44:167–175. doi: 10.1016/j.jhep.2005.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.