Summary
Cell therapy is a promising approach to generate an enteric nervous system (ENS) and treat enteric neuropathies. However, for translation to the clinic, it is highly likely that enteric neural progenitors will require manipulation prior to transplantation to enhance their ability to migrate and generate an ENS. In this study, we examine the effects of exposure to several factors on the ability of ENS progenitors, grown as enteric neurospheres, to migrate and generate an ENS. Exposure to glial-cell-line-derived neurotrophic factor (GDNF) resulted in a 14-fold increase in neurosphere volume and a 12-fold increase in cell number. Following co-culture with embryonic gut or transplantation into the colon of postnatal mice in vivo, cells derived from GDNF-treated neurospheres showed a 2-fold increase in the distance migrated compared with controls. Our data show that the ability of enteric neurospheres to generate an ENS can be enhanced by exposure to appropriate factors.
Keywords: enteric nervous system, enteric neural progenitors, enteric neurospheres, migration, transplantation, enteric neural crest
Highlights
-
•
Enteric neurospheres are likely to require manipulation for clinical applications
-
•
Exposure to GDNF increased the size and cell number in enteric neurospheres
-
•
GDNF-treated neurospheres showed enhanced migration after transplantation in vivo
-
•
Manipulation of enteric neurospheres can enhance the generation of enteric neurons
In this article, Stamp, McKeown, and colleagues examined the effects of several candidate factors on enteric neurosphere size and behavior following transplantation in vivo. They show that exposure of enteric neurospheres to GDNF enhances their ability to generate an enteric nervous system following transplantation.
Introduction
The enteric nervous system (ENS) is an extensive network of neurons within the bowel wall that arises from the neural crest. The ENS plays an essential role in regulating several gut functions including motility (Furness, 2012); consequently, congenital or acquired diseases of the ENS result in gastrointestinal motility disorders (Burns et al., 2016, Burns and Thapar, 2014, De Giorgio and Camilleri, 2004, De Giorgio et al., 2004, Knowles et al., 2010). Cell therapy has the potential to treat enteric neuropathies (Burns et al., 2016, Burns and Thapar, 2014, Cheng et al., 2015, Dettmann et al., 2014, Hotta et al., 2009, Kulkarni et al., 2012, Nishikawa et al., 2015, Pan et al., 2011, Rauch et al., 2006, Wilkinson et al., 2012).
Several different sources of donor cells have been investigated for generating an ENS in animal models (Burns et al., 2016). For example, recent studies showed that ENS progenitors can be derived from human pluripotent stem cells (PSCs) (Fattahi et al., 2016, Li et al., 2016), and when transplanted into a mouse model of Hirschsprung disease, a congenital enteric neuropathy, the progenitors colonized the entire colon and rescued mortality (Fattahi et al., 2016). ENS progenitors can be isolated from the bowel of infant and adult humans and laboratory animals (Almond et al., 2007, Becker et al., 2012, Bondurand et al., 2003, Hetz et al., 2014, Hotta et al., 2016b, Kruger et al., 2002, Lindley et al., 2008, Metzger et al., 2009b, Wilkinson et al., 2015), and following transplantation into the bowel of rodents they migrate and differentiate into different neurochemical types of neurons that are capable of firing action potentials (Hetz et al., 2014, Hotta et al., 2016a, Hotta et al., 2016b, Hotta et al., 2013). Patient-derived enteric neural progenitors harvested from healthy regions of the bowel are likely to be the safest source of enteric neurons for cell therapy. However, patient-derived enteric neural progenitors will probably require manipulation following isolation and prior to transplantation because (1) the distance that transplanted stem cells need to migrate in the human bowel to treat most enteric neuropathies is significantly greater than has been demonstrated in animal models, and (2) patient-derived cells may be defective in their ability to migrate and/or generate enteric neurons due to the causative genetic mutations that resulted in the disease (Hotta et al., 2009, Metzger et al., 2009b, Micci and Pasricha, 2007).
We examined the effects of exposure to several factors known to play roles in ENS development on the size of enteric neurospheres, and on the ability of cells derived from enteric neurospheres to migrate and generate an ENS when co-cultured with embryonic gut or following transplantation into the colon of postnatal mice in vivo. The factors examined were: (1) glial-cell-line-derived neurotrophic factor (GDNF), which is essential for the survival, proliferation, migration, and differentiation of enteric neural crest-derived cells (ENCCs), which give rise to the ENS during normal development (Laranjeira and Pachnis, 2009, Sasselli et al., 2012, Taraviras et al., 1999); (2) retinoic acid (RA), which promotes ENCC migration and the expression of the GDNF signaling receptor, RET, by ENCCs (Niederreither et al., 2003, Simkin et al., 2013, Wright-Jin et al., 2013); and (3) the 5-HT4 receptor agonist, RS67506, which promotes neurogenesis from endogenous (Belkind-Gerson et al., 2015, Liu et al., 2009) and transplanted (Hotta et al., 2016a) enteric neural progenitors. We show that growing enteric neurospheres in the presence of GDNF significantly increases neurosphere size and the distance migrated by neurosphere-derived cells when co-cultured with embryonic gut or when transplanted into the colon of postnatal mice in vivo.
Results
GDNF Increases the Size and Cell Number of Enteric Neurospheres
ENCCs from embryonic day 14.5 (E14.5) Ednrb-hKikGR mice were isolated by flow cytometry and cultured in 96-well low-attachment plates to allow them to form neurospheres (Hotta et al., 2013). For some experiments, the culture medium also contained GDNF (50 ng/mL) and/or RA (10 μM); previous studies have shown that these concentrations of GDNF and RA promote ENS development from ENCCs (Schriemer et al., 2016, Simkin et al., 2013, Taraviras et al., 1999). The mean volume of enteric neurospheres cultured in the presence of GDNF was significantly (14-fold) larger than in control neurospheres (Figure 1A). The volume of enteric neurospheres grown in the presence of RA was around 2-fold larger than that of controls, but this was not statistically significant due to high variability (Figure 1A). Neurospheres cultured in the presence of GDNF plus RA were significantly larger (4.8-fold) than control neurospheres, but were significantly smaller than neurospheres grown in the presence of GDNF alone (Figure 1A). Epidermal growth factor/basic fibroblast growth factor (EGF/bFGF) are routinely included in medium for both CNS and enteric neurospheres as they promote neural progenitor proliferation. To compare the effects of GDNF with EGF/bFGF, we cultured enteric neurospheres in medium containing GDNF but without EGF/bFGF. Neurospheres cultured in medium in which GDNF was substituted for EGF/bFGF were significantly larger than control neurospheres (medium containing EGF/bFGF but without added GDNF) (Figure 1A). Isolated ENCCs grown in medium that did not contain EGF/bFGF or GDNF did not form neurospheres. These experiments show that GDNF strongly enhances enteric neurosphere size.
Figure 1.
GDNF Enhances Enteric Neurosphere Size and Cell Number
(A) The effects of GDNF and/or RA on neurosphere volume. Significant differences were determined using a one-way ANOVA followed by Tukey's multiple comparison tests, and are shown by horizontal lines at the top of the graphs. GDNF and GDNF + RA significantly increased neurosphere volume compared with controls, although GDNF-treated neurospheres were also significantly larger than GDNF + RA-treated neurospheres. Data are shown as box-and-whisker plots in which the middle horizontal line shows the median, the top and bottom horizontal lines of the box show the upper and lower quartiles, respectively, and the vertical lines (whiskers) show the highest and lowest values. n indicates the number of neurospheres measured in each group, obtained from a minimum of three different experiments.
(B) Correlations between neurosphere volume and number of cells/neurosphere, determined by dissociating neurospheres to single cells, 1 and 2 weeks after neurospheres were generated. There is a strong correlation between neurosphere size and cell number; the gray line is the line of best fit for the 1-week data (control and GDNF data combined, r2 = 0.92) and the black line is the line of best fit for 2-week data (control and GDNF data combined, r2 = 0.99).
To examine the relationship between enteric neurosphere size and cell number, we dissociated neurospheres into single cells and counted the number of cells 7 and 14 days after ENCC isolation and plating. One week after plating with 10,000 cells/well, control enteric neural progenitors formed neurospheres comprising only 4,000–5,000 cells/well, while GDNF-treated neurospheres comprised around 55,000 cells/well (Figure 1B). For progenitors cultured under control conditions, neurosphere volume and cell number did not change significantly after a second week of culture, whereas GDNF-treated neurospheres increased in volume significantly during the second week, but not significantly in cell number/sphere (from 55,000 to 63,000 cells/sphere) (Figure 1B). There was a strong positive correlation between cell number and neurosphere volume for each of the two time points tested (Figure 1B).
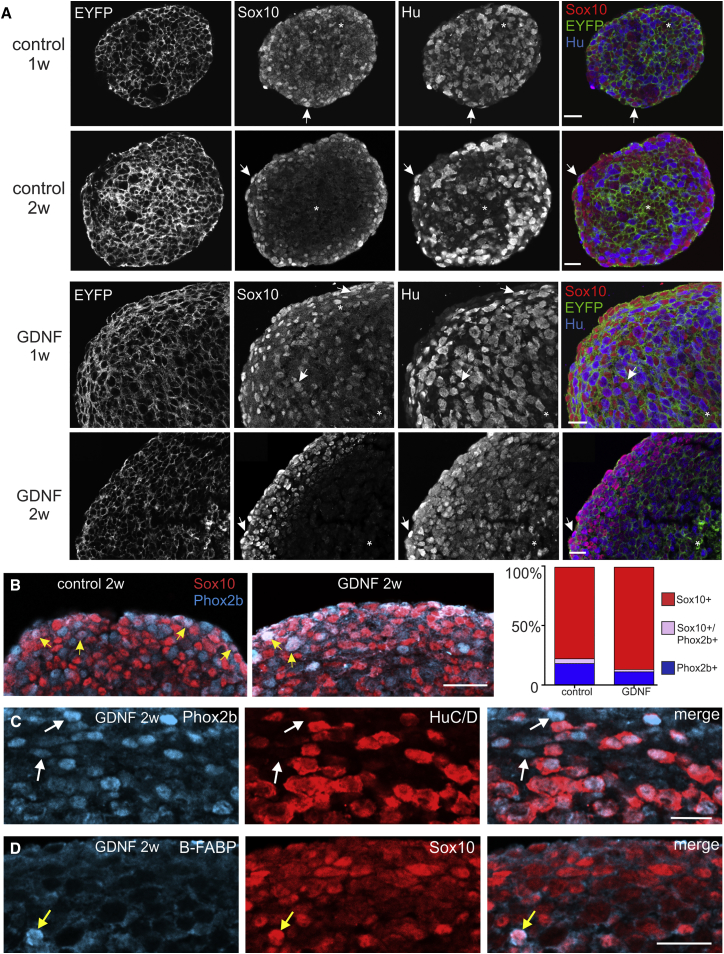
Neurospheres were cryosectioned 1 or 2 weeks after formation to examine the phenotype of neurosphere cells using antisera to the neural crest progenitor marker, SOX10, the pan-neuronal marker, HUC/D, the ENCC marker, PHOX2B, or the glial precursor marker, brain-specific fatty acid binding protein (B-FABP) (Kurtz et al., 1994, Pattyn et al., 1999, Young et al., 2003). For these experiments, neurospheres were generated from E14.5 Wnt1::Cre;ChR2-EYFP mice to maximize the number of fluorescent wavelengths available for immunofluorescence. We first confirmed that EYFP is expressed by all ENCCs in Wnt1::Cre;ChR2-EYFP mice; all SOX10+ and HUC/D+ cells in the E14.5 small intestine were EYFP+ (Figure S1). For control neurospheres, SOX10+ cells were evenly distributed after 1 week, but after 2 weeks in culture many of the SOX10+ cells were present close to the surface of the neurospheres (Figure 2A). HUC/D+ cells were scattered throughout the neurospheres after both 1 and 2 weeks (Figure 2A). Although GDNF-treated neurospheres were larger than controls, the relative distributions of SOX10+ and HUC/D+ cells within GDNF-treated neurospheres were similar to control neurospheres at both 1 and 2 weeks. In both control and GDNF-treated neurospheres, only a small number of cells showed HUC/D and SOX10 co-staining, and there were some cells that did not exhibit detectable SOX10 or HUC/D immunostaining (Figure 2A). The overlap between PHOX2B and HUC/D or SOX10 immunostaining was also examined. PHOX2B is expressed by all ENCCs within the gut (Corpening et al., 2008, Young et al., 1998) but does not appear to be expressed by “pre-enteric” neural crest cells (vagal neural crest cells prior to their entry into the gut) (Anderson et al., 2006). All HUC/D+ cells within neurospheres were also PHOX2B+, but there was also a small number of PHOX2B+/HUC/D− cells (Figure 2C). Although there is a high degree of overlap between PHOX2B and SOX10 expression by ENCCs in the embryonic gut (Young et al., 2003), there was very little overlap between SOX10 and PHOX2B immunostaining in 2-week control or GDNF-treated enteric neurospheres (Figure 2B). Quantification of SOX10+-only, SOX10+/PHOX2B+, and PHOX2B+-only cells of 2-week neurospheres revealed significantly more SOX10+-only cells in GDNF-treated neurospheres than in control neurospheres (Figure 2B; Chi-square test, p < 0.05, n = 3). None of the SOX10+ cells at the periphery of 2-week neurospheres showed staining for the glial precursor marker B-FABP, but a small number of SOX10+ cells in the centers of the neurospheres were B-FABP+ (Figure 2D). Thus, most of the SOX10+ cells within enteric neurospheres appear to be progenitors rather than glial precursors.
Figure 2.
Phenotype of Cells in Enteric Neurospheres
(A) Frozen sections through control and GDNF-treated enteric neurospheres showing immunostaining for SOX10 (red) and HUC/D (blue). In both control and GDNF-treated neurospheres, SOX10+ cells are evenly distributed throughout the neurospheres after 1 week (1w), but are concentrated near the periphery of the neurosphere after 2 weeks (2w) in culture. Some cells (asterisks) did not show detectable SOX10 or Hu immunostaining, and a small number of cells (arrows) showed both SOX10 and Hu staining.
(B) Periphery of 2-week (2w) control and GDNF-treated enteric neurospheres showing immunostaining for SOX10 (red) and PHOX2B (blue). There was a significantly higher proportion of SOX10+ only cells in GDNF-treated spheres (graph on right-hand side). Only a small number of cells (yellow arrows) were SOX10+/PHOX2B+.
(C) Two-week-old GDNF-treated neurospheres that had been immunostained using antibodies to PHOX2B and HUC/D. There was a high degree of overlap between HUC/D and PHOX2B expression, although a small number of PHOX2B+ cells were HUC/D negative (arrows).
(D) Only rare SOX10+ cells (yellow arrow) were immunoreactive for the glial precursor marker, B-FABP.
Scale bars, 20 μm.
To identify proliferating cells within neurospheres, we used Ki-67 antibodies were used. Ki-67+ cells were most abundant at the peripheries of both control and GDNF-treated neurospheres (Figure S2). This localization is consistent with a previous study that used thymidine analogs to identify proliferating cells in control mouse enteric neurospheres (Theocharatos et al., 2013). Immunolabeling of cryosectioned 7-day-old control and GDNF-treated neurospheres using antisera to activated caspase-3 showed that activated caspase-3+ cells were extremely rare in both control and GDNF neurospheres (data not shown).
Cells Derived from GDNF-Treated Neurospheres Migrate Further than Cells from Control Neurospheres When Co-cultured with Aneural Embryonic Gut
As a higher-throughput assay than in vivo studies, we first assessed the effects of GDNF and RA on the ability of neurosphere-derived cells to migrate and colonize explants of aneural embryonic gut. Single control neurospheres or neurospheres generated in the presence of GDNF and/or RA were placed in direct apposition with the oral end of explants of E11.5 hindgut removed prior to the arrival of endogenous ENCCs (“aneural gut”), and co-cultured for 1 week in medium containing 10% fetal calf serum but no added growth factors (Figures 3A and 3B). The 5-HT4 agonist, RS67506, was added to some co-cultures (Figure 3B). Neurospheres were exposed to GDNF and RA prior to co-culture because these factors have been shown to enhance the survival, proliferation, and expression of RET by ENCCs prior to their entry into the gut (Durbec et al., 1996, Simkin et al., 2013), while RS67506 was only included in the co-culture medium because it has been shown to promote neurogenesis of enteric neural progenitors within the gut wall (Liu et al., 2009).
Figure 3.
Migration and Phenotype of Enteric Neurosphere-Derived Cells Following Co-culture with Explants of Embryonic Aneural Gut Lacking Endogenous ENCCs
(A) Experimental timeline for co-culture experiments. Neurospheres were grown under control conditions or in the presence of glial-cell-line-derived neurotrophic factor (GDNF) and/or retinoic acid (RA) and grown for 2 weeks (pink arrow). Co-cultures were then established using control medium or medium containing the 5-HT4 agonist, RS67506 (green arrow).
(B) Diagram showing the co-culture set-up. Fluorescently labeled enteric neurospheres were placed on a filter paper support in direct apposition with the oral end of a segment of mid and distal colon removed from wild-type E11.5 mice, prior to the arrival of endogenous ENCCs, and grown as co-cultures for 7 days.
(C) The distance to the most distal neurosphere-derived cell from the edge of the neurosphere for different conditions. Significant differences were determined using a one-way ANOVA followed by Tukey's multiple comparison tests, and are shown by horizontal lines at the top of the graphs. Data are shown as box-and-whisker plots in which the middle horizontal line indicates the median, the top and bottom horizontal lines of the box indicate the upper and lower quartiles, respectively, and the vertical lines show the highest and lowest values. n indicates the total number of co-cultures analyzed for each group, from a minimum of three different experiments.
(D and E) Examples of co-cultures between a control neurosphere and an explant of aneural embryonic gut (D) and a GDNF-treated neurosphere and aneural embryonic gut (E) after 1 week of co-culture. The dotted line indicates the outline of gut explant. The most distal neurosphere-derived cells are indicated by white arrows.
(F) Aneural gut explant that had been co-cultured with a GDNF-treated neurosphere. Most EGFP+ cells within gut explants were SOX10+/PHOX2B+, although a small number of SOX10+/PHOX2B− (white arrow) and SOX10−/PHOX2B+ cells (yellow arrow) were also present.
(G) Although there were many HUC/D+ cells within the neurosphere, very few of the cells that colonized the gut explants were HUC/D; there is only a single HUC/D+ cell (white arrow) within the gut explant in this field of view.
The distances from the neurosphere to the most distal graft-derived cell in the gut explants for each of the different conditions are shown in Figure 3C. Cells from neurospheres generated in the presence of GDNF migrated significantly further than cells from control neurospheres (Figures 3C–3E). Cells from neurospheres generated in the presence of both GDNF and RA also migrated further than controls, although cells from RA-only-treated neurospheres did not migrate significantly further than cells from control neurospheres. The presence of RS67506 in the co-culture medium did not significantly increase the distance migrated by cells from control neurospheres or GDNF-treated neurospheres, although there was trend for longer migration of cells derived from GDNF-treated neurospheres. Cells from neurospheres generated in medium lacking EGF/bFGF but containing GDNF also migrated further than control neurospheres (grown in the presence of EGF/bFGF but not GDNF).
Phenotype of Neurosphere-Derived Cells that Colonize Co-cultured Gut Explants
Co-cultures using neurospheres generated from E14.5 Wnt1::Cre;ChR2-EYFP mice were immunostained using antisera to SOX10 and PHOX2B, SOX10 and HUC/D, or SOX10 and TUJ1; TUJ1 (TUBB3 or neuron-specific class III β-tubulin) labels neurites. The vast majority of control and GDNF-treated neurosphere-derived cells that had migrated into gut explants were SOX10+/PHOX2B+ (Figures 3F and S3A). Ninety-seven percent of cells within gut explants co-cultured with GDNF-treated neurospheres were SOX10+/PHOX2B+, 1% were SOX10+/PHOX2B−, and 2% were SOX10−/PHOX2B+ (n = 256 cells from four explants from two different experiments). SOX10+/PHOX2B+ cells are likely to be progenitors, SOX10+/PHOX2B− cells are likely to be glial precursors, and SOX10−/PHOX2B+ cells are likely to be neuronal precursors or neurons. Very few of the neurosphere-derived cells differentiated into HUC/D+ neurons within the gut under any conditions (Figures 3G and 4A–4E). In contrast, many cells that remained within the neurosphere were HUC/D+ (Figures 3G, 4A–4E, and S3B). Cells also migrated away from the neurospheres onto the filter paper support during the culture period; most of the cells that had dispersed from the neurosphere onto the paper support were SOX10+ but HUC/D− (Figure S3B). There were many TUJ1+ neurites within the gut explants (Figure S3C), which originated from neurons within the neurospheres on the filter paper supports, but TUJ1+ cell bodies were rare within the gut explants, which confirms data obtained with the HUC/D antisera. Graft-derived SOX10+ cells within the gut explants were found in close association with neurites (Figure S3C), suggesting that, like parasympathetic neuron precursors (Dyachuk et al., 2014, Espinosa-Medina et al., 2014), graft-derived ENCCs might be guided by neurites.
Figure 4.
Cells Derived from Neurospheres Generated in the Presence of GDNF and/or RA Gave Rise to More Complex Networks than Cells from Control Neurospheres
(A–E) Networks formed by cells derived from neurospheres generated under different conditions. Although many cells that remained within the neurospheres on the filter paper supports expressed HUC/D (magenta/white, black asterisks), only a small proportion of neurosphere-derived cells (green) within the gut explants expressed HUC/D (magenta, yellow arrows). Scale bars, 50 μm.
(F) The number of network interactions (two lines connecting on a skeletonized image) were counted per area of gut colonized (indicated by the red dashed line in C) for each condition. Treatment with GDNF significantly increased the number of network interactions per μm2. Significant differences were determined using a one-way ANOVA followed by Tukey's multiple comparison tests, and are shown by horizontal lines at the top of the graphs. Data are shown as box-and-whisker plots in which the middle horizontal line indicates the median, the top and bottom horizontal lines of the box indicate the upper and lower quartiles, respectively, and the vertical lines (whiskers) show the highest and lowest values. n indicates the total number of co-cultures analyzed for each group, from a minimum of three different experiments.
Exposure of Neurospheres to GDNF and/or RA Increases the Complexity of the Network Generated within Embryonic Gut Explants
During normal development, ENCCs give rise to a complex network within the gut wall (Watanabe et al., 2013). To analyze the network generated by different neurosphere-derived cells within embryonic gut explants, we skeletonized images of neurosphere-derived cells within the gut and quantified the density of skeleton interactions (Figure 4F). Neurospheres grown in the presence of GDNF or RA alone, or in combination, gave rise to networks with significantly more interactions/area than cells derived from control neurospheres (Figures 4A, 4B, 4D, and 4F). Moreover, cells derived from neurospheres grown in medium in which EGF/bFGF had been omitted and replaced by GDNF also showed more network interactions than cells derived from control neurospheres (Figures 4A and 4C). The addition of RS67506 to the co-culture medium did not increase the number of network interactions/area (Figures 4E and 4F).
Exposure of Neurospheres to GDNF Enhances Their Migration following Transplantation into the Colon In Vivo
Neurospheres grown under control conditions or in the presence of GDNF were transplanted into the distal colon of 2- to 3-week-old wild-type mice. After 4 weeks the recipient mice were euthanized, and the area occupied by graft-derived neurites and graft-derived cells was measured. Cells derived from neurospheres generated in the presence of GDNF occupied a significantly larger (2-fold) area than control neurospheres (t test, p < 0.05; Figures 5A and 5C). Neurites derived from neurospheres generated in the presence of GDNF also occupied a significantly larger (2-fold) area than control neurospheres (t test, p < 0.05; Figure 5B). The number of HUC/D+ cells per area of graft-derived cells was not significantly different for control GDNF-treated neurospheres (Figures 5D and 5E). These data suggest that GDNF-treated neurospheres generate more neurons because they contain a larger number of cells, rather than affecting the proportion of cells that differentiate into neurons. Graft-derived cells from both control and GDNF-treated neurospheres that were HUC/D− were SOX10+ (Figure S4).
Figure 5.
Extent of Migration and Neurite Projection of Enteric Neurosphere-Derived Cells in the Colon In Vivo
(A and B) Control or GDNF-treated neurospheres were transplanted into the colon of 2- to 3-week-old mice (n = 6 recipients for control neurospheres and n = 5 recipients for GDNF neurospheres). Four weeks later, the recipient mice were killed. The areas occupied by neurosphere-derived cells (A) and neurites (B) were significantly larger for neurospheres generated in the presence of GDNF (n = 5) compared with controls (n = 6; unpaired t tests, ∗p = 0.01 for area occupied by cell bodies, and p = 0.04 for area of fibers).
(C) Low-magnification images of control (left) and GDNF-treated (right) graft-derived cells in whole-mount preparations of external muscle of distal colon 4 weeks after transplantation of ENS progenitors. Each image shows less than 50% of the total outgrowth from the transplanted neurospheres (black asterisks). The dotted lines demarcate the area occupied by graft-derived cell bodies. Graft-derived neurites extend beyond the edges of each image.
(D) The number of Hu+ cells/area of graft-derived cells was not significantly different for control (n = 6) and GDNF-treated (n = 5) neurospheres. The middle horizontal line in each graph shows the median, the top and bottom horizontal lines of the box show the upper and lower quartiles, respectively, and the vertical lines show the highest and lowest values.
(E) Representative images of graft-derived cells (green) and neurons (magenta) in recipients into which control neurospheres (left) and GDNF-treated neurospheres (right) had been transplanted. Asterisks indicate graft-derived cells that do not express Hu.
The development of the neuronal nitric oxide synthase (nNOS) subtype of enteric neurons is promoted by RET signaling (Anderson et al., 2006, Uesaka and Enomoto, 2010, Wang et al., 2010, Yan et al., 2004). There was no significant difference in the proportion of graft-derived HUC/D+ neurons that showed nNOS immunostaining between recipients containing control and GDNF-treated neurospheres; in control neurosphere recipients, nNOS neurons constituted 45.1% ± 3.6% of HUC/D neurons, while in GDNF-treated neurosphere recipients nNOS neurons constituted 50.4% ± 3.6% (mean ± SEM, n = 3 of each type of recipient; minimum of 75 HUC/D+ cells counted per recipient, t test, p = 0.35, not significant).
Discussion
Although cell therapy is a promising approach for treating enteric neuropathies, it is commonly thought that enteric neural progenitors isolated from the human bowel will require biological and/or genetic manipulation prior to transplantation, owing to the size of the human bowel and proliferation and/or migration defects associated with the disease causing genetic mutation(s) (Hotta et al., 2009, Metzger et al., 2009b, Micci and Pasricha, 2007). In this study, we show that exposure to GDNF enhances the in vitro expansion of mouse enteric neural progenitors and that cells derived from GDNF-treated neurospheres migrate further within the gut wall than cells from control neurospheres in vitro and in vivo.
During development of the ENS, GDNF plays an essential role in survival, proliferation, migration, and neuronal differentiation (Laranjeira and Pachnis, 2009, Sasselli et al., 2012, Taraviras et al., 1999). Our data show that enteric neural progenitors grown as neurospheres also respond to GDNF, and we confirm a recent study reporting that GDNF-treated ENCCs generated larger neurospheres, although neurosphere size was not quantified in this study (Schriemer et al., 2016). We showed that GDNF promotes proliferation, as GDNF-treated neurospheres contained 12-fold more cells than control neurospheres. It is well established that GDNF promotes neuronal differentiation as well as proliferation of ENCCs (Heanue and Pachnis, 2007, Lake and Heuckeroth, 2013, Taraviras et al., 1999, Uesaka et al., 2016), and it is possible that repeated GDNF treatment could adversely affect progenitor maintenance and proliferation by promoting neuronal differentiation. However, in preliminary experiments, long-term GDNF treatment did not negatively affect the self-renewal ability of enteric neural progenitors, as GDNF-treated secondary and tertiary neurospheres generated from dissociated, GDNF-treated primary neurospheres were significantly larger than controls (Figure S5).
Within the embryonic gut, all ENCCs express PHOX2B; non-neuronal cells are SOX10+/PHOX2B+ while neurons are SOX10−/PHOX2B+ (Young et al., 2002, Young et al., 2003). Although cells comprising enteric neurospheres were isolated from the embryonic gut, surprisingly only around 5% of SOX10+ cells in both control and GDNF-treated neurospheres was also PHOX2B+. This suggests that PHOX2B is downregulated during the formation of neurospheres. There was a small, but significant, increase in the proportion of SOX10+/PHOX2B− cells in GDNF neurospheres compared with control neurospheres, which raises the possibility that SOX10+/PHOX2B− neurosphere cells are more proliferative than other neurosphere cells. It is surprising that exposure to GDNF did not increase the proportion of PHOX2B+ cells in neurospheres, as GDNF promotes the proliferation of SOX10+/PHOX2B+ ENCCs in vivo (Flynn et al., 2007). Moreover, the gut mesenchyme expresses GDNF (Natarajan et al., 2002), vagal neural crest-derived cells upregulate Phox2B after entering the foregut (Anderson et al., 2006) and Ret expression is regulated by PHOX2B (Pattyn et al., 1999). Our data show that PHOX2B is not essential for the proliferative effects of GDNF, and suggest that GDNF alone does not induce Phox2B in ENCCs after entering the gut. Most of the cells that colonized co-cultured explants of embryonic gut were SOX10+/PHOX2B+, and it is unclear whether PHOX2B is upregulated by SOX10+ cells within the gut environment or whether the only cells capable of colonizing the gut explants were the small number of SOX10+/PHOX2B+ cells present within neurospheres.
Although we showed that cells expressing activated caspase-3 were extremely rare in both control and GDNF-treated neurospheres after 1 week, it is possible that GDNF also promotes cell survival immediately after cell sorting, during the very early stages of neurosphere formation, as control neurospheres underwent a 50% reduction in cell number during neurosphere formation (from 10,000 to 5,000 cells).
ENCC number influences the distance they migrate along the embryonic gut during normal development (Barlow et al., 2008, Paratore et al., 2002, Peters-van der Sanden et al., 1993, Yntema and Hammond, 1954). In our study, cells from GDNF-treated neurospheres migrated further than control neurospheres following transplantation into the colon of postnatal recipient mice, demonstrating the importance of progenitor number in migration for regenerative medicine. If there was a linear correlation between cell number and migration, the 12-fold greater number of cells in GDNF-treated neurospheres might be expected to occupy a 3.5-fold larger area than control neurospheres; however, cells from GDNF neurospheres only colonized an area 2-fold larger than control neurospheres in the postnatal colon in vivo and in embryonic gut co-cultures. Although cells derived from GDNF-treated neurospheres migrated further than controls, the spread of GDNF-treated cells along the postnatal colon was still small compared with that recently described by human PSC-derived enteric neural progenitors (Fattahi et al., 2016). Thus, GDNF treatment alone is unlikely to be adequate to promote sufficient spread of transplanted enteric neural cells for cell therapy, and additional techniques will need to be developed, including methods to introduce greater numbers of cells to multiple locations along the colon (Burns et al., 2016) that would be used in combination with GDNF treatment.
While an increase in progenitor number is highly likely to contribute to the enhanced performance of cells derived from neurospheres exposed to GDNF, it is also possible that exposure to GDNF augments the ability of individual progenitors to migrate and form a network. We were unable to examine experimentally the relative contributions of cell number versus cell quality, as it is not possible to co-culture or transplant a similar number of control and GDNF-treated progenitors (it is not technically possible to co-culture 12 control neurospheres with a single embryonic gut explant or to transplant 12 control neurospheres into a single location in the mouse colon in vivo).
RA is known to play a role in ENCC migration and expression of the GDNF receptor, RET (Niederreither et al., 2003, Simkin et al., 2013, Wright-Jin et al., 2013). It was therefore surprising that RA treatment alone did not result in larger neurospheres or enhanced migration in embryonic hindgut co-cultures. Furthermore, combined treatment of neurospheres with GDNF and RA did not result in larger neurospheres or enhanced migration in embryonic gut explants than neurospheres treated with GDNF alone. It may be that the RET receptor is already maximally expressed by the cultured ENCCs and that addition of RA does not further upregulate its expression and, therefore, GDNF signaling.
In the current study, the 5-HT4 agonist, RS67506, was included in the co-culture medium because it has been shown to promote neurogenesis of enteric neural progenitors within the gut wall (Hotta et al., 2016a, Liu et al., 2009). We found no significant effect of RS67506 on migration or network formation in co-cultures, which might reflect differences in the behavior of enteric neural progenitors in the embryonic gut compared with the adult gut.
Of the numerous studies examining isolation and in vitro culture of rodent and human ENS progenitors, most use only bFGF and EGF growth factors in the culture media, usually in the presence of differing combinations of N2 and B27 supplements and/or chick embryo extract (Belkind-Gerson et al., 2015, Binder et al., 2015, Cheng et al., 2015, Dettmann et al., 2014, Gao et al., 2016, Hotta et al., 2013, Lindley et al., 2008, Metzger et al., 2009a). Few studies have included GDNF in the culture medium (Becker et al., 2012, Schriemer et al., 2016), and to date no study has made direct comparisons between established ENCC culture conditions and GDNF treatment. We have shown that GDNF treatment results in larger enteric neurospheres composed of significantly more cells, which display enhanced migratory capacity in both the embryonic and postnatal gut environment. Assuming that human enteric neural progenitors behave in a similar way to mouse progenitors, exposure to GDNF would represent a simple manipulation to expand human enteric neural progenitors prior to transplantation to treat enteric neuropathies. Unfortunately, the effects of RA or 5-HT4 activation were not additive to those of GDNF alone, at least in co-cultures with explants of embryonic gut.
Experimental Procedures
Mice
The following mice were used: C57BL/6 mice; Ednrb-hKikGR mice, in which all ENCCs express the fluorescent protein, KikGR (Nishiyama et al., 2012); and Wnt1::Cre;ChR2-EYFP mice, in which all ENCCs express EYFP, which were generated by mating Wnt1::Cre mice (Danielian et al., 1998) to Ai32(RCL-ChR2(H134R)/EYFP) Channel-rhodopsin-YFP (ChRd-YFP) mice (The Jackson Laboratory, stock no. 012569). Mice were time plug-mated. Pregnant mice were killed by cervical dislocation. Neurospheres were generated from E14.5 Ednrb-hKikGR or Wnt1::Cre;ChR2-EYFP mice. For in vivo studies, neurospheres were transplanted into 3- to 4-week-old C57BL/6 mice. All experiments were approved by the Anatomy & Neuroscience, Pathology, Pharmacology and Physiology Animal Ethics Committee of the University of Melbourne.
Generation and Culture of Neurospheres
ENCCs were isolated from E14.5 Ednrb-hKikGR or Wnt1::Cre;ChR2-EYFP mice as described previously (Hotta et al., 2013).
Analysis of Neurosphere Characteristics
The sizes of neurospheres were measured at 1 or 2 weeks after plating. Images were taken on a dissecting fluorescence microscope at 60× magnification. The radius of the spheres was measured using LSM Image Browser and the volume calculated. Cell number was determined by dissociating the spheres to a single-cell suspension by washing neurospheres in 0.1 M phosphate buffer (PB) and incubating in Accutase (STEMCELL Technologies) for 4–5 hr at 37°C, with gentle pipetting. For cryosectioning, neurospheres were fixed in 4% paraformaldehyde in PB, washed in PB, incubated in 30% sucrose in PB, transferred to a cryomold containing OCT compound (Tissue-Tek), frozen in liquid nitrogen, sectioned at 10 μm, and processed for immunofluorescence.
In Vitro Migration and Network Assay
Co-cultures between neurospheres and aneural E11.5 colon were established as described previously (Findlay et al., 2014) (see Supplemental Experimental Procedures).
In Vivo Transplantation of Ednrb-Kik Neurospheres to the Colon of Postnatal Mice
Neurospheres cultured for 7 days were transplanted into the distal colon of recipient wild-type mice (3–4 weeks of age) as previously described (Hotta et al., 2013). Four weeks after surgery, recipient mice were killed by cervical dislocation, the distal colon was removed, pinned, and fixed, and the mucosa removed as previously described (Hotta et al., 2013). Whole-mount preparations of external muscle were then processed for immunofluorescence (see Supplemental Experimental Procedures).
Measurements of Migration, Network, and Area of Co-culture Assays
Migration distance and network analysis were performed using Fiji/ImageJ (see Supplemental Experimental Procedures).
Measurement of Area Occupied by Graft-Derived Cells In Vivo
For determination of the area occupied by graft-derived cells plus fibers or by graft-derived cells only, tile scans of whole-mount preparations of recipient colon were taken using ×5 or ×10 objective lenses on a confocal microscope. The total area occupied by graft-derived cells plus fibers, or cells only, in each preparation was measured using ImageJ software. The density of HUC/D+ cells/area of graft-derived cells was determined using ImageJ.
Cell Counts
The proportion of the number of different cell types was determined from confocal microscope images obtained using a 20× objective using the Cell Counter plugin on ImageJ (Fiji).
Statistics
Data are displayed as box-and-whisker plots showing the median and interquartile ranges, and were analyzed using ANOVA with post hoc Tukey tests, two-tailed t tests, or Chi-square tests where appropriate. A p value of less than 0.05 was considered significant.
Author Contributions
Conception and design: S.J.McK., H.M.Y., L.A.S.; Financial support: S.J.McK., H.M.Y., L.A.S.; Collection of data: S.J.McK., M.M., A.J.B., H.M.Y., L.A.S.; Data analysis and interpretation: S.J.McK., M.M., H.M.Y., L.A.S.; Manuscript writing: S.J.McK., H.M.Y., L.A.S.; Final approval of manuscript: S.J.McK., M.M., A.J.B., H.M.Y., L.A.S.
Acknowledgments
This work was supported by NHMRC Project Grant APP1043397 to H.M.Y. and S.J.McK., NHMRC Project Grant APP1079234 to H.M.Y. and L.A.S., and NHMRC Senior Fellowship APP1002506 to H.M.Y. We thank Dr. Vanda Lennon (Mayo Clinic) for kindly providing the HUC/D antiserum, Dr. Thomas Müller (Max-Delbrück Center for Molecular Medicine, Berlin) for providing the B-FABP antiserum, Dr. Piers Emson (Cambridge University) for providing the nNOS antiserum, Professor Hideki Enomoto (Kobe University School of Medicine) for kindly providing the Ednrb-hKikGR mice and the PHOX2B antiserum, and Jan Morgan for excellent technical assistance.
Published: January 12, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.12.013.
Contributor Information
Sonja J. McKeown, Email: sonja.mckeown@monash.edu.
Lincon A. Stamp, Email: lstamp@unimelb.edu.au.
Supplemental Information
References
- Almond S., Lindley R.M., Kenny S.E., Connell M.G., Edgar D.H. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56:489–496. doi: 10.1136/gut.2006.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.B., Stewart A.L., Young H.M. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 2006;323:11–25. doi: 10.1007/s00441-005-0047-6. [DOI] [PubMed] [Google Scholar]
- Barlow A.J., Wallace A.S., Thapar N., Burns A.J. Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development. 2008;135:1681–1691. doi: 10.1242/dev.017418. [DOI] [PubMed] [Google Scholar]
- Becker L., Kulkarni S., Tiwari G., Micci M.A., Pasricha P.J. Divergent fate and origin of neurosphere-like bodies from different layers of the gut. Am. J. Physiol. 2012;302:G958–G965. doi: 10.1152/ajpgi.00511.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkind-Gerson J., Hotta R., Nagy N., Thomas A.R., Graham H., Cheng L., Solorzano J., Nguyen D., Kamionek M., Dietrich J. Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism. Inflamm. Bowel Dis. 2015;21:870–878. doi: 10.1097/MIB.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder E., Natarajan D., Cooper J., Kronfli R., Cananzi M., Delalande J.M., McCann C., Burns A.J., Thapar N. Enteric neurospheres are not specific to neural crest cultures: implications for neural stem cell therapies. PLoS One. 2015;10:e0119467. doi: 10.1371/journal.pone.0119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurand N., Natarajan D., Thapar N., Atkins C., Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130:6387–6400. doi: 10.1242/dev.00857. [DOI] [PubMed] [Google Scholar]
- Burns A.J., Thapar N. Neural stem cell therapies for enteric nervous system disorders. Nat. Rev. Gastroenterol. Hepatol. 2014;11:317–328. doi: 10.1038/nrgastro.2013.226. [DOI] [PubMed] [Google Scholar]
- Burns A.J., Goldstein A.M., Newgreen D.F., Stamp L., Schafer K.H., Metzger M., Hotta R., Young H.M., Andrews P.W., Thapar N. White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies. Dev. Biol. 2016;417:229–251. doi: 10.1016/j.ydbio.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L.S., Hotta R., Graham H.K., Nagy N., Goldstein A.M., Belkind-Gerson J. Endoscopic delivery of enteric neural stem cells to treat Hirschsprung disease. Neurogastroenterol. Motil. 2015;27:1509–1514. doi: 10.1111/nmo.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpening J.C., Cantrell V.A., Deal K.K., Southard-Smith E.M. A Histone2BCerulean BAC transgene identifies differential expression of PHOX2B in migrating enteric neural crest derivatives and enteric glia. Dev. Dyn. 2008;237:1119–1132. doi: 10.1002/dvdy.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian P.S., Muccino D., Rowitch D.H., Michael S.K., McMahon A.P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- De Giorgio R., Camilleri M. Human enteric neuropathies: morphology and molecular pathology. Neurogastroenterol. Motil. 2004;16:515–531. doi: 10.1111/j.1365-2982.2004.00538.x. [DOI] [PubMed] [Google Scholar]
- De Giorgio R., Guerrini S., Barbara G., Cremon C., Stanghellini V., Corinaldesi R. New insights into human enteric neuropathies. Neurogastroenterol. Motil. 2004;16:143–147. doi: 10.1111/j.1743-3150.2004.00491.x. [DOI] [PubMed] [Google Scholar]
- Dettmann H.M., Zhang Y., Wronna N., Kraushaar U., Guenther E., Mohr R., Neckel P.H., Mack A., Fuchs J., Just L. Isolation, expansion and transplantation of postnatal murine progenitor cells of the enteric nervous system. PLoS One. 2014;9:e97792. doi: 10.1371/journal.pone.0097792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbec P.L., Larsson-Blomberg L.B., Schuchardt A., Costantini F., Pachnis V. Common origin and developmental dependence on c-RET of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. doi: 10.1242/dev.122.1.349. [DOI] [PubMed] [Google Scholar]
- Dyachuk V., Furlan A., Shahidi M.K., Giovenco M., Kaukua N., Konstantinidou C., Pachnis V., Memic F., Marklund U., Muller T. Neurodevelopment. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science. 2014;345:82–87. doi: 10.1126/science.1253281. [DOI] [PubMed] [Google Scholar]
- Espinosa-Medina I., Outin E., Picard C.A., Chettouh Z., Dymecki S., Consalez G.G., Coppola E., Brunet J.F. Neurodevelopment. Parasympathetic ganglia derive from Schwann cell precursors. Science. 2014;345:87–90. doi: 10.1126/science.1253286. [DOI] [PubMed] [Google Scholar]
- Fattahi F., Steinbeck J.A., Kriks S., Tchieu J., Zimmer B., Kishinevsky S., Zeltner N., Mica Y., El-Nachef W., Zhao H. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531:105–109. doi: 10.1038/nature16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay Q., Yap K.K., Bergner A.J., Young H.M., Stamp L.A. Enteric neural progenitors are more efficient than brain-derived progenitors at generating neurons in the colon. Am. J. Physiol. 2014;307:G741–G748. doi: 10.1152/ajpgi.00225.2014. [DOI] [PubMed] [Google Scholar]
- Flynn B., Bergner A.J., Turner K.N., Young H.M., Anderson R.B. Effect of Gdnf haploinsufficiency on rate of migration and number of enteric neural crest-derived cells. Dev. Dyn. 2007;236:134–141. doi: 10.1002/dvdy.21013. [DOI] [PubMed] [Google Scholar]
- Furness J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- Gao T., Chen H., Liu M., Ge W., Yin Q. Prospective identification and culture of rat enteric neural stem cells (ENSCs) Cytotechnology. 2016;68:509–514. doi: 10.1007/s10616-014-9803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue T.A., Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat. Rev. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Hetz S., Acikgoez A., Voss U., Nieber K., Holland H., Hegewald C., Till H., Metzger R., Metzger M. In vivo transplantation of neurosphere-like bodies derived from the human postnatal and adult enteric nervous system: a pilot study. PLoS One. 2014;9:e93605. doi: 10.1371/journal.pone.0093605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta R., Natarajan D., Thapar N. Potential of cell therapy to treat pediatric motility disorders. Semin. Pediatr. Surg. 2009;18:263–273. doi: 10.1053/j.sempedsurg.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Hotta R., Stamp L.A., Foong J.P., Bergner A.J., McConnell S.N., Anderson R.B., Enomoto H., Newgreeen D.F., Obermayr F., Furness J.B. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J. Clin. Invest. 2013;123:1182–1191. doi: 10.1172/JCI65963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta R., Cheng L.S., Graham H.K., Nagy N., Belkind-Gerson J., Mattheolabakis G., Amiji M.M., Goldstein A.M. Delivery of enteric neural progenitors with 5-HT4 agonist-loaded nanoparticles and thermosensitive hydrogel enhances cell proliferation and differentiation following transplantation in vivo. Biomaterials. 2016;88:1–11. doi: 10.1016/j.biomaterials.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta R., Cheng L.S., Graham H.K., Pan W., Nagy N., Belkind-Gerson J., Goldstein A.M. Isogenic enteric neural progenitor cells can replace missing neurons and glia in mice with Hirschsprung disease. Neurogastroenterol. Motil. 2016;28:498–512. doi: 10.1111/nmo.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles C.H., De Giorgio R., Kapur R.P., Bruder E., Farrugia G., Geboes K., Lindberg G., Martin J.E., Meier-Ruge W.A., Milla P.J. The London classification of gastrointestinal neuromuscular pathology: report on behalf of the gastro 2009 international working group. Gut. 2010;59:882–887. doi: 10.1136/gut.2009.200444. [DOI] [PubMed] [Google Scholar]
- Kruger G., Mosher J., Bixby S., Joseph N., Iwashita T., Morrison S. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S., Becker L., Pasricha P.J. Stem cell transplantation in neurodegenerative disorders of the gastrointestinal tract: future or fiction? Gut. 2012;61:613–621. doi: 10.1136/gut.2010.235614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A., Zimmer A., Schnutgen F., Bruning G., Spener F., Muller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- Lake J.I., Heuckeroth R.O. Enteric nervous system development: migration, differentiation, and disease. Am. J. Physiol. 2013;305:G1–G24. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeira C., Pachnis V. Enteric nervous system development: recent progress and future challenges. Auton. Neurosci. 2009;151:61–69. doi: 10.1016/j.autneu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Li W., Huang L., Zeng J., Lin W., Li K., Sun J., Huang W., Chen J., Wang G., Ke Q. Characterization and transplantation of enteric neural crest cells from human induced pluripotent stem cells. Mol. Psychiatry. 2016 doi: 10.1038/mp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley R.M., Hawcutt D.B., Connell M.G., Almond S.N., Vannucchi M.G., Faussone-Pellegrini M.S., Edgar D.H., Kenny S.E. Human and mouse enteric nervous system neurosphere transplants regulate the function of aganglionic embryonic distal colon. Gastroenterology. 2008;135:205–216. doi: 10.1053/j.gastro.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Liu M.T., Kuan Y.H., Wang J., Hen R., Gershon M.D. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J. Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M., Bareiss P.M., Danker T., Wagner S., Hennenlotter J., Guenther E., Obermayr F., Stenzl A., Koenigsrainer A., Skutella T. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology. 2009;137:2063–2073.e4. doi: 10.1053/j.gastro.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Metzger M., Caldwell C., Barlow A.J., Burns A.J., Thapar N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. 2009;136:2214–2225. doi: 10.1053/j.gastro.2009.02.048. [DOI] [PubMed] [Google Scholar]
- Micci M.A., Pasricha P.J. Neural stem cells for the treatment of disorders of the enteric nervous system: strategies and challenges. Dev. Dyn. 2007;236:33–43. doi: 10.1002/dvdy.20975. [DOI] [PubMed] [Google Scholar]
- Natarajan D., Marcos-Gutierrez C., Pachnis V., de Graaff E. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- Niederreither K., Vermot J., Le Roux I., Schuhbaur B., Chambon P., Dolle P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development. 2003;130:2525–2534. doi: 10.1242/dev.00463. [DOI] [PubMed] [Google Scholar]
- Nishikawa R., Hotta R., Shimojima N., Shibata S., Nagoshi N., Nakamura M., Matsuzaki Y., Okano H.J., Kuroda T., Okano H. Migration and differentiation of transplanted enteric neural crest-derived cells in murine model of Hirschsprung's disease. Cytotechnology. 2015;67:661–670. doi: 10.1007/s10616-014-9754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama C., Uesaka T., Manabe T., Yonekura Y., Nagasawa T., Newgreen D.F., Young H.M., Enomoto H. Trans-mesenteric neural crest cells are the principal source of the colonic enteric nervous system. Nat. Neurosci. 2012;15:1211–1218. doi: 10.1038/nn.3184. [DOI] [PubMed] [Google Scholar]
- Pan W.K., Zheng B.J., Gao Y., Qin H., Liu Y. Transplantation of neonatal gut neural crest progenitors reconstructs ganglionic function in benzalkonium chloride-treated homogenic rat colon. J. Surg. Res. 2011;167:e221–230. doi: 10.1016/j.jss.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Paratore C., Eichenberger C., Suter U., Sommer L. SOX10 haploinsufficiency affects maintenance of progenitor cells in a mouse model of Hirschsprung disease. Hum. Mol. Genet. 2002;11:3075–3085. doi: 10.1093/hmg/11.24.3075. [DOI] [PubMed] [Google Scholar]
- Pattyn A., Morin X., Cremer H., Goridis C., Brunet J.F. The homeobox gene PHOX2B is essential for the development of autonomic neural crest derivatives. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- Peters-van der Sanden M.J., Kirby M.L., Gittenberger-de Groot A., Tibboel D., Mulder M.P., Meijers C. Ablation of various regions within the avian vagal neural crest has differential effects on ganglion formation in the fore-, mid- and hindgut. Dev. Dyn. 1993;196:183–194. doi: 10.1002/aja.1001960305. [DOI] [PubMed] [Google Scholar]
- Rauch U., Hansgen A., Hagl C., Holland-Cunz S., Schafer K.H. Isolation and cultivation of neuronal precursor cells from the developing human enteric nervous system as a tool for cell therapy in dysganglionosis. Int. J. Colorectal Dis. 2006;21:554–559. doi: 10.1007/s00384-005-0051-z. [DOI] [PubMed] [Google Scholar]
- Sasselli V., Pachnis V., Burns A.J. The enteric nervous system. Dev. Biol. 2012;366:64–73. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Schriemer D., Sribudiani Y., IJpma A., Natarajan D., MacKenzie K.C., Metzger M., Binder E., Burns A.J., Thapar N., Hofstra R.M. Regulators of gene expression in enteric neural crest cells are putative Hirschsprung disease genes. Dev. Biol. 2016;416:255–265. doi: 10.1016/j.ydbio.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Simkin J.E., Zhang D., Rollo B.N., Newgreen D.F. Retinoic acid upregulates RET and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut. PLoS One. 2013;8:e64077. doi: 10.1371/journal.pone.0064077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraviras S., Marcos-Gutierrez C.V., Durbec P., Jani H., Grigoriou M., Sukumaran M., Wang L.C., Hynes M., Raisman G., Pachnis V. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126:2785–2797. doi: 10.1242/dev.126.12.2785. [DOI] [PubMed] [Google Scholar]
- Theocharatos S., Wilkinson D.J., Darling S., Wilm B., Kenny S.E., Edgar D. Regulation of progenitor cell proliferation and neuronal differentiation in enteric nervous system neurospheres. PLoS One. 2013;8:e54809. doi: 10.1371/journal.pone.0054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka T., Enomoto H. Neural precursor death is central to the pathogenesis of intestinal aganglionosis in RET hypomorphic mice. J. Neurosci. 2010;30:5211–5218. doi: 10.1523/JNEUROSCI.6244-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka T., Young H.M., Pachnis V., Enomoto H. Development of the intrinsic and extrinsic innervation of the gut. Dev. Biol. 2016;417:158–167. doi: 10.1016/j.ydbio.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Wang H., Hughes I., Planer W., Parsadanian A., Grider J.R., Vohra B.P., Keller-Peck C., Heuckeroth R.O. The timing and location of glial cell line-derived neurotrophic factor expression determine enteric nervous system structure and function. J. Neurosci. 2010;30:1523–1538. doi: 10.1523/JNEUROSCI.3861-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Broders-Bondon F., Baral V., Paul-Gilloteaux P., Pingault V., Dufour S., Bondurand N. SOX10 and Itgb1 interaction in enteric neural crest cell migration. Dev. Biol. 2013;379:92–106. doi: 10.1016/j.ydbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.J., Edgar D.H., Kenny S.E. Future therapies for Hirschsprung's disease. Semin. Pediatr. Surg. 2012;21:364–370. doi: 10.1053/j.sempedsurg.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Wilkinson D.J., Bethell G.S., Shukla R., Kenny S.E., Edgar D.H. Isolation of enteric nervous system progenitor cells from the aganglionic gut of patients with Hirschsprung's disease. PLoS One. 2015;10:e0125724. doi: 10.1371/journal.pone.0125724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright-Jin E.C., Grider J.R., Duester G., Heuckeroth R.O. Retinaldehyde dehydrogenase enzymes regulate colon enteric nervous system structure and function. Dev. Biol. 2013;381:28–37. doi: 10.1016/j.ydbio.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Bergner A.J., Enomoto H., Milbrandt J., Newgreen D.F., Young H.M. Neural cells in the esophagus respond to glial cell line-derived neurotrophic factor and neurturin, and are RET-dependent. Dev. Biol. 2004;272:118–133. doi: 10.1016/j.ydbio.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Yntema C.L., Hammond W.S. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J. Comp. Neurol. 1954;101:515–541. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- Young H.M., Hearn C.J., Ciampoli D., Southwell B.R., Brunet J.F., Newgreen D.F. A single rostrocaudal colonization of the rodent intestine by enteric neuron precursors is revealed by the expression of PHOX2B, RET, and p75 and by explants grown under the kidney capsule or in organ culture. Dev. Biol. 1998;202:67–84. doi: 10.1006/dbio.1998.8987. [DOI] [PubMed] [Google Scholar]
- Young H.M., Jones B.R., McKeown S.J. The projections of early enteric neurons are influenced by the direction of neural crest cell migration. J. Neurosci. 2002;22:6005–6018. doi: 10.1523/JNEUROSCI.22-14-06005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H.M., Bergner A.J., Muller T. Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J. Comp. Neurol. 2003;456:1–11. doi: 10.1002/cne.10448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.