Abstract
Dogs have been under strong artificial selection as a consequence of their relationship with man. Differences between breeds are evident that could be reflected in seminal characteristics. The present study was to evaluate differences in sperm head morphometry between seven well-defined breeds of dog: the British Bulldog, Chihuahua, German Shepherd, Labrador Retriever, Spanish Mastiff, Staffordshire Terrier, and Valencian Rat Hunting dog. Semen samples were obtained by masturbation and smears stained with Diff-Quik. Morphometric analysis (CASA-Morph) produced four size and four shape parameters. Length, Ellipticity, and Elongation showed higher differences between breeds. MANOVA revealed differences among all breeds. Considering the whole dataset, principal component analysis (PCA) showed that PC1 was related to head shape and PC2 to size. Procluster analysis showed the British Bulldog to be the most isolated breed, followed by the German Shepherd. The PCA breed by breed showed the Chihuahua, Labrador Retriever, Spanish Mastiff, and Staffordshire Terrier to have PC1 related to shape and PC2 to size, whereas the British Bulldog, Valencia Rat Hunting dog, and German Shepherd had PC1 related to size and PC2 to shape. The dendrogram for cluster groupings and the distance between them showed the British Bulldog to be separated from the rest of the breeds. Future work on dog semen must take into account the large differences in the breeds’ sperm characteristics. The results provide a base for future work on phylogenetic and evolutionary studies of dogs, based on their seminal characteristics.
Keywords: Canis familiaris, cluster analysis, diversity, sperm morphometry
INTRODUCTION
What do we mean when we refer to a dog? As a consequence of the long common history between humans and dogs (Canis lupus familiaris), this species has been undergoing one of the faster and bigger artificial selection processes leading to a differentiation of well-defined brands among domestic animals. From this, can we really speak about “dog semen” without reference to the breed, or combine data from different brands without taking into account the part of the variance due to differences between them? From the studies in literature, results from different breeds are usually combined in the same study ignoring possible differences in the breeds.1
The study of reproductive differences and more specifically of semen quality between dog breeds is interesting at least from two points of view: for defining adequate breeding strategies2,3,4 and for analyzing speciation processes due to gamete barriers.5,6,7,8 The structure of sperm subpopulations has been established in a number of species, including the dog,9 and is also related to fertility in other species such as rams.10
Interbreeding studies are scarce and limited to animal production in species such as the boar11 and stallion.12 Although it is quite common to combine different breeds in the same work without any consideration about possible differences between them,13,14 this procedure could mask possible relevant differences among different brands, for example, comparing normozoospermic (implying the general concept of a dog) and teratozoospermic dogs, when differences could be explained as a consequence of samples taken from different breeds.15
The aim of the present work was to define the morphometric relationship between sperm morphological characteristics of several well-defined dog breeds by the use of the ISAS®v1 CASA-Morph system and advanced clustering techniques. It includes the analysis of cluster distance to define a morphometric sperm phylogeny.
MATERIALS AND METHODS
Collection and preparation of the samples
In this study, only the animals which had a clear pedigree as a true representative of their breeds were used. In total, 39 animals were included, comprising four British Bulldog, five Chihuahua, five German Shepherd, five Labrador Retriever, six Spanish Mastiff, seven Staffordshire Terrier, and seven Valencian Rat Hunting dogs. Sampling and analysis were carried out on the REPROVALCAN and Clínica Veterinaria Sangüeso veterinary clinics, located in Valencia (Spain). Following the routine semen extraction by manual masturbation after 14 days ejaculatory abstinence, one ejaculate was obtained in sterile sample cup from each dog. Once extracted, half an hour was necessary to complete the liquefaction (at 37°C) before analysis. After liquefaction, samples were analyzed microscopically to estimate sperm concentration. If necessary, the semen sample was diluted with CaniPlus Chill, sperm extender (MiniTub Ibérica S.L. Tarragona, Spain) to the concentration of 20–30 × 106 spermatozoa per milliliter.
For morphological analysis, 5 μl of each sample was deposited on a glass slide, smeared, and air dried for 1 h. Smears were stained with Diff-Quick (Medion Diagnostics, Düdingen, Switzerland), dipped for 25 s in each solution (fixative, solution I and solution II), washed free of excess colorant with tap water, air dried, and mounted with Eukitt (Sigma-Aldrich, Sant Louis, USA). Analyses were conducted using the morphometry module of an ISAS®v1 (Proiser R+D S.L., Paterna, Spain) system. The camera used was Proiser 782 m attached to a microscope UB203 (UOP/Proiser). Resolution of the analyzed images was 0.084 μm/pixel in both axes. Images from about 250 spermatozoa from each sample were captured and analyzed, to obtain eight morphometric parameter values: sperm head length (L, μm), width (W, μm), area (A, μm2), perimeter (P, μm), and the unitless shape factors Ellipticity (L/W), Elongation ([L − W]/[L + W]), Regularity (πLW/4A), and Rugosity (4πA/P2).
Statistical analysis
Data obtained from the analysis of all sperm morphometric parameters were first tested for normality and homoscedasticity by the Shapiro–Wilk and Kolmogorov–Smirnov tests, respectively. Because none of the parameters satisfied both criteria, nonparametric analyses were performed using the Kruskal–Wallis test.
Multivariate analysis of variance (MANOVA) was based on Wilk's lambda criterion,16 and cluster analysis was performed using the average linkage method.17 The multivariate linear model was: Y = XB + E, where X is the design matrix, B the matrix of regression parameters, and E the matrix of random deviations. E ~ N (0, ∑).
Principal component analysis (PCA) was performed on the morphometric data. We followed the criterion of selecting only the number of PCs with an eigenvalue (variance extracted for that particular principal component) >1 (Kaiser criterion) to select which components should be used in the next step of the analysis. The second step was to perform a two-step cluster procedure with the sperm-derived indices obtained after the PCA.18 All sperm morphometric measurements were clustered step-by-step for breed using a hierarchical clustering procedure (average linkage model and Euclidean2 distance), to classify and establish the breed groupings.19
The results are presented as mean ± standard deviation (s.d.). Statistical significance was considered when P < 0.05. All data were analyzed using InfoStat Software (version 2008, University of Córdoba, Córdoba, Argentina) for Windows.20
RESULTS
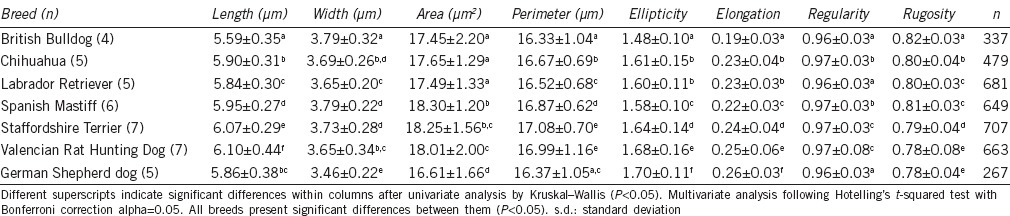
For comparison of the median values of each morphometric parameter independently between breeds, after Kruskal–Wallis analysis, the parameters showing the higher differences between breeds were Length, Ellipticity, and Elongation, while the most similar between them was regularity (Table 1). MANOVA analysis revealed significant differences among all the brands (Table 1).
Table 1.
Morphometric values of sperm head parameters (mean±s.d.) for each breed of dog
Principal components analysis yielded two PCs, both when all animals were considered as a whole population (Table 2) and when each breed was considered independently (Table 3). In the first case, PC1 was clearly related to the head shape of the spermatozoon and PC2 with the size (Table 2). Procluster graphic treatment showed the British Bulldog as the most isolated breed, followed by the German Shepherd, although they had different PC values (Figure 1).
Table 2.
Eigenvalues of each dog sperm head parameter in both PCs from considering all the breeds together as one population
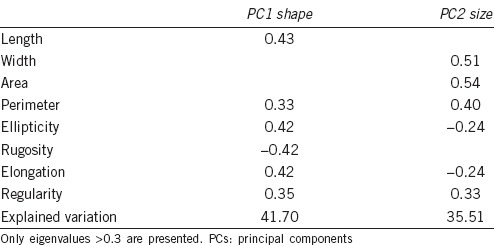
Table 3.
Eigenvalues of each dog sperm head parameter in both PCs, breed-by-breed
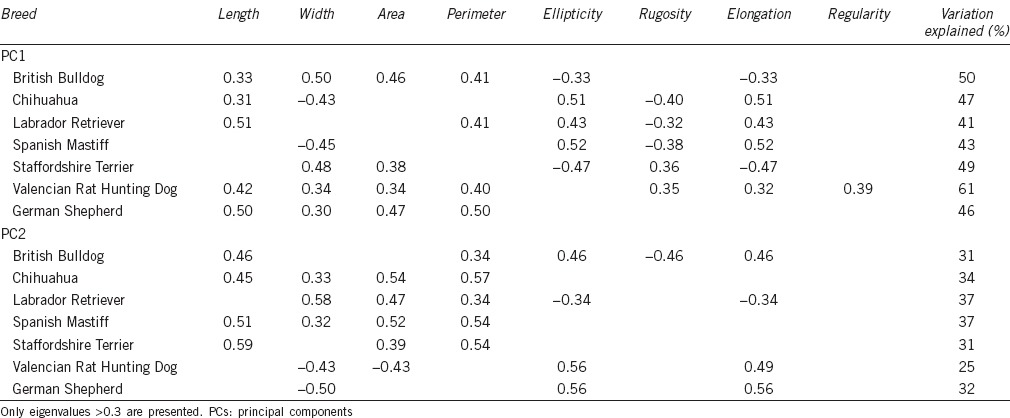
Figure 1.
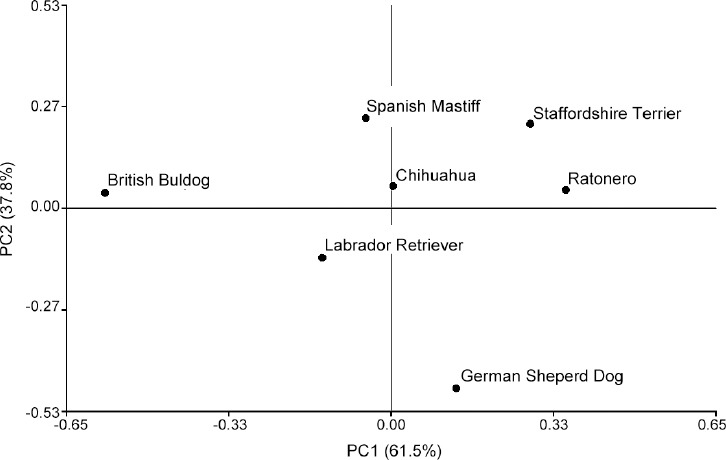
Diagram of breed distribution from PC centroid values (ordinate PC2, abscissa PC1) by procluster analysis.
Looking to PC by breed, some species (Chihuahua, Labrador Retriever, Spanish Mastiff, and Staffordshire Terrier) showed the whole population distribution, i.e., PC1 was related to the shape and PC2 to the size, while others (British Bulldog, Valencian Rat Hunting dog, and German Shepherd) presented the inverse pattern, i.e., PC1 was related to size and PC2 to shape (Table 3).
The construction of a dendrogram for the analysis of cluster groupings and the distance between them showed that the British Bulldog was separated from the rest of the breeds. From those brands, the German Shepherd was in a different group, showing a considerable distance. The Valencian Rat Hunting dog and Staffordshire Terrier were grouped in the same line. For the three remaining breeds, the Spanish Mastiff was independent of the Labrador Retriever and Chihuahua (Figure 2).
Figure 2.

Cluster-distance dendrogram, based on sperm head morphometric data.
DISCUSSION
Following the kind of the analyses made in the past, by just comparing median values of each parameter as independent variables, we can consider Length, Ellipticity, and Elongation as the most informative parameters to permit differentiation between the dog breeds.21 The Multivariate Analysis of Variance, a more advanced mathematical concept of the problem, showed differences among all the breeds, supporting the idea that dogs cannot be considered as one homogeneus concept.22,23 This makes it necessary to differentiate among breeds to obtain useful results when analyzing aspects such as freezability or other physiological stresses on dog spermatozoa, otherwise the variance due to the breed can cover up any variance associated with the process being analyzed.
Sperm design and function are two important determinants of male reproductive success24,25 and must be under strong natural selection,26 related to sperm competition.27,28 In the case of the domestic dog, natural selection has been replaced by strong artificial selection, and this pressure could cause a considerable stress on sperm function and structure, highly related to genetic effects.29 Natural sexual selection (relating to sperm competition and cryptic female choice) has influenced coevolution of oviduct length, testicular size, and sperm morphology in mammals.5 Further, spermatozoa must also overcome the barriers present in the female tract. All of the selective pressures, sperm competition and female tract selection, are likely to have influenced the evolution of the morphology and dimensions of the sperm cell itself.7
Sperm size must be involved in sperm transport either owing to the environment or through sperm selection and competence on their way to encounter the female gamete. In the boar, it has been demonstrated that spermatozoa back-flowing after artificial insemination are those with small head size and a short flagellum.29 This opens a new door for studying the significance of sperm subpopulations. Following what has been observed in natural populations, we can argue that in species such as the dog, where artificial selection has been so strong, the process of breed differentiation must also have presented some correlation of sperm traits and the evolution of female tract features. We have found that the morphometry of British Bulldog spermatozoa is completely isolated from that of other breeds. This could be related to the fact that this breed is particularly dependent on assisted reproduction.
It is highly accepted that all domestic dogs have come from the ancient gray wolf, with the recent evolution of modern dog breeds presenting a highly iterative process that drew on a limited genetic toolkit to create remarkable phenotypic diversity.31 It is interesting that it seems that all the domestic dogs have a unique origin (common ancestor), probably located in the Middle East.32 Current dogs can differ in size by two orders of magnitude and have extremely varied conformation, so it would seem that extreme artificial selection was a powerful force in the rapid development of the diversity in shape. It is critical to explain the genetic diversity of the founding population. If dogs had an origin only from a few wild canids then all this variation must be due to the mutations occurring during about 14 000 years.33 In contrast, if dogs originated from a large population of wild canids and had interbred with them for a long time during their evolutionary history, the high diversity could be explained by this historical influx of different genetic pools.34
The most plausible explanation is that the domestic dog is a genetically diverse species that most likely originated from a large founding stock possibly derived from wolf populations in different places and at different times. However, genetic isolation between some breeds (which happens during heavy breed selection) must have been sufficient to have caused divergence in allele frequency.35
We have studied a similar process in the case of South American camelids. In this case, two domesticated animals, the llama and alpaca, have been derived from the original wild species, guanaco and vicuña. Following the most common definition of species, given by Mayr,36 as the llama and alpaca can produce fertile hybrids, both “species” must be considered as one species. In fact, the level of hybridization in alpacas is close to 90%, but they and llamas are classified not only as different species but are also placed in different genera. Nevertheless, sperm head morphometry of both domestic camelids shows significant differences, indicating a possible process of gamete isolation.8
When discussing comparative sperm morphology, the first recompilation of data about mammalian spermatozoa37 should be considered. In a previous paper, four morphometric subpopulations were observed in mongrel dogs, by following a method similar to that used here.9 Reproductive management could also be improved by taking into consideration the specificity of each breed of dog.3 A good example of the evolution of sperm morphology within a phylogenetically close group has been shown in rodents.6,7,38 These studies have integrated information from evolutionary, physiological, and behavioral studies to address the changes in sperm morphology during evolution. Two main selective forces may have favored these changes: female selection within the reproductive tract, and sperm competition.7 In the case of dog evolution in well-defined breeds, sperm competition does not play a role because most of the reproduction was controlled if not assisted. Hence, it could be promising to look for sperm-female reproductive tract interactions.
It has been shown in Atlantic cod that spermatozoa with short heads maintain their swimming velocity for longer periods than those with long heads.24 Spermatozoa from different species acquire modifications adapted to their specific fertilization environment.39 Certainly, such results cannot be extrapolated to a mammalian species such as the dog, but these kinds of studies must be developed in the future for mammals.
Finally, it must be stressed that, associated with the high artificial selection of dog populations, there is considerable inbreeding, particularly in those breeds more difficult to reproduce. This was recently experimentally revealed in other species, such as trout.40 A comparison between pure breeds and mixed highly hybridized dogs could offer a good model for this kind of study. In addition, the analysis of motility and kinetics in combination with morphometric characteristics could offer a more holistic approach as proposed for fox spermatozoa.41 As a conclusion, future work related to the optimization of dog breeding, freezing/thawing processes, and general management of seminal doses must take into account differences between breeds. A study to find the relationships on the basis of morphometry of different breeds using clustering and cladistic techniques could be expedient.
AUTHOR CONTRIBUTIONS
CS and MC conceived and designed the experiments; MC, AA, MAM, and AGM performed the experiments; AV, JC, and CS analyzed the data; CS wrote the paper.
COMPETING INTERESTS
CS is Professor at Valencia University and acts as Scientific Director of Proiser R+D S.L Research and Development Laboratory. Neither he nor the other authors have interests that influenced the results presented in this paper.
ACKNOWLEDGMENTS
The authors would like to thank the owners of the dogs participating in the study. Some of the dogs are world champions, for which we are even more to be grateful, and also we would like to thank Dr. Sogol Fereidounfar and Dr. Trevor G. Cooper for critical reading and suggested corrections to the manuscript. AV was granted by the CONICIT and MICITT, Costa Rica.
REFERENCES
- 1.Dorado J, Gálvez MJ, Murabito MR, Muñoz-Serrano A, Hidalgo M. Identification of sperm subpopulations in canine ejaculates: effects of cold storage and egg yolk concentration. Anim Reprod Sci. 2011;127:106–13. doi: 10.1016/j.anireprosci.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Peña FJ, Núñez-Martínez I, Morán JM. Semen technologies in dog breeding: an update. Reprod Domest Anim. 2006;41(Suppl 2):21–9. doi: 10.1111/j.1439-0531.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- 3.Núñez-Martínez I, Morán JM, Peña FJ. Sperm indexes obtained using computer-assisted morphometry provide a forecast of the freezability of canine sperm. Int J Androl. 2007;30:182–9. doi: 10.1111/j.1365-2605.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer-Somi S, Aurich C. Use of a new computer-assisted sperm analyzer for the assessment of motility and viability of dog spermatozoa and evaluation of four different semen extenders for predilution. Anim Reprod Sci. 2007;102:1–13. doi: 10.1016/j.anireprosci.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Anderson MJ, Dixson AS, Dixson AF. Mammalian sperm and oviducts are sexually selected: evidence for co-evolution. J Zool. 2006;270:682–6. [Google Scholar]
- 6.Breed WG. The spermatozoon of Eurasian murine rodents: its morphological diversity and evolution. J Morphol. 2004;261:52–69. doi: 10.1002/jmor.10228. [DOI] [PubMed] [Google Scholar]
- 7.Roldan ER, Gomendio M, Vitullo AD. The evolution of eutherian spermatozoa and underlying selective forces: female selection and sperm competition. Biol Rev. 1992;67:551–93. doi: 10.1111/j.1469-185x.1992.tb01193.x. [DOI] [PubMed] [Google Scholar]
- 8.Soler C, Sancho M, García-Molina A, Núñez J, Parráguez VH, et al. Llama and alpaca comparative sperm head morphometric analysis. J Camelid Sci. 2014;7:48–58. [Google Scholar]
- 9.Núñez-Martínez I, Morán JM, Peña FJ. Two-step cluster procedure after principal component analysis identifies sperm subpopulations in canine ejaculates and its relation to cryoresistance. J Androl. 2006;27:596–603. doi: 10.2164/jandrol.05153. [DOI] [PubMed] [Google Scholar]
- 10.Maroto-Morales A, Ramón M, García-Álvarez O, Montoro V, Soler AJ, et al. Sperm head phenotype and male fertility in ram semen. Theriogenology. 2015;84:1536–41. doi: 10.1016/j.theriogenology.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 11.Martín-Hidalgo D, Barón FJ, Robina A, Bragado MJ, Hurtado de Lera et al. Inter- and intra-breed comparative study of sperm motility and viability in Iberian and Duroc boar semen during long-term storage in MR-A and XCell extenders. Anim Reprod Sci. 2013;139:109–44. doi: 10.1016/j.anireprosci.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Kavak A, Lundeheim N, Aidnik M, Einarsson S. Sperm morphology in Stonian and Tori breed stallions. Acta Vet Scand. 2004;45:11–8. doi: 10.1186/1751-0147-45-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holst BS, Rota A, Berg KA, Linde-Forsberg C, Rodriguez-Martinez H. Canine sperm head damage after freezing-thawing: ultrastructural evaluation and content of selected elements. Reprod Domest Anim. 1998;33:77–82. [Google Scholar]
- 14.Rijsselaere T, Van Soom A, Hoflack G, Maes D, de Kruif A. Automated sperm morphometry and morphology analysis of canine semen by the Hamilton-Thorne analyser. Theriogenology. 2004;62:1292–306. doi: 10.1016/j.theriogenology.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Dahlbom M, Anderson M, Vierula M, Alanko M. Morphometry of normal and teratozoospermic canine sperm heads using an image analyser: work in progress. Theriogenology. 1997;48:687–98. doi: 10.1016/s0093-691x(97)00284-7. [DOI] [PubMed] [Google Scholar]
- 16.Bray JH, Scott EM. Multivariate Analysis of Variance. Newbury Park, CA: SAGE Publications, Inc; 1985. [Google Scholar]
- 17.Aldenderfer MS, Blashfield RK. Cluster Analysis. Sage University Paper Series on Quantitative Applications in the Social Sciences, Series 07-044. Beverly Hills, Calif: Sage Publications; 1984. [Google Scholar]
- 18.Vicente-Fiel S, Palacin I, Santolaria P, Yániz JL. A comparative study of sperm morphometric subpopulations in cattle, goat, sheep and pigs using a computer-assisted fluorescence method (CASMA-F) Anim Reprod Sci. 2013;139:182–9. doi: 10.1016/j.anireprosci.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Peña FJ, Saravia F, García-Herreros M, Núñez-Martín I, Tapia JA, et al. Identification of sperm morphometric subpopulations in two different portions of the boar ejaculate and its relation to post-thaw quality. J Androl. 2005;26:716–23. doi: 10.2164/jandrol.05030. [DOI] [PubMed] [Google Scholar]
- 20.Balzarini MG, Gonzalez L, Tablada M, Casanoves F, Di Rienzo JA, et al. Infostat User's guide, Brujas Editorial, Córdoba, Argentina. 2008 [Google Scholar]
- 21.Soler C, Pérez-Sánchez F, Schulze H, Bergmann M, Oberpenning F, et al. Objective evaluation of the morphology of human epididymal sperm heads. Int J Androl. 2000;23:77–84. doi: 10.1046/j.1365-2605.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- 22.Peña AI, López-Lugilde L, Barrio M, Becerra JJ, Quintela LA, et al. Studies on the intracellular Ca [2]+ concentration of frozen-thawed dog spermatozoa: influence of equex from different sources, two thawing diluents and post-thaw incubation in capacitating conditions. Reprod Domest Anim. 2003;38:27–35. doi: 10.1046/j.1439-0531.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 23.Santana M, Batista M, Alamo D, González F, Niño T, et al. Influence of cool storage before freezing on the quality of frozen-thawed semen samples in dog. Reprod Domest Anim. 2013;48:165–70. doi: 10.1111/j.1439-0531.2012.02124.x. [DOI] [PubMed] [Google Scholar]
- 24.Tuset VM, Trippel EA, de Monserrat JJ. Sperm morphology and its influence on swimming speed in atlantic cod. J Appl Ichthyol. 2008;24:398–405. [Google Scholar]
- 25.Fitzpatrick JL, Garcia-Gonzalez F, Evans JP. Linking sperm length and velocity: the importance of intramale variation. Biol Lett. 2010;6:797–9. doi: 10.1098/rsbl.2010.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birkhead TR, Pizzari T. Post-copulatory sexual selection. Nat Rev Genet. 2002;3:262–73. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 27.Gómez Montoto L, Magaña C, Tourmente M, Martín-Coello J, Crespo C, et al. Sperm competition, sperm numbers and sperm quality in muroid rodents. PLoS One. 2011;6:e18173. doi: 10.1371/journal.pone.0018173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leivers S, Rhodes G, Simmons LW. Sperm competition in humans: mate guarding behavior negatively correlates with ejaculate quality. PLoS One. 2014;9:e108099. doi: 10.1371/journal.pone.0108099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birkhead, TR, Pellatt EJ, Brekke P, Yeates R, Castillo-Juarez H. Genetic effects on sperm design in the zebra finch. Nature. 2005;434:383–7. doi: 10.1038/nature03374. [DOI] [PubMed] [Google Scholar]
- 30.García-Vázquez FA, Hernández-Caravaca I, Yánez-Quintana W, Matás C, Soriano-Úbeda C, et al. Morphometry of boar sperm head and flagellum in semen backflow after insemination. Theriogenology. 2015;84:566–74. doi: 10.1016/j.theriogenology.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 31.von Holdt BM, Pollinger JP, Lohmueller KE, Han E, Parker HG, et al. Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature. 2010;464:898–903. doi: 10.1038/nature08837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard JA, Wayne RK, Wheeler J, Valadez R, Guillén S, et al. Ancient DNA evidence for old world origin of new world dogs. Science. 2002;298:1613–6. doi: 10.1126/science.1076980. [DOI] [PubMed] [Google Scholar]
- 33.Olsen SJ. Origins of the Domestic Dog. Tucson: University of Arizona Press; 1985. [Google Scholar]
- 34.Wayne RK, Nash WG, O’Brien SJ. Chromosomal evolution of the Canidae. II. Divergence from the primitive carnivore karyotype. Cytogenet Cell Genet. 1987;44:134–41. doi: 10.1159/000132357. [DOI] [PubMed] [Google Scholar]
- 35.Vilà C, Maldonado JE, Wayne RK. Pjylogenetic relationships, evolution, and genetic diversity of the domestic dog. J Heredity. 1999;90:71–7. doi: 10.1093/jhered/90.1.71. [DOI] [PubMed] [Google Scholar]
- 36.Mayr E. Systematics and the Origin of Species. New York: Columbia University Press; 1942. [Google Scholar]
- 37.Cummins JM, Woodwall PF. On sperm mammalian dimensions. J Reprod Fert. 1985;75:153–75. doi: 10.1530/jrf.0.0750153. [DOI] [PubMed] [Google Scholar]
- 38.Gallardo MH, Mondaca FC, Ojeda RA, Köhler N, Garrido O. Morphological diversity in the sperms of caviomorph rodents. J Neotrop Mammal. 2002;9:159–70. [Google Scholar]
- 39.Ramón M, Jiménez-Rabadán O, García-Álvarez O, Maroto-Morales A, Soler AJ, et al. Understanding sperm heterogeneity: biological and practical implications. Reprod Domest Anim. 2014;49(Suppl 4):30–6. doi: 10.1111/rda.12404. [DOI] [PubMed] [Google Scholar]
- 40.Johnson K, Butts IA, Smith JL, Wilson CC, Pitcher TE. The effects of inbreeding on sperm quality traits in captive-bred lake trout, Salvelinus namaycush (Walbaum, 1972) J Appl Ichtyol. 2015;31(Suppl 1):62–70. [Google Scholar]
- 41.Soler C, Contell J, Bori L, Sancho M, García-Molina A, et al. Sperm kinematic, head morphometric and kinetic-morphometric subpopulations in the blue fox (Alopex lagopus) Asian J Androl. 2016 doi: 10.4103/1008-682X.188445. doi: 10.4103/1008-682X.188445. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


