Abstract
This work provides information on the blue fox ejaculated sperm quality needed for seminal dose calculations. Twenty semen samples, obtained by masturbation, were analyzed for kinematic and morphometric parameters by using CASA-Mot and CASA-Morph system and principal component (PC) analysis. For motility, eight kinematic parameters were evaluated, which were reduced to PC1, related to linear variables, and PC2, related to oscillatory movement. The whole population was divided into three independent subpopulations: SP1, fast cells with linear movement; SP2, slow cells and nonoscillatory motility; and SP3, medium speed cells and oscillatory movement. In almost all cases, the subpopulation distribution by animal was significantly different. Head morphology analysis generated four size and four shape parameters, which were reduced to PC1, related to size, and PC2, related to shape of the cells. Three morphometric subpopulations existed: SP1: large oval cells; SP2: medium size elongated cells; and SP3: small and short cells. The subpopulation distribution differed between animals. Combining the kinematic and morphometric datasets produced PC1, related to morphometric parameters, and PC2, related to kinematics, which generated four sperm subpopulations – SP1: high oscillatory motility, large and short heads; SP2: medium velocity with small and short heads; SP3: slow motion small and elongated cells; and SP4: high linear speed and large elongated cells. Subpopulation distribution was different in all animals. The establishment of sperm subpopulations from kinematic, morphometric, and combined variables not only improves the well-defined fox semen characteristics and offers a good conceptual basis for fertility and sperm preservation techniques in this species, but also opens the door to use this approach in other species, included humans.
Keywords: integration of motility and morphology, principal component analysis, sperm morphometry, subpopulation
INTRODUCTION
Foxes have been domesticated in some cold countries (Finland, China, Russia, Argentina, Canada, and others), where its breeding is of high economical relevance. Most reproduction of these farmed foxes is by artificial insemination, but the process is not very technical, and only a few trials regarding general reproductive physiology and management have been proposed.1,2,3,4,5 Furthermore, sperm characteristics have only recently been studied.6,7 These species are sperm homomorphous with a low level of morphological sperm abnormalities (around 10%). In species with low sperm morpho-abnormalities, it would seem that morphological analysis is of little importance, but for this reason, the use of morphometry by CASA-Morph systems to find possible differences between ejaculates is obviously efficient and necessary.8,9,10,11,12
The purpose of the present work, with the aim of offering a scientific basis for this service, was to combine the multivariate analysis of both kinematic and morphometric data and the establishment of subpopulation structure based on all these parameters in the blue fox species.
MATERIALS AND METHODS
Individual semen samples from twenty individual blue Foxes (Alopex lagopus) were obtained by masturbation directly by technical personnel on five farms in the area of Vaasa (Finland). Samples were obtained from trained animals used for artificial insemination in a routine program. The whole sample was deposited in nonsterile sample tubes. Samples were analyzed at the same farm where they were obtained.
Kinematic and morphometric analyses were performed with the ISAS®v1 CASA-Mot and CASA-Morph systems (Proiser R+D, S.L., Paterna, Spain), comprising a UOP200i/Proiser microscope, the Proiser 782M video camera, and software. For motility analysis, raw samples were initially observed in the microscope, through a 10× negative phase-contrast objective to estimate the sperm concentration, after which 10 μl of raw sample was diluted with extender Safecell Plus (IMV, L’Aigle, France) to a working concentration of 20 × 106 cells ml−1. After thorough mixing, the samples were placed in an ISAS®D4C16 disposable counting chamber (Proiser R+D). Each one has four tracks with seven printed viewing squares, with a constant depth of 16 μm between slide and fixed cover slide. A sample volume of 3 μl is enough to fill the chamber completely, being distributed along the track by capillarity. When full, the slide could be immediately analyzed because time is not needed for stabilization of the fluid inside the chamber. Samples were analyzed with the ISAS®v1 motility module. Eight kinematic parameters were obtained, three velocities (curvilinear [VCL], straight-line [VSL], and averaged-path [VAP], μm s−1), three dimensionless motility indices (LIN [VSL/VCL], STR [VSL/VAP], and WOB [VAP/VCL]) and ALH (μm) and BCF (Hz). For each sample, seven fields, one inside each of the squares on the counting chamber, were captured and analyzed.7
For morphological analysis, 5 μl of each sample was deposited on a glass slide, smeared and air dried for one hour. Smears were stained with Diff-Quick (Medion Diagnostics, Düdingen, Switzerland), dipped for 25 s in each solution (fixative, solution I and solution II), washed free of excess colorant with water, air dried, and mounted with Eukitt (Sigma-Aldrich, Saint Louis, MO, USA). In the ISAS®v1 morphology module, about 200 cell images were captured at random and analyzed which generated four size parameters [length (L, μm), width (W, μm), area (A, μm2) and perimeter (P, μm)] and four derived dimensionless parameters of head shape [ellipticity (L/W), rugosity (4πA/P2), elongation ((L-W)/(L+W)), regularity (πLW/4A)].
Statistical analysis
To identify sperm subpopulations, clustering procedures were performed at first by each kinematic and morphometric parameter independently and then by the combination both datasets.12 In the three cases, the first step was to perform a principal component analysis (PCA) of the morphometric data. To select the number of principal components that should be used in the next step of analysis, the criterion of selecting only those components with an eigenvalue (variance extracted for that particular principal component) >1 (Kaiser criterion) was chosen. The second step was to perform a two-step cluster procedure with the sperm-derived indices obtained after the PCA to determine the subpopulation structure.
All sperm measurements within each ejaculate were clustered by kinematic, morphometric, and combined kinematic + morphometric parameter values using a nonhierarchical clustering procedure (k-means model and Euclidean distance), to classify the spermatozoa of the dataset into a reduced number of subpopulations according to their kinematic and sperm head morphometric values as has been described previously.9 The relative distribution frequency of spermatozoa belonging to each subpopulation by animal was analyzed by Chi-square and Mantel–Haenszel Chi-square tests.
The results are presented as mean ± standard deviation (s.d.). Statistical significance was considered at P < 0.05. All data were analyzed using InfoStat Software (v. 2008, University of Córdoba, Córdoba, Argentina) for Windows.13
RESULTS
Principal component analysis
The analysis was performed at three levels: kinematic, morphometric, and a combination of kinematic and morphometrics (Table 1).
Table 1.
PC analysis of fox spermatozoa based on kinetic (K), morphometric (M), and both sets of (T) data
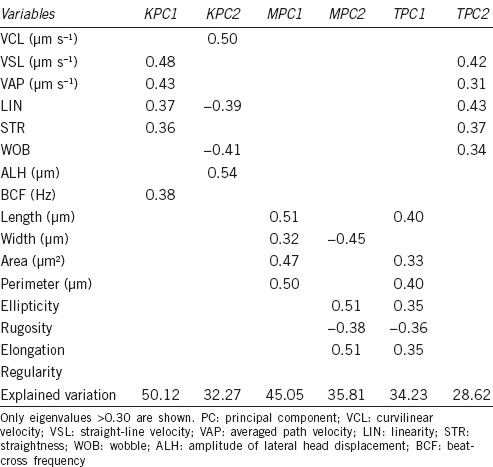
The eight kinematic parameters were reduced to two PCs. PC1 was related to linear variables (VSL, VAP, and LIN), explaining the 50.1% of the variance. PC2 was related to oscillatory movement (VCL and ALH), explaining 32.8% (Table 1).
The eight morphometric variables were also reduced to two PCs, being PC1, referring to size variables (Length, Area, and Perimeter) and explaining the 45.1%, and PC2, referring to elongation shape of the cells (Ellipticity and Elongation) for 35.8% of the total variance (Table 1).
Finally, considering all the variables together, again two PCs were found, even though explaining only 62.9% of the total variance. PC1 was related to morphometric parameters while PC2 was related to kinematic parameters (Table 1).
Kinematic subpopulation structure
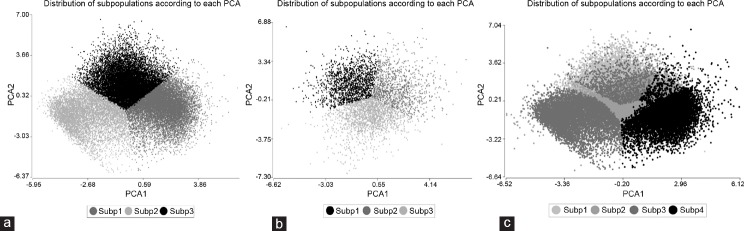
For the kinematic parameters, the whole population was divided into three independent subpopulations (Figure 1a). SP1 comprised 40.7% of the cells and was defined by fast and linear movement (with the highest VSL and an STR of 0.91); SP2 was less frequent at 22.2%, characterized by slow and nonoscillatory motility (indicating by the smallest ALH); and SP3, with 37.1% of the cells, was medium in speed and oscillatory (the highest VCL and ALH). The BCF increased from SP1 to SP3 (Table 2).
Figure 1.
Subpopulation (Subp) distribution according principal component analysis (PCA) for (a) kinematics; (b) morphometry; (c) kinetics and morphometry.
Table 2.
Kinematic sperm subpopulations in fox semen in all animals (A) and percentage of subpopulations in each male (B)
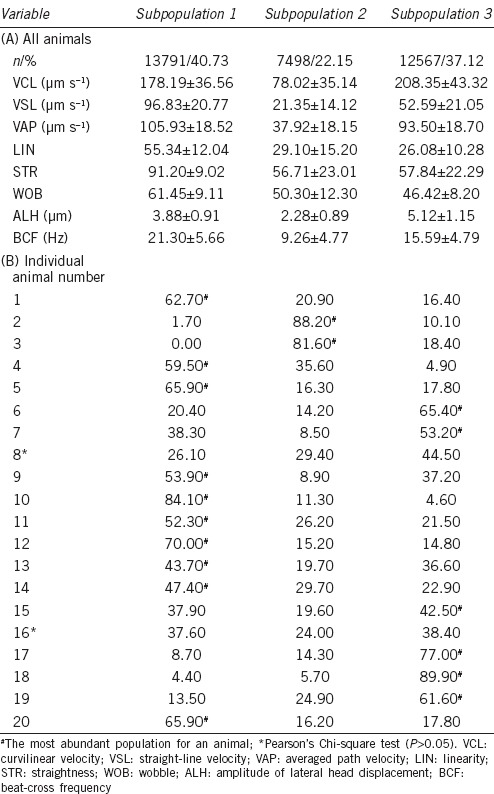
In almost all cases, the subpopulation distribution by animal was significantly different (χ2, P < 0.05) and only two animals (numbers 8 and 16) showed no differences in subpopulations. SP1 was predominant in ten animals, SP2 in two, and SP3 in six. In all cases, one subpopulation was clearly greater than the others (Table 2).
Morphometric subpopulation structure
The morphometric data also revealed three subpopulations (Figure 1b). SP1 comprised 35.3% of the cells and was characterized by large oval cells; SP2, less frequent at 26.7%, included medium size elongated cells; SP3 with 38.1% referred to small and short cells. The high level of regularity shown in all the subpopulations was remarkable (Table 3).
Table 3.
Morphometric sperm subpopulations in fox semen in all animals (A) and percentage of subpopulations in each male (B)
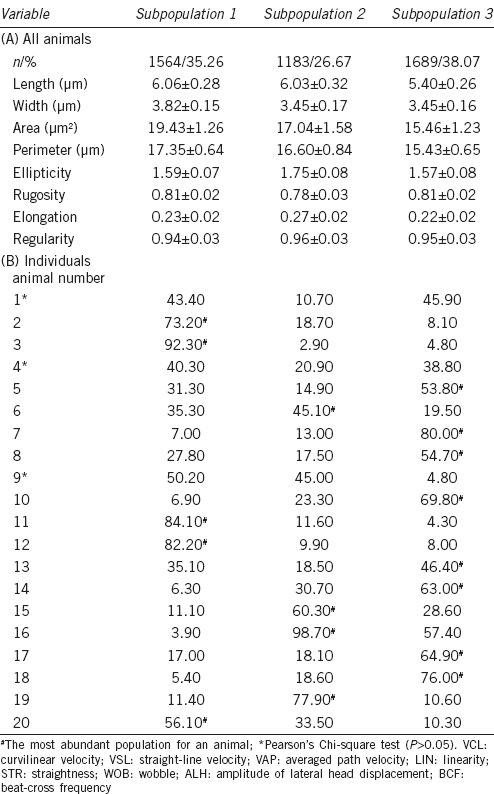
The subpopulation distribution by animal was significantly different (χ2, P < 0.05) in all cases although three animals (numbers 1, 4, and 9) showed two populations with similar frequency. SP1 was predominant in five animals, SP2 in four, and SP3 in eight (Table 3).
Combined kinematic and morphometric subpopulation structure
When both kinematic and morphometric variables were considered together, the total population could be divided into four subpopulations (Figure 1c). SP1 included 20.8% of cells assessed for motility and 29.7% of cells assessed for morphometry, with high oscillatory motility, large size, and short heads; SP2 was composed of medium velocity cells with small and short heads and comprising 32.1% of the motile and 26.9% of the morphologically assessed cells; SP3 with 21.0% of motile and 26.7% morphologically assessed cells, with slow motion and small and elongated cells; and SP4 was composed of high linear speed and large size elongated cells for 26.1% of motile and 16.7% of the morphologically assessed cells (Table 4).
Table 4.
Combined kinematic and morphometric sperm subpopulations in fox semen in all animals (A) and percentage of subpopulations in each male (B)
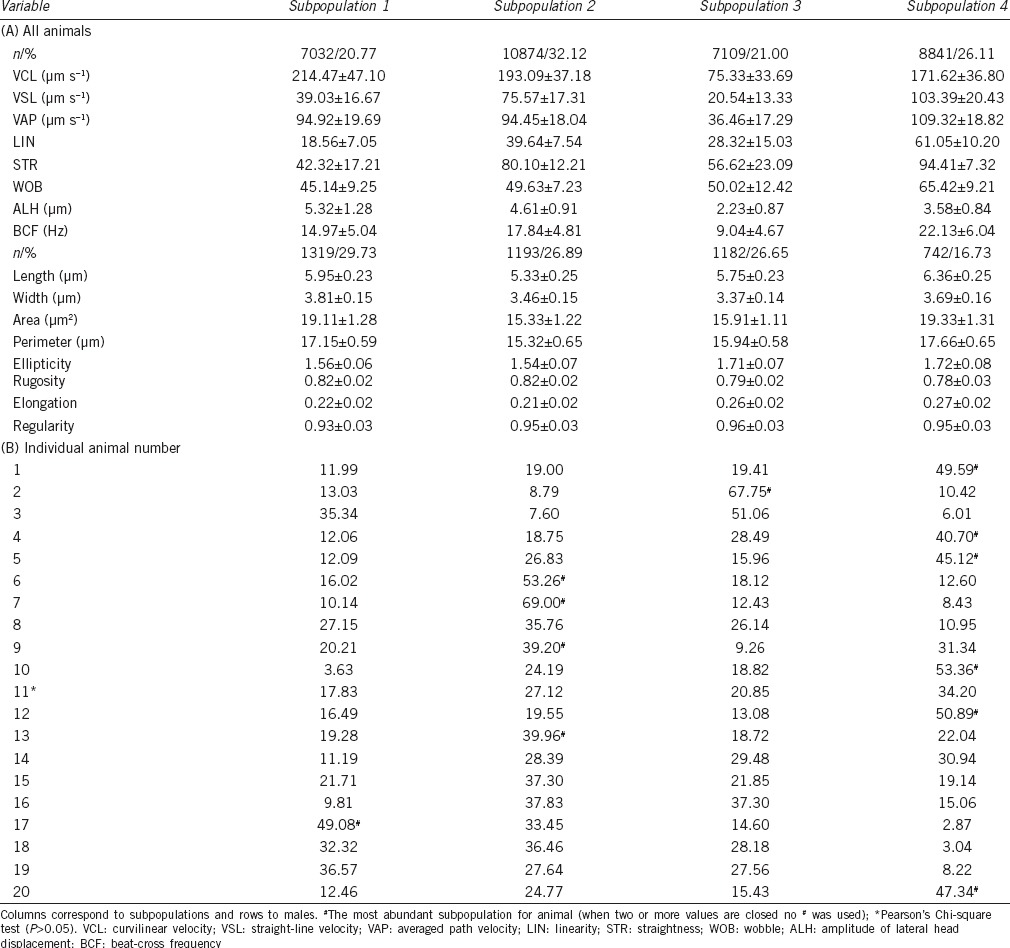
Subpopulation distribution was different (χ2, P < 0.05) in all animals but one (number 1). SP1 was the least frequent, being the biggest in only one case, whereas SP2 was most frequent in four, SP3 in two and the most frequent was SP4 being the most usual in six animals. Seven animals showed two subpopulations with equivalent frequencies (Table 4).
DISCUSSION
Until recently, semen was, and even is now, considered as a group of “equivalent” cells having the same role to win the race toward the oocyte, in something like a marathon. From this point of view, the concept of morphologically “normal” spermatozoa was developed and used for decades.14,15,16,17 The introduction of CASA technology has allowed the acquisition of kinematic and morphometric parameters that can be used for advanced multivariate statistics. By combining both CASA data and multivariate statistics, many publications in recent years have revealed the true structure of the sperm population in the semen is composed by different subpopulations with possible, but unknown, functional significance.18 These studies have been made using kinematic (boar,19 dog,20 fox,7 rabbit,21 solea,22 and stallion23,24) or morphometric parameters (boar, bull, goat,12 llama,25 ram,26,27 and red deer28). To the best of our knowledge, this study is the first work analyzing combined information of both kinetic and morphometric parameters, and offering a new approach to the evaluation of sperm subpopulations. Some previous papers have been published on the dog using both datasets, but not joining them as here.29,30 In any case, this holistic approach provides a much more complete explanation of phenomena related to sperm function.
The results obtained in the present study for motility subpopulation structure were completely in accordance with that published previously for the same species.7 This fact indicates that, independently of the animal, the structure is constant within the species. The three morphometric subpopulations defined in this species are in agreement with those described for other carnivore species such as the puma,31 but not with those for the dog, where four subpopulations have been found.29 This is the first time analysis with both kinds of metric parameters, kinematics and morphometric, have been performed. It revealed four sperm subpopulations, indicating that motility and morphology can be combined to provide a new perspective to assess what an ejaculate is and how it may function.
Even without following the holistic approach used here, it has been shown that sperm size and velocity subpopulations are interrelated, and that both are good indicators of fertility in red deer, when both sets of parameters are independently considered. Males show higher fertility when samples comprise higher percentages of spermatozoa with rapid and linear movement and of elongated shape.32 However, in another study, a clear correspondence between morphometric and kinematic sperm subpopulations was not observed in the ram.33 In the boar, it has been demonstrated that spermatozoa back-flowing after artificial insemination are those of low head size and short flagellum.34 In some birds, it has been shown that a strong positive correlation exists between sperm velocity and sperm flagellar length, the flagellum:head length ratio, tail length, and total sperm length.35,36 Both sperm length and velocity are heritable traits.11,37
Finally, it must be emphasized that in addition to the improvement in farm production of foxes, the information obtained on farmed foxes could be useful for its application to silver fox populations, and not only to this species but also to related ones. This was revealed in other species such as the red deer38 and brown bear.39,40
CONCLUSION
The establishment of sperm subpopulations by the use of kinematic, morphometric, and combined variables not only improves the well-defined fox semen characteristics, and offers a good conceptual basis for fertility and sperm preservation techniques in this species, but also opens the door to use this approach in other species, included humans.
COMPETING INTERESTS
CS is Professor at Valencia University and acts as Scientific Director of Proiser R+D S.L Research and Development Laboratory. Neither he nor the other authors have interests that influenced the results presented in this paper.
AUTHOR CONTRIBUTIONS
CS and JS conceived and designed the experiments; JC, LB, MS, and AG-M performed the experiments; JC, AV, and CS analyzed the data; CS wrote the paper.
ACKNOWLEDGMENTS
The authors are grateful to the farmers permitting samples to be taken, Sune Bergman, Ulf Eriksson, Peter Kastus, Leif Nyvall, and Anders Segervall. We also thank Dr. Sogol Fereidounfar for her critical reading and connections on the manuscript. AV was granted by CONICIT and MICITT, Costa Rica.
REFERENCES
- 1.Farstad W, Krogenæs A, Nagyová E, Hafne AL, Hyttel P. In vitro techniques in fox reproduction. Livest Prod Sci. 1993;36:23–7. [Google Scholar]
- 2.Ilukha VA, Harri M, Rekilä T. Reproductive success of farmed blue foxes. J Anim Breed Genet. 1997;114:465–74. doi: 10.1111/j.1439-0388.1997.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 3.Farstad W. Reprodcution in foxes: current research and future challenges. Anim Reprod Sci. 1998;53:35–42. doi: 10.1016/s0378-4320(98)00125-0. [DOI] [PubMed] [Google Scholar]
- 4.Berg KA, Wiger R, Dahl E, Torp T, Farstad W, et al. Seasonal changes in spermatogenic activity and plasma levels of FSH, LH and testosterone, and the effect of immunization against inhibin in the male silver fox (Vulpes vulpes) Int J Androl. 2001;24:284–94. doi: 10.1046/j.1365-2605.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 5.Pyykönen T, Ahola L, Hänninen S, Mononen J. A note on the reproductive success of primiparous blue fox vixens in social groups. Anim Reprod Sci. 2009;112:409–14. doi: 10.1016/j.anireprosci.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Stasiak K, Janicki B, Kupcewicz B. Biologic parameters of polar fox (Alopex lagopus L.) semen during the breeding season. Turk J Vet Anim Sci. 2010;34:327–31. [Google Scholar]
- 7.Soler C, García A, Contell J, Segervall J, Sancho M. Kinematics and subpopulations’ structure definition of blue fox (Alopex lagopus) sperm motility using the ISAS ® v1 CASA system. Reprod Domest Anim. 2014;49:560–7. doi: 10.1111/rda.12310. [DOI] [PubMed] [Google Scholar]
- 8.Sancho M, Pérez-Sánchez F, Tablado L, de Monserrat JJ, Soler C. Computer assisted morphometric analysis of ram sperm heads: evaluation of different fixative techniques. Theriogenology. 1998;50:27–37. doi: 10.1016/s0093-691x(98)00110-1. [DOI] [PubMed] [Google Scholar]
- 9.Peña FJ, Saravia F, García-Herreros M, Núñez-Martín I, Tapia JA, et al. Identification of sperm morphometric subpopulations in two different portions of the boar ejaculate and its relation to post-thaw quality. J Androl. 2005;26:716–23. doi: 10.2164/jandrol.05030. [DOI] [PubMed] [Google Scholar]
- 10.Yániz JL, Vicente-Fiel S, Capistrós S, Palacín I, Santolaria P. Automatic evaluation of ram sperm morphometry. Theiorgenology. 2012;77:1343–50. doi: 10.1016/j.theriogenology.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Lavara R, Vicente JS, Baselga M. Genetic variation in head morphometry of rabbit sperm. Theriogenology. 2013;80:313–8. doi: 10.1016/j.theriogenology.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Vicente-Fiel S, Palacin I, Santolaria P, Yániz JL. A comparative study of sperm morphometric subpopulations in cattle, goat, sheep and pigs using a computer-assisted fluorescence method (CASMA-F) Anim Reprod Sci. 2013;139:182–9. doi: 10.1016/j.anireprosci.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Balzarini MG, Gonzalez L, Tablada M, Casanoves F, Di Rienzo JA, et al. Infostat User's guide, Brujas Editorial, Córdoba, Argentina. 2008 [Google Scholar]
- 14.Rivera MM, Quintero-Moreno A, Barrera X, Palomo MJ, Rigau T, et al. Natural Mediterranean photoperiod does not affect the main parameters of boar-semen quality analysis. Theriogenology. 2005;64:934–46. doi: 10.1016/j.theriogenology.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Bellastella G, Cooper TG, Battaglia M, Ströse A, Torres I, et al. Dimensions of human ejaculated spermatozoa in Papanicolau-stained seminal and swim up smears obtained from the Integrated Semen Analysis System (ISAS ®) Asian J Androl. 2010;12:871–9. doi: 10.1038/aja.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 17.Dorado J, Gálvez MJ, Demyda-Peyrás S, Ortiz I, Morrell JM, et al. Differences in preservation of canine chilled semen using simple sperm, washing, single-layer centrifugation and modified swim-up preparation techniques. Reprod Fertil Dev. 2015 doi: 10.1071/RD15071. doi: 10.1071/RD15071. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Pastor F, Tozado EJ, Garde JJ, Anel L, de Paz P. Statistical series: opportunities and challenges of sperm motility subpopulation analysis. Theriogenology. 2011;75:783–95. doi: 10.1016/j.theriogenology.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Quintero-Moreno A, Rigau T, Rodríguez-Gil JE. Regression analyses and motility sperm subpopulation structure study as improving tools in boar semen quality analysis. Theriogenology. 2004;61:673–90. doi: 10.1016/s0093-691x(03)00248-6. [DOI] [PubMed] [Google Scholar]
- 20.Dorado J, Gálvez MJ, Murabito MR, Muñoz-Serrano A, Hidalgo M. Identification of sperm subpopulations in canine ejaculates: effects of cold storage and egg yolk concentration. Anim Reprod Sci. 2011;127:106–13. doi: 10.1016/j.anireprosci.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Quintero-Moreno A, Rigau T, Rodríguez-Gil JE. Multivariate cluster analysis regression procedures as tools to identify motile sperm subpopulations in rabbit semen to predict semen fertility and litter size. Reprod Domest Anim. 2007;42:312–9. doi: 10.1111/j.1439-0531.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 22.Martínez-Pastor F, Cabrita E, Soares F, Anel L, Dinis MT. Multivariate cluster analysis to study motility activation in Solea senegalensis spermatozoa: a model for marine teleosts. Reproduction. 2008;135:449–59. doi: 10.1530/REP-07-0376. [DOI] [PubMed] [Google Scholar]
- 23.Quintero-Moreno A, Mió J, Rigau AT, Rodríguez-Gil JE. Identification of sperm subpopulations with specific motility characteristics in stallion ejaculates. Theriogenology. 2003;59:1973–90. doi: 10.1016/s0093-691x(02)01297-9. [DOI] [PubMed] [Google Scholar]
- 24.Ortega-Ferrusola C, Macías García B, Suárez Rama V, Gallardo-Bolaños JM, González-Fernández L, et al. Identification of sperm subpopulations in stallion ejaculates: changes after cryopreservation and comparison with traditional statistics. Reprod Domest Anim. 2009;44:419–23. doi: 10.1111/j.1439-0531.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 25.Soler C, Sancho M, García A, Fuentes MC, Núñez J, et al. Ejaculate fractioning effect on llama sperm head morphometry as assessed by the ISAS ® CASA system. Reprod Domest Anim. 2014;49:71–8. doi: 10.1111/rda.12226. [DOI] [PubMed] [Google Scholar]
- 26.Bravo JA, Montanero J, Calero R, Roy TJ. Identification of sperm subpopulations with defined motility characteristics in ejaculates from Ile de France rams. Anim Reprod Sci. 2011;129:22–9. doi: 10.1016/j.anireprosci.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Martí JI, Aparicio IM, Leal CL, García-Herreros M. Seasonal dynamics of sperm morphometric subpopulations and its association with sperm quality parameters in ram ejaculates. Theriogenology. 2012;78:528–41. doi: 10.1016/j.theriogenology.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 28.Esteso MC, Fernández-Santos MR, Soler AJ, Montoro V, Martínez-Pastor F, et al. Identification of sperm-head morphometric subpopulations in iberian red deer epididymal sperm samples. Reprod Domest Anim. 2009;44:206–11. doi: 10.1111/j.1439-0531.2007.01029.x. [DOI] [PubMed] [Google Scholar]
- 29.Núñez Martínez I, Morán JM, Peña FJ. Two-step cluster procedure after principal component analysis identifies sperm subpopulations in canine ejaculates and its relation to cryoresistance. J Androl. 2006;27:596–603. doi: 10.2164/jandrol.05153. [DOI] [PubMed] [Google Scholar]
- 30.Núñez-Martínez I, Moran JM, Peña FJ. Sperm indexes obtained using computer-assisted morphometry provide a forecast of the freezability of canine sperm. Int J Androl. 2007;30:182–9. doi: 10.1111/j.1365-2605.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- 31.Cucho H, Alarcón V, Ordóñez C, Ampuero E, Meza A, et al. Puma (Puma concolor) epididymal sperm morphometry assessed by ISAS CASA system. Asian J Androl. 2016 doi: 10.4103/1008-682X.187584. doi: 10.4103/1008-682X.187584. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramón M, Soler AJ, Ortiz JA, García-Alvarez O, Maroto-Morales A, et al. Sperm population structure and male fertility: an intraspecific study on sperm design and velocity in red deer. Biol Reprod. 2013;89(110):1–7. doi: 10.1095/biolreprod.113.112110. [DOI] [PubMed] [Google Scholar]
- 33.Yániz JL, Palacín I, Vicente-Fiel S, Sánchez-Nadal JA, Santolaria P. Sperm population structure in high and low field fertility rams. Anim Reprod Sci. 2015;156:128–34. doi: 10.1016/j.anireprosci.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 34.García-Vázquez FA, Hernández-Caravaca I, Yánez-Quintana W, Matás C, Soriano-Úbeda C, et al. Morphometry of boar sperm head and flagellum in semen backflow after insemination. Theriogenology. 2015;84:566–74. doi: 10.1016/j.theriogenology.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Humphries S, Evans JP, Simmons LW. Sperm competition: linking form to function. BCM Evol Biol. 2008;8:319. doi: 10.1186/1471-2148-8-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzpatrick JL, García-Gonzalez F, Evans JP. Linking sperm length and velocity: the importance of intramale variation. Biol Lett. 2010;6:797–9. doi: 10.1098/rsbl.2010.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mossman J, Slate J, Humphries S, Birkhead T. Sperm morphology and velocity are genetically codetermined in the zebra finch. Evolution. 2009;63:2730–7. doi: 10.1111/j.1558-5646.2009.00753.x. [DOI] [PubMed] [Google Scholar]
- 38.Garde JJ, Martínez-Pastor F, Gomendio M, Malo AF, Soler AJ, et al. The application of reproductive technologies to natural populaitons of red deer. Reprod Domest Anim. 2006;41(Suppl 2):93–102. doi: 10.1111/j.1439-0531.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- 39.Anel L, Álvarez M, Martínez-Pastor F, Gomes S, Nicolás M, et al. Sperm cryopreservation in brown bear (Ursus arctos): preliminary aspects. Reprod Domest Anim. 2008;43(Suppl 4):9–17. doi: 10.1111/j.1439-0531.2008.01248.x. [DOI] [PubMed] [Google Scholar]
- 40.Álvarez-Rodríguez M, Álvarez M, López-Ureña E, Martínez-Rodríguez C, Borragan S, et al. Brown bear sperm double freezing: effect of elapsed time and use of PureSperm ® gradient between freeze-thaw cycles. Cryobiology. 2013;67:339–46. doi: 10.1016/j.cryobiol.2013.10.001. [DOI] [PubMed] [Google Scholar]