Abstract
This study was aimed to evaluate the effects of dietary n-6:n-3 ratio and Vitamin E on the membrane properties and motility characteristics of spermatozoa in boars. Forty Duroc boars were randomly distributed in a 2 × 2 factorial design with two n-6:n-3 ratios (14.4 and 6.6) and two Vitamin E levels (200 and 400 mg kg−1). During 16 weeks of treatment, fresh semen was collected at weeks 0, 8, 12, and 16 for measurements of motility characteristics, contents of fatty acids, membrane properties (membrane fluidity and membrane integrity), and lipid peroxidation of the spermatozoa. The semen was diluted in Beltsville Thawing Solution (BTS) extender and stored at 17°C, and the sperm motility was assessed at 12, 36, 72, and 120 h of storage. The 6.6 n-6:n-3 dietary ratio increased the contents of n-3 polyunsaturated fatty acids (PUFAs) and docosahexaenoic acid (DHA) and improved the membrane integrity and membrane fluidity of the spermatozoa, resulting in notably increased total motility, sperm progressive motility, and velocity parameters of fresh semen. Feeding diet with Vitamin E (400 mg kg−1) prevented sperm lipid peroxidation, and resulted in higher total motility and sperm progressive motility in fresh and liquid stored semen. In conclusion, the adjustment of n-6:n-3 ratio (6.6) and supply of Vitamin E (400 mg kg−1) successfully improved sperm motility characteristics and thus may be beneficial to the fertility of boars, which might be due to the modification of the physical and functional properties of spermatozoa membrane in response to dietary supplementation.
Keywords: boar, dietary n-6:n-3 ratio, fatty acid composition, lipid peroxidation, membrane properties, motility characteristics, Vitamin E
INTRODUCTION
Sperm production is the number of good-quality sperm cells ejaculated within a certain time, which is subsequently determined by the percentage of living and progressively motile sperm cells.1 Sperm production is a crucial determinant of the number of mating via artificial insemination.2 A major constraining factor of sperm production is the poor sperm motility3 resulted from a multistep process.4 Sperm motility is a central index of fertility strongly influenced by environmental factors, with diet being one of the most important modifying agents.5 Among dietary factors, numerous experimental data from human and animal studies indicate a positive effect of fish-derived n-3 polyunsaturated fatty acids (PUFAs) on sperm motility and fertility.6,7,8,9 For example, although the n-6:n-3 ratio in the fatty acid of porcine commercial diets is often >10:1 with no n-3 PUFA content, Mitre et al.7 found that n-3 PUFA-enriched diets that contained docosahexaenoic acid (DHA; 22:6n-3) reversed the n-6:n-3 ratio in spermatozoa and improved sperm motility. There was one research suggesting that dietary n-6:n-3 ratio in cockerels10 improved the fertilizing ability of spermatozoa. However, the underlying mechanism through which dietary n-6:n-3 ratio influences sperm functions remains unknown.
PUFAs of the n-6 and n-3 series are classified according to the distance of the first double bond to the methyl terminal. PUFAs cannot be synthesized de novo by animals and thus need to be provided in diets as they have been suggested to be important for the motility and functions of spermatozoa.11 As for n-6 fatty acids (FA), to the best of our knowledge, only three studies in chicken,8 bulls,12 and rams13 have reported a greater effect of dietary n-6 PUFA on the quality of spermatozoa. The porcine commercial diets provide adequate n-6 FA,6,14 which probably can satisfy the boar's requirement. Whereas dietary n-3 PUFA modifies spermatozoa FA profile7,15 and promotes the susceptibilities of membrane PUFAs to lipid peroxidation,16,17 resulting in changes of membrane integrity and function, and subsequently the changes of sperm motility and fertility.17,18 These results imply that dietary n-3 may help to improve sperm functions, especially motility, by modifying membrane properties as well as decreasing spermatozoa lipid peroxidation. As a fat-soluble antioxidant, Vitamin E can exert antioxidative effects and affect boar semen quality in vivo16 as well as in vitro.17 Therefore, Vitamin E was included in premix to reverse the negative impact of spermatozoa lipid peroxidation of PUFA addition.6,18
However, it is still unclear which component improves sperm quality due to the simultaneous supplementation of n-3 PUFA and Vitamin E, and the physiological effects of the altered membrane properties have not been investigated in boar by adjustment of dietary n-6:n-3 ratio and inclusion of Vitamin E. Thus, the experiments in this work were designed to find out the impacts of reducing the n-6:n-3 ratio to below 10:1 and the inclusion of Vitamin E in boar diets on the sperm motility characteristics, and the physical and functional properties of spermatozoa membrane.
MATERIALS AND METHODS
Animals and dietary treatments
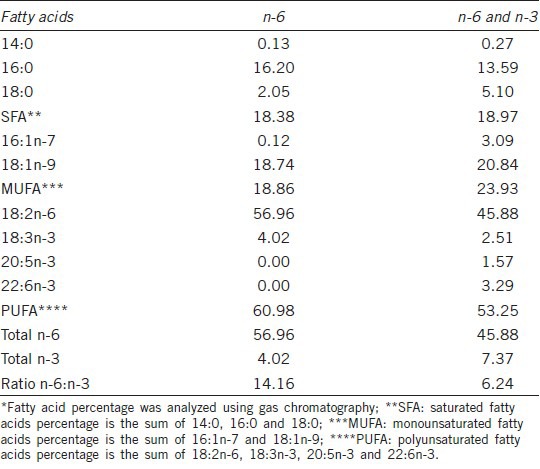
Forty Duroc boars were allocated by age (591.5 ± 9.2 d) and live weight (272.9 ± 47.6 kg) to four treatment groups, with 10 animals for each treatment. The basal diets were formulated to be isoenergetic and isonitrogenous and to meet the nutritional requirements19 (Table 1). The boars were provided ad libitum access to water. The experiment followed a 2 × 2 factorial (n-6:n-3 ratio and Vitamin E level) design. The experimental rations contained either soybean oil or soybean oil together with fish oil. The soybean oil (Shandong Bohi Industry Co., Ltd, Shandong, China) was the main source of n-6 FA leinoleic acid (LA, C18:2 n-6). The fish oil (Rongcheng Ayers Ocean Bio-Technology Co., Ltd., Rongcheng, Shandong, China) included 10% eicosapentaenoil acid (EPA, C20:5 n-3), 25% DHA and 35% total n-3 FA. The fish oil was stabilized with an antioxidant (500 ppm of Butylated Hydroxytoluene [BHT], Bayer, China). As the dietary n-6:n-3 ratio in commercial production of boars is usually higher than 10:1, the 14.4 n-6:n-3 ratio diet, which was composed of 60 g d−1 soybean oil and 2.5 kg d−1 basal diet, was used as the control diet; whereas 6.6 n-6:n-3 ratio diet consisted of 45 g d−1 soybean oil, 15 g d−1 fish oil and 2.5 kg d−1 basal diet. The FA profiles of 14.4 and 6.6 n-6:n-3 ratio diets are reported in Table 2. The second treatment was a dietary inclusion of Vitamin E (vE, a-tocopherol acetate, Zhejiang NHU Co., Ltd., Zhejiang, China) at two levels: 200 and 400 mg kg−1 of feed. The following experimental diets were formulated: 14.4 n-6:n-3 ratio diet with 200 mg vE kg−1; 14.4 n-6:n-3 ratio diet with 400 mg vE kg−1; 6.6 n-6:n-3 ratio diet with 200 mg vE kg−1; 6.6 n-6:n-3 ratio diet with 400 mg vE kg−1. The experiment lasted for 16 weeks.
Table 1.
Composition of basal diet
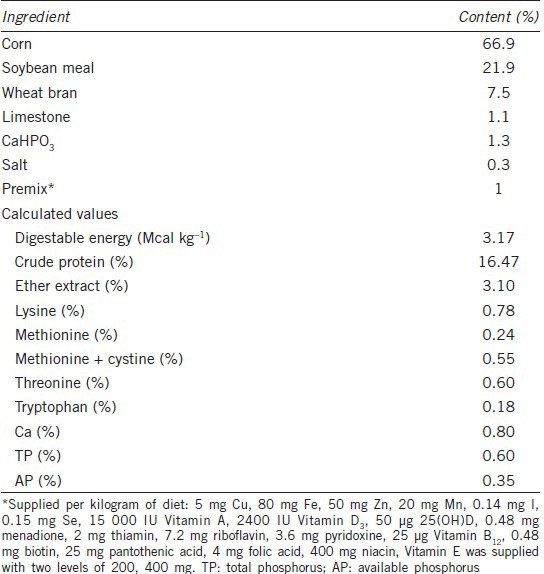
Table 2.
Fatty acids composition (g per 100 g of fatty acids) of the diets*
Semen evaluation
Semen was collected in thermo container (37°C) from all the boars (on 1 day in each week) at weeks 0, 8, 12 and 16 of the experiment by the gloved-hand technique.20 After collection, the semen was diluted (1:9, v/v) in a Beltsville Thawing Solution (BTS, Wuhan Jinli, Wuhan, China) to a final concentration of 2.5 × 109 spermatozoa dose−1. The diluted sperm-rich fraction was split into 80 ml semen doses. The first dose was stored at 17°C for 120 h, and aliquots were removed at hours 12, 36, 72, and 120 for motion analysis. The second dose was cooled and immediately transported at 17°C in an insulated container from the farm to the university laboratory within 24 h after extraction according to Yeste et al.18 to assess the sperm membrane properties (structural membrane integrity, function membrane integrity, and membrane fluidity). Aliquots of semen containing 3 × 10] and 3 × 108 sperm were centrifuged at 850 ×g for 20 min at 20°C. The obtained sperm pellets were re-suspended in 0.85% NaCl, washed twice by centrifugation and frozen at −80°C for fatty acid analysis and lipid peroxidation (malondialdehyde, MDA) assay.
Sperm motility
Before analyzing the sperm motility, semen samples were incubated at 37°C for 15 min. Then, a computer-assisted semen analyzer (CASA sperm analyzer; Songjiang, China) was used. The analysis was based on the examination of 30 consecutive digitalized images per second using a 10 × negative phase-contrast objective. Several fields of view were captured, and at least 1000 spermatozoa were counted in each analysis. The system provided data of the following 6 motility parameters: total motility (MOT, %); progressive motility (PMOT, %); curvilinear velocity (VCL, μm s−1), which is the average velocity measured over the actual point-to-point track followed by the cell; average path velocity (VAP, μm s−1), which corresponds to the average velocity of the smoothed cell path; straight line velocity (VSL, μm s−1), which represents the average velocity measured in a straight line from the beginning to the end of a track; and amplitude of lateral head displacement (ALH, μm).
Sperm membrane properties
Structural membrane integrity
The proportion of spermatozoa with intact plasma membrane was estimated using eosin Y (Solarbio, Beijing, China) staining with the observation of at least 200 spermatozoa at ×400 magnification. The unstained spermatozoa were considered to have an intact plasma membrane, whereas partly or completely purple spermatozoa were considered to have lost their plasma membrane.
Functional membrane integrity
Functional membrane was assessed using hypo-osmotic swelling test (HOS),21 which was performed by incubating an aliquot (50 μl) of semen sample with 1 ml of hypo-osmotic solution prepared by mixing 7.35 g sodium citrate 2H2O (Sinopharm, Beijing, China) and 13.51 g fructose (Sinopharm, Beijing, China) in 1 L of distilled H2O at 37°C for 30 min. After incubation, a minimum of 200 spermatozoa were evaluated for coiled tails by counting in at least five different fields under a phase-contrast microscope at ×400 magnification.
Membrane fluidity
Membrane fluidity determination followed the basic principle that the alterations in lipid packing change the mobility of a membrane-bound fluorophore. The mobility of the fluorophore can be monitored by exciting the fluorophore with a polarized light and measuring the emitted light in two planes parallel and perpendicular to the polarization plane of the excitation light. Fluorescence polarization (FP) is defined as the following ratio: r = (Ivv – G Ivh)/(Ivv + 2G Ivh), G = (Ihv/Ihh), where Ivv and Ivh are the fluorescence intensities measured in the parallel and perpendicular channels, and G is the grating correction factor. Membrane fluidization increases the mobility of the dye and decreases the intensity of the emitted parallel component. Fluorescence measurements (excitation λ = 365 nm; emission λ = 430 nm) were performed in a PerkinElmer LS 55 Luminescence Spectrometer equipped with polarizer. Aliquots of fresh spermatozoa (200–300 μl) were diluted in BTS and centrifuged for 20 min at 800 ×g. The washed spermatozoa were suspended at a concentration of 1 × 106 spermatozoa ml−1 in 3 ml of PBS (phosphate buffered saline) with the fluorescent lipophylic molecule 1,6-diphenyl-1,3,5-hexatriene (DPH, Sigma, USA, 10−6 mol l−1, prepared from a DPH stock solution of 2 × 10−3 mmol l−1 in tetrahydrofurane). The suspension was incubated for 30 min at room temperature.22 The DPH/phospholipid molar ratio was lower than 1/2000 in order to minimize the probe-to-probe interactions and probe-induced disturbance of the lipid bilayer. Sperm suspensions containing no DPH were similarly assessed to check light scattering. An increased value of r was interpreted to be a decrease of membrane fluidity, and the converse with decreased r value.
Lipid peroxidation assay
Cellular content of MDA (nmol 10−8 Sperm cells) was determined with commercial test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). Briefly, the measurement of MDA was accomplished through binding of thiobarbituric acid (TBA) to the MDA molecule, which produced a colorimetric reaction that can be measured on a spectrophotometer at a wavelength of 532 nm.
Sperm fatty acid analysis
Lipids were extracted from diets and sperm samples after homogenization in a suitable excess of chloroform–methanol (2:1, v/v).23 Fatty acid methyl esters (FAMEs) were prepared for gas chromatography determination using KOH/methanol.24 The CP-3800 gas chromatography (Varian Inc., USA) equipped with a 1177 injector, a flame ionization detector and a capillary chromatographic column CPSil88 (Varian Inc., USA) for FAME (50 m × 0.25 mm id 0.2 μm) was used in this experiment. The injector and detector temperatures were kept between 250°C and 270°C. Nitrogen was used as carrier gas. A total of 40 saturated, monounsaturated and polyunsaturated fatty acid standards (NU-CHEK, Prep, UK) were used. Peaks were identified by retention times relative to individual fatty acid standard.
Statistical analysis
All data were analyzed using Statistical Analysis Software (SAS, version 9.1, SAS Institute, Inc., Cary, NC, USA). Data were examined for normality and homogeneity of variance using the Kolmogorov–Smirnov normality test and Bartlett tests. Four transformations of data (square root [x], log10[x], arcsine square root[x] and 1/[x]) were effected when the distribution was not normal and/or heteroscedastic (x), and the most suitable transformation was chosen. The transformed data were used to calculate P values. The corresponding least squares mean and standard deviation of the nontransformed data were presented in the results for clarity. Data were subjected to analysis of variance (ANOVA) according to the GLM procedure of the Statistical Analysis System (SAS Institute, Inc., Cary, NC, USA). The model included dietary n-6:n-3 ratio, Vitamin E, two-way interaction of dietary n-6:n-3 ratio and Vitamin E as possible sources of variation. Vitamin E effect was not significant (P > 0.05) and thus was excluded in the final model for the fatty acid composition analysis of spermatozoa. Hence, the data were analyzed using an ANOVA for repeated measurements, with boar within dietary n-6:n-3 ratio as the subject of the repeated effect of time (i.e., week of experiment), and with dietary n-6:n-3 ratio, time, and two-way interaction of n-6:n-3 ratio and time as fixed effects. When ANOVA revealed a significant treatment effect, means were compared using Duncan's multiple range tests. Pearson correlations were calculated to determine the associations between viability, HOS, lipid peroxidation (MDA), CASA results and fatty acid composition in spermatozoa. In all statistical analyses, the significance level was set at 5%.
RESULTS
Semen motility characteristics
On the ground that the n-6:n-3 fatty acid ratio is generally higher than 10:1 in commercial porcine diets, 14.4 n-6:n-3 ratio diet was used as the control. To ascertain whether there was any effect of dietary n-6:n-3 ratio on sperm motility, CASA was used to evaluate the sperm motility characteristics. The results showed that the 6.6 dietary n-6:n-3 ratio resulted in considerably enhanced total motility, sperm progressive motility and kinematic parameters (VSL, VAP, and VCL) at week 12 and week 16 (Table 3). Compared with 200 mg kg−1 supplementation of Vitamin E, 400 mg kg−1 supplementation of Vitamin E notably increased the progressive sperm motility. In addition, to evaluate the influence of dietary n-6:n-3 ratio and Vitamin E on sperm motility in response to cooling (17°C), the semen was diluted with BTS at hours 12, 36, 72, and 120 at 17°C. The results showed that high Vitamin E diet resulted in higher total motility and sperm progressive motility than low Vitamin E diet (Figure 1), and dietary n-6:n-3 ratio had no effect on the motility of stored sperm (data not shown). The interaction between n-6:n-3 ratio and Vitamin E was not significant for all motility characteristics (P > 0.05).
Table 3.
Effect of dietary n-6:n-3 ratio (n6:n3) and Vitamin E on boar fresh sperm motility characteristics*
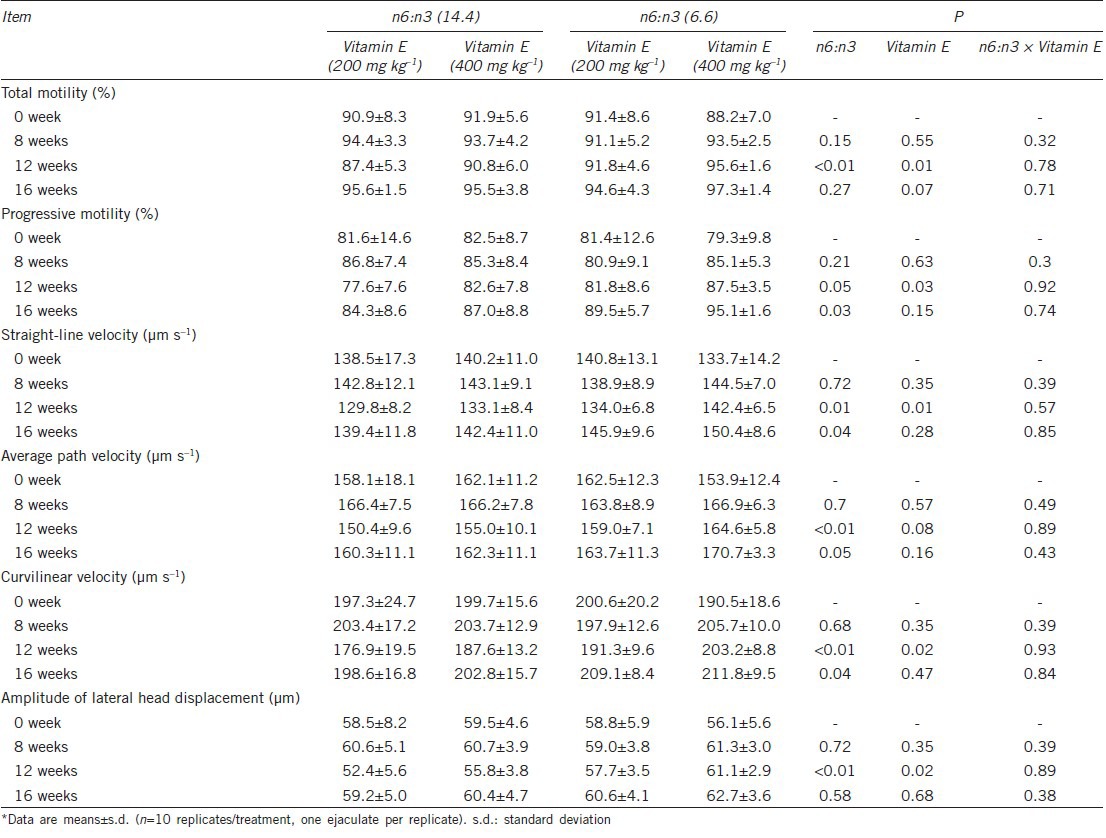
Figure 1.
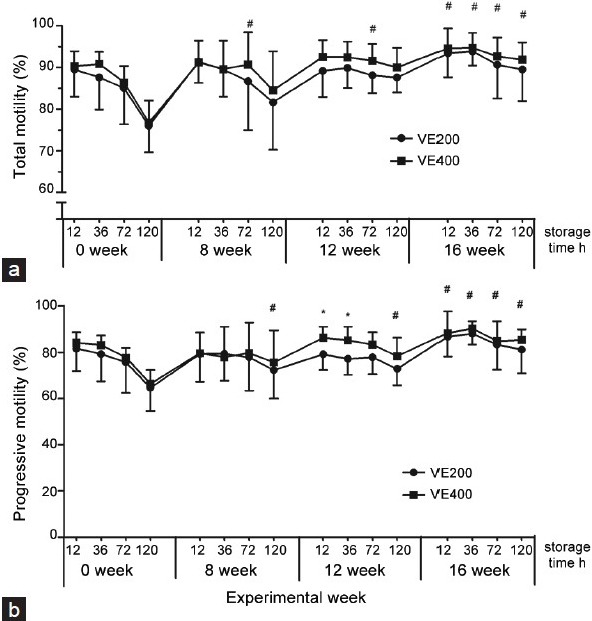
Changes in (a) total motility and (b) progressive motility of boar lipid stored sperm with dietary Vitamin E during 120-h storage at different experimental week (0, 8, 12 and 16 w). Data are presented as means ± s.d. (n = 10 replicates/vitamin group, one ejaculate per replicate). * P < 0.05, compared with Vitamin E diet (200 mg kg−1). #P < 0.05, compared with week 0 (within the same storage time).
Sperm membrane properties
The maintenance of membrane integrity and membrane fluidity of boar spermatozoa is important for achieving high sperm motility, because the motility of the spermatozoon partly depends on the transport of compounds across its membrane. At 0 and 8 week, sperm membrane integrity parameters and membrane fluidity did not differ among groups (P > 0.05, Figure 2). When the boars were fed for 12 and 16 weeks, the structural membrane integrity and functional membrane integrity were considerably increased in treatment groups, namely the groups with 6.6 ratio diet and Vitamin E (400 mg kg−1) diet. Increased membrane fluidity was found in the 6.6 n-6:n-3 group at 12 and 16 weeks and in the high Vitamin E group only at week 16. There was no significant effect of the interaction between dietary n-6:n-3 ratio and Vitamin E supplementation on sperm membrane properties (P > 0.05).
Figure 2.
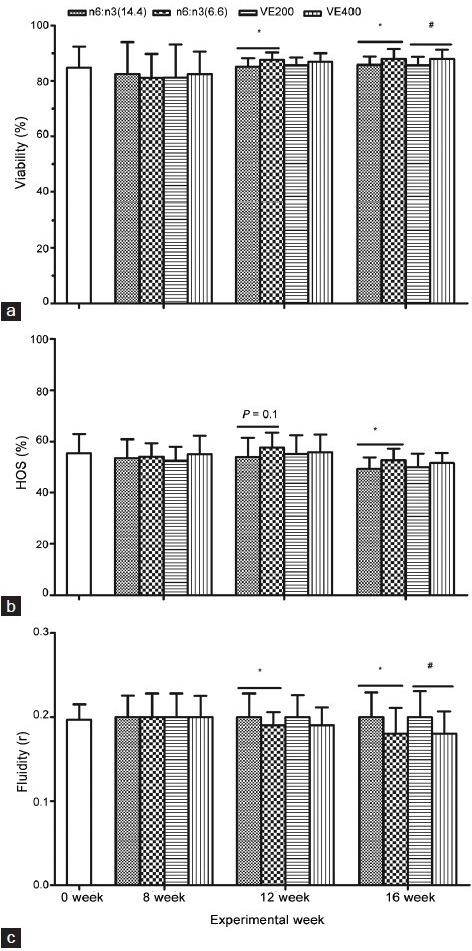
Effect of dietary n-6:n-3 ratio and Vitamin E on boar fresh sperm membrane properties including: (a) Viability (%); (b) HOS (%) and (c) fluidity (r) at different week of experiment (0, 8, 12 and 16 w). Data are main effect means ± s.d. (n = 8-12 replicates/treatment, one ejaculate per replicate). *Difference was from n6:n3 (6.6) versus n6:n3 (14.4) (P < 0.05). #Difference was from Vitamin E (VE) 400 versus Vitamin E (VE) 200 (P < 0.05).
Lipid peroxidation levels
The lipid peroxidation of sperm can lead to decreased motility and fertilizing ability of spermatozoa. The malondialdehyde (MDA) in the spermatozoa was assessed to validate the effect of dietary n-6:n-3 ratio and Vitamin E on the lipid peroxidation of sperm. Compared with low Vitamin E diet, high Vitamin E diet considerably reduced sperm lipid peroxidation, as measured by MDA levels in sperm. The sperm MDA level was not significantly different between the 6.6 and 14.4 n-6:n-3 groups (P > 0.05, Figure 3).
Figure 3.
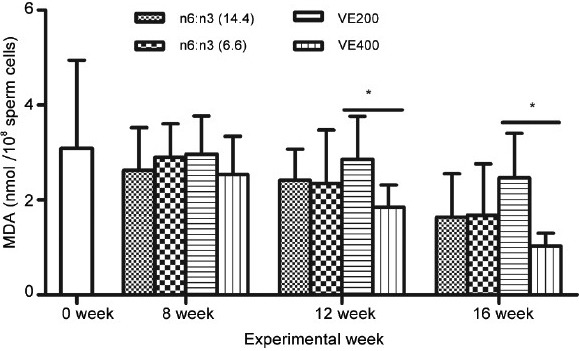
Effect of dietary n-6:n-3 ratio and Vitamin E on boar fresh sperm lipid peroxidation (MDA concentration, nmol 10−8 Sperm cells) at different week of experiment (0, 8, 12 and 16 w). Data are main effect means ± s.d. (n = 10 replicates/treatment, one ejaculate per replicate). *Difference was from Vitamin E (VE) 400 versus Vitamin E (VE) 200 (P < 0.05).
Sperm fatty acid composition
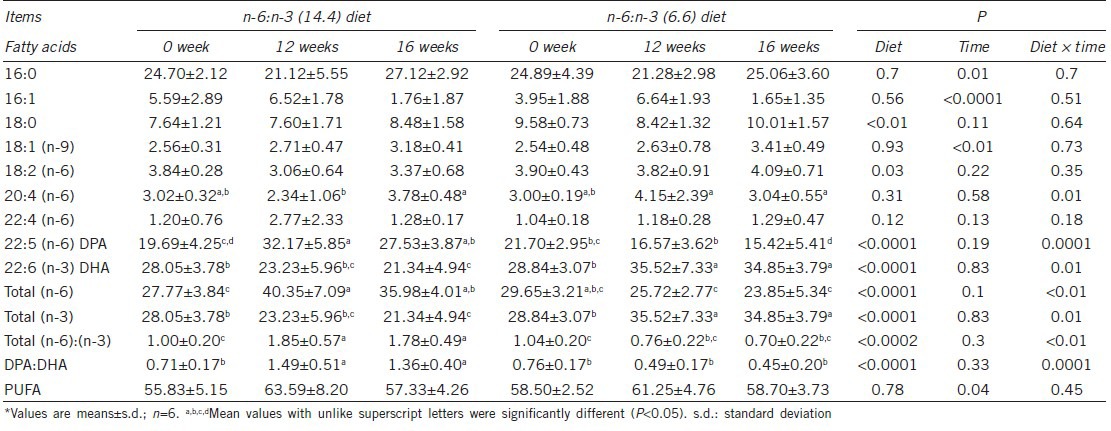
Dietary supplementation is known to interfere with the sperm fatty acid, which is a key regulatory factor of sperm membrane properties. To determine whether the sperm fatty acid was affected by the treatments in our experiments, we measured the sperm fatty acid composition. Vitamin E supplementation and the interaction between n-6:n-3 ratio and Vitamin E did not lead to any changes in fatty acid composition, which are thus not presented in Table 4. In general, the fatty acid composition of sperm was altered by dietary n-6:n-3 ratio as expected. Notably increased DHA and total n-3 PUFA in boar sperm were founded at the expense of DPA and total n-6 PUFA in 6.6 n-6:n-3 group compared with in 14.4 n-6:n-3 group at weeks 12 and 16 (Table 4). Compared with the boars consuming 14.4:1 diets, those consuming 6.6:1 diets had lower ratios of total n-6:n-3 and DPA: DHA in spermatozoa at weeks 12 and 16. The correlation analyses between all the variables demonstrated that the DHA content of spermatozoa was strongly positively correlated with viability, HOS and progressive motility (r = 0.38, 0.48, 0.35, P < 0.05; n = 36).
Table 4.
Fatty acid compositions (g per 100 g of fatty acids) of spermatozoa of boars fed diets differing in the ratio of n-6 to n-3 fatty acids from preexperiment*
DISCUSSION
The results of the present study confirm our hypothesis that 6.6 n-6:n-3 diet contributes to greater sperm motility than 14.4 n-6:n-3 diet, which is in agreement with the observations of boars in previous studies: the sperm motility was enhanced by dietary n-3 PUFA.6,7,25 However, contradicting results were reported in boars,15,26,27 which might be caused by variations in the quantities and sources of oil and duration of supplementation. Similar conflicting results about the effect of dietary supplementation with n-3 PUFA on sperm function have been reported in rabbit,28,29 human,9,26 and ram.27,30 Regardless of experimental factors, the control diets for boars contained high n-6 FA as they were corn-based (with a n-6:n-3 ratio higher than 10:1), except for the study by Rooke et al.6 which used barley- and wheat-based diet containing rapeseed components (with a n-6:n-3 ratio lower than 10:1). Refined fish oil (25% DHA and 10% EPA) was used for the dietary 6.6 n-6:n-3 ratio in the present study, and the inclusion content of n-3 FA (15 g d−1) was not within the range (40–300 g d−1) previously reported.7,15,18,31 CASA system assessment demonstrated that dietary 6.6 n-6:n-3 also yielded higher velocity (VSL, VAP, VCL), which has been proven to be useful for estimating the potential fertility of human32 and boars.33 Different velocity values of these studies may be attributed to the variations of software algorithms (to obtain VAP etc.) and acquisition settings.34
Changes in plasma membrane structure and functional activity appear to be an essential prerequisite for successful motility acquisition, fertilization and embryo development.35,36,37 The results of the present study are in agreement with those of the previous studies: the supplementation of n-3 PUFA could improve the functional membrane integrity (HOS values) of fresh semen in rabbit29 and ram,38 the structural membrane integrity of fresh semen in boar39 and the synaptic plasma membrane fluidity in rat.40 The reason for the improvement of the structural and functional membrane integrity of fresh boar semen found in the present study might be that dietary 6.6 n-6:n-3 ratio (n-3 enriched diet) increased the incorporation of DHA into the sperm head and the principle piece of sperm tail, and the DHA played a structural role and improved sperm membrane flexibility and stability against hypo-osmotic solution.41 More studies are needed to address the issues of how FAs are distributed in the heads and tails of the ejaculated boar spermatozoa and what are their corresponding fertilizing functions in each section. In addition, it is probable that DHA acyl-chain in sperm membrane phospholipid bilayers was increased by dietary 6.6 n-6:n-3 ratio, resulting in higher sperm flexibility, compressibility, deformability and elasticity and thus enhanced membrane fluidity of the fresh boar semen as measured by DPH polarization.42,43
The modification of the sperm membrane FA profile and lipid peroxidation appears to be closely associated with the changes of the physical and functional properties of spermatozoa membrane.44,45,46 In the present study, dietary 6.6 n-6:n-3 ratio successfully increased the n-3 PUFA and DHA contents of the sperm, though the total PUFA was not influenced, showing a noticeable responsiveness of boar sperm to dietary manipulation. Our findings are in agreement with those previously reported in boar,6,7 rabbit,29 ram38 and ovine.47 The boars consuming 14.4:1 n-6:n-3 diet displayed a sperm DPA: DHA ratio more than triple that of the boars consuming 6.6:1 n-6:n-3 diet at weeks 12 and 16. It is worthy to note that the optimization of DPA: DHA ratio in sperm may be expected to promote successful spermatozoa development and to facilitate the flagellar movement of the tail.43 Therefore, the above results suggest that the increased n-3 PUFA and DHA and optimized DPA: DHA ratio in sperm may partly explain the modification and stabilization of structural and functional properties of spermatozoa membrane as previously suggested.28,29,48 On the other hand, previous studies found that the spermatozoa of several species supplied with dietary n-3 PUFA are more easily subjected to lipid peroxidation,39,49 which negatively affects the membrane structural and functional properties and sperm motility.50,51 Our results show that the sperm lipid peroxidation (spontaneous MDA levels) had no statistical difference between 6.6 and 14.4 n-6:n-3 groups. In contrast, Strzezek et al.39 stated that dietary supplementation with n-3 PUFA to boar increased the sperm lipid peroxidation (induced MDA levels), which might mask the dietary effect on semen quality. Such differences possibly result from the different analyzed methods (spontaneous vs induced lipid peroxidation assay).52 The present study indicates that dietary n-6:n-3 ratio modifies membrane properties, which is independent of membrane lipid peroxidation.
The results of the present study also confirm the positive effect of high Vitamin E levels on the motility of the fresh and liquid-stored sperm. These findings are in agreement with previous observations in boars.17,53 The present study confirms the effect of high level Vitamin E on the prevention of lipid peroxidation in spermatozoa, which is in conformity with the result of Marin-Guzman et al.16 As for the changes of membrane properties, only the membrane structural integrity (head membrane) appears to be affected by Vitamin E supplementation, which is probably due to the different distributions of Vitamin E in the head, tail and plasma membrane of spermatozoa as shown in rat spermatozoa.36
CONCLUSIONS
Our results demonstrate that dietary 6.6 n-6:n-3 ratio influences the boar sperm fatty acid composition, enhances the sperm membrane properties, and increases sperm motility. Vitamin E (400 mg kg−1) diet prevents sperm lipid peroxidation, directly leading to the improvement of motility parameters. It is suggested that the adjustment of dietary n-6:n-3 ratio and supplementation of Vitamin E can improve sperm motility through modifying the physical and functional properties of spermatozoa membrane.
AUTHOR CONTRIBUTIONS
QL participated in the design of the study, performed the experiment and statistical analysis, and drafted the manuscript. YFZ participated in the statistical analysis and helped to draft the manuscript. RJD participated in the animal experimental studies. HKW participated in the design of the study. JP conceived of the study, and participated in its design and coordination and helped to draft the manuscript. SWJ participated in the design of the study and coordination. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This research was supported by National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2014BAD20B01), Natural Science Foundation of Hubei Province of China (2013CFA010), Innovation Position in Agricultural Science and Technology Innovation Center of Hubei Province (2011-620), and Fundamental Research Funds for the Central Universities (2662015PY014). We would like to thank Mr. Jiajian Tan, Mr. Jialian Li and Mr. Jiarong Ruan at Wuming boar stud for their support during the feeding trial. We also thank Yangxiang Joint Stock Company for providing boar feeding facilities. Finally, we are grateful for English revision from Prof. Zuoxiong Liu.
REFERENCES
- 1.Kemp B, Grooten H, Den Hartog L, Luiting P, Verstegen M. The effect of a high protein intake on sperm production in boars at two semen collection frequencies. Anim Reprod Sci. 1988;17:103–13. [Google Scholar]
- 2.Berndtson WE. Comparative reliability and sensitivity of different methods for assessing treatment effects on sperm production. Anim Reprod Sci. 2008;105:5–22. doi: 10.1016/j.anireprosci.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Knox R, Levis D, Safranski T, Singleton W. An update on North American boar stud practices. Theriogenology. 2008;70:1202–8. doi: 10.1016/j.theriogenology.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 4.Cooper T. Role of the epididymis in mediating changes in the male gamete during maturation. Adv Exp Med Biol. 1995;377:87. doi: 10.1007/978-1-4899-0952-7_6. [DOI] [PubMed] [Google Scholar]
- 5.Kunavongkrit A, Suriyasomboon A, Lundeheim N, Heard TW, Einarsson S. Management and sperm production of boars under differing environmental conditions. Theriogenology. 2005;63:657–67. doi: 10.1016/j.theriogenology.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Rooke J, Shao C, Speake B. Effects of feeding tuna oil on the lipid composition of pig spermatozoa and in vitro characteristics of semen. Reproduction. 2001;121:315–22. doi: 10.1530/rep.0.1210315. [DOI] [PubMed] [Google Scholar]
- 7.Mitre R, Cheminade C, Allaume P, Legrand P, Legrand AB. Oral intake of shark liver oil modifies lipid composition and improves motility and velocity of boar sperm. Theriogenology. 2004;62:1557–66. doi: 10.1016/j.theriogenology.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Surai P, Noble R, Sparks N, Speake B. Effect of long-term supplementation with arachidonic or docosahexaenoic acids on sperm production in the broiler chicken. J Reprod Fertil. 2000;120:257–64. [PubMed] [Google Scholar]
- 9.Safarinejad MR, Safarinejad S. The roles of omega-3 and omega-6 fatty acids in idiopathic male infertility. Asian J Androl. 2012;14:514–5. doi: 10.1038/aja.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanini SF, Torres CA, Bragagnolo N, Turatti JM, Silva MG, et al. Evaluation of the ratio of omega 6: omega 3 fatty acids and Vitamin E levels in the diet on the reproductive performance of cockerels. Arch Anim Nutr. 2003;57:429–42. doi: 10.1080/0003942032000161072. [DOI] [PubMed] [Google Scholar]
- 11.Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77:190–201. doi: 10.1095/biolreprod.107.060558. [DOI] [PubMed] [Google Scholar]
- 12.Adeel M, Ijaz A, Aleem M, Rehman H, Yousaf M, et al. Improvement of liquid and frozen-thawed semen quality of Nili-Ravi buffalo bulls (Bubalus bubalis) through supplementation of fat. Theriogenology. 2009;71:1220–5. doi: 10.1016/j.theriogenology.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Selvaraju S, Raju P, Rao SB, Raghavendra S, Nandi S, et al. Evaluation of maize grain and polyunsaturated fatty acid (PUFA) as energy sources for breeding rams based on hormonal, sperm functional parameters and fertility. Reprod Fertil Dev. 2012;24:669–78. doi: 10.1071/RD11229. [DOI] [PubMed] [Google Scholar]
- 14.Radomil L, Pettitt MJ, Merkies KM, Hickey KD, Buhr MM. Stress and dietary factors modify boar sperm for processing. Reprod Domest Anim. 2011;46:39–44. doi: 10.1111/j.1439-0531.2011.01865.x. [DOI] [PubMed] [Google Scholar]
- 15.Castellano CA, Audet I, Bailey JL, Laforest JP, Matte JJ. Dietary omega-3 fatty acids (fish oils) have limited effects on boar semen stored at 17 degrees C or cryopreserved. Theriogenology. 2010;74:1482–90. doi: 10.1016/j.theriogenology.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Marin-Guzman J, Mahan D, Chung Y, Pate J, Pope W. Effects of dietary selenium and Vitamin E on boar performance and tissue responses, semen quality, and subsequent fertilization rates in mature gilts. J Anim Sci. 1997;75:2994–3003. doi: 10.2527/1997.75112994x. [DOI] [PubMed] [Google Scholar]
- 17.Cerolini S, Maldjian A, Surai P, Noble R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim Reprod Sci. 2000;58:99–111. doi: 10.1016/s0378-4320(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 18.Yeste M, Barrera x, Coll D, Bonet S. The effects on boar sperm quality of dietary supplementation with omega-3 polyunsaturated fatty acids differ among porcine breeds. Theriogenology. 2011;76:184–96. doi: 10.1016/j.theriogenology.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 19.NRC. Nutrient Requirements of Swine. 10th Revised Edition. Washington, DC. USA: National Academy Press; 1998. [Google Scholar]
- 20.Hancock J, Hovell G. The collection of boar semen. Vet Rec. 1959;71:664–5. [Google Scholar]
- 21.Jeyendran R, Van der Ven H, Perez-Pelaez M, Crabo B, Zaneveld L. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–28. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 22.Giraud M, Motta C, Boucher D, Grizard G. Membrane fluidity predicts the outcome of cryopreservation of human spermatozoa. Hum Reprod. 2000;15:2160–4. doi: 10.1093/humrep/15.10.2160. [DOI] [PubMed] [Google Scholar]
- 23.Folch J, Lees M, Sloane-Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 24.Demirel G, Wachira A, Sinclair L, Wilkinson R, Wood J, et al. Effects of dietary n-3 polyunsaturated fatty acids, breed and dietary Vitamin E on the fatty acids of lamb muscle, liver and adipose tissue. Br J Nutr. 2004;91:551–65. doi: 10.1079/BJN20031079. [DOI] [PubMed] [Google Scholar]
- 25.Penny P, Noble R, Maldjian A, Cerolini S. Potential role of lipids for the enhancement of boar fertility and fecundity. Pig News Inf. 2000;21:119N–26N. [Google Scholar]
- 26.Conquer JA, Martin JB, Tummon I, Watson L, Tekpetey F. Effect of DHA supplementation on DHA status and sperm motility in asthenozoospermic males. Lipids. 2000;35:149–54. doi: 10.1007/BF02664764. [DOI] [PubMed] [Google Scholar]
- 27.Fair S, Doyle D, Diskin M, Hennessy A, Kenny D. The effect of dietary n-3 polyunsaturated fatty acids supplementation of rams on semen quality and subsequent quality of liquid stored semen. Theriogenology. 2014;81:210–9. doi: 10.1016/j.theriogenology.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Gliozzi T, Zaniboni L, Maldjian A, Luzi F, Maertens L, et al. Quality and lipid composition of spermatozoa in rabbits fed DHA and Vitamin E rich diets. Theriogenology. 2009;71:910–9. doi: 10.1016/j.theriogenology.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Mourvaki E, Cardinali R, Dal Bosco A, Corazzi L, Castellini C. Effects of flaxseed dietary supplementation on sperm quality and on lipid composition of sperm subfractions and prostatic granules in rabbit. Theriogenology. 2010;73:629–37. doi: 10.1016/j.theriogenology.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Matini Behzad A, Ebrahimi B, Alizadeh A, Esmaeili V, Dalman A, et al. Improvement in in vitro fertilization rate, decrease in reactive oxygen species and spermatozoa death incidence in rams by dietary fish oil. Reprod Domest Anim. 2014;49:599–605. doi: 10.1111/rda.12328. [DOI] [PubMed] [Google Scholar]
- 31.Estienne MJ, Harper AF, Crawford RJ. Dietary supplementation with a source of omega-3 fatty acids increases sperm number and the duration of ejaculation in boars. Theriogenology. 2008;70:70–6. doi: 10.1016/j.theriogenology.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Hirano Y, Shibahara H, Obara H, Suzuki T, Takamizawa S, et al. Andrology: relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J Assist Reprod Genet. 2001;18:215–20. doi: 10.1023/A:1009420432234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holt C, Holt WV, Moore HD, Reed HC, Curnock RM. Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: results of two fertility trials. J Androl. 1997;18:312–23. [PubMed] [Google Scholar]
- 34.Martínez-Pastor F, Tizado EJ, Garde JJ, Anel L, de Paz P. Statistical series: opportunities and challenges of sperm motility subpopulation analysis. Theriogenology. 2011;75:783–95. doi: 10.1016/j.theriogenology.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 35.Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. BBA Rev Biomembr. 2000;1469:197–235. doi: 10.1016/s0304-4157(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 36.Lenzi A, Gandini L, Picardo M, Tramer F, Sandri G, et al. Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (PUFA): scavenger mechanisms and possible scavenger therapies. Front Biosci. 2000;5:1–15. doi: 10.2741/lenzi. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-Martínez H. Laboratory semen assessment and prediction of fertility: still utopia.? Reprod Domest Anim. 2003;38:312–8. doi: 10.1046/j.1439-0531.2003.00436.x. [DOI] [PubMed] [Google Scholar]
- 38.Jafaroghli M, Abdi-Benemar H, Zamiri M, Khalili B, Farshad A, et al. Effects of dietary n-3 fatty acids and Vitamin C on semen characteristics, lipid composition of sperm and blood metabolites in fat-tailed Moghani rams. Anim Reprod Sci. 2014;147:17–24. doi: 10.1016/j.anireprosci.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Strzezek J, Fraser L, Kuklinska M, Dziekonska A, Lecewicz M. Effects of dietary supplementation with polyunsaturated fatty acids and antioxidants on biochemical characteristics of boar semen. Reprod Biol. 2004;4:271–87. [PubMed] [Google Scholar]
- 40.Hashimoto M, Hossain S, Shimada T, Shido O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol. 2006;33:934. doi: 10.1111/j.1440-1681.2006.04467.x. [DOI] [PubMed] [Google Scholar]
- 41.Gholami H, Chamani M, Towhidi A, Fazeli M. Effect of feeding a docosahexaenoic acid-enriched nutriceutical on the quality of fresh and frozen-thawed semen in Holstein bulls. Theriogenology. 2010;74:1548–58. doi: 10.1016/j.theriogenology.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 42.James P, Wolfe C, Ladha S, Jones R. Lipid diffusion in the plasma membrane of ram and boar spermatozoa during maturation in the epididymis measured by fluorescence recovery after photobleaching. Mol Reprod Dev. 1999;52:207–15. doi: 10.1002/(SICI)1098-2795(199902)52:2<207::AID-MRD12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 43.Speake B, Surai P, Rooke J, De Vriese S, Christophe A. Regulation of avian and mammalian sperm production by dietary fatty acids. In: Male Fertility and Lipid Metabolism. Champaign, Illinois: AOCS Press; 2003. pp. 96–117. [Google Scholar]
- 44.Haidl G, Opper C. Changes in lipids and membrane anisotropy in human spermatozoa during epididymal maturation. Hum Reprod. 1997;12:2720–3. doi: 10.1093/humrep/12.12.2720. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez JG, Storey BT. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995;42:334–46. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- 46.Jones R, Mann T, Sherins R. Adverse effects of peroxidized lipid on human spermatozoa. Proc R Soc Lond B Biol Sci. 1978;201:413–7. doi: 10.1098/rspb.1978.0053. [DOI] [PubMed] [Google Scholar]
- 47.Samadian F, Towhidi A, Rezayazdi K, Bahreini M. Effects of dietary n-3 fatty acids on characteristics and lipid composition of ovine sperm. Animal. 2010;4:2017–22. doi: 10.1017/S1751731110001308. [DOI] [PubMed] [Google Scholar]
- 48.Ladha S. Lipid heterogeneity and membrane fluidity in a highly polarized cell, the mammalian spermatozoon. J Membr Biol. 1998;165:1–10. doi: 10.1007/s002329900415. [DOI] [PubMed] [Google Scholar]
- 49.Cerolini S, Zaniboni L, Maldjian A, Gliozzi T. Effect of docosahexaenoic acid and a-tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology. 2006;66:877–86. doi: 10.1016/j.theriogenology.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 50.Jones R, Mann T. Damage to ram spermatozoa by peroxidation of endogenous phospholipids. J Reprod Fertil. 1977;50:261–8. doi: 10.1530/jrf.0.0500261. [DOI] [PubMed] [Google Scholar]
- 51.Sikka SC. Andrology lab corner*: role of oxidative stress and antioxidants in andrology and assisted reproductive technology. J Androl. 2004;25:5–18. doi: 10.1002/j.1939-4640.2004.tb02751.x. [DOI] [PubMed] [Google Scholar]
- 52.Mennella M, Jones R. Properties of spermatozoal superoxide dismutase and lack of involvement of superoxides in metal-ion-catalysed lipid-peroxidation and reactions in semen. Biochem J. 1980;191:289–97. doi: 10.1042/bj1910289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peña F, Johannisson A, Wallgren M, Martinez HR. Antioxidant supplementation of boar spermatozoa from different fractions of the ejaculate improves cryopreservation: changes in sperm membrane lipid architecture. Zygote. 2004;12:117–24. doi: 10.1017/s096719940400262x. [DOI] [PubMed] [Google Scholar]


