Abstract
In this study, we examined the relationship between sex hormone levels and lower urinary tract symptoms (LUTS) in men with benign prostatic hyperplasia (BPH) who underwent transurethral surgery. The study was conducted in 158 patients who came to our hospital for surgery. Clinical conditions were assessed by body mass index (BMI), digital rectal examination, International Prostate Symptom Score (IPSS) and transrectal ultrasound (TRUS). The levels of sex hormones (including total testosterone (TT), estradiol (E2), progesterone (P), luteinizing hormone (LH), follicle-stimulating hormone (FSH) and prolactin (PRL)) and prostate-specific antigen (PSA) were reviewed. Correlations were determined through statistical analysis. The mean age was 72.06 ± 8.68 years. The total IPSS was significantly associated with the TT level (r = −0.21, P= 0.01). Other sex hormone levels were not correlated with total IPSS. However, some ratios such as E2/TT (r = 0.23, P= 0.00) and FSH/LH (r = −0.17, P = 0.04) were associated with total IPSS. Further analysis showed that the nocturia was associated with age (r = 0.16, P= 0.04), BMI (r = 0.21, P = 0.01), and TT (r = −0.19, P= 0.02). Moreover, we divided the patients into two subgroups based on IPSS severity (<20 or ≥20). The mean TT level was in the normal range, but it was significantly related to the presence of severe LUTS. In summary, our study has shown that the severity of LUTS is associated with TT, E2/TT and FSH/LH in men who underwent prostate surgery. Increasing nocturia was observed in lower testosterone patients. Additional larger studies are needed to elucidate the potential mechanisms.
Keywords: aging, benign prostatic hyperplasia, lower urinary tract symptoms, sex hormones, testosterone
INTRODUCTION
Benign prostatic hyperplasia (BPH) and the associated lower urinary tract symptoms (LUTS) are very common conditions among urology patients and often require medical management.1 The exact cause of BPH is unknown, and the only two established factors associated with BPH are age and the androgenic disequilibrium.2,3,4,5,6 Many studies have suggested that sex steroids are involved in the development and maintenance of BPH and have tried to establish a relationship between sex steroids and BPH. Because sex hormones are thought to be an important determinant of prostate growth, they are also thought to contribute to the development and maintenance of LUTS secondary to BPH in aging men.2,3,4,7,8 However, the results on the potential relationships between serum sex hormones and BPH/LUTS have not been consistent.8,9,10,11,12,13 Moreover, few studies have focused on patients who underwent transurethral resection of prostate (TURP). Therefore, we conducted a retrospective study of aging men who underwent surgery to examine the possible associations between sex steroid hormones, age, body mass index (BMI), International Prostate Symptom Score (IPSS), and prostate volume.
MATERIALS AND METHODS
Patients and selection criteria
We retrospectively reviewed the clinical data from a research of “individuation therapy of prostate disease” in our team. A total of 280 men treated for symptomatic BPH/LUTS at xinhua Hospital (Shanghai, China) from December 2012 to June 2014 were evaluated. We excluded patients with malignancy, liver cirrhosis, obvious neuropathy, psychological disease, urinary tract infection, men taking hormones, antiandrogen agents, antifungal agents, or steroidal agents, and men who had previously undergone surgical or medical therapy for BPH. Patient age, BMI (calculated by weight in kilograms divided by the square of height in meters), medical history, digital rectal examination (DRE), IPSS, transrectal ultrasound (TRUS), serum prostate-specific antigen (PSA), and sex hormones (E2, FSH, LH, PRL, P, TT) were reviewed.
Indications of surgery
All patients were treated with monopolar TURP. The indications of TURP were provided as following: recurrent urinary retention, recurrent urinary tract infections, recurrent macroscopic hematuria, bladder stones or diverticula, or dilatation of the upper urinary tract with or without renal insufficiency (“Guidelines on benign prostatic hyperplasia version 2007” developed by Chinese Urological Association).
Laboratory examinations
Blood samples were taken between 8:00 and 11:00 a.m. to minimize the confounding effects of diurnal variations in the hormone concentrations before any rectal or urethral operations preoperatively.8,12,14 All patients underwent DRE and TRUS. The total prostate volume was calculated using the prostate ellipsoid formula as follows: (p/6) × width of maximum transverse dimension × length of maximum anteroposterior dimension × height of maximum superoinferior dimension.8,12,14 The prostate transitional zone volume was also calculated using the above formula. If the postoperative pathological results indicated prostate cancer, the subject was excluded from our study.
Utilized questionnaires
The IPSS, a validated 7-item questionnaire, was used to assess the patients’ LUTS before surgery (Chinese version was from “Guidelines on benign prostatic hyperplasia version 2007” developed by Chinese Urological Association). The 7 items have an ordered categorical response that can be scored from 0 to 5, with an overall score of 0-35.12,14 The severity of symptoms was classified as mild (7 or less), moderate (8 to 19) or severe (20 or greater).14 The IPSS storage subscores were evaluated by summing each patient's responses to questions 2 (frequency), 4 (urgency), and 7 (nocturia). The voiding symptoms scores were evaluated by summing each patient's responses to questions 1 (incomplete emptying), 3 (intermittency), 5 (weak stream), and 6 (straining).12,14
Statistical analaysis
The correlations between the different variables were first estimated using Spearman's correlation coefficients. Statistical significance was defined as P < 0.05. Spearman's rank correlation analysis was used to determine the relationship between LUTS (total IPSS, storage IPSS subscore, voiding IPSS subscore, nocturia IPSS subscore) and serum sex hormone levels. Moreover, we compared the IPSS severity groups with respect to TT. All statistical results were carried out using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patients’ characteristic
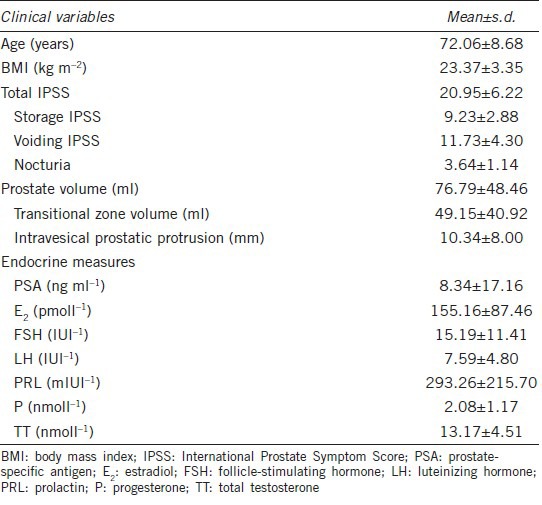
158 men with a mean age of 72.06 ± 8.68 years were included in our study. The endocrine parameters and clinical characteristics of the study population are shown in Table 1.
Table 1.
Patients’ characteristics (n=158)
IPSS and sex hormones
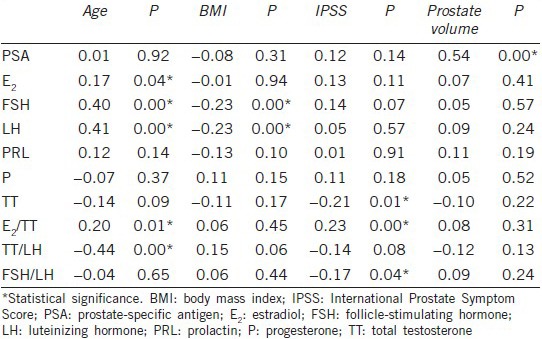
Based on the statistical analysis, the total IPSS was negatively correlated with TT (r = −0.21, P = 0.01) but was not correlated with any of the other sex hormones. Some interesting ratios such as E2/TT (r = 0.23, P = 0.00) and FSH/LH (r = −0.17, P = 0.04) were associated with total IPSS; however, there was no correlation between IPSS and the ratio of TT/LH (r = −0.14, P = 0.08) (Table 2).
Table 2.
Correlation of hormonal variables with age, BMI, IPSS, and prostate volume
IPSS subscores and sex hormones
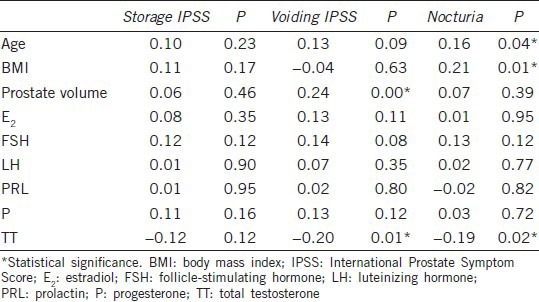
Further analysis showed that the IPSS storage symptom scores were not correlated with the sex hormone serum levels, age, BMI, or prostate volume. However, the IPSS voiding symptom scores were positively correlated with prostate volume (r = 0.24, P = 0.00) and negatively correlated with TT (r = −0.20, P = 0.01). The nocturia score was positively correlated with age (r = 0.16, P = 0.04) and BMI (r = 0.21, P = 0.01) and negatively correlated with TT (r = −0.19, P = 0.02) (Table 3).
Table 3.
Correlation of IPSS subscores with hormonal variables, age, BMI, and prostate volume
LUTS severity and TT
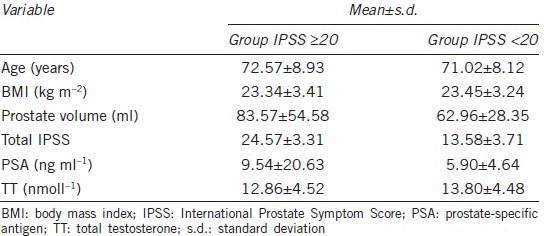
In addition, we divided the study sample according to LUTS severity: group I (7 < total IPSS <20) and group II (total IPSS ≥20) comprised 52 (32.91%) and 106 (67.09%) men, respectively (Table 4). The median level of TT in the two groups was 13.31 nmoll−1 and 12.42 nmoll−1, respectively. There was an increased risk of LUTS in men with lower serum concentrations of TT, although the mean level of testosterone was within the normal range.
Table 4.
Clinical and laboratory characteristics of patients according to IPSS severity
DISCUSSION
LUTS in aging
LUTS are common in men and women, especially in aged populations. The etiology of male LUTS is complex and multifactorial. One of the most common causes of LUTS in older men is BPH.15,16,17 It has a significant impact on the quality of life.18 The spectrum of LUTS includes storage, voiding, and postmicturition symptoms. Storage symptoms are more common and more bothersome to the patient than are voiding symptoms.15,16 Nocturia, a kind of bladder storage problems, is one of the most common LUTS. Many researchers are trying to find the impact factors of storage and voiding symptoms. The results, however, are inconsistent or mixed. Martin et al.19 found a relationship between storage symptoms and abdominal fat in a sample of men in Australia. However, Paick et al.20 reported that BMI was not associated with LUTS. In recently published reviews, incidence of nocturia increases with age, obesity (as BMI), but it is not strongly correlated with prostate volume as determined by TRUS, the same to our results.15,21
Sex hormones and BPH/LUTS
Previous studies have shown that plasma steroid hormones, especially androgens, have also been implicated in the development and maintenance of BPH.22 However, it is not clear how sex hormones contribute to the LUTS secondary to BPH in older men.23 Meanwhile, BPH/LUTS in some elderly men has been associated with diabetes, coronary heart disease, hypertension, obesity, and hypogonadism, among other conditions. These diseases may also affect the internal environment and are essentially characterized by an unbalance in circulating hormones.24 Recent data have revealed modifiable risk factors (e.g., sex steroid hormones, diet, physical activity and inflammation) that present new opportunities for treatment and prevention.1,7,25,26,27
In a cross-sectional study of 509 men aged 40–79 years, Liao et al.12 reported that most of the IPSS items were not significantly associated with serum sex hormone levels; the exception was nocturia, which showed a negative correlation with serum testosterone level. Kim et al.11 also found that TT level was significantly decreased in patients with ≥4 episodes of nocturia and that it was related to the presence of severe LUTS. The authors suggested that endogenous testosterone may have a beneficial role in lower urinary tract function and that a high frequency of nocturia may induce testosterone deficiency.11 Other findings have supported these results and suggested that testosterone deficiency may be a pathophysiological mechanism that connects LUTS and metabolic syndrome in men.14 In addition, some studies have indicated that LUTS occurs at an age at which plasma testosterone levels decline (in some men to hypogonadal levels).28,29 Normalizing testosterone levels in elderly men may improve LUTS, especially voiding disturbance.28,30,31
Although the mechanism of action is not yet understood, it may be explained by an overactivity of the autonomic nervous system. It is known that androgen receptors are present in the epithelium of the urethra and bladder. Testosterone seems to play a key role in the reflex activity of the autonomic nervous system in the pelvis, or it may interact with postsynaptic nongenomic receptors, which increase bladder capacity and compliance and decrease detrusor pressure at maximum flow in men, to suppress detrusor activity.32,33 Furthermore, human neurons in the wall of the bladder contain nitric oxide synthase. Similar to testosterone in the penis, testosterone in the bladder impacts nitric oxide synthase.28,34
In this study, TT level appeared to be the dominant predictor of LUTS, especially of the nocturia subscore (nocturia). We also found that some ratios such as E2/TT and FSH/LH were associated with the total IPSS. Although the mean TT level was within the normal range, it was associated with the presence of severe LUTS. In contrast, the findings of Favilla and his group differed from our results. They selected 127 men with symptomatic BPH who underwent surgery and found that TT level was the only significant predisposing factor for LUTS. They suggested that high risk LUTS were more likely to develop in men with greater TT serum concentrations, although the mean TT level was within the normal range.8
Moreover, other data reported no associations between serum sexual hormones and LUTS. Lee et al.10 collected clinical information from the health examinations of 2308 middle-aged eugonadal men and found no correlation between TT and IPSS. In another large-scale study, 5506 adults aged 30–79 years were randomly divided into groups whose blood results, anthropometric measures, lifestyle and psychosocial factors, comorbidities and urological symptoms were recorded. Statistical analysis showed that after adjusting for age, circulating sex hormone levels were generally not significant predictors of urological symptoms in the men.35
Limitations
Certain potential limitations of this study should also be noted. First, the sample size was relatively small; thus, the study population might not accurately represent the underlying population. Second, the study was hospital-based, and data from the general population would have been preferable to data from the sample of hospital patients. Third, the study only included Chinese men; thus, cultural and sociodemographic factors may have affected our results. Multicenter and longitudinal studies are needed for stronger conclusions to be made. Fourth, the serum hormone levels were only measured once, which is similar to most of the previous studies. The single measurement provides an imperfect estimate of a subject's usual hormonal status and can be influenced by both individual errors and analytical errors. Two samples that are taken on two separate days may provide a more accurate estimate. Fifth, urodynamic assessment may provide greater insight into the potential mode of action of sexual hormones in influencing LUTS. Sixth, we did not adjust for systemic diseases other than hypertension, coronary heart disease, and diabetes; other diseases that were unaccounted for could also lessen the strength of our study.
CONCLUSION
In summary, the present results have shown that the severity of LUTS is associated with TT, E2/TT and FSH/LH. In subscores of IPSS, only nocturia correlated with age, BMI and TT. Endogenous testosterone may play a beneficial role on lower urinary tract function. Further sufficiently powered studies need to be conducted in various samples to gain a better understanding of the relationship between LUTS and sex hormones.
AUTHOR CONTRIBUTIONS
YW and HP gathered the data, performed the statistical analysis, interpreted the results, and drafted and revised the manuscript. JQ and HFH conceived and designed the study. WMW and Dx coordinated and supervised the project and helped to draft the manuscript. LZ, ZQG, and QB participated in data collection. All authors have read and approved the final manuscript.
COMPETING INTEREST
All authors declare no competing interests.
ACKNOWLEDGMENTS
We gratefully acknowledge the help of Dr. Yun-Kai Zhu and Dr. Ya-Qing Chen for their excellent technical assistance in the ultrasound examinations. This work was supported by National Natural Science Foundation of China (Grant No. 81100410) and Shanghai Municipal Natural Science Foundation (Grant No. 10ZR1420200).
REFERENCES
- 1.Breyer BN, Sarma AV. Hyperglycemia and insulin resistance and the risk of BPH/LUTS: an update of recent literature. Curr Urol Rep. 2014;15:462. doi: 10.1007/s11934-014-0462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnard RJ, Aronson WJ. Benign prostatic hyperplasia: does lifestyle play a role? Phys Sportsmed. 2009;37:141–6. doi: 10.3810/psm.2009.12.1752. [DOI] [PubMed] [Google Scholar]
- 3.Montie JE, Pienta KJ. Review of the role of androgenic hormones in the epidemiology of benign prostatic hyperplasia and prostate cancer. Urology. 1994;43:892–9. doi: 10.1016/0090-4295(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths K, Eaton CL, Harper ME, Peeling B, Davies P. Steroid hormones and the pathogenesis of benign prostatic hyperplasia. Eur Urol. 1991;20(Suppl 1):68–77. doi: 10.1159/000471750. [DOI] [PubMed] [Google Scholar]
- 5.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–9. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis TR, Chughtai B, Kaplan SA. Testosterone and benign prostatic hyperplasia. Asian J Androl. 2015;17:212–6. doi: 10.4103/1008-682X.140966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82:184–99. doi: 10.1016/j.diff.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favilla V, Cimino S, Castelli T, Madonia M, Barbagallo I, et al. Relationship between lower urinary tract symptoms and serum levels of sex hormones in men with symptomatic benign prostatic hyperplasia. BJU Int. 2010;106:1700–3. doi: 10.1111/j.1464-410X.2010.09459.x. [DOI] [PubMed] [Google Scholar]
- 9.Cho KJ, Kim JC. Biomarkers for lower urinary tract dysfunction. Int J Urol. 2013;20:13–20. doi: 10.1111/j.1442-2042.2012.03216.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Kim Y, Park YW, Lee DG. Relationship between benign prostatic hyperplasia/lower urinary tract symptoms and total serum testosterone level in healthy middle-aged eugonadal men. J Sex Med. 2014;11:1309–15. doi: 10.1111/jsm.12489. [DOI] [PubMed] [Google Scholar]
- 11.Kim MK, Zhao C, Kim SD, Kim DG, Park JK. Relationship of sex hormones and nocturia in lower urinary tract symptoms induced by benign prostatic hyperplasia. Aging Male. 2012;15:90–5. doi: 10.3109/13685538.2012.659715. [DOI] [PubMed] [Google Scholar]
- 12.Liao CH, Chiang HS, Yu HJ. Serum testosterone levels significantly correlate with nocturia in men aged 40-79 years. Urology. 2011;78:631–5. doi: 10.1016/j.urology.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Trifiro MD, Parsons JK, Palazzi-Churas K, Bergstrom J, Lakin C, et al. Serum sex hormones and the 20-year risk of lower urinary tract symptoms in community-dwelling older men. BJU Int. 2010;105:1554–9. doi: 10.1111/j.1464-410X.2009.09090.x. [DOI] [PubMed] [Google Scholar]
- 14.Chang IH, Oh SY, Kim SC. A possible relationship between testosterone and lower urinary tract symptoms in men. J Urol. 2009;182:215–20. doi: 10.1016/j.juro.2009.02.123. [DOI] [PubMed] [Google Scholar]
- 15.Yukio H, Kellogg PJ, Karin SC, Debra EI, Naoya M, et al. Epidemiology and natural history of male lower urinary tract symptoms. Lower Urinary Tract Symptoms (LUTS): An International Consultation on Male LUTS. In: Chapple C, Abrams P, editors. Montreal, Canada: Société Internationaled’ Urologie (SIU); 2013. pp. 1–35. [Google Scholar]
- 16.Chapple CR, Wein AJ, Abrams P, Dmochowski RR, Giuliano F, et al. Lower urinary tract symptoms revisited: a broader clinical perspective. Eur Urol. 2008;54:563–9. doi: 10.1016/j.eururo.2008.03.109. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Hu H, xu K, Wang x, Na Y, et al. Prevalence, risk factors and the bother of lower urinary tract symptoms in China: a population-based survey. Int Urogynecol J. 2015;26:911–9. doi: 10.1007/s00192-015-2626-8. [DOI] [PubMed] [Google Scholar]
- 18.Patel ND, Parsons JK. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J Urol. 2014;30:170–6. doi: 10.4103/0970-1591.126900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin SA, Haren MT, Marshall VR, Lange K, Wittert GA, et al. Prevalence and factors associated with uncomplicated storage and voiding lower urinary tract symptoms in community-dwelling Australian men. World J Urol. 2011;29:179–84. doi: 10.1007/s00345-010-0605-8. [DOI] [PubMed] [Google Scholar]
- 20.Paick JS, Yang JH, Kim SW, Ku JH. Are age, anthropometry and components of metabolic syndrome-risk factors interrelated with lower urinary tract symptoms in patients with erectile dysfunction. A prospective study? Asian J Androl. 2007;9:213–20. doi: 10.1111/J.1745-7262.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 21.Osman NI, Chapple CR, Wein AJ. Nocturia: current concepts and future perspectives. Acta Physiol. 2013;207:53–65. doi: 10.1111/apha.12013. [DOI] [PubMed] [Google Scholar]
- 22.Platz EA, Kawachi I, Rimm EB, Longcope C, Stampfer MJ, et al. Plasma steroid hormones, surgery for benign prostatic hyperplasia, and severe lower urinary tract symptoms. Prostate Cancer Prostatic Dis. 1999;2:285–9. doi: 10.1038/sj.pcan.4500380. [DOI] [PubMed] [Google Scholar]
- 23.St Sauver JL, Jacobson DJ, McGree ME, Girman CJ, Klee GG, et al. Associations between longitudinal changes in serum estrogen, testosterone, and bioavailable testosterone and changes in benign urologic outcomes. Am J Epidemiol. 2011;173:787–96. doi: 10.1093/aje/kwq438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chavalmane AK, Comeglio P, Morelli A, Filippi S, Fibbi B, et al. Sex steroid receptors in male human bladder: expression and biological function. J Sex Med. 2010;7:2698–713. doi: 10.1111/j.1743-6109.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- 25.Antunes AA, Araujo LH, Nakano E, Muracca E, Srougi M. Obesity may influence the relationship between sex hormones and lower urinary tract symptoms. Int Braz J Urol. 2014;40:240–6. doi: 10.1590/S1677-5538.IBJU.2014.02.15. [DOI] [PubMed] [Google Scholar]
- 26.Vignozzi L, Morelli A, Sarchielli E, Comeglio P, Filippi S, et al. Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. J Endocrinol. 2012;212:71–84. doi: 10.1530/JOE-11-0289. [DOI] [PubMed] [Google Scholar]
- 27.Jung S, Lee EN, Lee SR, Kim MS, Lee MS. Tai chi for lower urinary tract symptoms and quality of life in elderly patients with benign prostate hypertrophy: a randomized controlled trial. Evid Based Complement Alternat Med 2012. 2012:1–7. doi: 10.1155/2012/624692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalinchenko S, Vishnevskiy EL, Koval AN, Mskhalaya GJ, Saad F. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male. 2008;11:57–61. doi: 10.1080/13685530801953994. [DOI] [PubMed] [Google Scholar]
- 29.Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11:1567–76. doi: 10.1111/jsm.12523. [DOI] [PubMed] [Google Scholar]
- 30.Shigehara K, Sugimoto K, Konaka H, Iijima M, Fukushima M, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male. 2011;14:53–8. doi: 10.3109/13685538.2010.518178. [DOI] [PubMed] [Google Scholar]
- 31.Amano T, Imao T, Takemae K, Iwamoto T, Nakanome M. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male. 2010;13:242–6. doi: 10.3109/13685538.2010.487552. [DOI] [PubMed] [Google Scholar]
- 32.Karazindiyanoglu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male. 2008;11:146–9. doi: 10.1080/13685530802290438. [DOI] [PubMed] [Google Scholar]
- 33.Koritsiadis G, Stravodimos K, Mitropoulos D, Doumanis G, Fokitis I, et al. Androgens and bladder outlet obstruction: a correlation with pressure-flow variables in a preliminary study. BJU Int. 2008;101:1542–6. doi: 10.1111/j.1464-410X.2008.07521.x. [DOI] [PubMed] [Google Scholar]
- 34.Yassin AA, El-Sakka AI, Saad F, Gooren LJ. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol. 2008;26:359–64. doi: 10.1007/s00345-008-0284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Litman HJ, Bhasin S, O’Leary MP, Link CL, McKinlay JB, et al. An investigation of the relationship between sex-steroid levels and urological symptoms: results from the Boston area community health survey. BJU Int. 2007;100:321–6. doi: 10.1111/j.1464-410X.2007.06938.x. [DOI] [PubMed] [Google Scholar]


