Dear Editor,
The patient is a 45-year-old male who presented to an outside clinic in 2014 for evaluation of painful ejaculation, infertility, and difficulty with urination for as long as he could remember. He reported no past significant medical or surgical history and no family history of infertility. He was given a trial of tamsulosin without improvement in his symptoms. Initial semen analysis demonstrated azoospermia. A subsequent MRI showed bilaterally enlarged seminal vesicles, and the patient then underwent transurethral resection of the ejaculatory ducts (TURED) for presumed ejaculatory duct obstruction (EDO), also without symptomatic improvement.
When the patient's symptoms persisted, he was referred to our clinic for further evaluation. On presentation, he denied a history of urinary tract or sexually transmitted infections, prostatitis, or prior related medical history, was taking no medications, and denied other surgical history beyond TURED. He complained of persistent pain with ejaculation as well as straining with urination. He denied dysuria, frequency, urgency, or gross hematuria, but did report occasional nocturia.
On physical examination, his testes were palpated to be 20 ml bilaterally, with grossly normal epididymides, vasa deferentia, and prostate gland. Semen analysis showed a volume of 0.5 ml and azoospermia and hormonal evaluation identified normal FSH, LH, and testosterone levels. The patient did not provide a second semen sample for analysis. Transrectal ultrasonography revealed massively dilated seminal vesicles (SVs), consistent with an MRI from his prior evaluation, which showed a left SV measuring 3.8 cm × 4.6 cm × 2.2 cm and a right SV measuring 3.1 cm × 4.2 cm × 2.6 cm.
The patient then underwent cystoscopic evaluation in the operating room for presumed EDO, with findings consistent with prior TURED. At the 6 o’clock position in the prostatic fossa were two vertically aligned ~5 French orifices, later confirmed to be markedly dilated ejaculatory ducts. No bladder abnormalities were visualized and both ureteral orifices were in their orthotopic positions. On contrast injection into the more distal ejaculatory duct orifice, an enlarged, dilated SV with a patent vas deferens was observed, with contrast refluxing the entire length of the left vas deferens (Figure 1a). The right vas deferens was not visualized on initial contrast injection, but was observed on later injection. The ejaculatory ducts were balloon dilated using a 5 French angioplasty balloon, and both ducts were cannulated and explored visually using a flexible ureteroscope, revealing markedly dilated SVs. Contrast injection demonstrated bilaterally enlarged SVs and no apparent vasal obstruction. A right vasotomy was performed and indigo carmine was injected, with subsequent appearance of blue dye in the bladder, confirming patency. Right testicular sperm aspiration was then performed, which revealed whole nonmotile sperm on microscopy.
Figure 1.
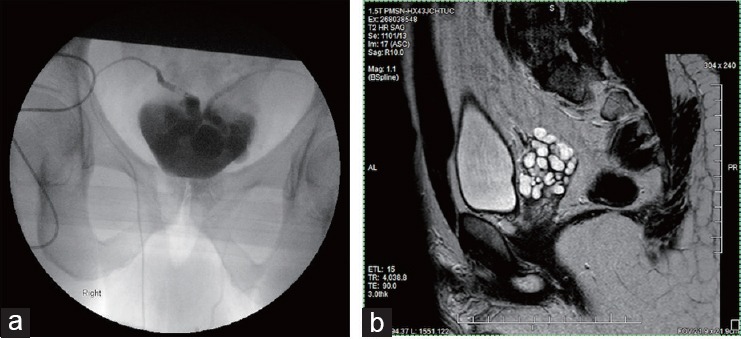
Imaging performed upon arrival to referral to our clinic, after the completion of TURED at outside facility. (a) Seminal vesiculogram showing marked dilation of the bilateral SVs as well as patency of the bilateral vasa deferentia. Communication between the right and left SVs was observed as well. (b) Pelvic MRI (T2 sagittal view) showing dilation of the R seminal vesicle.
A semen analysis performed 5 days postprocedure again demonstrated an ejaculate volume of 0.5 ml with azoospermia, indicating a failure of the surgical intervention. Repeat imaging showed persistent dilation of the seminal vesicles (Figure 1b), which lead to the diagnosis of functional ampullo-vesicular obstruction, as opposed to a physical obstruction. For the next several months, the patient continued to experience pain with ejaculation, which was localized to the epididymis. Eleven months after our original encounter, the patient returned to undergo sperm aspiration and bilateral epididymectomy, which resulted in complete resolution of his symptoms.
In this unusual case, a patient presented with symptoms of painful ejaculation and infertility, which are typical of ampullo-vesicular obstruction. However, the patient's symptoms failed to improve after a TURED procedure, and vesiculogram showed complete patency, with no evidence of physical obstruction along the ampullo-vesicular tract. This unusual finding led to the diagnosis of seminal vesicle dysfunction.
Congenital causes of EDO include congenital agenesis or atresia of the ejaculatory ducts,1 mutations in the cystic fibrosis transmembrane regulator (CFTR) gene,2 ectopic ureteral orifice opening directly into the ejaculatory duct(s), and Zinner syndrome, a cystic obstruction associated with renal agenesis.1,3 Much less commonly, EDO can result from functional defects of the vasa deferentia or the SVs in the absence of physical obstruction. Termed “seminal vesicle dysfunction,” this form of EDO results from failure of SV peristalsis. Ultimately, this disease is a diagnosis of exclusion, and can be made only after a physical obstruction has been ruled out via imaging and surgical interventions.
Ampullo-vesicular emptying defects have been observed in patients with spinal cord injuries, multiple sclerosis, and iatrogenic damage after retroperitoneal lymph node dissection or other pelvic surgery.4 There is some evidence from rat models that neuropathic changes resulting from diabetes mellitus may induce hypocontraction of the vas deferens and promote structural changes, resulting in SV dysfunction.5 Diabetic men with erectile dysfunction also exhibit concomitant bilateral enlargement of the SVs due to SV adynamia,6 which may improve with PDE5i treatment, although the mechanism of this effect is unknown.7 Several medications, including thiazide diuretics, α-adrenergic blockers, typical antipsychotics, and tricyclic antidepressants, are associated with impaired ejaculation and SV adynamia.8
Only ten cases of isolated functional ampullo-vesicular voiding defects in the absence of neurologic damage, diabetes mellitus, or physical obstruction have been reported.9 The etiology of these deficits is thought to be a deficit in local innervation, similar to those seen in megacolon and megaesophagus.10 In 1987, Colpi reported on six infertile men with ampullo-vesicular voiding deficiencies.9 Of these six patients, three opted for treatment with experimental therapies. One underwent unsuccessful implantation of an artificial spermatocele into the vas deferens. Another was asked to provide semen samples 5 min after treatment using several stimulant drugs, including midodrine, phentolamine, bethanechol, and emepronium, with no change in seminal parameters. The third patient was treated with 3 days of oral brompheniramine, midodrine, phenylpropanolamine, chlorpheniramine, and nethaprine, but the results of this treatment were not published. As such, there is currently no evidence of successful medical therapy using sympathomimetics or anticholinergics for the treatment of functional ampullo-vesicular dysfunction.
For patients presenting with infertility in the context of dilated seminal vesicles, we propose the following work-up, which is similar to that of the study by Colpi et al.10
Rule out obstruction with TURED procedure. Failure to improve after un-obstruction implies a diagnosis of functional obstruction
Consider medical therapy using experimental application of PDE5i for both pain and fertility
If the patient still desires fertility, proceed to sperm retrieval and ART
If pain persists, localize pain and consider local surgical therapies.
In summary, we describe a case of a patient with infertility and painful ejaculation and dilated seminal vesicles on imaging. The diagnosis of functional ampullo-vesicular voiding deficiency was made after surgical intervention failed to reduce the vesicular dilation or improve ejaculate volume and contents. Future work to define the etiologies of functional ampullo-vesicular obstruction is needed to better approach treatment in the few patients affected by this condition.
AUTHOR CONTRIBUTIONS
MDF conducted the literature review and drafted the manuscript. AWP and LIL conceived the case report after seeing the patient in clinic, operated on the patient, and captured the intraoperative images. JRC saw the patient in clinic, performed the TURED, and provided insight into his original presentation.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
AWP is a National Institutes of Health (NIH) K12 Scholar supported by a Male Reproductive Health Research Career (MRHR) Development Physician-Scientist Award (HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Program (to Dolores J. Lamb).
REFERENCES
- 1.Sandlow JI. In: Seminal vesicle and ejaculatory duct surgery. Glenn's Urologic Surgery. 7th ed. Goldstein M, editor. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 369. [Google Scholar]
- 2.Meschede D, Dworniczak B, Behre HM, Kleisch S, Claustres M, et al. CFTR gene mutations in men with bilateral ejaculatory-duct obstruction and anomalies of the seminal vesicles. Am J Hum Genet. 1997;61:1200–2. doi: 10.1086/301606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vohra S, Morgentaler A. Congenital anomalies of the vas deferens, epididymis, and seminal vesicles. Urology. 1997;49:313–21. doi: 10.1016/S0090-4295(96)00433-5. [DOI] [PubMed] [Google Scholar]
- 4.Phillips E, Carpenter C, Oates RD. Ejaculatory dysfunction. Urol Clin North Am. 2014;41:115. doi: 10.1016/j.ucl.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Tsounapi P, Honda M, Dimitriadis F, Shimizu S, Iguchi M, et al. PD36-11 the diabetes mellitus-induced dysfunction on seminal vesicles and vas deferens in the rat model. J Urol. 2015;193:769. [Google Scholar]
- 6.La Vignera S, Vieari E, Condorelli R, D’Agata R, Calogero AE. Ultrasound characterization of the seminal vesicles in infertile patients with type 2 diabetes mellitus. Eur J Radiol. 2011;80:64–7. doi: 10.1016/j.ejrad.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 7.La Vignera S, Vicari E, Condorelli R, D’Agata A, Calogero AE. Seminal vesicles and diabetic neuropathy: ultrasound evaluation after prolonged treatment with a selective phosphodiesterase-5 inhibitor. Andrology. 2013;1:245. doi: 10.1111/j.2047-2927.2012.00025.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith JF, Walsh TJ, Turek PJ. Ejaculatory duct obstruction. Urol Clin North Am. 2008;35:221–7. doi: 10.1016/j.ucl.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Colpi GM, Casella F, Zanollo A, Ballerini G, Balerna M, et al. Functional voiding disturbances of the ampullo-vesicular seminal tract: a cause of male infertility. Acta Eur Fertil. 1987;18:165. [PubMed] [Google Scholar]
- 10.Colpi GM, Negri L, Mariani M, Balerna M. Semen anomalies due to voiding defects of the ampullo-vesicular tract: infertility due to ampullo-vesicular voiding defects. Andrologia. 1990;22(Suppl 1):206. doi: 10.1111/j.1439-0272.1990.tb02086.x. [DOI] [PubMed] [Google Scholar]


