SUMMARY
Phosphoribosyl diphosphate (PRPP) is an important intermediate in cellular metabolism. PRPP is synthesized by PRPP synthase, as follows: ribose 5-phosphate + ATP → PRPP + AMP. PRPP is ubiquitously found in living organisms and is used in substitution reactions with the formation of glycosidic bonds. PRPP is utilized in the biosynthesis of purine and pyrimidine nucleotides, the amino acids histidine and tryptophan, the cofactors NAD and tetrahydromethanopterin, arabinosyl monophosphodecaprenol, and certain aminoglycoside antibiotics. The participation of PRPP in each of these metabolic pathways is reviewed. Central to the metabolism of PRPP is PRPP synthase, which has been studied from all kingdoms of life by classical mechanistic procedures. The results of these analyses are unified with recent progress in molecular enzymology and the elucidation of the three-dimensional structures of PRPP synthases from eubacteria, archaea, and humans. The structures and mechanisms of catalysis of the five diphosphoryltransferases are compared, as are those of selected enzymes of diphosphoryl transfer, phosphoryl transfer, and nucleotidyl transfer reactions. PRPP is used as a substrate by a large number phosphoribosyltransferases. The protein structures and reaction mechanisms of these phosphoribosyltransferases vary and demonstrate the versatility of PRPP as an intermediate in cellular physiology. PRPP synthases appear to have originated from a phosphoribosyltransferase during evolution, as demonstrated by phylogenetic analysis. PRPP, furthermore, is an effector molecule of purine and pyrimidine nucleotide biosynthesis, either by binding to PurR or PyrR regulatory proteins or as an allosteric activator of carbamoylphosphate synthetase. Genetic analyses have disclosed a number of mutants altered in the PRPP synthase-specifying genes in humans as well as bacterial species.
KEYWORDS: amino acid metabolism, diphosphoryl transfer, nucleotide metabolism, phosphoribosyl pyrophosphate, protein structure-function
INTRODUCTION
The compound 5-phospho-d-ribosyl-α-1-diphosphate (PRPP) is an important metabolite required in the biosynthesis of purine and pyrimidine nucleotides, the amino acids histidine and tryptophan, and the cofactors NAD and NADP (1–3). Furthermore, PRPP is utilized in the biosynthesis of methanopterin in certain archaeal species (4) and in the biosynthesis of polyprenylphosphate pentoses in Mycobacterium tuberculosis (5). By far the most abundant class of reactions using PRPP as a substrate result in the formation of N-glycosidic bonds, whereas a few reactions result in the formation of O- or C-glycosidic bonds. Kornberg and coworkers discovered PRPP in the mid-1950s while searching for a reaction that converted orotate to uridylate as well as the enzymes catalyzing these reactions (6, 7). The enzyme catalyzing the synthesis of PRPP, PRPP synthase (ATP:d-ribose 5-phosphate diphosphotransferase; EC 2.7.6.1) is ubiquitous among free-living organisms. Thus, only certain obligate intracellular parasites, such as some Chlamydia and Rickettsia species, lack a gene encoding a PRPP synthase. Therefore, in general, an organism contains at least one gene specifying PRPP synthase. A few bacterial species contain more than one prs gene. In contrast, many eukaryotic organisms contain more than one PRPP synthase-specifying gene. The yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe appear to contain five and three PRPP synthase orthologs, respectively (8–10), mammals (humans and rats) contain three PRPP synthase-encoding genes (11–13), and the plants Spinacea oleracea and Arabidopsis thaliana contain four and five PRPP synthase genes, respectively (14–16).
Although prs is believed to be an essential gene (17), a number of prs mutants have been isolated, primarily in Escherichia coli and Salmonella enterica serovar Typhimurium, that are conditional or specify mutant variants of PRPP synthase with altered enzymatic properties. Additionally, a few knockout mutant (Δprs) strains have been constructed in vitro. Insertion of these Δprs mutant alleles into strains with a specific genetic background revealed that an organism can be viable in the absence of PRPP synthase activity. Also, a number of naturally occurring variant PRPP synthases have been identified among patients with gout or uric acid overproduction (18).
The present review deals with all aspects of PRPP metabolism with an emphasis on biochemical, genetic, and physiological aspects of PRPP synthase and the utilization of PRPP in biosynthesis, primarily in microorganisms. The elucidation of high-resolution structures of PRPP synthase from a number of organisms makes this review timely. Previously, a few reviews have been published on PRPP synthases of microbial (1, 19), mammalian (18, 20), and plant (416) origins. These reviews are complemented by the present review.
Sequence analysis was performed with BLAST (21), the program packages of the Integrated Microbial Genomes website (http://img.jgi.doe.gov/) (22), and the National Center for Biotechnology Information data (http://www.ncbi.nlm.nih.gov/) (23), whereas amino acid sequence analysis and alignments were performed with Multalin (http://multalin.toulouse.inra.fr/multalin/) and DNA Strider (24, 25). Figures showing the three-dimensional structures were prepared with the Pymol molecular viewer (http://www.pymol.org) (26).
CHEMISTRY OF PRPP
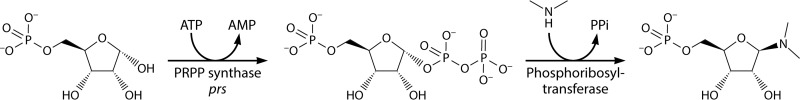
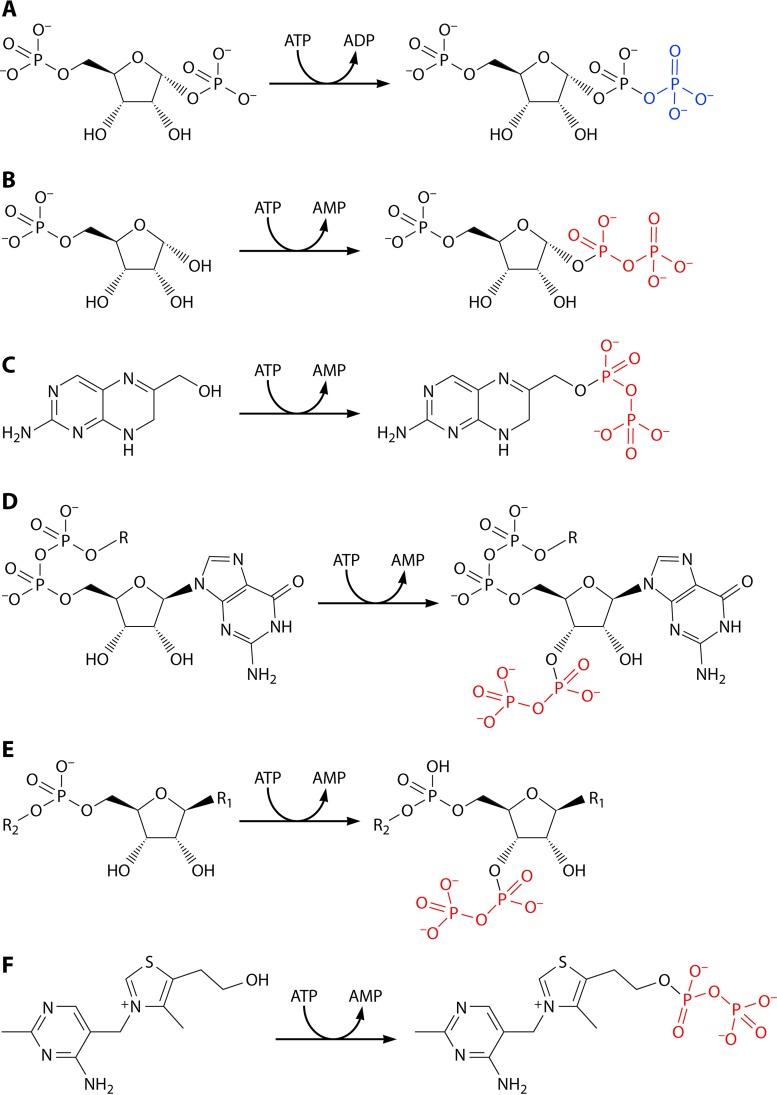
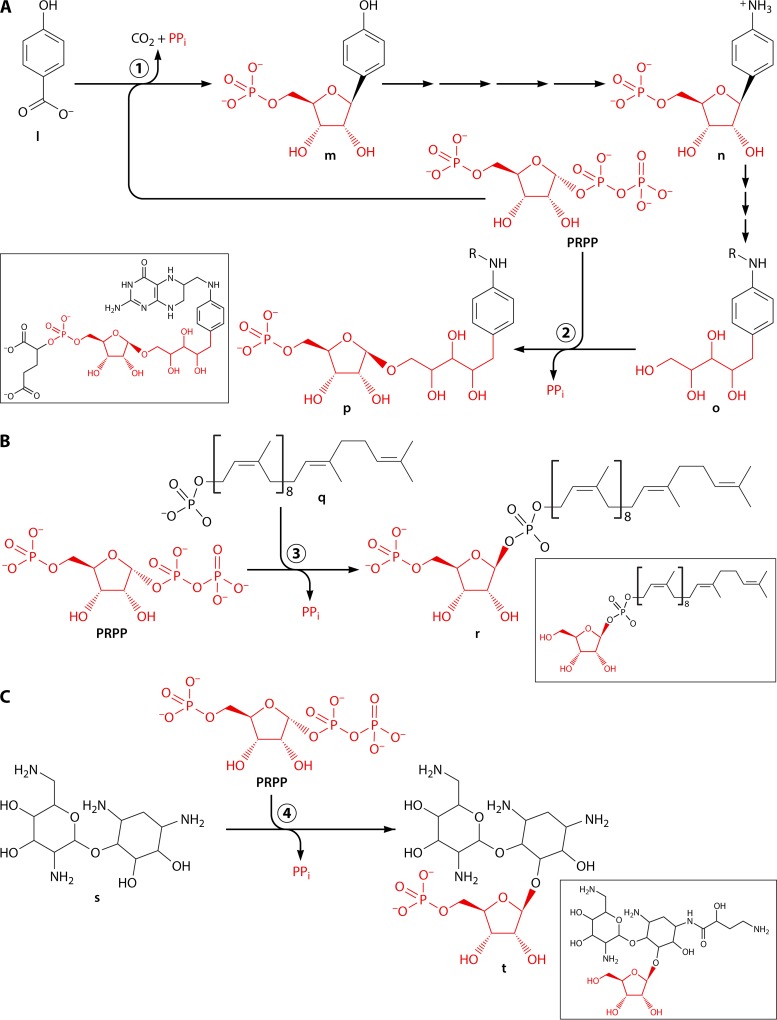
PRPP is synthesized by transfer, in a single step, of the β,γ-diphosphoryl group of ATP to the C-1 hydroxyl of α-d-ribose 5-phosphate, with the simultaneous formation of AMP by the following deceptively simple reaction: ribose 5-phosphate + ATP → PRPP + AMP (Fig. 1). The reaction is catalyzed by PRPP synthase (6, 7), which is encoded by a prs, prsA, or PRPS gene (13, 27, 28). PRPP may be regarded as an activated form of ribose 5-phosphate. Essentially all of the reactions utilizing PRPP as a substrate are substitutions, where usually nitrogen-containing aromatic bases replace the diphosphoryl group with simultaneous inversion of configuration at C-1 of the ribosyl moiety (Fig. 1). In the majority of these reactions, a ribonucleoside 5′-monophosphate is formed. Thus, “all” that happens in reactions such as those shown in Fig. 1 is an attachment of the nitrogenous compound to the ribosyl moiety and the formation of diphosphate (PPi). PPi, i.e., phosphoric acid anhydride, with a high negative free energy of hydrolysis, in turn is hydrolyzed to phosphate (Pi), which makes the phosphoribosyl transfer reaction thermodynamically irreversible.
FIG 1.
Biochemical function of PRPP. The β,γ-diphosphoryl group of ATP is transferred to ribose 5-phosphate with the generation of PRPP in a reaction catalyzed by PRPP synthase, which is encoded by a prs gene. PRPP is a substrate in a number of substitution reactions, most of which involve a nitrogen-containing compound. The reaction occurs at C-1 of the ribosyl moiety and proceeds with inversion of the configuration of this carbon and with PPi as a leaving group.
Immediately after its discovery, it was noted that PRPP was a stable compound as long as it was stored in the cold at neutral pH, whereas acidic or basic conditions and heating readily decomposed the compound. Divalent ions such as Mg2+, Mn2+, and Ba2+ encouraged decomposition. The degradation of PRPP, presumably by hydrolysis, appears to follow one of three pathways: (i) PRPP + H2O → ribose 5-phosphate + PPi, (ii) PRPP + H2O → 5-phosphoribosyl 1,2-cyclic phosphate + Pi (6, 7, 29), or (iii) PRPP + H2O → ribosyl 1,5-cyclic phosphate + PPi, with pathways i and ii representing the major pathways (30, 31). Degradation through pathway ii may furthermore continue according to the following scheme: 5-phosphoribosyl 1,2-cyclic phosphate → ribosyl 1-phosphate → ribose (32). The binding of divalent ions to PRPP is conveniently measured by 31P nuclear magnetic resonance, and this revealed that Mg2+ binds to both the 5-phosphate and the 1-diphosphate moieties of PRPP (33).
The standard free energy (ΔG°′) for the hydrolysis of PRPP (PRPP + H2O → ribose 5-phosphate + PPi) has been calculated to be approximately −35 kJ mol−1. This value should be compared to −47 kJ mol−1 for the hydrolysis of the α,β-phosphoanhydride bond of ATP (34). Consistently, the equilibrium constant for the diphosphoryl transfer reaction of PRPP synthase has been determined to be 29 (35).
Despite the lability of PRPP, procedures have been developed for the quantitative isolation of the compound. These include extraction of PRPP from cells with cold formic acid (36–38), cold perchloric acid (39), or boiling followed by immediate cooling (40), followed by chromatography or phosphoribosyltransferase-catalyzed conversion of PRPP to ribonucleoside 5′-monophosphate.
A chemically stable analog of PRPP has been synthesized for use as a ligand for various PRPP-binding proteins: 1-α-diphosphoryl-2,3-α-dihydroxy-4-β-cyclopentane-methanol 5-phosphate (cPRPP) (41, 42). Although this analog is an inhibitor of PRPP synthase activity, the compound has proven valuable as a PRPP analog in X-ray analyses of PRPP synthase.
BIOSYNTHESIS OF PRPP
PRPP Synthase and Phosphoribosyl Bisphosphate Phosphokinase
The synthesis of PRPP is catalyzed by two enzymes, PRPP synthase and phosphoribosyl bisphosphate phosphokinase. On a quantitative basis, PRPP synthase is by far the most important. Phosphoribosyl bisphosphate phosphokinase is a component of the phosphonate catabolic pathway and is present only in cells that thrive on phosphonate as a Pi source. Here, we shall deal first with PRPP synthase and then later with phosphoribosyl bisphosphate phosphokinase.
CLASSIFICATION AND PROPERTIES OF PRPP SYNTHASES
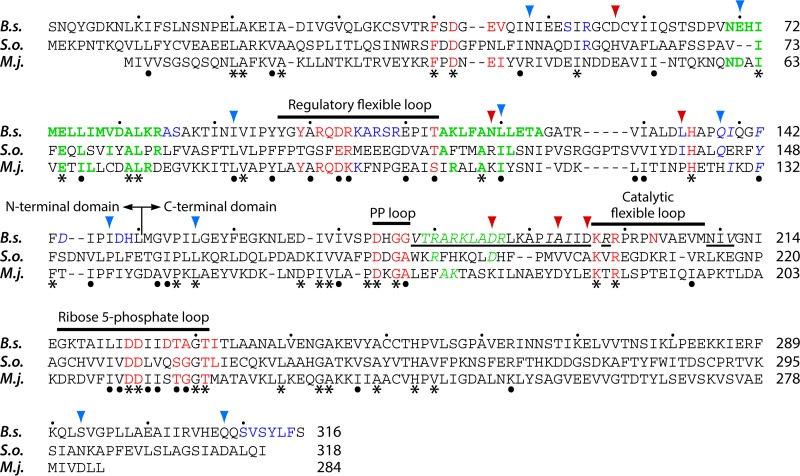
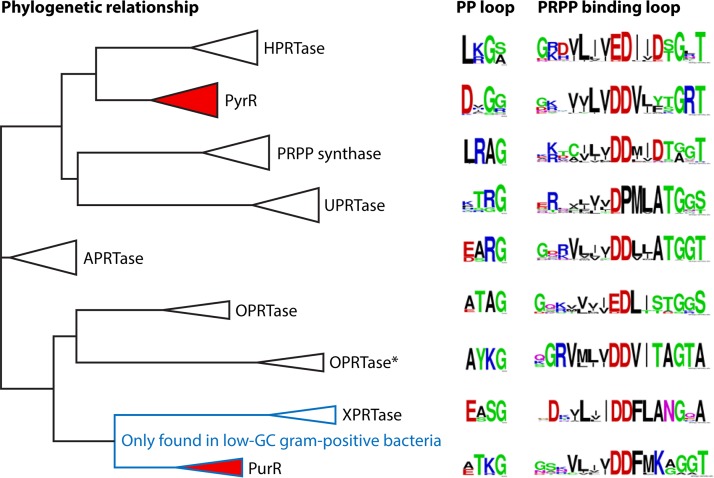
Alignment of the amino acid sequences from a variety of organisms revealed a high similarity among PRPP synthases. For example, the overall identity of PRPP synthase amino acid sequences of E. coli and humans is 47% (20), and that of PRPP synthases of E. coli and the Gram-positive organism Bacillus subtilis is 51% (43). In addition, a 15-amino-acid sequence is highly conserved among PRPP synthase and adenine phosphoribosyltransferase (44, 45). The latter enzyme catalyzes the following reaction: adenine + PRPP → AMP + PPi. This 15-amino-acid sequence of adenine phosphoribosyltransferase was designated the PRPP-binding site and later the ribose 5-phosphate-binding loop in PRPP synthase. Subsequently, studies of cDNA libraries of the plants S. oleracea and A. thaliana revealed the presence of four genes that specify a PRPP synthase. Two of these enzymes (isozymes 1 and 2) had primary structures similar to those of E. coli and humans, whereas two (isozymes 3 and 4) had primary structures quite different from those of E. coli and humans. The amino acid sequence identity of S. oleracea isozymes 1 and 2 is 88%, and that of isozymes 3 and 4 is 75%. In contrast, the amino acid sequence identity of isozymes 1 or 2 to isozymes 3 or 4 is modest, 22 to 25%. These deviations of amino acid sequence identities of S. oleracea PRPP synthase isozymes 3 and 4 from isozymes 1 and 2 and other “classical” PRPP synthases are also reflected in their physico-chemical properties (see below). It was therefore suggested that two different classes of PRPP synthases exist, classes I and II (46, 47). Additionally, PRPP synthases of archaeal origin show low amino acid sequence identity with class I or class II PRPP synthases. Thus, the amino acid sequence identities of B. subtilis and the thermophilic, methanogenic archaeon Methanocaldococcus jannaschii, the thermoacidophilic archaeon Sulfolobus solfataricus, or the thermoacidophilic, facultatively anaerobic, organotrophic archeaon Thermoplasma volcanium PRPP synthases are 26 to 28%. Analysis of the PRPP synthase of M. jannaschii revealed biochemical properties that appeared to be a mixture of the properties of class I and II PRPP synthases, and it was suggested to belong to a novel class of PRPP synthases, class III (48). In the present review, we instead designate these class III enzymes “archaeal PRPP synthases.” An alignment of representatives of PRPP synthases of class I (B. subtilis), class II (S. oleracea isozyme 4), and archaea (M. jannaschii) is shown in Fig. 2. Overall, 36 amino acid residues are identical among the three sequences, i.e., the amino acid sequence identity is 13% or less. The inclusion of an additional 26 conserved amino acid residues results in a similarity of approximately 21%. As indicated below, the conservation of amino acid residues identified as responsible for catalysis is very high, whereas amino acid residues involved in allosteric regulation of activity of B. subtilis PRPP synthase are nonconserved among the three classes, consistent with the fact that only class I PRPP synthases are allosterically regulated.
FIG 2.
Alignment of amino acid sequences of PRPP synthases from B. subtilis (B.s.), S. oleracea isozyme 4 (S.o.), and M. jannaschii (M.j.). The B. subtilis PRPP synthase amino acid sequence is numbered from the N-terminal serine, as the original N-terminal methionine is removed in the mature protein. Functional elements of the B. subtilis PRPP synthase are indicated by bars above the B. subtilis amino acid sequence. Amino acid residues identical in all three sequences are indicated by asterisks below the M. jannaschii PRPP synthase amino acid sequence, whereas conserved amino acid residues are indicated by dots. Conserved amino acids are valine, leucine, and isoleucine; phenylalanine and tyrosine; aspartate and glutamate; arginine and lysine; serine and threonine; and glycine and alanine. The division of the N- and C-terminal domains (150-Leu-Met-151) of B. subtilis PRPP synthase is indicated by a vertical line. B. subtilis PRPP synthase amino acid residues, which are located at the active site, are shown in red and those which are located at the allosteric, regulatory site are shown in blue, whereas those involved in subunit-subunit interactions are shown in green. Amino acid residues involved in formation of the bent dimer are shown in bold, whereas those involved in formation of the parallel dimer are shown in italics. Whenever amino acid residues of the PRPP synthase of S. oleracea or M. jannaschii are identical to those of the B. subtilis enzyme, the color code of the latter enzyme is applied to the S. oleracea and M. jannaschii residues as well. The underlined amino acid residues Val178-Asp196, Arg198, and Asn209-Val211 are involved in the formation of a tightly packed interface necessary for allosteric inhibition. Amino acid residues were selected on the basis of the three-dimensional structures previously published (49, 50, 54). Vertical arrowheads point to B. subtilis PRPP synthase amino acids, which are homologous to amino acids altered in the human PRPP synthase isozyme 1 due to point mutations in the PRSP1 gene. Red arrowheads point to amino acid alterations resulting in increased PRPP synthase activity, whereas blue arrowheads point to amino acid alterations resulting in decreased PRPP synthase activity. The amino acid alterations and properties of the human PRPP synthase variants are described further in the text and are summarized in Table 4.
Class I PRPP Synthases
The classical PRPP synthases, i.e., class I, are by far the most widely phylogenetically distributed PRPP synthases. These enzymes contain approximately 315 amino acid residues. PRPP synthase of the bacterial species S. enterica, E. coli, and B. subtilis, as well as those of humans and rats, are the best-studied PRPP synthases. Among the class I enzymes, B. subtilis and E. coli PRPP synthases and human PRPP synthase isozyme 1 have been crystallized, and high-resolution structures have been determined (49–52). A three-dimensional structure has been determined also for the PRPP synthase from the Gram-negative bacterium Burkholderia (Pseudomonas) pseudomallei strain 1710b (PDB code 3dah) (53). However, there are no biochemical data available for the latter enzyme. The crystal forms of PRPP synthase from the various organisms are summarized in Table 1. Some kinetic properties of PRPP synthases of various organisms are listed in Table 2.
TABLE 1.
Crystal forms of PRPP synthases and PRPP synthase-associated proteins
| Organism | Designation | Resolution (Å) | Ligands | PDB code | Reference(s) |
|---|---|---|---|---|---|
| B. subtilis | SO42− | 2.3 | Two SO42− (phosphate of ribose 5-phosphate, α-phosphate of ADP) | 1dkr | 49 |
| mADP | 2.2 | AMP (active site), methylene ADP (allosteric site) | 1dku | 49 | |
| Cd2+ | 2.8 | 2 Cd2+ (Mg2+ sites), AMP (active site), SO42− (phosphate of ribose 5-phosphate) | 1ibs | 50 | |
| AlF3 | 2.0 | 2 AMP, AlF3, 2 Mg2+, SO42− (α-phosphate of ADP in allosteric site); ATP analog at active site is pieced together by 3 molecules: AMP (α-phosphate)-AlF3 (β-phosphate)-AMP (γ-phosphate), with adenosyl moiety protruding into space between subunits | 54 | ||
| mADP-R5P | 2.1 | Methylene ADP and ribose 5-phosphate (active site), 2 Mg2+, SO42− (α-phosphate of ADP in allosteric site) | 54 | ||
| GDP | 1.8 | 4 Mg2+, Ca2+, GDP, GTP, 2 α,β-methylene ATP, 2 ribose 5-phosphate, glycerol | 54 | ||
| mGDP | 1.9 | 5 Mg2+, methylene GDP, 3 α,β-methylene ATP, 2 ribose 5-phosphate, 2 glycerol | 54 | ||
| B. pseudomallei | 2.3 | None | 3dah | 53 | |
| E. coli | PRPPS | 2.7 | Mg2+ | 4s2u | 52, 407 |
| M. jannaschii | Apo | 2.7 | None | 1u9y | 48 |
| Ternary | 2.9 | AMP, ribose 5-phosphate | 1u9z | 48 | |
| S. solfataricus | 2.8 | AMP | 4twb | 139 | |
| T. volcanium | ADP-SO42− | 1.5 | ADP, SO42− | 3lrt | 138 |
| ADP-SO42− | 1.8 | ADP, SO42− | 3nag | 138 | |
| mATP-SO42− | 1.9 | mATP, SO42− | 3lpn | 138 | |
| ADP-Mg2+-R5P | 1.9 | ADP, Mg2+, Pi, ribose 5-phosphate | 3mbi | 138 | |
| Human | hPRS1 wild typea | 2.6 | SO42− | 2h06 | 51 |
| hPRS1-ATP-SO42−-Cd2+a | 2.2 | AMP, SO42−, Cd2+ | 2hcr | 51 | |
| hPRS1 S132Ab | 2.2 | SO42− | 2h07 | 51 | |
| hPRS1 Y146Mc | 2.5 | SO42− | 2h08 | 51 | |
| —a | 2.6 | SO42− | 3efh | ||
| —a | 2.0 | SO42− | 3s5j | ||
| —d | 2.3 | Mg2+, SO42− | 4f8e | 124 | |
| PRS1a | 2.0 | SO42− | 3s5j | 118 | |
| E43Te | 3.0 | SO42− | 4lyg | 118 | |
| D65Nf | 2.1 | SO42− | 4lzn | 118 | |
| A87Tg | 3.3 | SO42− | 4LZO | 118 | |
| M115Th | 2.1 | SO42− | 4M0P | 118 | |
| Q133Pi | 2.7 | SO42− | 4M0U | 118 | |
| PAP39j | 2.7 | SO42− | 2c4k | ||
| PAP41k | 2.6 | 2ji4 |
PRPP synthase isozyme 1.
Ser132Ala mutant variant of PRPP synthase isozyme 1.
Tyr146Met mutant variant of PRPP synthase isozyme 1.
Asp52His mutant variant of PRPP synthase isozyme 1.
Glu43Thr mutant variant of PRPP synthase isozyme 1.
Asp65Asn mutant variant of PRPP synthase isozyme 1.
Ala87Thr mutant variant of PRPP synthase isozyme 1.
Met115Thr mutant variant of PRPP synthase isozyme 1.
Gln133Pro mutant variant of PRPP synthase isozyme 1.
PRPP synthase-associated protein 39.
PRPP synthase-associated protein 41.
TABLE 2.
Kinetic parameters of PRPP synthases of various organisms
| Organism (and enzyme) | PRPP synthase class |
Km (μM) |
Vmax or sp act (μmol/min/mg of protein) | Reference(s) | |||||
|---|---|---|---|---|---|---|---|---|---|
| R5P | ATP | dATP | GTP | CTP | UTP | ||||
| B. subtilis | I | 480 | 660 | 190 | 70 | ||||
| B. caldolyticus | I | 530 | 310 | 400 | 55 | ||||
| S. enterica | I | 160 | 50 | 130 | 56 | ||||
| E. coli | I | 203 | 113 | 181 | 57 | ||||
| M. tuberculosis | I | 8.2–71 | 1–25 | 1.4–530 | 77–79 | ||||
| B. amyloliquefaciens | I | 105 | 50 | 37 | 149 | ||||
| Human, isozyme 1 | I | 52 | 21 | 25 | 73 | ||||
| Human, isozyme 2 | I | 83 | 70a | 36 | 73 | ||||
| Rat, isozyme 1 | I | 40 | 44 | 39 | 71 | ||||
| Rat, isozyme 2 | I | 73 | 60 | 35 | 71 | ||||
| Rat, liver enzyme | I | 64 | 49 | 16 | 143 | ||||
| S. oleracea, isozyme 3 | II | 110 | 170 | 233 | 650 | 116 | 137 | 13.1b | 46 |
| S. oleracea, isozyme 4 | II | 48 | 7 | 84 | 490 | 680 | 500 | 16.2c | 47 |
| M jannaschii | Archaea | 2,800 | 2,600 | 2,200 | 48 | ||||
| T. kodakarensis | Archaea | 182 | 140 | ||||||
| P. calidifontis | Archaea | 60 | 80 | 480 | 422 | ||||
S0.5 value.
Value with ATP as diphosphoryl donor. Values varied between 1.2 and 6.9 with GTP, CTP, or UTP as the diphosphoryl donor.
Value with ATP as diphosphoryl donor. Values varied between 4.2 and 6.6 with GTP, CTP, or UTP as the diphosphoryl donor.
Eubacterial PRPP synthases.
In this section, we focus on the properties of PRPP synthase of the bacterial species B. subtilis, S. enterica, E. coli, and M. tuberculosis. Among these, a three-dimensional structure has been determined for B. subtilis and E. coli PRPP synthases. The biochemical properties of B. subtilis PRPP synthase are much less studied than are those of S. enterica and E. coli PRPP synthases. The latter two enzymes are identical except for two conservative amino acid substitutions (E. coli PRPP synthase serines 278 and 283 are threonine and alanine, respectively, in the S. enterica enzyme). Furthermore, the S. enterica and E. coli PRPP synthase amino acid sequences are 51% identical to that of the B. subtilis enzyme. It is therefore very likely that these three enzymes share identical three-dimensional structures, biochemical properties, and catalytic and regulatory properties. Below, we describe the three-dimensional structure of B. subtilis PRPP synthase and, when applicable, the properties of the enterobacterial PRPP synthases with reference to the structure of the B. subtilis enzyme.
(i) Three-dimensional structure of B. subtilis PRPP synthase.
A number of crystal forms, summarized in Table 1, are utilized for the description of the structure and catalysis of B. subtilis PRPP synthase: (a) SO42− PRPP synthase with one SO42− bound in the active site at the position corresponding to the phosphate moiety of ribose 5-phosphate and a second SO42− bound at the position corresponding to the α-phosphate of ADP at the regulatory site. No divalent metal ion was present, and the structure was determined to 2.3 Å resolution (PDB code 1dkr). (b) mADP PRPP synthase with methylene ADP bound at the active and regulatory sites. As before, no divalent metal ion was present, and the structure was determined to 2.2 Å resolution (PDB code 1dku) (49); (c) Cd2+ PRPP synthase with two Cd2+ bound in each monomer, presumably at the two Mg2+ sites, AMP bound at the active site, and SO42− bound in place of the phosphate moiety at the ribose 5-phosphate site. The structure was resolved to 2.8 Å resolution (PDB code 1ibs) (50). (d) AlF3 PRPP synthase; the crystal form contained in the active site an analog of ATP pieced together by three molecules: (i) AMP representing the AMP moiety of ATP, (ii) AlF3 with Al3+ representing the β-phosphorus, and the three F− representing the three oxygens, and (iii) a second AMP molecule whose phosphate represents the γ-phosphate of ATP. The remaining adenosyl moiety of the latter AMP molecule protrudes into an “empty space” between two subunits. Furthermore, the crystal form contained two Mg2+ (one ligated to the “triphosphate chain”), one ribose 5-phosphate at the active site, and one SO42− located at the position of the α-phosphate of ADP in the allosteric site. This structure was resolved to 2.8 Å resolution. Altogether, this complex is thought to resemble the transition state of PRPP synthase. (e) mADP-R5P PRPP synthase with one methylene ADP representing the ADP moiety of ATP as well one ribose 5-phosphate in the active site, and two Mg2+ and one SO42− representing the α-phosphate of ADP at the allosteric site in each monomer. This structure was resolved to 2.1 Å (54). The structure of the active site is described in more detail, including stereoscopic views, in the section “Mechanism of Catalysis,” below. Two additional crystal forms (GDP PRPP synthase and mGDP PRPP synthase) have been determined and have been particularly useful in elucidating the mechanism of allosteric regulation; these are also further described below.
(a) Tertiary structure.
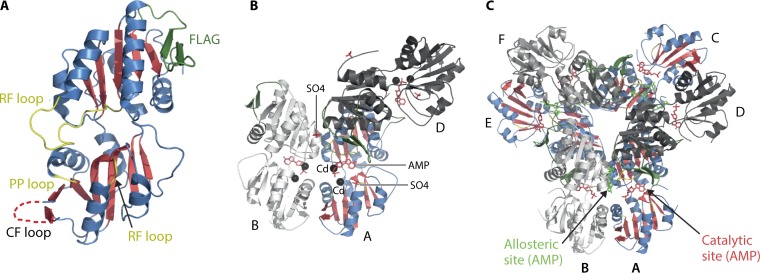
The PRPP synthase monomer is composed of two domains in a head-to-tail arrangement. The tertiary structures of the two domains are remarkably similar, although the amino acid sequence identity of the two domains (amino acid residues 1 to 150 and 152 to 292) is only 11%, with an additional 10% similar amino acid residues. Thus, the similarities in tertiary structure of the two domains are not at all predictable from their primary structures. Each domain possesses an α/β structure with a five-stranded parallel β-sheet at the center surrounded by four α-helices as well as one 310-helix in the N-terminal domain (Fig. 3A). Additionally, short antiparallel β-sheets, designated flag regions, flank both domains. This structure resembles that of type I phosphoribosyltransferases. It is likely, therefore, that type I phosphoribosyltransferases and PRPP synthase originated from the same ancestral gene and that “modern” PRPP synthases may have evolved from duplication of that ancestral gene that would have been half the size of the contemporary prs gene (54).
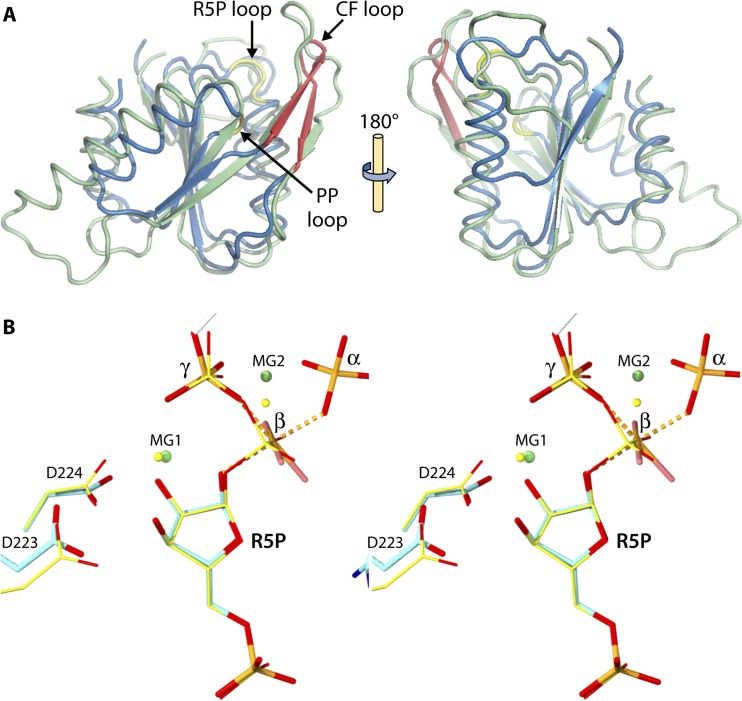
FIG 3.
Three-dimensional structure of B. subtilis PRPP synthase. (A) Monomer drawn on the basis of the SO42− PRPP synthase structure (PDB code 1dkr) (49). The N-terminal domain is at the top. Shown are the five-stranded parallel β-sheets (red), helices (blue), flag region (green), regulatory flexible (RF) loop, the ribose 5-phosphate (R5P) loop, and the PP loop (yellow). The unresolved catalytic flexible (CF) loop is shown as a dotted line. (B) Bent and parallel dimers drawn on the basis of the Cd2+ PRPP synthase structure (PDB code 1ibs) (50). Subunit A is colored similar to the monomer in panel A. Shown are the Cd2+ (black), AMP of the active site (red), and sulfate bound at the position of the phosphate moiety of ribose 5-phosphate and at the position of the α-phosphate of ADP of the allosteric site (red). (C) Hexameric propeller structure drawn on the basis of the mADP PRPP synthase structure (PDB code 1dku) (49). Subunit A (as well as subunits C and E) are colored as described for the monomer in panel A. Shown are the positions of the methylene ADP moieties (red) and methylene ADP molecules (green), both modeled to only AMP, of the ATP binding sites and the allosteric sites, respectively.
A number of regions along the amino acid sequence have been highlighted on the basis of their functions. These are called the regulatory flexible loop (Tyr97 to Thr113), the diphosphate (PP) loop (Asp174 to Gly177), the catalytic flexible loop (Lys197 to Met208), and the ribose 5-phosphate-binding loop (previously called the PRPP-binding site) (Gly216 to Thr231) (49, 50, 54) (Fig. 2).
There are two types of subunit interactions in PRPP synthase. First, interactions of the α3N and α4N helices of the N-terminal domain result in the formation of a bent head-to-head arrangement of two subunits, referred to as the bent dimer (Fig. 3B). The relevant amino acid residues involved in this interaction are Asn69, Glu70, Ile 72, Met73, Leu76, Ile77 (α3N), and Leu116 and Leu120 (α4N), many of which are highly conserved (Table 3, left column). Second, a subunit is aligned in a parallel manner with a neighboring subunit in an arrangement involving residues from both the N- and the C-terminal domains (Fig. 3B). These interactions include hydrophobic interactions and salt bridges of the α1C helix and the flag region of the C-terminal domain as well as interactions of the 310 helices and salt bridges of the N-terminal domain. Important residues in the formation of this type of dimer, referred to here as the parallel dimer, are Lys115, Gln138, Ile139, Phe142, Asp144, Val178, Asp186, Ile192, Ala193, Ile194, Arg198, and Val211 (Table 3, left column) (49). The two types of subunit interactions are shown in Fig. 3B, and it is easy to see how this “trimer” may be formally assembled into the hexameric, propeller-like structure shown in Fig. 3C. The enzyme thus consists of a trimer of dimers with all of the N-terminal domains forming an inner circle and the C-terminal domains forming the propeller blades at the outside, resulting in a 3-fold symmetry axis with perpendicular 2-fold axes (49).
TABLE 3.
Comparison of amino acid residues involved in dimer association of B. subtilis PRPP synthase with amino acids of PRPP synthases of other organisms
| Dimer type | Residue involved in indicated dimer association for speciesa |
||||||
|---|---|---|---|---|---|---|---|
| B. subtilis | E. coli | M. tuberculosis | Spinach isozyme 4 | M. jannaschii | T. volcanium | S. solfataricus | |
| Bent dimerb | N69 | N64 | N72 | I73 | N60 | E61 | D61 |
| E70 | D65 | R73 | F74 | D61 | V62 | K62 | |
| I72 | L67 | L75 | Q76 | I63 | E64 | L64 | |
| M73 | M68 | M76 | L77 | V64 | M65 | I65 | |
| L76 | L70 | L79 | I80 | I67 | T68 | F68 | |
| I77 | V72 | I80 | Y81 | L68 | L69 | L69 | |
| L116 | V112 | L119 | L122 | A107 | I106 | T108 | |
| L120 | F116 | L123 | V127 | I111 | I110 | I112 | |
| Parallel dimerc | K115 | K111 | R118 | R120 | R106 | Q105 | K107 |
| Q138 | Q134 | Q141 | Q144 | H128 | T128 | E130 | |
| I139 | I135 | I142 | E145 | I129 | L129 | E131 | |
| F142 | F138 | F145 | F139 | F132 | S132 | Y134 | |
| D144 | D140 | D147 | S150 | T134 | V144 | K136 | |
| V178 | V174 | V182 | G185 | V167 | L165 | L169 | |
| D186 | K182 | D190 | Q183 | K175 | A173 | E177 | |
| I192 | M189 | L197 | M199 | Y181 | H179 | Y183 | |
| A193 | A190 | A198 | V200 | D182 | F180 | S184 | |
| I194 | I192 | F199 | V201 | Y183 | F181 | Y185 | |
| R198 | R195 | R201 | V205 | T187 | K185 | E189 | |
| V211 | I208 | V218 | E207 | T200 | N198 | A202 | |
The annotated amino acid residues involved in the formation of the two types of dimer (bent and parallel) (see text for details) of B. subtilis PRPP synthase are listed in the left column according to the previously published three-dimensional structure (49). The B. subtilis PRPP synthase amino acid sequence was then aligned pairwise with the amino acid sequences of PRPP synthases of E. coli (accession no. U00096) (313); M. tuberculosis (accession no. AL123456) (408); S. oleracea isozyme 4 (15); M. jannaschii (accession no. L77117) (409); T. volcanium GSS1 (accession no. BA000011) (410), and S. solfataricus P2 (accession no. AE006641) (411). Amino acid residues at similar positions of PRPP synthases of the latter six organisms are listed in the other columns. Amino acid residues that are identical or conserved relative to the B. subtilis enzyme are shown in bold, whereas nonconserved residues are shown in lightface.
Interactions of the B. subtilis PRPP synthase N-terminal domain.
Interactions of the B. subtilis PRPP synthase N- and C-terminal domains.
(b) The active site.
The active site must accommodate the substrates ribose 5-phosphate and MgATP. In addition, an overwhelming volume of research data has shown that an additional so-called free Mg2+ is required for activity, because maximal activity is only obtained when Mg2+ is added to the reaction mixture in excess of the MgATP concentration for both bacterial (35, 45, 55–58) and mammalian (59, 60) PRPP synthases. Furthermore, most PRPP synthases may accept other divalent metal ions in place of Mg2+, and thus Mg2+ may be regarded as a pseudosubstrate (45, 56, 58, 61). In contrast, Ca2+ is an inhibitor of PRPP synthase activity, even in the presence of Mg2+ (45). In the crystal structure one Mg2+ (the MG1 site) coordinates to Asp174, a highly conserved residue of the C-terminal domain, to the oxygens of the hydroxyls of C-1, C-2, and C-3 of ribose 5-phosphate, to an oxygen of the γ-phosphate of ATP, and to a water molecule, which forms hydrogen bonds to Asp174 and Asp223. Thus Mg2+ ligates both of the substrates. A second Mg2+ (MG2) coordinates to His135 of the N-terminal domain, to oxygens of the α-, β-, and γ-phosphates of ATP, and to two water molecules, which form hydrogen bonds to the side chains of Asp103 and Arg104 and the carbonyl oxygen of Arg101. The effect of this intricate binding is a perfect alignment of ribose 5-phosphate and ATP for an in-line attack of the hydroxyl of C-1 of ribose 5-phosphate at the β-phosphorus of ATP (54), as described in detail below in “Mechanism of Catalysis.”
As described above, the two active site Mg2+ ligate to Asp174 of the C-terminal domain and His135 of the N-terminal domain. Similarly, the ATP-binding site is located at the interface of the N- and C-terminal domains of each subunit, but with contributions of amino acid residues of the N-terminal domain of a neighbor subunit of the parallel dimer, for example, subunit A and subunit D (Fig. 3B). The specific amino acid residues that are important in the binding of ATP in B. subtilis PRPP synthase are listed in Table S1, left column, in the supplemental material. In contrast, the ribose 5-phosphate-binding site is located within the C-terminal domain and is formed exclusively by amino acid residues of this domain. A subset of amino acid residues of the ribose 5-phosphate-binding site, Gly216 to Thr231, has been shown to directly interact with hydroxyls 2 and 3 (Asp223 and Asp224) or the phosphate moiety (Asp227, Thr228, Ala229, Thr231, and Ile232) (50, 54) (see Table S1, left column).
Chemical modification studies have confirmed the importance of some of the amino acid residues mentioned above. Thus, chemical modification of S. enterica PRPP synthase with 5′-(4-fluorosulfonylbenzoyl)adenosine completely inactivated the enzyme in a 1:1 molar ratio. ATP protected the enzyme against inactivation, and the site of modification was His130, which corresponds to His135 in B. subtilis PRPP synthase (62), providing evidence for the importance of this residue. Similarly, affinity labeling of E. coli PRPP synthase has been performed with the ATP analog 2′,3′-dialdehyde ATP. Three lysine residues were labeled, Lys181, Lys193, and Lys230. Only Lys193 is conserved, and it corresponds to Lys197 of B. subtilis PRPP synthase (63). As we discuss below, this Lys197 residue plays a very important role in the catalysis of PRPP synthase. Chemical modification with sulfhydryl reagents has been reported to cause inactivation of PRPP synthase, which is protected by the presence of ATP and Pi (64, 65), but cysteine residues have not been identified from the structural studies to be important in catalysis, which suggests that these treatments result in nonspecific inactivation of the enzyme.
The geometry of the two Mg2+-binding sites confirms a wealth of information on the binding properties of divalent cations to PRPP synthases of, in particular, S. enterica and E. coli. First, kinetic analysis of the S. enterica enzyme revealed the binding of both MgATP and free, i.e., enzyme-bound, Mg2+ (56). It was proposed that the enzyme-bound Mg2+ ligates to the α-phosphate of ATP, which is provided to the enzyme as β,γ-MgATP (58, 66). However, according to the crystal structure of B. subtilis PRPP synthase, the true substrate of the enzyme is the α,β,γ-tridentate complex of MgATP, which is consistent with the MG2 site described above. Additionally, analysis of E. coli PRPP synthase with an altered ribose 5-phosphate-binding site (Asp220Glu, Asp220Phe, and Asp221Ala) revealed that the effects on the values for the apparent maximal velocity (Vapp) and Km for ribose 5-phosphate were dependent on the divalent cation present, suggesting that the binding of ribose 5-phosphate also occurs via interaction with Mg2+ (61), which is consistent with the MG1 site described above. The crystal structure, furthermore, completes nuclear magnetic resonance analyses that attempted to elucidate the conformation of ATP at the active site of S. enterica PRPP synthase. Thus, paramagnetic line broadening of the C-1 proton or of 31P of ribose 5-phosphate by Cr(III) bound to ATP as the exchange-stable α,β,γ-tridentate complex was used to estimate the distances from the Cr atom (and presumably Mg2+) to the two atoms, at 6.7 to 8.0 Å. This is consistent with proximity of the ribose 5-phosphate C-1 hydroxyl and the β-phosphorus of ATP to the enzyme-bound divalent cation (67).
Steady-state kinetic analysis of the inhibition of S. enterica or E. coli PRPP synthase by substrate analogs revealed an ordered Bi-Bi mechanism with binding of Mg2+ first, followed by MgATP and then ribose 5-phosphate (41, 56, 57). The ordered kinetic mechanism was further confirmed by equilibrium dialysis. Thus, radioactive ATP and the inactive analog α,β-methylene ATP bound well to the free enzyme with dissociation constants in the micromolar range, whereas ribose 5-phosphate binding could not be detected unless α,β-methylene ATP was also included (68). Kinetic parameters of PRPP synthases from various bacilli and enteric organisms are listed in Table 2.
(c) The allosteric site.
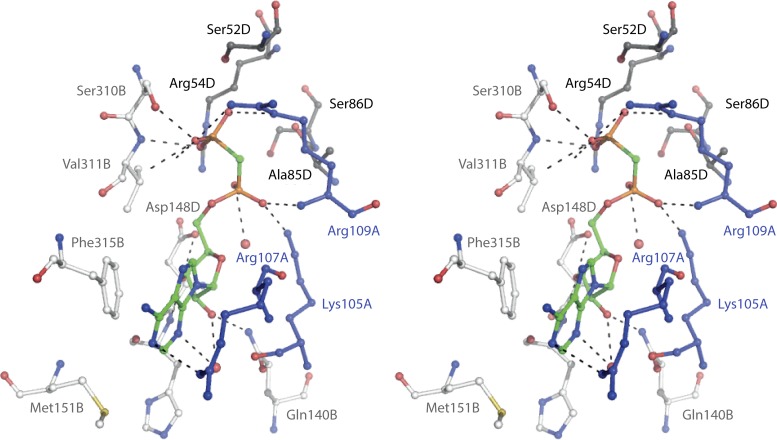
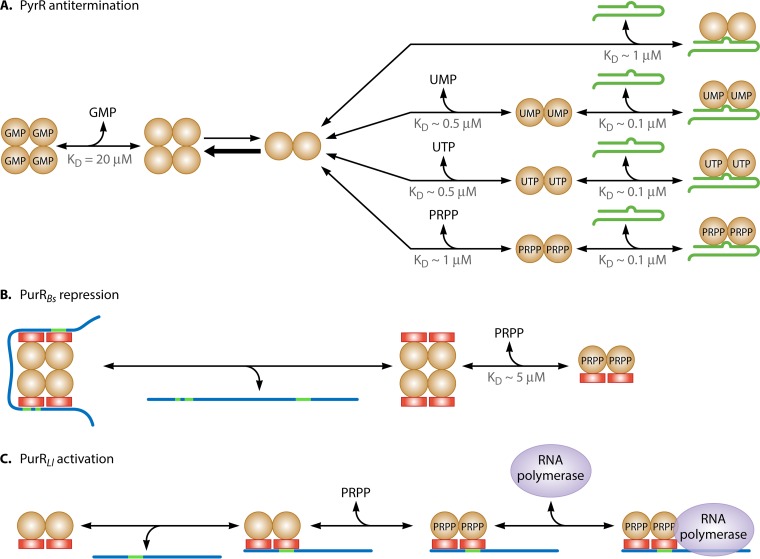
In addition to the crystal forms SO42− PRPP synthase and mADP-PRPP synthase described above, two additional crystal forms were found useful in elucidating the structure of the allosteric site. (f) GDP PRPP synthase (with four Mg2+, one Ca2+, one GDP, one GTP, two α,β-methylene ATP, and two ribose 5-phosphate molecules bound per asymmetric unit) resolved to 1.8 Å resolution; (g) mGDP PRPP synthase (with five Mg2+, one methylene GDP, three α,β-methylene ATP, and two ribose 5-phosphate molecules bound per asymmetric unit) resolved to 1.9 Å resolution (54). The allosteric site of B. subtilis PRPP synthase is composed of amino acid residues contributed by three subunits, such as A, B, and D (Fig. 3B) (49). The amino acid residues involved in the binding of the β-phosphate and the adenyl moiety of ADP are provided by subunit D (Ser52, Arg54, Ala85, and Ser86) and subunit B (Ser310, Val311, Ser312, and Phe315). Subunit B also provides amino acids for the so-called hydrophobic pocket (Leu134, Ile139, Gln140, Asp148, and His149). Finally, subunit A contains the regulatory flexible loop (Lys105, Ala106, Arg107, Ser108, and Arg109) (49, 54). These amino acid residues are listed in Table S2 in the supplemental material. A stereoscopic view of the allosteric site is shown in Fig. 4. We shall return to the mechanism of allosteric regulation below in the section “Regulation of PRPP Synthase Activity.”
FIG 4.
Allosteric site of B. subtilis PRPP synthase. Stereo view based on the mADP PRPP synthase structure (PDB code 1dku) (49). The site is occupied by methylene ADP. Amino acid residues contributing to methylene ADP binding are provide by three subunits, labeled A, B, and D, as in Fig. 3. Amino acid residues of subunit A are shown in blue, amino acid residues of subunit B are in light gray, and amino acid residues of subunit D are shown in dark gray.
In general, the activity of class I PRPP synthases is regulated by the presence of ribonucleoside diphosphates, primarily ADP, which inhibits the enzyme in competition with ATP as well as by binding to a second, allosteric site. In some cases, GDP is also an inhibitor, although GDP only binds to the allosteric site. Thus, a dual mechanism, involving competitive and allosteric inhibition, appears to control the activity of class I PRPP synthases. The complex inhibition pattern by ADP of the enzyme from S. enterica has been studied in particular detail. Kinetic analysis revealed that ADP competes with ATP at subsaturating ribose 5-phosphate concentrations and that ADP causes substrate inhibition by ribose 5-phosphate due to the binding at a site different from the active site (69). Direct binding was demonstrated by equilibrium dialysis. Thus, in the absence of ribose 5-phosphate, ADP binds to the same site as ATP, whereas in the presence of ribose 5-phosphate ADP binds to two sites per monomer (68). Presumably, these are the active and allosteric sites. Similarly, PRPP synthases from E. coli (45), B. subtilis, and mammalian sources have been shown to be subject to allosteric regulation by ADP, and the enzymes from B. subtilis, Bacillus caldolyticus, and mammals are also inhibited by GDP (55, 70–72).
As mentioned above, the activity of class I PRPP synthases requires the presence of Pi. In some cases, sulfate may replace Pi, although a 10-fold-higher concentration of sulfate is required (49, 71, 73). Removal of Pi from E. coli and S. enterica PRPP synthase causes irreversible inactivation or aggregation (35, 45, 74), although the presence of MgATP or MgPRPP stabilizes the enzyme from E. coli or S. enterica, respectively (35, 75). B. subtilis and mammalian PRPP synthases are stable but inactive upon Pi removal (49, 71, 73). Indeed, Pi and ADP have been shown to compete for the same binding site in B. subtilis and human PRPP synthases, with Pi acting as an allosteric activator and ADP as an allosteric inhibitor. Thus, certain mutant variants of PRPP synthase desensitized in inhibition by ribonucleoside diphosphates are reciprocally activated at lower concentrations of Pi (72), and the antitumor agent aminopyrimidopyrimidine ribonucleoside 5′-phosphate binds to the allosteric site of human PRPP synthase isozymes 1 and 2. The concentration needed for half-maximal binding increases with increasing Pi concentration (76). Finally, a sulfate ion was found in the B. subtilis SO42− PRPP synthase structure. Superimposition of the SO42− PRPP synthase and mADP PRPP structures revealed that the sulfate ion was located similarly to the β-phosphate of the methylene ADP molecule of the regulatory or allosteric ADP-binding site (49). Altogether, the data demonstrate that Pi and ADP compete for binding to the same site. Steady-state kinetic analysis revealed that Pi binds randomly to E. coli PRPP synthase, i.e., either before or after the ordered binding of Mg2+, MgATP, and ribose 5-phosphate. Similarly, ADP may bind to PRPP synthase in a random fashion, but with significantly different rate constants constituting the equilibria, so that the pathway where Mg2+, MgATP, and ribose 5-phosphate bind in the absence of Pi is much slower than when Pi is bound first. This phenomenon provokes what is termed kinetic cooperativity and thus the sigmoid saturation curves frequently obtained for Pi activation with class I PRPP synthases (75).
As expected from the 51% identity of the amino acid sequences of B. subtilis and E. coli PRPP synthases, the amino acid residues involved in the formation of dimers as well as the active site and the allosteric site (Table 3; see also Tables S1 and S2 in the supplemental material) are highly conserved. The identity of this subset of amino acids is 61%. These data were furthermore confirmed by analysis of the three-dimensional structure of E. coli PRPP synthase (52).
(ii) M. tuberculosis PRPP synthase.
Some attention has been devoted to studies of PRPP synthase of M. tuberculosis in searching for possible targets for drug treatment of tuberculosis. Three research groups have characterized this enzyme (77–79). All three groups agree that the quaternary structure of the active enzyme is a hexamer and that allosteric inhibition is exhibited by the ribonucleoside diphosphates ADP and GDP, whereas there is some disagreement about the kinetic properties of the enzyme (Table 2). In addition, one research group established GTP, UTP, and CTP as diphosphoryl donors, with kcat/Km ratios of 3.8, 3.0, and 2.5 M−1 s−1, respectively, compared to 26 M−1 s−1 for ATP (78). Interestingly, analytical ultracentrifugation revealed that the apo-enzyme exists as a trimer as well as a hexamer. The presence of ADP greatly shifted the oligomeric state toward the hexamer, whereas the presence of ATP had no effect on the oligomerization state (79). A comparison of the amino acid residues involved in dimer formation, bent as well as parallel dimers, of M. tuberculosis and B. subtilis PRPP synthases is shown in Table 3. It is evident that essentially all of the amino acids involved in dimer formation of B. subtilis PRPP synthase are retained in the M. tuberculosis enzyme. Additionally, a comparison of the amino acid residues involved in the binding of the substrates MgATP, ribose 5-phosphate, and Mg2+ for the two enzymes reveals a high degree of conservation, as does a comparison of the amino acid residues involved in the formation of the allosteric site between the two enzymes (see Tables S1 and S2 in the supplemental material). All of these data are consistent with the identification of the M. tuberculosis enzyme as a class I PRPP synthase. The amino acids involved in the binding of GTP and the pyrimidine ribonucleoside 5′-triphosphates CTP and UTP as substrates for PRPP synthase have not been mapped, so the propensity of M. tuberculosis PRPP synthase to utilize these latter ribonucleoside 5′-triphosphates remains unexplained. Other class I PRPP synthases do not utilize GTP, CTP, or UTP.
(iii) Other bacterial PRPP synthases.
Although most prokaryotic species contain a single gene encoding PRPP synthase, the genomes of almost 100 species have been annotated as containing two PRPP synthase orthologs. Biochemical data for PRPP synthase are available for only a few of these organisms. One of these is Lactococcus lactis, which contains two PRPP synthase-encoding orthologs, prsA and prsB, and for which some physiologic data exist. The two genes are separated by approximately 300 kbp. The sequence identities of the prsA- and prsB-specified amino acid sequences with that of B. subtilis PRPP synthase are 65% (L. lactis PrsA), and 52% (L. lactis PrsB), whereas the amino acid sequence identity between the two L. lactis Prs sequences is 49%. Comparison of amino acid sequence of L. lactis prsA- and prsB-specified gene products and the established active site amino acid residues of B. subtilis PRPP synthase reveals that PrsA and B. subtilis PRPP synthase are nearly identical, whereas in PrsB the amino acid residues of the catalytic flexible loop vary considerably from those of B. subtilis PRPP synthase. Specifically, the catalytically important Lys197 and Arg199 residues of B. subtilis PRPP synthase are replaced by Tyr198 and Asp200 in L. lactis PrsB (see Table S3 in the supplemental material). Complementation analysis revealed that L. lactis prsA complements an E. coli prs allele, whereas prsB does not, which demonstrated that prsA encodes a functional PRPP synthase. The function of the prsB gene product remains to be established (P. Bennedsen and J. Martinussen, The Technical University of Denmark, unpublished results).
The Gram-negative bacterium Thermus thermophilus also contains two PRPP synthase-specifying orthologs. However, both of these genes specify active PRPP synthases (417).
A few bacterial species have three annotated PRPP synthase orthologs. In Roseiflexus castenholzii (DSM 13941), one amino acid sequence has a histidine residue corresponding to B. subtilis Arg199 as well as an additional six amino acid residues in the catalytic flexible loop, and there is 43% amino acid sequence identity with B. subtilis PRPP synthase. The other two amino acid sequences have 42% and 51% amino acid sequence identity with B. subtilis PRPP synthase. Similarly, in Sphingobium japonicum strain UT26S, one sequence has an aspartate at the position of the B. subtilis Lys197 and there is a 9-amino-acid insertion in the catalytic flexible loop. In Pseudomonas stutzeri strain A 1501, the amino acid sequence identity with B. subtilis PRPP synthase varies between 23% and 49% and in Clostridium beijerinckii strain NCIMB 8052 these values are 27 and 62%, whereas in Rhodoferax ferrireducens strain T118 these values are 24 and 54%. No biochemical studies of any of these putative PRPP synthases are currently available.
Yeast PRPP synthases.
Among yeast species, PRPP synthases have been analyzed only in S. cerevisiae and S. pombe, but presumably many of the physiologic properties of PRPP metabolism for these two species are similar to those of other yeast species. Although yeast PRPP synthases share typical class I properties, they also contain properties different from the PRPP synthases described so far.
(i) S. cerevisiae.
S. cerevisiae contains five PRPP synthase-specifying orthologs, PRS1 to PRS5 (8, 9). Inspection of the amino acid sequences reveals that PRS2, PRS3, and PRS4 appear to encode typical class I enzymes. Also, PRS1 and PRS5 may encode class I enzymes, but PRS1- and PRS5-specified amino acid sequences contain so-called nonhomologous regions (NHRs), which are insertions relative to the sequences specified by PRS2, PRS3, or PRS4 (Fig. 5). Prs1 contains a single NHR (NHR1) of 105 amino acids in length, whereas Prs5 contains two NHRs of 110 amino acids (NHR5-1) and 63 amino acids (NHR5-2). For Prs1, immunochemical analysis confirmed that the NHR1 is actually present in the polypeptide found in yeast cells (8, 80, 81). The Prs1 NHR1 is inserted after Ser199, whereas the Prs5 NHR5-2 is inserted after Ser306. These two positions, Ser199 and Ser306, correspond to Arg202 of the B. subtilis PRPP synthase, and thus the NHRs are inserted in the catalytic flexible loop. In contrast, the Prs5 NHR5-1 sequence is inserted after Pro100, which corresponds to Asp103 of the B. subtilis PRPP synthase, and thus the NHR5-2 is inserted in the regulatory flexible loop. The identities of the amino acid sequences specified by PRS2, PRS3, and PRS4 with that of B. subtilis PRPP synthase range between 42 and 46%, whereas for the PRS1- and PRS5-specified sequences the identities are 29 and 24%, respectively. After removal of the NHR amino acid sequences in silico, the latter values increase to 41% (Prs1 ΔNHR1) and 39% (Prs5 ΔNHR5-1 ΔNHR5-2).
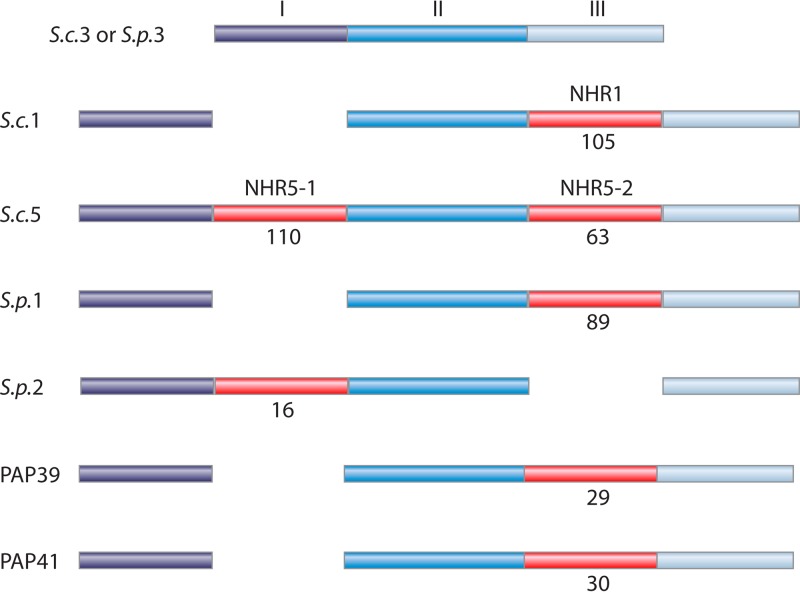
FIG 5.
Positions of nonhomologous regions. Each line of bars represents polypeptides of a PRPP synthase, with the amino terminus at the left end. S.c.1, S. cerevisiae Prs1; S.c.3, S. cerevisiae Prs3; S.c.5, S. cerevisiae Prs5; S.p.1, S. pombe Prs1; S.p.2, S. pombe Prs2; S.p.3, S. pombe Prs3; PAP39, human PRPP synthase-associated protein 39; PAP41, human PRPP synthase-associated protein 41. S. cerevisiae and S. pombe Prs3 are shown at the top as a bar consisting of three segments (I, II, and III) represented in different shades of blue. This bar could represent most class I PRPP synthases. The left and right ends of segment II are located within the regulatory and catalytic flexible loops, respectively. An NHR is shown in red. The number of amino acid residues of each NHR is shown below the bars. The three NHRs of S. cerevisiae Prs1 and -5 are designated NHR1, NHR5-1, and NHR5-2. According to this nomenclature, S. cerevisiae Prs1 has the structure segment 1–segment 2–NHR1–segment 3; S. cerevisiae Prs5 has the structure segment 1–NHR5-1–segment 2–NHR1–segment 3; S. pombe PRPP synthase 1 has the structure segment I–segment II–NHR–segment III; S. pombe PRPP synthase 2 has the structure segment I–NHR–segment II–segment III; human PAP39 and -41 have the structure segment I–segment II–NHR–segment III.
(a) Interaction of PRS gene products.
Unlike most other cloned prs genes, the gene products of each of the five S. cerevisiae PRS genes have no PRPP synthase activity in vivo when their genes are harbored and expressed individually in an E. coli host strain (82). In contrast, the formation of active PRPP synthase requires the expression of two or more PRS genes, as established by deletion analysis of PRS genes in S. cerevisiae or by expression of PRS genes in E. coli. Single-knockout mutations in PRS1, PRS2, PRS3, PRS4, or PRS5 result in viable phenotypes (80, 83). Additionally, all 10 combinations of two PRS gene knockouts result in viable phenotypes except the Δprs1 Δprs5 and Δprs3 Δprs5 double knockout mutants, both of which are lethal (83). The latter double mutant, Δprs3 Δprs5 strain, is lethal because the Prs1 polypeptide is unstable in a Δprs3 genetic background. Thus, strains with the genotype Δprs3 Δprs5 are phenotypically prs1 prs3 prs5 (84). Among the five possible combinations of deletion of three PRS genes, only Δprs1 Δprs2 Δprs3, Δprs1 Δprs3 Δprs4, and Δprs2 Δprs4 Δprs5 deletants are viable, whereas Δprs1 Δprs2 Δprs4 and Δprs2 Δprs3 Δprs4 mutants are lethal; thus, S. cerevisiae cannot exist with only a single PRS gene. These results were confirmed by analysis of complementation by relevant combinations of PRS genes of an E. coli Δprs allele. This analysis furthermore showed that the simultaneous expression of PRS1 and PRS2 or the simultaneous expression of PRS1 and PRS4 resulted in PRPP synthase with very low activity in vivo, presumably too low to promote growth of S. cerevisiae Δprs3 Δprs4 Δprs5 or Δprs2 Δprs3 Δprs5 deletants. Although complementation of the E. coli Δprs allele by combinations of two PRS genes revealed the production of active PRPP synthase in vivo, in vitro PRPP synthase activity could be detected only in cell extract of the strain that expressed both PRS1 and PRS3 (82). The conclusions from this analysis are that active PRPP synthase requires either PRS1 or PRS5 in addition to at least one more PRS gene product, but not just any PRS gene product. Thus, an active enzyme consists of the PRS1 and PRS3 gene products, or an active enzyme consists of the PRS5 PRS2, the PRS5 PRS4, or the PRS5 PRS2 PRS4 gene products (83).
Physical interactions of Prs polypeptides was furthermore demonstrated by yeast two-hybrid analysis. The results of this analysis showed interactions between Prs1 and Prs3, between Prs1 and Prs2, and between Prs1 and Prs4. These interactions were demonstrated in a reciprocal manner, i.e., with each PRS gene fused to the binding domain as well as to the activating domain. Additionally, some nonreciprocal interactions were also observed between Prs5 and Prs2 and between Prs5 and Prs4 (83). Studies of synthetic lethality of combinations of prs deletions also demonstrated interactions between Prs1 and Prs3 and between Prs1 and Prs5 (84, 85). These Prs polypeptide interactions, demonstrated by genetic and physical analyses after manually manipulating the relevant DNA molecules, have been by and large confirmed by high-throughput analyses with two-hybrid analysis (86–88), protein fragment complementation (89, 90), or affinity capture mass spectrometry (91, 92).
The protein-protein interaction analyses revealed an extensive interaction between the various PRS gene products. Evidently, all of the Prs polypeptides interact with one another. Presumably, each Prs polypeptide may be able to form a hexameric structure like that of B. subtilis PRPP synthase, as well as mixed hetero-hexamers and eventually larger molecular structures similar to those described for mammalian PRPP synthases (see below).
(b) Enzyme structure.
S. cerevisiae PRPP synthase appears to be a large enzyme. PRPP synthase activity of wild-type S. cerevisiae strain YN94-2 eluted as a single symmetrical peak corresponding to a molecular mass of at least 900 kDa based on exclusion chromatography (see Fig. S1A in the supplemental material). Also, the enzyme eluted as a single peak during ion exchange chromatography (Fig. S1B). These results may indicate the presence of a single enzyme form containing all five PRS gene products, although the existence of multiple forms with nearly identical size and charge properties cannot be excluded. Additionally, PRPP synthase activities of extracts of the Δprs2, Δprs3, or Δprs4 strains eluted with molecular masses identical to that of the wild type, whereas no PRPP synthase activity could be determined in the exclusion chromatography eluates of either a Δprs1 or a Δprs5 strain, even though activity could be easily determined in the cell extracts before loading them to the column (Fig. S1A). Thus, PRPP synthase lacking either of the Prs1 and Prs5 polypeptides may be unstable under the conditions of exclusion chromatography, and the presence of an additional PRS gene product(s) apparently stabilizes the enzyme complex (93).
Amino acid residues important in active site formation, i.e., residues involved in the binding of the adenyl moiety and the triphosphate chain of ATP as well as binding of ribose 5-phosphate, and the catalytic flexible loop of B. subtilis PRPP synthase were compared to those of each of the five S. cerevisiae PRS-specified amino acid sequences (Table S3). The similarity of these selected B. subtilis PRPP synthase amino acid residues with each of those of S. cerevisiae was 71% (PRS1-specified amino acid residues), 88% (PRS2-, PRS3-, and PRS4-specified amino acid residues), or 50% (PRS5-specified amino acid residues). Little is known of the enzymatic properties of S. cerevisiae PRPP synthase activity. Some activity remains following dialysis of an S. cerevisiae crude extract against Pi-free buffer and in assays under Pi-free conditions, and only 18% of the activity remained when the enzyme was assayed in the presence of 2 mM ADP, relative to 100% in the absence of ADP (82). These properties differ from those of bacterial PRPP synthases.
(c) Physiological function.
The complexity of S. cerevisiae PRPP synthase polypeptides may reflect their physiological function. Evidence for involvement of PRS-specified polypeptides in processes other than catalysis of PRPP synthesis originates from characterization of (i) prs mutants and (ii) mutants defective in other processes and from analyses of (iii) protein-protein interactions with PRS-specified polypeptides. First, Δprs3 mutant strains are highly pleiotropic and it was concluded that PRS3 is required for cell wall integrity, cell cycle arrest following deprivation of nutrients, proper ion homeostasis, and proper actin cytoskeleton organization and cell size homeostasis (94–96). The cell wall integrity signaling pathway includes the protein Slt2 and the transcription factor Rlm1 of the protein kinase C (Pkc1) mitogen-activated protein kinase cascade (97). Strains with prs lesions are sensitive to the purine analog 1,3,7-trimethylxanthine (caffeine), which interferes with Pkc1 activated cell wall integrity signaling and the mitogen-activated protein kinase cascade (98), providing evidence for Prs polypeptides being involved in cell wall integrity signaling (99). Second, the pps1-1 lesion of S. cerevisiae, isolated after screening for an elongated cell morphology, is an allele of PRS1 (100). Third, evidence of Prs polypeptides being involved in the stress and cell wall integrity signaling pathway comes from yeast two-hybrid analysis of Prs polypeptides with Slt2 (85, 101, 102) and by coimmunoprecipitation analysis (418).
A number of other protein-protein interactions with Prs polypeptides as partners have been identified. Rim11, a homolog of mammalian glycogen synthase kinase-3β, interacts with Prs2, Prs3, and Prs5, as shown by affinity-capture mass spectrometry (103) and confirmed by manual yeast two-hybrid analysis with the RIM11 gene and each of the PRS genes (104). High throughput analyses revealed a number of protein-protein interactions of Prs polypeptides with other polypeptides (86, 88), although their physiological functions have not been established (102). Interestingly, the interaction of the protein kinase Rim11 with Prs5 may be responsible for the phosphorylation of the latter protein (104). Phosphoproteome analysis by mass spectrometry revealed three phosphorylation sites in Prs5, 361-KTTpSTSpSTpSS-370 (where p indicates phosphorylation of the following serine residues) (105). This sequence is located within NHR5-2.
From the data provided above, it appears very likely that yeast PRS-specified polypeptides share functions in addition to the catalysis of PRPP formation. Further investigations are necessary to establish the detailed mechanisms of this behavior.
(d) Function of NHRs.
The function of the NHRs has been studied to some extent. Deletion of NHR1 prevented the interaction with Slt2 (84). It is therefore possible that yeast PRPP synthase may be a bifunctional enzyme involved in nucleotide and amino acid biosynthesis as well as in maintaining cell wall integrity, the latter function involving NHR1 (84, 85, 101, 102).
Finally, the deletion of NHR5-1 resulted in a polypeptide with altered interaction with other Prs polypeptides. The simultaneous expression in an E. coli Δprs host strain of PRS3 and the prs5 ΔNHR5-1 mutant or of PRS3 and the prs5 ΔNHR5-1 ΔNHR5-2 mutant resulted in complementation of the E. coli Δprs allele, and thus production of active PRPP synthase, whereas no complementation was observed with the simultaneous expression of the wild-type alleles PRS3 and PRS5. This result may indicate that the NHR5-1 prevents the formation of an active enzyme rather than preventing interaction of Prs3 and Prs5, as interaction of these polypeptides has been previously established (89, 106) (B. Hove-Jensen, unpublished result).
(e) Ribonucleotide pool sizes and PRPP synthase activity.
The apparent interaction of Prs polypeptides with proteins of other cellular processes suggests an involvement of Prs polypeptides in linking metabolism to various stress situations. It remains to be established, however, if other physiological phenomena, such as perturbation of the pool sizes of ribonucleotides or PRPP, are also involved in the transmission of these metabolic effects. Single PRS knockouts are either unaffected in growth rate, as observed for the mutant strains harboring Δprs2, Δprs4, and Δprs5 or reduced in growth rate, as observed for mutant strains with Δprs1 and Δprs3 (8, 81, 106). Along with reduced growth rates, the pool sizes of adenylate nucleotides (AMP, ADP, and ATP) were reduced to approximately 50% of those of wild-type strains in Δprs1 and Δprs3 strains, whereas less-reduced adenylate nucleotide pool sizes were observed in Δprs2, Δprs4, and Δprs5 strains (106). Correspondingly, PRPP synthase activity was lower in Δprs1 or Δprs3 strains than in Δprs2 or Δprs4 strains (81). A Δprs1 Δprs3 strain (i.e., synthesizing Prs2, Prs4, and Prs5) contained approximately 40% of the adenylate nucleotides and 2% to 3% of the PRPP synthase activity found with the wild-type strain. A Δprs2 Δprs4 Δprs5 strain (i.e., synthesizing Prs1 and Prs3) had an almost-normal growth rate, 90% adenylate nucleotide pools, and 16% PRPP synthase activity compared to the values for the wild-type strain (83). The relatively low PRPP synthase activities determined in the various mutant strains may indicate that nonoptimal assay conditions were employed or that PRPP synthase may be unstable in vitro when one or more PRS gene products are absent, and thus these results may not accurately reflect PRPP synthase activity in vivo. Altogether, a hierarchy exists among prs lesions. Thus, Δprs3 or Δprs1 deletants appear to be more severely affected than Δprs3, Δprs4, or Δprs5 deletants.
In summary, much focus has been devoted to the analysis of Prs polypeptide interactions with one another as well as with other polypeptides. However, the enzymology of yeast PRPP synthase is far from solved. The characterization in the future of a few mutants suggested from studies of the well-characterized bacterial PRPP synthases might contribute significantly to our understanding of the oligomerization of this enzyme. The B. subtilis PRPP synthase amino acid residues His135, Asp174, and Lys197 are all very important for catalysis. These amino acid residues are identical in the five yeast Prs polypeptides except for Prs1, which has Asn171 and Arg196 rather than aspartate and lysine residues, respectively, that are found in B. subtilis PRPP synthase (Table S3). Thus, a replacement of the Lys197 analogs with alanine followed by complementation and enzyme activity analysis might prove valuable in establishing which Prs polypeptide, i.e., PRPP synthase subunit, is important for enzyme activity. It is generally believed that an active yeast PRPP synthase is composed of Prs1 and Prs3 or of Prs2, Prs4, and Prs5 (83). It is furthermore very likely that these polypeptides form a propeller-like hexameric structure similar to that of B. subtilis PRPP synthase. It would therefore be of interest to analyze variants, such as Prs2Lys197Ala Prs4 Prs5, Prs2 Prs4Lys197Ala Prs5, Prs2 Prs4 Prs5Lys308Ala, Prs1Arg196Ala Prs3, or Prs1 Prs3Lys196Ala, as well as some double variants for complementation and enzymatic properties. Indeed, the multiple, heterologous subunits composition of yeast PRPP synthase provide a unique system to study alterations in a single subunit of the hexameric enzyme, which is not possible with other PRPP synthases, which are also hexamers but are composed of a single type of subunit.
S. cerevisiae PRPP synthase is an intriguing enzyme, with its expression of more than one ortholog necessary for PRPP synthase activity. Also, the presence of NHRs imposes interesting questions about the structure and function of PRPP synthase. Do NHRs have ordered structures or are they intrinsically disordered regions? Could they confer a Hub protein character to PRPP synthase, thus facilitating heterologous protein interactions such as those described above?
(ii) S. pombe.
S. pombe contains three PRPP synthase orthologs, PRS1, PRS2, and PRS3, which presumably specify class I PRPP synthases, as judged from their deduced amino acid sequences. The three genes are unlinked with PRS1 located in chromosome I, PRS2 in chromosome II, and PRS3 in chromosome III. As PRS-specified polypeptides of S. cerevisiae, two of the PRS-specified polypeptides of S. pombe contain NHRs (Fig. 5). Similar to the NHRs of S. cerevisiae Prs1 and Prs5, the NHRs of S. pombe are located within the catalytic flexible loop (Prs1 NHR1) or within the regulatory flexible loop (Prs2 NHR2). Amino acid sequence alignments with B. subtilis PRPP synthase and S. pombe PRS-specified amino acid sequences revealed an identity for Prs1 of 29% (37% when the NHR1 is removed), for Prs2 of 39% (42% when the NHR2 is removed), and for Prs3, 45%. A comparison of amino acid residues presumed to be important for active site formation and catalysis is included in Table S3. Specifically, these amino acid residues of Prs3 are highly conserved, with 88% identity compared to 63% and 75% of those of Prs1 and Prs2, respectively. In addition, only Prs2 and Prs3 contain both of the important lysine and arginine residues of the catalytic flexible loop.
The three S. pombe PRS genes have been cloned in a plasmid vector and expressed in an E. coli Δprs strain for complementation analysis (Table S4). The results showed that neither PRS1, PRS2, nor PRS3 alone complemented the prs null allele. In contrast, simultaneous expression of PRS3 and PRS1 or PRS2 resulted in complementation, whereas simultaneous expression of PRS1 with PRS2 did not result in complementation. Furthermore, very low PRPP synthase activity could be measured in extracts of cells harboring PRS1 PRS3 or PRS2 PRS3. The activity, however, increased more than 10-fold in extracts of cells harboring PRS1 PRS3 in one plasmid and PRS2 in a second compatible plasmid or, vice versa, PRS2 PRS3 in one plasmid and PRS1 in a second compatible plasmid. These data suggest that the presence of all three PRS gene products may have a stabilizing effect on the enzyme activity (Hove-Jensen, unpublished data).
Heterozygous diploid deletion mutations have been constructed for essentially all of the genes of S. pombe. Strains containing deletion mutations in PRS1 (also designated SPAC4A8.14) or PRS2 (also designated SPBC3D6.06c) are viable. In contrast, PRS3 (also designated SPCC1620.06c) is essential (107). Additionally, two amino acids, Tyr168 and Ser172, of the PRS3-specified amino acid sequence were shown to be phosphorylated (108). Ser172 corresponds to Ser172 of B. subtilis PRPP synthase, a fairly conserved amino acid residue. It remains to be established if phosphorylation affects the activity of PRPP synthase.
The combined conclusion of the complementation and deletion analyses with S. pombe PRS genes is that PRPP synthase activity requires the PRS3 gene product as well as the gene product of either PRS1 or PRS2.
(iii) PRPP synthases of other fungi.
The association of multiple PRPP synthase subunits occurs in other species of fungi as well. The filamentous fungus Aspergillus nidulans contains a mycelium of multinucleate cells that are partitioned by septa (reviewed by Harris [109]). Briefly, the formation of septa is regulated by a protein kinase cascade that involves a sepH-specified protein kinase, which is a positive regulator of the septation initiation network. Thus, a phenotype of sepH strain mutations is a reduction or lack of septation. A number of suppressors of sepH mutation has been isolated and characterized, among which are altered levels of PRPP synthase activity. Those authors offered a somewhat speculative model, in which competition of PRPP synthase and SepH for ATP might be the trigger of septation (110).
A. nidulans contains three PRPP synthase orthologs: PRS1 (located on chromosome I), PRS2 (on chromosome VI), and PRS3 (on chromosome VII). Prs1 (GenBank accession no. CBF69369.1; 489 amino acid residues) resembles S. cerevisiae Prs1, as there is an addition of 169 amino acid residues inserted within the catalytic flexible loop; Prs2 (435 amino acid residues; GenBank no. CBF83270.1) has an insertion of 116 amino acid residues at position 115, i.e., far from the catalytic flexible loop, and Prs2 may represent a new variant of PRPP synthases; and Prs3 (320 amino acid residues; GenBank no. CBF85908.1) appears to be a “normal” PRPP synthase polypeptide. Each of the three amino acid sequences reveals all of the characteristic features for class I PRPP synthases, i.e., amino acids involved in subunit-subunit interactions, substrate binding and catalysis, and allosteric regulation. Yeast two-hybrid analyses revealed interactions of Prs1 with Prs2, Prs1 with Prs3, and Prs2 with Prs3. Thus, the enzyme may contain all three polypeptides in an unknown stoichiometry (110).
The filamentous fungus Eremothecium (Ashbya) gossypii contains four PRPP synthase-specifying orthologs (111, 112). Two of these genes, both located on chromosome VII, have been analyzed: Agl080c (accession no. NP_986586), a homolog of S. cerevisiae PRS2 or PRS4, and Agr371c (accession no. NP_987037), a homolog of S. cerevisiae PRS3. The two other orthologs are homologs of S. cerevisiae PRS1 (accession no. NP_984943) and PRS5 (accession no. NP_984410). Both of these latter E. gossypii gene products have NHRs at positions similar to those of S. cerevisiae Prs1 and Prs5, although their lengths vary. All four E. gossypii genes may specify typical class I PRPP synthases (112). Similar to the situation with S. cerevisiae, the knockout of the PRS3 homolog had more dramatic effects on various growth parameters than that of knockout of the PRS2/PRS4 homolog. The presence of only four PRS orthologs in E. gossypii, contrary to five in S. cerevisiae, may indicate some redundancy in the PRS2 and PRS4 genes of S. cerevisiae, as only one of these is present in E. gossypii. It was furthermore shown that introduction of the amino acid alteration Leu133Ile or Leu132Ile into the PRS2/PRS4 or the PRS3 homolog, respectively, resulted in reduction of feedback inhibition of PRPP synthase activity. Similar effects were observed with His196Gln or His195Gln alterations of the PRS2/PRS4 or the PRS3 homolog, respectively. These amino acid alterations of PRPP synthase were introduced to desensitize the pathway for riboflavin biosynthesis for which purine nucleotides, and thus PRPP, are precursors (112). We shall return to explain the effect of the amino acid alterations on PRPP synthase activity in the section “Regulation of PRPP Synthase Activity.”
It is obvious from the description above that the physiology of yeast PRPP synthase differs from that of bacteria. Although also belonging to the class I PRPP synthases, major differences exist, such as the presence of the amino acid sequences of NHR, the requirement of heteromeric oligomerization, and the involvement of PRS-specified polypeptides in cellular processes other than PRPP synthesis.
Mammalian PRPP synthases and PRPP synthase-associated proteins.
As described above, yeasts contain PRPP synthase-specifying homologs with NHRs inserted at specific positions as well as “ordinary” PRPP synthase-specifying homologs without these insertions. Similar proteins are found in mammalian organisms, where apparent PRPP synthases have insertions similar to the yeast NHRs. In mammals, PRPP synthase polypeptides with an insertion have been designated PRPP synthase-associated proteins, or PAP. The descriptions below for each type of PRPP synthase and PAP complement previously published reviews (18, 20).
(i) PRPP synthase.
Among PRPP synthases of mammalian origin, only those of humans and rats have been characterized. Both organisms contain three PRPP synthase genes, PRPS1 to PRPS3, that encode PRPP synthase isozymes 1 to 3. PRPS1 and PRPS2 are located on the X chromosome, whereas PRPS3 is located on chromosome 7. The latter gene is expressed only in testes, whereas both of the former genes are expressed in all the tissues examined (11).
(a) Enzyme characteristics.
Human and rat PRPP synthases also belong to the class I PRPP synthases, and as such their properties resemble the microbial class I PRPP synthases with respect to their restricted diphosphoryl donor capacity (ATP and dATP) and allosteric regulation by Pi and ADP (18, 20). The amino acid sequence identity among the three human PRPP synthase isozymes is 91% to 95%, whereas the identities of B. subtilis PRPP synthase and the three human isozymes are 45% or 46%. Exclusion chromatography of PRPP synthase activity isolated from rat liver revealed a protein complex with a molecular mass of 1,000 kDa or more. The protein complex consisted of four different polypeptides, the two catalytic PRPP synthase isozymes 1 and 2 as well as the two so-called PAP-39 and PAP-41 peptides, and was estimated to contain 20 molecules of isozyme 1, five of isozyme 2, and eight and one of PAP-39 and PAP-41, respectively (113). Previous characterization of human PRPP synthase may have been performed on mixtures of isozyme 1 and 2, although erythrocytes, the source of the enzyme in those early studies, contain predominantly isozyme 1 (114), and similar to the rat liver enzyme, human PRPP synthase was isolated as large oligomeric structures (115, 116). Human isozymes 1 and 2 differ in 15 amino acid residues, and human and rat isozyme 1 are identical, whereas human and rat isozyme 2 differ in only 3 amino acid residues.
Although native PRPP synthase isolated from tissues may better represent the physiological state of the enzyme, efforts have been spent also to characterize the individual isozymes. Isozymes 1 and 2 of both humans and rats have been purified and characterized after expression of their recombinant genes in E. coli (71, 73). As expected from the close identity of the four enzymes, they shared many similarities in physico-chemical properties. They all oligomerize to very large structures with molecular masses around 1,000 kDa (human isozymes 1 and 2, rat isozyme 1) or approximately 550 kDa (rat isozyme 2), as determined by size exclusion chromatography. Even though they are quite similar in primary structure, there are also differences between human isozymes 1 and 2. Different purification procedures were employed for the two isozymes: isozyme 2 was much more labile to heat inactivation than isozyme 1, isozyme 1 was saturated by lower concentrations of ATP, ribose 5-phosphate, and Mg2+ than isozyme 2, and isozyme 1 was more prone to inhibition by ribonucleoside diphosphates than isozyme 2. Comparison of human and rat enzymes revealed very few differences (71, 73). Some kinetic properties of PRPP synthases of humans and rats are listed in Table 2.
(b) Three-dimensional structure.
The human PRPP synthase isozyme 1 three-dimensional structure has been determined from a number of crystal forms (Table 1). (i) hPRS1 wild-type PRPP synthase has three SO42− ions bound, one at the phosphate position of ribose 5-phosphate, one at the position of the α-phosphate of ADP at the allosteric site, and one at a new, third position at the parallel dimer interface; its structure was determined at 2.0 Å resolution (PDB code 2h06). (ii) ATP-SO42−-Cd2+ PRPP synthase (also wild type), with three SO42− bound as in the hPRS1 wild-type PRPP synthase crystal form, ATP (modeled to only AMP) at the active site, and one Cd2+ per monomer; its structure was determined at 2.2 Å resolution (PDB code 2hcr) (51). (iii) PRS1 (wild-type PRPP synthase) with one SO42− bound; its structure was determined at 2.0 Å resolution (PDB code 3s5j) (117, 118). In addition, three-dimensional structures of some mutant variants have been determined as well and are described below. From the 46% identity among B. subtilis and human PRPP synthase isozyme 1 amino acid sequences, it comes as no surprise that the three-dimensional structures of the two enzymes are essentially identical. Superimposition of the hPRS1 wild-type PRPP synthase structure and various B. subtilis PRPP synthase structures revealed root mean square deviation values of 1.0 to 1.2 Å. As expected, also the human PRPP synthase isozyme 1 has the hexameric propeller structure of B. subtilis PRPP synthase (Fig. 3C) (51). In addition, the structure of human PRPP synthase isozyme 1 has been visualized by negative stain electron microscopy. The apo- and the ADP-bound forms have a three-leaf clover structure, into which the crystal structure of isozyme 1 can dock (118).
(c) Mutant PRPP synthases.
A number of variants of human PRPP synthase isozyme 1 have been discovered because of their severe clinical effects on the affected individuals. As reviewed previously (119), these pathological effects are caused either by increased activity of PRPP synthase isozyme 1, i.e., “superactivity” of the enzyme or overexpression of the PRPS1 gene, which results in an array of physiological effects, including hyperuricemia, gouty arthritis, or neurosensory defects, or by reduced activity of PRPP synthase isozyme 1, resulting in neuropathy, deafness, or intellectual disabilities. Historically, these altered PRPP synthases were described by the somewhat-imprecise designations “gain of function” or “loss of function.” Sixteen mutant variants affecting 15 amino acid positions have been discovered and are summarized in Table 4. (No mutant forms of PRPP synthase isozyme 2 or PAP have been described). The loss of function of PRPS1 variants (i.e., reduced activity in Table 4) are the variants Asp64Asn, Ala86Thr, Met114Thr, Gln132Pro, Val141Leu, Leu151Pro, Ile289Thr, and Gly305Arg, which had 40 to 70% of the specific activity of normal PRPP synthase (120–122). In Fig. 2, each of these amino acid positions is indicated by blue arrowheads, which point to the homologous position in B. subtilis PRPP synthase. It is evident that none of these amino acid alterations are within positions that are directly involved in catalysis, which presumably would produce inactive enzyme forms. Asp64 and Met114, equivalent to B. subtilis PRPP synthase Glu70 and Leu120, respectively, are located at stretches of amino acids involved in the formation of the bent dimer. Val141, equivalent to B. subtilis PRPP synthase Ile147, is located close to sequences that are involved the formation of the parallel dimer, and Gln132, Ile289, and Gly305, equivalent to B. subtilis PRPP synthase Gln138, Ser293, and Gln308 are within or close to positions involved in allosteric regulation of PRPP synthase activity. Finally, Ala86, equivalent to B. subtilis PRPP synthase Ile92, is located close to the regulatory flexible loop. Altogether, the amino acid positions causing reduced activity of PRPP synthase isozyme 1 are located at positions secondary to catalysis, and their effect instead may be to alter the secondary structure, resulting in poorer enzymatic function.
TABLE 4.
Human PRPP synthase isozyme 1 variantsa
| Effect and amino acid alteration | Homologous position in B. subtilis PRPP synthase | Enzyme property | Reference(s) |
|---|---|---|---|
| Increased activityb | |||
| Asp51His | Asp57 | Reduced inhibition by ADP, increased stimulation by Pi | 72 |
| Asn113Ser | Asn119 | Reduced inhibition by ADP, increased stimulation by Pi | 7 |
| Leu128Ile | Leu134 | Reduced inhibition by ADP, increased stimulation by Pi | 72 |
| Asp182His | Asp186 | Reduced inhibition by ADP, increased stimulation by Pi | 72 |
| Ala189Val | Ala193 | Reduced inhibition by ADP, increased stimulation by Pi | 72 |
| His192Leu | Asp196 | Reduced inhibition by ADP, increased stimulation by Pi, reduced stability | 72, 123 |
| Reduced activity | |||
| Glu42Asp | Asn48 | Not characterized | 120 |
| Asp64Asn | Glu70 | Reduced sp act in crude erythrocyte extract | 121 |
| Ala86Thr | Ile92 | Reduced sp act in crude erythrocyte extract | 121 |
| Met114Thr | L119 | Reduced sp act in crude fibroblast extract | 120 |
| Gln132Pro | Gln138 | Reduced sp act in crude erythrocyte and fibroblast extracts | 122 |
| Val141Leu | Ile147 | Reduced activation by Pi | 412 |
| Leu151Pro | Leu156 | Reduced sp act in crude erythrocyte and fibroblast extracts | 122 |
| Ile289Thr | Ser293 | Reduced sp act in crude erythrocyte extract | 121 |
| Gly305Arg | Gln308 | Reduced sp act in crude erythrocyte extract | 121 |
| Acute lymphoblastic leukemia relapse specificc | |||
| Val52Ala | Cys58 | 148 | |
| Ile71Val | Ile77 | 148 | |
| Cys76Ser | Leu82 | 148 | |
| Ser102Asn | Ser108 | 148 | |
| Asn113Asp | Asn119 | 148 | |
| Asp138Gly | Asp144 | 148 | |
| Asn143Ser | Asp148 | 148 | |
| Gly173Glu | Gly177 | 148 | |
| Lys175Asn | Thr179 | 148 | |
| Asp182Glu | Asp186 | 148 | |
| Ala189Thr/Val | Ala193 | 148 | |
| Leu190Ile | Ile194 | 148 | |
| Thr302Ser | Val305 | 148 | |
| Tyr310Cys | Tyr313 | 148 |
Amino acid residues are numbered from the N-terminal proline residue, as the original N-terminal methionine is removed in the mature protein (72).
A comprehensive review of the various diseases caused by mutations in the human PRPP synthase isozyme 1-encoding gene was published previously (413).
Relapse-specific PRPP synthase variants have not been characterized enzymatically.
The gain-of-function variants (i.e., with increased activity in Table 4) are the variants Asp51His, Asn113Ser, Leu128Ile, Asp182His, Ala189Val, and His192Leu. These variant forms of PRPP synthase have increased activity primarily because of their reciprocal effects on allosteric regulation by ADP and Pi, namely, reduced inhibition by the former and increased stimulation by the latter (72, 123). Studies of these PRPP synthase mutant variants have proven valuable in the elucidation of the allosteric regulation of the enzyme, and we shall return to a further description of them in the section “Regulation of PRPP Synthase Activity.”
The structure of three mutant variants has been determined. One is the Asp51His variant, which has SO42− bound at the position of the α-phosphate of ADP at the allosteric site as well as Mg2+ (PDB code 4f8e) (124). Patients containing the Asp51His variant suffer from hyperuricemia and severe gout. The other structures are those of the Ser131Ala (PDB code 2h07) and Tyr145Met (PDB code 2h08) variants, each with three SO42− bound as described for the hPRS1 wild-type PRPP synthase structure. The authors used the observation of these SO42− ions as indicative of a second allosteric Pi-binding site (51).
(d) PRPP synthase isozyme 2 and Myc-driven cancers.
As mentioned above, no mutants of human PRPP synthase isozyme 2, specified by the PRPS2 gene, have been described. Remarkably, however, the activity of isozyme 2 has been shown to be critical in developing and maintaining cancers caused by Myc transcription factor. The eukaryotic translation initiation factor 4E is also involved in the development of this type of cancer. The PRPS2 gene, but not the PRPS1 gene, contains a pyrimidine-rich translational element in the 5′-untranslated region. Myc-driven hyperactivation results in an interaction of the eukaryotic translation initiation factor 4E and possibly other factors with the pyrimidine-rich translational element and results in an increase of translation of PRPS2-specific mRNA, which results in increased PRPP synthase isozyme 2 synthesis and, thus, increased nucleotide production. The increase in nucleotide synthesis inhibits PRPP synthase isozyme 1, whereas isozyme 2 is less sensitive to feedback inhibition by ribonucleotides. Also, the lack of the pyrimidine-rich translational element within the PRPS1 mRNA prevents the stimulation of synthesis of isozyme 1. Altogether, compelling evidence shows that PRPP synthase isozyme 2 plays an important role in the metabolism of cells with Myc-driven cancers (125).
In addition, PRPP synthase isozyme 2, but not isozyme 1, has been shown to be prone to arginylation (419). This process has severe effects impacting PRPP synthase activity and stability (420).
(ii) PAP.
Mammalian PAP-39 and PAP-41 may be variants of the S. cerevisiae and S. pombe PRS1-specified gene products described previously, i.e., PRPP synthase-like proteins containing an NHR. The amino acid sequence of human PRPP synthase isozyme 1 is 41% identical to both the PAP-39 and PAP-41 sequences; the amino acid sequence of PAP-39 is 76% identical to that of PAP-41. The NHRs are both located within the catalytic flexible loop and are 29 and 30 amino acid residues in length, respectively. Thus, the additions are located at a position similar to NHR1 of S. cerevisiae Prs1, NHR5-2 of S. cerevisiae Prs5, and the NHR of S. pombe Prs1, but they differ in length (Fig. 5). The identity of B. subtilis PRPP synthase and PAP-39 and PAP-41 is 31% and 34%, respectively. After removal of the additions, these values increase to only 34% and 37%, respectively. Inspection of important amino acid residues revealed that the ribose 5-phosphate-binding site is well-preserved in PAP-39 and PAP-41. In contrast, residues involved in the binding of ATP and residues important for catalysis are poorly conserved. Specifically, the two important residues of the catalytic flexible loop (Lys197 and Arg199 in B. subtilis) are absent in the two PAPs. Data for PAP-39 are included in Table S3 in the supplemental material. Originally, the PAPs were identified as subunits that copurified with rat PRPP synthase. Interaction of PRPP synthase and PAP has been demonstrated by several methodologies, such as cross-linking and coimmunoprecipitation. The PAP could be removed from PRPP synthase isolated from rat liver by exclusion chromatography in the presence of 1 M magnesium chloride. Both of these latter processes yielded PRPP synthase with increased activity, which suggested that the PAPs play a negative regulatory role on PRPP synthase activity (113). Furthermore, a protein devoid of PRPP synthase activity was obtained after expression of the PAP-39-specifying gene in E. coli. Partial reconstitution of rat PRPP synthase activity could be achieved by coexpression of recombinant genes specifying PRPP synthase isozymes 1 and 2 and PAP-39 in E. coli, which resulted in an active oligomeric enzyme eluting near the void volume, i.e., an enzyme consisting of a large number of subunits (126).
Mammalian and yeast PRPP synthase structures resemble one another in interesting ways. Both mammalian and yeast PRPP synthases have constituent subunits of “ordinary” PRPP synthase polypeptides, i.e., isozymes 1 and 2 of humans or rats and Prs2, Prs3, and Prs5 of S. cerevisiae, as well as the NHR-containing polypeptides PAP-39 and PAP-41 of humans or rats and Prs1 and Prs5 of S. cerevisiae, as described above. Indeed, the presence of multiple, heterogenous subunits, including subunits with NHR sequences, may be widespread among eukaryotic organisms. The studies with yeast suggest that such heterologous structures may play roles in cellular regulation that are not yet well defined.
The crystal structures of human PAP-39 and PAP-41 have been determined at 2.7 and 2.6 Å resolution (PDB codes 2c4k and 2ji4, respectively). We found that superimposition of these two structures with that of B subtilis PRPP synthase (PDB code 1dku) revealed root mean square deviation values of 1.1 and 0.97 Å for PAP-39 and PAP-41, respectively, which indicates that the three-dimensional structures of the PAPs and human and B. subtilis PRPP synthases are essentially identical.
Other class I PRPP synthases.
Some interest has been devoted to PRPP synthase of the mosquito Culex pipiens pallens. This mosquito is the major vector for various roundworms causing lymphatic filariasis (elephantiasis) and for the Japanese encephalitis virus. An important insecticide used in controlling C. pipiens pallens and other insects as well is the pyrethroid ester deltamethrin. Many insect species develop deltamethrin resistance by knockout mutations, and increased expression of specific genes may assist in providing resistance. Expression of the PRPS1 gene is one example of this phenomenon. The PRPS1-encoded enzyme is a typical class I PRPP synthase. The exact mechanism of the contribution of overexpression of PRPS1 to deltamethrin resistance remains to be established (127).
Planarians, with Dugesia japonica as an example, are studied because of their remarkable ability to regenerate from injuries such as lost body parts. A PRPP synthase-specifying ortholog (DjPRPS) of D. japonica has been cloned. The deduced amino acid sequence reveals a typical class I PRPP synthase, with 37% identity to the amino acid sequence of B. subtilis PRPP synthase (128).
Class II PRPP Synthases
S. oleracea isozymes 3 and 4 were proposed to constitute a second class of PRPP synthases, class II, based on a comparison of the biochemical properties of the enzymes (14, 15). Both isozymes 3 and 4 are active in the absence of Pi, and both enzymes are inhibited by the ribonucleoside diphosphates ADP and GDP in a competitive manner, i.e., they compete with ATP for binding at the active site. Thus, no allosteric inhibition by either of these ribonucleoside diphosphates was detected. Kinetic parameters are summarized in Table 2. Both of the S. oleracea isozymes are able to use ATP, dATP, GTP, CTP, and UTP as diphosphoryl donors. The apparent Michaelis-Menten constants for the other diphosphoryl donors for isozyme 3 and isozyme 4 varied between 85 and 680 μM. Exclusion chromatography of isozyme 4 revealed a molecular mass of 110 kDa. Identical results were obtained in Tris- or phosphate ion-based buffer and in the presence or absence of MgATP. Recombinant S. oleracea isozyme 3 eluted identically to isozyme 4 in exclusion chromatography. With a calculated molecular mass of the subunit as 35.4 kDa, this value of 110 kDa is consistent with a homotrimeric quaternary structure (46, 47). Some controversy exists on this point, as characterization of sugarcane class II PRPP synthase revealed a molecular mass of 214 kDa, i.e., a value consistent with a hexameric quaternary structure. Like S. oleracea PRPP synthase isozymes 3 and 4, the activity of sugarcane PRPP synthase is independent of Pi (129).
Amino acid sequence alignments of PRPP synthase isozyme 3 or 4 with the amino acid sequence of B. subtilis PRPP synthase revealed a conservation of amino acids along the polypeptide. Thus, a small part of the regulatory flexible loop (Phe97 to Thr114 of isozyme 4), the PRPP-binding site consisting of the PP loop (Asp183 to Ala186 of isozyme 4) and the ribose 5-phosphate-binding loop (Gly222 to Thr237 of isozyme 4) are extensively conserved. The catalytic flexible loop (Lys204 to Leu215) is less conserved, but the apparently indispensable residues, Lys204 and Arg206, are retained. The amino acid residues important for binding of the adenine moiety of ATP, Phe41, Asp43, and Gln47, and the catalytically important residue His141 are also conserved (Fig. 2 and Tables S1 and S2). Altogether, it is therefore likely that the mechanism of catalysis of class II PRPP synthases is identical or similar to that of class I PRPP synthases.
The amino acid residues involved in the formation of the two types of subunit-subunit interactions identified in B. subtilis PRPP synthase, i.e., the bent dimer and the parallel dimer, are poorly conserved in class II PRPP synthases (Table 3). In class II PRPP synthases represented by the S. oleracea PRPP synthase isozyme 4, only 3 of the 8 residues involved in the formation of the B. subtilis PRPP synthase bent dimer are conserved, and only 4 of the 12 residues involved in the formation of the B. subtilis PRPP synthase parallel dimer are conserved. It is likely, therefore, that the quaternary structure of class II PRPP synthases differs from that of class I enzymes. Also, the amino acid residues identified as involved in allosteric regulation of B. subtilis PRPP synthase are poorly conserved in S. oleracea PRPP synthase isozyme 4; among the 20 residues listed in Table S2, only 6 are conserved, which is consistent with the lack of allosteric regulation of class II PRPP synthases.
The amino acid sequences of S. oleracea PRPP synthase isozyme 2 (class I) and isozyme 3 (class II) contain extensions of 76 and 87 amino acids, respectively, at the N terminus compared to isozyme 4. For isozyme 2, this extension contains an amino acid sequence that indicates its localization in chloroplasts, whereas for isozyme 3 the extension contains an amino acid sequence that indicates its localization in mitochondria. Indeed, in vitro expression of PRS2 and synthesis of isozyme 2 in the presence of chloroplasts showed that this isozyme was transported to chloroplasts (15). Unlike S. oleracea PRPP synthase isozymes 2 and 3, the amino acid length of isozyme 4 is similar to that of other PRPP synthases (318 amino acids compared to 316 of B. subtilis PRPP synthase), and thus isozyme 4 is believed to be located in the cytoplasm (15).
Interestingly, previous analyses of plant PRPP synthases provide some additional information on the class II enzymes. PRPP synthase from spinach leaves has been partially purified. The activity was independent of Pi. In fact, Pi inhibited the activity with half-maximal inhibition at 28 μM. The apparent Km values for ATP, ribose 5-phosphate, and Mg2+ were 36 μM, 10 μM, and 1 mM, respectively. ATP was by far the best diphosphoryl donor, but GTP, UTP, and CTP also were diphosphoryl donors. The PRPP synthase activity was found predominantly in the cytosol (>95% of the total activity), with minor contributions by the nuclear, chloroplast, and mitochondrial fractions (1 to 2%) (130). These data have to be reevaluated in light of the present knowledge of multiple isozymes in spinach. Rubber tree (Hevea brasiliensis) latex PRPP synthase has been partially purified and characterized. Exclusion chromatography revealed a molecular mass of 200 kDa. The authors estimated the subunit molecular mass as 56 kDa following gel electrophoresis under denaturing conditions. Actually, a band corresponding to a polypeptide of molecular mass 35 kDa was also visible in the photograph of the gel. This size, 35 kDa, is the “standard” size of PRPP synthases. Activity was obtained in the absence of Pi. ATP functioned as a diphosphoryl donor, whereas neither GTP, CTP, nor UTP functioned as a diphosphoryl donor. Apparent Km values for ATP and ribose 5-phosphate were determined to be 0.2 M and 40 μM, respectively (131). As before, the data should be reevaluated with the current knowledge of the multiple forms of PRPP synthases. It remains unknown if the latex fraction contains a specific PRPP synthase. Finally, native PRPP synthase has been characterized in partially purified or crude preparations from black gram (Vigna mungo) hypocytols (416, 421).
BLAST analysis with the amino acid sequences of S. oleracea or A. thaliana PRPP synthase isozyme 3 or 4 as the queries revealed the presence of these enzymes also in green algae species, such as Chlamydomonas reinhardtii (accession no. XP_001694483 [an isozyme 3-like sequence]) (132), Chlorella variabilis (XP_005848636 [isozyme 3-like]) (133), Coccomyxa subellipsoidea (XP_005643144.1 [353-amino-acid sequence, isozyme 4-like]) (134), Ostreococcus lucimarinus (XP_001421441.1 [305-amino-acid sequence, isozyme 4-like] and XP_001421590 [304-amino-acid sequence, isozyme 4-like]) (135), Ostreococcus tauri (XP_003083594.1 [396-amino-acid sequence, isozyme 3-like] and XP_003083246.1 [552-amino-acid sequence]) (136), and Volvox carteri (XP_002955183 [398-amino-acid sequence, isozyme 3-like]) (137). BLAST analysis with S. oleracea PRPP synthase isoenzyme 1 as the query revealed the presence of class I PRPP synthase also in O. lucimarinus, C. variabilis, C. subellipsoidea, and V. carteri. Thus, in general the class II PRPP synthases seem to be an addition to the classical, class I PRPP synthases. It should be added that the completion of the A. thaliana genome sequencing revealed the presence of a gene encoding a class I PRPP synthase isozyme 5 (16).
Archaeal PRPP Synthases
The biochemical characterization of PRPP synthase of M. jannaschii persuaded the authors to propose the existence of a third class of PRPP synthases. The activity of this enzyme is activated by Pi and it has restricted diphosphoryl donor capacity (ATP and dATP only), which are properties of class I PRPP synthases. However, ADP binds to only the active site, and the enzyme appears to be unregulated by an allosteric mechanism involving purine ribonucleoside diphosphates, which is a property of class II PRPP synthases. Additionally, the quaternary structure of M. jannaschii PRPP synthase is tetrameric (48). In light of the three-dimensional structures of PRPP synthase of T. volcanium and S. solfataricus, which are dimers, it seems more reasonable to define this class of PRPP synthases as “archaeal PRPP synthases” rather than class III synthases. Amino acid sequence alignments of PRPP synthase of the three archaeal species, M. jannaschii, T. volcanium, and S. solfataricus, with the amino acid sequence of B. subtilis PRPP synthase revealed conservation of amino acids along the polypeptide. The regulatory flexible loop, the PP loop and the ribose 5-phosphate-binding loop are extensively conserved; the catalytic flexible loop is less conserved, but the apparent indispensable lysine and arginine residues are retained. The amino acid residues important for binding of the adenine moiety and the catalytically important histidine residues are also conserved (Table S1). As with the class II PRPP synthases, it is likely that the archaeal PRPP synthases and class I PRPP synthases share identical catalytic mechanisms.
M. jannaschii.
The amino acid sequences involved in various functions are conserved in M. jannaschii PRPP synthase, i.e., the regulatory flexible loop, Leu88 to Ser104, the PP loop, Asp163 to Ala166, the catalytic flexible loop, Lys186 to Ala197, and the ribose 5-phosphate-binding loop, Asp205 to Thr220 (Table S1). Also, the two-domain structure of the M. jannaschii PRPP synthase subunit resembles that of the B. subtilis enzyme.
Biochemical characterization of PRPP synthase from M. jannaschii revealed properties unlike those described above for class I and class II PRPP synthases (Table 2). Apparently, the poor affinity for both substrates may be compensated by a very large Vmax value. Additionally, the activity of M. jannaschii PRPP synthase is stimulated by Pi, diphosphoryl donors are ATP and dATP, ADP is a competitive inhibitor, and ADP binds to a single site per subunit, hence, the active site. These properties partly point to a class I enzyme, i.e., restricted diphosphoryl donor capacity and activation by Pi, and partly to a class II enzyme, i.e., lack of allosteric regulation. The quaternary structure of M. jannaschii PRPP synthase was furthermore found to be tetrameric (48).
The structure of M. jannaschii PRPP synthase, as deduced from X-ray crystallographic analysis, is considerably more compact than that of B. subtilis PRPP synthase, primarily because of truncations at the N- and C-terminal ends as well as one shorter loop in the interior of the sequence. As with B. subtilis PRPP synthase, the structure of M. jannaschii PRPP synthase revealed two different types of subunit-subunit interactions; these were revealed by the structure of the apo-PRPP synthase structure (PDB code 1u9y) and the structure of the AMP-R5P PRPP synthase complex (with AMP and ribose 5-phosphate bound; PDB code 1u9z). The tetrameric M. jannaschii PRPP synthase could be superimposed easily with two of the subunits of B. subtilis PRPP synthase to form almost identical bent dimers. Consistent with the bent dimer organization of M. jannaschii PRPP synthase, 6 of the 8 amino acid residues selected (Table 3) were identical or conserved among B. subtilis and M. jannaschii PRPP synthases. Interestingly, the interface of the bent dimer contains residues involved in the binding of the adenine moiety of ATP, namely, Phe32B (Phe40D of B. subtilis PRPP synthase), Asp34B (Asp42D), Glu36B (Glu44D), and Arg92A (Arg101A). The rest of the active site, i.e., the binding of the remaining part of ATP and ribose 5-phosphate, is provided entirely by one subunit of a bent dimer, indicating that substrate recognition and catalytic machinery of B. subtilis and M. jannaschii PRPP synthases are identical. The conservation of amino acid residues involved in substrate binding and catalysis is apparent from the amino acid comparison summarized in Table S1. In contrast, the other subunit-subunit interaction of M. jannaschii did not superimpose with the linear dimers of the B. subtilis enzyme. Only 4 of 12 amino acid residues involved in the parallel dimer formation of B. subtilis PRPP synthase were identical or conserved (Table 3). The allosteric effectors of B. subtilis PRPP synthase, ADP and Pi, bind to residues at the interface of the parallel dimer, which is not conserved among the two enzymes, and very few if any amino acid residues involved in allosteric regulation of B. subtilis PRPP synthase are conserved in M. jannaschii PRPP synthase (Table S2). This is consistent with the lack of allosteric regulation in vitro of M. jannaschii PRPP synthase, which binds only a single molecule of ADP per subunit (48).
T. volcanium.
As described above for M. jannaschii PRPP synthase, amino acids involved in the formation of the bent dimer are relatively well conserved relative to the homologous amino acid residues of B. subtilis PRPP synthase, whereas the amino acid residues involved in the formation of the parallel dimer are much less conserved. Amino acid residues involved in substrate binding and catalysis are well conserved (Table S1), whereas only a few amino acid residues involved in allosteric regulation of B. subtilis PRPP synthase are conserved in T. volcanium PRPP synthase (Table S2).
Structures of several crystal forms of T. volcanium PRPP synthase have been described. (i) ADP-SO42− PRPP synthase, with ADP and SO42− present at the active site representing ATP and the phosphoryl moiety of ribose 5-phosphate, determined at 1.5 Å resolution (PDB code 3lrt). The active site is located in the cleft between the two domains as in the B. subtilis enzyme. The binding of ADP is well defined; the adenine moiety is stacked between Arg91 and Phe32 and N6 is bound to Asp34 and Glu36, and the ribose hydroxyls are bound to Glu92, His93, and Tyr96. In addition, ADP interacts with the side chains of amino acid residues Asp34, Glu36, Arg91, and Ser214 through water molecules. The crystal form has an open conformation, i.e., the catalytic flexible loop is open, resulting in full accessibility of the active site for substrates. The side chain nitrogens of the catalytic flexible loop Lys184 are located 14 to 16 Å away from the β-phosphate oxygen of ADP (138). (ii) mATP-SO42− PRPP synthase, determined at 1.8 Å resolution (PDB code 3lpn). In this crystal form, the ATP analog α,β-methylene ATP replaces ADP of the ADP-SO42− PRPP synthase described above. The binding of α,β-methylene ATP, including the triphosphate chain, is well defined and shows His93 interacting with the oxygens of the β- and γ-phosphates and Tyr96, His124, and Asp125 interacting with the γ-phosphate. The catalytic flexible loop remained open (138). (iii) ADP-Mg2+-R5P PRPP synthase, determined at 1.8 Å resolution (PDB code 3mbi). Here, MgADP and ribose 5-phosphate are located at the active site. Mg2+ coordinates the polypeptide through His124, the α- and β-phosphates, as well as three water molecules. Thus, the binding of this Mg2+ resembles that of the MG2 site of B. subtilis PRPP synthase, except for the coordination of Mg2+ to the γ-phosphate rather than water in the B. subtilis enzyme. Furthermore, the catalytic flexible loop is closed, i.e., it is bent approximately 45° toward the bound ligands, MgADP and ribose 5-phosphate, compared to the open form; this results in the placement of Lys184 and Arg186 close to the β-phosphate of ADP (and presumably of ATP), and in shielding of the active site and the transition state from the solvent (138). The importance of this closure of the catalytic flexible loop for catalysis is discussed below in the section “Mechanism of Catalysis.”
The crystal structure as well as molecular sieving revealed that T. volcanium PRPP synthase is a dimer, which does not form higher-symmetry oligomers (hexamers or tetramers) like those described for the class I PRPP synthases (138). Also, the crystal structure with α,β-methylene ATP/ADP and ribose 5-phosphate present revealed a dimeric quaternary structure. However, molecular sieving in the presence of ATP (or α,β-methylene ATP) and ribose 5-phosphate has not been performed, so formation of a tetramer resembling that of M. jannaschii PRPP synthase under some conditions cannot be ruled out.
S. solfataricus.
S. solfataricus PRPP synthase resembles PRPP synthases from M. jannaschii and T. volcanium with respect to conservation or lack of conservation of amino acid residues involved in dimer formation (Table 3), substrate binding and catalysis (Table S1), and allosteric regulation (Table S2). Structural information for S. solfataricus PRPP synthase is available from analysis of a crystal form with AMP and sulfate representing the phosphoryl group of ribose 5-phosphate at 2.8 Å resolution (PDB code 4twb). The enzyme appears to be active as a dimer like that described for T. volcanium PRPP synthase. Amino acid sequence identity of S. solfataricus and T. volcanium PRPP synthases is 29%. The fold of the subunit resembles that of other PRPP synthases, and the binding of the nucleotide substrate ATP (modeled to only the AMP portion) is highly conserved in comparison to the PRPP synthases of T. volcanium, M. jannaschii, and B. subtilis (139).
Other archaeal PRPP synthases.
The gene encoding PRPP synthase of the hyperthermophilic archaeon Thermococcus kodakarensis has been expressed in E. coli and PRPP synthase purified to near homogeneity. Recombinant T. kodakarensis PRPP synthase accepts ATP, CTP, and GTP as diphosphoryl donors, with ATP as the preferred diphosphoryl donor. The enzyme eluted as a monomer in exclusion chromatography (140). Chromatography was performed without the presence of substrates. It is therefore possible that the active enzyme consists of more than one subunit. Indeed, comparison of amino acid residues involved in the formation of the bent dimer of M. jannaschii with those of T. kodakarensis revealed an almost exact match, suggesting that active T. kodakarensis PRPP synthase may constitute a tetramer similar to that of M. jannaschii PRPP synthase. PRPP synthase from the hyperthermophilic archaeon Pyrobaculum calidifontis has been characterized and shown to resemble that of T. kodakarensis (422). The similarities of the amino acid sequence of T. kodakarensis PRPP synthase with those of M. jannaschii, S. sulfolobus, and T. volcanium are 52 to 58% (identity, 32 to 40%), whereas with B. subtilis PRPP synthase the values are 47% (similarity) and 28% (identity).
The genomes of the following archaeal species appear to contain two prs genes: Archaeoglobus fulgidus, Archaeoglobus veneficus, Ferroglobus placidus, Halorhabdus utahensis, Methanocella conradii, Thermofilum pendens, and Thermogladius cellulolyticus. No biochemical information is available on the properties of the corresponding proteins. Amino acid sequence comparisons of two putative A. fulgidus PRPP synthases and B. subtilis PRPP synthase revealed a high degree of conservation of amino acid residues involved in substrate binding and catalysis. Specifically, the two important lysine and arginine residues are present in both sequences: Lys172 and Arg175 in sequence 1, Lys186 and Arg 188 in sequence 2 (Table S3). Indeed, amino acid sequence comparisons revealed that A. veneficus, F. placidus, M. conradii, T. pendens, and T. cellulolyticus may encode two bona fide PRPP synthases, as evaluated by the presence of conserved or identical amino acids at positions homologous to B. subtilis PRPP synthase residues Phe40, His135, and Asp174 of the ribose 5-phosphate-binding loop and Lys197 and Arg199 of the catalytic flexible loop. In contrast, one of the putative PRPP synthases of H. utahensis has aspartate and threonine rather than lysine and arginine in the catalytic flexible loop, and thus it very likely does not represent an active PRPP synthase.
In spite of large variations in protein structure among class I, class II, and archaeal PRPP synthases as a result of the different oligomerization properties described above, it is obvious that the catalytic mechanism essentially is the same among the three PRPP synthase classes, as evaluated by the conservation of the catalytically important amino acid residues in the three classes.
MECHANISM OF CATALYSIS
The three-dimensional structure of the important catalytic flexible loop, Lys197 to Met208 of B. subtilis PRPP synthase, Lys186 to Ala197 of the M. jannaschii enzyme, Lys188 to Ile199 of the S. solfataricus enzyme, and Lys194 to Met205 of human PRPP synthase isozyme 1, remained unresolved in studies of the structures of these enzymes (48–51, 139). However, the solution of the T. volcanium PRPP synthase structure revealed that the binding of the substrate ribose 5-phosphate and the substrate analog MgADP resulted in a closed conformation of the catalytic flexible loop, in which strands β10 and β11 move 17 Å toward the substrates and bring the important catalytic residues, Lys184 and Arg186, into proximity with ribose 5-phosphate and the triphosphate chain of the nucleotide (Fig. 6A). According to this structure, Lys184 and Arg186 bind to oxygen(s) of the β-phosphate of ATP. Additionally, Mg2+ coordinates to a nitrogen of His124 as well as to oxygens of the α- and β-phosphates of ADP and, presumably, of ATP. In the open conformation, the C-5′, the α-phosphorus, and the β-phosphorus of ADP or ATP are essentially linearly arranged, whereas in the closed conformation the β-phosphate is bent approximately 90° relative to the C-5′ and the α-phosphorus and thus locates the β-phosphorus in an ideal position for nucleophilic attack by the O-1 of ribose 5-phosphate (Fig. 6B) (138). Only a single Mg2+ was identified at the active site of T. volcanium PRPP synthase, and biochemical information as to the number of Mg2+ necessary for catalysis has not been published. This is important because dual roles for Mg2 have been identified in the reaction mechanism of other PRPP synthases.
FIG 6.
Catalytic mechanism of PRPP synthase. (A) Closure of the catalytic flexible loop of T. volcanium PRPP synthase by superimposition of the open and closed structures (PDB codes 3lrt and 3mbi, respectively). Structural elements are colored as described for Fig. 3A. A 17-Å movement of the catalytic flexible loop, consisting of the β10 and β11 strands, results in the closed conformation necessary for catalysis. (B) Close-up view of the binding of substrates at the active site of T. volcanium PRPP synthase, with open and closed catalytic flexible loops. In the open conformation, the triphosphate chain of ATP, modeled here to only ADP, forms a more or less linear arrangement. In the closed conformation, the triphosphate chain, again modeled to only ADP, bends with the β-phosphate, resulting in a position ideal for attack of O-1 of ribose 5-phosphate on the β-phosphorus. An Mg2+ of the closed conformation is shown as a black sphere (138). (C) Stereo view of the binding of ribose 5-phosphate, Mg2+, and the transition state analog AlF3 to the active site of B. subtilis PRPP synthase, AlF3 PRPP synthase. (Reproduced from reference 54 with permission.) α indicates the α-phosphate of ATP provided by an AMP molecule; β indicates the β-phosphate of ATP provided by Al3+ (bound to three F− ions); γ indicates the γ-phosphate of ATP provided by the phosphate of a second AMP molecule. The two Mg2+ are indicated by MG1 and MG2. Relevant amino acid residues His135, Asp174, Lys197, and Arg199 are included as well. (D) Stereo view of the binding of ribose 5-phosphate, α,β-methylene ATP, Mg2+, and Ca2+ to the active site of B. subtilis PRPP synthase in the GDP PRPP synthase complex. (Reproduced from reference 54 with permission.) Ca2+ (designated CA1) coordinates to the hydroxyls at C-1, C-2, and C-3 of ribose 5-phosphate, oxygen of the β- and γ-phosphates of α,β-methylene ATP, Asp174, as well as a water molecule. The Mg2+ (designated MG2) coordinates to the oxygen of C-2′ of the ribosyl moiety as well as oxygen of the α- and γ-phosphates of α,β-methylene ATP, as well as to three water molecules. Thus, there is no coordination to oxygen of the β-phosphate of α,β-methylene ATP.
Additional information was obtained from studies of two structures of activated B. subtilis PRPP synthase. In the first of these structures, a transition-state analog of phosphoryl transfer reactions, AlF3, was employed in the presence of the product AMP and the substrate ribose 5-phosphate, which generated a transition-state bound complex (AlF3 PRPP synthase structure) (Fig. 6C). Here, Al3+ represents the β-phosphorus, whereas the three F− ions represent the three oxygens of the β-phosphate, and the α- and γ-phosphates were provided by two AMP molecules as described before. In the second of these structures, the substrate analog α,β-methylene ATP and the substrate ribose 5-phosphate were employed to generate an active-state quaternary complex (mATP-R5P PRPP synthase structure). In the former structure containing AlF3, the catalytic flexible loop was closed and revealed contacts between Lys197 and Arg199 with the β-phosphoryl group of ATP, whereas in the second, the mATP-R5P structure, the catalytic flexible loop was open. Thus, the contacts between Lys197 and Arg199 observed in the AlF3 structure appear to be lost in the active-state quaternary complex (mATP-R5P), where the flexible catalytic loop remains unresolved. Thus, loop closure appears to be transient and specific for only the transition state. Al3+ of the AlF3 structure has an octahedral coordination with the C-1 oxygen of ribose 5-phoshate, the three F− ions, and also one oxygen from each of the α- and γ-phosphoryl groups. The distances from the Al3+, which corresponds to the β-phosphorus of ATP, to the C-1 oxygen of ribose 5-phosphate and the oxygen of the α-phosphoryl group are 2.0 and 1.9 Å, respectively, and thus these coordinations have partial bond character. In the AlF3 structure, Mg2+ is located on both sides of the triphosphate chain. As observed with T. volcanium PRPP synthase, the binding of Mg2+ at the MG2 site of B. subtilis PRPP synthase results in bending of the triphosphate chain of ATP. This bent conformation is caused by the tridentate conformation, α,β,γ-MgATP. Furthermore, the Mg2+ of the MG1 site coordinates to the γ-phosphate (as well as to ribose 5-phosphate and Asp174), and thus the two Mg2+ are responsible for a tight conformation of the triphosphate chain of ATP, and a perfect inline geometry of the C-1 oxygen of ribose 5-phosphate, the β-phosphate, and the α-phosphate is created. Furthermore, the bent conformation of the triphosphate chain results in electrostatic repulsion among the phosphoryl groups, whereas the coordination of Mg2+ to a nitrogen of His135 may withdraw charge from the phosphoryl groups coordinated to the Mg2+ at the MG2 site (54).
Although the Al3+-generated transition state adopts a hexacoordinated octahedral bipyrimidal structure rather than the pentacoordinated trigonal-bipyrimidal transition state expected for a phosphotransfer mechanism, the data are in agreement with an associative nucleophilic substitution, SN2, mechanism. This mechanism is supported by the perfect inline orientation of the nucleophile (the C-1 oxygen of ribose 5-phosphate), the partial bond character between the attacking nucleophile and the transferred group (represented by Al3+), and a transient charge of −2 (provided by the two “free” γ-phosphoryl oxygens and represented by fluorides). An effect of the two basic amino acid residues of the catalytic flexible loop, Lys197 and Arg199, may be stabilization of the transient negative charge. The SN2 mechanism is supported by previous studies of S. enterica PRPP synthase, which showed that the product of the reaction of ATP with [1-18O]ribose 5-phosphate was [18O]PRPP rather than [18O]AMP (66, 141).
REGULATION OF PRPP SYNTHASE ACTIVITY
All PRPP synthases analyzed, class I from microbes (S. enterica [69], B. subtilis [70], E. coli [45, 75], humans [72, 142], and rats [143, 144]), class II (spinach isozymes 3 and 4 [46, 47]), and also the archaeon M. jannaschii (48), are inhibited in a competitive manner by ADP, which binds in competition with ATP at the active site. Superimposed on the competition of ADP and ATP at the active site is allosteric inhibition by ADP. This allosteric inhibition is well documented. However, let us first mention some results of a steady-state kinetic analysis of the binding of allosteric effectors to E. coli PRPP synthase, which showed that Pi and ADP compete for binding to the same site (75). Additionally, Pi is required for the activity of the class I PRPP synthases of S. enterica (35, 56), E. coli (45), B. subtilis (70), B. caldolyticus (55), humans (73), and rats (71), as well as for the stability in the absence of MgATP of S. enterica and E. coli PRPP synthases (35, 45). Finally, Pi can be replaced as an activator by high concentrations of SO42− (49, 71, 73). Altogether, it is therefore more correct to say that that PRPP synthase is allosterically regulated with Pi as an allosteric activator and ADP as an allosteric inhibitor (75).
Allosteric inhibition of PRPP synthase by ADP was demonstrated by detailed steady-state kinetic analysis. A distinctive feature of allosteric binding by ADP is that it requires the active site to be fully occupied by both ATP or ADP and ribose 5-phosphate. This was concluded from the observation that ADP inhibition becomes stronger as the enzyme approaches saturation with ribose 5-phosphate; this is explained by the ordered binding of substrates in which ribose 5-phosphate binds last, i.e., ADP binding at the active (ATP) site induces apparent substrate inhibition by ribose 5-phosphate (69). The presence of a separate ADP-binding site was demonstrated directly by equilibrium binding dialysis. Thus, ATP (actually the unreactive analog α,β-methylene ATP) binds to a single site per molecule, the active site, in the presence of ribose 5-phosphate. In the absence of ribose 5-phosphate, ADP binds to the same site as ATP, whereas in the presence of ribose 5-phosphate ADP binds to the additional allosteric site to yield two bound ADPs per monomer (68).
The synthesis of PRPP was further studied in vivo. Purine starvation of S. enterica resulted in accumulation of PRPP, whereas purine nucleotides were depleted, suggesting that these conditions caused reduction of an inhibitor of PRPP synthesis. This inhibitor could very well be ADP (145). Also, the addition of adenine to cultures of S. enterica caused a dramatic reduction in the PRPP pool, which only slowly returned to the previous level. Adenine and PRPP react in vivo to form AMP followed by the formation ADP, which causes inhibition of PRPP synthase. Therefore, the PRPP pool only slowly returns to the level before adenine addition (146).
PRPP synthase from some organisms such as B. subtilis, B. caldolyticus, and mammals are also allosterically inhibited by GDP (55, 70, 71, 73).
Characterization of several mutants of human PRPP synthase isozyme 1 demonstrated the existence and physiological importance of the allosteric site in human PRPP synthase. The mutations were identified in the human PRPS1 gene in individuals with inherited defects in purine metabolism (72). The positions of the amino acid replacements identified, Asp51His, Asn113Ser, Leu128Ile, Asp182His, Ala189Val, and His192Gln, are indicated by arrows above the deduced B. subtilis PRPP synthase amino acid sequence in Fig. 2. The affected individuals exhibit hyperuricemia, gouty arthritis, and in some cases neurosensory defects, with an X-linked pattern of inheritance. Six different mutations in human PRPP synthase isozyme 1 have been characterized at the molecular level. The kinetics of inhibition of the purified recombinant mutant enzymes by ADP and GDP confirmed that they were much less sensitive to inhibition than the wild-type isozyme and that the pattern of inhibition by ADP with respect to ATP was altered from a complex pattern of inhibition of the wild-type enzyme to simple competition. Thus, the existence of a distinct allosteric mode of inhibition by ADP and GDP was demonstrated. The fact that desensitization to allosteric inhibition results in metabolic defects that are readily explained by overproduction of PRPP in human cells is strong evidence that this inhibition is critical for normal regulation of PRPP synthesis. The mutations that destroy allosteric inhibition in human PRPP synthase are scattered throughout the primary sequence but are localized in the three-dimensional structure almost exclusively in the interface between dimers in the hexamer.
In molecular terms, the mechanism of allosteric inhibition may be described by comparing the three-dimensional structures of various PRPP synthase complexes. Thus, the B. subtilis SO42−, ADP, GDP, and mGDP PRPP synthase complexes are characterized by tight packing of both the N- and C-terminal domains and represent an inactive form (the T-state) of the enzyme, whereas the B. subtilis AlF3 and mATP-R5P PRPP synthase complexes are less tightly packed and represent an active form (the R-state) of the enzyme. Of particular importance is a comparison of the crystal structures of the transition-state complex, AlF3 PRPP synthase, and the inhibited-state quaternary complex, mGDP PRPP synthase. The latter complex contained Mg2+, α,β-methylene ATP, and ribose 5-phosphate in the active site as well as methylene GDP in the allosteric site. The binding of ribose 5-phosphate, the adenine base of ATP, the γ-phosphate, and also Mg2+ of the MG1 site is virtually identical in the two complexes. In contrast, Mg2+ at the MG2 site was displaced, and the torsional angle of the glycosidic bond of ATP differed by a 55° rotation among the two structures, 72° (in mGDP PRPP synthase) versus 127° (in AlF3 PRPP synthase). This rotation displaces the α- and β-phosphates of the nucleotide bound to the active site and appears to prevent the inline arrangement of the C-1 oxygen of ribose 5-phosphate and the β- and α-phosphates of ATP necessary for nucleophilic attack. The important amino acid residues involved in these alterations are located within the regulatory flexible loop (Tyr97 to Thr113) (Fig. 2). Thus, for the binding of the ribose moiety of ATP, a shift in hydrogen bonding of the C-2′ oxygen and Gln102 to hydrogen bonding of the C-3′ oxygen and Glu110 occurred. Additionally, the MG2-coordinated water molecules changed from coordinating with Arg101, Asp103, and Arg104 to coordinate only with Asp103, whereas Arg104 changed to form hydrogen bonds to oxygens of the α-phosphoryl. Arg101 no longer formed hydrogen bonds in the T-state (54).
As expected from the analysis of the mutant variants of human PRPP synthase isozyme 1 described above, these altered substrate-binding capabilities are accompanied by transitions in the hexameric structure. Reciprocal interactions between two C-terminal domain amino acid residues, Val178 to Asp195, Arg198, and Asn209 to Val211 result in a tightly packed interface. This stretch of amino acid residues straddles the catalytic flexible loop (Lys197 to Met208). Particularly important is a hydrophobic pocket consisting of Ile192, Ile194, and Val211. Altogether, the interactions prevent loop closure. The binding of substrates at the active site as well as SO42− (and hence presumably Pi) at the allosteric site decouples these C-terminal amino acid interactions and permits loop closure. On the other hand, when ADP or GDP is bound at the allosteric site, interactions of the C-2′ hydroxyl of the bound ADP or GDP with Gln140, which interacts with the imidazole moiety of His149 through a water molecule and hence interacts with Leu134, results in maintaining the hydrophobic pocket and the tight C-terminal domain interaction. All these interactions are lost in the transition-state-like complex, AlF3 (54).
Site-directed mutagenesis of the B. subtilis prs gene was performed to generate mutant variants with amino acid alterations similar to the naturally occurring mutations in human PRPP synthase isozyme 1 describe above (54). As observed for the human counterparts, the kinetic properties of Pi activation among the mutant variants were generally shifted toward an increased affinity for Pi. The mutant B. subtilis enzymes displayed an approximate 10-fold decrease or more with the half-saturation concentrations for Pi compared to the wild-type form, except for the Asp57His variant (homologous to the human Asp51His variant), whose Pi dependence on activity was similar to that of the wild type, and Asp196Gln (homologous to human His192Gln), whose activity appeared independent of Pi within the range of Pi concentrations applied.
The B. subtilis PRPP synthase variants described above were also analyzed with respect to inhibition by the ribonucleoside diphosphate GDP, which is less complex than ADP inhibition, as GDP only binds to the regulatory site and not the active site. When inhibition by GDP of each of the mutant PRPP synthases was analyzed at a Pi concentration corresponding to the individual half-saturation concentration value for Pi, similar KiGDP values were obtained for the wild type and the following variants: Asn119Ser (homologous to human Asn113Ser), Leu134Ile (homologous to human Leu128Ile), Asp186His (homologous to human Asp182His), and Ala193Val (homologous to human Ala189Val). This observation indicates that PRPP synthase may represent the V-system of the Monod, Wyman and Changeux model for allosteric systems (147), where the activator (Pi) and the inhibitor (GDP) compete for binding to the same site to alter the equilibrium between the R and T conformation of the enzyme. The mutant enzymes described above that have an increased affinity for Pi all have changed equilibria between conformers that further the R-conformer compared to the wild-type enzyme.
Mapping of each of the amino acid substitutions in the structure (Fig. 2) that influence this equilibrium between the active and inactive conformer shows that three amino acid residues, Asp186, Ala193, and Asp186 are located within the region of the C-terminal domain interaction, which is important for transition of the allosteric signal, i.e., the binding of ribonucleoside diphosphate at the allosteric site. Additionally, Asn199 is located at the N-terminal interface of the bent dimer, and likewise may perturb the binding of ribonucleoside diphosphate at the allosteric signal, as described above. Finally, Leu134 is involved in the transmission of the allosteric signal. Almost all of the amino acid substitutions are located on the interface within a dimer or between dimers in the hexamer. These positions are in accordance with the existence of an equilibrium between the active and the inactive form of class I PRPP synthase that can be altered by side chain substitutions that stabilize any of the two conformers, the active R or the inactive T conformer.
A large number of mutations have furthermore been identified in the gene specifying human PRPP synthase isozyme 1 of patients suffering from relapse of acute lymphoblastic leukemia. Negative feedback-defective PRPP synthase resulted from mutations that arose after treatment with 6-mercaptopurine and 6-thioguanine as chemotherapeutics. Twenty-nine lesions affecting 14 amino acid positions have been identified. Some of these positions were already known from the previous analysis of PRPP synthase from patients with gout or uric acid overproduction, Asn113, Asp182, and Ala189. At the latter position, two variants were identified (Ala189Thr [10 patients] and Ala189Val [1 patient]). Mapping of the amino acid alterations on the three-dimensional structure of human PRPP synthase isozyme 1 revealed the location of the variations at the allosteric site (Ser102, Asn143, Thr302) or at the dimer interface (Asn113, Gly173, Lys175, Asp182, Ala189, Leu190). The conclusion from this analysis is that the cytotoxic effect of administering compounds such as 6-mercaptopurine and 6-thioguanine may be counteracted by mutations that accelerate the production of PRPP and hence accelerate the de novo synthesis of purine nucleotides, which competes with the cytotoxic compounds (148).
Fortuitously, the presence of Ca2+ during crystallization of B. subtilis PRPP synthase allowed important conclusions about the mechanism of inhibition by this ion (54). Ca2+ was identified as a hepta-coordinating metal ion at the MG1 site of the GDP PRPP synthase complex, whereas Mg2+ occupied the MG2 site (Fig. 6D). In contrast to this Mg2+/Ca2+ arrangement, two Mg2+ were found at the other active site of the asymmetric unit of the GDP PRPP synthase complex. The ligands coordinated to Ca2+ resembled those coordinated to Mg2+: Asp174, oxygens of C-1, C-2, and C-3 of ribose 5-phosphate, oxygen of the γ-phosphate, and a water molecule, with an additional seventh coordination to oxygen of the β-phosphate of ATP. In particular, the latter coordination of Ca2+ causes perturbation of the inline arrangement of the C-1 oxygen of ribose 5-phosphate and the β- and α-phosphates of ATP described above (54). Previous kinetic analysis revealed that Ca2+, which does not support PRPP synthase activity alone, is an inhibitor of PRPP synthase activity even in the presence of a very large excess of Mg2+ (56, 57).
The detailed molecular description of the regulation of PRPP formation has prompted the application of this knowledge for the improvement of commercially important products of biochemical pathways for which PRPP is an intermediate. The previously discussed improvement of riboflavin synthesis by E. gossypii harboring feedback-resistant variants of PRPP synthase is one example (112). Another example is the production of purine nucleosides by Bacillus amyloliquefaciens. The following PRPP synthase variants were constructed (with the equivalent variant of human PRPP synthase isozyme shown in parentheses): Asp58His (Asp51His), Asn119Ser (Asn113Ser), and Leu134Ile (Leu128Ile). As expected, the B. amyloliquefaciens PRPP synthase variants Asn119Ser and Leu134Ile were activated at lower Pi concentrations and were relatively more resistant to inhibition by ADP and GDP than the wild type, and indeed, both B. amyloliquefaciens and B. subtilis strains carrying the mutations specifying Asn119Ser or Leu134Ile had improved production of purine nucleosides, presumably due to increased purine de novo synthesis. Kinetic constants of B. amyloliquefaciens PRPP synthase are summarized in Table 2 (149).
PHOSPHORIBOSYL BISPHOSPHATE PHOSPHOKINASE
Mutants that are defective in the prs gene have been isolated in E. coli and S. enterica as described below. Strains with knockout mutations (Δprs) have a simultaneous requirement for five metabolites: purine and pyrimidine nucleosides, histidine, tryptophan, and NAD (2, 3). Mutants that suppress the requirement for NAD can be easily isolated. These mutants retain the other four requirements; their genetic defect maps to the pstSCAB-phoU operon (150, 151). Lesions in this operon result in constitutive expression of the Pho regulon operons, which are involved in the assimilation of Pi and the acquisition of Pi from other sources (152). Genetic analysis revealed that suppression of the NAD requirement phenotype originated from increased expression of the phnN cistron of the tetrakaidecacistronic phnC-P operon (150). The gene products of this operon are involved in the catabolism of phosphonates (152). It was furthermore shown that the phnN cistron specifies a phosphokinase with the ability to use ribosyl 1,5-bisphosphate as a substrate: ribosyl 1,5-bisphosphate + ATP → PRPP + ADP, i.e., a ribosyl bisphosphate phosphokinase (EC 2.7.4.23) (Fig. 7A). It was shown that the enzyme was able to convert ribosyl 1,5-bisphosphate to a product that functioned as a substrate for purified xanthine phosphoribosyltransferase and that the product was XMP according to the following chemical equation: xanthine + PRPP → XMP + PPi (151). Subsequently, it was shown that ribosyl 1,5-bisphosphate is an intermediate in phosphonate catabolism (153). However, ribosyl 1,5-bisphosphate is also a metabolite in cells that are not thriving on phosphonate, although the origin and metabolic role of the compound under these conditions remain unclear. Even though the reaction product of ribosyl bisphosphate phosphokinase is PRPP, the amount synthesized is insufficient to replace the activity of PRPP synthase, even in cells growing with phosphonate as their Pi source (B. Hove-Jensen, unpublished result) or in cells that express phnN in a multicopy plasmid (151). The amino acid sequences of ribosyl bisphosphate phosphokinase and PRPP synthase show only low similarity, approximately 25%. Among the amino acid residues of B. subtilis PRPP synthase with annotated functions from the crystal structure, very few, if any at all, can be recognized in the amino acid sequence of ribosyl bisphosphate phosphokinase. Likewise, the similarity of ribosyl bisphosphate phosphokinase to type I phosphoribosyltransferase and type II phosphoribosyltransferase is very low, 16% with adenine phosphoribosyltransferase (type I) and with quinolinate phosphoribosyltransferase (type II). Thus, the evolutionary origin and mechanism of phosphoribosyl bisphosphate phosphokinase remain enigmatic; a deeper understanding awaits the determination of the three-dimensional structure of the enzyme. The physiologic importance of phosphoribosyl bisphosphate phosphokinase in the context of phosphonate catabolism has been discussed and previously reviewed (154, 155).
FIG 7.
Reactions catalyzed by diphosphoryltransferases and alternative biosynthesis of PRPP. In some cases, ATP may be replaced by dATP. The diphosphoryl and phosphoryl moieties of the products are shown in red and blue, respectively. (A) Reaction catalyzed by phosphoribosyl bisphosphate phosphokinase. The substrate is ribosyl 1,5-bisphosphate, the product is PRPP. (B) Reaction catalyzed by PRPP synthase. The substrate is ribose 5-phosphate, the product is PRPP. (C) Reaction catalyzed by 2-amino-4-hydroxy-6-hydroxymethyldihydropterin diphosphokinase. The substrate is 2-amino-4-hydroxy-6-hydroxymethyldihydropterin, and the product is 2-amino-4-hydroxy-6-hydroxymethyldihydropterin diphosphate. (D) Reaction catalyzed by GTP/GDP 3′-diphosphokinase (stringent factor). R may be a hydrogen or a phosphoryl group, i.e., the substrate is GDP or GTP, respectively, and the product is guanosine 3′-diphosphate 5′-diphosphate (ppGpp) or guanosine 3′-diphosphate 5′-triphosphate (pppGpp), respectively. (E) Reaction catalyzed by nucleotide diphosphokinase. R1 may be an adenyl, a guanyl, or a hypoxanthyl univalent radical, whereas R2 may be a hydrogen, a phosphoryl, or a diphosphoryl moiety. (F) Reaction catalyzed by thiamine diphosphokinase. The substrate is thiamine, and the product is thiamine diphosphate.
DIPHOSPHORYL, NUCLEOTIDYL, AND PHOSPHORYL TRANSFER
In spite of a large number of di- or triphosphate-containing compounds, very few enzymes catalyze the transfer of an intact diphosphoryl group, i.e., attack the β-phosphorus of ribonucleoside triphosphates, in contrast to enzymes that catalyze the transfer of a phosphoryl group, i.e., attack the γ-phosphorus, and enzymes that catalyze the transfer of a nucleotidyl group, i.e., attack the α-phosphorus. These latter two groups of enzymes are much more abundant than the diphosphoryltransferases. Below, we give examples of the mechanism of catalysis of each of these three enzyme classes as a means of comparing catalytic strategies for nucleophilic substitutions on the α-, β-, and γ-phosphorus atoms of nucleoside triphosphate substrates.
Substitution Reactions at α-, β-, or γ-Phosphates
Diphosphoryl transfer.
The diphosphoryltransferases other than PRPP synthase are 2-amino-4-hydroxy-6-hydroxymethyldihydropterin diphosphokinase (ATP:2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropterin 6′-diphosphotransferase; EC 2.7.6.3) encoded by folK in E. coli (156), the stringent factor or GTP/GDP 3′-diphosphokinase (ATP:nucleoside-5′-phosphate pyrophosphotransferase; EC 2.7.6.5) encoded by relA in E. coli (157, 158), and in some organisms thiamine diphosphokinase (ATP:thiamine diphosphotransferase; EC 2.7.6.2) (159) and nucleotide diphosphokinase (ATP:nucleoside-5′-phosphate diphosphotransferase; EC 2.7.6.4) (160) (Fig. 7B to F). In addition, a number of other enzymes, primarily diphosphohydrolases, catalyze reactions with nucleophilic attacks on the β-phosphorus of (deoxy)ribonucleoside triphosphates with the release of PPi and the corresponding (deoxy)ribonucleoside 5′-monophosphate, and these are members of the so-called Nudix enzyme superfamily, as reviewed previously (161–163).
(i) Hydroxymethyldihydropterin diphosphokinase.
In addition to PRPP synthase, the crystal structure of a number of enzymes catalyzing diphosphoryl transfer reactions has been determined. These allow the comparison of the active sites and catalytic mechanism of these enzymes. Hydroxymethyldihydropterin diphosphokinase of E. coli contains 159 amino acid residues, is monomeric with a molecular mass of 18 kDa, and catalyzes the diphosphorylation of 6-hydroxymethyl 7,8-dihydropterin, the committed step in folate biosynthesis (Fig. 7C). The structure of E. coli hydroxymethyldihydropterin diphosphokinase with a variety of ligands has been determined: these include the ternary complex with a substrate analog and a substrate, enzyme-Mg2+-α,β-methylene ATP-hydroxydihydropterin complex (PDB code 1q0n) (164). Additional reports on crystal or solution structures of hydroxymethyldihydropterin diphosphokinase of E. coli and Haemophilus influenzae have been published (165–167). Structural and mechanistic properties of this enzyme have been previously reviewed (168). Only 23 amino acid residues are identical between E. coli hydroxymethyldihydropterin diphosphokinase and PRPP synthase, and the folds of the two polypeptides are very different. The former enzyme adopts a fold comprised of four α-helices and six β-strands, β1α1β2β3α2β4β5β6α3α4. Substrate recognition is mediated by three loop regions located between β1 and α1 (loop L1), between β2 and β3 (loop L2), and between α2 and β4 (loop L3). ATP binds first to hydroxymethyldihydropterin diphosphokinase, which results in large structural changes in the three loops, in particular L2 and L3, and allows the binding of the second substrate, hydroxymethyldihydropterin (164, 169, 170). Furthermore, two Mg2+ are bound within the active site (Fig. 8A). One Mg2+, MgA, coordinates to oxygens of the α- and β-phosphates of ATP, to Asp95 and Asp97 as well as to two water molecules. A second Mg2+, MgB, coordinates to oxygens of the β- and γ-phosphates of ATP, to the hydroxyl of the substrate hydroxymethyldihydropterin, to Asp95 and Asp97, as does the first Mg2+, and finally to a water molecule. MgB thus coordinates both substrates. This arrangement of Mg2+, substrate, and amino acid residues places the hydroxymethyldihydropterin hydroxyl at a distance of 3.2 Å from the β-phosphorus of ATP (164). The triphosphate chain of ATP and the hydroxyl of hydroxymethyldihydropterin are organized in a head-to-head arrangement, much like that of ribose 5-phosphate and ATP of PRPP synthase, with the γ-phosphate “bent” away rather than in a linear arrangement, which allows attack of the hydroxymethyldihydropterin hydroxyl on the β-phosphate of ATP (Fig. 8A).
FIG 8.
Diphosphoryl, nucleotidyl, and phosphoryl transfer reactions. Wat, water molecules. (A) The active site of the ternary complex of E. coli hydroxymethyldihydropterin diphosphokinase. AMPCPP, α,β-methylene ATP; HP, hydroxydihydropterin (PDB code 1q0n) (164). (B) The active site of DNA polymerase β (PDB code 1bpy) (176). (C) The active site of cAMP protein kinase A. AMPPNP, β,γ-imido ATP (PDB code 4hpu) (201).
(ii) Thiamine diphosphokinase.
Thiamine diphosphate is the active cofactor in a number of C—C bond formation and scission processes (171). Some organisms synthesize thiamine diphosphate by diphosphoryl transfer of the β,γ-diphosphoryl group of ATP to thiamine (Fig. 7F), whereas others phosphorylate thiamine phosphate in a single phosphoryl transfer reaction. The crystal structure of mouse thiamine diphosphokinase, which utilizes the former mechanism, has been solved; the enzyme structure contains AMP and pyrithiamine diphosphate, i.e., the products of the reaction of ATP with pyrithiamine, a thiamine analog which contains a substituted pyrimidine moiety rather than a substituted thiazole moiety. The structure also contains one Mg2+, which coordinated with oxygens of the AMP-phosphate to the α-phosphate of pyrithiamine diphosphate and to the carboxyls of Asp46, Asp71, Asp73, and Asp100 (PDB code 2f17). The coordination of Mg2+ to the α-phosphate of pyrithiamine diphosphate is consistent with (but does not demonstrate) a bent structure of the triphosphate chain of the substrate ATP, as was observed with PRPP synthase and hydroxymethyldihydropterin diphosphokinase (172). However, those authors also suggested that the data permitted a linear arrangement of the triphosphate chain and a more or less perpendicular orientation of the pyrithiamine, with the hydroxyl group oriented for nucleophilic attack on a β-phosphate oxygen (172). The structures of thiamine diphosphokinases from a few other organisms have been solved. These include the structure of the Streptococcus mutans enzyme with thiamine diphosphate and two Mg2+ present but without an adenylyl moiety present (PDB code 3ihk), Candida albicans with thiaminyl imidodiphosphate present (173), and S. cerevisiae with thiamine present (PDB code 1ig0) (174). The exact architecture of the active site with substrates and Mg2+ cannot presently be clearly established.
(iii) GTP/GDP 3′-diphosphokinase.
The structure of the third diphosphoryltransferase, GTP/GDP 3′-diphosphokinase (Fig. 7D), is poorly characterized, presumably because the C terminus associates with the ribosome. This association may hamper crystallization because that region may be disordered when not bound. However, important conclusions have been obtained from the studies of the bifunctional RelA/SpoT homolog (RelSeq) of Streptococcus dysgalactiae subsp. equisimilis, in which the diphosphoryltransferase (RelA) and diphosphohydrolytic (SpoT) activities are located in the same polypeptide but in different domains (175). The diphosphoryltransferase domain of RelSeq bears some structural resemblance to the so-called palm domain of human DNA polymerase β, in which two Mg2+ intricately position the substrates, primer, and deoxyribonucleoside triphosphate through coordination to the 3′-hydroxyl of the nucleophilic primer and to oxygens of each of the α-, β-, and γ-phosphates of the incipient deoxyribonucleoside triphosphate, and by means of coordination to three invariably conserved aspartate residues (Asp190, Asp192, and Asp256) (176). Unlike DNA polymerase β and PRPP synthase, RelSeq appears to carry out catalysis by the assistance of only a single Mg2+. Thus, the dependence of the diphosphoryl transfer reaction on the Mg2+ concentration parallels the ribonucleoside triphosphate concentration; that is, there is no stimulation of diphosphoryl transfer activity by excess Mg2+ (177). RelSeq contains only two of the three acidic residues: Asp264, which is homologous to Asp190 of DNA polymerase, and Glu323, which is homologous to Asp256. The lack of a homolog of the third aspartate residue may imply an inability to bind a second Mg2+ (175). However, this view that GTP/GDP 3′-diphosphokinase requires only a single Mg2+ has been challenged. The activity of monofunctional E. coli GTP/GDP 3′-diphosphokinase is stimulated by Mg2+ in excess of the ribonucleoside triphosphate (ATP and GTP) concentration; this is unlike the activity of the RelA/SpoT bifunctional M. tuberculosis GTP/GDP 3′-diphosphokinase, which was inhibited at increased Mg2+ concentrations. Analysis of mutant variants of these two GTP/GDP 3′-diphosphokinases revealed the importance of the amino acid sequence 306-Glu-Any-Asp-Asp-309 in E. coli GTP/GDP 3′-diphosphokinase (homologous to 348-Arg-Any-Lys-Asp-351 in M. tuberculosis GTP/GDP 3′-diphosphokinase). After swapping these motifs, the activity of E. coli GTP/GDP 3′-diphosphokinase is inhibited by excess Mg2+, whereas the activity of M. tuberculosis GTP/GDP 3′-diphosphokinase chimera is stimulated by Mg2+ (178). It is possible that the mechanism of diphosphoryl transfer of bifunctional GTP/GDP 3′-diphosphokinases, such as those of S. dysgalactiae or M. tuberculosis, may deviate from that of PRPP synthase and hydroxymethyldihydropterin diphosphokinase by the apparent requirement of only a single Mg2+, whereas the mechanism of diphosphoryl transfer of monofunctional GTP/GDP 3′-diphosphokinases, such as that of E. coli, may resemble other diphosphoryltransferases in requiring two Mg2+ for activity.
(iv) MutT.
For the sake of completeness, we include a brief description of one member of the Nudix hydrolases, MutT, encoded by the mutT gene in E. coli. Like the diphosphoryltransferases, MutT catalyzes a nucleophilic substitution reaction on the β-phosphorus but uses water as the nucleophile. MutT contains 129 amino acid residues, has a molecular mass of 15 kDa, and is active as a monomer. The solution structure has been determined (179). The enzyme is also designated 8-oxo-dGTP diphosphohydrolase, because 8-oxo-dGTP appears to be the best substrate in vitro and is probably also the most important substrate in vivo. Other (deoxy)ribonucleoside triphosphates are also hydrolyzed, albeit at much higher Km values (180). 8-oxo-dGTP results from oxidation of dGTP and may be misincorporated into DNA opposite of deoxyadenylate residues, which would result in AT → GC transversions (181). The frequency of such transversions increased by 100- to 1,000-fold in a mutT strain (182). Like PRPP synthase and other diphosphoryltransferases, MutT requires two divalent metal ions (Mg2+ or Mn2+, with a preference for Mg2+) for activity. One ion is enzyme bound, and the other is bound to the (deoxy)ribonucleoside triphosphate. The enzyme-bound Mg2+/Mn2+ coordinates to the nucleophilic water, to Gly38, Glu56, Glu57, and Glu98 and to a second water molecule, whereas the nucleotide-bound Mg2+/Mn2+ coordinates to oxygens of the β- and γ-phosphates (162, 183). Catalysis involves the abstraction of a proton of the nucleophilic water by Glu53, followed by attack on the β-phosphorus and displacement of 8-oxo-dGMP and MgPPi (162). The biochemistry, physiology, and genetics of MutT as well as other members of the Nudix enzyme family have been previously reviewed (161, 162).
Nucleotidyl transfer.
Nucleotidyltransferases catalyze reactions of the following type: acceptor + (deoxy)ribonucleoside-P-P-P → acceptor-P-(deoxy)ribonucleoside + PPi. Examples include reactions catalyzed by nucleic acid polymerases (DNAn + dNTP → DNAn+1 + PPi, or RNAn + NTP → RNAn+1 + PPi,) or glucose 1-phosphate uridylyltransferase (glucose 1-phosphate + UTP → UDP-glucose + PPi). Indeed, nucleic acid polymerases and sugar phosphate nucleotidyltransferases (sometimes called sugar nucleoside diphosphate diphosphorylases) are the most prominent members of this class of enzymes. These reactions have substitutions with nucleophilic attack on the α-phosphorus of ribonucleoside triphosphate or deoxyribonucleoside triphosphate in common. The structure of the active sites of RNA and DNA polymerases are quite similar. Here, we return to the human DNA polymerase β, briefly described above, to further describe the active site and the mechanism of catalysis, which involves the intricate positioning of two Mg2+ by means of coordination to three conserved aspartate residues, as originally suggested by Steitz and Steitz for DNA polymerase I and other nucleic acid polymerases (184). Figure 8B shows the binding of the two Mg2+ to the active site of DNA polymerase β. MgA coordinates to oxygens of the α-, β-, and γ-phosphates to Asp190 and Asp192 and to a water molecule, whereas MgB coordinates to an oxygen of the α-phosphate to the two carboxylate oxygens of Asp190, and to Asp192 and Asp256. The sixth coordination of MgB was not detected. Presumably, this coordination normally involves the 3′-hydroxyl of the deoxyribosyl that receives the nucleotidyl moiety. This 3′-hydroxyl is missing in 2′,3′-dideoxyribonucleotides and, possibly, the crystal packaging does not allow space for a water molecule (176). Similar geometric arrangements are found in other DNA polymerases, such as DNA polymerase II (185). Extensive reviews of the structure and function of DNA polymerase β and other DNA polymerases have been previously published (186, 187). The binding of two Mg2+ by a triad of aspartate residues is found also in RNA polymerases. Reviews of the importance of Mg2+ in DNA and RNA synthesis have been previously published (188, 189).
An example of a sugar phosphate nucleotidyltransferases is the glmU gene product, a bifunctional enzyme that catalyzes the acetylation of 2-amino-glucose 1-phosphate and the uridylylation of N-acetyl-2-aminoglucose 1-phosphate to yield UDP-N-acetylglucosamine. The two activities are located in different domains, with the uridylyltransferase allocated to the N-terminal domain (190). UDP-N-acetylglucosamine is a precursor of cell wall synthesis in many bacterial and fungal species as well as a substrate in glycosylation reactions in eukaryotic species (191). The crystal structure of GlmU of M. tuberculosis contains two Mg2+. One (MgA2+) coordinates to Asn239 and Asp114, to an oxygen of the phosphate of N-acetylglucosamine 1-phosphate, and to an oxygen of the α-phosphate of UTP, and thus MgA2+ bridges the two substrates. The other (MgB2+) coordinates to oxygens of the α-, β-, and γ-phosphates of UTP and to three water molecules. Thus, MgB2+ apparently does not coordinate to any amino acid residue (and, formally, there is no MgB2+-binding site). Rather, the uridylylation domain of GlmU appears to harbor a single enzyme-bound Mg2+ (MgA2+), whereas the second Mg2+ enters as MgUTP during the catalytic cycle. This two-Mg2+-mediated mechanism is believed to be general in sugar nucleotide synthesis (192), as similar active site arrangements are observed for 2-keto-3-deoxymanno-octulonate cytidylyltransferase of E. coli (PDB code 3k8d) (193), glucose 1-phosphate uridylyltransferase of Corynebacterium glutamicum (PDB code 2pa4) (194), 2-C-methylerythritol 4-phosphate cytidylyltransferase of Mycobacterium smegmatis (PDB code 2xwl), and M. tuberculosis (PDB code 2xwn) (195).
An exception to this two-Mg2+-mediated mechanism is N-acetylglucosamine uridylyltransferase of C. albicans, which contains only a single Mg2+, presumably coordinated with oxygens of the α-, β-, and γ-phosphates of UTP, i.e., MgB2+ in analogy with M. tuberculosis GlmU. Furthermore, the function of MgA2+ may be performed by Lys421, because the ε-N of this residue coordinates oxygens of the α- and β-phosphates of UDP-N-acetylglucosamine after product formation and, thus, it also presumably coordinates to oxygens of the phosphate of N-acetylglucosamine and of the α-phosphate of UTP during catalysis. Arg116 may also participate in the function performed by MgA2+ with other sugar phosphate nucleotidyltransferases (196).
Phosphoryl transfer.
In general, enzymes catalyzing phosphoryl transfer reactions also require two Mg2+, as demonstrated from studies of Pyrococcus furiosus UMP kinase (PDB code 2bmu) (197), Staphylococcus aureus tagatose 6-phosphate kinase (PDB code 2jg1) (198), Bifidobacterium longum N-acetylhexosamine kinase (PDB code 4wh3) (199), mammalian cyclin-dependent kinase 2 (PDB code 3qhw) (200), or cyclic AMP (cAMP)-dependent protein kinase A (PDB codes 1l3r and 4hpu) (201, 202). We shall use protein kinase A for a more detailed description. Crystals of the enzyme were obtained as a complex with reaction-inert β,γ-imido ATP, Mg2+, and a serine-containing peptide substrate. Of the two Mg2+, MgA2+ coordinates to oxygens of the α- and γ-phosphates, to the bridging N of the β- and γ-phosphates of β,γ-imido ATP, to Asn171 and Asp184, as well as to a water molecule, whereas the other, MgB2+, coordinates to oxygens of the β- and γ-phosphates, to both of the oxygens of Asp184, as well as to two water molecules (Fig. 8C). Unlike the examples of diphosphoryl and nucleotidyl transfer reactions described above, there is apparently no coordination between Mg2+ and the nucleophilic hydroxyl of the serine residue of the peptide substrate. However, this oxygen appears to be held firmly in place by hydrogen bonding to Lys168 and Asp166 (202).
The number of Mg2+ involved in kinase-catalyzed reactions may vary from enzyme to enzyme. Typically, nucleoside diphosphokinases contain only a single Mg2+. Adenylate kinase catalyzes the reversible reactions AMP + ATP → 2 ADP or dAMP + ATP → dADP + ADP (203). The enzyme contains a loop, which closes upon substrate binding and thus prevents nucleophilic attack by water on the ATP triphosphate chain. The structure of the E. coli enzyme has been probed with β,γ-imido ATP (PDB code 1ank). The phosphoryl group of AMP and the phosphate chain of β,γ-imido ATP face one another in an almost colinear arrangement. One oxygen of the phosphoryl group of AMP is in close proximity to the γ-phosphorus of β,γ-imido ATP, and thus it is ideally placed for an inline attack with the subsequent formation of ADP (204). This colinear structure of the phosphoryl groups resembles the structure of adenylate kinase ligated to the inhibitor di(adenosine-5′)pentaphosphate, except for the presence of an additional phosphoryl group (205). Mg2+, which is necessary for adenylate kinase activity, does not coordinate to side chains of the enzyme but rather coordinates to two oxygens of di(adenosine-5′)pentaphosphate as well as to four water molecules (206). This binding of Mg2+ to di(adenosine-5′)pentaphosphate is consistent with β,γ-MgATP as the substrate for adenylate kinase (207) and Mg2+ coordination to oxygens of both of the β-phosphates of two ADP molecules of M. tuberculosis adenylate kinase, i.e., the reaction in the direction of ATP synthesis (PDB code 2cdn) (208). Furthermore, in comparison with diphosphoryl and nucleotidyl transfer reactions described above, adenylate kinase may represent an alternative means of performing the function played by the second Mg2+ in other phosphotransferases. The stabilizing and neutralizing effect of a second Mg2+ may be replaced by one or more arginine and/or lysine residues, such as those found near the triphosphate chain and AMP in adenylate kinase (PDB codes 1ank and 2cdb) (204, 208).
Catalytic strategies.
The triphosphate chain of (deoxy)ribonucleoside triphosphates is quite electron rich and, hence, negatively charged, so that chemical reactions involving this chain require special conditions. In addition, an activated, i.e., deprotonated, nucleophile may contribute additional negative charge to the transition state. As described above, these difficulties are circumvented at least in part by the presence of Mg2+ in the active site. Figure 9 shows a schematic representation of part of the active sites of enzymes or groups of enzymes that catalyze substitution reactions at the α-, β-, or γ-phosphates of (deoxy)ribonucleoside triphosphates and that contain two Mg2+. The triphosphate chain, the coordination of each Mg2+, and the nucleophile are shown for each enzyme in Fig. 9. Additional amino acid residues may be involved in the binding of the substrates but are not shown in Fig. 9. The two Mg2+ may have multiple functions that include exact positioning of the substrates for transition-state formation, electrostatic stabilization of the nucleophile and the transition state, and electrostatic stabilization of the product(s) or leaving group(s). As described before for PRPP synthase, one Mg2+ (MgB [Fig. 9]) presumably is involved in the stabilization of the nucleophile, i.e., the acceptor of the diphosphoryl group, the C-1 hydroxyl of ribose 5-phosphate. Similarly, MgB of nucleotidyltransferases may participate in stabilization of the nucleophiles, i.e., hexose phosphate (Fig. 9C) and deprotonated DNA primer (Fig. 9D) (189). In contrast, no coordination is observed between MgB and the γ-phosphate-attacking nucleophilic serine residue in protein kinase A (Fig. 9A), and nucleophile positioning involves amino acid residues. As in the case of MgB and PRPP synthase, MgB of nucleotidyltransferases binds the nucleophile and may stabilize the deprotonated nucleophile (Fig. 9C and D). Interestingly, MgB of PRPP synthase and the nucleotidyltransferases coordinate both of the substrates, i.e., ribose 5-phosphate and ATP in PRPP synthase (Fig. 9B), N-acetylglucosamine 1-phosphate and UTP in N-acetylglucosamine 1-phosphate uridylyltransferase (Fig. 9C), and the DNA template and deoxyribonucleoside triphosphate in DNA polymerases (Fig. 9D). The γ-phosphate of nucleoside triphosphates carries two negative charges at physiologic pH, whereas the α- and β-phosphates carry one negative charge each. These charges are neutralized by Mg2+. The second Mg2+, MgA, in the enzymes under discussion typically binds the substrate (deoxy)ribonucleoside triphosphate. Thus, the form bound to the enzyme in all the cases shown appears to be the tridentate α,β,γ-MgATP. The MgA therefore not only partly neutralizes the triphosphate chain but also provides the proper conformation of the substrate to the enzyme. The general belief is that the functioning of MgA includes a stabilization of the transition state. It is noteworthy that the two MgA and MgB are localized on both sides of the triphosphate chain, except in the nucleic acid polymerases, where the two Mg2+ are located on the same side of the triphosphate chain.
FIG 9.
Catalytic strategies. The coordination of Mg2+ (shown as blue spheres) of the active site to the substrates, to amino acid residues, and to water molecules (shown as red spheres) is schematically illustrated by blue lines. (A) cAMP-dependent protein kinase A (PDB code 4hpu) (201). (B) PRPP synthase. R5P, ribose 5-phosphate. MgA was designated MG2, and MgB was designated MG1 before (54). (C) Hexose 1-phosphate nucleotidyltransfrease, based on the structure of N-acetylglucosamine 1-phosphate uridylyltransferase (PDB code 4g87). MgB coordinates to aspartate and asparagine residues (192). (D) DNA polymerase based on the structure of DNA polymerase β (PDB code 1bpy) (176). The three acidic residues are usually aspartates, but occasionally one aspartate is replaced by a glutamate (185, 186). The structure is valid also for RNA polymerases (187).
UTILIZATION OF PRPP
The concentration of PRPP is approximately 0.5 mM in cells of S. enterica growing exponentially in minimal salts medium with glucose as carbon source. This value can be compared to the concentrations of ATP, GTP, UTP and CTP, which are 3, 0.9, 0.9, and 0.5 mM, respectively (19). PRPP is utilized in nucleotide, amino acid, and cofactor biosynthetic pathways. The relative consumption of PRPP for each of these processes in E. coli has been estimated. Thus, purine and pyrimidine nucleotide biosyntheses each consume 30 to 40% of the PRPP synthesized, whereas histidine and tryptophan biosyntheses each consume 10 to 15% of the PRPP synthesized, and NAD and NADP biosyntheses consume approximately 1% of the PRPP synthesized (1).
Reactions at the Anomeric Carbon of PRPP
The reactions occurring at the anomeric carbon of PRPP greatly outnumber the reactions occurring at the diphosphoryl chain. The reactions at the anomeric carbon are catalyzed by phosphoribosyltransferases, whereas hydrolases catalyze the reactions at the diphosphoryl chain. Phosphoribosyltransferases catalyze reactions in which N-, C-, or O-glycosidic bonds are formed with the concomitant formation of PPi (Fig. 1). We shall review first here the reactions occurring at the anomeric carbon and then the reactions occurring at the diphosphate chain.
Comparison of phosphoribosyltransferase and PRPP synthase structures.
The structure of phosphoribosyltransferases vary to some extent. The most widespread structure, present in the so-called type I phosphoribosyltransferases, consists of a core of a five-stranded β-sheet surrounded by a number of helices and additionally, a loop structure, a hood, which participates in the binding of the nucleobase substrate, and a (catalytic) flexible loop, which remains unresolved until the binding of PRPP, when it closes the active site for catalysis (209, 210). The type I phosphoribosyltransferases utilize purine or pyrimidine bases or glutamine-derived ammonia as the phosphoribosyl acceptor. Remarkably, in spite of only approximately 20% amino acid sequence identity among type I phosphoribosyltransferases and PRPP synthase, the three-dimensional structures of type I phosphoribosyltransferases strongly resemble those of each domain of PRPP synthase, as shown in Fig. 10A for T. volcanium PRPP synthase and Toxoplasma gondii hypoxanthine-guanine phosphoribosyltransferase. We shall use the T. gondii hypoxanthine-guanine phosphoribosyltransferase as a structural “prototype” of type I phosphoribosyltransferases, as many of the structural features of this enzyme apply to most other type I phosphoribosyltransferases as well. The homology of the central five-stranded β-sheet and the surrounding helices is evident in both structures, as is also the homologous position of the catalytic flexible loop of PRPP synthase and the flexible loop of the phosphoribosyltransferase.
FIG 10.
Similarity of the folds of the PRPP synthase domain and type I phosphoribosyltransferases. (A) Superimposition of the C-terminal domain (amino acids 151 to 286) of T. volcanium PRPP synthase (blue; PDB code 3mbi) (138) and T. gondii hypoxanthine-guanine phosphoribosyltransferase (green; PDB code 1fsg) (212). The PP, ribose 5-phosphate (R5P), and catalytic flexible (CF) loops are indicated. (B) Substrate binding at the active sites of B. subtilis PRPP synthase and T. gondii hypoxanthine-guanine phosphoribosyltransferase. (Reproduced from reference 54 with permission.) The B. subtilis PRPP synthase transition-state analog (Fig. 6C) consisting of the phosphoryl moiety of AMP (α), AlF3 (β), the phosphoryl moiety of a second AMP molecule (γ), and ribose 5-phosphate, as well as Asp223 and Asp224 are shown as thick lines, and the two Mg2+ are shown as green spheres (54). Residues from the structure of T. gondii hypoxanthine-guanine phosphoribosyltransferase in complex with 9-deazaguanine (not shown), PRPP, and Mg2+, as well as Glu146 and Asp147 (PDB code 1fsg) (212), are shown as thin yellow lines or spheres superimposed on the AlF3 PRPP synthase structure.
A comparison of amino acid residues of PRPP synthase and various type I phosphoribosyltransferases reveals only a single conserved region, a series of hydrophobic residues, usually three, followed by one or two aspartate residues, two or three hydrophobic residues, and a four-residue sequence characteristic of sharp turns in the polypeptide chain, such as Thr-Gly-Gly-Thr (44, 45, 211). This region in B. subtilis PRPP synthase (216-Gly-Lys-Thr-Ala-Ile-Leu-Ile-Asp-Asp-Ile-Ile-Asp-Thr-Ala-Gly-Thr-232) is the ribose 5-phosphate-binding loop described above (45), whereas the corresponding region in T. gondii hypoxanthine-guanine phosphoribosyltransferase (139-Asp-Lys-His-Val-Leu-Ile-Val-Glu-Asp-Ile-Val-Asp-Thr-Gly-Phe-Thr-154) is the PRPP-binding loop (212). Additionally, the PP loop 79-Lys-Glu-80 of T. gondii hypoxanthine-guanine phosphoribosyltransferase and amino acid residues of the flexible catalytic loop (residues 114 to 134 in T. gondii hypoxanthine-guanine phosphoribosyltransferase) are involved in the binding of PRPP or MgPRPP in other phosphoribosyltransferases; collectively, these sequences constitute the PRPP-binding site (209, 210). The three-dimensional structures of the ribose 5-phosphate-binding loop of PRPP synthase and the PRPP-binding loop of phosphoribosyltransferases are very much alike, as shown in Fig. 10B, which shows a superimposition of parts of the active site of B. subtilis PRPP synthase and T. gondii hypoxanthine-guanine phosphoribosyltransferase: the binding of ribose 5-phosphate, the β- and γ-phosphates of ATP, and two Mg2+ at the active site of B. subtilis PRPP synthase and the binding of PRPP and Mg2+ at the active site of T. gondii hypoxanthine-guanine phosphoribosyltransferase (54, 212).
N-Glycosidic bond formation.
By far the most abundant reactions involving PRPP are formation of N-glycosidic bonds. The prototype of reactions involving PRPP is shown in Fig. 1, and examples of the products containing carbons of PRPP are shown in Fig. 11. A nitrogen-containing compound displaces the diphosphoryl group of PRPP in a nucleophilic reaction with inversion of the anomeric carbon (for example, adenine + PRPP → AMP + PPi). In general, the nitrogen-containing compounds are aromatic bases. In purine and pyrimidine nucleotide biosynthesis, the ribosyl group of PRPP remains the ribosyl or deoxyribosyl group of the nucleotides. Similarly, in cofactor (NAD and NADP) biosynthesis, the ribosyl group of PRPP remains the ribosyl group of the cofactors. In contrast, in tryptophan and histidine biosynthesis, only some of the ribosyl carbons are built into the products. Thus, in tryptophan biosynthesis, C-2 and C-3 of the indolyl moiety originate from C-1 and C-2, respectively, of the ribosyl moiety of PRPP, whereas five of the six carbons of histidine originate from the ribosyl moiety of PRPP with the carboxyl group originating from C-5 of PRPP. The nitrogen-containing compounds utilized by phosphoribosyltransferases may be the purine bases adenine, hypoxanthine, xanthine, or guanine, the pyrimidine bases may be orotate or uracil, the pyridine bases may be quinolinate or nicotinate, or the bases may be anthranilate or ATP. A variant among phosphoribosyltransferases, amidophosphoribosyltransferase, the first enzyme of de novo purine biosynthesis, utilizes glutamine-derived ammonia. Altogether, 10 enzymes utilize PRPP for N-glycosidic bond formation, with 11 products in organisms, including E. coli and B. subtilis (Fig. 11).
FIG 11.
N-Glycosidic bond formation with PRPP. PRPP and atoms of the products derived from PRPP are shown in red. Each reaction produces an N-glycosidic 5′-phosphoribosyl compound and PPi. The reaction with quinolinate also produces carbon dioxide. The compounds a to e are products of de novo reactions: a, 5-phosphoribosyl 1-amine; b, orotidine 5′-monophosphate; c, 5′-phosphoribosylnicotinate; d, 5′-phosphoribosyl-ATP; e, 5′-phosphoribosylanthranilate. The compounds f to k are products of salvage reactions: f, AMP; g, IMP; h, XMP; i, GMP; j, UMP; k, 5′-phosphoribosylnicotinate.
As we shall see below, PRPP is a very versatile compound for the delivery of phosphoribosyl moieties. A large number of phosphoribosyltransferases have been described. Although they all use PRPP as phosphoribosyl donor and, presumably, they follow similar chemical mechanisms, they differ widely in their three-dimensional structures. The enzymes range from the type I phosphoribosyltransferases, which are primarily involved in purine and pyrimidine nucleotide biosynthesis, type II phosphoribosyltransferases, which are involved in pyridine nucleotide biosynthesis, to type III and type IV phosphoribosyltransferases, which are involved in histidine and tryptophan biosynthesis, respectively. The three-dimensional structures of a large number of phosphoribosyltransferases have been determined. Detailed reviews on the biochemistry of phosphoribosyltransferases have been published (209, 210, 213).
(i) Purine nucleotide biosynthesis.
Nucleotide biosynthesis occurs via two sets of reactions: de novo reactions, in which nucleotides are generated by the addition of atoms one by one to the starting material, and the so-called salvage or auxiliary reactions, by which preformed nucleobases are utilized for nucleotide biosynthesis.
(a) Purine de novo synthesis.
De novo purine nucleotide biosynthesis commences with PRPP. The purine ring is built on the phosphoribosyl moiety, atom by atom, beginning with the addition to C-1 of the ribosyl moiety of the nitrogen of glutamine-derived NH3, which subsequently becomes N-9 of the complete purine moiety. This reaction is catalyzed by amidophosphoribosyltransferase (5-phospho-β-d-ribosylamine:diphosphate phospho-α-d-ribosyltransferase [glutamate-amidating]; EC 2.4.2.14), which is encoded by purF in E. coli: glutamine + PRPP + H2O → glutamate + 5-phosphoribosyl 1-amine + PPi (Fig. 11, compound a) (214). Amidophosphoribosyltransferase contains a domain that catalyzes the hydrolysis of glutamine to form glutamate and ammonia. Subsequently, the ammonia is transferred through a channel 20 Å long to the phosphoribosyltransferase domain active site, where PRPP is already positioned for further reaction (215). The process of ammonia channeling in amidophosphoribosyltransferases has been previously reviewed (216). Although it is unusual for a phosphoribosyltransferase to have an additional enzyme activity, such as glutaminase, amidophosphoribosyltransferase has a typical type I phosphoribosyltransferase fold with the five-stranded β-sheets surrounded by two helices on either side, a hood, a catalytic flexible loop, as well as an MgPRPP-binding site consisting of the PRPP-binding loop and a PP loop. The enzyme binds PRPP in a manner similar to that described above for T. gondii hypoxanthine-guanine phosphoribosyltransferase (Fig. 10B).
(b) Purine salvage synthesis.
The purine salvage or auxiliary reactions consist of several enzymes that phosphoribosylate adenine, or the 6-oxopurines hypoxanthine, xanthine, and guanine. The products of these phosphoribosylation reactions are AMP, IMP, XMP, and GMP, respectively (Fig. 11, compounds f to i). In general, in most organisms there is a single phosphoribosyltransferase that is specific for adenine and at least one additional phosphoribosyltransferase with specificity toward 6-oxopurine. E. coli and S. enterica both contain an apt-specified adenine phosphoribosyltransferase, hpt-specified hypoxanthine phosphoribosyltransferase, and gpt-specified xanthine-guanine phosphoribosyltransferase (19), whereas S. solfataricus contains a pgT-1-specified adenine phosphoribosyltransferase and a pgT-2-specified 6-oxopurine phosphoribosyltransferase with affinity for hypoxanthine, xanthine, and guanine (217). The purine phosphoribosyltransferase-catalyzed reactions are responsible for the utilization of the purine compounds adenine, hypoxanthine, xanthine, and guanine as purine sources for purine auxotrophic strains.
The specificities of purine phosphoribosyltransferases are not readily deduced from their amino acid sequences. The PRPP-binding loop of adenine phosphoribosyltransferase (EC 2.4.2.7) (adenine + PRPP → AMP + PPi) of E. coli is 126-Asp-Asp-Leu-Leu-Ala-Thr-131. The sequence Asp-Asp-hyd-hyd-Ala-Thr (hyd indicates hydrophobic residues) is found in a large majority of adenine phosphoribosyltransferases. An exception is S. solfataricus adenine phosphoribosyltransferase, whose sequence is 95-Asp-Asp-Ile-Thr-Asp-Thr-100. On the other hand, hypoxanthine and hypoxanthine-guanine phosphoribosyltransferases contain the homologous sequence Glu-Asp-Ile-Ile-Asp-Thr/Ser. That is, a glutamate residue replaces the first acidic residue of the Asp-Asp dipeptide and the underlined Asp replaces an alanine residue of adenine phosphoribosyltransferase, exemplified by Ala130 of the E.coli adenine phosphoribosyltransferase. E. coli gpt-specified xanthine-guanine phosphoribosyltransferase instead has the sequence 88-Asp-Asp-Leu-Val-Asp-Thr-93, i.e., an Asp-Asp dipeptide as in adenine phosphoribosyltransferase and Asp92 as in oxopurine phosphoribosyltransferase (217).
The lengths of the adenine phosphoribosyltransferases show some heterogeneity. The enzyme has a basal size of approximately 180 amino acid residues, as seen in the enzymes from E. coli, the purine de novo synthesis-lacking protozoan parasite Giardia lamblia, S. cerevisiae, and humans, whereas the enzymes from the protozoan parasite Leishmania donovani and the archeon S. solfataricus have 237 and 210 amino acid residues, respectively. Nevertheless, all of them share the common core structure of type I phosphoribosyltransferases (218–220). The additional amino acid residues of S. solfataricus and L. donovani adenine phosphoribosyltransferases protrude from both the N and C termini relative to the 180-amino-acid adenine phosphoribosyltransferases and may contribute to structural variations of the hood (221, 222). The structures have been determined with the apo-forms or with adenine or AMP bound in the active site. Particularly interesting is the structure of the G. lamblia enzyme with the purine analog 9-deazaadenine and MgPRPP bound at the active site. In this structure, the hydroxyls of the ribose moiety coordinate to Mg2+ and the 124-Glu-Asp-125 acidic dipeptide, whereas the 5-phosphate is bound to the 128-Ala-Thr-Gly-Gly-Thr-132 subset of the PRPP-binding loop, and the diphosphate chain ligates to Mg2+ and to Arg63, which is part of the PP loop. Superimposition of the 9-deazaadenine and MgPRPP-containing structure and an AMP-containing structure revealed a 2.1 Å movement of the anomeric carbon of PRPP during catalysis, whereas the 5- or 5′-phosphate and adenine moieties essentially remained fixed (218).
As already mentioned, the specificity of phosphoribosyltransferases with affinity for 6-oxopurine varies depending on the organism. Any given 6-oxopurine phosphoribosyltransferase has some affinity for all three 6-oxopurines (hypoxanthine, xanthine, and guanine), but in general has a preference for one or two of the compounds. The discrimination between 6-amino and 6-oxo purines is based on hydrogen bonding to the nitrogen versus oxygen of the purine ring (209, 213). Thus, the allocation of a phosphoribosyltransferase to the EC 2.4.2.8 (hypoxanthine phosphoribosyltransferase) class as opposed to the EC 2.4.2.22 (xanthine phosphoribosyltransferase) class may be a somewhat superficial and misleading description. The binding of PRPP to the 6-oxypurine phosphoribosyltransferases is similar to that described above for adenine phosphoribosyltransferase; that is, it involves a PRPP-binding site consisting of a PRPP-binding loop with a carboxylate dipeptide, Asp-Asp or Glu-Asp, a PP loop, and a Thr/Ser-Gly-Glu-Thr consensus sequence involved in the binding of the 5-phosphate moiety.
(ii) Pyrimidine nucleotide biosynthesis.
Unlike de novo purine nucleotide biosynthesis, with de novo pyrimidine nucleotide biosynthesis the pyrimidine moiety is first synthesized and the product, orotate, and PRPP are assembled to form orotidine 5′-monophosphate (Fig. 11, compound b) in a reaction catalyzed by orotate phosphoribosyltransferase (orotidine-5′-phosphate:diphosphate phospho-α-d-ribosyl-transferase, EC 2.4.2.10; orotate + PRPP → orotidine 5′-monophosphate + PPi). Orotate phosphoribosyltransferase, a typical type I phosphoribosyltransferase, contains the “usual” structural elements, a five-stranded β-sheet surrounded by a number of helices, usually four, a hood structure involved in the binding of the substrate orotate, and a flexible catalytic loop, which closes the active site on catalysis. The structures of orotate phosphoribosyltransferase from a variety of sources, including S. enterica (223), E. coli (224), S. mutans (225), Plasmodium falciparum (226), and L. donovani (227) have been published.
The three-dimensional structure of S. enterica orotate phosphoribosyltransferase in complex with orotate and MgPRPP revealed an asymmetric structure of the dimer, because both substrates bind to the active site of one subunit (B), whereas only orotate was bound to the A subunit. The flexible catalytic loop of subunit A closes the active site of subunit B, whose flexible catalytic loop remains unstructured and presumably open. Orotate is bound by amino acids of the hood, and PRPP is bound in the PRPP-binding loop, which includes the 124-Asp-Asp-125 dipeptide (coordinating to hydroxyls of ribose C-2 and C-3), the sequence 128-Thr-Ala-Gly-Thr-131 (binding the 5-phosphate), and Tyr72 and Lys73 (the PP loop). A single Mg2+ coordinates the oxygens of ribosyl C-1, C-2, and C-3, an oxygen of the β-phosphate as well as two water molecules. On catalytic flexible loop closure, lysine, arginine, and histidine residues of this loop bind to the diphosphate chain, and, together with the Mg2+ they participate in neutralizing the diphosphate chain during catalysis (228). The structure reveals the presence of two shared active sites at the interface of the dimer, which has been also demonstrated by formation of a heterodimer composed of one subunit defective in PRPP binding and one subunit defective in the catalytic flexible loop (229).
The structure of S. cerevisiae orotate phosphoribosyltransferase resembles that of S. enterica (230). As mentioned, the dimeric structure is asymmetric. This asymmetry, together with the movement of the catalytic flexible loops during (i.e., before and after) catalysis, as well as kinetic analysis results prompted the authors to suggest an alternating active site catalytic mechanism, i.e., only one active site is functional at any given time (230, 231).
Unlike the purine salvage reactions, pyrimidine salvage involves the activity of only a single phosphoribosyltransferase: uracil phosphoribosyltransferase (UMP:diphosphate phospho-α-d-ribosyltransferase; EC 2.4.2.9; uracil + PRPP → UMP + PPi) (Fig. 11, compound j). Uracil phosphoribosyltransferase allows organisms to utilize uracil formed endogenously by nucleic acid catabolism or supplied by the environment. Uracil phosphoribosyltransferase requires Mg2+ for activity. In general, the activity of the enzyme is allosterically activated by GTP. GTP causes association of subunits to tetramers, as observed for uracil phosphoribosyltransferase from E. coli (232) and T. gondii (233). Alternatively, GTP functions as an allosteric activator of the oligomeric uracil phosphoribosyltransferase of Sulfolobus species (234–237). The enzyme from M. tuberculosis differs from the aforementioned in being unaffected by GTP (238, 239), and the enzyme from Giardia intestinalis dimerizes in the presence of GTP (240).
The PRPP-binding motif of uracil phosphoribosyltransferase differs markedly from that of other phosphoribosyltransferases or PRPP synthase in that the typical acidic dipeptide (Asp-Asp or Glu-Asp) is replaced by Asp-Pro (highlighted in bold in the sequence below), as illustrated here with the E. coli uracil phosphoribosyltransferase amino acid sequence: 123-Glu-Arg-Met-Ala-Leu-Ile-Val-Asp-Pro-Met-Leu-Ala-Thr-Gly-Gly-Ser-138 (241). The Asp-Pro dipeptide appears to be universal among uracil phosphoribosyltransferases, as it is present in all the enzymes from the organisms mentioned above as well as yeasts (242), bacilli (243, 244), and lactococci (245). Analysis of a mutant variant that had this particular proline residue replaced by an aspartate revealed a 100-fold increase in the Km value for uracil and a 50-fold reduction in the kcat value. The Km value for PRPP was reduced in the mutant, whereas the kcat/KmPRPP value was almost unchanged. It was therefore concluded by the authors that the proline residue of the PRPP-binding loop of uracil phosphoribosyltransferase “is of little importance for binding of PRPP to the free enzyme, but is critical for binding of uracil to the enzyme-PRPP complex and for the catalytic rate” (246).
Two variant forms of uracil phosphoribosyltransferase are found in Gram-positive organisms, such as B. caldolyticus and B. subtilis. Both genes were shown to complement E. coli upp lesions and therefore encode active enzymes. One of these (upp) specifies uracil phosphoribosyltransferase, whereas the other (pyrR) specifies a regulatory protein involved in regulation of pyrimidine gene expression, PyrR (247, 248). We shall return to PyrR below in the section “PRPP as Mediator of Metabolic Regulation.”
Finally, a phosphoribosyltransferase activity for dioxotetrahydropyrimidine (dioxotetrahydropyrimidine phosphoribosyltransferase; EC 2.4.2.20) has been characterized (249). Whether it has a separate activity or is a variant of other pyrimidine phosphoribosyltransferases remains unresolved.
(iii) NAD biosynthesis.
The phosphoribosyltransferases of NAD biosynthesis are members of the so-called type II phosphoribosyltransferases. De novo pyridine nucleotide biosynthesis resembles de novo pyrimidine nucleotide biosynthesis in that the aromatic base is synthesized prior to phosphoribosylation (250). The reaction with PRPP and quinolinate is catalyzed by quinolinate phosphoribosyltransferase (β-nicotinate-d-ribonucleotide:diphosphate phospho-α-d-ribosyltransferase [carboxylating]; EC 2.4.2.19) encoded by nadC in E. coli: quinolinate + PRPP → 5′-phospho β-d-ribosylnicotinate + PPi + CO2. 5′-Phosphoribosylnicotinate is also designated nicotinate mononucleotide (Fig. 11, compund c). The reaction is unusual in producing carbon dioxide (251, 252). In pyridine cofactor salvage synthesis, nicotinate phosphoribosyltransferase (5-phospho-α-d-ribose 1-diphosphate:nicotinate ligase [ADP, diphosphate-forming]; EC 6.3.4.21), encoded by pncB in E. coli, catalyzes the reaction nicotinate + PRPP (+ ATP) → 5′-phospho-β-d-ribosyl-nicotinate + PPi (+ ADP + Pi). This reaction is unusual in that the enzyme is stimulated by ATP in a stoichiometric manner. That is, the enzyme is phosphorylated by ATP at His219, which results in the formation of a high-affinity form with Km values for nicotinate and PRPP 200-fold lower than those of the unphosphorylated enzyme. Also, the coupling of ATP hydrolysis to the phosphoribosyltransferase reaction causes a change in the equilibrium value, from 0.67 to 1,100 (253–255). Mutational replacement of His219 completely abrogates phosphorylation and stimulation by ATP (256). Mammalian organisms such as mice and humans contain yet a different pyridine phosphoribosyltransferase, nicotinamide phosphoribosyltransferase (nicotinamide-d-ribonucleotide:diphosphate phosphor-α-d-ribosyltransferase; EC 2.4.2.12): nicotinamide + PRPP → 5′-phospho-β-d-ribosyl-nicotinamide (nicotinamide mononucleotide, NMN) + PPi (257, 258). As with purine and pyrimidine nucleotide biosynthesis, PRPP is used in both the de novo reactions of NAD synthesis (quinolinate phosphoribosyltransferase) and in NAD salvage reactions (nicotinate and nicotinamide phosphoribosyltransferases).
(a) Quinolinate phosphoribosyltransferase.
The structures of quinolinate phosphoribosyltransferase from a number of sources have been determined. These sources include M. tuberculosis (259), S. enterica (211), Helicobacter pylori (260), Thermotoga maritima (261), and S. cerevisiae (262). The structures of these enzymes resemble one another. The structure of the ternary complex of M. tuberculosis quinolinate phosphoribosyltransferase with a quinolinate analog (phthalate) and a PRPP analog (5-phosphoribosyl-1-[β-methylene]diphosphate, i.e., the two phosphorus atoms are connected by a methylene moiety) reveals the binding of quinolinate and PRPP together with two divalent metal ions (in this case, Mn2+). In the ternary complex, the ribosyl moiety of PRPP is hydrogen bonded to Glu201 and to Asp222 through the C-2 and C-3 hydroxyls. The two divalent metal ions coordinate to the same two hydroxyls, to the diphosphate chain or to water molecules; the ions do not coordinate to enzyme amino acid residues. The diphosphate chain forms hydrogen bonds to arginine, lysine, and aspartate residues. The 5-phosphate is hydrogen bonded to, among other residues, Gly270 and Gly249. Thus, although the fold of quinolinate phosphoribosyltransferase (a type II phosphoribosyltransferase) is very different from the fold of type I phosphoribosyltransferases, some similarities are evident. PRPP binding involves two acidic residues (Glu201 and Asp222), which is reminiscent of the Asp-Asp dipeptide of type I phosphoribosyltransferases and of PRPP synthase; the 5-phosphate binding involves glycine residues, and diphosphate binding involves several basic residues. Although different in structure, similar arrangements have evolved for the binding of the common substrate PRPP, which suggests a common theme in the design of active sites.
(b) Nicotinate phosphoribosyltransferase.
The structure of nicotinate phosphoribosyltransferase of the archaeon Thermoplasma acidophilum revealed a three-domain arrangement, where the structures of the N-terminal and central domains resemble the two domains of quinolinate phosphoribosyltransferases and a unique C-terminal domain is added. Only a few differences were observed in the number of β-strands between nicotinate and quinolinate phosphoribosyltransferases. The phosphate group of PRPP binds to T. acidophilum nicotinate phosphoribosyltransferase in a mode similar to the binding of the PRPP analog to M. tuberculosis quinolinate phosphoribosyltransferase, and the structures of the active sites of the two enzymes are very similar in spite of little sequence conservation between them (263).
The C-terminal domain, approximately 100 amino acid residues in length, is composed of seven β-strands and one helix. Enzymological data for T. acidophilum nicotinate phosphoribosyltransferase are not available, so it remains unknown if the activity of this enzyme is stimulated by ATP. It is possible that the C-terminal domain participates in phosphorylation and ATP hydrolysis and, by analogy, the phosphorylation site may be present in the central domain, although a His219 homolog is not found in T. acidophilum nicotinate phosphoribosyltransferase. The C-terminal domain of S. enterica nicotinate phosphoribosyltransferase is exposed to the solvent; trypsin treatment removed 24 C-terminal amino acid residues and inactivated the ATPase activity. This inactivation was prevented by ATP binding, which provides evidence for the involvement of the C-terminal domain in energy coupling (264).
(c) Nicotinamide phosphoribosyltransferase.
Vertebrates lack nicotinamide deamidase and, thus, do not produce nicotinate from nicotinamide. Rather, they salvage nicotinamide by means of nicotinamide phosphoribosyltransferase.
The sandwich and α/β-barrel structures described above for M. tuberculosis quinolinate phosphoribosyltransferase are repeated in human nicotinamide phosphoribosyltransferase. The position of 5′- or 5-phosphate maps identically in structures with 5′-phospho β-d-ribosylnicotinamide or with PRPP bound, whereas the ribose moieties are displaced relative to one another. The 5-phosphate of PRPP is hydrogen bound to Gly383 and Gly384 (both of the A subunit), which are part of the 381-Gly-Ser-Gly-Gly-Gly-385 loop that is similar to the corresponding loop of T. acidophilum nicotinate phosphoribosyltransferase (263). The diphosphate of PRPP is hydrogen bonded to Arg39 (B subunit), Arg40 (B subunit), Arg196 (A subunit), and Lys400 (B subunit), and the C-2 and C-3 hydroxyls are hydrogen bonded to Arg311 and Asp313 (both of the A subunit). Thus, the binding of PRPP to human nicotinamide phosphoribosyltransferase is homologous to the binding of PRPP to the other type II phosphoribosyltransferases (265).
As expected, nicotinamide phosphoribosyltransferases from mouse and rat have structures similar to that of the human enzyme (266, 267). There is extensive structural resemblance between the type II phosphoribosyltransferases, namely, quinolinate, nicotinate, and nicotinamide phosphoribosyltransferases. Chappie and coworkers have offered a theory for the evolution of the type II phosphoribosyltransferases, in which nicotinamide phosphoribosyltransferase evolved from nicotinate phosphoribosyltransferase, which in turn evolved from quinolinate phosphoribosyltransferase (268).
Studies of human nicotinamide phosphoribosyltransferase have attracted interest, because the enzyme has been identified as a growth factor for early-stage B cells (hence it is called “pre-B cell colony-enhancing factor”) and has been associated with binding to and activation of the insulin receptor and has been also called “visfatin.” For a review of the many apparent functions of human nicotinamide phosphoribosyltransferase, consult the article by Garten and colleagues (269). It is unclear if the many functions may be ascribed simply to variations in the NAD pool. However, because of the increased synthesis of nicotinamide phosphoribosyltransferase in tumor tissues, studies have been directed toward the isolation of inhibitors of the enzyme. Several of these inhibitors could be converted enzymatically to phosphoribosylated derivatives. Analysis of the structure of a ribosylated inhibitor bound to nicotinamide phosphoribosyltransferase confirmed the binding of its 5-phosphoribosyl moiety (270).
(iv) Histidine biosynthesis.
In histidine biosynthesis, all of the five carbons of PRPP are retained. The carboxylate, the α-C and the β-C of the amino propanoic side chain of histidine are generated from C-5, C-4, and C-3, respectively, of PRPP, whereas C-4 and C-5 of the imidazole moiety are derived from C-2 and C-1 of PRPP. In the first step of histidine biosynthesis, PRPP and ATP react with the formation of PPi and N-(5′-phospho-β-d-ribosyl)-ATP, which has an N-glycosidic bond of C-1 of the ribosyl moiety to N-1 of ATP (Fig. 11, compound d) (271). The reaction is catalyzed by ATP phosphoribosyltransferase (ATP phosphoribosyltransferase; EC 2.4.2.17), which is encoded by hisG in E. coli (272, 273).
There are two subfamilies of ATP phosphoribosyltransferases. They have different quaternary structures (hexa- or octameric) (274–276) but similar molecular architectures of their catalytic domains. The E. coli ATP phosphoribosyltransferase polypeptide contains three domains. Domain I consists of four parallel and two antiparallel β-strands flanked by two α-helices on each side; domain II is constructed of four parallel and one antiparallel β-strand flanked by one helix on one side and two helices on the other side. Domain III is a histidine-binding domain (277). Domains I and II constitute the catalytic fold of ATP phosphoribosyltransferase. Thus, although this fold appears to be reminiscent of the type I phosphoribosyltransferase and PRPP synthase domain fold, it represents a unique fold. Furthermore, ATP phosphoribosyltransferases lack the hood and the catalytic flexible loop characteristic of type I phosphoribosyltransferases. Additionally, the active form of both types of enzymes are dimers or higher-order oligomers.
The active form of E. coli ATP phosphoribosyltransferase is a dimer of the three-domain polypeptide. Activity is inhibited by AMP, which prevents the binding of ATP and PRPP. The binding of the inhibitors histidine and eventually also guanosine 3′-diphosphate 5′-diphosphate result in the formation of a hexameric, inactive quaternary structure (277–279). There is only 27% sequence identity between E. coli guanine-xanthine phosphoribosyltransferase and ATP phosphoribosyltransferase. A PRPP-binding loop located within domain II, however, is easily identified in the ATP phosphoribosyltransferase sequence 162-Gly-Leu-Ala-Asp-Ala-Ile-Cys-Asp-Leu-Val-Ser-Thr-Gly-Ala-Thr-176. The structure of ATP phosphoribosyltransferase from M. tuberculosis is similar to that of E. coli; in this case, the PRPP -binding loop was identified as 147-Gly-Val-Ala-Asp-Ala-Ile-Ala-Asp-Val-Val-Gly-Arg-Thr-Leu-Ser-162 (280). ATP phosphoribosyltransferases, such as those of E. coli and M. tuberculosis, are sometimes designated HisGL, with L designating “long” form. This is in contrast to HisGS, with S designating “short” form in, for example, L. lactis. HisGS ATP phosphoribosyltransferases lack domain III and instead associate with a distinct polypeptide, HisZ, to form active octameric oligomers, whereas the isolated HisG and HisZ polypeptides associate into inactive dimers. The catalytic fold of HisGS ATP phosphoribosyltransferases is almost identical to that of HisGL ATP phosphoribosyltransferases, and the active site is located, once again, in a cleft between domain I and II. PRPP-binding loops have been identified in ATP phosphoribosyltransferases of L. lactis (148-Gly-Leu-Ala-Asp-Ala-Ile-Val-Asp-Ile-Val-Glu-Thr-Gly-Asn-Thr-162) (281) and of the hyperthermophilic bacterium T. maritima (140-Gly-Leu-Ser-Asp-Leu-Ile-Val-Asp-Ile-Thr-Glu-Thr-Gly-Arg-Thr-155) (282). Mutations that replaced Thr159 or Thr162 of L. lactis ATP phosphoribosyltransferase confirmed the importance of this sequence in PRPP binding (283).
(v) Tryptophan biosynthesis.
Contrary to the situation in histidine biosynthesis, only two carbons of PRPP are retained in tryptophan. C-2 and C-3 of the indole moiety of tryptophan originate from C-1 and C-2, respectively, of PRPP. The tryptophan biosynthetic intermediate anthranilate reacts with PRPP in a reaction catalyzed by anthranilate phosphoribosyltransferase (EC 2.4.2.18), encoded by trpD, which results in the formation of N-(5-phospho-β-d-ribosyl)-anthranilate (Fig. 11, compound e) and PPi. The anthranilate phosphoribosyltransferase isolated from E. coli is bifunctional: it also contains glutamine chorismate amidotransferase activity. Consequently, the E. coli trpD gene product is considerably larger, a total of 531 amino acid residues, than monofunctional anthranilate phosphoribosyltransferases, such as those from S. solfataricus or M. tuberculosis, which have 345 or 370 amino acid residues, respectively (284, 285). Other tryptophan biosynthetic enzymes from various species have been shown to harbor two activities.
The overall three-dimensional structure of anthranilate phosphoribosyltransferase is similar among all of the species so far analyzed, i.e., the enterobacterium Pectobacterium carotovorum (286), S. solfataricus (284), M. tuberculosis (285), Xanthomonas campestris (PDB code 4hkm), T. thermophilus (PDB code 1v8g), and a cyanobacterial species (Nostoc sp.) (PDB code 1vqu). The two-domain structure consists of a smaller N-terminal domain of six helices (the α-domain) and a larger C-terminal domain of six parallel β-sheets and one antiparallel β-sheet, as well as eight or more helices (the α/β-domain). Although the five-stranded parallel β-sheet characteristic of type I phosphoribosyltransferases is recognizable, anthranilate phosphoribosyltransferase contains additional structural features and thus constitutes a separate fold sometimes designated type IV phosphoribosyltransferase (287). The enzyme is active as a homodimer; the α-domain is responsible for the dimerization. Binding of PRPP and anthranilate is mediated in a cleft of the α/β-domain. The binding of PRPP is promoted by two Mg2+ or Mn2+, which accounts for a requirement of Mg2+ for activity (288). The binding of PRPP has been well mapped in the structures of S. solfataricus and M. tuberculosis anthranilate phosphoribosyltransferase. The amino acid residues involved are the sequence 79-Gly-Thr-Gly-Gly-Asp-83, Lys106 and 223-Asp-Glu-224 of the S. solfataricus enzyme (107-Gly-Thr-Gly-Gly-Asp-111, Lys136 and 251-Asp-Glu-252, respectively, of the M. tuberculosis enzyme). Remarkably, in spite of this sequence conservation, the binding of PRPP in the two enzymes is very different. In common, one divalent cation (MG1) coordinates to the α- and β-phosphates of PRPP. Another divalent cation (MG2) coordinates to the two acidic residues (Asp-Glu). The Gly-Thr-Gly-Gly-Asp seqence “wraps around” PRPP with the amino-terminal glycine binding to the diphosphate chain and the carboxy-terminal aspartate or glycine binding to the 5-phosphate. Specifically, Gly79 forms hydrogen bond to the β-phosphate in the S. solfataricus enzyme. Lys106 of the latter enzyme binds to the β-phosphate, whereas Lys135 of the M. tuberculosis enzyme binds to the α-phosphate. Thus, this sequence may be compared to the PP loop of type I phosphoribosyltransferases and of PRPP synthases. Among the differences in PRPP binding between the two enzymes are the binding properties of Asp-Glu, which bind to MG2 and to the oxygen of C-5 (Asp residue only) in S. solfataricus anthranilate phosphoribosyltransferase. MG2 furthermore coordinates to the 5-phosphate and to the oxygens of C-4 and C-5 of ribose 5-phosphate (289). The corresponding aspartate and gluatmate residues of the M. tuberculosis enzyme are coordinated to MG1 (Glu252) or to MG2 (Asp251). The PRPP divalent cation coordination activities of the latter enzyme are directed exclusively toward the diphosphate chain (285). The conserved glycine-rich sequence 79-Gly-Thr-Gly-Gly-Asp-83 resembles the subsequence Asp-Asp-Xxx-Xxx-Thr-Gly-Gly-Thr of several phosphoribosyltransferases and the glycine-containing subsequence of the PRPP-binding loop of PRPP synthase (228-Thr-Ala-Gly-Thr-232, 234-Ser-Gly-Gly-Thr-237 or 217-Thr-Gly-Gly-Thr-220 of B. subtilis, S. solfataricus, or M. jannaschii PRPP synthase, respectively). These sequences of phosphoribosyltransferases and PRPP synthases are involved in the binding of the 5-phosphate moiety of PRPP or ribose 5-phosphate.
Each subunit of anthranilate phosphoribosyltransferase binds two anthranilate molecules in addition to one PRPP molecule and two Mg2+. Three sites for anthranilate are responsible for tunnel movement of anthranilate, with a concomitant 180° flipping of the compound toward MgPRPP, which at this point is presumably already present. However, anthranilate may bind to the outer end of the tunnel before MgPRPP is bound, and binding of the latter compound may participate in tunnel formation (290). Interestingly, in S. solfataricus anthranilate phosphoribosyltransferase Gly79 also forms a hydrogen bond to the anthranilate nitrogen. Thus, this residue may be particularly important in positioning anthranilate and PRPP for inline attack of anthranilate on C-1 of PRPP (289).
Tryptophan biosynthesis in M. tuberculosis has attracted much attention, as trp mutant strains of this organism are avirulent (291). Since the organism's host, humans, are unable to synthesize tryptophan, a search for inhibitors of tryptophan biosynthetic enzymes, including for anthranilate phosphoribosyltransferase, has been conducted in hopes of identifying an antimicrobial agent (287, 290, 292).
(vi) Phosphoribosyl transfer without participation of PRPP.
For the sake of completeness, the cobT-encoded nicotinate nucleotide 5,6-dimethylbenzimidazole phosphoribosyltransferase (EC 2.4.2.21) of S. enterica and E. coli should be mentioned at this point. Although this enzyme is a phosphoribosyltransferase, it does not utilize PRPP as a substrate. Rather, the 5-phosphoribosyl moiety is detached from the nicotinamide mononucleotide and is added to the 5,6-dimethylbenzimidazole, and thus one N-glycoside bond is replaced by a second N-glycoside bond (293, 294). A variant of this enzyme (EC 2.4.2.55) has been characterized from the bacterium Sporomusa ovata. In contrast to nicotinate nucleotide:5,6-dimethylbenzimidazole phosphoribosyltransferase, the S. ovata enzyme (EC 2.4.2.55) also utilizes phenol derivatives as acceptors in the transfer of the 5-phosphoribosyl moiety (295).
C-Glycosidic bond formation: tetrahydromethanopterin biosynthesis.
Tetrahydromethanopterin is one of a number of cofactors involved in methanogenesis and sulfate reduction in a variety of archaeal species (296, 297) and methylotrophic bacteria (298, 299). The pathway of tetrahydromethanopterin biosynthesis has been deduced from the structures of a series of metabolites meticulously solved by White and colleagues (4, 300–303). The function of methanopterin is analogous to that of dihydrofolate, i.e., it is a carrier of one-carbon molecules, including formyl, methylene, and methyl groups. Both methanopterin and folic acid contain a substituted pteridyl moiety as well as an aryl component apparently derived from 4-aminobenzoate. However, methanopterin contains both deoxyribulosyl and phosphoribosyl moieties that are not present in folate. Both of these pentose moieties are derived from PRPP. The two PRPP-utilizing biochemical reactions of methanopterin biosynthesis are shown in Fig. 12A. Initially, and in what is regarded as the first committed step in tetrahydromethanopterin biosynthesis, 4-hydroxybenzoate condenses with PRPP with the formation of 5′-phospho-β-d-ribosyl 4-hydroxybenzene as well as PPi and carbon dioxide (Fig. 12A, reaction 1). The product 5′-phospho-β-d-ribosyl 4-hydroxybenzene contains a carbon-carbon bond between the ribosyl and aryl moieties (Fig. 12A, compound m). The enzyme catalyzing this reaction has affinity for both 4-hydroxy- and 4-aminobenzoate (304, 305). In vitro and in vivo analyses used 4-aminobenzoate to elucidate the metabolic pathway. The enzyme has been designated 4-(β-d-ribofuranosyl)aminobenzene-5′-phosphate synthase, and in M. jannaschii it is encoded by the MJ1427 gene. Although 4-hydroxybenzoate rather than 4-aminobenzoate appears to be the origin of the aryl moiety of methanopterin, at least in M. jannaschii (304), we shall use the designation aminobenzoate phosphoribosyltransferase for this enzyme, because most studies have used 4-aminobenzoate as the substrate. Indeed, the product of the aminobenzoate phosphoribosyltransferase-catalyzed reaction with 4-hydroxybenzoate as the substrate, 5′-phospho-β-d-ribosyl 4-hydroxybenzene, is transformed in a subsequent enzymatic step to the amino derivative 5′-phospho-β-d-ribosyl 4-aminobenzene (Fig. 12A, compound n).
FIG 12.
C- and O-glycosidic bond formation with PRPP. The phosphoribosyl donor PRPP is shown in red, and atoms derived from PRPP in the intermediates and products are also shown in red. (A) Biosynthesis of tetrahydromethanopterin. Compound labels: l, 4-hydroxybenzoate; m, 5′-phospho-β-d-ribosyl 4-hydroxybenzene; n, 5′-phospho-β-d-ribosyl 4-aminobenzene; o, N-[(7,8-dihydropterin-6-yl)methyl]-4-(1-deoxy-d-ribulosyl)aminobenzene; p, 1-(4-{N-[(7,8-dihydropterin-6-yl)methyl]amino}phenyl)-5-(5-phospho-α-d-ribulosyl)-1-deoxyribitol. Enzyme 1, 4-aminobenzoate phosphoribosyltransferase (5-phospho-α-d-ribose 1-diphosphate:4-aminobenzoate 5-phospho-β-d-ribofuranosyltransferase [decarboxylating], EC 2.4.2.54); enzyme 2, 1-(4-{N-[(7,8-dihydropterin-6-yl)methyl]amino}phenyl)-5-(5-phospho-α-d-ribulosyl)-1-deoxyribitol synthase. This enzyme activity has not been identified. The four reactions leading from compound m to compound n in effect convert a hydroxy group to an amino group and involve the formation of phosphate ester and the addition of an aspartyl residue and the removal of Pi and fumarate (304). The three reactions leading from compound n to compound o involve the attachment of a pterin derivative (R = N-[7,8-dihydropterin-6-yl]methyl) to the nitrogen of compound n followed by opening of the ribosyl moiety and isomerization to a ribulose derivative and dephosphorylation to form compound o. The boxed compound is the product of the pathway for 5,6,7,8-tetrahydromethanopterin (with a complete structure of the pteridyl moiety), the active cofactor in transformation of carbon dioxide to methane in methanogenic Archaea (301), and is formed from compound p by attachment of a glutamyl moiety to the phosphate group followed by dehydrogenation of the pteridyl moiety. (B) Biosynthesis of arabinosyl monophosphodecaprenol. Compound labels: q, decaprenyl phosphate; r, 5-phospho-β-d-ribosyl 1-O-monophosphodecaprenol (decaprenylphospho-β-d-ribosyl 5-phosphate). Enzyme 3, decaprenyl phosphate phosphoribosyltransferase (5-phospho-α-d-ribosyl 1-diphosphate:decaprenyl-phosphate 5-phosphoribosyltransferase; EC 2.4.2.45). The boxed compound is the arabinosyl donor arabinosyl monophosphodecaprenol, which is formed from compound r by dephosphorylation of the ester at C-5 of the ribosyl moiety followed by epimerization. The latter two reactions occur outside the cell (5). (C) Biosynthesis of butirosin. Compound labels: s, neamine; t, 5″-phosphoribostamycin. Enzyme 4, neamine phosphoribosyltransferase. The boxed compound is butirosin. Two isomers are synthesized; one contains a ribosyl moiety (shown) and a second contains an arabinosyl moiety (not shown). Other aminoglycoside antibiotics derived from neamine, i.e., neomycin B, paromomycin, and lividomycin B, lack the 4-amino-2-hydroxybutyryl side chain but contain an N-acetylaminoglucosyl moiety attached to C-3″ of the ribosyl moiety, and the pseudodisaccharides of the three compounds are differently decorated with hydroxyl and amino groups (318).
The aminobenzoate phosphoribosyltransferases of the methanogenic archaea Methanosarcina thermophila, M. jannaschii, and Methanothermobacter thermautotrophicus as well as the hyperthermophilic, nonmethanogenic (sulfate-reducing) archaeon A. fulgidus have been purified and studied. Edman degradation of aminobenzoate phosphoribosyltransferase isolated from cell extracts of M. thermophila strain TM-1 was used to establish the sequence of the 20 N-terminal amino acid residues, which were then used to identify aminobenzoate phosphoribosyltransferase-encoding genes in a variety of organisms (299). The molecular mass of the native enzyme is 63.5 kDa, and that of the subunit is 32.6 kDa, i.e., the enzyme is a homodimer. PPi and 5′-phospho-β-d-ribosyl 4-aminobenzene have been demonstrated to be products of the reaction. The reaction requires Mg2+ or Mn2+, the latter ion being the most efficient. The optimal pH is 4.8, and the optimal temperature is 50°C. Some kinetic constants were determined; the apparent Km4-aminobenzoate was 58 μM and the apparent KmPRPP was 3.6 mM. Pyridoxal phosphate is suggested to be involved in the reaction, but data are inconclusive in this respect, and it has been suggested that pyridoxal phosphate may be involved in the reaction in a way that does not require the carbonyl of pyridoxal phosphate (301, 305).
The M. jannaschii aminobenzoate phosphoribosyltransferase, encoded by the gene MJ1427, resembles that of M. thermophila (306). Product inhibition studies established the reaction as an ordered Bi-Ter mechanism with binding of PRPP followed by 4-aminobenzoate, and release of CO2, then 4-(β-d-ribofuranosyl)aminobenzene-5′-phosphate, and finally PPi. As with the enzyme from M. thermophila, the affinity for PRPP is low (KmPRPP, 1.5 mM), whereas that for 4-aminobenzoate is lower (Km4-aminobenzoate, 0.15 mM). A kcat value was determined to be 0.23 s−1. Phosphoribosyltransferase-catalyzed reactions are thought to proceed with the intermediate formation of a ribooxocarbenium ion as well as a negatively charged nucleophile. Aminobenzoate phosphoribosyltransferase poses a special problem for this generalization. How does the enzyme stabilize the negative charge at C-1 of the nucleophile when the substrate already carries a negatively charged carboxyl group (4-aminobenzoate or 4-hydrozybenzoate)? No prosthetic group, neither pyridoxal 5-phosphate nor a pyruvoyl moiety, appear to be present, indicating that enzyme side chains are only involved in the catalytic process (306).
In A. fulgidus, the gene AF2089 encodes aminobenzoate phosphoribosyltransferase. Following cloning and expression of the gene in E. coli, the enzyme was partly purified. It has a temperature optimum at 70°C and maximal activity at pH 5.3. Gel filtration revealed a molecular mass of 57 kDa, corresponding to a homodimer, because the molecular mass of the subunit was established as 35.5 kDa (307). Finally, a procedure for the purification of aminobenzoate phosphoribosyltransferase from M. thermautotrophicus has been published (307).
Some archaeal species appear to contain two aminobenzoate phosphoribosyltransferase-encoding genes. These are Pyrococcus abyssi, where the aminobenzoate phosphoribosyltransferases (sequence designations PAB0141 and PAB1694) show 30% amino acid sequence identity, Pyrococcus horikoshii (sequence designations PH0227 and PH1228), whose enzymes show 29% amino sequence identity, and Aeropyrum pernix (sequence designations APE2425 and APE1512), whose enzymes show 28% amino acid sequence identity (299).
None of the aminobenzoate phosphoribosyltransferases contains a classical PRPP-binding site, as is the case for type II phosphoribosyltransferases. As mentioned, the binding of PRPP to type II phosphoribosyltransferases occurs by interaction of a few amino acid residues to the phosphate moieties of PRPP. Amino acid sequence comparison of aminobenzoate phosphoribosyltransferases with M. tuberculosis or T. acidophilum nicotinate and quinolinate phosphoribosyltransferases did not reveal conserved amino acid residues between these phosphoribosyltransferases.
O-Glycosidic bond formation. (i) Tetrahydromethanopterin biosynthesis.
Another reaction of archaeal methanopterin biosynthesis utilizes PRPP as a substrate; interestingly, this reaction results in the formation of an O-glycosidic bond (Fig. 12A, reaction 2). The phosphoribosyl acceptor substrate of this reaction is N-[(7,8-dihydropterin-6-yl)methyl]-4-(1-deoxy-d-ribulosyl)aminobenzene (Fig. 12A, compound o), and the product is 1-(4-{N-[(7,8-dihydropterin-6-yl)methyl]amino}phenyl)-5-(5-phospho-α-d-ribulosyl)-1-deoxyribitol (Fig. 12A, compound p), i.e., C-1 of the phosphoribosyl moiety is attached to a hydroxyl group of the ribulosyl moiety of compound o, with inversion of the anomeric carbon of the phosphoribosyl moiety, as usual (301). The enzyme catalyzing this reaction has not been identified. One additional reaction is necessary to convert compound p to the final cofactor (Fig. 12A) and it is described in the figure legend.
(ii) Arabinosyl monophosphodecaprenol biosynthesis.
Arabinogalactan is an important constituent of the cell wall of mycobacteria, such as M. tuberculosis and M. smegmatis. Arabinogalactan contains d-arabinosyl and d-galactosyl moieties. Polymerization of the arabinosyl moieties is catalyzed by a number of arabinosyltransferases, for which the donor is a lipid-linked arabinosyl donor, β-d-arabinosyl monophosphodecaprenol (reviewed previously in references 5 and 308). Tracer methodology applied to membrane preparation of M. smegmatis to study the origin of the arabinosyl derivatives revealed that ribulose 5-phosphate was a precursor, but that ribulose 5-phosphate was not converted directly to arabinose 5-phosphate (309). Rather, the pentosyl phosphate donor was shown to be PRPP. Thus, following incubation of M. smegmatis membrane preparations with [14C]PRPP, the production of [14C]ribosyl phosphopolyprenol, [14C]arabinosyl phosphopolyprenol, 5-phospho-[14C]ribosyl phosphopolyprenol, and 5-phospo-[14C]arabinosyl phosphopolyprenol was demonstrated, which showed the involvement of PRPP in the synthesis of arabinose derivatives for cell wall formation in this organism Fig. 12B (310). Subsequently, an M. tuberculosis gene (Rv3806c) encoding an enzyme that phosphoribosylates decaprenyl phosphate was identified, cloned, and expressed in E. coli. The enzyme is an integral membrane protein; membrane fractions of an E. coli strain hosting Rv3806c were able to convert PRPP and decaprenyl phosphate into 5-phospho-β-d-ribosyl 1-phosphoryldecaprenol (311). The enzyme is designated 5-phospho-α-d-ribosyl-1-diphosphate:decaprenyl-phosphate 5-phosphoribosyltransferase. In analogy with other phosphoribosyltransferases, we shall use the name decaprenyl phosphate phosphoribosyltransferase.
Inspection of the amino acid sequence of decaprenyl phosphate phosphoribosyltransferase does not reveal the classical PRPP-binding site that is characteristic of PRPP synthases and type I phosphoribosyltransferases. As mentioned, decaprenyl phosphate phosphoribosyltransferase is a membrane-bound enzyme, which is consistent with both a membrane-soluble substrate (decaprenyl phosphate) and a soluble substrate (PRPP). A topology with nine transmembrane domains was suggested for the structure of M. tuberculosis decaprenyl phosphate phosphoribosyltransferase by molecular modeling studies. According to this model, the active site may involve the cytoplasmic loop II. From a search of amino acid sequence alignments with other phosphoribosyltransferases, a sequence, 73-Asn-Asp-x-x-Asp-77 (where x indicates any amino acid), was suggested as important for PRPP binding and enzymatic function (312). Indeed, the M. tuberculosis decaprenyl phosphate phosphoribosyltransferase amino acid sequence 69-Val-Tyr-Leu-Val-Asn-Asp-Val-Arg-Asp-77 resembles a large part of the PRPP binding loop of E. coli hypoxanthine-guanine phosphoribosyltransferase, 95-Val-Leu-Ile-Val-Glu-Asp-Ile-Ile-Asp-103, as well as the corresponding amino acid sequence of other phosphoribosyltransferases (213, 313). These amino acids are located within the cytoplasmic loop II and the junction of this loop and the transmembrane 2-domain. Analysis of the decaprenyl phosphate phosphoribosyltransferase replacement mutants Asn73Gln and Asp77Glu revealed significantly increased Km values for PRPP, providing evidence that this sequence is involved in PRPP binding (312).
(iii) Aminoglycoside antibiotic biosynthesis.
A third reaction that forms an O-glycosidic bond with PRPP as the phosphoribosyl donor occurs in the biosynthesis of the aminoglycoside antibiotic butirosin. The 26-ORF btr gene cluster specifies the biosynthetic pathway for buritosin in Bacillus circulans (314, 315). The btrL cistron encodes neamine phosphoribosyltransferase (neamine:5-phospho-α-d-ribose 1-diphosphate phosphoribosyltransferase; EC 2.4.2.49), which phosphoribosylates neamine with the formation of 5″-phosphoribostamycin (Fig. 12C) (316). The B. circulans neamine phosphoribosyltransferase contains a well-conserved PRPP-binding site, 212-Asp-Ile-Val-Ile-Leu-Glu-Asp-Gln-Pro-His-Thr-Gly-Gly-Thr-225, which may be compared to the E. coli hypoxanthine phosphoribosyltransferase sequence, 94-Asp-Val-Leu-Ile-Val-Glu-Asp-Ile-Ile-Asp-Ser-Gly-Asn-Thr-107. Neamine phosphoribosyltransferase contains 604 amino acid residues and is much larger than type I phosphoribosyltransferases (316), such as E. coli hypoxanthine and guanine-xanthine phosphoribosyltransferases, which contain 178 and 152 amino acids, respectively. It is also larger than type II phosphoribosyltransferases, such as E. coli quinolinate and nicotinate phosphoribosyltransferases, which contain 297 and 400 amino acids, respectively. It is possible that the neamine phosphoribosyltransferase is a bifunctional enzyme, although a second activity has not been discovered. This putative second activity is not the phosphatase that hydrolyzes the phosphate ester of 5″-phosphoribostamycin to ribostamycin, as this activity is encoded by the btrP cistron (316).
In addition to butirosin, a number of other aminoglycoside antibiotics contain a pseudodisaccharide resembling that of butirosin as well as a ribosyl moiety. Examples of these antibiotics are neomycin B, paromomycin, and lividomycin B. The neomycin B biosynthesis-specifying gene cluster of Streptomyces fradiae contains the neoL reading frame (accession number CAF33322), whose deduced amino acid sequence shows only 22% amino acid sequence identity with that of neamine phosphoribosyltransferase. However, certain stretches of amino acids along the sequences appear highly conserved, among these is the PRPP-binding site (317). Similarly, a putative neamine phosphoribosyltransferase may be present in Streptomyces lividus, because an ORF (accession number CAG38706) of the lividomycin B biosynthetic cluster of S. lividus is 29% identical to the B. circulans neamine phosphoribosyltransferase amino acid sequence. Details on the genetics of aminoglycoside antibiotic biosynthesis have been previously reviewed (318).
Reactions at the Diphosphoryl Moiety of PRPP
A few enzymes have been shown to hydrolyze the phosphoric anhydride bond of the diphosphoryl moiety of PRPP: PRPP + H2O → ribosyl 1,5-bisphosphate + Pi. A number of archaeal species, such as M. jannaschii, Methanosarcina acetivorans, A. fulgidus, and T. kodakarensis, contain a form III ribulose 1,5-bisphosphate carboxylase/oxygenase, which catalyzes the carboxylation of ribulose 1,5-bisphosphate. Usually ribulose 1,5-bisphosphate is formed by ATP-dependent phosphorylation of ribulose 5-phosphate. However, in M. jannaschii and M. acetivorans, ribulose 1,5-bisphosphate originates from PRPP, and it has been suggested that PRPP is hydrolyzed to ribose 5-phosphate followed by NAD+-dependent oxidation to ribulose 1,5-bisphosphate. Alternatively, PRPP may be transformed to ribosyl 1,2-cyclic phosphate and then to ribulose 1,5-bisphosphate. The physiologic function of this pathway remains to be established (319). In addition, certain diphosphoryl (Nudix) hydrolases are able to hydrolyze PRPP with the formation of ribosyl 1,5-bisphosphate and Pi (320). Finally, a PRPP pyrophosphatase activity has been identified in macrophages exposed to hypoxia (321).
Cofactor Biosynthesis
Thiamine diphosphate.
Thiamine diphosphate synthesis utilizes 5′-phosphoribosyl 5-aminoimidazole as a precursor. This compound is also a precursor of purine nucleotide biosynthesis. 5′-Phosphoribosyl 5-aminoimidazole is converted to a pyrimidine moiety by thiC-encoded phosphomethylpyrimidine synthase. As such, some of the carbons of PRPP end up in the pyrimidine moiety of thiamine diphosphate. Thus, C-2 of PRPP becomes the methyl group of the pyrimidine moiety, C-4 of PRPP becomes C-5 of the pyrimidine moiety, and C-5 of PRPP becomes the methylene group that connects the pyrimidine and thiazole moieties of thiamine diphosphate. C-1 and C-3 of PRPP are lost as formate and carbon dioxide (322).
Flavins.
The ribitol moiety of the flavin-containing cofactors FAD and FMN are derived from the ribosyl moiety of GTP, and thus originally from PRPP (323, 324).
Pterins.
The pterin moiety of folate derivatives are synthesized from GTP. In tetrahydrofolate, the C-7, C-6, and the methylene attached to C-6 of the pterin moiety are derived from C-1, C-2, and C-3 of PRPP. C-4 and C-5 of PRPP are lost as glyoxylate. The synthesis of pterin and methanopterin follow identical pathways at this stage (325, 326). Also, the molybdopterin is synthesized from GTP with similar utilization of carbons of PRPP (327).
Carbon-Phosphorus Lyase Pathway
As mentioned previously, PRPP can be synthesized in a reaction catalyzed by phosphoribosyl bisphosphate phosphokinase. This reaction is part of the carbon-phosphorus lyase pathway, by which phosphonates are catabolized by means of a radical-mediated process. This catabolic pathway is specified by the 14-cistron operon phnCDEFGHIJKLMNOP found in numerous microorganisms, including E. coli (154). Phosphoribosyl bisphosphate phosphokinase is specified by the phnN cistron and catalyzes the following reaction: ribosyl 1,5-bisphospate + ATP → PRPP + AMP, which is the final chemical reaction specified by the phnC-D operon (151, 153). Thus, catabolism of phosphonates by the carbon-phosphorus lyase pathway produces PRPP, in which the phosphorus of phosphonate is represented by the α-phosphate at the C-1 position. The utilization of phosphonate-derived phosphorus requires one or more of the above-described phosphoribosyltransferases, which produces PPi, which can by hydrolyzed to Pi by diphosphatase (154, 328).
PRPP AS MEDIATOR OF METABOLIC REGULATION
Until now this review has presented enzymes that produce PRPP and enzymes that consume PRPP with the formation of N-, O-, or C-glycosidic bonds. The following section is dedicated to the regulatory functions played by PRPP. These functions include binding to the PyrR regulator, which is involved in translational control of pyrimidine biosynthesis gene expression, binding to the PRPP responsive PurR regulator of Gram-positive organisms with low GC content, and allosteric activation by PRPP of carbamoylphosphate synthetase.
The Enigma of PRPP as a Regulator of Metabolic Activity
Bacterial enzymes that utilize PRPP generally have Km values at least 1 order of magnitude lower than the normal intracellular PRPP concentration. Thus, the PRPP concentration is unlikely to be a rate-determining factor for phosphoribosyltransferases. No regulatory function, such as allosteric inhibition, of phosphoribosyltransferase activity by PRPP at a metabolic level under normal growth conditions has been identified experimentally. Traditionally, PRPP pool sizes are expressed as millimoles per gram (dry weight); this unit is dependent on the cell composition and is not directly translatable into the intracellular molar concentration for comparisons between different species. However, it appears safe to state that the PRPP concentration in bacterial cells is in the millimolar range (329, 330). Bacteria regulate their PRPP pools in response to exogenous purine sources. Thus, the PRPP pool of B. subtilis, L. lactis, E. coli, or S. enterica is reduced 2- to 4-fold by the addition of adenine or hypoxanthine (Table 5). This reduction in PRPP pool size may be caused by allosteric and isosteric regulation of PRPP synthase by purine nucleotides, i.e., ADP and GDP, as described above.
TABLE 5.
PRPP pool sizes in various bacterial species
| Bacterial species | Purine added | PRPP pool sizea | Reference |
|---|---|---|---|
| B. subtilis | None | 1.1 ± 0.1 | 356 |
| Adenine | 0.6b | 356 | |
| L. lactis | None | 2.6 ± 0.6 | 414 |
| Hypoxanthine | 0.7 ± 0.2 | 414 | |
| E. coli | None | 4.4 | 415 |
| Hypoxanthine | 1.2 | 415 | |
| S. enterica | None | 1.2 | 36 |
| Hypoxanthine | 0.3 | 36 |
The pool size units (means ± standard errors) are millimoles per gram dry weight.
No repression was observed with hypoxanthine for this species.
The situation is different in mammals, where the PRPP concentration is rate limiting for purine biosynthesis de novo and for salvage reactions (331), which suggests that the rates of PRPP-utilizing enzymes may be determined by substrate saturation kinetics. The PRPP concentration in mammalian cells is much lower than that of bacteria, 5 to 30 μM, although there are variations throughout the cell cycle (331, 332).
We are therefore faced with the enigma of explaining how the high intracellular PRPP levels of microbial organisms are sensed by regulator proteins, which presumably should be saturated with PRPP under most physiological conditions. The enigma has been only partly resolved, and further research will be needed to fill in the missing information. Details of the mechanism of PRPP-mediated regulation of pyrimidine and purine nucleotide synthesis by the PyrR and PurR regulatory proteins are described in the next section.
Regulation of Pyrimidine Metabolism by the RNA-Binding PyrR Protein
Pyrimidine biosynthetic genes are present in most organisms, and their expression is subject to control by different mechanisms. Regulation of bacterial pyrimidine metabolism has been extensively reviewed previously (333–335). Here we shall concentrate on a description of the structure of PyrR and the involvement of PRPP in PyrR-mediated regulation of pyrimidine biosynthesis in B. subtilis, B. caldolyticus, and L. lactis.
Three-dimensional structure of PyrR.
The three-dimensional structure of PyrR from three microbial sources has been published: B. subtilis (336), B. caldolyticus (337), and M. tuberculosis (338). The three-dimensional structure of T. thermophilus PyrR has also been solved (PDB code 1ufr). The overall structures of the four PyrR proteins are identical and contains the typical type I phosphoribosyltransferase fold, a five-stranded β-sheet surrounded by three helices, and the typical sequence elements are easily identified: a PRPP-binding site, i.e., a PRPP-binding loop (100-Val-Ile-Leu-Val-Asp-Asp-Val-Leu-Phe-Thr-Gly-Arg-Thr-112 of B. caldolyticus PyrR), a PP loop (37-Gly-Ile-Lys-Thr-Arg-41 of B. caldolyticus PyrR), a flexible loop (Thr71 to Asn83 of B. caldolyticus PyrR), and a hood structural element. The binding of UMP and GMP to B. caldolyticus PyrR involves identical amino acid residues, Asp104, Asp105, Thr109, and Arg137. The 104-Asp-Asp-105 dipeptide of the PRPP-binding loop binds to the nucleotide via Mg2+. Altogether, the binding of PRPP and nucleotides to PyrR is similar to that of type I phosphoribosyltransferases. PyrR from B. caldolyticus has been proposed to exist as a dimer or tetramer (337, 339, 340), although B. subtilis PyrR has been shown to exist in a hexameric state (341). The dimerization pattern of PyrR is different from that of type I phosphoribosyltransferases, and the RNA-binding activity of PyrR has been proposed to be due to the exposure of a region of basic amino acid residues in the dimer. Effectors of the quaternary structure are PRPP, uridine ribonucleotides, GTP, and pyr leader mRNA. A close resemblance of the crystal structure of GMP-bound B. caldolyticus PyrR to an evolutionarily distant hypoxanthine-guanine phosphoribosyltransferase suggests that the two proteins share ancestry (337). However, PyrR from both B. subtilis and B. caldolyticus have a low uracil phosphoribosyltransferase activity that can support slow growth of a pyrimidine requiring strain on uracil (248, 342).
Mechanism of PRPP-mediated regulation by PyrR.
The B. subtilis pyr gene cluster encodes all of the enzymes required for the biosynthesis of UMP, a uracil transporter, and the regulatory protein PyrR (342). Transcription is controlled by pyrimidine availability through an attenuation mechanism. There are three terminator-antiterminator structures in pyr mRNA. PyrR binds to a conserved region in the pyr operon leader resulting in disruption of an antiterminator that is the dominant RNA structure in the absence of PyrR binding. The negative effect of PyrR is observed only when pyrimidine precursors are available in the growth medium (341–343).
The active RNA binding form of PyrR appears to be a dimer (Fig. 13A) which is capable of recognizing and binding a specific RNA structure present in the leader mRNA transcripts of PyrR-regulated genes (340). Dimer formation and RNA binding occurs when UMP, UTP, or PRPP is bound to PyrR, resulting in transcriptional termination. GTP counteracts the binding of UTP (and possibly UMP), resulting in multimer formation and presumably transcriptional readthrough because the RNA-binding sites are sequestered in the tetramer. UMP, UTP, and GTP bind to PyrR at physiological concentrations, while PRPP binding has an apparent dissociation constant at subphysiological concentrations, as discussed above (340).
FIG 13.
Mechanism of PRPP-mediated regulation of transcription by PyrR and PurR. PyrR and PurR are shown as orange spheres. (A) Model of PyrR regulation based on the combined data from B. subtilis and B. caldolyticus. RNA (green hairpin) binds to dimeric PyrR, which is stabilized by UMP, UTP, or PRPP. The tetrameric conformation is stabilized by GMP and does not bind RNA. See the text for details. (B) Model of PurRBs repression. DNA (blue line) containing one strong (solid green line) and one weak (green punctuated line) PurBox binds to two PurRBs dimers (forming a weak tetramer) in the absence of PRPP. In the presence of PRPP, the DNA binding is prevented and the tetramerization is lost. (C) Model of PurRLl activation. PurRLl binds to PurBox sequences, irrespective of PRPP binding, presumably as a dimer. Binding of PRPP is hypothesized to expose a binding site for RNA polymerase. RNA polymerase is positioned correctly relative to the −10 region of the promoter.
The physiological relevance of PRPP binding to L. lactis PyrR was demonstrated by transcriptomic analysis of nucleotide metabolism. L. lactis pyr gene expression is regulated by PyrR-dependent attenuation with a mechanism similar to that of B. subtilis (344–346). The mRNA levels under PyrR attenuation control showed an inverse correlation with the intracellular PRPP concentrations (347), in accordance with an increased RNA binding and attenuation upon PRPP binding to PyrR. It is reasonable to suspect that PyrR from L. lactis also binds RNA as a dimer and that dimers are stabilized in relation to tetramers by binding of PRPP. An increase in PRPP concentration is a consequence of purine shortage and hence a diminished demand for pyrimidine synthesis; thus, the inverse correlation of PRPP and pyr gene expression serves a valuable physiological function.
Proteins with homology to PyrR are found among many bacterial species. With few exceptions, PyrR is found among Firmicutes, Cyanobacteria, and Actinobacteria. Apparently, PyrR is absent from the alphaproteobacteria, but it is present in some orders of the other classes of proteobacteria. PyrR is absent from Enterobacteriales but present in the Pseudomonadales order. B. subtilis has two different enzymatic activities that catalyze the formation of UMP from uracil and PRPP, PyrR and uracil phosphoribosyltransferase, encoded by upp (248). PyrR and uracil phosphoribosyltransferase share 23% identity, which suggests that PyrR, aside from its close ancestry to the hypoxanthine-guanine phosphoribosyltransferases, also shares ancestry with uracil phosphoribosyltransferases.
Regulation of Purine Metabolism by PRPP-Responsive, DNA-Binding PurR Proteins
PRPP-responsive PurR transcriptional regulators are found only in low-GC Gram-positive bacteria, but the members of this small protein family differ widely in their mechanism of regulation. The B. subtilis PurR, here designated PurRBs, is a negative regulator, whereas the L. lactis PurR, here designated PurRLl, is an activator of purine metabolism gene expression. In contrast to the PRPP-responsive PurR regulators from Gram-positive bacteria, a LacI-type PurR repressor protein is present in E. coli and a variety of other bacterial species. The LacI-type PurR proteins do not respond to PRPP, but they repress the transcription of purine genes when bound to the corepressors hypoxanthine or guanine (348, 349). In the following sections, we shall describe in more detail the structure and function of B. subtilis and L. lactis PurR.
Three-dimensional structure of B. subtilis PurR.
B. subtilis PurR contains 285 amino acid residues and has a homodimeric quaternary structure (350). A C-terminal domain of the subunit (residues 77 to 285) has the characteristic fold of type I phosphoribosyltransferases and PRPP synthases described previously, a central parallel β sheet flanked by α-helices. Hood and flexible loop structural elements are also present. The C-terminal domain of PurRBs also contains a PRPP-binding site, i.e., a PRPP-binding loop (196-Gly-Ser-Asn-Val-Leu-Ile-Ile-Asp-Asp-Phe-Met-Lys-Ala-Gly-Gly-Thr-211) and a PP loop (139-Thr-Lys-Gly-Ile-142) (351).
Gel mobility shift analysis showed that binding of PRPP to PurRBs changes its affinity toward its DNA-binding sites (350, 352). X-ray structural analysis of PurRBs crystals containing the PRPP analog cPRPP revealed that the compound binds to the PRPP and PP loops analogous to PRPP binding in type 1 phosphoribosyltransferases. Contacting amino acid residues were Lys140 and Asp203/Asp204 of the PP- and PRPP-binding loop, respectively, as well as residues in the flexible loop (353). Curiously, the binding of cPRPP occurred without the participation of Mg2+, which may suggest relatively weak binding of cPRPP. DNA binding of two mutant variants of PurRBs, Asp203Ala and Asp204Ala, could not be inhibited by PRPP in vitro, and the alterations caused a superrepression phenotype of pur gene expression consistent with poor binding of PRPP to the altered PurRBs proteins (352). Altogether, binding of PRPP to PurRBs strongly resembles the binding of PRPP to type I phosphoribosyltransferases.
In spite of the high sequence similarity to type I phosphoribosyltransferases, PurRBs is inactive as a phosphoribosyltransferase and does not even bind purine bases. This deficiency is explained by the interactions of the large side chains of well-conserved Tyr102 and Phe205, which prevent the binding of a nucleophile in proper position relative to C-1 of PRPP. The presence of these bulky amino acid side chains also explains the inability of compounds containing nucleobases to inhibit PurRBs-PurBox interactions (351). Interestingly, xanthine phosphoribosyltransferases contain phenylalanine in the analogous position to Phe205, which indicates that the Phe205-Tyr102 interaction is crucial to blocking access of purines to the active site of PurR.
The N-terminal domain (residues 1 to 74) of PurRBs forms a winged-helix domain, a subdivision of the DNA binding helix-turn-helix domains (351), and is attached to the C-terminal domain by a three-residue linker. This domain is responsible for the binding of PurRBs to so-called PurBox sites located upstream of the PurR-regulated operons (353). Functionally, PurRBs may be regarded as a crippled phosphoribosyltransferase which has lost the catalytic activity but retained the PRPP-binding capability and has gained an N-terminal DNA-binding domain.
Mechanism of PRPP-mediated regulation of purine biosynthesis in B. subtilis.
The addition of adenine to cultures of B. subtilis causes repression of transcription of a large number of operons, including genes for purine nucleotide de novo biosynthetic enzymes, genes specifying purine transport proteins, genes for enzymes in purine nucleotide interconversion, and certain cofactor biosynthetic genes (350, 354, 355). Similar to the situation in enteric organisms (146), the addition of adenine to cultures of B. subtilis causes depletion of the PRPP pool (356). Induction of expression of PurR-regulated genes by PRPP binding has been evaluated in PurRBs-DNA-binding assays in vitro (350). PurRBs binds to a region 20 to 150 bp upstream of the B. subtilis pur operon (357). This DNA region contains the operator region −81AAACACGAACATTA (PurBox1)-16 nucleotides-TATCGTTCGATAAT−38 (PurBox2), numbered relative to the transcription start site. PurBox1 and PurBox2 have similarity to the consensus PurBox (AWWWCCGAACWWT), although PurBox2 is inverted (354, 358). Functional dissection has defined a minimal control region and revealed the importance of the two PurBoxes in the overall regulatory process. Based on the affinity of PurRBs for the PurBoxes, PurBox1 was designated “strong” and PurBox2 was designated “weak.” High-affinity binding of PurR to the control region was shown to require at least one strong PurBox, whereas the induction of PurRBs repression by PRPP requires at least one weak PurBox. The binding of PurRBs to the operator is cooperative with a stoichiometry of two PurRBs dimers binding to the operator, which suggests that one PurRBs dimer binds to PurBox1 and another PurRBs dimer to PurBox2, with wrapping of the DNA around the two PurRBs dimers (Fig. 13B) (353).
In B. subtilis a guanine-responsive attenuation mechanism is superimposed on the repression of transcription initiation by the PurR regulatory system, which results in a complex regulatory pattern. Thus, the addition of guanine to cultures of B. subtilis causes premature transcription termination in the mRNA leader region of purE (the upstream cistron of the major 12-cistron pur operon) (359). This premature transcription termination is caused by the purine riboswitch mechanism, which regulates the pur, xpt, pbuG, and yxjA operons (360). This riboswitch mechanism involves the binding of guanine or hypoxanthine to the untranslated leader sequences but does not involve PRPP. Details of the mechanisms of riboswitches have been previously reviewed (361, 362).
Mechanism of PRPP-mediated regulation of purine biosynthesis in L. lactis.
Overall, the effect of purine addition has the same effect on L. lactis as that on B. subtilis: the PRPP level drops and the expression levels of genes of the PurR regulon are lowered (363). However, a different mechanism of action became apparent when the L. lactis purR gene was identified among purine auxotrophic strains obtained after transposon mutagenesis. The purR insertion mutation caused reduced transcription of purD, which was complemented by purR+. These and other data showed that L. lactis purR encodes an activator of purine gene expression. PurRLl contains 271 amino acid residues, close to the length of PurRBs (285 amino acids). Remarkably, PurRLl and PurRBs are 48% identical (69% similar), and yet their mechanisms are very different. Like PurRBs, PurRLl contains a helix-turn-helix domain at the N-terminal end. It also contains the typical type I phosphoribosyltransferase structural elements, a PRPP-binding site, i.e., a PRPP-binding loop (194-Gly-Gln-Asn-Val-Leu-Ile-Val-Asp-Asp-Phe-Met-Lys-Gly-Ala-Gly-Thr-209), a PP loop (137-Thr-Lys-Gly-Ile-140), and a flexible loop (358).
PurRLl recognizes PurBox sequences similar to the consensus PurBox sequence, AWWWCCGAACWWT (364). PurRLl binds to PurBoxes under both activating (high PRPP) and nonactivating conditions. Activation of transcription requires that the PurBox sequence is located at an exact position relative to the −10 promoter region, presumably in order to bind and position RNA polymerase correctly for open complex formation (358) (Fig. 13C). The standard −35 promoter elements of constitutive promoters are usually absent from PurR-activated promoters. In L. lactis, different topologies of PurBoxes have been identified in which the binding sites are located in tandem or head to head, which suggests that PurR may form different multimeric conformations (363).
In vivo kinetics of the PRPP-modulated activation of transcription by PurRLl was studied by a combination of temporal mRNA and intracellular PRPP quantifications during a metabolic downshift (347). Saturation curves for the coupled process of PRPP binding to PurRLl and subsequent activation of mRNA synthesis were obtained for all genes belonging to the PurR regulon in L. lactis. Interestingly, most saturation curves appeared sigmoid, suggesting mechanisms employing cooperativity, but more classical Michaelis-Menten curve forms were apparent for some PurR-activated genes involved in purine salvage and folate metabolism (347). To our knowledge, this work represents the first example of a kinetic analysis of the action of a regulatory protein performed in a living organism.
Evolution of PurR.
PurR proteins from various organisms share extensive sequence similarity, but the differences in regulatory mechanisms between PurRBs and PurRLl show that they have diverged considerably during evolution. Figure 14 shows the phylogenetic relationship among members of the PurR family. The branching pattern of the PurR phylogeny resembles the branching pattern for the whole-genome bacterial phylogeny (365), indicating that the PurR protein was present during most of the evolution of the low-GC branch of Gram-positive bacteria. The Bacillus branch and the Streptococcus branch share an ancestor, which is not shared by any other species. It is apparent that the bacilli and streptococci use the B. subtilis type PurBox, while all the others use the L. lactis type PurBox (Fig. 14A), except the clostridia, for which no information is available. According to the unrooted phylogenetic tree, it is likely that the activating PurRLl type evolved first and that the PurRBs type diverged early in the evolution of the low-GC Gram-positive bacteria. As a consequence, the L. lactis PurBox must simultaneously have evolved into the B. subtilis PurBox. When the sequences in the logo plots for the two PurBox types are compared (Fig. 14B), it is easily recognized that the B. subtilis PurBox consists of two L. lactis type PurBox sequences located as inverted repeats with a spacing of 16 nucleotides, as previously described (354).
FIG 14.
Phylogenetic analysis of PurR from low-GC Gram-positive bacteria. (A) PurR sequences from representatives of the major bacterial lineages in the low-GC Gram-positive bacteria were aligned using the Clustal Omega program, and a phylogenetic tree was constructed using the ClustalW2 phylogeny program. (B) The type of PurBox used by each species was identified at the RegPrecise website (http://regprecise.lbl.gov/RegPrecise/collection_tffam.jsp?tffamily_id=53), and the two types of logo plots were constructed from the PurBox sequences presented for Streptococcaceae and Bacillales, respectively, using the weblogo service (http://weblogo.berkeley.edu/logo.cgi).
To further analyze the relationship of PurR with enzymes containing a type I phosphoribosyltransferase fold, a phylogenetic analysis was performed for homologs of B. subtilis class 1 phosphoribosyltransferases with all the major bacterial lineages, spanning an evolution of at least 3 billion years. Included in the analysis are also logo plots of conserved amino acids in the PP loop and the PRPP-binding loop (Fig. 15). It is apparent that PurR and xanthine phosphoribosyltransferase are close relatives (366), that PyrR and hypoxanthine-guanine phosphoribosyltransferases are also closely related (337), and that they share ancestry with uracil phosphoribosyltransferases (336). As noted in Fig. 15, PurR and xanthine phosphoribosyltransferase homologs are only found in the low-GC Gram-positive bacterial lineage. PurR and adenine phosphoribosyltransferase has previously been inferred to be structurally related, based upon the common structure of the flexible loop, which forms a two-stranded antiparallel β-ribbon structure (210). A similar structure is also present in the flexible loop of B. subtilis xanthine phosphoribosyltransferase (366). Thus, PurR, xanthine phosphoribosyltransferase, and adenine phosphoribosyltransferase are all structurally related.
FIG 15.
Common phylogeny of PyrR, PurR, type 1 phosphoribosyltransferases, and class I PRPP synthases in all major bacterial lineages. Homologous protein sequences were deduced and identified from representative genome sequences of all major bacterial lineages for the following proteins: adenine phosphoribosyltransferase (APRTase), hypoxanthine-guanine phosphoribosyltransferase (HPRTase), orotate phosphoribosyltransferase (OPRTase), uracil phosphoribosyltransferase (UPRTase), and xanthine phosphoribosyltransferase (XPRTase). The collected sequences were aligned using the Clustal Omega program, and a phylogenetic tree was constructed using the ClustalW2 phylogeny program. (Left) Phylogenetic tree, with protein sequences belonging to same enzyme families grouped together as triangles, starting at the point of the first branching of the individual protein sequences and labeled with the enzyme abbreviations shown above. The triangle marked OPRTase* is a group of orotate phosphoribosyltransferase sequences that appear to form a separate branch with a distinct set of amino acid sequences in the PP- and PRPP-binding loops. Sequences with homology to the xanthine phosphoribosyltransferase and PurR proteins were only found among the low-GC Gram-positive bacteria, as indicated. (Middle) Logo plot of the frequency of amino acids in the PP loop identified in the Clustal Omega alignment and constructed using the weblogo service (http://weblogo.berkeley.edu/logo.cgi). (Right) Logo plot of the frequency of amino acids in the PRPP-binding loop identified in the Clustal Omega alignment and constructed using the weblogo service.
If PurR arose by evolution from a xanthine phosphoribosyltransferase, perhaps it could have exploited some of the peculiarities of this enzyme. Of special interest is the fact that the active form of xanthine phosphoribosyltransferase is a dimer and that binding of PRPP is necessary for stabilization of the dimeric conformation (366). Most other type I phosphoribosyltransferases have active tetrameric rather than dimeric structures. Dimer formation could be important for PRPP modulation of PurRLl as it is for PurRBs, perhaps by exposing interaction sites needed for RNA polymerase capture.
Search for a common mechanism for PRPP modulation of PurR conformation.
PurR is a family of proteins that share extensive similarity but which are divided into at least two phylogenetic branches with different PurR functionalities, a B. subtilis-like branch and an L. lactis-like branch. Members of both branches appear to have rigid DNA-binding domains with hydrophobic cores that are not readily modulated by PRPP binding, and both appear to harbor extensive positively charged domains that may bind the DNA backbone and result in protection of large DNA stretches. Yet, regulators from one family represented by PurRLl appear to bind to single-PurBox motifs with unknown stoichiometry. Presumably, the binding of PRPP and closure of the flexible loop expose a binding site that is used to recruit RNA polymerase. Regulators from the second family, represented by PurRBs, recognize two PurBoxes in a stoichiometry of one PurR dimer per PurBox and in a conformation that involves formation of PurR tetramers (Fig. 13B and C). Occupancy of the PRPP-binding site reduces unspecific DNA binding and prevents PurR repression of transcription initiation. It is likely that the closing of the flexible loop upon PRPP binding, which is also shared by all type I phosphoribosyltransferases, is the mechanistic basis for both effects. There is currently no evidence that all PurR regulators in one family share a mechanism, and it is not clear whether PurRLl and PurRBs may substitute for one another. However, B. subtilis-like tandem PurBoxes are found only in the Bacillus and Staphylococcus branches and single PurBoxes are found in the Lactococcus, Streptococcus, and Lactobacillus branches. A search for evolutionary “crossover” organisms with PurR proteins that possess both repression and activation abilities may be very useful in this respect.
Allosteric Activation of Carbamoylphosphate Synthetase Activity by PRPP
Carbamoyl phosphate is a precursor for the synthesis of pyrimidine nucleotides and the amino acid arginine. While bacterial species like E. coli and S. enterica contain only a single carbamoyl phosphate synthetase, other bacterial species, like B. subtilis, contain specific enzymes for each pathway, pyrimidine nucleotides and arginine. The activity of pyrimidine nucleotide-specific enzyme was found to be stimulated by PRPP (367, 368).
In animals and fungi, two or three of the six enzymes of de novo pyrimidine nucleotide biosynthesis are grouped in di- or trifunctional enzymes. Animals contain the trifunctional fusion of carbamoylphosphate synthase, aspartate transcarbamoylase, and dihydroororase activities, called CAD. CAD thus catalyzes the ATP-dependent conversion of glutamine, bicarbonate, and aspartate to dihydroorotate. The function and structure of CAD have been previously reviewed (369, 370). Dihydroorotate in turn is oxidized to orotate, which is then phosphoribosylated to orotidine 5′-monophosphate, a reaction catalyzed by orotate phosphoribosyltransferase. In the present context, the important activity of CAD is carbamoyl phosphate synthase. The activity of this enzyme is regulated by UTP and PRPP. UTP is a feedback inhibitor, whereas PRPP is an allosteric activator of the enzyme, as found for the B. subtilis pyrimidine-specific carbamoyl phosphate synthetase (368, 371). CAD activity is modulated further by protein phosphorylation mediated by the mitogen-activated protein kinase (372), cAMP-dependent protein kinase (372), and mTORC1 (373). The expression of the cad genes is also subject to regulation. Upon transition from G1 to S phase, the human cad mRNA level increased approximately 10-fold, which is mediated by the global regulator c-MYC (373, 374).
prs GENES, MUTANTS, AND GENE REGULATION
PRPP synthase is believed to be present in all free-living organisms. A number of prs mutants with alterations in the enzyme have been isolated and characterized. In addition, a few knockout mutant alleles have been constructed in vitro and, following recombination, gene conversions have yielded null alleles. Also, mutant variants of PRPP synthase have been characterized in a wide variety of organisms, including humans. We review here the properties of these mutant variants of bacterial origin.
E. coli and S. enterica
E. coli prs-1 and prs-2.
A number of prs mutants have been isolated in E. coli. First, the prs-1 allele appeared in a genetic selection designed to isolate mutants with improved utilization of guanosine as a purine source (28). Briefly, five mutants were characterized; all of them had a lesion in the gsk gene as well as a lesion in a gene specifying proteins of an early part of the purine nucleotide biosynthesis de novo pathway, i.e., mutants with the genotype purF gsk and purM gsk. The appearance of the prs-1 gsk mutant strain may be explained along this line by extending the purine nucleotide biosynthesis de novo pathway to include prs in addition to purF, purD, purL, etc. The prs-1 allele specifies Asp128Ala. This residue is only two residues away from His130, equivalent to His135 of B. subtilis PRPP synthase. As described before, His135 is important for the coordination of the MG2 site, i.e., maintaining the α,β,γ-tridentate complex of MgATP. Consistent with this, PRPP synthase specified by prs-1 had a 27-fold increase in the Km for ATP, whereas the Km for ribose 5-phosphate was essentially unaltered (375).
A temperature-labile PRPP synthase, specified by the prs-2 allele, was isolated by localized mutagenesis of the hemA region (2). The prs-2 allele contained two mutations, one responsible for a Gly8Ser alteration, the other a cytidylate-to-thymidylate transition that results in an alteration close to the Shine-Dalgarno sequence. The latter mutation resulted in increased synthesis of PRPP synthase and thus in part compensated for a poorly functioning enzyme. The Gly8Ser alteration was responsible for the temperature-labile PRPP synthase (376).
In vitro-generated knockout prs alleles.
A prs knockout mutation would render the strain prototrophic for purine, pyrimidine, and pyridine nucleotides as well as histidine and tryptophan. Any scheme to isolate such a strain must include the following considerations. NAD, or its metabolite, nicotinamide mononucleotide, may be utilized intact without catabolism to satisfy the pyridine nucleotide requirement (377). The requirement of PRPP for tryptophan and histidine synthesis can be eliminated by the addition of these two amino acids to the growth medium. In contrast, PRPP is normally essential for both de novo and salvage purine and pyrimidine nucleotide reactions. PRPP consumption in de novo synthesis of purine and pyrimidine nucleotides is mediated by purF-specified amidophosphoribosyltransferase and pyrE-specified orotate phosphoribosyltransferase, whereas the salvage reactions of purine and pyrimidine nucleotides are mediated by adenine, hypoxanthine, xanthine, guanine, and uracil phosphoribosyltransferases. Some organisms, such as E. coli and S. enterica, also contain nucleoside kinases, which phosphorylate nucleosides, such as inosine, guanosine, and uridine to IMP, GMP, and UMP, respectively. Uptake of these nucleosides via these kinase reactions would eliminate the requirement for PRPP. However, nucleosides are predominantly phosphorolyzed by nucleoside phosphorylases, such as purine nucleoside phosphorylase I (deoD) with specificity for adenosine, inosine, and guanosine, purine nucleoside phosphorylase II (xapA) with specificity for inosine, xanthosine and guanosine, and uridine phosphorylase (udp) with specificity for uridine (19). Thus, knockout of deoD and udp is necessary to permit the salvage of nucleosides by phosphorylation of guanosine by guanosine kinase (gsk) and of uridine by uridine kinase (udk). With respect to guanosine utilization, yet another genetic lesion was necessary to permit isolation of a Δprs strain. This lesion, for example gsk-3, improved the activity of guanosine kinase by rendering the enzyme insensitive to feedback inhibition by GTP and presumably GDP (378; Hove-Jensen, unpublished data). Altogether, a background strain, a deoD udp gsk-3 mutant strain, was used for the successful recombination of the in vitro-generated, plasmid-borne Δprs allele into the chromosome of E. coli. (The growth medium included guanosine, uridine, histidine, tryptophan, and NAD.) Two alleles, Δprs-3::Kanr and Δprs-4::Kanr, were recombined into the chromosome (3). Also, the temperature sensitivity of the prs-2 allele could be rescued in the deoD udp gsk-3 genetic background (2).
dnaR.
PRPP synthase has been shown to be involved in DNA replication, but it is unclear whether the effect is direct or indirect. A genetic lesion of E. coli, dnaR130, was shown to be an allele of the prs gene, here designated prs-130. A strain harboring prs-130 was temperature sensitive for initiation of DNA replication, whereas elongation was unaffected. Only replication initiating at the chromosome-borne chromosomal origin of replication, oriC, was affected by prs-130. Thus, replication of an oriC-harboring plasmid, stable DNA synthesis, i.e., replication independent of RNase HI (specified by rnhA), and replication of bacteriophage λ were all unaffected by prs-130. The fact that bacteriophage λ DNA replication was unaffected by temperature and that identical bacteriophage λ burst sizes of prs-130 and prs+ strains were observed prompted the researcher to suggest that the temperature-sensitive DNA replication of the prs-130 strain was not caused by lack of precursors for nucleic acids or proteins. Also, the decline in PRPP synthase activity specified by prs-130 paralleled the decline in DNA replication upon temperature shift. The prs-130 lesion is located within a 274-bp HindIII-AvaII DNA fragment of the prs coding sequence. The entire prs-130 allele has been sequenced, but the sequence cannot be found in the available databases (379, 380).
A number of mutant alleles of genes other than prs were able to suppress the temperature sensitivity caused by the prs-130 allele. These extragenic suppressors of prs-130 include dnaA (381), certain rpoB alleles specifying rifampin-resistant variants of the β-subunit of RNA polymerase (382), and rpe, specifying d-ribulose 5-phosphate 3-epimerase. Suppression of prs-130 by mutations in the dnaA gene prompted the study author to suggest a direct physical interaction of DnaA and PRPP synthase, whereas the appearance of prs-130 suppressors in RNA polymerase suggested involvement of PRPP synthase in a transcriptional event in DNA replication at oriC. Remarkably, d-ribulose 5-phosphate 3-epimerase (specified by the rpe gene) also suppressed the temperature-sensitive phenotype of the prs-130 strain (383). Ribulose phosphate epimerase catalyzes the interconversion of ribulose 5-phosphate and xylulose 5-phosphate, and the enzyme is part of the nonoxidative pentose-phosphate pathway (384), which involves ribose 5-phosphate, the precursor of PRPP. This fact leads us to ask whether knockout of rpe would result in an increase of ribose 5-phosphate isomerase activity? This activity catalyzes the interconversion of ribulose 5-phosphate and ribose 5-phosphate, which might lead to improved PRPP synthesis.
Altogether, these various observations demonstrate an involvement of PRPP synthase in some aspects of DNA synthesis in E. coli, but the detailed biochemistry of these effects remain unclear.
ssrA.
The ssrA gene specifies the SsrA RNA, which together with the SmpB protein is involved in rescuing stalled ribosomes. SsrA RNA functions both as a tRNA and as an mRNA molecule; SsrA RNA function results in the synthesis of an Ssr polypeptide fusion that is destined for degradation and the liberation of the stalled ribosome (385, 386). Two temperature-sensitive mutants were isolated in an ΔssrA genetic background. Both mutations mapped within the prs gene, one of which specified Cys215Tyr. The temperature-sensitive phenotype was suppressed by reinsertion of a wild-type ssrA gene (387). Similar mutants were isolated involving the thyA gene (388), and thus the restoration of the activity of SsrA is not restricted to misexpression of the prs gene (386).
However, using SmpB as bait in pulldown assays, SsrA RNA and a number of polypeptides were copurified. One of these polypeptides was PRPP synthase. Half-maximal binding of PRPP synthase to SsrA RNA was obtained at 1 to 2 μM PRPP synthase, which was compared to half-maximal binding of ribosomal protein S1 to SsrA RNA at 0.030 μM, which indicates relatively poor binding of PRPP synthase to SsrA RNA. The binding of PRPP synthase is therefore relatively unspecific, so that physiologically significant binding of PRPP synthase to an SsrA RNA particle, if it occurs at all, may require additional protein factors (389).
S. enterica prs-100.
The prs-100 allele, previously designated prsB (390), was isolated by combined screening of ethyl methanesulfonate-mutagenized cells for temperature-sensitive growth and lack of PRPP synthase activity (390). The prs-100 lesion causes an Arg78Cys alteration and is located within a stretch of amino acid residues that are involved in the formation of the bent dimer (Table 3, amino acid residues Leu76 and Ile77). This finding supported the conclusion of the authors, “that the mutation alters the enzyme's kinetic properties through substantial structural alterations rather than by specific perturbation of substrate binding or catalysis” (27).
Thiamine biosynthesis.
A genetic screen for mutants of S. enterica with improved biosynthesis of thiamine was conducted in a strain with purF and gnd lesions, i.e., a strain lacking amidophosphoribosyltransferase and glucose 6-phosphate dehydrogenase activities. Normally, amidophosphoribosyltransferase (specified by purF) is essential for thiamine diphosphate synthesis. Six thiamine-prototrophic mutants arose at a low frequency (approximately 4 × 10−8). All of the mutants had decreased activity of PRPP synthase, which presumably resulted in an increase in the ribose 5-phosphate pool and thus stimulation of the amidophosphoribosyltransferase-independent, spontaneous (i.e., nonenzymatic) amination of ribose 5-phosphate to 5-phosphoribosyl 1-amine and subsequent thiamine diphosphate synthesis. One mutation mapped within the Shine-Dalgarno sequence, whereas the remaining five mapped within the prs coding region. The in vitro PRPP synthase activity of these latter mutants was 20 to 60% of the wild-type activity. Interestingly, one PRPP synthase variant, Asp224Ala, i.e., an alteration within the PRPP-binding loop, retained approximately 30% of the wild-type PRPP synthase activity. As pointed out by the study authors, the selection procedure yielded only mutants with residual activity of PRPP synthase. Because the mutations were acquired by positive selection (growth in the absence of thiamine) and all of the mutations negatively affected the activity of PRPP synthase, this procedure could be valuable in a screen for mutations that test the importance of various amino acid residues in PRPP synthase activity (391).
prs Gene Organization and Regulation of prs Gene Expression
E. coli and S. enterica.
The prs gene is a member of the purine regulon, because prs gene expression is under the control of the purR gene product (a LacI type protein). The PurBox sequence of the prs gene is identical and located at identical positions in E. coli and S. enterica: −56AAGAAAACGTTTTCGC−41 (with the first cytidylate residue of the transcript numbered +1). Four nucleotides separate the PurBox and the −35 region. PurR caused 3-fold repression of prs gene expression in vivo in experiments that measured expression of prs-lacZ operon fusions with or without the presence of purR. Furthermore, electrophoretic mobility shift analysis of PurR binding to DNA containing the prs PurBox demonstrated that PurR bound to the DNA. Finally, protection of the prs PurBox by PurR was demonstrated by DNase I footprinting (392). The synthesis of PRPP synthase is derepressed approximately 10-fold during pyrimidine starvation (393), and uridine derivatives specifically cause repression of PRPP synthase synthesis (394).
The genetic organization of the prs region in E. coli and S. enterica is identical. The prs gene is located downstream of the lolB gene (encoding an outer membrane lipoprotein required for localization of lipoproteins) (395) and ispE (encoding 4-diphosphocytidyl 2-C-methylerythritol kinase) (396). The prs gene is transcribed from two promoters. A larger transcript is initiated two genes upstream of prs and comprises lolB-ispE-prs. A smaller transcript initiates within the ispE coding sequence and contains the last 152 nucleotides of the ispE coding sequence in both organisms. The transcription start site of the smaller transcript and a rho-independent termination site downstream of the prs coding sequence have been mapped (45, 397). The smaller transcript is 20-fold more abundant than the larger transcript. Pyrimidine-mediated prs gene regulation occurs via increased amounts of the smaller transcript (397). The coding region of the prs gene is preceded by a leader sequence 302 nucleotides long in E. coli and 417 nucleotides long in S. enterica (45, 397, 398). Comparison of the two leader sequences revealed 149 almost-identical nucleotides of the 5′ end of the transcripts of the two organisms, followed by 115 nucleotides in the S. enterica prs leader that are not present in the E. coli prs leader and 152 nucleotides which are almost identical in the leaders of the two transcripts, i.e., an a-b-c arrangement in S. enterica and an a-c arrangement in E. coli.
Altogether, the expression of the prs genes of E. coli and S. enterica is controlled by both purine and pyrimidine compounds. Purine-mediated regulation occurs by means of the PurR repressor binding to the PurBox located within the upstream ispE gene. Several lines of experimental results have provided evidence for the involvement of the prs leader in regulation of the synthesis of PRPP synthase. First, two prs-galK operon fusions were analyzed for production of galactokinase activity. The first gene fusion contained the prs promoter as well as 24 of the 5′ nucleotides of the prs transcript; the second contained the prs promoter, the entire prs leader, and 13 nucleotides of the prs coding sequence. Cells containing gene fusion one had 5 times as much galactokinase activity as cells containing gene fusion two. Thus, nucleotides of the prs leader contain a structure(s) that reduces the production of galactokinase of the gene fusion, and presumably of PRPP synthase under native conditions (399). In another study, a mutant variant of RNA polymerase (rpoBC) of S. enterica that was selected as resistant to pyrimidine fluoroanalogs was constitutive in expression of the pyrE gene specifying orotate phosphoribosyltransferase. The activity of orotate phosphoribosyltransferase was elevated 40-fold, and the activity of PRPP synthase was also elevated, but only approximately 2-fold (400). The defective RNA polymerase was shown to increase the coupling of transcription and translation at the pyrE attenuator (401). It is possible, therefore, that the increase in prs gene expression is also caused by increased coupling of transcription and translation within the prs leader. In other experiments, whole-transcriptome shotgun sequencing has revealed a short transcript originating from the E. coli prs promoter. Although the ends of this transcript have not been mapped, the analysis provides additional evidence for the existence of a prematurely terminated prs transcript (M. A. Sørensen, University of Copenhagen, personal communication). Indeed, a number of potential leaders may be formed from the prs leader, and quite a number of secondary structures, including hairpins and loops, are predicted from the nucleotide sequence. One possible secondary structure of the S. enterica prs leader is shown in Fig. 16. Unlike the pyrE attenuator, which is followed by eight uridylate residues and has been shown to be regulated by premature transcription termination in the leader sequence (402), the putative E. coli prs leader stem-loops are followed by none or a few uridylate residues. This may explain the difference in changes in pyrimidine-mediated regulation of the two operons: 40-fold in pyrE gene expression and 2.3-fold in prs gene expression.
FIG 16.
Possible secondary structure of the S. enterica prs leader. The sequence ranges from nucleotide 1, indicated by 5′, to nucleotide 417, which is immediately followed by the initiator codon-specifying guanylate-uridylate-guanylate triplet and is indicated by 3′ (45). The ΔG of the structure is −540 kJ mol−1. The leader prs sequences of S. enterica and E. coli are identical except for the framed sequence, which is present only in the S. enterica prs leader. The nucleotides circled in red represent a possible leader peptide, and the red arrows point to the nucleotides of a possible Shine-Dalgarno sequence. Possible leader peptide amino sequences are indicated for E. coli and S. enterica. Identical amino acid residues of the two possible leader peptides are underlined.
Gram-positive organisms.
Genetic mapping and nucleotide sequencing has shown that the prs gene of B. subtilis is located between the gcaD (tms-26) and ctc genes (43, 403). The former gene encodes the bifunctional enzyme α-d-glucosamine 1-phosphate acetyltransferase, N-acetylglucosamine 1-phosphate uridylyltransferase (404), whereas the latter gene encodes a general stress protein similar to E. coli ribosomal protein L25 (405). In vegetative cells of B. subtilis, gcaD, prs, and ctc are expressed as a tricistronic operon from a promoter immediately upstream of gcaD (406). Similar arrangements of gcaD-prs-cts are found in other Gram-positive organisms, Clostridiaceae, Corynebacteriaceae, Listeriaceae, and Staphylococcaceae species. On the other hand, Streptococcaceae such as Streptococcus pyogenes, Enterococcus faecalis, and L. lactis have other more heterogeneous genetic organization patterns for prs genes. Streptococcaceae generally contain two prs genes, as described previously for L. lactis. In contrast to the situation in enterobacteria, the regulation of prs gene expression in Gram-positive organisms is essentially unknown.
FUTURE PERSPECTIVES
Although extensive efforts have been spent on elucidation of the various aspects of PRPP metabolism, a number of important aspects remain to be disclosed. Perhaps most important is the function or functions of the NHR found in many eukaryotic PRPP synthases and the functions of the PAPs in human and rat PRPP synthases. For example, purification of PRPP synthase from crude extracts of wild-type S. cerevisiae should be possible and allow elucidation of the quaternary structure of the native enzyme or enzymes. Additionally, the fact that S. cerevisiae PRPP synthase is a multimeric enzyme consisting of different subunits, such as Prs1 and Prs3 and possibly higher-order structures, makes possible studies of mutants with alterations of various amino acid residues in one subunit while the other subunit(s) retains the wild-type phenotype. Candidates for such an analysis are the His135, Asp174, and Lys197 residues (amino acid numbers refer to B. subtilis PRPP synthase positons). Similar experiments may be possible with mammalian PRPP synthase(s). As mentioned previously, there is compelling evidence for the involvement of yeast PRPP synthase polypeptides in a variety of cellular processes other than amino acid and nucleotide syntheses, and there is some evidence that NHRs play roles in this involvement. PAPs may also be involved in cellular processes other than nucleotide synthesis, and studies in mammalian cell lines might reveal roles of the PAPs in other such physiological processes and cross-regulation. Studies of mouse cell lines (and live animals) with the PAPs knocked out, individually or together, could be of very great interest in this respect. As described above, a specific role for human PRPP synthase isozyme 2 in cancer cells has been documented (125). Although PAPs were not implicated, they could very well participate in mediating the observed effects.
Another aspect of interest is the three-dimensional structure of class II PRPP synthases. The available data on their quaternary structure are conflicting. On one hand, circular dichroism and gel filtration analyses predict the structure of sugarcane PRPP synthase to be hexameric, similar to that of B. subtilis PRPP synthase (129), whereas recombinant spinach PRPP synthase isozymes 3 and 4 eluted as trimers in gel filtration chromatography (47).
Furthermore, the three-dimensional structures of PRPP-utilizing enzymes other than those for purine, pyrimidine, pyridine, histidine, and tryptophan biosynthesis, i.e., enzymes catalyzing the formation of O- and C-glycosidic bonds, are worth solving, as they may contribute additional information regarding the versatility of PRPP in intermediary metabolism.
Regulation of PRPP synthase activity in vivo is presently poorly understood. In vitro, the activity is intricately regulated by the energy charge, with ATP being a substrate, AMP a product, and ADP a potent negative, allosteric, and isosteric (i.e., competitive at the ATP site) regulator of PRPP synthase activity. Understanding how these properties contribute to physiological in vivo regulation requires further investigation.
Supplementary Material
ACKNOWLEDGMENTS
The work of K.R.A. is funded by The Danish Diabetes Academy, which is supported by the Novo Nordisk Foundation, a Sapere Aude grant from the Danish Council for Independent Research, and a grant from the Lundbeck Foundation.
Biographies

Bjarne Hove-Jensen began graduate studies at the Enzyme Division, Institute of Biological Chemistry B, University of Copenhagen, in 1976, where he worked on various aspects of purine nucleotide salvage metabolism in E. coli. He serendipitously discovered a mutant with an altered phosphoribosyl diphosphate synthase. After receiving his Ph.D. degree in Biochemistry in 1983 at the University of Copenhagen, he continued studies of nucleotide metabolism in various organisms with an emphasis on the physiological role of PRPP synthase. He became Associate Professor of Biochemistry in 1988 at the University of Copenhagen. Fortuitously, around 2000 he discovered that PRPP is also an intermediate in the catabolic pathway by which phosphonic acid-phosphorus is assimilated by the carbon phosphorus-lyase pathway. Important parts of his research project were conducted on sabbaticals with Dr. Switzer at the University of Illinois at Urbana-Champaign, at Queen's University, Kingston, Ontario, Canada, and at Aarhus University. He is now Associate Professor Emeritus at Aarhus University and continues studies of the physiological role of PRPP.

Kasper R. Andersen received his Ph.D. degree in structural biology in 2009 at Aarhus University. He worked with Nobel Laureate V. Ramakrishnan and his laboratory at the Medical Research Council, Laboratory of Molecular Biology, Cambridge, UK, in an effort to explore the mechanism of mRNA cleavage by the bacterial toxin RelE. From the crystal structures of RelE bound to the ribosomal A-site in both the pre- and postcleaved state, this group of researchers described how the RelE endonuclease performs its ribosome-dependent mRNA cleavage. In 2010, Dr. Andersen began postdoctoral studies at Massachusetts Institute of Technology, where he determined the structure of the largest nucleoporins, Nup188 and Nup192, by using a combination of X-ray crystallography and electron microscopy, and he revealed that the structures have distant similarity to nuclear transport receptors. In 2014, he returned to Denmark as an Assistant Professor at Aarhus University, where he studies kinase-mediated signaling.

Mogens Kilstrup received his Master's degree in Biochemistry in 1986 and his Ph.D. degree in molecular biology in 1990, both at the Institute of Biological Chemistry B, University of Copenhagen. He worked on multivalent regulation of pyrimidine gene expression in enteric bacteria. He discovered the guanine-responsive PurR regulator and characterized the first purR mutant of E. coli. After a short postdoctoral period, he was employed at the Technical University of Denmark, where he studied mechanisms of stress response in L. lactis. Dr. Kilstrup became Associate Professor and Reader at DTU-Bioengineering, the Technical University of Denmark, in 2000 and 2008, respectively. Dr. Kilstrup and Dr. Martinussen collaborate and have jointly discovered and characterized the first activating PRPP-responsive PurR regulator protein of L. lactis, and they have published the first in vivo kinetic analysis of allosteric interactions in bacteria by quantifying and correlating the intracellular concentrations of PRPP and all pur mRNA levels during a purine downshift.

Jan Martinussen received his Ph.D. degree from the Department of Molecular Biology, University of Southern Denmark, in 1992. His research area was the control of gene expression in E. coli by using the cAMP/CRP and CytR/DeoR regulons and the hok/sok antisense system as model systems. From 1992 to 1997, he was a postdoctoral researcher at the Danish Center for Lactic Acid Bacteria, the Technical University of Denmark, where he investigated pyrimidine nucleotide metabolism in L. lactis. He became Associate Professor at DTU-Bioengineering, Technical University of Denmark, in 1997, and his work has ever since been focused on the genetics and physiology of Gram-positive organisms, with an emphasis on nucleotide metabolism in lactic acid bacteria.

Robert L. Switzer received his Ph.D. degree in Biochemistry from the University of California, Berkeley, in 1966 under the direction of H. A. Barker. He began his studies on the regulation and mechanistic enzymology of bacterial PRPP synthase at the suggestion of Earl R. Stadtman while a postdoctoral fellow at the National Institutes of Health from 1966 to 1968. After joining the Department of Biochemistry at the University of Illinois at Urbana-Champaign in 1968, he continued research on PRPP synthase for more than 25 years, a period that included fruitful collaborations with Dr. Hove-Jensen and Dr. Michael Becker. He also led research on the regulation of numerous enzymes and genes of bacterial pyrimidine and purine biosynthesis. His group discovered the RNA-binding regulatory protein PyrR. He remained on the University of Illinois Department of Biochemistry faculty until retirement as Professor Emeritus in 2002. He was a Guggenheim Fellow and is a Fellow of the American Academy of Microbiology.

Martin Willemoës received his Ph.D. degree from the University of Copenhagen in 1996 while working with Dr. Hove-Jensen on structure-function analysis of the PRPP-binding motif in PRPP synthase and the enzyme kinetics of allosteric regulation of this enzyme. As both Assistant and Associate Professor at the Centre for Crystallographic Studies, Department of Chemistry, University of Copenhagen (1999 to 2003) and at the Section for Biomolecular Sciences, Department of Biology (from 2005), he has worked with structure-function analyses of catalysis and regulation of enzymes of pyrimidine nucleotide metabolism. Over the last 7 years, his research interests have gradually moved toward the area of protein and enzyme design as a member of the Linderstrøm-Lang Centre for Protein Science at the Department of Biology, University of Copenhagen.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/MMBR.00040-16.
REFERENCES
- 1.Jensen KF. 1983. Metabolism of 5-phosphoribosyl 1-pyrophosphate (PRPP) in Escherichia coli and Salmonella typhimurium, p 1–25. In Munch-Petersen A. (ed), Metabolism of nucleotides, nucleosides and nucleobases in microorganisms. Academic Press, Inc., London, United Kingdom. [Google Scholar]
- 2.Hove-Jensen B. 1988. Mutation in the phosphoribosylpyrophosphate synthetase gene (prs) that results in simultaneous requirements for purine and pyrimidine nucleosides, nicotinamide nucleotide, histidine, and tryptophan in Escherichia coli. J Bacteriol 170:1148–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hove-Jensen B. 1989. Phosphoribosylpyrophosphate (PRPP)-less mutants of Escherichia coli. Mol Microbiol 3:1487–1492. doi: 10.1111/j.1365-2958.1989.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 4.Graham DE, White RH. 2002. Elucidation of methanogenic coenzyme biosyntheses: from spectroscopy to genomics. Nat Prod Rep 19:133–147. doi: 10.1039/b103714p. [DOI] [PubMed] [Google Scholar]
- 5.Wolucka BA. 2008. Biosynthesis of d-arabinose in mycobacteria: a novel bacterial pathway with implications for antimycobacterial therapy. FEBS J 275:2691–2711. doi: 10.1111/j.1742-4658.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- 6.Kornberg A, Lieberman I, Simms ES. 1955. Enzymatic synthesis and properties of 5-phosphoribosylpyrophosphate. J Biol Chem 215:389–402. [PubMed] [Google Scholar]
- 7.Khorana HG, Fernandes JF, Kornberg A. 1958. Pyrophosphorylation of ribose 5-phosphate in the enzymatic synthesis of 5-phosphorylribose 1-pyrophosphate. J Biol Chem 230:941–948. [PubMed] [Google Scholar]
- 8.Carter AT, Narbad A, Pearson BM, Beck KF, Logghe M, Contreras R, Schweizer M. 1994. Phosphoribosylpyrophosphate synthetase (PRS): a new gene family in Saccharomyces cerevisiae. Yeast 10:1031–1044. doi: 10.1002/yea.320100805. [DOI] [PubMed] [Google Scholar]
- 9.Mannhaupt G, Vetter I, Schwarzlose C, Mitzel S, Feldmann H. 1996. Analysis of a 26 kb region on the left arm of yeast chromosome XV. Yeast 12:67–76. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, Stewart A, Sgouros J, Peat N, Hayles J, Baker S, Basham D, Bowman S, Brooks K, Brown D, Brown S, Chillingworth T, Churcher C, Collins M, Connor R, Cronin A, Davis P, Feltwell T, Fraser A, Gentles S, Goble A, Hamlin N, Harris D, Hidalgo J, Hodgson G, Holroyd S, Hornsby T, Howarth S, Huckle EJ, Hunt S, Jagels K, James K, Jones L, Jones M, Leather S, McDonald S, McLean J, Mooney P, Moule S, Mungall K, Murphy L, Niblett D, Odell C, Oliver K, O'Neil S, Pearson D, Quail MA, et al. 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415:871–880. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- 11.Taira M, Iizasa T, Yamada K, Shimada H, Tatibana M. 1989. Tissue-differential expression of two distinct genes for phosphoribosyl pyrophosphate synthetase and existence of the testis-specific transcript. Biochim Biophys Acta 1007:203–208. doi: 10.1016/0167-4781(89)90040-7. [DOI] [PubMed] [Google Scholar]
- 12.Roessler BJ, Bell G, Heidler S, Seino S, Becker M, Palella TD. 1990. Cloning of two distinct copies of human phosphoribosylpyrophosphate synthetase cDNA. Nucleic Acids Res 18:193. doi: 10.1093/nar/18.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taira M, Ishijima S, Kita K, Yamada K, Iizasa T, Tatibana M. 1987. Nucleotide and deduced amino acid sequences of two distinct cDNAs for rat phosphoribosylpyrophosphate synthetase. J Biol Chem 262:14867–14870. [PubMed] [Google Scholar]
- 14.Krath BN, Eriksen TA, Poulsen TS, Hove-Jensen B. 1999. Cloning and sequencing of cDNAs specifying a novel class of phosphoribosyl diphosphate synthase in Arabidopsis thaliana. Biochim Biophys Acta 1430:403–408. doi: 10.1016/S0167-4838(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 15.Krath BN, Hove-Jensen B. 1999. Organellar and cytosolic localization of four phosphoribosyl diphosphate synthase isozymes in spinach. Plant Physiol 119:497–506. doi: 10.1104/pp.119.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin X, Kaul S, Rounsley S, Shea TP, Benito MI, Town CD, Fujii CY, Mason T, Bowman CL, Barnstead M, Feldblyum TV, Buell CR, Ketchum KA, Lee J, Ronning CM, Koo HL, Moffat KS, Cronin LA, Shen M, Pai G, Van Aken S, Umayam L, Tallon LJ, Gill JE, Adams MD, Carrera AJ, Creasy TH, Goodman HM, Somerville CR, Copenhaver GP, Preuss D, Nierman WC, White O, Eisen JA, Salzberg SL, Fraser CM, Venter JC. 1999. Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature 402:761–768. doi: 10.1038/45471. [DOI] [PubMed] [Google Scholar]
- 17.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio Collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker MA. 2001. Phosphoribosylpyrophosphate synthetase and the regulation of phosphoribosylpyrophosphate production in human cells. Prog Nucleic Acid Res Mol Biol 69:115–148. doi: 10.1016/S0079-6603(01)69046-9. [DOI] [PubMed] [Google Scholar]
- 19.Jensen KF, Dandanell G, Hove-Jensen B, Willemoës M. 2008. Nucleotides, nucleosides, and nucleobases. EcoSal Plus doi: 10.1128/ecosalplus.3.6.2. [DOI] [PubMed] [Google Scholar]
- 20.Tatibana M, Kita K, Taira M, Ishijima S, Sonoda T, Ishizuka T, Iizasa T, Ahmad I. 1995. Mammalian phosphoribosyl-pyrophosphate synthetase. Adv Enzyme Regul 35:229–249. doi: 10.1016/0065-2571(94)00017-W. [DOI] [PubMed] [Google Scholar]
- 21.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz VM, Chen IM, Palaniappan K, Chu K, Szeto E, Grechkin Y, Ratner A, Jacob B, Huang J, Williams P, Huntemann M, Anderson I, Mavromatis K, Ivanova NN, Kyrpides NC. 2012. IMG: the Integrated Microbial Genomes database and comparative analysis system. Nucleic Acids Res 40:D115–D122. doi: 10.1093/nar/gkr1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. 2013. GenBank. Nucleic Acids Res 41:D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corpet F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marck C. 1988. DNA Strider: a C program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res 16:1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLano WL. 2002. Unraveling hot spots in binding interfaces: progress and challenges. Curr Opin Struct Biol 12:14–20. doi: 10.1016/S0959-440X(02)00283-X. [DOI] [PubMed] [Google Scholar]
- 27.Post DA, Switzer RL. 1991. prsB is an allele of the Salmonella typhimurium prsA gene: characterization of a mutant phosphoribosylpyrophosphate synthetase. J Bacteriol 173:1978–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hove-Jensen B, Nygaard P. 1982. Phosphoribosylpyrophosphate synthetase of Escherichia coli. Identification of a mutant enzyme. Eur J Biochem 126:327–332. [DOI] [PubMed] [Google Scholar]
- 29.Remy CN, Remy WT, Buchanan JM. 1955. Biosynthesis of the purines. VIII. Enzymatic synthesis and utilization of alpha-5-phosphoribosylpyrophosphate. J Biol Chem 217:885–895. [PubMed] [Google Scholar]
- 30.Dennis AL, Puskas M, Stasaitis S, Sandwick RK. 2000. The formation of a 1-5 phosphodiester linkage in the spontaneous breakdown of 5-phosphoribosyl-alpha-1-pyrophosphate. J Inorg Biochem 81:73–80. doi: 10.1016/S0162-0134(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 31.Meola M, Yamen B, Weaver K, Sandwick RK. 2003. The catalytic effect of Mg2+ and imidazole on the decomposition of 5-phosphoribosyl-alpha-1-pyrophosphate in aqueous solution. J Inorg Biochem 93:235–242. doi: 10.1016/S0162-0134(02)00578-0. [DOI] [PubMed] [Google Scholar]
- 32.Trembacz H, Jezewska MM. 1990. The route of non-enzymic and enzymic breakdown of 5-phosphoribosyl 1-pyrophosphate to ribose 1-phosphate. Biochem J 271:621–625. doi: 10.1042/bj2710621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smithers GW, O'Sullivan WJ. 1982. 31P nuclear magnetic resonance study of phosphoribosyldiphosphate and its interaction with magnesium ions. J Biol Chem 257:6164–6170. [PubMed] [Google Scholar]
- 34.Frey PA, Arabshahi A. 1995. Standard free energy change for the hydrolysis of the alpha,beta-phosphoanhydride bridge in ATP. Biochemistry 34:11307–11310. doi: 10.1021/bi00036a001. [DOI] [PubMed] [Google Scholar]
- 35.Switzer RL. 1969. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. I. Purification and properties of the enzyme from Salmonella typhimurium. J Biol Chem 244:2854–2863. [PubMed] [Google Scholar]
- 36.Jensen KF, Houlberg U, Nygaard P. 1979. Thin-layer chromatographic methods to isolate 32P-labeled 5-phosphoribosyl-alpha-1-pyrophosphate (PRPP): determination of cellular PRPP pools and assay of PRPP synthetase activity. Anal Biochem 98:254–263. doi: 10.1016/0003-2697(79)90138-6. [DOI] [PubMed] [Google Scholar]
- 37.Michelsen O, Villadsen IS. 1979. Chromatographic methods for the determination of alpha- and beta-5-phospho-d-ribose-alpha-1-pyrophosphate pools in bacteria. Anal Biochem 98:264–272. doi: 10.1016/0003-2697(79)90139-8. [DOI] [PubMed] [Google Scholar]
- 38.Villadsen IS, Michelsen O. 1977. Regulation of PRPP and nucleoside tri- and tetraphosphate pools in Escherichia coli under conditions of nitrogen starvation. J Bacteriol 130:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagnara AS, Finch LR. 1972. Quantitative extraction and estimation of intracellular nucleoside triphosphates of Escherichia coli. Anal Biochem 45:24–34. doi: 10.1016/0003-2697(72)90004-8. [DOI] [PubMed] [Google Scholar]
- 40.Bagnara AS, Mitchell A, Sin IL, Finch LR. 1973. A sensitive method for estimating 5-phosphoribosyl 1-pyrophosphate in Escherichia coli. Anal Biochem 54:535–544. doi: 10.1016/0003-2697(73)90385-0. [DOI] [PubMed] [Google Scholar]
- 41.Parry RJ, Burns MR, Skae PN, Hoyt JC, Pal B. 1996. Carbocyclic analogues of D-ribose-5-phosphate: synthesis and behavior with 5-phosphoribosyl alpha-1-pyrophosphate synthetases. Bioorg Med Chem 4:1077–1088. doi: 10.1016/0968-0896(96)00090-9. [DOI] [PubMed] [Google Scholar]
- 42.Parry RJ, Burns MR, Jiralerspong S, Alemany L. 1997. Synthesis of (+)-(1S)-1-pyrophosphoryl-(2R,3R)-2,3-dihydroxy-(4S)-4-(phosphoryloxymethyl)cyclopentane, a stable, optically-active carbocyclic analog of 5-phosphoribosyl-1-pyrophosphate (PRPP). Tetrahedron 53:7077–7088. doi: 10.1016/S0040-4020(97)00387-6. [DOI] [Google Scholar]
- 43.Nilsson D, Hove-Jensen B, Arnvig K. 1989. Primary structure of the tms and prs genes of Bacillus subtilis. Mol Gen Genet 218:565–571. doi: 10.1007/BF00332425. [DOI] [PubMed] [Google Scholar]
- 44.Hershey HV, Taylor MW. 1986. Nucleotide sequence and deduced amino acid sequence of Escherichia coli adenine phosphoribosyltransferase and comparison with other analogous enzymes. Gene 43:287–293. doi: 10.1016/0378-1119(86)90218-0. [DOI] [PubMed] [Google Scholar]
- 45.Hove-Jensen B, Harlow KW, King CJ, Switzer RL. 1986. Phosphoribosylpyrophosphate synthetase of Escherichia coli. Properties of the purified enzyme and primary structure of the prs gene. J Biol Chem 261:6765–6771. [PubMed] [Google Scholar]
- 46.Krath BN, Hove-Jensen B. 2001. Class II recombinant phosphoribosyl diphosphate synthase from spinach. Phosphate independence and diphosphoryl donor specificity. J Biol Chem 276:17851–17856. doi: 10.1074/jbc.M010172200. [DOI] [PubMed] [Google Scholar]
- 47.Krath BN, Hove-Jensen B. 2001. Implications of secondary structure prediction and amino acid sequence comparison of class I and class II phosphoribosyl diphosphate synthases on catalysis, regulation, and quaternary structure. Protein Sci 10:2317–2324. doi: 10.1110/ps.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadziola A, Jepsen CH, Johansson E, McGuire J, Larsen S, Hove-Jensen B. 2005. Novel class III phosphoribosyl diphosphate synthase: structure and properties of the tetrameric, phosphate-activated, non-allosterically inhibited enzyme from Methanocaldococcus jannaschii. J Mol Biol 354:815–828. doi: 10.1016/j.jmb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Eriksen TA, Kadziola A, Bentsen AK, Harlow KW, Larsen S. 2000. Structural basis for the function of Bacillus subtilis phosphoribosyl-pyrophosphate synthetase. Nat Struct Biol 7:303–308. doi: 10.1038/74069. [DOI] [PubMed] [Google Scholar]
- 50.Eriksen TA, Kadziola A, Larsen S. 2002. Binding of cations in Bacillus subtilis phosphoribosyldiphosphate synthetase and their role in catalysis. Protein Sci 11:271–279. doi: 10.1110/ps.28502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Lu Y, Peng B, Ding J. 2007. Crystal structure of human phosphoribosylpyrophosphate synthetase 1 reveals a novel allosteric site. Biochem J 401:39–47. doi: 10.1042/BJ20061066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timofeev VI, Abramchik YA, Zhukhlistova NE, Mauravieva TI, Esipov RS, Kuranova IP. 2016. Three-dimensional structure of phosphoribosyl pyrophosphate synthetase from E. coli at 2.71 Å resolution. Crystallogr Rep 61:44–54. doi: 10.1134/S1063774516010247. [DOI] [Google Scholar]
- 53.Baugh L, Gallagher LA, Patrapuvich R, Clifton MC, Gardberg AS, Edwards TE, Armour B, Begley DW, Dieterich SH, Dranow DM, Abendroth J, Fairman JW, Fox D III, Staker BL, Phan I, Gillespie A, Choi R, Nakazawa-Hewitt S, Nguyen MT, Napuli A, Barrett L, Buchko GW, Stacy R, Myler PJ, Stewart LJ, Manoil C, Van Voorhis WC. 2013. Combining functional and structural genomics to sample the essential Burkholderia structome. PLoS One 8:e53851. doi: 10.1371/journal.pone.0053851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nygaard FB. 2001. The molecular mechanism of catalysis and allosteric regulation in the phosphoribosyldiphosphate synthase from Bacillus subtilis. PhD thesis University of Copenhagen, Copenhagen, Denmark. [Google Scholar]
- 55.Hove-Jensen B, McGuire JN. 2004. Surface exposed amino acid differences between mesophilic and thermophilic phosphoribosyl diphosphate synthase. Eur J Biochem 271:4526–4533. doi: 10.1111/j.1432-1033.2004.04412.x. [DOI] [PubMed] [Google Scholar]
- 56.Switzer RL. 1971. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. 3. Kinetic studies of the reaction mechanism. J Biol Chem 246:2447–2458. [PubMed] [Google Scholar]
- 57.Willemoës M, Hove-Jensen B. 1997. Binding of divalent magnesium by Escherichia coli phosphoribosyl diphosphate synthetase. Biochemistry 36:5078–5083. doi: 10.1021/bi962610a. [DOI] [PubMed] [Google Scholar]
- 58.Gibson KJ, Switzer RL. 1980. Structure of the divalent cation.nucleotide complex at the active site of phosphoribosylpyrophosphate synthetase. J Biol Chem 255:694–696. [PubMed] [Google Scholar]
- 59.Roth DG, Shelton E, Deuel TF. 1974. Purification and properties of phosphoribosyl pyrophosphate synthetase from rat liver. J Biol Chem 249:291–296. [PubMed] [Google Scholar]
- 60.Murray AW, Wong PC. 1967. 5-Phosphoribosyl pyrophosphate synthetase from Ehrlich ascites-tumour cells: effect of magnesium and ATP concentration on the enzymic activity. Biochem Biophys Res Commun 29:582–587. doi: 10.1016/0006-291X(67)90525-6. [DOI] [PubMed] [Google Scholar]
- 61.Willemoës M, Nilsson D, Hove-Jensen B. 1996. Effects of mutagenesis of aspartic acid residues in the putative phosphoribosyl diphosphate binding site of Escherichia coli phosphoribosyl diphosphate synthetase on metal ion specificity and ribose 5-phosphate binding. Biochemistry 35:8181–8186. doi: 10.1021/bi9528560. [DOI] [PubMed] [Google Scholar]
- 62.Harlow KW, Switzer RL. 1990. Chemical modification of Salmonella typhimurium phosphoribosylpyrophosphate synthetase with 5′-(p-fluorosulfonylbenzoyl)adenosine. Identification of an active site histidine. J Biol Chem 265:5487–5493. [PubMed] [Google Scholar]
- 63.Hilden I, Hove-Jensen B, Harlow KW. 1995. Inactivation of Escherichia coli phosphoribosylpyrophosphate synthetase by the 2′,3′-dialdehyde derivative of ATP. Identification of active site lysines. J Biol Chem 270:20730–20736. [DOI] [PubMed] [Google Scholar]
- 64.Roberts MF, Switzer RL, Schubert KR. 1975. Inactivation of Salmonella phosphoribosylpyrophosphate synthetase by oxidation of a specific sulfhydryl group with potassium permanganate. J Biol Chem 250:5364–5369. [PubMed] [Google Scholar]
- 65.Harlow KW, Switzer RL. 1990. Sulfhydryl chemistry of Salmonella typhimurium phosphoribosylpyrophosphate synthetase: identification of two classes of cysteinyl residues. Arch Biochem Biophys 276:466–472. doi: 10.1016/0003-9861(90)90746-L. [DOI] [PubMed] [Google Scholar]
- 66.Li TM, Mildvan AS, Switzer RL. 1978. Studies of the stereochemistry and of the role of metal ions in the mechanism of phosphoribosylpyrophosphate synthetase from Salmonella typhimurium. J Biol Chem 253:3918–3923. [PubMed] [Google Scholar]
- 67.Li TM, Switzer RL, Mildvan AS. 1979. Kinetic and magnetic resonance studies of the interaction of the Cr-ATP complex with phosphoribosylpyrophosphate synthetase from Salmonella typhimurium. Arch Biochem Biophys 193:1–13. doi: 10.1016/0003-9861(79)90001-8. [DOI] [PubMed] [Google Scholar]
- 68.Gibson KJ, Schubert KR, Switzer RL. 1982. Binding of the substrates and the allosteric inhibitor adenosine 5′-diphosphate to phosphoribosylpyrophosphate synthetase from Salmonella typhimurium. J Biol Chem 257:2391–2396. [PubMed] [Google Scholar]
- 69.Switzer RL, Sogin DC. 1973. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. V. Inhibition by end products and regulation by adenosine diphosphate. J Biol Chem 248:1063–1073. [PubMed] [Google Scholar]
- 70.Arnvig K, Hove-Jensen B, Switzer RL. 1990. Purification and properties of phosphoribosyl-diphosphate synthetase from Bacillus subtilis. Eur J Biochem 192:195–200. doi: 10.1111/j.1432-1033.1990.tb19214.x. [DOI] [PubMed] [Google Scholar]
- 71.Ishijima S, Kita K, Ahmad I, Ishizuka T, Taira M, Tatibana M. 1991. Expression of rat phosphoribosylpyrophosphate synthetase subunits I and II in Escherichia coli. Isolation and characterization of the recombinant isoforms. J Biol Chem 266:15693–15697. [PubMed] [Google Scholar]
- 72.Becker MA, Smith PR, Taylor W, Mustafi R, Switzer RL. 1995. The genetic and functional basis of purine nucleotide feedback-resistant phosphoribosylpyrophosphate synthetase superactivity. J Clin Invest 96:2133–2141. doi: 10.1172/JCI118267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nosal JM, Switzer RL, Becker MA. 1993. Overexpression, purification, and characterization of recombinant human 5-phosphoribosyl-1-pyrophosphate synthetase isozymes I and II. J Biol Chem 268:10168–10175. [PubMed] [Google Scholar]
- 74.Schubert KR, Switzer RL, Shelton E. 1975. Studies of the quaternary structure and the chemical properties of phosphoribosylpyrophosphate synthetase from Salmonella typhimurium. J Biol Chem 250:7492–7500. [PubMed] [Google Scholar]
- 75.Willemoës M, Hove-Jensen B, Larsen S. 2000. Steady state kinetic model for the binding of substrates and allosteric effectors to Escherichia coli phosphoribosyl-diphosphate synthase. J Biol Chem 275:35408–35412. doi: 10.1074/jbc.M006346200. [DOI] [PubMed] [Google Scholar]
- 76.Fry DW, Becker MA, Switzer RL. 1995. Inhibition of human 5-phosphoribosyl-1-pyrophosphate synthetase by 4-amino-8-(beta-d-ribofuranosylamino)-pyrimido[5,4-d]pyrimidine-5′-monophosphate: evidence for interaction at the ADP allosteric site. Mol Pharmacol 47:810–815. [PubMed] [Google Scholar]
- 77.Lucarelli AP, Buroni S, Pasca MR, Rizzi M, Cavagnino A, Valentini G, Riccardi G, Chiarelli LR. 2010. Mycobacterium tuberculosis phosphoribosylpyrophosphate synthetase: biochemical features of a crucial enzyme for mycobacterial cell wall biosynthesis. PLoS One 5:e15494. doi: 10.1371/journal.pone.0015494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Breda A, Martinelli LK, Bizarro CV, Rosado LA, Borges CB, Santos DS, Basso LA. 2012. Wild-type phosphoribosylpyrophosphate synthase (PRS) from Mycobacterium tuberculosis: a bacterial class II PRS? PLoS One 7:e39245. doi: 10.1371/journal.pone.0039245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alderwick LJ, Lloyd GS, Lloyd AJ, Lovering AL, Eggeling L, Besra GS. 2011. Biochemical characterization of the Mycobacterium tuberculosis phosphoribosyl-1-pyrophosphate synthetase. Glycobiology 21:410–425. doi: 10.1093/glycob/cwq173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carter AT, Beiche F, Narbad A, Hove-Jensen B, Schweizer LM, Schweizer M. 1995. Are all four yeast PRS genes essential? Biochem Soc Trans 23:621S. doi: 10.1042/bst023621s. [DOI] [PubMed] [Google Scholar]
- 81.Carter AT, Beiche F, Hove-Jensen B, Narbad A, Barker PJ, Schweizer LM, Schweizer M. 1997. PRS1 is a key member of the gene family encoding phosphoribosylpyrophosphate synthetase in Saccharomyces cerevisiae. Mol Gen Genet 254:148–156. doi: 10.1007/s004380050402. [DOI] [PubMed] [Google Scholar]
- 82.Hove-Jensen B. 2004. Heterooligomeric phosphoribosyl diphosphate synthase of Saccharomyces cerevisiae: combinatorial expression of the five PRS genes in Escherichia coli. J Biol Chem 279:40345–40350. doi: 10.1074/jbc.M405480200. [DOI] [PubMed] [Google Scholar]
- 83.Hernando Y, Carter AT, Parr A, Hove-Jensen B, Schweizer M. 1999. Genetic analysis and enzyme activity suggest the existence of more than one minimal functional unit capable of synthesizing phosphoribosyl pyrophosphate in Saccharomyces cerevisiae. J Biol Chem 274:12480–12487. doi: 10.1074/jbc.274.18.12480. [DOI] [PubMed] [Google Scholar]
- 84.Ugbogu EA, Wippler S, Euston M, Kouwenhoven EN, de Brouwer AP, Schweizer LM, Schweizer M. 2013. The contribution of the nonhomologous region of Prs1 to the maintenance of cell wall integrity and cell viability. FEMS Yeast Res 13:291–301. doi: 10.1111/1567-1364.12033. [DOI] [PubMed] [Google Scholar]
- 85.Wang K, Vavassori S, Schweizer LM, Schweizer M. 2004. Impaired PRPP-synthesizing capacity compromises cell integrity signalling in Saccharomyces cerevisiae. Microbiology 150:3327–3339. doi: 10.1099/mic.0.27373-0. [DOI] [PubMed] [Google Scholar]
- 86.Ito T, Chiba T, Ozawa R, Yoshida M, Hattori M, Sakaki Y. 2001. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc Natl Acad Sci U S A 98:4569–4574. doi: 10.1073/pnas.061034498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu H, Braun P, Yildirim MA, Lemmens I, Venkatesan K, Sahalie J, Hirozane-Kishikawa T, Gebreab F, Li N, Simonis N, Hao T, Rual JF, Dricot A, Vazquez A, Murray RR, Simon C, Tardivo L, Tam S, Svrzikapa N, Fan C, de Smet AS, Motyl A, Hudson ME, Park J, Xin X, Cusick ME, Moore T, Boone C, Snyder M, Roth FP, Barabasi AL, Tavernier J, Hill DE, Vidal M. 2008. High-quality binary protein interaction map of the yeast interactome network. Science 322:104–110. doi: 10.1126/science.1158684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, Qureshi-Emili A, Li Y, Godwin B, Conover D, Kalbfleisch T, Vijayadamodar G, Yang M, Johnston M, Fields S, Rothberg JM. 2000. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- 89.Tarassov K, Messier V, Landry CR, Radinovic S, Serna Molina MM, Shames I, Malitskaya Y, Vogel J, Bussey H, Michnick SW. 2008. An in vivo map of the yeast protein interactome. Science 320:1465–1470. doi: 10.1126/science.1153878. [DOI] [PubMed] [Google Scholar]
- 90.Schlecht U, Miranda M, Suresh S, Davis RW, St Onge RP. 2012. Multiplex assay for condition-dependent changes in protein-protein interactions. Proc Natl Acad Sci U S A 109:9213–9218. doi: 10.1073/pnas.1204952109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gavin AC, Aloy P, Grandi P, Krause R, Boesche M, Marzioch M, Rau C, Jensen LJ, Bastuck S, Dumpelfeld B, Edelmann A, Heurtier MA, Hoffman V, Hoefert C, Klein K, Hudak M, Michon AM, Schelder M, Schirle M, Remor M, Rudi T, Hooper S, Bauer A, Bouwmeester T, Casari G, Drewes G, Neubauer G, Rick JM, Kuster B, Bork P, Russell RB, Superti-Furga G. 2006. Proteome survey reveals modularity of the yeast cell machinery. Nature 440:631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- 92.Collins SR, Kemmeren P, Zhao XC, Greenblatt JF, Spencer F, Holstege FC, Weissman JS, Krogan NJ. 2007. Toward a comprehensive atlas of the physical interactome of Saccharomyces cerevisiae. Mol Cell Proteomics 6:439–450. doi: 10.1074/mcp.M600381-MCP200. [DOI] [PubMed] [Google Scholar]
- 93.Hove-Jensen B, Krath BN, Merritt H, Schweizer M. 2000. Saccharomyces cerevisiae phosphoribosyl diphosphate synthase may exist as pentaoligomer, abstr X-20 100th Gen Meet Am Soc Microbiol, Los Angeles, CA American Society for Microbiology, Washington, DC. [Google Scholar]
- 94.Binley KM, Radcliffe PA, Trevethick J, Duffy KA, Sudbery PE. 1999. The yeast PRS3 gene is required for cell integrity, cell cycle arrest upon nutrient deprivation, ion homeostasis and the proper organization of the actin cytoskeleton. Yeast 15:1459–1469. doi:. [DOI] [PubMed] [Google Scholar]
- 95.Care A, Vousden KA, Binley KM, Radcliffe P, Trevethick J, Mannazzu I, Sudbery PE. 2004. A synthetic lethal screen identifies a role for the cortical actin patch/endocytosis complex in the response to nutrient deprivation in Saccharomyces cerevisiae. Genetics 166:707–719. doi: 10.1534/genetics.166.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jorgensen P, Nishikawa JL, Breitkreutz BJ, Tyers M. 2002. Systematic identification of pathways that couple cell growth and division in yeast. Science 297:395–400. doi: 10.1126/science.1070850. [DOI] [PubMed] [Google Scholar]
- 97.Levin DE. 2011. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuranda K, Leberre V, Sokol S, Palamarczyk G, Francois J. 2006. Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol Microbiol 61:1147–1166. doi: 10.1111/j.1365-2958.2006.05300.x. [DOI] [PubMed] [Google Scholar]
- 99.Schneiter R, Carter AT, Hernando Y, Zellnig G, Schweizer LM, Schweizer M. 2000. The importance of the five phosphoribosyl-pyrophosphate synthetase (Prs) gene products of Saccharomyces cerevisiae in the maintenance of cell integrity and the subcellular localization of Prs1p. Microbiology 146:3269–3278. doi: 10.1099/00221287-146-12-3269. [DOI] [PubMed] [Google Scholar]
- 100.Blacketer MJ, Madaule P, Myers AM. 1994. The Saccharomyces cerevisiae mutation elm4-1 facilitates pseudohyphal differentiation and interacts with a deficiency in phosphoribosylpyrophosphate synthase activity to cause constitutive pseudohyphal growth. Mol Cell Biol 14:4671–4681. doi: 10.1128/MCB.14.7.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vavassori S, Wang K, Schweizer LM, Schweizer M. 2005. In Saccharomyces cerevisiae, impaired PRPP synthesis is accompanied by valproate and Li+ sensitivity. Biochem Soc Trans 33:1154–1157. doi: 10.1042/BST0331154. [DOI] [PubMed] [Google Scholar]
- 102.Vavassori S, Wang K, Schweizer LM, Schweizer M. 2005. Ramifications of impaired PRPP synthesis in Saccharomyces cerevisiae. Biochem Soc Trans 33:1418–1420. doi: 10.1042/BST0331418. [DOI] [PubMed] [Google Scholar]
- 103.Ho Y, Gruhler A, Heilbut A, Bader GD, Moore L, Adams SL, Millar A, Taylor P, Bennett K, Boutilier K, Yang L, Wolting C, Donaldson I, Schandorff S, Shewnarane J, Vo M, Taggart J, Goudreault M, Muskat B, Alfarano C, Dewar D, Lin Z, Michalickova K, Willems AR, Sassi H, Nielsen PA, Rasmussen KJ, Andersen JR, Johansen LE, Hansen LH, Jespersen H, Podtelejnikov A, Nielsen E, Crawford J, Poulsen V, Sorensen BD, Matthiesen J, Hendrickson RC, Gleeson F, Pawson T, Moran MF, Durocher D, Mann M, Hogue CW, Figeys D, Tyers M. 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415:180–183. doi: 10.1038/415180a. [DOI] [PubMed] [Google Scholar]
- 104.Kleineidam A, Vavassori S, Wang K, Schweizer LM, Griac P, Schweizer M. 2009. Valproic acid- and lithium-sensitivity in prs mutants of Saccharomyces cerevisiae. Biochem Soc Trans 37:1115–1120. doi: 10.1042/BST0371115. [DOI] [PubMed] [Google Scholar]
- 105.Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, White FM. 2002. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat Biotechnol 20:301–305. doi: 10.1038/nbt0302-301. [DOI] [PubMed] [Google Scholar]
- 106.Hernando Y, Parr A, Schweizer M. 1998. PRS5, the fifth member of the phosphoribosyl pyrophosphate synthetase gene family in Saccharomyces cerevisiae, is essential for cell viability in the absence of either PRS1 or PRS3. J Bacteriol 180:6404–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim DU, Hayles J, Kim D, Wood V, Park HO, Won M, Yoo HS, Duhig T, Nam M, Palmer G, Han S, Jeffery L, Baek ST, Lee H, Shim YS, Lee M, Kim L, Heo KS, Noh EJ, Lee AR, Jang YJ, Chung KS, Choi SJ, Park JY, Park Y, Kim HM, Park SK, Park HJ, Kang EJ, Kim HB, Kang HS, Park HM, Kim K, Song K, Song KB, Nurse P, Hoe KL. 2010. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol 28:617–623. doi: 10.1038/nbt.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson-Grady JT, Villen J, Gygi SP. 2008. Phosphoproteome analysis of fission yeast. J Proteome Res 7:1088–1097. doi: 10.1021/pr7006335. [DOI] [PubMed] [Google Scholar]
- 109.Harris SD. 2001. Septum formation in Aspergillus nidulans. Curr Opin Microbiol 4:736–739. doi: 10.1016/S1369-5274(01)00276-4. [DOI] [PubMed] [Google Scholar]
- 110.Zhong G, Wei W, Guan Q, Ma Z, Wei H, Xu X, Zhang S, Lu L. 2012. Phosphoribosyl pyrophosphate synthetase, as a suppressor of the sepH mutation in Aspergillus nidulans, is required for the proper timing of septation. Mol Microbiol 86:894–907. doi: 10.1111/mmi.12026. [DOI] [PubMed] [Google Scholar]
- 111.Dietrich FS, Voegeli S, Brachat S, Lerch A, Gates K, Steiner S, Mohr C, Pohlmann R, Luedi P, Choi S, Wing RA, Flavier A, Gaffney TD, Philippsen P. 2004. The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces cerevisiae genome. Science 304:304–307. doi: 10.1126/science.1095781. [DOI] [PubMed] [Google Scholar]
- 112.Jiménez A, Santos MA, Revuelta JL. 2008. Phosphoribosyl pyrophosphate synthetase activity affects growth and riboflavin production in Ashbya gossypii. BMC Biotechnol 8:67. doi: 10.1186/1472-6750-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kita K, Otsuki T, Ishizuka T, Tatibana M. 1989. Rat liver phosphoribosyl pyrophosphate synthetase: existence of the purified enzyme as heterogeneous aggregates and identification of the catalytic subunit. J Biochem 105:736–741. [DOI] [PubMed] [Google Scholar]
- 114.Becker MA, Heidler SA, Nosal JM, Switzer RL, LeBeau MM, Shapiro LJ, Palella TD, Roessler BJ. 1991. Human phosphoribosylpyrophosphate synthetase (PRS) 2: an independent active, X chromosome-linked PRS isoform. Adv Exp Med Biol 309B:129–132. doi: 10.1007/978-1-4615-7703-4_29. [DOI] [PubMed] [Google Scholar]
- 115.Becker MA, Meyer LJ, Huisman WH, Lazar C, Adams WB. 1977. Human erythrocyte phosphoribosylpyrophosphate synthetase. Subunit analysis and states of subunit association. J Biol Chem 252:3911–3918. [PubMed] [Google Scholar]
- 116.Fox IH, Kelley WN. 1971. Human phosphoribosylpyrophosphate synthetase. Distribution, purification, and properties. J Biol Chem 246:5739–5748. [PubMed] [Google Scholar]
- 117.Tang W, Li X, Zhu Z, Tong S, Li X, Zhang X, Teng M, Niu L. 2006. Expression, purification, crystallization and preliminary X-ray diffraction analysis of human phosphoribosyl pyrophosphate synthetase 1 (PRS1). Acta Crystallogr Sect F Struct Biol Cryst Commun 62:432–434. doi: 10.1107/S1744309106009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen P, Liu Z, Wang X, Peng J, Sun Q, Li J, Wang M, Niu L, Zhang Z, Cai G, Teng M, Li X. 2015. Crystal and EM structures of human phosphoribosyl pyrophosphate synthase I (PRS1) provide novel insights into the disease-associated mutations. PLoS One 10:e0120304. doi: 10.1371/journal.pone.0120304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duley JA, Christodoulou J, de Brouwer AP. 2011. The PRPP synthetase spectrum: what does it demonstrate about nucleotide syndromes? Nucleosides Nucleotides Nucleic Acids 30:1129–1139. doi: 10.1080/15257770.2011.591747. [DOI] [PubMed] [Google Scholar]
- 120.Kim HJ, Sohn KM, Shy ME, Krajewski KM, Hwang M, Park JH, Jang SY, Won HH, Choi BO, Hong SH, Kim BJ, Suh YL, Ki CS, Lee SY, Kim SH, Kim JW. 2007. Mutations in PRPS1, which encodes the phosphoribosyl pyrophosphate synthetase enzyme critical for nucleotide biosynthesis, cause hereditary peripheral neuropathy with hearing loss and optic neuropathy (cmtx5). Am J Hum Genet 81:552–558. doi: 10.1086/519529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X, Han D, Li J, Han B, Ouyang X, Cheng J, Li X, Jin Z, Wang Y, Bitner-Glindzicz M, Kong X, Xu H, Kantardzhieva A, Eavey RD, Seidman CE, Seidman JG, Du LL, Chen ZY, Dai P, Teng M, Yan D, Yuan H. 2010. Loss-of-function mutations in the PRPS1 gene cause a type of nonsyndromic X-linked sensorineural deafness, DFN2. Am J Hum Genet 86:65–71. doi: 10.1016/j.ajhg.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Brouwer AP, Williams KL, Duley JA, van Kuilenburg AB, Nabuurs SB, Egmont-Petersen M, Lugtenberg D, Zoetekouw L, Banning MJ, Roeffen M, Hamel BC, Weaving L, Ouvrier RA, Donald JA, Wevers RA, Christodoulou J, van Bokhoven H. 2007. Arts syndrome is caused by loss-of-function mutations in PRPS1. Am J Hum Genet 81:507–518. doi: 10.1086/520706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garcia-Pavia P, Torres RJ, Rivero M, Ahmed M, Garcia-Puig J, Becker MA. 2003. Phosphoribosylpyrophosphate synthetase overactivity as a cause of uric acid overproduction in a young woman. Arthritis Rheum 48:2036–2041. doi: 10.1002/art.11058. [DOI] [PubMed] [Google Scholar]
- 124.Chen P, Li J, Ma J, Teng M, Li X. 2013. A small disturbance, but a serious disease: the possible mechanism of D52H-mutant of human PRS1 that causes gout. IUBMB Life 65:518–525. doi: 10.1002/iub.1154. [DOI] [PubMed] [Google Scholar]
- 125.Cunningham JT, Moreno MV, Lodi A, Ronen SM, Ruggero D. 2014. Protein and nucleotide biosynthesis are coupled by a single rate-limiting enzyme, PRPS2, to drive cancer. Cell 157:1088–1103. doi: 10.1016/j.cell.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ishijima S, Asai T, Kita K, Sonoda T, Tatibana M. 1997. Partial reconstitution of mammalian phosphoribosylpyrophosphate synthetase in Escherichia coli cells. Coexpression of catalytic subunits with the 39-kDa associated protein leads to formation of soluble multimeric complexes of various compositions. Biochim Biophys Acta 1342:28–36. [DOI] [PubMed] [Google Scholar]
- 127.Hong S, Zhou D, Chen C, Wang W, Lv Y, Ye Y, Zou P, Yv J, Chang X, Yv X, Shi L, Ma L, Sun Y, Zhang D, Shen B, Zhu C. 2013. Ribose-phosphate pyrophosphokinase 1 (PRPS1) associated with deltamethrin resistance in Culex pipiens pallens. Parasitol Res 112:847–854. doi: 10.1007/s00436-012-3205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shi C, Dong Z, Zhang H, Cheng F, Chen G, Liu D. 2015. Cloning and characterization of DjPRPS gene in freshwater planarian Dugesia japonica. Turk J Biochem 40:58–65. doi: 10.5505/tjb.2015.61482. [DOI] [Google Scholar]
- 129.Sculaccio SA, Napolitano HB, Beltramini LM, Oliva G, Carrilho E, Thiemann OH. 2008. Sugarcane phosphoribosyl pyrophosphate synthetase: molecular characterization of a phosphate-independent PRS. Plant Mol Biol Rep 26:301–315. doi: 10.1007/s11105-008-0043-6. [DOI] [Google Scholar]
- 130.Ashihara H. 1977. Characterisation of phosphoribosylpyrophosphate synthetase from spinach leaves. Z Pflanzenphysiol 81:379–392. [Google Scholar]
- 131.Gallois R, Prevot JC, Clement A, Jacob JL. 1997. Purification and characterization of phosphoribosylpyrophosphate synthetase from rubber tree latex. Plant Physiol 115:847–852. doi: 10.1104/pp.115.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Marechal-Drouard L, Marshall WF, Qu LH, Nelson DR, Sanderfoot AA, Spalding MH, Kapitonov VV, Ren Q, Ferris P, Lindquist E, Shapiro H, Lucas SM, Grimwood J, Schmutz J, Cardol P, Cerutti H, Chanfreau G, Chen CL, Cognat V, Croft MT, Dent R, Dutcher S, Fernandez E, Fukuzawa H, Gonzalez-Ballester D, Gonzalez-Halphen D, Hallmann A, Hanikenne M, Hippler M, Inwood W, Jabbari K, Kalanon M, Kuras R, Lefebvre PA, Lemaire SD, Lobanov AV, Lohr M, Manuell A, Meier I, Mets L, Mittag M, Mittelmeier T, et al. 2007. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blanc G, Duncan G, Agarkova I, Borodovsky M, Gurnon J, Kuo A, Lindquist E, Lucas S, Pangilinan J, Polle J, Salamov A, Terry A, Yamada T, Dunigan DD, Grigoriev IV, Claverie JM, Van Etten JL. 2010. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 22:2943–2955. doi: 10.1105/tpc.110.076406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blanc G, Agarkova I, Grimwood J, Kuo A, Brueggeman A, Dunigan DD, Gurnon J, Ladunga I, Lindquist E, Lucas S, Pangilinan J, Proschold T, Salamov A, Schmutz J, Weeks D, Yamada T, Lomsadze A, Borodovsky M, Claverie JM, Grigoriev IV, Van Etten JL. 2012. The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol 13:R39. doi: 10.1186/gb-2012-13-5-r39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Palenik B, Grimwood J, Aerts A, Rouze P, Salamov A, Putnam N, Dupont C, Jorgensen R, Derelle E, Rombauts S, Zhou K, Otillar R, Merchant SS, Podell S, Gaasterland T, Napoli C, Gendler K, Manuell A, Tai V, Vallon O, Piganeau G, Jancek S, Heijde M, Jabbari K, Bowler C, Lohr M, Robbens S, Werner G, Dubchak I, Pazour GJ, Ren Q, Paulsen I, Delwiche C, Schmutz J, Rokhsar D, Van de Peer Y, Moreau H, Grigoriev IV. 2007. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc Natl Acad Sci U S A 104:7705–7710. doi: 10.1073/pnas.0611046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Derelle E, Ferraz C, Rombauts S, Rouze P, Worden AZ, Robbens S, Partensky F, Degroeve S, Echeynie S, Cooke R, Saeys Y, Wuyts J, Jabbari K, Bowler C, Panaud O, Piegu B, Ball SG, Ral JP, Bouget FY, Piganeau G, De Baets B, Picard A, Delseny M, Demaille J, Van de Peer Y, Moreau H. 2006. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc Natl Acad Sci U S A 103:11647–11652. doi: 10.1073/pnas.0604795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prochnik SE, Umen J, Nedelcu AM, Hallmann A, Miller SM, Nishii I, Ferris P, Kuo A, Mitros T, Fritz-Laylin LK, Hellsten U, Chapman J, Simakov O, Rensing SA, Terry A, Pangilinan J, Kapitonov V, Jurka J, Salamov A, Shapiro H, Schmutz J, Grimwood J, Lindquist E, Lucas S, Grigoriev IV, Schmitt R, Kirk D, Rokhsar DS. 2010. Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science 329:223–226. doi: 10.1126/science.1188800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cherney MM, Cherney LT, Garen CR, James MN. 2011. The structures of Thermoplasma volcanium phosphoribosyl pyrophosphate synthetase bound to ribose-5-phosphate and ATP analogs. J Mol Biol 413:844–856. doi: 10.1016/j.jmb.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 139.Andersen RW, Leggio LL, Hove-Jensen B, Kadziola A. 2015. Structure of dimeric, recombinant Sulfolobus solfataricus phosphoribosyl diphosphate synthase: a bent dimer defining the adenine specificity of the substrate ATP. Extremophiles 19:407–415. doi: 10.1007/s00792-014-0726-x. [DOI] [PubMed] [Google Scholar]
- 140.Rashid N, Morikawa M, Imanaka T. 1997. Gene cloning and characterization of recombinant ribose phosphate pyrophosphokinase from a hyperthermophilic archaeon. J Biosci Bioeng 83:412–418. [Google Scholar]
- 141.Miller GA Jr, Rosenzweig S, Switzer RL. 1975. Oxygen-18 studies of the mechanism of pyrophosphoryl group transfer catalyzed by phosphoribosylpyrophosphate synthetase. Arch Biochem Biophys 171:732–736. doi: 10.1016/0003-9861(75)90086-7. [DOI] [PubMed] [Google Scholar]
- 142.Fox IH, Kelley WN. 1972. Human phosphoribosylpyrophosphate synthetase. Kinetic mechanism and end product inhibition. J Biol Chem 247:2126–2131. [PubMed] [Google Scholar]
- 143.Sonoda T, Kita K, Ishijima S, Ishizuka T, Ahmad I, Tatibana M. 1997. Kinetic and regulatory properties of rat liver phosphoribosylpyrophosphate synthetase complex are partly distinct from those of isolated recombinant component catalytic subunits. J Biochem 122:635–640. doi: 10.1093/oxfordjournals.jbchem.a021800. [DOI] [PubMed] [Google Scholar]
- 144.Roth DG, Deuel TF. 1974. Stability and regulation of phosphoribosyl pyrophosphate synthetase from rat liver. J Biol Chem 249:297–301. [PubMed] [Google Scholar]
- 145.Sadler WC, Switzer RL. 1977. Regulation of Salmonella phosphoribosylpyrophosphate synthetase activity in vivo. Deductions from pool measurements. J Biol Chem 252:8504–8511. [PubMed] [Google Scholar]
- 146.Bagnara AS, Finch LR. 1973. Relationships between intracellular contents of nucleotides and 5-phosphoribosyl 1-pyrophosphate in Escherichia coli. Eur J Biochem 36:422–427. doi: 10.1111/j.1432-1033.1973.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 147.Monod J, Wyman J, Changeux JP. 1965. On the nature of allosteric transitions: a plausible model. J Mol Biol 12:88–118. doi: 10.1016/S0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 148.Li B, Li H, Bai Y, Kirschner-Schwabe R, Yang JJ, Chen Y, Lu G, Tzoneva G, Ma X, Wu T, Li W, Lu H, Ding L, Liang H, Huang X, Yang M, Jin L, Kang H, Chen S, Du A, Shen S, Ding J, Chen H, Chen J, von Stackelberg A, Gu L, Zhang J, Ferrando A, Tang J, Wang S, Zhou BB. 2015. Negative feedback-defective PRPS1 mutants drive thiopurine resistance in relapsed childhood ALL. Nat Med 21:563–571. doi: 10.1038/nm.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zakataeva NP, Romanenkov DV, Skripnikova VS, Vitushkina MV, Livshits VA, Kivero AD, Novikova AE. 2012. Wild-type and feedback-resistant phosphoribosyl pyrophosphate synthetases from Bacillus amyloliquefaciens: purification, characterization, and application to increase purine nucleoside production. Appl Microbiol Biotechnol 93:2023–2033. doi: 10.1007/s00253-011-3687-3. [DOI] [PubMed] [Google Scholar]
- 150.Hove-Jensen B. 1996. Phosphoribosyl diphosphate synthetase-independent NAD de novo synthesis in Escherichia coli: a new phenotype of phosphate regulon mutants. J Bacteriol 178:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hove-Jensen B, Rosenkrantz TJ, Haldimann A, Wanner BL. 2003. Escherichia coli phnN, encoding ribose 1,5-bisphosphokinase activity (phosphoribosyl diphosphate forming): dual role in phosphonate degradation and NAD biosynthesis pathways. J Bacteriol 185:2793–2801. doi: 10.1128/JB.185.9.2793-2801.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wanner BL. 1996. Phosphorus assimilation and control of the phosphate regulon, p 1357–1381. In Neidhardt FC, Curtis R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 153.Hove-Jensen B, McSorley FR, Zechel DL. 2011. Physiological role of phnP-specified phosphoribosyl cyclic phosphodiesterase in catabolism of organophosphonic acids by the carbon-phosphorus lyase pathway. J Am Chem Soc 133:3617–3624. doi: 10.1021/ja1102713. [DOI] [PubMed] [Google Scholar]
- 154.Hove-Jensen B, Zechel DL, Jochimsen B. 2014. Utilization of glyphosate as phosphate source: biochemistry and genetics of bacterial carbon-phosphorus lyase. Microbiol Mol Biol Rev 78:176–197. doi: 10.1128/MMBR.00040-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Seweryn P, Van LB, Kjeldgaard M, Russo CJ, Passmore LA, Hove-Jensen B, Jochimsen B, Brodersen DE. 2015. Structural insights into the bacterial carbon-phosphorus lyase machinery. Nature 525:68–72. doi: 10.1038/nature14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Richey DP, Brown GM. 1969. The biosynthesis of folic acid. IX. Purification and properties of the enzymes required for the formation of dihydropteroic acid. J Biol Chem 244:1582–1592. [PubMed] [Google Scholar]
- 157.Block R, Haseltine AW. 1975. Purification and properties of stringent factor. J Biol Chem 250:1212–1217. [PubMed] [Google Scholar]
- 158.Sy J, Ogawa Y, Lipmann F. 1973. Nonribosomal synthesis of guanosine 5′,3′-polyphosphates by the ribosomal wash of stringent Escherichia coli. Proc Natl Acad Sci U S A 70:2145–2148. doi: 10.1073/pnas.70.7.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leuthardt F, Nielsen H. 1952. Phosphorylation biologique de la thiamine. Helv Chim Acta 35:1196–1209. doi: 10.1002/hlca.19520350415. [DOI] [Google Scholar]
- 160.Oki T, Yoshimoto A, Sato S, Takamatsu A. 1975. Purine nucleotide pyrophosphotransferase from Streptomyces morookaensis, capable of synthesizing pppApp and pppGpp. Biochim Biophys Acta 410:262–272. doi: 10.1016/0005-2744(75)90228-4. [DOI] [PubMed] [Google Scholar]
- 161.McLennan AG. 2006. The Nudix hydrolase superfamily. Cell Mol Life Sci 63:123–143. doi: 10.1007/s00018-005-5386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mildvan AS, Xia Z, Azurmendi HF, Saraswat V, Legler PM, Massiah MA, Gabelli SB, Bianchet MA, Kang LW, Amzel LM. 2005. Structures and mechanisms of Nudix hydrolases. Arch Biochem Biophys 433:129–143. doi: 10.1016/j.abb.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 163.Bessman MJ, Frick DN, O'Handley SF. 1996. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J Biol Chem 271:25059–25062. doi: 10.1074/jbc.271.41.25059. [DOI] [PubMed] [Google Scholar]
- 164.Blaszczyk J, Shi G, Yan H, Ji X. 2000. Catalytic center assembly of HPPK as revealed by the crystal structure of a ternary complex at 1.25 Å resolution. Structure 8:1049–1058. doi: 10.1016/S0969-2126(00)00502-5. [DOI] [PubMed] [Google Scholar]
- 165.Stammers DK, Achari A, Somers DO, Bryant PK, Rosemond J, Scott DL, Champness JN. 1999. 2.0 Å X-ray structure of the ternary complex of 7,8-dihydro-6-hydroxymethylpterinpyrophosphokinase from Escherichia coli with ATP and a substrate analogue. FEBS Lett 456:49–53. doi: 10.1016/S0014-5793(99)00860-1. [DOI] [PubMed] [Google Scholar]
- 166.Xiao B, Shi G, Chen X, Yan H, Ji X. 1999. Crystal structure of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase, a potential target for the development of novel antimicrobial agents. Structure 7:489–496. doi: 10.1016/S0969-2126(99)80065-3. [DOI] [PubMed] [Google Scholar]
- 167.Hennig M, Dale GE, D'Arcy A, Danel F, Fischer S, Gray CP, Jolidon S, Muller F, Page MG, Pattison P, Oefner C. 1999. The structure and function of the 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase from Haemophilus influenzae. J Mol Biol 287:211–219. doi: 10.1006/jmbi.1999.2623. [DOI] [PubMed] [Google Scholar]
- 168.Bermingham A, Derrick JP. 2002. The folic acid biosynthesis pathway in bacteria: evaluation of potential for antibacterial drug discovery. Bioessays 24:637–648. doi: 10.1002/bies.10114. [DOI] [PubMed] [Google Scholar]
- 169.Xiao B, Shi G, Gao J, Blaszczyk J, Liu Q, Ji X, Yan H. 2001. Unusual conformational changes in 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase as revealed by X-ray crystallography and NMR. J Biol Chem 276:40274–40281. doi: 10.1074/jbc.M103837200. [DOI] [PubMed] [Google Scholar]
- 170.Blaszczyk J, Shi G, Li Y, Yan H, Ji X. 2004. Reaction trajectory of pyrophosphoryl transfer catalyzed by 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase. Structure 12:467–475. doi: 10.1016/j.str.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 171.Bunik VI, Tylicki A, Lukashev NV. 2013. Thiamin diphosphate-dependent enzymes: from enzymology to metabolic regulation, drug design and disease models. FEBS J 280:6412–6442. doi: 10.1111/febs.12512. [DOI] [PubMed] [Google Scholar]
- 172.Liu JY, Timm DE, Hurley TD. 2006. Pyrithiamine as a substrate for thiamine pyrophosphokinase. J Biol Chem 281:6601–6607. doi: 10.1074/jbc.M510951200. [DOI] [PubMed] [Google Scholar]
- 173.Santini S, Monchois V, Mouz N, Sigoillot C, Rousselle T, Claverie JM, Abergel C. 2008. Structural characterization of CA1462, the Candida albicans thiamine pyrophosphokinase. BMC Struct Biol 8:33. doi: 10.1186/1472-6807-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Baker LJ, Dorocke JA, Harris RA, Timm DE. 2001. The crystal structure of yeast thiamin pyrophosphokinase. Structure 9:539–546. doi: 10.1016/S0969-2126(01)00615-3. [DOI] [PubMed] [Google Scholar]
- 175.Hogg T, Mechold U, Malke H, Cashel M, Hilgenfeld R. 2004. Conformational antagonism between opposing active sites in a bifunctional RelA/SpoT homolog modulates (p)ppGpp metabolism during the stringent response. Cell 117:57–68. doi: 10.1016/S0092-8674(04)00260-0. [DOI] [PubMed] [Google Scholar]
- 176.Sawaya MR, Prasad R, Wilson SH, Kraut J, Pelletier H. 1997. Crystal structures of human DNA polymerase beta complexed with gapped and nicked DNA: evidence for an induced fit mechanism. Biochemistry 36:11205–11215. doi: 10.1021/bi9703812. [DOI] [PubMed] [Google Scholar]
- 177.Mechold U, Murphy H, Brown L, Cashel M. 2002. Intramolecular regulation of the opposing (p)ppGpp catalytic activities of RelSeq, the Rel/Spo enzyme from Streptococcus equisimilis. J Bacteriol 184:2878–2888. doi: 10.1128/JB.184.11.2878-2888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sajish M, Tiwari D, Rananaware D, Nandicoori VK, Prakash B. 2007. A charge reversal differentiates (p)ppGpp synthesis by monofunctional and bifunctional Rel proteins. J Biol Chem 282:34977–34983. doi: 10.1074/jbc.M704828200. [DOI] [PubMed] [Google Scholar]
- 179.Abeygunawardana C, Weber DJ, Gittis AG, Frick DN, Lin J, Miller AF, Bessman MJ, Mildvan AS. 1995. Solution structure of the MutT enzyme, a nucleoside triphosphate pyrophosphohydrolase. Biochemistry 34:14997–15005. doi: 10.1021/bi00046a006. [DOI] [PubMed] [Google Scholar]
- 180.Maki H, Sekiguchi M. 1992. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 355:273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- 181.Yanofsky C, Cox EC, Horn V. 1966. The unusual mutagenic specificity of an E. coli mutator gene. Proc Natl Acad Sci U S A 55:274–281. doi: 10.1073/pnas.55.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bhatnagar SK, Bessman MJ. 1988. Studies on the mutator gene, mutT of Escherichia coli. Molecular cloning of the gene, purification of the gene product, and identification of a novel nucleoside triphosphatase. J Biol Chem 263:8953–8957. [PubMed] [Google Scholar]
- 183.Frick DN, Weber DJ, Gillespie JR, Bessman MJ, Mildvan AS. 1994. Dual divalent cation requirement of the MutT dGTPase. Kinetic and magnetic resonance studies of the metal and substrate complexes. J Biol Chem 269:1794–1803. [PubMed] [Google Scholar]
- 184.Steitz TA, Steitz JA. 1993. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci U S A 90:6498–6502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang F, Yang W. 2009. Structural insight into translesion synthesis by DNA Pol II. Cell 139:1279–1289. doi: 10.1016/j.cell.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Beard WA, Wilson SH. 2014. Structure and mechanism of DNA polymerase beta. Biochemistry 53:2768–2780. doi: 10.1021/bi500139h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McHenry CS. 2011. DNA replicases from a bacterial perspective. Annu Rev Biochem 80:403–436. doi: 10.1146/annurev-biochem-061208-091655. [DOI] [PubMed] [Google Scholar]
- 188.Borukhov S, Lee J. 2005. RNA polymerase structure and function at lac operon. C R Biol 328:576–587. doi: 10.1016/j.crvi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 189.Yang W, Lee JY, Nowotny M. 2006. Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity. Mol Cell 22:5–13. doi: 10.1016/j.molcel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 190.Gehring AM, Lees WJ, Mindiola DJ, Walsh CT, Brown ED. 1996. Acetyltransfer precedes uridylyltransfer in the formation of UDP-N-acetylglucosamine in separable active sites of the bifunctional GlmU protein of Escherichia coli. Biochemistry 35:579–585. doi: 10.1021/bi952275a. [DOI] [PubMed] [Google Scholar]
- 191.Roseman S. 2001. Reflections on glycobiology. J Biol Chem 276:41527–41542. doi: 10.1074/jbc.R100053200. [DOI] [PubMed] [Google Scholar]
- 192.Jagtap PK, Verma SK, Vithani N, Bais VS, Prakash B. 2013. Crystal structures identify an atypical two-metal-ion mechanism for uridyltransfer in GlmU: its significance to sugar nucleotidyl transferases. J Mol Biol 425:1745–1759. doi: 10.1016/j.jmb.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 193.Heyes DJ, Levy C, Lafite P, Roberts IS, Goldrick M, Stachulski AV, Rossington SB, Stanford D, Rigby SE, Scrutton NS, Leys D. 2009. Structure-based mechanism of CMP-2-keto-3-deoxymanno-octulonic acid synthetase: convergent evolution of a sugar-activating enzyme with DNA/RNA polymerases. J Biol Chem 284:35514–35523. doi: 10.1074/jbc.M109.056630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Thoden JB, Holden HM. 2007. Active site geometry of glucose-1-phosphate uridylyltransferase. Protein Sci 16:1379–1388. doi: 10.1110/ps.072864707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Björkelid C, Bergfors T, Henriksson LM, Stern AL, Unge T, Mowbray SL, Jones TA. 2011. Structural and functional studies of mycobacterial IspD enzymes. Acta Crystallogr D Biol Crystallogr 67:403–414. doi: 10.1107/S0907444911006160. [DOI] [PubMed] [Google Scholar]
- 196.Maruyama D, Nishitani Y, Nonaka T, Kita A, Fukami TA, Mio T, Yamada-Okabe H, Yamada-Okabe T, Miki K. 2007. Crystal structure of uridine-diphospho-N-acetylglucosamine pyrophosphorylase from Candida albicans and catalytic reaction mechanism. J Biol Chem 282:17221–17230. doi: 10.1074/jbc.M611873200. [DOI] [PubMed] [Google Scholar]
- 197.Marco-Marin C, Gil-Ortiz F, Rubio V. 2005. The crystal structure of Pyrococcus furiosus UMP kinase provides insight into catalysis and regulation in microbial pyrimidine nucleotide biosynthesis. J Mol Biol 352:438–454. doi: 10.1016/j.jmb.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 198.Miallau L, Hunter WN, McSweeney SM, Leonard GA. 2007. Structures of Staphylococcus aureus D-tagatose-6-phosphate kinase implicate domain motions in specificity and mechanism. J Biol Chem 282:19948–19957. doi: 10.1074/jbc.M701480200. [DOI] [PubMed] [Google Scholar]
- 199.Sato M, Arakawa T, Nam YW, Nishimoto M, Kitaoka M, Fushinobu S. 2015. Open-close structural change upon ligand binding and two magnesium ions required for the catalysis of N-acetylhexosamine 1-kinase. Biochim Biophys Acta 1854:333–340. doi: 10.1016/j.bbapap.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 200.Bao ZQ, Jacobsen DM, Young MA. 2011. Briefly bound to activate: transient binding of a second catalytic magnesium activates the structure and dynamics of CDK2 kinase for catalysis. Structure 19:675–690. doi: 10.1016/j.str.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bastidas AC, Deal MS, Steichen JM, Guo Y, Wu J, Taylor SS. 2013. Phosphoryl transfer by protein kinase A is captured in a crystal lattice. J Am Chem Soc 135:4788–4798. doi: 10.1021/ja312237q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Madhusudan Akamine P, Xuong NH, Taylor SS. 2002. Crystal structure of a transition state mimic of the catalytic subunit of cAMP-dependent protein kinase. Nat Struct Biol 9:273–277. doi: 10.1038/nsb780. [DOI] [PubMed] [Google Scholar]
- 203.Saint Girons I, Gilles AM, Margarita D, Michelson S, Monnot M, Fermandjian S, Danchin A, Barzu O. 1987. Structural and catalytic characteristics of Escherichia coli adenylate kinase. J Biol Chem 262:622–629. [PubMed] [Google Scholar]
- 204.Berry MB, Meador B, Bilderback T, Liang P, Glaser M, Phillips GN Jr. 1994. The closed conformation of a highly flexible protein: the structure of E. coli adenylate kinase with bound AMP and AMPPNP. Proteins 19:183–198. doi: 10.1002/prot.340190304. [DOI] [PubMed] [Google Scholar]
- 205.Müller CW, Schulz GE. 1992. Structure of the complex between adenylate kinase from Escherichia coli and the inhibitor Ap5A refined at 1.9 Å resolution. A model for a catalytic transition state. J Mol Biol 224:159–177. [DOI] [PubMed] [Google Scholar]
- 206.Berry MB, Phillips GN Jr. 1998. Crystal structures of Bacillus stearothermophilus adenylate kinase with bound Ap5A, Mg2+ Ap5A, and Mn2+ Ap5A reveal an intermediate lid position and six coordinate octahedral geometry for bound Mg2+ and Mn2+. Proteins 32:276–288. doi:. [DOI] [PubMed] [Google Scholar]
- 207.Krishnamurthy H, Lou H, Kimple A, Vieille C, Cukier RI. 2005. Associative mechanism for phosphoryl transfer: a molecular dynamics simulation of Escherichia coli adenylate kinase complexed with its substrates. Proteins 58:88–100. doi: 10.1002/prot.20301. [DOI] [PubMed] [Google Scholar]
- 208.Bellinzoni M, Haouz A, Grana M, Munier-Lehmann H, Shepard W, Alzari PM. 2006. The crystal structure of Mycobacterium tuberculosis adenylate kinase in complex with two molecules of ADP and Mg2+ supports an associative mechanism for phosphoryl transfer. Protein Sci 15:1489–1493. doi: 10.1110/ps.062163406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schramm VL, Grubmeyer C. 2004. Phosphoribosyltransferase mechanisms and roles in nucleic acid metabolism. Prog Nucleic Acid Res Mol Biol 78:261–304. doi: 10.1016/S0079-6603(04)78007-1. [DOI] [PubMed] [Google Scholar]
- 210.Sinha SC, Smith JL. 2001. The PRT protein family. Curr Opin Struct Biol 11:733–739. doi: 10.1016/S0959-440X(01)00274-3. [DOI] [PubMed] [Google Scholar]
- 211.Eads JC, Ozturk D, Wexler TB, Grubmeyer C, Sacchettini JC. 1997. A new function for a common fold: the crystal structure of quinolinic acid phosphoribosyltransferase. Structure 5:47–58. doi: 10.1016/S0969-2126(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 212.Heroux A, White EL, Ross LJ, Kuzin AP, Borhani DW. 2000. Substrate deformation in a hypoxanthine-guanine phosphoribosyltransferase ternary complex: the structural basis for catalysis. Structure 8:1309–1318. doi: 10.1016/S0969-2126(00)00546-3. [DOI] [PubMed] [Google Scholar]
- 213.Craig SP III, Eakin AE. 2000. Purine phosphoribosyltransferases. J Biol Chem 275:20231–20234. doi: 10.1074/jbc.R000002200. [DOI] [PubMed] [Google Scholar]
- 214.Messenger LJ, Zalkin H. 1979. Glutamine phosphoribosylpyrophosphate amidotransferase from Escherichia coli. Purification and properties. J Biol Chem 254:3382–3392. [PubMed] [Google Scholar]
- 215.Krahn JM, Kim JH, Burns MR, Parry RJ, Zalkin H, Smith JL. 1997. Coupled formation of an amidotransferase interdomain ammonia channel and a phosphoribosyltransferase active site. Biochemistry 36:11061–11068. doi: 10.1021/bi9714114. [DOI] [PubMed] [Google Scholar]
- 216.Smith JL. 1998. Glutamine PRPP amidotransferase: snapshots of an enzyme in action. Curr Opin Struct Biol 8:686–694. doi: 10.1016/S0959-440X(98)80087-0. [DOI] [PubMed] [Google Scholar]
- 217.Hansen MR, Jensen KS, Rasmussen MS, Christoffersen S, Kadziola A, Jensen KF. 2014. Specificities and pH profiles of adenine and hypoxanthine-guanine-xanthine phosphoribosyltransferases (nucleotide synthases) of the thermoacidophile archaeon Sulfolobus solfataricus. Extremophiles 18:179–187. doi: 10.1007/s00792-013-0595-8. [DOI] [PubMed] [Google Scholar]
- 218.Shi W, Sarver AE, Wang CC, Tanaka KS, Almo SC, Schramm VL. 2002. Closed site complexes of adenine phosphoribosyltransferase from Giardia lamblia reveal a mechanism of ribosyl migration. J Biol Chem 277:39981–39988. doi: 10.1074/jbc.M205596200. [DOI] [PubMed] [Google Scholar]
- 219.Shi W, Tanaka KS, Crother TR, Taylor MW, Almo SC, Schramm VL. 2001. Structural analysis of adenine phosphoribosyltransferase from Saccharomyces cerevisiae. Biochemistry 40:10800–10809. doi: 10.1021/bi010465h. [DOI] [PubMed] [Google Scholar]
- 220.Silva CH, Silva M, Iulek J, Thiemann OH. 2008. Structural complexes of human adenine phosphoribosyltransferase reveal novel features of the APRT catalytic mechanism. J Biomol Struct Dyn 25:589–597. doi: 10.1080/07391102.2008.10507205. [DOI] [PubMed] [Google Scholar]
- 221.Phillips CL, Ullman B, Brennan RG, Hill CP. 1999. Crystal structures of adenine phosphoribosyltransferase from Leishmania donovani. EMBO J 18:3533–3545. doi: 10.1093/emboj/18.13.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jensen KF, Hansen MR, Jensen KS, Christoffersen S, Poulsen JC, Molgaard A, Kadziola A. 2015. Adenine phosphoribosyltransferase from Sulfolobus solfataricus is an enzyme with unusual kinetic properties and a crystal structure that suggests it evolved from a 6-oxopurine phosphoribosyltransferase. Biochemistry 54:2323–2334. doi: 10.1021/bi501334m. [DOI] [PubMed] [Google Scholar]
- 223.Scapin G, Ozturk DH, Grubmeyer C, Sacchettini JC. 1995. The crystal structure of the orotate phosphoribosyltransferase complexed with orotate and alpha-d-5-phosphoribosyl-1-pyrophosphate. Biochemistry 34:10744–10754. doi: 10.1021/bi00034a006. [DOI] [PubMed] [Google Scholar]
- 224.Henriksen A, Aghajari N, Jensen KF, Gajhede M. 1996. A flexible loop at the dimer interface is a part of the active site of the adjacent monomer of Escherichia coli orotate phosphoribosyltransferase. Biochemistry 35:3803–3809. doi: 10.1021/bi952226y. [DOI] [PubMed] [Google Scholar]
- 225.Liu CP, Xu R, Gao ZQ, Xu JH, Hou HF, Li LQ, She Z, Li LF, Su XD, Liu P, Dong YH. 2010. Structure of orotate phosphoribosyltransferase from the caries pathogen Streptococcus mutans. Acta Crystallogr Sect F Struct Biol Cryst Commun 66:498–502. doi: 10.1107/S1744309110009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kumar S, Krishnamoorthy K, Mudeppa DG, Rathod PK. 2015. Structure of Plasmodium falciparum orotate phosphoribosyltransferase with autologous inhibitory protein-protein interactions. Acta Crystallogr Sect F Struct Biol Commun 71:600–608. doi: 10.1107/S2053230X1500549X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.French JB, Yates PA, Soysa DR, Boitz JM, Carter NS, Chang B, Ullman B, Ealick SE. 2011. The Leishmania donovani UMP synthase is essential for promastigote viability and has an unusual tetrameric structure that exhibits substrate-controlled oligomerization. J Biol Chem 286:20930–20941. doi: 10.1074/jbc.M111.228213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Grubmeyer C, Hansen MR, Fedorov AA, Almo SC. 2012. Structure of Salmonella typhimurium OMP synthase in a complete substrate complex. Biochemistry 51:4397–4405. doi: 10.1021/bi300083p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ozturk DH, Dorfman RH, Scapin G, Sacchettini JC, Grubmeyer C. 1995. Locations and functional roles of conserved lysine residues in Salmonella typhimurium orotate phosphoribosyltransferase. Biochemistry 34:10755–10763. doi: 10.1021/bi00034a007. [DOI] [PubMed] [Google Scholar]
- 230.Gonzalez-Segura L, Witte JF, McClard RW, Hurley TD. 2007. Ternary complex formation and induced asymmetry in orotate phosphoribosyltransferase. Biochemistry 46:14075–14086. doi: 10.1021/bi701023z. [DOI] [PubMed] [Google Scholar]
- 231.McClard RW, Holets EA, MacKinnon AL, Witte JF. 2006. Half-of-sites binding of orotidine 5′-phosphate and alpha-d-5-phosphorylribose 1-diphosphate to orotate phosphoribosyltransferase from Saccharomyces cerevisiae supports a novel variant of the Theorell-Chance mechanism with alternating site catalysis. Biochemistry 45:5330–5342. doi: 10.1021/bi051650o. [DOI] [PubMed] [Google Scholar]
- 232.Jensen KF, Mygind B. 1996. Different oligomeric states are involved in the allosteric behavior of uracil phosphoribosyltransferase from Escherichia coli. Eur J Biochem 240:637–645. doi: 10.1111/j.1432-1033.1996.0637h.x. [DOI] [PubMed] [Google Scholar]
- 233.Schumacher MA, Bashor CJ, Song MH, Otsu K, Zhu S, Parry RJ, Ullman B, Brennan RG. 2002. The structural mechanism of GTP stabilized oligomerization and catalytic activation of the Toxoplasma gondii uracil phosphoribosyltransferase. Proc Natl Acad Sci U S A 99:78–83. doi: 10.1073/pnas.012399599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Linde L, Jensen KF. 1996. Uracil phosphoribosyltransferase from the extreme thermoacidophilic archaebacterium Sulfolobus shibatae is an allosteric enzyme, activated by GTP and inhibited by CTP. Biochim Biophys Acta 1296:16–22. doi: 10.1016/0167-4838(96)00045-3. [DOI] [PubMed] [Google Scholar]
- 235.Jensen KF, Arent S, Larsen S, Schack L. 2005. Allosteric properties of the GTP activated and CTP inhibited uracil phosphoribosyltransferase from the thermoacidophilic archaeon Sulfolobus solfataricus. FEBS J 272:1440–1453. doi: 10.1111/j.1742-4658.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- 236.Christoffersen S, Kadziola A, Johansson E, Rasmussen M, Willemoes M, Jensen KF. 2009. Structural and kinetic studies of the allosteric transition in Sulfolobus solfataricus uracil phosphoribosyltransferase: permanent activation by engineering of the C-terminus. J Mol Biol 393:464–477. doi: 10.1016/j.jmb.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 237.Arent S, Harris P, Jensen KF, Larsen S. 2005. Allosteric regulation and communication between subunits in uracil phosphoribosyltransferase from Sulfolobus solfataricus. Biochemistry 44:883–892. doi: 10.1021/bi048041l. [DOI] [PubMed] [Google Scholar]
- 238.Villela AD, Ducati RG, Rosado LA, Bloch CJ, Prates MV, Goncalves DC, Ramos CH, Basso LA, Santos DS. 2013. Biochemical characterization of uracil phosphoribosyltransferase from Mycobacterium tuberculosis. PLoS One 8:e56445. doi: 10.1371/journal.pone.0056445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ghode P, Jobichen C, Ramachandran S, Bifani P, Sivaraman J. 2015. Structural basis of mapping the spontaneous mutations with 5-flurouracil in uracil phosphoribosyltransferase from Mycobacterium tuberculosis. Biochem Biophys Res Commun 467:577–582. doi: 10.1016/j.bbrc.2015.09.133. [DOI] [PubMed] [Google Scholar]
- 240.Dai YP, Lee CS, O'Sullivan WJ. 1995. Properties of uracil phosphoribosyltransferase from Giardia intestinalis. Int J Parasitol 25:207–214. doi: 10.1016/0020-7519(94)00090-B. [DOI] [PubMed] [Google Scholar]
- 241.Andersen PS, Smith JM, Mygind B. 1992. Characterization of the upp gene encoding uracil phosphoribosyltransferase of Escherichia coli K12. Eur J Biochem 204:51–56. doi: 10.1111/j.1432-1033.1992.tb16604.x. [DOI] [PubMed] [Google Scholar]
- 242.Kern L, de Montigny J, Jund R, Lacroute F. 1990. The FUR1 gene of Saccharomyces cerevisiae: cloning, structure and expression of wild-type and mutant alleles. Gene 88:149–157. doi: 10.1016/0378-1119(90)90026-N. [DOI] [PubMed] [Google Scholar]
- 243.Kadziola A, Neuhard J, Larsen S. 2002. Structure of product-bound Bacillus caldolyticus uracil phosphoribosyltransferase confirms ordered sequential substrate binding. Acta Crystallogr D Biol Crystallogr 58:936–945. doi: 10.1107/S0907444902005024. [DOI] [PubMed] [Google Scholar]
- 244.Jensen HK, Mikkelsen N, Neuhard J. 1997. Recombinant uracil phosphoribosyltransferase from the thermophile Bacillus caldolyticus: expression, purification, and partial characterization. Protein Expr Purif 10:356–364. doi: 10.1006/prep.1997.0755. [DOI] [PubMed] [Google Scholar]
- 245.Martinussen J, Hammer K. 1994. Cloning and characterization of upp, a gene encoding uracil phosphoribosyltransferase from Lactococcus lactis. J Bacteriol 176:6457–6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lundegaard C, Jensen KF. 1999. Kinetic mechanism of uracil phosphoribosyltransferase from Escherichia coli and catalytic importance of the conserved proline in the PRPP binding site. Biochemistry 38:3327–3334. doi: 10.1021/bi982279q. [DOI] [PubMed] [Google Scholar]
- 247.Ghim SY, Neuhard J. 1994. The pyrimidine biosynthesis operon of the thermophile Bacillus caldolyticus includes genes for uracil phosphoribosyltransferase and uracil permease. J Bacteriol 176:3698–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Martinussen J, Glaser P, Andersen PS, Saxild HH. 1995. Two genes encoding uracil phosphoribosyltransferase are present in Bacillus subtilis. J Bacteriol 177:271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hatfield D, Wyngarden JB. 1964. 3-Ribosylpurines. I. Synthesis of (3-ribosyluric acid) 5′phosphate and (3-ribosylxanthine) 5′phosphate by a pyrimidine ribonucleotide pyrophosphorylase. J Biol Chem 239:2680–2586. [PubMed] [Google Scholar]
- 250.Osterman A. 2009. Biogenesis and homeostasis of nicotinamide adenine dinucleotide cofactor. EcoSal Plus doi: 10.1128/ecosalplus36310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bhatia R, Calvo KC. 1996. The sequencing expression, purification, and steady-state kinetic analysis of quinolinate phosphoribosyl transferase from Escherichia coli. Arch Biochem Biophys 325:270–278. doi: 10.1006/abbi.1996.0034. [DOI] [PubMed] [Google Scholar]
- 252.Hughes KT, Dessen A, Gray JP, Grubmeyer C. 1993. The Salmonella typhimurium nadC gene: sequence determination by use of Mud-P22 and purification of quinolinate phosphoribosyltransferase. J Bacteriol 175:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Vinitsky A, Grubmeyer C. 1993. A new paradigm for biochemical energy coupling. Salmonella typhimurium nicotinate phosphoribosyltransferase. J Biol Chem 268:26004–26010. [PubMed] [Google Scholar]
- 254.Gross J, Rajavel M, Segura E, Grubmeyer C. 1996. Energy coupling in Salmonella typhimurium nicotinic acid phosphoribosyltransferase: identification of His-219 as site of phosphorylation. Biochemistry 35:3917–3924. doi: 10.1021/bi9517906. [DOI] [PubMed] [Google Scholar]
- 255.Gross JW, Rajavel M, Grubmeyer C. 1998. Kinetic mechanism of nicotinic acid phosphoribosyltransferase: implications for energy coupling. Biochemistry 37:4189–4199. doi: 10.1021/bi972014w. [DOI] [PubMed] [Google Scholar]
- 256.Rajavel M, Lalo D, Gross JW, Grubmeyer C. 1998. Conversion of a cosubstrate to an inhibitor: phosphorylation mutants of nicotinic acid phosphoribosyltransferase. Biochemistry 37:4181–4188. doi: 10.1021/bi9720134. [DOI] [PubMed] [Google Scholar]
- 257.Dietrich LS, Muniz O. 1972. Inhibition of nicotinamide phosphoribosyltransferase by pyridine nucleotides. Biochemistry 11:1691–1695. doi: 10.1021/bi00759a025. [DOI] [PubMed] [Google Scholar]
- 258.Preiss J, Handler P. 1957. Enzymatic synthesis of nicotinamide mononucleotide. J Biol Chem 225:759–770. [PubMed] [Google Scholar]
- 259.Sharma V, Grubmeyer C, Sacchettini JC. 1998. Crystal structure of quinolinic acid phosphoribosyltransferase from Mycobacterium tuberculosis: a potential TB drug target. Structure 6:1587–1599. doi: 10.1016/S0969-2126(98)00156-7. [DOI] [PubMed] [Google Scholar]
- 260.Kim MK, Im YJ, Lee JH, Eom SH. 2006. Crystal structure of quinolinic acid phosphoribosyltransferase from Helicobacter pylori. Proteins 63:252–255. doi: 10.1002/prot.20834. [DOI] [PubMed] [Google Scholar]
- 261.Schwarzenbacher R, Jaroszewski L, von Delft F, Abdubek P, Ambing E, Biorac T, Brinen LS, Canaves JM, Cambell J, Chiu HJ, Dai X, Deacon AM, DiDonato M, Elsliger MA, Eshagi S, Floyd R, Godzik A, Grittini C, Grzechnik SK, Hampton E, Karlak C, Klock HE, Koesema E, Kovarik JS, Kreusch A, Kuhn P, Lesley SA, Levin I, McMullan D, McPhillips TM, Miller MD, Morse A, Moy K, Ouyang J, Page R, Quijano K, Robb A, Spraggon G, Stevens RC, van den Bedem H, Velasquez J, Vincent J, Wang X, West B, Wolf G, Xu Q, Hodgson KO, Wooley J, Wilson IA. 2004. Crystal structure of a type II quinolic acid phosphoribosyltransferase (TM1645) from Thermotoga maritima at 2.50 Å resolution. Proteins 55:768–771. doi: 10.1002/prot.20029. [DOI] [PubMed] [Google Scholar]
- 262.di Luccio E, Wilson DK. 2008. Comprehensive X-ray structural studies of the quinolinate phosphoribosyl transferase (BNA6) from Saccharomyces cerevisiae. Biochemistry 47:4039–4050. doi: 10.1021/bi7020475. [DOI] [PubMed] [Google Scholar]
- 263.Shin DH, Oganesyan N, Jancarik J, Yokota H, Kim R, Kim SH. 2005. Crystal structure of a nicotinate phosphoribosyltransferase from Thermoplasma acidophilum. J Biol Chem 280:18326–18335. doi: 10.1074/jbc.M501622200. [DOI] [PubMed] [Google Scholar]
- 264.Rajavel M, Gross J, Segura E, Moore WT, Grubmeyer C. 1996. Limited proteolysis of Salmonella typhimurium nicotinic acid phosphoribosyltransferase reveals ATP-linked conformational change. Biochemistry 35:3909–3916. doi: 10.1021/bi951791y. [DOI] [PubMed] [Google Scholar]
- 265.Takahashi R, Nakamura S, Nakazawa T, Minoura K, Yoshida T, Nishi Y, Kobayashi Y, Ohkubo T. 2010. Structure and reaction mechanism of human nicotinamide phosphoribosyltransferase. J Biochem 147:95–107. doi: 10.1093/jb/mvp152. [DOI] [PubMed] [Google Scholar]
- 266.Kim MK, Lee JH, Kim H, Park SJ, Kim SH, Kang GB, Lee YS, Kim JB, Kim KK, Suh SW, Eom SH. 2006. Crystal structure of visfatin/pre-B cell colony-enhancing factor 1/nicotinamide phosphoribosyltransferase, free and in complex with the anti-cancer agent FK-866. J Mol Biol 362:66–77. doi: 10.1016/j.jmb.2006.06.082. [DOI] [PubMed] [Google Scholar]
- 267.Khan JA, Tao X, Tong L. 2006. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat Struct Mol Biol 13:582–588. doi: 10.1038/nsmb1105. [DOI] [PubMed] [Google Scholar]
- 268.Chappie JS, Canaves JM, Han GW, Rife CL, Xu Q, Stevens RC. 2005. The structure of a eukaryotic nicotinic acid phosphoribosyltransferase reveals structural heterogeneity among type II PRTases. Structure 13:1385–1396. doi: 10.1016/j.str.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 269.Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. 2015. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 11:535–546. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 270.Oh A, Ho YC, Zak M, Liu Y, Chen X, Yuen PW, Zheng X, Liu Y, Dragovich PS, Wang W. 2014. Structural and biochemical analyses of the catalysis and potency impact of inhibitor phosphoribosylation by human nicotinamide phosphoribosyltransferase. Chembiochem 15:1121–1130. doi: 10.1002/cbic.201402023. [DOI] [PubMed] [Google Scholar]
- 271.Ames BN, Martin RG, Garry BJ. 1961. The first step of histidine biosynthesis. J Biol Chem 236:2019–2026. [PubMed] [Google Scholar]
- 272.Bruni CB, Musti AM, Frunzio R, Blasi F. 1980. Structural and physiological studies of the Escherichia coli histidine operon inserted into plasmid vectors. J Bacteriol 142:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Voll MJ, Appella E, Martin RG. 1967. Purification and composition studies of phosphoribosyladenosine triphosphate:pyrophosphate phosphoribosyltransferase, the first enzyme of histidine biosynthesis. J Biol Chem 242:1760–1767. [PubMed] [Google Scholar]
- 274.Bovee ML, Champagne KS, Demeler B, Francklyn CS. 2002. The quaternary structure of the HisZ-HisG N-1-(5′-phosphoribosyl)-ATP transferase from Lactococcus lactis. Biochemistry 41:11838–11846. doi: 10.1021/bi020243z. [DOI] [PubMed] [Google Scholar]
- 275.Sissler M, Delorme C, Bond J, Ehrlich SD, Renault P, Francklyn C. 1999. An aminoacyl-tRNA synthetase paralog with a catalytic role in histidine biosynthesis. Proc Natl Acad Sci U S A 96:8985–8990. doi: 10.1073/pnas.96.16.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lohkamp B, Coggins JR, Lapthorn AJ. 2000. Purification, crystallization and preliminary X-ray crystallographic analysis of ATP-phosphoribosyltransferase from Escherichia coli. Acta Crystallogr D Biol Crystallogr 56:1488–1491. doi: 10.1107/S0907444900011306. [DOI] [PubMed] [Google Scholar]
- 277.Lohkamp B, McDermott G, Campbell SA, Coggins JR, Lapthorn AJ. 2004. The structure of Escherichia coli ATP-phosphoribosyltransferase: identification of substrate binding sites and mode of AMP inhibition. J Mol Biol 336:131–144. doi: 10.1016/j.jmb.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 278.Morton DP, Parsons SM. 1977. Inhibition of ATP phosphoribosyltransferase by AMP and ADP in the absence and presence of histidine. Arch Biochem Biophys 181:643–648. doi: 10.1016/0003-9861(77)90270-3. [DOI] [PubMed] [Google Scholar]
- 279.Morton DP, Parsons SM. 1977. Synergistic inhibition of ATP phosphoribosyltransferase by guanosine tetraphosphate and histidine. Biochem Biophys Res Commun 74:172–177. doi: 10.1016/0006-291X(77)91390-0. [DOI] [PubMed] [Google Scholar]
- 280.Cho Y, Sharma V, Sacchettini JC. 2003. Crystal structure of ATP phosphoribosyltransferase from Mycobacterium tuberculosis. J Biol Chem 278:8333–8339. doi: 10.1074/jbc.M212124200. [DOI] [PubMed] [Google Scholar]
- 281.Champagne KS, Sissler M, Larrabee Y, Doublie S, Francklyn CS. 2005. Activation of the hetero-octameric ATP phosphoribosyl transferase through subunit interface rearrangement by a tRNA synthetase paralog. J Biol Chem 280:34096–34104. doi: 10.1074/jbc.M505041200. [DOI] [PubMed] [Google Scholar]
- 282.Vega MC, Zou P, Fernandez FJ, Murphy GE, Sterner R, Popov A, Wilmanns M. 2005. Regulation of the hetero-octameric ATP phosphoribosyl transferase complex from Thermotoga maritima by a tRNA synthetase-like subunit. Mol Microbiol 55:675–686. doi: 10.1111/j.1365-2958.2004.04422.x. [DOI] [PubMed] [Google Scholar]
- 283.Champagne KS, Piscitelli E, Francklyn CS. 2006. Substrate recognition by the hetero-octameric ATP phosphoribosyltransferase from Lactococcus lactis. Biochemistry 45:14933–14943. doi: 10.1021/bi061802v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mayans O, Ivens A, Nissen LJ, Kirschner K, Wilmanns M. 2002. Structural analysis of two enzymes catalysing reverse metabolic reactions implies common ancestry. EMBO J 21:3245–3254. doi: 10.1093/emboj/cdf298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lee CE, Goodfellow C, Javid-Majd F, Baker EN, Shaun Lott J. 2006. The crystal structure of TrpD, a metabolic enzyme essential for lung colonization by Mycobacterium tuberculosis, in complex with its substrate phosphoribosylpyrophosphate. J Mol Biol 355:784–797. doi: 10.1016/j.jmb.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 286.Kim C, Xuong NH, Edwards S, Madhusudan Yee MC, Spraggon G, Mills SE. 2002. The crystal structure of anthranilate phosphoribosyltransferase from the enterobacterium Pectobacterium carotovorum. FEBS Lett 523:239–246. doi: 10.1016/S0014-5793(02)02905-8. [DOI] [PubMed] [Google Scholar]
- 287.Castell A, Short FL, Evans GL, Cookson TV, Bulloch EM, Joseph DD, Lee CE, Parker EJ, Baker EN, Lott JS. 2013. The substrate capture mechanism of Mycobacterium tuberculosis anthranilate phosphoribosyltransferase provides a mode for inhibition. Biochemistry 52:1776–1787. doi: 10.1021/bi301387m. [DOI] [PubMed] [Google Scholar]
- 288.Ivens A, Mayans O, Szadkowski H, Wilmanns M, Kirschner K. 2001. Purification, characterization and crystallization of thermostable anthranilate phosphoribosyltransferase from Sulfolobus solfataricus. Eur J Biochem 268:2246–2252. doi: 10.1046/j.1432-1327.2001.02102.x. [DOI] [PubMed] [Google Scholar]
- 289.Marino M, Deuss M, Svergun DI, Konarev PV, Sterner R, Mayans O. 2006. Structural and mutational analysis of substrate complexation by anthranilate phosphoribosyltransferase from Sulfolobus solfataricus. J Biol Chem 281:21410–21421. doi: 10.1074/jbc.M601403200. [DOI] [PubMed] [Google Scholar]
- 290.Cookson TV, Castell A, Bulloch EM, Evans GL, Short FL, Baker EN, Lott JS, Parker EJ. 2014. Alternative substrates reveal catalytic cycle and key binding events in the reaction catalysed by anthranilate phosphoribosyltransferase from Mycobacterium tuberculosis. Biochem J 461:87–98. doi: 10.1042/BJ20140209. [DOI] [PubMed] [Google Scholar]
- 291.Smith DA, Parish T, Stoker NG, Bancroft GJ. 2001. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect Immun 69:1142–1150. doi: 10.1128/IAI.69.2.1442-1150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Evans GL, Gamage SA, Bulloch EM, Baker EN, Denny WA, Lott JS. 2014. Repurposing the chemical scaffold of the anti-arthritic drug Lobenzarit to target tryptophan biosynthesis in Mycobacterium tuberculosis. Chembiochem 15:852–864. doi: 10.1002/cbic.201300628. [DOI] [PubMed] [Google Scholar]
- 293.Trzebiatowski JR, O'Toole GA, Escalante-Semerena JC. 1994. The cobT gene of Salmonella typhimurium encodes the NaMN:5,6-dimethylbenzimidazole phosphoribosyltransferase responsible for the synthesis of N1-(5-phospho-alpha-d-ribosyl)-5,6-dimethylbenzimidazole, an intermediate in the synthesis of the nucleotide loop of cobalamin. J Bacteriol 176:3568–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lawrence JG, Roth JR. 1995. The cobalamin (coenzyme B12) biosynthetic genes of Escherichia coli. J Bacteriol 177:6371–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chan CH, Escalante-Semerena JC. 2011. ArsAB, a novel enzyme from Sporomusa ovata activates phenolic bases for adenosylcobamide biosynthesis. Mol Microbiol 81:952–967. doi: 10.1111/j.1365-2958.2011.07741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.DiMarco AA, Bobik TA, Wolfe RS. 1990. Unusual coenzymes of methanogenesis. Annu Rev Biochem 59:355–594. doi: 10.1146/annurev.bi.59.070190.002035. [DOI] [PubMed] [Google Scholar]
- 297.Maden BE. 2000. Tetrahydrofolate and tetrahydromethanopterin compared: functionally distinct carriers in C1 metabolism. Biochem J 350:609–629. doi: 10.1042/bj3500609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Vorholt JA, Chistoserdova L, Stolyar SM, Thauer RK, Lidstrom ME. 1999. Distribution of tetrahydromethanopterin-dependent enzymes in methylotrophic bacteria and phylogeny of methenyl tetrahydromethanopterin cyclohydrolases. J Bacteriol 181:5750–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Scott JW, Rasche ME. 2002. Purification, overproduction, and partial characterization of beta-RFAP synthase, a key enzyme in the methanopterin biosynthesis pathway. J Bacteriol 184:4442–4448. doi: 10.1128/JB.184.16.4442-4448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.White RH. 1990. Biosynthesis of methanopterin. Biochemistry 29:5397–5404. doi: 10.1021/bi00474a027. [DOI] [PubMed] [Google Scholar]
- 301.White RH. 1996. Biosynthesis of methanopterin. Biochemistry 35:3447–3456. doi: 10.1021/bi952308m. [DOI] [PubMed] [Google Scholar]
- 302.White RH. 1998. Methanopterin biosynthesis: methylation of the biosynthetic intermediates. Biochim Biophys Acta 1380:257–267. doi: 10.1016/S0304-4165(97)00148-7. [DOI] [PubMed] [Google Scholar]
- 303.White RH. 1993. Structures of the modified folates in the thermophilic archaebacteria Pyrococcus furiosus. Biochemistry 32:745–753. doi: 10.1021/bi00054a003. [DOI] [PubMed] [Google Scholar]
- 304.White RH. 2011. The conversion of a phenol to an aniline occurs in the biochemical formation of the 1-(4-aminophenyl)-1-deoxy-d-ribitol moiety in methanopterin. Biochemistry 50:6041–6052. doi: 10.1021/bi200362w. [DOI] [PubMed] [Google Scholar]
- 305.Rasche ME, White RH. 1998. Mechanism for the enzymatic formation of 4-(beta-d-ribofuranosyl)aminobenzene 5′-phosphate during the biosynthesis of methanopterin. Biochemistry 37:11343–11351. doi: 10.1021/bi973086q. [DOI] [PubMed] [Google Scholar]
- 306.Dumitru RV, Ragsdale SW. 2004. Mechanism of 4-(beta-d-ribofuranosyl)aminobenzene 5′-phosphate synthase, a key enzyme in the methanopterin biosynthetic pathway. J Biol Chem 279:39389–39395. doi: 10.1074/jbc.M406442200. [DOI] [PubMed] [Google Scholar]
- 307.Bechard ME, Chhatwal S, Garcia RE, Rasche ME. 2003. Application of a colorimetric assay to identify putative ribofuranosylaminobenzene 5′-phosphate synthase genes expressed with activity in Escherichia coli. Biol Proced Online 5:69–77. doi: 10.1251/bpo48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Crick DC, Mahapatra S, Brennan PJ. 2001. Biosynthesis of the arabinogalactan-peptidoglycan complex of Mycobacterium tuberculosis. Glycobiology 11:107R–118R. doi: 10.1093/glycob/11.9.107R. [DOI] [PubMed] [Google Scholar]
- 309.Scherman M, Weston A, Duncan K, Whittington A, Upton R, Deng L, Comber R, Friedrich JD, McNeil M. 1995. Biosynthetic origin of mycobacterial cell wall arabinosyl residues. J Bacteriol 177:7125–7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Scherman MS, Kalbe-Bournonville L, Bush D, Xin Y, Deng L, McNeil M. 1996. Polyprenylphosphate-pentoses in mycobacteria are synthesized from 5-phosphoribose pyrophosphate. J Biol Chem 271:29652–29658. doi: 10.1074/jbc.271.47.29652. [DOI] [PubMed] [Google Scholar]
- 311.Huang H, Scherman MS, D'Haeze W, Vereecke D, Holsters M, Crick DC, McNeil MR. 2005. Identification and active expression of the Mycobacterium tuberculosis gene encoding 5-phospho-alpha-d-ribose-1-diphosphate: decaprenyl-phosphate 5-phosphoribosyltransferase, the first enzyme committed to decaprenylphosphoryl-d-arabinose synthesis. J Biol Chem 280:24539–24543. doi: 10.1074/jbc.M504068200. [DOI] [PubMed] [Google Scholar]
- 312.Huang H, Berg S, Spencer JS, Vereecke D, D'Haeze W, Holsters M, McNeil MR. 2008. Identification of amino acids and domains required for catalytic activity of DPPR synthase, a cell wall biosynthetic enzyme of Mycobacterium tuberculosis. Microbiology 154:736–743. doi: 10.1099/mic.0.2007/013532-0. [DOI] [PubMed] [Google Scholar]
- 313.Blattner FR, Plunkett G III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 314.Kudo F, Numakura M, Tamegai H, Yamamoto H, Eguchi T, Kakinuma K. 2005. Extended sequence and functional analysis of the butirosin biosynthetic gene cluster in Bacillus circulans SANK 72073. J Antibiot (Tokyo) 58:373–379. doi: 10.1038/ja.2005.47. [DOI] [PubMed] [Google Scholar]
- 315.Ota Y, Tamegai H, Kudo F, Kuriki H, Koike-Takeshita A, Eguchi T, Kakinuma K. 2000. Butirosin-biosynthetic gene cluster from Bacillus circulans. J Antibiotics 53:1158–1167. doi: 10.7164/antibiotics.53.1158. [DOI] [PubMed] [Google Scholar]
- 316.Kudo F, Fujii T, Kinoshita S, Eguchi T. 2007. Unique O-ribosylation in the biosynthesis of butirosin. Bioorg Med Chem 15:4360–4368. doi: 10.1016/j.bmc.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 317.Huang F, Haydock SF, Mironenko T, Spiteller D, Li Y, Spencer JB. 2005. The neomycin biosynthetic gene cluster of Streptomyces fradiae NCIMB 8233: characterisation of an aminotransferase involved in the formation of 2-deoxystreptamine. Org Biomol Chem 3:1410–1418. doi: 10.1039/b501199j. [DOI] [PubMed] [Google Scholar]
- 318.Kudo F, Eguchi T. 2009. Biosynthetic genes for aminoglycoside antibiotics. J Antibiot (Tokyo) 62:471–481. doi: 10.1038/ja.2009.76. [DOI] [PubMed] [Google Scholar]
- 319.Finn MW, Tabita FR. 2004. Modified pathway to synthesize ribulose 1,5-bisphosphate in methanogenic archaea. J Bacteriol 186:6360–6366. doi: 10.1128/JB.186.19.6360-6366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Fisher DI, Safrany ST, Strike P, McLennan AG, Cartwright JL. 2002. Nudix hydrolases that degrade dinucleoside and diphosphoinositol polyphosphates also have 5-phosphoribosyl 1-pyrophosphate (PRPP) pyrophosphatase activity that generates the glycolytic activator ribose 1,5-bisphosphate. J Biol Chem 277:47313–47317. doi: 10.1074/jbc.M209795200. [DOI] [PubMed] [Google Scholar]
- 321.Kawaguchi T, Veech RL, Uyeda K. 2001. Regulation of energy metabolism in macrophages during hypoxia. Roles of fructose 2,6-bisphosphate and ribose 1,5-bisphosphate. J Biol Chem 276:28554–28561. doi: 10.1074/jbc.M101396200. [DOI] [PubMed] [Google Scholar]
- 322.Begley TP, Downs DM, Ealick SE, McLafferty FW, Van Loon AP, Taylor S, Campobasso N, Chiu HJ, Kinsland C, Reddick JJ, Xi J. 1999. Thiamin biosynthesis in prokaryotes. Arch Microbiol 171:293–300. doi: 10.1007/s002030050713. [DOI] [PubMed] [Google Scholar]
- 323.Haase I, Grawert T, Illarionov B, Bacher A, Fischer M. 2014. Recent advances in riboflavin biosynthesis. Methods Mol Biol 1146:15–40. doi: 10.1007/978-1-4939-0452-5_2. [DOI] [PubMed] [Google Scholar]
- 324.Fischer M, Bacher A. 2010. Biosynthesis of riboflavin. EcoSal Plus doi: 10.1128/ecosalplus.3.6.3.2. [DOI] [PubMed] [Google Scholar]
- 325.White RH. 2001. Biosynthesis of the methanogenic cofactors. Vitam Horm 61:299–337. doi: 10.1016/S0083-6729(01)61010-0. [DOI] [PubMed] [Google Scholar]
- 326.Green JM, Matthews RG. 2007. Folate biosynthesis, reduction, and polyglutamylation and the interconversion of folate derivatives. EcoSal Plus doi: 10.1128/ecosalplus.3.6.3.6. [DOI] [PubMed] [Google Scholar]
- 327.Mendel RR, Leimkühler S. 2014. The biosynthesis of the molybdenum cofactors. J Biol Inorg Chem 20:337–347. doi: 10.1007/s00775-014-1173-y. [DOI] [PubMed] [Google Scholar]
- 328.Chin JP, McGrath JW, Quinn JP. 2016. Microbial transformations in phosphonate biosynthesis and catabolism, and their importance in nutrient cycling. Curr Opin Chem Biol 31:50–57. doi: 10.1016/j.cbpa.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 329.Schneider DA, Gourse RL. 2004. Relationship between growth rate and ATP concentration in Escherichia coli: a bioassay for available cellular ATP. J Biol Chem 279:8262–8268. doi: 10.1074/jbc.M311996200. [DOI] [PubMed] [Google Scholar]
- 330.Yaginuma H, Kawai S, Tabata KV, Tomiyama K, Kakizuka A, Komatsuzaki T, Noji H, Imamura H. 2014. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci Rep 4:6522. doi: 10.1038/srep06522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Fridman A, Saha A, Chan A, Casteel DE, Pilz RB, Boss GR. 2013. Cell cycle regulation of purine synthesis by phosphoribosyl pyrophosphate and inorganic phosphate. Biochem J 454:91–99. doi: 10.1042/BJ20130153. [DOI] [PubMed] [Google Scholar]
- 332.Becker MA, Raivio KO, Seegmiller JE. 1979. Synthesis of phosphoribosylpyrophosphate in mammalian cells. Adv Enzymol Relat Areas Mol Biol 49:281–306. [DOI] [PubMed] [Google Scholar]
- 333.Turnbough CL Jr, Switzer RL. 2008. Regulation of pyrimidine biosynthetic gene expression in bacteria: repression without repressors. Microbiol Mol Biol Rev 72:266–300. doi: 10.1128/MMBR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Switzer RL, Turner RJ, Lu Y. 1998. Regulation of the Bacillus subtilis pyrimidine biosynthetic operon by transcriptional attenuation: control of gene expression by an mRNA-binding protein. Prog Nucleic Acid Res Mol Biol 62:329–367. doi: 10.1016/S0079-6603(08)60512-7. [DOI] [PubMed] [Google Scholar]
- 335.Kilstrup M, Hammer K, Ruhdal Jensen P, Martinussen J. 2005. Nucleotide metabolism and its control in lactic acid bacteria. FEMS Microbiol Rev 29:555–590. doi: 10.1016/j.fmrre.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 336.Tomchick DR, Turner RJ, Switzer RL, Smith JL. 1998. Adaptation of an enzyme to regulatory function: structure of Bacillus subtilis PyrR, a pyr RNA-binding attenuation protein and uracil phosphoribosyltransferase. Structure 6:337–350. doi: 10.1016/S0969-2126(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 337.Chander P, Halbig KM, Miller JK, Fields CJ, Bonner HK, Grabner GK, Switzer RL, Smith JL. 2005. Structure of the nucleotide complex of PyrR, the pyr attenuation protein from Bacillus caldolyticus, suggests dual regulation by pyrimidine and purine nucleotides. J Bacteriol 187:1773–1782. doi: 10.1128/JB.187.5.1773-1782.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kantardjieff KA, Vasquez C, Castro P, Warfel NM, Rho BS, Lekin T, Kim CY, Segelke BW, Terwilliger TC, Rupp B. 2005. Structure of pyrR (Rv1379) from Mycobacterium tuberculosis: a persistence gene and protein drug target. Acta Crystallogr D Biol Crystallogr 61:355–364. doi: 10.1107/S090744490403389X. [DOI] [PubMed] [Google Scholar]
- 339.Perica T, Kondo Y, Tiwari SP, McLaughlin SH, Kemplen KR, Zhang X, Steward A, Reuter N, Clarke J, Teichmann SA. 2014. Evolution of oligomeric state through allosteric pathways that mimic ligand binding. Science 346:1254346. doi: 10.1126/science.1254346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Jorgensen CM, Fields CJ, Chander P, Watt D, Burgner JW II, Smith JL, Switzer RL. 2008. pyr RNA binding to the Bacillus caldolyticus PyrR attenuation protein: characterization and regulation by uridine and guanosine nucleotides. FEBS J 275:655–670. doi: 10.1111/j.1742-4658.2007.06227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Turner RJ, Bonner ER, Grabner GK, Switzer RL. 1998. Purification and characterization of Bacillus subtilis PyrR, a bifunctional pyr mRNA-binding attenuation protein/uracil phosphoribosyltransferase. J Biol Chem 273:5932–5938. doi: 10.1074/jbc.273.10.5932. [DOI] [PubMed] [Google Scholar]
- 342.Turner RJ, Lu Y, Switzer RL. 1994. Regulation of the Bacillus subtilis pyrimidine biosynthetic (pyr) gene cluster by an autogenous transcriptional attenuation mechanism. J Bacteriol 176:3708–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Bonner ER, D'Elia JN, Billips BK, Switzer RL. 2001. Molecular recognition of pyr mRNA by the Bacillus subtilis attenuation regulatory protein PyrR. Nucleic Acids Res 29:4851–4865. doi: 10.1093/nar/29.23.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Andersen PS, Martinussen J, Hammer K. 1996. Sequence analysis and identification of the pyrKDbF operon from Lactococcus lactis including a novel gene, pyrK, involved in pyrimidine biosynthesis J Bacteriol 178:5005–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Martinussen J, Hammer K. 1998. The carB gene encoding the large subunit of carbamoylphosphate synthetase from Lactococcus lactis is transcribed monocistronically. J Bacteriol 180:4380–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Martinussen J, Schallert J, Andersen B, Hammer K. 2001. The pyrimidine operon pyrRPB-carA from Lactococcus lactis. J Bacteriol 183:2785–2794. doi: 10.1128/JB.183.9.2785-2794.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Jendresen CB, Dimitrov P, Gautier L, Liu M, Martinussen J, Kilstrup M. 2014. Towards in vivo regulon kinetics: PurR activation by 5-phosphoribosyl-alpha-1-pyrophosphate during purine depletion in Lactococcus lactis. Microbiology 160:1321–1331. doi: 10.1099/mic.0.077933-0. [DOI] [PubMed] [Google Scholar]
- 348.Houlberg U, Jensen KF. 1983. Role of hypoxanthine and guanine in regulation of Salmonella typhimurium pur gene expression. J Bacteriol 153:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Choi KY, Zalkin H. 1992. Structural characterization and corepressor binding of the Escherichia coli purine repressor. J Bacteriol 174:6207–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Weng M, Nagy PL, Zalkin H. 1995. Identification of the Bacillus subtilis pur operon repressor. Proc Natl Acad Sci U S A 92:7455–7749. doi: 10.1073/pnas.92.16.7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sinha SC, Krahn J, Shin BS, Tomchick DR, Zalkin H, Smith JL. 2003. The purine repressor of Bacillus subtilis: a novel combination of domains adapted for transcription regulation. J Bacteriol 185:4087–4098. doi: 10.1128/JB.185.14.4087-4098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Weng M, Zalkin H. 2000. Mutations in the Bacillus subtilis purine repressor that perturb PRPP effector function in vitro and in vivo. Curr Microbiol 41:56–59. doi: 10.1007/s002840010091. [DOI] [PubMed] [Google Scholar]
- 353.Bera AK, Zhu J, Zalkin H, Smith JL. 2003. Functional dissection of the Bacillus subtilis pur operator site. J Bacteriol 185:4099–4109. doi: 10.1128/JB.185.14.4099-4109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Saxild HH, Brunstedt K, Nielsen KI, Jarmer H, Nygaard P. 2001. Definition of the Bacillus subtilis PurR operator using genetic and bioinformatic tools and expansion of the PurR regulon with glyA, guaC, pbuG, xpt-pbuX, yqhZ-folD, and pbuO. J Bacteriol 183:6175–6183. doi: 10.1128/JB.183.21.6175-6183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Ebbole DJ, Zalkin H. 1989. Bacillus subtilis pur operon expression and regulation. J Bacteriol 171:2136–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Saxild HH, Nygaard P. 1991. Regulation of levels of purine biosynthetic enzymes in Bacillus subtilis: effects of changing purine nucleotide pools. J Gen Microbiol 137:2387–2394. doi: 10.1099/00221287-137-10-2387. [DOI] [PubMed] [Google Scholar]
- 357.Shin BS, Stein A, Zalkin H. 1997. Interaction of Bacillus subtilis purine repressor with DNA. J Bacteriol 179:7394–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kilstrup M, Martinussen J. 1998. A transcriptional activator, homologous to the Bacillus subtilis PurR repressor, is required for expression of purine biosynthetic genes in Lactococcus lactis. J Bacteriol 180:3907–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Ebbole DJ, Zalkin H. 1988. Detection of pur operon-attenuated mRNA and accumulated degradation intermediates in Bacillus subtilis. J Biol Chem 263:10894–10902. [PubMed] [Google Scholar]
- 360.Mandal M, Boese B, Barrick JE, Winkler WC, Breaker RR. 2003. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell 113:577–586. doi: 10.1016/S0092-8674(03)00391-X. [DOI] [PubMed] [Google Scholar]
- 361.Gilbert SD, Montange RK, Stoddard CD, Batey RT. 2006. Structural studies of the purine and SAM binding riboswitches. Cold Spring Harb Symp Quant Biol 71:259–268. doi: 10.1101/sqb.2006.71.015. [DOI] [PubMed] [Google Scholar]
- 362.Mandal M, Breaker RR. 2004. Gene regulation by riboswitches. Nat Rev Mol Cell Biol 5:451–463. doi: 10.1038/nrm1403. [DOI] [PubMed] [Google Scholar]
- 363.Jendresen CB, Martinussen J, Kilstrup M. 2012. The PurR regulon in Lactococcus lactis: transcriptional regulation of the purine nucleotide metabolism and translational machinery. Microbiology 158:2026–2038. doi: 10.1099/mic.0.059576-0. [DOI] [PubMed] [Google Scholar]
- 364.Kilstrup M, Jessing SG, Wichmand-Jorgensen SB, Madsen M, Nilsson D. 1998. Activation control of pur gene expression in Lactococcus lactis: proposal for a consensus activator binding sequence based on deletion analysis and site-directed mutagenesis of purC and purD promoter regions. J Bacteriol 180:3900–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ciccarelli FD, Doerks T, von Mering C, Creevey CJ, Snel B, Bork P. 2006. Toward automatic reconstruction of a highly resolved tree of life. Science 311:1283–1287. doi: 10.1126/science.1123061. [DOI] [PubMed] [Google Scholar]
- 366.Arent S, Kadziola A, Larsen S, Neuhard J, Jensen KF. 2006. The extraordinary specificity of xanthine phosphoribosyltransferase from Bacillus subtilis elucidated by reaction kinetics, ligand binding, and crystallography. Biochemistry 45:6615–6627. doi: 10.1021/bi060287y. [DOI] [PubMed] [Google Scholar]
- 367.Potvin B, Gooder H. 1975. Carbamyl phosphate synthesis in Bacillus subtilis. Biochem Genet 13:125–143. doi: 10.1007/BF00486011. [DOI] [PubMed] [Google Scholar]
- 368.Paulus TJ, Switzer RL. 1979. Characterization of pyrimidine-repressible and arginine-repressible carbamyl phosphate synthetases from Bacillus subtilis. J Bacteriol 137:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Jones ME. 1980. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem 49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- 370.Evans DR, Guy HI. 2004. Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway. J Biol Chem 279:33035–33038. doi: 10.1074/jbc.R400007200. [DOI] [PubMed] [Google Scholar]
- 371.Shoaf WT, Jones ME. 1973. Uridylic acid synthesis in Ehrlich ascites carcinoma. Properties, subcellular distribution, and nature of enzyme complexes of the six biosynthetic enzymes. Biochemistry 12:4039–4051. [DOI] [PubMed] [Google Scholar]
- 372.Sigoillot FD, Evans DR, Guy HI. 2002. Growth-dependent regulation of mammalian pyrimidine biosynthesis by the protein kinase A and MAPK signaling cascades. J Biol Chem 277:15745–15751. doi: 10.1074/jbc.M201112200. [DOI] [PubMed] [Google Scholar]
- 373.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. 2013. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Miltenberger RJ, Sukow KA, Farnham PJ. 1995. An E-box-mediated increase in cad transcription at the G1/S-phase boundary is suppressed by inhibitory c-Myc mutants. Mol Cell Biol 15:2527–2535. doi: 10.1128/MCB.15.5.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Bower SG, Harlow KW, Switzer RL, Hove-Jensen B. 1989. Characterization of the Escherichia coli prsA1-encoded mutant phosphoribosylpyrophosphate synthetase identifies a divalent cation-nucleotide binding site. J Biol Chem 264:10287–10291. [PubMed] [Google Scholar]
- 376.Post DA, Switzer RL, Hove-Jensen B. 1996. The defective phosphoribosyl diphosphate synthase in a temperature-sensitive prs-2 mutant of Escherichia coli is compensated by increased enzyme synthesis. Microbiology 142:359–365. doi: 10.1099/13500872-142-2-359. [DOI] [PubMed] [Google Scholar]
- 377.Foster JW, Moat AG. 1980. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol Rev 44:83–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Petersen C. 1999. Inhibition of cellular growth by increased guanine nucleotide pools. Characterization of an Escherichia coli mutant with a guanosine kinase that is insensitive to feedback inhibition by GTP. J Biol Chem 274:5348–5356. [DOI] [PubMed] [Google Scholar]
- 379.Sakakibara Y. 1992. dnaR function of the prs gene of Escherichia coli in initiation of chromosome replication. J Mol Biol 226:989–996. doi: 10.1016/0022-2836(92)91047-S. [DOI] [PubMed] [Google Scholar]
- 380.Sakakibara Y. 1992. Novel Escherichia coli mutant, dnaR, thermosensitive in initiation of chromosome replication. J Mol Biol 226:979–987. doi: 10.1016/0022-2836(92)91046-R. [DOI] [PubMed] [Google Scholar]
- 381.Sakakibara Y. 1993. Cooperation of the prs and dnaA gene products for initiation of chromosome replication in Escherichia coli. J Bacteriol 175:5559–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Sakakibara Y. 1995. Suppression of thermosensitive initiation of DNA replication in a dnaR mutant of Escherichia coli by a rifampin resistance mutation in the rpoB gene. J Bacteriol 177:733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Sakakibara Y. 1997. Involvement of the ribulosephosphate epimerase gene in the dnaA and dnaR functions for initiation of chromosome replication in Escherichia coli. Mol Microbiol 24:793–801. doi: 10.1046/j.1365-2958.1997.3851746.x. [DOI] [PubMed] [Google Scholar]
- 384.Sprenger GA. 1995. Genetics of pentose-phosphate pathway enzymes of Escherichia coli K-12. Arch Microbiol 164:324–330. doi: 10.1007/BF02529978. [DOI] [PubMed] [Google Scholar]
- 385.Muto A, Ushida C, Himeno H. 1998. A bacterial RNA that functions as both a tRNA and an mRNA. Trends Biochem Sci 23:25–29. doi: 10.1016/S0968-0004(97)01159-6. [DOI] [PubMed] [Google Scholar]
- 386.Janssen BD, Hayes CS. 2012. The tmRNA ribosome-rescue system. Adv Protein Chem Struct Biol 86:151–191. doi: 10.1016/B978-0-12-386497-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Ando H, Kitabatake M, Inokuchi H. 1996. 10Sa RNA complements the temperature-sensitive phenotype caused by a mutation in the phosphoribosyl pyrophosphate synthetase (prs) gene in Escherichia coli. Genes Genet Syst 71:47–50. doi: 10.1266/ggs.71.47. [DOI] [PubMed] [Google Scholar]
- 388.Nakano H, Goto S, Nakayashiki T, Inokuchi H. 2001. Temperature-sensitive mutations in various genes of Escherichia coli K12 can be suppressed by the ssrA gene for 10Sa RNA (tmRNA). Mol Genet Genomics 265:615–621. doi: 10.1007/s004380100453. [DOI] [PubMed] [Google Scholar]
- 389.Karzai AW, Sauer RT. 2001. Protein factors associated with the SsrA-SmpB tagging and ribosome rescue complex. Proc Natl Acad Sci U S A 98:3040–3044. doi: 10.1073/pnas.051628298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Pandey NK, Switzer RL. 1982. Mutant strains of Salmonella typhimurium with defective phosphoribosylpyrophosphate synthetase activity. J Gen Microbiol 128:1863–1871. [DOI] [PubMed] [Google Scholar]
- 391.Koenigsknecht MJ, Fenlon LA, Downs DM. 2010. Phosphoribosylpyrophosphate synthetase (PrsA) variants alter cellular pools of ribose 5-phosphate and influence thiamine synthesis in Salmonella enterica. Microbiology 156:950–959. doi: 10.1099/mic.0.033050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.He B, Choi KY, Zalkin H. 1993. Regulation of Escherichia coli glnB, prsA, and speA by the purine repressor. J Bacteriol 175:3598–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.White MN, Olszowy J, Switzer RL. 1971. Regulation and mechanism of phosphoribosylpyrophosphate synthetase: repression by end products. J Bacteriol 108:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Olszowy J, Switzer RL. 1972. Specific repression of phosphoribosylpyrophosphate synthetase by uridine compounds in Salmonella typhimurium. J Bacteriol 110:450–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Matsuyama S, Yokota N, Tokuda H. 1997. A novel outer membrane lipoprotein, LolB (HemM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J 16:6947–6955. doi: 10.1093/emboj/16.23.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Lange BM, Croteau R. 1999. Isopentenyl diphosphate biosynthesis via a mevalonate-independent pathway: isopentenyl monophosphate kinase catalyzes the terminal enzymatic step. Proc Natl Acad Sci U S A 96:13714–13719. doi: 10.1073/pnas.96.24.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Post DA, Hove-Jensen B, Switzer RL. 1993. Characterization of the hemA-prs region of the Escherichia coli and Salmonella typhimurium chromosomes: identification of two open reading frames and implications for prs expression. J Gen Microbiol 139:259–266. doi: 10.1099/00221287-139-2-259. [DOI] [PubMed] [Google Scholar]
- 398.Bower SG, Hove-Jensen B, Switzer RL. 1988. Structure of the gene encoding phosphoribosylpyrophosphate synthetase (prsA) in Salmonella typhimurium. J Bacteriol 170:3243–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Hove-Jensen B. 1985. Cloning and characterization of the prs gene encoding phosphoribosylpyrophosphate synthetase of Escherichia coli. Mol Gen Genet 201:269–276. doi: 10.1007/BF00425670. [DOI] [PubMed] [Google Scholar]
- 400.Jensen KF, Neuhard J, Schack L. 1982. RNA polymerase involvement in the regulation of expression of Salmonella typhimurium pyr genes. Isolation and characterization of a fluorouracil-resistant mutant with high, constitutive expression of the pyrB and pyrE genes due to a mutation in rpoBC. EMBO J 1:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Jensen KF, Fast R, Karlstrom O, Larsen JN. 1986. Association of RNA polymerase having increased Km for ATP and UTP with hyperexpression of the pyrB and pyrE genes of Salmonella typhimurium. J Bacteriol 166:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Poulsen P, Jensen KF, Valentin-Hansen P, Carlsson P, Lundberg LG. 1983. Nucleotide sequence of the Escherichia coli pyrE gene and of the DNA in front of the protein-coding region. Eur J Biochem 135:223–229. doi: 10.1111/j.1432-1033.1983.tb07641.x. [DOI] [PubMed] [Google Scholar]
- 403.Nilsson D, Hove-Jensen B. 1987. Phosphoribosylpyrophosphate synthetase of Bacillus subtilis. Cloning, characterization and chromosomal mapping of the prs gene Gene 53:247–255. [DOI] [PubMed] [Google Scholar]
- 404.Mengin-Lecreulx D, van Heijenoort J. 1994. Copurification of glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase activities of Escherichia coli: characterization of the glmU gene product as a bifunctional enzyme catalyzing two subsequent steps in the pathway for UDP-N-acetylglucosamine synthesis. J Bacteriol 176:5788–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Schmalisch M, Langbein I, Stulke J. 2002. The general stress protein Ctc of Bacillus subtilis is a ribosomal protein. J Mol Microbiol Biotechnol 4:495–501. [PubMed] [Google Scholar]
- 406.Hilden I, Krath BN, Hove-Jensen B. 1995. Tricistronic operon expression of the genes gcaD (tms), which encodes N-acetylglucosamine 1-phosphate uridyltransferase, prs, which encodes phosphoribosyl diphosphate synthetase, and ctc in vegetative cells of Bacillus subtilis. J Bacteriol 177:7280–7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Timofeev VI, Abramchik YA, Zhukhlistova NE, Kuranova IP. 2015. Crystallization and preliminary X-ray diffraction study of phosphoribosyl pyrophosphate synthetase from E. coli. Crystallogr Rep 60:683–688. [Google Scholar]
- 408.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 409.Bult CJ, White O, Olsen GJ, Zhou L, Fleischmann RD, Sutton GG, Blake JA, FitzGerald LM, Clayton RA, Gocayne JD, Kerlavage AR, Dougherty BA, Tomb JF, Adams MD, Reich CI, Overbeek R, Kirkness EF, Weinstock KG, Merrick JM, Glodek A, Scott JL, Geoghagen NS, Venter JC. 1996. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 410.Kawashima T, Amano N, Koike H, Makino S, Higuchi S, Kawashima-Ohya Y, Watanabe K, Yamazaki M, Kanehori K, Kawamoto T, Nunoshiba T, Yamamoto Y, Aramaki H, Makino K, Suzuki M. 2000. Archaeal adaptation to higher temperatures revealed by genomic sequence of Thermoplasma volcanium. Proc Natl Acad Sci U S A 97:14257–14262. doi: 10.1073/pnas.97.26.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.She Q, Singh RK, Confalonieri F, Zivanovic Y, Allard G, Awayez MJ, Chan-Weiher CC, Clausen IG, Curtis BA, De Moors A, Erauso G, Fletcher C, Gordon PM, Heikamp-de Jong I, Jeffries AC, Kozera CJ, Medina N, Peng X, Thi-Ngoc HP, Redder P, Schenk ME, Theriault C, Tolstrup N, Charlebois RL, Doolittle WF, Duguet M, Gaasterland T, Garrett RA, Ragan MA, Sensen CW, Van der Oost J. 2001. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc Natl Acad Sci U S A 98:7835–7840. doi: 10.1073/pnas.141222098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Moran R, Kuilenburg AB, Duley J, Nabuurs SB, Retno-Fitri A, Christodoulou J, Roelofsen J, Yntema HG, Friedman NR, van Bokhoven H, de Brouwer AP. 2012. Phosphoribosylpyrophosphate synthetase superactivity and recurrent infections is caused by a p.Val142Leu mutation in PRS-I. Am J Med Genet 158A:455–460. doi: 10.1002/ajmg.a.34428. [DOI] [PubMed] [Google Scholar]
- 413.Mittal R, Patel K, Mittal J, Chan B, Yan D, Grati M, Liu XZ. 2015. Association of PRPS1 mutations with disease phenotypes. Dis Markers 2015:127013. doi: 10.1155/2015/127013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Jendresen CB, Kilstrup M, Martinussen J. 2011. A simplified method for rapid quantification of intracellular nucleoside triphosphates by one-dimensional thin-layer chromatography. Anal Biochem 409:249–259. doi: 10.1016/j.ab.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 415.Nygaard P, Smith JM. 1993. Evidence for a novel glycinamide ribonucleotide transformylase in Escherichia coli. J Bacteriol 175:3591–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Ashihara H. 2016. Biosynthesis of 5-phosphoribosyl-1-pyrophosphate in plants: a review. Eur Chem Bull 5:314–323. [Google Scholar]
- 417.Esipov RS, Abramchik YA, Fateev IV, Muravyova TI, Artemova KG, Konstantinova ID, Kuranova IP, Miroshnikov AI. 2016. Recombinant phosphoribosyl pyrophosphate synthetase from Thermus thermophilus HB27: isolation and properties. Russ J Bioorg Chem 42:512–521. doi: 10.1134/s1068162016040075. [DOI] [Google Scholar]
- 418.Ugbogu EA, Wang K, Schweizer LM, Schweizer M. 2016. Metabolic gene products have evolved to interact with the cell wall integrity pathway in Saccharomyces cerevisiae. FEMS Yeast Res pii:fow092. doi: 10.1093/femsyr/fow092. [DOI] [PubMed] [Google Scholar]
- 419.Wong CCL, Xu T, Rai R, Bailey AO, Yates JR III, Wolf YI, Zebronski H, Kashina A. 2007. Global analysis of posttranslational protein arginylation. PLoS Biol 5:e258. doi: 10.1371/journal.pbio.0050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Zhang F, Patel DM, Colavita K, Rodionova I, Buckley B, Scott DA, Kumar A, Shabalina SA, Saha S, Chernov M, Osterman AL, Kashina A. 2015. Arginylation regulates purine nucleotide bisynthesis by enhancing the activity of phosphoribosyl pyrophosphate synthase. Nat Commun 6:7517. doi: 10.1038/ncomms8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Ashihara H, Komamine A. 1974. Regulatory properties of a plant phosphoribosylpyrophosphate synthetase. Plant Sci Lett 2:119–123. [Google Scholar]
- 422.Bibi T, Perveen S, Aziz I, Bashir Q, Rashid N, Imanaka T, Akhtar M. 2016. Pcal_1127, a highly stable and efficient ribose-5-phosphate pyrophosphokinase from Pyrobaculum calidifontis. Extremophiles 20:821–830. doi: 10.1007/s00792-016-0869-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.