Summary
We previously showed the efficacy of multiple research cell lines (RCLs) of human CNS neural stem cells (HuCNS-SCs) in mouse and rat models of thoracic spinal cord injury (SCI), supporting a thoracic SCI clinical trial. Experts recommend in vivo preclinical testing of the intended clinical cell lot/line (CCL) in models with validity for the planned clinical target. We therefore tested the efficacy of two HuCNS-SC lines in cervical SCI: one RCL, and one CCL intended for use in the Pathway Study of cervical SCI in man. We assessed locomotor recovery and sensory function, as well as engraftment, migration, and fate. No evidence of efficacy of the CCL was observed; some data suggested a negative impact of the CCL on outcomes. These data raise questions about the development and validation of potency/comparability assays for clinical testing of cell products, and lack of US Food and Drug Administration requirements for in vivo testing of intended clinical cell lines.
Keywords: human neural stem cells (hNSC), spinal cord injury (SCI), cervical, efficacy, good manufacturing practices (GMP), RIGOR, FDA guidelines for cell therapies
Graphical Abstract
Highlights
-
•
Human CNS stem cells (HuCNS-SCs) have been used in multiple clinical trials
-
•
Research cell lines of HuCNS-SCs are efficacious in spinal cord injury (SCI) models
-
•
The clinical cell line (CCL) of HuCNS-SC was not efficacious in a cervical SCI model
-
•
Despite lack of in vivo efficacy, the CCL was used in the Pathways clinical study
Anderson and colleagues report that preclinical testing of a human neural stem cell line intended for use in a human clinical trial of cervical spinal cord injury (SCI) failed to show efficacy in an animal model of SCI, whereas a research-grade cell line did show efficacy. The human trial proceeded despite the negative data.
Introduction
The first prospective study of spinal cord injury (SCI) prevalence in the USA revised the estimated number of individuals living with SCI upward by 5-fold to 1.3 million (Christopher and Dana Reeve Foundation, 2008). The average age at time of SCI is 34 years, resulting in a lifetime of paralysis associated with a host of medical complications. The impact of SCI in economic terms is highly disproportionate to the incidence of injury, rising to an average lifetime cost of several million dollars for individuals sustaining high-level cervical injuries. Critically, the majority of clinical SCI cases are at the cervical level (52.4%) (Christopher and Dana Reeve Foundation, 2008), making potential therapeutic interventions in cervical SCI rodent models a high priority.
Selection of a target clinical population is one key to translation of cell therapeutics. Two critical variables are treatment timing and vertebral level (thoracic versus cervical), both of which affect the incidence of spontaneous recovery in man (Fawcett et al., 2007). Rodent contusion models reproduce the principal pathophysiological features of clinical SCI with sensitive and relevant outcome measures (Stokes and Jakeman, 2002, Nishi et al., 2007). With respect to timing, the timeline of pathophysiological events following SCI in animal models versus the human condition is debatable; although many suggest that transplantation 9 days post-injury (DPI) in the rodent corresponds to the sub-acute clinical setting, while transplantation 30–60 DPI corresponds to the early chronic clinical setting (Houle and Tessler, 2003, Fawcett et al., 2007). Focusing enrollment for an SCI trial on chronic (>3 months post-SCI) cervical SCI subjects, compared with acute thoracic subjects, could reduce the enrollment required to attain statistical power to discriminate an improvement of 10 AIS (American Spinal Injury Association Impairment Scale) motor points dramatically from 250 to 25 AIS A subjects or from 1,100 to 50 AIS B subjects (Fawcett et al., 2007). Further, the larger pool of chronic SCI individuals may facilitate subject accrual, while an increased delay between injury and enrollment may improve the informed consent process (Anderson and Cummings, 2016).
With respect to vertebral level, there are compelling reasons to drive toward clinical trials focused on cervical SCI in more chronic cases. Many, however, have cautioned against proceeding to clinical trial for cervical SCI based on preclinical data in thoracic SCI models (Kwon et al., 2013). One reason for hesitation is increased recognition that cervical and thoracic injuries have a number of profound differences. For example, functional motor impairment across levels changes due to the anatomical characteristics of the spinal cord. Disruption of spinal circuitry due to systemic autonomic and immune effects is also level specific. Autonomic dysreflexias, particularly abnormal cardiovascular control, affect 50%–70% of human SCI patients with injury above T6 (Krassioukov and Claydon, 2006) but are rare when the injury is below this level. Accordingly, the impact of modulation of sprouting or connectivity via cell transplantation therapies could exert unanticipated effects in the case of high thoracic and cervical SCI, which would not be evident in low thoracic SCI models. In parallel, disruption of descending sympathetic outflow specifically associated with cervical and high-level SCI has been shown to exert clinically significant and chronic splenic atrophy and immune suppression (Lucin et al., 2007, Zhang et al., 2013). Of note, SCI subjects in most cell therapeutic clinical trials would receive at least transient pharmacological immunosuppressive agents. In summary, these data demonstrate that injury level is a key variable in establishing not only efficacy but also safety in preclinical testing of investigational agents.
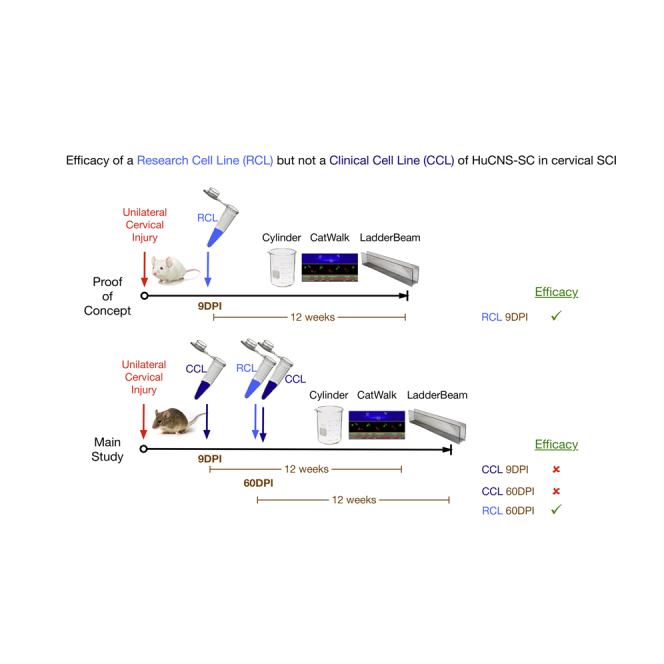
One therapeutic approach for SCI is cell transplantation. For this approach, cell survival is also a critical variable for preclinical models. The advantages of constitutively immunodeficient mice versus immunosuppression in immunocompetent mice in achieving maximal donor cell engraftment for xenotransplantation studies are significant (Anderson et al., 2011). This issue is particularly critical for establishing safety of a stem cell therapy, as tumor formation is impaired by a host immunorejection response (Dressel et al., 2008, Anderson et al., 2011). Accordingly, we have reported transplantation of multiple research-grade cell lines (RCLs) into both mouse and rat models of thoracic contusion SCI (Cummings et al., 2005, Hooshmand et al., 2009, Salazar et al., 2010, Piltti et al., 2013a, Piltti et al., 2013b, Sontag et al., 2013, Sontag et al., 2014, Piltti et al., 2015). However, guidance from both the neurotransplantation and SCI fields emphasizes in vivo preclinical testing of the intended clinical cell lot/line (CCL) prior to proceeding in man. In the present study, we therefore tested the efficacy of human CNS-derived neural stem cell lines (HuCNS-SC) in an immunodeficient mouse model of cervical SCI. Two lines were tested: one derived as an RCL (HuCNS-SC RCL), the other intended for use in human cervical SCI under a funded NIH U01 (in what became the Pathway Study trial, NCT02163876). We assessed locomotor recovery and sensory function, as well as cell engraftment, migration, and neural lineage fate.
Results
Transplantation of HuCNS-SC RCLs into a Unilateral Cervical SCI Model 9 DPI Results in Engraftment of Donor Human Cells and Functional Locomotor Recovery 12 Weeks Post-transplant
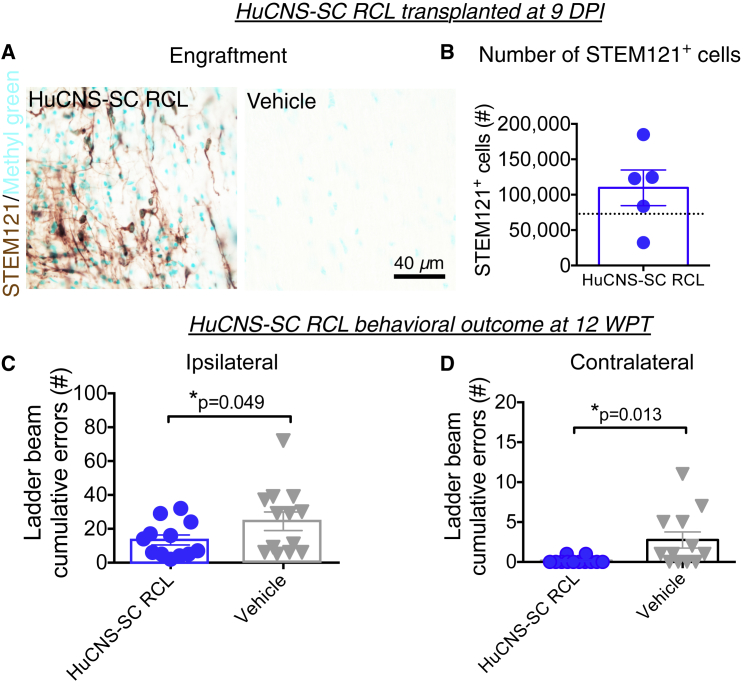
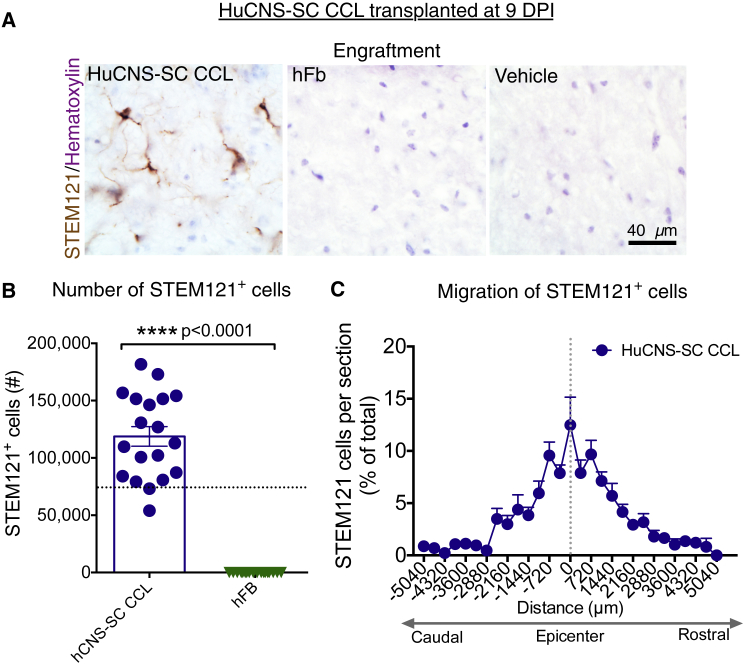
Based on our previously results transplanting multiple RCLs into both mouse and rat models of thoracic contusion SCI (Cummings et al., 2005, Hooshmand et al., 2009, Salazar et al., 2010, Piltti et al., 2013a, Piltti et al., 2013b, Sontag et al., 2013, Sontag et al., 2014, Piltti et al., 2015), we investigated transplantation of the HuCNS-SC RCL at 9 DPI following unilateral cervical contusion SCI in Rag2γ mice. All mice received a unilateral right-sided contusion injury as described in the Experimental Procedures; accordingly, the right side of the cord is ipsilateral to the injury. A total of 75,000 HuCNS-SC RCLs were transplanted into four parenchymal sites (N = 12); mice in the control group received vehicle injections (N = 13). Bilateral transplantation was selected to parallel previous thoracic studies because of the potential for contralateral demyelination (Arvanian et al., 2009), and because HuCNS-SC transplanted only ipsilateral to the injury do not cross to the contralateral spinal cord. All of the Rag2γ mice receiving transplants exhibited engraftment, as identified by immunohistochemical staining for the human cytoplasm-specific antibody STEM121 (Figure 1A). Blinded, unbiased quantification of the number of STEM121+/methyl green+ cells using an optical fractionator probe revealed an average of 109,695 donor human cells/animal at 12 weeks post-transplant (WPT) (Figure 1B).
Figure 1.
HuCNS-SC RCLs Exhibit Engraftment and Improve Locomotor Recovery in a 9 DPI Transplantation Paradigm
(A) Representative images from cervical SCI groups receiving HuCNS-SC RCL transplant (left) or vehicle control (right) 9 DPI. Animals were sacrificed 12 WPT. Sections were immunostained with a human-specific cytoplasmic marker STEM121 (brown) and counterstained with methyl green.
(B) Blinded, unbiased stereological quantification 12 WPT revealed an average of 109,695 ± 25,197 human cells in the RCL group (n = 9). Dashed line denotes transplant dose of 75,000 cells.
(C and D) Animals receiving RCL 9 DPI (n = 12) exhibited a significant decrease in horizontal ladder beam errors at 12 WPT for both ipsilateral (13.4 ± 3.0 versus 24.5 ± 5.5 for controls) and contralateral (0.2 ± 0.1 versus 3.8 ± 1.4 for controls) forepaws (Student's one-tailed t test, ∗p < 0.05) compared with controls (n = 13 ipsilateral, 12 contralateral). Data shown as means ± SEM.
In locomotor assessment on a horizontal ladder beam task 12 WPT, mice receiving the HuCNS-SC RCL demonstrated a significant reduction in the number of ipsilateral forelimb errors compared with vehicle control (Figure 1C), as well as complete normalization of contralateral forelimb errors to pre-injury baseline (Figure 1D), demonstrating proof of concept for disease-modifying activity of HuCNS-SC RCL to improve locomotor function after cervical SCI.
Comparison of HuCNS-SC RCL and HuCNS-SC CCL Lines for Engraftment, Fate, Locomotor Recovery, and Sensory Parameters in a Unilateral Cervical SCI Model 60 DPI
We next sought to evaluate parameters important in establishing clinically relevant efficacy for cervical SCI: first, delayed transplantation, using a 60 DPI time point; second, in vivo comparability between the HuCNS-SC RCL employed above and the HuCNS-SC CCL intended for the human clinical trial in cervical SCI; third, a data profile that included assessment of allodynia and hyperalgesia and employed adult as opposed to aged mice. Groups were expanded to include injured only (no cell or vehicle injection) as an injection control and human fibroblast (hFB) transplant as a cellular control. Surgical and post-operative exclusions are described under Experimental Procedures and in Figures S1A–S1D.
RCL and CCL Engraftment in the 60 DPI Cohort
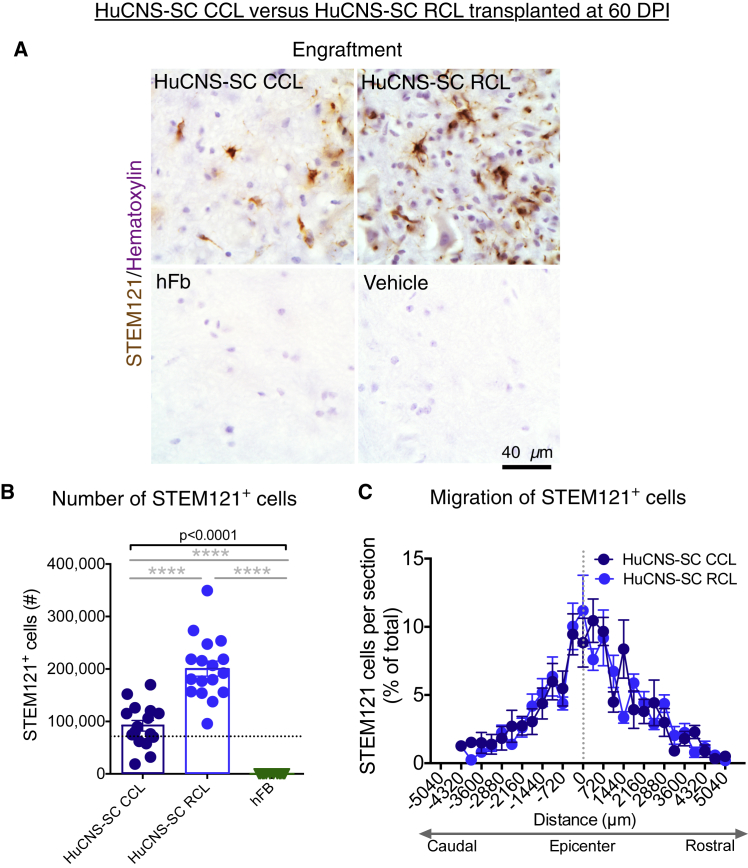
Engraftment data are shown for groups that received human cells (Figure 2A). All young adult Agouti Rag2γ(c) hybrid mice that received donor human RCL or CCL transplants exhibited engraftment. No sustained engraftment was observed in mice that received the hFB cellular control. Blinded, unbiased stereological quantification using the optical fractionator probe revealed an average of 91,701 human cells/animal for the CCL and 200,754 human cells/animal for the RCL, which represented significantly greater engraftment of the RCL (Figure 2B; one-way ANOVA, p < 0.0001; post hoc Tukey's test of CCL versus RCL, p < 0.0001). However, the number of human cells was equivalent for the CCL 60 DPI cohort compared with the RCL 9 DPI proof-of-concept cohort data shown in Figure 1 (Figure 1B RCL 9 DPI 109,695 cells, Figure 2B CCL 60 DPI 91,701 cells; Student's two-tailed t test, p > 0.4), suggesting that there was adequate engraftment of the CCL to support recovery of function if the donor cells acted via the same or similar mechanisms. Stereology was used to analyze the migration of the CCL and RCL; no differences were observed in the rostral-caudal extent of migration between the two cell lines (Figure 2C).
Figure 2.
HuCNS-SC CCLs Exhibit Reduced Engraftment Compared with RCLs in a 60 DPI Paradigm
(A) Representative images from cervical SCI groups that received either a CCL, RCL, or hFb transplant, or vehicle control 60 DPI. Animals were sacrificed 12 WPT. Sections were immunostained with STEM121 (brown) and counterstained with hematoxylin (purple).
(B) Blinded quantification 12 WPT revealed an average of 91,701 human cells for the CCL group (n = 16), 200,754 for RCL group (n = 17), and no surviving human cells for the hFB group (n = 12) A one-way ANOVA revealed significant differences in survival (solid bar p < 0.0001; post hoc Tukey's test of CCL versus RCL and hFb, ∗∗∗∗p < 0.0001). Dashed line denotes transplant dose of 75,000 cells.
(C) No difference was found in the rostral-caudal extent of human cell migration between the CCL (n = 16) and RCL (n = 17) at 12 WPT (unpaired t tests with Holm-Sidak multiple comparison correction, p > 0.05). Dashed vertical line indicates injury epicenter. Data shown as means ± SEM.
RCL and CCL Fate in the 60 DPI Cohort
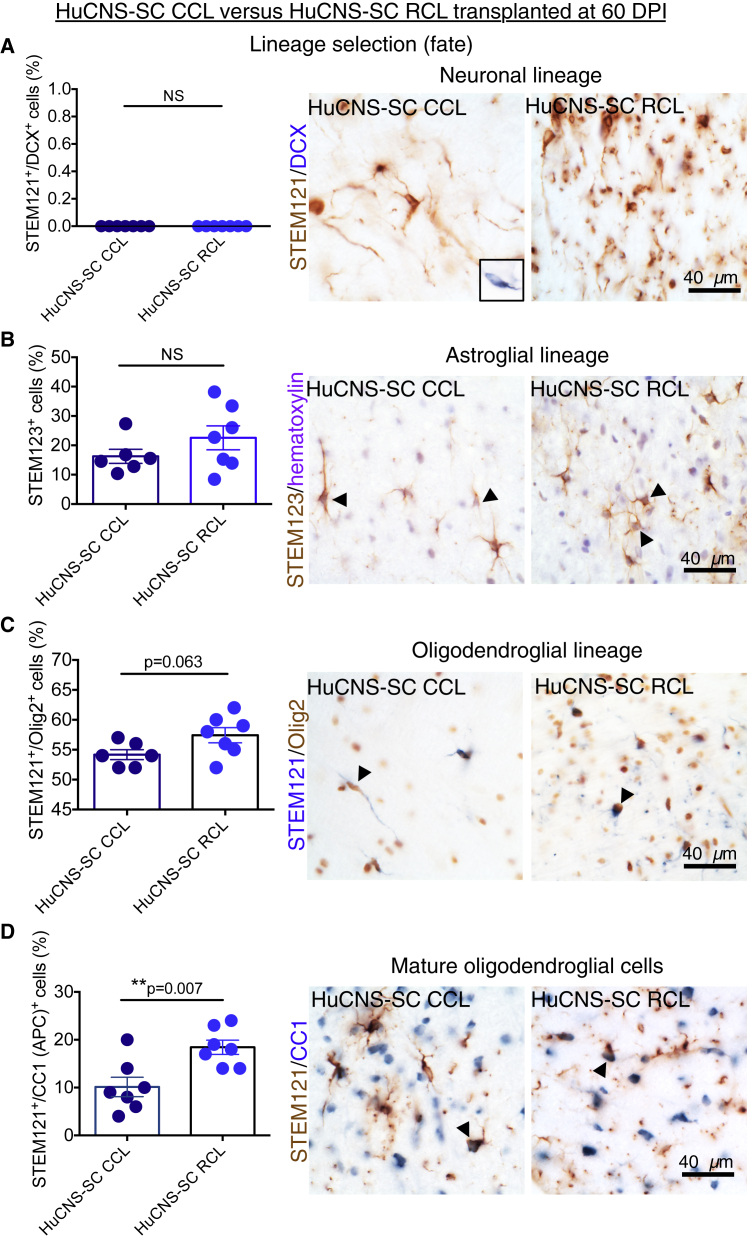
Cell fate data are shown only for groups that received HuCNS-SCs. We previously reported that multiple different HuCNS-SC RCLs exhibit robust differentiation along the oligodendroglial lineage after transplantation into rodent models of SCI 9, 30, or 60 DPI (Cummings et al., 2005, Hooshmand et al., 2009, Salazar et al., 2010, Piltti et al., 2013a, Piltti et al., 2013b, Sontag et al., 2013). In contrast, these RCLs exhibit limited potential to generate oligodendroglial lineage cells in vitro (Sontag et al., 2013) and limited terminal differentiation into CC1-positive mature oligodendrocytes in the uninjured CNS in vivo (Sontag et al., 2014). Accordingly, we assessed the cell lineage fate of the RCL and CCL in this 60 DPI unilateral cervical model (Figure 3) in a random subset of animals from each group (N = 7/group) using double-labeling immunohistochemistry for STEM121+ and doublecortin (DCX), nuclear Olig2, and APC/CC1, as described in the Experimental Procedures. Astroglial fate was determined by staining for the human-specific GFAP marker STEM123. Quantification was conducted via blinded, unbiased stereology using the optical fractionator probe in StereoInvestigator. All data are expressed as the proportion of STEM121+/marker+ donor human cells relative to total STEM121+ cells in the same animal.
Figure 3.
Lineage Analysis of HuCNS-SC CCL and RCL in the 60 DPI Paradigm
Data for proportional human cell fate were collected by blinded, unbiased stereology.
(A) Co-immunostaining for STEM121 (brown) and the early neuronal marker DCX (blue) revealed no STEM121+/DCX+ cells in either the CCL (n = 7) or RCL (n = 7) groups (Student's two-tailed t test, p > 0.05, n.s.). Inset shows positive control for DCX in the hippocampus.
(B) Immunostaining for human-specific GFAP (STEM123, brown) with hematoxylin counterstaining (purple) revealed no significant difference in STEM123 proportion between the CCL (n = 6) and RCL (n = 7) groups (Student's two-tailed t test, p > 0.2).
(C) Immunostaining for STEM121 (blue) and the oligodendroglial nuclear marker Olig2 (brown) revealed that the largest proportion of STEM121+ cells were also nuclear Olig2+ and there was a trend for a decrease in Olgi2+ cells in CCL (n = 5) versus RCL (n = 7) transplants (Student's two-tailed t test, p = 0.06).
(D) Immunostaining for STEM121 (brown) and the mature oligodendroglial marker CC1 (blue) revealed a significant decrease in the proportion of STEM121+/CC1+ cells in CCL (n = 7) versus RCL (n = 7) transplants (Student's two-tailed t test, p < 0.007).
Arrowheads indicate double-positive cells. Data shown as means ± SEM.
In contrast with previous studies in thoracic SCI at sub-acute and chronic time points, no evidence for neuronal lineage differentiation of donor cells, as evidenced by the lack of detection of STEM121+/DCX+ profiles, was observed (Figure 3A). These data were consistent with previous observations of the RCL transplanted at 9 DPI in this cervical SCI model (not shown), in which an extremely small percentage of DCX+ neurons was observed and suggest that local cues in the cervical microenvironment are either less permissive or fail to drive neuronal fate. Approximately 16% of CCL 60 DPI donor cells exhibited STEM123+ immunolabeling, with no significant differences in proportional astroglial fate observed when RCL 60 DPI (23%) and CCL 60 DPI groups were compared (Figure 3B). This percentage was consistent with previous observations in thoracic SCI in which RCL were transplanted 30–60 DPI (Salazar et al., 2010, Piltti et al., 2013a, Piltti et al., 2013b); in parallel, the majority of donor cells exhibited oligodendroglial lineage markers, regardless of group. However, there was a non-significant trend for a reduction in the STEM121+/nuclear Olig2+ proportion in CCL versus RCL cells (Figure 3C), and analysis of a mature oligodendroglial lineage marker (CC1) revealed approximately half the number of STEM121+/CC1+ donor human cells in CCL 60 DPI versus RCL 60 DPI transplanted animals (Figure 3D; 10% versus 18%, Student's two-tailed t test, p < 0.007). These data suggest that there were differences between the CCL and RCL in response to the injured microenvironment 60 DPI, which may have limited or delayed the generation of myelinating oligodendrocytes in CCL transplants.
Locomotor Recovery in the 60 DPI Cohort
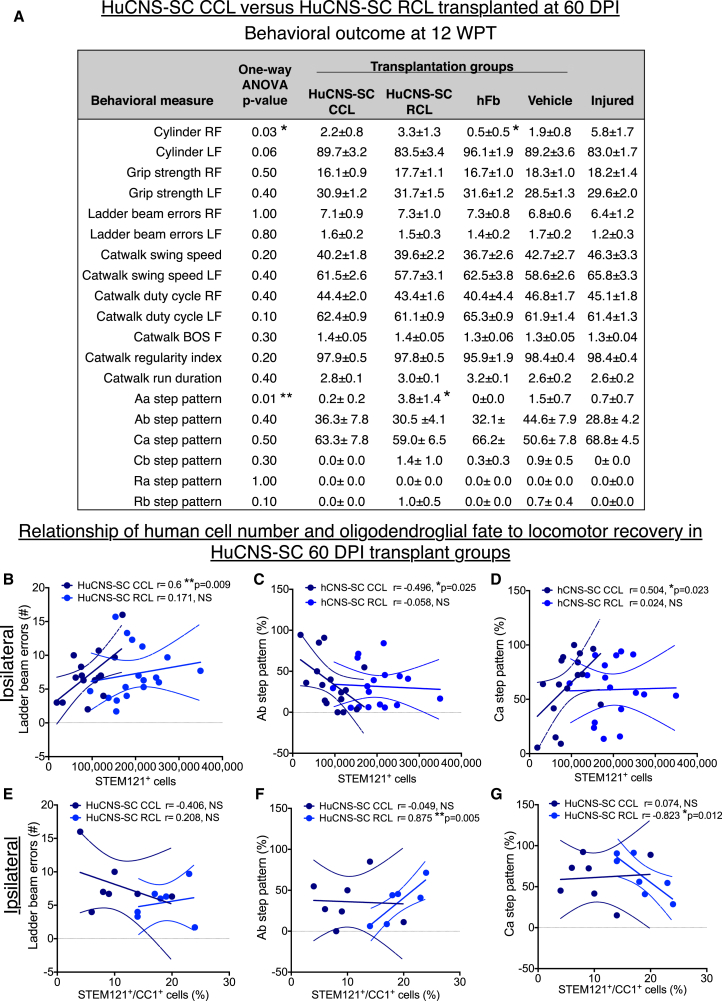
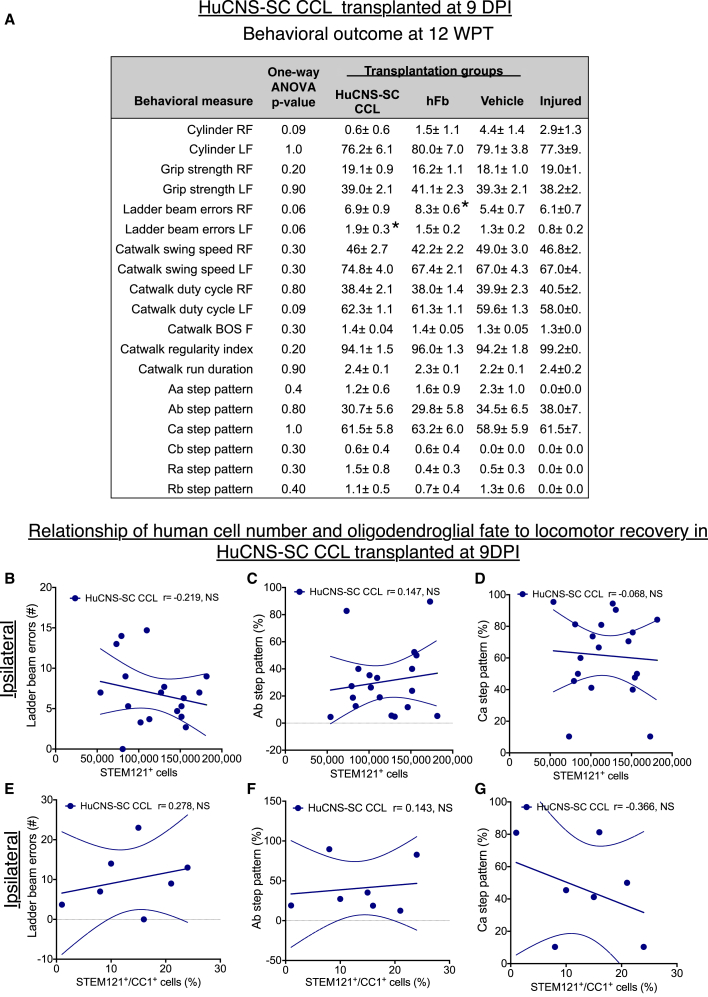
No statistical differences were observed between injured only (no injection) and vehicle injection groups in any behavioral task; statistical comparisons shown were therefore based on comparison of groups receiving human cells versus vehicle injection. Recovery of function was assessed on four locomotor tasks: grip strength, cylinder reaching (percentage paw placement), horizontal ladder beam, and CatWalk step kinematic analysis. A summary of behavioral data is contained in Figure 4A. Although the RCL exhibited significant improvements in CatWalk Aa step pattern (coordination of alternating right-fore, right-hind, left-fore, left-hind) (Hamers et al., 2006) compared with the CCL at 12 WPT (Figure 4A; one-way ANOVA p = 0.01, post hoc Tukey's multiple comparison t test p < 0.05), there were no significant differences compared with vehicle controls. No evidence for recovery of function was observed for the CCL in any of these tasks at 12 WPT or indeed at any time point (Figures 4 and S3). To test whether there was a relationship between the lack of observed locomotor recovery and 12 WPT engraftment, correlation analyses were performed for each of the principal locomotor measures collected for both the RCL and CCL. Although we have previously reported a reduction in hindlimb errors on the horizontal ladder beam that was correlated with increasing total STEM121+ RCL engraftment after thoracic SCI (Hooshmand et al., 2009), we observed the opposite here; that is, an increase in right forelimb (ipsilateral) ladder errors was associated with an increase in the number of engrafted CCL cells (Figure 4B, dark blue circles; Pearson r = 0.6, p = 0.009). No correlation was observed between ipsilateral ladder errors and RCL engraftment (Figure 4B, light blue circles; Pearson r = 0.171, not significant [n.s.]). These data suggest a negative effect on locomotor recovery specific for the CCL, and failure of the RCL to produce robust locomotor recovery at this dose in young animals when transplantation was delayed to 60 DPI.
Figure 4.
Analysis of Locomotor Recovery in HuCNS-SC 60 DPI Cohort Transplantation Groups versus Vehicle Controls 12 WPT
(A) Table showing average ± SEM values for cylinder reaching (percentage paw placement), grip strength, horizontal ladder beam errors, and CatWalk step kinematic analysis for each experimental group. RF, ipsilateral forelimb; LF, contralateral forelimb. Where one-way ANOVA reached significance (∗p ≤ 0.05, ∗∗p ≤ 0.01), Tukey's multiple comparison t tests were conducted. Significant differences were found in ipsilateral cylinder reaching between the hFB and Injured only groups (∗p ≤ 0.05), and in Aa step pattern between the RCL, CCL, and hFB groups (∗p ≤ 0.05). n = 15–16 for CCL, n = 16–17 for RCL, n = 11–12 for hFB, n = 15–17 for Vehicle, and n = 9–10 for Injured only (see Figure S1D for exact numbers). BOS, base of support.
(B–G) Pearson correlations were conducted between the number of human STEM121+ cells and (B) ipsilateral forelimb ladder beam errors, (C) Ab step pattern, and (D) Ca step pattern. Pearson correlation were also conducted between STEM121+/CC1+ human oligodendroglial cell proportion and (E) ipsilateral forelimb ladder beam errors, (F) Ab step pattern, and (G) Ca step pattern. Dashed lines indicate confidence intervals of 95%. n = 7 for CCL and n = 7 for RCL in CC1+ correlational analyses.
The finding of impaired function on the ladder beam was paralleled in kinematics analysis of gait on the CatWalk (Figures 4C and 4D). In young adult mice, the predominant pre-SCI step pattern was Ab (80%), while the predominant post-SCI step pattern shifted to Ca (60%). We focused on correlations of proportions of Ab and Ca patterns, anticipating that an improvement in locomotor recovery would be associated with an increase in Ab step pattern and a decrease in Ca step patterns. In contrast, CCL-treated animals exhibited a decrease in Ab step pattern and an increase in Ca step pattern in association with an increase in the number of engrafted CCL cells (Figure 4C, %Ab Pearson r = −0.496, p = 0.025; Figure 4D, %Ca Pearson r = 0.504, p = 0.023). No correlation was observed between Ab or Ca step pattern and RCL engraftment (Figure 4C, %Ab Pearson r = −0.0580, n.s.; Figure 4D, %Ca Pearson r = 0.0239, n.s.). Accordingly, these data suggest a negative effect on locomotor recovery specific for the CCL, and failure of the RCL to produce robust locomotor recovery in young animals when transplantation was delayed to 60 DPI.
Based on observed differences in CC1+ oligodendroglial differentiation between the RCL and CCL, we also conducted correlation analyses for ladder beam errors, Ab step pattern, and Ca step pattern for these cell lines. No significant relationships were found between ladder beam errors and proportion of STEM121+/CC1+ cells for either the RCL or CCL (Figure 4E, RCL Pearson r = 0.208, n.s.; CCL Pearson r = −0.406, n.s.). Similarly, the CCL exhibited no relationship between proportion of STEM121+/CC1+ cells for either Ab or Ca step patterns (Figure 4F, Ab Pearson r = −0.049, n.s.; Figure 4G Ca Pearson r = −0.074, n.s.). However, analysis of these relationships for the RCL was significant (Figure 4F, Ab Pearson r = −0.875, p = 0.005; Figure 4G, Ca Pearson r = −0.823, p = 0.012), suggesting that the potential to exert donor-cell-driven repair in chronic cervical SCI may be associated with in vivo capacity to generate terminally differentiated oligodendrocytes.
Sensory Assessment in the 60 DPI Cohort
A significant concern is the potential for cell engraftment, or specific lineage selection, to induce or exacerbate neuropathic pain syndromes in SCI (Hofstetter et al., 2005, Macias et al., 2006). Accordingly, animals were assessed for mechanical allodynia using Von Frey testing and hyperalgesia using Hargreaves testing. Critically, although reduced, allodynia/hyperalgesia can be detected in constitutively immunodeficient animals (Moalem et al., 2004, Kleinschnitz et al., 2006). No changes were observed between any groups in these measures either immediately prior to sacrifice at 12 WPT or in two-way ANOVA across time (Figure S4), suggesting neither a cell-based impairment of sensory function nor initiation of a neuropathic pain syndrome.
Evaluation of the CCL for Engraftment, Fate, Locomotor Recovery, and Sensory Parameters in a Unilateral Cervical SCI Model 9 DPI
While RCL transplantation into the cervical spinal cord 9 DPI resulted in recovery of locomotor function in aged Rag2γ mice, the effect of RCL transplantation into the cervical spinal cord 60 DPI in young Agouti Rag2γ(c) hybrid mice was attenuated, and correlational analyses suggest CCL transplantation into this model produced some decrements in function. Together, the data suggest that less benefit was achieved after transplantation into a delayed/chronic cervical SCI paradigm, and that the RCL and CCL exerted different effects after cervical SCI. To investigate this variation, we evaluated cell engraftment, fate, locomotor recovery, and sensory function following CCL transplantation into the cervical spinal cord 9 DPI in a separate cohort of young Agouti Rag2γ(c) hybrid mice.
CCL Engraftment in the 9 DPI Cohort
All Agouti Rag2γ(c) hybrid mice receiving CCL at 9 DPI exhibited engraftment 12 WPT, while no sustained engraftment was observed in mice receiving the hFB cellular control (Figure 5A). Blinded, unbiased stereological quantification revealed an average of 118,757 human cells/animal (Figure 5B), which was significantly greater compared with the 60 DPI CCL cohort Figure 2B; a comparison is shown in Figure S2A (18,757 versus 91,701, Student's two-tailed t test, p < 0.05). This contrasts with previous observations in thoracic SCI, where we observe no significant differences in surviving cells quantified 12–16 WPT after human neural stem cells transplantation at 0, 9, or 30 DPI. However, comparison of migration between the 9 (Figure 5C) and 60 DPI (Figure 2C) CCL cohorts revealed no differences in the rostral-caudal extent of migration at any distance from the lesion epicenter (unpaired t test with Holm-Sidak multiple comparison correction, n.s.).
Figure 5.
HuCNS-SC CCL Exhibit Engraftment and Rostral-Caudal Migration in a 9 DPI Paradigm
(A) Representative images from cervical SCI groups that received CCL or hFb transplant, or vehicle control 9 DPI. Animals were sacrificed 12 WPT. Sections were immunostained with STEM121 (brown) and counterstained with hematoxylin.
(B) Blinded, unbiased quantification 12 WPT revealed an average of 118,757 human cells for the CCL group (n = 19) and no human cells for the hFB group (n = 18) (Student's two-tailed t test, p < 0.0001). Dashed line indicates the transplant dose of 75,000 cells.
(C) Rostral-caudal extent of human cell migration for the CCL at 12 WPT. Dashed vertical line indicates injury epicenter (n = 19). Data shown as means ± SEM.
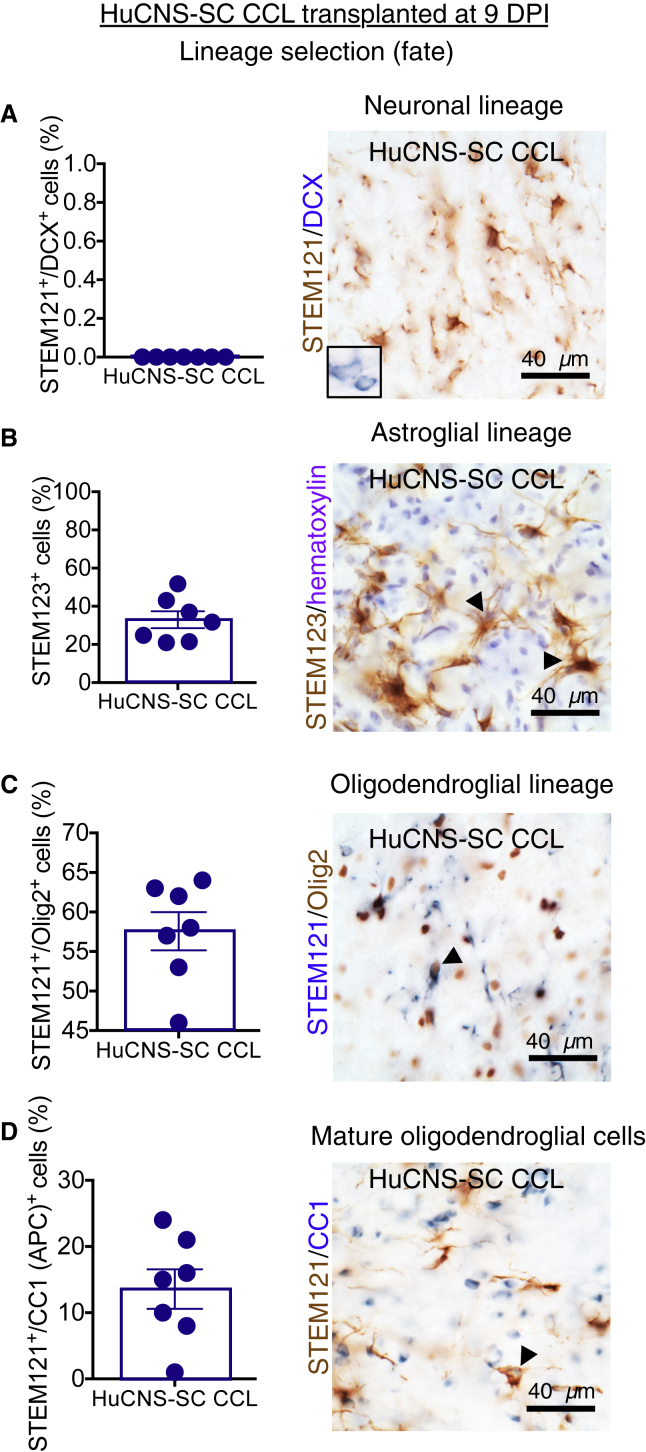
CCL Fate in the 9 DPI Cohort
Analysis of human cell fate was conducted as described for 60 DPI cohorts. As for the 60 DPI CCL cohort, no evidence of neuronal lineage differentiation was observed (Figure 6A). Approximately 33% of human CCL 9 DPI were positive for the human astroglial marker STEM123 (Figure 6B). In parallel with the 60 DPI cohort, the largest proportion of human cells exhibited oligodendroglial lineage markers and were positive for either STEM121+/Olig2+ (Figure 6C; 55%) or STEM121+/CC1+ (Figure 6D; 10%). No differences were observed between the 9 DPI and 60 DPI CCL cohorts in neuronal or oligodendroglial lineage fate proportions (Figure S2B, Student's two-tailed t test, p > 0.05). However, comparison of the CCL 9 DPI and 60 DPI cohorts revealed a doubling of STEM123+ astroglial cells in the 9 DPI cohort (Figure S2B, Student's two-tailed t test, p < 0.009). Differences in the post-injury cervical SCI microenvironment associated with transplantation timing and/or variation between CCL shipments, corresponding to the 9 DPI and 60 DPI cohort transplantation dates, may have influenced CCL fate. In comparison, previous experience with RCL has not demonstrated an increase in astroglial fate in mice receiving human cells at 30 versus 9 DPI (Cummings et al., 2005, Salazar et al., 2010).
Figure 6.
Lineage Analysis of HuCNS-SC CCL in the 9 DPI Paradigm
Data for proportional human cell fate were collected by blinded, unbiased stereology
(A) Immunostaining for STEM121 (brown) and DCX (blue) revealed no STEM121+/DCX+ cells in CCL 9 DPI animals. Inset shows positive control for DCX in cortex.
(B) Immunostaining for human-specific GFAP (STEM123, brown) with hematoxylin counterstaining (purple) revealed 33% ± 4.4% of CCL 9 DPI were positive for this astroglial marker.
(C) Immunostaining for STEM121 (blue) and Olig2 (brown) revealed that the largest proportion of STEM121+ cells in the 9 DPI transplant group were positive for this oligodendroglial marker (57.6% ± 2.4%).
(D) Immunostaining for STEM121 (brown) and CC1 (blue) revealed 13.6% ± 3% of CCL 9 DPI were positive for this mature oligodendroglial marker.
Arrowheads indicate double-positive cells. n = 7 for all lineage tests. Data shown as means ± SEM.
Locomotor Recovery in the 9 DPI Cohort
In contrast to the 9 DPI RCL cohort, no evidence for recovery of function was observed for the 9 DPI CCL cohort in any locomotor assessment at 12 WPT or any time point (Figures 7 and S5). A summary of behavioral data is shown in Figure 7A. Analysis of locomotor recovery versus engraftment at 12 WPT did not reveal significant correlations between the number of engrafted STEM121+ CCL cells and right forelimb (ipsilateral) errors on the horizontal ladder beam (Figure 7A; Pearson r = −0.218, n.s.) or in any CatWalk step patterns, including Ab (Figure 7B; Pearson r = 0.147, n.s.) or Ca (Figure 7C; Pearson r = −0.068, n.s.). Similarly, analysis of locomotor recovery versus fate did not reveal significant correlations between the number of engrafted STEM121+/CC1+ CCL cells and right forelimb (ipsilateral) errors on the horizontal ladder beam (Figure 7D; Pearson r = 0.278, n.s.) or in any CatWalk step patterns, including Ab (Figure 7E; Pearson r = 0.143, n.s.) or Ca (Figure 7F; Pearson r = −0.366, n.s.). Together, these data suggest that the CCL failed to promote functional locomotor recovery in this animal model of cervical SCI; however, no significant decrements in function were observed on correlational analyses.
Figure 7.
Locomotor Recovery in HuCNS-SC 9 DPI Cohort Transplantation Groups versus Vehicle Controls 12 WPT
Data for proportional human cell fate were collected by blinded, unbiased stereology.
(A) Average ± SEM values for cylinder reaching (percentage paw placement), grip strength, horizontal ladder beam errors, and CatWalk kinematic analysis for each group. RF, ipsilateral forelimb; LF, contralateral forelimb. No significant differences were found between groups using one-way ANOVA p = 0.06; however Tukey's multiple comparison revealed significant an increase in ipsilateral forelimb ladder beam errors in the hFB group compared with vehicle group (p < 0.05) and in contralateral forelimb ladder beam errors in CCL 9 DPI group compared with injured only group (p < 0.05), denoted by ∗. n = 18–19 for CCL, n = 17–18 for hFB, n = 18–20 for Vehicle, and n = 9–10 for Injured only (see Figure S1D for exact numbers).
(B–G) Pearson correlations were conducted between the number of human STEM121+ cells and (B) ipsilateral forelimb ladder beam error number, (C) Ab step pattern, and (D) Ca step pattern (n = 18). Pearson correlations were also run between STEM121+/CC1+ human oligodendroglial cell proportion and (E) ipsilateral forelimb ladder beam errors, (F) Ab step pattern, and (G) Ca step pattern (n = 7). No significant differences were observed, suggesting neither improvements nor decrements in function at 9 DPI. Dashed lines indicate confidence intervals of 95%.
Sensory Parameter Assessment in the 9 DPI Cohort
All animals were assessed for mechanical allodynia using Von Frey testing and hyperalgesia via Hargreaves testing. No changes were observed between any groups in either two-way ANOVA across time or immediately prior to sacrifice 12 WPT (Figure S6). Accordingly, these data suggest that CCL transplantation 9 DPI neither altered sensory function nor initiated a neuropathic pain syndrome.
Discussion
HuCNS-SC CCLs have been tested in human clinical trials in several paradigms. An HuCNS-SC line was authorized by the US Food and Drug Administration (FDA) for testing in the lysosomal storage disorder neuronal ceroid lipofuscinosis (NCL) (NCT00337636). Six patients were transplanted and the study was completed in 2009. The results suggested that the direct transplantation of HuCNS-SCs into the CNS was safe (Selden et al., 2013). An HuCNS-SC line was also authorized for testing in the lethal disorder Pelizaeus-Merzbacher disease (NCT01005004). Four patients received this HuCNS-SC line and the study was completed in 2012 (Gupta et al., 2012), again supporting a favorable safety profile. In 2010, an HuCNS-SC line was authorized by Swissmedic for a phase I/II trial in thoracic SCI (NCT01321333) in Zurich (Health Canada and the FDA later adding two North American sites). An HuCNS-SC line was transplanted into 12 SCI patients; the trial was completed in April 2015. Interim data were presented at the fourth Joint International Spinal Cord Society and American Spinal Injury Association meeting in Montreal (May 14, 2015). However, as of September 30, 2016, final results and/or a peer-reviewed publication of this study has yet to be published. The principal objective of the current study was to test the efficacy of the HuCNS-SC CCL intended for use in the human cervical SCI Pathway Study under an NIH-funded U01 in a unilateral cervical contusion injury model, assessing locomotor recovery and sensory function, as well as cell engraftment, migration, and neural lineage fate.
Although we have previously demonstrated evidence for recovery of motor function in both thoracic and cervical models using several different research cell lines (RCL), no evidence in support of the efficacy of the tested intended HuCNS-SC CCL was detected in the current cervical SCI study. In contrast, correlative measures of total donor cell number and locomotor function suggested, in some cases, a negative impact of engraftment of the CCL on functional outcome in this animal model. A clinical trial testing this HuCNS-SC CCL was initiated in December 2014 for cervical SCI (NCT02163876) after preliminary analysis of the dataset in the present report was reported to StemCells Inc. A press release of interim 6-month data for the trial (November 18, 2015) reported improvements in motor strength in 4/5 subjects, in contrast to the animal data presented here. Subsequently, citing a lack of significant improvements and the lack of a trend for improvements over time, StemCells Inc. terminated the Pathway Study on May 31, 2016.
These facts raise several issues for stem cell transplantation. There has been extensive discussion regarding the validity of animal models in clinical translation (van der Worp et al., 2010), based on the failure of animal models of disease to predict efficacy in the clinical trial setting. Publication bias and overprediction of efficacy is suggested to account for as much as a third of this discrepancy (Sena et al., 2010), and methodological/RIGOR flaws in animal studies have also been raised as significant concerns (Lapchak, 2012, Chang et al., 2015). It can be difficult to determine the applicability of endpoints in an animal model to the human condition, or the timing of a therapeutic in rodents versus timing in man (Henderson et al., 2013). Conversely, poorly designed clinical studies may play a role in overestimating or underestimating clinical impact (Moller, 2014). Accordingly, publication of preclinical data from carefully controlled and properly blinded animal studies is key for advancing the field. Multiple RCLs have previously been shown to improve recovery of function in multiple thoracic SCI models. In addition, the RCL employed in the proof-of-concept cohort here did demonstrate efficacy in this cervical SCI model. Moreover, in our preclinical animal studies, all RIGOR recommendations and other standards were met. One interpretation of these data is that these observations derive from variation between cell lines and/or in cell manufacture/processing and not in the model itself or in the experimental execution of the model.
For stem cell therapies, generation of cell lots and lines with consistent potency and comparability is recognized by the field and the FDA as a significant issue for clinical translation (Hyun et al., 2008, Lo et al., 2008). Inconsistencies in scale-up and production of good manufacturing practice (GMP) CCLs, failure to develop potency/comparability assays based on demonstrable clinically significant activity rather than simplicity, and failure of potency/comparability assays to offer adequate analysis of clinically significant endpoints are issues that have been addressed extensively in the context of mesenchymal stem cell products (MSCs). MSCs represent an exemplar in which the failure of pivotal clinical trials has been linked to these factors (Galipeau, 2013, Chinnadurai et al., 2015). Accordingly, in addition to controversies over the validity of animal models and quality of preclinical design, an additional variable may be the development and validation of potency/comparability assays for clinical testing of cell products, including the lack of FDA requirements for in vivo testing. Critically, failure to address and define the source of variation between research, process development, and clinical cell lots and lines, or to conduct in vivo testing of all cell product lots and lines used for clinical transplantation, is an issue for not only efficacy, but safety, as the in vivo factors controlling donor differentiation, cell division, and tumorigenesis remain poorly defined, and a focus on in vitro assays that are poorly linked to efficacy may fail to detect critical variations that result in an altered risk profile. We suggest that this raises concerns regarding both the adequacy of current standards for demonstration of potency and comparability between therapeutic cell lots and lines, as well as a potential issue for informed consent during patient enrollment (Anderson and Cummings, 2016).
In response to these data, StemCells Inc. noted that the CCL cells tested herein are not the cells that were tested in the Pathway Study, because cells were sent to the University of California, Irvine (UCI) from the “process development laboratory” and not produced under current GMP conditions/manufacturing requirements of a clinical trial. StemCell Inc.'s response is contrary to the milestones of the NIH-funded U01 for testing of the “intended CCL”. However, we do not have access to the safety/toxicology profile submitted to gain FDA authorization for the trial nor the final clinical product administered in the Pathway Study. If one accepts that the CCL cells used herein do not share sufficient comparability with the final clinical product to be considered as “representative”, then we believe the Pathway Study went forward in the absence of in vivo efficacy testing. Conversely, if one views the “process development laboratory” and final clinical product as substantially similar, then we would argue that the Pathway Study went forward with cells that failed to yield preclinical efficacy. Many scientists are unaware that in vivo preclinical testing of the final clinical product is not required by the FDA. As we have noted (Anderson and Cummings, 2016), FDA guidance states that because “human-derived cellular therapy products intended for clinical administration in animals may not be informative” (due to the species specific nature of some paradigms or products), “testing of an analogous product may be a suitable alternative” (Center for Biologics Evaluation and Research, 2013).
Of course, when the final analysis and follow-up are completed for the Pathway Study, the key issue will be whether the final clinical product demonstrated a positive or negative safety and tolerability profile, as well as preliminary evidence related to efficacy outcome measures in humans. If negative safety data or a failure in efficacy are ultimately observed in the clinical trial, it is likely that hindsight will call out the failure to match preclinical and clinical studies in terms of the tested product. Conversely, if positive safety and/or efficacy profiles were to have been observed in the clinical trial, one might have asserted that preclinical models of cervical SCI in rodents are not predictive, and/or that the details of cell manufacturing significantly affect outcome in both animals and humans. In the end, we have no way to conduct an objective assessment of preclinical versus clinical discrepancies, which raises a more general problem for translational research supported by academic-industry partnerships.
Are the cells used in the NCL trial, the Pelizaeus-Merzbacher Disease trial, or the Zurich thoracic SCI trial derived from the same donor or by the same manufacturing process as those used in the Pathway Study? Because all cell lines, whether from different donors or different manufacturing preparations, have been designated as “HuCNS-SC” by StemCells Inc., it is impossible for a subject enrolling in a trial using this product to fully understand the basis on which the trial was founded, and come to an informed individual decision on participation. We suggest that open disclosure of specific stem cell product designations (e.g., standardized clinical cell line reference numbers), should be required, negotiated as a part of academic-industry collaborations, as well as for publication of preclinical and clinical data, and institutional review board approval of consent documents. This is a standard that we are unable to meet due to nondisclosure restrictions; accordingly, multiple different cell lines are referred to herein by the common appellation HuCNS-SC. For clarity in this regard, it should be stated that the CCL referenced in this article is not the same line as the CCL reported on by Marsh et al. (2017), or any cell line previously reported on by our laboratory.
Finally, it is important to address the relationship between translational/preclinical research and clinical trial success rate (Roberts et al., 2012, Perrin, 2014). Although many factors may contribute, including lack of alignment between animal models and human disease, the lack of correspondence between preclinical research and clinical trial success may also suggest a need for conducting sufficient basic research to understand the mechanism(s) of action (MOA) of a particular cell therapy and enable potency/comparability assay design that can robustly detect variation between cell lots and lines. Until optimized in vitro assays are available, this may require in vivo testing of final clinical products that have completed full-release testing. It has been argued that the MOA should not be required in order to proceed with testing a drug or cell therapy in man. This position posits that were we to wait until every aspect of a particular cell line were understood, we would never proceed to clinical testing. In fact, with respect to evidentiary standards for drug testing and approval, the prevailing opinion is that “theories about MOA of a drug or disease mechanisms play important parts in drug development and approval, but they are entirely subsidiary to the fundamental questions that must be answered in the course of drug approval; namely, is a drug effective, and is it safe in use.” (Katz, 2004). While this perspective has a logical degree of practicality, we suggest that the failure (or disincentive) to understand MOA may be an alternative reason for failure in translational medicine and clinical trials. Finally, since the Pathway Study failed to show efficacy in humans, we will never know if this failure was because neural stem cells, in general, are not effective for human SCI or, rather, that the wrong cell line was tested prematurely in humans.
Experimental Procedures
All studies were in accordance with the Institutional Animal Care and Use Committee and Human Stem Cell Research Oversight at UCI. All data were maintained under good laboratory practice-like protocols, with an assigned data monitor. Animal care, behavior, and analysis were performed by investigators blinded to group, and random group allotment was used. Inclusion/exclusion criteria were established in advance (see Supplemental Experimental Procedures). Additional detail on experimental procedures, randomization, blinding, exclusions, cells, injuries and surgeries, transplantation, and analyses is contained in Supplemental Experimental Procedures.
HuCNS-SC and hFb
Sorted HuCNS-SC lines were provided by StemCells Inc.; details about cells are described in the Supplemental Experimental Procedures. RCL and CCL cells were shipped overnight to UCI. Fresh RCL or CCL shipments were received each surgery day. Cell yield, viability, and preparation data were recorded for each vial received (Table S1). Human mesenchymal stromal cell hFbs (Cell Applications) were thawed and cultured at UCI in DMEM with 10% fetal bovine serum and glutamine for 7 days prior to transplantation.
Contusion Injuries
For the proof-of-concept cohort, we used 18-month-old, commercially available, Rag2γ(c) female mice (Taconic). For the main study, 10 to 12 week old female Agouti Rag2γ(c) hybrid mice were used (StemCells Inc.). Animals were anesthetized, spinal cords at C5 vertebral level were exposed by laminectomy, stabilized, and unilateral 30-kDa contusion injuries with 5 s dwell time were administrated with a 1 mm diameter tip using an IH Impactor (Precision Systems & Instrumentation). Animals received standard post-operative care, including buprenorphine, lactated Ringer's, bladder care, and antibiotics.
Transplantation
Mice were anesthetized 9 or 60 DPI and a total volume of 1 μL (250 nL per injection site) of cell suspension (75,000 cells) or vehicle was injected via two rostral injections and two caudal injections 0.75 mm from midline.
Exclusions
Thirty-three animals entered the proof-of-concept study cohort; 25 completed the study. A total of 147 animals entered the main study cohorts; 139 completed the study. There were no animal exclusions due to engraftment failure or histological issues. Pre- and post-injury animal exclusions, Grubbs exclusions, and final numbers for statistical analysis are detailed in Figures S1A–S1D.
Assessment of Locomotor and Sensory Function
All tasks were assessed prior to injury (baseline) and at 3, 8, and 12 WPT. The horizontal ladder beam task was performed as described (Salazar et al., 2010) using CatWalk XT (Noldus v9.0). Forelimb-use asymmetry was assessed using a cylinder task. Forepaw grip strength was measured for each paw alone and together in five trials per mouse using a Dunnett-style grip strength meter (Pawar et al., 2015). Mechanical allodynia was assessed using a Von Frey test (Salazar et al., 2010). Thermal hyperalgesia was assessed using a Hargreaves test (Piltti et al., 2013a, Piltti et al., 2013b).
Histology and Stereological Quantification
At 12 WPT, mice were terminally anesthetized, and perfused, and cord segments were dissected (C1–T2 roots), post-fixed, cryoprotected with 20% sucrose, and flash frozen (Hooshmand et al., 2009). Coronal 30 μm sections were cryostated and immunostained. The primary antibodies and secondary antibodies used are listed in Table S2. Final animal numbers for analysis are listed in Figure S1. Quantification was via unbiased stereology. Migration of human cells was analyzed as percentage of the cells per section relative to total number of human cells. A random set of animals were selected for proportional counting of cell fate (N = 7/group).
Statistics
All data are means ± SEM; statistics were performed using Prism v6 (GraphPad Software). Comparisons between groups were analyzed using one-way ANOVA combined with Tukey's post hoc t tests or Student's one or two-tailed t tests. Locomotor and sensory function were compared using two-way repeated measures ANOVA combined with multiple comparison corrected/Bonferroni post hoc t tests. Migration was analyzed using unpaired two-tailed t tests with Holm-Sidak multiple comparison correction. Correlation between human cells and ladder beam errors or percentage of step patterns were assessed using a Pearson correlation coefficient. A p value of ≤0.05 was considered to be significant. In the figure legends, n denotes the number of individual mice.
Author Contributions
A.J.A. designed experiments, wrote the manuscript, trained and supervised staff, and contributed to data analysis and finalization of figures for publication. K.M.P. supervised and conducted cell preparation for transplantation, prepared figures for publication, and contributed to data analysis and to manuscript writing. M.J.H. supervised cell transplantation, maintained experimental blinding for surgeries and behavioral analyses, and contributed to data analysis and manuscript writing. R.A.N. supervised all surgeries and behavioral testing, and contributed to experimental planning and data analysis. B.J.C. designed experiments, and contributed to final data analysis, preparation of figures for publication, and manuscript writing. K.M.P., M.J.H., and R.A.N. contributed equally.
Acknowledgments
We thank staff of the Christopher and Dana Reeve Foundation Core (CDRF core), especially B. Ott, J. Lucero, O. Mendez, H. Liu, and C. Worne. This study was supported by an NIH grant (U01-NS079420), the CDRF (AAC-2005), and a canceled Sponsored Research Agreement (STEM-1000643) from StemCells Inc. This work was supported, in part, by a Sponsored Research Agreement (STEM-1000634) from StemCells Inc. to A.J.A. and B.J.C. However, this Sponsored Research Agreement was canceled during the collection/analysis of the data.
Published: February 14, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.12.018.
Supplemental Information
References
- Anderson A.J., Cummings B.J. Achieving informed consent for cellular therapies: a preclinical translational research perspective on regulations versus a dose of reality. J. Law Med. Ethics. 2016;44:394–401. doi: 10.1177/1073110516667937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.J., Haus D.L., Hooshmand M.J., Perez H., Sontag C.J., Cummings B.J. Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient? Regen. Med. 2011;6:367–406. doi: 10.2217/rme.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanian V.L., Schnell L., Lou L., Golshani R., Hunanyan A., Ghosh A., Pearse D.D., Robinson J.K., Schwab M.E., Fawcett J.W., Mendell L.M. Chronic spinal hemisection in rats induces a progressive decline in transmission in uninjured fibers to motoneurons. Exp. Neurol. 2009;216:471–480. doi: 10.1016/j.expneurol.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Biologics Evaluation and Research . US Department of Health and Huma Services, US Food and Drug Administration and Center for Biologics Evaluation and Research, Office of Communication, Outreach, and Development (OCOD), US Government; 2013. Guidance for Industry - Preclinical Assessment of Investigational Cellular and Gene Therapy Products; p. 35. [Google Scholar]
- Chang J., Phelan M., Cummings B.J. A meta-analysis of efficacy in pre-clinical human stem cell therapies for traumatic brain injury. Exp. Neurol. 2015;273:225–233. doi: 10.1016/j.expneurol.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Chinnadurai R., Ng S., Velu V., Galipeau J. Challenges in animal modelling of mesenchymal stromal cell therapy for inflammatory bowel disease. World J. Gastroenterol. 2015;21:4779–4787. doi: 10.3748/wjg.v21.i16.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher and Dana Reeve Foundation (2008). One degree of separation. https://www.christopherreeve.org/living-with-paralysis/stats-about-paralysis.
- Cummings B.J., Uchida N., Tamaki S.J., Salazar D.L., Hooshmand M., Summers R., Gage F.H., Anderson A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel R., Schindehutte J., Kuhlmann T., Elsner L., Novota P., Baier P.C., Schillert A., Bickeboller H., Herrmann T., Trenkwalder C. The tumorigenicity of mouse embryonic stem cells and in vitro differentiated neuronal cells is controlled by the recipients' immune response. PLoS One. 2008;3:e2622. doi: 10.1371/journal.pone.0002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J.W., Curt A., Steeves J.D., Coleman W.P., Tuszynski M.H., Lammertse D., Bartlett P.F., Blight A.R., Dietz V., Ditunno J. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- Galipeau J. The mesenchymal stromal cells dilemma–does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Gupta N., Henry R.G., Strober J., Kang S.M., Lim D.A., Bucci M., Caverzasi E., Gaetano L., Mandelli M.L., Ryan T. Neural stem cell engraftment and myelination in the human brain. Sci. Transl. Med. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers F.P., Koopmans G.C., Joosten E.A. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma. 2006;23:537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- Henderson V.C., Kimmelman J., Fergusson D., Grimshaw J.M., Hackam D.G. Threats to validity in the design and conduct of preclinical efficacy studies: a systematic review of guidelines for in vivo animal experiments. PLoS Med. 2013;10:e1001489. doi: 10.1371/journal.pmed.1001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter C.P., Holmstrom N.A., Lilja J.A., Schweinhardt P., Hao J., Spenger C., Wiesenfeld-Hallin Z., Kurpad S.N., Frisen J., Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Hooshmand M.J., Sontag C.J., Uchida N., Tamaki S., Anderson A.J., Cummings B.J. Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: correlation of engraftment with recovery. PLoS One. 2009;4:e5871. doi: 10.1371/journal.pone.0005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle J.D., Tessler A. Repair of chronic spinal cord injury. Exp. Neurol. 2003;182:247–260. doi: 10.1016/s0014-4886(03)00029-3. [DOI] [PubMed] [Google Scholar]
- Hyun I., Lindvall O., Ahrlund-Richter L., Cattaneo E., Cavazzana-Calvo M., Cossu G., De Luca M., Fox I.J., Gerstle C., Goldstein R.A. New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell. 2008;3:607–609. doi: 10.1016/j.stem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Katz R. FDA: evidentiary standards for drug development and approval. NeuroRx. 2004;1:307–316. doi: 10.1602/neurorx.1.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C., Hofstetter H.H., Meuth S.G., Braeuninger S., Sommer C., Stoll G. T cell infiltration after chronic constriction injury of mouse sciatic nerve is associated with interleukin-17 expression. Exp. Neurol. 2006;200:480–485. doi: 10.1016/j.expneurol.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Krassioukov A., Claydon V.E. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog. Brain Res. 2006;152:223–229. doi: 10.1016/S0079-6123(05)52014-4. [DOI] [PubMed] [Google Scholar]
- Kwon B.K., Soril L.J., Bacon M., Beattie M.S., Blesch A., Bresnahan J.C., Bunge M.B., Dunlop S.A., Fehlings M.G., Ferguson A.R. Demonstrating efficacy in preclinical studies of cellular therapies for spinal cord injury - how much is enough? Exp. Neurol. 2013;248:30–44. doi: 10.1016/j.expneurol.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Lapchak P.A. Scientific Rigor recommendations for optimizing the clinical applicability of translational research. J. Neurol. Neurophysiol. 2012;3:e111. doi: 10.4172/2155-9562.1000e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo B., Kriegstein A., Grady D. Clinical trials in stem cell transplantation: guidelines for scientific and ethical review. Clin. Trials. 2008;5:517–522. doi: 10.1177/1740774508096705. [DOI] [PubMed] [Google Scholar]
- Lucin K.M., Sanders V.M., Jones T.B., Malarkey W.B., Popovich P.G. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp. Neurol. 2007;207:75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias M.Y., Syring M.B., Pizzi M.A., Crowe M.J., Alexanian A.R., Kurpad S.N. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp. Neurol. 2006;201:335–348. doi: 10.1016/j.expneurol.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Marsh S., Yeung S., Torres M., Lau L., Davis J., Capela A., Monuki E., Poon W., Blurton-Jones M. Human neural stem cells fail to terminally differentiate, cause tumor-like growths, and provide no cognitive benefits in an immune-deficient transgenic model of Alzheimer's disease. Stem Cell Rep. 2017;8:235–248. doi: 10.1016/j.stemcr.2016.12.019. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem G., Xu K., Yu L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience. 2004;129:767–777. doi: 10.1016/j.neuroscience.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Moller D.R. Negative clinical trials in sarcoidosis: failed therapies or flawed study design? Eur. Respir. J. 2014;44:1123–1126. doi: 10.1183/09031936.00156314. [DOI] [PubMed] [Google Scholar]
- Nishi R.A., Liu H., Chu Y., Hamamura M., Su M.Y., Nalcioglu O., Anderson A.J. Behavioral, histological, and ex vivo magnetic resonance imaging assessment of graded contusion spinal cord injury in mice. J. Neurotrauma. 2007;24:674–689. doi: 10.1089/neu.2006.0204. [DOI] [PubMed] [Google Scholar]
- Pawar K., Cummings B.J., Thomas A., Shea L.D., Levine A., Pfaff S., Anderson A.J. Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: association with recovery of forelimb function. Biomaterials. 2015;65:1–12. doi: 10.1016/j.biomaterials.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin S. Preclinical research: make mouse studies work. Nature. 2014;507:423–425. doi: 10.1038/507423a. [DOI] [PubMed] [Google Scholar]
- Piltti K.M., Salazar D.L., Uchida N., Cummings B.J., Anderson A.J. Safety of epicenter versus intact parenchyma as a transplantation site for human neural stem cells for spinal cord injury therapy. Stem Cells Transl. Med. 2013;2:204–216. doi: 10.5966/sctm.2012-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltti K.M., Salazar D.L., Uchida N., Cummings B.J., Anderson A.J. Safety of human neural stem cell transplantation in chronic spinal cord injury. Stem Cells Transl. Med. 2013;2:961–974. doi: 10.5966/sctm.2013-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltti K.M., Avakian S.N., Funes G.M., Hu A., Uchida N., Anderson A.J., Cummings B.J. Transplantation dose alters the dynamics of human neural stem cell engraftment, proliferation and migration after spinal cord injury. Stem Cell Res. 2015;15:341–353. doi: 10.1016/j.scr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.F., Fischhoff M.A., Sakowski S.A., Feldman E.L. Perspective: transforming science into medicine: how clinician-scientists can build bridges across research's “valley of death”. Acad. Med. 2012;87:266–270. doi: 10.1097/ACM.0b013e3182446fa3. [DOI] [PubMed] [Google Scholar]
- Salazar D.L., Uchida N., Hamers F.P., Cummings B.J., Anderson A.J. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One. 2010;5:e12272. doi: 10.1371/journal.pone.0012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden N.R., Al-Uzri A., Huhn S.L., Koch T.K., Sikora D.M., Nguyen-Driver M.D., Guillaume D.J., Koh J.L., Gultekin S.H., Anderson J.C. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. J. Neurosurg. Pediatr. 2013;11:643–652. doi: 10.3171/2013.3.PEDS12397. [DOI] [PubMed] [Google Scholar]
- Sena E.S., van der Worp H.B., Bath P.M., Howells D.W., Macleod M.R. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag C.J., Nguyen H.X., Kamei N., Uchida N., Anderson A.J., Cummings B.J. Immunosuppressants affect human neural stem cells in vitro but not in an in vivo model of spinal cord injury. Stem Cells Transl. Med. 2013;2:731–744. doi: 10.5966/sctm.2012-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag C.J., Uchida N., Cummings B.J., Anderson A.J. Injury to the spinal cord niche alters the engraftment dynamics of human neural stem cells. Stem Cell Rep. 2014;2:620–632. doi: 10.1016/j.stemcr.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes B.T., Jakeman L.B. Experimental modelling of human spinal cord injury: a model that crosses the species barrier and mimics the spectrum of human cytopathology. Spinal Cord. 2002;40:101–109. doi: 10.1038/sj.sc.3101254. [DOI] [PubMed] [Google Scholar]
- van der Worp H.B., Howells D.W., Sena E.S., Porritt M.J., Rewell S., O'Collins V., Macleod M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Guan Z., Reader B., Shawler T., Mandrekar-Colucci S., Huang K., Weil Z., Bratasz A., Wells J., Powell N.D. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J. Neurosci. 2013;33:12970–12981. doi: 10.1523/JNEUROSCI.1974-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.