Abstract
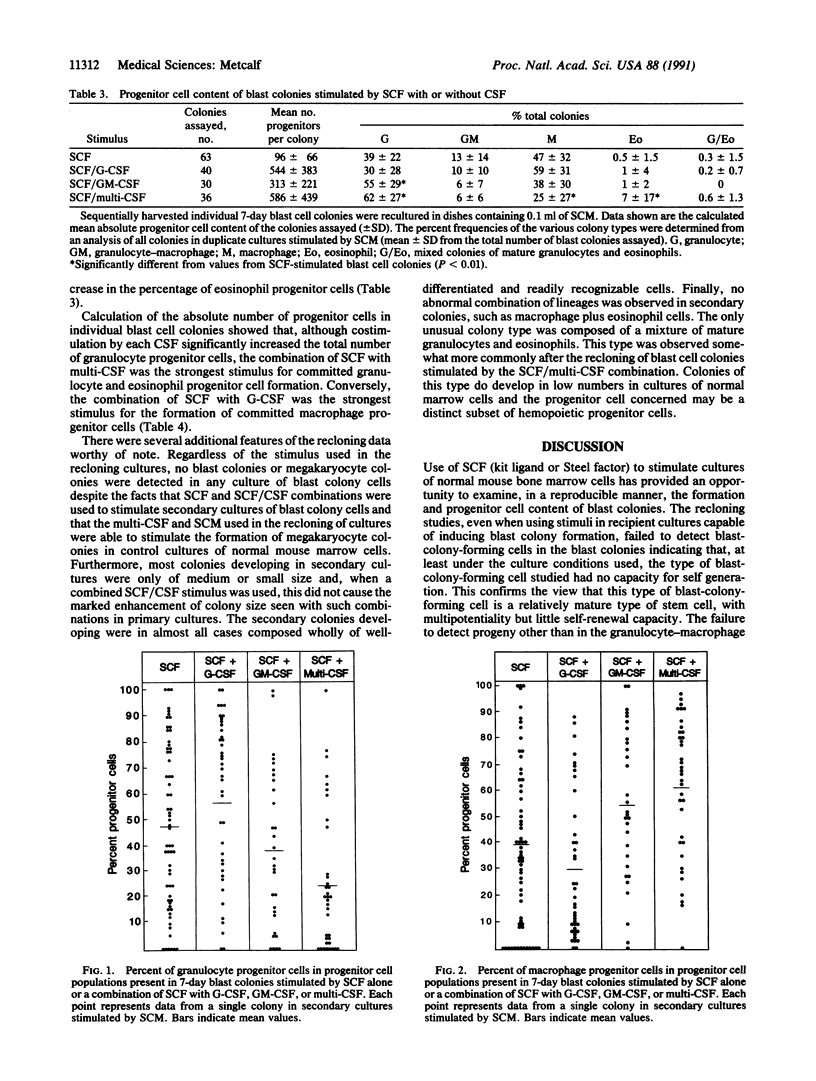
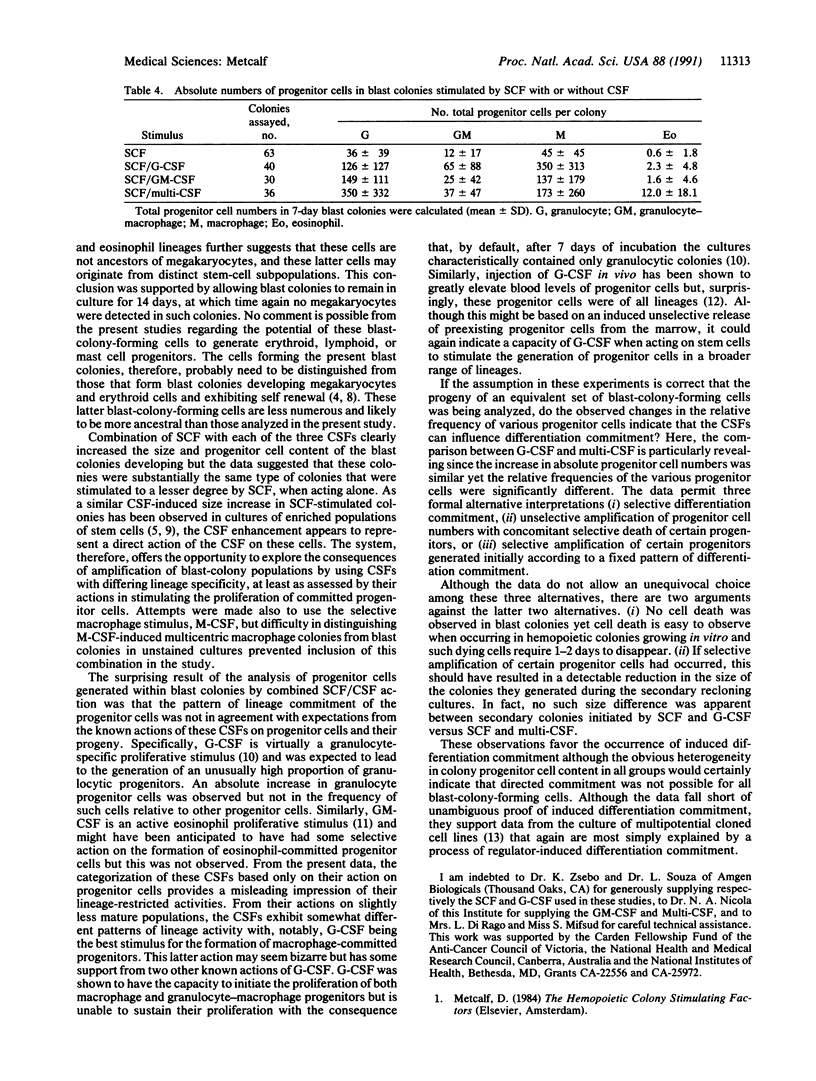
In clonal cultures of normal mouse marrow cells, combination of granulocyte, granulocyte-macrophage, or multipotential colony-stimulating factor (G-CSF, GM-CSF, or multi-CSF, respectively) with stem cell factor (SCF) did not alter the number of blast colonies stimulated to develop compared with SCF alone but induced an up to 25-fold increase in their mean cell content and an up to 6-fold increase in their mean progenitor cell content. Costimulation of blast colony formation by SCF plus G-CSF did not change the relative frequency of progenitor cells of different types within the colonies compared with colonies stimulated by SCF alone. However, combination of GM-CSF or multi-CSF with SCF significantly increased the relative frequency of granulocytic progenitors and, for multi-CSF, also of eosinophil progenitor cells. These changes in the relative frequencies of progenitor cells committed to the various lineages support the hypothesis that hemopoietic regulators have some ability to induce selective lineage commitment in the progeny of multipotential cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. M., Lyman S. D., Baird A., Wignall J. M., Eisenman J., Rauch C., March C. J., Boswell H. S., Gimpel S. D., Cosman D. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990 Oct 5;63(1):235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- Bernstein I. D., Andrews R. G., Zsebo K. M. Recombinant human stem cell factor enhances the formation of colonies by CD34+ and CD34+lin- cells, and the generation of colony-forming cell progeny from CD34+lin- cells cultured with interleukin-3, granulocyte colony-stimulating factor, or granulocyte-macrophage colony-stimulating factor. Blood. 1991 Jun 1;77(11):2316–2321. [PubMed] [Google Scholar]
- Dührsen U., Villeval J. L., Boyd J., Kannourakis G., Morstyn G., Metcalf D. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988 Dec;72(6):2074–2081. [PubMed] [Google Scholar]
- Heyworth C. M., Dexter T. M., Kan O., Whetton A. D. The role of hemopoietic growth factors in self-renewal and differentiation of IL-3-dependent multipotential stem cells. Growth Factors. 1990;2(2-3):197–211. doi: 10.3109/08977199009071506. [DOI] [PubMed] [Google Scholar]
- Keller G. M., Phillips R. A. Detection in vitro of a unique, multipotent hemopoietic progenitor. J Cell Physiol Suppl. 1982;1:31–36. doi: 10.1002/jcp.1041130408. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Burgess A. W., Johnson G. R., Nicola N. A., Nice E. C., DeLamarter J., Thatcher D. R., Mermod J. J. In vitro actions on hemopoietic cells of recombinant murine GM-CSF purified after production in Escherichia coli: comparison with purified native GM-CSF. J Cell Physiol. 1986 Sep;128(3):421–431. doi: 10.1002/jcp.1041280311. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Direct proliferative actions of stem cell factor on murine bone marrow cells in vitro: effects of combination with colony-stimulating factors. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6239–6243. doi: 10.1073/pnas.88.14.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hemopoietic cells. J Cell Physiol. 1983 Aug;116(2):198–206. doi: 10.1002/jcp.1041160211. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The Florey Lecture, 1991. The colony-stimulating factors: discovery to clinical use. Philos Trans R Soc Lond B Biol Sci. 1991 Jul 29;333(1266):147–173. doi: 10.1098/rstb.1991.0065. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3843–3847. doi: 10.1073/pnas.79.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M., Ihle J. N. Permissive role of interleukin 3 (IL-3) in proliferation and differentiation of multipotential hemopoietic progenitors in culture. J Cell Physiol. 1985 Aug;124(2):182–190. doi: 10.1002/jcp.1041240203. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Wypych J., McNiece I. K., Lu H. S., Smith K. A., Karkare S. B., Sachdev R. K., Yuschenkoff V. N., Birkett N. C., Williams L. R. Identification, purification, and biological characterization of hematopoietic stem cell factor from buffalo rat liver--conditioned medium. Cell. 1990 Oct 5;63(1):195–201. doi: 10.1016/0092-8674(90)90300-4. [DOI] [PubMed] [Google Scholar]



