Abstract
Introduction
The ease of imaging the retinal vasculature, and the evolving evidence suggesting this microvascular bed might reflect the cerebral microvasculature, presents an opportunity to investigate cerebrovascular disease and the contribution of microvascular disease to dementia with fundus camera imaging.
Methods
A systematic review and meta-analysis was carried out to assess the measurement of retinal properties in dementia using fundus imaging.
Results
Ten studies assessing retinal properties in dementia were included. Quantitative measurement revealed significant yet inconsistent pathologic changes in vessel caliber, tortuosity, and fractal dimension. Retinopathy was more prevalent in dementia. No association of age-related macular degeneration with dementia was reported.
Discussion
Inconsistent findings across studies provide tentative support for the application of fundus camera imaging as a means of identifying changes associated with dementia. The potential of fundus image analysis in differentiating between dementia subtypes should be investigated using larger well-characterized samples. Future work should focus on refining and standardizing methods and measurements.
Keywords: Fundus, Dementia, Alzheimer's disease, Retinal imaging, Retinal vasculature
1. Introduction
Dementia poses a major global medical, economic, and public health challenge [1], [2], [3]. Given this worldwide burden there is currently great interest in finding early and easily accessible biomarkers of dementia to ultimately aid prevention. An ideal biomarker for dementia screening should be reliable, predictive, reproducible, noninvasive, simple to perform, and inexpensive [4]. Novel biomarkers, including structural and functional neuroimaging, genetic factors, and biochemical analysis of blood and cerebrospinal fluid, have been examined. Despite this research focus, there remains an ongoing need for sensitive biomarkers for dementia. Increasingly, studies have found evidence that cerebrovascular disease and systemic vascular factors such as type 2 diabetes and hypertension are associated with increased risk of dementia [5], [6]. Alzheimer's disease (AD), the most common form of dementia, is known to have a vascular component with small-vessel disease, microinfarction, and cerebral amyloid angiopathy contributing to the pathogenesis [6], [7]. Despite the evidence of a vascular component, difficulties in directly visualizing the cerebral microvasculature in vivo have hindered efforts to demonstrate the involvement of cerebral vessels in dementia.
Anatomically and developmentally, the retina is an extension of the brain [8]. Because of the homology between the retinal and cerebral microvasculature [9], the retinal vasculature has potential to be used as a proxy measure whereby the condition of retinal vessels may reflect the condition of the cerebral vasculature. This has distinct advantages because of the ease with which the retina can be noninvasively visualized and photographed, offering a “window” to study brain microvascular and neuronal pathology [10], [11]. Different retinal imaging modalities, such as fundus camera imaging and optical coherence tomography (OCT), to measure changes in retinal nerve fiber layer and retinal ganglion cell loss, and fluorescein angiography have been applied in the management and research of systemic diseases. Advancements in retinal imaging technology have led to promising findings, particularly OCT where a recent review demonstrated that the measurement of retinal nerve fiber layer thickness, as a reflection of axonal loss, provides a promising method to aid in the diagnosis of various neurodegenerative diseases, including AD [12]. Although all imaging modalities merit further study, this review chose to focus on the use of fundus camera imaging.
Retinal microvascular abnormalities in relation to cognitive dysfunction and dementia have been described in review articles previously [13], [14], [15]. These reviews found evidence to support the hypothesis that retinal microvascular abnormalities are associated with dementia [14], [15] or cognitive impairment/dementia both in diabetic patients and the general population [13]. Retinal abnormalities were most consistently associated with poorer verbal memory, information processing speed, and executive function in population-based samples of middle age and older people [13]. Heringa et al. [14] reported stronger associations between retinal microvascular changes and dementia in cross-sectional studies (odds ratio [OR] range, 1.17–5.57) than in longitudinal studies where no consistent associations between retinal morphology and dementia or cognitive impairment were found (OR and hazard ratio [HR] range, 0.77–1.55). Cheung et al. [15] noted that although various studies have found an association between retinal vascular changes and dementia, the results across these studies were variable. The findings were inconclusive because of heterogeneity of study design in terms of retinal parameters, imaging methods, and outcomes. These previous reviews have examined the extent to which retinal properties relate to cognitive ability and dementia [13], [14], [15]. To our knowledge, no comprehensive review has been published on the specific utility of fundus camera imaging as a method of identifying and measuring a wide range of retinal changes, specific to dementia and its various subtypes. For the purposes of this review, we define fundus imaging as the use of fundus camera photography to measure, observe, and quantify microvascular retinal features and abnormalities.
The direct visualization of the retina using fundus imaging offers an opportunity to assess the potential for abnormalities and changes in retinal microvasculature to serve as biomarkers of microvascular pathology in subtypes of dementia. Fundus photography, with high sensitivity, specificity, and interexamination and intraexamination agreement [16], is typically used to determine three different types of retinal properties: retinopathy, variation in vessel caliber, and changes in the global geometric branching network [17]. Furthermore, the digital output from modern camera systems lends itself to image processing methods for computer-assisted programs to objectively quantify important features of the retina and its vasculature with increasing accuracy and reliability [18]. We aimed to conduct a systematic review of the literature to examine the application of fundus camera imaging and analysis in dementia, including AD, vascular dementia (VaD), frontotemporal dementia, and dementia with Lewy bodies.
2. Methods
2.1. Search strategy
Published studies were identified through systematic searches of the Medical Literature Analysis and Retrieval System Online (MEDLINE, including work in progress from 1946), PubMed (from 1950), and the Excerpta Medica Database (EMBASE, from 1980) for all human studies published until March 2016, in all languages. Search filters included were keyword, title, and abstract information. The Medical Subject Heading search terms were “retina,” or “fundus,” or “retinal vasculature,” or “retinal microvasculature,” or “retinal vascular,” or “retinal vessel,” or “retinopathy” and in combination with “dementia,” or “Alzheimer,” or “Lewy bodies,” or “cognition,” or “cognitive”. Articles with any combination of any of the retinal terms and any dementia or cognition term were reviewed. We also searched Google Scholar for all studies published before and including March 2016. References of relevant articles were hand-searched and a forward citation search was performed to identify further studies.
2.2. Inclusion and exclusion criteria
This review aimed to include all published studies applying fundus camera imaging to examine the association between retinal vasculature/retinopathy and any form of dementia. Inclusion criteria were (1) original study; (2) written in English; (3) assessment of retinal parameters using fundus imaging; (4) diagnosis of AD, frontotemporal dementia, dementia with Lewy bodies, or VaD; and (5) diagnosis of dementia based on established criteria such as the National Institute of Neurological, Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association [19].
The following studies were excluded: (1) review studies; (2) single-case reports; (3) nonhuman research; (4) non-English language studies; (5) conference presentations or summaries; (6) studies without details of dementia diagnosis criteria; and (7) studies examining retinal integrity through a method other than fundus photography, for example, a laser Doppler instrument or OCT.
2.3. Data extraction
All identified studies were screened by title and abstract by two independent reviewers. Irrelevant and duplicate articles were removed, and the remaining articles were assessed for agreement with the inclusion and exclusion criteria by full-text review (Fig. 1). Data extracted from studies at this stage included title, year of publication, authors, study aim, study type, number of patients and control subjects, mean age, diagnostic criteria, participant selection criteria, method of fundus imaging and image analysis used, results, and conclusions.
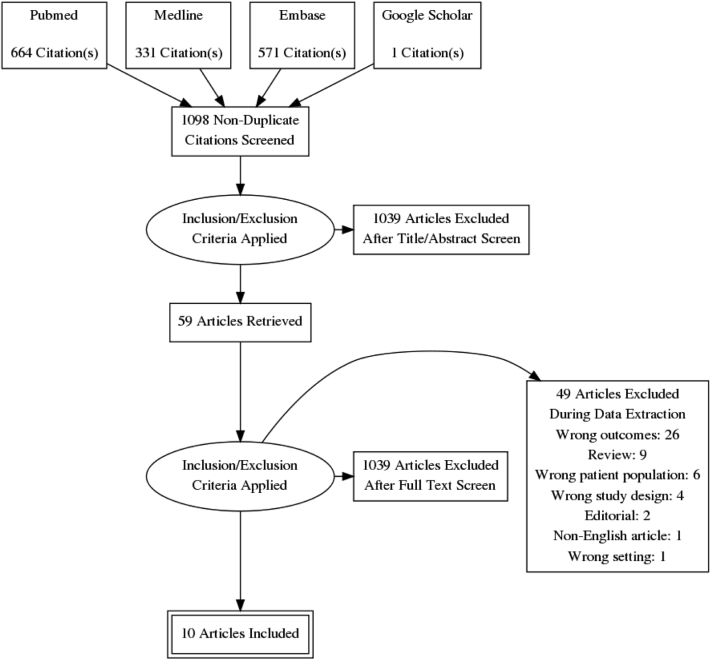
Fig. 1.
Flow diagram for manuscript selection.
2.4. Statistical analysis
Review Manager Software Version 5.3 (Cochrane, Oxford) and R v. 2.15.2 were used for the meta-analysis of continuous and categorical outcomes, calculating the summary estimates including 95% confidence intervals (CIs). Extracted data (means, standard deviations [SD], and sample sizes) were used to calculate the mean difference (MD) using an inverse variance random-effects model. A random-effects generic inverse variance method was used to plot summary odds ratios (sORs) and 95% CIs of adjusted ratios for categorical measures. We tested for heterogeneity between study results with the χ2 test for heterogeneity with an alpha level for significance set at P = .05. Stratified analysis was carried out on dementia subgroups where possible.
3. Results
One thousand five hundred sixty-six studies were identified in the literature search. One additional study was identified from Google Scholar. Four hundred sixty-nine were duplicates and were therefore removed. The remaining 1098 were screened by title and abstract only. Of these, 59 were considered to be potentially relevant and were assessed by full-text review.
Ten studies met the inclusion criteria. The populations sampled came from the US (2), UK (2), Iceland (1), the Netherlands (3), Australia (1), and Singapore (1). Multiple articles from the same study population were included only if different retinal properties or outcome measures were examined in separate articles. Table 1 describes the characteristics of the studies reviewed.
Table 1.
Included studies using fundus imaging methods to examine retinal associations with dementia
| Study | Study design and total sample size | Dementia outcome number of cases | Mean (SD) age∗ | Male (%) | Retinal measures | Type of fundus/camera model | Software/grading | One/both eyes | Region measured | Statistical analysis | Additional adjustments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Klaver et al. [20]; Rotterdam Study | Prospective population-based study. (n = 1438) | AD (n = 62 incident cases) | No AMD = 80.5 (4.4); stage 1 = 80 (4.0); stage 2 = 81.3 (4.5); stage 3 = 82.2 (5.6); stage 4 = 84.5 (4.8) | No AMD = 35.6%; stage 1 = 32.1%; stage 2 = 36.4%; stage 3 = 32.6%; stage 4 = 31.3% | AMD | Topcon TRV-50VT fundus camera, 35° field | Grading of fundus transparencies according to the international classification system | Not reported | Macular area | Cox proportional hazards regression analysis | Age, sex, smoking, atherosclerosis, APOE ε4 carrier status |
| Baker et al. [21]; Cardiovascular Health Study | Population-based cross-sectional study (n = 2211) | AD (n = 99); VaD (n = 11); mixed AD VaD (n = 49); other types (n = 5) | 78 | 40% | Retinopathy; AVN; FAN; retinal vascular caliber | Canon CR-45UAF, nonmydriatic fundus camera, 45° field | Observer graded. Caliber was measured and summarized | One (50% right, 50% left) | Centered between the OD and macula. Caliber measured one disc diameter from the OD margin | ANCOVA; logistic regression | Age, sex, race, field center, education, internal carotid intimamedia thickness, weight, hypertension, diabetes, smoking, cerebral MRI signs |
| Baker et al. [22]; Cardiovascular Health Study | Population-based cross-sectional study (n = 2088) | Dementia (n = 135); AD (n = 86) | With AMD: 80 (4.7); no AMD: 78 (4.2) | With AMD: 41%; no AMD 39.7% | AMD | Canon CR-45UAF, nonmydriatic fundus camera, 45° field | Observer graded. | One (50% right, 50% left) | Grading was performed by the superimposition of a circular grid over the macular area | Logistic regression | Age, sex, ethnicity, study center, education, systolic BP, total cholesterol level, diabetes, smoking, APOE status based on six common genotypes (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4, ε4/ε4) |
| Qiu et al. [23]; AGES-Reykjavik Study | Population-based cross-sectional study (n = 3906) | AD (n = 66); VaD (n = 31); possible AD and VaD (n = 20) | 76 | 42% | Retinopathy; AVN; FAN, | 6.3-Megapixel Canon CR6 nonmydriatic camera; 45° field | Observer graded | Both | Two images, centered on the OD and the macula | Linear regression; logistic regression | Age, sex, education, visual acuity, depressive symptoms, smoking, hypertension, diabetes, BMI, use of anticoagulants, brain infarcts, load of subcortical and periventricular white matter hyperintensities, cerebral microbleeds |
| de Jong et al. [24]; Rotterdam Study | Population-based prospective study (n = 5553) | Incident dementia: AD (n = 519); VaD (n = 73); other subtypes (n = 63) | 68 | 41% | CRVE; CRAE | Topcon, 20° field | Retinal Vessel Measurement System | One (best quality image of either eye) | Centered on the OD | Cox proportional hazards models | Age, sex, systolic BP, antihyperintensive medication, serum total cholesterol, serum C-reactive protein, smoking, diabetes mellitus, coronary heart disease, stroke, CRAE/CRVE adjusted for fellow vessel caliber |
| Schrijvers et al. [25]; Rotterdam Study | Population-based cross-sectional and prospective study (n = 6078) | AD (n = 149); VaD (n = 29); other subtypes (n = 17) | 69 | 41% | Retinopathy | Topcon TRV-50VT, 35° field | Observer graded | Both | Centered on the macula |
Logistic regression; Cox proportional hazards models | Age, sex, stroke, systolic BP, use of antihypertensive medication, education, smoking, diabetes, total cholesterol, C-reactive protein, coronary heart disease, APOE ε4 carrier status |
| Frost et al. [26]; Australian Imaging, Biomarkers and Lifestyle (AIBL) Flagship Study of Ageing | Case-control study (n = 25:123) | AD (n = 25) | Control: 71.6; AD: 72.4 | Control: 45%; AD: 48% | CRAE; CRVE; AVR; FD; BSTD; BC; AF; JE; LDR; tortuosity; Num1stB | Canon CR-1 nonmydriatic camera, 45° field | SIVA | Not reported | Centered on OD; 0.5–1.0 disc diameters or 0.5–2.0 disc diameters away from the disc margin | ANCOVA; receiver-operating characteristic (ROC) curve analysis | Age, sex, hypertension, diabetes, smoking, APOE ε4 carrier status |
| Cheung et al. [10] Singapore Epidemiology of Eye Disease (SEED) program |
Case-control study (n = 136:290) | AD (n = 136) | Control: 73.9; AD: 74.8 | Control: 53%; AD: 47% | CRAE; CRVE; FD; tortuosity; BA | Canon CR-DGi 10D or Canon CR-1 40D, 45° field | SIVA | Both | Two images of each eye: one centered at the OD and the other centered at the fovea. Measurements taken between 0.5 and 2.0 disc diameters away from the disc margin | Independent t test or χ2 test; logistic regression | Age, sex, ethnicity, smoking, hypertension, hypercholesterolemia, diabetes, history of myocardial infarction, CRAE/CRVE adjusted for fellow vessel caliber |
| Williams et al. [27] | Case-control study (n = 258:322) | AD (n = 258) | Control: 76.6; AD: 80.1 | Control: 39%; AD: 37% | AMD | Slit-lamp mounted Canon CR-DGi | Observer graded | One (grade from worst eye or from only gradable image) | 6000 μm AREDS grid centered on fovea | Logistic regression | Age, smoking, recent illness, APOE ε4 carrier status |
| Williams et al. [28] | Case-control study (n = 213:294) | AD (n = 213) | Control: 76.3; AD: 79.6 | Control: 40%; AD: 36% | CRAE; CRVE; FD; tortuosity BA | 500 Canon CR-DGi | SIVA | One (right image were available, otherwise left) | 0.5 and 2.0 disc diameters away from the disc margin | independent t test or χ2 test; logistic regression | Age, sex, mean arterial BP, smoking, hypercholesterolemia, diabetes mellitus, history of cardiovascular disease, cerebrovascular disease, medications (aspirin/clopidogrel, beta blockers, calcium channel blockers, diuretics, nonsteroidal antiinflammatory drugs, thyroxine), CRAE/CRVE adjusted for fellow vessel caliber |
Abbreviations: AD, Alzheimer's disease; AF, asymmetry factor; AMD, age-related macular degeneration; ANCOVA, analysis of covariance; APOE, apolipoprotein E ε4 carriership defined as the presence of at least one APOE ε4 allele; AREDS, Age-Related Eye Disease Study; AVN, arteriovenous nicking; AVR, arteriovenous ratio; BA, branching angle; BC, branching coefficient; BMI, body mass index; BP, blood pressure; BSTD, standard deviation of vessel width in Zone B; CRAE/CRVE, central retinal arterial/venular equivalent; FAN, focal arteriolar narrowing; FD, fractal dimension; JE, Junctional exponent deviation; LDR, length to diameter ratio; MRI, magnetic resonance; Num1stB, number of first branching vessels in zone C; OD, optic disc; SD, standard deviation; SIVA, Singapore I Vessel Assessment; VaD, vascular dementia.
For longitudinal studies age at baseline.
3.1. Study design and population
The 10 studies included comprised three prospective cohort studies [20], [24], [25], three population-based cross-sectional studies [21], [22], [23], and four case-control studies [10], [26], [27], [28]. Although several articles had overlapping samples, each article assessed different retinal features. These articles were therefore all included. Across the 10 studies the number of unique participants (i.e., without overlap across studies) was 13,349. The number of dementia cases per article varied from 25 to 655 [24], [28]. Table 1 describes the characteristics of the studies reviewed.
3.2. Measurement
Studies assessed retinal parameters through visual grading or with the application of computer-assisted programs. Retinopathy, arteriovenous nicking (AVN), focal arteriolar narrowing (FAN), and age-related macular degeneration (AMD) were visually graded. Definitions and grading of retinopathy differed across studies. Retinopathy was identified by three ophthalmologically trained physicians in the Rotterdam Study by the presence of one or more dot/blot hemorrhages, microaneurysms, or cotton wool spots or evidence of laser treatment for retinopathy [25]. The Cardiovascular Health Study examined images for signs of microaneurysms, retinal hemorrhages, cotton wool spots, hard exudates, macular edema, intraretinal microvascular abnormalities, venous beading, new vessels at the disc or elsewhere, and vitreous hemorrhage [21]. Retinopathy was identified by three certified graders in the Age Gene/Environment Susceptibility (AGES)-Reykjavik Study on the basis of presences of retinal blot hemorrhages, microaneurysms, soft exudates, and other less common lesions such as hard exudates, macular edema, and optic disc swelling [23]. AMD was identified and graded according to the international classification grading systems [29], [30] by Baker et al. [22] and Williams et al. [27].
Quantitative retinal measurements performed using computer-assisted methods included central retinal arterial equivalent (CRAE); central retinal venular equivalent (CRVE); arteriovenous ratio (AVR); SD of vessel width in zone B (BSTD); length to diameter ratio (LDR); curvature tortuosity; bifurcation angle; junctional exponent deviation; fractal dimension (FD); number of first branching arterioles; branching coefficient (BC); and asymmetry factor. Most studies assessed vessel width as continuous variables. See Table 2 for definitions of retinal parameter terminology.
Table 2.
Retinal parameters assessed by reviewed studies
| Retinal measure | Description | Study |
|---|---|---|
| Age-related macular degeneration (AMD) | Degenerative disorder of the macula characterized generally by extensive drusen, often associated with pigmentary abnormalities (Coleman et al., 2008∗) | Klaver et al. [20]; Baker et al. [22]; Williams et al. [27] |
| Retinopathy | Disease of the retina that results in impairment or loss of vision-symptoms include microaneurysms, hemorrhages, hard exudates, and cotton wool spots | Baker et al. [21]; Qiu et al. [23]; Schrijvers et al. [25] |
| Focal arteriolar narrowing (FAN) | Presence of localized areas of arteriolar constriction—definite: arteriole ≥40 μm in diameter and ≥250 μm in length, with caliber of constricted area ≤1/2 that of proximal and distal segment; probable: constricted vessel <40 μm in diameter or <250 μm in length (Qiu et al., 2009†) | Baker et al. [21]; Qiu et al. [23] |
| Arteriovenous nicking | Arteriole crossing a venule resulting in the compression of the venule with bulging on either side of the crossing. Definite: tapering or narrowing of the venule on three or four sides of the crossing; probable: narrowing on only two sides of the crossing (Qiu et al., 2009†) | Baker et al. [21]; Qiu et al. [23] |
| Central retinal arteriolar equivalent caliber (CRAE) | Summary measures of vascular equivalent caliber representing the equivalent single-vessel parent width for the six largest arterioles and venules. Based on the Knudston-Parr-Hubbard formula (Knudston et al., 2003∗∗) | de Jong et al. [24]; Frost et al. [26]; Cheung et al., [10]; Williams et al. [28] |
| Central retinal venular equivalent (CRVE) caliber | ||
| Arteriovenous ratio (AVR) | The ratio between summarized arteriolar caliber measurement (CRAE) with respect to the summarized venular caliber (CRVE). CRAE/CRVE = AVR | Frost et al. [26] |
| Fractal dimension of arteriolar network (FDa) | Global summary measure of branching complexity of the retinal vascular tree reflecting how thoroughly the branching pattern fills two-dimensional spaces. Larger values represent a more complex pattern. Calculated from the skeletonized line tracing using the box-counting method | Frost et al. [26]; Cheung et al., [10]; Williams et al. [28] |
| Fractal dimension of venular network (FDv) | ||
| Curvature tortuosity arteriole | Integral of the curvature squared along the vessel path, normalized by the total path length. Measurements are summarized to represent the average tortuosity of arterioles and venules separately with smaller values reflecting straighter vessels | Frost et al. [26]; Cheung et al., [10]; Williams et al. [28] |
| Curvature tortuosity venule | ||
| Number of first branching arterioles | The number of arterioles and venules with a first bifurcation, branch, or daughter vessel, in zone C | Frost et al. [26] |
| Number of first branching venules | ||
| Branching coefficient arteriole | Calculated from average Num1st (number of first branching vessels) measurements. Branching coefficient reflects the relationship between parent vessels and branches. Defined as the summed square of the mean vessel widths of each branch or daughter vessel divided by the square of the mean width of the parent vessel | Frost et al. [26] |
| Branching coefficient venule | ||
| Junctional exponent deviation for arterioles | Calculated from average Num1st measurements. Junctional exponent deviation reflects the deviation from the optimum ratio of vessel widths at a bifurcation | Frost et al. [26] |
| Junctional exponent deviation for venules | ||
| Length to diameter ratio arteriole (LDRa) | Length to diameter ratio is the length of the vessel from the midpoint of one bifurcation to the midpoint of the next bifurcation. It is expressed as a ratio to the diameter of the parent vessel at the first bifurcation | Frost et al. [26] |
| Length to diameter ratio venular (LDRv) |
Coleman HR, Chan CC, Ferris FL, Chew EY. Age-related macular degeneration. The Lancet 2003;372 (9652):1835–45.
Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Current Eye Research 2003; 27(3): 143–149
Qiu C, Cotch MF, Sigurdsson S, Klein R, Jonasson F, Klein BE, et al. Microvascular lesions in the brain and retina: the age, gene/environment susceptibility—Reykjavik Study. Annals of Neurology 2009;65 (5):569–76.
Retinal photographs were taken after pharmacological pupil dilation, except in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing [26] where images were taken using a nonmydriatic camera in a darkened room and in the Cardiovascular Health Study [21], [22] in which images were taken after 5 minutes of dark adaptation. Methods of fundus photography and image analysis varied across studies (see Table 1).
Singapore “I” Vessel Assessment (SIVA) software was used to automatically extract retinal vascular structure and calculate quantitative measures from retinal images in three studies [10], [26], [28]. Quantitative measurement in the Rotterdam Study [24] was performed using Retinal Vessel Measurement System. The remaining studies used observer grading methods [20], [21], [22], [23], [25], [27].
3.3. Associations between retinal parameters and dementia
Associations between retinal parameters and dementia outcomes are presented in Table 3, Table 4. These tables report results adjusted for demographics and vascular risk factors (details of adjustments are provided in Table 1). Results from cross-sectional and prospective cohort studies are presented separately. Associations of increased retinal parameter values with dementia were indicated by “+,” associations of decreased retinal parameter values with dementia were indicated by “−,” and no statistically significant association between retinal parameters and dementia was indicated by “=.” In text both uncorrected and adjusted results are provided where possible.
Table 3.
Associations between retinal parameters measured using fundus imaging and dementia in cross-sectional studies
| Study | Dementia outcome | Focal arteriolar narrowing, arteriovenous nicking, and retinal vessel caliber |
FD, tortuosity, branching pattern and geometry |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinopathy∗ |
AMD∗ |
FAN∗ |
AVN∗ |
CRAE |
CRVE |
AVR |
FD |
Tortuosity |
BA |
BC |
AF |
||||||
| a |
v |
a |
v |
a |
v |
a |
v |
||||||||||
| OR (95% CI) or direction of association | |||||||||||||||||
| Baker et al. [21] | Dementia | 1.17 (0.62–2.22) | … | 1.99 (1.11–3.56) | 1.48 (0.74–2.96) | 1.42 (0.74–2.73)† | =† | … | … | … | … | … | … | … | … | … | … |
| Mixed AD VaD | 3.57 (1.31–9.75) | ||||||||||||||||
| AD | 1.38 (0.64–2.97) | ||||||||||||||||
| Baker et al. [22] | Dementia | … | 0.98 (0.57–1.69) | … | … | … | … | … | … | … | … | … | … | … | … | … | … |
| AD | 1.09 (0.57–2.08) | ||||||||||||||||
| Qiu et al. [23] | Dementia | 1.35 (0.89–2.04) | … | = | = | … | … | … | … | … | … | … | … | … | … | … | … |
| AD | 1.22 (0.73–2.04) | ||||||||||||||||
| VaD | 1.95 (1.04–3.62) | ||||||||||||||||
| Schrijvers et al. [25] | Dementia | 1.92 (1.24–2.98) | … | … | … | … | … | … | … | … | … | … | … | … | … | … | … |
| AD | 1.89 (1.15–3.10) | ||||||||||||||||
| VaD | 2.00 (0.71–5.63) | ||||||||||||||||
| Frost et al. [26] | AD | … | … | … | … | − | − | = | − | − | = | − | = | = | + | + | + |
| Cheung et al., [10] | AD | … | … | … | … | − | − | … | − | − | + | + | = | … | … | … | … |
| 1.22 (0.78–1.91) | 2.01 (1.27–3.19) | 1.35 (1.08–1.68) | 1.47 (1.17–1.84) | 1.8 (1.48–2.53) | 1.94 (1.48–2.53) | ||||||||||||
| Williams et al. [28] | AD | … | … | … | … | + | = | … | − | − | − | = | = | … | … | … | … |
| 1.11 (0.83–1.47) | 0.99 (0.75–1.32) | 0.92 (0.74–1.14) | 0.77 (0.62–0.97) | 0.78 (0.63–0.97) | 1.01 (0.82–1.24) | ||||||||||||
| Williams et al. [27] | AD | … | = | … | … | … | … | … | … | … | … | … | … | … | … | … | … |
Abbreviations: a, arteriolar; AD, Alzheimer's disease; AF, asymmetry factor; AMD, age-related macular degeneration; AVN, arteriovenous nicking; AVR, arteriovenous ratio; BA, branching angle; BC, branching coefficient; CI, confidence intervals; CRAE/CRVE, central retinal arterial/venular equivalent; FAN, focal arteriolar narrowing; FD, fractal dimension; OR, odds ratio; v, venular; VaD, vascular dementia; …, outcome measure not evaluated; =, no association between presence of retinal parameter and outcome measure.
NOTE. Results for vessel caliber were recorded in such a way that a negative coefficient means narrowing is associated with dementia, positive coefficient means widening is associated with dementia.
Measure dichotomized: present versus absent.
Retinal vessel caliber not measured using CRAE/CRVE, caliber categorized into quintiles, result reported for first quintile; results for FD/tortuosity/BA/BC/AF presented such that a negative coefficient means reduced retinal parameter is associated with AD, positive coefficient means increased retinal parameter associated with AD. Bold values indicate significant associations.
Table 4.
Associations between retinal parameters and dementia in longitudinal studies
| Study | Dementia outcome | AMD |
Retinopathy |
Vessel caliber |
|
|---|---|---|---|---|---|
| CRAE |
CRVE |
||||
| RR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Schrijvers et al. [25] | Dementia | 1.15 (0.89–1.50) | |||
| AD | 1.15 (0.86–1.55) | ||||
| VaD | 0.90 (0.39–2.11) | ||||
| De Jong et al. [24] | Dementia | 1.05 (0.96–1.16) | 1.11 (1.00–1.22) | ||
| AD | 1.02 (0.91–1.14) | 1.06 (0.95–1.19) | |||
| VaD | 1.33 (0.99–1.78) | 1.44 (1.10–1.89) | |||
| Klaver et al. [20] | AD | 1.5 (0.6–3.5) | |||
Abbreviations: AD, Alzheimer's disease; AMD, age-related macular degeneration; CI, confidence interval; CRAE/CRVE, central retinal arterial/venular equivalent; HR, hazard ratio; RR, relative risk; VaD, vascular dementia.
NOTE. Bold values indicate significant associations.
3.4. Cross-sectional studies: Retinopathy and AMD
3.4.1. Retinopathy
Neither the Cardiovascular Health Study [21] nor the AGES-Reykjavik Study [23] found an association between retinopathy and dementia in uncorrected (OR, 1.34; 95% CI, 0.90–1.99 [data not provided in the Cardiovascular Health Study]) or multivariate-adjusted models (OR, 1.17; 95% CI, 0.62–2.22; OR, 1.35; 95% CI, 0.89–2.04, respectively) in all subjects. In stratified multivariable models, among persons with hypertension, retinopathy was associated with dementia (OR, 2.10; 95% CI, 1.04–4.24). No association was found in those without hypertension [21]. Analyses stratified by diabetes status found an association between retinopathy and dementia in those without diabetes (OR, 1.96; 95% CI, 0.96–4.02). No association was found in those with diabetes (OR, 0.32; 95% CI, 0.07–1.44) [21].
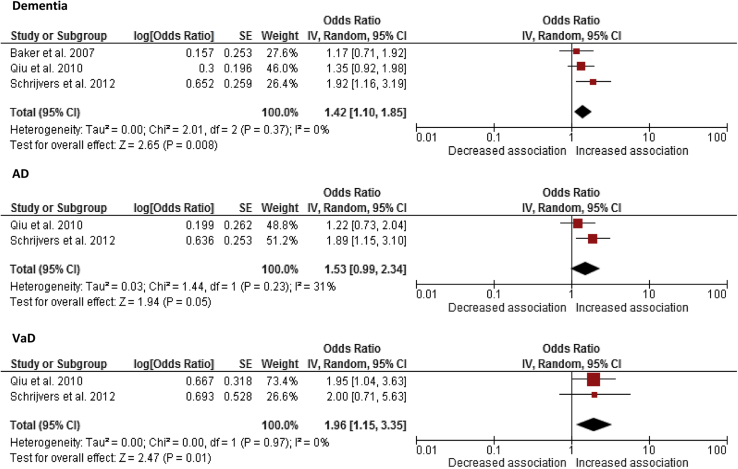
Schrijvers et al. [25] found a significant association between retinopathy and dementia in a population-based sample of individuals aged 55 years and older (age and sex–adjusted OR, 2.04; 95% CI, 1.34–3.09) with a multivariable-adjusted OR = 1.92 (95% CI, 1.24–2.98). sOR of dementia in the presence versus absence of retinopathy was 1.42 (95% CI, 1.10–1.85; Fig. 2), with the 95% CI from two studies crossing zero. There was no significant heterogeneity between the three articles (χ2 P = .37).
Fig. 2.
Meta-analysis providing unadjusted summary odds ratios (sORs) showing association of retinopathy and dementia, Alzheimer's disease (AD) and vascular dementia (VaD). The size of the square denotes the weight attributed to each article, and the horizontal lines represent the 95% confidence interval (CI). A diamond represents sOR with the width representing the 95% CI. Abbreviation: SE, standard error.
The cross-sectional AGES-Reykjavik Study found that those with retinopathy lesions had an increased risk of VaD (age, sex, and education–adjusted OR, 1.98; 95% CI, 1.10–3.56; multivariate-adjusted OR, 1.95; 95% CI, 1.04–3.62) but not AD (age, sex, and education–adjusted OR, 1.20; 95% CI, 0.73–1.98; multivariate-adjusted OR, 1.22; 95% CI, 0.73–2.04) [23]. The Rotterdam Study found increased risk of AD with retinopathy (age and sex–adjusted OR, 1.80; 95% CI, 1.11–2.91; multivariate-adjusted OR, 1.89; 95% CI, 1.15–3.10). The association between retinopathy and VaD (OR, 3.01; 95% CI, 1.26–7.21) did not persist after full adjustment (OR, 2.0; 95% CI, 0.71–5.63) [25]. Retinopathy was not significantly associated with AD or “mixed” AD VaD when assessed separately in the Cardiovascular Health Study (data not reported in study article) [21]. sOR of AD and VaD in retinopathy was 1.53 (95% CI, 0.99–2.34; Fig. 2) and 1.96 (95% CI, 1.15–3.35; Fig. 2), respectively, with no significant heterogeneity between articles (AD, χ2 P = .23; VaD, χ2 P = 0.97).
3.4.2. Age-related macular degeneration
Williams et al. [27] found an association between the most advanced cases of AMD (grade 3: geographic atrophy or neovascular AMD) and AD when uncorrected (OR, 2.5; 95% CI, 1.3–5.0) [27]. The association was lost following adjustment for potential confounding variables (age, smoking, APOE ε4 carrier status, and recent illness) (OR, 1.38; 95% CI, 0.6–3.2). Earlier stages of AMD were not associated with AD in unadjusted (data not provided) or adjusted models (grade 1: OR, 0.65; 95% CI, 0.4–1.1; grade 2: OR, 1.00; 95% CI, 0.5–1.9).
Early AMD (presence of soft drusen/retinal pigment epithelial depigmentation/combination of soft drusen with increased retinal pigment/depigmentation in the absence of exudative AMD/pure geographic atrophy) was not associated with dementia (adjusted for age, sex, ethnicity, study center: OR, 0.77; 95% CI, 0.47–1.27; fully adjusted model: OR, 0.98; 95% CI, 0.57–1.69) or AD (OR, 0.81; 95% CI, 0.45–1.48 and OR, 1.09; 95% CI, 0.57–2.08) [22]. Late AMD was not assessed because of the rarity of these lesion types.
3.5. Longitudinal studies: Retinopathy and AMD
Retinopathy and AMD in relation to incident dementia 2 to 11 years later was examined (see Table 4). Retinopathy was not significantly associated with increased risk of dementia (age and sex–adjusted HR, 1.15; 95% CI, 0.88–1.48; multivariate-adjusted HR, 1.15; 95% CI, 0.89–1.50), AD (age and sex–adjusted HR, 1.12; 95% CI, 0.83–1.50; multivariate-adjusted HR, 1.15; 95% CI, 0.86–1.55), or VaD (age and sex–adjusted HR, 0.97; 95% CI, 0.42–2.23; multivariate-adjusted HR, 0.90; 95% CI, 0.39–2.11) after a follow-up of 11.6 years [25]. Klaver et al. [20] found an increased risk of incident AD after 25.2 months in those with advanced stage AMD (indistinct or reticular drusen with pigmentary irregularities/the presence of either atrophic or neovascular end-stage macular degeneration) at baseline (age and sex–adjusted relative risk [RR], 2.1; 95% CI, 1.1–4.3). However, the association was attenuated following adjustment for smoking and atherosclerosis (RR, 1.5; 95% CI, 0.6–3.5). Risk of AD did not increase for those with earlier AMD in age and sex–adjusted model (RR, 1.0; 95% CI, 0.6–1.9) or following adjustment for smoking and atherosclerosis (RR, 1.0; 95% CI, 0.6–3.5).
3.6. Cross-sectional studies: Retinal vascular caliber, FAN, or AVN
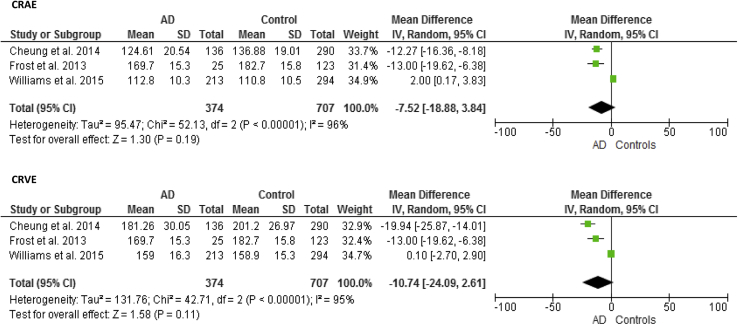
Associations between arteriolar widths and dementia were inconsistent (see Table 3). Using CRAE as a summary measure of vessel caliber, Frost et al. [26] and Cheung et al. [10] found arteriolar narrowing in AD (area under the curve [AUC] = 0.612, SD = 0.082; age, sex, and ethnicity–adjusted OR, 2.02; 95% CI, 1.59–2.58, respectively), whereas Williams et al. [28] found evidence of arteriolar widening in AD (age and sex–adjusted OR, 1.37; 95% CI, 1.08–1.75). However, associations were lost once cardiovascular risk factors were controlled for OR, 1.22; 95% CI, 0.78–1.91 [10] and OR, 1.1; 95% CI, 0.83–1.47 [28]. Meta-analysis of the three studies found a reduction in arteriolar width in AD using CRAE (MD, −7.52; 95% CI, −18.88 to 3.84; Fig. 3) with significant heterogeneity between articles (χ2 P < .001). The Cardiovascular Health Study failed to find a significant association between arteriolar caliber and dementia (multivariate-adjusted OR, 1.42; 95% CI, 0.74–2.96) [21]. Different measures of arteriolar caliber were also applied by Frost et al. [26]. Increasing SD of arteriolar widths (AUC = 0.595, SD = 0.070, P = .0086) and arteriolar attenuation (AUC = 0.651, SD = 0.068, P = .049) in AD was found using a measure of the SD of vessel width (arteriolar) in zone B (BSTDa) and arteriolar LDR.
Fig. 3.
Meta-analysis of Alzheimer's disease (AD) versus control subjects: CRAE and CRVE. The size of the square denotes the weight attributed to each article, and the horizontal lines represent the 95% confidence interval (CI). A diamond represents the summary mean difference with the width representing the 95% CI. Unadjusted results reported. Abbreviations: CRAE, central retinal arterial equivalent; CRVE, central retinal venular equivalent; SD, standard deviation.
Associations between venular caliber and dementia were also mixed (see Table 3). Frost et al. [26] found evidence of narrower venular widths in AD using CRVE (AUC = 0.703, SD = 0.067, P = .0049) and increasing SD of vessel widths (venular) (AUC = 0.541, SD = 0.081, P = .0089) using BSTDv. Cheung et al. [10] also found that patients with AD (n = 136) had narrower venular calibers measured using CRVE (P < .001; age, sex, and ethnicity–adjusted OR, 2.17; 95% CI, 1.69–2.79; multivariate-adjusted OR, 2.01, 95% CI, 1.27–3.19). Williams et al. [28] did not find a significant difference between CRVE in patients with AD (n = 213) and control subjects (n = 294) (d = 0.006, P = .951) (multivariable-adjusted OR, 0.99; 95% CI, 0.75–1.32, P = .960). Although not wholly consistent, there was a general reduction in venular calibers in AD using CRVE (MD, −10.74; 95% CI, −24.09 to 2.61; Fig. 3) with significant heterogeneity between articles (χ2 P < .001). Using venular LDR Frost et al. [26] found no difference in caliber between AD and control subjects (P > .05). The only study using AVR found no significant difference between patients with AD and the control group (P > .05) [26].
Neither study examining the association between AVN and dementia found any significant associations (multivariable-adjusted OR, 1.48; 95% CI, 0.74–2.96) [21] (data not provided) [23].
FAN associations were mixed (see Table 3). Although Qiu et al. [23] failed to find an association of FAN with dementia (data not provided), the Cardiovascular Health Study found a relationship (multivariate-adjusted OR, 1.99; 95% CI, 1.11–3.56) that appeared to be driven by hypertension and diabetes [21]. Those with evidence of FAN were more likely to have “mixed AD VaD” (OR, 3.57; 95% CI, 1.31–9.75) but not AD (OR, 1.38; 95% CI, 0.64–2.97).
3.7. Longitudinal studies: Retinal vascular caliber, FAN, or AVN
The prospective Rotterdam Study examined associations between retinal vascular calibers and incident dementia, AD (with or without cerebrovascular disease), and VaD [24] (Table 4). Increased risk of dementia with retinal venular widening was found (age and sex–adjusted HR, 1.09; 95% CI, 1.01–1.18; multivariate-adjusted HR, 1.11; 95% CI, 1.00–1.22) [24]. Stratified analyses revealed the increased risk was driven by the association with VaD (n = 73) (HR, 1.31; 95% CI, 1.06–1.64; multivariate-adjusted HR, 1.44; 95% CI, 1.10–1.89). Venular width was not significantly associated with increased risk of AD in stratified analyses following exclusion of those with cardiovascular disease (n = 47) (HR, 1.16; 95% CI, 0.82–1.64).
No association of arteriolar width with risk of dementia (HR, 0.99; 95% CI, 0.91–1.07), AD (HR, 0.98; 95% CI, 0.89–1.14), or VaD (HR, 1.06; 95% CI, 0.84–1.33) was found in age and sex–adjusted models [24]. Narrower arterioles were related to an increased risk of VaD but only after adjustment for venular calibers (HR, 1.40; 95% CI, 1.05–1.86).
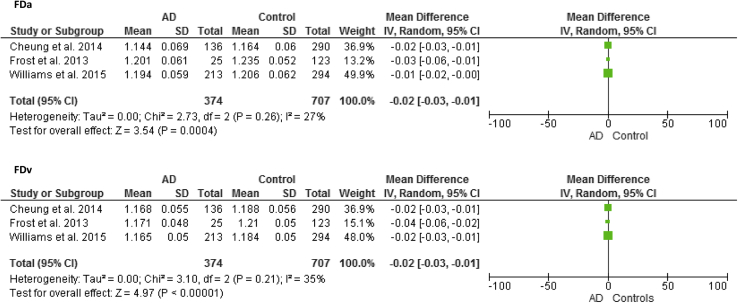
3.8. Cross-sectional studies: FD, branching pattern, and geometry
Three articles examined the link between FD and AD [10], [26], [28], each reporting a reduction in arteriolar and venular FD indicating a sparser network in AD (MD, −0.02; 95% CI, −0.03 to −0.01) (Fig. 4) with no significant heterogeneity between articles (arteriolar FD χ2 P = .26; venular FD χ2 P = .21). Patients with AD demonstrated reduced complexity of the branching pattern in comparison with control subjects with lower arteriolar (AUC = 0.644, SD = 0.075, P = .021) and venular FDs (AUC = 0.716, SD = 0.074, P = .0033) [26]. A smaller number of first branching vessels in zone C were found in AD (arteriolar; AUC = 0.675, SD = 0.142, P = .022; venular; AUC = 0.660, SD = 0.121), again indicating reduced complexity of branching [26]. Reduced FD in patients with AD with significant results for venular (d = 0.4, P = .001; multivariate-adjusted OR per SD decrease, 1.47; 95% CI, 1.17–1.84), arteriolar (d = 0.3, P = .002; OR, 1.35, 95% CI, 1.08–1.68), and total FD (d = 0.4, P < .001; OR, 1.54; 95% CI, 1.23–1.93) was also found in the Singapore Epidemiology of Eye Disease study [10]. Likewise, Williams et al. [28] found significantly lower fractal total (d = 0.3, P = .001), arteriolar (d = 0.2, P = .024), and venular (d = 0.4, P < .001) dimensions in AD. Those with lower venular FD (multivariate-adjusted OR per SD increase, 0.77; 95% CI, 0.62–0.97, P = .025) were more likely to have AD. The association between total and arteriolar FD and AD did not persist in the final models (OR, 0.85; 95% CI, 0.68–1.06, P = .141; OR, 0.92; 95% CI, 0.74–1.14, P = .436).
Fig. 4.
Meta-analysis of Alzheimer's disease (AD) versus control subjects: Arteriolar fractal dimension (FDa) and venular fractal dimension (FDv). The size of the square denotes the weight attributed to each article. Unadjusted results reported. Abbreviation: SD, standard deviation.
Neither study examining branching angle found a difference in arteriolar or venular angle between AD and control groups (d = 0.07, P = .521; d = 0.01, P = .922, respectively) [10] (d = 0.006, P = .523; d = 0.04, P = .599, respectively) [28]. Another measure of circulatory optimality, junctional exponent deviation, also failed to identify an association between AD and branching angles (data not provided) [26]. Higher venular BC values in AD (AUC = 0.55, SD = 0.084, P = .035), indicating reduced optimality of branching geometry, were found [26]. Further evidence of reduced branching geometry optimality with greater arteriolar and venular asymmetry factor values in AD (AUC = 0.578, SD = 0.081, P = .037; AUC = 0.616, SD = 0.074, P = .047, respectively) was found [26].
Inconsistent associations between tortuosity and dementia were found [10], [26], [28] (see Table 3). More tortuous arterioles and venules were observed in AD than in control subjects (d = 0.2, P < .001) in the Singapore Epidemiology of Eye Disease study [10]. Those with higher arteriolar and venular tortuosity were more likely to have AD (multivariable-adjusted OR per SD increase, 1.8; 95% CI, 1.48–2.53; OR, 1.94; 95% CI, 1.48–2.53, respectively). Frost et al. [26] found less tortuous venules (AUC = 0.706, SD = 0.073, P = .042) in AD with no significant difference in arteriolar tortuosity between patients with AD (n = 25) and healthy control subjects (n = 123) (data not provided). Conversely, Williams et al. [28] found lower arteriolar tortuosity in AD (d = 0.2, P = .030, multivariable-adjusted OR, 0.78; 95% CI, 0.63–0.97, P = .27), and not venules (d = 0.07, P = .458).
4. Discussion
The aim of this review was to assess the relationship between microvascular changes in the retina and dementia with the use of fundus camera imaging. Despite considerable heterogeneity in both retinal parameters and study design, the cross-sectional studies reviewed found evidence of some consistent cross-sectional associations with dementia. The heterogeneity of retinal measurements in the few longitudinal studies included in this review precluded the comparison of findings across prospective studies.
The most consistent finding was decreased FD in AD [10], [26], [28]. A decreased FD indicating a sparser branching density has also been observed in stroke [31], [32], cognitive dysfunction [33], and hypertension [18]. Pathophysiologically, a less complex retinal microvascular network is a result of retinal vessel rarefaction and collapse, which may cause retinal hypoxia [34]. Destruction and obstruction of the small perforating cerebral vessels have been reported [35] indicating that corresponding pathologic mechanisms are responsible for microvascular changes in the retina and brain. These findings provide evidence for the role of microvascular pathology in the development of dementia. However, the effect sizes reported here are small to medium (d = 0.2–0.4). In the largest study, the association of arteriolar fractal analysis was lost in the fully adjusted model [28]. Potential differences between arteriolar and venular fractal networks should be assessed using data from larger studies and in different patient populations.
Vessel caliber measurements present conflicting results; both narrower [10], [26] and wider arterioles [28] were associated with AD. The finding of narrower venules in AD [10], [26] was not replicated by Williams et al. [28]. The prospective Rotterdam Study found wider venular calibers were related to an increased risk of dementia, largely driven by the association with risk of VaD [24]. The association between narrower arteriolar caliber and AD in the Singapore Epidemiology of Eye Disease program study was lost after adjustment for cardiovascular risk factors [10]. Narrower arterioles are strongly associated with hypertension [11], [36], which could be responsible for the attenuation in association. Wider retinal venules in VaD could reflect inflammation, cerebral hypoperfusion, and subsequent ischemia, whereas narrower venules in AD may reflect increased venular wall thickness as a result of collagen deposition in cerebral veins [37]. However, evidence has also been reported supporting the involvement of inflammation in the development of AD [38]. It is possible that opposing changes in diameter caused by inflammation and collagen deposition may decrease the likelihood of detecting meaningful changes and effects. The different mechanisms underlying the association of changes to arteriolar and venular caliber emphasize the importance of analyses according to dementia subtypes and in adjusting for the opposing effect of fellow vessel caliber. Contrasting effects of hypertension causing arteriolar narrowing and ischemia and endothelial dysfunction causing arteriolar widening may lead to spurious associations of vessel caliber with dementia. Narrower arteriolar caliber was associated with an increased risk of VaD only following adjustment for venular calibers [24]. Most studies adjusted for the opposing effects of fellow vessel caliber [10], [24], [28]. Frost et al. [26] do not report adjusting for opposing vessel type. Inconsistent associations of vessel caliber could also be attributed to natural variation because of pulsation during the cardiac cycle and by vasomotion with arteriolar and venular caliber found to vary by up to 17% and 11%, respectively [39].
Tortuosity has been proposed as a means of identifying those at risk of microvascular complications by detecting initial vascular changes [28], [40]. Results were inconsistent with both increased [10] and decreased tortuosity [26], [28] associated with AD. Although the underlying mechanisms for its onset and development remain unclear, retinal tortuosity has been associated with hypertension, retinopathy, cerebral vessel disease, stroke, and ischemic heart disease [18], [41], [42], [43]. The finding of increased tortuosity in AD is in line with previous findings in stroke, hypertension, and cerebrovascular disease [40], [44], [45]. Reduced tortuosity has been associated with increasing risk of death from ischemic heart disease possibly resulting from endothelial dysfunction and a widespread impairment of perfusion or oxygenation in the microvasculature [42]. Reduced arteriolar tortuosity has also been found in diabetic retinopathy [17]. The accuracy of tortuosity measurement may be related to the length of the vessel segments [46]. For example, when assessing tortuosity of an entire vessel segment labeled as tortuous, smaller straight (i.e., less tortuous) subsections within the segment may complicate classification by influencing the overall tortuosity of the vessel making it appear less tortuous. All three studies used SIVA to calculate tortuosity where automatically segmented vessels are typically short enough to be classed as either entirely tortuous or nontortuous. Short-term changes in tortuosity in response to environmental effects have also been reported [47]. Tortuosity discrepancies may also be because of differences in race between the studies (Chinese, Indian, and Malay in the Cheung et al. study [10] vs. white Caucasians in Frost et al. [26] and Williams et al. [28]).
Retinopathy lesions including microaneurysms and retinal hemorrhages reflect a breakdown of the retinal blood barrier [48], [49]. Results from cross-sectional [23], [25] and longitudinal studies [25] suggest that although a marker of microvascular pathology retinopathy is more prevalent in dementia, the onset does not precede the development of dementia. Although retinopathy may reflect underlying cerebral vascular pathology, it is more likely to reflect advanced cerebral microvascular states and as such may not be useful as a diagnostic tool or biomarker for risk of development, as the condition is unlikely to develop before the clinical stages of dementia [25]. The inconsistent findings could reflect the different definitions and grading of retinopathy across studies. Although the three studies mostly used common features for diagnosis, it is noteworthy that the only study failing to identify a significant association used the most comprehensive range of retinopathy signs including intraretinal microvascular abnormalities, venous beading, vitreous hemorrhage, and new vessels at the disc or elsewhere [21]. Neither cross-sectional nor longitudinal associations between AMD and dementia were found [20], [22], [27].
An additional motivation of this review was to establish whether fundus image analysis could differentiate between dementia subtypes. Most studies meeting the inclusion criteria focused exclusively on AD [10], [20], [26], [27], [28] with limited numbers of VaD across studies (n = 102). The few studies with sufficient numbers for stratified analysis revealed different outcomes by diagnosis: larger venular calibers associated with increased risk of VaD but not with AD [24]; FAN was associated with mixed AD VaD but not with AD [21]; and retinopathy was associated with AD but not with VaD [25] with the opposite finding for Qiu et al. [23]. Although the limited number of cases of VaD (n = 29) could explain the insignificant association in the Rotterdam Study [25], results provide some evidence of the utility of fundus imaging in the differentiation of dementia subtypes. Fundus imaging should be applied to assess the role of microvascular pathology in larger samples of other forms of dementia, particularly VaD.
Accurate and meaningful interpretation of retinal parameters requires not only precise quantification and calculation but also an understanding of potential effects of axial length and ocular refractive error. Dimensional parameters such as CRAE and CRVE are subject to magnification effects and refractive error. The Blue Mountains Eye Study and Beaver Dam Eye Study found an association between myopic refraction and reduced retinal FD and smaller retinal vessel diameters, respectively [50], [51]. The Blue Mountains Eye Study found that correcting for refractive error appeared to increase the statistical power to detect associations with retinal vessel caliber [51]. Only one study reviewed adjusted for magnification effects using refractive data [24]. Data on axial length were not included in the reviewed studies. Longer axial length has been associated with the narrowing of arterioles and venules, increased arteriolar and venular BCs, and less tortuous arterioles [52]. Patton et al. [53] likewise found an association between increased axial length and narrowing of retinal vessels. No effect of axial length on AVR, junctional exponents, and bifurcation angles was found. Future studies using dimensional measures should adjust for refraction and axial length. Dimensionless measures that are not subject to the influence of these effects such as AVR, junction exponent, tortuosity, and LDRs have been calculated.
Most of the quantitative measurements were assessed and calculated using the same semiautomated software, SIVA. Despite using the software according to the standardized protocol to measure common retinal vascular properties in similar patient groups, results were inconsistent [10], [26], [28]. Moderate to high intergrader reliability of quantitative measurements using SIVA has been reported with coefficient of variations ranging from 1.76% for venular caliber to 17.66% for venular tortuosity [18]. In addition to SIVA, a number of software packages are available (e.g., VAMPIRE [54], ARIA [55], IVAN [University of Wisconsin, Madison]), each implementing different algorithms for detection and measurement of retinal vascular features. Although the uniformity of quantitative retinal measurement methods in the reviewed studies facilitates comparison, additional studies using different software and measurement methods are required to provide a more comprehensive view of retinal vascular status in dementia. Furthermore, a comparison of retinal vascular parameters measured using the same fundus images with different software packages is required.
Some methodological issues warrant consideration, chiefly the limited number and heterogeneity of reviewed studies, including design, population, covariates, retinal parameters, and outcome measures. The review of the association between many retinal parameters and dementia was therefore restricted to a descriptive comparison. In addition, studies with negative findings may be under-represented because of publication bias. The studies reviewed neither report dementia severity nor do they include histopathologic confirmation of diagnosis. Different levels of disease severity and diagnostic inaccuracy could account for some of the inconsistencies between studies. Although all used standard clinical diagnosis criteria, studies have shown these criteria to routinely fail in accurately differentiating between AD and non-AD dementia [56]. A study of 15,367 patients found that 16.6% of patients with VaD were misdiagnosed with AD [57]. It is possible that the AD samples in the studies reviewed include patients with VaD, which would introduce variability to the outcomes. Studies also vary in the number of potential confounding variables adjusted for. Participants with ungradable retinal photographs were excluded from most studies. Excluded participants were more likely to have vascular risk factors [21], [22], [24]. Because of the association of these risk factors with both dementia and retinal abnormalities the observed associations may have been falsely attenuated as a result of selection bias.
Although not wholly consistent, the findings of this review provide evidence to support the hypothesis that changes in retinal microvasculature identified using fundus imaging are associated with dementia. The measurement of retinal microvasculature, particularly FD, using fundus camera imaging appears to offer a promising means of noninvasively identifying retinal microvascular abnormalities associated with dementia. However, the strength of the relation between retinal microvascular properties and dementia is modest, which limits the prognostic value at the population level. Yet, imaging the retinal microvasculature may be used to increase understanding of the mechanisms underlying the pathogenesis of dementia, provide enhanced differentiation of dementia subtypes, and as a potential means of improving detection of those at an increased risk of dementia. Future research should explore the diagnostic and prognostic contribution of combining measures of the retinal vasculature with other clinical and imaging biomarkers in a multimodal approach, for example, metrics derived from retinal OCT. Analysis of combined retinal vascular parameters (e.g., vessel calibers, FD, and tortuosity), instead of individual markers, may also provide increased sensitivity and specificity for different subtypes of dementia. In addition, dynamic functional measures of retinal microcirculation including flowmetry [58], retinal oximetry [59], and dynamic vessel assessments [60] could provide further valuable insights into cerebral hemodynamics in dementia. Greater measurement harmonization between studies with a consensus on standards for scientific reporting of retinal microvascular changes related to dementia is required. Measures taken to optimize image analysis through the introduction of standard operating procedures related to imaging techniques, camera systems, and measurement could help to reduce the variability and increase the clinical utility of retinal imaging. Further studies with common objective and standardized retinal measurements are required in larger samples of patients with different forms of dementia to increase understanding of the specificity of retinal microvascular abnormalities. In addition, more prospective data are needed to determine the most appropriate parameters for application as diagnostic tools or biomarkers for risk of development of dementia. These issues need to be addressed before a conclusion on the true clinical utility of fundus camera imaging in dementia can be reached.
Research in context.
-
1.
Systematic review: We searched Medical Literature Analysis and Retrieval System Online, Excerpta Medica Database, PubMed, and Google Scholar for all human studies published until March 2016. Studies were systematically evaluated and meta-analysis of pooled results was performed.
-
2.
Interpretation: This is the most comprehensive systematic review focusing specifically on the use of fundus camera imaging in dementia. Significant pathologic changes in retinal parameter were found. Results across studies were inconsistent potentially because of varied methodologies applied and the lack of standardized methods and measurements.
-
3.
Future directions: The potential of fundus camera image analysis in differentiating between dementia subtypes should be the subject of further research of larger well-powered samples of well-characterized dementia types with sufficient numbers for stratified analyses. A particular focus on VaD is warranted because of previous findings of an association of retinal vessel width and major risk factors for VaD, cerebral small vessel disease, and stroke.
Acknowledgments
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) grant “Multi-modal retinal biomarkers for vascular dementia” (EP/M005976/1).
Conflicts of interest: The authors do not have any conflicts of interest.
References
- 1.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Thies W., Bleiler L. Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7:208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Comas-Herrera A., Northey S., Wittenberg R., Knapp M., Bhattacharyya S., Burns A. Future costs of dementia-related long-term care: exploring future scenarios. Int Psychogeriatr. 2011;23:20–30. doi: 10.1017/S1041610210000025. [DOI] [PubMed] [Google Scholar]
- 4.Wright C.F., Hall A., Matthews F.E., Brayne C. Biomarkers, dementia, and public health. Ann N Y Acad Sci. 2009;1180:11–19. doi: 10.1111/j.1749-6632.2009.04942.x. [DOI] [PubMed] [Google Scholar]
- 5.Wiesmann M., Kiliaan A.J., Claassen J.A. Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab. 2013;33:1696–1706. doi: 10.1038/jcbfm.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knopman D.S. Dementia and cerebrovascular disease. Mayo Clin Proc. 2006;81:223–230. doi: 10.4065/81.2.223. [DOI] [PubMed] [Google Scholar]
- 7.Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.London A., Benhar I., Schwartz M. The retina as a window to the brain from eye research to CNS disorders. Nat Rev Neurol. 2011;9:44–53. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 9.Patton N., Aslam T., MacGillivray T., Pattie A., Deary I.J., Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206:319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung C.Y., Ong Y.T., Ikram M.K., Ong S.Y., Li X., Hilal S. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimers Dement. 2014;10:135–142. doi: 10.1016/j.jalz.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Ikram M.K., De Jong F.J., Van Dijk E.J., Prins N.D., Hofman A., Breteler M.M. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain. 2006;129:182–188. doi: 10.1093/brain/awh688. [DOI] [PubMed] [Google Scholar]
- 12.Thomson K.L., Yeo J.M., Waddell B., Cameron J.R., Pal S. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement (Amst) 2015;1:136–143. doi: 10.1016/j.dadm.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding J., Strachan M.W., Reynolds R.M., Frier B.M., Deary I.J., Gerald F.F. Diabetic retinopathy and cognitive decline in older people with type 2 diabetes the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:2883–2889. doi: 10.2337/db10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heringa S.M., Bouvy W.H., van den Berg E., Moll A.C., Kappelle L.J., Biessels G.J. Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: a systematic review. J Cereb Blood Flow Metab. 2013;33:983–995. doi: 10.1038/jcbfm.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung C.Y., Chen C., Wong T.Y. Ocular fundus photography as a tool to study stroke and dementia. Semin Neurol. 2015;35:481–490. doi: 10.1055/s-0035-1563570. [DOI] [PubMed] [Google Scholar]
- 16.Pérez M.A., Bruce B.B., Newman N.J., Biousse V. The use of retinal photography in non-ophthalmic settings and its potential for neurology. Neurologist. 2012;18:350–355. doi: 10.1097/NRL.0b013e318272f7d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung C.Y., Ikram M.K., Sabanayagam C., Wong T.Y. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension. 2012;60:1094–1103. doi: 10.1161/HYPERTENSIONAHA.111.189142. [DOI] [PubMed] [Google Scholar]
- 18.Cheung C.Y., Tay W.T., Mitchell P., Wang J.J., Hsu W., Lee M.L. Quantitative and qualitative retinal microvascular characteristics and blood pressure. J Hypertens. 2011;29:1380–1391. doi: 10.1097/HJH.0b013e328347266c. [DOI] [PubMed] [Google Scholar]
- 19.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Klaver C.C., Ott A., Hofman A., Assink J.J., Breteler M.M., de Jong P.T. Is age-related maculopathy associated with Alzheimer's disease?: the Rotterdam Study. Am J Epidemiol. 1999;150:963–968. doi: 10.1093/oxfordjournals.aje.a010105. [DOI] [PubMed] [Google Scholar]
- 21.Baker M.L., Larsen E.K., Kuller L.H., Klein R., Klein B.E., Siscovick D.S. Retinal microvascular signs, cognitive function, and dementia in older persons the Cardiovascular Health Study. Stroke. 2007;38:2041–2047. doi: 10.1161/STROKEAHA.107.483586. [DOI] [PubMed] [Google Scholar]
- 22.Baker M.L., Wang J.J., Rogers S., Klein R., Kuller L.H., Larsen E.K. Early age-related macular degeneration, cognitive function, and dementia: the Cardiovascular Health Study. Arch Ophthalmol. 2009;127:667–673. doi: 10.1001/archophthalmol.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu C., Cotch M.F., Sigurdsson S., Jonsson P.V., Jonsdottir M.K., Sveinbjrnsdottir S. Cerebral microbleeds, retinopathy, and dementia the AGES-Reykjavik Study. Neurology. 2010;75:2221–2228. doi: 10.1212/WNL.0b013e3182020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Jong F.J., Schrijvers E.M., Ikram M.K., Koudstaal P.J., de Jong P.T., Hofman A. Retinal vascular caliber and risk of dementia the Rotterdam Study. Neurology. 2011;76:816–821. doi: 10.1212/WNL.0b013e31820e7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrijvers E.M., Buitendijk G.H., Ikram M.K., Koudstaal P.J., Hofman A., Vingerling J.R. Retinopathy and risk of dementia the Rotterdam Study. Neurology. 2012;79:365–370. doi: 10.1212/WNL.0b013e318260cd7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frost S., Kanagasingam Y., Sohrabi H., Vignarajan J., Bourgeat P., Salvado O. Retinal vascular biomarkers for early detection and monitoring of Alzheimer's disease. Transl Psychiatry. 2013;3:e233. doi: 10.1038/tp.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams M.A., Silvestri V., Craig D., Passmore A.P., Silvestri G. The prevalence of age-related macular degeneration in Alzheimer's disease. J Alzheimers Dis. 2014;42:909–914. doi: 10.3233/JAD-140243. [DOI] [PubMed] [Google Scholar]
- 28.Williams M.A., McGowan A.J., Cardwell C.R., Cheung C.Y., Craig D., Passmore P. Retinal microvascular network attenuation in Alzheimer's disease. Alzheimers Dement (Amst) 2015;1:229–235. doi: 10.1016/j.dadm.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bird A.C., Bressler N.M., Bressler S.B., Chisholm I.H., Coscas G., Davis M.D. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 30.Klein R., Davis M.D., Magli Y.L., Segal P., Klein B.E., Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki R., Azemin M.C., Kumar D.K., Tan A.G., Liew G., Wong T.Y. Fractal dimension of the retinal vasculature and risk of stroke: a nested case-control study. Neurology. 2011;76:1766–1767. doi: 10.1212/WNL.0b013e31821a7d7d. [DOI] [PubMed] [Google Scholar]
- 32.Doubal F.N., MacGillivray T.J., Patton N., Dhillon B., Dennis M.S., Wardlaw J.M. Fractal analysis of retinal vessels suggests that a distinct vasculopathy causes lacunar stroke. Neurology. 2010;74:1102–1107. doi: 10.1212/WNL.0b013e3181d7d8b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung C.Y., Ong S., Ikram M.K., Ong Y.T., Chen C.P., Venketasubramanian N. Retinal vascular fractal dimension is associated with cognitive dysfunction. J Stroke Cerebrovasc Dis. 2014;23:43–50. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Hammes H.P., Feng Y., Pfister F., Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomita Y., Kubis N., Calando Y., Dinh A.T., Méric P., Seylaz J. Long-term in vivo investigation of mouse cerebral microcirculation by fluorescence confocal microscopy in the area of focal ischemia. J Cereb Blood Flow Metab. 2005;25:858–867. doi: 10.1038/sj.jcbfm.9600077. [DOI] [PubMed] [Google Scholar]
- 36.Liew G., Mitchell P., Wong T.Y., Lindley R.I., Cheung N., Kaushik S. Retinal microvascular signs and cognitive impairment. J Am Geriatr Soc. 2009;57:1892–1896. doi: 10.1111/j.1532-5415.2009.02459.x. [DOI] [PubMed] [Google Scholar]
- 37.Berisha F., Feke G.T., Trempe C.L., McMeel J.W., Schepens C.L. Retinal abnormalities in early Alzheimer's disease. Invest Ophthalmol Vis Sci. 2007;48:2285–2289. doi: 10.1167/iovs.06-1029. [DOI] [PubMed] [Google Scholar]
- 38.Tuppo E.E., Arias H.R. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Knudtson M.D., Klein B.E., Klein R., Wong T.Y., Hubbard L.D., Lee K.E. Variation associated with measurement of retinal vessel diameters at different points in the pulse cycle. Br J Ophthalmol. 2004;88:57–61. doi: 10.1136/bjo.88.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benitez-Aguirre P., Craig M.E., Sasongko M.B., Jenkins A.J., Wong T.Y., Wang J.J. Retinal vascular geometry predicts incident retinopathy in young people with type 1 diabetes a prospective cohort study from adolescence. Diabetes Care. 2011;34:1622–1627. doi: 10.2337/dc10-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dougherty G., Johnson M.J., Wiers M.D. Measurement of retinal vascular tortuosity and its application to retinal pathologies. Med Biol Eng Comput. 2010;48:87–95. doi: 10.1007/s11517-009-0559-4. [DOI] [PubMed] [Google Scholar]
- 42.Witt N., Wong T.Y., Hughes A.D., Chaturvedi N., Klein B.E., Evans R. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47:975–981. doi: 10.1161/01.HYP.0000216717.72048.6c. [DOI] [PubMed] [Google Scholar]
- 43.Kwa V.I., Van der Sande J.J., Stam J., Tijmes N., Vrooland J.L. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology. 2002;59:1536–1540. doi: 10.1212/01.wnl.0000033093.16450.5c. [DOI] [PubMed] [Google Scholar]
- 44.Ong Y.T., Hilal S., Cheung C.Y., Xu X., Chen C., Venketasubramanian N. Retinal vascular fractals and cognitive impairment. Dement Geriatr Cogn Dis Extra. 2014;4:305–313. doi: 10.1159/000363286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen C.G., Rudnicka A.R., Nightingale C.M., Mullen R., Barman S.A., Sattar N. Retinal arteriolar tortuosity and cardiovascular risk factors in a multi-ethnic population study of 10-year-old children; the Child Heart and Health Study in England (CHASE) Arterioscler Thromb Vasc Biol. 2011;31:1933–1938. doi: 10.1161/ATVBAHA.111.225219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hart W.E., Goldbaum M., Côté B., Kube P., Nelson M.R. Measurement and classification of retinal vascular tortuosity. Int J Med Inform. 1999;53:239–252. doi: 10.1016/s1386-5056(98)00163-4. [DOI] [PubMed] [Google Scholar]
- 47.MacCormick I.J., Somner J., Morris D.S., MacGillivray T.J., Bourne R.R., Huang S.S. Retinal vessel tortuosity in response to hypobaric hypoxia. High Alt Med Biol. 2012;13:263–268. doi: 10.1089/ham.2011.1097. [DOI] [PubMed] [Google Scholar]
- 48.Wong T.Y., Klein R., Sharrett A.R., Nieto F.J., Boland L.L., Couper D.J. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons the Atherosclerosis Risk in Communities Study. Stroke. 2002;33:1487–1492. doi: 10.1161/01.str.0000016789.56668.43. [DOI] [PubMed] [Google Scholar]
- 49.Lindley R.I., Wang J.J., Wong M.C., Mitchell P., Liew G., Hand P. Retinal microvasculature in acute lacunar stroke: a cross-sectional study. Lancet Neurol. 2009;8:628–634. doi: 10.1016/S1474-4422(09)70131-0. [DOI] [PubMed] [Google Scholar]
- 50.Li H., Mitchell P., Liew G., Rochtchina E., Kifley A., Wong T.Y. Lens opacity and refractive influences on the measurement of retinal vascular fractal dimension. Acta Ophthalmol. 2010;88:e234–e240. doi: 10.1111/j.1755-3768.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- 51.Wong T.Y., Knudtson M.D., Klein R., Klein B.E., Meuer S.M., Hubbard L.D. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111:1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 52.Lim L.S., Cheung C.Y., Lin X., Mitchell P., Wong T.Y., Mei-Saw S. Influence of refractive error and axial length on retinal vessel geometric characteristics. Invest Ophthalmol Vis Sci. 2011;52:669–678. doi: 10.1167/iovs.10-6184. [DOI] [PubMed] [Google Scholar]
- 53.Patton N., Maini R., MacGillivary T., Aslam T.M., Deary I.J., Dhillon B. Effect of axial length on retinal vascular network geometry. Am J Ophthalmol. 2005;140:648–653. doi: 10.1016/j.ajo.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 54.Perez-Rovira A, MacGillivray T, Trucco E, Chin KS, Zutis K, Lupascu C, et al. VAMPIRE: vessel assessment and measurement platform for images of the REtina. In: Engineering in medicine and biology society, EMBC, 2011 annual international conference of the IEEE 2011 Aug 30 (pp. 3391–3394). IEEE. [DOI] [PubMed]
- 55.Bankhead P., Scholfield C.N., McGeown J.G., Curtis T.M. Fast retinal vessel detection and measurement using wavelets and edge location refinement. PLoS One. 2012;7:e32435. doi: 10.1371/journal.pone.0032435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beach T.G., Monsell S.E., Phillips L.E., Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71:266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirson N., Hunter C., Desai U., Cummings A., San Roman A., Faries D. Excess costs associated with misdiagnosis of Alzheimer's disease among US Medicare beneficiaries with vascular dementia or Parkinson's disease. Alzheimers Dement. 2013;9:P845–P846. [Google Scholar]
- 58.Harazy J.M., Raff U., Welzenbach J., Ott C., Ritt M., Lehmann M. New software analyses increase the reliability of measurements of retinal arterioles morphology by scanning laser Doppler flowmetry in humans. J Hypertens. 2011;29:777–782. doi: 10.1097/HJH.0b013e328343c27a. [DOI] [PubMed] [Google Scholar]
- 59.Einarsdottir A.B., Hardarson S.H., Krisjansdottir J.V., Bragason D.T., Snaedal J., Stefansson E. Retinal oximetry imaging in Alzheimer's Disease. J Alzheimers Dis. 2015;49:79–83. doi: 10.3233/JAD-150457. [DOI] [PubMed] [Google Scholar]
- 60.Lim M., Sasongko M.B., Ikram M.K., Lamoureux E., Wang J.J., Wong T.Y. Systemic associations of dynamic retinal vessel analysis: a review of current literature. Microcirculation. 2013;20:257–268. doi: 10.1111/micc.12026. [DOI] [PubMed] [Google Scholar]