Abstract
Background
Non-small-cell lung cancer (NSCLC) is a leading cause of death. Interventions to reduce mortality in patients with NSCLC represent a patient-important field of research. Little is known about interventions used outside the Western world for NSCLC. One intervention widely used in Asia is astragalus-based herbal preparations.
Methods
We conducted a comprehensive systematic review of all published randomized clinical trials (RCTs) evaluating astragalus-based herbal preparations in NSCLC patients. We searched independently, in duplicate, 6 English language electronic databases and 2 Chinese-language databases. We abstracted data independently, in duplicate on studies reporting of methods, survival outcomes, tumor responses, and performance score responses. We applied a random-effects meta-analysis and report outcomes as relative risks (RR) with 95% confidence intervals (CIs).
Results
We included 65 RCTs enrolling 4751 patients. All trials included the herbal preparations plus platinum-based chemotherapy versus chemotherapy alone. We pooled 7 studies (n = 529) reporting on survival at 6 months and found a pooled RR of 0.54 (95% CI, 0.45 to 0.65, P ≤ 0.0001). We included 20 trials (n = 1520) on survival at 12 months and found a pooled RR of 0.65 (95% CI, 0.54 to 0.79, P ≤ 0.0001). This effect was consistent at 24 and 36 months. When we applied a composite endpoint of any tumor treatment response, we pooled data from 57 trials and found a pooled RR of 1.35 in favor of herbal treatment (95% CI, 1.26 to 1.44, P ≤ 0.0001). Statistical heterogeneity was low across trials.
Limitations
The quality of reporting the RCTs was generally poor. There is also reason to believe that studies reported as randomized may not be.
Conclusions
We found a large treatment effect of adding astragalus-based herbal treatment to standard chemotherapy regimens. There is a pressing need for validation of these findings in well-conducted RCTs in a Western setting.
Keywords: astralagus, non-small-cell lung cancer, herbal preparations
Introduction
Lung cancer is the leading cause of cancer death worldwide.1,2 In the United States, lung cancer is the leading cause of death, where it is estimated that 219,440 new cases of lung and bronchus cancer will be diagnosed in 2009; leading to 159,390 lung cancer-related deaths.3 Only 15% of all lung cancer patients are alive 5 years or more after diagnosis.3
The World Health Organization divides lung cancer into 2 major classes: non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). NSCLC accounts for over 85% of all lung cancer cases, and it includes 2 major types: (1) non-squamous carcinoma (including adenocarcinoma, large-cell carcinoma, other cell types) and (2) squamous cell (epidermoid) carcinoma.4
The clinical guidelines for patients with NSCLC include combinations of cytotoxic chemotherapy and targeted biologic therapies, such as bevacizumab and erlotinib.4 Unfortunately, these current treatments involve non-specific, non-selective cytotoxic chemotherapy, which results in only a modest increase in survival and causes significant toxicity to the patient.5 According to a meta-analysis of 33 Phase III randomized controlled trials (RCTs), platinum-based chemotherapy offers symptomatic relief and modest improvements in survival (rarely >2 months).6 Data from 3 RCTs showed that platinum-based chemotherapy provided a median survival time of approximately 7 to 10 months.7–9
In China, traditional Chinese medicines (TCM), which are herbal and/or animal/insect-based combinations, are frequently combined with chemotherapy for the treatment of cancer.10 One commonly used herbal compound, astragalus, appears to have a number of immunomodulatory properties.11–13 Astragalus appears to have antitumor activity where its potentiates LAK cell activity in vitro when used in combination with IL-214. Astragalus appears to restore in vitro T-cell function, which is suppressed in cancer patients.15 A meta-analysis of 34 RCTs found that Chinese medicines containing the herb astragalus (Astragalus membranaceus) may increase effectiveness of platinum-based chemotherapy when combined with chemotherapy.16 When compared to chemotherapy alone, astragalus-based Chinese medicines reduced risk of death at 12 months (risk ratio [RR] = 0.67; 95% confidence interval [CI], 0.52 to 0.87), improved tumor response data (RR = 1.34; 95% CI, 1.24 to 1.46), reduced risk of death at 24 months (RR = 0.58; 95% CI, 0.49 to 0.68), increased tumor response (RR = 1.76; 95% CI, 1.23 to 2.53) and stabilized or improved Karnofsky performance status (RR = 1.28; 95% confidence interval [CI], 1.12 to 1.46).16
There are a large number of published RCTs on astragalus-based Chinese medicines combined with platinum-based chemotherapy.16 Although a meta-analysis was conducted on NSCLC treatments using astragalus-based Chinese medicines along with platinum-based chemotherapy,16 this study did not included Chinese language studies in its search strategy. In a previous meta-analysis on TCM treatments for hepatocellular carcinoma, 44/45 RCTs extracted were in Chinese compared to 1/45 RCTs in English; thereby illustrating the importance of including Chinese manuscripts in the analysis.10
Our objective is to systematically review the scientific literature for RCTs on astragalus-based Chinese medicines combined with platinum-based chemotherapy and to meta-analyze the pooled data from these RCTs. Should the results be favorable, astragalus-chemotherapy combination treatment may provide an important step forward for new interventions for patients with NSCLC.
Methods
Study inclusion criteria
We included any study that randomized patients with advanced NSCLC, provided the treatment group with Chinese herbal medicines containing the herb astragalus in combination with standard platinum-based chemotherapy, provided the control group with platinum-based chemotherapy alone, and reported data on at least one of our outcomes of interest (survival, tumor response, or performance status) with sufficient detail to permit calculation of the risk ratios of each outcome. We excluded pharmacokinetic studies and non-randomized trials. We excluded studies that reported only laboratory values rather than clinical responses. We also excluded direct comparisons of TCM formulations.
Search strategy
PW and EM worked independently, in duplicate, searching the following English electronic databases: MEDLINE (1966 to June 2009), AMED (1985 to June 2009), Alt Health Watch (1995 to June 2009), CINAHL (1982 to June 2009), Nursing and Allied Health Collection: Basic (1985 to June 2009), Cochrane Database of Systematic Reviews (2008). In addition, PW and YL, both fluent in Mandarin and Cantonese, searched the Chinese databases CNKI (1979 to June 2009) and Wan Fang (1994 to June 2009) independently. No language restrictions were placed on the searches.
Three reviewers (PW, EM and YL) assessed eligibility based on the full text papers and conducted data extraction, independently, using a standard pre-piloted form. Disagreements were resolved by consensus or by a third reviewer. If the required information was not available in the published article, we obtained additional information in correspondence with the authors. We included all evaluated outcome measures including: survival at 6, 12, 24, and 36 months, disease stage, Karnofsky performace (KP), and the response evaluation criteria in solid tumors (RECIST). The response is categorized as complete response (CR), partial response (PR) outcomes, stable disease (SD), progressive disease (PD) and as CR + PR as a composite for response rate.
In addition, we extracted data on trial quality, protocol, and outcomes assessed. We assessed quality through the reporting of the following criteria: sequence generation and allocation concealment. We also noted the language in which the paper was written and the setting the studies were conducted. These criteria were not used for weighting covariates in the meta-analysis; instead, these were considered a priori explanations for study heterogeneity.
All inclusion and exclusion criteria and the categorization of outcomes were made before any meta-analysis of the data. Our decision to group together for this meta-analysis those studies using platinum-based chemotherapy was based on the fact that this therapy is currently a standard treatment for advanced NSCLC. Following the example set by D’Addario et al17 and the Cochrane Collaboration’s Non–Small-Cell Lung Cancer Collaborative Group,18 platinum-based chemotherapy was grouped together as a therapeutic class when assessing efficacy of treatment for NSCLC. Each stage of the planning, design, analysis, and reporting of this meta-analysis was conducted in accordance with the QUOROM Statement guidelines.19
Analysis of outcomes
Survival
Given that all of the studies identified in our systematic search reported crude survival data as the number of patients in each treatment group who died by 6, 12, 24, or 36 months, we calculated the probability of failure (death) as the number of patients who had died by each time point divided by the total number of patients enrolled at the start of the trial for each treatment group. This approach is intentionally conservative: if some patients dropped out of the study, retaining them in the denominator as we have done would lower the estimate of effectiveness. This is analogous to an intention-to-treat analysis.20 The risk ratios of treatment failure (death) at each time point was calculated as the proportion who died in the astragalus-based herbal medicine plus platinum-based chemotherapy treatment group, compared to the proportion in the platinum-based chemotherapy group. Thus, RR less than 1 favors the combination regimen.
Objective tumor response
Given that most of the studies identified in our systematic search reported tumor response at conclusion of treatment using RECIST,21 we calculated the probability of tumor response as the number of patients experiencing any response (complete response plus partial response) divided by the total number of patients in each treatment group (complete response plus partial response plus no change plus progressive disease). The RR of tumor response was calculated as the probability of tumor response in the astragalus-based herbal medicine plus platinum-based chemotherapy treatment group, divided by this proportion in the platinum-based chemotherapy group. Thus, RR more than 1 favors the combination regimen. This is the approach for meta-analysis of tumor response recommended by Sutton et al.22
Performance status
Many of the studies identified in our systematic search reported performance status using the Karnofsky performance scale,23 with most using a 10-point change as the cutoff for improved or worse performance status, and a few others using a 20-point change as the cutoff. We therefore calculated the probability of improved or stable performance status as the proportion of improved or stable performance status: (>10-point increase plus no change) divided by the total (>10-point increase, plus no change, plus >10-point decrease). The RR of improved or stable performance status was calculated as the proportion of improved or stable performance status in the astragalus-based herbal medicine plus platinum-based chemotherapy treatment group, divided by this proportion in the platinum-based chemotherapy group. Thus, RR more than 1 favors the combination regimen.
Analysis
We used the random-effects model of DerSimonian and Laird24 to estimate the summary RR for each of the four outcomes: risk of death (at 6, 12, 24, and 36 months), tumor response, performance status, and severe chemotherapy toxicity. We used the I2 statistic to assess between-study heterogeneity and interpreted the outcome as <50% as non-problematic heterogeneity. To assess publication bias, we used the Begg-Mazumdar test, which examines the association between the effect estimates of individual studies and their variances; significant correlation between these two factors identifies publication bias.25
We applied the RR and 95% CI as our primary effect measure in this analysis. For analysis examining response, favorable results for the TCM intervention are in the direction greater than 1. In circumstances of zero outcome events in either arm of a trial, we used the Haldane method and added 1 to each arm, as suggested by Sheehe.26 We first pooled studies on all interventions versus all controls using the DerSimonian-Laird random effects method.27 This method recognizes and anchors studies as a sample of all potential studies, and incorporates an additional between-study component to the estimate of variability. We calculated the I2 statistic for each analysis as a measure of the proportion of the overall variation that is attributable to between-study heterogeneity.28 Forest plots are displayed for the primary analysis, showing individual study effect measures with 95% CIs and the overall DerSimmonian-Laird pooled estimate. We conducted a meta-regression analysis using the unrestricted maximum likelihood method to determine if the a priori covariates of TCM formulation yielded differing effects. We examined publication bias visually and through the Begg-Mazumdar tests. We calculated the optimal information size (OIS) required to determine adequate power across trials. We used Stats Direct and Comprehensive Meta-Analysis (Version 2) for all statistical procedures. All P values are 2-sided and a P value < 0.05 was considered significant. PW and EM conducted the analysis.
Results
Included studies
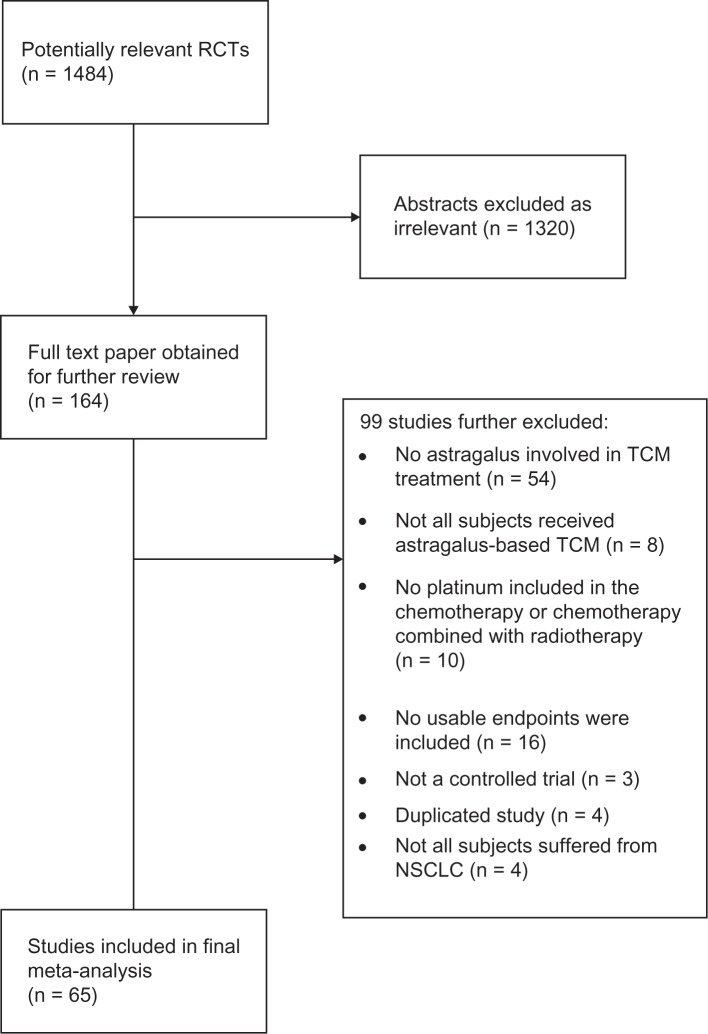
Our systematic search identified 1484 potentially relevant abstracts, of which 164 were identified as requiring full-text article retrieval (Figure 1). Close screening of these 221 studies identified 65 studies that met our inclusion criteria, containing a total of 4751 patients. Most studies were small (median 67, interquartile range 56 to 83). Studies poorly reported methodological issues including sequence generation (25%), allocation concealment (3%), and reporting of adverse events (67%). Table 1 provides the study characteristics.
Figure 1.
Flow diagram of included studies.
Table 1.
Included study characteristics
| Study | No of patients | Protocola | Ingredients | Disease stageb |
|---|---|---|---|---|
| Cao86 | 76 | CAP/NP + astragalus combination | Adenophora verticillata, Ophiopogonis japonicis, Schisandra chinensis, Astragalus membranaceus (astralagus), Oldenlandia diffusa, Eriobotrya japonica, Fritillaria cirrhosa, Arisaema amurense | III/IV |
| Chen32 | 43 | CAP + astragalus combination | Codonopsis pilosula, Astragalus membranaceus, Atractylodis macrocephala, Poria cocos, Pinellia ternata, Citrus reticulata, Dioscoreae opposita, Oldenlandia diffusa, Houttuynia cordata, Patrina villosa, Scutellaria barbata, Agrimonia pilosa, Ziziphus jujube | III/IV |
| Chen58 | 56 | NP + Yi Qi Yan Yin | Adenophora, Liriope, Rehmanniae praeparatum, astragalus, Radix pseudostellariae, Poria, Ligustrum, hawthorn, Chinese sage, Oldenlandia diffusa | III/IV |
| Cheng75 | 60 | FDH + Ai Di injection | Panax ginseng, astragalus, Eleutherococcus senticosus, Mylabirs cichoii | III/IV |
| Chu80,a | 128 | EAP + radiation + astragalus combination | Astragalus, Panax ginseng, Atractylodis macrocephala, Psoralea corylifolia, Asparagus cochinchinensis, Ophiopogonis japonicis, Scrophularia ningpoensis, Rehmannia glutinosa, Sparganium stoloniferum, Curcuma zeodoraria, Manis pentadactyla, Ostrea gigis shell, Trichosanthes kirilowii, Arisaema amurense, Scutellaria barbata, Oldenlandia diffusa | III/IV |
| Fan85 | 112 | CAP + astragalus combination | Codonopsis pilosulae, Atractylodis macrocephala, Glycyrrhiza uralensis, Astragalus, Ligustricum lucidum, Poria cocos, Salvia miltiorrhiza, Prunus persica | III/IV |
| Fei90 | 68 | MVP + astragalus combination | Astragalus, Codonopsis pilosulae, Rehmannia glutinosae, Asparagus cochinchinensis, Ophiopogonis japonicis, Scrophulariae ningpoensis, Cimicifuga foetida, Houttuynia cordata, Smilax glabra, Aloe vera | III/IV |
| Feng63 | 60 | GP + Feiliuping II | Astragalus, American ginseng, peach seed, safflower, petiolate paris [root], Oldenlandia diffusa | III/IV |
| Gao89 | 96 | MVP/CAP + astragalus | Astragalus | III/IV |
| Gao72 | 67 | GCN + Ai Di injection | Panax ginseng, astragalus, Eleutherococcus senticosus, Mylabirs cichoi | III/IV |
| Hong38 | 47 | NP + Ai Di injection | Panax ginseng, astragalus, Eleutherococcussenticosus, Mylabirs cichoi | III/IV |
| Jia87 | 68 | MVP/NP + astragalus combination | Curcuma longa, Curcuma aromatica, Snake, Prunellae vulgaris, Concha ostrea, Oldenlandia diffusa, astragalus, Panax qinquefolium | III/IV |
| Jin46 | 90 | MVP + astragalus combination | Astragalus, Polygonatum chinense, Ligustricum lucidum, Ganoderma lucidum, Salvia chinensis, Paris polyphylla, Drynaria fortunei, Citrus reticulata | III/IV |
| Kong66 | 52 | GP + Zhenqifuzhen capsule | Astragalus, Ligustrum | III/IV |
| Li81 | 114 | CE/CAP + astragalus combination | Astragalus, Polygonatum chinense, Panax ginseng, Agrimonia pilosa, Houttuynia cordata, Rheum palmatum, Polyporus umbellatus, Lobelia chinensis, Oldenlandia diffusa, Arisaema amurense, Coix lachryma, Prunus persica, Trichosanthes kirilowii, Prunella vulgaris | III/IV |
| Li78 | 90 | CE/CAP + astragalus combination | Astragalus, Adenophora verticillata, Lilium brownii, Ophiopogonis japonicis, Coix lachryma, Scutellaria barbata, Akebia trifoliata, Selaginella doederleinii, Agrimoniae pilosa, Polistes japonicus, Fritillaria thunbergii, Houttuynia cordata, Pinellia ternate, Glycyrrhizae | III/IV |
| Li54 | 35 | NP + Jian Pi Wen Shen | Astragalus, Atractylodes, Poria, Epimedium, cistanche, greater selaginella, Chinese sage | II–IV |
| Li56 | 70 | NP + Jian Pi Yi Shen | Pantax ginseng, astragalus, Poria, Atractylodes, dry orange peel, amomum fruit, Red peony root, Ligustrum, Cuscuta, eclipta, babchi seed, Encommiae ulmoide, Epimedium, Wolfberry, Polygonatum, hawthorn, gizzard lining, liquorice root | III, IV |
| Li61 | 83 | NP + TCM | Astragalus, Atractylodes, Adenophora, dendrobium, ligustrum, Wolfberry, lily, Chinese angelica, rehmanniae vaporata, hemlock parsley | III/IV |
| Li65 | 40 | NP/VP + Yi Qi Hua Tan | Campanumaea pilosula, astragalus, Trichosanthes rind, fritillary | III/IV |
| Lin52 | 202 | NP/MVP + Shan-dan capsule | Ginseng, astragalus, Atractylodes, gizzard lining, Trichosanthes, Pinellia, magnolia, Hovenia dulcis, Curcuma, salvia | I–IV |
| Liu34 | 77 | MAP + Jin Fu Kang | Astragalus, Adenophora verticillata, Ophiopogonis japonicis, Ligustricum lucidum, Selaginella doederleinii, Paris polyphylla | |
| Liu88* | 190 | CAP/MVP + Jin Fu Kang | Astragalus, Adenophora verticillata, Ophiopogonis japonicis, Ligustricum lucidum, Selaginella doederleinii, Paris polyphylla | II, III or IV |
| Liu35 | 144 | MAP + Jin Fu Kang | Astragalus, Adenophora verticillata, Ophiopogonis japonicis, Ligustricum lucidum, Selaginella doederleinii, Paris polyphylla | |
| Liu79 | 65 | EAP/CAP + astragalus combination | Astragalus, Pseudostellaria heterophylla, Adenophora verticillata, Atractylodis macrocephala, Rehmannia glutinosae, Coix lachryma, Poria cocos, Curcuma zeodoaria, Saliva miltiorrhiza, Panax notoginseng, Citrus aurantium, Cremastra variabilis, Prunus armeniaca | III/IV |
| Liu48 | 78 | CAP/MVP/CE + astragalus combination | Pseudostellaria heterophylla, astragalus, Atractylodis macrocephala, Poria cocos, Ophiopogonis japonicis, Oldenlandia diffusa, Scutellaria barbata, Taraxicum mongolicum, Paris polyphylla, Fritillaria thunbergii, Ligustricum lucidum, Buthus martensi, Scolopendra subspinipes, Hirudo nipponica, Coix lachryma, Glycyrrhizae uralensis | |
| Liu59 | 40 | NP/GP + Yi Qi Yan Yin | Astragalus, Adenophora, asparagus, Liriope, Ligustrum, greater selaginella, Chinese sage, paris [root] | III/IV |
| Liu69 | 60 | NP + Fuzhenguben | American ginseng, astragalus, Salvia, Chinese angelica, Heartleaf Houttuynia Herb, dry orange peel, Thunberg fritillary bulb | III/IV |
| Lu74 | 73 | NP + Ai Di injection | Panax ginseng, astragalus, Eleutherococcus senticosus, Mylabirs cichoi | III/IV |
| Lu42 | 96 | PAC + Ai Di injection | Panax ginseng, astragalus, Eleutherococcus, senticosus, Mylabirs cichoi | III/IV |
| Lu70 | 58 | NP + Zhongyao Zengmian | Black ant, Acanthopanax, raw oyster shell, astragalus, polygonatum [root], Epimedium herb, mulberry, Barbary wolfberry fruit, Campanumaea pilosula, atractylodes, Poria, Salvia | III/IV |
| Luo62 | 50 | TAX + DDP + Shenqifuzhen injection | Campanumaea pilosula, astragalus | III/IV |
| Luo43 | 108 | EP + TCM | Astragalus, Atractylodes, Dried longan pulp, Rehmanniae praeparatum, white peony root, Radix rehmanniae, dry orange peel, costustoot, hemlock parsley | III/IV |
| Mao92 | 60 | MVP + TCM | Astragalus, Chinese angelica, Atractylodes, Poria, polygonatum, Ligustrum, paris [root], ornus, dried orange peel | I–II |
| Shen36 | 80 | NP + TCM | Stragalus, Atractylodes, Adenophora, asparagus, Liriope, almond, Radix stemonae, Trichosanthes rind, raw arisaema [root], Shizandra berry, Chinese sage, Oldenlandia diffusa, prunella, raw oyster shell, Bulbus Fritillariae | IIIb/IV |
| Shen41 | 72 | NP + TCM | Astragalus, Atractylodes polygonatum, Adenophora, Liriope, umbilicaria, ligustrum, babchi seed, Spatholobus stem, Oldenlandia diffusa | III/IV |
| Sui33* | 80 | MVP + Astragalus combination | Lilium brownii, Rehmannia glutinosa, Scrophularia ningpoensis, Angelicae sinensis, Ophiopogonis japonicis, Paeonia lactiflora, Adenophora verticillata, Astragalus, Ligustricum lucidum, Paris polyphylla, Oldenlandia diffusa, Houttuynia cordata, Fritillaria cirrhosa, Cremastra variabilis (with individualized additions) | II, III or IV |
| Sun82 | 74 | CE-CAP/MVP/TC + Astragalus combination | Ganoderma lucidum, Pseudostellaria heterophylla, Coix lachryma, Atractylodis macrocephala, astragalus, Lycium chinense, Curcuma zeodoaria, Scolopendra subspinipes, Smilax glabra | III/IV |
| Tian93 | 40 | NP + Fu Zhen Jie Du | Astragalus, Adenophora, asparagus, Liriope, Ligustrum, epimedium, Oldenlandia diffusa | III/IV |
| Wang71 | 32 | NP/MVP + Ai Di injection | Panax ginseng, astragalus, Eleutherococcus senticosus, Mylabirs cichoi | III/IV |
| Wang77 | 98 | NP + Ai Di injection | Panax ginseng, astragalus, Eleutherococcus senticosus, Mylabirs cichoi | III/IV |
| Wang44 | 93 | MOP + astragalus combination | Adenophora verticillata, Asparagus cochinchinensis, Ophiopogonis japonicis, Pseudostellaria heterophylla, Astragalus, Curcuma zeodoaria, Atractylodis macrocephala, C. aromatica, Paeonia rubra, Paeonia lactiflora, Oldenlandia diffusa, Scutellaria barbata | |
| Wang31 | 58 | Cisplatin + astragalus combination | Astragalus, Panax ginseng, Lilium brownii, Adenophora verticillata, Ophiopogonis japonici, Fritillaria cirrhosa, Morus alba, Trichosanthes kirilowii, Scutellariae baicalensis, Paris polyphylla, Scutellaria barbata, Solanum nigrum, Lepidium apetalum, Atractylodis macrocephalae, Poria cocos, Phaseolus carcaratus, Ziziphus jujube | III/IV |
| Wang83 | 58 | CAP/MVP + astragalus combination | Panax ginseng, astragalus, Asparagus cochinchinensis, Ophiopogonis japonicis, Adenophora verticillata, Angelicae sinensis, Dioscoreae opposita, Dendrobium nobile, Polygonatum chinense, Schisandra chinensis, Ziziphus spinosa, Glycyrrhizae uralensis, Citrus reticulate | III/IV |
| Wang50 | 60 | NP + Ai Di injection | Panax ginseng, astragalus, Eleutherococcussenticosus, Mylabirs cichoi | III/IV |
| Wang40 | 46 | GP/TP + TCM | Astragalus, polygonatum, umbilicaria, babchi seed, Atractylodes | II–IV |
| Wen67 | 78 | NP + TCM | Astragalus, Campanumaea pilosula, Polygonatum, Ligustrum, Spatholobus stem, ass hide glue (melt), Pinellia | II–IV |
| Weng30 | 34 | CAP + astragalus combination | Codonopsis pilosulae, astragalus, Atractylodis macrocephala, Poria cocos, Pinellia ternata, Citrus reticulata, Dioscorea opposita, Oldenlandia diffusa, Houttuynia cordata, Patrina villosa, Scutellaria barbata, Agrimonia pilosa, Ziziphus jujube | |
| Xie68 | 60 | NP + TCM | Ginseng, Atractylodes, Poria, liquorice root, astragalus, Chinese angelica, Spatholobus stem, Pyrrosia [leaf ], Barbed skullcap herb | III/IV |
| Xu57 | 40 | TP + Ke Liu Wan | Curcuma, Mylabris, Gecko, Vietnamese Sophora Root, leech, ground beetle, astragalus | II–IV |
| Xu60 | 72 | GP/TP + TCM | Astragalus, Adenophora, Liriope, apricot kernel, Thunberg Fritillary Bulb, wild buckwheat rhizome, curcuma, Chinese honeylocust fruit, coix seed, Oldenlandia diffusa | IV |
| Xue37 | 72 | GP + TCM | Astragalus, Pseudostellaria root, Atractylodes, Poria, Pinellia, dry orange peel, mulberry root bark, Rehmanniae vaporata, heartleaf Houttuynia herb, Oldenlandia diffusa | IIIb/IV |
| Yang51 | 56 | GP + Ai Di injection | Panax ginseng, Astragalus, Eleutherococcussenticosus, Mylabirs cichoi | III/IV |
| Yang64 | 40 | GP + Ke Liu Wan | Astragalus, Mylabris, leech | III/IV |
| Yu47† | 92 | MAP + radiation + astragalus combination | Astragalus, Pseudostellaria heterophylla, Poria cocos, Amomum xanthioides, Salvia miltiorrhiza, Paeonia rubra, Spathalobus suberectus, Schisandra chinensis, Glycyrrhizae uralensis (with individualized additions) | III/IV |
| Zhang76 | 50 | NP + Ai Di injection | Panax ginseng, Astragalus, Eleutherococcus senticosus, Mylabirs cichoi | III/IV |
| Zhang73 | 98 | NP + Ai Di injection | Panax ginseng, Astragalus, Eleutherococcus senticosus, Mylabirs cichoi | III/IV |
| Zhang91 | 60 | EP + Ai Di injection | Panax ginseng, Astragalus, Eleutherococcus senticosus, Mylabirs cichoi | III/IV |
| Zhang55 | 70 | GP + Jian Pi Zi Yin | Astragalus, Atractylodes, Poria, cinnamomvine, coix seed, mix-fried licorice, pseudostellaria root, Adenophora, Liriope, Ligustrum, dry toad’s skin, Thunberg Fritillary Bulb, Buthus, notoginseng, Curcuma | II–IV |
| Zhang39 | 60 | MVP + Bu Qi Huo Xue | Astragalus, Radix pseudostellariae, Atractylodes, amomum fruit, Poria, salvia, Spatholobus stem, earthworm, red peony root, ligustrum, Oldenlandia diffusa, bulbus fritilariae, mix-fried licorice | III/IV |
| Zhong94 | 48 | GP + Yi Fei Jian | Poria, astragalus, Atractylodes, Pinellia, Hovenia dulcis, sweetflag, Hovenia dulcis, Platycodon root, greater selaginella, dry toad’s skin, Wolfberry, ligustrum, epimedium, mix-fried turtle shell, Shizandra berry | III/IV |
| Zhou53 | 60 | NP + Fu Zhen Bu Xu | Astragalus, Campanumaea pilosula, Poria, Atractylodes, Dioscoreae, Chinese angelica, white peony root, ass hide glue, wolfberry, Ligustrum, Semen psoraleae, cinnamon, Rehmanniae, Liriope, Adenophora, Dendrobium, Pinellia, dried orange peel, unibract fritillary bulb, liquorice root | II–IV |
| Zhou84 | 63 | CAP + astragalus combination | Panax ginseng, Atractylodis macrocephala, Poria cocos, astragalus, Polygonatum chinense, Ophiopogonis japonicis, Cordyceps chinensis, Lycium chinense, Ephedra sinica, Prunus armeniaca, Trionyx sinensis, Prunellae vulgaris, Oldenlandia diffusa, Scutellaria barbata, P. notoginseng | III/IV |
| Zhou45 | 91 | Cisplatin + astragalus combination | Adenophora verticillata, Ophiopogonis japonicus, Pseudostellaria heterophylla, Paris Polyphylla, astragalus, Scutellaria barbata, Curcuma zeodoaria, Atractylodis macrocephala, Paeonia rubra, Paeonia lactiflora, Oryza sativa, Hordeum vulgare, Massa fermenta, Crataegus pinnatifida, Cremastra variabilis, Curcuma aromatica, Citrus reticulata | III/IV |
| Zou49 | 60 | MAP + astragalus | Astragalus | III/IV |
In studies that included stage II patients, all patients received systemic therapy, and no patients received surgery.
In studies in which patients received radiation in addition to chemotherapy, all patients in both groups received radiation and chemotherapy. The only difference between the two groups was whether they received astragalus-based herbal medicine.
Abbreviations: CAP, cyclophosphamide, doxorubicin, cisplatin; NP, vinorelbine, cisplatin; FDH, hydroxy camptothecin, fluorouracil, leucovorin, cisplatin; EAP, etoposide, doxorubicin, cisplatin; MVP, mitomycin-C, vindesine, cisplatin; TC, paclitaxel, carboplatin; GCN, gemcitabine, cisplatin, vinorelbine; CE, cisplatin, etoposide; MAP, mitomycin, doxorubicin, cisplatin; EP, etoposide, cisplatin; MOP, mitomycin, vincristine, cisplatin.
Survival
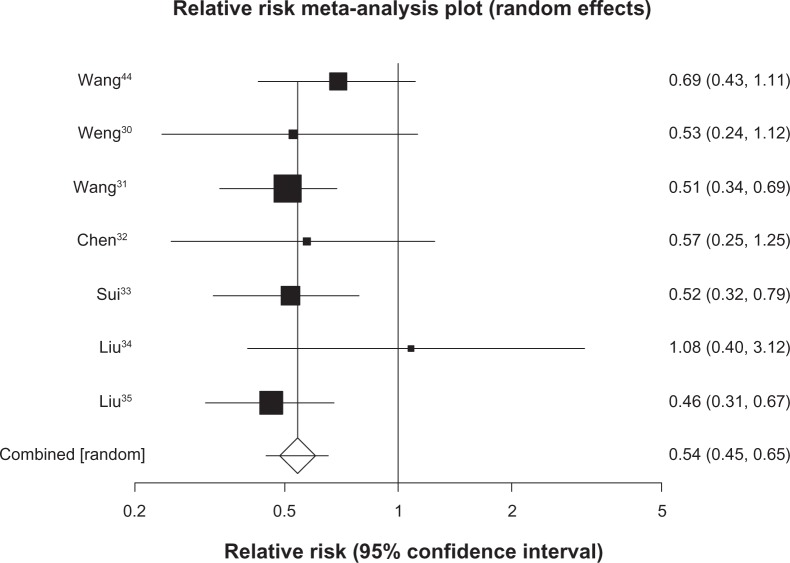
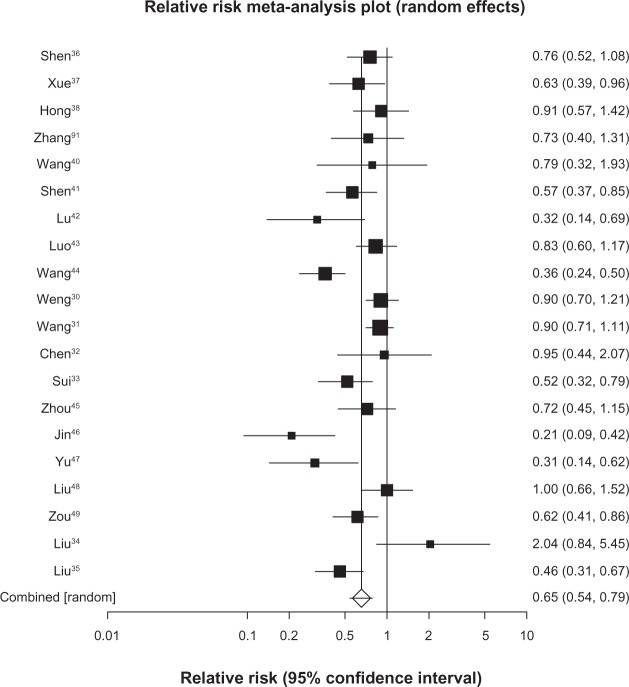
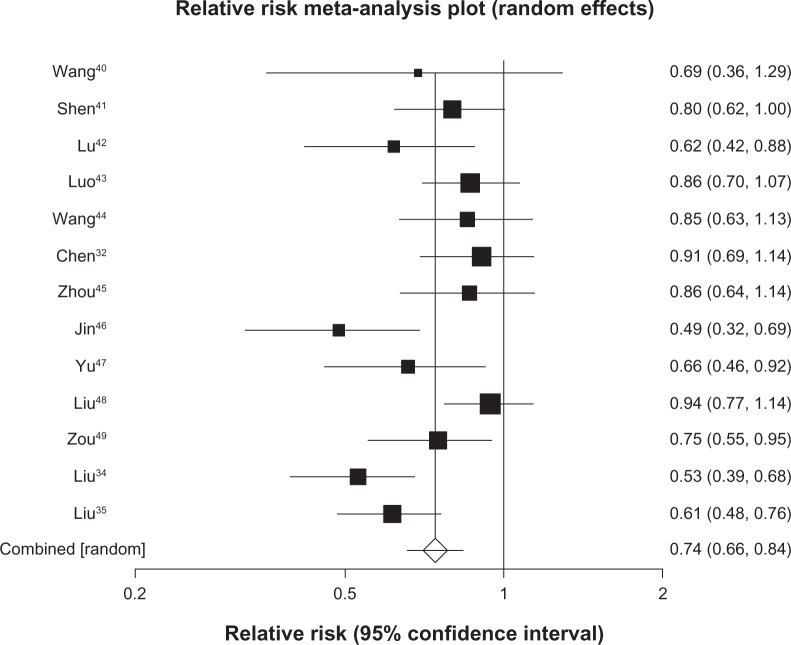
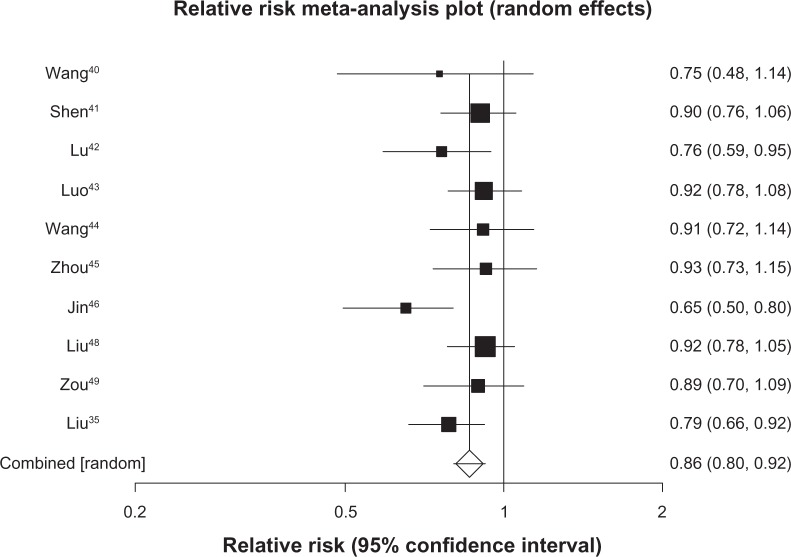
We pooled 7 studies29–35 (n = 529) reporting on survival at 6 months and found a pooled RR of 0.54 (95% CI, 0.45 to 0.65, P ≤ 0.0001, I2 = 0%, 95% CI, 0% to 58%, P = 0.74, see Figure 2). We included 20 trials30–49 (n = 1520) in our analysis of survival at 12 months and found a pooled RR of 0.65 (95% CI, 0.54 to 0.79, P ≤ 0.0001, I2 = 74%, 95% CI, 57% to 82%, P < 0.0001, see Figure 3). As 12-month survival was our primary outcome, we applied the publication bias assessment and found no evidence of publication bias (Kendall’s tau = –0.157, P = 0.31). We included 13 trials32,34,35,40–49 (n = 1090) with survival rates reported at 24 months and found a pooled RR of 0.74 (95% CI, 0.66 to 0.84, P ≤ 0.0001, I2 = 64%, 23% to 78%, P = 0.0008, see Figure 4). We included data from 10 trials35,40–46,48,49 (n = 878) reporting on survival at 36 months and found a pooled RR of 0.86 (95% CI, 0.80–0.92, P ≤ 0.0001, I2 = 29%, 95% CI, 0% to 65%, P = 0.17, see Figure 5).
Figure 2.
Six-month survival with astragalus-based herbs and platinum-based chemotherapy versus platinum-based chemotherapy alone.
Figure 3.
Twelve-month survival with astragalus-based herbs and platinum-based chemotherapy versus platinum-based chemotherapy alone.
Figure 4.
Twenty-four-month survival with astragalus-based herbs and platinum-based chemotherapy versus platinum-based chemotherapy alone.
Figure 5.
Thirty-six-month survival with astragalus-based herbs and platinum-based chemotherapy versus platinum-based chemotherapy alone.
Tumor response
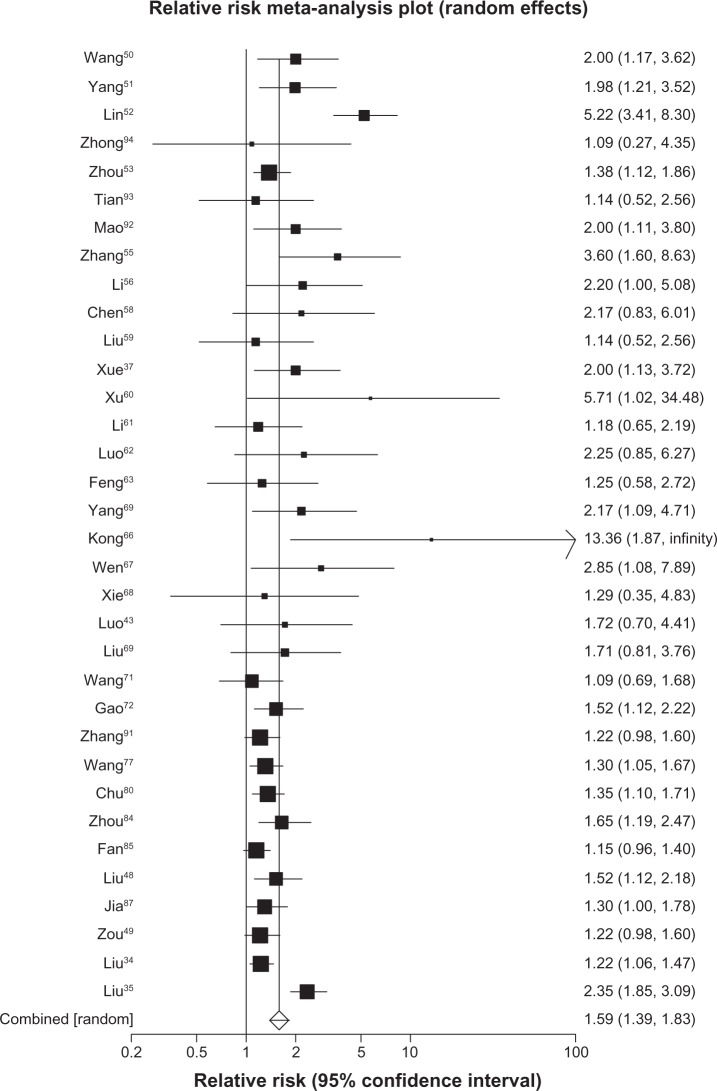
We were able to include data from 27 trials37,39–43,50–70 (n = 1759) reporting on complete responses to treatment and found a pooled RR of 1.43 in the direction of favorable outcomes for herbal-based treatment (95% CI, 0.98 to 2.10, P = 0.07, I2 = 0%, 95% CI, 0% to 42%, P ≤ 0.99). The same 27 trials reported on partial response to treatment and found a pooled RR of 1.35 favoring herbal treatment (95% CI, 1.19 to 1.53, P ≤ 0.0001, I2 = 0%, 95% CI, 0% to 38%, P = 0.99). When we applied a composite endpoint of any treatment response we pooled data from 57 trials30,32–35,37,39–45,48–50,87–90 and found a pooled RR of 1.35 in favor of herbal treatment (95% CI, 1.26 to 1.44, P ≤ 0.0001, I2 = 0%, 0% to 28%, P = 0.99, see Figure 6).
Figure 6.
Tumor response with astragalus-based herbs and platinum-based chemotherapy versus platinum-based chemotherapy alone.
Performance status
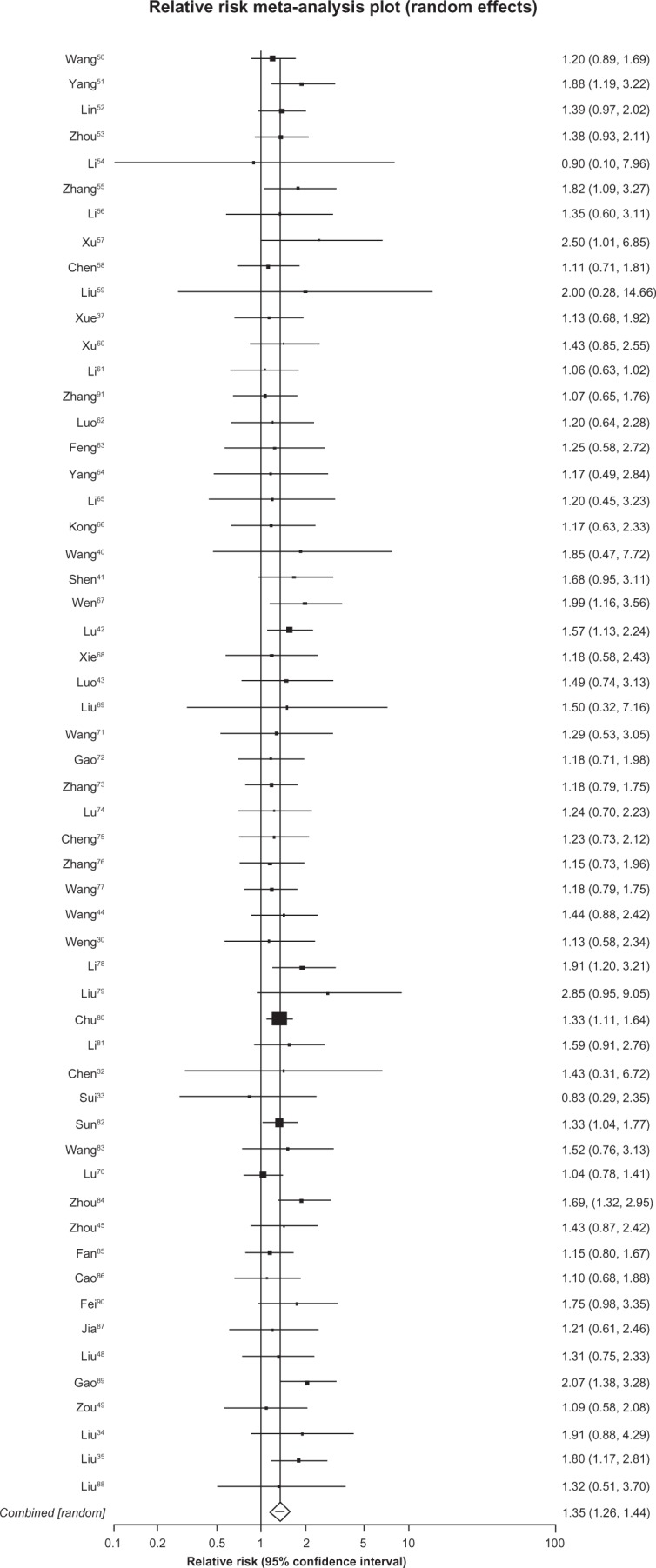
We included data from 35 trials35,37,43,48–53,55,56,58–64,66–72,77,80,84,85,87,88,91–94 (n = 2650) assessing stable or improved Karnofsky scores and found a pooled RR of 1.58 (95%, 1.39 to 1.81, P ≤ 0.0001, I2 = 69%, 55% to 78%, P ≤ 0.0001, see Figure 7).
Figure 7.
Stable/improved Karnofsky performance status with astragalus-based herbs and platinum-based chemotherapy versus platinum-based chemotherapy alone.
Discussion
Our findings should be of interest to cancer researchers and funding agencies. We found consistent evidence of improved survival and tumor response in astragalus-based herbal medicine therapy combined with platinum-based chemotherapy compared with platinum-based chemotherapy alone, the standard of care. While there is reason to be cautious of the quality of the included clinical trials, due to their small sample sizes and inadequate reporting of methodological issues, there has been a consistent direction of treatment effect that warrants further examination by experienced clinical trialists in a transparent manner.
Our study has both strengths and limitations to consider. Strengths include our extensive searching and identification of Chinese clinical trials that few systematic review groups may be able to accomplish. Our analysis used a broad approach that considered all astragalus containing herbal combinations as comparatively similar, thus allowing much greater power to detect an effect over a single trial. It is possible that specific combination exert a differing therapeutic effect; however we were unable to identify such specific formulations. The limitations of our study are predominantly related to the need for caution in interpreting the clinical trials. There is consistent evidence that publication bias may exist in Chinese medical journals and thus, only positive trials are published.95 Although we recognize favorable outcomes may be more likely to be published, our analysis actually does not find that only positive trials are published. If one examines the forest plots, on average, most trial are negative, but when pooled, become positive. A recent evaluation, by Wu et al, found that many studies labelled as RCTs with Chinese journals were, in fact, not randomized.96 In our own experience, we recognize many Chinese clinical trialists have not been exposed to appropriate clinical epidemiology training. Yet even if our analysis includes predominantly non-randomized studies, the consistency of therapeutic effect warrants further examination. Other systematic reviews of published studies from Chinese journals have identified specific journals with better study quality, and also found trends in improvement of study quality over time.97 We assessed publication bias in our primary outcome (survival at 12 months) and did not find statistical evidence of bias, although funnel plots and statistical tests cannot identify the absence of publication bias. The reporting of quality features including how trials were randomized and how allocation concealment was achieved adds further caution to our study interpretation. While the inadequate reporting of these items is undesirable, there is, as yet, conflicting evidence that the reporting of these issues affects the magnitude of treatment effect.98–100
Given the consistency of treatment effect, large number of trials, importance of the disease and the caution about study quality it seems only reasonable that a clinical trial should be conducted in a Western setting that can ensure adequate sample size and concealed allocation to study arms. Such a clinical trial would provide strong inferences into the believability of our meta-analysis findings and could massively impact drug development. However, until a trial is conducted, we recommend counseling interested patients to maintain cautious optimism on any treatment effect and discuss with their oncology physician about potential costs and harms.
Our study builds on an existing collaboration between researchers in China and in North America. We recognize that important and effective drugs have been discovered by examining the Chinese medical literature for existing clinical trials. Artemisin-based therapy for malaria and oseltamivir (Tamiflu®) for influenza are two compelling examples.101,102 We have previously used this approach for examining potentially effective interventions for hepatocellular cancers and found evidence of existing interventions that have never been evaluated in the West, despite compelling evidence of effectiveness. We believe that this approach represents a low-cost approach to identifying potentially effective new opportunities for drug development.
Additional research is needed to further understand the specific immunologic and cytotoxic mechanisms that astragalus may affect as an adjunct to chemotherapy for the treatment of advanced NSCLC.
Acknowledgments
This study was generously supported by the Lotte and John Hecht Memorial Foundation. We thank Y Liang for searching assistance.
Footnotes
Disclosure
The authors declare no potential conflicts of interest.
References
- 1.Carney DN. Lung cancer – time to move on from chemotherapy. N Engl J Med. 2002;346:126–128. doi: 10.1056/NEJM200201103460211. [DOI] [PubMed] [Google Scholar]
- 2.Chute JP, Chen T, Feigal E, Simon R, Johnson BE. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol. 1999;17:1794–1801. doi: 10.1200/JCO.1999.17.6.1794. [DOI] [PubMed] [Google Scholar]
- 3.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 4.NCCN National Comprehensive Cancer Network (NCCN) – Clinical Practice Guidelines: Non-small Cell Lung Cancer v.2. 2009. Available from http://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf.
- 5.Burris HA., 3rd Shortcomings of current therapies for non-small-cell lung cancer: unmet medical needs. Oncogene. 2009;28(Suppl 1):S4–S13. doi: 10.1038/onc.2009.196. [DOI] [PubMed] [Google Scholar]
- 6.Breathnach OS, Freidlin B, Conley B, et al. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol. 2001;19:1734–1742. doi: 10.1200/JCO.2001.19.6.1734. [DOI] [PubMed] [Google Scholar]
- 7.Kelly K, Crowley J, Bunn PA, Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19:3210–3218. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 8.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 9.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 10.Wu P, Dugoua JJ, Eyawo O, Mills EJ. Traditional Chinese Medicines in the treatment of hepatocellular cancers: a systematic review and meta-analysis. J Exp Clin Cancer Res. 2009;28:112. doi: 10.1186/1756-9966-28-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu DT, Wong WL, Mavligit GM. Immunotherapy with Chinese medicinal herbs. II. Reversal of cyclophosphamide-induced immune suppression by administration of fractionated Astragalus membranaceus in vivo. J Clin Lab Immunol. 1988;25:125–129. [PubMed] [Google Scholar]
- 12.Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004;320:1103–1111. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Hersh EM, Lee SL, McLaughlin M, Loo TL, Mavligit GM. Preliminary observations on the effects of the Chinese medicinal herbs Astragalus membranaceus and Ligustrum lucidum on lymphocyte blastogenic responses. J Biol Response Mod. 1983;2:227–237. [PubMed] [Google Scholar]
- 14.Chu DT, Lepe-Zuniga J, Wong WL, LaPushin R, Mavligit GM. Fractionated extract of Astragalus membranaceus, a Chinese medicinal herb, potentiates LAK cell cytotoxicity generated by a low dose of recombinant interleukin-2. J Clin Lab Immunol. 1988;26:183–187. [PubMed] [Google Scholar]
- 15.Chu DT, Wong WL, Mavligit GM. Immunotherapy with Chinese medicinal herbs. I. Immune restoration of local xenogeneic graft-versus-host reaction in cancer patients by fractionated Astragalus membranaceus in vitro. J Clin Lab Immunol. 1988;25:119–123. [PubMed] [Google Scholar]
- 16.McCulloch M, See C, Shu XJ, et al. Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non-small-cell lung cancer: meta-analysis of randomized trials. J Clin Oncol. 2006;24:419–430. doi: 10.1200/JCO.2005.03.6392. [DOI] [PubMed] [Google Scholar]
- 17.D’Addario G, Pintilie M, Leighl NB, Feld R, Cerny T, Shepherd FA. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol. 2005;23:2926–2936. doi: 10.1200/JCO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 18.Non-Small Cell Lung Cancer Collaborative Group Chemotherapy for non-small cell lung cancer. Cochrane Database System Review. 2004:CD002139. doi: 10.1002/14651858.CD002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 20.Heritier SR, Gebski VJ, Keech AC. Inclusion of patients in clinical trial analysis: the intention-to-treat principle. Med J Aust. 2003;179:438–440. doi: 10.5694/j.1326-5377.2003.tb05627.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Orgnaization; 1979. [Google Scholar]
- 22.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-Analysis in Medical Research. Chichester, United Kingdom: John Wiley & Sons; 2000. [Google Scholar]
- 23.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45:2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 26.Sheehe PR. Combination of log relative risk in retrospective studies of disease. Am J Public Health Nations Health. 1966;56:1745–1750. doi: 10.2105/ajph.56.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Wang JX, Zhu CL. A clinical observation of the effect of supplementing Qi and nourishing yin prescription combined with MOP on the stage III IV of the nonsmall cell lung cancer. Shi Yong Zhong Xi Yi Jie He Za Zhi. 1997;10:1839–1840. [Google Scholar]
- 30.Weng JY. Chinese and Western medicine in the treatment of 19 cases of advanced nonsmall cell lung cancer. Zhejiang Zhong Liu. 1998;4:129. [Google Scholar]
- 31.Wang YQ. Integrated Chinese and Western medicine combined treatment for primary-stage lung cancer with pleural effusion. Liaoning Zhongyi Zazhi. 2000;27:129. [Google Scholar]
- 32.Chen GP, Weng JY. Treatment value of Chinese medicine combined with chemotherapy for excision of lung cancer. Zhejiang Zhong Xi Yi Jie He Za Zhi. 2000;10:407–408. [Google Scholar]
- 33.Sui DJ, Zhou LN, Li G. Fuzhengjian to treat middle to late-stage lung cancer in 40 patients. Shanxi Zhongyi. 2001;17:16–17. [Google Scholar]
- 34.Liu JX, Shi ZM, Xu ZH, et al. Clinical observations of Jin Fu Kang Kou Fu Ye in treating non-small-cell lung cancer. Zhong Yi Za Zhi. 1997;38:727–729. [Google Scholar]
- 35.Liu JX, Shi ZM, Li HG, et al. Clinical observations of lung nourishing anti-tumor beverage in treating non-small-cell lung cancer in 271 cases. Shanghai Zhong Yi Yao Za Zhi. 2001;2:4–6. [Google Scholar]
- 36.Shen J. The survival rate in 80 non-small cell lung cancer patients when using TCM combined with chemotherapy. World Health Digest Chinese Traditional Medicine. 2008;7:172–173. [Google Scholar]
- 37.Xue YB, Zhou XY, Shi HC. Clinical observation in 36 non-small cell lung cancer treating with chemotherapy and TCM. Jiangsu Zhongyiyao. 2008;40:42–44. [Google Scholar]
- 38.Hong Z, LX P, Chen JF, Huang FL, Zheng XL. Traditional Chinese Medicine Ai Di Parenteral solution with NP regimen for reversal of multidrug resistance in non-small cell lung cancer with P-gp overexpression. Journal of Clinical Medicine in Practice. 2005;9:30–33. [Google Scholar]
- 39.Zhang WQ, Du FM, Zhao MC, Zhao SF, Zhao YM. Buqihuoxue protocol combined with chemotherapy in advanced non-small cell lung cancer. Zhejiang Zhongxiyi Jiehe Zazhi. 2005;15:340–341. [Google Scholar]
- 40.Wang ZQ, Xu ZY, Zhou WD, Deng HB, Zhang M, Zhang H. Clinical research of TCM combined with western medicine in non-small cell lung cancer treatment. Traditional ChineseMedicine Journal. 2006;5:41–43. [Google Scholar]
- 41.Shen DM, Xu F. Clinical observation of combined Chinese and western medicine in treating non-small cell lung cancer. Shanghai Journal of Traditional Chinese Medicine. 2005;39:5–6. [Google Scholar]
- 42.Lu XC, Liu JL, Cui HY, Wang LQ, Wu S, Zhang QX. Clinical research of combined Chinese and western medicine in non-small cell lung cancer treatment. Modern Oncology. 2005;13:832–834. [Google Scholar]
- 43.Luo XL, Qing DM, Li JC, Gong HW. TCM treatment in 174 old patients with non-small cell lung cancer. Zhongliu Fangzhi Yanjiu. 2004;31:657–658. [Google Scholar]
- 44.Wang JX, Zhu CL. A clinical observation of the effect of supplementing Qi and nourishing yin prescription combined with MOP on the stage III IV of the nonsmall cell lung cancer. Shi Yong Zhong Xi Yi Jie He Za Zhi. 1997;10:1839–1840. [Google Scholar]
- 45.Zhou HF. Chinese herbal medicine Yi Qi Yang Yin Tang combined with vinorelbine-cisplatin chemotherapy in the treatment of 46 patients with stage III and IV non-small-cell lung cancer. Zhejiang Zhongyi Zazhi. 2003;38:474. [Google Scholar]
- 46.Jin CJ, Li LN, Cui Q, et al. Clinical observation of chemotherapy and Chinese Medicine in treating advanced non-small-cell lung cancer. Shanghai Zhong Yi Yao Za Zhi. 2003;37:16–17. [Google Scholar]
- 47.Yu LL, Han ZY. Clinical observation of Chinese herbal medicine combined with interventional chemoradiotherapy in the treatment of non-small-cell lung cancer. Zhongguo Zhongxiyi Jiehe Zazhi. 2003;23:56–57. [Google Scholar]
- 48.Liu SS. Clinical research of Fei Yi Liu He Ji in the treatment of primary bronchopulmonary cancer. Shan Dong Zhongyiyao Daxue Xuebue. 2004;28:99–102. [Google Scholar]
- 49.Zou YH, Liu XM. Effect of astragalus injection combined with chemotherapy on quality of life in patients with advanced non-small cell lung cancer [Article in Chinese] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23:733–735. [PubMed] [Google Scholar]
- 50.Wang WR. Ai Di injection combined with NP in the treatment of advanced non-small cell lung cancer. Jiangxi Zhongyiyao Daxue Xuebao. 2008;10:125–126. [Google Scholar]
- 51.Yang QR, Chen WZ, Huang JD, Chen XL. Ai Di injection combined with chemotherapy in the treatment of middle to late-stage of lung cancer. Guangming Zhongyi. 2008;23:1760–1761. [Google Scholar]
- 52.Lin HS, Li DR. Phase II clinical trial for evaluating the adjuvant chemotherapy for non-small cell lung cancer with Shen-Dan capsule and related Traditional Chinese Medicine. Chinese Journal of New Drugs. 2007;16:1791–1795. [Google Scholar]
- 53.Zhou Q. Clinical observation of treating 30 lung cancer patients with Fuzhenbuxu Decoction. Henan Zhongyi. 2009;29:369–370. [Google Scholar]
- 54.Li CJ, Liu JX, Liu LS, Sun JL, Li HG. Regulatory effect of jianpiwenshen recipein combining with chemotherapy on the blood level of es-tradiol in patients with lung cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:26–29. [PubMed] [Google Scholar]
- 55.Zhang HT, Fang ST, Huang ZY, Liu LW. Clinical observation in treating non-small cell lung cancer with chemotherapy and Jianpiyangyin and Huataiquyu Decoction. Hubei Zhongyi Zazhi. 2008;30:11–12. [Google Scholar]
- 56.Li DF, Zhang H, Zhou M. Clinical study of treating middle to late stage non-small cell lung cancer using chemotherapy combined with Jianpiyishen Decoction. Journal of Sichuan of Traditional Chinese Medicine. 2008;26:51–53. [Google Scholar]
- 57.Xu L, Li AY, Wang JX. FACT-L clinical research of Keliuwan combined with TP in advanced non-small cell lung cancer treatment. Jilin Zhongyiyao. 2008;28:794–796. [Google Scholar]
- 58.Chen GY, Cui L, Liu JJ. Clinical study of treating middle to late stage of non-small cell lung cancer with chemotherapy and Yiqiyangyin Decoction. Jiangsu Zhongyiyao. 2007;39:34–35. [Google Scholar]
- 59.Liu LS, Liu JX, Li CJ, Tian JH, Shi ZM. Clinical Effect of YiqiYangyin Jiedu Decoction in Treating Patients with Advanced Non-small Cell Lung Cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28:352–355. [PubMed] [Google Scholar]
- 60.Xu YQ, Ding R, Zhang Y, Luo Y, Xu JW. Clinical observation in non-small cell treatment with TCM and chemotherapy. Liaoning Zhongyi Zazhi. 2007;34:916–917. [Google Scholar]
- 61.Li L, Qing YK, J G, Liu Q, Zhang ZW. Chemotherapy using NP combined with TCM in the treatment of 44 patients with advanced nonsmall – cell lung cancer. Zhongguo Zhongxiyi Jiehe Waike Zazhi. 2005;11:489–490. [Google Scholar]
- 62.Luo SZ, Long JH, Yu XY. The clinical observation of advanced non-small cell lung cancer treated with Shenqifuzhen injections combined with PTX and DDP. Cancer Research and Clinic. 2006;18:181–183. [Google Scholar]
- 63.Feng L, Hua JB, Piao BK. Clinical Study of Feiliuping on Quality of Life of Lung Cancer Patients. Chinese Journal of Information on TCM. 2006;13:12–13. [Google Scholar]
- 64.Yang C, Wang RP. Clinical observation of Keliuwan in advanced non-small cell lung cancer treatment. Chinese Archives of Traditional Chinese Medicine. 2004;22:2090–2091. [Google Scholar]
- 65.Li M. Clinical observation of Yiqihuatan methods in non-small cell lung cancer treatment. Journal of Hebei TCM and Pharmacology. 2007;22:12–14. [Google Scholar]
- 66.Kong YZ, Sun Y, Len JX, Yang QL. Zhenqifuzhen capsule combined with chemotherapy in non-small cell lung cancer treatment. Jiaoning Zhongyi Zazhi. 2005;32:872–873. [Google Scholar]
- 67.Wen HY, Wang XS, Chen MX. A randomised controled study of combined Chinese and western medicine in treating non-small cell lung cancer. Liaoning Zhongyi Zazhi. 2007;34:75–76. [Google Scholar]
- 68.Xie Y, He WG. TCM Jianji II combined with NP chemotherapy in advanced non-small cell lung cancer treatment. Cancer Research and Clinic. 2006;18:701–703. [Google Scholar]
- 69.Liu F. Clinical Observation to Non-small cellular lung cancer treated with fuzheng guben decoction and chemotherapy. Zhejiang Zhongyiyao Daxue Xuebao. 2007;31:316–318. [Google Scholar]
- 70.Lu YX, Bai GD, Huang DP, Qin B, Liu M. Zhongyao Zengmian Decoction combined with chemotherapy in the treatment of advanced non-small cell lung cancer. 2008;31:12–14. [Google Scholar]
- 71.Wang C, Wang LM, Xie GR. Clinical observation of Aidi injectable liquid simultaneously used with chemotherapy to treat non-small-cell lung cancer. Tianjin Zhongyi. 2002;19:61. [Google Scholar]
- 72.Gao P. Clinical observation of Ai Di Zhu She Ye combined with chemotherapy in treating late stage non-small cell lung cancer. Henan Zhongyi. 2003;10:45–46. [Google Scholar]
- 73.Zhang NS, Yang DZ, Niu RG, et al. Analysis of 98 cases of middle and late stage non-small-cell lung cancer treated with Ai Di Zhu She Ye combined with chemotherapy. Zhongyiyao Xuekan. 2003;21:1599. [Google Scholar]
- 74.Lu J, Lu L, Fang J. Analysis of 75 patients with late stage non-small-cell lung cancer treated with Ai Di Zhu She Ye and chemotherapy. Shiyong Zhongyi Neike Zazhi. 2003;17:136. [Google Scholar]
- 75.Cheng WC, Ma L, Jin C, et al. Observation of the effects of Ai Di Zhu She Ye combined with Irinotecan protocol in the treatment of middle and late stage non-small-cell lung cancer. Zhongliu Yanjiu Yu Linchuang. 2003;15:199–200. [Google Scholar]
- 76.Zhang LH, Wang FZ, Liu GR. Observation of the effect of Chinese herbal medicine Ai Di Zhu She Ye combined with chemotherapy on short-term quality of life in late stage nonsmall cell lung cancer. Zhongguo Shiyong Neike Zazhi. 2003;23:427–428. [Google Scholar]
- 77.Wang DJ, Chen YL, Ren J, et al. A randomized clinical study on the efficacy of Aidi injection combined with chemotherapy in the treatment of advanced nonsmall cell lung cancer. Zhongguo Feiai Zazhi. 2004;7:247–249. doi: 10.3779/j.issn.1009-3419.2004.03.16. [DOI] [PubMed] [Google Scholar]
- 78.Li TS. Clinical study of Fei Bao Dan combined with chemotherapy in the treatment of advanced non-small-cell lung cancer. Zhongguo Zhongyiyao Xinxi Zazhi. 1999;6:50. [Google Scholar]
- 79.Liu Q. Observations of effectiveness of Yi Qi San Jie Fang combined with chemotherapy in treating 35 cases of middle and late state non-small-lung cancer. Shanxi Zhongyi. 1999;15:26–27. [Google Scholar]
- 80.Chu GT, Cao YS, Zheng AP. 64 cases of non-small-cell lung cancer treated with Fu Zheng Qu Yu Tang combined with chemoradiotherapy. He Nan Zhongyiyao Xuekan. 1999;14:31–32. [Google Scholar]
- 81.Li DY, Ou CM, Li GD, et al. Clinical observations of Fu Zheng Pai Du Kang Ai Fang in increasing effectiveness and reducing toxicity of chemotherapy in non-small-cell lung cancer. Zhongguo Zhongxiyi Jiehe Zazhi. 2000;20:208–209. [Google Scholar]
- 82.Sun SX, Wang XM, Yu RC. Analysis of Chinese medicine to benefit qi and tonify blood in the treatment of late-stage non-small-cell lung cancer. Zhongguo Zhongyiyao Xinxi Zazhi. 2002;9:57–58. [Google Scholar]
- 83.Wang ZL. Clinical observation of Yi Qi Yang Yin Gu Ben Tang to reduce side effects of chemotherapy in patients with non-small-cell lung cancer. Jiangxi Zhongyiyao. 2002;33:40. [Google Scholar]
- 84.Zhou H, Zhang DC. Zhang Shi Kang Ai San No. 5 combined with chemotherapy the treatment of late stage lung cancer. Hubei Zhongyi Zazhi. 2003;25:27–28. [Google Scholar]
- 85.Fan YF, Xu QP, Jiang N, et al. Clinical study of tumor response with integrated Chinese Western medicine in the treatment of non-small-cell lung cancer. Fujian Zhongyiyao. 2003;34:6–7. [Google Scholar]
- 86.Cao Y, Yuan SH, Qiao ZB, et al. Clinical research on Yi Qi Yang Yin Fang combined with chemotherapy in the treatment of late stage non-small-cell lung cancer. Zhongguo Zhongyi Jichu Yixue Zazhi. 2003;9:32–33. 595. [Google Scholar]
- 87.Jia YJ, Shi FM, Jia CS, et al. Clinical research of Xiao Yan Tang in the treatment of late stage non-small-cell lung cancer. Tianjin Zhongyiyao. 2004;21:108–110. [Google Scholar]
- 88.Liu JX, Pan MQ, Li YH, et al. Clinical study of Jin Fu Kang oral liquid for treating non-small-cell lung cancer. Zhongliu. 2001;21:463–465. [Google Scholar]
- 89.Gao CR, Xia HP, Shi JG, et al. Clinical observation of Shenqi pills combined with chemotherapy to treat non-small-cell lung cancer. Zhongguo Zhongxiyi Jiehe Zazhi. 2001;21:908. [Google Scholar]
- 90.Fei CB, Wang YM, Wang LL, et al. Fu Zheng Chinese medicine combined with chemotherapy in the treatment of non-small-cell lung cancer. Liaoning Zhongyi Zazhi. 2003;30:266–267. [Google Scholar]
- 91.Zhang XH, Li J, Sun CY, et al. Clinical research on Ai Di Zhu She Ye combined with chemotherapy in the treatment of middle and late stage non-small-cell lung cancer. Zhonghua Zhongxiyi Zazhi. 2004;5:230–231. [Google Scholar]
- 92.Mao CH. Effects of chemotherapy and TCM on immune function and quality of life of patients after the operation of lung cancer at nonage. Guiding Journal of TCM. 2007;13:16–17. [Google Scholar]
- 93.Tian JH, Liu LS, CJ L, Shi ZM. Effects of fuzheng jiedu decoction on quality of life of patients with non-small cell lung cancer. ACTA Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai. 2007;21:34–37. [Google Scholar]
- 94.Zhong Y, Zhou RY, Wu LY, Wang YP, Lin HY. Study of clinical effect of feiyi decoction in treating NSCL of elderly patients combined with chemothreapy. Liaoning Zhongyi Zazhi. 2008;38:1722–1724. [Google Scholar]
- 95.Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials. 1998;19:159–166. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]
- 96.Wu T, Li Y, Bian Z, Liu G, Moher D. Randomized trials published in some Chinese journals: how many are randomized? Trials. 2009;10:46. doi: 10.1186/1745-6215-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu GP, Gao SW. Quality of clinical trials of Chinese herbal drugs, a review of 314 published papers [Article in Chinese] Zhongguo Zhong Xi Yi Jie He Za Zhi. 1994;14:50–52. [PubMed] [Google Scholar]
- 98.Schulz KF, Chalmers I, Grimes DA, Altman DG. Assessing the quality of randomization from reports of controlled trials published in obstetrics and gynecology journals. JAMA. 1994;272:125–128. [PubMed] [Google Scholar]
- 99.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 100.Balk EM, Bonis PA, Moskowitz H, et al. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. JAMA. 2002;287:2973–2982. doi: 10.1001/jama.287.22.2973. [DOI] [PubMed] [Google Scholar]
- 101.Pittler MH, Ernst E. Artemether for severe malaria: a meta-analysis of randomized clinical trials. Clin Infect Dis. 1999;28:597–601. doi: 10.1086/515148. [DOI] [PubMed] [Google Scholar]
- 102.Matheson NJ, Harnden AR, Perera R, Sheikh A, Symmonds-Abrahams M. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database Syst Rev. 2007:CD002744. doi: 10.1002/14651858.CD002744.pub2. [DOI] [PubMed] [Google Scholar]