Abstract
Lung cancer (LC) has become the leading cancer-related cause of death in the US and in developed European countries in the last decade. Its incidence is still growing in females and in smokers. Surgery remains the treatment of choice whenever feasible, but unfortunately, many patients have an advanced LC at presentation and one-third of potentially operable patients do not receive a tumor resection because of their low compliance for intervention due to their compromised cardiopulmonary functions and other comorbidities. For these patients the alternative therapeutic options are stereotactic radiotherapy or percutaneous radiofrequency. When surgery is planned, an anatomical resection (segmentectomy, lobectomy, bilobectomy, pneumonectomy, sleeve lobectomy) is usually performed; wedge resection (considered as a nonanatomical one) is generally the accepted option for unfit patients. The recent increase in discovering small and peripheral LCs and/or ground-glass opacities with screening programs has dramatically increased surgeons’ interest in limited resections. The role of these resections is discussed. Also, recent improvements in molecular biology techniques have increased the chemotherapic options for neoadjuvant LC treatment. The role and the importance of targeted chemotherapy is also discussed.
Keywords: lung cancer, adenocarcinoma, surgery, radiofrequency, radiotherapy, chemotherapy
Introduction
Lung cancer (LC) is a very significant and important public health problem with approximately 170,000 new cases diagnosed in the United States (US) per year.1 It has become the leading cause of cancer death in the US and around the world in recent years: its incidence and mortality are highest in the US and in the developed European countries and lower in undeveloped countries of Central and South America, Asia, and Africa.1 LC deaths will continue to rise worldwide due to the increasing use of tobacco.2–4
The World Health Organization (WHO) separates histological tumor types into 2 categories: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC).5 Histological NSCLC’s subtypes are shown in Table 1. SCLC belongs to the spectrum of neuroendocrine lung tumors (Table 2).6
Table 1.
NSCLC histological subtypes
| Squamous cell carcinoma |
| Adenocarcinoma |
| Large cell carcinoma |
| Adenosquamous carcinoma |
| Sarcomatoid carcinoma |
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. World Health Organization Classification of Tumours. Pathology and Genetics. Tumours of the Lung, Pleura, Thymus and Heart. IARC Presse: Lyon, France; 2004.5
Table 2.
The spectrum of neuroendocrine (NE) proliferations and tumors
| NE cell hyperplasia and tumorlets |
| NE cell hyperplasia with fibrosis and/or inflammation |
| NE cell hyperplasia adjacent to carcinoids |
| Diffuse idiopathic NE cell hyperplasia with or without airway fibrosis |
| Tumorlets |
| Tumors with NE morphology |
| Typical carcinoid |
| Atypical carcinoid |
| Large-cell neuroendocrine carcinoma |
| Small-cell carcinoma |
| NSCLC with NE differentiation |
| Other tumors with NE properties |
| Pulmonary blastoma |
| Primitive neuroectodermal tumor |
| Desmoplastic round cell rhabdoid phenotype |
| Paraganglioma |
Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. WHO histological classification of tumours. Histological typing of lung and pleural tumours. 3rd edition Springer-Verlag: Berlin.6
In 50% of cases NSCLC is a disseminated disease with a gloomy prognosis and a median survival <1 year; the overall 5-year survival does not exceed 15%. Patients with advanced disease have a very poor prognosis (Stage IIIB inoperable patients’ 5-year survival rate is <10% and <2% for Stage IV diseases).
Surgical resection remains the mainstay of therapy for NSCLC, when feasible; unfortunately many patients present with an advanced disease at the time of diagnosis (late diagnosis is, in fact, very common), or do not receive tumor resection because they do not tolerate surgery due to compromised cardiopulmonary functions.
This review paper analyzes and discusses treatment options that have recently become available for NSCLC management, and covers surgery, stereotactic radiotherapy, radiofrequency, and targeted chemotherapy.
Surgery
Stage I and II NSCLC patients should be approached as potential candidates for surgery and the majority of them undergo the intervention. Patients with Stage IIIA or IIIB NSCLC are not eligible for an immediate operation: they must be considered for a preoperative multimodal therapy (chemotherapy, radiotherapy or both in combination), and then reconsidered for surgery in case of the effectiveness of the treatment.
A complete tumor resection is the goal of surgery for operable NSCLCs; incomplete resections, instead, not only do not have any therapeutic advantage, but may also reduce any of the potential benefits gained with a postoperative chemo/radiotherapy.
Anatomic interventions (segmentectomy, lobectomy/bilobectomy, bronchoplastic lobectomy and pneumonectomy) are the standard of care for operable LCs. The 5-year survival rates for Stage I NSCLC following lobectomy are reported to be between 60% and 70%.6 Nonanatomic resections, such as wedge resections, are historically recommended for those patients with a poor cardiac or respiratory function or in cases of multiple synchronous or metachronous tumors only.
New trends of early diagnosis of LC (eg, LC screening protocol with multislice spiral thoracic CT scan or high-resolution computed tomographic [HR-CT] thoracic scanning) may be available to surgeons in 3% to 5% of the population, with a small-sized peripheral solitary pulmonary nodule (SPN) or a ground glass opacity (GGO) lesion which may be benign, premalignant or malignant. A GGO lesion is so defined when the internal density of the nodule, at the CT scan, is low and the bronchovascular structures in the GGO area can be still visualized (Figure 1). On the other hand, in a solid lesion the internal density of the solid nodule is so high that it obscures the bronchovascular structures (Figures 2 and 3). The GGO classification was outlined by Asamura et al in 2003.7 Many of these malignant SPNs are more likely to have adenocarcinoma histology, especially bronchioloalveolar carcinoma (BAC); BAC (mucinous and nonmucinous) is a subset of lung adenocarcinoma, which shows growth of tumoral cells along pre-existing alveolar structures (lepidic growth) without evidence of stromal, vascular, or pleural neoplastic invasion (Figure 4).5
Figure 1.
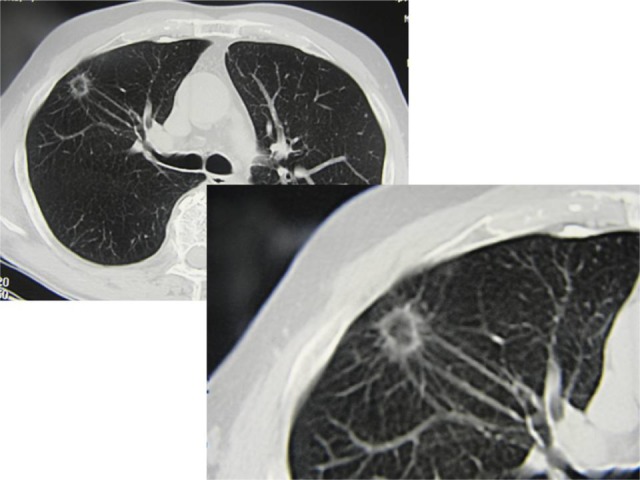
Occasionally discovered asymptomatic ground glass opacity in the right lung: surgical resection (segmentectomy) revealed T1aN0 bronchioloalveolar lung carcinoma.
Figure 2.
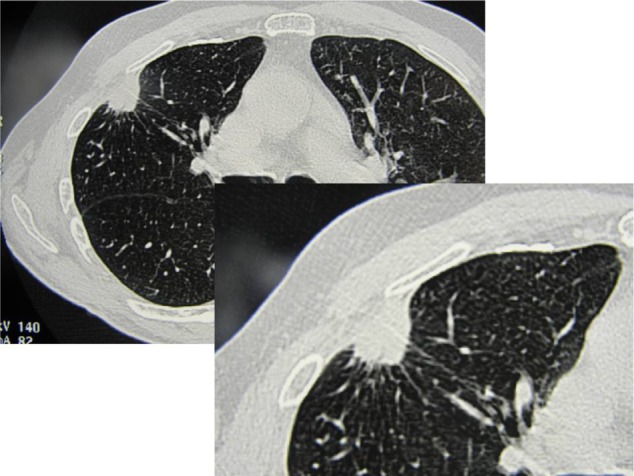
Occasionally discovered solid neoplastic lesion in the right lung in a male, former smoker, patient: surgical resection (segmentectomy) revealed T1aN0 squamous cell carcinoma.
Figure 3.
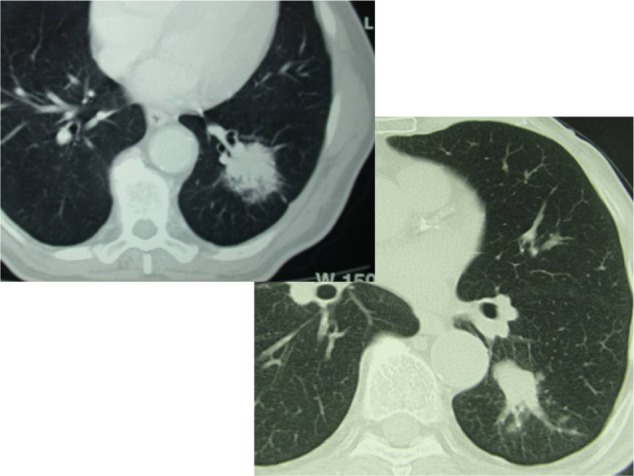
Partially solid solitary pulmonary nodule in the left lung in two nonsmoker male patients: left lower lobectomy was performed in both and histology showed T2aN0 G2 adenocarcinoma.
Figure 4.
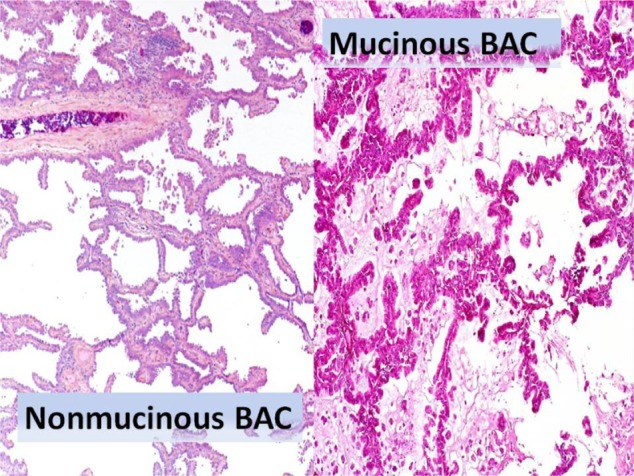
Bronchioloalveolar carcinoma (BAC, mucinous and non-mucinous variants) histologic specimens. BAC arises from alveolar walls as a noninvasive tumor with lepidic spread (along alveolar septa); no stromal, vascular, or pleural invasion is seen. Microscopically BAC is composed of tall, columnar cells lining alveolar septa and projecting into spaces with papillary projections, but underlying lung architecture is preserved; variable anaplasia but usually well differentiated. Non-mucinous type is composed of cuboidal cells with bright eosinophilic cytoplasm, prominent nucleoli, and nuclear atypia; apical spouts and hobnail cells are often present. Mucinous type is composed of well-differentiated columnar cells containing mucin that line respiratory spaces; tumor cells are associated with bronchioles, not bronchi; demarcation between normal and tumor cells is usually sharp.
Positive positron emission tomography (PET) scan lesions should be considered for surgery, especially if a high standardized uptake value is observed. Transthoracic fine needle aspiration biopsy is suggested for suspected malignant lesions >10 mm in maximum size, or in 5- to 10-mm diameter lesions whose size increases in repeated CT scans.
In case of proven LC, fit patients who tolerate lobectomy usually undergo surgery. Lobectomy is in fact considered the treatment of choice even for early stage NSCLCs since Ginsberg and Rubinstein8 published in 1995 the results of a Lung Cancer Study Group randomized trial comparing the role of limited pulmonary resections versus lobectomy for early-stage NSCLCs. The authors demonstrated an increased risk for local tumor recurrence rate (17.5%), a 30% increase in the overall death rate, a 50% increase in the observed death with cancer rate, and a lower 5-year survival rate in patients receiving nonanatomic lung resections.8 Local tumor recurrences were increased following wedge resections compared with segmentectomies. According to this publication, sublobar lung resections were reserved for patients with limited physiological reserves, especially in Europe and North America.
In 1996, Jang et al9 reported that the focal GGO areas on HR-CT could be interpreted as an early sign of localized BAC.
In Japan and in Western countries, recently improved anatomopathological and radiological studies on GGOs,7,9–14 with a favorable histology and prognosis, increased the screening of high-risk patients and created a large cohort of individuals with small pulmonary lesions different from those evaluated in the Ginsberg and Rubinstein study. The concomitant improvements in chemotherapeutic regimens and the growing population of older patients with significant clinical comorbidities, which contraindicate extended pulmonary resections, pushed many researchers to extend limited pulmonary resection in those patients.
Limited surgical resections have some theoretical advantages: the respiratory function may be preserved in the postoperative course; the possible postoperative complications may be reduced, and the feasibility of a second resection in case of tumor’s local recurrence may be preserved.15–18 Recent advances in both surgical and anesthesiological techniques (eg, muscle-sparing thoracotomy, totally video-assisted thoracoscopic surgery [VATS] lobectomy or segmentectomy, application of brachytherapy, or intraoperative radiotherapy) have made it possible to extend such pulmonary resections to a greater number of patients with an early-stage NSCLC.19–22
Five- and 10-year survival rates are excellent for fit patients with early-stage LC treated with anatomical resections but, unfortunately, a subset of patients suffer from cardiopulmonary diseases and other concomitant medical comorbidities and therefore are not good candidates for standard resections. For these, other therapeutic options are: external beam radiotherapy or more recent approaches such as radiofrequency ablation or stereotactic radiosurgery.
Although many studies have been recently published,23–27 at present there is no universally accepted criterion defining the term “poor pulmonary function” in those patients with LC who do not tolerate lobectomy (see Nomenclature section for the current clinical criteria identifying those patients).
Retrospective series reporting the poor outcome of unfit patients with untreated LC have been published: the median survival in these patients ranged between 11 and 14.2 months in the papers of McGarry et al and Kyasa and Jazieh,28,29 and most of these patients died from LC. Older retrospective series report 5-year survival rates ranging from 31% to 68%.29–31
Only a few prospective trials have been published comparing limited resection with lobectomy in patients with early-stage NSCLC and severe pulmonary impairment.
In 2005 the results of a prospective Phase II trial limited to patients with poor pulmonary function were published.32 High-risk patients (see Nomenclature section for their characteristics) underwent video-assisted wedge resection followed by local radiotherapy (maximum 56 Gy) in case of pathological T1N0NSCLC. Thirty-two of 58 patients (55.2%) were T1N0 and received a complete treatment; median survival was 32 months (there was 1 postoperative death) and 5-year survival rate was 29%. Local recurrence rate was 29%.
If the usefulness of the external beam radiation is demonstrated to be useful in decreasing local recurrence rate after limited lung resections,31–34 the next problem is delivering the correct radiation since there could be some limitations, such as irregular stapler lines at the site of surgical tumor resection, physiological lung movements, and the patient’s compliance.35 A possible solution may be the placement of large hemoclips at the apex of the wedge resection which will facilitate the postoperative radiotherapy delivery.
Inadequate resection margins have been attributed to recurrence in many solid tumors, including NSCLC: the incidence of tumor recurrence after sublobar resections in NSCLC may be related to the margins obtained during the resection. It is not yet clear how significantly this affects long-term survival in those patients with poor respiratory function and important medical comorbidities, and in those who have received wedge resections, since they do not tolerate a lobectomy. In contrast, when limited resections are performed in fit patients who would otherwise tolerate anatomic procedures, the increased local recurrence risk affects long-term survival, enhancing the role of adequate resection margins. The presence of negative cytologic margins alone is not sufficient to reduce the recurrence risk: as El-Sherif et al,36 Sawabata et al,37 and Schuchert et al38 recently demonstrated, a tumor margin of at least 2 cm is needed. Many authors in the past 10 years have supported segmentectomy instead of wedge resection for its effectiveness in achieving the 2 cm margin free goal.
El-Sherif et al39 reported their 13 years’ experience comparing the results of lobectomy and sublobar resections in Stage I NSCLC. Overall survival was better in the lobectomy group of patients and similar tumor recurrence rates were registered (28% in the lobectomy group, 29% in the sublobar resection group). Patients who received a limited resection had a similar disease-free survival after lobectomy if they were Stage IA, but a lower disease-free survival if staged IB. Kraev et al40 compared the outcome of 215 patients receiving lobectomy with 74 patients that underwent wedge resection for Stage I NSCLC: there was a significant benefit on long-term survival for patients with tumors less than 3 cm in diameter who underwent lobectomy.
The use of adjuvant brachytherapy as an alternative to external radiotherapy after limited resections41–43 has been recently reported. In a nonrandomized study of 200 patients comparing limited resections with or without brachytherapy, local recurrence rate significantly decreased from 18% to 2% in patients treated with 125I seeds41 Birdas et al35 compared the outcome of lobectomy and sublobar resections plus brachytherapy in 167 patients with Stage IB NSCLC and found equivalent local recurrence and survival rates in the two groups of patients. Blasberg et al43 reported their recent experience with Da Vinci robotic 125I brachytherapy seed placement in 11 high-risk patients who underwent limited pulmonary resections: perioperative mortality and local recurrence rates were 0% and 9%, respectively (1 of 11).
In conclusion, we are convinced that the option of surgery should be considered every time LC is diagnosed. Patients with potentially resectable LC and poor respiratory/cardiac function should be evaluated by a multidisciplinary lung cancer clinic working team that includes an experienced thoracic surgeon, a pulmonologist, a cardiologist, a medical oncologist, a radiation oncologist, and a chest radiologist. Smoking patients must receive intensive smoking-cessation counseling and be enrolled in a pulmonary rehabilitation program prior to a possible surgical choice. A correct preoperative diagnostic assessment (PET scan, transbronchial fine needle biopsy, mediastinoscopy/anterior mediastinotomy, if necessary) is mandatory to determine the tumor’s clinical stage as accurately as possible. Open or VATS surgery should be proposed when feasible; alternatively, the patient must be offered another treatment protocol that may include stereotactic radiotherapy or radiofrequency ablation in association with chemotherapy.
Stereotactic body radiation therapy
Until recently, patients unfit for surgery typically underwent conventionally fractionated radiotherapy with a total dose of 60 to 70 Gy delivered in a 6- to 7-week period; the poor outcomes with conventional radiotherapy are reflected in Surveillance, Epidemiology, and End Results (SEER) data44 showing a lung cancer 5-year survival rate of <15%. Locally uncontrolled tumor is the predominant pattern of failure in patients irradiated with conventional radiotherapy at conventional doses. In order to improve local tumor control and, hence, overall survival (OS), dose-escalation represents a very important issue. Nevertheless, dose-escalation by conventionally fractionated radiotherapy is limited by two main factors: a prolonged overall treatment time, resulting in a considerable amount of tumor repopulation, and an increased radiation dose delivered to the functional lung tissue, with a possible further functional impairment, even if regional lymph nodes may be excluded from radiation volumes in stage I NSCLC. Stereotactic body radiation therapy (SBRT) is an emerging option that may improve local control and survival; it is a technique characterized by the use of accurate repositioning which allows the administration of a few large fractions that are able to destroy the neoplastic target through radio-ablation. First reports on SBRT’s efficacy were published by Baumann et al,45 and a recent update of their results in 57 patients shows 60% 3-year OS, 88% cause-specific survival, and 52% progression-free survival (PFS) rates.45 Fakiris et al46 (an Indiana University group, a US SBRT pioneer center) recently published their experience with 70 patients showing 43% OS, 88% 3-year local-control rate, and 82% cause-specific survival rates. An Italian study by Ricardi et al47 recently reported data from Phase II prospective trials on 62 patients with 28 months median follow-up, which showed a 3-year OS rate of 57.1% with 87.8% local-control rate and 72.5% cause-specific survival rate. Koto et al48 reported a 71.7% 3-year overall-survival rate and an 83.5% cause-specific survival rate. Moreover, Radiation Therapy Oncology Group (RTOG) 0236 trial mature results on 59 patients, with 34.4 months as median follow-up time, showed 55.8% 3-year overall-survival and 97.6% local-control rates.49 A correct histological diagnosis was available in nearly 60% of patients; no statistical differences in survival were observed when comparing SBRT in proved neoplastic patients with those without a pathologic diagnosis. Even if not statistically confirmed in all of these trials, smaller tumors tend to have a better outcome. Few reports are available on operable patients refusing surgery and treated with SBRT: the most recent of these is the paper by Onishi et al,50 reporting a multi-institutional Japanese experience on 87 operable patients in which the 5-year OS rate was 92% for T1 and 73% for T2 tumors with 55 months median follow-up.
SBRT toxicity rate is very low;50 acute toxicities include acute radiation pneumonitis in a minority of patients (<5%) and skin reactions only when high doses are delivered with a limited number of fields and skin doses exceed 50% of prescription dose. Typical radiological findings of late pulmonary toxicity (eg, postactinic fibrosis) are very common, without important effect on pulmonary function and/or patient quality of life. When the targeted lesions are very close to the chest wall, specific chronic SBRT-related toxicities include thoracic pain and/or rib fractures; brachial plexopathy is very frequent in SBRT treatment of apical tumors. At present, central tumors are not eligible for SBRT treatment due to low radiation tolerance of the great vessels, main bronchus, and heart’s low radiation tolerance. Innovative techniques and fractionation schedules are mandatory in order to overcome these limitations.
In conclusion, SBRT is a very effective treatment in nonsurgical patients, as well shown by several Phase II studies in which a prolonged follow-up is achieved. SBRT appears to be superior to the conventional radiation therapy.
The role of SBRT as a primary therapy alternative to surgery has to be determined in recent trials ongoing in The Netherlands and United States comparing the results of stage IA NSCLC patients treated with surgery versus SBRT. Dose-escalation protocols (RTOG 0618 trial) are now exploring the role of dose-intensification in the treatment of large NSCLC, in which the local control rates are inferior. Even if data on SBRT dose-escalation protocols late effects are not yet available, its toxicity appears to be really low. Correct patient selection criteria, treatment planning, and delivery standardization of the radiation doses are crucial issues for SBRT treatment to become a widespread application in NSCLC early-stage patients.
Percutaneous thermal ablation with radiofrequency
Percutaneous thermal ablation with radiofrequency (RF) is a well-established interventional procedure for loco regional treatment of many tumors (hepatic, renal, colorectal, and bone metastases) as an alternative treatment to surgery in inoperable patients.51–54
The use of RF in the treatment of lung cancer was firstly proposed by Dupuy et al55 in 2000 and nowadays it is recognized as an efficient treatment in unresectable primary tumors or for those selected patients with metastatic disease in whom symptom palliation is required.56–59 In the past 10 years, several studies based upon an overall experience of over 1000 patients treated with curative or palliative intent have demonstrated that RF ablation in regional anesthesia is a minimally invasive technique, is safe and effective, and is associated with low morbidity and mortality rates, short hospitalization, and improved quality of life.60–62
Indications
Peripheral and small-sized tumors (≤3–3.5 cm) are ideal for RF treatment; tumors >3 cm may in fact present a low percentage of complete necrosis after RF. This evidence strictly correlates with an average time progression of the disease of 45 months for tumors <3 cm (P < 0.002).63 Ideal RF candidates are early-stage NSCLC patients with high surgical risk due to comorbidities (cardiac or respiratory) for whom conventional radiotherapy offers a 5-year survival rate of not more than 10% to 30%.64
RF in stage II NSCLCs could be proposed in combination with chemotherapy due to the possible lymph node neoplastic involvement, untreatable by RF.64 Advanced-stage NSCLC patients (IIIB or IV, due to the presence of satellite nodules), or those presenting with a progression of disease after chemo-radiotherapy may also benefit from RF.65,66 Symptomatic advanced stage LCs with pleura and/or chest wall invasion may also benefit from RF, used for antalgic purposes.
Contraindications to RF are: central tumors (located close to trachea and/or main bronchi), the presence of severe COPD or lung atelectasis, or an untreatable coagulopathy. A tumor close to the heart or great vessels is not a contraindication to RF per se, but in order to avoid possible high risks, the procedure must be performed by experienced radiologists.
Technique
RF ablation (RFA) is based on the use of interstitial hyperthermia to determine tumoral tissue destruction and death. Biologic tissues heated to 50°C for more than few minutes undergo coagulative necrosis, but if heated up to more than 105°C they are carbonized and produce gas. The optimum temperature for RFA ranges from 60°C to 100°C. The goal of lung RFA is to obtain a complete coagulative necrosis of tumoral cells with a 0.5- to 1-cm free margin (the so called “surgical margin”).
An RFA system consists of three components (a radio-frequency generator, active needle electrode pads, and a ground), and several SAB systems are available. A wide choice of needles is also available (such as cooled-tip electrode needles and expandable electrode-needles with multiple exit laterally deployable electrodes). The choice of RFA systems and needles may vary according to the operator’s individual experience, the location and size of the tumor, and the desired volume of necrosis.60,62,63
Chan et al recently published a meta-analysis of 46 published series of primary LCs and lung metastases treated with RF (an overall total of 2905 procedures performed in 1584 patients) showing that there is a tendency to perform RF under conscious sedation using multilined expandable electrodes.67
RF is usually performed percutaneously, rarely during thoracotomy. In order to obtain precise localization of a lesion and its relationship with the electrode-needle, the procedure is carried out under computed tomography (CT) guidance; however it does not permit a real time visualization of the needle’s advancement within the lesion. The needle path can be monitored only using a CT fluoroscopy, which subjects both patient and operator to a high dose of radiation.62,63
Follow-up
Radiological follow-up is usually scheduled with repeated chest CT scans (1, 3, 6, and 12 months after RF) to assess treatment response and to identify possible persistent disease or tumor recurrence. PET-CT seems to be more sensitive than CT scan and it may be proposed as the most suitable method for early detection of relapse, according to some recent published experiences.65–67
Results
Patients treated with RF present with encouragingly short- and medium-term survival rates: 1-year and 2-year survival rates of 78% to 95% and 57% to 84%, respectively.62–64,68,69 Although it may be difficult to assess cancer-specific survival in high-risk patients with severe comorbidities, Chan et al reviewed studies published between 2002 and 2009, observing 82% (range 58%–100%) cancer-specific survival rate with 89.8 ± 8.6 months mean follow-up.67 A potential RF with conventional radiotherapy synergistic effect in the treatment of Stage I–II NSCLC is emerging from the studies of Dupuy et al (24 patients in stage I)70 and Grieco et al (41 patients, stage I and II).71 Chan et al reported that primary LCs recur more frequently than lung metastases (22.2% vs 18.1%, P > 0.05).67
Complications
RFA of lung malignancy is a relatively safe treatment with low mortality (0%–5.6%) and 24.6% morbidity rates.67 Pneumothorax is the most common complication (30%–60% of cases), but only 10% of these require chest tube placement.62,63 Limited pleural effusion sometimes occurs (14.8% of cases);67 thoracentesis or chest tube are required in only 1% to 7% of cases.62 Immediate self-limiting intraparenchymal hemorrhage occurs in 10% of patients.63 Pneumonia, lung abscesses, hemoptysis, and pain exacerbation of chronic obstructive pulmonary lung disease (COPD) have been described after RFA of lung tumors.62
Conclusions
RFA is becoming an accepted treatment in selected inoperable patients with primary LC or pulmonary metastases. Patients with lesions <3 cm are ideal candidates for RF: these lesions, in fact, have the higher chance to achieve a complete tumor necrosis increasing the progression-free survival.
RFA’s local tumor control is lower than surgery but RF seems to be more effective than conventional radiation therapy in the treatment of high-risk patients. Moreover, initial encouraging results demonstrate that RFA in combination with RT provides a potential synergistic effect, especially for treating larger tumors, improving local survival compared to conventional RT alone.
Targeted chemotherapy for LC patients
Only recently has adjuvant chemotherapy become the standard of care for patients with completely resected stage II and III NSCLC with a good performance status.72 Long-term safety and efficacy data from two adjuvant cisplatin-based chemotherapy trials have been published. However, current data suggest that chemotherapy has reached a therapeutic plateau and that new combinations of currently available cytotoxic agents are unlikely to confer clinically relevant improvements in survival.73,74
This plateau highlights the need for novel approaches to improve the outcome of patients with advanced disease. Promising results from clinical treatment trials identify a spectrum of targeted cancer therapies in several broad categories. These include both small-molecule inhibitors of either key receptors or enzyme-binding sites, as well as intravenously delivered monoclonal antibodies that block a specific binding interaction between ligands and their receptors.75 An improved understanding of molecular processes underlying tumor biology has led to the development of therapies that target these processes. Intracellular signaling pathways related to the vascular endothelial growth factor receptor (VEGFR) and the epidermal growth factor receptor (EGFR) both play a central role in tumorigenesis as well as tumor growth, and are therefore rational targets for anticancer drug development.
Various agents that target these pathways are in clinical development. Some of these have already changed treatment practice in NSCLC, most notably the monoclonal antibody (mAb) bevacizumab in combination with chemotherapy in the first-line setting and the small-molecule tyrosine-kinase inhibitors erlotinib and gefitinib in the second-line setting.76
Antibody against VEGF
A successful ligand-targeting strategy comes from the VEGF-targeting mAb bevacizumab and ranibizumab.77 Bevacizumab is a recombinant humanized mAb that binds to VEGF. Recently, the addition of bevacizumab to paclitaxel and carboplatin was shown to give a significant survival benefit; however, this efficacy benefit was seen with an increased risk of treatment-related deaths.78
To establish whether the benefits of bevacizumab were extended in combination with other chemotherapy doublets, the randomized Phase III trial Avastin in Lung (AVAiL; BO17704) evaluated PFS in 1043 patients who received first-line bevacizumab (7.5 or 15 mg/kg) or placebo combined with cisplatin (80 mg/m2) and gemcitabine (1250 mg/m2) (CG). Both bevacizumab doses in combination with chemotherapy significantly improved PFS versus placebo.78 (Bevacizumab 7.5 mg/kg + CG: hazard ratio [HR] 0.75 [0.62–0.91], median 6.7 months, P = 0.0026, response rate 34%, P < 0.0001. Bevacizumab 15 mg/kg + CG: HR 0.82 [0.68–0.98], median 6.5 months, P = 0.0301, response rate 30%, P = 0.0017. Placebo + CG: median 6 months, response rate 20%. OS not yet reached). Bevacizumab, in combination with platinum-based chemotherapy, has received US Food and Drug Administration and European Medicines Agency approval for the first-line treatment of predominantly non-squamous advanced NSCLC. The National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for NSCLC also recommend bevacizumab in combination with chemotherapy as first-line therapy,79 while the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines state that the addition of bevacizumab to carboplatin/paclitaxel should be considered a therapeutic option in a clinically selected subset of patients.80,81
Targeting the EGFR pathway in first-line NSCLC
Tyrosine kinase inhibitors
The two proto-oncogenes currently known to be more commonly mutated in lung adenocarcinoma are K-Ras and EGFR.82
Erlotinib and gefitinib are orally active inhibitors of the intracellular tyrosine-kinase domain of the EGFR. Both selectively inhibit EGFR tyrosine kinase, reducing autophosphorylation and thereby disrupting EGFR signalling.83,84 This leads to inhibition of cell proliferation, blockade of cell cycle progression, and stimulation of apoptosis, with consequent inhibition of tumor growth. Erlotinib and gefitinib also potentiate the antitumor effects of cytotoxic chemotherapy.85,86 Erlotinib may be considered in patients selected by clinical or molecular markers.87
Pathologists observed that EGFR mutations are particularly frequent in adenocarcinoma with papillary and acinar subtypes and that those patients with a solid-pattern adenocarcinoma harboring EGFR mutations had a shorter PFS after treatment with erlotinib.88 A shorter PFS (10 months) after treatment with erlotinib in solid adenocarcinomas has been observed.89
A meta-analysis examined five small trials of first-line treatment with erlotinib or gefitinib monotherapy in patients in whom EGFR mutations were assessed.90 Ninety percent of the EGFR-mutant patients were Caucasians. The majority of EGFR-mutant patients were women (81%), had adenocarcinoma (89%), and had no history of tobacco use (58%). Of the 84 patients harboring a sensitizing EGFR mutation, treated with erlotinib or gefitinib, 56 patients (67%) achieved an objective response, with a median PFS of 11.8 months and a median OS of 23.9 months. For 83 patients with wild-type EGFR and wild-type K-Ras, the response rate was 5%, the median PFS was 3.1 months, and the median survival was 11.8 months. Finally, in 41 patients with wild-type EGFR and mutated K-Ras, response was 0%, PFS 3.3 months, and median survival 13 months. In the group of 59 patients with three or four clinical predictors (eg, Asian, female, never-smoker, adenocarcinoma), EGFR mutation status was able to divide these patients into two clear subsets: 38 patients with sensitizing mutations (response 76%, PFS 12.9 months, and median survival 23.8 months) and 21 patients without a sensitizing mutation (response 0%, PFS 1.8 months, and median survival 14.8 months).90
The Spanish Lung Cancer Group evaluated the feasibility of large-scale screening of EGFR mutations in advanced stage NSCLC patients and analyzed the association between EGFR mutations and clinical outcomes to erlotinib (150 mg daily).90 From April 2005 to November 2008, a total of 2105 NSCLC patients from 129 institutions was prospectively screened for EGFR mutations, and patients who had an EGFR mutation were treated with erlotinib. The most frequently detected mutations were deletion of exon 19 (62.2%) and L858R (37.8%). Mutations in the EGFR gene were detected in 350 pretreatment tumor samples of 2105 patients (16.6%). The overall response rate was 70.6% (including 12.2% complete responses), 19.3% of patients were stable, and 10.2% experienced progressive disease. A better response was associated with an exon 19 mutation (n = 135) than with the L858R mutation (n = 82, odds ratio [OR]: 3.08; 95% confidence interval [CI]: 1.63–5.81; P = 0.001) and with an age of 61 to 70 years (OR: 2.55; P = 0.006). No significant difference in PFS related to the type of mutation was observed. Median PFS was 14 months (95% CI: 11.3–16.7). Median OS was 28 months (95% CI: 22.7–31.3) for patients receiving first-line therapy. As described,87 patients with exon 19 deletions had a longer PFS (14.6 vs 9.7 months; P = 0.02) and OS (30.8 vs 14.8 months; P < 0.001) than those with the L858R mutation.90 The Iressa versus Carboplatin/Paclitaxel in Asia (Iressa Pan-Asia Study) was a Phase III trial that compared gefitinib with carboplatin and paclitaxel in Asian patients with adenocarcinoma who were light smokers (defined as ≤10 pack-years and quit >15 years ago) or never-smokers. The primary endpoint was the noninferiority of gefitinib compared with carboplatin and paclitaxel on PFS. The trial met the primary endpoint and demonstrated the superiority of gefitinib (n = 609) compared with carboplatin and paclitaxel (n = 608) (HR 0.74; 95% CI: 0.65–0.85; P < 0.001; median PFS of 5.7 and 5.8 months, respectively), and higher objective response rate (43% vs 32.2%; OR: 1.59; 95% CI: 1.25–2.01; P < 0.001). Significantly, more patients in the gefitinib arm than those in the carboplatin and paclitaxel arm experienced a clinically relevant improvement in quality of life. OS in an early analysis (450 patients have died [37%]) revealed similar survival among patients in the gefitinib arm compared with carboplatin and paclitaxel treatment arm (HR: 0.91; 95%: CI 0.76 –1.10; median survival of 18.6 and 17.3 months, respectively). There was a significant interaction between treatment and EGFR mutation and PFS. In the EGFR mutation-positive subgroup, the PFS was significantly longer among patients receiving gefitinib than among those receiving carboplatin and paclitaxel (HR: 0.48; 95% CI: 0.36–0.64; P < 0.001). In Asian patients with EGFR mutations, gefitinib treatment attained a significantly better response and PFS than chemotherapy.91
Two randomized Phase III trials compared the efficacy of erlotinib (150 mg/day) or placebo combined with six cycles of chemotherapy in patients with previously untreated stage IIIB/IV NSCLC (TALENT trial = 1172 patients; TRIBUTE trial = 1079 patients). The addition of erlotinib to chemotherapy did not improve median OS compared with chemotherapy alone in either the TALENT (9.9 vs 10.2 months, respectively; HR: 1.06; 95% CI: 0.90–1.23) or the TRIBUTE (10.6 vs 10.5 months, respectively; HR: 0.995; 95% CI: 0.86–1.16) trials.92,93
Two randomized Phase III studies (INTACT 1, INTACT 2) investigating the addition of gefitinib to platinum-based chemotherapy, did not show any survival benefit with the addition of gefitinib.94,95 The lack of an additive effect with EGFR tyrosine kinase inhibitors (TKIs) and chemotherapy may relate to a mechanistic interaction; for example, the antiproliferative effects of EGFR TKIs may render tumor cells less sensitive to cytotoxic agents.
There is a great interest in whether biomarkers can be used to predict which patients will benefit most from treatment with EGFR TKIs. Retrospective evidence comes from the BR.21 trial, in which tumor biopsy samples were used to investigate whether response and survival with erlotinib was associated with EGFR expression, EGFR gene amplification and mutations.96,97 The presence of an EGFR mutation may increase tumor responsiveness to erlotinib, but there was no evidence of an effect of mutation status on survival (patients with wild-type and mutated EGFR both had prolonged survival). Patients with EGFR-positive tumors and tumors with a high EGFR gene copy number may benefit most from erlotinib in terms of survival, but prospective validation of the biomarkers is required before the tests can be used in routine clinical practice. It is remarkable that the INTEREST trial failed to achieve its coprimary endpoint of demonstrating an OS benefit for gefitinib versus docetaxel in patients with high EGFR gene copy number (HR: 1.09; 95% CI: 0.78–1.51; P = 0.62; ITT population); there was no evidence that EGFR FISH-positive patients had superior OS on gefitinib compared with docetaxel.98 The analysis of FISH-positive patients was based on small sample sizes: 85 and 89 patients in the gefitinib and docetaxel arms, respectively.
MERIT, a trial investigating potential relationships between tumor biomarkers and clinical benefit from erlotinib, is the largest prospective genomic profiling trial conducted to date in advanced NSCLC. In baseline tumor biopsy samples of 264 patients, no binary markers for clinical benefit were identified. However, exploratory Affymetrix analysis identified three candidate markers of response on chromosome 7: EGFR, phosphoserine phosphatise, and RAPGEF5 (which encodes guanine nucleotide exchange factor).99,100
The clinical efficacy of gefitinib or erlotinib is limited by the development of acquired drug resistance that is believed to be caused by the gatekeeper T790M mutation, which is detected in 50% of clinically resistant patients.101,102 The T790M mutation has also been detected in pretreatment specimens.103 The T790M mutation was associated with a strikingly short PFS, a median of 7.7 months in patients with the T790M mutation and 16.5 months in those without the mutation (HR for progression for the T790M allele, 11.5; P < 0.001).104 SARMS (Scorpion Amplification Refractory Mutation System) in combination with another method identified T790M in 70% of post-treatment biopsy specimens and in plasma DNA in 54% of patients with prior response to gefitinib or erlotinib and in 29% of patients with prior stable disease.105 This highlights the need for large, robust, prospective placebo-controlled trials in order to definitively identify potential biomarkers. Preclinical data suggest that sequential or pulse dosing of erlotinib may prove more effective than concurrent administration.106 The effectiveness of sequential administration is currently being investigated in a Phase III trial (SATURN, large-scale prospective study), in which patients with stage IIIB/IV NSCLC receive four cycles of a standard platinum-based doublet followed by either erlotinib or placebo, to determine whether any of the prospective biomarkers reliably predicts a response and, more importantly, survival.
Pharmacogenomic selection in NSCLC
Other novel approaches may also have potential in advanced NSCLC. There is a growing interest in pharmacogenomics – the optimization of chemotherapy that takes the tumor genome into account – to better select patients whose disease will respond to treatment and thereby improve the therapeutic index.107 Despite recent advances, more research efforts are warranted to improve patients’ survival and to minimize toxicity profiles, and there is still a strong need for the identification of novel biomarkers of drug activity that can help the clinicians in the therapeutic decision-making.108 Tailored chemotherapy on the basis of tumor pharmacogenomics may represent a promising novel approach to treat NSCLC patients. For example, ongoing research aims to generate predictive genomic signatures for chemotherapy response. Molecular biological studies have already identified genetic aberrations that are potentially useful for customizing treatment; mRNA transcripts involved in DNA repair pathways, such as ERCC1 and BRCA1, have been shown to confer selective resistance to cisplatin or taxanes.109,110
ERCC1
Many efforts have been made in recent years to identify molecular markers capable of predicting clinical resistance toward scisplatin and its derivates. ERRC1, a DNA-repair protein belonging to the nucleotide excision repair complex, is a structure-specific DNA repair endonuclease responsible for the 5′-incision of damaged DNA.111 Low ERCC1 mRNA expression levels were associated with better clinical outcomes of patients with advanced stage NSCLC after cisplatin/gemcitabine therapy.112 On the other hand, there is an inverse correlation between ERCC1 protein expression and clinical outcomes (low level and worst prognosis) of patients with stage I who underwent surgical resection, but received no other form of treatment before and after surgery.113 This paradox probably indicates that an intact DNA repair mechanism may reduce the accumulation of genetic aberrations that are thought to contribute to the tumor’s malignant potential.113
Nevertheless, the determination of ERCC1 is a promising strategy for the stratification of the patients to be treated with platinum-based therapies.
BRCA1
Breast cancer susceptibility gene 1 (BRCA1) is recruited to the sites of DNA breaks, playing a central role in DNA repair and in cell-cycle checkpoint control. Binding of the mediator of DNA damage checkpoint 1 (MDC1) protein to the phosphorylated tail of histone H2AX facilitates the formation of BRCA1 nuclear foci at double-strand breaks.114 The receptor-associated protein 80 (RAP80) acts upstream of BRCA1 and is required for the accumulation of BRCA1 to sites of DNA breaks.115–117 Abraxas recruits RAP80 to form a complex with BRCA1. Both Abraxas and RAP80 are required for DNA damage repair, and cells depleted of Abraxas or RAP80 exhibit hypersensitivity to irradiation.115
A wealth of data indicate that BRCA1 confers sensitivity to apoptosis induced by antimicrotubule drugs (paclitaxel and vincristine), but induces resistance to DNA-damaging agents (cisplatin and etoposide) and radiotherapy.118,119 This differential modulating effect of BRCA1 mRNA expression was also observed in tumor cells isolated from malignant effusions of NSCLC and gastric cancer patients, where BRCA1 mRNA levels negatively correlated with cisplatin sensitivity and positively with docetaxel sensitivity.120
Two retrospective studies, in NSCLC121 and ovarian cancer122 patients, found that low or intermediate BRCA1 mRNA levels correlated with a significantly longer survival following platinum-based chemotherapy, while survival in patients with higher BRCA1 expression increased following taxane-based chemotherapy.122 In the multivariate analysis, low levels of BRCA1 mRNA were associated with significantly better PFS and OS.123 BRCA1 overexpression confers aggressive behavior in transgenic models of small-cell and squamous-cell lung carcinomas, as well as in a subset of lung adenocarcinomas harboring the intrinsic T/t-antigen cancer signature.124 Poor prognosis has also been associated with BRCA1 over expression in early NSCLC.125 In the Phase II study, 2-year survival was 41% in patients with the lowest levels of BRCA1, 16% in those with intermediate levels, and 0% in those with the highest levels.126 Moreover, in a study of 96 stage IV NSCLC patients treated with docetaxel plus gemcitabine, we observed that as BRCA1 mRNA levels increased, the probability of response increased and the risk of progression decreased. For patients with the highest BRCA1 levels, the response rate was 58.6%, compared with 13.8% for those with intermediate levels and 27.6% for those with the lowest levels.127
RAP80 levels influenced median survival. In patients with low BRCA1 levels receiving cisplatin plus gemcitabine, median survival was not reached in patients with low RAP80 levels, while it was 8 months for patients with intermediate RAP80 and 7 months for those with high RAP80 (P = 0.006). It has been hypothesized that if RAP80 were elevated, it could cause resistance to cisplatin-based chemotherapy even in the presence of low BRCA1 levels. RAP80 mRNA levels strongly influence the predictive value of low levels of BRCA1 in patients customized to receive cisplatin plus gemcitabine.128
Thymidylate synthase
Pemetrexed is a potent inhibitor of thymidylate synthase (TS, main target)129,130 and other folate-dependent enzymes, including dihydrofolate reductase and glycinamide ribonucleotide formyltransferase. Thymidylate synthase is an enzyme that catalyzes the methylation of deoxyuridylate to deoxythimidylate using 5,10-methylenetetrahydrofolate as a cofactor. This function maintains the tymidine-5-prime monophosphate pool critical for DNA replication and repair.131 Pemetrexed is currently approved in combination with cisplatin for first-line treatment of malignant pleural mesothelioma.132
Preclinical data have indicated that an overexpression of thymidylate synthase correlates with reduced sensitivity to pemetrexed.133,134 A recent study in chemotherapy-naïve patients with adenocarcinoma or squamous cell carcinoma of the lung demonstrated that the thymidylate synthase gene and protein baseline expression were significantly higher in squamous cell carcinoma compared with adenocarcinoma (P < 0.0001).135 In addition, thymidylate synthase and S phase kinase–associated protein (Skp2) are transcriptionally regulated in the S phase of the cell cycle by the transcription factor E2F-1.136,137 Like thymidylate synthase, elevated expression of Skp2 has been more commonly found in patients with squamous cell carcinoma of the lung rather than in patients with adenocarcinoma.138
In a recent large randomized trial, chemotherapy-naive patients with locally advanced or metastatic NSCLC experienced similar median OS with pemetrexed/cisplatin and gemcitabine/cisplatin overall (10.3 months in each arm), but in a preplanned analysis patients with adenocarcinoma or large cell carcinoma treated with pemetrexed/cisplatin experienced significantly improved OS compared with those receiving gemcitabine/cisplatin.139 In contrast, patients with squamous cell histology did not experience improved OS with pemetrexed/cisplatin. These findings could be explained by the lower expression of thymidylate synthase, the pemetrexed specific target, in adenocarcinoma compared with squamous cell carcinoma.135 This is the first evidence of survival differences between platinum doublets according to tumor histology.
The OS analyses by treatment arm for each of three histologic groups (large-cell carcinoma, adenocarcinoma, and squamous) demonstrated that cisplatin/pemetrexed in patients with adenocarcinoma and large-cell carcinoma resulted in significantly better survival than cisplatin/gemcitabine (adenocarcinoma: n = 847, 12.6 vs 10.9 months, respectively; HR: 0.84; 95% CI: 0.71–0.99; P = 0.03; large-cell carcinoma: n = 153, 10.4 vs 6.7 months, respectively; HR: 0.67; 95% CI: 0.48–0.96; P = 0.03; non-squamous: n = 1000, 11.8 vs 10.4 months, respectively; HR: = 0.81; 95% CI: 0.70–0.94; P = 0.005).139 The use of TS is a good predictive marker of treatment efficacy to antifolate drugs and indicates that both Real-Time PCR and immunohistochemistry may be used to assess TS expression level.108
The results of pharmacogenomics research are preliminary to date, and the clinical applications of this research remain to be demonstrated.
Poly(ADP-ribose) polymerase family
Nuclear processes involving access to or modification of the genome, such as transcription and DNA repair, require a host of structural and regulatory proteins. Poly(ADP-ribose) polymerase-1 (PARP-1), a ubiquitous and abundant nuclear protein and the founding member of the PARP family, has a number of distinct biochemical activities that make it well suited for both structural and regulatory roles across the genome.140,142
The known functions of the PARP family members span a wide range of cellular processes, including DNA repair, transcription, cellular signaling, cell-cycle regulation, and mitosis.140,142,145 This diverse array of processes plays key roles in a wide variety of biological outcomes, including differentiation, development, stress responses, inflammation, and cancer.
PARP-1 contributes in many unique ways to the molecular biology of nuclear processes, playing key roles in the maintenance of genomic integrity, the regulation of chromatin structure and transcription, and the establishment of DNA methylation patterns, as well as a host of other processes (eg, mitotic apparatus function, cell death pathways).140,141
The earliest functions ascribed to PARP-1 were related to DNA repair and the maintenance of genomic integrity, and much of the PARP-1 literature has been devoted to this aspect of PARP-1 biology.146 PARP-1 has been implicated in at least three distinct DNA repair pathways: base excision repair (BER), single-strand break (SSB) repair, and double-strand break (DSB) repair.147,148
In response to low levels of DNA damage, PARP-1 promotes cell survival and DNA repair. With severe DNA damage, PARP-1 promotes cell death through at least two distinct pathways: 1) energy failure-induced necrosis, which results from depletion of NAD+ (and ultimately ATP); and 2) apoptosis-inducing factor-dependent apoptosis.139,147 Thus PARP-1 has a vital role in determining cellular outcomes in response to DNA damage. As it might have been expected, PARP-1 interacts physically and functionally with other key DNA damage detection and response proteins, including the ATM kinase and p53.147
The development of specific, potent, effective, and safe PARP inhibitors has become an area of active research and much recent excitement in the PARP field.149 The focus has been on competitive inhibitors of PARP catalytic activity that may be useful as research tools, as well as clinical therapies. These include compounds derived from isoquinolines, phenanthridines, and phthalazines, as well as other structural derivatives, and a number of them are currently being tested in clinical trials as cancer therapies.145,146 Although quinazolinone and quinoxaline derivatives may be more selective for PARP-1 and PARP-2, respectively,140 increasing specificity is an important area of focus for the future. PARP inhibitors are likely to be useful for treating a wide variety of diseases related to genome integrity (eg, cancers),150 as well as stress and inflammatory responses (eg, cardiovascular disease).151
A number of clinical trials are now underway examining the safety and efficacy of PARP inhibitors as treatments for a variety of cancers, including breast, uterine, and ovarian cancers. In many cases, the efficacy of the inhibitors may be due to synthetic lethality between PARP inhibition and a genetic lesion in the cancer cells. For example, p53-deficient breast cancer cells treated with PARP inhibitors lose resistance to doxorubicin, a clinically active antitumor anthracycline antibiotic that promotes apoptosis.152 The goal of this approach is to target cells defective in one DNA repair pathway by inhibiting another. A clinical trial based on this approach has shown selective antitumor activity for the PARP inhibitor, olaparib, in breast and ovarian cancers containing BRCA1 and BRCA2 mutations at safely administrable doses with minimal side effects.153
BRCA mutation carriers are recognized to have a susceptibility to DNA DSB and increased cancer risk related to a deficit in homologous repair (HR) of DNA.154 Capitalizing on this vulnerability, targeted therapies are being tested that overload the capacity of cancer cells to perform HR. Such an opportunity is presented by inhibiting PARP, an enzyme that is involved in base excision repair of SSB in DNA.155 An increase in SSB secondary to PARP inhibition has been demonstrated as a mechanism to increase DSB and overwhelm HR. PARP inhibitors have been demonstrated to dramatically reduce poly(ADP)-ribosylation in a clinical setting144 and an initial treatment trial using the PARP inhibitor, olaparib, has demonstrated single-agent responses in the cancers of patients with BRCA-related tumors.153 This area of investigation suggests that PARP inhibitors could be used in prevention, particularly if PARP inhibitors used in clinical prevention trials are found to eliminate cancer risk signatures.
In many cases, however, we lack a clear mechanistic understanding of how PARP-1 contributes to the nuclear processes in which it participates. Many questions and issues remain to be addressed in future studies. For example, our knowledge of PARP-1 structure is incomplete. In addition, our understanding of the physiological functions of PARP-1 is limited. More sophisticated and specific animal models, such as tissue-specific knockout mice, will be required to address this issue. Finally, more specific PARP inhibitors will be required both as tools and therapeutics.
Conclusion
The need for novel approaches to improve the outcome of patients with advanced disease is well known. Effective and better-tolerated treatment strategies for patients with advanced NSCLC are clearly needed. Targeted agents have the potential for increased selectivity and thereby reduced toxicity compared with standard chemotherapies. The introduction of targeted agents, with different modes of action and toxicity profiles to chemotherapy, represents a real break through in the treatment of NSCLC.
In the first-line setting, bevacizumab-based therapy is the first treatment in more than a decade to show a survival benefit beyond that achieved with standard chemotherapy. The safety profile of bevacizumab should be considered within the context of the significant survival advantage conferred over chemotherapy alone in this setting. Bevacizumab with chemotherapy, in view of hemorrhagic complications, is currently considered suitable for patients with non-squamous disease, minimal baseline hemoptysis, and no central nervous system metastases with the additional exclusion criterion of radiological evidence of tumors invading or abutting major blood vessels, as a further safety precaution. Despite the promising success of these agents, targeting VEGF signaling appears to be insufficient to inhibit tumor angiogenesis permanently. Often tumors treated with anti-VEGF therapy develop resistance by selection of ‘hypoxia-resistant’ cells or by activating alternative angiogenic pathways,156 thus suggesting that an alternative therapy to target angiogenesis needs to be identified.
The Phase III trials reviewed here provide compelling evidence that targeted therapies can provide valuable improvements in outcomes in both the first- and second-line treatment settings. Erlotinib significantly prolongs survival and improves the quality of life of patients who have relapsed during prior therapy for advanced disease, while gefitinib has recently demonstrated similar efficacy to chemotherapy in the second-line setting. It has become clear that the presence of mutations in the EGFR TK domain best predicts those who have a dramatic benefit from EGFR TK inhibitors. It is better to restrict the use of EGFR TK inhibitors in the front-line setting to only those who have an EGFR TK mutation in the tumor.157
Recent advances have identified a subgroup of patients harboring EGFR mutations (nearly 15% in Caucasians and up to 50% in Asians) in whom targeted therapy with EGFR TKIs yields dramatic responses in 70% of cases (including 12% complete responses) and median PFS > 1 year (even longer in women and in patients with del 19), 20% of whom were disease-free at 3 years, and median survival was 27 months. It is advisable to implement EGFR mutation screening as a standard practice.89 It is plausible that several genetically defined subclasses of EGFR mutations could help to improve current clinical outcomes by combining erlotinib or gefitinib with other targeted drugs. EGFR mutations by immunohistochemistry could be only an ancillary procedure, since the T790M mutation cannot yet be tested by immunohistochemistry. Genetic tool kits should be implemented to test both the activating EGFR mutations (del 19 and L858R) and the T790M mutation, which is present at low levels in pre-treatment tumor samples.128 At present, any cytology cell block specimen with ≥25% of tumor cells should be deemed adequate for EGFR mutational analysis by direct sequencing.148 In general, pleural fluids have the most material of all cytologic samples. The absolute amount of cancer on the sections is less critical than the homogeneity of the sections, and by dissecting ten 5-µm sections, adequate DNA could be obtained from regions of cancer as small as 2 mm and with as few as 40 cancer cells.158 Molina-Vila and colleagues159 have developed a method that permits detection of del 19, L858R, and T790M in samples containing as few as eight tumor cells (in 10 µL of buffer), approximately 5 pg of DNA per microliter of crude extract. In addition to direct sequencing, EGFR mutations can be assessed by common fragment analysis of PCR-mediated amplification products (del 19), by real-time PCR (L858R), or by the peptide nucleic acid locked nucleic acid PCR clamp method; SARMS has also been used, as has the DxS EGFR29 mutation-detection kit. Monoclonal antibodies against del 19 and L858R have shown a sensitivity of 92% by immunohistochemistry.160 In future, the assessment of EGFR mutations should be performed in accredited central laboratories or outsourced to accredited commercial laboratories.128
Maintenance chemotherapy with pemetrexed is a new option in the management of patients with metastatic NSCLC.157
It could be argued that the efficacy to date of cisplatin/pemetrexed in non-squamous histology should enable it to be a preferred regimen for future studies testing molecular targeted therapies in non-squamous histology.139 There is much to look forward to in the context of targeting the insulin-like growth factor system and developing personalized therapies to reduce the metastatic potential of many cancers.
Finally, these targets represent factors that are not only clinically relevant but also may play a critical role in early tumor development prior to the evolution of frank invasive malignancy. This possibility qualifies these targets for consideration in the development of cancer prevention interventions. Small-molecule oral agents with few side effects and the absence of serious long-term toxicities have a greater chance of finding clinical application in cancer prevention. As such, these targets represent factors that also may play a critical role early in tumor development prior to the evolution of frank invasive malignancy. This possibility qualifies these targets for consideration in the development of cancer prevention interventions.161
Nomenclature and definitions
Early-stage lung cancer is a NSCLC limited to the lung parenchyma without invasion of the surrounding structures and without lymph nodal or systemic metastases.
Anatomic tumor resection (eg, segmentectomy, lobectomy/bilobectomy, bronchoplastic lobectomy, and pneumonectomy) is a surgical resection in which lung’s anatomy is resected. The resection is performed by isolating, dissecting out, and ligating the arteries, the veins, and the bronchi for the segment(s), the lobe(s), or the entire lung. A lobar and mediastinal lymph node dissection is an integral part of the operation whenever a LC is resected.
Nonanatomic tumor resection (eg, wedge resection) is a resection in which vessels and bronchi are not identified and hilar dissection is not performed.
Limited or lesser resection is a lung resection less than a standard lobectomy. Thus both wedge resection and segmentectomy have been considered within the term of limited pulmonary resection even if the second is considered as an anatomic one.
Patient with poor respiratory function is a patient which presents with a PCO2 > 45 mmHg, PO2 < 50 mmHg (without supplemental O2), has a predicted postoperative forced expiration volume in 1 second (FEV1) <0.8 l or <40% predicted values, carbon monoxide diffusing capacity in the lung <50%, maximum oxygen consumption of <15 mL/kg ⋅ min, or has a poor exercise performance status (eg, unable to climb a flight of stairs without resting).
Local tumor recurrence is the presence of LC within the ipsilateral hemithorax (including the mediastinum) after the tumor resection.
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.American Cancer Society . Cancer facts and figures 1999. Atlanta: American Cancer Society; 1999. [Google Scholar]
- 2.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 3.Doll R, Hill AB. Smoking and cancer of the lung: preliminary report. Br Med J. 1950;4682:739–748. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, World Health Organization Classification of Tumours . Tumours of the Lung, Pleura, Thymus and Heart. IARC Presse; Lyon, France: 2004. Pathology and Genetics. [Google Scholar]
- 6.Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E, World Health Organization International Histological Classification of Tumors . Histological typing of Lung and Pleural Tumors. 3rd ed. Berlin, Germany: Springer Verlag; 1999. [Google Scholar]
- 7.Asamura H, Suzuki K, Watanabe S, Matsuno Y, Maeshima A, Tsuchida R. A clinicopathological study of resected subcentimeter lung cancers: a favorable prognosis for Ground Glass Opacity lesions. Ann Thorac Surg. 2003;76:1016–1022. doi: 10.1016/s0003-4975(03)00835-x. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–662. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 9.Jang HJ, Lee KS, Kwon OJ, Rhee CH, Shim YM, Han J. Bronchioloalveolar carcinoma: focal area of ground-glass attenuation at thin-section CT as an early sign. Radiology. 1996;199:485–488. doi: 10.1148/radiology.199.2.8668800. [DOI] [PubMed] [Google Scholar]
- 10.Nakata M, Sawada S, Yamashita M, et al. Objective radiologic analysis of ground-glass opacity aimed at curative limited resection for small peripheral non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;129:1226–1231. doi: 10.1016/j.jtcvs.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Yamato Y, Tsuchida M, Watanabe T, et al. Early results of a prospective study of limited resection for bronchioloalveolar adenocarcinoma of the lung. Ann Thorac Surg. 2001;71:971–974. doi: 10.1016/s0003-4975(00)02507-8. [DOI] [PubMed] [Google Scholar]
- 12.Yamada S, Kohno T. Video-assisted thoracic surgery for pure ground-glass opacities 2 cm or less in diameter. Ann Thorac Surg. 2004;77:1911–1915. doi: 10.1016/j.athoracsur.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Sakurai H, Dobashi Y, Mizutani E, et al. Bronchioloalveolar carcinoma of the lung 3 centimeters or less in diameter: a prognostic assessment. Ann Thorac Surg. 2004;78:1728–1733. doi: 10.1016/j.athoracsur.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Bhure UN, Lardinois D, Kalff V, et al. Accuracy of CT parameters for assessment of tumor size and aggressiveness in lung adenocarcinoma with bronchioloalveolar elements. Br J Radiol. 2010;83:843–849. doi: 10.1259/bjr/13711326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lederle FA. Lobectomy versus limited resection in T1N0 lung cancer. Ann Thorac Surg. 1996;62:1249–1250. doi: 10.1016/0003-4975(96)85176-9. [DOI] [PubMed] [Google Scholar]
- 16.Keenan RJ, Landrenau RJ, Maley RH, Jr, et al. Segmental resection spares pulmonary function in patients with Stage I lung cancer. Ann Thorac Surg. 2004;78:228–233. doi: 10.1016/j.athoracsur.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Koike T, Togashi K, Shirato T, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination. Ann Thorac Surg. 2009;88:1106–1111. doi: 10.1016/j.athoracsur.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 18.Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for Stage I non-small cell lung cancer in the elderly. Ann Thorac Surg. 2009;87:1662–1666. doi: 10.1016/j.athoracsur.2009.02.097. [DOI] [PubMed] [Google Scholar]
- 19.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–2018. doi: 10.1016/j.athoracsur.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Handy JR, Asaph JW, Douville EC, Ott GY, Grunkemeier GL, Wu YX. Does video-assisted thoracoscopic lobectomy for lung cancer provides improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg. 2010;37:451–455. doi: 10.1016/j.ejcts.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 21.Shaw JP, Dembitzer FR, Wisnivesky JP, et al. Video-assisted thoracoscopic lobectomy: state of the art and future directions. Ann Thorac Surg. 2008;85:705–709. doi: 10.1016/j.athoracsur.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 22.Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg. 2010;140:19–25. doi: 10.1016/j.jtcvs.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 23.BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001;56:89–108. doi: 10.1136/thorax.56.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines, 2nd ed. Chest. 2007;132:161S–177S. doi: 10.1378/chest.07-1359. [DOI] [PubMed] [Google Scholar]
- 25.Brunelli A, Charloux A, Bolliger CT, et al. The European Respiratory Society and European Society of Thoracic Surgeons joint task force on fitness for radical therapy: ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 26.Charloux A, Brunelli A, Bolliger CT, et al. European Respiratory Society and European Society of Thoracic Surgeons joint Task Force on fitness for radical therapy: Lung function evaluation before surgery in lung cancer patients: how are recent advances put into practice? A survey among members of the European Society of Thoracic Surgeons (ESTS) and of the Thoracic Oncology Section of the European Respiratory Society (ERS) Interact. Cardiovasc Thorac Surg. 2009;9:925–931. doi: 10.1510/icvts.2009.211219. [DOI] [PubMed] [Google Scholar]
- 27.Brunelli A. Risk assessment for pulmonary resections. Semin Thorac Cardiovasc Surg. 2010;22:2–13. doi: 10.1053/j.semtcvs.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 28.McGarry RC, Song G, des Rosiers P, Timmerman R. Observation-only management of early stage, medically inoperable lung cancer. Chest. 2002;121:1155–1158. doi: 10.1378/chest.121.4.1155. [DOI] [PubMed] [Google Scholar]
- 29.Kyasa MJ, Jazieh AR. Characteristics and outcome of patients with unresectable early-stage non-small cell lung cancer. South Med J. 2002;95:1149–1152. [PubMed] [Google Scholar]
- 30.Pastorino U, Valente M, Bendini V, Pagnoni A, Ravasi G. Effect of chronic cardiopulmonarydisease on survival after resection for stage Ia lung cancer. Thorax. 1982;37:680–683. doi: 10.1136/thx.37.9.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller JI, Hatcher CR. Limited resection of bronchogenic carcinoma in the patient with marked impairment of pulmonary function. Ann Thorac Surg. 1987;44:340–343. doi: 10.1016/s0003-4975(10)63785-x. [DOI] [PubMed] [Google Scholar]
- 32.Erret LE, Wilson J, Chiu RC, Munro DD. Wedge resection as an alternative procedure for peripheral bronchogenic carcinoma in poor-risk patients. J Thorac Cardiovasc Surg. 1985;90:656–661. [PubMed] [Google Scholar]
- 33.Landrenau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1N0M0) non-small cell lung cancer. J Thorac Cardiovasc Surg. 1997;113:691–698. doi: 10.1016/S0022-5223(97)70226-5. [DOI] [PubMed] [Google Scholar]
- 34.Shennib H, Bogart J, Herndon JE, II, et al. The Cancer and Leukemia Group B and The eastern Cooperative Oncology Group Video-assisted resection and local radiotherapy for peripheral lung cancer in high-risk patients: The Cancer and Leukemia Group B (CALGB) 9335, a phase II, multi-institutional cooperative group study. J Thorac Cardiovasc Surg. 2005;129:813–818. doi: 10.1016/j.jtcvs.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Birdas TJ, Koehler RP, Colonias A, et al. Sublobar resection with brachytherapy versus lobectomy for Stage IB non-small cell lung cancer. Ann Thorac Surg. 2006;81:434–438. doi: 10.1016/j.athoracsur.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 36.El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol. 2007;14:2400–2405. doi: 10.1245/s10434-007-9421-9. [DOI] [PubMed] [Google Scholar]
- 37.Sawabata N, Matsumura A, Ohota M, et al. Cytologically malignant margins of wedge resected stage I non-small cell lung cancer. Ann Thorac Surg. 2002;74:1953–1957. doi: 10.1016/s0003-4975(02)03993-0. [DOI] [PubMed] [Google Scholar]
- 38.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–932. doi: 10.1016/j.athoracsur.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 39.El-Sherif A, Gooding WE, Santos R, et al. Wedge resection versus lobectomy for Stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg. 2006;82:408–416. doi: 10.1016/j.athoracsur.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 40.Kraev A, Rassias D, Vetto J, et al. Wedge resection versus lobectomy: 10 year survival in Stage I primary lung cancer. Chest. 2007;131:136–140. doi: 10.1378/chest.06-0840. [DOI] [PubMed] [Google Scholar]
- 41.Santos R, Colonias A, Parda D, et al. Comparison between sublobar resection and 125 Iodine brachytherapy after sublobar resection in high risk patients with Stage I non-small cell lung cancer. Surgery. 2003;134:691–697. doi: 10.1016/s0039-6060(03)00327-1. [DOI] [PubMed] [Google Scholar]
- 42.Fernando HC, Santos RS, Benfield JR, et al. Lobar and sublobar resection with and without brachytherapy for small stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;129:261–267. doi: 10.1016/j.jtcvs.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 43.Blasberg JD, Belsey SJ, Scwartz GS, et al. Robotic brachytherapy and sublobar resection for T1 non-small cell lung cancer in high-risk patients. Ann Thorac Surg. 2010;89:360–367. doi: 10.1016/j.athoracsur.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 44.Wisnivesky JP, Bonomi M, Henschke C, Iannuzzi M, McGinn T. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest. 2005;128:1461–1467. doi: 10.1378/chest.128.3.1461. [DOI] [PubMed] [Google Scholar]
- 45.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 46.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 47.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2010;68:72–77. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Koto M, Takai Y, Ogawa Y, et al. A phase II study on stereotactic body radiotherapy for stage I non small-cell lung cancer. Radiother Oncol. 2007;85:429–434. doi: 10.1016/j.radonc.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 49.Timmermann R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small cell lung cancer Can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2009.07.1751. In press. [DOI] [PubMed] [Google Scholar]
- 51.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 52.Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, Gandini G. Radio-frequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31:948–956. doi: 10.1007/s00270-008-9362-0. [DOI] [PubMed] [Google Scholar]
- 53.Gervais DA, McGovern FJ, Arellano RS, Mc-Dougal WS, Mueller PR. Renal cellcarcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226:417–424. doi: 10.1148/radiol.2262012062. [DOI] [PubMed] [Google Scholar]
- 54.Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22:300–306. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 55.Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radio frequency ablation of malignancies in the lung. Am J Roentgenol. 2000;174:57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 56.Herrera LJ, Fernando HC, Perry Y, et al. Radiofrequency ablation of pulmonary malignant tumors in non-surgical candidates. J Thorac Cardiovasc Surg. 2003;125:929–937. doi: 10.1067/mtc.2003.18. [DOI] [PubMed] [Google Scholar]
- 57.Hiran C, Fernando MD. Radiofrequency ablation to treat non small cell lung cancer and pulmonary metastases. Ann Thorac Surg. 2008;85:780–784. doi: 10.1016/j.athoracsur.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 58.Simon CJ, Dupuy DE, Di Petrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 59.Shu Yan Huo A, Lawson Morris D, King J, Glenn D. Use of percutaneous radiofrequency ablation in pulmonary metastases from renal cell carcinoma. Ann Surg Oncol. 2009;16:3169–3175. doi: 10.1245/s10434-009-0664-5. [DOI] [PubMed] [Google Scholar]
- 60.Roy AM, Bent C, Fotheringham T. Radiofrequency ablation of lung lesions: practical applications and tips. Curr Probl Diagn Radiol. 2009;38:44–52. doi: 10.1067/j.cpradiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Steinke K, Sewell PE, Dupuy D, et al. Pulmonary radiofrequency ablation – an international study survey. Anticancer Res. 2004;24:339–343. [PubMed] [Google Scholar]
- 62.Casal RF, Tam AL, Eapen GA. Radiofrequency ablation of lung tumors. Clin Chest Med. 2010;31:151–163. doi: 10.1016/j.ccm.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Crocetti L, Lencioni R. Radiofrequency ablation of pulmonary tumors. Eur J Radiol. 2010;75:23–27. doi: 10.1016/j.ejrad.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Pua BB, Thornton RH, Solomon SB. Ablation of pulmonary malignancy: current status. J Vasc Interv Radiol. 2010;21:S223–S232. doi: 10.1016/j.jvir.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 65.Simon CJ, Dupuy DE, Di Petrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 66.Iguchi T, Hiraki T, Gobara H. Percutaneous radiofrequency ablation of lung tumors close to the heart or aorta: evaluation of safety and effectiveness. J Vasc Interv Radiol. 2007;18:733–740. doi: 10.1016/j.jvir.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 67.Chan VO, McDermott S, Malone DE, Dodd JD. Percutaneous radio-frequency ablation of lung tumors; evaluation of the literature using evidence-based techniques. J Thorac Imaging. 2010 Sep 8; doi: 10.1097/RTI.0b013e3181e48d5e. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 68.Pennathur A, Abbas G, Landreneau RJ, Luketich JD. Radiofrequency ablation for the treatment of stage I non small cell lung neoplasm. Semin Thorac Cardiovasc Surg. 2008;20:279–284. doi: 10.1053/j.semtcvs.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Pennathur A, Abbas G, Schuchert MJ, Landreneau RJ, Luketich JD. Image-guided radiofrequency ablation for the treatment of early-stage non-small cell lung neoplasm in high-risk patients. Semin Thorac Cardiovasc Surg. 2010;22:53–58. doi: 10.1053/j.semtcvs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dupuy DE, Di Petrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotheraphy for medically inoperable stage I non small cell lung cancer. Chest. 2006;129:738–745. doi: 10.1378/chest.129.3.738. [DOI] [PubMed] [Google Scholar]
- 71.Grieco CA, Simon CJ, Mayo-Smith WW, Di Petrillo TA, Ready NE, Dupuy DE. Percutaneous image-guided thermal ablation and radiation therapy: outcomes of combined treatment for 41 patients with inoperable stage I/II non-small-cell lung cancer. J Vasc Interv Radiol. 2006;17:1117–1124. doi: 10.1097/01.RVI.0000228373.58498.6E. [DOI] [PubMed] [Google Scholar]
- 72.Pisters KM, Evans WK, Azzoli CG, et al. Cancer care Ontario and American society of clinical oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectablenon small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–5518. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 73.Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant cisplatin based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 74.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson KA, Brown PH. Drug development for cancer chemoprevention: focus on molecular target. Semin Oncol. 2010;37:345–358. doi: 10.1053/j.seminoncol.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 76.Reck M, Crinò L. Advances in anti-VEGF and anti-EGFR therapy for advanced non small cell lung cancer. Lung Cancer. 2009;63:1–9. doi: 10.1016/j.lungcan.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Lien S, Lowman HB. Therapeutic anti-VEGF antibodies. Hand Exp Pharmacol. 2008;181:131–150. doi: 10.1007/978-3-540-73259-4_6. [DOI] [PubMed] [Google Scholar]
- 78.Manegold C, von Pawel J, Zatloukal P, et al. Efficacy and safety results from BO17704, a randomised, placebo-controlled phase III study of bevacizumab in combination with cisplatin and gemcitabine in patientswith advanced or recurrent non-squamous non-small cell lung cancer (NSCLC) Eur J Cancer (Suppl) 2007;5:357. Abstract 6503. [Google Scholar]
- 79.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer v2, 2008.
- 80.Socinski MA, Crowell R, Hensing TE, et al. Treatment of non-small cell lung cancer, stage IV: ACCP evidence-based clinical practice guidelines, 2nd ed. Chest. 2007;132:S277–S289. doi: 10.1378/chest.07-1381. [DOI] [PubMed] [Google Scholar]
- 81.Sandler A, Gray R, Perry MC, et al. Paclitaxelcarboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 82.Guha U, Chaerkady R, Marimuthu A, et al. Comparisons of tyrosine phosphorylated proteins in cells expressing lung cancer-specific alleles of EGFR and KRAS. Proc Natl Acad Sci U S A. 2008;105:14112–14117. doi: 10.1073/pnas.0806158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838–4848. [PubMed] [Google Scholar]
- 84.Yano S, Yamaguchi M, Dong RP. EGFR tyrosine kinase inhibitor “gefitinib (Iressa)” for cancer therapy. Nippon Yakurigaku Zasshi. 2003;122:491–497. doi: 10.1254/fpj.122.491. [DOI] [PubMed] [Google Scholar]
- 85.Pollack VA, Savage DM, Baker DA, et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999;291:739–748. [PubMed] [Google Scholar]
- 86.Knight LA, Di NF, Whitehouse P, et al. The in vitro effect of gefitinib (‘Iressa’) alone and in combination with cytotoxic chemotherapy on human solid tumours. BMC Cancer. 2004;4:83. doi: 10.1186/1471-2407-4-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol. 2008;26:863–869. doi: 10.1200/JCO.2007.13.2720. [DOI] [PubMed] [Google Scholar]
- 88.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–827. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 89.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 90.Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated nonsmall cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Canc Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 92.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 93.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 94.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-smallcell lung cancer: a phase III trial–INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 95.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small cell lung cancer: a phase III trial–INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 96.Marchetti A, Felicioni L, Buttitta F. Assessing EGFR mutations. N Engl J Med. 2006;354:526–528. doi: 10.1056/NEJMc052564. [DOI] [PubMed] [Google Scholar]
- 97.Tsao MS, Sakurada A, Cutz JC. Erlotinib in lung cancer – molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 98.Douillard JY, Kim E, Hirsh V, et al. Gefitinib (IRESSA) versus docetaxel in patients with locally advanced or metastatic non-small cell lung cancer pre-treated with platinum-based chemotherapy: a randomized, open-label phase III study (INTEREST) J Thorac Oncol. 2007;2(Suppl 4):S305. Abstract PRS-02. [Google Scholar]
- 99.Tan EH, Ramlau R, Pluzanska A, et al. MERIT: a prospective study of putative relationships between tumour biomarkers and clinical benefit from erlotinib in advanced non-small cell lung cancer (NSCLC) J Thoracic Oncol. 2007;2(Suppl 4):S394. Abstract D2-04. [Google Scholar]
- 100.Reck M, Milanowski J, Au SK, et al. Analysis of possible relationships between tumour biomarkers and clinical benefit from erlotinib in advanced non-small cell lung cancer (NSCLC): data from the prospective MERIT study. Eur J Cancer Suppl. 2007;5:360. Abstract 6512. [Google Scholar]
- 101.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10(4):281–289. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou W, Ercan D, Chen L, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009;462(7276):1070–1074. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inukai M, Toyooka S, Ito S, et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res. 2006;66:7854–7858. doi: 10.1158/0008-5472.CAN-06-1951. [DOI] [PubMed] [Google Scholar]
- 104.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuang Y, Rogers A, Yeap BY, et al. Noninvasive detection of EGFR T790M in gefitinib or erlotinib resistant non-small cell lung cancer. Clin Cancer Res. 2009;15:2630–2636. doi: 10.1158/1078-0432.CCR-08-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davies AM, Ho C, Lara PN, Jr, Mack P, Gumerlock PH, Gandara DR. Pharmacodynamic separation of epidermal growth factor receptor tyrosine kinase inhibitors and chemotherapy in non-small-cell lung cancer. Clin Lung Cancer. 2006;7:385–388. doi: 10.3816/CLC.2006.n.021. [DOI] [PubMed] [Google Scholar]
- 107.Rosell R, Cuello M, Cecere F, et al. Treatment of non-small-cell lung cancer and pharmacogenomics: where we are and where we are going. Curr Opin Oncol. 2006;18:135–143. doi: 10.1097/01.cco.0000208786.91947.eb. [DOI] [PubMed] [Google Scholar]
- 108.Ceppi P, Papotti M, Scagliotti GV. New strategies for targeting the therapy of NSCLC: the role of ERCC1 and TS. Adv Med Sci. 2010;55:22–25. doi: 10.2478/v10039-010-0017-4. [DOI] [PubMed] [Google Scholar]
- 109.Rosell R, Cecere F, Santarpia M, Reguart N, Taron M. Predicting the outcome of chemotherapy for lung cancer. Curr Opin Pharmacol. 2006;6:323–331. doi: 10.1016/j.coph.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 110.Santarpia M, Altavilla G, Salazar F, Taron M, Rosell R. From the bench to the bed: individualizing treatment in non-small-cell lung cancer. Clin Transl Oncol. 2006;8:71–76. doi: 10.1007/s12094-006-0161-2. [DOI] [PubMed] [Google Scholar]
- 111.Sancar A. Mechanisms of DNA excision repair. Science. 1994;266:1954–1956. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- 112.Lord RV, Brabender J, Gandara D, et al. Low ERCC1 expression correlates with prolonged survival after cisplatinum plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002;8:2286–2291. [PubMed] [Google Scholar]
- 113.Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Eng J Med. 2007;356:800–808. doi: 10.1056/NEJMoa065411. [DOI] [PubMed] [Google Scholar]
- 114.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 115.Wang B, Matsuoka S, Ballif BA, et al. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science. 2007;316:1194–1198. doi: 10.1126/science.1139476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sobhian B, Shao G, Lilli DR, et al. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science. 2007;316:1198–1202. doi: 10.1126/science.1139516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim H, Chen J, Yu X. Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science. 2007;316:1202–1205. doi: 10.1126/science.1139621. [DOI] [PubMed] [Google Scholar]
- 118.Lafarge S, Sylvain V, Ferrara M, Bignon YJ. Inhibition of BRCA1 leads to increased chemoresistance to microtubule-interfering agents, an effect that involves the JNK pathway. Oncogene. 2001;20:6597–6606. doi: 10.1038/sj.onc.1204812. [DOI] [PubMed] [Google Scholar]
- 119.Abbott DW, Thompson ME, Robinson-Benion C, et al. BRCA1 expression restores radiation resistance in BRCA1-defective cancer cells through enhancement of transcription-coupled DNA repair. J Biol Chem. 1999;274:18808–18812. doi: 10.1074/jbc.274.26.18808. [DOI] [PubMed] [Google Scholar]
- 120.Wang L, Wei J, Qian X, et al. ERCC1 and BRCA1 mRNA expression levels in metastatic malignant effusions is associated with chemosensitivity to cisplatin and/or docetaxel. BMC Cancer. 2008;8:97. doi: 10.1186/1471-2407-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taron M, Rosell R, Felip E, et al. BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet. 2004;13:2443–2449. doi: 10.1093/hmg/ddh260. [DOI] [PubMed] [Google Scholar]
- 122.Quinn JE, James CR, Stewart GE, et al. BRCA1 mRNA Expression levels predict for overall survival in ovarian cancer after chemotherapy. Clin Cancer Res. 2007;13:7413–7420. doi: 10.1158/1078-0432.CCR-07-1083. [DOI] [PubMed] [Google Scholar]
- 123.Margeli M, Cirauqui B, Castella E, et al. The prognostic value of BRCA1 mRNA expression levels following neoadjuvant chemotherapy in breast cancer. PloS One. 2010 doi: 10.1371/journal.pone.0009499. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deeb KK, Michalowska AM, Yoon CY, et al. Identification of an integrated SV40 T/t-antigen cancer signature in aggressive human breast, prostate, and lung carcinomas with poor prognosis. Cancer Res. 2007;67:8065–8080. doi: 10.1158/0008-5472.CAN-07-1515. [DOI] [PubMed] [Google Scholar]
- 125.Rosell R, Skrzypski M, Jassem E, et al. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One. 2007;2:e1129. doi: 10.1371/journal.pone.0001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosell R, Perez-Roca L, Sanchez JJ, et al. Customized treatment in non-small-cell lung cancer based on EGFR mutations and BRCA1 mRNA expression. PLoS One. 2009;4:e5133. doi: 10.1371/journal.pone.0005133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boukovinas I, Papadaki C, Mendez P, et al. Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PLoS One. 2008;3:e3695. doi: 10.1371/journal.pone.0003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosell R, Moran T, Viteri S, et al. Optimization of genetics to create therapies for metastatic (stage IV) non-small-cell lung cancer. Exp Opin Pharmacother. 2010;11:1683–1693. doi: 10.1517/14656566.2010.482101. [DOI] [PubMed] [Google Scholar]
- 129.Taylor EC, Kuhnt D, Shih C, et al. A dideazatetrahydrofolate analogue lacking a chiral center at C-6, N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-)ethyl]benzoyl]-L-glutamic acid, is an inhibitor of thymidylate synthase. J Med Chem. 1992;35:4450–4454. doi: 10.1021/jm00101a023. [DOI] [PubMed] [Google Scholar]
- 130.Schultz RM, Patel VF, Worzalla JF, et al. Role of thymidylate synthase in the antitumor activity of the multitargetedantifolate, LY231514. Anticancer Res. 1999;19:437–443. [PubMed] [Google Scholar]
- 131.Shih C, Habeck LL, Mendelsohn LG, et al. Multiple folate enzyme inhibition: mechanism of a novel pyrrolopyrimidine-based antifolate LY231514 (MTA) Adv Enzyme Regul. 1998;38:135–152. doi: 10.1016/s0065-2571(97)00017-4. [DOI] [PubMed] [Google Scholar]
- 132.Vogelzang NJ, Rusthoven JJ, Symanowski, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 133.Sigmond J, Backus HH, Wouters D, et al. Induction of resistance to the multitargetedantifolatepemetrexed (ALIMTA) in Wi Dr human colon cancer cells is associated with thymidylate synthase overexpression. Biochem Pharmacol. 2003;66:431–438. doi: 10.1016/s0006-2952(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 134.Giovannetti E, Mey V, Nannizzi S, et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol. 2005;68:110–118. doi: 10.1124/mol.104.009373. [DOI] [PubMed] [Google Scholar]
- 135.Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006;107:1589–1596. doi: 10.1002/cncr.22208. [DOI] [PubMed] [Google Scholar]
- 136.Huang C, Liu D, Nakano J, et al. E2F-1 overexpression associated with TS and surviving gene expressions in non-small-cell lung cancer. J Clin Oncol. 2007;25:426s. (Suppl):Abstr 7669. [Google Scholar]
- 137.Sowers R, Toguchida T, Qin J, et al. mRNA expression levels of E2F transcription factors correlate with dihydrofolatereductase, reduced folate carrier, and thymidylate synthase mRNA expression in osteosarcoma. Mol Cancer Ther. 2003;2:535–541. [PubMed] [Google Scholar]
- 138.Salon C, Merdzhanova G, Brambilla C, et al. E2F-1, Skp2 and cyclin E oncoproteins are upregulated and directly correlated in high-grade neuroendocrine lung tumors. Oncogene. 2007;26:6927–6936. doi: 10.1038/sj.onc.1210499. [DOI] [PubMed] [Google Scholar]
- 139.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 140.Hassa PO, Hottiger MO. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci. 2008;13:3046–3082. doi: 10.2741/2909. [DOI] [PubMed] [Google Scholar]
- 141.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 142.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADPribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 143.Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 144.Chang P, Jacobson MK, Mitchison TJ. Poly(ADP-ribose) is required for spindle assembly and structure. Nature. 2004;432:645–649. doi: 10.1038/nature03061. [DOI] [PubMed] [Google Scholar]
- 145.Hakme A, Wong HK, Dantzer F, Schreiber V. The expanding field of poly(ADP-ribosyl)ation reactions ‘Protein Modifications: Beyond the Usual Suspects’ Review Series. EMBO Rep. 2008;9:1094–1100. doi: 10.1038/embor.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.D’Amours D, Desnoyers S, D’Silva I, Poirier GG. Poly(ADP-ribosyl) ation reactions in the regulation of nuclear functions. Biochem J. 1999;342:249–268. [PMC free article] [PubMed] [Google Scholar]
- 147.Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003;31:446–454. doi: 10.1016/s0301-472x(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 148.Woodhouse BC, Dianov GL. Poly ADP-ribose polymerase-1: an international molecule of mystery. DNA Repair. 2008;7:1077–1086. doi: 10.1016/j.dnarep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 149.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ratnam K, Low JA. Current development of clinical inhibitors of poly(ADP-ribose) polymerase in oncology. Clin Cancer Res. 2007;13:1383–1388. doi: 10.1158/1078-0432.CCR-06-2260. [DOI] [PubMed] [Google Scholar]
- 151.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in c cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–260. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Munoz-Gamez JA, Martin-Oliva D, Aguilar-Quesada R, et al. PARP inhibition sensitizes p53-deficient breast cancer cells to doxorubicin-induced apoptosis. Biochem J. 2005;386:119–125. doi: 10.1042/BJ20040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 154.McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 155.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 156.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 157.Stinchcombe TE, Bogart J, Wigle DA, Govindan R. Annual review of advances in lung cancer clinical research. A report for the year 2009. J Thorac Oncol. 2010;5:935–939. doi: 10.1097/JTO.0b013e3181e3a2e6. [DOI] [PubMed] [Google Scholar]
- 158.Smouse JH, Cibas ES, Janne PA, et al. EGFR mutations are detected comparably in cytologic and surgical pathology specimens of nonsmall cell lung cancer. Cancer Cytopathol. 2009;117:67–72. doi: 10.1002/cncy.20011. [DOI] [PubMed] [Google Scholar]
- 159.Molina-Vila MA, Bertran-Alamillo J, Reguart N, et al. A sensitive method for detecting EGFR mutations in non-small cell lung cancer samples with few tumor cells. J Thorac Oncol. 2008;3:1224–1235. doi: 10.1097/JTO.0b013e318189f579. [DOI] [PubMed] [Google Scholar]
- 160.Yu J, Kane S, Wu J, et al. Mutation-specific antibodies for the detection of EGFR mutations in nonsmall-cell lung cancer. Clin Cancer Res. 2009;15:3023–3028. doi: 10.1158/1078-0432.CCR-08-2739. [DOI] [PubMed] [Google Scholar]
- 161.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]


