Abstract
MET, the receptor for hepatocyte growth factor, has been identified as a novel promising target in various human malignancies, including lung cancer. Research studies have demonstrated that MET signaling plays important physiologic roles in embryogenesis and early development, whereas its deregulation from an otherwise quiescent signaling state in mature adult tissues can lead to upregulated cell proliferation, survival, scattering, motility and migration, angiogenesis, invasion, and metastasis in tumorigenesis and tumor progression. The MET pathway can be activated through ligand (hepatocyte growth factor, HGF) or MET receptor overexpression, genomic amplification, MET mutations, and alternative splicing. A number of novel therapeutic agents that target the MET/hepatocyte growth factor pathway have been tested in early-phase clinical studies with promising results. Phase III studies of MET targeting agents have recently been initiated. This paper will review the MET signaling pathway and biology in lung cancer, and the recent clinical development and advances of MET/hepatocyte growth factor targeting agents. Emphasis will be placed on discussing various unanswered issues and key strategies needed to optimize further clinical development of MET targeting personalized lung cancer therapy.
Keywords: MET, HGF, lung cancer, targeted therapy
Introduction
Molecular targeted therapy in lung cancer has made significant progress in the past decade.1 Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), gefitinib and erlotinib, were the first kinase targeted therapy approved in advanced nonsmall cell lung cancer (NSCLC) second/third-line treatment. More recent data has also provided strong rationale for their first-line use in patients harboring EGFR-TKI sensitizing mutations (eg, L858R and exon 19 deletions).2–6 The paradigm of molecularly matched targeted therapy is further strengthened by the successful application of another small molecule kinase inhibitor crizotinib, a dual anaplastic lymphoma kinase (ALK) and MET inhibitor, in NSCLC patients who harbor the ALK 2p23 chromosomal rearrangement that results in the fusion oncoprotein EML4-ALK.7–9 However, both EGFR-TKI and ALK inhibitors only target a small portion of the Western NSCLC population (15% and 3%–6.7%, respectively), and resistance inevitably occurs in those with targeted therapy despite initial response. Development of other novel molecular targeted therapies is still urgently needed to further impact lung cancer and improve overall survival (OS).
Lung cancer is now considered to be a collection of heterogeneous molecular disease subgroups with different underlying genetic/genomic alterations, even if they share similar or identical histological features. Hence, the quest for more novel targets to be inhibited in lung cancer continues beyond EGFR and ALK. The MET signaling cascade primarily regulates and coordinates an invasive genetic signaling program in embryogenesis and early development. On the other hand, while in adult tissues, MET signaling is typically in quiescence except in processes such as wound healing. In human cancers, a deregulated MET pathway is commonly involved in tumorigenesis, tumor invasion, metastasis, and tumor progression.10–14 Research studies have demonstrated the interaction between MET and other oncogenic pathways, and the role of MET signaling in EGFR-TKI resistance.15–17 MET is a multifaceted receptor tyrosine kinase that has been under intensive preclinical investigation for over 25 years. MET is now known to be a novel “druggable” target within the human kinome, with quite promising results of early-phase clinical investigations of MET targeting agents emerging. This review will provide a concise summary of the current understanding of MET biology, signaling, targeted therapeutic agents, and the updated status of MET targeted clinical trial studies (Tables 1 and 2) in lung cancer.
Table 1.
MET targeting agents in lung cancer clinical trials
| Drug | Targets | Company | Phase | Combination | Line of treatment | Type of cancer | Trial status | Locations | ClinicalTrial.org number |
|---|---|---|---|---|---|---|---|---|---|
| Tivantinib (ARQ 197) |
MET (non-ATP competitive inhibitor) |
ArQule/Daiichi-Sankyo/Kyowa Hakko Kirin | II | Erlotinib | 2nd | Stage IIIB/IV NSCLC | Complete | International | NCT00777309 |
| II | Erlotinib | 2nd or 3rd | Stage IIIB/IV KRAS (+) NSCLC | Active | United States | NCT01395758 | |||
| III | Erlotinib | 2nd or 3rd | Stage IIIB/IV nonsquamous NSCLC | Active | International | NCT01244191 | |||
| III | Erlotinib | 2nd or 3rd | Stage IIIB/IV nonsquamous wild-type EGFR NSCLC | Active | Japan | NCT01377376 | |||
| Onartuzumab (MetMAb) |
MET (one-arm mAb) |
Genentech/Roche | II | Erlotinib | 2nd or 3rd | Advanced NSCLC | Active, not recruiting | – | NCT00854308 |
| III | Erlotinib | 2nd or 3rd | Stage IIIB/IV, MET IHC (+)NSCLC | Active | International | NCT01456325 | |||
| II | Gemcitabine + cisplatin/carboplatin | 1st | Stage IIIB/IV squamous | Not yet recruiting | – | NCT00854308 | |||
| II | Bevacizumab + platinum + paclitaxel/pemetrexed | 1st | Stage IIIB/IV nonsquamous NSCLC | Not yet recruiting | – | NCT01496742 | |||
| Crizotinib (PF-2341066) |
MET, ALK | Pfizer | I | PF-00299804 (pan-HER inhibitor) | – | Stage IIIB/IV NSCLC | Active | United States and Australia | NCT01121575 |
| I/II | Erlotinib | 2nd or 3rd | Stage IIIB/IV lung adenocarcinoma | Active | United States | NCT00965731 | |||
| III | None (open label, vs standard chemotherapy) | 2nd | NSCLC harbors ALK translocation | Active | International | NCT00932893 | |||
| III | None (open label, vs cisplatin/carboplatin + pemetrexed) | 1st | Nonsquamous NSCLC harbors ALK translocation | Active | International | NCT00932893 | |||
| Cabozantinib (XL184, BMS-907351) |
MET, VEGFR2, RET, KIT, AXL, FLT3 | Exelixis/Bristol-Myers Squibb | Ib/II | Erlotinib | – | Stage IIIB/IV NSCLC with disease progression on erlotinib (phase II) | Active, not recruiting | United States | NCT00596648 |
| II | None (open label, vs placebo) | – | Advanced malignancies including lung cancer | Active, not recruiting | International | NCT00940225 | |||
| Ficlatuzumab (AV-299) |
HGF Ab | AVEO Pharmaceuticals | Ib/II | Gefitinib | – | Asian patients with IIIB/IV lung adenocarcinoma (phase II) | Active, not recruiting | Asia | NCT01039948 |
| Foretinib (XL880, EXEL-2880, GSK1363089) |
MET, VEGFR2, AXL | Exelixis/GlaxoSmithKline | I/II | Erlotinib | 2nd or 3rd | Stage IIIB/IV NSCLC on erlotinib | Active | Canada | NCT01068587 |
| PF-04217903 | MET | Pfizer | I | None | – | Advanced cancer | Complete | – | NCT00706355 |
| SGX523 | MET | SGX Pharmaceuticals | I | None | – | Advanced cancer | Terminated | – | NCT00607399 |
| AMG 102 | HGF (HuMAb) |
Amgen | Ib/II | Cisplatin/etoposide | 1st | Extensive stage SCLC | Active, not recruiting | NCT00791154 | |
| I/II | Erlotinib | 2nd or 3rd | Stage IIIB/IV NSCLC | Active | United States | NCT01233687 | |||
| Amuvatinib (MP470) MGCD265 |
MET, c-KIT, PDGFR, FLT3 MET | Astex Pharmaceuticals MethylGene | II | Platinum/etoposide | 2nd or 3rd | SCLC | Active | United States and Poland | NCT01357395 |
| I/II | Erlotinib | 2nd or 3rd | Stage IIIB/IV NSCLC | Active | United States | NCT00975767 |
Abbreviations: Ab, antibody; ALK, anaplastic lymphoma kinase; ATP, adenosine triphosphate; FLT3, Fms-like tyrosine kinase 3; HER, human epidermal growth factor receptor; HGF, hepatocyte growth factor; IHC, immunohistochemistry; MAb, monoclonal antibody; NSCLC, nonsmall cell lung cancer; PDGFR, platelet-derived growth factor receptor; SCLC, small cell lung cancer; VEGFR2, vascular endothelial growth factor receptor 2; vs, versus.
Table 2.
Results of completed phase II MET/hepatocyte growth factor targeted therapy clinical trials
| Trial name | Endpoint | Sample size | Major conclusions | Side effects | Reference |
|---|---|---|---|---|---|
| ARQ197-209 (NCT00777309) | Primary: PFS Secondary: OS, ORR, safety | ET, n = 84; EP, n = 83 | 1. In ITT patients: PFS was better in ET (median 3.8 m vs 2.3 m, P = 0.24; planned multivariable Cox regression HR 0.68, P < 0.05) 2. OS: no difference between ET and EP (median 8.5 m vs 6.9 m, adjusted HR 0.87, P = 0.5) 3 ORR (PR only): 7/74 (10%) in ET and 5/72 (7%) in EP 4. Planned exploratory analysis: • PFS was better in the ET arm in KRAS mutant group (median 2.3 m vs 1.0 m, P = 0.006, n = 10 and n = 5) • Patients with nonsquamous histology (n = 117) showed benefit from ET in both PFS (adjusted HR 0.61, P = 0.04) and OS (adjusted HR 0.58, P = 0.04), by applying the proportional hazards model generated for ITT population • Patients with EGFR wild-type tumors (n = 99) demonstrated a trend towards a benefit from ET in PFS (HR 0.70; P = 0.12) and OS (HR 0.76, P = 0.25) • Among the ITT population, median time to new metastatic lesions was longer in ET (7.3 m vs 3. 6 m, HR 0.49, P < 0.01). This effect was more pronounced in patients with nonsquamous histology |
Rash, diarrhea, fatigue, and anemia were similar in two groups | 34 |
| XL184-202 Phase Ib/II study of cabozantinib (XL184) with or without erlotinib in NSCLC with acquired erlotinib resistance (NCT00596648) | Objectives: safety, pharmacokinetics, MTD, and ORR | 54 in phase Ib; 36 patients assessable for response | 1. The overall safety and tolerability profile of cabozantinib and erlotinib appear acceptable 2. Three patients with prior erlotinib therapy had ≥30% reduction in tumor measurements on at least one postbaseline scan, including three with a complete or PR 3. Prolonged SD ≥4 months has been observed in some patients including one patient for ≥9 months and one patient with EGFR T790M |
Diarrhea (26%), fatigue (15%), dyspnea (12%), and hypoxia (9%) | 86 |
| Randomized discontinuation trial of cabozantinib (XL184) (NCT00940225) | Primary: ORR, safety | 398/483 enrolled patients; nine types of solid tumor were evaluable for the lead-in stage | 1. Soft tissue ORR 9% in 8/9 indications, 10% in NSCLC. ODC (PR or SD) at week twelve of 40% or higher were observed in six different solid tumors, including NSCLC. 69% with at least one postbaseline scan had tumor regression 2. Complete or partial resolution of bone scan lesions was observed in 59/68 (86.8%) patients with prostate or breast cancer or melanoma |
Most common related AEs grade ≥3: fatigue (9%), hand-foot syndrome (8%), and hypertension (5%) | 87 |
| OAM4558 g (NCT00854308) | Primary: PFS Secondary: OS, safety | ME, n = 64; Placebo plus erlotinib, n = 64 | 1. 54% of patients had MET IHC expression and associated with a worse outcome (OS HR 2.52) 2. In MET IHC 2+/3+ group: improved PFS (median 3.0 m vs 1.5 m, HR 0.47, P = 0.01) and OS (median 12.6 m vs 4.6 m, HR 0.37, P = 0.002) in ME group 3. In MET IHC −/1+ group: worse OS in ME group (HR 3.02, P = 0.021) 4. An OS benefit from ME was observed in MET FISH+ as well as in FISH−/IHC+; removing patients with EGFR mutation did not alter results |
Erlotinib-related toxicities were comparable between treatment arms | 96 |
Abbreviations: AE, adverse effect; EP, erlotinib plus placebo; ET, erlotinib plus tivantinib; FISH, fluorescence in situ hybridization; HR, hazard ratio; IHC, immunohistochemistry; ITT, intention-to-treat; m, month; ME, MetMAb plus erlotinib; MTD, maximum tolerated dose; NSCLC, nonsmall cell lung cancer; ODC, overall disease control; ORR, overall response rate; OS, overall survival; PFS, progression free survival; PR, partial response; SD, stable disease; vs, versus.
MET receptor: structure and function
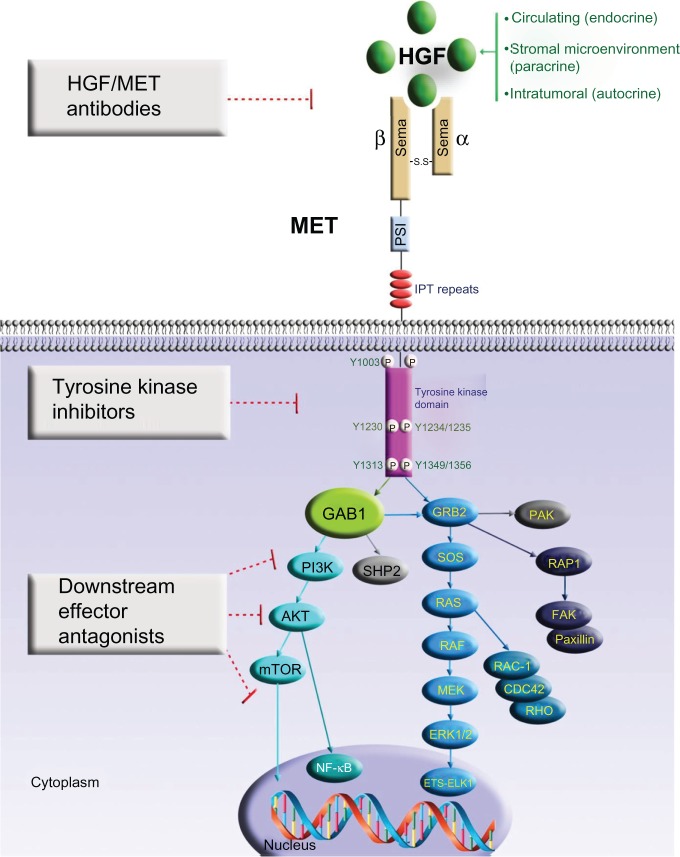
MET is a receptor tyrosine kinase composed of the α-chain (50 kDa) and the transmembrane β-chain (140 kDa) subunit linked by a covalent disulphide bond. The β-chain of MET has seven conserved subdomains, which have functional significance and homology with other cell signaling proteins. The amino-terminal semaphorin (or Sema) domain has a seven-bladed β-propeller fold – also found in the plexin family of semaphorin receptors – that serves as a key element for ligand binding and receptor homodimerization.18–20 Following the Sema domain in the carboxyl-terminal is the plexin–semaphorin–integrin domain, and then four immunoglobulin-like–plexin–transcription factor repeats. MET transmembrane domain consists of a single α-helix. The cytoplasmic tyrosine kinase domain contains a number of key serine and tyrosine phosphorylation sites important in the recruitment of the Src homology region 2 domain containing signaling transducers and intermediaries. The illustrative representation of the structure and functional domains of MET, and its key signaling cascades is shown in Figure 1. The natural ligand for MET receptor is hepatocyte growth factor (HGF), also called scatter factor (SF), which is produced by stromal and mesenchymal cells and acts primarily on MET-expressing epithelial cells in an endocrine and/or paracrine fashion.21 The tumoral source of HGF has also been found to mediate an autocrine oncogenic signaling in cancer.14,22 HGF was originally identified as a mitogen for hepatocytes and epithelial cells. HGF/MET autocrine activation in HGF-transgenic mice or MET-transgenic mice in vivo with promotion of hepatocarcinogenesis has also been reported.23–25 Upon ligand binding to HGF, MET is phosphorylated at multiple residues, with subsequent catalytic activation of signaling transduction cascades pivotal in cell proliferation, survival, angiogenesis, morphogenesis, cell scattering, motility, migration, epithelial-mesenchymal transition, and invasion. Importantly, it is known to have indispensible functions in embryogenesis and organogenesis under its physiological genetic invasive programming.26 The phosphorylation of the major autophosphorylation sites Y1230, Y1234, and Y1235 – located within the activation loop of the tyrosine kinase domain – activates the intrinsic catalytic kinase activity of MET. As a result, an activated docking site in the kinase domain further recruits intracellular adaptor molecules through the Src homology region 2 domains and other recognition motifs, such as growth factor receptor-bound protein 2-associated adaptor protein 1 (GAB1) (key signaling adaptor coordinator of the cellular responses to MET). Downstream signaling of the growth factor receptor-bound protein 2-mitogen-activated protein kinase (GRB2-MAPK) cascade, the phosphatidylinositol 3-kinase–mammalian target of rapamycin (PI3K–mTOR) pathway, and the signal transducer and activator of transcription (STAT) pathway are eventually activated, mediating various cellular functions.27,28
Figure 1.
MET receptor signaling and strategies of therapeutic inhibition in lung cancer.
Notes: Aberrant hepatocyte growth factor stimulation of MET in human cancer can occur by aberrant autocrine (intratumoral), paracrine (stromal microenvironmental), or endocrine (circulatory) loop signal activation. Upon hepatocyte growth factor binding to the Sema domain, the MET receptor dimerizes, which leads to autophosphorylation of intracellular kinase domain tyrosine residues Y1230/Y1234/Y1235, followed by the phosphorylation of Y1349 and Y1356. MET activation results in the recruitment and activation of downstream adaptor proteins and kinase targets. This results in a multitude of effects such as increased cell proliferation, cell cycle progression, scattering, motility, survival, extracellular matrix remodeling, and changes in metabolism. MET signaling is important in mediating tumor addiction/dependence and tumor expedience. Therapeutic intervention strategies to inhibit MET receptor oncogenic signaling cascade include blocking ligand-receptor interaction, preventing receptor dimerization, blocking MET kinase intrinsic activity, and inhibiting specific downstream signal transducers.
Abbreviations: AKT, protein kinase B; CDC42, cell division control protein 42 homolog; ERK, extracellular signal-regulated kinase; ETS-Elk1, E 26-like transcription factor 1; FAK, focal adhesion kinase; GAB1, growth factor receptor-bound protein 2-associated adaptor protein 1; GRB2, growth factor receptor-bound protein 2; HGF, hepatocyte growth factor; IPT, immunoglobulin-like–plexin transcription factor; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; P, phosphorylation; PAK, p21-activated kinase; PI3K, phosphatidylinositol 3-kinase; PSI, plexin–semaphorin–integrin; RAC-1, Ras-related C3 botulinum toxin substrate 1; RAF, rapidly accelerated fibrosarcoma; RAP1, Ras-related protein 1; RAS, rat sarcoma oncogene homolog; RHO, Ras homolog; SHP2, Src homology region 2-containing protein tyrosine phosphatase 2; SOS, Sos protein.
MET is also known to crosstalk with a number of other signaling pathways.29 The crosstalk between MET and EGFR/ERBB family receptors is particularly important in lung cancer.30,31 Crosstalk between MET and v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) signaling has been implicated by both preclinical and clinical findings recently.32–34 Matsubara et al determined molecular predictors of sensitivity to the MET inhibitor PHA-665752 in lung carcinoma cells, and identified that high phospho-MET and dependence of the protein kinase B and extracellular signal-regulated kinase signaling (ERK) pathways on MET activation may predict drug sensitivity to MET inhibitor, especially in KRAS-mutated cell lines.35 Furthermore, MET inhibitor PHA-665752 has been shown to reverse lung premalignancy induced by mutant KRAS (KrasLA1 mice) in vivo.36
MET Pathway in lung cancer
MET alterations in lung cancer
Past studies have shown that MET alterations occur in numerous human hematologic and solid malignancies (http://www.vai.org/met). The MET pathway has been found to be deregulated and activated in a number of human malignancies, including lung cancer. MET signaling can be altered through ligand or receptor overexpression, genomic amplification, mutations, or alternative splicing. These alterations lead to signaling deregulation which can be mediated through ligand (HGF)-independent receptor activation or through its ligand (HGF)-dependent activation via autocrine (intratumoral HGF), paracrine (mesenchymal or microenvironmental HGF), or endocrine (circulatory HGF) loop signaling cascades (Figure 1).
MET receptor is often overexpressed in both SCLC and NSCLC (particularly in nonsquamous NSCLC).14,37–40 Using a phosphoproteomic approach, Rikova et al characterized tyrosine kinase signaling across 41 NSCLC cell lines and over 150 NSCLC tumor samples and established that MET is the top most highly tyrosine-phosphorylated receptor tyrosine kinase in NSCLC tumor samples (ranked third in cell lines).41 The study findings lend further support to the role of MET as a primary “driver” oncogenic kinase in NSCLC. Furthermore, recent tumor microarray expression analysis of MET/HGF in human cancers demonstrated that both MET and HGF are commonly expressed in human solid cancers, including lung cancer. Seventy-two percent (29/40) of lung cancer tissue was found to express MET, and 40% (16/40) had MET receptor overexpression.14 Moreover, phospho-MET expression was found to be the highest in lung cancer, followed by ovarian, breast, renal, and colon cancers.14
MET gene (Gene ID: 4233) is located in the 7q31 locus of chromosome 7 and has a total length of 125,982 base pairs, consisting of 21 exons separated by 20 introns.42,43 MET gene mutations and copy number variations have been reported in a variety of human tumor tissues, especially in lung cancer.14,39,40,44–48 The MET receptor mutations in lung cancer were mainly found clustered in the nontyrosine kinase domain, namely in the juxtamembrane domain and Sema domain. MET kinase domain mutations have been found to be somatically selected in the metastatic tissues, compared with the primary solid cancers.49 Previous studies characterizing the juxtamembrane domain mutations (R988C, T1010I, alternative spliced juxtamembrane-skipping variant) demonstrated that these are activating oncogenic variants, with enhanced oncogenic signaling, tumorigenicity, cell motility, and migration.40,50 Both somatic and germline variants of MET have been reported in various human cancers, including renal cell carcinoma (in which the first MET kinase domain mutations were identified both in hereditary and sporadic diseases) and thoracic malignancies.39,40,50,51 Their relative significance and relevance in lung cancer biology and progression remains to be further defined. By using quantitative real-time polymerase chain reaction assay for MET amplification, multiple studies have reported primary MET amplification to be in the wide range of 2%–21% in NSCLC lung adenocarcinomas, particularly in TKI-naive cohorts.44,45,47,48 Using fluorescence in situ hybridization (FISH) assay, the Lung Cancer Mutation Consortium reported 4.1% of adenocarcinoma (n = 295) have MET amplification >2.2 (defined by MET/chromosome 7 enumeration probe), while 9.6% ALK 2p23 translocation was identified in ALK break apart FISH assay.52 The role of MET amplification in lung cancer progression remains controversial. Cappuzzo et al tested MET gene copy number in 447 NSCLC patients and found that a high MET gene copy number (at least five copies/cell) was negatively associated with survival (hazard ratio [HR] 0.66, P = 0.04).53 In another study that utilized quantitative real-time polymerase chain reaction method – instead of FISH – on formalin-fixed paraffin embedded (FFPE) archival tumor tissue-extracted DNA samples, Kanteti et al demonstrated that a MET gene copy number of more than four in lung adenocarcinoma tissue samples was associated with a trend of better prognosis (median survival 39 months versus 16 months, P = 0.06).45
Lung cancer EGFR-TKI resistance: role of MET and HGF
EGFR-TKIs such as gefitinib and erlotinib have demonstrated clinical efficacies in NSCLC, especially under the circumstance of tumors bearing EGFR activating mutations (eg, L858R, exon 19 deletions). Nevertheless, subsequent acquired resistance invariably emerges after initial response, leading to cancer mortality. Secondary emergence of MET gene amplification was reported in EGFR-TKI resistant cell clones (HCC827-GR) derived upon chronic escalating gefitinib exposure, observed in four out of 18 (22%) lung cancer specimens with acquired EGFR-TKI resistance.17,16 Bean et al reported a similar MET amplification rate (nine out of 43, 21%) in patients with acquired EGFR-TKI resistance, compared with only 3% (two out of 62) in untreated EGFR-mutant patients.15 However, 44% (four out of nine) patients found with MET amplification had concurrent T790M EGFR TKI-resistant mutation. Two of the most recent tissue rebiopsy studies on genetic analysis of acquired EGFR-TKI-resistant NSCLC reported, respectively, 11% (four out of 37) and 5% (two out of 37) of samples found with a high MET gene copy number,54,55 frequencies that are lower than anticipated based on earlier data. Rho et al demonstrated that MET activation – instead of MET gene amplification – in PC-9 EGFR-TKI-resistant cells sustained activation of MET by HGF enhanced tumor cell migration and invasion abilities.56 However, their work showed that MET activation was secondary to increasing passage number without exposure to EGFR-TKI, and was not related to EGFR-TKI resistance.56 A previous study demonstrated that in primary erlotinib-resistant H1975 lung adenocarcinoma cells (T790M/L858R-EGFR) expressing wild-type MET without genomic amplification, SU11274 (MET inhibitor) – when in combination with erlotinib – effectively inhibited H1975 cells in vitro and in vivo with abrogation of cytoskeletal functions and regression of the xenograft growth.31 Together, the studies above provide a rationale to develop clinical studies of MET inhibitor, in combination with EGFR-TKI in NSCLC (especially TKI-resistant patients), both as primary or secondary strategies to prevent or overcome EGFR-TKI resistance.
Recent studies showed that drug-sensitive cells with mutant EGFR (HCC827, PC-9 [with deletion exon 19] against erlotinib; and H1975 [with L858R/T790M] against CL-387785) exhibited an “adaptive” tumor resistance with 100-fold higher half maximal inhibitory concentration to escape the EGFR-TKIs,57,58 as early as within the first 6–9 days of drug exposure.57 Previous work showed that these early resistant cells that existed in a quiescent state evaded the TKIs through a MET-independent survival mechanism with newly addicted dependence on the mitochondrial B-cell lymphoma 2/B-cell lymphoma-extra large (BCL-2/BCL-xL) antiapoptotic pathway, which can be further targeted by B-cell lymphoma 2 domain 3 (BH3)-mimetic agents to inhibit the BCL2/BCL-xL signal path.57 Hence, the role of MET in acquired EGFR-TKI resistance might be more relevant in the late stages of resistance development, but not necessarily so in the early emergence of the adaptive drug-resistance state.
HGF overexpression may also be involved in acquired EGFR-TKI resistance in NSCLC.59–61 High level HGF expression was detected in 61% of lung cancer tumors with acquired EGFR-TKI resistance, more frequent than EGFR T790M (52%) secondary mutation or MET amplification (9%) in Japanese patients.59 An in vivo study showed HGF induced EGFR-TKI resistance of lung adenocarcinoma cells by restoring the PI3K/AKT signaling pathway via phosphorylation of MET, but not EGFR or ERBB3,60 and transient inhibition induced apoptosis and overcame HGF-mediated EGFR-TKI resistance.62 A new MET kinase inhibitor, E7050, was found to reverse the HGF-induced resistance to reversible (gefitinib), irreversible (BIBW2992), and mutant-selective (WZ4002) EGFR-TKI resistance.63
Role of MET/HGF signaling in tumor hypoxia and stem/progenitor cells
Hypoxia in tissue favors both “stemness” and “invasive growth” in normal embryonic development, as well as in tumor growth. It has been shown that hypoxia induces MET expression and activation via hypoxia inducible factor-1α mediated transcriptional upregulation, and amplified HGF signaling, resulting in MET-HGF synergized induction of invasion. The inhibition of MET expression prevented hypoxia-induced invasion growth.64
HGF and MET are highly expressed in various stem and progenitor cells, but only expressed in a low level in their mature cells, indicating their important physiologic role in cell differentiation and normal embryonic development.26 MET has been postulated to be a potential marker of an expanding cell population that undergoes an aberrant differentiation program and that retains stem cell properties.10,65 MET e xpression was found to colocalize at the lung bronchoalveolar duct junction, where the bronchoalveolar stem cells were identified.14,66 Recently, De Bacco et al reported that MET expression increased in response to ionizing radiation through the ataxia telangiectasia mutated–nuclear f actor κ-light-chain-enhancer (ATM/NFκB) of activated B cells signaling pathway, leading to radioresistance and cancer invasion.67 Hence, it would be important to ascertain if MET expression is truly elevated in cancer stem cells and contributes to cancer stem cell-mediated therapeutic resistance and tumor invasion.68
MET signaling has also been implicated in promoting angiogenesis, and HGF itself is known as a potent proangiogenic factor.69,70 Inhibiting the MET pathway using MET inhibitor in a preclinical murine model demonstrated the antiangiogenic effect in addition to direct antitumor effects.71
Current clinical data of MET/HGF targeting agents in lung cancer
Early preclinical MET inhibitors that have been studied include geldanamycin, K252a, SU11274, and PHA-665752.33,39,72,73 It has been demonstrated that small molecule MET inhibitors SU11274 and PHA-665752 inhibited cell viability and growth and induced apoptosis by inhibiting MET/HGF signaling on various in vitro and in vivo lung cancer models.31,33,36,39 Currently, there are three major treatment strategies in HGF/MET inhibition under clinical development: MET-TKI inhibition, MET antibody, and HGF antibody. Many of these agents have already been approved for human clinical trials (Tables 1 and 2).
MET TKIs
Tivantinib (ARQ 197)
Tivantinib is uniquely the first nonadenosine triphosphate-competitive small molecule that selectively targets MET, with the mechanism of action locking the kinase in a “closed” and “inactive” conformation when bound to the drug. An in vivo study demonstrated its antitumor activity in various cancers (colon, gastric, and breast cancers).74 The safety and antitumor activity in various solid tumors were also demonstrated in phase I clinical trials.75,76 Tivantinib is mainly metabolized by cytochrome P450 2C19 (CYP2C19). The ratio of the poor metabolizer of CYP2C19 in Asians is reported to be around 20%, while it is very low in Caucasians. A phase I trial in Japanese patients found that tivantinib was well tolerated in poor metabolizer patients as well as extensive metabolizer patients. CYP2C19 genotypes clearly affected the exposure to tivantinib, a finding that led to different recommended phase II doses: 360 mg twice daily for extensive metabolizer patients and 240 mg twice daily for poor metabolizer patients.77 A number of phase II trials have been launched to investigate the effects of ARQ 197 in various malignancies, including lung cancer. ARQ 197–209 is a global randomized placebo-controlled phase II clinical trial comparing erlotinib (150 mg daily) plus tivantinib (360 mg twice daily) with erlotinib plus placebo in advanced NSCLC patients. The primary endpoint is progression-free survival (PFS), and the secondary endpoints are overall response rate, overall survival (OS), and subset analysis. The trial result has been reported.34 The trial enrolled 167 patients who were randomized to erlotinib plus tivantinib (n = 84) or erlotinib plus placebo (n = 83). Median PFS was 3.8 months in the erlotinib plus tivantinib arm and 2.3 months in the erlotinib plus placebo arm (HR 0.81, P = 0.24). The planned multivariable Cox regression model in the intention-to-treat population adjusting for key prognostic factors yielded significant improvement in PFS with HR 0.68 (95% confidence interval 0.47–0.98, P < 0.05). The difference in OS in the two arms was not statistically significant (median 8.5 months versus 6.9 months, adjusted HR 0.87, P = 0.5).
More importantly, a planned subset analysis demonstrated significant improvement in both PFS (adjusted HR 0.61, 95% confidence interval 0.39–0.98, P = 0.04) and OS (adjusted HR 0.58, 95% confidence interval 0.34–0.99, P = 0.04) in patients with nonsquamous histology who were treated with erlotinib plus tivantinib. This exploratory analysis suggests that nonsquamous tumors may indeed be enriched for MET expression, which is supported by existing literature that MET/HGF pathway activation more frequently occurs in nonsquamous NSCLC and plays a pivotal role in tumor invasion, progression, and metastasis.39,78 Preliminary biomarker analysis in this phase II trial showed that among nonsquamous tumors 75% were MET-positive by immunohistochemistry (IHC), compared with only 12% of squamous tumors. Interestingly, the exploratory analyses also showed that PFS was significantly better in the ARQ 197 arm in patients with KRAS mutant (median PFS 2.3 months versus 1.0 month, HR 0.18, P = 0.006, n = 10 and n = 5, respectively), with a similar trend (not statistically significant) in patients with EGFR wild-type. The side effects, including rash, diarrhea, fatigue, and anemia were similar in the two groups.34 Finally, ARQ 197 was also found in an exploratory analysis of time-to-development of new metastases to significantly delay new tumor metastases among patients treated with erlotinib plus tivantinib compared with the erlotinib plus placebo arm (median 7.3 months versus 3.6 months, HR 0.49, P < 0.01 in the intention-to-treat population), consistent with the notion that MET “oncogene expedience” can be targeted therapeutically. Interestingly, this effect was also observed to be more pronounced in the nonsquamous patients.34
The findings from the ARQ 197–209 trial facilitated further tivantinib clinical trial designs. A global randomized phase III trial (NCT01244191) of ARQ 197 in combination with erlotinib versus erlotinib plus placebo for advanced nonsquamous NSCLC patients who have received one or two prior systemic anticancer therapies is now ongoing.79 There is also a parallel randomized phase III study in Asia (Japan, Korea, and Taiwan; ARQ 197-006) for wild-type EGFR advanced nonsquamous NSCLC with erlotinib plus tivantinib versus erlotinib plus placebo arms – with ARQ 197 doses according to the CYP2C19 polymorphism (NCT 01377376) – that has already started recruiting (since June 2011). In addition, a phase II randomized open-label study of erlotinib plus tivantinib versus single agent chemotherapy (pemetrexed, docetaxel, or gemcitabine) in previously treated KRAS mutation positive patients with locally advanced or metastatic NSCLC began in July 2011 (ARQ 197-218, NCT01395758), with a primary outcome of PFS. The results from these trials will greatly improve the understanding of the role of MET inhibitor in NSCLC and appropriate patient selection.
To this end, it is of interest to note that in the phase I clinical trial of ARQ 197 in solid tumors,76 pharmacokinetic and pharmacodynamic studies were completed along with treatment evaluation using serial biopsies, dynamic contrast-enhanced magnetic resonance imaging, and circulating endothelial cell and circulating tumor cell enumeration. ARQ 197 decreased phosphorylated MET, total MET, and phosphorylated focal adhesion kinase and increased terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick-end labeling staining in tumor biopsies (n = 15). Circulating endothelial cells decreased in 58.1% (25/43) of patients, but no significant changes in dynamic contrast-enhanced magnetic resonance imaging parameters were observed after ARQ 197 treatment. Of 15 patients with detectable circulating tumor cells, eight (53.3%) had ≥30% decline in circulating tumor cells after treatment.
Crizotinib (PF-2341066, Xalkori®)
Crizotinib has recently been approved by the Food and Drug Administration (Xalkori) in the United States for patients with NSCLC whose tumor harbors ALK 2p23 translocation and oncogenic fusion (most common as EML4-ALK fusion), as determined by the parallelly approved Vysis LSI dual color probe ALK 2p23 break apart rearrangement FISH assay (Abbott Laboratories, Abbott Park, IL). Crizotinib has known MET kinase inhibitory activity in addition to that of ALK. Interestingly, the drug was initially developed as a MET inhibitor preclinically and subsequently in a phase I trial.80,81 It potently inhibited MET phosphorylation and MET dependent proliferation, migration, or invasion of human tumor cell in vitro. In addition, it also potently inhibited HGF-stimulated endothelial cell survival or invasion and serum-stimulated tubulogenesis in vitro, indicating that its cytoreductive antitumor efficacy may be mediated by direct effects on tumor cell growth or survival as well as antiangiogenic mechanisms.82 Subsequently, the clinical development of crizotinib was steered towards focusing primarily on ALK-rearranged NSCLC, ultimately leading to the drug’s recent Food and Drug Administration approval in August 2011. However, in a recent case report, an NSCLC patient with de novo MET amplification but no ALK rearrangement achieved a rapid and durable response to crizotinib, indicating that it is also a bona fide MET inhibitor clinically.83 Dramatic clinical improvement and radiographic regression were also observed in patients with MET amplified esophagogastric adenocarcinoma84 and glioblastoma multiforme85 upon treatment with crizotinib. Two phase I/II trials of crizotinib in combination with erlotinib or PF-00299804 (pan-human EGFR inhibitor) in advanced NSCLC, regardless of ALK 2p23 translocation status, are currently ongoing.
Cabozantinib (XL184/BMS-907351)
Cabozantinib is a multitargeted inhibitor with activities mainly against MET and vascular endothelial growth factor receptor 2 (VEGFR2), and with additional inhibitory activities toward RET, AXL, KIT, and FMS-like tyrosine kinase 3 (FLT3). Cabozantinib has shown promising antitumor effect in various solid tumors, including NSCLC, in clinical trials. The phase Ib/II trial of cabozantinib with and without erlotinib in patients with NSCLC and acquired erlotinib resistance has been reported.86 The overall safety and tolerability profile of cabozantinib and erlotinib appear acceptable without evidence of a cabozantinib/erlotinib drug–drug interaction. Six out of 36 patients assessable for response, including at least three with prior erlotinib therapy, had ≥30% reduction in tumor measurements on at least one postbaseline scan, including three with a complete or partial response (one with MET amplification). Prolonged stable disease of ≥4 months has been observed in some patients including one for >9 months and one with EGFR T790M.86
The phase II randomized discontinuation trial of cabozantinib (XL184) in patients with advanced solid tumors (NCT00940225) has been completed and the interim result was recently presented.87 Of the 483 enrolled patients, 398 with nine different types of solid tumors were evaluable and evidence of soft tissue tumor regression was observed in all tumor types. The most common related adverse events (grade ≥3) were fatigue (9%), hand-foot syndrome (8%), and hypertension (5%). Dose reductions for adverse events occurred in 41% of patients. In soft tissue tumors, the overall response rate (complete or partial response) was 9% in eight out of nine indications. Rates of disease control (partial response or stable disease) at week twelve of ≥40% were observed in six different solid tumors, including NSCLC (40%, partial response in six out of 47 [13%]). Interestingly, all the patients with mutations of EGFR (four out of 23) or KRAS (three out of 23) had partial response or stable disease, and none of the nonresponders had EGFR or KRAS mutations. However, the sample size was too small to draw any conclusion between the response rate and EGFR/KRAS mutation status. The MET amplification status was not reported in this interim report. Quite interestingly, there were soft tissue and visceral tumor regression and resolution of bone lesions on a bone scan observed across multiple tumor types, supporting the notion of targeting MET pathway as the key player of “oncogene expedience” in addition to “oncogene addiction/dependence.”87
Foretinib (XL880, EXEL-2880, GSK1363089)
Foretinib is a multitargeted small molecule kinase inhibitor that targets MET and members of the VEGFR kinase families, with additional inhibitory activity toward KIT, FLT3, platelet-derived growth factor receptor-β, TIE-2, RON, and AXL.88 In vivo, these effects produce significant dose-dependent inhibition of tumor burden in an experimental model of lung metastasis.88 A phase I study of foretinib has been conducted in metastatic or unresectable solid tumor patients.89 A phase I/II clinical trial of foretinib with and without erlotinib in locally advanced or metastatic NSCLC is currently ongoing in Canada.
Other MET-TKIs
PF-04217903 is an orally available, adenosine triphosphate-competitive small molecule inhibitor of MET that demonstrated selectivity of >1000-fold for MET compared with a screening panel of >150 protein kinases.90 A phase I trial of PF-04217903 in advanced solid tumors was completed in 2011 and the results are expected to be reported soon.
Amuvatinib (MP470) is another oral multitargeted TKI that inhibits the mutant forms of KIT, platelet-derived growth factor receptor-α (PDGFR-α), and MET, as well as DNA repair. A phase I trial has demonstrated that it is well tolerated in combination with different antitumor chemotherapies.91 The role of amuvatinib in SCLC is currently being studied in a phase II trial.
Other MET-TKIs including SGX523 and MGCD265 are under early clinical development also.
MET antibodies
Onartuzumab (MetMAb)
MetMAb is a recombinant, humanized, monovalent (one-armed) monoclonal antibody designed to bind to MET and inhibit HGF binding without inducing MET dimerization.92 This is in contrast to traditional bivalent antibodies to MET, which potentially activate – rather than inhibit – MET signaling by inducing MET dimerization.93,94 Its safety and recommended dose have been established in patients with solid tumor in a phase Ib trial.95 Recently, a global randomized, double-blind phase II study comparing MetMAb plus erlotinib to placebo plus erlotinib in second/third-line NSCLC (OAM4558 g) has been completed. The updated efficacy results were presented at the 2011 American Society of Clinical Oncology annual meeting. NSCLC patients (N = 128) were equally randomized to receive MetMAb plus erlotinib or placebo plus erlotinib. Fifty-four percent (54%) of patients had MET IHC expression (2+ or 3+) NSCLC, which was associated with a worse outcome (OS HR 2.52, placebo plus erlotinib cohort). In the MET IHC 2+ or 3+ group (n = 65), MetMAb resulted in a statistically and clinically significant improvement in both PFS (median 3.0 months versus 1.5 months, HR 0.47, P = 0.01) and OS (median 12.6 months versus 4.6 months, HR 0.37, P = 0.002).96 In the MET IHC low/negative group, OS was worse in the MetMAb plus erlotinib group (HR 3.02, P = 0.021). Erlotinib-related toxicities were comparable between treatment arms. The design of the MetMAb phase II trial incorporated the development of a companion diagnostic test for assaying MET receptor expression level by IHC, and raised the prospect of it being a predictive biomarker. In the MetMAb trial, according to the trial investigators, the OS benefit (or the trend of OS benefit) was not exclusive to EGFR mutations or MET FISH+ (at least five copies) (P = 0.19) and was observed in FISH−/IHC+ (P = 0.09) patients, suggesting IHC is a more sensitive predictor of benefit for MetMAb. Removing patients with EGFR mutations did not alter results, albeit with the P value of survival benefit becoming statistically insignificant (P = 0.29) in the analysis likely due to a small sample size.96
A complete and durable (lasting 2 years) response of MetMAb was observed in a 48 year-old female with chemorefractory metastatic gastric cancer to the liver who was treated in a phase I clinical trial with MetMAb.97 These results lend support for further investigation of MetMAb as a potential personalized MET targeting cancer therapy.98 In light of the negative survival data seen in the MetMAb plus erlotinib arm in patients with low MET expression in the phase II study, a phase III study of MetMAb in combination with erlotinib has recently been launched in MET IHC+ advanced NSCLC. The role of MetMAb in combination with a traditional platinum doublet in the first-line setting in advanced NSCLC will also be studied in proposed phase II clinical trials (NCT00854308, NCT01496742).
CE-355621
CE-355621 is a novel antibody against MET. Its antitumor efficacy was studied in preclinical study,99 but it has not been reported in clinical study.
HGF antibodies
Ficlatuzumab (AV-299)
Ficlatuzumab is a humanized anti-HGF immunoglobulin G1 monoclonal antibody with potent MET signaling inhibition through blocking of its sole ligand HGF. Clinical data from phase I studies of ficlatuzumab indicate a favorable tolerability profile in combination with EGFR inhibitors,100,101 and partial response was observed in five out of twelve patients with NSCLC.100 A phase II trial evaluating ficlatuzumab in combination with gefitinib as a first-line therapy for patients with never or light smoking history NSCLC in Asia was completed in 2011 and the results are expected to be reported soon.
AMG 102
AMG 102 is another novel, fully humanized monoclonal antibody that selectively targets HGF. Its tolerance and toxicities have been studied in combination with antiangiogenesis targeted therapies in adult patients with advanced solid tumors and clinical response was observed.102 A phase I/II trial of AMG 102 with erlotinib or cytotoxic chemotherapy in advanced NSCLC in currently ongoing.
Personalized MET targeted lung cancer therapy: future directions
The role of MET/HGF in human cancer growth and invasion has been extensively studied and the recent clinical studies on MET inhibitors or antibodies in lung cancer patients are rather promising. However, a number of important questions remain to be fully addressed in the optimization of MET target therapy as outlined below. Addressing these critical areas of MET inhibition strategies in lung cancer would help to bring MET targeted agents to full clinical fruition and to impact patient outcomes more meaningfully.
First, the optimal patient subgroup that would most benefit from MET inhibitor treatment remains to be determined. Several biomarkers, including HGF serum level and tumor MET gene amplification and overexpression, have been studied in both preclinical models and ongoing clinical trials. A reliable and validated predictive biomarker for MET targeted therapy is urgently needed. Nonetheless, a confounding issue here is that many MET targeting agents in clinical trial studies now are multitargeted inhibitors, with the exception of tivantinib and MetMAb. This creates analytical complexities in response correlation. Abnormally high HGF levels have been associated with advanced disease and poor outcomes for several cancers.103,104 Plasma HGF concentrations have been reported to correlate with response to XL184.105 Recently, a case report of durable complete response of gastric cancer with MetMAb found the patient had a remarkably high serum HGF level before MetMAb treatment and experienced a rapid and sustained drop in HGF level posttreatment.97,98 MET amplification has been shown to correlate with drug sensitivity in preclinical models,106 and is incorporated in the phase II and ongoing phase III ARQ 197 clinical trials. MET overexpression as determined by IHC staining is showing good promise as a predictive biomarker, illustrated by the MetMAb phase II study results. Further work in developing this as a companion diagnostic test is ongoing. Optimizing and standardizing MET expression and amplification analysis, whether FISH, quantitative real-time polymerase chain reaction, or FFPE IHC, would be crucial in future clinical development. Novel technological platforms might be useful in this regard, as in the example of multiplexed expression assays and laser microdissection mass spectrometry-based quantitative formalin-fixed paraffin embedded tumor tissue proteomics.107 A better and more sophisticated bioinformatics algorithm would be highly helpful in defining MET activation and thus molecular-candidate patient selection. Moreover, the noninvasive detection of in vivo MET expression in a murine xenograft model by radiolabeling MET antibody (MetSeek™)108 or MET inhibitor ([C11]SU11274) in positron emission tomography imaging109 also shows promise. Developing a MET target imaging platform for patient selection, monitoring therapeutic response, and progression under MET inhibitor therapy would have tremendous clinical utilities. Furthermore, combining MET inhibitor clinical development with novel analytic platforms of assaying circulating tumor cells, circulating endothelial cells, and angiogenesis imaging modality, eg, dynamic contrast-enhanced magnetic resonance imaging, would be quite useful.
Secondly, the best strategy for using MET targeted inhibition as a single agent or in combination with other targeted agents, especially EGFR-TKI, remains to be determined. Currently, most of the phase II and III trials testing MET agents are being conducted in combination with erlotinib (Tables 1 and 2). Recent data suggests that MET inhibition either alone or in combination with EGFR-TKI may indeed have a role in primary therapy of EGFR-TKI naive NSCLC patients. In addition, a number of potential opportunities exist in the combination of MET inhibition with other targeted therapy, such as HGF targeting agents, other targeted kinases agents, or downstream signaling effector inhibitors, eg, PI3K, MEK, and BCL-2/BCL-xL, which require further investigation. To this end, more sophisticated bioinformatics and pathway analysis may be of benefit to achieve these goals, especially when incorporated into upcoming MET targeting agent clinical studies.
Finally, it is important to understand both the potential acquired resistance mechanisms against MET targeting inhibition and strategies to overcome its resistance. Prior experience in other targeted therapeutics, such as erlotinib against mutant EGFR and crizotinib against EML4-ALK, suggests that acquired resistance is thus far unavoidable.55,110 Proactive efforts in identifying potential resistance mechanisms in MET targeted inhibition would be needed to accelerate the discovery of newer cotargeting strategies to dampen drug resistance. Efforts to derive a deeper understanding of tumor biology and molecular pathways in progressing disease from rebiopsy tissues in sites of resistant disease could have a profound impact in advancing the rational therapeutic strategies in MET targeting therapy.
Conclusion
In summary, the important role of the MET pathway in lung cancer and other human malignancies has been demonstrated in extensive preclinical studies, and MET targeting agents have already entered into the arena of cancer clinical investigations. Currently, multiple MET targeting agents are being actively tested for clinical efficacy in lung cancer, including two phase III randomized clinical studies (tivantinib and onartuzumab). Earlier phase studies have shown promising results. However, in order to fully impact the clinical outcome in lung cancer, a number of questions remain to be further addressed in MET targeted therapeutics. These include optimal patient selection, predictive biomarker development, better strategies of single agent or in combination with other targeted agents, and resistance mechanisms. With concerted efforts in translational and clinical development of the MET targeting agents to continue, it will not be unrealistic to expect MET targeting therapies to finally come to full clinical fruition in the foreseeable future to impact lung cancer clinical outcome.
Footnotes
Disclosure
PCM received consulting honorarium and research funding (clinical trial) from Daiichi-Sankyo/ArQule. YF reports no conflicts of interest in this work.
References
- 1.Ray MR, Jablons D, He B. Lung cancer therapeutics that target signaling pathways: an update. Expert Rev Respir Med. 2010;4(5):631–645. doi: 10.1586/ers.10.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 7.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 8.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3(1):13–17. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 9.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6(8):637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- 11.Bredin CG, Liu Z, Klominek J. Growth factor-enhanced expression and activity of matrix metalloproteases in human non-small cell lung cancer cell lines. Anticancer Res. 2003;23(6C):4877–4884. [PubMed] [Google Scholar]
- 12.Cooper CS, Tempest PR, Beckman MP, Heldin CH, Brookes P. Amplification and overexpression of the met gene in spontaneously transformed NIH3T3 mouse fibroblasts. EMBO J. 1986;5(10):2623–2628. doi: 10.1002/j.1460-2075.1986.tb04543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao H, Hung W, Tremblay E, et al. Constitutive activation of met kinase in non-small-cell lung carcinomas correlates with anchorage-independent cell survival. J Cell Biochem. 2002;86(4):665–677. doi: 10.1002/jcb.10239. [DOI] [PubMed] [Google Scholar]
- 14.Ma PC, Tretiakova MS, MacKinnon AC, et al. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer. 2008;47(12):1025–1037. doi: 10.1002/gcc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104(52):20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cappuzzo F, Janne PA, Skokan M, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol. 2009;20(2):298–304. doi: 10.1093/annonc/mdn635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 18.Antipenko A, Himanen JP, van Leyen K, et al. Structure of the semaphorin-3 A receptor binding module. Neuron. 2003;39(4):589–598. doi: 10.1016/s0896-6273(03)00502-6. [DOI] [PubMed] [Google Scholar]
- 19.Gherardi E, Youles ME, Miguel RN, et al. Functional map and domain structure of MET, the product of the c-met protooncogene and receptor for hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A. 2003;100(21):12039–12044. doi: 10.1073/pnas.2034936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Love CA, Harlos K, Mavaddat N, et al. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol. 2003;10(10):843–848. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- 21.Jeffers M, Rong S, Vande Woude GF. Enhanced tumorigenicity and invasion-metastasis by hepatocyte growth factor/scatter factor-met signalling in human cells concomitant with induction of the urokinase proteolysis network. Mol Cell Biol. 1996;16(3):1115–1125. doi: 10.1128/mcb.16.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Q, Bradley R, Kang L, et al. Hepatocyte growth factor (HGF) autocrine activation predicts sensitivity to MET inhibition in glioblastoma. Proc Natl Acad Sci U S A. 2012;109(2):570–575. doi: 10.1073/pnas.1119059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horiguchi N, Takayama H, Toyoda M, et al. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene. 2002;21(12):1791–1799. doi: 10.1038/sj.onc.1205248. [DOI] [PubMed] [Google Scholar]
- 24.Tward AD, Jones KD, Yant S, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci U S A. 2007;104(37):14771–14776. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153(5):1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8(10):404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 27.Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77(2):261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 28.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 29.Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19(10):542–551. doi: 10.1016/j.tcb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Guo A, Villen J, Kornhauser J, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105(2):692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Z, Du R, Jiang S, et al. Dual MET-EGFR combinatorial inhibition against T790M-EGFR-mediated erlotinib-resistant lung cancer. Br J Cancer. 2008;99(6):911–922. doi: 10.1038/sj.bjc.6604559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long IS, Han K, Li M, et al. Met receptor overexpression and oncogenic Ki-ras mutation cooperate to enhance tumorigenicity of colon cancer cells in vivo. Mol Cancer Res. 2003;1(5):393–401. [PubMed] [Google Scholar]
- 33.Ma PC, Schaefer E, Christensen JG, Salgia R. A selective small molecule c-MET inhibitor, PHA665752, cooperates with rapamycin. Clin Cancer Res. 2005;11(6):2312–2319. doi: 10.1158/1078-0432.CCR-04-1708. [DOI] [PubMed] [Google Scholar]
- 34.Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol. 2011;29(24):3307–3315. doi: 10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 35.Matsubara D, Ishikawa S, Oguni S, Aburatani H, Fukayama M, Niki T. Molecular predictors of sensitivity to the MET inhibitor PHA665752 in lung carcinoma cells. J Thorac Oncol. 2010;5(9):1317–1324. doi: 10.1097/JTO.0b013e3181e2a409. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Wislez M, Fujimoto N, et al. A selective small molecule inhibitor of c-Met, PHA-665752, reverses lung premalignancy induced by mutant K-ras. Mol Cancer Ther. 2008;7(4):952–960. doi: 10.1158/1535-7163.MCT-07-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olivero M, Rizzo M, Madeddu R, et al. Overexpression and activation of hepatocyte growth factor/scatter factor in human non-small-cell lung carcinomas. Br J Cancer. 1996;74(12):1862–1868. doi: 10.1038/bjc.1996.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maulik G, Kijima T, Ma PC, et al. Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res. 2002;8(2):620–627. [PubMed] [Google Scholar]
- 39.Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65(4):1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 40.Ma PC, Kijima T, Maulik G, et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 2003;63(19):6272–6281. [PubMed] [Google Scholar]
- 41.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 42.Duh FM, Scherer SW, Tsui LC, Lerman MI, Zbar B, Schmidt L. Gene structure of the human MET proto-oncogene. Oncogene. 1997;15(13):1583–1586. doi: 10.1038/sj.onc.1201338. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y. The human hepatocyte growth factor receptor gene: complete structural organization and promoter characterization. Gene. 1998;215(1):159–169. doi: 10.1016/s0378-1119(98)00264-9. [DOI] [PubMed] [Google Scholar]
- 44.Beau-Faller M, Ruppert AM, Voegeli AC, et al. MET gene copy number in non-small cell lung cancer: molecular analysis in a targeted tyrosine kinase inhibitor naive cohort. J Thorac Oncol. 2008;3(4):331–339. doi: 10.1097/JTO.0b013e318168d9d4. [DOI] [PubMed] [Google Scholar]
- 45.Kanteti R, Yala S, Ferguson MK, Salgia R. MET, HGF, EGFR, and PXN gene copy number in lung cancer using DNA extracts from FFPE archival samples and prognostic significance. J Environ Pathol Toxicol Oncol. 2009;28(2):89–98. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22(4):309–325. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- 47.Onitsuka T, Uramoto H, Ono K, et al. Comprehensive molecular analyses of lung adenocarcinoma with regard to the epidermal growth factor receptor, K-ras, MET, and hepatocyte growth factor status. J Thorac Oncol. 2010;5(5):591–596. doi: 10.1097/JTO.0b013e3181d0a4db. [DOI] [PubMed] [Google Scholar]
- 48.Onozato R, Kosaka T, Kuwano H, Sekido Y, Yatabe Y, Mitsudomi T. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol. 2009;4(1):5–11. doi: 10.1097/JTO.0b013e3181913e0e. [DOI] [PubMed] [Google Scholar]
- 49.Di Renzo MF, Olivero M, Martone T, et al. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene. 2000;19(12):1547–1555. doi: 10.1038/sj.onc.1203455. [DOI] [PubMed] [Google Scholar]
- 50.Kong-Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res. 2006;66(1):283–289. doi: 10.1158/0008-5472.CAN-05-2749. [DOI] [PubMed] [Google Scholar]
- 51.Krishnaswamy S, Kanteti R, Duke-Cohan JS, et al. Ethnic differences and functional analysis of MET mutations in lung cancer. Clin Cancer Res. 2009;15(18):5714–5723. doi: 10.1158/1078-0432.CCR-09-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varella-Garcia M, Iafrate J, Pao W, et al. ALK fusion and MET amplification as molecular biomarkers and therapeutic targets in advanced lung adenocarcinomas in the Lung Cancer Mutation Consortium; Paper presented at: 14th World Conference on Lung Cancer; July 3–7, 2011; Amsterdam, The Netherlands. [Google Scholar]
- 53.Cappuzzo F, Marchetti A, Skokan M, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol. 2009;27(10):1667–1674. doi: 10.1200/JCO.2008.19.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17(5):1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rho JK, Choi YJ, Lee JK, et al. The role of MET activation in determining the sensitivity to epidermal growth factor receptor tyrosine kinase inhibitors. Mol Cancer Res. 2009;7(10):1736–1743. doi: 10.1158/1541-7786.MCR-08-0504. [DOI] [PubMed] [Google Scholar]
- 57.Fan W, Tang Z, Yin L, et al. Met-independent lung cancer cells evading EGFR kinase inhibitors are therapeutically susceptible to BH3 mimetic agents. Cancer Res. 2011;71(13):4494–4505. doi: 10.1158/0008-5472.CAN-10-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yano S, Yamada T, Takeuchi S, et al. Hepatocyte growth factor expression in EGFR mutant lung cancer with intrinsic and acquired resistance to tyrosine kinase inhibitors in a Japanese cohort. J Thorac Oncol. 2011;6(12):2011–2017. doi: 10.1097/JTO.0b013e31823ab0dd. [DOI] [PubMed] [Google Scholar]
- 60.Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68(22):9479–9487. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 61.Yamada T, Matsumoto K, Wang W, et al. Hepatocyte growth factor reduces susceptibility to an irreversible epidermal growth factor receptor inhibitor in EGFR-T790M mutant lung cancer. Clin Cancer Res. 2010;16(1):174–183. doi: 10.1158/1078-0432.CCR-09-1204. [DOI] [PubMed] [Google Scholar]
- 62.Donev IS, Wang W, Yamada T, et al. Transient PI3K inhibition induces apoptosis and overcomes HGF-mediated resistance to EGFR-TKIs in EGFR mutant lung cancer. Clin Cancer Res. 2011;17(8):2260–2269. doi: 10.1158/1078-0432.CCR-10-1993. [DOI] [PubMed] [Google Scholar]
- 63.Wang W, Li Q, Takeuchi S, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancer. Clin Cancer Res. 2012;18(6):1663–1671. doi: 10.1158/1078-0432.CCR-11-1171. [DOI] [PubMed] [Google Scholar]
- 64.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3(4):347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Li A, Glas M, et al. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc Natl Acad Sci U S A. 2011;108(24):9951–9956. doi: 10.1073/pnas.1016912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 67.De Bacco F, Luraghi P, Medico E, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst. 2011;103(8):645–661. doi: 10.1093/jnci/djr093. [DOI] [PubMed] [Google Scholar]
- 68.Guryanova OA, Bao S. How scatter factor receptor c-MET contributes to tumor radioresistance: ready, set, scatter! J Natl Cancer Inst. 2011;103(8):617–619. doi: 10.1093/jnci/djr103. [DOI] [PubMed] [Google Scholar]
- 69.Rosen EM, Goldberg ID. Scatter factor and angiogenesis. Adv Cancer Res. 1995;67:257–279. doi: 10.1016/s0065-230x(08)60715-0. [DOI] [PubMed] [Google Scholar]
- 70.Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci U S A. 2003;100(22):12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puri N, Khramtsov A, Ahmed S, et al. A selective small molecule inhibitor of c-Met, PHA665752, inhibits tumorigenicity and angiogenesis in mouse lung cancer xenografts. Cancer Res. 2007;67(8):3529–3534. doi: 10.1158/0008-5472.CAN-06-4416. [DOI] [PubMed] [Google Scholar]
- 72.Sattler M, Pride YB, Ma P, et al. A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 2003;63(17):5462–5469. [PubMed] [Google Scholar]
- 73.Christensen JG, Schreck R, Burrows J, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63(21):7345–7355. [PubMed] [Google Scholar]
- 74.Munshi N, Jeay S, Li Y, et al. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther. 2010;9(6):1544–1553. doi: 10.1158/1535-7163.MCT-09-1173. [DOI] [PubMed] [Google Scholar]
- 75.Rosen LS, Senzer N, Mekhail T, et al. A phase I dose-escalation study of tivantinib (ARQ 197) in adult patients with metastatic solid tumors. Clin Cancer Res. 2011;17(24):7754–7764. doi: 10.1158/1078-0432.CCR-11-1002. [DOI] [PubMed] [Google Scholar]
- 76.Yap TA, Olmos D, Brunetto AT, et al. Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J Clin Oncol. 2011;29(10):1271–1279. doi: 10.1200/JCO.2010.31.0367. [DOI] [PubMed] [Google Scholar]
- 77.Nishina T, Hirashima T, Sugio K, et al. The effect of CYP2C19 polymorphism on the tolerability of ARQ 197: results from phase I trial in Japanese patients with metastatic solid tumors [abstract] J Clin Oncol. 2011;29(Suppl):2516. [Google Scholar]
- 78.Tsao MS, Yang Y, Marcus A, Liu N, Mou L. Hepatocyte growth factor is predominantly expressed by the carcinoma cells in non-small-cell lung cancer. Hum Pathol. 2001;32(1):57–65. doi: 10.1053/hupa.2001.21133. [DOI] [PubMed] [Google Scholar]
- 79.Scagliotti GV, Novello S, Schiller JH, et al. Rationale and design of MARQUEE: a phase III, randomized, double-blind study of tivantinib plus erlotinib versus placebo plus erlotinib in previously treated patients with locally advanced or metastatic, nonsquamous, non-small-cell lung cancer. Clin Lung Cancer. 2012 Mar 21; doi: 10.1016/j.cllc.2012.01.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 80.Cui JJ, Tran-Dube M, Shen H, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK) J Med Chem. 2011;54(18):6342–6363. doi: 10.1021/jm2007613. [DOI] [PubMed] [Google Scholar]
- 81.Kwak EL, Camidge DR, Clark J, et al. Clinical activity observed in a phase I dose escalation trial of an oral c-met and ALK inhibitor, PF-02341066 [abstract] J Clin Oncol. 2009;27(Suppl 15):3509. [Google Scholar]
- 82.Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67(9):4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 83.Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6(5):942–946. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 84.Lennerz JK, Kwak EL, Michael M, et al. Identification of a small and lethal subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib by MET amplification [abstract] J Clin Oncol. 2011;29(Suppl):4130. doi: 10.1200/JCO.2011.35.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chi A, Kwak E, Clark J, et al. Clinical improvement and rapid radiographic regression induced by a MET inhibitor in a patient with MET-amplified glioblastoma [abstract] J Clin Oncol. 2011;29(Suppl):2072. [Google Scholar]
- 86.Wakelee HA, Gettinger SN, Engelman JA, et al. A phase Ib/II study of XL184 (BMS 907351) with and without erlotinib (E) in patients (pts) with non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol. 2010;28(Suppl 15):3017. [Google Scholar]
- 87.Gordon MS, Vogelzang NJ, Schoffski P, Daud A, Spira AI. Activity of cabozantinib (XL184) in soft tissue and bone: results of a phase II randomized discontinuation trial (RDT) in patients (pts) with advanced solid tumors [abstract] J Clin Oncol. 2011;29(Suppl):3010. [Google Scholar]
- 88.Qian F, Engst S, Yamaguchi K, et al. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res. 2009;69(20):8009–8016. doi: 10.1158/0008-5472.CAN-08-4889. [DOI] [PubMed] [Google Scholar]
- 89.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16(13):3507–3516. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 90.Zou HY, Li Q, Lee JH, et al. Sensitivity of selected human tumor models to PF-04217903, a novel selective c-Met kinase inhibitor. Mol Cancer Ther. 2012;11(4):1036–1047. doi: 10.1158/1535-7163.MCT-11-0839. [DOI] [PubMed] [Google Scholar]
- 91.Sankhala KK, Tolcher AW, Mita MM, et al. Amuvatinib (MP-470), an oral dual inhibitor of mutant kinases and DNA repair: final results from a 100-patient, 5-arm phase Ib trial in combination with five standard of care (SOC) anticancer regimens [abstract] J Clin Oncol. 2011;29(Suppl):3074. [Google Scholar]
- 92.Martens T, Schmidt NO, Eckerich C, et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res. 2006;12(20 Pt 1):6144–6152. doi: 10.1158/1078-0432.CCR-05-1418. [DOI] [PubMed] [Google Scholar]
- 93.Ohashi K, Marion PL, Nakai H, et al. Sustained survival of human hepatocytes in mice: a model for in vivo infection with human hepatitis B and hepatitis delta viruses. Nat Med. 2000;6(3):327–331. doi: 10.1038/73187. [DOI] [PubMed] [Google Scholar]
- 94.Prat M, Crepaldi T, Pennacchietti S, Bussolino F, Comoglio PM. Agonistic monoclonal antibodies against the Met receptor dissect the biological responses to HGF. J Cell Sci. 1998;111(Pt 2):237–247. doi: 10.1242/jcs.111.2.237. [DOI] [PubMed] [Google Scholar]
- 95.Berinstein NL, Morse M, Kaufman H, et al. A phase I study of the safety and immunogenicity of a therapeutic vaccine, DPX-0907 in patients with advanced-stage ovarian, breast, or prostate cancer [abstract] J Clin Oncol. 2011;29(Suppl):e13050. [Google Scholar]
- 96.Spigel DR, Ervin TJ, Ramlau R, et al. Final efficacy results from OAM4558 g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC [abstract] J Clin Oncol. 2011;29(Suppl):7505. [Google Scholar]
- 97.Catenacci DV, Henderson L, Xiao SY, et al. Durable complete response of metastatic gastric cancer with anti-Met therapy followed by resistance at recurrence. Cancer Discov. 2011;1(7):573–579. doi: 10.1158/2159-8290.CD-11-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng Y, Ma PC. Anti-MET targeted therapy has come of age: the first durable complete response with MetMAb in metastatic gastric cancer. Cancer Discov. 2011;1(7):550–554. doi: 10.1158/2159-8290.CD-11-0289. [DOI] [PubMed] [Google Scholar]
- 99.Tseng JR, Kang KW, Dandekar M, et al. Preclinical efficacy of the c-Met inhibitor CE-355621 in a U87 MG mouse xenograft model evaluated by 18F-FDG small-animal PET. J Nucl Med. 2008;49(1):129–134. doi: 10.2967/jnumed.106.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tan E, Park K, Lim WT, et al. Phase Ib study of ficlatuzumab (formerly AV-299), an anti-hepatocyte growth factor (HGF) monoclonal antibody (MAb) in combination with gefitinib (G) in Asian patients (pts) with NSCLC [abstract] J Clin Oncol. 2011;29(Suppl):7571. [Google Scholar]
- 101.Patnaik A, Weiss GJ, Papadopoulos K, et al. Phase I study of SCH 900105 (SC), an anti-hepatocyte growth factor (HGF) monoclonal antibody (MAb), as a single agent and in combination with erlotinib (E) in patients (pts) with advanced solid tumors [abstract] J Clin Oncol. 2010;28(Suppl 15):2525. [Google Scholar]
- 102.Rosen PJ, Sweeney CJ, Park DJ, et al. AMG 102, an HGF/SF antagonist, in combination with anti-angiogenesis targeted therapies in adult patients with advanced solid tumors [abstract] J Clin Oncol. 2008;26(Suppl):3570. [Google Scholar]
- 103.Tanimoto S, Fukumori T, El-Moula G, et al. Prognostic significance of serum hepatocyte growth factor in clear cell renal cell carcinoma: comparison with serum vascular endothelial growth factor. J Med Invest. 2008;55(1–2):106–111. doi: 10.2152/jmi.55.106. [DOI] [PubMed] [Google Scholar]
- 104.Bharti A, Ma PC, Maulik G, et al. Haptoglobin alpha-subunit and hepatocyte growth factor can potentially serve as serum tumor biomarkers in small cell lung cancer. Anticancer Res. 2004;24(2C):1031–1038. [PubMed] [Google Scholar]
- 105.Muller T, DePrimo S, McGrath G, et al. Pharmacodynamic and correlative biomarker analyses in clinical trials of XL184, an oral, potent inhibitor of MET, VEGFR2, and RET [abstract] Mol Cancer Ther. 2009;8(Suppl 12):B269. [Google Scholar]
- 106.Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A. 2006;103(7):2316–2321. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel V, Hood BL, Molinolo AA, et al. Proteomic analysis of laser-captured paraffin-embedded tissues: a molecular portrait of head and neck cancer progression. Clin Cancer Res. 2008;14(4):1002–1014. doi: 10.1158/1078-0432.CCR-07-1497. [DOI] [PubMed] [Google Scholar]
- 108.Hay RV, Cao B, Skinner RS, et al. Nuclear imaging of Met-expressing human and canine cancer xenografts with radiolabeled monoclonal antibodies (MetSeek) Clin Cancer Res. 2005;11(19 Pt 2):7064s–7069s. doi: 10.1158/1078-0432.CCR-1004-0014. [DOI] [PubMed] [Google Scholar]
- 109.Wu C, Tang Z, Fan W, et al. In vivo positron emission tomography (PET) imaging of mesenchymal-epithelial transition (MET) receptor. J Med Chem. 2010;53(1):139–146. doi: 10.1021/jm900803q. [DOI] [PubMed] [Google Scholar]
- 110.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra117. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]