Abstract
Objectives
An economic evaluation was conducted in conjunction with a prospective, multicenter, randomized trial, to compare pemetrexed with erlotinib in pretreated patients with metastatic non-small cell lung cancer (NSCLC) in Greece.
Methods
The effectiveness of treatments examined was comparable; thus, cost minimization analysis was conducted to evaluate which option is less costly. Patient-level resource utilization data were combined with unit cost data, which were aggregated to compute the total treatment cost for each patient. The analysis was conducted with respect to the individual incurring the cost. Due to the limited life-expectancy of the patients, discounting was unnecessary. Since data were right censored, the Bang and Tsiatis method was employed to identify unbiased estimators of the mean cost per treatment arm, while other methods were employed for sensitivity analysis. To analyze uncertainty and to construct uncertainty intervals (UI), stochastic analysis was performed based on 5000 bootstrap replications.
Results
The one-year survival rate was 28.3% in the pemetrexed arm and 31.7% in the erlotinib arm, while the corresponding median survival over the follow-up period was 7.1 and 6.7 months, respectively (P = 0.765). Total cost in the pemetrexed arm was €10508 (95% UI: €9552–€11488), while in the erlotinib arm the cost was €9563 (95% UI: €8499–€10711); thus, no statistically significant difference was found between the comparators (P = 0.206). Results remained constant for all sensitivity analyses.
Conclusions
There is no survival or cost difference between erlotinib and pemetrexed; thus, these therapies are equivalent. Further studies are needed to determine whether other parameters, such as quality of life, differ among treatment options.
Keywords: erlotinib, economic evaluation, lung cancer, pemetrexed
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death in both men and women in Western countries and accounts for approximately 80%–85% of new cases of lung carcinoma each year.1 Systemic chemotherapy remains the cornerstone of treatment for metastatic NSCLC, as it prolongs survival and palliates symptoms compared with best supportive care alone.2 Doublet chemotherapy regimens are considered the standard of care because they are superior to single-agent treatments, while three-drug combinations do not appear to offer any benefit in terms of overall survival compared to two-drug regimens.3 Platinum-based doublets are preferred over platinum-free regimens because they are associated with a minimal survival benefit.4
Nevertheless, NSCLC patients will inevitably experience tumor progression; patients with a good performance status (PS) and may be eligible for second-line treatment. Docetaxel, pemetrexed, and erlotinib are considered standard second-line therapies for patients with a good performance status.5–7 Docetaxel has received approval as a second-line treatment based on the results of a randomized phase III trial demonstrating prolonged overall survival (OS) compared to a placebo.7 A more recent, non-inferiority phase III study compared docetaxel with pemetrexed as second-line therapy in NSCLC.6 No significant difference was observed in OS or 1-year survival, but pemetrexed was associated with a more favorable toxicity profile. Erlotinib significantly prolonged progression-free survival (PFS) and OS compared to a placebo in a phase III trial.5 Although erlotinib and pemetrexed are both considered standard second-line treatments, they have never been compared.
Recently, the Lung Cancer Working Group of the Hellenic Oncology Research Group (HORG) conducted a prospective, multicenter, randomized, phase III trial to compare pemetrexed and erlotinib in pretreated patients with metastatic NSCLC in Greece.8 Given the high incidence of NSCLC and the significant morbidity and mortality associated with it, this disease imposes a heavy burden on the health system and society. Therapies are also expensive and have varying impacts. In this context, an economic evaluation was conducted in conjunction with a clinical trial to assess the economic impact of therapeutic alternatives. Here, we present the results of this economic analysis, which are critically important during the current financial crisis.
Methods
Patients and therapies
According to the clinical protocol of the study, patients were recruited if they were older than 18 years of age and had histologically or cytologically confirmed NSCLC stage IIIB (wet) or IV, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2, had progressed during or after first-line chemotherapy (platinum based for those who were ≤65 years), or had a negative Comprehensive Geriatric Assessment (non-frail patients) for those who were ≥65 years; all patients had a life expectancy ≥3 months and adequate hematological, renal, and liver profiles. Patients were excluded if they had any severe pathological comorbidity or mental disorder that could prevent their compliance with the treatment protocol. “Frail” patients who were ≥65 years were also excluded. After July 2008, when a phase III trial by Scaglioti et al9 demonstrated survival differences based on histologic type, only patients with adenocarcinomas were randomized. Nonetheless, all eligible patients were included in economic analysis. According to our pre-specified protocol, the primary endpoint of the trial was to compare time to tumor progression (TTP) between treatments, while secondary objectives included comparing OS, safety, and treatment cost of the two alternative cancer regimens. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and good clinical practice guidelines were observed. All patients were required to provide written informed consent and the study was approved by the ethics and scientific committees of each participating institution.
Eligible patients were centrally registered and stratified according to age, PS, disease stage and progress in prior chemotherapy and were randomly assigned to either pemetrexed (Alimta; Eli Lilly, Indianapolis, IN, USA) 500 mg/m2 on day 1 and every 3 weeks or erlotinib (Tarceva; Roche Ltd, Basel, Switzerland) 150 mg × 1 continuously. Patients in the pemetrexed group received corticosteroids, vitamin B12, and folic acid according to standard recommendations. Concurrent use of pemetrexed with non-steroidal anti-inflammatory drugs was not allowed. Granulocyte colony-stimulating factor (G-CSF) was not used prophylactically in any participant. The chemotherapy regimen was planned to be delivered for six cycles, except in cases in which persistent toxicity delayed chemotherapy and led to removing the patient from the study. Patients included in the analysis were reasonably well-balanced regarding all important clinical characteristics (Table 1).
Table 1.
Demographic data of patients in the two treatment groups
| Pemetrexed (n = 166) |
Erlotinib (n = 166) |
P-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | |||||
| Median | 66 | 65 | |||
| Min-max | 42–86 | 37–83 | |||
| Sex | |||||
| Male | 138 | 83.1 | 135 | 81.3 | 0.667 |
| Female | 28 | 16.9 | 31 | 18.7 | |
| Performance status | |||||
| 0 | 37 | 22.3 | 44 | 26.5 | 0.065 |
| 1 | 98 | 59.0 | 104 | 62.7 | |
| 2 | 31 | 18.7 | 18 | 10.8 | |
| Histological subtypes | |||||
| Squamous | 36 | 21.7 | 39 | 23.5 | |
| Non-squamous | 94 | 56.6 | 89 | 53.6 | |
| Large-cell | 7 | 4.2 | 4 | 2.4 | |
| Mixed | 2 | 1.2 | 4 | 2.4 | |
| Undifferentiated | 14 | 8.4 | 10 | 6.0 | |
| BAC | 2 | 1.2 | 1 | 0.6 | |
| Unknown | 11 | 6.6 | 19 | 11.4 | |
| Stage | |||||
| IIIB | 19 | 11.4 | 12 | 7.2 | 0.187 |
| IV | 147 | 88.6 | 154 | 92.8 | |
| Line therapy | |||||
| 2nd | 101 | 60.8 | 89 | 53.6 | 0.183 |
| .2nd | 65 | 39.2 | 77 | 46.4 | |
| Response to previous treatment | |||||
| Response | 36 | 21.7 | 26 | 15.7 | 0.159 |
| No Response | 130 | 78.3 | 140 | 84.3 | |
| Interval from previous therapy (median, min-max) | 1.6 | 1.0–34.0 | 1.4 | 1.0–17.0 | |
Abbreviation: BAC, Bronchioloalveolar carcinoma.
Analytical approach
The present economic evaluation was conducted in conjunction with a clinical trial involving 332 patients who were randomized into two different treatment groups. Treatment effectiveness was quantified for the economic study in terms of mean patient survival, which followed the standard approach for this type of study.10–12 Survival was calculated as the time from randomization to death from any cause or loss to follow-up or to the end of the follow-up period. The perspective of economic evaluation was that of the sickness funds; thus, only direct health care costs reimbursed by payers were considered. Remaining costs, such as transportation costs or missed days of work, were ignored in the present analysis. Given the limited life-expectancy of patients, costs were estimated without discounting. Because effectiveness analysis showed no difference in survival, only the mean total therapy cost was reimbursed in each alternative case for cost-minimization analysis.
Costing methodology
Patient-level resource utilization data were combined with unit cost data and these data were then aggregated to compute total treatment cost reimbursed by payers for each patient. Particularly, patient data concerning chemotherapy doses delivered were multiplied by drug prices to compute chemotherapy cost. The costing methodology was performed on a pragmatic basis, taking into account drug wastage because in cases of surplus, the drugs were not used for another patient. Body surface area was estimated to be 1.775 (±0.19) and 1.760 (±0.17) for erlotinib and pemetrexed, respectively.
The administration cost reflects several factors, such as hospitalization due to toxicity, use of erythropoiesis-stimulating agents (ESAs) or G-CSF, and other medication delivered during chemotherapy administration or subsequently for treating adverse events. Hospitalization cost was computed as the product of the number of inpatient days and the cost per day for each patient. Visits to oncologists occurred for treatment administration and in case of adverse events. For patients undergoing radiotherapy, total cost was calculated based upon the duration of treatment and the cost per session.
Examination cost for each cycle accounted for diagnostic imaging, such as computed tomography (CT)-scans, X-rays, and laboratory tests, such as full blood cell count with differential and platelet count as well as full biochemistry tests (SGPT, SGOT, γ-GT, albumin, bilirubin, sodium, potassium, lactate dehydrogenase, alkaline phosphatase, uric acid, and serum creatinine). Drug prices were obtained from the price bulletin issued by the Greek Ministry of Health.13 There was no variation in the prices of resources since these are common across all public hospitals in the country. Table 2 depicts unit price data used for economic analysis.
Table 2.
Unit price data used to estimate treatment cost
| Chemotherapy and cost of drugs | Cost (€) | Examinations | Cost (€) |
|---|---|---|---|
| Pemetrexed Pd Inf 1 Vial × 500 Mg | 963.93 | CT scan | 80.60 |
| Erlotinib FC TabsBt 30 × 150 Mg | 1652.25 | X-ray | 15.00 |
| Lenograstim Ps Inj Sol 33.6 Miu (263 Mcg)/VialBt × 5 Vials + 5 Pf.Syr × 1 Ml Solv | 294.93 | Hb | 4.00 |
| Epoetin AInj Sol 4 Vials × 40,000 Iu/Ml | 869.73 | ESR | 2.00 |
| Cisplatin Inj So Inf 1 Vial 100 Ml × 1 Mg/Ml | 25.52 | SGOT | 9.00 |
| Vinorelbine Sol Iv Inf 10 Mg/Ml Btx 1 Vial x 1Ml | 12.63 | SGPT | 9.00 |
| Gemcitabine Pd Sol Inf 200 Mg/Vial Bt × 1 Vial × 200 Mg | 67.12 | γ-Gt | 5.00 |
| Docetaxel Inj Co Inf 1 Vial × 20 Mg/0.5 Ml | 119.09 | LHD | 4.80 |
| Paclitaxel Cs Sol Inf (Concentrate) 6 Mg/Ml Bt × 1 Vial 30 Mg/5 Ml | 63.07 | Creatine | 4.10 |
| Bevacizumab Cs Sol Inf 25 Mg/MlBt × 1 Vial × 16 Ml (400 Mg/16 Ml) | 1043.08 | Albumin | 5.90 |
| Carboplatin Inj Lyo + Solv Fl 150 Mg/15 Ml | 37.38 | Bilirubin | 2.90 |
| Irinotecan Cs Sol Inf 20 Mg/MlBt × 1 Vial × 5 Ml (glass bottle) | 121.37 | Na | 5.20 |
| Ondansetron FC Tabl Bt 15 × 4 Mg | 25.20 | K | 5.20 |
| Ondansetron Injsol Bt1 Ampx 8 Mg/4 Ml | 6.96 | LDH | 4.80 |
| Radiotherapy | ALP | 5.00 | |
| Radiotherapy planning | 290.60 | Other resources | |
| Radiotherapy cost per session | 14.00 | Hospitalization | 73.37 |
| Outpatient visit | 5.00 |
Abbreviations: ALP, alkaline phosphatase; CT, computed tomography; ESR, erythrocyte sedimentation rate; γ-Gt, γ-glutamyl transpeptidase; FC, film-coated; Hb, hemoglobin; K, potassium; LDH, lactate dehydrogenase; Na, sodium; SGOT, serum glutamic-oxaloacetic transaminase; SGPT, serum glutamic pyruvate transaminase.
Analysis
In economic analyses, the mean cost is rarely normally distributed, but instead is skewed with a long right tail due to the small number of oncology patients that have costly adverse events, while for some patents the cost is very low since they die within 2 months. Hence, testing a hypothesis is problematic using conventional approaches (ie, confidence intervals based on the central limit theorem).14 Furthermore, right-censored data often occur in randomized clinical trials and failure to address this censoring can lead to inconsistent estimates.15 In this study, survival time and, more importantly, cost data were right-censored by 30.7% and 21.7% for pemetrexed and erlotinib, respectively, due to loss of follow-up or because some patients were still alive at the end of the study. To evaluate such cases, a wide range of parametric and non-parametric approaches are available. According to various recommendations,10,14 the non-parametric Bang and Tsiatis16 and the Lin et al17 methods are appropriate approaches to address this censoring. In our case, the base case result was estimated using the non-parametric Bang and Tsiatis method, but the Lin et al 1997, Zhao and Tian18 and the Carides et al “two stage regression”19 approaches were employed for sensitivity analysis. In short, the Bang and Tsiatis method can be used to estimate the weighted cost for each uncensored patient based on the inverse probability of being censored at the time of death. The consistent Kaplan-Meier estimator is used to predict the probability of censoring for these patients, and total cost is derived from the weighted average of costs divided by the total sample size. Similarly, in the Lin et al method, cost estimation is derived from the average of non-censored cases weighted by the probability of surviving at the time of death. A weakness of the abovementioned estimators is that they ignore censored cases in cost calculation. In contrast, the Zhao and Tian method takes into account all cases with longer lifetimes (censored or uncensored) to adjust the cost of censored cases. This estimator generalizes the Bang and Tsiatis estimator by adding an efficiency term, thus achieving a decrease in variance, particularly in cases of heavy censoring. The “two-stage-regression” estimator is a parametric approach in which the total cumulative cost is modeled as a function of failure time. Expected cost is estimated as a function of failure time, and estimated expected cost at a given point is weighted by the Kaplan-Meier probability of death at this point.20 There are several variations of this method, but only a simple linear model was employed in the present analysis.
For uncertainty, the bootstrap method was used to derive standard error estimates for total cost in both treatment groups. The bootstrap method was preferred as a reasonable alternative to parametric inference, as it avoids parametric assumptions and extremely complicated formulas for calculating standard errors.21 Thus, from the original dataset containing cost and failure time, 5000 new datasets for all variables were drawn using random sampling with replacement to estimate uncertainty intervals (UI) using the percentile method and thus to conduct hypothesis testing. All clinical data, which were collected prospectively through clinical report forms, were stored in a main electronic trial database. Statistical and economic analysis was conducted using SPSS (version 16.0) software and the Visual Basic language of Microsoft Office 2003.
Results
The one-year survival rate was 28.3% in the pemetrexed group and 31.7% in the erlotinib group, while corresponding median survival during the follow-up period was 7.1 and 6.7 months, respectively (P = 0.765). Median time to progression was 2.9 months (95% CI: 2.9–3.5) and 3.6 months (95% CI: 2.8–4.3) for the pemetrexed and erlotinib groups, respectively (P = 0.136). No differences were observed in tumor recurrence (90.4% vs 90.2%, P = 0.560). Mean overall survival was 10.39 months (95% CI: 8.44–12.32) and 10.94 months (95% CI: 9.33–12.00, (P = 0.75) for the pemetrexed and erlotinib groups, respectively. Similar overall response rates were also observed in the two groups (11.4% vs 9%, respectively; P = 0.469). According to univariate analysis, the only statistically significant prognostic factors for overall survival were PS (2 vs 0+1; hazard ratio, HR 2.517, 95% CI: 1.780–3.560; P < 0.001) and tumor stage (IV vs IIIB; HR 2.039, 95% CI: 1.243–3.345; P < 0.001). Therapy was not a predictive factor and treatments resulted in comparable survival rates and median survival for the follow-up period. Because there were no statistically significant differences in the primary clinical outcome measures in the two groups, we compared treatment costs.
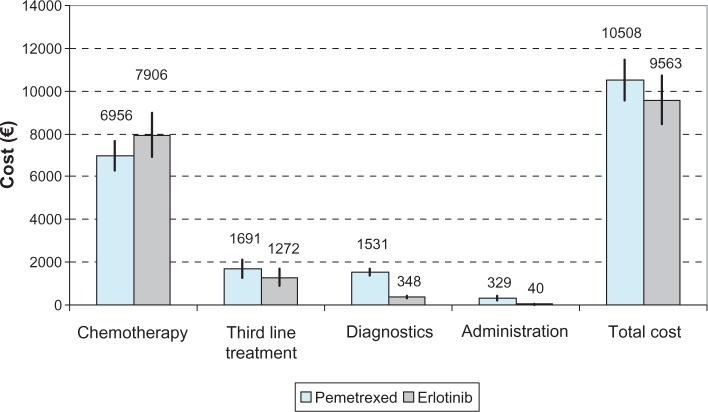
Details of each component of total treatment cost are depicted in Figure 1 and Table 3. Probabilistic sensitivity analysis revealed no statistically significant difference between the costs of the two comparators. Total cost was €10508 (95% UI: €9552–€11488) in the pemetrexed group and €9563 (95% UI: €8499–€10711) in the erlotinib group, a difference of €945 (95% UI: €–513–€2383; P = 0.206). The main factor driving total treatment cost in both arms was the cost of chemotherapy. Particularly, the mean cost of chemotherapy in patients receiving pemetrexed was €6956 (95% UI: €6292–€7646), while the cost for those receiving erlotinib was €7900 (95% UI: €6945–€8986), revealing a non-significant difference of €944 (95% UI: €–2197–€263; P = 0.120).
Figure 1.
Cost (€) per item and treatment arm.
Note: The black line represents the 95% uncertainty intervals.
Table 3.
Treatment costs per patient and therapy group
| B-mean (% of total cost) (B-95% LUI B-95% UUI) | Pemetrexed | Erlotinib | Difference | P-value |
|---|---|---|---|---|
| Chemotherapy | 6956 (66.2%) (6292–7646) | 7900 (82.6%) (6945–8986) | -944 (-2197–263) | 0.120 |
| 3rd line treatment | 1692 (16.1%) (1270–2115) | 1275 (13.3%) (904–1662) | 417 (-150–982) | 0.160 |
| Diagnostics | 1363 (13.0%) (1170–1562) | 310 (3.2%) (265–355) | 1053 (885–1231) | <0.001 |
| Administration | 497 (4.7%) (299–611) | 79 (0.8%) (39–111) | 419 (267–587) | <0.001 |
| Total cost | 10508 (9552–11488) | 9563 (8499–10711) | 945 (-513–2383) | 0.206 |
Notes: B indicates 5000 bootstrap replications; P-value based on a two-tailed bootstrap approximation.
Abbreviations: LUI, Lower Uncertainty Interval; UUI, Upper Uncertainty Interval.
The cost of third-line drugs was estimated to be €1692 (95% UI: €1270–€2115) in the pemetrexed group and €1275 (95% UI: €904–€1662) in the erlotinib group, but the difference was not statistically significant (P = 0.160). Diagnostic and administration costs were statistically significantly lower for the erlotinib group than the pemetrexed group. Chemotherapy accounted for 66.2% of the total treatment cost in the pemetrexed group, followed by third-line costs, which accounted for 16.1%. In contrast, in the erlotinib group, drug cost represented 82.2% of the total treatment cost. Notably, the cost of therapy in some extreme cases was as high as €55,000, while it was lower than €2000 for the entire duration of the study for those who died within 2 months.
Sensitivity analysis
Similar results were observed when other non-parametric models were employed. In both the Zhao and Tian and the Lin et al 1997 models, correction biases for total cost and standard deviations of the various components were similar to those presented above. In the Zhao and Tian approach, mean total treatment cost in patients receiving pemetrexed was €9260 (95% UI: €8175–€10458), while for patients receiving erlotinib this value was €9702 (95% UI: €8795–€10697), a non-statistically significant difference of €442 (95% UI: €–1908–€1031; P = 0.56).18 According to the Lin et al 1997 model, the total cost of pemetrexed was estimated to be €10586 (95% UI: €9573–€111608), while the cost of erlotinib was €10122 (95% UI: €8863–€11498).17 The true difference of total cost was estimated as €464 (95% UI: €–3114–€2996, P = 0.89). Table 4 shows the results of regression analysis based on the two-stage regression model, which includes the independent variable of time measured in terms of monthly units. The coefficient b0 reflects the constant and b1 represents the effect of time. Both estimators were statistically significant (P < 0.001 and P < 0.001, respectively). Taking into account the mean survival per therapy group, the total cost of pemetrexed was estimated to be €11425 (95% UI €8883–€13957), while this value was €10623 (95% UI €8205–€13051) in the erlotinib group.
Table 4.
Estimated regression parameters for the two-stage regression model
| Coefficients | Std err | t-statistic | Probability | 95% LCI | 95% UCI | |
|---|---|---|---|---|---|---|
| Pemetrexed R2 = 28.2% | ||||||
| b1 | 442 | 65 | 6.76 | <0.001 | 312 | 571 |
| b0 | 6835 | 602 | 11.35 | <0.001 | 5643 | 8028 |
| Erlotinib R2 = 35.3% | ||||||
| b1 | 489 | 58 | 8.45 | <0.001 | 375 | 604 |
| b0 | 5275 | 592 | 8.92 | <0.001 | 4104 | 6445 |
Note: b1 expresses the “time” estimator and is measured in months.
Abbreviations: LCI, lower confidence Interval; UCI, upper confidence interval; Std err, standard error.
Frequently, in economic analysis such as this one, there is no correction for censoring; instead, bootstrapping is used directly to draw data sets from the original matrix of censored and uncensored patients. Even in this case, analysis failed to demonstrate a statistically significant difference concerning total treatment cost (P > 0.05).
Discussion
The amount spent each year on treating advanced/metastatic NSCLC is significant. This is due to multiple factors, such as disease prevalence, the considerable share of patients undergoing chemotherapy, treatment length, high health technologies prices, and therapy-induced toxicities.20,22 Since there are no clinical trials directly comparing the efficacy of a cytotoxic agent (pemetrexed) with small molecular-targeted therapy (erlotinib), a clinical study and a corresponding economic evaluation were undertaken to assess clinical differences and the economic burden of comparators from a payer perspective in Greece.
Apart from efficacy and safety, comprehensive sets of resource utilization data were gathered from participating centers during the trial. In the context of the analysis, direct medical costs were estimated. Perspective, indirect costs such as productivity losses were not taken into account. Our analysis showed that the two alternatives have comparable survival times; thus, we analyzed their cost differences to determine the less costly treatment option.
In the presence of proper censoring, the Bang and Tsiatis method was used to estimate total therapy cost. For the sensitivity analysis, two other non-parametric approaches and one parametric approach were used to determine whether the results would remain constant. According to our results, all available estimators of cost were similar, failing to demonstrate differences in between the two treatments. Under conditions of light-censoring of data, the Bang and Tsiatis, Zhao and Tian, and Lin et al methods are identical and represent consistent estimators or actual cost.23 A two-stage regression model also gave similar results, providing a pattern of cost accumulation at any given point of survival time. Other parametric analyses24–26 were not used because individual covariates (sex, age, PS, etc) are frequently incorporated in statistical modeling based on their clinical, rather than economic, importance.20
A statistical limitation of all analyses undertaken was that the cost histories in specific time intervals were not available and thus the accumulation pattern of cost was unknown. Thus, all the methods used were calculated in a simplified form, where only the total cost at the time or death or censoring was considered. Furthermore, for the application of the Zhao and Tian method, a strong assumption was adopted that cost and survival were linearly correlated during the course of the trial.
Additionally, another limitation concerning the nature of the two treatments compared, was that pemetrexed is indicated for non-squamous NSCLC, whereas most guidelines recommend the use of erlotinib for epidermal growth factor receptor (EGFR)-positive patients. This raises a question regarding the comparability of these treatments. This heterogeneity among the intended patient populations may have influenced the results of the analysis.
It must be highlighted that frail patients were excluded from participation in the study. Frequently, classic clinical trials under-enlist or reject frail patients. Frail older adults are weak, often have many complex medical problems, have a lower ability for independent living, may have impaired mental abilities, and have significant difficulty complying with specific protocols.
Additionally, quality-of-life analysis was not considered in the present study. Thus, due to a lack of specific data in Greece, the study ignored potential differences between the two treatments regarding the health-related quality of life (HRQoL). Oral EGFR inhibitors such as erlotinib may have a much different toxicity profile than cytotoxic chemotherapy, including pemetrexed, and these differences are likely to affect quality-of-life outcomes. For instance, the INTEREST trial showed better HRQoL outcomes in patients treated with gefitinib (a similar EGFR inhibitor) versus docetaxel, another second-line chemotherapy option for NSCLC.27 In addition, cost-effectiveness analyses have compared erlotinib, pemetrexed, and docetaxel in the second-line setting, where a calculation of quality-adjusted life years (QALYs) was possible because the authors used health utilities for oral versus iv therapy for NSCLC. One such economic evaluation showed that erlotinib was superior to both docetaxel and pemetrexed.28 Nonetheless, in the analysis, erlotinib, docetaxel, and pemetrexed yielded 0.42, 0.41, and 0.41 QALYs, respectively, indicating that these treatments are nearly equivalent from a clinical perspective. Additionally, the authors concluded that their results should be confirmed through controlled clinical trials.
The findings in the literature are relatively consistent depending on the model’s assumptions.
In Greece, an economic evaluation was undertaken in combination with a randomized phase III study to evaluate treatment with docetaxel-gemcitabine (DG) relative to vinorelbine-cisplatin (VC) as a front-line treatment for patients with advanced/metastatic non-small cell lung cancer.20 The analysis revealed that the treatments showed comparable survival rates, while VC was associated with a lower cost; however, it was also more toxic than the DG regimen, a significant parameter that should be considered when clinical decisions are made. True cost was estimated using similar advanced right-censored techniques to correct for bias in cost calculation.
A second economic evaluation was undertaken alongside a multicenter randomized phase III trial to compare the DG combination with docetaxel monotherapy in untreated patients with advanced/metastatic non-small cell lung cancer.29 The authors concluded that DG is a cost-effective treatment option in relation to docetaxel monotherapy for patients with NSCLC in the Greek National Health System (NHS) setting.
A health economic model constructed for the United Kingdom (UK) was used to evaluate the cost of second-line treatment of NSCLC, comparing docetaxel and best supportive care.30 The base case cost-effectiveness analysis reported a cost per life-year gained of 13,863 pounds sterling for docetaxel 75 mg/m2. Sensitivity analysis showed that the number of treatment cycles per patient, which affects total treatment cost, had the most influence on cost per life-year gained in the base case scenario. The model concluded that docetaxel was a cost-effective second-line treatment for pretreated NSCLC in terms of survival gains for a reasonable increase in costs. In another analysis in the UK, the use of erlotinib instead of docetaxel or pemetrexed was considered a cost-saving option from the NHS perspective.31
A pharmaco-economic review based on data provided by several countries showed that erlotinib may be a cost-saving treatment relative to chemotherapy with docetaxel or pemetrexed for NSCLC.32 Estimated total direct costs with erlotinib were lower than those with docetaxel and pemetrexed because of the generally lower drug acquisition, administration, and adverse event management costs associated with erlotinib. Sensitivity analyses consistently showed that these results were robust under several assumptions and scenarios.
Another cost-effectiveness analysis was conducted to compare costs and effectiveness in patients who received second-line erlotinib with those in patients who received docetaxel in Canada.33 The analysis showed that erlotinib and docetaxel were statistically equivalent regarding treatment cost and overall survival. In clinical practice, docetaxel appeared to be the more frequently prescribed option. The authors concluded that the choice of whether to use erlotinib or docetaxel should be based on factors relating to patient preference rather than costs or effectiveness.
In another analysis, a comparison of clinical and economic outcomes among patients receiving second-line monotherapy with erlotinib, docetaxel, and pemetrexed for NSCLC was conducted for a large network of outpatient community clinics in the USA. The authors concluded that there were no significant differences in OS and PFS between patients receiving erlotinib, docetaxel, and pemetrexed.34 Nevertheless, erlotinib and docetaxel were associated with a statistically significant lower cost and resource use relative to pemetrexed. Similar results were obtained in the case of Portugal.35
In the present evaluation, clinical and also the economic consequences of two therapies, pemetrexed and erlotinib, were considered equivalent, and either treatment can be used. However, in actual clinical settings, the most important issue when selecting treatments is the “right patients for the right treatment.” The use of reliable biomarkers will allow selection of patients who are likely to benefit from a particular treatment, while protecting patients from toxicity and healthcare systems from the significant costs of ineffective agents. In this study, pemetrexed was restricted to patients who had tumors with a non-squamous histology.36 Similarly, although erlotinib was shown to have a benefit in the general population,5 this benefit was primarily driven by the presence of an EGFR mutation.37
Our results are only relevant to Greece particularly the basis of the resources and drug prices used. Total treatment cost is driven primarily by the cost of chemotherapy; thus, the results are sensitive to the cost of drugs. Hence, if any cost parameters change, the conclusions of this study may also change. Our economic analysis was based upon a specific treatment protocol and thus it cannot be extrapolated to other dosage schemes. Finally, in the absence of appropriate data, we confined our analysis to the sickness fund perspective and not to society overall. Future studies could be conducted for a broader analysis.
Conclusion
According to survival analysis, there is no evidence for survival differences between the treatment with pemetrexed and erlotinib. Additionally, this economic evaluation provides further decision criteria based on cost considerations. Pemetrexed and erlotinib regimens represent two equivalent approaches for managing patients with pretreated non-small cell lung cancer. Thus, other clinical parameters may guide the final preference in clinical practice.
Acknowledgments
No funding was received for this research. The authors would like to thank Mr Philip Lees, Technical Editor of the Hellenic Journal of Cardiology, for his critical assistance with the English text.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.NSCLC Meta-Analyses Collaborative Group Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26(28):4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delbaldo C, Michiels S, Syz N, Soria JC, Le CT, Pignon JP. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysis. JAMA. 2004;292(4):470–484. doi: 10.1001/jama.292.4.470. [DOI] [PubMed] [Google Scholar]
- 4.Azzoli CG, Baker S, Jr, Temin S, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27(36):6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. Journal of Clinical Oncology. 2004;22(9):1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. Journal of Clinical Oncology. 2000;18(10):2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 8.Vamvakas L, Agelaki S, Kentepozidis N, et al. Pemetrexed (MTA) compared with erlotinib (ERL) in pretreated patients with advanced non-small cell lung cancer (NSCLC): Results of a randomized phase III Hellenic Oncology Research Group trial. J Clin Oncol. 2010;28(7s) Abstr 7519. [Google Scholar]
- 9.Scagliotti GV, Parikh P, von PJ, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 10.Ramsey S, Willke R, Briggs A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health. 2005;8(5):521–533. doi: 10.1111/j.1524-4733.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 11.Gold M. Panel on cost-effectiveness in health and medicine. Med Care. 1996;34(Suppl 12):DS197–DS199. [PubMed] [Google Scholar]
- 12.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 13.Greek Ministry of Health Drug price bulletin. [Accessed at July 24, 2011]. http://www.yyka.gov.gr/
- 14.O’Hagan A, Stevens JW. On estimators of medical costs with censored data. J Health Econ. 2004;23(3):615–625. doi: 10.1016/j.jhealeco.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Doshi JA, Glick HA, Polsky D. Analyses of cost data in economic evaluations conducted alongside randomized controlled trials. Value Health. 2006;9(5):334–340. doi: 10.1111/j.1524-4733.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- 16.Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika. 2000;87(2):329–343. [Google Scholar]
- 17.Lin DY, Feuer EJ, Etzioni R, Wax Y. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53:113–128. [PubMed] [Google Scholar]
- 18.Zhao H, Tian L. On estimating medical cost and incremental cost-effectiveness ratios with censored data. Biometrics. 2001;57(4):1002–1008. doi: 10.1111/j.0006-341x.2001.01002.x. [DOI] [PubMed] [Google Scholar]
- 19.Carides GW, Heyse JF, Iglewicz B. A regression-based method for estimating mean treatment cost in the presence of right-censoring. Biostatistics. 2000;1(3):299–313. doi: 10.1093/biostatistics/1.3.299. [DOI] [PubMed] [Google Scholar]
- 20.Maniadakis N, Fragoulakis V, Pallis AG, Simou E, Georgoulias V. Economic evaluation of docetaxel-gemcitabine versus vinorelbine-cisplatin combination as front-line treatment of patients with advanced/metastatic non-small-cell lung cancer in Greece: a cost-minimization analysis. Ann Oncol. 2010;21(7):1462–1467. doi: 10.1093/annonc/mdp551. [DOI] [PubMed] [Google Scholar]
- 21.Raikou M, McGuire A. Estimating medical care costs under conditions of censoring. J Health Econ. 2004;23(3):443–470. doi: 10.1016/j.jhealeco.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12(10):933–980. doi: 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Bang H, Wang H, Pfeifer PE. On the equivalence of some medical cost estimators with censored data. Stat Med. 2007;26(24):4520–4530. doi: 10.1002/sim.2882. [DOI] [PubMed] [Google Scholar]
- 24.Lin DY. Linear regression analysis of censored medical costs. Biostatistics. 2000;1(1):35–47. doi: 10.1093/biostatistics/1.1.35. [DOI] [PubMed] [Google Scholar]
- 25.Lin DY. Proportional means regression for censored medical costs. Biometrics. 2000;56(3):775–778. doi: 10.1111/j.0006-341x.2000.00775.x. [DOI] [PubMed] [Google Scholar]
- 26.Lin DY. Regression analysis of incomplete medical cost data. Stat Med. 2003;22(7):1181–1200. doi: 10.1002/sim.1377. [DOI] [PubMed] [Google Scholar]
- 27.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372(9652):1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 28.Carlson JJ, Reyes C, Oestreicher N, Lubeck D, Ramsey SD, Veenstra DL. Comparative clinical and economic outcomes of treatments for refractory non-small cell lung cancer (NSCLC) Lung Cancer. 2008;61(3):405–415. doi: 10.1016/j.lungcan.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Maniadakis N, Fragoulakis V, Pallis A, Prezerakos P, Georgoulias V. Economic evaluation of docetaxel/gemcitabine versus docetaxel as frontline treatment of patients with advanced/metastatic non-small cell lung cancer in Greece. Lung Cancer. 2007;58(2):275–281. doi: 10.1016/j.lungcan.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Holmes J, Dunlop D, Hemmett L, Sharplin P, Bose U. A cost-effectiveness analysis of docetaxel in the second-line treatment of non-small cell lung cancer. Pharmacoeconomics. 2004;22(9):581–589. doi: 10.2165/00019053-200422090-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lewis G, Peake M, Aultman R, et al. Cost-effectiveness of erlotinib versus docetaxel for second-line treatment of advanced non-small-cell lung cancer in the United Kingdom. J Int Med Res. 2010;38(1):9–21. doi: 10.1177/147323001003800102. [DOI] [PubMed] [Google Scholar]
- 32.Lyseng-Williamson KA. Erlotinib: a pharmacoeconomic review of its use in advanced non-small cell lung cancer. Pharmacoeconomics. 2010;28(1):75–92. doi: 10.2165/10482880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Cromwell I, van der Hoek K, Melosky B, Peacock S. Erlotinib or docetaxel for second-line treatment of non-small cell lung cancer: a real-world cost-effectiveness analysis. J Thorac Oncol. 2011;6(12):2097–2103. doi: 10.1097/JTO.0b013e31822f657a. [DOI] [PubMed] [Google Scholar]
- 34.Nadler E, Forsyth M, Satram-Hoang S, Reyes C. Costs and clinical outcomes among patients with second-line non-small cell lung cancer in the outpatient community setting. J Thorac Oncol. 2012;7(1):212–218. doi: 10.1097/JTO.0b013e3182307f33. [DOI] [PubMed] [Google Scholar]
- 35.Araujo A, Parente B, Sotto-Mayor R, et al. An economic analysis of erlotinib, docetaxel, pemetrexed and best supportive care as second or third line treatment of non-small cell lung cancer. Rev Port Pneumol. 2008;14(6):803–827. [PubMed] [Google Scholar]
- 36.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist. 2009;14(3):253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 37.Pallis AG, Fennell DA, Szutowicz E, Leighl NB, Greillier L, Dziadziuszko R. Biomarkers of clinical benefit for anti-epidermal growth factor receptor agents in patients with non-small-cell lung cancer. Br J Cancer. 2011;105(1):1–8. doi: 10.1038/bjc.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]