Abstract
Introduction
Blood protein analysis of total tau (t-tau) may be a practical screening biomarker for chronic traumatic encephalopathy (CTE), a neurodegenerative tauopathy associated with repetitive head impact (RHI) exposure. We examined plasma t-tau in symptomatic former National Football League (NFL) players compared with controls and the relationship between RHI exposure and later-life plasma t-tau.
Methods
Ninety-six former NFL players (age 40–69) and 25 same-age controls underwent blood draw to determine plasma t-tau levels. The cumulative head impact index (CHII) quantified RHI exposure. Subjects completed measures of clinical function.
Results
A higher CHII predicted greater plasma t-tau in the former NFL players (P = .0137). No group differences in plasma t-tau emerged, but a concentration ≥3.56 pg/mL was 100% specific to former NFL players. Plasma t-tau did not predict clinical function.
Discussion
Greater RHI exposure predicted higher later-life plasma t-tau concentrations, and further study on plasma t-tau as a candidate screening biomarker for CTE is warranted.
Keywords: American football, Chronic traumatic encephalopathy, Repetitive head impacts, Tau, Total tau, Plasma, Head trauma
1. Introduction
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease only found in individuals with a history of exposure to repetitive head impacts (RHI), such as former American football players [1], [2]. The pathognomonic lesion of CTE is the perivascular deposition of hyperphosphorylated tau (p-tau) at the depths of the cortical sulci [3]. P-tau deposition is initially seen in frontotemporal brain regions, progresses to the medial temporal lobes (MTLs), and eventually becomes widespread. CTE presents with a constellation of cognitive, behavior, and mood deficits and, in some cases, motor signs [4]. Although CTE can only be diagnosed by neuropathological examination [3], clinical research diagnostic criteria have been proposed [5]. Yet, in vivo biomarkers that can detect the presence of CTE pathology during life have not yet been identified, precluding the ability to accurately diagnose CTE at this time. Biomarkers have become the gold standard in the diagnosis of neurodegenerative diseases, such as Alzheimer's disease (AD), and play a key role in understanding disease biology and determining therapeutic efficacy [6], [7], [8], [9]. A similar framework is being adopted for CTE [5].
A number of potential neuroimaging biomarkers for CTE have been identified (e.g., volumetric magnetic resonance imaging [MRI], diffusion-tensor imaging [DTI], MR spectroscopy) [5], but none of these methods assess the pathology underlying CTE, i.e., p-tau burden. Positron-emission tomography (PET) tau-specific radioligands have emerged as an optimal biomarker for detecting tauopathies, like AD [6], [10], with the expectation that PET imaging will serve as the gold standard diagnostic biomarker for CTE. The pragmatism of PET imaging, however, is problematic as it is expensive, time demanding, and involves exposure to radiation. Cerebrospinal fluid (CSF) protein markers of neurodegeneration (e.g., total tau [t-tau] and p-tau) are a practical alternative to PET imaging that is an accepted diagnostic tool in AD [6], [11], [12]. CSF protein analysis still requires a lumbar puncture, a procedure that is often viewed as invasive, and is feared by many patients.
The development of ultrasensitive blood immunoassays makes it now possible to detect low abundance proteins, such as tau, in the periphery. Blood analysis of tau protein is a time efficient, noninvasive, and reliable procedure, making it a candidate screening biomarker for neurodegenerative tauopathies, such as AD [11], [13], [14], [15], [16], [17]. Plasma exosomal tau has recently been proposed as a biomarker for CTE [18], but the techniques for isolation of brain-derived exosomes in blood are technically challenging and can lead to significant variability in the quality (e.g., purity, efficiency) of the extracted exosome [19]. Plasma tau is a more appealing option to the clinician and clinical researcher. Plasma assays of p-tau are still being developed and refined. However, plasma t-tau has been supported as a diagnostic tool for AD [6], [11], [20], [21], [22] but not without conflicting reports [23]. It has been theorized that significant axonal damage is required before peripheral increases in t-tau are observed in participants with AD [21].
Because tau is predominantly expressed in neuronal axons [24], plasma t-tau may be sensitive to the diffuse axonal injury that occurs during concussion [25] and RHI [26], [27], [28], [29], [30]. The utility of plasma t-tau in the setting of head trauma and RHI exposure is beginning to be explored. In a multicenter cohort study of professional Swedish ice hockey players [31], plasma t-tau concentrations were found to be elevated after a concussion and correlated with duration of postconcussion symptoms. Military personnel with a self-reported traumatic brain injury (TBI) also exhibited higher concentrations of plasma t-tau relative to controls, and plasma t-tau levels were higher for those with a medical record history of TBI and for those with three or more TBIs versus fewer than three TBIs [32]. Greater total postconcussive symptoms correlated with higher peripheral t-tau concentrations [32]. Regarding RHI, increases in plasma t-tau have been reported in a sample of 30 Olympic boxers immediately after a bout where there were no knock-outs [33], providing evidence for the acute effects of RHI on plasma t-tau levels. Exposure to RHI may also lead to chronic elevations in plasma t-tau levels given that RHI-related axonal abnormalities can persist over time [34], have been observed in former NFL players [34], [35], [36], [37], and are a common pathological feature in CTE [2].
The potential for plasma t-tau to serve as screening biomarker for CTE remains unknown partially because no study has examined the relationship between RHI exposure and later-life plasma t-tau concentrations. The objective of this study was to examine plasma t-tau concentrations in former NFL players presumably at risk for CTE, compared with same-age controls. In the former NFL group, we investigated the relationship between RHI exposure, using a previously reported cumulative head impact index (CHII) [38], and later-life plasma t-tau concentrations. This study additionally examined the association between plasma t-tau levels and performance on neuropsychological and behavioral/mood tests in the former NFL players. Optimal methodology for identifying a clinical biomarker involves inclusion of a sample with the clinical diagnosis of the disease of interest (suggesting a high probability of disease presence) [39]. Here, CTE is the target disease, but it cannot be diagnosed during life, and the extent of disease and if it is clinically present in the former NFL players are unknown. This is problematic given that significant axonal degeneration may be necessary for plasma t-tau to be elevated [21]. Therefore, one primary objective of this study was to identify a plasma t-tau concentration that had a high specificity to the former NFL group; a highly specific cutoff is one important criterion in the performance evaluation of a biomarker [39].
2. Methods
2.1. Participants
The original sample included 124 subjects (96 former NFL players and 28 same-aged controls) from a study examining in vivo biomarkers for CTE, entitled “Diagnosing and Evaluating Traumatic Encephalopathy Using Clinical Tests” (DETECT). Recruitment for DETECT began in 2011 and concluded in 2015. Inclusion criteria for the former NFL players included male, aged 40–69 years, a minimum of two seasons in the NFL and a minimum of 12 years of organized football, and had self-reported complaints of cognitive, behavioral, and/or mood symptoms at the time of telephone screen. Former NFL players must also not have had a history of concussion within one year before study entry. The same-age control group was required to have no history of participation in contact sports, service in the military, self-reported TBI or concussion, or cognitive, behavioral, and/or mood symptoms at telephone screen. Exclusion criteria for all participants included general MRI and/or lumbar puncture contraindications, presence of another central nervous system disease, and/or a primary language other than English. Participants enrolled in the DETECT study completed a single 2- to 3-day study visit, which involved administration of a battery of neuropsychological tests, neurological and psychiatric evaluations, blood draw, a history interview, and other examinations not relevant to the present study. Blood draw was completed on day 1, and neuropsychological testing was typically performed on day 3. All study protocols were approved by the Boston University Medical Center Institutional Review Board. Participants provided written informed consent before participation.
2.1.1. Sample size for present study
One former NFL player was not included in analyses that examined clinical test performance because of evidence of intentional poor effort (based on failure on multiple performance validity tests, neuropsychological scores at floor, and external evidence). This case, however, was included in analyses examining group differences in plasma t-tau and the association between RHI exposure and plasma t-tau. The sample of 28 controls was reduced to 25 after 3 were excluded because of a history of TBI (n = 2) and participation in football (n = 1), information that was not disclosed during the initial study screening. There were two controls with a history of youth soccer play and one who participated in youth amateur wrestling, but these participants were not excluded because of their brief participation in these sports and lack of concussion history. Of note, whereas contact sport history was an exclusion criterion for controls, a majority of the controls had a history of noncontact sport participation. Baseball (28.0%, n = 7) and swimming (52.0%, n = 13) were the most common primary sports played. Basketball (8.0%, n = 2) and crew (4.0%, n = 1) were additional primary sports played by controls.
2.2. Measures
2.2.1. Plasma t-tau
Nonfasting blood samples were collected with no significant differences between the groups in time of collection. Blood was collected into plastic dipotassium EDTA tubes and processed according to standard procedures, with plasma aliquoted and frozen at −80°C. Frozen plasma aliquots were shipped on dry ice to Quanterix (Lexington, MA, USA) where they were assayed in batch. Plasma t-tau concentrations were measured in duplicate from each sample with the Simoa HD-1 analyzer (Quanterix; Lexington) using human Tau kits (101444) at the Quanterix laboratories by Quanterix staff who were blind to group membership. All samples were detectable with an average coefficient of variation of 4%. This assay has been described elsewhere [22]. This assay uses a sandwich of two specific monoclonal antibodies, a capture antibody to the mid-domain of human tau and a detection antibody in the N-terminus.
2.2.2. Cumulative head impact index
The CHII was used to quantitate RHI exposure retrospectively in the former NFL players. A detailed description on the development of the CHII has been provided elsewhere [38]. The CHII is derived from self-reported football history (number of seasons played, position[s] played, levels played), and estimated head impact frequencies based on published helmet accelerometer studies. The CHII was originally developed in a sample of former youth, high school, and college football players, for which there are available helmet accelerometer studies to estimate frequency of head impacts. There are no helmet accelerometer studies at the professional football level; thus, college-level estimates of head impact frequencies were applied to the present sample for their professional football exposure. The CHII was computed for each former NFL player, and a higher CHII reflects greater exposure to RHI.
2.2.3. Neuropsychological and neuropsychiatric measures
All participants completed a comprehensive neuropsychological test battery that assessed the major cognitive domains (i.e., attention, executive function, verbal and visual episodic memory, language, visuospatial function) and semistructured interviews and self-report measures of neuropsychiatric function. A list of the tests administered as part of DETECT and the respective clinical domains assessed has been described previously [40]. Neuropsychological test raw scores were transformed to standard scores using normative data calibrated for age, gender, and/or education. As described in Alosco et al. [40], principal component analysis was performed to generate four clinical factor composite scores: behavioral/mood, psychomotor speed/executive function, verbal memory, and visual memory. These clinical composite scores were included in the present study.
2.3. Statistical analysis
Analysis of variance adjusting for age examined differences in plasma t-tau concentrations between the former NFL players and controls. Moses test of extreme reaction compared the range of plasma t-tau levels between the groups. Receiver-operating characteristic (ROC) curve was performed to identify the plasma t-tau concentration that resulted in 100% specificity for distinguishing the former NFL players from controls. Partial correlations controlling for age and body mass index (BMI) then examined the relationship between plasma t-tau levels and the CHII. BMI was included as a covariate because peripheral tau can be found in muscle [41]. A final set of partial correlations adjusting for age and BMI then determined the association between plasma t-tau and the psychomotor speed/executive function, verbal memory, visual memory, and behavior/mood factor composite scores among the former NFL players. Sample size for these analyses was reduced because of missing data on the neuropsychological and/or behavior/mood tests that make up the composites. For all analyses, the significance level was set at an alpha of 0.05.
3. Results
3.1. Plasma t-tau levels and ROC curve analyses
Table 1 shows demographic, athletic history, RHI exposure variables, and clinical characteristics, for the former NFL players and controls. In the full sample, bivariate correlations showed that plasma t-tau was not associated with age (P = .395), years of education (P = .635), or BMI (P = .099). Mann-Whitney U test also showed no significant difference between Caucasians and African Americans for plasma t-tau concentrations (P = .152). The mean (standard deviation [SD]) plasma t-tau in former NFL players was 2.53 (1.01) pg/mL, whereas for controls it was 2.46 (0.57) pg/mL, a nonsignificant difference after controlling for age (t = −0.42, P = .6719). However, the Moses test showed that the former NFL players exhibited a significantly greater range in plasma t-tau levels compared with controls, with a tendency for the former NFL players to have higher plasma t-tau levels in the upper end of the range (range for former NFL players: 0.77–6.19, range for controls: 1.35–3.46, P = .042). A plasma t-tau concentration of 3.56 pg/mL was 100% specific to the former NFL players (sensitivity = 12.50%). Twelve of the former NFL players had a plasma t-tau concentration ≥3.56, and there were no controls with this plasma t-tau level (see Fig. 1). In fact, only five controls had a plasma t-tau >3.0 pg/mL. Lineman (offensive and defensive) had higher plasma t-tau levels relative to nonlinemen (t(94) = 2.58, P = .011).
Table 1.
Sample characteristics
| NFL (n = 96) | Control (n = 25) | P-value | |
|---|---|---|---|
| Age, mean (SD) years | 55.16 (7.95) | 57.04 (6.63) | .278 |
| Education, mean (SD) years | 16.41 (0.97) | 17.32 (2.10) | .043 |
| African American∗, n (%) (N = 95 for NFL) | 42 (44.2) | 1 (4.0) | <.001 |
| Duration of football play, mean (SD) years | 18.23 (3.48) | — | — |
| CHII, mean (SD) | 20,326 (6936.72) | — | — |
| Years since retirement∗, mean (SD) (N = 94) | 26.49 (8.94) | — | — |
| Primary position group, n (%) | — | — | |
| Offensive line | 28 (29.2) | — | — |
| Running back | 8 (8.3) | — | — |
| Tight end | 5 (5.2) | — | — |
| Offensive skill | 1 (1.0) | — | — |
| Defensive line | 15 (15.6) | — | — |
| Linebacker | 21 (21.9) | — | — |
| Defensive back | 18 (18.8) | — | — |
| Body mass index, mean (SD), kg/m2 | 33.38 (5.08) | 27.94 (3.77) | <.001 |
Abbreviations: CHII, cumulative head impact index; SD, standard deviation.
Sample size reduced because of missing data. Years since retirement was calculated through a subtraction of retirement year from year of study examination.
Fig. 1.
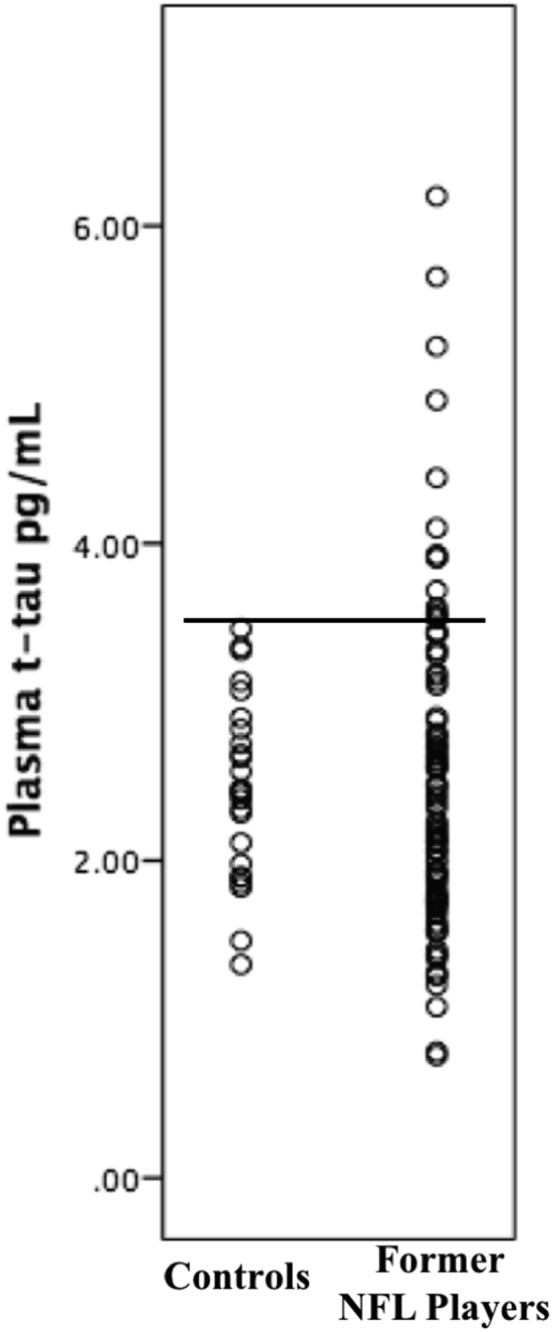
Distribution of plasma total tau (t-tau) in former NFL players. There are no group differences in mean plasma t-tau. There is a significant difference in the range of plasma t-tau values (P = .042), and plasma t-tau levels above the black bolded line reflect those 100% specific to the former NFL players, which corresponds to ≥3.56 pg/mL. Y-axis values are plasma t-tau concentrations in pg/mL units.
3.2. Plasma t-tau, RHI exposure, and clinical test performance
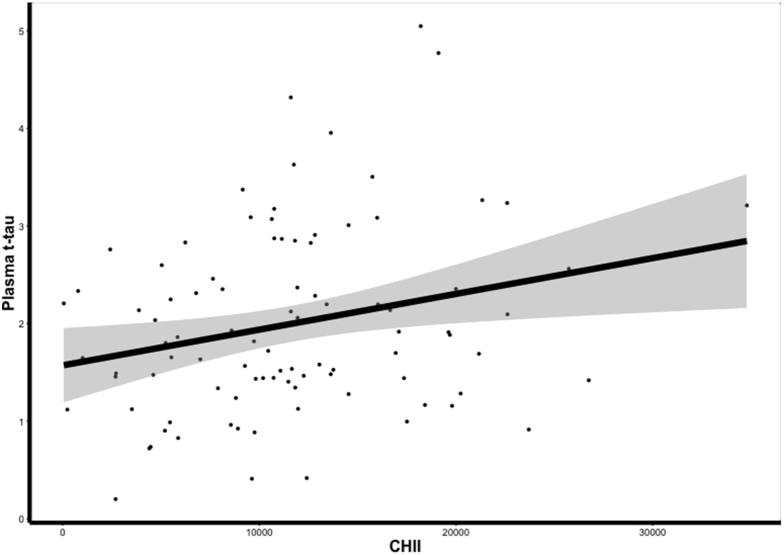
The mean CHII was 20.326 (SD = 6936.72). After controlling for age and BMI, there was a significant correlation between plasma t-tau levels and the CHII (r = 0.25, P = .0137) in the former NFL players; there was no significant association with years of football played (r = 0.12, P = .2656). Greater exposure to RHI was associated with higher concentrations of plasma t-tau (see Fig. 2). The 12 former NFL players with a plasma t-tau concentration ≥3.56 pg/mL had a higher CHII (F (3,92) = 5.767, P = .018; mean [SD] = 25,338.48 [7968.51] versus 19,610.87 [6519.94]), after controlling for age and BMI. Plasma t-tau levels were not related to any of the clinical factor composite scores (P > .10 for all).
Fig. 2.
Greater exposure to repetitive head impacts is associated with higher later-life concentrations in plasma total tau (t-tau). The scatter plot depicts the relationship of the residuals between the cumulative head impact index (CHII) and plasma t-tau after controlling for age and body mass index, which was significant (P = .0137). Because the residuals resulted in negative values on the x-axis, a constant value was added to y- and x-axis values to facilitate interpretation. X-axis values are scores on the CHII, with higher scores representing greater exposure to repetitive head impact. Y-axis values are plasma t-tau concentrations in pg/mL units. Shaded region represents standard error.
4. Discussion
The present study found that greater exposure to RHI correlated with higher levels of later-life plasma t-tau concentrations in a sample of 96 former NFL players. Although there were no differences in plasma t-tau levels between the former NFL players and same-age controls, the former NFL players exhibited more extreme plasma t-tau concentrations, with 12 having a plasma t-tau level ≥3.56 pg/mL (highest concentration of 6.19 pg/mL). No control subject had a plasma t-tau level ≥3.56 pg/mL, making this concentration 100% specific to the former NFL players. Former NFL players with a plasma t-tau concentration ≥3.56 pg/mL also had significantly greater exposure to RHI compared with former NFL players with plasma t-tau levels below 3.56 pg/mL. Although 3.56 pg/mL had poor sensitivity (12.5%), this is not unexpected given sensitivity is sacrificed when specificity is high. Recent diagnostic guidelines for AD emphasize the need for incorporation of high specificity in the development of biomarkers [42]. Regardless, an ideal biomarker has both high sensitivity and specificity [39]. A highly specific biomarker should be used in combination with sensitive biomarkers to maximize disease detection and differentiation from aging and similar neurological conditions.
The present association between greater RHI exposure and higher later-life plasma t-tau concentrations extends research linking sports-related concussion [31], [43] and RHI [32], [33] with acute elevations in plasma t-tau concentrations. Both acute and chronic elevations in plasma t-tau after exposure to RHI may be related to white matter changes. Tau is a protein highly expressed in neuronal unmyelinated cortical axons, where it facilitates the stability of microtubules [24], [44]. T-tau is elevated in the plasma after a concussion, potentially a consequence of diffuse axonal injury that occurs during this injury [25]. In fact, plasma t-tau levels may be useful in monitoring concussion recovery, as it is correlated with duration of postconcussion symptoms [31]. RHI may aggravate diffuse axonal injury and prevent recovery [45]. Exposure to RHI has been associated with acute white matter abnormalities that persist over time [26], [27], [28], [29], [30], [34], [35], [36], [37] and have been observed in former NFL players [35]. In addition to chronic axonal injury, RHI-related elevations in later-life plasma t-tau may also be capturing axonal degeneration and neuronal loss related to a neurodegenerative disease, such as CTE. RHI exposure is a necessary risk factor for CTE [1], [2], [3], and CTE is characterized by an abnormal perivascular accumulation of p-tau in neurons and astroglia at the depths of the cortical sulci [2], [3]. P-tau disperses throughout the cortex with disease progression and extends into the MTL, diencephalon, and brainstem. Beta-amyloid deposition is found in 52% of CTE cases, tends to be seen as diffuse (and not neuritic) plaques, and is associated with the presence of the ε4 allele of the APOE gene and age [2], [46]. White matter microstructural changes are also a common pathological feature of CTE. Unfortunately, there is no way to discern whether elevations in plasma t-tau in this sample are secondary to chronic axonopathy from recurrent head trauma or a neurodegenerative process. T-tau is also a general marker of cortical axonal damage that has limited diagnostic use in differentiating among different tauopathies (e.g., AD, frontotemporal lobar degeneration) and pathology other than neurodegenerative tauopathies (e.g., cerebrovascular disease) [47], [48]. P-tau antibodies are more specific to tauopathies [48], and once plasma assays of p-tau are refined, examining plasma p-tau (in isolation and in combination with plasma amyloid and t-tau) and RHI exposure will improve understanding on how plasma t-tau relates to CTE.
In the context of the association between RHI exposure and later-life plasma t-tau, the overall lack of plasma t-tau differences between the former NFL players and controls could be related to below threshold levels of pathology in the former NFL group. Axonal degeneration in this sample of former NFL players may not have been severe enough to result in elevated concentrations of peripheral t-tau. This claim is supported by research in AD. Participants with MCI do not exhibit significantly higher levels of plasma t-tau compared with controls, whereas plasma t-tau concentrations are elevated in AD dementia [21], [22]. But, even in AD dementia, the range of plasma t-tau levels overlap with those in MCI and normal cognition groups [21]. These findings have been interpreted as plasma t-tau being a late marker of AD, with substantial axonal degeneration required before reaching abnormal concentrations [21]. The presence of CTE neuropathology in the current sample of former NFL players is unknown, and the extent of disease may be minimal and certainly below clinical threshold because of the lack of association between plasma t-tau and clinical tests. Although exposure to RHI is believed to be necessary for the development of CTE [1], [2], it is likely not sufficient because not all individuals exposed to RHI develop later-life clinical impairments. It is certainly possible that many participants in this sample may be without meaningful CTE neuropathology, potentially contributing to the null group effects. Clinical function may also be more sensitive to p-tau burden, particularly in the early stages of disease [49]. Interestingly, however, plasma t-tau concentrations ≥3.56 pg/mL was specific to the former NFL group. This value approaches (but is still lower) than plasma t-tau concentrations reported in studies among participants with MCI (e.g., 4.34 and 4.68 pg/mL) that used the same Quanterix Simoa-HD1 tau assay platform as in our study [21], [22]. It could be speculated that the former NFL players with a plasma t-tau concentration ≥3.56 pg/mL may be in the early stages of a neurodegenerative process, which could be CTE given the greater history of RHI exposure in this subset.
Because a clinical diagnosis of CTE cannot be made at this time, conclusions regarding plasma t-tau as a biomarker for CTE cannot be made. Identifying a specific cutoff is a necessary step in establishing a clinical biomarker [39], but only 12 of the former NFL players had concentrations ≥3.56 pg/mL. Once CTE can be diagnosed in vivo using gold standard biomarkers of p-tau burden (i.e., PET imaging), it will permit the opportunity to recruit a sample with clinically diagnosed CTE, thereby allowing for determination of the sensitivity and specificity of plasma t-tau to CTE relative to normal controls and other neurodegenerative diseases. It will also allow for determination of disease severity, permitting an empirical test of our hypothesis that the lack of differences in plasma t-tau between the former NFL players and controls in this sample was because of below threshold levels of pathology. Ultimately, plasma t-tau as a screening biomarker for CTE will only be confirmed once antemortem plasma t-tau is correlated with postmortem CTE pathology. These data likely would not be available in the short term. Future work could improve understanding on the clinical research utility of plasma t-tau by correlating it with other proposed biomarkers of CTE, like PET imaging of p-tau burden and MRI-DTI, and with provisional clinical research diagnostic criteria for CTE [5].
Several additional shortcomings of the present study deserve attention. We examined plasma t-tau in isolation and analysis of a comprehensive panel of plasma proteins (e.g., amyloid beta, different tau isoforms, apoE, interleukin 16, neurofilament proteins) has been shown to yield better diagnostic accuracy, including distinguishing prodromal AD from dementia [50], [51]. The present study was cross-sectional. Longitudinal research in participants at high risk for CTE that implement neuroimaging modalities that can track disease progression (e.g., PET imaging) will clarify how plasma t-tau changes with disease progression and when disease reaches threshold to increase t-tau in the blood. The correspondence between blood analytes and pathological changes in the brain remains unknown, emphasizing the need for clinicopathological correlation studies. The CHII retrospectively estimated RHI exposure. Head impact frequencies is one variable that comprises the CHII that is based on helmet accelerometer studies among college football players [38]. The CHII was developed in football players that played in a more modern era relative to the current sample. The number of head impacts and their association with plasma t-tau may have been underestimated in this sample. The present sample included a relatively demographically homogeneous sample of former professional football players, limiting the generalizability of our findings to amateur football players, other contact sport athletes, and more broadly, the general population.
In conclusion, greater exposure to RHI correlated with higher later-life plasma t-tau concentrations in this sample of former NFL players. Blood analysis of t-tau is relatively inexpensive and noninvasive, making it a potentially useful approach for screening for the presence of disease in the clinic and research setting. However, further study is needed to determine plasma t-tau as a candidate screening biomarker for CTE, including repeating the present study in a sample clinically diagnosed with CTE (once possible) and research that examines plasma t-tau in conjunction with other plasma proteins in subjects at high risk for CTE, longitudinal examinations, and studies that correlate plasma t-tau with PET imaging of tau.
Research in Context.
-
1.
Systematic Review: The authors reviewed the literature using PubMed and references of research articles. No study has examined the association between exposure to repetitive head impacts (RHI) and later-life plasma total tau (t-tau) levels. However, plasma t-tau has diagnostic utility in Alzheimer's disease, and RHI has been linked with acute elevations in plasma t-tau. These studies are appropriately cited.
-
2.
Interpretation: Blood protein analysis of t-tau is inexpensive and noninvasive and may be able to detect later-life pathological changes associated with RHI exposure.
-
3.
Future directions: To determine whether plasma t-tau can be a screening biomarker for chronic traumatic encephalopathy (CTE), future work should 1) repeat this study once CTE can be clinically diagnosed, 2) examine the association between plasma t-tau with other proposed biomarkers of CTE (e.g., positron-emission tomography tau imaging), 3) conduct longitudinal examinations, and 4) investigate the correlation between antemortem plasma t-tau and postmortem CTE pathology.
Acknowledgments
Sources of funding: This work was supported by grants from the National Institutes of Health (NIH, R01 NS 078337, R56 9500304025, U01 NS093334). This publication was also supported by the National Center for Advancing Translational Sciences, NIH, through BU-CTSI grant number 1UL1TR001430. Michael L. Alosco and research reported in this publication is supported by the NIH under grant number 1F32NS096803-01. Christine Baugh is currently supported by the National Institutes of Mental Health under award number T32MH019733. The plasma total tau assays were performed at Quanterix (Lexington, MA, USA) at no cost. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. There is no sponsor. Conflicts of interest: Robert A. Stern has received research funding from Avid Radiopharmaceuticals, Inc. (Philadelphia, PA, USA). He is a member of the Mackey-White Committee of the NFL Players Association and is a paid member of the Medical Science Committee for the National Collegiate Athletic Association (NCAA) Student-Athlete Concussion Injury Litigation. He is a paid consultant to Avanir Pharmaceuticals, Inc. (Aliso Viejo, CA, USA) and Biogen (Cambridge, MA, USA). He receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. (Lutz, FL, USA) and compensation from expert legal opinion. Robert C. Cantu is a paid consultant to the NFL Head Neck and Spine Committee and National Operating Committee on Standards for Athletic Equipment. He is a member of the Mackey-White Committee of the NFL Players Association and is a paid member of the Medical Science Committee for the NCAA Student-Athlete Concussion Injury Litigation. He receives royalties from book publications and compensation from expert legal opinion. Christine Baugh has received research funding from the National Collegiate Athletic Association and the Harvard Football Players Health Study that is funded by the National Football League Players' Association. Andreas Jeromin, Nate Estochen, and Linan Song are employed by Quanterix. Andreas Jermoin is an advisor to Quanterix Corporation and holds stock options. For the remaining authors, there are no conflicts of interest to declare.
References
- 1.Bieniek K.F., Ross O.A., Cormier K.A., Walton R.L., Soto-Ortolaza A., Johnston A.E. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130:877–889. doi: 10.1007/s00401-015-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee A.C., Cairns N.J., Dickson D.W., Folkerth R.D., Keene C.D., Litvan I. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern R.A., Daneshvar D.H., Baugh C.M., Seichepine D.R., Montenigro P.H., Riley D.O. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montenigro P.H., Baugh C.M., Daneshvar D.H., Mez J., Budson A.E., Au R. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6:68. doi: 10.1186/s13195-014-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Feldman H.H., Frisoni G.B. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattsson N., Carrillo M.C., Dean R.A., Devous M.D., Sr., Nikolcheva T., Pesini P. Revolutionizing Alzheimer's disease and clinical trials through biomarkers. Alzheimers Dement (Amst) 2015;1:412–419. doi: 10.1016/j.dadm.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vellas B., Carrillo M.C., Sampaio C., Brashear H.R., Siemers E., Hampel H. Designing drug trials for Alzheimer's disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013;9:438–444. doi: 10.1016/j.jalz.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson K.A., Schultz A., Betensky R.A., Becker J.A., Sepulcre J., Rentz D. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsson B., Lautner R., Andreasson U., Ohrfelt A., Portelius E., Bjerke M. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 12.Kang J.H., Korecka M., Figurski M.J., Toledo J.B., Blennow K., Zetterberg H. The Alzheimer's Disease Neuroimaging Initiative 2 Biomarker Core: A review of progress and plans. Alzheimers Dement. 2015;11:772–791. doi: 10.1016/j.jalz.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apostolova L.G., Hwang K.S., Avila D., Elashoff D., Kohannim O., Teng E. Brain amyloidosis ascertainment from cognitive, imaging, and peripheral blood protein measures. Neurology. 2015;84:729–737. doi: 10.1212/WNL.0000000000001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olazaran J., Gil-de-Gomez L., Rodriguez-Martin A., Valenti-Soler M., Frades-Payo B., Marin-Munoz J. A blood-based, 7-metabolite signature for the early diagnosis of Alzheimer's disease. J Alzheimers Dis. 2015;45:1157–1173. doi: 10.3233/JAD-142925. [DOI] [PubMed] [Google Scholar]
- 15.Wang G., Zhou Y., Huang F.J., Tang H.D., Xu X.H., Liu J.J. Plasma metabolite profiles of Alzheimer's disease and mild cognitive impairment. J Proteome Res. 2014;13:2649–2658. doi: 10.1021/pr5000895. [DOI] [PubMed] [Google Scholar]
- 16.Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Cedarbaum J. 2014 Update of the Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2015;11:e1–e120. doi: 10.1016/j.jalz.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doecke J.D., Laws S.M., Faux N.G., Wilson W., Burnham S.C., Lam C.P. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012;69:1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern R.A., Tripodis Y., Baugh C.M., Fritts N.G., Martin B.M., Chaisson C. Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy. J Alzheimers Dis. 2016;51:1099–1109. doi: 10.3233/JAD-151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor D.D., Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Chiu M.J., Chen Y.F., Chen T.F., Yang S.Y., Yang F.P., Tseng T.W. Plasma tau as a window to the brain-negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer's disease. Hum Brain Mapp. 2014;35:3132–3142. doi: 10.1002/hbm.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zetterberg H., Wilson D., Andreasson U., Minthon L., Blennow K., Randall J. Plasma tau levels in Alzheimer's disease. Alzheimers Res Ther. 2013;5:9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dage J.L., Wennberg A.M., Airey D.C., Hagen C.E., Knopman D.S., Machulda M.M. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016;12:1226–1234. doi: 10.1016/j.jalz.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shanthi K.B., Krishnan S., Rani P. A systematic review and meta-analysis of plasma amyloid 1-42 and tau as biomarkers for Alzheimer's disease. SAGE Open Med. 2015;3 doi: 10.1177/2050312115598250. 2050312115598250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trojanowski J.Q., Schuck T., Schmidt M.L., Lee V.M. Distribution of tau proteins in the normal human central and peripheral nervous system. J Histochem Cytochem. 1989;37:209–215. doi: 10.1177/37.2.2492045. [DOI] [PubMed] [Google Scholar]
- 25.Giza C.C., Hovda D.A. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75 Suppl 4:S24–S33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chappell M.H., Ulug A.M., Zhang L., Heitger M.H., Jordan B.D., Zimmerman R.D. Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study. J Magn Reson Imaging. 2006;24:537–542. doi: 10.1002/jmri.20656. [DOI] [PubMed] [Google Scholar]
- 27.Koerte I.K., Ertl-Wagner B., Reiser M., Zafonte R., Shenton M.E. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA. 2012;308:1859–1861. doi: 10.1001/jama.2012.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipton M.L., Kim N., Zimmerman M.E., Kim M., Stewart W.F., Branch C.A. Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology. 2013;268:850–857. doi: 10.1148/radiol.13130545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merchant-Borna K., Asselin P., Narayan D., Abar B., Jones C.M., Bazarian J.J. Novel Method of Weighting Cumulative Helmet Impacts Improves Correlation with Brain White Matter Changes After One Football Season of Sub-concussive Head Blows. Ann Biomed Eng. 2016;44:3679–3692. doi: 10.1007/s10439-016-1680-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L., Ravdin L.D., Relkin N., Zimmerman R.D., Jordan B., Lathan W.E. Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? AJNR Am J Neuroradiol. 2003;24:52–57. [PMC free article] [PubMed] [Google Scholar]
- 31.Shahim P., Tegner Y., Wilson D.H., Randall J., Skillback T., Pazooki D. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014;71:684–692. doi: 10.1001/jamaneurol.2014.367. [DOI] [PubMed] [Google Scholar]
- 32.Olivera A., Lejbman N., Jeromin A., French L.M., Kim H.S., Cashion A. Peripheral Total Tau in Military Personnel Who Sustain Traumatic Brain Injuries During Deployment. JAMA Neurol. 2015;72:1109–1116. doi: 10.1001/jamaneurol.2015.1383. [DOI] [PubMed] [Google Scholar]
- 33.Neselius S., Zetterberg H., Blennow K., Randall J., Wilson D., Marcusson J. Olympic boxing is associated with elevated levels of the neuronal protein tau in plasma. Brain Inj. 2013;27:425–433. doi: 10.3109/02699052.2012.750752. [DOI] [PubMed] [Google Scholar]
- 34.Bazarian J.J., Zhu T., Zhong J., Janigro D., Rozen E., Roberts A. Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PLoS One. 2014;9:e94734. doi: 10.1371/journal.pone.0094734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Multani N., Goswami R., Khodadadi M., Ebraheem A., Davis K.D., Tator C.H. The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J Neurol. 2016;263:1332–1341. doi: 10.1007/s00415-016-8141-0. [DOI] [PubMed] [Google Scholar]
- 36.Strain J., Didehbani N., Cullum C.M., Mansinghani S., Conover H., Kraut M.A. Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology. 2013;81:25–32. doi: 10.1212/WNL.0b013e318299ccf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strain J.F., Didehbani N., Spence J., Conover H., Bartz E.K., Mansinghani S. White Matter Changes and Confrontation Naming in Retired Aging National Football League Athletes. J Neurotrauma. 2016 doi: 10.1089/neu.2016.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montenigro P.H., Alosco M.L., Martin B.M., Daneshvar D.H., Mez J., Chaisson C.E. Cumulative Head Impact Exposure Predicts Later-Life Depression, Apathy, Executive Dysfunction, and Cognitive Impairment in Former High School and College Football Players. J Neurotrauma. 2016 doi: 10.1089/neu.2016.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Humpel C. Identifying and validating biomarkers for Alzheimer's disease. Trends Biotechnol. 2011;29:26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alosco M.L., Jarnagin J., Tripodis Y., Platt M., Martin B., Chaisson C.E. Olfactory Function and Associated Clinical Correlates in Former National Football League Players. J Neurotrauma. 2016 doi: 10.1089/neu.2016.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lubke U., Six J., Villanova M., Boons J., Vandermeeren M., Ceuterick C. Microtubule-associated protein tau epitopes are present in fiber lesions in diverse muscle disorders. Am J Pathol. 1994;145:175–188. [PMC free article] [PubMed] [Google Scholar]
- 42.Jack C.R., Jr., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier T.B., Bergamino M., Bellgowan P.S., Teague T.K., Ling J.M., Jeromin A. Longitudinal assessment of white matter abnormalities following sports-related concussion. Hum Brain Mapp. 2016;37:833–845. doi: 10.1002/hbm.23072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gendron T.F., Petrucelli L. The role of tau in neurodegeneration. Mol Neurodegener. 2009;4:13. doi: 10.1186/1750-1326-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stein T.D., Montenigro P.H., Alvarez V.E., Xia W., Crary J.F., Tripodis Y. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015;130:21–34. doi: 10.1007/s00401-015-1435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hesse C., Rosengren L., Andreasen N., Davidsson P., Vanderstichele H., Vanmechelen E. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–190. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- 48.Hampel H., Blennow K., Shaw L.M., Hoessler Y.C., Zetterberg H., Trojanowski J.Q. Total and phosphorylated tau protein as biological markers of Alzheimer's disease. Exp Gerontol. 2010;45:30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glodzik L., de Santi S., Tsui W.H., Mosconi L., Zinkowski R., Pirraglia E. Phosphorylated tau 231, memory decline and medial temporal atrophy in normal elders. Neurobiol Aging. 2011;32:2131–2141. doi: 10.1016/j.neurobiolaging.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiu M.J., Yang S.Y., Horng H.E., Yang C.C., Chen T.F., Chieh J.J. Combined plasma biomarkers for diagnosing mild cognition impairment and Alzheimer's disease. ACS Chem Neurosci. 2013;4:1530–1536. doi: 10.1021/cn400129p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo L.H., Alexopoulos P., Wagenpfeil S., Kurz A., Perneczky R. Alzheimer's Disease Neuroimaging I, Plasma proteomics for the identification of Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27:337–342. doi: 10.1097/WAD.0b013e31827b60d2. [DOI] [PMC free article] [PubMed] [Google Scholar]