Abstract
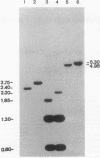
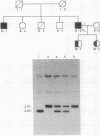
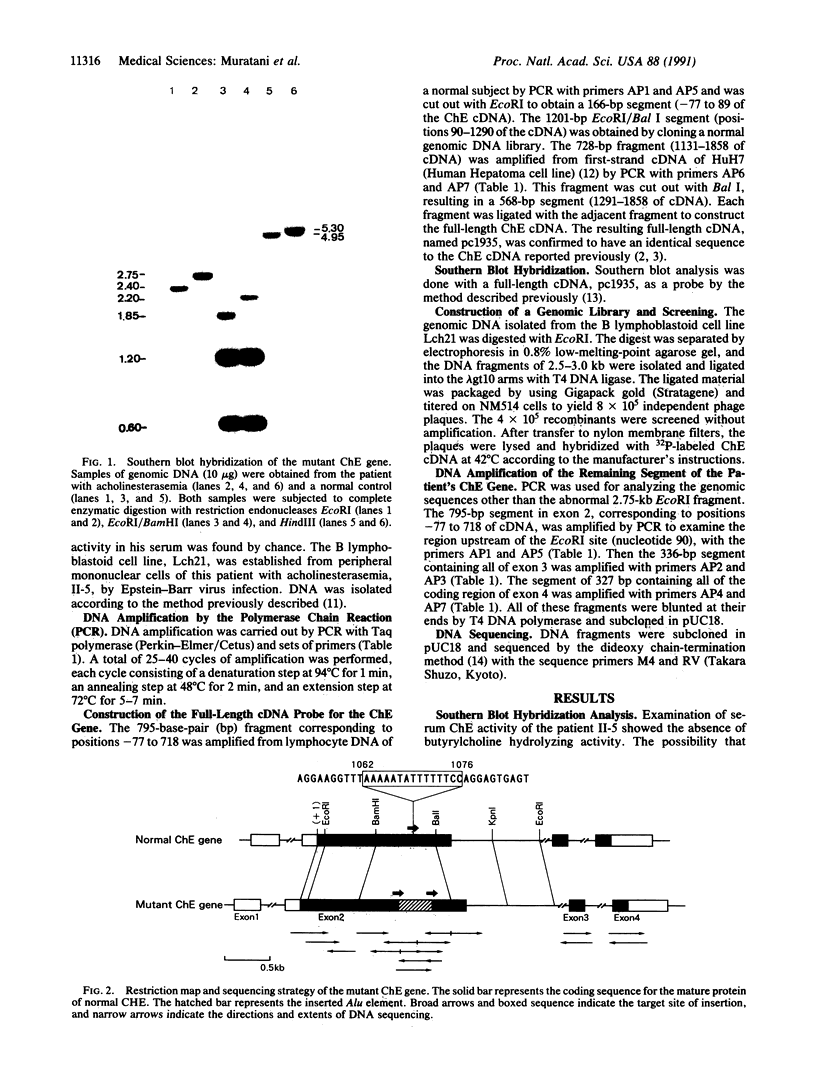
The human cholinesterase (ChE) gene from a patient with acholinesterasemia was cloned and analyzed. By using ChE cDNA as a probe, four independent clones were isolated from a genomic library constructed from the patient's DNA. Sequencing analysis of all of the four clones revealed that exon 2 of the ChE gene was disrupted by a 342-base-pair (bp) insertion of Alu element, including a poly(A) tract of 38 bp, which showed 93% sequence homology with a current type of human Alu consensus sequence. Southern blot analysis showed that the Alu insertion occurred in both alleles of the patient and was inherited in the patient's family. This Alu insertion was flanked by 15-bp of target site duplication in exon 2 corresponding to positions 1062-1076 of ChE cDNA, indicating that an Alu element could have been integrated by retrotransposition. Thus, this case provides an important clue to the mechanism of inactivation of a gene by integration of a retrotransposon.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arpagaus M., Kott M., Vatsis K. P., Bartels C. F., La Du B. N., Lockridge O. Structure of the gene for human butyrylcholinesterase. Evidence for a single copy. Biochemistry. 1990 Jan 9;29(1):124–131. doi: 10.1021/bi00453a015. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Baron W. F., Stout D. B., Davidson E. H. Sources and evolution of human Alu repeated sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4770–4774. doi: 10.1073/pnas.85.13.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Wong C., Youssoufian H., Scott A. F., Phillips D. G., Antonarakis S. E. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature. 1988 Mar 10;332(6160):164–166. doi: 10.1038/332164a0. [DOI] [PubMed] [Google Scholar]
- Krolewski J. J., Rush M. G. Some extrachromosomal circular DNAs containing the Alu family of dispersed repetitive sequences may be reverse transcripts. J Mol Biol. 1984 Mar 25;174(1):31–40. doi: 10.1016/0022-2836(84)90363-2. [DOI] [PubMed] [Google Scholar]
- Kunisada T., Yamagishi H. Sequence organization of repetitive sequences enriched in small polydisperse circular DNAs from HeLa cells. J Mol Biol. 1987 Dec 20;198(4):557–565. doi: 10.1016/0022-2836(87)90199-9. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Goldthwait D. A., Samols D. Identification of Alu transposition in human lung carcinoma cells. Cell. 1988 Jul 15;54(2):153–159. doi: 10.1016/0092-8674(88)90547-8. [DOI] [PubMed] [Google Scholar]
- McGuire M. C., Nogueira C. P., Bartels C. F., Lightstone H., Hajra A., Van der Spek A. F., Lockridge O., La Du B. N. Identification of the structural mutation responsible for the dibucaine-resistant (atypical) variant form of human serum cholinesterase. Proc Natl Acad Sci U S A. 1989 Feb;86(3):953–957. doi: 10.1073/pnas.86.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTiernan C., Adkins S., Chatonnet A., Vaughan T. A., Bartels C. F., Kott M., Rosenberry T. L., La Du B. N., Lockridge O. Brain cDNA clone for human cholinesterase. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6682–6686. doi: 10.1073/pnas.84.19.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Nogueira C. P., McGuire M. C., Graeser C., Bartels C. F., Arpagaus M., Van der Spek A. F., Lightstone H., Lockridge O., La Du B. N. Identification of a frameshift mutation responsible for the silent phenotype of human serum cholinesterase, Gly 117 (GGT----GGAG). Am J Hum Genet. 1990 May;46(5):934–942. [PMC free article] [PubMed] [Google Scholar]
- Prody C. A., Zevin-Sonkin D., Gnatt A., Goldberg O., Soreq H. Isolation and characterization of full-length cDNA clones coding for cholinesterase from fetal human tissues. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3555–3559. doi: 10.1073/pnas.84.11.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Haber J. E., Botstein D. Lethal disruption of the yeast actin gene by integrative DNA transformation. Science. 1982 Jul 23;217(4557):371–373. doi: 10.1126/science.7046050. [DOI] [PubMed] [Google Scholar]
- Singer M. F. SINEs and LINEs: highly repeated short and long interspersed sequences in mammalian genomes. Cell. 1982 Mar;28(3):433–434. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- Soreq H., Zamir R., Zevin-Sonkin D., Zakut H. Human cholinesterase genes localized by hybridization to chromosomes 3 and 16. Hum Genet. 1987 Dec;77(4):325–328. doi: 10.1007/BF00291419. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]